Abstract
Angiogenesis, the development of new blood vessels from existing ones, is a critical process in wound healing and skeletal muscle hypertrophy. It also leads to pathological conditions such as retinopathy and tumor genesis. Metformin, the first-line treatment for type 2 diabetic mellitus, has a specific regulatory effect on the process of angiogenesis. Anti-angiogenesis can inhibit the occurrence and metastasis of tumors and alleviate patients’ symptoms with polycystic ovary syndrome. Moreover, promoting angiogenesis effect can accelerate wound healing and promote stroke recovery and limb ischemia reconstruction. This review reorganizes metformin in angiogenesis, and the underlying mechanism in alleviating disease to bring some inspiration to relevant researchers.
Keywords: Metformin, angiogenesis, anti-angiogenesis
Introduction
Diabetes is an incurable common chronic disease, and there are over 100 million diabetes cases in China, the highest in the world.1 As the first-line treatment for type 2 diabetic mellitus (T2DM), the endless emergence of metformin’s relevant studies has shown that it repeatedly distinguished itself in other disease therapy such as anti-cancer, anti-aging, weight-losing, and other areas of the human incomparably expectations. It is almost neck and neck with the previous generation of miracle drug, aspirin, absolutely worthy of the title of a new generation of the miracle drug.
Metformin is a type of medicine associated with a traditional medicinal plant, lilacs, also known as goat’s rue used by the French to treat polyuria since the Middle Ages. The main active ingredient of lilacs, guanidine, was shown to lower blood glucose in 1918. After the 1920s, some guanidine derivatives were compounded by chemical methods. Then, it started to treat diabetes. However, due to lactic acid toxicity, especially after insulin, the clinical application of the guanidine derivatives was suspended. After the 1940s, metformin anti-malarial function drew the researchers’ attention, and some people also tried to use metformin to treat the flu.2 Obviously, both of the two quite exciting functions of metformin were not confirmed. But its hypoglycemic position and treating non-insulin-dependent diabetes passed formal clinical trials and was approved by the US Food and Drug Administration (FDA) in 1995.3
Metformin has been found to have other therapeutic effects other than its hypoglycemic effects, including, but not limited to, inhibiting or delaying certain cancers, treating polycystic ovarian disease, improving blood vessel dilation, and reducing inflammation. However, there is a lack of information about the role of metformin in regulating angiogenesis. This review reorganizes the potential role of metformin in angiogenesis to inspire those interested in it.
The role of metformin in anti-angiogenesis
The anti-tumor effect of metformin via anti-angiogenesis
Angiogenesis refers to the physiological process of new blood vessels forming from the existing ones. It is a complicated and orderly process, which needs pro-angiogenesis factors and inhibitors coordinating with each other. In normal circumstances, they are in equilibrium. Once the balance is disturbed, the vascular system will be activated, resulting in excessive angiogenesis or vascular degradation caused by inhibiting the vascular system.
On account of the abnormal structure and function of the new blood vessels in tumor tissues and imperfect vascular matrix, these microvessels are prone to leakage. Therefore, tumor cells do not need to go through a complex invasion process to penetrate blood vessels directly into the bloodstream and form metastasis at a distance. More and more studies have shown that angiogenesis is slow and sparse in benign tumors. In contrast, most malignancies have dense angiogenesis and rapid growth. Therefore, angiogenesis plays a critical role in the occurrence and metastasis of tumors, and inhibition of this process may significantly prevent tumor growth and metastasis.4
Recent studies have shown that metformin has a significant inhibitory effect on tumor angiogenesis. However, the potential mechanism of this anti-angiogenic effect remains a mystery. In this part, we collected some reports on metformin inhibiting tumor angiogenesis in vitro and in vivo. The pathological feature of metastatic breast cancer is angiogenesis and abnormal blood vessel morphology. Wang et al.5 found that for metastatic breast cancer models, metformin can significantly reduce the microvascular density (MVD), enhance vascular normalization, inhibit tumor angiogenesis, and the downregulation of platelet-derived growth factor B (PDGF-B) plays a crucial role in this process. Metformin reduces pulmonary metastasis of primary tumors and increases chemotherapy sensitivity through the vascular effect of metformin. Moschetta et al.6 demonstrated the effectiveness of metformin in controlling the angiogenesis process of breast tumors through vascular endothelial growth factor (VEGF) and hypoxia-inducible factor-1 (HIF-1), which are the most essential angiogenic markers. Coincidentally, Wang et al.7 found that metformin reduced phosphorylation levels of human epidermal growth factor receptor 2 (HER2) protein, significantly inhibited HER2 signaling–induced tumor angiogenesis, and markedly lessened the expression of HIF1-targeted genes, including vascular endothelial growth factor A (VEGFA), ultimately resulting in smaller tumor vessel size and reduced microvessel density. They further demonstrated that metformin ameliorated tumor hypoxia and restrained hypoxia-inducible factor-1α (HIF-1α) induced expressions of angiogenesis-associated factors (AAFs) through elevating tumor blood perfusion, thus suppressing the excessive tumor angiogenesis.8 Abo-Elmatty et al.9 have clarified that metformin combined with carboplatin can effectively reduce the expression and MVD of the angiogenic mediator “VEGF” and inhibit angiogenesis and proliferation, consistent with the conclusions of previous studies. Also, Yang and his partner10 verified the anti-angiogenic activity of metformin in vivo by a human esophageal squamous cell carcinoma (ESCC) patient-derived xenograft (PDX) mouse model for the first time.
Orecchioni et al.11 revealed that when human MDA-MB-436 triple negative breast cancer cells were cultured alone, metformin inhibited the production of several angiogenic-related proteins such as insulin-like growth factor binding protein-2 (IGFBP-2), platelet-derived growth factor-AA (PDGF-AA), VEGF, angiogenin, matrix metalloprotein-9 (MMP-9), and endostatin. In vivo, metformin reduced the microvascular density of the tumor and altered the perivascular/endothelial cell ratio, resulting in abnormal phenotypes of tumor blood vessels. In pancreatic ductal adenocarcinoma (PDAC), metformin suppressed tumor angiogenesis and enhanced the chemo-sensitivity of gemcitabine via inactivating pancreatic stellate cells (PSCs).12 The results of Qu and Yang13 suggested that metformin could inhibit hepatocellular carcinoma (HCC) angiogenesis through targeting hepatic stellate cells (HSCs) through the AMP-activated protein kinase (AMPK) pathway. Also, a research reported that a patient with a 9-year history of hepatic hemangioma took metformin (750 mg/day) for about 3 years due to hyperglycemia. Surprisingly, the tumor volume gradually decreased, indicating that metformin treatment had a strong inhibitory effect on the proliferation of hepatic hemangioma cells. Thus, Ono et al.14 speculated that the anti-angiogenic effect of metformin may be one of the reasons for tumor shrinkage. Similarly, Miyoshi et al.15 also supported the point that metformin can inhibit angiogenesis in hepatocyte carcinoma cell lines. Nevertheless, they hold that the function of metformin in VEGF gene expression regulation may demonstrate a dual effect. This dual effect probably depends on the type of cell, the duration of curative, the dosage, and other different signaling pathways.15 A study surprisingly reported VEGF upregulation upon metformin treatment, together with increased angiogenesis and tumor growth acceleration.16 Rattan et al.17 confirmed for the first time that metformin treatment inhibited both angiogenesis and metastatic spread of ovarian cancer. And the activation of the AMPK/mTOR pathway induced by metformin was accompanied by decreased microvessel density and VEGF expression.17 Liao et al.18 demonstrated that high levels of luteinizing hormone (LH) promoted ovarian cancer angiogenesis through the PI3K/AKT/mTOR pathway. Metformin can inhibit the mTOR signaling pathway, block the expression of VEGF and Slit2 induced by LH, and further inhibit tumor angiogenesis. Together, these studies confirmed the dual role of metformin in VEGF regulation.
The therapeutic effect on other diseases of metformin via anti-angiogenesis
In a recent study, Virtanen et al.19 recruited 20 pregnant women to investigate the effect of metformin on angiogenesis in the present of maternal sera. They found that when the metformin level was 5 and 50 µg/mL (therapeutic concentrations), there was no change in angiogenesis in comparison to the control group. But when the concentration of metformin reaches 600 µg/mL, it shows a potent inhibition of angiogenesis. This study concluded that metformin may prevent pre-eclampsia by restoring the angiogenic imbalance in maternal sera.19 Endothelial dysfunction of pre-eclampsia and other hypertensive pregnancy disorders (HPDs) results in hypoxia and increases blood pressure. Moreover, Sun et al.20 confirmed that metformin plays an endothelial protective effect by restoring the gestational diabetes mellitus (GDM)–induced angiogenesis impairment may lead to the downregulation of p65 and upregulation of nuclear factor-erythroid 2-related factor 2 (Nrf2). However, it is also described that metformin directly affects endothelial cells, decreasing their proliferation and differentiation potential.21 It is thought that endothelial dysfunction plays a critical role in angiogenic incompetence. Endothelial dysfunction refers to the inability of the endodermis of small arteries in the body to function normally, which leads to vasospasm, abnormal constriction, thrombosis, and angiogenesis.22 In a sense, the improvement of endothelial dysfunction contributes to the formation of new blood vessels and the maintenance of vascular integrity, and plays an essential role in the endothelialization process and vascular repair after the body is damaged.23 Soobryan et al.24 found that metformin can treat endothelial dysfunction in patients with hyperglycemia by enhancing the nitric oxide system, endothelin-derived hyperpolarization factor, and Sirtuin 1. In summary, metformin provides another way to treat these pregnancy disorders.24 However, given the high dose of metformin in this cell research, we suppose that, in clinical conversion, there is still a long way to go for the anti-angiogenic effects of metformin applied in the clinic, due to potential dosage (such as toxicity and adverse reactions).
Metformin is widely accepted as an activator of AMPK. Soraya and his colleagues25 found that oral administration of metformin at 50 mg/kg could significantly (p < 0.01) reduce angiogenesis in granulomatous tissue by 34%, with inhibition of angiogenesis partially dependent on AMPK. Similarly, Dallaglio et al.26 have shown that metformin has anti-angiogenic activity and can inhibit capillary network formation in endothelial cells, which is partly dependent on AMPK. Also, metformin inhibited angiogenesis in Matrigel pellets in vivo and prevented the increase in MVD in obese mice fed with a high-fat diet.26 Ni et al.27 found that the decrease of endothelial progenitor cells’ (EPCs) growth was concentration-dependent with metformin. Their research reported that metformin inhibited the angiogenic capacity of EPCs, and the mechanism involved AMPK-mediated autophagy pathway activity and increased p27 expression.27 But Wang and Sun28 regarded that the inhibitory effect of metformin on angiogenesis was primarily due to the skewing effect of tumor-associated macrophages (TAMs) polarization in the microenvironment.
The prominent pathological feature of polycystic ovary syndrome (PCOS) is abnormal angiogenesis. Tan et al.29 found that the concentration of thrombospondin-1 (TSP-1), a novel anti-angiogenic adipokine, is lower in patients with PCOS, and the treatment of metformin could increase the level of TSP-1 in serum. Coincidentally, Di Pietro et al.30 found that metformin had an improved effect on ovarian angiogenesis. The improved effect may cause the re-accumulation of small follicles observed in PCOS rats, reduce the formation of cysts, and improve the follicular development and the percentage of corpus luteum.30 Thus, in clinical practice, metformin has been introduced into PCOS treatment, playing the dual role of controlling hyperglycemia and improving ovarian angiogenesis.
Han et al.31 investigated the effects and the potential mechanism of metformin on retinal vascular endothelium, mainly characterized by angiogenesis and inflammation. Their data showed that metformin inhibited angiogenesis in a dose-dependent manner in human retinal vascular endothelial cell culture, and its effects of anti-angiogenic and anti-inflammatory were potent.31 Metformin exerts anti-angiogenesis effects and delays the normal vessel formation in the recovery phase of oxygen-induced retinopathy (OIR) in mice, likely by suppressing the levels of Flk1 (VEGF receptor-2).32
Ying treated human umbilical vein endothelial cells (HUVECs) with metformin, and found that it could suppress Smad1/5 phosphorylation and angiogenesis induced by bone morphogenetic proteins-9 (BMP9), whose mechanism was the suppression of BMP9 signaling mediated by upregulation of Smurf1 (Smad ubiquitination regulatory factor 1). It also suppressed the angiogenesis induced by BMP9 in mice Matrigel plug. These findings revealed that metformin significantly reduced choroidal neovascularization.33 HUVECs were studied by Luo et al.34 revealed that miR-21 has been confirmed to be associated with the process of angiogenesis and strikingly downregulated by metformin in a time and dose-dependent manner. In return, the inhibition of miR-21 promoted metformin’s action of anti-angiogenesis. Moreover, metformin can increase the expression of miR-145 and miR-23b in ovarian cell lines and decrease the vasculogenic potential of ovarian cancer cells induced by nerve growth factor (NGF).35 Then, due to many actions of metformin in different pathologies, we supposed that the effect of metformin was attributable to the regulation of many miRs.
In athymic BALB/c nu/nu mouse xenograft models (induced by injecting BIU-87 cells) treated with metformin combined with cisplatin, it markedly inhibited AKT/mTOR signaling pathway and its angiogenesis.36 The analysis of Mathews Samuel et al.37 in mouse microvascular endothelial cells (MMECs) supports the possibility that metformin combined with 2-deoxyglucose (2DG) may be an appropriate anti-proliferative and anti-angiogenic treatment strategy. In gastric cancer (GC) cells, metformin was found to efficiently inhibit protein tyrosine phosphatase receptor delta (PTPRD)-loss-induced angiogenesis and reverse the decrease in PTPRD expression.38 To sum up, metformin could play different roles in various diseases and pathological processes by inhibiting angiogenesis.
The role of metformin in promoting angiogenesis
Metformin, a widely accepted AMPK activator, improves in utero pulmonary hypertension (IPH)—pulmonary arterial endothelial cells (PAECs) angiogenesis, increases phosphorylation of endothelial NO synthase (eNOS) serine (1179), and decreases the eNOS–caveolin-1 association. Metformin also increases MnSOD activity and expression of both eNOS and MnSOD. In their research, AMPK has been confirmed to restore angiogenesis in IPH and increase nitric oxide bioavailability. However, whether metformin is beneficial to the treatment of pulmonary hypertension needs further study.39
Multiple factors may influence the occurrence of the disease; metformin may change one of the factors. Nevertheless, in the end, whether it can treat a disease is also restricted by other factors. For example, Liu et al.40 confirmed that metformin activated AMPK pathway and promoted the eNOS phosphorylation. Thus, they concluded that metformin promoted the focal angiogenesis after neurogenesis and attenuated ischemia-induced brain injury in mice after middle cerebral artery occlusion, which suggested that metformin was a potential new drug for the treatment of ischemic stroke.40 However, metformin increased the number of neural stem cells in mice, which must be done by estrogen to repair brain damage, so this improvement was significant in female mice but not in male mice.41 Fortunately, Venna et al.42 found that metformin can directly increase VEGF expression and improve MVD. Metformin can even be sufficient for those who have been injured for 24 h. Furthermore, it also can promote recovery and improve stroke behavior. Nevertheless, when the treatment is performed in AMPK α-2 knockout mice, it has no apparent role in the angiogenesis, which suggests that the effect of metformin promotes post-stroke recovery by enhancing angiogenesis mediated by AMPK.42 Metformin improves cognitive function by activating neurogenesis in the subependymal zone/subventricular zone, restoring vascular integrity, producing a richer cerebral blood flow, and so on. Its mechanism enhanced glycolysis by increasing mRNA expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and finally increased the angiogenesis and neurogenic potential of neural stem cells.43 Besides, metformin the activation of AMPK–FOXO3 signaling and inhibition of VEGF also plays a neuroprotective role in other models of Parkinson’s disease (PD) and neurodegenerative diseases.44
Takahashi et al.45 found that metformin-treated WT mice accelerated limb perfusion after surgery of posterior limb ischemia, which significantly increased the phosphorylation levels of AMPK and eNOS in the muscle tissues of WT mice after ischemia. In eNOS-deficient knockout mice, metformin-induced AMPK phosphorylation in ischemic tissues was significantly increased. However, it did not affect the recovery of ischemic limb blood flow after the operation. Their study suggests that metformin promotes evacuation through AMPK/eNOS-related mechanisms in tissue ischemia, which is beneficial to patients with severe limb ischemia.45 The combination of simvastatin and metformin significantly improved limb angiogenesis, increased VEGF expression and capillary density (CD31(+)), improved foot function, and slowed disease progression in diabetic HLI mice.46
In patients with type 2 diabetes mellitus (T2DM), wound healing is widely believed to be due to hyperglycemia that causes dysfunction of EPCs. Metformin has shown the potential to improve endothelial function and wound healing processes. However, the potential mechanisms of metformin for the positive effect remain to be unknown. Yu and his team47 designed experiments to verify whether metformin could accelerate wound healing by improving the EPCs—impaired function in streptozotocin-induced diabetic mice. They found that metformin accelerated wound closure in diabetic mice, stimulated angiogenesis, and increased the number of circulating EPCs. Besides, metformin restored the impaired angiogenesis and migration of bone marrow endothelial progenitor cells (BM-EPCs) in diabetic mice.47 Ahmed et al.48 took hyperglycemic db/db mice as the T2DM model and observed that metformin treatment could promote wound healing, improve angiogenesis, and increase the number of circulating EPCs in db/db mice. In vitro, metformin treatment reversed the dysfunction of BM-EPC function reflected by angiogenesis and significantly increased nitric oxide (NO) production while decreasing O2-levels in BM-EPCs from db/db mice, which was consistent with the previous study. Also, they found that the expression of TSP-1 in BM-EPCs cultured in vitro was significantly reduced after treatment with metformin. In summary, their study confirms that metformin contributes to wound healing and improves BM-EPC function in diabetic mice, which may be dependent on the AMPK/eNOS pathway.48
Moreover, Xu et al.49 confirmed that metformin upregulated the RAS guanyl releasing protein 1 (RasGRP1)-dependent VEGF signaling and ameliorated the impaired angiogenesis caused by high glucose in HUVECs. Zolali et al.50 also selected HUVECs to assess the effect of metformin on endocan biogenesis under diabetic conditions. Their data suggest that metformin promotes the angiogenic potential of endothelial cells, which is the leading cause of diabetic foot ulcers, possibly due to endothelin dynamics regulation under high glucose conditions.50
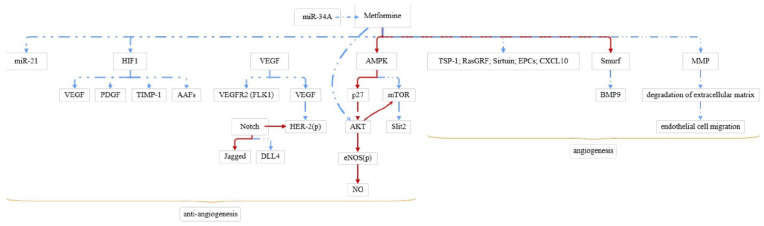
Decreased angiogenesis leads to persistent pulmonary hypertension (PPHN) in neonates. AMPK agonist metformin increased mitochondrial complex protein and number, in vitro angiogenesis, and Jagged 1 (Jag1) level, while decreased delta-like 4 (DLL4) level in PAEC of PPHN. In vivo injection of metformin can increase the pulmonary vascular density of PPHN. Thus, metformin may improve PPHN to some scale.44 Bakhashab et al.51 revealed that metformin had a dual effect by simultaneously increasing VEGFA and reducing chemokine (C-X-C motif) ligand 10 (CXCL10) and tissue inhibitor of metalloproteinase 1 (TIMP1) in CD34(+) cells in a model of the diabetic state combined with hypoxia. The presence of metformin increased Sirtuin 1 expression and attenuated angiogenic impairment in high-glycemic MMECs. On the contrary, the over-expression of miR-34a mimic could prevent metformin-mediated protection. These results indicate that miR-34a has an anti-angiogenic effect in MMECs by regulating Sirtuin 1 expression, regulated by metformin. In conclusion, miR-34a is a target of metformin-mediated vascular protection.52 Moreover, professor Wu and his colleagues53 found that the treatment of metformin for implanted flap model C57BL/6 mice can effectively improve the post-operative flap survival area, blood flow, and the number of microvessels and reduced tissue edema. These effects were attributable to increased autophagy mediated by activation of the AMPK–mTOR–TFEB signaling pathway.53 The molecular mechanisms of metformin’s effect are shown in Figure 1.
Figure 1.
The molecular mechanisms of metformin promote/inhibit angiogenesis.
“ ” mean
decrease and “
” mean
decrease and “ ” mean
increase.
” mean
increase.
Discussion
The various effects of metformin in different conditions described above seem so promising. However, whether a drug can genuinely become a therapeutic drug depends on several factors. The first thing is to make trails by cell or animal experiments to study its mechanism. Even if the results are very encouraging, it still needs a proper clinical test to confirm a specific treatment effect. In reality, most animal experiments can only show cytological evidence of effectiveness. Also, it should be pointed out that, given that metformin could be used in clinical practice, we need to know the critical point: not all human body data belongs to the clinical trial data. Observational studies included retrospective and prospective trials, such as a case mentioned above, which is a retrospective study. A patient with hepatic hemangioma took metformin for about 3 years, and the follow-up showed that the hemangioma gradually decreased and disappeared.14 Metformin has been shown to reduce the risk of gastric cancer in prospective studies.54 These are just epidemiological studies and not interventional clinical trials.
In other words, metformin is not the intervention measures set deliberately by researchers. The result of metformin applied to the observed patients only showed its correlation with disease improvement, which cannot necessarily indicate its curative effect. Furthermore, the curative effect of metformin must be proved by interventional clinical trials. So, the interpretation of the epidemiological data needs to be taken cautiously. According to the classification of evidence-based evidence, the reliability of a single case report and retrospective study is weak, while some prospective study is more reliable, and the most reliable research is interventional clinical trials. And what we called phase 3 clinical trials were designed by following the standard of randomized, double-blind, controlled, multi-center studies. Until clinical trials are completed, all studies are almost as unreliable as legends.
From what has been discussed above, we may find a cruel reality: metformin may indeed have many functions, but only a small part through preliminary clinical trials proved that it could make a difference. Most relevant research to clinical therapy is not very mature and still needs further study to verify the effect. Even if metformin has a particular effect, real results may also be minimal. Why was metformin not as a miracle as expected? In fact, this phenomenon is not specific to metformin; a lot of research and discovery will fall into the same routine: a promising beginning and a fruitless conclusion. Most importantly, the occurrence of a disease can be determined by several factors. One of the factors may be changed by metformin application, which does not imply it can be used in clinical practice as a curative drug. A case mentioned above, Liu et al.40 confirmed that metformin promoted the focal angiogenesis after middle cerebral artery occlusion and nerve generated. However, metformin increases the number of neural stem cells in mice, which must be done by estrogen to repair brain damage, so this improvement is significant in female mice but not in male mice.41
Although metformin is the commonly used medicine for diabetes, do not let its aura blind you to its potential adverse reactions. It is not a panacea and can cause severe lactic acidosis syndrome. The use of metformin in clinical practice will be cautious in patients with chronic kidney disease. Another side effect of metformin is the secondary shortage of vitamin B12.55 About a third of people who take metformin will reduce the intestinal absorption of vitamin B12, which leads to the deficiency of vitamin B12. Then, it causes or contributes to a host of other conditions, such as memory decline, impaired attention, neuron degeneration, and anemia.56 Patients taking metformin in clinical need to be taken care of the mean corpuscular volume (MCV) in blood routine examination. The usual range of the index is 76~96 fl. Above this range, undoubtedly it is anemia caused by vitamin B12 deficiency.
Finally, to return to the question at the beginning of this article, does metformin have a chance of becoming the next panacea? It can only be said that scientific research is a process of “hypothesis boldly and verify carefully.” We need to look forward to more possibilities. However, before obtaining evidence from clinical trials, its bright spot only stays at the stage of possibility, and we have to be alert to potential adverse reactions.
Conclusion
These studies above have sufficiently demonstrated that metformin occupied a significant position in the process of regulating angiogenesis. Therefore, studying the biological effects of metformin is of significant theoretical and practical value, particularly for treating the disease with abnormal angiogenesis and the reconstruction of vascular function. With the physiological functions, pharmacological features, and clinical value of metformin has been revealed, we humans research for the mechanism of metformin inhibiting angiogenesis and promoting angiogenesis-related signal transduction pathways has made significant progress.
In a word, we concluded that metformin might have different and conflicting results in diverse physiological and pathological conditions, just like a double-edged sword. Metformin may create both promoting and inhibiting effects on angiogenesis, and its role depends mainly on its action pathway, the type of cells being investigated, and the nature and conditions of the disease. Under what circumstances can it be used to promote or inhibit angiogenesis? It is still an unsolved problem.
Limitation
This review reorganized the role of metformin in angiogenesis, and the underlying mechanism of metformin in alleviating disease via mediating the angiogenesis process. It may bring a new view of reference for overcoming angiogenesis-related diseases. However, most of the relevant studies are still not mature and need further investigation.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Hua Luo  https://orcid.org/0000-0003-4099-6570
https://orcid.org/0000-0003-4099-6570
References
- 1. Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA 2017; 317: 2515–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garcia EY. Flumamine, a new synthetic analgesic and anti-flu drug. J Philipp Med Assoc 1950; 26(7): 287–293. [PubMed] [Google Scholar]
- 3. Bailey CJ. Metformin: historical overview. Diabetologia 2017; 60(9): 1566–1576. [DOI] [PubMed] [Google Scholar]
- 4. Kurelac I, Umesh Ganesh N, Iorio M, et al. The multifaceted effects of metformin on tumor microenvironment. Semin Cell Dev Biol 2020; 98: 90–97. [DOI] [PubMed] [Google Scholar]
- 5. Wang JC, Li GY, Wang B, et al. Metformin inhibits metastatic breast cancer progression and improves chemosensitivity by inducing vessel normalization via PDGF-B downregulation. J Exp Clin Cancer Res 2019; 38: 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moschetta MG, Leonel C, Maschio-Signorini LB, et al. Evaluation of angiogenesis process after metformin and LY294002 treatment in mammary tumor. Anticancer Agents Med Chem 2019; 19(5): 655–666. [DOI] [PubMed] [Google Scholar]
- 7. Wang J, Li G, Wang Y, et al. Suppression of tumor angiogenesis by metformin treatment via a mechanism linked to targeting of HER2/HIF-1α/VEGF secretion axis. Oncotarget 2015; 6: 44579–44592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang JC, Li GY, Li PP, et al. Suppression of hypoxia-induced excessive angiogenesis by metformin via elevating tumor blood perfusion. Oncotarget 2017; 8: 73892–73904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abo-Elmatty DM, Ahmed EA, Tawfik MK, et al. Metformin enhancing the antitumor efficacy of carboplatin against Ehrlich solid carcinoma grown in diabetic mice: effect on IGF-1 and tumoral expression of IGF-1 receptors. Int Immunopharmacol 2017; 44: 72–86. [DOI] [PubMed] [Google Scholar]
- 10. Yang Y, Jin G, Liu H, et al. Metformin inhibits esophageal squamous cell carcinoma-induced angiogenesis by suppressing JAK/STAT3 signaling pathway. J Transl Med 2017; 8: 74673–74687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Orecchioni S, Reggiani F, Talarico G, et al. The biguanides metformin and phenformin inhibit angiogenesis, local and metastatic growth of breast cancer by targeting both neoplastic and microenvironment cells. Int J Cancer 2015; 136: E534–E544. [DOI] [PubMed] [Google Scholar]
- 12. Qian W, Li J, Chen K, et al. Metformin suppresses tumor angiogenesis and enhances the chemosensitivity of gemcitabine in a genetically engineered mouse model of pancreatic cancer. Life Sci 2018; 208: 253–261. [DOI] [PubMed] [Google Scholar]
- 13. Qu H, Yang X. Metformin inhibits angiogenesis induced by interaction of hepatocellular carcinoma with hepatic stellate cells. Cell Biochem Biophys 2015; 71(2): 931–936. [DOI] [PubMed] [Google Scholar]
- 14. Ono M, Sawada K, Okumura T. A case of liver hemangioma with markedly reduced tumor size after metformin treatment: a case report. Clin J Gastroenterol 2017; 10(1): 63–67. [DOI] [PubMed] [Google Scholar]
- 15. Miyoshi H, Kato K, Iwama H, et al. Effect of the anti-diabetic drug metformin in hepatocellular carcinoma in vitro and in vivo. Int J Oncol 2014; 45: 322–332. [DOI] [PubMed] [Google Scholar]
- 16. Martin MJ, Hayward R, Viros A, et al. Metformin accelerates the growth of BRAF V600E-driven melanoma by upregulating VEGF-A. Cancer Discov 2012; 2(4): 344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rattan R, Graham RP, Maguire JL, et al. Metformin suppresses ovarian cancer growth and metastasis with enhancement of cisplatin cytotoxicity in vivo. Neoplasia 2011; 13(5): 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liao H, Zhou Q, Gu Y, et al. Luteinizing hormone facilitates angiogenesis in ovarian epithelial tumor cells and metformin inhibits the effect through the mTOR signaling pathway. Oncol Rep 2012; 27(6): 1873–1878. [DOI] [PubMed] [Google Scholar]
- 19. Virtanen A, Huttala O, Tihtonen K, et al. Therapeutic doses of metformin do not have impact on angiogenesis in presence of sera from pre-eclamptic, IUGR and healthy pregnancies. Pregnancy Hypertens 2020; 22: 7–13. [DOI] [PubMed] [Google Scholar]
- 20. Sun CC, Lai YN, Wang WH, et al. Metformin ameliorates gestational diabetes mellitus-induced endothelial dysfunction via downregulation of p65 and upregulation of Nrf2. Front Pharmacol 2020; 11: 575390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garrido MP, Vera C, Vega M, et al. Metformin prevents nerve growth factor-dependent proliferative and proangiogenic effects in epithelial ovarian cancer cells and endothelial cells. Ther Adv Med Oncol 2018; 10: 1758835918770984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ungvari Z, Tarantini S, Kiss T, et al. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat Rev Cardiol 2018; 15(9): 555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takamiya M, Okigaki M, Jin D, et al. Granulocyte colony-stimulating factor-mobilized circulating c-Kit+/Flk-1+ progenitor cells regenerate endothelium and inhibit neointimal hyperplasia after vascular injury. Arterioscler Thromb Vasc Biol 2006; 26(4): 751–757. [DOI] [PubMed] [Google Scholar]
- 24. Soobryan N, Murugesan S, Pandiyan A, et al. Angiogenic dysregulation in pregnancy-related hypertension—a role for metformin. Reprod Sci 2018; 25(11): 1531–1539. [DOI] [PubMed] [Google Scholar]
- 25. Soraya H, Esfahanian N, Shakiba Y, et al. Anti-angiogenic effects of metformin, an AMPK activator, on human umbilical vein endothelial cells and on granulation tissue in rat. Iran J Basic Med Sci 2012; 15(6): 1202–1209. [PMC free article] [PubMed] [Google Scholar]
- 26. Dallaglio K, Bruno A, Cantelmo AR, et al. Paradoxic effects of metformin on endothelial cells and angiogenesis. Carcinogenesis 2014; 35(5): 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ni HZ, Liu Z, Sun LL, et al. Metformin inhibits angiogenesis of endothelial progenitor cells via miR-221-mediated p27 expression and autophagy. Future Med Chem 2019; 11(17): 2263–2272. [DOI] [PubMed] [Google Scholar]
- 28. Wang JC, Sun X. Metformin’s antitumour and anti-angiogenic activities are mediated by skewing macrophage polarization. J Cell Mol Med 2018; 22: 3825–3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tan BK, Adya R, Chen J, et al. Metformin decreases angiogenesis via NF-kappaB and Erk1/2/Erk5 pathways by increasing the antiangiogenic thrombospondin-1. Cardiovasc Res 2009; 83: 566–574. [DOI] [PubMed] [Google Scholar]
- 30. Di Pietro M, Parborell F, Irusta G, et al. Metformin regulates ovarian angiogenesis and follicular development in a female polycystic ovary syndrome rat model. Endocrinology 2015; 156(4): 1453–1463. [DOI] [PubMed] [Google Scholar]
- 31. Han J, Li Y, Liu X, et al. Metformin suppresses retinal angiogenesis and inflammation in vitro and in vivo. PLoS ONE 2018; 13(3): e0193031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Joe SG, Yoon YH, Choi JA, et al. Anti-angiogenic effect of metformin in mouse oxygen-induced retinopathy is mediated by reducing levels of the vascular endothelial growth factor receptor Flk-1. PLoS ONE 2015; 10(3): e0119708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ying Y, Ueta T, Jiang S, et al. Metformin inhibits ALK1-mediated angiogenesis via activation of AMPK. Oncotarget 2017; 8: 32794–32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Luo M, Tan X, Mu L, et al. MiRNA-21 mediates the antiangiogenic activity of metformin through targeting PTEN and SMAD7 expression and PI3K/AKT pathway. Sci Rep 2017; 7: 43427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garrido MP, Salvatierra R, Valenzuela-Valderrama M, et al. Metformin reduces NGF-induced tumour promoter effects in epithelial ovarian cancer cells. Pharmaceuticals 2020; 13: 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang D, Wu X. In vitro and in vivo targeting of bladder carcinoma with metformin in combination with cisplatin. Oncol Lett 2015; 10: 975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mathews Samuel S, Satheesh NJ, Ghosh S, et al. Treatment with a combination of metformin and 2-deoxyglucose upregulates thrombospondin-1 in microvascular endothelial cells: implications in anti-angiogenic cancer therapy. Cancer 2019; 11: 1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bae WJ, Ahn JM, Byeon HE, et al. PTPRD-inactivation-induced CXCL8 promotes angiogenesis and metastasis in gastric cancer and is inhibited by metformin. J Exp Clin Cancer Res 2019; 38: 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Teng RJ, Du J, Afolayan AJ, et al. AMP kinase activation improves angiogenesis in pulmonary artery endothelial cells with in utero pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2013; 304: L29–L42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu Y, Tang G, Zhang Z, et al. Metformin promotes focal angiogenesis and neurogenesis in mice following middle cerebral artery occlusion. Neurosci Lett 2014; 579: 46–51. [DOI] [PubMed] [Google Scholar]
- 41. Ruddy RM, Adams KV, Morshead CM. Age- and sex-dependent effects of metformin on neural precursor cells and cognitive recovery in a model of neonatal stroke. Sci Adv 2019; 5(9): eaax1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Venna VR, Li J, Hammond MD, et al. Chronic metformin treatment improves post-stroke angiogenesis and recovery after experimental stroke. Eur J Neurosci 2014; 39(12): 2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhu X, Shen J, Feng S, et al. Metformin improves cognition of aged mice by promoting cerebral angiogenesis and neurogenesis. Aging 2020; 12: 17845–17862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. El-Ghaiesh SH, Bahr HI, Ibrahiem AT, et al. Metformin protects from rotenone–induced nigrostriatal neuronal death in adult mice by activating AMPK-FOXO3 signaling and mitigation of angiogenesis. Front Mol Neurosci 2020; 13: 84. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45. Takahashi N, Shibata R, Ouchi N, et al. Metformin stimulates ischemia-induced revascularization through an eNOS dependent pathway in the ischemic hindlimb mice model. J Vasc Surg 2015; 61(2): 489–496. [DOI] [PubMed] [Google Scholar]
- 46. Goggi JL, Haslop A, Boominathan R, et al. Imaging the proangiogenic effects of cardiovascular drugs in a diabetic model of limb ischemia. Contrast Media Mol Imaging 2019; 2019: 2538909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yu JW, Deng YP, Han X, et al. Metformin improves the angiogenic functions of endothelial progenitor cells via activating AMPK/eNOS pathway in diabetic mice. Cardiovasc Diabetol 2016; 15: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ahmed FW, Glanville M, Al-Qahtani MH, et al. Metformin accelerates wound healing in type 2 diabetic db/db mice. Int J Mol Sci 2017; 16: 8691–8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xu J, Liu M, Yu M, et al. RasGRP1 is a target for VEGF to induce angiogenesis and involved in the endothelial-protective effects of metformin under high glucose in HUVECs. IUBMB Life 2019; 71(9): 1391–1400. [DOI] [PubMed] [Google Scholar]
- 50. Zolali E, Rezabakhsh A, Nabat E, et al. Metformin effect on endocan biogenesis in human endothelial cells under diabetic condition. Arch Med Res 2019; 50(5): 304–314. [DOI] [PubMed] [Google Scholar]
- 51. Bakhashab S, Ahmed FW, Schulten HJ, et al. Metformin improves the angiogenic potential of human CD34+ cells co-incident with downregulating CXCL10 and TIMP1 gene expression and increasing VEGFA under hyperglycemia and hypoxia within a therapeutic window for myocardial infarction. Cardiovasc Diabetol 2016; 15: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Arunachalam G, Lakshmanan AP, Samuel SM, et al. Molecular interplay between microRNA-34a and Sirtuin1 in hyperglycemia-mediated impaired angiogenesis in endothelial cells: effects of metformin. J Pharmacol Exp Ther 2016; 356(2): 314–323. [DOI] [PubMed] [Google Scholar]
- 53. Wu H, Ding J, Li S, et al. Metformin promotes the survival of random-pattern skin flaps by inducing autophagy via the AMPK-mTOR-TFEB signaling pathway. Int J Biol Sci 2019; 15(2): 325–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cheung KS, Chan EW, Wong AYS, et al. Metformin use and gastric cancer risk in diabetic patients after helicobacter pylori eradication. J Natl Cancer Inst 2019; 111: 484–489. [DOI] [PubMed] [Google Scholar]
- 55. Aroda VR, Edelstein SL, Goldberg RB, et al. Long-term metformin use and vitamin B12 deficiency in the diabetes prevention program outcomes study. J Clin Endocrinol Metab 2016; 101(4): 1754–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu KW, Dai LK, Jean W. Metformin-related vitamin B12 deficiency. Age Ageing 2006; 35: 200–201. [DOI] [PubMed] [Google Scholar]