Abstract
Background:
Burosumab, a recombinant anti-FGF23 monoclonal antibody, was recently introduced as a treatment for X-linked hypophosphatemia (XLH). Burosumab normalizes blood phosphate levels, thereby healing rickets, decreasing leg bowing, and reducing pain. We aimed to explore the body composition and cardiometabolic health of pediatric patients with XLH treated with burosumab.
Methods:
This observational real-life study was conducted on growing children and adolescents. The outcome measures included changes in sex- and age-adjusted anthropometric and body composition parameters [fat mass (FM), fat-free mass (FFM), appendicular skeletal muscle mass (ASMM), muscle-to-fat ratio (MFR)], blood pressure, laboratory evaluation, and radiographic rickets severity [Thacher Rickets Severity Score (TRSS)]. Body composition was assessed by bioelectrical impedance analysis (BIA). Percentiles for FFM% and ASMM% were calculated according to BIA pediatric reference curves. The delta variable was calculated as the variable at 12 months minus the variable at baseline.
Results:
A total of 15 pediatric patients with XLH are treated in our clinic; included in the analyses were 7 children and adolescents (3 males, mean age 8.7 ± 3.2 years) with XLH without comorbidities. Baseline BIA revealed an unfavorable physique, with increased body fat percentage in five patients and decreased muscle mass in six. Indices of lean body mass significantly increased after 6 and 12 months of treatment: FFM(kg) (p = 0.001, p = 0.046, respectively) and ASMM(kg) (p = 0.012, p = 0.034, respectively), without any significant change in FM(kg). The percentile of ASMM% increased significantly after 6 months of treatment (p = 0.006) and stabilized thereafter. TRSS improved significantly after 12 months of therapy (p = 0.005). Age was positively correlated with delta TRSS (r = 0.814, p = 0.026), and delta TRSS was negatively correlated with delta MFR (r = −0.826, p = 0.022).
Conclusions:
There was a heretofore unrecognized improvement in body composition of growing children and adolescents with XLH who were treated with burosumab. These findings highlight the need to initiate burosumab treatment at a younger age when rickets is less severe.
Keywords: body composition, burosumab therapy, children and adolescents, muscle-to-fat ratio, rickets severity score (RSS), X-linked hypophosphatemia (XLH)
Introduction
X-linked hypophosphatemia (XLH) is a rare inherited disease caused by an inactivating mutation in the phosphate-regulating endopeptidase homolog X-linked (PHEX) gene.1,2 PHEX is expressed primarily in osteoblasts where it encodes an enzyme that degrades local small integrin-binding ligand N-linked glycoproteins (SIBLING proteins), particularly osteopontin.3 An intact PHEX also suppresses the production of the serum phosphatonin fibroblast growth factor 23 (FGF23).4,5 The upregulation of FGF23 due to PHEX mutation results in hyperphosphaturia, hypophosphatemia, and decreased levels of calcitriol due to the inhibitory effect on 25-hydroxyvitamin D3 1-α-hydroxylase.6 Increased FGF23 levels have been linked to adverse cardiometabolic sequelae. FGF23 was suggested as a contributing factor to the development and progression of cardiac morbidity and mortality in patients with chronic renal failure; however, the pathogenesis of myocardial damage in such settings is multifactorial, and the role of FGF23 cannot be determined in isolation.7,8 Moreover, local myocardial FGF23 production was demonstrated in cases of myocardial damage.7 Uncertainty still exists on whether FGF23 is the cause, or the result of myocardial disease, as reflected by studies in both animal models and in humans. A mice model for XLH did not demonstrate cardiac hypertrophy when elevated circulating FGF23 was concurrent with hypophosphatemia9 and anti-FGF23 neutralizing antibodies did not prevent cardiac hypertrophy in mice with chronic kidney disease.10 These findings suggest that other factors as well, contribute to the decreased life expectancy in adult patients with XLH, since the effect of FGF23 on survival in FGF23-mediated hypophosphatemic diseases is not well established.11 Signal transduction pathways coupling insulin and insulin-like growth factor 1 (IGF1) levels with FGF23 production have also been described.12 The escalating rate of overweight and obesity in young patients with XLH13 together with the life-long exposure to increased FGF23 levels may harbor a greater risk for cardiometabolic morbidity.
Burosumab (Crysvita®, Ultragenyx), a recombinant anti-FGF23 monoclonal antibody, was introduced as a treatment for XLH in 2018.14,15 Burosumab normalizes blood phosphate levels, thereby healing rickets, decreasing leg bowing, and reducing pain. Since its introduction, data on the beneficial effects of this treatment on children’s growth and on their biochemical profile have been accumulating, but data on the impact of this treatment on metabolic health are lacking. In January 2019, burosumab was approved by the Israeli healthcare basket committee, and it has become the treatment of choice for pediatric patients with XLH. Our aims of this study were to explore the effects of burosumab treatment on the body composition and cardiometabolic health parameters of children and adolescents with XLH.
Methods
Patients
This observational real-life study was conducted in the Pediatric Metabolic Bone Disease Unit in the authors’ tertiary medical center. The study protocol was approved by the Tel Aviv Sourasky Medical Center Institutional Review Board (201910556) and the parents of the participants provided written informed consent. The data were handled in accordance with the principles of Good Clinical Practice.
A total of 15 pediatric patients with XLH are currently being treated with burosumab in the medical center. All of the patients with a clinical diagnosis are referred to genetic analysis of PHEX sequencing for XLH confirmation. Excluded from the study were three children who had been <5 years of age at the initiation of burosumab, two adolescents who completed linear growth prior to burosumab initiation, and one noncompliant child. Also excluded were two patients with comorbid conditions that could affect linear growth (one girl with central precocious puberty treated with analogue of gonadotropin-releasing hormone agonist and one boy with septo-optic dysplasia treated with growth hormone replacement therapy). The seven growing children and adolescents included in the analysis had completed 1 year of burosumab treatment.
Burosumab was administered according to the recommended treatment protocol every 2 weeks, and the dose was adjusted (between 0.8 mg/kg and 2 mg/kg) to achieve a serum phosphorus level at the low end of the normal range for age and for healing of rickets.14 Oral phosphate supplement and calcitriol were discontinued 1 week before starting burosumab.15,16
Study protocol
The study protocol consisted of multi-professional clinic visits at three time points during burosumab therapy (baseline, at 6 months, and at 12 months). Each visit included blood pressure and anthropometric measurements, physical examination, and body composition assessment. Fasting blood was drawn for glucose levels, liver panel (alanine transaminase, aspartate aminotransferase, gamma-glutamyl transpeptidase), and lipid panel [total cholesterol, high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), and triglycerides]. The routine laboratory surveillance included: serum concentrations of phosphate, alkaline phosphatase, 1,25-dihydroxyvitamin D and intact parathyroid hormone. X-ray imaging (wrists and knees) for evaluation of the rickets score was performed at baseline and at 12 months after initiating burosumab treatment.
Auxological assessment
Growth surveillance included the anthropometric parameters of measuring weight (in light clothing and by means of a standard calibrated scale) and height (by means of a commercial Harpenden–Holtain stadiometer). Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Anthropometric variables (height and BMI values) were converted to sex- and age-specific z-scores according to the CDC2000 Growth Charts for the United States.17 Blood pressure was measured according to clinical practice guidelines, and percentiles for systolic and diastolic blood pressure were calculated according to height, sex, and age.18 The physical examination of the patients included pubertal stage assessment, which was performed according to Tanner and Marshall staging by a pediatric endocrinologist.19,20
Body composition analysis
Body composition in children older than 5 years was measured by bioelectrical impedance analysis (BIA) using a Tanita Body Composition Analyzer, Tanita MC-780 MA and GMON Professional Software, which have been clinically proven to be accurate and reliable, and to provide highly reproducible results.21 The BIA report included the following information: fat mass (FM), fat mass%, muscle mass, fat-free mass (FFM) and skeletal muscle mass in four limbs. The BIA report also included sex and age-adjusted normal range for fat percentage and muscle mass and a graphical representation of the individual’s physique rating. Calculated measures included FFM% as [FFM(kg)/weight(kg)] × 100, appendicular skeletal muscle mass (ASMM) as the sum of muscle mass of four limbs, ASMM% as [ASMM(kg)/weight(kg)] × 100, and muscle-to-fat ratio (MFR) as ASMM(kg)/FM(kg). Percentiles for FFM% and ASMM% were calculated according to BIA pediatric reference curves.22 Delta MFR was calculated as MFR at 12 months of treatment minus the MFR at baseline.
Rickets severity score
Rickets severity was assessed with the Thacher Rickets Severity Score (TRSS),23 which is a 10-point score for radiographs of wrists and knees for assessment of the degree of growth plate widening, metaphyseal fraying and cupping, and the proportion of the growth plate affected by disease. The score progressed in half-point increments from zero (normal) to 10 points (severe). Skeletal X-ray imaging interpretation was performed by a metabolic bone disease specialist (LZ). Delta TRSS represented the improvement in radiographic manifestations of rickets, and it was calculated as the TRSS at baseline minus the TRSS at 12 months of treatment.
Statistics
The data were analyzed with the Statistical Package for the Social Sciences software version 25 (SPSS Inc., Chicago, IL, USA). All statistical tests were two-sided. The Kolmogorov–Smirnov test and the Shapiro–Wilk test were applied to test the normal distribution of continuous parameters. The data are expressed as means ± standard deviation (SD) since the variables were normally distributed. The paired t-test was used for comparing the means of variables at different time points. Pearson correlations were applied to determine correlations between delta TRSS and other variables. A p-value ⩽0.05 was considered significant.
Results
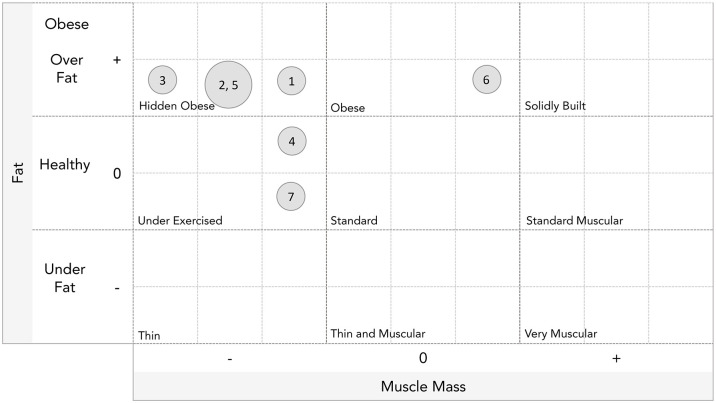
The baseline characteristics of seven children and adolescents (three males) diagnosed with XLH prior to the initiation of burosumab treatment are presented in Table 1. The mean age at burosumab initiation was 8.7 ± 3.2 years. Overall, five patients (71.4%) completed genetic analysis with the confirmation of a heterozygous PHEX mutation; five patients were prepubertal and two girls had Tanner stage 3. Body composition measurements of the seven patients revealed that five of them (71.4%) had body fat percentages above the normal range for sex and age, while six of them (85.7%) had muscle mass below the normal range adjusted for sex, age, and height at baseline. The physique ratings of patients at burosumab initiation are presented in Figure 1. All of the study patients had normal sex-, age- and height-adjusted systolic and diastolic blood pressure levels (mean ± SD: 100 ± 8 mmHg and 57 ± 6 mmHg, respectively). Fasting blood glucose levels and lipid profiles were within the normal range (mean ± SD: glucose: 85.1 ± 7.3 mg/dl, total cholesterol: 150.1 ± 28.8 mg/dl, triglycerides: 81.6 ± 25.0 mg/dL, LDL-c: 91.4 ± 20.1 mg/dl, and HDL-c: 50.9 ± 5.7 mg/dl,) as were liver function tests.
Table 1.
Baseline characteristics of nine pediatric patients with XLH.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Age | 5 yrs 1 mo | 5 yrs 7 mos | 6 yrs 4 mos | 9 yrs 2 mos | 9 yrs 11 mos | 11 yrs 6 mos | 13 yrs 4 mos |
| Sex | F | M | M | F | M | F | F |
| PHEX gene mutation | c.1783A>T(het); pLys595* | c.146delIT; p.His487Glnfs*28 | c.565C>T(het); pGin189X(STOP), mosaicism50% | Not done | c.488C>A(hemi); p.Ser163*(STOP) | Not done | c.1735G>A (het); p.Gly579Arg; rs875989883 |
| XLH in the nuclear family | Mother and sister | Mother | Mother and brother | ||||
| Age at diagnosis | 1 yrs 6 mos | 7 mos | 2 yrs | 2 yrs 6 mos | 2 yrs 6 mos | 6 yrs | 1 yrs |
| Height SDS | −2.14 | −2.01 | −0.19 | −1.00 | −1.40 | −2.42 | −2.07 |
| Weight SDS | −0.51 | −0.28 | 0.39 | 0 | 0.41 | 0.42 | −1.76 |
| BMI SDS | 1.31 | 1.58 | 0.84 | 0.49 | 1.34 | 1.77 | −0.91 |
| Pubertal stage, Tanner | 1 | 1 | 1 | 1 | 1 | 3 | 3 |
| Fat% (normal range) | 25.6 (15–25) | 22.4 (13–20) | 21.6 (13–20) | 23.4 (16–27) | 26.9 (13–22) | 29.7 (16–29) | 21.2 (16–29) |
| Muscle mass, kg (normal range) | 11.5 (13.3–16.8) | 13.6 (17.9–22.7) | 16.2 (22.8–28.9) | 20.7 (22.9–28.9) | 22.9 (28.1–35.6) | 28.8 (23.5–29.7) | 25.6 (28.6–36.2) |
| ASMM, kg | 3.7 | 4.0 | 5.5 | 8.1 | 8.9 | 11.2 | 10.3 |
| Muscle-to-fat ratio | 0.9 | 1.0 | 1.1 | 1.2 | 1.0 | 0.9 | 1.4 |
| Serum phosphate (mg/dl) | 2.1 | 3.7 | 3.4 | 2.2 | 2.6 | 2.6 | 2.8 |
| Serum alkaline phosphatase (U/l) | 290 | 471 | 321 | 586 | 667 | 511 | 609 |
| Serum 1,25(OH)2D (ng/ml) | 23 | – | 21 | 28 | 28.1 | 25.7 | 16 |
| Serum PTH (pg/ml) | 44.5 | 50 | 43.2 | 74.2 | 21.3 | 32 | 46.6 |
| Clinical symptoms | Leg pain, Dolichocephaly Chiari I | Leg pain and genu varum | Leg pain | Leg pain and genu varum | Leg pain | Leg pain and genu varum | Leg pain and genu varum |
| Dental findings | None | Recurrent dental abscess | None | None | Dental abscess | None | None |
Normal ranges for laboratory values: serum phosphate 3.6–5.8 mg/dl; serum alkaline phosphatase 100–350 U/l; serum 1,25(OH)2D 20–100 ng/ml; serum PTH 12–65 pg/ml.
1,25(OH)2D, 1,25-dihydroxyvitamin D; ASMM, appendicular skeletal muscle mass; BMI, body mass index; F, female; IM, intramuscular; M, male; mo, month; PTH, parathyroid hormone; rGH, recombinant growth hormone; SDS, standard deviation score; XLH, X-linked hypophosphatemia; yrs, years.
Figure 1.
Physique rating of seven children and adolescents with XLH at baseline, as assessed by the Tanita Body Composition Analyzer. The physique rating is an indicator obtained by balancing the amount of body fat and muscle. The number in each circle corresponds to the patient number in Table 1.
XLH, X-linked hypophosphatemia.
Table 2 presents the patients’ rickets severity score and their anthropometric and body composition dynamics at three time points (baseline, and 6 and 12 months). The mean height SD score (SDS) of the patients improved significantly during the first 6 months of treatment (p = 0.023), with stabilization during the second period of treatment (p = 0.548). The mean BMI SDS of the patients decreased significantly during the first 6 months of treatment (p = 0.006), with stabilization being observed during the second period of treatment (p = 0.366). The proportion of patients with an unfavorable physique rating had decreased by 12 months of treatment: two patients (28.6%) had body fat percentages above the normal range adjusted for sex and age and four patients (57.1%) had muscle mass below the normal range adjusted for sex, age, and height. The significant change in body composition parameters at 6 and at 12 months of treatment was in FFM(kg) (p = 0.001, p = 0.046, respectively) and in ASMM(kg) (p = 0.012, p = 0.034, respectively), without any significant change in FM(kg). The ASMM percentile increased significantly at 6 months of treatment (p = 0.006) and stabilized thereafter.
Table 2.
Twelve-month surveillance of 7 burosumab-treated XLH patients.
| Baseline | 6 months | 12 months | p a | p b | |
|---|---|---|---|---|---|
| Age, years (range) | 8.7 ± 3.2 (5.1–13.3) | 9.3 ± 3.1 (5.7–13.8) | 9.8 ± 3.1 (6.4–14.3) | ||
| Anthropometric measurements | |||||
| Rickets severity score | 2.29 ± 1.07 | Not done | 0.43 ± 0.73 | 0.005 | |
| Height, cm | 121.3 ± 16.3 | 125.4 ± 16.3 | 128.3 ± 15.4 | <0.001 | <0.001 |
| Height SDS | −1.59 ± 0.81 | −1.41 ± 0.77 | −1.37 ± 0.77 | 0.023 | 0.548 |
| Weight, kg | 28.5 ± 9.6 | 29.7 ± 9.6 | 31.6 ± 10.9 | 0.001 | 0.063 |
| Weight SDS | −0.21 ± 0.78 | −0.31 ± 0.79 | −0.31 ± 0.75 | 0.060 | 0.990 |
| BMI | 19.1 ± 3.2 | 18.5 ± 2.9 | 18.7 ± 3.5 | 0.072 | 0.661 |
| BMI SDS | 0.92 ± 0.92 | 0.66 ± 0.96 | 0.56 ± 0.84 | 0.006 | 0.366 |
| Body composition analysis | |||||
| Fat mass, kg | 7.0 ± 3.1 | 7.2 ± 2.9 | 7.9 ± 4.1 | 0.313 | 0.231 |
| Fat mass, % | 24.40 ± 3.13 | 24.06 ± 2.18 | 24.24 ± 3.96 | 0.645 | 0.822 |
| Fat-free mass, kg | 21.1 ± 6.7 | 22.5 ± 6.9 | 23.5 ± 7.2 | 0.001 | 0.046 |
| Fat-free mass, % | 74.26 ± 2.93 | 75.82 ± 2.57 | 75.20 ± 3.65 | 0.175 | 0.497 |
| Fat-free mass percentile | 11.00 ± 9.98 | 18.86 ± 15.96 | 21.71 ± 14.82 | 0.068 | 0.518 |
| ASMM, kg | 7.4 ± 3.0 | 8.0 ± 3.2 | 8.4 ± 3.3 | 0.012 | 0.034 |
| ASMM, % | 25.28 ± 3.09 | 26.18 ± 3.01 | 26.32 ± 2.22 | 0.130 | 0.722 |
| ASMM percentile | 8.14 ± 8.45 | 22.00 ± 16.43 | 25.57 ± 19.60 | 0.006 | 0.356 |
| Muscle-to-fat ratio (range) | 1.06 ± 0.20 (0.87–1.56) | 1.10 ± 0.15 (0.88–1.54) | 1.11 ± 0.18 (0.78–1.63) | 0.420 | 0.824 |
Data are expressed as mean ± standard deviation (SD) or number (percent).
pa represents a comparison between the variable at baseline and after 6 months of treatment.
pb represents a comparison between the variable at 6 months and at 12 months of treatment.
Bold indicates significant.
ASMM, appendicular skeletal muscle mass; BMI, body mass index; SDS, standard deviation score; XLH, X-linked hypophosphatemia.
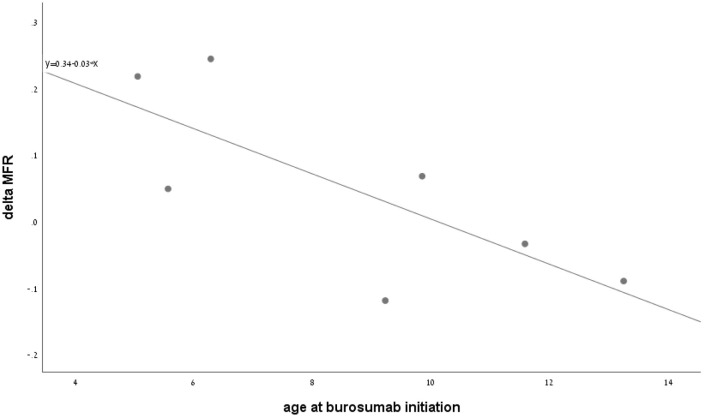
TRSS improved significantly at 12 months of treatment (p = 0.005). Age was significantly correlated with delta TRSS (r = 0.814, p = 0.026), and delta TRSS was significantly correlated with delta MFR (r = −0.826, p = 0.022). Figure 2 presents the correlation between age at initiation of burosumab treatment and delta MFR.
Figure 2.
The correlation between age at burosumab initiation and delta muscle-to-fat ratio after 1 year of therapy.
Discussion
The findings of this analysis revealed that XLH pediatric patients improved their body composition under burosumab treatment as evidenced by decreased adiposity with a simultaneous increase in muscle mass. The 12 months of treatment with burosumab improved linear growth and severity of the rickets. The greater improvement in rickets score under burosumab treatment was associated with older age and with a less pronounced improvement in the composite index of MFR. These findings highlight the need to initiate burosumab treatment at a younger age when rickets is less severe.
Increased prevalence of obesity has been reported in pediatric patients with XLH.13 The observation that one-third of XLH patients are overweight or obese was based on anthropometric measurements with BMI calculations, which is the most widely used method of classifying weight status in the pediatric population.24,25 However, since BMI does not distinguish muscle from adipose tissue, it may therefore underdiagnose subjects with abnormal body composition.26,27,28,29 BMI may be an even poorer predictor of body fat and fat distribution when used in patients with skeletal dysplasia.30 Increased body fat percentage and increased abdominal obesity beginning in early life are strong predictors and key factors in the development of early-onset type 2 diabetes and metabolic syndrome.31 A relatively decreased muscle mass and inactivity are also linked to metabolic derangements.28,32 The results of the current study revealed that 71.4% of the children and adolescents with XLH had body fat percentages above the normal range, and that most of them had muscle mass below the normal range. The combination of increased adiposity with a relatively low muscle mass, termed ‘sarcopenic obesity’, may yield normal values that disguise an abnormal BMI.
During normal growth, children are expected to follow the same percentile, therefore, the improvement observed in the percentile of ASMM% in the current study patients may be attributed to the improvement in their medical condition. Of note, during the year of burosumab treatment, muscle mass increased without a concurrent increase in FM. This is an unexpected, albeit favorable finding, since the study cohort was comprised of prepubertal children and adolescent girls who, unlike adolescent boys, are not expected to undergo an increase only in muscle mass.22 This clinical course led to a decrease in the proportion of patients with an unfavorable body physique after 1 year of burosumab treatment (28.6% of the patients had fat percentage above the normal range and one-half of them had muscle mass below the desired level). This double beneficial effect translated into an increase in the MFR, which had been described as a sensitive detector for metabolic syndrome.29 Of note, none of the current study patients had evidence of elevated blood pressure, impaired fasting glucose levels, or dyslipidemia. Metabolic surveillance is nevertheless warranted in these patients, since abnormal body composition reportedly may precede the presentation of metabolic complications.31
It is well established that burosumab treatment improves linear growth of patients with XLH.15,16,33,34 The profound improvement in height SDS of the current patients was already observed at 6 months of treatment, followed by stabilization, but body composition continued to improve along the entire year of treatment, thus supporting the need for a longer follow-up to determine whether this effect persists or diminishes over time. The conventional treatment with phosphate and calcitriol supplements, even when optimized, was found to be associated with incomplete healing of rickets and persistent short stature.15 Therefore, we speculate that optimizing the dose of phosphate and calcitriol supplements is not expected to change the body composition of patients with XLH.
There was an inverse relationship between radiographic rickets severity at the initiation of burosumab treatment and the beneficial effect on body composition. XLH is a progressive medical condition with deterioration in radiographic bone morphology and in skeletal deformities.34 According to the natural history of the disease, rickets is less severe at a younger age, and a greater improvement in body composition will be observed in patients with decreasing rickets severity. This finding further highlights the need to initiate burosumab treatment as early as possible, when rickets is less severe.
There are multiple plausible explanations for the current findings. Patients treated with burosumab report decreased bone pain, which enables them to engage in physical activity. Physical activity, in turn, contributes to some FFM accretion in prepubertal children, an effect that is accentuated in adolescent boys due to the anabolic effect of testosterone.35 The increase in FFM contributes to an increase in energy expenditure, thereby resulting in loss of FM if an appropriate dietary regimen is followed. Since the majority of the current cohort were prepubertal children and adolescent girls, an increase in physical activity may not be the sole explanation.
Phosphorus deficiency has been linked to the pathogenesis of obesity. Specifically, a decline in hepatic ATP production as a result of hypophosphatemia affects central nervous system pathways, resulting in increased appetite, decreased thermogenesis, and decreased energy expenditure.36,37 Burosumab treatment reportedly normalized blood phosphorous levels of patients after years of persistent hypophosphatemia despite conventional treatment with oral phosphate supplements and vitamin D supplementation. FGF23, a key player in the pathogenesis of XLH, was found to be associated with abdominal obesity in adults,38 although it is not clear whether FGF23 is the cause or the result of obesity. It is tempting to consider that the decrease in its level, combined with decreased fat percentage and increased muscle mass, may limit the risk of metabolic complications in the future.
The present study has several limitations and strengths that warrant clarification. There are no responses to questionnaires on physical activity habits and nutritional consumption, which could contribute to the data interpretation. Another limitation is the small study population. The interpretation of the skeletal X-rays was not blinded to the timing of TRSS evaluation and therefore could introduce bias. However, the utilization of a standardized TRSS scoring method enabled the comparison between rickets severity of patients in different stages of growth. The utilization of BIA for body composition assessment could be inferior to a DEXA scan due to lack of normal reference charts, thus limiting the ability to calculate SDS of fat percentage or muscle mass.39 However, BIA is performed without exposure to radiation, it is easy to perform, and it has a relatively high reproducibility.40 The major strengths of our study are the multi-professional collaboration in the treatment and surveillance of our patients, and the uniformity of growth measurements, physical examinations, and radiographic imaging interpretations that were performed by trained medical personnel throughout the study.
In conclusion, the present study demonstrated a heretofore unrecognized important beneficial change in the body composition of children and adolescents with XLH who were treated with burosumab, as revealed by decreased adiposity with simultaneously increased muscle mass. Studies on larger patient populations with long-term surveillance are needed for further delineation of burosumab’s impact on body composition and its cardiometabolic implications.
Acknowledgments
The authors thank all of the participating patients and their families and the multidisciplinary team that cares for XLH patients treated in the Metabolic Bone Clinic. We thank Esther Eshkol for editorial assistance. Parts of these data were presented in abstract form in the International Conference on Children’s Bone Health, June 2019, Salzburg, Austria.
Footnotes
Author contributions: AB made substantial contributions to the conception and design of the study, acquisition, analysis, and interpretation of data and drafting the initial manuscript. YL made substantial contributions to the design of the study and reviewed it critically for important intellectual content. RK and LK contributed to the discussion and reviewed the manuscript critically for important intellectual content. LZ made substantial contributions to the conception and design of the study, the interpretation of data, and critical revision of the manuscript for important intellectual content. All authors gave final approval of the version to be published. AB is the guarantor of this work, and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Data availability: The data used to support the findings of this study are available from the corresponding author upon request.
ORCID iD: Avivit Brener  https://orcid.org/0000-0002-2742-8264
https://orcid.org/0000-0002-2742-8264
Contributor Information
Avivit Brener, Pediatric Endocrinology and Diabetes Unit, Dana-Dwek Children’s Hospital, Tel Aviv Sourasky Medical Center, 6 Weizmann Street, Tel Aviv, 6423906, Israel; Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Yael Lebenthal, Pediatric Endocrinology and Diabetes Unit, Dana-Dwek Children’s Hospital, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel; Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Roxana Cleper, Pediatric Nephrology Unit, Dana-Dwek Children’s Hospital, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel; Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Livia Kapusta, Pediatric Cardiology Unit, Dana-Dwek Children’s Hospital, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel; Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel; Department of Paediatric Cardiology, Amalia Children’s Hospital, Radboud University Medical Centre, Nijmegen, The Netherlands.
Leonid Zeitlin, Pediatric Orthopedic Department, Dana-Dwek Children’s Hospital, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel; Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
References
- 1. Rowe PS. The PEX gene: its role in X-linked rickets, osteomalacia, and bone mineral metabolism. Exp Nephrol 1997; 5: 355–363. [PubMed] [Google Scholar]
- 2. Zheng B, Wang C, Chen Q, et al. Functional characterization of PHEX gene variants in children with X-linked hypophosphatemic rickets shows no evidence of genotype-phenotype correlation. J Bone Miner Res. Epub ahead of print 24 April 2020. DOI: 10.1002/jbmr.4035. [DOI] [PubMed] [Google Scholar]
- 3. Barros NM, Hoac B, Neves RL, et al. Proteolytic processing of osteopontin by PHEX and accumulation of osteopontin fragments in Hyp mouse bone, the murine model of X-linked hypophosphatemia. J Bone Miner Res 2013; 28: 688–699. [DOI] [PubMed] [Google Scholar]
- 4. Yu X, Kennedy RH, Liu SJ. JAK2/STAT3, not ERK1/2, mediates interleukin-6-induced activation of inducible nitric-oxide synthase and decrease in contractility of adult ventricular myocytes. J Biol Chem 2003; 278: 16304–16309. [DOI] [PubMed] [Google Scholar]
- 5. Murali SK, Andrukhova O, Clinkenbeard EL, et al. Excessive osteocytic Fgf23 secretion contributes to pyrophosphate accumulation and mineralization defect in Hyp mice. PLoS Bio 2016; 14: e1002427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuro-O M, Moe OW. FGF23-αKlotho as a paradigm for a kidney-bone network. Bone 2017; 100: 4–18. [DOI] [PubMed] [Google Scholar]
- 7. Stöhr R, Schuh A, Heine GH, et al. FGF23 in cardiovascular disease: innocent bystander or active mediator? Front Endocrinol (Lausanne) 2018; 9: 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011; 121: 4393–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu ES, Thoonen R, Petit E, et al. Increased circulating FGF23 does not lead to cardiac hypertrophy in the male Hyp mouse model of XLH. Endocrinology 2018; 159: 2165–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shalhoub V, Shatzen EM, Ward SC, et al. FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J Clin Invest 2012; 122: 2543–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hawley S, Shaw NJ, Delmestri A, et al. Prevalence and mortality of individuals with X-linked hypophosphatemia: a United Kingdom real-world data analysis. J Clin Endocrinol Metab 2020; 105: e871–e878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bär L, Feger M, Fajol A, et al. Insulin suppresses the production of fibroblast growth factor 23 (FGF23). Proc Natl Acad Sci U S A 2018; 115: 5804–5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhukouskaya VV, Rothenbuhler A, Colao A, et al. Increased prevalence of overweight and obesity in children with X-linked hypophosphatemia. Endocr Connect 2020; 9: 144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haffner D, Emma F, Eastwood DM, et al. Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia. Nat Rev Nephrol 2019; 15: 435–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carpenter TO, Whyte MP, Imel EA, et al. Burosumab therapy in children with X-linked hypophosphatemia. N Engl J Med 2018; 378: 1987–1998. [DOI] [PubMed] [Google Scholar]
- 16. Imel EA, Glorieux FH, Whyte MP, et al. Burosumab versus conventional therapy in children with X-linked hypophosphataemia: a randomised, active-controlled, open-label, phase 3 trial. Lancet 2019; 393: 2416–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000. CDC growth charts for the United States: methods and development. Vital Health Stat 11 2002; 246: 1–190. [PubMed] [Google Scholar]
- 18. Flynn JT, Kaelber DC, Baker-Smith CM, et al.; Subcommittee on Screening and Management of High Blood Pressure in Children. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 2017; 140: e20171904. [DOI] [PubMed] [Google Scholar]
- 19. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child 1970; 45: 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969; 44: 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee SY, Gallagher D. Assessment methods in human body composition. Curr Opin Clin Nutr Metab Care 2008; 11: 566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McCarthy H, Cole T, Fry T, et al. Body fat reference curves for children. Int J Obes 2006; 30: 598–602. [DOI] [PubMed] [Google Scholar]
- 23. Thacher TD, Pettifor JM, Tebben PJ, et al. Rickets severity predicts clinical outcomes in children with X-linked hypophosphatemia: utility of the radiographic rickets severity score. Bone 2019; 122: 76–81. [DOI] [PubMed] [Google Scholar]
- 24. Cole TJ, Bellizzi MC, Flegal KM, et al. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000; 320: 1240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maynard LM, Wisemandle W, Roche AF, et al. Childhood body composition in relation to body mass index. Pediatrics 2001; 107: 344–350. [DOI] [PubMed] [Google Scholar]
- 26. McCarthy HD. Body fat measurements in children as predictors for the metabolic syndrome: focus on waist circumference. Proc Nutr Soc 2006; 65: 385–392. [DOI] [PubMed] [Google Scholar]
- 27. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med 2006; 23: 469–480. [DOI] [PubMed] [Google Scholar]
- 28. Hunter GR, Singh H, Carter SJ, et al. Sarcopenia and its implications for metabolic health. J Obes 2019; 2019: 8031705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramírez-Vélez R, Carrillo HA, Correa-Bautista JE, et al. Fat-to-muscle ratio: a new anthropometric indicator as a screening tool for metabolic syndrome in young Colombian people. Nutrients 2018; 10: 1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fredwall SO, Maanum G, Johansen H, et al. Current knowledge of medical complications in adults with achondroplasia: a scoping review. Clin Genet 2020; 97: 179–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Magge SN, Goodman E, Armstrong SC; Committee on Nutrition; Section on Endocrinology; Section on Obesity. The metabolic syndrome in children and adolescents: shifting the focus to cardiometabolic risk factor clustering. Pediatrics 2017; 140: e20171603. [DOI] [PubMed] [Google Scholar]
- 32. Bowden Davies KA, Pickles S, Sprung VS, et al. Reduced physical activity in young and older adults: metabolic and musculoskeletal implications. Ther Adv Endocrinol Metab 2019; 10: 2042018819888824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Santos Rodríguez F. X-linked hypophosphataemic rickets and growth. Adv Ther 2020; 37(Suppl. 2): 55–61. [DOI] [PubMed] [Google Scholar]
- 34. Kinoshita Y, Fukumoto S. X-linked hypophosphatemia and FGF23-related hypophosphatemic diseases: prospect for new treatment. Endocr Rev 2018; 39: 274–291. [DOI] [PubMed] [Google Scholar]
- 35. Westerterp KR. Changes in physical activity over the lifespan: impact on body composition and sarcopenic obesity. Obes Rev 2018; 19(Suppl. 1): 8–13. [DOI] [PubMed] [Google Scholar]
- 36. Obeid OA. Low phosphorus status might contribute to the onset of obesity. Obes Rev 2013; 14: 659–664. [DOI] [PubMed] [Google Scholar]
- 37. Bassil MS, Obeid OA. Phosphorus supplementation recovers the blunted diet-induced thermogenesis of overweight and obese adults: a pilot study. Nutrients 2016; 8: 801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hu X, Ma X, Luo Y, et al. Associations of serum fibroblast growth factor 23 levels with obesity and visceral fat accumulation. Clin Nutr 2018; 37: 223–228. [DOI] [PubMed] [Google Scholar]
- 39. Tompuri TT, Lakka TA, Hakulinen M, et al. Assessment of body composition by dual-energy X-ray absorptiometry, bioimpedance analysis and anthropometrics in children: the physical activity and nutrition in children study. Clin Physiol Funct Imaging 2015; 35: 21–33. [DOI] [PubMed] [Google Scholar]
- 40. Kyle UG, Earthman CP, Pichard C, et al. Body composition during growth in children: limitations and perspectives of bioelectrical impedance analysis. Eur J Clin Nutr 2015; 69: 1298–1305. [DOI] [PubMed] [Google Scholar]