Abstract
Emerging evidence indicates that males and females display different neurobiological responses to chronic stress which contribute to varied behavioral adaptations. In particular, pyramidal neurons undergo dendritic atrophy and synapse loss in the prefrontal cortex (PFC) of male, but not female, mice. Our recent work shows that chronic stress also provokes microglia-mediated neuronal remodeling, which contributes to synaptic deficits in the PFC and associated behavioral consequences in males. Separate studies indicate that chronic stress promotes astrocyte dystrophy in the PFC which is associated with behavioral despair. Notably, these prior reports focused primarily on stress effects in males. In the present studies, male and female mice were exposed to 14 or 28 days of chronic unpredictable stress (CUS) to assess molecular and cellular adaptations of microglia, astrocytes, and neurons in the medial PFC. Consistent with our recent work, male, but not female, mice displayed behavioral and cognitive deficits with corresponding perturbations of neuroimmune factors in the PFC after 14 days of CUS. Fluorescence-activated cell sorting and gene expression analyses revealed that CUS increased expression of select markers of phagocytosis in male PFC microglia. Confocal imaging in Thy1-GFP(M) mice showed that CUS reduced dendritic spine density, decreased GFAP immunolabeling, and increased microglia-mediated neuronal remodeling only in male mice. After 28 days of CUS, both male and female mice displayed behavioral and cognitive impairments. Interestingly, there were limited stress effects on neuroimmune factors and measures of microglial phagocytosis in the PFC of both sexes. Despite limited changes in neuroimmune function, reduced GFAP immunolabeling and dendritic spine deficits persisted in male mice. Further, GFAP immunolabeling and dendritic spine density remained unaltered in the PFC of females. These findings indicate that chronic stress causes sex-specific and temporally dynamic changes in microglial function which are associated with different neurobiological and behavioral adaptations. In all, these results suggest that microglia-mediated neuronal remodeling, astrocyte dystrophy, and synapse loss contribute to stress-induced PFC dysfunction and associated behavioral consequences in male mice.
Keywords: Stress, Sex differences, Microglia, Astrocytes, Prefrontal cortex
Highlights
-
•
Male but not female mice show behavioral and cognitive deficits after CUS (14 days).
-
•
CUS-induced synapse loss was linked to neuronal remodeling by microglia in the PFC.
-
•
Prolonged CUS (28 days) caused behavioral impairments in both males and females.
-
•
At 28 days spine deficits remained, but microglial engulfment was limited in males.
-
•
Spine density and microglia function were unaltered in females after prolonged CUS.
1. Introduction
Stress is experienced when an organism encounters real or perceived threats to homeostasis. In response, behavior and physiological processes are adjusted to promote survival and reestablish homeostasis (Herman, 2013; Ulrich-Lai and Herman, 2009). Although this response is initially adaptive, chronic or prolonged stress produces deleterious effects on molecular and cellular neurobiology (Duman et al., 2016; Krishnan and Nestler, 2008). Separate studies showed that chronic stress drives structural remodeling of neurons (i.e., dendritic atrophy; synaptic loss) (Duric et al., 2010; Ota et al., 2014; Radley et al., 2006), reductions in glial fibrillary acidic protein (GFAP) indicating astrocyte dystrophy (Banasr and Duman, 2008), and morphological changes in ionized calcium-binding adaptor (IBA)-1 suggesting microglia activation (Tynan et al., 2010) in brain regions mediating complex behaviors, such as the prefrontal cortex (PFC). Each of these neurobiological effects have been implicated in stress-induced behavioral and cognitive impairments. Indeed viral-mediated over-expression of neuronal REDD1, a negative regulator of synaptogenesis increased by chronic stress, reduced synapse density in the PFC and drove anhedonia and passive stress-coping (Ota et al., 2014). Other studies showed that the behavioral effects of chronic stress can be recapitulated by depleting astrocytes in the PFC (Banasr and Duman, 2008). Further studies showed that the antibiotic minocycline, which suppresses microglia activation, can mitigate stress-induced working memory deficits (Hinwood et al., 2012). While these findings demonstrate cell-specific effects of chronic stress, it is becoming increasingly clear that interactions between various cells in the brain contribute to the neurobiological and behavioral effects of stress. For example, work from our lab showed that chronic stress exposure increased neuronal colony-stimulating factor (CSF)-1 in the PFC; a key regulator of microglia function (Bohlen et al., 2017). Further studies showed that CSF1 signaling provoked microglia-mediated neuronal remodeling, which contributed to synapse loss in the PFC and associated behavioral consequences (Horchar and Wohleb, 2019; Wohleb et al., 2018). These studies support the notion that dynamic, multi-cellular adaptations contribute to stress effects on behavior and cognition (Arnsten, 2015; Wohleb, 2016).
Notably, stress research has been limited by a focus on the neurobiology of stress in male rodents (Shansky and Morrison, 2009). This is relevant because recent work indicates that chronic stress promotes divergent neurobiological effects in males and females. For example, dendritic atrophy of neurons and morphological changes in microglia are more pronounced in males as compared to females (Bollinger et al, 2016, 2017; Garrett and Wellman, 2009). Our prior work expounded on these studies and showed that PFC microglia in males and females show different phenotypic responses to chronic stress (Wohleb et al., 2018). However, those studies were not designed to directly compare molecular and functional changes in microglia between sexes. Further, it remains unclear if differences in stress responses between males and females persist or if similar processes emerge after extended exposure to chronic stress. These research questions are significant because the neurobiological effects of chronic stress resemble pathophysiological features of psychiatric disorders, particularly Major Depressive Disorder (MDD). Indeed preclinical stress models can provide valuable insight, as exposure to psychological stress is known to influence the onset and severity of MDD (Gilman et al., 2013, Kendler et al., 1999, McLaughlin et al., 2010). In line with preclinical findings, postmortem studies have demonstrated that individuals with MDD have a lower density of dendritic spines and astrocytes in the medial PFC (Cotter et al., 2001; Duman et al., 2016; Kang et al., 2012). Recently, RNA sequencing revealed that men and women with MDD have divergent molecular changes in the PFC. Specifically, reduced synapse-associated gene expression was found in men, while women had an enrichment of these genes (Seney et al., 2018). Moreover, microglia-associated transcripts were increased in men and decreased in women, indicating that sex differences are not restricted to neurons. Altogether these data imply that distinct neurobiological pathways contribute to the pathophysiology of men and women suffering from MDD. These molecular changes may be linked to sex-specific risk factors (i.e., trauma exposure) and may underlie differences in reported symptoms and incidence of MDD in men and women (Otte et al., 2016). Further studies into the varied neurobiological responses to stress may provide insight into the sex differences observed in these clinical conditions.
In the present studies, mice were exposed to varied durations of chronic unpredictable stress (CUS) to determine how sex influences neurobiological and behavioral responses to stress. Our results indicate that male and female mice initially show diverging behavioral effects after 14 days of CUS, but show similar responses following 28 days of CUS. Notably, behavioral consequences in male mice were associated with increased microglia-mediated neuronal remodeling and synaptic deficits in the PFC at 14 days. Additionally, we observed a transient increase in microglia-mediated neuronal remodeling in the medial PFC of male mice, whereas microglial activity remained relatively unchanged in females. These results suggest that chronic stress differentially regulates neuron-microglia interactions in the PFC of male and female mice, leading to divergent effects on synaptic plasticity and behavior. Further, these studies indicate that structural remodeling of neurons by microglia is not persistent and that synaptic deficits in the PFC and associated behavioral consequences are maintained by other molecular and cellular processes.
2. Materials and methods
2.1. Animals
Studies were performed using adult male and female mice (6–10 weeks old). Mice were group-housed (4/cage) in 11.5″x 7.5″x 6″ polypropylene cages under a 12 h light-dark cycle (light on: 08:00–20:00) with ad libitum access to water and rodent chow. Transgenic (and wild-type littermate) Thy1-GFP-M mice were obtained from in-house breeders (Jackson Laboratories; Tg(Thy1-EGFP)MJrs/J; #007788). Wild-type C57BL/6 mice were purchased from Jackson Laboratories (#000664). Mice were randomly assigned to experimental groups. Animal experiments were carried out in accordance with the National Institutes of Health guide for the care and use of laboratory animals and studies were approved by the University of Cincinnati Institutional Animal Care and Use Committee. As such, all efforts were made to minimize animal suffering and reduce the number of animals used.
2.2. Chronic unpredictable stress (CUS)
CUS was performed as previously described (Wohleb et al., 2018). In brief, mice were exposed to two random daily stressors for 14 or 28 consecutive days. Stressors included cage rotation, physical restraint, isolation, radio noise, food or water deprivation, light on overnight, light off during day, rat odor, stroboscopic light, crowding, wet bedding, no bedding, or tilted cage. This well-established stress paradigm causes persistent elevations in circulating glucocorticoids (Kreisel et al., 2014; Ota et al., 2014; Horchar and Wohleb, 2019).
2.3. Behavioral and cognitive testing
The open field test (OFT), forced swim test (FST), and temporal order recognition task (TOR) were conducted as previously described (Wohleb et al., 2018; Yuen et al., 2012). All behavioral tests were performed in a normally lit room (white light), during the light phase of the circadian cycle (08:00–11:00). The light intensity during behavioral testing was approximately 300 lux. Mice were placed in the room 30 min prior to testing to habituate. For the OFT, individual mice were placed in an empty, white Plexiglas™ arena (22 × 30 × 14 cm). Activity was recorded for 10 min and videos were analyzed using Noldus EthoVision XT 12. For the FST, mice were placed in a 2-L beaker of water (24° ±1 °C) for 10 min and time spent immobile was recorded (Can et al., 2011). For the TOR, mice were placed in a plastic arena with two plastic Lego™ trees secured to the bottom of the arena for 5 min. Thirty minutes after the initial stage, mice were placed in the same arena with Lego™ blocks for 5 min. An hour after the second stage, mice were placed in the arena with one block and one tree, counterbalanced to prevent bias. The time spent exploring each of these objects was measured. A discrimination index was calculated as: difference between time spent exploring the tree or the block divided by the total time spent exploring the tree and the block. Mice were exposed to all behavior/cognitive tests prior to sacrifice for gene expression analyses. All behavioral analyses were performed by a well-trained experimenter blinded to experimental conditions.
2.4. RNA isolation and quantitative real-time PCR
Mice were sacrificed via rapid cervical dislocation and the PFC was dissected into a micro-centrifuge tube and placed into dry ice. Tissue was stored at −80 °C until further processing. RNA was extracted from whole PFC using TRIzol Reagent according to manufacturer's protocol (Invitrogen). RNA was extracted from microglia using a Single Cell RNA Purification Kit (Norgen Biotek Corp., Thorold, Canada). For each sample RNA concentration and quality were assessed using a Nanodrop (consistent mRNA quality was observed with 260/280 ratios > 1.8). Samples were reverse transcribed using a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, #4368814). Quantitative real-time PCR was conducted using a 7900 Real-Time PCR System (Applied Biosystems), with samples run in duplicate as previously described (Wohleb et al., 2018). The cycle threshold (CT) was determined for all genes, normalized to housekeeping gene levels (Gapdh), and fold change was standardized to male-controls using the 2−ΔΔCT method (Livak and Schmittgen, 2001). All primers were tested for efficiency with varied concentrations of cDNA and analyses of the melting curve before use in assays. The housekeeping gene Gapdh was used for all assays as we did not observe any differences in relative expression (CT) across experimental conditions. Primers used for this study are listed in Table 1.
Table 1.
List of primers used for qRT-PCR.
| Gene | Forward | Reverse |
|---|---|---|
| Gapdh | GAAGGTCGGTGTGAACGGATTTGGC | GATGGGCTTCCCGTTGATGACAAGC |
| Csf1 | CGAGTCAACAGAGCAACCAA | TGTCAGTCTCTGCCTGGATG |
| Il34 | TACAGCCACCTCTGCTTGTG | GCAAGATACGGCATTTGGTT |
| Cx3cl1 | ATCCGCCACATTGGAAGACC | ACAGCAGGACTCGGCCAAAC |
| Tgfb1 | CCCTATATTTGGAGCCTGGA | CTTGCGACCCACGTAGTAGA |
| Tnfa | CGACGTGGAACTGGCAGAAG | GAGGGAGGCCATTTGGGAAC |
| Bdnf | TTGTTTTGTGCCGTTTACCA | GGTAAGAGAGCCAGCCACTG |
| Vegf | GCCTCGGTTCCAGAAGGGAGAGG | GTCCAGTGCACCCAAGAGAGCAG |
| Gfap | CGAGCGTGCAGAGATGATGG | CTGTTGGCCGTAAGCTGGTC |
| Aldh1l1 | AAAGCGGGACTCATCCTCTTTGG | CTTGAAGAACTGGGAGGCTGG |
| Csf1r | GACCCTGAATCTCCCGGAAG | GGTACAACGGTAGGTCCCAG |
| Cx3cr1 | AGCCACTGTTGCCTCAACCC | AATGCTGTCCTGCCTGCTCCTC |
| Tgfbr1 | GCGGGGAGAAGAAGTTGCTG | CGTCCATGTCCCATTCTCTTTG |
| Cd11b | TCCGGTAGCATCAACAACAT | GGTGAAGTGAATCCGGAACT |
| Trem2 | CCAGTCCTTGAGGGTGTCAT | AGAGTGATGGTGACGGTTCC |
| Apoe | AACTGACGGCACTGATGGAGGAC | GGTTGCGTAGATCCTCCATGTCGG |
| P2ry12 | CACGGATTCCCTACACCCTG | GGGTGCTCTCCTTCACGTAG |
| Mertk | GAGACTCCCAGTCAACCACAGG | AAACGCAACAGGAGGTAGGAG |
2.5. Percoll gradient enrichment of microglia
Mice were sacrificed via rapid cervical dislocation, at which point the frontal cortex was dissected out and placed in ice-cold 1 × PBS. Samples were then passed through a 70 μm cell strainer. Homogenates were centrifuged at 1200×g for 5 min. Supernatants were removed and cell pellets were re-suspended in 70% isotonic Percoll (GE Healthcare, Uppsalla, Sweden, #17089102). A discontinuous Percoll density gradient was layered as follows: 30% and 0% isotonic Percoll. The gradient was centrifuged for 20 min at 2000×g and enriched microglia were collected from the interphase between the 70% and 30% Percoll layers. Enriched microglia were labeled with antibodies for flow cytometry and sorted based on CD11b/CD45 expression using a BioRad S3e cytometer/cell sorter (Hercules, CA, U.S.A.).
2.6. Fluorescence-activated cell sorting (FACS)
Following Percoll enrichment, samples were stained for surface antigens, as previously described (Horchar and Wohleb, 2019). In brief, Fc receptors were blocked with anti-CD16/CD32 antibody (BioLegend, San Diego, CA, U.S.A., #553141). Cells were washed and then incubated with conjugated antibodies (FITC-CX3CR1, #149020 Biolegend; PE-CD115, #565249 BD Biosciences; PE-CF594-CD45, #562420 BD Biosciences; PerCp-Cy5.5-CD11b, #550993 BD Biosciences) for 1 h at 4οC. Cells were washed and then re-suspended in FACS buffer for analysis. Non-specific binding was assessed using isotype-matched antibodies. Antigen expression was determined using a BioRad S3e four-color cytometer/cell sorter. Microglia were identified based on cell surface markers (CD11b+/CD45lo) and were collected for RNA isolation. Other myeloid cells (i.e., macrophages/monocytes: CD11b+/CD45hi) were excluded from the sorted sample. Data were analyzed using FlowJo software (Ashland, OR, U.S.A.).
2.7. Immunohistology
Mice were anesthetized with a high dose of sodium pentobarbital solution (Fatal-Plus) and transcardially perfused with sterile PBS and 4% paraformaldehyde (PFA). Brains were post-fixed in 4% PFA for 24 h and incubated in 30% sucrose for an additional 24 h. Frozen brains were sectioned at 40 μm using a Leica CM3050 S cryostat. Free-floating sections were washed, then blocked for 1 h at room temperature. Sections were washed, then incubated with primary antibodies: rabbit anti-IBA1 (Wako, #019–19741) or rat anti-GFAP (Invitrogen, #13–0300) overnight at 4 °C. Sections were washed and incubated with conjugated secondary antibody overnight at 4 °C (Alexa Fluor 546 donkey anti-rabbit, #A10040; Alexa Fluor 546 goat anti-rat, #A11081, Fisher Scientific). Following secondary antibody incubation, sections were washed, mounted on Superfrost(+) slides (Fisher Scientific) with Fluoromount-G, and coverslipped (Fisher Scientific).
2.8. Quantitative immunofluorescence
Confocal images were obtained on a Nikon C2+ microscope equipped with CFI Plan Apochromat Lambda Series Objectives. Images were captured with Nikon C2si+ camera and analyzed using Nikon Elements software. In Thy1-GFP(M) mice, layer I of the medial PFC was identified and apical dendritic segments were imaged using 40x objective lens with a 2.5x zoom (NA 1.4) with z-stack sampling: 0.075 μm. For each sample, 6–8 dendritic segments were analyzed in NeuronStudio as previously described (Wohleb et al., 2018). Proportional area of GFAP+ or IBA-1+ material in images taken with the 20x objective was measured using standardized threshold parameters with ImageJ software (NIH, Bethesda, Maryland). IBA-1+ cells were counted by a trained researcher blinded to animal condition (4–6 images per sample). To quantify the number and volume of GFP + inclusions within IBA-1+ microglia (16–25 per sample) or GFAP + astrocytes, confocal images (40x; zoom 2.5x), NA 0.95, z-stack sampling: 0.2 μm) were examined in 3-dimensional space and orthogonal z-stacks with Nikon Elements Image Analysis software. Confocal imaging with these settings provides sufficient resolution to detect synaptic inclusions (average inclusion diameter: 0.25 μm) (Weinhard et al., 2018).
2.9. Statistical analysis
Data were analyzed using GraphPad Prism 8.1.2 (La Jolla, California). Significant main effects and interactions were determined using two-way ANOVA (sex × stress). Sidak's multiple comparisons test was used to assess baseline sex-differences and stress effects by comparing opposite-sex control groups, as well as same-sex control and CUS groups. The relationship between histological parameters were determined using the Pearson's correlation coefficient.
3. Results
3.1. Male, but not female, mice display altered stress coping and working memory impairments after 14 days of CUS
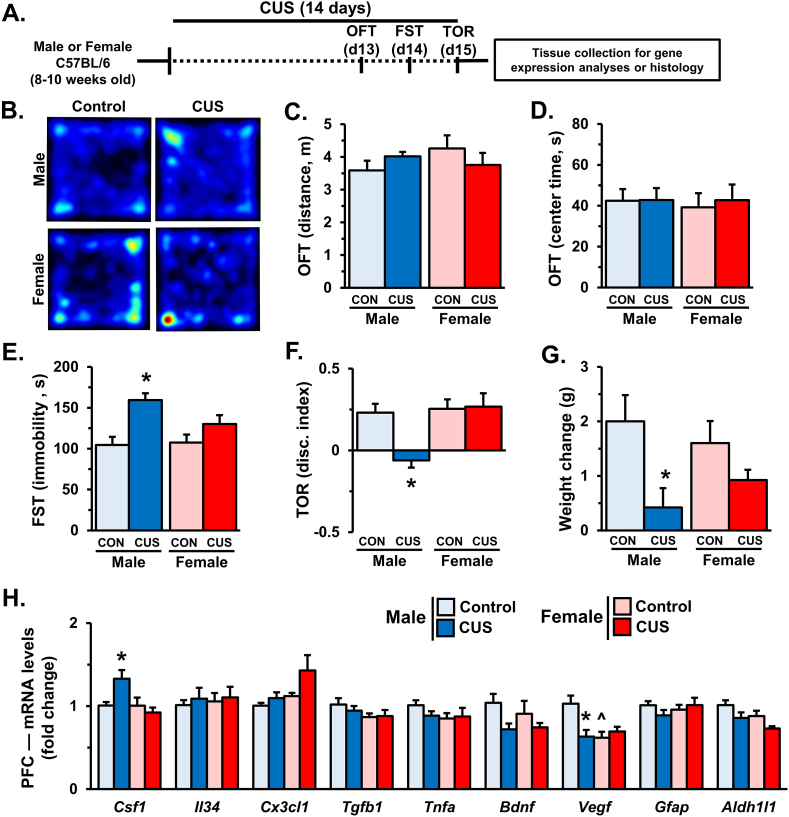
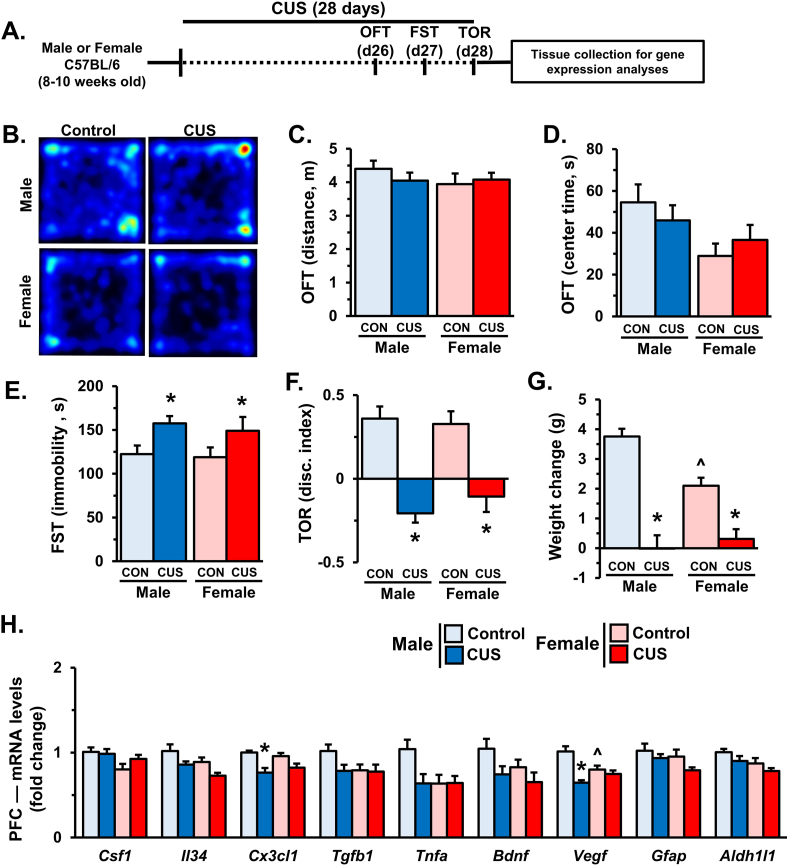
To examine the effects of 14 days CUS on behavioral and cognitive performance, mice were tested in the open-field test (OFT), forced swim test (FST), and temporal object recognition task (TOR) on subsequent days (Fig. 1A). Representative heat maps show that male and female mice had comparable locomotion and time spent in the center of the OFT following 14 days of CUS (Fig. 1B–D). There was an effect of CUS on immobility in the FST (stress: F1,25 = 9.052, p < 0.006), with post-hoc analyses indicating heightened immobility in male but not female mice (p < 0.02, Fig. 1E). Similarly, CUS impaired temporal object discrimination in males but not females (interaction: F1,25 = 5.807, p < 0.03; Fig. 1F). At the end of these experiments mice were weighed to assess physiological effects of stress. Overall, 14 days CUS decreased weight gain (stress: F1,37 = 9.754, p < 0.004). Follow-up comparisons indicate this was driven by reduced weight gain in male mice exposed to CUS (p < 0.02, Fig. 1G).
Fig. 1.
Male, but not female, mice show behavioral changes after 14 days of CUS. Male and female C57BL/6 mice were exposed to 14 days of chronic unpredictable stress (CUS) or handled as controls. Mice were assessed in the open field test (OFT), forced swim test (FST), and temporal object recognition test (TOR) on subsequent days (n = 7–8/group). A) Schematic showing experimental approach and timeline. B) Representative heat maps from the OFT. Distance traveled (C) and time spent in the center of the OFT (D) are shown. E) Immobility during the FST. F) Discrimination index as measured by the TOR. Two hours after testing, weight change (G) was calculated (n = 9–12/group), and the prefrontal cortex (PFC) was dissected for mRNA analyses. H) Relative fold change of mRNA levels in the PFC of male and female were determined using qRT-PCR (n = 7–8/group). Bars represent the mean ± S.E.M. Based on post-hoc analyses, means significantly different than same-sex controls are denoted (*, p < 0.05) while those different from opposite sex controls are noted by (^, p < 0.05).
Following behavioral testing, the PFC was dissected to assess the expression of neuroimmune genes, neurotrophic factors, and astrocyte-specific transcripts (Fig. 1H). Consistent with prior studies (Wohleb et al., 2018), 14 days of CUS increased Csf1 mRNA levels in the PFC of male mice (interaction: F1,28 = 7.459, p < 0.02). Control females showed higher baseline Cx3cl1 expression than their male counterparts (sex: F1,28 = 4.758, p < 0.04), however post hoc analyses did not show significant differences between groups. Moreover, there were no significant sex differences in- or stress effects on- Il34, Tgfb1, or Tnfa expression in the PFC. Exposure to 14 days of CUS reduced Bdnf transcript in the PFC (stress: F1,28 = 5.313, p < 0.03) across groups, but no significant differences were observed in post hoc comparisons. We observed effects of stress and sex on Vegf expression (interaction: F1,28 = 9.203, p < 0.006) however, post hoc comparisons revealed that the effect of CUS was specific to males (p < 0.004), and that females had lower baseline Vegf expression compared to control males (p < 0.003). There were no observed differences in mRNA expression of the astrocyte marker Gfap; but overall females showed lower levels of Aldh1ll (sex: F1,27 = 5.03, p < 0.04) and stress modestly decreased Aldh1l1 transcript across groups (stress: F1,27 = 6.862, p < 0.02). Altogether these data indicate that 14 days of CUS promotes sex-specific molecular changes in the PFC that correspond with divergent behavioral responses.
3.2. Exposure to 14 days of CUS causes varied molecular adaptations in PFC microglia of male and female mice
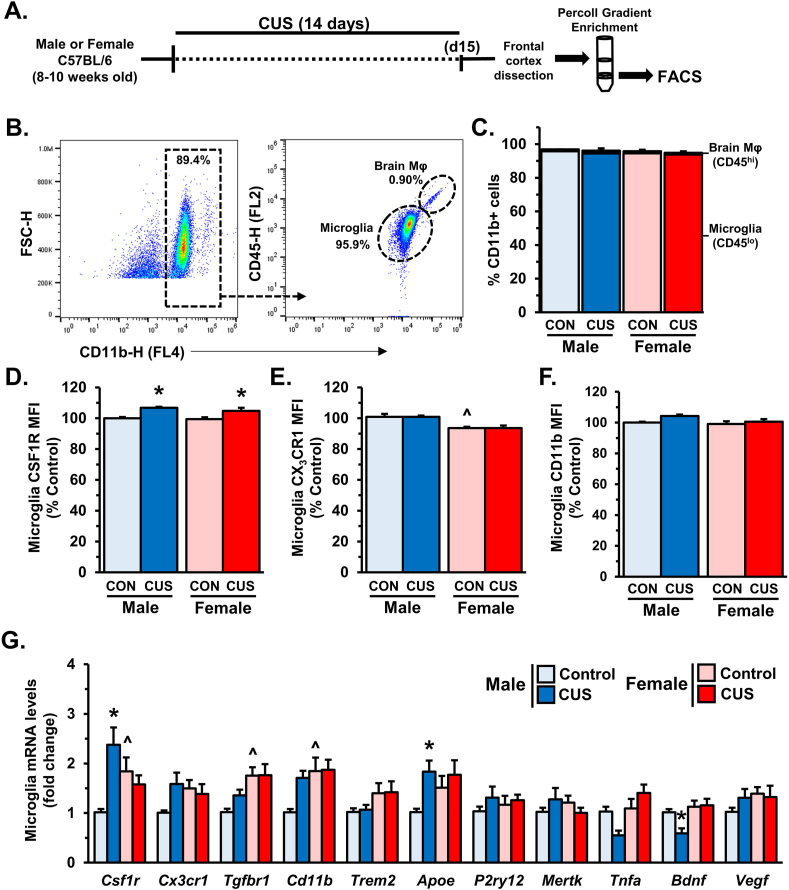
In subsequent experiments, fresh samples of frontal cortex were dissected from male or female mice after 14 days of CUS or control conditions. Following Percoll density gradient enrichment, microglia (CD11b+/CD45lo) were collected by fluorescence-activated cell sorting (FACS) for gene expression analyses (Fig. 2A). Representative dot plots show the gating strategy used for all samples (Fig. 2B). Simultaneous flow cytometry confirmed that the proportion of microglia and macrophages (CD11b+/CD45hi) in the PFC did not differ in male or female mice after 14 days of CUS (Fig. 2C). Analyses of mean fluorescent intensity (MFI) showed that 14 days of chronic stress exposure increased cell surface expression of CSF1R (stress: F1,12 = 22.46, p < 0.0006) in both male (p < 0.009) and female (p < 0.04) mice (Fig. 2D). Our analyses also showed that females had less cell surface expression of CX3CR1 (sex: F1,12 = 24.26; p < 0.0005, Fig. 2E); but this baseline difference was not affected by stress. Further, 14 days of CUS modestly increased surface expression of CD11b on PFC microglia across groups (stress: F1,28 = 4.604; p < 0.05, Fig. 2F).
Fig. 2.
Diverging molecular adaptations to CUS in male and female microglia. Male and female C57BL/6 mice were exposed to 14 days of chronic unpredictable stress (CUS) or handled as control (CON). A) Two hours after the final stressor frontal cortex was dissected for microglia enrichment through Percoll gradient isolation. Following enrichment microglia were sorted (FACS) based on CD11b+/CD45lo expression. B) Gating strategy for FACS is shown. C) Relative quantities of microglia (CD11b+/CD45lo) and brain macrophages (CD11b+/CD45hi). Surface levels of CSF1R (CD115) (D), CX3CR1 (E), and CD11b (F) were determined (n = 4–8/group). Following FACS, sorted microglia were used for transcriptional analyses via qRT-PCR (n = 6–8/group) (G). Bars represent the mean ± S.E.M. Means significantly different than same-sex controls based on ANOVA are denoted (*, p < 0.05). Means significantly different from opposite sex controls based on ANOVA are noted by (^, p < 0.05).
After FACS, mRNA was isolated from purified microglia for gene expression analyses (Fig. 2G). Similar to prior findings, 14 days of CUS increased Csf1r expression in PFC microglia (interaction: F1,26 = 12.46, p < 0.002) in male mice (p < 0.002). Of note, post hoc comparisons showed that control females had higher Csf1r transcript compared to control males (p < 0.05). There was also a subtle sex-dependent change in Cx3cr1 expression following CUS (interaction: F1,26 = 4.14, p = 0.05) that could be attributed to baseline differences in females and a modest increase in males after CUS (p = 0.08). Moreover, females showed higher expression of Tgfbr1 (sex: F1,26 = 12.56; p < 0.002), Cd11b (sex: F1,26 = 6.538; p < 0.02), and Trem2 (sex: F1,24 = 4.477, p < 0.05) as compared to males. Further analyses revealed that 14 days of CUS increased Apoe expression in microglia (stress: F1,25 = 5.783; p < 0.03), which was most pronounced in males (p = 0.05). Other microglia-enriched genes, P2ry12 and Mertk, were unchanged after 14 days of CUS. Levels of Tnfa were differentially regulated in male and female mice following CUS (interaction: F1,28 = 7.688; p < 0.01); with males showing a slight decrease in Tnfa expression (p = 0.07). Interestingly CUS exposure altered microglial Bdnf expression (interaction: F1,26 = 4.316; p < 0.05), with males showing significant reductions in Bdnf levels (p < 0.03). However, unlike our results from whole PFC, microglial Vegf was not altered by CUS. These data indicate that the molecular profile of microglia varies between sexes, and chronic stress causes distinct molecular changes in microglia from males and females.
3.3. Exposure to 14 days of CUS decreases spine density in the PFC of male, but not female, mice
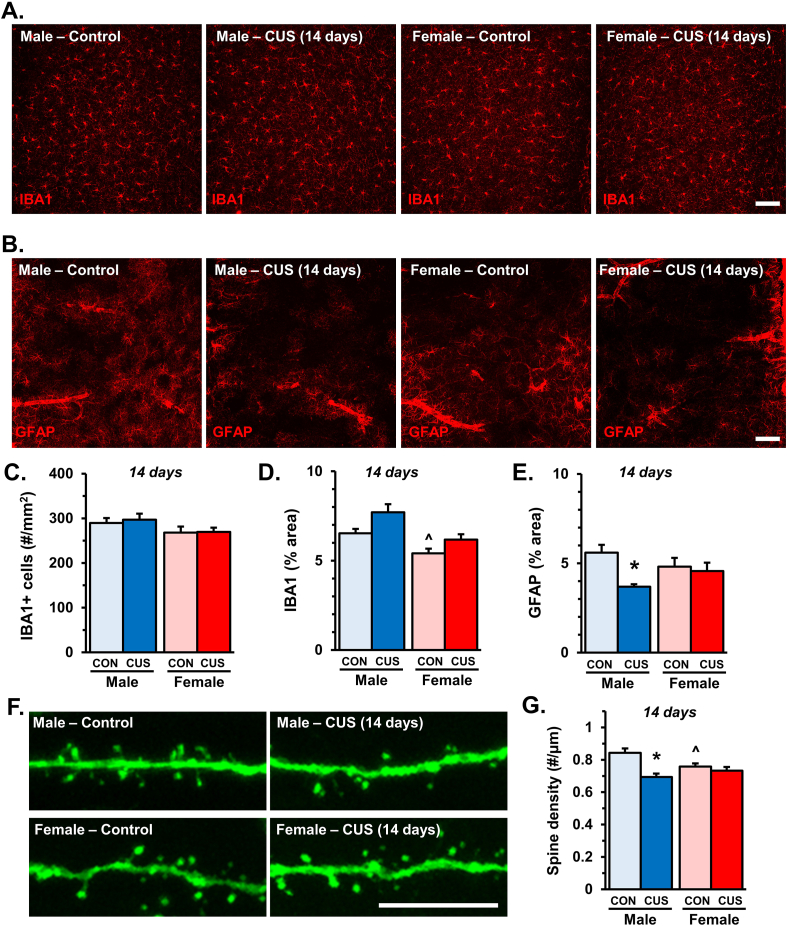
Following the same 14 day CUS paradigm described above, PFC sections were taken from Thy1-GFP(M) mice and treated with antibodies against IBA-1 or GFAP to assess the effects of CUS on the morphology and distribution of microglia and astrocytes respectively (Fig. 3A and B). Image analyses showed no difference in the number of IBA-1+ microglia within the medial PFC (Fig. 3C). Despite this, females had lower baseline IBA-1+ proportional area (sex: F1,18 = 14.31, p < 0.001), and CUS exposure caused a modest increase in both males and females (stress: F1,18 = 7.72; p < 0.01, Fig. 3D). Analyses of GFAP+ proportional area showed that CUS reduced labeling of this astrocyte marker (stress: F1,18 = 4.956; p < 0.04), and was most pronounced in males (p < 0.04, Fig. 3E). We then assessed the effects of CUS on the apical spine density of Thy1-GFP+ pyramidal neurons (Fig. 3F). As previously reported (Wohleb et al., 2018), 14 days of CUS caused sex-specific reductions in apical spine density (interaction: F1,18 = 7.68; p < 0.02; Fig. 3G). In particular, after CUS male mice showed significant synapse loss as compared to same-sex controls (p < 0.0006). Interestingly, we found that apical spine density was positively correlated with GFAP+ proportional area in males (r(9) = 0.7; p < 0.02; Fig. S2). Post-hoc analyses also indicated that female mice had fewer apical dendritic spines than control males (p < 0.04), however apical spine density was not affected by CUS in females. These findings indicate that male and female mice have varied cellular neurobiology in the medial PFC, and chronic stress promotes sex-specific glial alterations that correspond with synaptic deficits.
Fig. 3.
Exposure to 14 days of CUS reduces apical spine density in male mice. Male and female Thy1-GFP(M) mice were exposed to 14 days of chronic unpredictable stress (CUS) or handled as control (CON). Two hours after the final stressor brains were collected for immunohistology. Representative confocal images of (A) IBA-1 and (B) GFAP immunofluorescence in the medial PFC of male and female CON or CUS mice (n = 5–6/group). C) Number of IBA-1+ microglia per area (mm2) in the medial PFC. D) Quantification of IBA-1+ proportional (%) area in medial PFC. E) Proportional area of GFAP+ immunofluorescence in the medial PFC. In the same samples apical dendrites were identified in layer I of medial PFC and dendritic spine density was analyzed. F) Representative confocal images of layer I apical dendrites in the medial PFC of male and female CON or CUS mice. G) Quantification of average dendritic spine density is shown (n = 5–6/group). Bars represent the mean ± S.E.M. Based on post-hoc analyses, means significantly different than same-sex controls are denoted (*, p < 0.05) while those different from opposite sex controls are noted by (^, p < 0.05).
3.4. Exposure to 14 days of CUS increased microglial engulfment of neuronal elements in the PFC of male mice
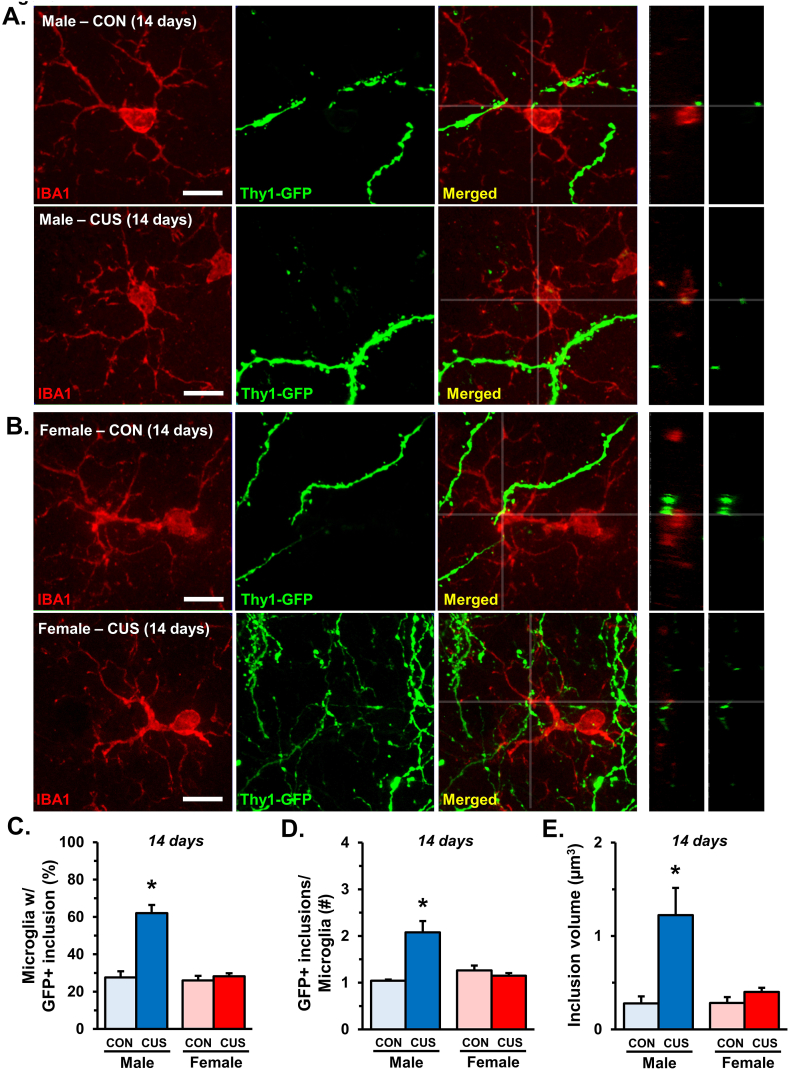
Our previous work showed that CUS drives microglia-mediated neuronal remodeling in the medial PFC of male mice (Horchar and Wohleb, 2019; Wohleb et al., 2018). To validate this work, confocal imaging and 3-dimensional analyses were used to examine co-localization of Thy1-GFP+ neuronal elements within IBA-1+ microglia in layer I of the medial PFC. Representative images of microglia and neuronal inclusions are shown (Fig. 4A and B). Image analyses indicated that 14 days of CUS increased the proportion of microglia with GFP+ neuronal elements in layer I of the medial PFC (interaction: F1,18 = 25.81; p < 0.0001) exclusively in male mice (p < 0.0001; Fig. 4C). Further, Pearson's correlations indicated that there was a significant negative correlation between the proportion of microglia with GFP+ elements and apical spine density in male mice (r(9) = −0.7; p < 0.02; Fig. S2). We also found a greater number of neuronal elements per microglia (interaction: F1,18 = 18.64; p < 0.0005) in male mice exposed to CUS (p < 0.0001; Fig. 4D). Additional analyses revealed that the volume of these GFP+ inclusions was increased (interaction: F1,18 = 8.113; p < 0.02) in male CUS mice (p < 0.0008; Fig. 4E). We also analyzed confocal images of GFAP+ astrocytes for GFP+ inclusions but found no clear evidence that these cells engulfed neuronal elements in the PFC (data not shown). These data provide further evidence that chronic stress drives functional changes in microglia that increase phagocytosis of neuronal elements in the PFC of male mice.
Fig. 4.
Male mice show increased microglial engulfment of neuronal material after 14 days of CUS. Male and female C57BL/6 mice were exposed to 14 days of chronic unpredictable stress (CUS) or handled as control. Two hours after the final stressor brains were collected for immunohistology. Confocal images obtained from layer I of the medial PFC of (A) male and (B) female mice are shown (n = 5–6/group). Side panels show orthogonal cross-section at designated coordinates. White scale bars represent 10 μm. C) Proportion of microglia in layer I of medial PFC with GFP + inclusions. D) Average number of GFP + inclusions in microglia with neuronal elements. E) Total volume of inclusions (μm3) per microglia in layer I of the medial PFC. Bars represent the mean ± S.E.M. Based on post-hoc analyses, means significantly different than same-sex controls are denoted (*, p < 0.05) while those different from opposite sex controls are noted by (^, p < 0.05).
3.5. Both male and female mice show altered stress coping and working memory impairments after exposure to 28 days of CUS
Following an extended period of CUS (28 days; Fig. 5A), neither male nor female mice showed a discernible difference in overall locomotion in the OFT (Fig. 5B and C), though males overall spent more time in the area's center (sex: F1,23 = 5.522; p < 0.03, Fig. 5D). However, stress increased immobility in the FST (stress: F1,34 = 19.92; p < 0.0001) for both males and females compared to same-sex controls (p < 0.02, Fig. 5E). Similarly, exposure to 28 days of CUS reduced discrimination index in the TOR (stress: F1,33 = 43.01; p < 0.0001) in both males and females compared to unstressed controls (p < 0.0009; Fig. 5F). Additionally, both sexes showed attenuated weight gain following CUS (interaction: F1,35 = 8.781; p < 0.006; Fig. 5G), and male controls gained more weight than their female counterparts by the end of the 28 day paradigm (p < 0.005).
Fig. 5.
Male and female mice show altered stress-coping and working memory impairments after prolonged CUS exposure. Male and female C57BL/6 mice were exposed to 28 days of chronic unpredictable stress (CUS) or handled as controls. Mice were assessed in the open field test (OFT), forced swim test (FST), and temporal object recognition test (TOR) on subsequent days (n = 9–11/group). A) Schematic showing experimental approach and timeline. B) Representative heat maps from the OFT. Distance traveled (C) and time spent in the center of the OFT (D) are shown. E) Immobility during the FST. F) Discrimination index as measured by the TOR. Two hours after testing, weight change (G) was calculated (n = 9–11/group), and the prefrontal cortex (PFC) was dissected for mRNA analyses. H) Relative fold change of mRNA levels in the PFC of male and female mice are shown for: Csf1, Il34, Cx3cl1, Tgfb1, Tnfa, Bdnf, Vegf, Gfap, and Aldh1l1 (n = 7–8/group). Bars represent the mean ± S.E.M. Based on post-hoc analyses, means significantly different than same-sex controls are denoted (*, p < 0.05) while those different from opposite sex controls are noted by (^, p < 0.05).
After behavioral testing, PFC samples were collected for gene expression analyses (Fig. 5H). Of note, there were no differences in Csf1 transcript following 28 days of CUS. Exposure to CUS caused a modest decrease in Cx3cl1 expression (stress: F1,27 = 18.91; p < 0.0003), which was significant in males (p < 0.003). Levels of Il34 and Tgfb1 remain unchanged in the PFC, but there was a modest decrease in Tnfa levels following CUS (interaction: F1,27 = 4.009, p = 0.055). Further, CUS exposure caused a broad reduction in Bdnf transcript (stress: F1,28 = 5.289; p < 0.03), but post hoc analyses did not show significant differences between groups. Prolonged CUS altered levels of Vegf in the PFC (interaction: F1,28 = 11.72; p < 0.002); with females having lower expression overall (p < 0.009) and males showing decreased expression after CUS (p < 0.0001). Similar to earlier time points, there were no observed effects of sex or stress on the astrocyte marker Gfap in the PFC, but females showed lower levels of Aldh1ll (sex: F1,27 = 5.941; p < 0.03). These data suggest that prolonged CUS causes similar behavioral and cognitive effects in males and females despite being associated with dynamic, sex-specific molecular changes in the PFC.
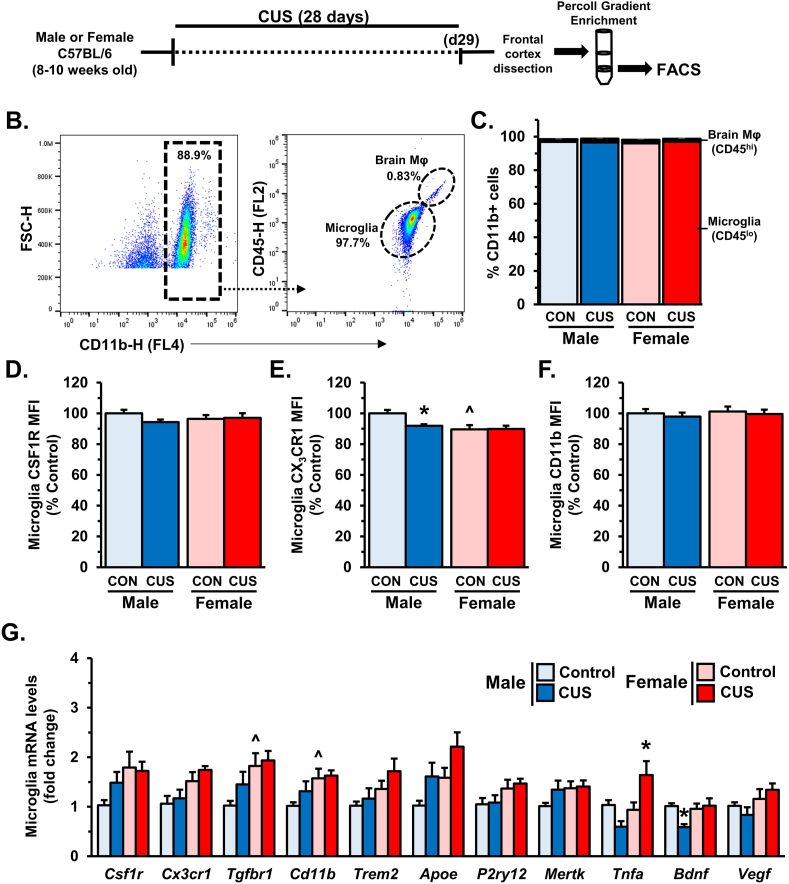
3.6. Molecular adaptations in PFC microglia are attenuated in males following 28 days of CUS
As in section 3.2, microglia were isolated through FACS to assess the effects of prolonged CUS on their molecular profile (Fig. 6A–C). Analysis of MFI showed no difference in the relative surface expression of CSF1R after 28 days of CUS (Fig. 6D). Baseline sex-differences in microglial CX3CR1 surface expression were again observed (sex: F1,27 = 8.552, p < 0.007), with females showing lower relative MFI compared to control males (p < 0.007; Fig. 6E). Further, we found that 28 days of CUS reduced CX3CR1 surface expression in male mice compared to controls (p < 0.05). In contrast, we observed no effects of sex or stress on microglial CD11b surface expression (Fig. 6F).
Fig. 6.
Molecular adaptations in PFC microglia are attenuated in males following 28 days of CUS. Male and female C57BL/6 mice were exposed to 14 days of chronic unpredictable stress (CUS) or handled as control (CON). A) Two hours after the final stressor frontal cortex was dissected for microglia enrichment through Percoll gradient isolation. Following enrichment microglia were sorted (FACS) based on CD11b+/CD45lo expression. B) Gating strategy for FACS is shown. C) Relative quantities of microglia (CD11b+/CD45lo) and brain macrophages (CD11b+/CD45hi). Surface levels of CSF1R (CD115) (D), CX3CR1 (E), and CD11b (F) were determined (n = 7–8/group). Following FACS, sorted microglia were used for transcriptional analyses via qRT-PCR (n = 7–8/group) (G). Bars represent the mean ± S.E.M. Based on post-hoc analyses, means significantly different than same-sex controls are denoted (*, p < 0.05) while those different from opposite sex controls are noted by (^, p < 0.05).
Following FACS, mRNA was isolated from purified microglia for gene expression analyses (Fig. 6G). Interestingly, there was no effect of CUS exposure on Csf1r levels, however females overall had increased microglial Csf1r expression (sex: F1,28 = 13.08, p < 0.02). Similar to the earlier time point, PFC microglia from females showed differential expression of Cx3cr1 (sex: F1,27 = 11.32, p < 0.003), Tgfbr1 (sex: F1,27 = 8.78, p < 0.006), Cd11b (sex: F1,28 = 7.94, p < 0.009), and Trem2 (sex: F1,27 = 5.22, p < 0.03). There were broad effects on microglial expression of Apoe (stress: F1,26 = 6.81, p < 0.02 and sex: F1,26 = 6.284; p < 0.02) and P2ry12 (sex: F1,28 = 6.25; p < 0.02); but post hoc comparisons did not show any significant differences between groups. Further, no differences were observed in Mertk expression. In contrast, levels of Tnfa transcript varied with sex and stress (interaction: F1,28 = 10.59; p < 0.004), with females showing increased expression after 28 days of CUS (p < 0.03). Furthermore we found that microglial Bdnf expression was altered by this paradigm (interaction: F1,28 = 4.782; p < 0.04). Notably, Bdnf expression was significantly reduced in PFC microglia from stressed males (p < 0.02). Vegf expression was found to vary with sex (F1,27 = 5.209; p < 0.04), although no significant differences were found in post-hoc comparisons. Collectively these results suggest that stress-induced molecular adaptations in microglia are dynamic; changing over the course of stress exposure.
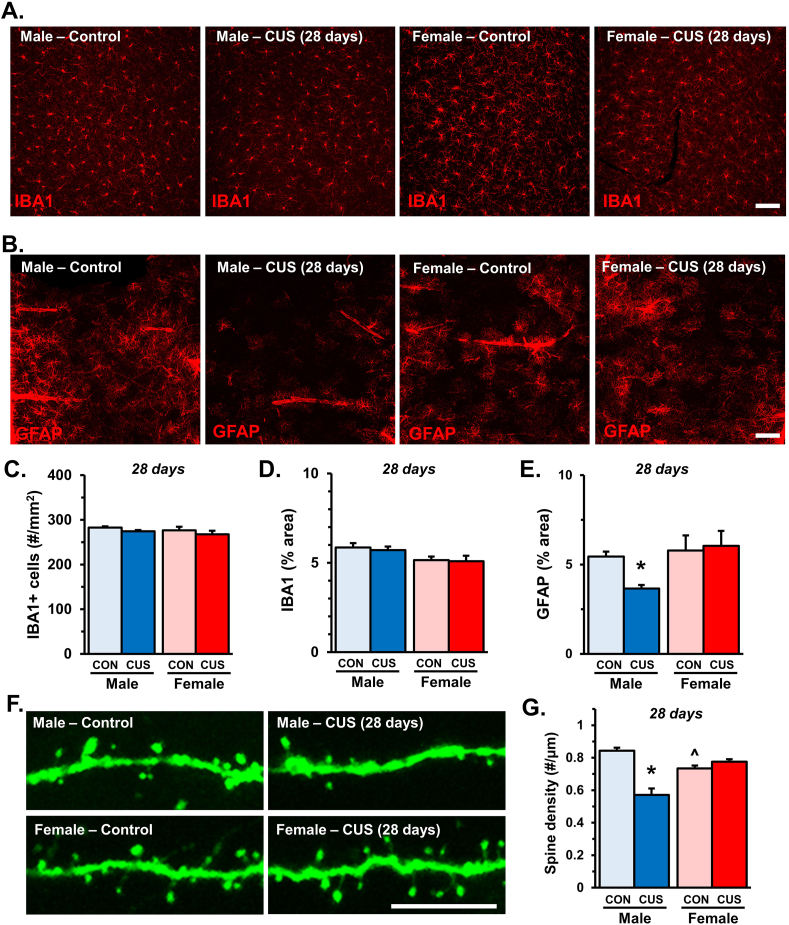
3.7. Male mice show reduced GFAP labeling and apical spine density in the PFC after 28 days of CUS
Further studies used immunohistology to assess the morphology and distribution of microglia and astrocytes in the medial PFC of Thy1-GFP(M) mice following 28 days of CUS (Fig. 7A and B). There were no differences in the density of IBA-1+ cells in the medial PFC and overall, females showed reduced IBA-1 proportional area (sex: F1,17 = 5.18; p < 0.04). Moreover, there was no effect of stress on IBA-1 proportional area (Fig. 7C and D). Additional analyses showed that prolonged CUS exposure decreased GFAP+ proportional area (stress: F1,17 = 7.48; p < 0.01) within the medial PFC of male mice (p < 0.02; Fig. 7E). Exposure to 28 days of CUS differentially affected apical spine density on Thy1-GFP+ pyramidal neurons (interaction: F1,17 = 41.3; p < 0.0001, Fig. 7F). In particular, males but not females, showed decreased apical spine density in the medial PFC after CUS (p < 0.0001; Fig. 7G), which again correlated with reduced GFAP+ area (r(8) = 0.7; p < 0.04; Fig. S2). Similar to earlier time points, post hoc comparisons showed that female controls had lower apical spine density as compared to male controls (p < 0.02). Again these results demonstrate that stress alters the cellular neurobiology of the medial PFC in a sex-dependent manner.
Fig. 7.
Reduced GFAP labeling and apical spine density in male mice following 28 days of CUS. Male and female Thy1-GFP(M) mice were exposed to 14 days of chronic unpredictable stress (CUS) or handled as control (CON). Two hours after the final stressor brains were collected for immunohistology. Representative images of (A) IBA-1 and (B) GFAP immunofluorescence in the medial PFC of male and female CON or CUS mice (n = 5–6/group). C) Number of IBA-1+ microglia per area (mm2) in the medial PFC. D) Quantification of IBA-1+ proportional (%) area in medial PFC. E) Proportional area of GFAP+ immunofluorescence in the medial PFC. In the same samples apical dendrites were identified in layer I of medial PFC and dendritic spine density was analyzed. F) Representative confocal images of layer I apical dendrites in the medial PFC of male and female CON or CUS mice. G) Quantification of average dendritic spine density is shown (n = 5–6/group). Bars represent the mean ± S.E.M. Based on post-hoc analyses, means significantly different than same-sex controls are denoted (*, p < 0.05) while those different from opposite sex controls are noted by (^, p < 0.05).
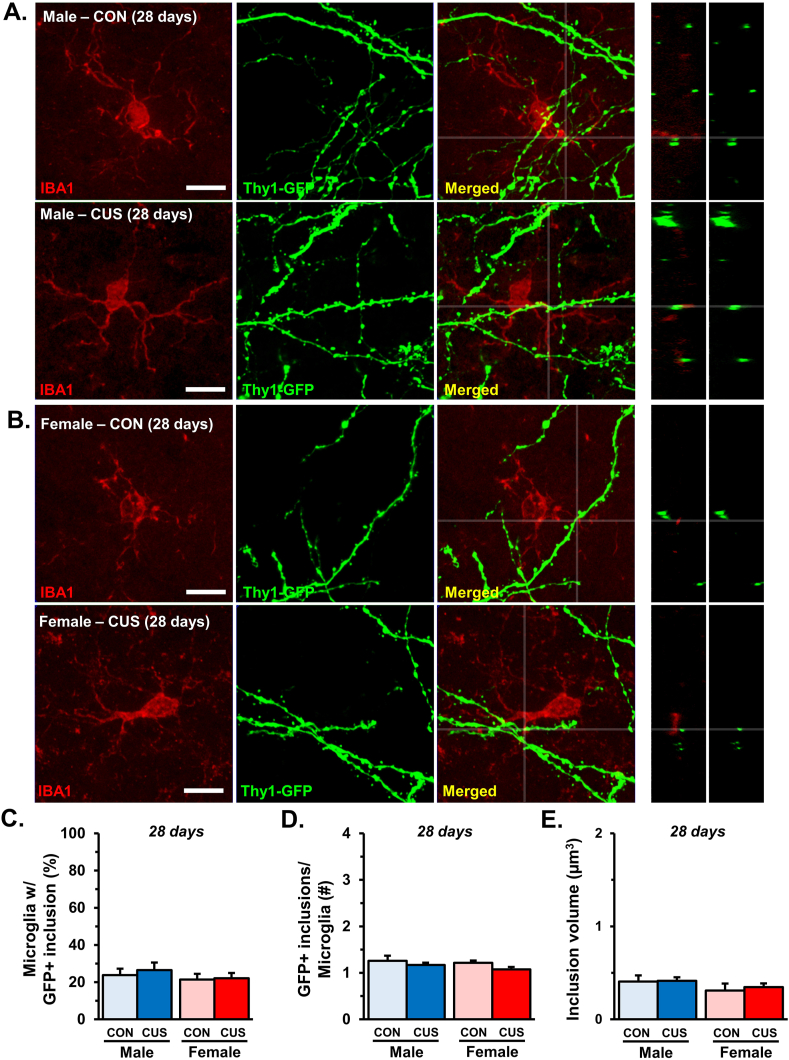
3.8. Microglia do not display increased phagocytosis of neuronal elements after 28 days of CUS
Using confocal imaging and 3-dimensional analysis (as in section 3.4), neuron-microglia interactions were examined in layer I of the medial PFC after prolonged CUS (Fig. 8A and B). Strikingly, we found that 28 days of CUS did not affect the proportion of IBA-1+ cells with GFP+ neuronal elements (Fig. 8C). In line with this, we found no differences in the number of GFP+ inclusions per microglia, nor the volume of these inclusions (Fig. 8D and E). Similar to 14 days of CUS, we did not observe any GFP + inclusions in GFAP + astrocytes in the PFC (data not shown). These results suggest that, despite sustained synaptic deficits, microglia-mediated neuronal remodeling does not persist in male mice exposed to 28 days of CUS.
Fig. 8.
Exposure to 28 days of CUS is not associated with altered microglia-mediated neuronal remodeling. Male and female C57BL/6 mice were exposed to 28 days of chronic unpredictable stress (CUS) or handled as control. Two hours after the final stressor brains were collected for immunohistology. Confocal images obtained from layer I of the medial PFC of (A) male and (B) female mice are shown (n = 5–6/group). Side panels show orthogonal cross-section at designated coordinates. White scale bars represent 10 μm. C) Proportion of microglia in layer I of medial PFC with GFP + inclusions. D) Average number of GFP + inclusions in microglia with neuronal elements. E) Total volume of inclusions (μm3) per microglia in layer I of the medial PFC. Bars represent the mean ± S.E.M. Based on post-hoc analyses, means significantly different than same-sex controls are denoted (*, p < 0.05) while those different from opposite sex controls are noted by (^, p < 0.05).
4. Discussion
Substantial progress has been made in understanding the neurobiological pathways driving behavioral and cognitive symptoms associated with psychiatric disorders. Despite these efforts, conditions such as MDD remain a leading cause of disability associated with profound socioeconomic burdens (Kessler, 2012; Simon, 2003). The use of preclinical stress models can lend valuable insight into the molecular and cellular mechanisms contributing to behavioral and cognitive impairment (Bale et al., 2019). Indeed preclinical models provide strong evidence that chronic stress exposure drives synaptic loss on pyramidal neurons in the medial PFC, and these synaptic deficits promote behavioral and cognitive impairments (Goldwater et al., 2009; Radley et al., 2006). As noted, multiple cell type-specific adaptations have been implicated in stress-associated structural remodeling of neurons and alterations in glial function (Krishnan and Nestler, 2010; Vaishnav Krishnan and Nestler, 2008). Expounding on these studies, research from our lab and others demonstrates that neuron-microglia interactions shape synaptic and behavioral responses to chronic stress (Horchar and Wohleb, 2019; Milior et al., 2016; Wohleb et al., 2018). Of note, these studies indicate that chronic stress alters microglia function in a sex-specific manner; supporting earlier findings that chronic stress has sex-dependent effects on microglial morphology and brain expression of certain immune factors (Bollinger et al, 2016, 2017). This is intriguing as other studies suggest chronic stress causes more pronounced dendritic atrophy in the PFC of males compared to females (Garrett and Wellman, 2009; Moench et al., 2020; Shansky et al., 2004). In this context, we sought to examine neuron-microglia interactions in the PFC and associated behavioral effects in males and females after varied durations of chronic stress.
Here we show that male mice display increased passive coping and impaired temporal object recognition following 14 days of CUS, whereas stressed female mice perform similarly to controls. Further we demonstrate that while stress alters microglial function and causes synaptic loss on apical dendrites in the PFC of males, these neurobiological changes were not observed in females. After 28 days of CUS exposure, female mice display similar behavioral changes to stressed males, yet apical spine density and markers of microglia-mediated neuronal remodeling remain unchanged. These studies replicate and expound on our prior work showing that 14 days of CUS increases microglia-mediated neuronal remodeling via CSF-1 and glucocorticoid signaling in male mice (Horchar and Wohleb, 2019; Wohleb et al., 2018). After 14 days of CUS we observed behavioral consequences in males that coincided with increased PFC expression of Csf1, alongside increased levels of Csf1r transcript and CSF1R surface protein on PFC microglia. We also found that PFC microglia in males had increased expression of Cd11b and Apoe, which have been described as markers of disease-associated microglia (Keren-Shaul et al., 2017; Krasemann et al., 2017). These changes in microglia phenotype were linked to reduced apical dendritic spine density and increased microglial engulfment of neuronal elements in the medial PFC of males. Interestingly, after extended CUS exposures (28 days) the phagocytic activity of microglia in males returned to baseline but behavioral and synaptic effects persisted. The neuroimmune profile in the PFC of males reflected these functional changes as levels of Csf1 in the medial PFC as well as Csf1r, Cd11b, and Apoe in microglia were normalized. These data support the notion that prolonged stress exposure leads to dynamic changes in microglia phenotype and function (Kreisel et al., 2014). Our findings also revealed that microglial phagocytosis of neuronal elements in the medial PFC may be a distinct neurobiological response contributing to behavioral effects in males. It is possible that stress-induced structural changes in other corticolimbic circuits mediate the behavioral changes observed in females after 28 days of CUS (Carvalho-Netto et al., 2011). Additionally, our studies focused on changes in dendritic spine density on Thy1-GFP+ pyramidal neurons, but it could be that chronic stress promotes remodeling of other neuronal compartments by microglia (i.e., pre-synaptic terminals) (Weinhard et al., 2018). Future research will be needed to establish the role of microglia and in sex-specific pre- and post-synaptic modifications across corticolimbic circuitry.
Another important finding is that microglia showed baseline sex differences in expression of Csf1r, Tgfbr1, and Cd11b transcripts, and CX3CR1 protein on the cell surface. While these findings are novel they are not altogether surprising, as innate immune cells show numerous sex differences throughout the periphery (Klein and Flanagan, 2016). This is also consistent with recent reports that microglia display different molecular and functional profiles in males and females in physiological (Guneykaya et al., 2018) and pathological conditions (Sorge et al., 2015; Villa et al., 2018). Accumulating evidence indicates that hormonal differences in males and females regulate molecular pathways in neurons and microglia, which likely shape the neurobiological effects of stress (Arambula and McCarthy, 2020). Despite the limited effects of CUS on female microglia it is notable that they did display increased levels of Tnfa after 28 days of CUS. This is relevant as TNFα has been previously implicated in synaptic scaling by enhancing surface expression of AMPA receptors (Beattie et al., 2002; Stellwagen and Malenka, 2006). Other studies show that female mice maintain AMPA receptor expression and function in the medial PFC following chronic stress (Wei et al., 2014). Thus, it is plausible that microglial TNFα helps preserve synaptic connections in the PFC of female mice. Further studies are needed to fully appreciate the mechanisms mediating the divergent effects of chronic stress in males and females.
It is possible that these distinct and dynamic microglia responses are related to fluctuations in neuronal activity and synaptic plasticity in response to stress. Of note, initial stress exposure increases glutamatergic activity in the PFC, leading to increased NMDA and AMPA receptor mediated currents in pyramidal neurons (Bagley and Moghaddam, 1997; Yuen et al., 2009). However prolonged stress is associated with reduced NMDA and AMPA receptor-mediated currents and loss of apical dendritic spines on PFC pyramidal neurons, which may help to preserve neuronal integrity and avoid excitotoxic insult (Luczynski et al., 2015; Popoli et al., 2012; Yuen et al., 2012). These changes in neuronal activity with stress are relevant because microglia are highly attuned to activity-dependent neuronal cues and can regulate neuronal function through structural remodeling or release of soluble factors (Bollinger and Wohleb, 2019; Eyo and Wu, 2013). For instance, elevated neuronal activity is shown to increase CSF1 signaling that can, in turn, promote microglial phagocytosis (Iaccarino et al., 2016; Luo et al., 2013; Wohleb et al., 2018). In this context, it is plausible that microglia facilitate stress-induced neuronal remodeling in an activity-dependent manner. Indeed our recent work showed that dampening PFC activity during CUS exposure decreased transcript levels of Csf1 in the PFC and Csf1r in microglia, and reduced microglia-mediated neuronal remodeling (Bollinger et al., 2020). Collectively, this work suggests that stress-induced neuronal remodeling by microglia in the PFC is a coordinated process directed by neuron-microglia interactions. This process may involve phagocytosis of intact synaptic or dendritic compartments as well as clearance of debris removed by other means (Brown and Neher, 2014). In either case, microglia-mediated neuronal remodeling in chronic stress may not be inherently pathological as microglia try to preserve neuronal integrity and promote homeostasis.
Another relevant point is that microglia can release soluble factors that may contribute to stress-induced structural remodeling of neurons and synaptic deficits in the PFC. In particular, we observed that PFC microglia have reduced Bdnf expression in male mice after 14 and 28 days of CUS. This is important as prior research shows that mice with deficient BDNF signaling via the Val66Met polymorphism develop stress-induced behavioral changes and synaptic deficits earlier than wild-type littermates (Yu et al., 2012). It is important to note that the Val66Met polymorphism is ubiquitous and impairs BDNF secretion from all cells over the entire lifespan (Egan et al., 2003). More recent studies using an inducible microglia-specific knockout of BDNF indicate that microglial BDNF is important for learning-dependent synaptogenesis (Parkhurst et al., 2013). In this context, future studies are needed to understand the role of microglial BDNF in the neurobiology of stress.
In summary, these experiments suggest that chronic stress elicits different neurobiological adaptations in the PFC of male and female mice. While we found that 14 days of CUS drives behavioral changes, synaptic loss, and microglia-mediated neuronal remodeling in the PFC of male mice, these changes were absent in females. Further, although female mice showed behavioral changes after 28 days of CUS, we did not find evidence of synaptic loss or neuronal remodeling. These results indicate that, despite converging behavioral responses, male and female mice display diverging neurobiological adaptations to chronic stress. These findings lend insight into sex-specific microglia responses to stress that may have implications for psychiatric disorders. Indeed our preclinical studies align with a recent report showing sex-specific transcriptional changes in the postmortem dorsolateral PFC of individuals diagnosed with MDD. In particular, males had increased expression of microglia-associated genes and reduced levels of synapse-related genes, while females showed opposing gene expression profiles (Seney et al., 2018). This study also found evidence for a temporally dynamic microglial response to stress in the male PFC, as increased microglia-mediated neuronal remodeling observed at 14 days of CUS was not observed at 28 days. These findings support the idea that stress-induced changes in microglia function may be adaptive and faciliate structural remodeling of hyperactivated neurons, with the emergence of behavioral consequences occurring as an inadvertent result. In the end, these studies provide novel insight into the dynamic role of microglia in the neurobiological and behavioral adaptations to chronic stress.
Author contributions
E.S.W. designed research studies; E.S.W., S.C.W., and J.L.B. conducted experiments and acquired data; E.S.W. provided resources and materials; E.S.W. and S.C.W. analyzed data, E.S.W., S.C.W., and J.L.B. wrote and edited the manuscript.
Data availability
Data will be made available on request
Funding and disclosure
This work was supported by the Brain & Behavior Research Foundation (NARSAD Young Investigator Grant; 25488), University of Cincinnati Neurobiology Research Center Pilot Award, and the National Institutes of Health (R01-MH123545; R21-MH120614; F32-MH123051). The authors have declared no conflict of interest exists.
CRediT authorship contribution statement
Samuel C. Woodburn: Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Project administration, Visualization. Justin L. Bollinger: Investigation, Manuscript review. Eric S. Wohleb: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Data curation, Manuscript review, Supervision, Funding acquisition.
Acknowledgements
We would like to thank Matthew Horchar for his early efforts on these studies.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100312.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Arambula S.E., Mccarthy M.M. Neuroendocrine-immune crosstalk shapes sex-specific brain development. Endocrinology. 2020;161:1–13. doi: 10.1210/endocr/bqaa055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten A.F.T. Stress weakens prefrontal networks: molecular insults to higher cognition. Nat. Neurosci. 2015;18:1376–1385. doi: 10.1038/nn.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley J., Moghaddam B. Temporal dynamics of glutamate efflux in the prefrontal cortex and in the hippocampus following repeated stress: effects of pretreatment with saline or diazepam. Neuroscience. 1997;77:65–73. doi: 10.1016/s0306-4522(96)00435-6. [DOI] [PubMed] [Google Scholar]
- Bale T.L., Abel T., Akil H., Carlezon W.A., Moghaddam B., Nestler E.J., Ressler K.J., Thompson S.M. The critical importance of basic animal research for neuropsychiatric disorders. Neuropsychopharmacology. 2019 doi: 10.1038/s41386-019-0405-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M., Duman R.S. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol. Psychiatr. 2008;64:863–870. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie E.C., Stellwagen D., Morishita W., Bresnahan J.C., Byeong K.H., Von Zastrow M., Beattie M.S., Malenka R.C. Control of synaptic strength by glial TNFα. Science (80-. ) 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Bohlen C.J., Bennett F.C., Tucker A.F., Collins H.Y., Mulinyawe S.B., Barres B.A. Diverse requirements for microglial survival, specification, and function revealed by defined-medium cultures. Neuron. 2017;94:759–773.e8. doi: 10.1016/j.neuron.2017.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger J.L., Bergeon Burns C.M., Wellman C.L. Differential effects of stress on microglial cell activation in male and female medial prefrontal cortex. Brain Behav. Immun. 2016;52:88–97. doi: 10.1016/j.bbi.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger J.L., Collins K.E., Patel R., Wellman C.L. Behavioral stress alters corticolimbic microglia in a sex- and brain region-specific manner. PloS One. 2017;12 doi: 10.1371/journal.pone.0187631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger J.L., Horchar M.J., Wohleb E.S. Diazepam limits microglia-mediated neuronal remodeling in the prefrontal cortex and associated behavioral consequences following chronic unpredictable stress. Neuropsychopharmacology. 2020:1–11. doi: 10.1038/s41386-020-0720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger J.L., Wohleb E.S. The formative role of microglia in stress-induced synaptic deficits and associated behavioral consequences. Neurosci. Lett. 2019;711 doi: 10.1016/j.neulet.2019.134369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G.C., Neher J.J. Microglial phagocytosis of live neurons. Nat. Rev. Neurosci. 2014;15:209–216. doi: 10.1038/nrn3710. [DOI] [PubMed] [Google Scholar]
- Can A., Dao D.T., Arad M., Terrillion C.E., Piantadosi S.C., Gould T.D. The mouse forced swim test. JoVE. 2011 doi: 10.3791/3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-Netto E.F., Myers B., Jones K., Solomon M.B., Herman J.P. Sex differences in synaptic plasticity in stress-responsive brain regions following chronic variable stress. Physiol. Behav. 2011;104:242–247. doi: 10.1016/j.physbeh.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter D., Mackay D., Landau S., Kerwin R., Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch. Gen. Psychiatr. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- Duman R.S., Aghajanian G.K., Sanacora G., Krystal J.H. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat. Med. 2016;22:238–249. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duric V., Banasr M., Licznerski P., Schmidt H.D., Stockmeier C.A., Simen A.A., Newton S.S., Duman R.S. A negative regulator of MAP kinase causes depressive behavior. Nat. Med. 2010;16:1328–1332. doi: 10.1038/nm.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan M.F., Kojima M., Callicott J.H., Goldberg T.E., Kolachana B.S., Bertolino A., Zaitsev E., Gold B., Goldman D., Dean M., Lu B., Weinberger D.R. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/S0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Eyo U.B., Wu L.-J.J. Bidirectional microglia-neuron communication in the healthy brain. Neural Plast. 2013 doi: 10.1155/2013/456857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett J.E., Wellman C.L. Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience. 2009;162:195–207. doi: 10.1016/j.neuroscience.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S.E., Trinh N.H., Smoller J.W., Fava M., Murphy J.M., Breslau J. Psychosocial stressors and the prognosis of major depression: a test of Axis IV. Psychol. Med. 2013;43:303–316. doi: 10.1017/S0033291712001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldwater D.S., Pavlides C., Hunter R.G., Bloss E.B., Hof P.R., McEwen B.S., Morrison J.H. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009;164:798–808. doi: 10.1016/j.neuroscience.2009.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guneykaya D., Ivanov A., Perez Hernandez D., Haage V., Wojtas B., Meyer N., Maricos M., Jordan P., Buonfiglioli A., Gielniewski B., Ochocka N., Comert C., Friedrich C., Suarez Artiles L., Kaminska B., Mertins P., Beule D., Kettenmann H., Wolf S.A. Transcriptional and translational differences of microglia from male and female brains. Cell Rep. 2018;24:2773–2783.e6. doi: 10.1016/j.celrep.2018.08.001. [DOI] [PubMed] [Google Scholar]
- Herman J.P. Neural control of chronic stress adaptation. Front. Behav. Neurosci. 2013;7:1–12. doi: 10.3389/fnbeh.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinwood M., Morandini J., Day T.A., Walker F.R. Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cerebr. Cortex. 2012;22:1442–1454. doi: 10.1093/cercor/bhr229. [DOI] [PubMed] [Google Scholar]
- Horchar M.J.M.J., Wohleb E.S.E.S. Glucocorticoid receptor antagonism prevents microglia-mediated neuronal remodeling and behavioral despair following chronic unpredictable stress. Brain Behav. Immun. 2019;81 doi: 10.1016/j.bbi.2019.06.030. [DOI] [PubMed] [Google Scholar]
- Iaccarino H.F., Singer A.C., Martorell A.J., Rudenko A., Gao F., Gillingham T.Z., Mathys H., Seo J., Kritskiy O., Abdurrob F., Adaikkan C., Canter R.G., Rueda R., Brown E.N., Boyden E.S., Tsai L.H. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature. 2016 doi: 10.1038/nature20587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H.J., Voleti B., Hajszan T., Rajkowska G., Stockmeier C.A., Licznerski P., Lepack A., Majik M.S., Jeong L.S., Banasr M., Son H., Duman R.S. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat. Med. 2012;18:1413–1417. doi: 10.1038/nm.2886. https://www.nature.com/articles/nm.2886#supplementary-information [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler, Karkowski, Prescott Causal relationship between stressful life events and the onset of major depression. Am. J. Psychiatr. 1999;156(6) doi: 10.1176/ajp.156.6.837. 837–831. [DOI] [PubMed] [Google Scholar]
- Keren-Shaul H., Spinrad A., Weiner A., Matcovitch-Natan O., Dvir-Szternfeld R., Ulland T.K., David E., Baruch K., Lara-Astaiso D., Toth B., Itzkovitz S., Colonna M., Schwartz M., Amit I. A unique microglia type Associated with restricting development of Alzheimer's disease. Cell. 2017;169:1276–1290.e17. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- Kessler R.C. The costs of depression. Psychiatr. Clin. 2012;35:1–14. doi: 10.1016/j.psc.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- Krasemann S., Madore C., Cialic R., Baufeld C., Fatimy R. El, Beckers L., Loughlin E.O., Xu Y., Fanek Z., Greco D.J., Smith S.T., Tweet G., Humulock Z., Zrzavy T., Conde-sanroman P., Gacias M., Weng Z., Chen H., Tjon E., Hartmann K., Madi A., Ulrich J., Glatzel M. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity. 2017;47:566–581. doi: 10.1016/j.immuni.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisel T., Frank M.G., Licht T., Reshef R., Ben-Menachem-Zidon O., Baratta M.V., Maier S.F., Yirmiya R. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol. Psychiatr. 2014;19:699–709. doi: 10.1038/mp.2013.155. [DOI] [PubMed] [Google Scholar]
- Krishnan V., Nestler E.J. Linking molecules to mood. Am. J. Psychiatr. 2010;167:1305–1320. doi: 10.1176/appi.ajp.2009.10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V., Nestler E.J. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan Vaishnav, Nestler E.J. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luczynski P., Moquin L., Gratton A. Chronic stress alters the dendritic morphology of callosal neurons and the acute glutamate stress response in the rat medial prefrontal cortex. Stress. 2015;18:654–667. doi: 10.3109/10253890.2015.1073256. [DOI] [PubMed] [Google Scholar]
- Luo J., Elwood F., Britschgi M., Villeda S., Zhang H., Ding Z., Zhu L., Alabsi H., Getachew R., Narasimhan R., Wabl R., Fainberg N., James M.L., Wong G., Relton J., Gambhir S.S., Pollard J.W., Wyss-Coray T. Colony-stimulating factor 1 receptor (CSF1R) signaling in injured neurons facilitates protection and survival. J. Exp. Med. 2013;210:157–172. doi: 10.1084/jem.20120412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Conron K.J., Koenen K.C., Gilman S.E. Childhood adversity, adult stressful life events, and risk of past-year psychiatric disorder: a test of the stress sensitization hypothesis in a population-based sample of adults. Psychol. Med. 2010;40:1647–1658. doi: 10.1017/S0033291709992121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milior G., Lecours C., Samson L., Bisht K., Poggini S., Pagani F., Deflorio C., Lauro C., Alboni S., Limatola C., Branchi I., Tremblay M.E., Maggi L. Fractalkine receptor deficiency impairs microglial and neuronal responsiveness to chronic stress. Brain Behav. Immun. 2016;55:114–125. doi: 10.1016/j.bbi.2015.07.024. [DOI] [PubMed] [Google Scholar]
- Moench K.M., Breach M.R., Wellman C.L. Prior stress followed by a novel stress challenge results in sex-specific deficits in behavioral flexibility and changes in gene expression in rat medial prefrontal cortex. Horm. Behav. 2020;117 doi: 10.1016/j.yhbeh.2019.104615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota K.T., Liu R.J., Voleti B., Maldonado-Aviles J.G., Duric V., Iwata M., Dutheil S., Duman C., Boikess S., Lewis D.A., Stockmeier C.A., DiLeone R.J., Rex C., Aghajanian G.K., Duman R.S. REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat. Med. 2014;20:531–535. doi: 10.1038/nm.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte C., Gold S.M., Penninx B.W., Pariante C.M., Etkin A., Fava M., Mohr D.C., Schatzberg A.F. Major depressive disorder. Nat. Rev. Dis. Prim. 2016 doi: 10.1038/nrdp.2016.65. [DOI] [PubMed] [Google Scholar]
- Parkhurst C.N., Yang G., Ninan I., Savas J.N., Yates J.R., 3rd, Lafaille J.J., Hempstead B.L., Littman D.R., Gan W.-B.B., Yates J.R., Iii, Lafaille J.J., Hempstead B.L., Littman D.R., Gan W.-B.B. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli M., Yan Z., McEwen B.S., Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley J.J., Rocher A.B., Miller M., Janssen W.G.M., Liston C., Hof P.R., McEwen B.S., Morrison J.H. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cerebr. Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Seney M.L., Huo Z., Cahill K., French L., Puralewski R., Zhang J., Logan R.W., Tseng G., Lewis D.A., Sibille E. Opposite molecular signatures of depression in men and women. Biol. Psychiatr. 2018;84:18–27. doi: 10.1016/j.biopsych.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky R.M., Glavis-Bloom C., Lerman D., McRae P., Benson C., Miller K., Cosand L., Horvath T.L., Arnsten A.F. Estrogen mediates sex differences in stress-induced prefrontal cortex dysfunction. Mol. Psychiatr. 2004;9:531–538. doi: 10.1038/sj.mp.4001435. [DOI] [PubMed] [Google Scholar]
- Shansky R.M., Morrison J.H. Stress-induced dendritic remodeling in the medial prefrontal cortex: effects of circuit, hormones and rest. Brain Res. 2009;1293:108–113. doi: 10.1016/j.brainres.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon G.E. Social and economic burden of mood disorders. Biol. Psychiatr. 2003 doi: 10.1016/S0006-3223(03)00420-7. [DOI] [PubMed] [Google Scholar]
- Sorge R.E., Mapplebeck J.C.S., Rosen S., Beggs S., Taves S., Alexander J.K., Martin L.J., Austin J.S., Sotocinal S.G., Chen D., Yang M., Shi X.Q., Huang H., Pillon N.J., Bilan P.J., Tu Y., Klip A., Ji R.R., Zhang J., Salter M.W., Mogil J.S. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015;18:1081–1083. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D., Malenka R.C. Synaptic scaling mediated by glial TNF-α. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Tynan R.J., Naicker S., Hinwood M., Nalivaiko E., Buller K.M., Pow D.V., Day T.A., Walker F.R. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav. Immun. 2010;24:1058–1068. doi: 10.1016/j.bbi.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai Y.M., Herman J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa A., Gelosa P., Castiglioni L., Cimino M., Rizzi N., Pepe G., Lolli F., Marcello E., Sironi L., Vegeto E., Maggi A. Sex-specific features of microglia from adult mice. Cell Rep. 2018;23:3501–3511. doi: 10.1016/j.celrep.2018.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J., Yuen E.Y., Liu W., Li X., Zhong P., Karatsoreos I.N., McEwen B.S., Yan Z. Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition. Mol. Psychiatr. 2014;19:588–598. doi: 10.1038/mp.2013.83. [DOI] [PubMed] [Google Scholar]
- Weinhard L., Di Bartolomei G., Bolasco G., Machado P., Schieber N.L., Neniskyte U., Exiga M., Vadisiute A., Raggioli A., Schertel A., Schwab Y., Gross C.T. Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat. Commun. 2018;9 doi: 10.1038/s41467-018-03566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb E.S. Neuron-microglia interactions in mental health disorders: “For better, and for worse. Front. Immunol. 2016 doi: 10.3389/fimmu.2016.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb E.S., Terwilliger R., Duman C.H., Duman R.S. Stress-induced neuronal colony stimulating factor 1 provokes microglia-mediated neuronal remodeling and depressive-like behavior. Biol. Psychiatr. 2018;83:38–49. doi: 10.1016/j.biopsych.2017.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Wang D.D., Wang Y., Liu T., Lee F.S., Chen Z.Y. Variant brain-derived neurotrophic factor Val66Met polymorphism alters vulnerability to stress and response to antidepressants. J. Neurosci. 2012;32:4092–4101. doi: 10.1523/JNEUROSCI.5048-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen E.Y., Liu W., Karatsoreos I.N., Feng J., McEwen B.S., Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc. Natl. Acad. Sci. U. S. A. 2009;106:14075–14079. doi: 10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen E.Y., Wei J., Liu W., Zhong P., Li X., Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73:962–977. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request