Abstract
Background:
Eculizumab, a humanized monoclonal antibody targeted to terminal complement protein C5, is approved in Japan for treatment of patients with anti-acetylcholine receptor antibody-positive (AChR+) generalized myasthenia gravis (gMG) whose symptoms are difficult to control with high-dose intravenous immunoglobulin (IVIg) therapy or plasmapheresis.
Methods:
This interim analysis of mandatory post-marketing surveillance in Japan assessed the safety and effectiveness of eculizumab at 26 weeks after treatment initiation in patients with AChR+ gMG.
Results:
Data were available for 40 adult patients in Japan [62.5% (25/40) female; mean age at eculizumab initiation, 51.0 years]. Fifteen patients had a history of thymoma. Six patients were excluded from the effectiveness analysis set due to participation in the open-label extension part of the phase III, randomized, double-blind, placebo-controlled REGAIN study [ClinicalTrials.gov identifier: NCT02301624]. After 26 weeks’ follow up, 32 patients (80%) were continuing eculizumab treatment. Adverse drug reactions were reported by seven patients [most frequently headache (n = 3)]. One death was reported during eculizumab treatment (relationship unclear as determined by the treating physician) and there was one death 45 days after the last dose (considered unrelated). No meningococcal infections were reported. Mean (standard deviation) changes from baseline in Myasthenia Gravis-Activities of Daily Living (MG-ADL) and Quantitative Myasthenia Gravis (QMG) scores were −3.7 (2.61) (n = 27) and −5.6 (3.50) (n = 26), respectively, at 12 weeks, and −4.3 (2.72) (n = 26) and −5.6 (4.02) (n = 24), respectively, at 26 weeks. Improvements in MG-ADL and QMG scores were generally similar in patients with/without a history of thymoma. Frequency of IVIg use decreased following eculizumab initiation.
Conclusion:
In a real-world setting, eculizumab was effective and well tolerated for the treatment of AChR+ gMG in adult Japanese patients whose disease was refractory to IVIg or plasmapheresis. These findings are consistent with the efficacy and safety results from the global phase III REGAIN study of eculizumab.
Keywords: eculizumab, effectiveness, myasthenia gravis, safety, thymoma
Introduction
Generalized myasthenia gravis (gMG) is a rare autoimmune disease affecting the neuromuscular junction, which is characterized by fluctuating severe muscle weakness and fatigue.1 Patients with gMG can experience difficulties with speech, swallowing, vision, and mobility, resulting in disability and impairment of quality of life.2 The reported prevalence of myasthenia gravis (MG) is 107–278 per million,3–7 and is increasing in both Western countries and Japan, particularly in people older than 60–65 years.8–11 In Japan, the prevalence of MG was 11.8 per 100,000 of the population in 2006,7 and the majority of cases are gMG.12
Up to half of patients with gMG in Japan have insufficiently controlled disease (i.e. inadequately controlled symptoms and/or insufficient reduction of oral corticosteroid treatment).13,14 Furthermore, some patients are unable to tolerate corticosteroids and conventional immunosuppressants.15,16 Refractory disease is associated with significantly higher risk of exacerbation, myasthenic crisis, inpatient hospitalization, and emergency room visits, and significantly longer duration of hospital stay.15,17 Guidelines recommend repeated treatment with high-dose intravenous immunoglobulin (IVIg) and/or plasmapheresis to achieve the target of minimal manifestations status in patients with refractory gMG who are experiencing symptoms despite immunosuppressive therapies.18,19 Data from a retrospective analysis suggest that early intervention with these therapies combined with a low-dose corticosteroid regimen might be effective for maintaining minimal manifestations status.14 However, IVIg and plasmapheresis do not target the specific pathologic mechanisms underlying the disease, and a proportion of patients with refractory gMG do not respond to these therapies, even after aggressive and repeated application.14,20
Autoantibodies to the acetylcholine receptor (AChR) are detected in 80–90% of patients with gMG.7,11,12,21 These autoantibodies initiate pathogenic processes at the post-synaptic membrane of the neuromuscular junction, including complement-mediated membrane damage and inflammation.22 Studies using animal models identified a role for complement protein C5 in the pathogenesis of MG.23,24 Eculizumab (Soliris®; Alexion Pharma GK, Tokyo, Japan), a humanized monoclonal antibody targeted to terminal complement protein C5, was approved in Japan in December 2017 for the treatment of patients with anti-AChR antibody-positive (AChR+) gMG whose symptoms are difficult to control with high-dose IVIg or plasmapheresis.25 The efficacy and safety of eculizumab were demonstrated in the global phase III REGAIN study [ClinicalTrials.gov identifier: NCT01997229].26 Subanalysis of data from REGAIN and its open-label extension [OLE; ClinicalTrials.gov identifier: NCT02301624]27 showed a consistent efficacy and safety profile between the Japanese and White [45.5% (40/88) from Europe; 43.2% (38/88) from North America; 11.4% (10/88) from South America; 17.0% (15/88) Hispanic or Latino] patients included in the OLE.28 The entry criteria for the REGAIN study defined a more restricted patient population compared with the patients who are eligible to receive eculizumab in a real-world setting with regard to disease severity, prior treatment, and thymoma history. Therefore, efficacy and safety in the broader group of patients receiving eculizumab in clinical practice in Japan have not been fully established.
As the number of Japanese patients who received eculizumab in the clinical studies was limited, the Japanese Pharmaceuticals and Medical Devices Agency require the manufacturer to conduct mandatory post-marketing surveillance of patients treated with eculizumab since its approval to understand the characteristics of the patients treated and to obtain additional effectiveness and safety data in clinical practice. We report an interim analysis of safety and effectiveness data for adult patients in Japan with AChR+ gMG who were treated with eculizumab between 2017 and 2019, including those with and without a history of thymoma.
Methods
Study design and participants
The regulatory-mandated post-marketing surveillance includes all adult patients in Japan with AChR+ gMG treated with eculizumab in a real-life setting since its approval in December 2017. Patients who were receiving eculizumab during the REGAIN study OLE were switched to treatment with commercially available drug as soon as possible following approval. The protocol for the post-marketing surveillance was confirmed by the Japanese Pharmaceuticals and Medical Devices Agency (22 January 2018) and the study is being conducted in accordance with Good Post-Marketing Study Practice ordinance.29 Local ethical approval was not required for this study.
Patients receive the recommended dose and schedule of eculizumab (intravenous infusion), that is, 900 mg once weekly for 4 weeks, followed by 1200 mg 1 week later, and 1200 mg every 2 weeks thereafter.25 Meningococcal vaccination is required ⩾2 weeks before the first dose of eculizumab.
Data are extracted from each patient’s medical chart and documented in a case report form (CRF), collected every 6 months for the first 2 years and then yearly thereafter. Interim analyses of safety and effectiveness were conducted after 26 weeks of therapy with eculizumab (data cut-off: October 2019). Post-marketing surveillance is ongoing; the follow-up period for individual patients extends to 3 years (or 8 weeks after treatment discontinuation, if before 3 years).
Outcomes
Data are collected on patient demographics and disease characteristics at eculizumab initiation, duration of eculizumab therapy and number of doses received, reasons for eculizumab discontinuation, and number and type of other MG therapies (IVIg and plasmapheresis) received during the 6 months before and 6 months after eculizumab initiation.
Adverse events (AEs) and adverse drug reactions (ADRs) are classified according to the Japanese version of the Medical Dictionary for Regulatory Activities. ADRs are defined as those AEs for which a relationship to eculizumab treatment was not excluded in the opinion of the patient’s physician and confirmed by the sponsor based on information provided by the physician. Serious ADRs are those that result in death, life-threatening events, hospitalization or prolonged hospitalization for treatment, persistent or significant disability or dysfunction, congenital anomalies, or any other important medical events. Clinical exacerbation is defined as MG crisis, death, or MG exacerbation requiring use of rescue medication/therapy (e.g. plasmapheresis).
MG-Activities of Daily Living (MG-ADL) and Quantitative MG (QMG) assessment scores are recorded at baseline and weeks 12 and 26 after eculizumab initiation. Effectiveness was assessed based on changes from baseline in MG-ADL and QMG scores in all patients and according to thymoma history, and on MG-ADL and QMG responder rates (reduction from baseline in total score of ⩾3 MG-ADL or ⩾5 QMG points).
Statistical analyses
Safety was assessed in the safety analysis set (patients with ⩾1 completed CRF). Effectiveness outcomes were assessed in the effectiveness analysis set, which included all patients with ⩾1 completed CRF, with the exception of those who had received eculizumab in REGAIN or the REGAIN OLE. Analyses of effectiveness (changes from baseline in MG-ADL and QMG scores and responder rates) were also performed in subgroups of patients with and without a history of thymoma.
All analyses were descriptive. Continuous variables were summarized descriptively using means, standard deviations (SDs), 95% confidence intervals, medians, and ranges. Missing data were not imputed. Categorical variables were presented as counts and percentages and were based on observed data.
Results
Patients
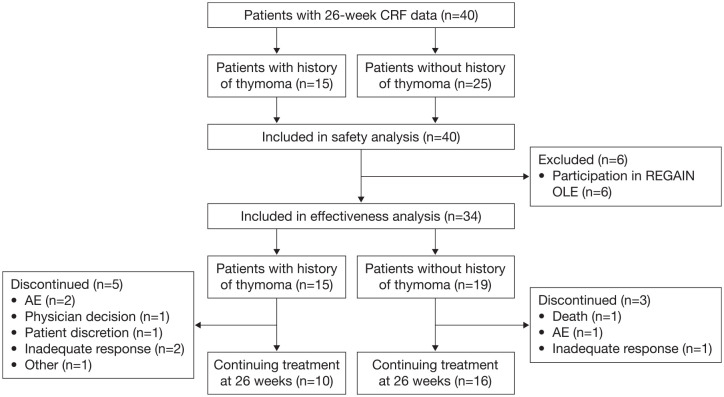
Data collected over 26 weeks of eculizumab therapy were available for a total of 40 adult patients in Japan (Figure 1), all of whom were included in the safety analysis set. Six patients participated in the REGAIN OLE; safety data for these patients were only included for the period after they left the OLE, having switched to treatment with commercially available drug (i.e. safety data collected during their treatment in the OLE were not included in the safety analysis) and these patients were excluded from the effectiveness analysis set. Most patients were female [62.5% (25/40); Table 1]. Mean age at eculizumab initiation was 51.0 years. All patients had anti-AChR antibodies and had been vaccinated against Neisseria meningitidis before initiating treatment with eculizumab, in accordance with the prescribing information in Japan.25 Mean MG-ADL and QMG scores at baseline were 8.8 and 15.1, respectively, and 40.0% (16/40), 42.5% (17/40), and 17.5% (7/40) of patients had Myasthenia Gravis Foundation of America (MGFA) disease classification of II, III and IV, respectively, at eculizumab initiation. A history of thymoma was reported in 37.5% (15/40) of patients. All patients had previously received corticosteroids and/or immunosuppressants, and 92.5% (37/40) had received IVIg and/or plasmapheresis.
Figure 1.
Patient disposition.
Patients may be counted for more than one reason for discontinuation.
AE, adverse event; CRF, case report form; OLE, open-label extension.
Table 1.
Patient demographics and disease characteristics at the start of eculizumab treatment (safety analysis set).
| Adult patients with gMG who received eculizumab (n = 40) | |
|---|---|
| Age (years) | |
| Mean (SD) | 51.0 (17.4) |
| Median (range) | 55.5 (19–84) |
| ⩾65, n (%) | 10 (25.0) |
| Female, n (%) | 25 (62.5) |
| BMI (kg/m2), mean (SD) | 23.4 (4.7) |
| Inpatient before eculizumab initiation, n (%) | 9 (22.5) |
| History of thymoma, n (%) | 15 (37.5) |
| Thymus surgery, n (%) | 25 (62.5) |
| Extended thymectomy | 21 (84.0)a |
| Thymectomy | 3 (12.0)a |
| Unknown type of thymectomy | 1 (4.0)a |
| Meningococcal vaccination, n (%) | 40 (100.0) |
| Anti-AChR positive, n (%) | 40 (100.0) |
| Severity (MGFA classification) at first dose, n (%) | |
| IIa | 10 (25.0) |
| IIb | 6 (15.0) |
| IIIa | 9 (22.5) |
| IIIb | 8 (20.0) |
| IVa | 2 (5.0) |
| IVb | 5 (12.5) |
| V | 0 |
| MG-ADL total score, mean (SD) | 8.8 (5.3) |
| QMG total score, mean (SD) | 15.1 (7.1) |
| Previous exacerbation, including crisis, n (%) | 28 (70.0) |
| Previous treatment, n (%) | |
| Corticosteroids | 39 (97.5) |
| Immunosuppressantsb | 39 (97.5) |
| Corticosteroids and/or immunosuppressantsb | 40 (100.0) |
| Cholinesterase inhibitors | 27 (67.5) |
| IVIgc | 35 (87.5) |
| Plasmapheresisd | 20 (50.0) |
| IVIgc and/or plasmapheresisd | 37 (92.5) |
Expressed as percentage of patients with history of thymus surgery.
Prior immunosuppressants included azathioprine, ciclosporin, and tacrolimus.
Time from last treatment to first dose of eculizumab ranged from 2 to 1953 days.
Time from last treatment to first dose of eculizumab ranged from 3 to 1641 days.
AChR, acetylcholine receptor; BMI, body mass index; gMG, generalized myasthenia gravis; IVIg, intravenous immunoglobulin; MG-ADL, Myasthenia Gravis Activities of Daily Living score; MGFA, Myasthenia Gravis Foundation of America; QMG, Quantitative Myasthenia Gravis score; SD, standard deviation.
Exposure and treatment status
The mean duration of eculizumab treatment was 28.8 weeks, with some patients receiving therapy for up to 1 year (Table 2). At the data cut-off (26 weeks), 80% (32/40) of patients were continuing therapy. Of eight patients (20%) who discontinued eculizumab treatment during the 26-week follow up, seven (18% of total) had discontinued by 12 weeks. The most common reasons for discontinuation were AEs and inadequate response, each reported by three patients (Figure 1). Rates of discontinuation were numerically higher in patients with, versus those without, a history of thymoma (Table 2). Two deaths were reported (see below for details).
Table 2.
Eculizumab exposure and treatment status at 12 weeks and end of follow up for all patients and according to thymoma history (safety analysis set).
| Adult patients with gMG who received eculizumab | |||
|---|---|---|---|
| All (n = 40) | With history of thymoma (n = 15) | Without history of thymoma (n = 25) | |
| Overall treatment duration, weeks | |||
| Mean (SD) | 28.8 (15.9) | 25.8 (16.4) | 30.5 (15.6) |
| Median (range) | 26.0 (0–52) | 26.0 (1–52) | 26.0 (0–52) |
| Total cumulative dose, mg, mean (SD) | 16,940.6 (9194.1) | 15,913.5 (9788.8) | 17,556.8 (8967.4) |
| Treatment status at Week 12, n (%) | |||
| Continuation | 33 (82.5) | 11 (73.3) | 22 (88.0) |
| Discontinuation | 7 (17.5) | 4 (26.7) | 3 (12.0) |
| Final treatment status, n (%) | |||
| Ongoing | 32 (80.0) | 10 (66.7) | 22 (88.0) |
| Discontinued | 8 (20.0) | 5 (33.3) | 3 (12.0) |
gMG, generalized myasthenia gravis; SD, standard deviation.
Safety
A total of 16 patients experienced AEs (Table 3). Seven patients experienced 12 ADRs comprising headache (three events), nasopharyngitis, influenza-like illness, intestinal perforation, vomiting, musculoskeletal stiffness, erythema, pruritus, acute myocardial infarction, and ventricular fibrillation (one event each). Six of the seven patients experiencing ADRs were aged <65 years and all seven had a history of MG exacerbation, including myasthenic crisis. Four patients experienced serious ADRs, including one patient each with nasopharyngitis, influenza-like illness, and intestinal perforation, and another patient with both acute myocardial infarction and ventricular fibrillation. No meningococcal infections were reported. The rates of AEs, serious AEs, ADRs, serious ADRs, and non-meningococcal infections were numerically lower in those patients with versus without a history of thymoma (Table 3).
Table 3.
Overview of AEs for all patients and according to thymoma history (safety analysis set).
| Patients, n (%) | Adult patients with gMG who received eculizumab | ||
|---|---|---|---|
| All (n = 40) | With history of thymoma (n = 15) | Without history of thymoma (n = 25) | |
| AE | 16 (40.0) | 4 (26.7) | 12 (48.0) |
| ADR | 7 (17.5) | 2 (13.3) | 5 (20.0) |
| Serious AE | 10 (25.0) | 3 (20.0) | 7 (28.0) |
| Serious ADR | 4 (10.0) | 1 (6.7) | 3 (12.0) |
| Death | 2 (5.0)a | 1 (6.7) | 1 (4.0) |
| Meningococcal infection | 0 | 0 | 0 |
| Non-meningococcal infection | 6 (15.0) | 0 | 6 (24.0) |
| Non-meningococcal infection ADRs | 0 | 0 | 0 |
| Infusion reaction | 1 (2.5) | 0 | 1 (4.0) |
One patient who died had already discontinued treatment due to an AE, therefore the death was not included as a reason for discontinuation.
ADR, adverse drug reaction; AE, adverse event; gMG, generalized myasthenia gravis.
Two deaths were reported during the 26-week follow-up period. One 72-year-old male patient with type 2 diabetes and hypertension died 10 days after the first eculizumab infusion because of ventricular fibrillation and acute myocardial infarction (causal relationship with treatment stated by the physician to be unclear). One 62-year-old male patient who received one infusion of eculizumab died 45 days after discontinuation due to asphyxia during an MG crisis. The patient had a history of MG exacerbations, but no history of MG crises, and the event was not considered to be related to eculizumab treatment.
Effectiveness
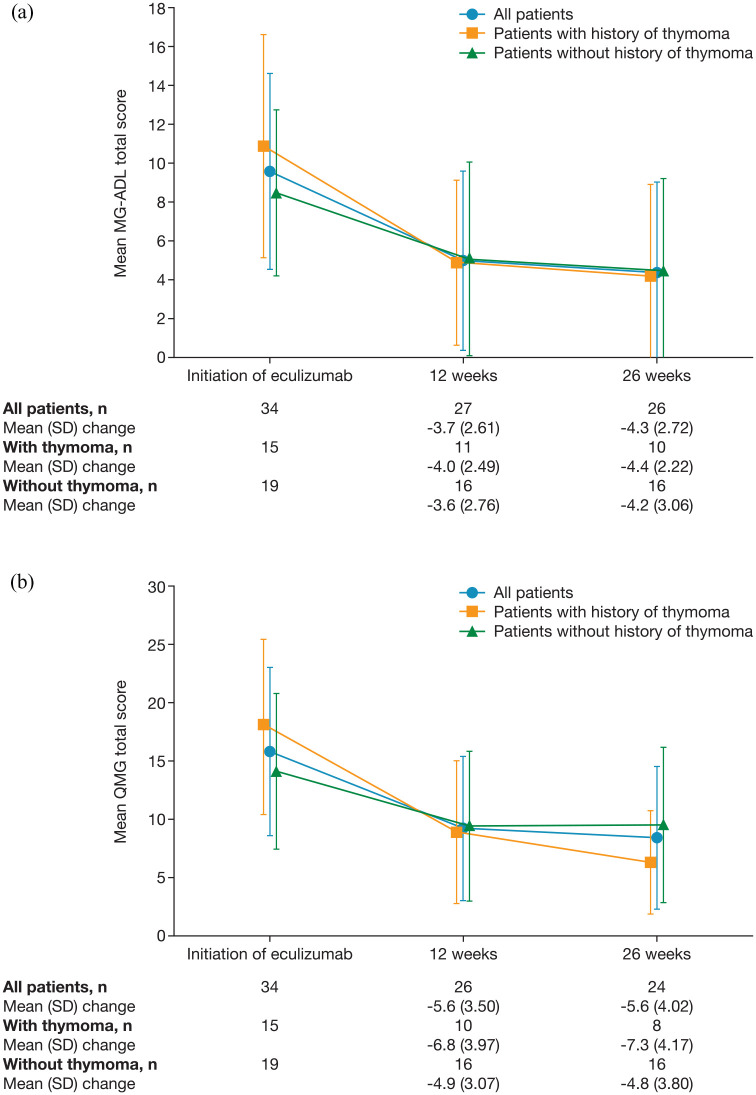
In the effectiveness analysis set, mean MG-ADL and QMG total scores decreased after initiation of eculizumab [Figure 2(a) and (b)]. The mean (SD) change from baseline in MG-ADL total score was −3.7 (2.61) (n = 27) at 12 weeks and −4.3 (2.72) (n = 26) at 26 weeks. The mean (SD) change from baseline in QMG total score was −5.6 (3.50) (n = 26) at 12 weeks and −5.6 (4.02) (n = 24) at 26 weeks.
Figure 2.
Total scores for (a) MG-ADL and (b) QMG at eculizumab initiation and after 12 and 26 weeks of treatment in all patients with generalized myasthenia gravis and in subgroups of patients with and without a history of thymoma (effectiveness analysis set).
Data in graphs have been offset for clarity.
MG-ADL, Myasthenia Gravis Activities of Daily Living score; QMG, Quantitative Myasthenia Gravis score; SD, standard deviation.
In patients with and without a history of thymoma, the mean (SD) changes from baseline in MG-ADL were −4.0 (2.49) (n = 11) and −3.6 (2.76) (n = 16), respectively, after 12 weeks of eculizumab treatment and −4.4 (2.22) (n = 10) and −4.2 (3.06) (n = 16), respectively, after 26 weeks of treatment [Figure 2(a)]. Mean (SD) changes from baseline in QMG in patients with and without a history of thymoma were −6.8 (3.97) (n = 10) and −4.9 (3.07) (n = 16), respectively, at 12 weeks and −7.3 (4.17) (n = 8) and −4.8 (3.80) (n = 16), respectively, at 26 weeks [Figure 2(b)].
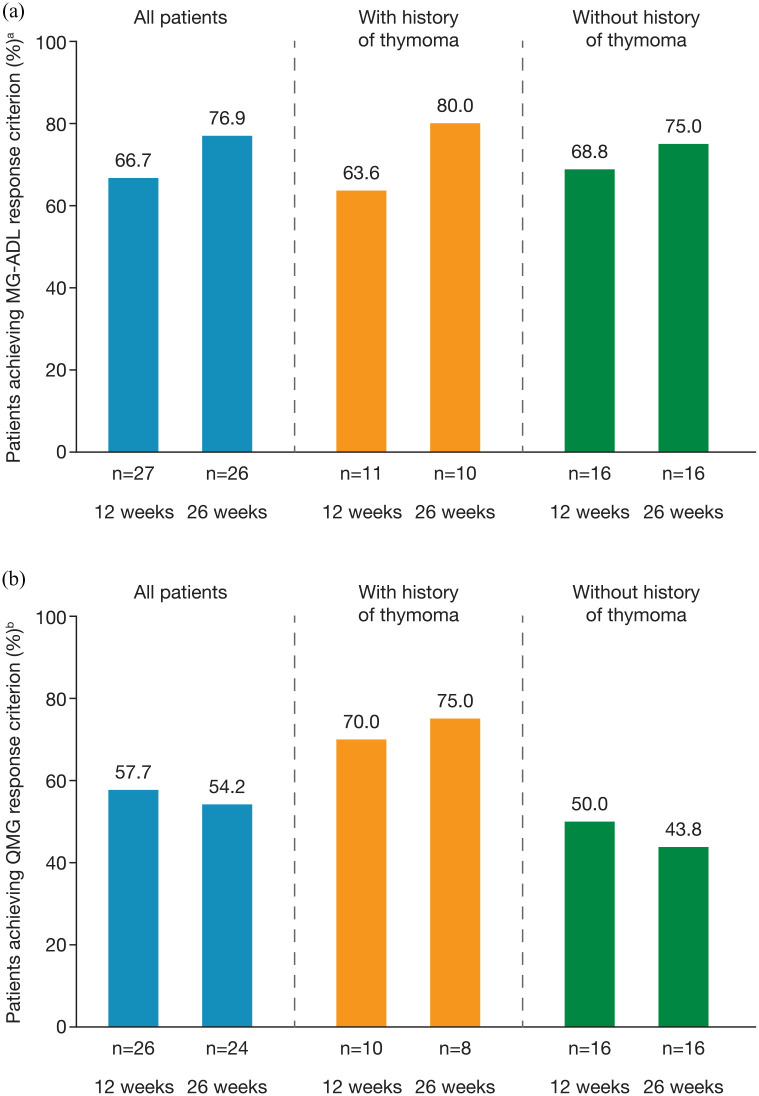
Regardless of thymoma history, a clinically meaningful MG-ADL response (⩾3-point improvement from baseline) was recorded for approximately two thirds of patients [overall 66.7% (18/27)] at 12 weeks and ⩾75% of patients [overall 76.9% (920/26)] at 26 weeks [Figure 3(a)]. QMG responder rates (⩾5-point improvement from baseline) in the effectiveness analysis set were ⩾50% at both 12 [57.7% (15/26)] and 26 weeks [54.2% (13/24); (Figure 3(b)]. QMG responder rates were higher in patients with a history of thymoma [70% (7/10) and 75% (6/8) at 12 and 26 weeks, respectively] than in patients without a history of thymoma [50% (8/16) and 44% (7/16) of patients at 12 and 26 weeks, respectively] (Figure 3b). Of 16 patients who had assessments for both MG-ADL and QMG responder status at 12 and 26 weeks, 14 (88%) maintained or improved their response (as defined by their MG-ADL or QMG score) between the two timepoints.
Figure 3.
(a) MG-ADL respondersa and (b) QMG respondersb after 12 and 26 weeks of eculizumab treatment in all patients with generalized myasthenia gravis and in subgroups of patients with and without a history of thymoma (effectiveness analysis set).
a⩾3-point improvement in MG-ADL total score after eculizumab initiation.
b⩾5-point improvement in QMG total score after eculizumab initiation.
MG-ADL, Myasthenia Gravis Activities of Daily Living score; QMG, Quantitative Myasthenia Gravis score.
A total of 6 of 34 patients in the effectiveness analysis set experienced a clinical deterioration/exacerbation during follow up (five instances of rescue-medication/therapy use, one of MG crisis, and one death).
Use of IVIg
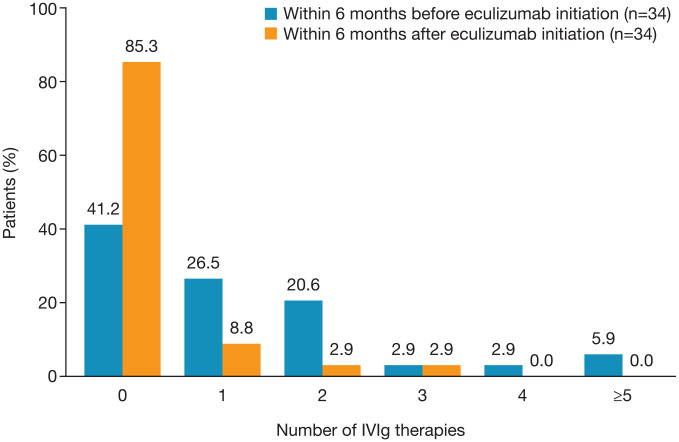
The mean (SD) and median (range) number of IVIg treatments administered in the effectiveness analysis set decreased from 1.2 (1.49) and 1.0 (0–6), respectively, within the 6 months before eculizumab initiation to 0.2 (0.65) and 0 (0–3), respectively, during the 6 months after initiation. The proportion of patients receiving ⩾1 IVIg treatment decreased from 59% (20/34) in the 6 months before eculizumab initiation to 15% (5/34) during the 6 months after initiation (Figure 4).
Figure 4.
Proportion of patients receiving no/one/two/three/four/at least five IVIg treatments before and after eculizumab initiation (effectiveness analysis set).
IVIg, intravenous immunoglobulin.
Discussion
This interim analysis of post-marketing surveillance data demonstrated that eculizumab was effective and well tolerated for the treatment of AChR+ gMG in adult Japanese patients with treatment-refractory gMG in a real-world setting. After 26 weeks of follow up, most patients were continuing eculizumab therapy [80% (32/40)] and had achieved a clinically relevant and sustained improvement, and there was a reduction in the number of IVIg treatments required.
The findings of this analysis are consistent with the efficacy and safety results from the phase III REGAIN study of eculizumab and the subanalysis of data from Japanese patients included in REGAIN and its OLE.26–28 The magnitude of improvement in MG-ADL and QMG scores after 26 weeks of eculizumab treatment reported here (−4.3 and −5.6, respectively) is similar to that reported after 26 weeks of treatment in the REGAIN overall study population (−4.1 and −4.6, respectively26), despite the different settings of these analyses, and is greater than that reported in Japanese patients at 26 weeks after switching from placebo treatment to open-label eculizumab (approximately −2.0 and −2.5, respectively28). In addition, sustained clinically relevant improvements were achieved by more than two thirds of patients for MG-ADL and more than half of patients for QMG, based on established responder criteria, consistent with the findings of REGAIN.26 The population in this analysis comprised patients with a range of MGFA disease severity classifications; however, overall, patients had a greater treatment burden at baseline than those enrolled in the REGAIN study. All patients had received corticosteroids and/or immunosuppressants and a higher proportion of patients in this cohort versus REGAIN had previously received IVIg or plasmapheresis. Additionally, 70% (28/40) of patients in the real-world setting had previously experienced an exacerbation, including myasthenic crisis, which may suggest a greater disease burden. Patients with a history of thymoma or who had undergone thymectomy within 12 months of the study were excluded from REGAIN.26 Thymoma-associated MG is significantly associated with greater disease severity12 and is often refractory to treatment.15 In this post-marketing surveillance, 37.5% (15/40) of the cohort had a history of thymoma and 62.5% (25/40) had previous thymectomy/extended thymectomy (including the 15 patients with a history of thymoma).
The effectiveness of eculizumab, based on MG-ADL total score, was generally comparable in patients with gMG with/without thymoma history. However, compared with the patient subgroup with thymoma, the subgroup without thymoma had a smaller magnitude of change in QMG total score between baseline and week 26 and a notably smaller proportion met the QMG responder criterion. Several factors may have contributed to this, including the small numbers of patients in the subgroups, and underlying clinical differences. Consistent with previous studies,12 patients with thymoma had higher MG-ADL and QMG scores (more severe disease) at baseline and, therefore, a high responder rate based on score reduction was not unexpected. The potentially greater effectiveness of eculizumab reflected in the QMG outcomes in thymoma-associated MG might also be explained in part by the presence of anti-striational antibodies, which are often detected in thymoma-associated MG30,31 and have been implicated in complement activation and muscle dysfunction.32 Differences in QMG and MG-ADL outcomes in patients treated with eculizumab were also noted in the REGAIN study. Such differences may be related to the nature of the assessments: while the patient-reported MG-ADL is based on the past week’s experience, the physician-reported QMG is a point-in-time measure. The two instruments may also differ in their sensitivity to the response to treatment in different muscle groups.33
No new safety findings were identified during 26 weeks of follow up compared with the eculizumab clinical trial program in MG26–28 and the post-marketing surveillance of eculizumab in other indications in Japan.34,35 All patients had been vaccinated against meningococcus in accordance with the prescribing recommendations,25 and no cases of meningitis were reported. Two patients died during the follow-up period; one death was considered unrelated to eculizumab treatment, while the relationship of the other death was unclear. Serious ADRs were infrequent. The most common ADR was headache, which was consistent with AEs reported in the REGAIN study and its OLE,26,27 and with studies of eculizumab in patients with paroxysmal nocturnal hemoglobinuria, atypical hemolytic uremic syndrome, and neuromyelitis optica spectrum disorder.36–38
Epidemiological data from Japan show an increase in late-onset gMG, with a growing proportion of patients aged ⩾65 years.7 These patients are at greater risk of AEs when receiving corticosteroids and immunosuppressants, and Japanese guidelines for the treatment of gMG highlight the importance of considering patients’ quality of life when selecting treatment strategies.1 The findings of our analysis provide further support for the use of eculizumab therapy for patients with AChR+ gMG whose symptoms are difficult to control with high-dose IVIg or plasmapheresis. An observational analysis of 12 patients with gMG who received eculizumab in Japan since its approval has also demonstrated clinical benefits, including corticosteroid reductions, in patients with refractory gMG with or without a history of thymoma, and those with early-onset gMG.39
This was an observational study, and the findings are limited by the small number of patients who had received eculizumab since its approval in Japan, and the lack of a control group. Effectiveness data are further limited by a small number of discontinuations during the follow-up period. Longer-term follow up of a larger cohort of patients is ongoing and will provide more information on the real-world safety and effectiveness of eculizumab, as well as its effect on patient-reported outcomes, such as quality of life and fatigue. Additional analyses may also help inform whether corticosteroids and other MG therapies can be reduced with eculizumab treatment to further improve quality of life.
Conclusion
This first analysis of data from the post-marketing surveillance of eculizumab in a real-world setting in adult patients with AChR+ gMG in Japan demonstrates its safety and effectiveness in patients with and without a history of thymoma.
Acknowledgments
The authors thank Sivani Paskaradevan (Alexion Pharmaceuticals Inc.) for critical review of the manuscript. The statistical analysis was performed by Yuta Takahashi of Seven to One, Inc., funded by Alexion Pharma GK. Editorial assistance was provided by Dr Kirsteen Munn of Anthemis Consulting Ltd., funded by Alexion Pharmaceuticals Inc.
Footnotes
Conflict of interest statement: Hiroyuki Murai has served as a paid consultant for Alexion Pharmaceuticals, arGEN-X BVBA, and Ra Pharmaceuticals, and has received speaker honoraria from the Japan Blood Products Organization and research support from the Ministry of Health, Labour and Welfare, Japan. Shigeaki Suzuki has received personal fees from Alexion Pharmaceuticals, the Japan Blood Products Organization, and Asahi Kasei Medical. Miki Hasebe was an employee of Alexion Pharma GK at the time the analysis was conducted and owns stock in Alexion Pharma. Yuji Fukamizu is an employee of Alexion Pharma GK and owns stock in Alexion Pharma. Ema Rodrigues is an employee of Alexion Pharmaceuticals, Inc. Kimiaki Utsugisawa has served as a paid consultant for UCB Pharma, Ra Pharmaceuticals, and arGEN-X BVBA, and has received speaker honoraria from Alexion Pharmaceuticals.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: research funding for this analysis was provided by Alexion Pharma GK.
Data availability statement: Alexion will consider requests for disclosure of clinical study participant-level data provided that participant privacy is assured through methods like data de-identification, pseudonymization, or anonymization (as required by applicable law), and if such disclosure was included in the relevant study, informed consent form or similar documentation. Qualified academic investigators may request participant-level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion-sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion clinical trials disclosure and transparency policy at http://alexion.com/research-development.
Link to data request form (https://alexion.com/contact-alexion/medical-information).
ORCID iDs: Hiroyuki Murai  https://orcid.org/0000-0003-1612-1440
https://orcid.org/0000-0003-1612-1440
Shigeaki Suzuki  https://orcid.org/0000-0003-4388-4574
https://orcid.org/0000-0003-4388-4574
Contributor Information
Hiroyuki Murai, Department of Neurology, International University of Health and Welfare, 852 Hatakeda, Narita 286-8520, Japan.
Shigeaki Suzuki, Department of Neurology, Keio University School of Medicine, Tokyo, Japan.
Miki Hasebe, Amgen KK, Tokyo, Japan; formerly of Alexion Pharma GK, Tokyo, Japan.
Yuji Fukamizu, Alexion Pharma GK, Tokyo, Japan.
Ema Rodrigues, Alexion Pharmaceuticals, Inc., Boston, MA, USA.
Kimiaki Utsugisawa, Department of Neurology, Hanamaki General Hospital, Hanamaki, Japan.
References
- 1. Murai H. Japanese clinical guidelines for myasthenia gravis: putting into practice. Clin Exp Neuroimmunol 2015; 6: 21–31. [Google Scholar]
- 2. Boscoe AN, Xin H, L’Italien GJ, et al. Impact of refractory myasthenia gravis on health-related quality of life. Clin Neuromusc Dis 2019; 20: 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fang F, Sveinsson O, Thormar G, et al. The autoimmune spectrum of myasthenia gravis: a Swedish population-based study. J Intern Med 2015; 277: 594−604. [DOI] [PubMed] [Google Scholar]
- 4. Park SY, Lee JY, Lim NG, et al. Incidence and prevalence of myasthenia gravis in Korea: a population-based study using the national health insurance claims database. J Clin Neurol 2016; 12: 340−344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heldal AT, Owe JF, Gilhus NE, et al. Seropositive myasthenia gravis: a nationwide epidemiologic study. Neurology 2009; 73: 150–151. [DOI] [PubMed] [Google Scholar]
- 6. Cetin H, Fulop G, Zach H, et al. Epidemiology of myasthenia gravis in Austria: rising prevalence in an ageing society. Wien Klin Wochenschr 2012; 124: 763–768. [DOI] [PubMed] [Google Scholar]
- 7. Murai H, Yamashita N, Watanabe M, et al. Characteristics of myasthenia gravis according to onset-age: Japanese nationwide survey. J Neurol Sci 2011; 305: 97–102. [DOI] [PubMed] [Google Scholar]
- 8. Maddison P, Ambrose PA, Sadalage G, et al. Prospective study of the incidence of myasthenia gravis in the East Midlands of England. Neuroepidemiology 2019; 53: 93–99. [DOI] [PubMed] [Google Scholar]
- 9. Matsuda M, Dohi-Iijima N, Nakamura A, et al. Increase in incidence of elderly-onset patients with myasthenia gravis in Nagano Prefecture, Japan. Intern Med 2005; 44: 572–577. [DOI] [PubMed] [Google Scholar]
- 10. Matsui N, Nakane S, Nakagawa Y, et al. Increasing incidence of elderly onset patients with myasthenia gravis in a local area of Japan. J Neurol Neurosurg Psychiatry 2009; 80: 1168–1171. [DOI] [PubMed] [Google Scholar]
- 11. Yoshikawa H, Iwasa K, Adachi Y, et al. Ten-year chronological clinical profiles of myasthenia Gravis in Japan – epidemiological analyses of the national database established by the policy of intractable diseases of Japan. J Neurologic Sci 2017; 381: 58. [Google Scholar]
- 12. Akaishi T, Suzuki Y, Imai T, et al. Response to treatment of myasthenia gravis according to clinical subtype. BMC Neurology 2016; 16: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kawaguchi N, Kuwabara S, Nemoto Y, et al. Treatment and outcome of myasthenia gravis: retrospective multi-center analysis of 470 Japanese patients, 1999–2000. J Neurol Sci 2004; 224: 43–47. [DOI] [PubMed] [Google Scholar]
- 14. Utsugisawa K, Nagane Y, Akaishi T, et al. Early fast-acting treatment strategy against generalized myasthenia gravis. Muscle Nerve 2017; 55: 794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schneider-Gold C, Hagenacker T, Melzer N, et al. Understanding the burden of refractory myasthenia gravis. Ther Adv Neurologic Disord 2019; 12: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Utsugisawa K, Suzuki S, Nagane Y, et al. Health-related quality-of-life and treatment targets in myasthenia gravis. Muscle Nerve 2014; 50: 493–500. [DOI] [PubMed] [Google Scholar]
- 17. Murai H, Hasebe M, Murata T, et al. Clinical burden and healthcare resource utilisation associated with myasthenia gravis: assessments from a Japanese claims database. Clin Exp Neuroimmunol 2019; 10: 61–68. [Google Scholar]
- 18. Japanese Committee of Clinical Guidelines for Myasthenia Gravis. Japanese clinical guidelines for myasthenia gravis 2014 [in Japanese]. Tokyo: Nankodo, 2014. [Google Scholar]
- 19. Sanders DB, Wolfe GI, Benatar M, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology 2016; 87: 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nagane Y, Suzuki S, Suzuki N, et al. Early aggressive treatment strategy against myasthenia gravis. Eur Neurol 2011; 65: 16–22. [DOI] [PubMed] [Google Scholar]
- 21. Meriggioli MN, Sanders DB. Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol 2009; 8: 475–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morgan BP, Chamberlain-Banoub J, Neal JW, et al. The membrane attack pathway of complement drives pathology in passively induced experimental autoimmune myasthenia gravis in mice. Clin Exp Immunol 2006; 146: 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Christadoss P. C5 gene influences the development of murine myasthenia gravis. J Immunol 1988; 140: 2589–2592. [PubMed] [Google Scholar]
- 24. Zhou Y, Gong B, Lin F, et al. Anti-C5 antibody treatment ameliorates weakness in experimentally acquired myasthenia gravis. J Immunol 2007; 179: 8562–8567. [DOI] [PubMed] [Google Scholar]
- 25. Pharmaceuticals and Medical Devices Agency. Soliris intravenous infusion 300 mg [in Japanese], https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/870056_6399424A1023_1_16 (2020, accessed 13 January 2021).
- 26. Howard JF, Jr, Utsugisawa K, Benatar M, et al.; REGAIN study group. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol 2017; 16: 976–986. [DOI] [PubMed] [Google Scholar]
- 27. Muppidi S, Utsugisawa K, Benatar M, et al.; Regain study group. Long-term safety and efficacy of eculizumab in generalized myasthenia gravis. Muscle Nerve 2019; 60: 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murai H, Uzawa A, Suzuki Y, et al.; REGAIN study group. Long-term efficacy and safety of eculizumab in Japanese patients with generalized myasthenia gravis: a subgroup analysis of the REGAIN open-label extension study. J Neurol Sci 2019; 407: 116419. [DOI] [PubMed] [Google Scholar]
- 29. Ministry of Health Labour and Welfare. Ministerial ordinance No. 171: ordinance of the Ministry of Health on standards for the implementation of surveys and tests after the manufacture and sale of pharmaceuticals [in Japanese], https://www.mhlw.go.jp/web/t_doc?dataId=81aa6623&dataType=0&pageNo=1 (2004, accessed 16 June 2020).
- 30. Suzuki S, Utsugisawa K, Nagane Y, et al. Classification of myasthenia gravis based on autoantibodies status. Arch Neurol 2007; 64: 1121–1124. [DOI] [PubMed] [Google Scholar]
- 31. Suzuki S, Nishimoto T, Kohno M, et al. Clinical and immunological predictors of prognosis for Japanese patients with thymoma-associated myasthenia gravis. J Neuroimmunol 2013; 258: 61–66. [DOI] [PubMed] [Google Scholar]
- 32. Romi F, Skeie GO, Gilhus NE, et al. Striational antibodies in myasthenia gravis: reactivity and possible clinical significance. Arch Neurol 2005; 62: 442–446. [DOI] [PubMed] [Google Scholar]
- 33. De Meel RHP, Verschuuren JJGM, Tannemaat MR. Distinct representation of muscle weakness in QMG and MG-ADL. Lancet Neurol 2018; 17: 204–205. [DOI] [PubMed] [Google Scholar]
- 34. Kato H, Miyakawa Y, Hidaka Y, et al. Safety and effectiveness of eculizumab for adult patients with atypical hemolytic-uremic syndrome in Japan: interim analysis of post-marketing surveillance. Clin Exp Nephrol 2018; 23: 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ninomiya H, Obara N, Chiba S, et al. Interim analysis of post-marketing surveillance of eculizumab for paroxysmal nocturnal hemoglobinuria in Japan. Int J Hematol 2016; 104: 548–558. [DOI] [PubMed] [Google Scholar]
- 36. Hillmen P, Muus P, Roth A, et al. Long-term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol 2013; 162: 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Licht C, Greenbaum LA, Muus P, et al. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int 2015; 87: 1061–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pittock SJ, Berthele A, Fujihara K, et al. Eculizumab in aquaporin-4-positive neuromyelitis optica spectrum disorder. N Engl J Med 2019; 381: 614–625. [DOI] [PubMed] [Google Scholar]
- 39. Oyama M, Okada K, Masuda M, et al. Suitable indications of eculizumab for patients with refractory generalized myasthenia gravis. Ther Adv Neurol Disord. Epub ahead of print 18 March 2020. DOI: 10.1177/1756286420904207. [DOI] [PMC free article] [PubMed] [Google Scholar]