Abstract
Introduction
Mechanical thrombectomy (MT) increases functional independence in patients with acute ischaemic stroke with anterior circulation large vessel occlusion (LVO), and the probability to achieve functional independence decreases by 20% for each 1-hour delay to reperfusion. Therefore, we aim to investigate whether direct angiosuite transfer (DAT) is superior to standard imaging/emergency department-based management in achieving 90-day functional independence in patients presenting with an acute severe neurological deficit likely due to LVO and requiring emergent treatment with MT.
Methods and analysis
DIRECT ANGIO (Effect of DIRECT transfer to ANGIOsuite on functional outcome in patient with severe acute stroke treated with thrombectomy: the randomised DIRECT ANGIO Trial) trial is an investigator-initiated, multicentre, prospective, randomised, open-label, blinded endpoint (PROBE) study. Eligibility requires a patient ≤75 years, pre-stroke modified Rankin Scale (mRS) 0–2, presenting an acute severe neurological deficit and admitted within 5 hours of symptoms onset in an endovascular-capable centre. A total of 208 patients are randomly allocated in a 1:1 ratio to DAT or standard management. The primary outcome is the rate of patients achieving a functional independence, assessed as mRS 0–2 at 90 days. Secondary endpoints include patients presenting confirmed LVO, patients eligible to intravenous thrombolysis alone, patients with intracerebral haemorrhage and stroke-mimics, intrahospital time metrics, early neurological improvement (reduction in National Institutes of Health Stroke Scale by ≥8 points or reaching 0–1 at 24 hours) and mRS overall distribution at 90 days and 12 months. Safety outcomes are death and intracerebral haemorrhage transformation. Medico-economics analyses include health-related quality of life and cost utility assessment.
Ethics and dissemination
The DIRECT ANGIO trial was approved by the ethics committee of Ile de France 1. Study began in April 2020. Results will be published in an international peer-reviewed medical journal.
Trial registration number
Keywords: interventional radiology, stroke medicine, clinical trials
Strengths and limitations of this study.
DIRECT ANGIO trial is the first multicentre randomised clinical trial to directly compare direct angiosuite transfer (DAT) versus standard management for highly suspected patients with anterior circulation large vessel occlusion ischaemic stroke.
DIRECT ANGIO aims to provide further evidence of the clinical benefit of DAT, as well as socioeconomic positive impact for the global health system.
The multicentre setting and large pragmatic inclusions criteria compatible with current clinical practice and recommendations will allow external validity.
Primary outcome measure will allow evaluation of functional independence at 90 days.
Secondary outcomes will measure the safety and medico-economic impact of DAT.
Introduction
Background and rationale
Strokes remain a large cause of death and disability, and prevalence of large vessel occlusion (LVO) among patients with suspected acute ischaemic stroke ranged from 13% to 52%, with overall prevalence of 30.0%.1 Mechanical thrombectomy (MT) has become the standard of care for reperfusion therapies in acute anterior LVO strokes,2 and is strongly dependent on time with 20% decreased probability of functional independence for each 1-hour delay to reperfusion.3 The Highly Effective Reperfusion evaluated in Multiple Endovascular Stroke Trials (HERMES) meta-analysis demonstrated that prognosis is directly related to combined ischaemic core volume with age and expected imaging-to-reperfusion time after successful reperfusion.4 While the stroke network reorganisation reduced symptoms-to-needle time and increased accessibility to endovascular-capable centres, it is currently crucial to achieve fast triage and initiation of endovascular therapy. To date, patients with a suspected stroke are first admitted in the radiology/emergency department and secondary transfer to the angiosuite for MT if LVO is confirmed. This approach results in prolonged delays in delivering definitive therapy in the setting of LVO, whereas the angiosuite has imaging facilities to rule out intracranial haemorrhage (ICH) and confirm proximal arterial occlusion (cone beam CT (CBCT)), and therefore the ability to triage patients. Retrospective studies reported a clinical benefit of a direct angiosuite transfer (DAT).5–7 The aim of DIRECT ANGIO (Effect of DIRECT transfer to ANGIOsuite on functional outcome in patient with severe acute stroke treated with thrombectomy: the randomised DIRECT ANGIO Trial) trial is to compare the effectiveness and safety of DAT versus conventional management in patients presenting an acute severe neurological deficit and thus mainly due to LVO eligible to MT.
Objectives
Primary objective
The primary objective of the study is to determine whether DAT compared with conventional management is associated with improved 90-day functional independence in patients presenting with prehospital acute severe neurological deficit likely to require treatment with MT. Functional independence is defined as a modified Rankin Scale (mRS) score of 0–2 at 90 days.
Secondary objectives
The study will also explore the feasibility, efficacy and safety of DAT, as well as cost-utility assessment.
Trial design
The DIRECT ANGIO trial is a prospective, multicentre, randomised, open-label, blinded-endpoint (PROBE), two-arm, clinical trial to compare the effectiveness and safety of DAT versus standard management in patients with acute prehospital severe neurological deficit suspected to LVO of the anterior circulation.
Consort diagram
Figure 1 shows the shows the Consolidated Standards of Reporting Trials (CONSORT) diagram of the DIRECT ANGIO trial.8
Figure 1.
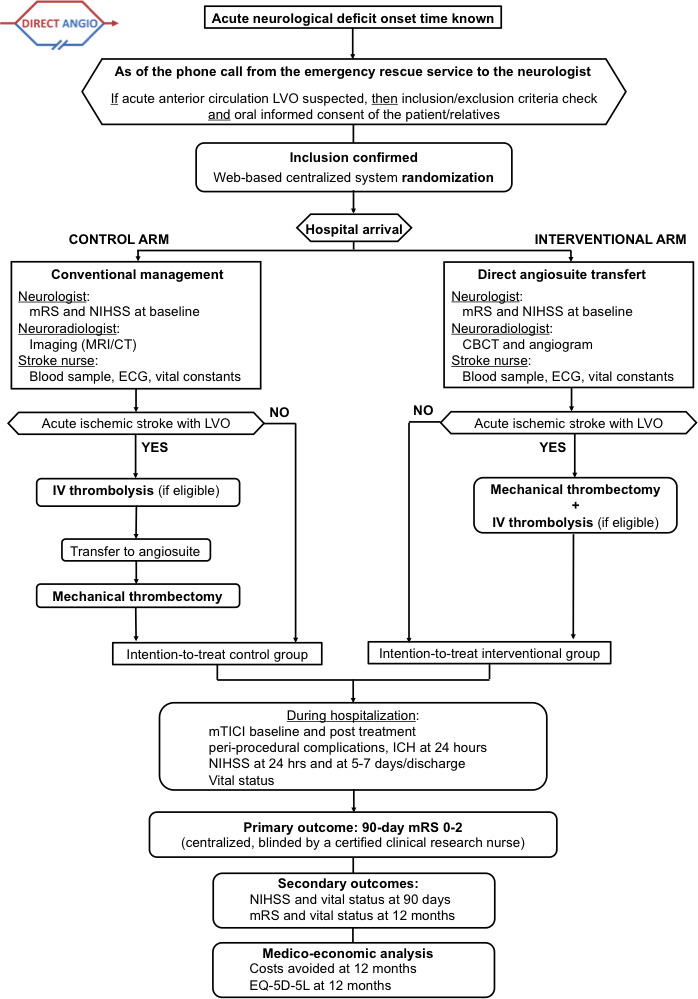
CONSORT (Consolidated Standards of Reporting Trials) diagram of the DIRECT ANGIO trial illustrating the randomisation and flow of patients in the study. CBCT, cone beam CT; EQ-5D-5L, 5 dimensions and 5 levels EuroQol questionnaire; ICH, intracerebral haemorrhage; IV, intravenous; LVO, large vessel occlusion; mRS, modified Rankin Scale; mTICI, modified Thrombolysis In Cerebral Infarction; NIHSS, National Institutes of Health Stroke Scale.
Methods and analysis
Participants, interventions and outcomes
This manuscript was written in accordance with the Standard Protocol Items: Recommendations for Interventional Trials guidelines.9
Study setting
The DIRECT ANGIO trial takes place in nine comprehensive stroke centres in France (CHRU-Nancy, CHU Besançon, CH Colmar, CHU Strasbourg, CHU Reims, Foch, CHRU Montpellier, CHU Limoges and CHU Bordeaux).
Eligibility criteria
Inclusion criteria
Adult patients ≤75 years old, pre-stroke mRS 0–2, with acute severe neurological deficit at prehospital stage and directly admitted at an endovascular-capable centre within 5 hours of symptoms onset and who meet all eligibility criteria are considered for study enrolment. Secondary transfer patients are not eligible in the trial. As the study objective is to target a completely autonomous population that can be assumed to have neither cognitive problems, nor a history of stroke age was limited to 75 years old. Table 1 lists the inclusion and exclusion criteria.
Table 1.
DIRECT ANGIO inclusion and exclusion criteria
| Inclusion | Exclusion |
|
|
*New criteria of the version 3.0.
mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale.
As of the phone call from the emergency rescue service, inclusion and exclusion criteria are checked and then the patient randomise during the hospital travel (before admission). We are modifying the timing of randomisation. Randomisation will be performed after severe stroke confirmation by a neurologist immediately after admission (version 3.0). Oral informed consent will be sought via telephone conversations from patient or from their relatives. Emergency consent procedure may be considered if consent is not possible by the subject or a proxy. Written informed consent for continuation will be then collected as soon as possible, within 3 months.
Interventions
Experimental arm (DAT approach)
On arrival in angiosuite and after rapid neurological examination (using National Institutes of Health Stroke Scale (NIHSS) and mRS scores) and blood sample, the patient undergoes CBCT in order to exclude non-ischaemic stroke and angiogram to confirm LVO (tandem, intracranial internal carotid artery, M1 or proximal M2 segment of the middle cerebral artery, basilar artery or P1 segment of the posterior cerebral artery). Several managements can be performed:
Whatever the NIHSS score at admission, patients with no ICH and with LVO were treated with MT and, if eligible, with intravenous recombinant tissue plasminogen activator (IV rt-PA) as soon as possible. A low Alberta Stroke Program Early CT Scale (ASPECTS) or low collateral score was not an exclusion criterion for MT.
Patients with no ICH and with a distal vessel occlusion were treated with IV rt-PA alone, if eligible.
Patients with no ICH and with no arterial occlusion were started on IV rt-PA, if eligible, and received an additional stroke imaging (MRI or CT) to decide on further treatment.
Patients with an ICH and no occlusion were treated as per institutional standards.
Patients with an ICH and LVO were treated with MT after an individualised case discussion between neurologist, neuroradiologist and patient or his/her proxy.
However, the subject will remain in the intention-to-treat population.
Control arm (conventional approach)
Arrival is in the imaging/emergency department, and after neurological examination and blood sample, the patient undergoes stroke imaging (MRI or CT). After LVO confirmation, the patient is treated with IV rt-PA, if eligible, and transfer to angiosuite for MT as soon as possible. In the setting of no LVO, the patients are treated as per institutional standards.
Clinical assessment
Baseline characteristics include prestroke mRS score, symptoms and intrahospital time metrics. Neurological deficit is assessed using the NIHSS score at baseline, after 24 (±6) hours, at 5–7 days (or discharge if earlier) and at 90 (±15) days. At 90 (±15) days and 12 (±1) months, outcome assessment is also evaluated with the mRS score and health-related quality of life (EQ-5D-5L).10 11
Imaging protocol
Baseline imaging (MRI or CT for the standard management group and CBCT and angiogram for the DAT group) is performed. Baseline imaging, angiographic imaging before, and at the end of endovascular procedure as well as follow-up imaging at 24 (±6) hours for ICH are assessed by an independent core laboratory. The core laboratory evaluates the findings on the baseline imaging for the ASPECTS (range 0–10, with 1 point subtracted for any evidence of early ischaemic change in each defined region on the CT scan or diffusion-weighted imaging sequence),12 baseline vessel imaging (CT angiogram or MR angiogram) for the location of the occlusion. The core laboratory assessed also angiographic outcomes on digital subtraction angiography, using the modified Thrombolysis in Cerebral Infarction (mTICI) score, which ranges from 0 (no reperfusion) to 3 (complete reperfusion).13 Radiological outcome measures will be centrally analysed, blinded to treatment allocation.
Outcomes
Primary outcome
The proportion of patients with functional independence defined as mRS score 0–2 at 90 (±15) days between DAT and conventional admission in patients ≤75 years old presenting an acute severe neurological deficit at prehospital stage and directly admitted at an endovascular-capable centre within 5 hours of onset.
Secondary outcomes
-
Secondary feasibility endpoints:
Rate and site of LVO.
Intrahospital time metrics (admission to imaging/needle/puncture/reperfusion, imaging to puncture/reperfusion and puncture to reperfusion).
-
Secondary efficacy endpoints:
Quality of reperfusion according to the mTICI score.
Procedural complications (embolus in a new territory, perforation and dissection).
Clinical status with the NIHSS score at 24 (±6) hours, 5–7 days (or discharge if earlier), 90 (±15) days.
Blinded 12 (±1)-month mRS score.
-
Secondary safety endpoints:
Rate of patients eligible to IV rt-PA alone.
Rate of ICH.
Rate of stroke mimics.
Rate of patients requiring secondary stroke imaging.
Rate of intracerebral haemorrhagic transformation of ischaemic stroke according to the European Cooperative Acute Stroke Study III classification.14
Rate of mortality at 90 (±15) days and 12 (±1) months.
Rate of decompressive hemicraniectomy.
-
Cost-utility assessment include health-related quality of life assessment at 90 (±15) days and 12 (±1) months and assessment of costs from the time of randomisation to the 12-month follow-up:
Costs of hospitalisation.
Institutionalised living.
Outpatient care.
Informal care provided by relatives.
Cost of lost productivity.
Recruitment
The patients are expected to be included during a 30-month period starting in April 2020.
2016–2017: Protocol, approvals from ethics committee (CPP IDF I) and the French Medicine Agency (Agence Nationale de Sécurité du Médicament et des produits de santé, ANSM); trial tool development (online case report form (CRF) and randomisation system).
2020–2023: Inclusion of patients.
2023–2024: Cleaning and closure of the database, data analyses, writing of the manuscript and submission for publication.
Trial status
The current protocol is 2.0. Study started enrolment in 27 April 2020. To date (31 January 2021), seven patients have been randomised in the study (one centre open).
Patient and public involvement
Patients will not be invited to comment on study design or conduction of the trial.
Methods: assignment of interventions
Allocation and sequence generation
After inclusion, the patients are randomised in two arms using a web-based centralised system with a 1:1 ratio to either DAT or standard management (figure 1). The randomisation sequence is provided by an independent statistician (who did not take part in assessing the patients at any point in the study) using computer-generated random numbers and stratified by centre and delay from onset to hospital admission (before or after 2.5 hours). The randomisation sequence is implemented in the electronic case report form system to ensure a centralised real-time randomisation procedure. Subjects are enrolled and randomised by emergency physicians, neurologists or neuroradiologists.
Blinding
For the primary outcome, a centralised certified clinical research nurse from the trial centre, who will be unaware of the treatment group assignments, records the mRS at 90 days and 1 year by telephone with the patient, proxy or healthcare provider.8 9 All neuroimaging readings including determination of the ASPECTS score, arterial occlusion site, clot burden score and haemorrhagic transformation are performed by the imaging core laboratory, which is also blinded to procedure allocation.
Methods: data collection, management and analysis
Data collection and management
Similarly to other studies,15 the entire study is conducted using eCRFs, where all clinical data on enrolled subjects are entered (single-keyed) by the site personnel. The eCRF was developed using CleanWeb (Tentelemed) software. The essential data necessary for monitoring the primary and secondary endpoints are identified and managed at regular intervals throughout the trial. Data are monitored by members of the CIC 1433 Technological Innovation of Nancy University Hospital using the predefined rules and queries are automatically edited. Lastly, overall automated monitoring is performed by the data manager after completion of data entry. In cases of discrepancies, queries can be edited to resolve the problems encountered.
Patient withdrawal
Evaluated procedure is tested during the management of endovascular thrombectomy. Nevertheless, the participant can withdraw consent at any time without the need for further explanation. Data will be destroyed and a new patient will be randomised for the complete sample size.
Statistical methods
Sample size estimation
Based on the literature,2 5–7 we expect a 90-day mRS 0–2 rate of 30% in the control arm. We assume that DAT approach (intervention arm) will be associated with an absolute increase of 20% (corresponding to a 90-day mRS 0–2 rate of 50%) due to 1-hour delay to reperfusion reduction. To detect this effect size, with a two-sided test at the 0.05 level of significance, and a power of 80%, 93 subjects per arm will be required. To account for an anticipated rate of 10% dropout (ie, patients lost to follow-up and without LVO), we planned to include a total of 208 subjects (104 per arm).
Interim analysis
One interim analysis is planned once 50% of patients have been included, for the study to be stopped early owing either to compelling evidence of efficacy (using a prespecified Haybittle–Peto efficacy boundary with an alpha level of 0.001) or of futility. The independent data and safety monitoring board (DSMB) could recommend stopping the study if prolongation of the trial clearly compromises patient safety (in case of serious adverse reactions (SARs) or suspected unexpected serious adverse reactions (SUSARs)). The steering committee will be responsible to continue, hold or stop the study based on the DSMB recommendations.
Statistical analyses
Statistical analyses will be carried out independently by the CIC 1433 Technological Innovation of Nancy University hospital under the responsibility of Professor Jacques Felblinger, where statisticians and investigators will be aware of the treatment group allocation. Baseline characteristics will be described for each treatment group; categorical variables will be expressed as frequencies and percentages and quantitative variables will be expressed as means±SD or medians (IQR) for non-Gaussian distribution. Normality of distributions will be assessed graphically and by using the Shapiro-Wilk test. No formal statistical comparisons of baseline characteristics will be done; clinical importance of any imbalance will be noted. All analyses will be performed using all randomised participants based on their original group of randomisation, according to the intention-to-treat principle. The intention-to-treat analysis will analyse all included patients and patients will be analysed according to the randomisation scheme. This analysis will include all patients with LVO but also with ICH, stroke mimics, ischaemic stroke without LVO at admission, independently of receiving MT or not. A per-protocol analysis will be considered only for primary endpoint as a secondary analysis. Per-protocol population will include all randomised patients excluding those without LVO strokes. Furthermore, the costs avoided analysis will take into account a cost difference between the two randomisation arms at 12 (±1) months and will extrapolate this difference over an expected lifetime using a Markov model. The cost-utility analysis will also be performed with health-related quality of life estimated with EQ-5D-5L questionnaire. The CONSORT statement recommendations will be applied for drafting the final report.
Methods: monitoring
Data monitoring
Before the start of the study, neurological and neuroradiological medical and paramedical teams are trained at each site for the study protocol by study coordinators. Physicians are in charge of patient screening and inclusion. Data will be collected in a web-based eCRF by trial personnel. Each centre will only have access to site-specific data. Each patient will receive a unique trial identification number. Only the investigators and research team will have access to any protected health information of study participants and any study data.
Data monitoring and quality control will be conducted in each centre after the first 10 inclusions then after the next 20 inclusions and at the end of the study by official representatives of the study promoter (Department of Clinical Research and Innovation, Nancy University Hospital). Data will be handled according to the French law. All original records (including consent forms, reports of SUSARs and relevant correspondences) will be archived at trial sites for 15 years. The clean trial database file will be anonymised and maintained for 15 years. Only the principal investigators and the statistician will have access to the final dataset.
Harms
Every adverse event that could be related to the trial will be reported to the trial coordinating centre. According to the French law, all suspected serious adverse events will be reported to the ANSM. The DSMB will also be informed. DSMB is independent from the trial investigators and will perform an ongoing review of safety parameters and study conduct. The members of the DSMB are not participants of the DIRECT ANGIO consortium and not involved in the clinical trial. The DSMB is composed of one neuroradiologist, one pharmacovigilance specialist and one methodologist, who are not participating in the study and are not affiliated with the sponsor and who have skills and expertise in clinical neuroscience and clinical research. The DSMB will be responsible for safeguarding the interests of trial participants, assessing the safety of the interventions during the trial and for monitoring the overall conduct of the trial. DSMB could also formulate recommendations related to the recruitment/retention of participants, their management, improving adherence to protocol-specified regimens, and the procedures for data management and quality control. No formal criteria are set to stop the study. However, recommendations for pausing or stopping the study could be made by DSMB in case of SARs and SUSAR. The scientific committee will be responsible for promptly reviewing the DSMB recommendations and to decide whether to continue, hold or stop the study, and to determine whether amendments to the protocol are needed.
Ethics and dissemination
Any change to eligibility criteria, outcomes and analyses will be communicated to investigators, the ethics committee and the ANSM to obtain their approval. The DIRECT ANGIO trial was approved by the ethics committee (CPP) of Ile de France 1.
Consent or assent
Whenever possible to include the patient, written inform consent will be searched. Nevertheless, related to neurological injury and emergency, the patient may be unable to provide written informed consent. In this case, written informed consent could be obtained from the patient next of kin if immediately available. Otherwise, an emergency consent procedure is used with investigator signature countersigned by an independent physician. As soon as possible after recovery, written informed consent form will be obtained.
Dissemination
Results will be published in an international peer-reviewed medical journal.
Discussion
Few studies evaluated the impact of DAT in the management of suspected LVO stroke patients, especially in the setting of primary admission. DIRECT ANGIO is a French PROBE, two-arm randomised trial comparing DAT versus standard management for patients with acute severe neurological deficit before hospital admission and thus suspected to anterior circulation LVO eligible to MT. Randomisation is performed before hospital admission and within 5 hours of onset. Primary endpoint is the 90-day functional independence. The study will provide efficacy and safety data as well as socioeconomic evidence for the DAT management for patients with acute severe stroke.
Supplementary Material
Acknowledgments
DIRECT ANGIO boards and institutions: DSMB, CHRU Nancy, CIC-IT, CIC-EC, DRI.
Footnotes
Twitter: @TDesmettre
Collaborators: DIRECT ANGIO Investigators: CHRU-Nancy: Benjamin Gory, Isabelle Costa, Serge Bracard, René Anxionnat, Marc Braun, Anne-Laure Derelle, Romain Tonnelet, Liang Liao, François Zhu, Emmanuelle Schmitt, Sophie Planel, Sébastien Richard, Lisa Humbertjean, Gioia Mione, Jean-Christophe Lacour, Nolwenn Riou-Comte, Gabriela Hossu, Marine Beaumont, Mitchelle Bailang, Gérard Audibert, Marie Reitter, Agnès Masson, Lionel Alb, Adriana Tabarna, Marcela Voicu, Iona Podar, Madalina Brezeanu. CHU Besançon: Alessandra Biondi, Giovanni Vitale, Fortunato Di Caterino, Guillaume Charbonnier, Panagiotis Primikiris, Thierry Moulin, Elisabeth de Bustos Medeiros, Benjamin Bouamra, Louise Bonnet, Fabrice Vuillier, Thibaut Desmettre CH Colmar: Pablo A Lebedinsky, Mariano Musacchio, Frederico Bolognini, Francis Vuillemet, Nicolas Kempf, CH Mulhouse: Elie Cohen-Khallas, Andreas Fickl, Eric Schluck, Mathilde Goudot, Sylvie Courtois, Sohrab Mostoufizadehghalamfarsa, Alessandro La Porta. CHU Reims: Sébastien Soize, Solène Moulin, Laurent Pierot, Pierre-François Manceau, Maher Sahnoun, Christophe Gelmini, Nathalie Caucheteux, Vi Tuan Hua, Isabelle Serre, Mickaël Hoang. CHU Bordeaux: Catherine Pradeau, Gaultier Marnat, Florent Gariel, Xavier Barreau, Jérôme Berge, Louis Veunac, Patrice Menegon, Igor Sibon, Ludovic Lucas, Stéphane Olindo, Pauline Renou, Sharmila Sagnier, Mathilde Poli, Sabrina Debruxelles, Jean-Marc Mene, Musa Sesay. CHU Montpellier: Vincent Costalat, Caroline Arquizan, Cyril Dargazanli, Grégory Gascou, Pierre-Henri Lefèvre, Imad Derraz, Carlos Riquelme, Nicolas Gaillard, Isabelle Mourand, Lucas Corti. Fondation Adolphe de Rothschild: Michel Piotin, Raphael Blanc, Hocine Redjem, Simon Escalard, Jean-Philippe Desilles, Gabriele Ciccio, Stanislas Smajda, Mikael Mazighi, Mikael Obadia, Candice Sabben, Ovide Corabianu, Thomas de Broucker, Didier Smadja, Sonia Alamowitch, Olivier Ille, Eric Manchon, Pierre-Yves Garcia, Guillaume Taylor, Malek Ben Maacha. Hôpital Foch: Adrien Wang, Serge Evrard, Maya Tchikviladze, Nadia Ajili, Bertrand Lapergue, David Weisenburger, Lucas Gorza, Oguzhan Coskun, Arturo Consoli, Federico Di Maria, Georges Rodesh, Morgan Leguen, Julie Gratieux, Fernando Pico, Haja Rakotoharinandrasana, Philippe Tassan, Roxanna Poll, Sylvie Marinier. CHU Limoges: Aymerci Rouchaud, Suzana Saleme, Charbel Mounayer, Francisco Macian-Montoro, Dominique Cailloce.
Contributors: NR-C, ACh, SR, LN, FG, GH and BG are members of DIRECT ANGIO scientific committee and contributed to the conception and design of the research protocol. NR-C, ACh, SR and BG provided critical skills concerning trial interventions and procedures. NR-C and BG wrote the first version of the protocol. BG wrote this manuscript. GH designed the statistical analysis plan. NR-C, FZ, ACh, SR, FG, GH and BG are involved in acquisition, analysis and interpretation of the data. NR-C, FZ, SR, LN, GA, HA, VC, CA, OB, ACo, BL, TL, AR, FM, DC, AB, TM, TD, GM, IS, XC, APL, FV, NK, LP, SM, PL, MM, RB, CS, ES, SB, RA and BG are involved in acquisition of the data. All authors revised the final protocol and approved this submission.
Funding: DIRECT ANGIO trial was supported by funding from French Ministry of Health (Programme Hospitalier de Recherche Clinique Inter Régional (PHRC IR 2018) and from the university hospital of Nancy (principal investigator: BG. Funding amount: 295 990 euros).
Disclaimer: The funder had no role in study design, study conduction, writing or submitting the manuscript.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting or dissemination plans of this research. Refer to the Methods and analysis section for further details.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
DIRECT ANGIO Investigators:
Benjamin Gory, Isabelle Costa, Serge Bracard, René Anxionnat, Marc Braun, Anne-Laure Derelle, Romain Tonnelet, Liang Liao, François Zhu, Emmanuelle Schmitt, Sophie Planel, Sébastien Richard, Lisa Humbertjean, Gioia Mione, Jean-Christophe Lacour, Nolwenn Riou-Comte, Gabriela Hossu, Marine Beaumont, Mitchelle Bailang, Gérard Audibert, Marie Reitter, Agnès Masson, Lionel Alb, Adriana Tabarna, Marcela Voicu, Iona Podar, Madalina Brezeanu, Alessandra Biondi, Giovanni Vitale, Fortunato Di Caterino, Guillaume Charbonnier, Panagiotis Primikiris, Thierry Moulin, Elisabeth de Bustos Medeiros, Benjamin Bouamra, Louise Bonnet, Fabrice Vuillier, Thibaut Desmettre, Pablo A Lebedinsky, Mariano Musacchio, Frederico Bolognini, Francis Vuillemet, Nicolas Kempf, Elie Cohen-Khallas, Andreas Fickl, Eric Schluck, Mathilde Goudot, Sylvie Courtois, Sohrab Mostoufizadehghalamfarsa, Alessandro La Porta, Sébastien Soize, Solène Moulin, Laurent Pierot, Pierre-François Manceau, Maher Sahnoun, Christophe Gelmini, Nathalie Caucheteux, Vi Tuan Hua, Isabelle Serre, Mickaël Hoang, Catherine Pradeau, Gaultier Marnat, Florent Gariel, Xavier Barreau, Jérôme Berge, Louis Veunac, Patrice Menegon, Igor Sibon, Ludovic Lucas, Stéphane Olindo, Pauline Renou, Sharmila Sagnier, Mathilde Poli, Sabrina Debruxelles, Jean-Marc Mene, Musa Sesay, Vincent Costalat, Caroline Arquizan, Cyril Dargazanli, Grégory Gascou, Pierre-Henri Lefèvre, Imad Derraz, Carlos Riquelme, Nicolas Gaillard, Isabelle Mourand, Lucas Corti, Michel Piotin, Raphael Blanc, Hocine Redjem, Simon Escalard, Jean-Philippe Desilles, Gabriele Ciccio, Stanislas Smajda, Mikael Mazighi, Mikael Obadia, Candice Sabben, Ovide Corabianu, Thomas de Broucker, Didier Smadja, Sonia Alamowitch, Olivier Ille, Eric Manchon, Pierre-Yves Garcia, Guillaume Taylor, Malek Ben Maacha, Adrien Wang, Serge Evrard, Maya Tchikviladze, Nadia Ajili, Bertrand Lapergue, David Weisenburger, Lucas Gorza, Oguzhan Coskun, Arturo Consoli, Federico Di Maria, Georges Rodesh, Morgan Leguen, Julie Gratieux, Fernando Pico, Haja Rakotoharinandrasana, Philippe Tassan, Roxanna Poll, Sylvie Marinier, Aymerci Rouchaud, Suzana Saleme, Charbel Mounayer, Francisco Macian-Montoro, and Dominique Cailloce
References
- 1. Waqas M, Rai AT, Vakharia K, et al. Effect of definition and methods on estimates of prevalence of large vessel occlusion in acute ischemic stroke: a systematic review and meta-analysis. J Neurointerv Surg 2020;12:260–5. 10.1136/neurintsurg-2019-015172 [DOI] [PubMed] [Google Scholar]
- 2. Bracard S, Ducrocq X, Mas JL, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol 2016;15:1138–47. 10.1016/S1474-4422(16)30177-6 [DOI] [PubMed] [Google Scholar]
- 3. Saver JL, Goyal M, van der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 2016;316:1279–88. 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 4. Campbell BCV, Majoie CBLM, Albers GW, et al. Penumbral imaging and functional outcome in patients with anterior circulation ischaemic stroke treated with endovascular thrombectomy versus medical therapy: a meta-analysis of individual patient-level data. Lancet Neurol 2019;18:46–55. 10.1016/S1474-4422(18)30314-4 [DOI] [PubMed] [Google Scholar]
- 5. Psychogios M-N, Behme D, Schregel K, et al. One-stop management of acute stroke patients: minimizing door-to-reperfusion times. Stroke 2017;48:3152–5. 10.1161/STROKEAHA.117.018077 [DOI] [PubMed] [Google Scholar]
- 6. Ribo M, Boned S, Rubiera M, et al. Direct transfer to angiosuite to reduce door-to-puncture time in thrombectomy for acute stroke. J Neurointerv Surg 2018;10:221–4. 10.1136/neurintsurg-2017-013038 [DOI] [PubMed] [Google Scholar]
- 7. Jadhav AP, Kenmuir CL, Aghaebrahim A, et al. Interfacility transfer directly to the neuroangiography suite in acute ischemic stroke patients undergoing thrombectomy. Stroke 2017;48:1884–9. 10.1161/STROKEAHA.117.016946 [DOI] [PubMed] [Google Scholar]
- 8. Schulz KF, Altman DG, Moher D, et al. Consort 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. Spirit 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–7. 10.1161/01.STR.19.5.604 [DOI] [PubMed] [Google Scholar]
- 11. EuroQol Group . EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 12. Barber PA, Demchuk AM, Zhang J, et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. aspects Study Group. Alberta stroke programme early CT score. Lancet 2000;355:1670–4. 10.1016/s0140-6736(00)02237-6 [DOI] [PubMed] [Google Scholar]
- 13. Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 2013;44:2650–63. 10.1161/STROKEAHA.113.001972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–29. 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 15. Chabanne R, Fernandez-Canal C, Degos V, et al. Sedation versus general anaesthesia in endovascular therapy for anterior circulation acute ischaemic stroke: the multicentre randomised controlled AMETIS trial study protocol. BMJ Open 2019;9:e027561. 10.1136/bmjopen-2018-027561 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


