Abstract
Preexisting heart failure (HF) in patients with sepsis is associated with worse clinical outcomes. Core sepsis management includes aggressive volume resuscitation followed by vasopressors (and potentially inotropes) if fluid is inadequate to restore perfusion; however, large fluid boluses and vasoactive agents are concerning amid the cardiac dysfunction of HF. This review summarizes evidence regarding the influence of HF on sepsis clinical outcomes, pathophysiologic concerns, resuscitation targets, hemodynamic interventions, and adjunct management (ie, antiarrhythmics, positive pressure ventilatory support, and renal replacement therapy) in patients with sepsis and preexisting HF. Patients with sepsis and preexisting HF receive less fluid during resuscitation; however, evidence suggests traditional fluid resuscitation targets do not increase the risk of adverse events in HF patients with sepsis and likely improve outcomes. Norepinephrine remains the most well-supported vasopressor for patients with sepsis with preexisting HF, while dopamine may induce more cardiac adverse events. Dobutamine should be used cautiously given its generally detrimental effects but may have an application when combined with norepinephrine in patients with low cardiac output. Management of chronic HF medications warrants careful consideration for continuation or discontinuation upon development of sepsis, and β-blockers may be appropriate to continue in the absence of acute hemodynamic decompensation. Optimal management of atrial fibrillation may include β-blockers after acute hemodynamic stabilization as they have also shown independent benefits in sepsis. Positive pressure ventilatory support and renal replacement must be carefully monitored for effects on cardiac function when HF is present.
Keywords: sepsis, septic shock, heart failure, fluids, resuscitation, vasopressors, inotropes, antiarrhythmics
Introduction
Sepsis/septic shock and heart failure (HF) contribute to substantial morbidity and mortality.1 The mortality rate of septic shock is approximately 40%,2 while HF diagnosis confers 50% mortality by 5 years.3–5 Sepsis and HF were ranked first and second, respectively, as conditions with the highest 30-day readmission rates among Medicare patients in 2018,6 and notably, sepsis/septic shock are responsible for a quarter of all HF patient deaths.7,8
The pathophysiology of these disease states results in overlapping and competing hemodynamics and treatment effects. Indeed, sepsis management is characterized by aggressive volume resuscitation with crystalloid fluids and hemodynamic support with vasopressors, which may appear antithetical to conventional HF management that promotes preload and afterload reduction.9
Currently, no recommendations exist for managing patients with sepsis/septic shock and HF, and limited evidence describes the impact of concomitant sepsis and HF on treatment and outcomes. This review discusses the influence of preexisting HF on sepsis outcomes, describes relevant pathophysiology, and assesses hemodynamic monitoring, interventions, and adjunct therapy in this subset of patients.
Methodology
A literature search was performed to identify studies including patients with sepsis/septic shock and HF. The PubMed database was searched for English-language studies published between January 1995 and February 2020 using combinations of the search terms heart failure, congestive heart failure, left ventricular dysfunction, cardiac dysfunction, sepsis, septic shock, severe sepsis, fluids, vasopressors, inotropes, arrhythmias, ventilatory support, and renal replacement therapy. Studies that reported patients presenting with sepsis/septic shock and HF were included. Study designs included were prospective, retrospective, observational, or interventional. References within original research articles, review articles, editorials, abstracts, meta-analyses, and systematic reviews were screened for inclusion.
As HF is a clinical definition, this terminology is not synonymous with defects such as cardiomyopathy and left ventricular dysfunction (LVD) but often includes such defects as its precipitating factors. Table 1 provides definitions used.10 For the purposes of this review, HF refers to the broad category of all subtypes and any mention of a specific variation such as HF with preserved ejection fraction (HFpEF) or HF with reduced ejection fraction (HFrEF) is noted.
Table 1.
Definitions.10
| Heart failure (HF) | Clinical syndrome that results from any structural or functional impairment of ventricular filling or ejection of blood |
|---|---|
| Heart failure reduced ejection fraction (HFrEF) | Ejection fraction (EF) ≤40% |
| Systolic HF | Synonym for HFrEF |
| Heart failure preserved ejection fraction (HFpEF) | EF ≥ 50% |
| Cardiomyopathy | Disease of the myocardium primarily manifested as dilated ventricles or hypertrophic ventricles resulting in inadequate function |
| Left ventricular dysfunction (LVD) | Broad term referring to a problem with the left ventricle supplying the body with adequate blood (often manifested as low EF) |
| Cardiac dysfunction | A milieu term for cardiac-related issues such as HF, cardiomyopathy, LVD |
| Preexisting HF | Heart failure that was present on patient’s admission to the hospital for sepsis and did not occur while the patient was hospitalized |
Prognosis and Outcome Differences
Clinical reasoning suggests underlying cardiac dysfunction of HF will worsen outcomes in a hemodynamically unstable state such as sepsis/septic shock, and data support this hypothesis, especially in HFrEF.11–13 Alon et al revealed a mortality increase in HF patients admitted for sepsis compared to patients without HF admitted for sepsis (51% vs 41%; P = .015).13 A retrospective review of 174 patients (87 with HFrEF and 87 without HFrEF) presenting with sepsis showed HFrEF conferred higher in-hospital mortality (57.5% vs 34.5%; P = .002).11 Ishak Gabra et al evaluated patients with sepsis and preexisting HFrEF and HFpEF and observed trends toward increased mortality.12 Neither HFrEF (odds ratio [OR]: 1.88; P = .06) nor HFpEF (OR: 1.56; P = .25) were statistically associated with increased 28-day mortality when adjusted for severity of illness, but HFrEF was associated with increased new-onset arrhythmias (52% vs 23%; P = .0001).12 Prabhu et al observed increased mortality among patients with sepsis with EF <50%, wherein 44% (n = 14/32) of nonsurvivors had depressed EF compared to 12% (n = 4/34) of survivors (P = .005).14 The average initial EF in the nonsurvivor group was also lower (53% vs 63%; P = .029). Long-term outcomes of sepsis may also worsen in HF patients. Patients with severe sepsis/septic shock and comorbid HF had a 75% mortality rate 1 year postdischarge,15 which is larger than the severe sepsis 1-year mortality rate (40%-44%)16,17 and 10-year mortality rates for severe sepsis (67%) and nonseptic critical illness (57%).18
In contrast, Ouellette and Shah observed no association between EF and clinical outcomes in patients with sepsis.19 Rates of mortality (32% vs 24%; P = .12) and intubation (49% vs 50%; P = .687) were similar between patients with a reduced EF (average EF = 35%) and controls (average EF = 60%). Furthermore, a meta-analysis determined that new LVD was not a sensitive (48%) or specific (65%) predictor of in-hospital 30-day mortality20; however, preexisting cardiac dysfunction was not distinguished from septic cardiomyopathy, limiting its description of preexisting HF.
Many of these studies were restricted to patients with sepsis with a reduced EF where the impact of septic myocardial depression was not differentiated from preexisting cardiac dysfunction (Table 2), making examination of the true impact of preexisting HF on patients with sepsis difficult. The impact of HF directly on sepsis outcomes is further confounded as nearly half of HF patients are plagued by frailty, the combination of aging and multiple complex disease states producing additive deterioration causing greater morbidity and mortality.21,22 Frailty is also independently associated with increased mortality in sepsis.23 Frailty appears more often in HFpEF than in HFrEF,24 which is surprising considering the greater negative outcomes suggested in sepsis and HFrEF. Frailty likely contributes to the increased mortality of sepsis with preexisting HF beyond only poor cardiac performance across the HF spectrum emphasizing the importance of complete medical care beyond just circulatory management.
Table 2.
Patient Outcomes in Sepsis With Preexisting HF.
| Reference | Study type | Population and number (n) | Outcome | HF-related results |
|---|---|---|---|---|
| Abou Dagher et al11 | Retrospective | Patients with preexisting HF (LVEF < 40%) presenting with sepsis, n = 174 (87 with HF) | The effect of preexisting HFrEF on mortality | Patients with HF had a higher in-hospital mortality (57.5% vs 34.5%; P = .002) and had higher odds of death (OR: 2.45; 95% CI: 1.22-4.88; P .01) |
| Ishak Gabra et al12,a | Retrospective | Septic shock, n =2 26 (96 with HF) | The effect of preexisting HF on mortality | HFrEF was not significantly associated with increased 28-day mortality (OR: 1.88; 95% CI: 0.98-3.63; P = .06). HFpEF was not as strongly associated with 28-day mortality (OR: 1.56; P = .25) |
| Alkhalaf et al15,a | Retrospective | Severe sepsis/septic, n = 195 | HF effects on sepsis survivors’ 1-year outcomes and performance status | High rate of 1-year mortality (>70%) noted in patients with HF and sepsis |
| Ouellette and Shah19 | Retrospective, matched cohort | Sepsis stratified into with low EF (n = 197) and control (n = 197), n = 394 | Differences in outcomes based on presence or absence of preexisting LVD | No difference in mortality (32% vs 24%; P = .12) or intubation (49% vs 50%; P = .687) between the low EF group and normal EF group |
Abbreviations: EF, ejection fraction; HF, heart failure; HFpEF, heart failure preserved ejection fraction; HFrEF, heart failure reduced ejection fraction; LVEF, left ventricular ejection fraction; LVD, left ventricular dysfunction; OR, odds ratio.
Abstract.
In summary, the cumulative data suggest sepsis and HF combine to worsen clinical outcomes, and the possibility of septic myocardial dysfunction occurring in a patient with pre-existing HF adds to the combined disease concerns.25 While worsened clinical outcomes are likely not exclusively imparted by poor heart function, examining specialized management of patients with sepsis with preexisting HF is of critical importance considering the importance of cardiac function to hemodynamic interventions.
Pathophysiologic Considerations
During sepsis, the host-mediated immune response creates vascular endothelial cell dysfunction leading to increased capillary permeability and a fluid shift from the vasculature to the interstitial space, and septic shock occurs when fluid fails to stabilize hemodynamics.2 The ideal intervention for sepsis focuses on replenishing intravascular volume with fluid boluses that increase preload to augment cardiac output (CO) and thus restore end-organ perfusion. Sepsis directly impacts heart function by enhancing cytokine activation of nitric oxide synthase, which decreases peak systolic calcium levels impairing cellular contraction resulting in negative inotropy.26 In a normally functioning heart, sepsis-induced tachycardia coupled with decreased afterload from vasodilation may offset the decreased preload and impaired myocardial contractility, resulting in no change or a net increase in CO.27 Whether or not these compensatory effects have deleterious consequences in someone with preexisting cardiac dysfunction has not been described but raises concern given the increased mortality in sepsis with preexisting HF. Sepsis also independently induces cardiac dysfunction (ie, septic myocardial depression) independent of HF,28 which may have compounding negative consequences with preexisting HF.
Heart failure is characterized by a cycle of decreasing CO and neurohormonal compensation leading to cardiac damage and fluid retention.29 Inadequate oxygenation leads to sympathetic nervous system-induced catecholamine release that can exert direct cardiotoxic effects and increase myocardial workload.30,31 Sympathetic nervous system activation further stimulates renin–angiotensin–aldosterone system upregulation, which triggers arterial vasoconstriction, expands intravascular and interstitial volume through sodium and water retention, and leads to pathologic cardiac remodeling.32,33 In chronic HF, decreased capillary endothelial permeability causes intravascular albumin loss and decreased hydrostatic pressure.32 Fluid follows the pressure gradient into the interstitial space, which results in new fluid homeostasis through subsequent expansion of the intravascular volume to match the expanded interstitial space. Over time, increased preload stretches myofibrils past their inherent elasticity, resulting in impaired contraction, stroke volume (SV), and CO.
As the majority of intravascular volume rests in the venous system (85%), this chronic overexpansion creates a state primed for acute or chronic HF development upon critical illness, wherein catecholamine surge constricts the venous system leading to fluid transudation, most consequentially, into the pulmonary alveolar space.33,34 Overlap exists between the hemodynamic effects of sepsis and HF, but whether this necessitates treatment variation remains unknown. Table 3 summarizes proposed disease state intervention interaction effects on hemodynamics.
Table 3.
Interaction of Disease State and Sepsis Management on Hemodynamics.a
| CO |
|||||
|---|---|---|---|---|---|
| Stroke volume |
|||||
| Disease/intervention | MAP | SVR | HR | Preload | Contractility |
| HF | ↓ | ↑ | ↑ | ↑ | ↔↓ |
| Sepsis | ↓ | ↓ | ↑ | ↓ | ↑ |
| HF + sepsis | ↓ | ↔ | ↑ | ↓ | ↕↔ |
| +Norepinephrine | ↑ | ↑ | ↑ | ↔ | ↔↑ |
| +Intravenous fluids | ↕↔ | ↔ | ↔↓ | ↑ | ↕↔ |
| +Inotrope | ↕↔ | ↔ | ↑ | ↔ | ↑ |
Abbreviations: CO, cardiac output; HF, heart failure; HR, heart rate; MAP, mean arterial pressure; SVR, systemic vascular resistance; ↑, increased; ↓, decreased; ↔, neutral effect; ↕, varying effect with possible increase or decrease.
Hemodynamic effects of HF and sepsis and their combined interaction. Norepinephrine, intravenous fluids, and inotrope hemodynamic effects on the combined state of HF and sepsis.
Volume resuscitation.
Naturally, concern exists in volume resuscitating patients with sepsis with HF. Large fluid boluses (ie, 30 mL/kg) may lead to fluid overload followed by pulmonary edema requiring mechanical ventilation (MV).35,36 This concern is of particular importance as fluid overload has been linked to increased mortality in critically ill patients.35,37,38 Patients with HF have increased circulating blood volume and/or dilated ventricles with increased preload at baseline,33 and opposite of sepsis, reducing preload pharmacologically through venous dilation is often employed to treat patients with severe HF.10,39 The influence of the combined effects of these fluid and hemodynamic dysregulations warrants concern.
Vasopressors.
While vasopressors are used ubiquitously in septic shock, their use in cardiogenic is more nuanced. Current HF recommendations promote strategies to reduce ventricular afterload (to improve CO and reduce myocardial oxygen demand) and optimize cardiac preload (increasing CO without affecting myocardial oxygen demand).39,40 Vasopressors increase afterload, which may decrease CO, and compensatory endogenous inotropy may increase myocardial oxygen consumption.41,42 Considering CO in HF generally benefits more from reduced afterload, it raises concern that too much vasopressor-mediated afterload increase may especially impair cardiac function in HF. This concern may be lessened as acute HF developing to cardiogenic shock can elicit decreases in systemic vascular resistance secondary to cytokine release, which can mimic the underlying pathology of sepsis and provide an incentive for vasopressor use in patients with both septic and cardiogenic shock features.43
Additionally, vasopressors may cause fluid redistribution from the venous system and promote a fluid shift into the pulmonary space.33,34 Considering HF patients have chronically elevated intravascular volumes, there is risk for an outsized fluid shift stimulated by vasopressor-induced contraction of the overfilled venous resevoir.34 These mechanisms form a framework for typically avoiding vasopressors in HF patients; however, in the setting of sepsis and chronic HF, management is confounded.
Inotropic agents.
Sepsis with preexisting HF may present a mixed picture of cardiogenic and septic shock, possibly through sepsis-induced myocardial dysfunction or by hypoperfusion from sepsis stimulating over demand of the impaired cardiac muscle and subsequent acute HF.27 This cardiac dysfunction may incentivize approaches favoring inotropes to increase CO, especially once extensive fluid resuscitation and vasoconstriction has failed to correct perfusion.9
Resuscitation Monitoring and Targets
Acute sepsis management uses measurements of oxygenation and hemodynamic status, and these measures may be confounded in patients with preexisting HF. Table 4 summarizes proposed effects of HF on common sepsis resuscitation parameters.
Table 4.
Impact of HF on Sepsis Resuscitation Parameters.
| Resuscitation parameter | Impact of HF on parameters in sepsis | Mechanism of HF confounding |
|---|---|---|
| Lactate | ↑ | Possibly inherently elevated in patients with chronic severe HF (EF < 20%) |
| Capillary refill time | ↔↑ | Likely accurate unless severe edema present in which case it may be elevated |
| ScVO2 | ↓ | Chronic low CO may adapt patients to low oxygenation causing inherent low oxygenation |
| PPV/SVV | ↑↔ | Most profound in right HF secondary to pulmonary hypertension failure from increased RV afterload |
| CVP | ↑ | Mainly in right HF due to elevated RV pressure, and chronic HF may lead to impaired ventricular compliance causing venous congestion elevating CVP |
Abbreviations: CO, cardiac output; CVP, central venous pressure; EF, ejection fraction; HF, heart failure; PPV, pulse pressure variation; RV, right ventricle; ScVO2, central venous oxygenation; SVV, stroke volume variation.
The impact of HF on general interpretability of common resuscitation parameters used in sepsis and septic shock with proposed mechanism of confounding. The arrows indicate a change from the value in a patient presenting with sepsis without preexisting HF (eg, lactate may be additively elevated in patients with sepsis with HF compared to patients with sepsis without preexisting HF).
Lactate.
Lactate is the only clinical parameter recommended to guide initial sepsis resuscitation as an assessment of adequate oxygenation.9 The most recent sepsis definition employs lactate greater than 2 mmol/L to indicate hypoxia from septic shock.2 Lactate may develop during sepsis from mechanisms other than hypoperfusion, specifically adrenergic overstimulation (β-2 agonism) by endogenous catecholamines or exogenous epinephrine.44,45 Additionally, as lactate is also produced from anaerobic metabolism occurring secondary to hypoperfusion, chronically impaired oxygen delivery in HF may confound measurement. In a cohort of hospitalized advanced HF patients not in a state of shock, 25% had elevated plasma lactate (defined as >2.1 mmol/L).46 Given the severity of the patients in this cohort (left ventricular ejection fraction [LVEF] < 20% and requiring left ventricular assist device implantation), broadly attributing elevated lactate to chronic HF when evaluating patients with sepsis is unfounded, but it may predispose some patients with severe HF to higher measurements. As such, consistently elevated lactate levels in adequately resuscitated HF patients should be interpreted with caution, especially in those with severe HF.
Capillary refill time.
Capillary refill time (CRT)-guided resuscitation was recently compared to lactate-guided resuscitation in a multicenter clinical trial of 424 patients with septic shock, and the conclusions showed outcome differences, but a trend toward mortality reduction using the CRT-guided strategy was observed (34.9% vs 43.4%; P = .06).47 Heart failure was not an exclusion for the study, but the rate of preexisting HF was not reported.47 Capillary refill time has also been associated with inadequate perfusion in cardiogenic shock states.48 Capillary refill time may be prolonged in HF patients with low CO, and the degree of hand edema may confound measurement.49 Given that CRT has shown comparable and potentially superior lactate-guided resuscitation, CRT may be more reliable in patients with severe HF (eg, EF< 20%) where lactate may be constitutively elevated.46 Surviving Sepsis guidelines have not endorsed protocolized CRT-guided resuscitation,9,50 but CRT provides a useful bedside assessment in sepsis with preexisting HF patients considering its ease of use and efficacy provided clinical features allow for reliable testing.
Venous oxygenation.
Mixed venous oxygenation (SvO2), and its surrogate marker, central venous oxygen saturation (ScvO2), have been used to monitor oxygenation changes in response to treatment during sepsis, targeting values over 65% and 70%, respectively.51,52 Multiple studies have shown an association between early ScvO2 < 70% and mortality in sepsis/septic shock,53,54 and SvO2 < 60% in acute HF was associated with higher mortality.55 Ouellette and Shah found that among patients with sepsis with preexisting LVD, those who died had lower initial ScvO2 (61% vs 70%; P = .002).19 However, the utility of ScvO2 as a therapeutic target has been questioned over the past several decades and was removed from the most recent Surviving Sepsis Guidelines.9,56
In sepsis, CO is presumably normal or elevated while tissues are unable to extract oxygen secondary to microcirculation disruption.57 The perseverance of CO and low oxygen extraction in sepsis can cause misleadingly elevated SvO2/ScvO2. Velissaris et al reported as many as 50% of patients with ScvO2 > 70% at baseline to be fluid responsive during sepsis, indicating these patients were in need of volume replenishment despite apparently adequate oxygenation.58 Cardiogenic shock states present with relatively lower venous oxygenation,57 creating a common concern that low CO in HF may mask the compensatory increase in CO during sepsis and thus confound venous oxygenation measurements. Patients with chronic low CO may maintain adequate tissue oxygenation in states of low mixed venous oxygenation through supranormal oxygen extraction adaptations,59,60 confounding the ability to target SvO2/ScvO2 in HF patients with sepsis. Indeed, this phenomenon has been reported in patients with chronic SvO2 as low as 40%.60 Given these considerations, SvO2/ScvO2 should not be routinely used to guide resuscitation in sepsis with preexisting HF.
Pulse pressure variation and stroke volume variation.
Pulse pressure variation (PPV) and stroke volume variation (SVV) rely on cyclical intrathoracic pressure changes during MV to assess fluid resuscitation status. These dynamic readings are superior predictors of fluid response when compared to the static measurement of central venous pressure (CVP).61 Both PPV and SVV measures increase in CO during inspiration, and more profound increases in CO suggest fluid responsiveness reflected by higher PPV and SVV. Target PPV values vary drastically, and PPV cannot reliably predict fluid responsiveness when between 4% and 17%, the range reported in most intensive care unit (ICU) patients.62 Stroke volume variation may be even less reliable than PPV and requires more specialized monitoring equipment.63 In HF, the presence of right ventricular (RV) dysfunction or LVD may diminish the accuracy of PPV.64 When RV SV is impaired due to increased RV afterload or RV contractile dysfunction, and not due to decreased RV preload, PPV may be falsely indicative of fluid responsiveness.65 However, the HF spectrum has varying degrees of RV and LVD, and this variation is likely most relevant in right HF secondary to pulmonary hypertension.66 Heart failure may lead to exaggerated PPV and SVV, as the pleural pressure increases likely have a greater magnitude of effect when applied to dysfunctional ventricles. Nevertheless, PPV and SVV may informative fluid response predictors in patients with systolic HF without a dysfunctional RV, and more research is needed to determine the impact of severely impaired LV function on the accuracy of these parameters.
Central venous pressure.
The CVP assessment was removed from the 2016 Surviving Sepsis Guidelines citing limited ability to predict volume status within the normal CVP range of 8 to 12 mm Hg as it is only predictive of response at extreme ranges.9 Central venous pressure may only identify adequate fluid resuscitation in 54% of patients with sepsis67 and has been unreliable outside of the initial 12 hours of septic shock resuscitation to predict both volume status and fluid responsiveness.68 Furthermore, CVP has demonstrated low correlations to both intravascular volume and change in cardiac index.69 Central venous pressure may be even less reliable in patients with sepsis with preexisting HF, as filling pressures estimating preload depend on ventricular compliance,70 which is altered in chronic HF.32 Generally, RV dysfunction produces a falsely elevated CVP, whereas LVD and pulmonary edema alone may not drastically alter CVP.71,72 Patients can present with overly dilated LVs while having CVPs below 8 mm Hg, which may predispose to CVP-guided fluid overload and unintentional induction of acute HF.73 The lack of overall usefulness in guiding sepsis resuscitation and the potential for enhanced unreliability in HF make CVP an ineffective tool for guiding resuscitation.
Point-of-care ultrasound.
The cardiac dysfunction inherent to patients with sepsis with preexisting HF may prompt clinicians to seek initial ultrasound guidance during resuscitation to predict fluid responsiveness. Intensivist assessments prior to cardiologist interpreted ultrasound incorrectly identified LV function in 40% and RV function in 50% of patients suggesting expert involvement, which is not always readily available, may be important for this practice.74 Indeed, cardiologist interpreted point-of-care ultrasound improved clinician confidence in decision-making concerning fluid use.74 However, point-of-care ultrasound in the emergency department prior initiation of interventions in septic shock has been associated with increased odds of death compared to no ultrasound or ultrasound after intervention.75 Prior ultrasound was associated with less aggressive fluid resuscitation possibly leading to poorer outcomes. Interestingly, sensitivity analysis found no differences in adjusted mortality in patients receiving vasopressors.75 Given the potential for observer error and potential harm, point-of-care ultrasound should not be used prior to guideline-recommended fluid resuscitation in the absence of expert interpretation. Future prospective studies are needed to assess whether point-of-care ultrasound could provide a more nuanced approach to resuscitation specifically in patients with sepsis with preexisting HF.
Fluid Use
Surviving sepsis guidelines recommend administration of a 30 mL/kg bolus of crystalloid fluid within 3 hours of presentation to correct hypotension in patients with sepsis, regardless of comorbid conditions.9 Early goal-directed therapy (EGDT) provided the foundation of delivering large fluid boluses to all patients with sepsis.51 However, EGDT and its targets have been called into question by large trials refuting their efficacy and suggesting increased intensity of care without clinical benefits.76–78 A recent meta-analysis by the PRISM investigators showed no mortality benefit (overall and in a subgroup of underlying cardiovascular dysfunction) and higher hospitalization costs with EDGT.79 Although the tenants of sepsis management have undergone a reorientation, the literature on fluid use in HF patients with sepsis is primarily limited to conflicting observational and retrospective studies (Table 5). Evidence attributing traditional fluid use to worse outcomes in HF patients with sepsis is sparse; however, providing guideline-recommended fluids during acute resuscitation is supported by the majority of the literature as it likely decreases mortality without the risk of fluid overload and other adverse events.
Table 5.
Fluid Utilization and Associated Outcomes.
| Reference | Study type | Population and number (n) | Outcome | HF-related results |
|---|---|---|---|---|
| Singh et al80,a | Retrospective | Severe sepsis and septic shock, n = 505 (151 with EF < 40%) | The effect of the amount of fluid administered accordingly in patients with low EF | Receipt of >3 L of fluid with EF <40% was associated with increased hospital stay (OR: 1.9) and mortality (OR: 1.7). No significant levels reported |
| Khan et al81 | Retrospective | Sepsis and septic shock, n = 208 (71% preexisting HF) | The impact of ≥30 mL/kg or <30 mL/kg of fluid in the first 6 hours after sepsis diagnosis on respiratory outcomes | No differences in mechanical intubation within 72 hours between the <30 mL/kg group and the ≥30 mL/kg group (35% vs. 33%; P = .34) |
| Truong et al82 | Retrospective | Septic shock, n = 1027 | Compliance and outcomes associated with 30 mL/kg fluid resuscitation within 6 hours | HF was associated with decreased compliance with 30 mL/kg fluid resuscitation (40.9% vs 47.3%; P = .014). Compliance was not associated with differences in mortality |
| Duttuluri et al83,a | Retrospective | Severe sepsis and septic shock with a history of HF, n = 333 | Impact of failure to meet 30 mL/kg during resuscitation | Failure to meet 30 mL/kg did not affect overall mortality rate (25.6% failure vs 21 % nonfailure; P = .36) Hypotensive HF patients had a mortality benefit from adequate fluid resuscitation (23% vs 43%; P = .0015) and lower intubation rates (46% vs 65%; P = .008). |
| Leisman et al84 | Prospective observational | Sepsis and septic shock, n = 3686 | Factors associated with positive fluid response | HF was predictive of fluid refractoriness (OR: 1.43; 95% CI: 1.20-1.72; P < .001) 56% of HF patients were initially fluid responsive |
| Leisman et al85 | Prospective observational | Severe sepsis and septic shock; subgroup with HF, n = 1045 | Impact of compliance with a 3-hour sepsis bundle | Patients with HF had high rates of bundle noncompliance (77%). Bundle compliance was associated with decreased mortality in HF patients (OR: 0.67; 95% CI: 0.47-0.95; P = .026) |
| Liu et al86 | Retrospective | Hemodynamically stable patients with sepsis with intermediate lactate values in the ED, n = 18 122; with history of HF (4144) | Effect of 3-hour sepsis bundle implementation on outcomes | Bundle compliant patients with HF had lower mortality risk (14.8% vs 1 1.6%; P = .03) Patients with HF and/or kidney disease received more total fluid postbundle implementation (1.4 vs 1.7 L; P < .01) |
| Seymour et al87 | Retrospective | Sepsis and septic shock, n = 49 331; with HF (10 092) | Associations between time until completion of a 3-hour sepsis bundle and risk-adjusted mortality | Each hour in time to completion of initial IV fluid bolus did not affect mortality in HF patients (OR: 1.00; 95% CI: 0.97-1.04) Each hour delayed for the 3-hour bundle increased hospital mortality in HF patients (OR: 1.06; 95% CI: 1.04-1.09) |
| Kelm et al88 | Retrospective cohort | Severe sepsis and septic shock, n = 405; n = 40 with HF | Clinical evidence of fluid overload after EGDT | Fluid overload on day 1 was significantly higher in HF patients: 31 (1 1.4%) vs 9 (6.8%); P > .05 |
| Akhter et al89,a | Retrospective cohort | Patients with sepsis with HF and/or ESRD, n = unknown | Effect of 30 mL/kg volume resuscitation on outcomes | Fluid resuscitation with 30 mL/kg was less likely in patients with HF and/or ESRD (13.8% vs 21%; P = .02) There was no difference between intubation rates or mortality in either group |
Abbreviations: ED, emergency department; EGDT, early goal-directed therapy; EF, ejection fraction; ESRD, end-stage renal disease; HF, heart failure; OR, odds ratio.
Abstract
One retrospective study suggested increased mortality in patients with severe sepsis/septic shock and HFrEF given >3 L of fluid.80 Whether the association with mortality could be attributed to the presence of systolic dysfunction or more severe sepsis is unclear. Of note, 3 L of fluid would likely be reached with fluid administered in excess of the 30 mL/kg initial bolus, as an average weight of 70 kg would confer only about 2 L of fluid. Additionally, the time frame of fluid administration was not reported, making it difficult to apply this volume cutoff.80 Leisman et al reported HF as predictive of the fluid refractory phenotype, but even among HF patients, over half were initially fluid responsive.84 Delayed fluid administration was the most predictive factor of the refractory phenotype,84 and delayed fluid administration has been reported in HF by observational studies.90,91 This factor may imply that HF was predictive due to delayed fluid administration. Kelm et al examined fluid overload after EGDT in 405 patients with septic shock and found no significant increase in day 1 or persistent fluid overload among a subgroup of 60 patients with sepsis and HF, although the study was not designed to assess this outcome.88 Among a septic cohort with 71% of preexisting HF, Khan et al found no differences in the rates of intubation between receipt of <30 mL/kg and ≥30 mL/kg of resuscitation fluid in the first 6 hours.81 This study was the first to directly assess the impact of traditional fluid boluses on intubation rates as a primary outcome, and the lack of harm from fluids tempers concern for fluid resuscitation-induced pulmonary edema.
A large retrospective study assessing multiple populations showed that a subgroup of 1045 HF patients with sepsis had reduced mortality when compliant with a 3-hour sepsis bundle that included 30 mL/kg fluid bolus initiated within 30 minutes (60-day in-hospital mortality OR: 0.67; 95% CI: 0.47–0.95; P = .026).85 The benefit was surprisingly accentuated in the HF subgroup compared to the overall cohort85; however, these results may not be attributed solely to volume resuscitation as the timely effects of bundle implementation are not strictly related to fluids and may be due to earlier antimicrobials, source control, and additional treatment measures. Liu et al examined 4144 patients with HF in separate periods of pre- and postbundle implementation and found bundle compliance decreased mortality among HF patients.86 Postbundle mortality for patients with HF was reduced (11.6% vs 14.8%; P = .03). The mortality reduction was exclusive to patients with HF and/or kidney disease and was not seen when these patients were excluded from analyses.86 Shah and Ouellette reported EGDT compliance decreased in-hospital mortality among patients with sepsis with a reduced EF (16.7% vs 36.3%; P < .05).92 Collectively, data demonstrate compliance with goal-directed therapy and fluid targets likely improves morbidity and mortality in HF patients with sepsis.
Noncompliance with sepsis bundles or guideline-recommended therapy shows either a neutral or deleterious effect in sepsis with preexisting HF, and broadly adjusting fluid goals based on the presence of HF without careful clinical assessment is unwarranted. Suggesting neutral outcomes, a study of 1027 patients with septic shock recently found failure to administer an initial 30 mL/kg fluid bolus did not affect mortality (OR: 1.03; 95% CI: 0.76-1.41).82 This finding, along with concern for excess fluids increasing ICU mortality, continued to rebut the “one size fits all” approach of fluid resuscitation, but no subgroups of HF were analyzed. Seymour et al observed an increase in mortality for each hour delay of a 3-hour sepsis bundle among HF patients, but this outcome was not observed for each hour delay in fluid bolus administration.87 The results are limited by nonstandardized fluid initiation times, and whether time 0 was related to fluids initiated during triage or floor order entries is unknown. In contrast, Kuttab et al showed failure to complete 30 mL/kg by 3 hours was associated with increased in-hospital mortality regardless of comorbidities (OR: 1.52; 95% CI: 1.03-2.24).90 However, no significant mortality difference was observed with failure to meet 30 mL/kg by 3 hours in HF (OR: 1.48, 95% CI: 0.68-3.21), although the study was not powered to assess this subgroup.90 A cohort of 333 patients with severe sepsis/septic shock with preexisting HF found failure to meet 30 mL/kg was not associated with increased mortality (25.6% vs 21%; P = .36).83 However, in the group of patients who had HF and were hypotensive, adequate fluid resuscitation was associated with lower mortality (23% vs 43%; P = .0015) and lower intubation rates (46% vs 65%; P = .008).83 These results highlight the importance of maintaining guideline-directed fluid targets in HF patients with sepsis as inadequate resuscitation has demonstrated at best, neutral, and worse detrimental outcomes.
Despite the evidence supporting guideline-directed fluid resuscitation, clinicians tend to under-resuscitate patients with HF likely due to concerns for volume overload. Wardi et al recently surveyed critical care clinicians, and 43% of respondents did not believe 30 mL/kg fluid boluses were appropriate for patients with sepsis with HFrEF, while only 11% deemed it inappropriate in patients without HFrEF (P < .01).93 Unsurprisingly, failure to reach fluid resuscitation of 30 mL/kg has been observed in patients with HF.82,89–91 Kuttab et al found decreased odds of reaching a goal of 30 mL/kg of fluid by 3 hours in patients with HF (14.3 mL/kg in patients with HF vs 30 mL/kg in patients without HF; OR: 0.42 [0.29-0.60]).90 Leisman et al associated HF with longer times to initiation of fluids (β = 20 minutes; CI, 14 to 25; P < .001) and a lower volume of fluid resuscitation (β = −14 mL/kg; CI, −17 to −12; P < .001).91 Abou Dagher et al found patients with sepsis and pre-existing HF received less intravenous (IV) fluid in the first 24 hours (2.75 ± 2.28 L vs 3.67 ± 2.82 L; P = .038).11 While clinical concern may cause under-resuscitation in HF patients with sepsis, available data do not support this practice, and the vast majority of the literature advocates against under resuscitation.
After the acute resuscitation phase of sepsis, therapeutic strategies during the optimization, stabilization, and evacuation phases of fluid management remain unclear.94 The pilot study Restrictive IV Fluid Trial in Severe Sepsis and Septic Shock examined the effect of conservative fluid management (<60 mL/kg over the first 72 hours) compared to usual care in 109 patients with severe sepsis/septic shock.95 No mortality difference was observed between the conservative fluid approach and usual care (21.8% vs 22.8%; P = .99). Less fluid administration was observed during the first 24 hours after randomization (7.8 vs 16.6 mL/kg; P = .02); however, each group had roughly 9 hours from triage to randomization in which both received >30 mL/kg of fluid (34.4 vs 36.2 mL/kg; P = .49),95 indicating that the discussion of conservative fluid management occurs after the initial 30 mL/kg bolus. Hjortrup et al prospectively examined a conservative fluid strategy versus standard care through 5 days after initial resuscitation and found reduced incidence of worsening kidney function in the restrictive group (37% vs 54%; P = .03).96 These results provide insight into opportunities to safely mitigate excess fluid administration without compromising the clinical benefits of acute resuscitation, an attractive strategy for HF patients.
Data have shown higher fluid balances during hospitalization correlate with increased mortality,38,68,97 but patients with markers of severe HF were excluded from these analyses. Interestingly, intensive fluid restriction over several days has not demonstrated improved outcomes in nonseptic acutely decompensated HF patients.98,99 A recent meta-analysis of de-resuscitation in critically ill patients showed that a conservative fluid approach resulted in less ICU and ventilator days without increasing adverse events but did not affect mortality; however, the review excluded studies that included patients with HF.100 The impact of optimizing the de-resuscitation and fluid restrictive phases after initial resuscitation in sepsis with preexisting HF presents an intriguing clinical question that remains to be answered, but current evidence points to possible benefits without harm with a conservative approach adopted after initial resuscitation.
In summary, patients presenting with sepsis and preexisting HF should not be withheld within the recommended 30 mL/kg bolus of crystalloid fluid in the acute resuscitation phase. Achieving 30 mL/kg of fluid by 3 hours is a reasonable goal in sepsis with preexisting HF, as it has improved outcomes in patients with sepsis.82 However, caution is advised in patients with advanced HF, and research on these specific subgroups (eg, EF < 15%) is warranted due to the lack of conclusive evidence and risk of disproportionate consequences from volume resuscitation. After acute fluid resuscitation, a conservative fluid strategy is reasonable in HF patients, but more research is needed in protocolizing a conservative fluid strategy.
Pharmacological Considerations for Sepsis With HF
The Surviving Sepsis guidelines recommend norepinephrine (NE) as the first-line vasopressor in sepsis and septic shock followed by vasopressin and possibly epinephrine or dopamine as second- or third-line options. Low-quality recommendations are provided for dopamine, epinephrine, and dobutamine use in select patient populations, and evidence has been primarily based on improvements in hemodynamic parameters and a neutral effect on outcomes.9 Due to the lack of evidence concerning vasopressors within patients with sepsis with preexisting HF, data are primarily extracted from subgroups within studies on sepsis, septic shock, and other shock presentations. Figure 1 summarizes proposed optimal interventions in resuscitation in septic shock with preexisting HF in relation to both fluid and pharmacologic therapy.
Figure 1.
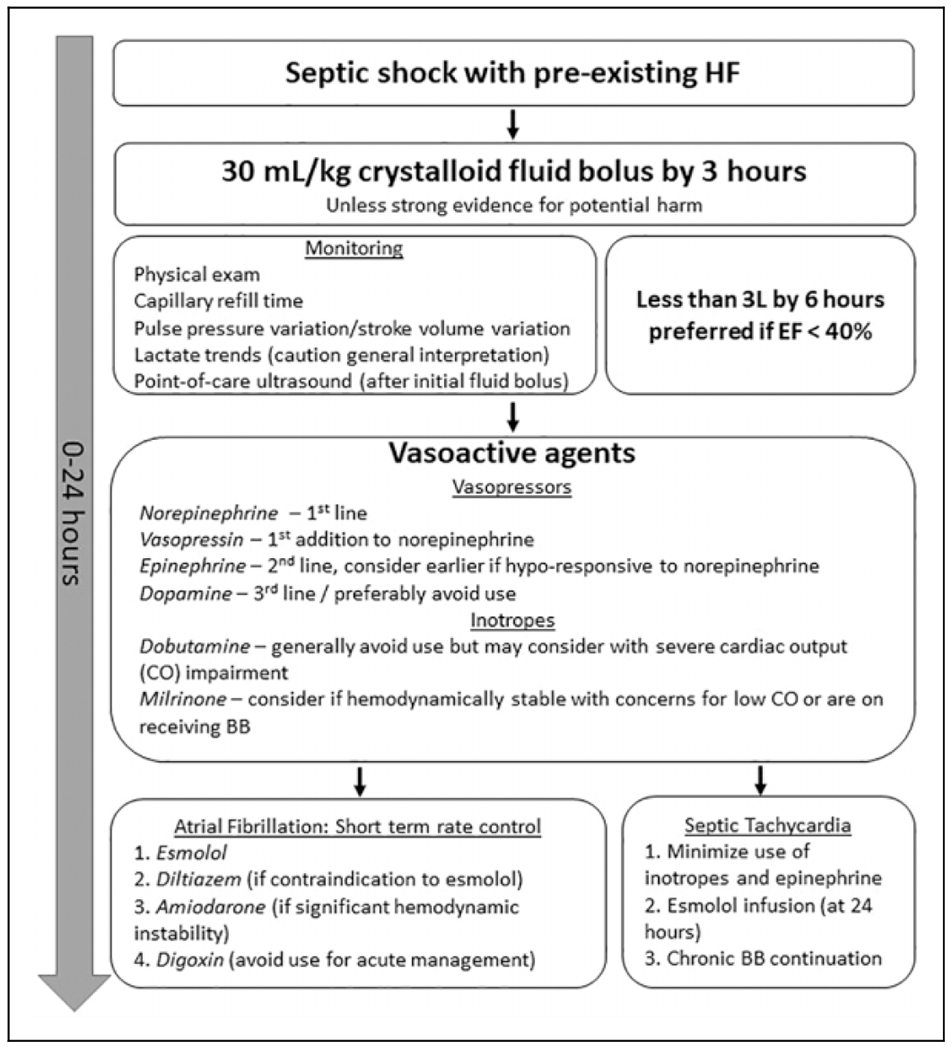
Depiction of proposed optimal interventions for septic shock with preexisting HF in the first 24 hours of management. Fluid resuscitation should consist of 30 mL/kg administered in the first 3 hours unless obvious findings indicating a high risk of harm are present. Monitoring should include careful physical assessment and indices of volume resuscitation status. Administering less than 3 L of fluid in patients with HFrEF over the first 6 hours provides a reasonable conservative goal; however, fluid should not be withheld when multiple indices and clinical status indicate it would be highly beneficial. Preferred vasopressors agents are NE, vasopressin, and possibly epinephrine, while dopamine should be avoided when possible. Inotropes are not universally recommended, but dobutamine may be considered in patients with persistently low CO and low EF. Milrinone may be more efficacious in patients currently receiving a BB. If AF is present, esmolol and diltiazem may be used in the short term, and amiodarone may be more hemodynamically favorable in whom even small changes in blood pressure are concerning. Digoxin should not be used routinely for acute AF management. Septic tachycardia from adrenergic overstimulation and increased cardiac workload may be mitigated by avoiding inotropic stimulation and epinephrine, and by esmolol infusion started 24 hours after hemodynamic optimization, or continuation of home BB. AF, atrial fibrillation; BB, β-blocker; CO, cardiac output; EF, ejection fraction; HF, heart failure; HFrEF, heart failure reduced ejection fraction; NE, norepinephrine
Norepinephrine.
As the first-line vasopressor in sepsis,9 NE has the most supportive evidence. Norepinephrine mediates arterial constriction via α-1 adrenergic agonism, and limited positive inotropy is observed through β-1 stimulation.101 Furthermore, NE may directly stimulate coronary vessel dilation in failing hearts.102 This mixed action has created interest in the role of NE in hemodynamic support during cardiogenic shock.
The Sepsis Occurrence in Acutely Ill Patients (SOAP) II trial demonstrated superiority of NE over dopamine in 280 patients with cardiogenic shock through a comparative reduction in 28-day mortality (P = .03) and lower incidence of arrhythmias within all variations of shock (ie, hypovolemic, septic, and cardiogenic).103 In keeping with these results, a 2015 meta-analysis prompted guidelines to recommend NE over dopamine as first line in septic shock as it demonstrated lower mortality (risk ratio [RR]: 0.89; 95% CI: 0.81-0.98) and lower risk of arrhythmias (RR: 0.48; 95% CI: 0.40-0.58).104 The data observed a lower relative heart rate (HR) of 19 beats per minute when comparing NE to dopamine, which may minimize increases in myocardial oxygen demand. Another recent meta-analysis compared NE to dopamine for cardiogenic shock and again concluded NE was associated with lower 28-day mortality and arrhythmic events regardless of etiology of cardiogenic shock.105 Norepinephrine was prospectively evaluated against epinephrine for managing cardiogenic shock secondary to myocardial infarction in 57 patients, and NE reduced refractory shock (7% vs 32%; P = .008) and had lower HR, less lactatemia, and less acidosis.106
Even with aspects of HF pathophysiology suggesting diminished efficacy, NE may have beneficial effects on cardiac function. Norepinephrine increased CO among patients with septic shock and an average baseline EF of 47% who had positive responses to passive leg raise (ie, volume responsive with cardiac preload reserve).107 Similarly, Maas et al concluded that SVV > 8.7% was predictive of NE-induced increase in CO.108 However, HF patients commonly lack the cardiac preload reserve detected in volume response assessments,109 and this preload reserve deficiency creates concern for reduced NE effectiveness. On the other hand, increased venous return via vasoconstriction may decrease the need for fluid administration through fluid recruitment from the venous vasculature reservoir.110 Despite the mechanistic confounding in HF, an observational study showed NE improved cardiac index and SV index in patients with EF < 45%.111 Interestingly, a small group of patients experienced decreases in cardiac index after NE, which may represent a group in whom the increased afterload unmasks underlying systolic dysfunction of HF.111 Hamzaoui et al showed NE increased LVEF in 38 patients with septic shock (49%-56%; P < .05) and in a subgroup of these patients with LVEF ≥45% (36% to 44%; P < .05).112 Taken together, these data demonstrate that volume-responsive patients likely experience a more profound increase in CO due to NE. However, even amid concern for low volume responsiveness in HF, NE has improved EF in HFrEF. Whether actively recruiting the existing excess fluid in the vasculature of HF patients through vasoconstriction could replace some early fluid administration is unknown. This principle may allow earlier use of NE in patients for whom fluid overload is a concern by utilizing the increased volume in the venous capacitance of HF patients. The Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis (CLOVERS) trial is currently in recruitment and will begin to address this line of thinking as it compares early fluid strategy to early vasopressor strategy in sepsis.113 In summary, NE has been found to be safe and effective in patients with sepsis and cardiac dysfunction and should remain the first-line vasopressor in patients with septic shock and HF.
Vasopressin.
Vasopressin is recommended as a second-line vasopressor in septic shock9 but has no recommendations concerning use in acute HF or cardiogenic shock.10 Heart failure-mediated renin-angiotensin-aldosterone system activation leads to chronically elevated levels of endogenous vasopressin,114 and vasopressin antagonism has been applied as a therapeutic strategy in HF.10 In contrast, septic shock has shown endogenous vasopressin deficiency,115 especially in the late phase of shock.116 These competing effects may decrease the efficacy of vasopressin in patients with septic shock and HF as preexisting elevated levels of vasopressin in HF may reduce the degree of deficiency in sepsis and, thus, the benefits of vasopressin replacement. The application of vasopressin agonism in HF appears confounded by pathophysiologic principles, but its role in septic shock outweighs these theoretical concerns.
As a potent vasoconstrictor, concern exists for vasopressin’s negative impact on coronary perfusion, which has been demonstrated in animal models.117,118 Additionally, caution should be held in patients with mixed septic and cardiogenic shock as vasopressin-mediated vasoconstriction through V1-receptor activation increases afterload significantly with no direct inotropic effects (unlike NE), thus further reducing CO.119 However, no human data have indicated increased cardiac injury or reduced CO among patients treated with vasopressin.120–123 The Vasppressin and Septic Shock Trial (VASST) compared vasopressin to NE added to existing vasoactive agents and found that the addition of vasopressin reduced mortality in a subgroup of patients with less severe sepsis (NE requirements: 5-14 μg/min).124 A post hoc analysis of the cardiopulmonary effects of vasopressin in the VASST trial (vasopressin vs NE in septic shock) showed vasopressin did not diminish cardiac index compared to NE even in the subset of patients with the lowest CO.121 Additionally, vasopressin was associated with a lower HR compared to NE.121 The VASST trial excluded patients with underlying severe HF (New York Heart Association [NYHA] class III or IV) limiting conclusions from the largest vasopressin study to date.124 Notably, vasopressin induces profound vasoconstriction in the fluid reservoir of the splanchnic vasculature,125 and the active recruitment of this volume in HF patients raises caution, although no evidence supports this concern. In summary, given the lack of human data on adverse effects and the efficacy when combined with NE, vasopressin should remain the agent of choice to add to NE in sepsis with preexisting HF.
Epinephrine.
Epinephrine is recommended as a second-line vasopressor to supplement NE in the management of septic shock with weak quality of evidence.9 While epinephrine is used in septic shock, its utility in low output states of acute HF has been disputed.126 Through β-1-mediated inotropy, epinephrine increases SV, EF, and CO,127 all ideal for patients with HF. Epinephrine has demonstrated comparative outcomes to NE in septic shock but has concerns for metabolic and splanchnic adverse effects not seen with NE.128–130
In a retrospective cohort, Sato et al suggested increased arrhythmias and mortality with the use of epinephrine in septic shock (mortality HR: 4.79; 95% CI: 2.12-10.82; P < .001).131 In a clinical trial of ICU patients requiring NE or epinephrine, no difference in time to goal mean arterial pressure (MAP) or mortality was found in patients with severe sepsis and circulatory failure.132 However, epinephrine produced more metabolic side effects and tachycardia.132 Levy et al compared epinephrine to NE plus dobutamine for the management of cardiogenic shock not caused by myocardial infarction (90% of patients had a history of HF) and found similar increases in global hemodynamic effects (eg, cardiac index, MAP).133 Epinephrine increased HR, arrhythmias, lactate, and inadequate gastric mucosal perfusion compared to NE/dobutamine.133 A recent trial of epinephrine versus NE in patients with cardiogenic shock due to acute myocardial infraction observed more refractory shock in the epinephrine group resulting in early trial termination (37% vs 7%; P = .008).106 Heart rate, cardiac double product, and lactic acidosis increased significantly with epinephrine, while remaining unchanged with NE.106
Elevated lactate from epinephrine is commonly viewed as an adverse event; however, a more nuanced appreciation of lactate is warranted. The lactate generated from epinephrine comes from β2-receptor induction of aerobic glycolysis and not exclusively from hypoxia.45 Exogenous lactate was shown to augment CO in acutely decompensated HFrEF.134 Additionally, elevated lactate after epinephrine was predictive of better outcomes in nonspecific135 and septic shock.136 Lactate may not be an entirely negative consequence of epinephrine, and future studies should investigate benefits with epinephrine based on initial lactate levels.
In summary, limited data support an application for epinephrine in sepsis and septic shock with preexisting HF. Epinephrine has shown arrhythmogenic side effects without hemodynamic benefits over other agents when managing cardiogenic shock states and should be avoided, if possible, in the management of sepsis with preexisting HF.
Dopamine.
Dopamine is recommended as a last-line vasopressor for septic shock due to heightened risk of arrhythmias compared to other vasopressors.9 Dopamine’s inotropic and vasopressor qualities have made it appealing for hemodynamic management101; however, evidence has suggested harmful cardiac effects complicating its use in HF patients with sepsis. De Backer et al concluded dopamine was associated with greater mortality and more arrhythmias compared to NE in septic shock.137 As previously described, dopamine was inferior to NE in the SOAP II trial and in meta-analyses of nonspecific shock, septic, and cardiogenic shock.103–105 The mechanism for these adverse outcomes may be attributed to the increase in HR and higher incidence of arrhythmias, specifically atrial fibrillation (AF), with dopamine compared to NE.103,137 Increased mortality was not seen in septic or hypovolemic shock in the SOAP II trial, suggesting specific deleterious cardiac effects.103 Patients with severe HF have increased risk for developing cardiogenic shock in the midst of sepsis, further directing the choice of vasopressor away from dopamine.138
Dopamine has been prospectively evaluated in acute HF patients with average EFs of 30% to 35%.139,140 These trials did not demonstrate increased cardiac adverse effects of dopamine, but a major limitation in extrapolating these data to septic HF patients is the dose used in these HF trials was 5 μg/kg/min.139,140 The dose in SOAP II represented dopamine dosing for sepsis/septic shock and ranged from approximately 12 to 16 μg/kg/min over the first 7 days.103 Considering dopamine dosing greatly effects targeted receptor profiles, the more adverse effects observed from dopamine at higher doses (specifically arrhythmias) may limit the safety findings of the trials in acute HF. Further, Mebazaa et al observed that dopamine had the highest association with mortality among vasoactive agents when used in acute HF, mostly due to higher in-hospital mortality. 141 Notably, a recent meta-analysis of dopamine in critically ill patients with cardiac dysfunction found dopamine did not affect mortality; however, the trials included had doses in the 2 to 10 μg/kg/min range, with most being below 5 μg/kg/min.142
In summary, the data on dopamine for sepsis with preexisting HF are limited with an apparent dose-dependent effect on adverse outcomes, suggesting greater adverse effects with the higher doses used in septic shock. Due to the adverse effects in septic and cardiogenic shock, suggested harm in acute HF, and lack of benefit among critically ill patients with cardiac dysfunction, dopamine should be generally avoided in sepsis with preexisting HF.
Dobutamine.
Dobutamine is recommend to treat persistent hypotension despite fluids and vasopressors in patients with sepsis9 and historically in the context of improving ScvO2 to >70% in EGDT.51 Dobutamine is also used in severe HF to support patients with low CO.10 These applications stem from its strong inotropic effects augmenting oxygen delivery, MAP, and CO.143,144
Despite an attractive mechanism, dobutamine has largely failed to improve oxygenation and outcomes among patients with sepsis, but some recent studies suggest a benefit. Arguing against dobutamine use, Sato et al associated dobutamine with higher mortality and incidence of AF in septic shock.131 Hernandez et al found dobutamine failed to improve perfusion parameters in septic shock, despite an increase in HR, CO, and EF.145 Hayes et al146 found no clinical improvement in cardiac index or oxygenation status from dobutamine among critically ill patients (72% sepsis/septic shock) and associated dobutamine with increased hospital mortality (54% vs 34%; P = .04). Additionally, treatment with NE and as-needed dobutamine based on cardiac index did not improve survival or outcomes when compared to NE alone in patients with septic shock.129 Concerning HF-related outcomes, a meta-analysis of acute decompensated HF patients comparing dobutamine and nesiritide found lower survival rate (OR: 0.48; 95% CI: 0.36-0.63; P < .001) and greater readmission rate (OR: 0.52; 95% CI: 0.36-0.73; P < .001) with dobutamine.147
In support of dobutamine, a recent meta-analysis found that NE plus dobutamine was associated with decreased 28-day mortality (highest surface under the cumulative ranking [SUCRA] of 85.9% compared to NE plus epinephrine [74.6%], epinephrine [72.5%], vasopressin [66.1%], and NE [59.8%]), especially among patients with sepsis with low CO.148 The SUCRA compares the interventions to the hypothetical best intervention for the outcome, and the higher the percentage, the closer the intervention is to the hypothetical best intervention.149 The SUCRA ranking method may be influenced by clinician preference/familiarity and variable levels of data quality warranting cautious interpretation.150 Despite the absence of clear outcomes data, patients with sepsis with preexisting HF have more dobutamine use than those with sepsis and no HF.11,15 Whether dobutamine has a specific impact on outcomes for patients with preexisting HF remains unknown.
In summary, even though dobutamine presents an intuitive option to treat persistent hypotension in septic HF with low CO from LVD, evidence does not show clear clinical benefit. Evidence largely supports a negative or neutral effect on outcomes from dobutamine. Recent data suggest an application for dobutamine when combined with NE in patients with sepsis/septic shock and low CO. Dobutamine may be considered a second-line vasoactive agent as adjunct to NE for sepsis with preexisting HF presenting with concerns for reduced CO (ie, severe HFrEF) but should not be used regularly in HFpEF.
Milrinone.
Milrinone’s inotropic effects are often used to improve CO in severe HF.10 However, milrinone causes greater vasodilation than dobutamine making it an unlikely choice in sepsis.151 Indeed, in a trial of milrinone in sepsis, increases in NE and epinephrine were needed during milrinone infusion to counter vasodilation.152 Milrinone has demonstrated superior correlation with venous oxygenation compared to dobutamine in severe HF,153 but a prospective clinical trial in acute HF showed milrinone worsened hypotension, increased arrhythmias, and possible mortality increase.154 Interestingly, Sato et al reported milrinone was not associated with increased mortality in septic shock (HR: 0.885; 95% CI: 0.44-1.79; P = .734), unlike epinephrine and dobutamine.131 However, milrinone was associated with increased incidence of AF similar to both epinephrine and dobutamine.131 Milrinone showed no detrimental effects and possibly enhanced cardiac function when used in combination with β-blockers (BBs) in sepsis to combat concerns for catecholamine-induced myocardial depression by avoiding dobutamine.155,156 Unlike dobutamine, phosphodiesterase inhibitor efficacy is not altered by concomitant BBs,157 but the specific application of milrinone use preferentially to dobutamine in patients on chronic BB therapy is not established.
In summary, the hypotensive effects in sepsis and lack of efficacy in acute HF patients of milrinone suggest it should not be used during the acute resuscitative phase but may be used cautiously after stabilization if there are concerns for catecholamine toxicity and reduced dobutamine efficacy (eg, concomitant BB).
Considerations With BBs and Angiotensin Inhibition
Patients with chronic HF are likely managed with chronic BBs, angiotensin-converting enzyme inhibitors (ACE-Is), angiotensin receptor blockers (ARBs), or angiotensin receptor neprilysin inhibitors (ARNi).10,158 β-Blockers have been of interest in sepsis as evidence has shown profound overstimulation of adrenergic receptors contributes to cardiac dysfunction.159 During sepsis, BBs attenuate inflammatory cytokines, improve cardiac function, counteract metabolic dysregulation, and prevent negative consequence from sympathetic overstimulation.160,161 While the majority of the studies have assessed infusions of fast-acting BBs (eg, esmolol) for this application, the question of how to manage concurrent home BB therapy remains, particularly in patients who may benefit from dobutamine. Additionally, ACE-Is reduce pro-inflammatory mediators and acute lung injury in an experimental sepsis animal model,162 although their evidence is far less developed for this role in therapy. The potential beneficial effects of medication continuation are often overshadowed by fear of negative hemodynamic consequences.
β-Blockers.
Patients presenting with BB home therapy may have upregulated β-receptors,163 which may lead to altered doses of vasoactive agents needed to meet goal hemodynamics. In the setting of upregulated β-receptors, abruptly withdrawing the BB may expose an abnormally high number of receptors to agonism from endogenous catecholamines during sepsis and potentially augment effects of β-agonist vasoactive agents. In contrast, continuing BB may impede binding of vasoactive agents to β-receptors to an unknown degree and decrease efficacy. Interestingly, in the failing heart, carvedilol does not upregulate β-receptors due to a different binding pattern than metoprolol.164,165 Differences in the hemodynamic effects between metoprolol and carvedilol have been observed with concomitant dobutamine.166 In the presence of metoprolol, the inotropic effects of dobutamine were slightly blunted, whereas carvedilol almost completely blunted dobutamine’s inotropic effects.166 Additionally, only high-dose dobutamine (15-20 μg/kg/min) increased CO in the presence of chronic carvedilol blockade.157 The greater dobutamine blunting effect of carvedilol possibly occurs due to the lack of upregulated β-receptors available for dobutamine binding and the strong binding of carvedilol to the β-receptors preventing displacement by dobutamine.167 Sepsis also causes downregulation of β-receptors,160 and given the absence of upregulated β-receptors with carvedilol, patients on concurrent carvedilol may require higher doses of dobutamine, although higher doses of dobutamine increase arrhythmia risk.168 The effect of concurrent BBs on the arrhythmogenic potential of dobutamine in sepsis is unknown. Interestingly, BB use prevented dobutamine-induced ventricular arrhythmias in decompensated HF with EF<35%.169
The clinical significance of interplay between the β-receptor upregulation and sepsis management is unknown, and current guidelines give no recommendations for the management of chronic BBs in sepsis.9 Interestingly, one study showed that previously prescribed BB decreased mortality in patients with septic shock from 22.1% to 17.7% (OR: 0.78; 95% CI: 0.66-0.93; P =0.005).170 The authors hypothesized the benefit was from prophylactic BB inhibition of septic cardiac depression before the development of sepsis or septic shock.170 Additionally, long-term BB therapy has been associated with significantly lower lactate levels during sepsis and septic shock.171 Whether lower lactate is due to β-blockade inhibiting β-2-mediated glycolysis or a sepsis protective effect is unknown, but caution is warranted when interpreting lactate levels in the presence of BBs. The authors noted a trend toward decreased mortality in the BB group at 28 days (35% vs 49%; P = .08), while the trial was not powered to examine mortality.171 DeMott et al sought to quantify dose changes in vasopressors based on chronic antihypertensive treatment and found that concurrent BB therapy did not affect vasopressor dosing at 48 hours.172
While previous BB use does not compel continuation during sepsis, it does raise an interesting clinical question, and BB discontinuation in sepsis has been a constant debate. Fuchs et al found a survival benefit associated with continuing BB therapy during the acute phase of severe sepsis and septic shock in a retrospective secondary analysis of a single-center study.173 Continued BB therapy was associated with decreased hospital (P = .03), 28-day (P = .04), and 90-day mortality rates (40.7% vs 52.7%; P = .046).173 A multicenter observational study (ClinicalTrials.gov Identifier: NCT03190408)174 was completed that examines antihypertensive therapy and the effects on outcomes in patients with septic shock, and the results are waiting to be reported. The data may be confounded as BB continuation in sepsis and septic shock could be a surrogate marker for patients with more stable blood pressure limiting the applicability of these data in the absence of prospective evaluation.
In acute decompensated HF, BB discontinuation has been associated with increased hospital and short-term mortality.175 Additionally, BB discontinuation was associated with significantly higher mortality among acute HF patients treated with milrinone.176 Caution is warranted in interpreting these findings as it is unknown if the benefits are from BB continuation during the acute setting or due to higher rates of continuation on discharge. One clinical trial demonstrated no differences in safety outcomes from keeping versus stopping BB in acute decompensated HF patients with an average EF of 32% but was not powered to detect mortality differences.177 This result may encourage continuation given the lack of adverse events and the benefits observed in other studies. In summary, given the suggested benefits of BB blocker continuation in sepsis and acute decompensated HF, continuation of chronic BBs in the setting of sepsis and HF should be strongly considered.
Angiotensin inhibitors.
Limited data exist on implications of managing antecedent ACE-Is, ARBs, and ARNi therapy. Demott et al demonstrated that prehospital ACE-I was associated with the shortest time on vasopressors (19 hours) compared to BB (24 hours), BB, ACE-I (30 hours), and no therapy (30 hours).172 A retrospective study of over 50 000 patients with sepsis found the presepsis ACE-I/ARBs were associated with reduced 30- and 90-day mortality, while presepsis BBs were not.178 Similarly, another large database review of over 30 000 patients admitted for sepsis revealed that ACE-I and ARBs were associated with lower hospital mortality (adjusted OR: 0.93; 95% CI: 0.88-0.98 and adjusted OR: 0.85; 95% CI: 0.81-0.90, respectively).179 The retrospective natures of these reports limits conclusions due to observational and nonrandomized design and lack of granular clinical characteristics. The inability to detect sepsis-related mortality prevents differentiation of the protective effect of ACE-Is and ARBs on cardiovascular disease (eg, myocardial infraction).
Both ACE-Is and ARBs can elevate serum potassium levels,10 and hyperkalemia has been associated with mortality in critically ill patients.180 Additionally, sepsis often induces renal dysfunction that leads to elevated potassium levels.181 These concerns may warrant discontinuation of ACE-Is and ARBs in the setting of sepsis and HF and restart upon stabilized renal function. No clinical data exist on the effects of neprilysin inhibitors limiting discussion of ARNi continuation. Interestingly, neprilysin was protective in a mouse model of septic shock,182 and the impact of inhibited neprilysin during human sepsis may warrant investigation.
In summary, ACE-Is and ARBs often warrant discontinuation due to unstable blood pressures and renal dysfunction in sepsis, and the data supporting their continuation are limited compared to BBs. Nonetheless, presepsis ACE-I and ARB use appears to offer protection in sepsis, and they should be restarted as soon as possible after stabilization to resume evidence-based HF management.
Management of AF
Heart failure, impaired LVEF, and sepsis are independently and additively associated with higher incidence of arrhythmias, specifically AF.183–186 Acute-onset AF represents up to 70% of supraventricular arrhythmias in sepsis,187,188 and management considerations with preexisting HF are limited to applying data from long-term HF studies and subacute settings.189,190 The hemodynamic consequences of antiarrhythmic drugs are a common concern in sepsis and septic shock, and following suit, AF guidelines recommend only electrical cardioversion in hemodynamically unstable patients.190 Even after acute hemodynamic stabilization, medication-induced blood pressure changes should be avoided and the hemodynamic effects of drug therapies considered carefully. After stabilization, rate control may be preferred in ICU patients as the majority will convert to normal sinus rhythm with resolution of acute illness.191 Indeed, one study of critically ill patients with AF found 81% reverted to normal sinus rhythm when treated with rate control alone.192 However, Gillinov et al found no clinical benefits favoring rate or rhythm control in ICU patients following cardiac surgery,193 and failure to restore sinus rhythm in patients with sepsis may be associated with greater in-hospital mortality.194 The hypersympathetic tone in sepsis may promote irregular ventricular conduction of AF impulses independently worsening CO and hemodynamics.195 Conventional wisdom may point clinicians toward agents considered hemodynamically favorable (eg, amiodarone and digoxin) compared to agents with negative inotropy (eg, BBs and calcium channel blockers [CCBs]); however, current evidence appears to favor BBs in the management AF in hemodynamically stabilized patients with sepsis with preexisting HF.
β-Blockers.
The evidence for AF management with rate control through BBs supports their application as a first-line option. The strongest support comes from Walkey et al, suggesting BBs were associated with the lowest hospital mortality as initial IV AF treatment in a cohort of 39 693 patients with sepsis with AF.196 β-Blockers were associated with lower mortality compared to CCBs, digoxin, and amiodarone (RR: 0.92, 0.79, 0.64, respectively; P < .05). The association with lower mortality was maintained for BBs in subgroups of patients on vasopressors, with preexisting HF and with new-onset and chronic AF.196 However, this study is limited due to the use of International Classification of Disease (ICD)-9 codes and administrative data that lack information on disease severity and specific clinical characteristics. Additionally, initial IV AF treatment was included if given during the first 14 days of sepsis admission preventing data application to the acute resuscitation setting. Even though patients were well matched through propensity scores, the lack of granular data and wide window of administration limit applicability, as clinicians may have avoided BBs in patients with more profound hypotension electing to use other agents (eg, amiodarone or digoxin). These concerns warrant caution in interpreting the mortality reduction and outline the need for prospective assessment. Nonetheless, this study provides strong support that BBs may be the most appropriate agents in HF patients to control AF during sepsis hospitalization. A recent systematic review of BBs early in sepsis found a likely mortality benefit, with two-thirds of the 14 included trials (13 prospective, 1 retrospective) reporting mortality reductions.197 Esmolol was used as the intervention in 12 of the 14 trials. However, nearly all the trials had exclusion criteria related to severe HF and cardiac dysfunction (eg, LVEF<35%, dobutamine use, NYHA class III or IV).197
The concern for BB use in sepsis is primarily negative inotropy in hemodynamically unstable patients, but BBs may have neutral hemodynamic effects after initial sepsis resuscitation despite the CO reduction.155,197–200 Gore and Wolfe showed patients with sepsis receiving esmolol infusion maintained peripheral blood flow and oxygenation despite a 20% reduction in CO.198 Balik et al showed esmolol to reduce tachycardia after preload correction in septic shock did not adversely affect hemodynamic effects; however, the study included patients with EFs approximately 60% and normal cardiac function.199 Du et al assessed esmolol in patients with sepsis without LVD and observed increased SV, decreased HR, lower lactate, and no effect on MAP.200 Esmolol was shown to reduce systolic BP by only 5 mm Hg in patients with severely impaired EF (~27%).201 A dose-dependent reduction in CO and EF occurred, showing small reductions with 4 and 8 mg/min and larger reductions with 16 mg/min. After infusion cessation, all parameters returned to normal in 10 to 30 minutes.201 Morelli et al found esmolol infusion titrated to lower the HR 24 hours after hemodynamic stabilization in patients with sepsis was associated with reduced mortality and less NE use without increasing adverse events.202 The highest dose of esmolol in this study was 6.7 mg/min with an average dose of 3.3 mg/min,202 suggesting the doses with minimal effects in patients with low EF are the doses used effectively in sepsis (ie, ≤8 mg/min).201 The same study group examined the specific cardiac effects of esmolol infusion started 24 hours after adequate resuscitation in septic shock and found an increased SV, unchanged CO and LVEF, and reduced NE requirements.203 The neutral effect of CO is hypothesized to be from reducing arterial stiffness and subsequently increasing LV SV, even despite the reduced contractility from BBs. However, these results should be applied cautiously and based on individual cardiac function as EF does not reveal broad spectrum of cardiac function. Data beyond esmolol are limited, but enteral metoprolol in combination with milrinone safely improved hemodynamic parameters in patients with sepsis, but the study excluded those with overt HF.155 Most important to the hemodynamic safety discussion, a systematic review of 14 studies (13 clinical trials) reported no statistical changes in blood pressure from BB use in sepsis among all studies.197
In summary, BB evidence is limited by extrapolation from related clinical studies, but the suggested mortality benefits in patients with sepsis, hemodynamic safety in both sepsis and HF, and possible independent benefit in sepsis pathology make them, specifically the rapid-acting esmolol, a first line for AF in HF patients with sepsis after acute hemodynamic stabilization. Attenuation of tachycardia from BBs may prevent the development of new-onset AF as unopposed tachycardia causes shortening of the atrial refractory period promoting AF development, which has important implications for overall hemodynamic stability.204 Future studies should assess the ability of both short-acting (esmolol) and chronic long-term BB therapy to prevent the development of AF.
Calcium channel blockers.
The use of CCB is complicated by the recommendation to avoid their use in HFrEF205; however, these recommendations do not completely extend to patients with HFpEF and are based on chronic use and long-term follow-up data. Interestingly, Lee et al showed CCBs prior to sepsis admission were associated with a slight mortality reduction.206 However, as previously stated, CCBs showed greater mortality than BBs in patients with septic shock with AF and history of HF.196 Diltiazem was slower than esmolol to achieve sinus rhythm at 2 hours compared to esmolol in postoperative noncardiac surgery patients, of which 80% were experiencing AF (33% vs 59%; P < .05).207 Diltiazem elicited greater ventricular rate reduction compared to metoprolol in hemodynamically stable emergency department patients with AF without causing hypotension.208 However, patients with severe HF were excluded from the trial.208 A similar emergency department trial demonstrated greater ventricular rate goal with diltiazem over metoprolol through 30 minutes, and diltiazem did not increase hypotension.209 In acute exacerbations of HFrEF, diltiazem achieved rapid control of AF with rapid ventricular rate but did not convert to sinus rhythm and was associated with acute transient decreases in systolic (−17 mm Hg) and diastolic (−12 mm Hg) blood pressure.210 Hirschy et al retrospectively suggested no differences between diltiazem and metoprolol in HR control, bradycardia, or hypotension in HFrEF with AF and rapid ventricular rate.211 These studies are hardly applicable to septic admissions, but they suggest short-term acute rate control with CCBs over BBs is a reasonable option provided hemodynamic stabilization. Diltiazem may serve as a second-line option for rate control in the management of AF in HFpEF patients with sepsis with contraindications to BBs.
Evidence comparing CCB to other agents in the acute setting of AF is limited to small trials lacking description of cardiac dysfunction. Siu et al compared diltiazem to amiodarone or digoxin in non-ICU hospitalized patients with AF and rapid ventricular response and showed diltiazem was superior in time to and achievement of HR <90 beats per minute.212 In a prospective trial of 60 critically ill patients with atrial arrhythmias (95% AF), diltiazem bolus followed by diltiazem continuous infusion was compared to amiodarone bolus or amiodarone bolus plus infusion.213 Diltiazem and the 2 amiodarone regimens were comparable in controlling HR at 4 hours, but diltiazem was superior in controlling 24-hour HR. However, diltiazem caused significantly more hypotension than amiodarone.213 Of note, diltiazem infusions may complicate the disposition of patients leaving the emergency department, and diltiazem IV bolus followed by oral immediate release diltiazem has been associated with both lower HR and treatment failure compared to IV bolus then infusion.214 Given the recommendations to avoid CCBs in HFrEF and lack of strong evidence supporting its use over BBs in sepsis with preexisting HF, acute CCB use should be reserved for patients with HFpEF with contraindications to BB and used primarily in the form of IV boluses followed by oral therapy provided clinical status allows oral therapy. Some data have demonstrated diltiazem efficacy and safety in HFrEF, but larger studies are needed to fully assess the risks in this population.
Amiodarone.
Amiodarone can achieve both rate control and is frequently used in patients with sepsis do to its neutral effects on hemodynamics,186,195 and patients with AF and HFrEF are commonly managed with amiodarone.215 A study of IV amiodarone in critically ill patients with moderately reduced EFs (40% ± 16%) refractory to electrical and pharmacologic cardioversion demonstrated general hemodynamic safety as evidenced by lower HR and higher systolic blood pressure shortly after amiodarone initiation.216 Additionally, amiodarone boluses had both higher and faster success rates compared to digoxin boluses in controlling acute AF among patients with contraindications to BBs and CCBs.217
Amiodarone carries the potential for chemical cardioversion and thus concerns for cardioembolic events.218 Anticoagulation strategies have been investigated as a therapy independently for sepsis, as disseminated intravascular coagulation may increase mortality,219 but have largely shown no benefit with increased bleeding.220–222 However, a possible benefit may exist among the most severely ill and those with sepsis-induced coagulopathies.223 Notably, patients with AF during severe sepsis have over double the risk for in-hospital stroke.188 In a national study examining stroke and bleeding risk associated with anticoagulation in sepsis with AF, Walkey et al found 35% of patients received parenteral anticoagulation with no impact on the rates of in-hospital ischemic stroke.224 However, anticoagulation was associated with increased clinically significant bleeding.224 Given the lack of guidance on anticoagulation for AF during sepsis, it may be prudent to hold anticoagulation in the absence of planned cardioversion during the acute and subacute phases of sepsis management.
Digoxin.
Digoxin’s negative chronotropic and positive inotropic effects are attractive to minimize hypotension during AF treatment in sepsis. Early studies in sepsis suggested the inotropic effects may improve LV function.225 In fact, American Heart Association/American College of Cardioology/Heart Rhythm Society (AHA/ACC/HRS) guidelines recommend digoxin as a second-line agent in the acute management of AF amid cardiac dysfunction in HF or acute coronary syndrome, specifically in those with severe LVD and hemodynamic instability.215 However, digoxin concentrations >1 to 1.2 ng/mL are associated with increased mortality in patients with preexisting HF, and the optimal range for patients with HF and LVEF <45% is 0.5 to 0.8 ng/mL.226,227 A post hoc analysis of the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial found only digoxin serum concentrations <0.9 ng/mL conclusively showed no increase in mortality.228 Additionally, digoxin has a long, unpredictable half-life (~36 hours) and is altered by renal impairment, further complicating its use in patients with sepsis.229 Sepsis-induced kidney injury may couple with digoxin to synergistically raise serum potassium levels, increasing the risk of arrhythmias.230,231 Digoxin’s efficacy may be confounded in sepsis, as high sympathetic activity has been suggested to reduce digoxin effectiveness.232 Digoxin was less effective than amiodarone in a small study of critically ill patients with cardiac dysfunction, but 2 of the 26 patients in the amiodarone group developed severe bradycardia or HF aggravation, prompting the authors to recommend digoxin as first line in hemodynamically unstable patients with AF.233 A recent retrospective study of 180 patients with sepsis (86% with AF) who received digoxin within 24 hours of ICU admission found improvement in hemodynamic parameters through 24 hours after digoxin without significant adverse events.234 The study identified only 180 patients over a 10-year period implying sparse digoxin use in this patient population, but the results support negligible hemodynamic consequences in patients with sepsis with AF. However, the small sample size and 10-year period make these findings difficult to apply. While digoxin shows positive hemodynamic effects, the narrow therapeutic index for HF patients, monitoring required, and lack of clear-cut outcomes data make digoxin a second- or third-line choice in patients whom BBs and/or amiodarone are unable to control AF.
Considerations in Supportive Care
Supportive care often provides the foundation of ICU management as robust evidence lacks across many fronts. Positive pressure ventilatory support (PPVS) and renal replacement therapy (RRT) are foundational to caring for critically ill patients and their effects on cardiac function and fluid status deserve discussion.
Positive Pressure Ventilatory Support.
Clinical outcomes regarding the interplay of HF with PPVS in patients with sepsis remain unknown, and PPVS management depends on extensive clinical expertise, but some data are germane to the discussion. The 2016 European Society of Cardiology (ESC) HF guidelines recommend maintenance of oxygen saturation (SpO2) >90% and supports PPVS in patients with hemodynamic instability as seen in sepsis.39 Sepsis guidelines restrict specific PPVS recommendations to acute respiratory distress syndrome (ARDS), favoring low tidal volume strategies.9 In a retrospective assessment of PPVS in patients with sepsis, cardiac dysfunction was not associated with either progression to ARDS or worse outcomes in ARDS.235
Altering thoracic pressure, specifically through positive end-expiratory pressure (PEEP), impacts ventricular function. In normal inspiration, intrathoracic pressure decreases to a greater extent than intraluminal aortic pressure, thus increasing LV afterload. With the application of PPVS, this effect is reversed and a potential increase in CO is seen.236 However, the normal increase in intrathoracic pressure decreases the CO from the RV and thus preload is diminished in the LV. This preload reduction may reduce CO in patients with normal LV function. However, since HF patients are chronically hypervolemic, the effect of preload reduction on CO is likely negligible and CO augmentation may be improved by reduced LV afterload obtained though PPVS.236,237 Positive end-expiratory pressure (normally 5-10 mm Hg) is key to this application as it may increase CO in HF through a reduction in LV afterload, LV dilation, and myocardial oxygen demand.236 Concerns for extensive PEEP in cardiac dysfunction have suggested increased cardiac surface pressure from PEEP may reduce coronary blood flow.238 Nonetheless, in the afterload-dependent state of HF, the reduced afterload afforded by PEEP likely has larger benefits than negative impacts on LV preload,239 making PEEP a controllable factor to improve cardiac function in septic HF patients with LVD. Relevant to earlier discussion of CVP, as PPVS increases thoracic cavity pressure, it can complicate cardiac filling pressures used to assess fluid status (eg, CVP),240 further decreasing the limited efficacy of this parameter in these patients.
In summary, PPVS, and specifically PEEP, should be incorporated into general hemodynamic assessment of the HF patient with sepsis. Future studies evaluating the role of PPVS in this unique patient population may be warranted.
Renal Replacement Therapy.
Supportive care with RRT for those on chronic dialysis and new acute kidney injury to manage associated fluid and electrolyte disturbances is common practice in the ICU, and RRT’s impact on fluids in HF patients with sepsis warrants consideration. Recently, Barbar et al demonstrated no difference in outcomes between early RRT (within 12 hours of kidney failure) and delayed RRT (after 48 hours if still indicated) in patients with acute kidney injury in early septic shock.241 Additionally, a meta-analysis of 11 randomized controlled trials evaluating early and late RRT in critical illness found no difference in mortality but showed a higher incidence of catheter-associated infections and hypophosphatemia in the early RRT group.242 Another meta-analysis of early versus late RRT in critical illness after cardiac surgery suggested benefit with early RRT; however, the studies had a high level of heterogeneity (I2 = 56%), and the authors specifically cite the absence of septic shock pathophysiology as a potential explanation for the benefit in these patients.243 Taken together, these data do not support early RRT in patients with sepsis.
In HF, RRT functions as a powerful fluid removal mechanism, normally through ultrafiltration. The Ultrafiltration versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Congestive Heart Failure (UNLOAD) trial showed superiority of ultrafiltration RRT to diuretics over 48 hours when started within 24 hours of hospitalization.244 The Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF) found ultrafiltration was no better than diuretic therapy but worsened renal function in patients hospitalized for HF with cardiorenal syndrome.245 These results are hardly generalizable as patients were excluded for sepsis, hemodynamic instability, and vasopressors use. Heart failure promotes hemodynamic concerns as the presence of cardiac dysfunction is strongly associated with intradialytic hypotension.246 Intradialytic hypotension occurs from failure to induce a compensatory increase in both CO and systemic vascular resistance, and as these 2 parameters are extensively dysregulated or require pharmacological augmentation during sepsis,247 concern for hypotension increases. Additionally, LVD is directly associated with higher incidences of intradialytic hypotension.248
In summary, early RRT in sepsis has been well evaluated and does not demonstrate benefit over delayed strategies and should not be used specifically in sepsis with preexisting HF. Renal replacement therapy in sepsis with preexisting HF may be used as supportive care relevant to sepsis-induced kidney injury but should not employed to manage HF fluid status. The risk of intradialytic hypotension is likely enhanced in HF patients with sepsis, specifically with HFrEF, and careful application of RRT should be implemented in these patients.
Conclusion
Sepsis and HF are common complications in critically ill patients and create an additively complex pathological state. Clinicians may hesitate to implement guideline directed therapy, specifically related to fluid resuscitation. Nevertheless, deviation from traditional sepsis management based solely on preexisting HF causes worse clinical outcomes and no data currently support this practice among HF patients with sepsis. Guideline fluid and vasopressor recommendations are safe and likely confer benefit to HF patients as they do to non-HF patients, and their lack of implementation likely worsens outcomes. Beyond first-line sepsis management, dobutamine may offer additional benefit in patients with septic HF through CO modulation. In general, inotropes are associated with poorer outcomes, but niche applications may exist. Additionally, continuing home BB therapy during sepsis may improve outcomes, but hemodynamic instability may warrant discontinuation and later restart. Short-acting BB use during sepsis to decrease tachycardia and/or treat AF has continually been shown as a promising strategy and warrants large clinical trials. Both PPVS and RRT likely have more pronounced impacts in HF patients with sepsis. Moving forward, examination of HF severity in sepsis through specific reporting of patient demographics is critical, whether through EF measurement or other HF classification measure (eg, American Heart Association staging). Until research reveals otherwise, adherence to guideline-directed management of sepsis remains the standard for sepsis with preexisting HF.
Acknowledgments
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Bloom MW, Greenberg B, Jaarsma T, et al. Heart failure with reduced ejection fraction. Nat Rev Dis Primers. 2017;3:17058. [DOI] [PubMed] [Google Scholar]
- 2.Singer M, Deutschman CS, Seymour CW, et al. The Third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016; 193(3):259–272. [DOI] [PubMed] [Google Scholar]
- 4.Vincent J-L, Jones G, David S, Olariu E, Cadwell KK. Frequency and mortality of septic shock in Europe and North America: a systematic review and meta-analysis. Critical Care. 2019; 23(1):196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roger VL. Epidemiology of heart failure. Circ Res. 2013;113(6): 646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajj J, Blaine N, Salavaci J, Jacoby D. The “centrality of sepsis”: a review on incidence, mortality, and cost of care. Healthcare (Basel). 2018;6(3):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker AMN, Drozd M, Hall M, et al. Prevalence and predictors of sepsis death in patients with chronic heart failure and reduced left ventricular ejection fraction. J Am Heart Assoc. 2018;7(20): e009684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaduganathan M, Patel RB, Michel A, et al. Mode of death in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2017;69(5):556–569. [DOI] [PubMed] [Google Scholar]
- 9.Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. [DOI] [PubMed] [Google Scholar]
- 10.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2013; 62(16):e147–239. [DOI] [PubMed] [Google Scholar]
- 11.Abou Dagher G, Hajjar K, Khoury C, et al. Outcomes of patients with systolic heart failure presenting with sepsis to the emergency department of a tertiary hospital: a retrospective chart review study from Lebanon. BMJ Open. 2018;8(7):e022185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishak Gabra N, Kim B, Iyengar R, et al. Outcomes of patients with chronic heart failure and septic shock. Chest. 2017;152(4):A377. [Google Scholar]
- 13.Alon D, Stein GY, Korenfeld R, Fuchs S. Predictors and outcomes of infection-related hospital admissions of heart failure patients. PLoS One. 2013;8(8):e72476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prabhu MM, Yalakala SK, Shetty R, Thakkar A, Sitapara T. Prognosis of left ventricular systolic dysfunction in septic shock patients. J Clin Diagn Res. 2015;9(3):OC05–OC08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alkhalaf M, Abd-Aziz N, Arabi Y, Tangiisuran B. Impact of congestive heart failure on severe sepsis and septic shock survivors: outcomes and performance status after 1-year hospital discharge. Critical Care. 2012;16(suppl 1):P400–P400. [Google Scholar]
- 16.Karlsson S, Varpula M, Ruokonen E, et al. Incidence, treatment, and outcome of severe sepsis in ICU-treated adults in Finland: the Finnsepsis Study. Intensive Care Med. 2007;33(3):435–443. [DOI] [PubMed] [Google Scholar]
- 17.Prescott HC, Langa KM, Liu V, Escobar GJ, Iwashyna TJ. Increased 1-year healthcare use in survivors of severe sepsis. Am J Respir Crit Care Med. 2014;190(1):62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linder A, Guh D, Boyd JH, Walley KR, Anis AH, Russell JA. Long-term (10-year) mortality of younger previously healthy patients with severe sepsis/septic shock is worse than that of patients with nonseptic critical illness and of the general population. Crit Care Med. 2014;42(10):2211–2218. [DOI] [PubMed] [Google Scholar]
- 19.Ouellette DR, Shah SZ. Comparison of outcomes from sepsis between patients with and without pre-existing left ventricular dysfunction: a case-control analysis. Critical Care. 2014; 18(2):R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sevilla Berrios RA, O’Horo JC, Velagapudi V, Pulido JN. Correlation of left ventricular systolic dysfunction determined by low ejection fraction and 30-day mortality in patients with severe sepsis and septic shock: a systematic review and meta-analysis. J Crit Care. 2014;29(4):495–499. [DOI] [PubMed] [Google Scholar]
- 21.Denfeld QE, Winters-Stone K, Mudd JO, Gelow JM, Kurdi S, Lee CS. The prevalence of frailty in heart failure: a systematic review and meta-analysis. Int J Cardiol. 2017;236:283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vitale C, Spoletini I, Rosano GM. Frailty in heart failure: implications for management. Card Fail Rev. 2018;4(2):104–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahalingam M, Moore JX, Donnelly JP, Safford MM, Wang HE. Frailty syndrome and risk of sepsis in the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort. J Intensive Care Med. 2019;34(4):292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ather S, Chan W, Bozkurt B, et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59(11):998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kakihana Y, Ito T, Nakahara M, Yamaguchi K, Yasuda T. Sepsis-induced myocardial dysfunction: pathophysiology and management. J Intensive Care. 2016;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinugawa K, Takahashi T, Kohmoto O, et al. Nitric oxide-mediated effects of interleukin-6 on [Ca2+]i and cell contraction in cultured chick ventricular myocytes. Circ Res. 1994;75(2): 285–295. [DOI] [PubMed] [Google Scholar]
- 27.Vincent JL. Understanding cardiac output. Crit Care. 2008; 12(4):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parker MM, Shelhamer JH, Bacharach SL, et al. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med. 1984;100(4):483–490. [DOI] [PubMed] [Google Scholar]
- 29.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348(20): 2007–2018. [DOI] [PubMed] [Google Scholar]
- 30.Ellison GM, Torella D, Karakikes I, et al. Acute beta-adrenergic overload produces myocyte damage through calcium leakage from the ryanodine receptor 2 but spares cardiac stem cells. J Biol Chem. 2007;282(15):11397–11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaney E, Shaw A. Pathophysiology of fluid retention in heart failure. Contrib Nephrol. 2010;164:46–53. [DOI] [PubMed] [Google Scholar]
- 32.Delgado RM 3rd, Willerson JT. Pathophysiology of heart failure: a look at the future. Tex Heart Inst J. 1999;26(1):28–33. [PMC free article] [PubMed] [Google Scholar]
- 33.Miller WL.Fluid volume overload and congestion in heart failure: time to reconsider pathophysiology and how volume is assessed. Circ Heart Fail. 2016;9(8):e002922. [DOI] [PubMed] [Google Scholar]
- 34.Fallick C, Sobotka PA, Dunlap ME. Sympathetically mediated changes in capacitance: redistribution of the venous reservoir as a cause of decompensation. Circ Heart Fail. 2011;4(5):669–675. [DOI] [PubMed] [Google Scholar]
- 35.Bouchard J, Soroko SB, Chertow GM, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76(4):422–427. [DOI] [PubMed] [Google Scholar]
- 36.Murphy CV, Schramm GE, Doherty JA, et al. The importance of fluid management in acute lung injury secondary to septic shock. Chest. 2009;136(1):102–109. [DOI] [PubMed] [Google Scholar]
- 37.Koonrangsesomboon W, Khwannimit B. Impact of positive fluid balance on mortality and length of stay in septic shock patients. Indian J Crit Care Med. 2015;19(12):708–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen Y, Huang X, Zhang W. Association between fluid intake and mortality in critically ill patients with negative fluid balance: a retrospective cohort study. Critical Care. 2017;21(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Rev Esp Cardiol (Engl Ed). 2016;69(12):1167. [DOI] [PubMed] [Google Scholar]
- 40.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Card Fail. 2017;23(8):628–651. [DOI] [PubMed] [Google Scholar]
- 41.Beurton A, Ducrocq N, Auchet T, et al. Beneficial effects of norepinephrine alone on cardiovascular function and tissue oxygenation in a pig model of cardiogenic shock. Shock. 2016;46(2): 214–218. [DOI] [PubMed] [Google Scholar]
- 42.Squara P, Hollenberg S, Payen D.Reconsidering vasopressors for cardiogenic shock: everything should be made as simple as possible, but not simpler. Chest. 2019;156(2):392–401. [DOI] [PubMed] [Google Scholar]
- 43.Bellumkonda L, Gul B, Masri SC. Evolving concepts in diagnosis and management of cardiogenic shock. Am J Cardiol. 2018; 122(6):1104–1110. [DOI] [PubMed] [Google Scholar]
- 44.James JH, Wagner KR, King JK, et al. Stimulation of both aerobic glycolysis and Na(+)-K(+)-ATPase activity in skeletal muscle by epinephrine or amylin. Am J Physiol. 1999;277(1): E176–186. [DOI] [PubMed] [Google Scholar]
- 45.Levy B, Desebbe O, Montemont C, Gibot S. Increased aerobic glycolysis through beta2 stimulation is a common mechanism involved in lactate formation during shock states. Shock. 2008; 30(4):417–421. [DOI] [PubMed] [Google Scholar]
- 46.Adamo L, Nassif ME, Novak E, LaRue SJ, Mann DL. Prevalence of lactic acidaemia in patients with advanced heart failure and depressed cardiac output. Eur J Heart Fail. 2017;19(8): 1027–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hernandez G, Ospina-Tascon GA, Damiani LP, et al. Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-day mortality among patients with septic shock: the ANDROMEDA-SHOCK randomized clinical trial. JAMA. 2019;321(7):654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Beuningen A, Metkus T, Schulman S. 138: Bedside assessment of peripheral perfusion in cardiogenic shock. Critical Care Med. 2016;44(12):112. [Google Scholar]
- 49.Scott WA, Iannaccone ST. Intensive care management, including cardiorespiratory care. In: Darras BT, Jones HR, Ryan MM, De Vivo DC, eds. Neuromuscular Disorders of Infancy, Childhood, and Adolescence (Second Edition). Academic Press; 2015: 904–912. Chap 44. [Google Scholar]
- 50.Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med. 2018;44(6):925–928. [DOI] [PubMed] [Google Scholar]
- 51.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377. [DOI] [PubMed] [Google Scholar]
- 52.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. [DOI] [PubMed] [Google Scholar]
- 53.Protti A, Masson S, Latini R, et al. Persistence of central venous oxygen desaturation during early sepsis is associated with higher mortality: a retrospective analysis of the ALBIOS trial. Chest. 2018;154(6):1291–1300. [DOI] [PubMed] [Google Scholar]
- 54.Boulain T, Garot D, Vignon P, et al. Prevalence of low central venous oxygen saturation in the first hours of intensive care unit admission and associated mortality in septic shock patients: a prospective multicentre study. Crit Care. 2014;18(6):609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gallet R, Lellouche N, Mitchell-Heggs L, et al. Prognosis value of central venous oxygen saturation in acute decompensated heart failure. Arch Cardiovasc Dis. 2012;105(1):5–12. [DOI] [PubMed] [Google Scholar]
- 56.Nebout S, Pirracchio R. Should we monitor ScVO(2) in critically Ill patients? Cardiol Res Pract. 2012;2012:370697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vincent JL, De Backer D. Circulatory shock. N Engl J Med. 2013; 369(18):1726–1734. [DOI] [PubMed] [Google Scholar]
- 58.Velissaris D, Pierrakos C, Scolletta S, De Backer D, Vincent JL. High mixed venous oxygen saturation levels do not exclude fluid responsiveness in critically ill septic patients. Crit Care. 2011; 15(4):R177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Beest P, Wietasch G, Scheeren T, Spronk P, Kuiper M. Clinical review: use of venous oxygen saturations as a goal—a yet unfinished puzzle. Crit Care. 2011;15(5):232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schlichtig R, Cowden WL, Chaitman BR. Tolerance of unusually low mixed venous oxygen saturation. Adaptations in the chronic low cardiac output syndrome. Am J Med. 1986;80(5):813–818. [DOI] [PubMed] [Google Scholar]
- 61.Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002;121(6): 2000–2008. [DOI] [PubMed] [Google Scholar]
- 62.Biais M, Ehrmann S, Mari A, et al. Clinical relevance of pulse pressure variations for predicting fluid responsiveness in mechanically ventilated intensive care unit patients: the grey zone approach. Crit Care. 2014;18(6):587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rathore A, Singh S, Lamsal R, Taank P, Paul D. Validity of pulse pressure variation (PPV) compared with stroke volume variation (SVV) in predicting fluid responsiveness. Turk J Anaesthesiol Reanim. 2017;45(4):210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He HW, Liu DW. The pitfall of pulse pressure variation in the cardiac dysfunction condition. Crit Care. 2015;19:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Michard F Changes in arterial pressure during mechanical ventilation. Anesthesiology. 2005;103(2):419–428; quiz 449–415. [DOI] [PubMed] [Google Scholar]
- 66.Wyler von Ballmoos M, Takala J, Roeck M, et al. Pulse-pressure variation and hemodynamic response in patients with elevated pulmonary artery pressure: a clinical study. Crit Care. 2010; 14(3): R111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Osman D, Ridel C, Ray P, et al. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med. 2007;35(1):64–68. [DOI] [PubMed] [Google Scholar]
- 68.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39(2):259–265. [DOI] [PubMed] [Google Scholar]
- 69.Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134(1):172–178. [DOI] [PubMed] [Google Scholar]
- 70.Raper R, Sibbald WJ. Misled by the wedge? The Swan-Ganz catheter and left ventricular preload. Chest. 1986;89(3):427–434. [DOI] [PubMed] [Google Scholar]
- 71.Forrester JS, Diamond G, McHugh TJ, Swan HJ. Filling pressures in the right and left sides of the heart in acute myocardial infarction. A reappraisal of central-venous-pressure monitoring. N Engl J Med. 1971;285(4):190–193. [DOI] [PubMed] [Google Scholar]
- 72.Berlin DA, Bakker J. Starling curves and central venous pressure. Crit Care. 2015;19:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sasai T, Tokioka H, Fukushima T, et al. Reliability of central venous pressure to assess left ventricular preload for fluid resuscitation in patients with septic shock. J Intensive Care. 2014; 2(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sekiguchi H, Harada Y, Villarraga HR, Mankad SV, Gajic O. Focused cardiac ultrasound in the early resuscitation of severe sepsis and septic shock: a prospective pilot study. J Anesth. 2017;31(4):487–493. [DOI] [PubMed] [Google Scholar]
- 75.Mosier JM, Stolz U, Milligan R, et al. Impact of point-of-care ultrasound in the emergency department on care processes and outcomes in critically ill nontraumatic patients. Critical Care Explorations. 2019;1(6):e0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pro CI, Yealy DM, Kellum JA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014; 370(18):1683–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.ARISE Investigators, ANZICS Clinical Trials Group, Peake SL, Delaney A, Bailey M, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16): 1496–1506. [DOI] [PubMed] [Google Scholar]
- 78.Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015; 372(14):1301–1311. [DOI] [PubMed] [Google Scholar]
- 79.Investigators P, Rowan KM, Angus DC, et al. Early, goal-directed therapy for septic shock—a patient-level meta-analysis. N Engl J Med. 2017;376(23):2223–2234. [DOI] [PubMed] [Google Scholar]
- 80.Singh H, Iskandir M, Sachdev S, Simmons B, Rabines A, Hassen GW. The effect of initial volume resuscitation for sepsis in patients with congestive heart failure: is it associated with higher mortality. J Cardiac Failure. 2016;22(8): S54–S55. [Google Scholar]
- 81.Khan RA, Khan NA, Bauer SR, et al. Association between volume of fluid resuscitation and intubation in high-risk patients with sepsis, heart failure, end-stage renal disease, and cirrhosis. Chest. 2020;157(2):286–292. [DOI] [PubMed] [Google Scholar]
- 82.Truong TN, Dunn AS, McCardle K, et al. Adherence to fluid resuscitation guidelines and outcomes in patients with septic shock: reassessing the “one-size-fits-all” approach. J Crit Care. 2019;51:94–98. [DOI] [PubMed] [Google Scholar]
- 83.Duttuluri M, Rose K, Shapiro J, et al. Fluid Resuscitation Dilemma in Patients with Congestive Heart Failure Presenting with Severe Sepsis/Septic Shock. D45. Critical Care: Circulatory Hemodymanics, Shock, Cardiovascular Disease, and Fluid Management. American Thoracic Society; 2016: A7048–A7048. [Google Scholar]
- 84.Leisman DE, Doerfler ME, Schneider SM, Masick KD, D’Amore JA, D’Angelo JK. Predictors, prevalence, and outcomes of early crystalloid responsiveness among initially hypotensive patients with sepsis and septic shock. Crit Care Med. 2018;46(2):189–198. [DOI] [PubMed] [Google Scholar]
- 85.Leisman DE, Doerfler ME, Ward MF, et al. Survival benefit and cost savings from compliance with a simplified 3-hour sepsis bundle in a series of prospective, multisite, observational cohorts. Crit Care Med. 2017;45(3):395–406. [DOI] [PubMed] [Google Scholar]
- 86.Liu VX, Morehouse JW, Marelich GP, et al. Multicenter implementation of a treatment bundle for patients with sepsis and intermediate lactate values. Am J Respir Crit Care Med. 2016;193(11): 1264–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kelm DJ, Perrin JT, Cartin-Ceba R, Gajic O, Schenck L, Kennedy CC. Fluid overload in patients with severe sepsis and septic shock treated with early goal-directed therapy is associated with increased acute need for fluid-related medical interventions and hospital death. Shock. 2015;43(1):68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Akhter M, Hallare M, Roontiva A, Stowell J. 154 fluid resuscitation of septic patients at risk for fluid overload. Ann Emerg Med. 2017;70(4): S61–S62. [Google Scholar]
- 90.Kuttab HI, Lykins JD, Hughes MD, et al. Evaluation and predictors of fluid resuscitation in patients with severe sepsis and septic shock. Crit Care Med. 2019;47(11):1582–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leisman DE, Goldman C, Doerfler ME, et al. Patterns and outcomes associated with timeliness of initial crystalloid resuscitation in a prospective sepsis and septic shock cohort. Crit Care Med. 2017;45(10):1596–1606. [DOI] [PubMed] [Google Scholar]
- 92.Shah S, Ouellette DR. Early goal-directed therapy for sepsis in patients with preexisting left ventricular dysfunction: a retrospective comparison of outcomes based upon protocol adherence. Chest. 2010;138(4):897A. [Google Scholar]
- 93.Wardi G, Villar J, Gross E, et al. 122 Analysis of a multi-center survey to assess fluid resuscitation practice in patients with sepsis and heart failure. Ann Emerg Med. 2017;70(4):S50. [Google Scholar]
- 94.Malbrain M, Van Regenmortel N, Saugel B, et al. Principles of fluid management and stewardship in septic shock: it is time to consider the four D’s and the four phases of fluid therapy. Ann Intensive Care. 2018;8(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Corl KA, Prodromou M, Merchant RC, et al. The Restrictive IV Fluid Trial in Severe Sepsis and Septic Shock (RIFTS): a randomized pilot study. Crit Care Med. 2019;47(7):951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hjortrup PB, Haase N, Bundgaard H, et al. Restricting volumes of resuscitation fluid in adults with septic shock after initial management: the classic randomised, parallel-group, multicentre feasibility trial. Intensive Care Med. 2016;42(11):1695–1705. [DOI] [PubMed] [Google Scholar]
- 97.Micek ST, McEvoy C, McKenzie M, Hampton N, Doherty JA, Kollef MH. Fluid balance and cardiac function in septic shock as predictors of hospital mortality. Crit Care. 2013;17(5): R246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aliti GB, Rabelo ER, Clausell N, Rohde LE, Biolo A, Beck-da-Silva L. Aggressive fluid and sodium restriction in acute decompensated heart failure: a randomized clinical trial. JAMA Intern Med. 2013;173(12):1058–1064. [DOI] [PubMed] [Google Scholar]
- 99.Travers B, O’Loughlin C, Murphy NF, et al. Fluid restriction in the management of decompensated heart failure: no impact on time to clinical stability. J Card Fail. 2007;13(2):128–132. [DOI] [PubMed] [Google Scholar]
- 100.Silversides JA, Major E, Ferguson AJ, et al. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. Intensive Care Med. 2017;43(2):155–170. [DOI] [PubMed] [Google Scholar]
- 101.Stratton L, Berlin DA, Arbo JE. Vasopressors and inotropes in sepsis. Emerg Med Clin North Am. 2017;35(1):75–91. [DOI] [PubMed] [Google Scholar]
- 102.Sun D, Huang A, Mital S, et al. Norepinephrine elicits beta2-receptor-mediated dilation of isolated human coronary arterioles. Circulation. 2002;106(5):550–555. [DOI] [PubMed] [Google Scholar]
- 103.De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362(9):779–789. [DOI] [PubMed] [Google Scholar]
- 104.Avni T, Lador A, Lev S, Leibovici L, Paul M, Grossman A. Vasopressors for the treatment of septic shock: systematic review and meta-analysis. PLoS One. 2015;10(8): e0129305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rui Q, Jiang Y, Chen M, Zhang N, Yang H, Zhou Y. Dopamine versus norepinephrine in the treatment of cardiogenic shock: a PRISMA-compliant meta-analysis. Medicine (Baltimore). 2017; 96(43): e8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Levy B, Clere-Jehl R, Legras A, et al. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2018;72(2):173–182. [DOI] [PubMed] [Google Scholar]
- 107.Monnet X, Jabot J, Maizel J, Richard C, Teboul JL. Norepinephrine increases cardiac preload and reduces preload dependency assessed by passive leg raising in septic shock patients. Crit Care Med. 2011;39(4):689–694. [DOI] [PubMed] [Google Scholar]
- 108.Maas JJ, Pinsky MR, de Wilde RB, de Jonge E, Jansen JR. Cardiac output response to norepinephrine in postoperative cardiac surgery patients: interpretation with venous return and cardiac function curves. Crit Care Med. 2013;41(1): 143–150. [DOI] [PubMed] [Google Scholar]
- 109.Little RC, Little WC. Cardiac preload, afterload, and heart failure. Arch Intern Med. 1982;142(4):819–822. [PubMed] [Google Scholar]
- 110.Perner A, De Backer D. Understanding hypovolaemia. Intensive Care Med. 2014;40(4):613–615. [DOI] [PubMed] [Google Scholar]
- 111.Hamzaoui O, Georger JF, Monnet X, et al. Early administration of norepinephrine increases cardiac preload and cardiac output in septic patients with life-threatening hypotension. Crit Care. 2010;14(4): R142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hamzaoui O, Jozwiak M, Geffriaud T, et al. Norepinephrine exerts an inotropic effect during the early phase of human septic shock. Br J Anaesth. 2018;120(3):517–524. [DOI] [PubMed] [Google Scholar]
- 113.Self WH, Semler MW, Bellomo R, et al. Liberal versus restrictive intravenous fluid therapy for early septic shock: rationale for a randomized trial. Ann Emerg Med. 2018;72(4): 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Szatalowicz VL, Arnold PE, Chaimovitz C, Bichet D, Berl T, Schrier RW. Radioimmunoassay of plasma arginine vasopressin in hyponatremic patients with congestive heart failure. N Engl J Med. 1981;305(5):263–266. [DOI] [PubMed] [Google Scholar]
- 115.Landry DW, Levin HR, Gallant EM, et al. Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation. 1997;95(5):1122–1125. [DOI] [PubMed] [Google Scholar]
- 116.Sharshar T, Blanchard A, Paillard M, Raphael JC, Gajdos P, Annane D. Circulating vasopressin levels in septic shock. Crit Care Med. 2003;31(6):1752–1758. [DOI] [PubMed] [Google Scholar]
- 117.Maturi MF, Martin SE, Markle D, et al. Coronary vasoconstriction induced by vasopressin. Production ofmyocardial ischemia in dogs by constriction of nondiseased small vessels. Circulation. 1991;83(6):2111–2121. [DOI] [PubMed] [Google Scholar]
- 118.Walker BR, Childs ME, Adams EM. Direct cardiac effects of vasopressin: role of V1-and V2-vasopressinergic receptors. Am J Physiol. 1988;255(2 Pt 2): H261–265. [DOI] [PubMed] [Google Scholar]
- 119.Dunser MW, Mayr AJ, Ulmer H, et al. The effects of vasopressin on systemic hemodynamics in catecholamine-resistant septic and postcardiotomy shock: a retrospective analysis. Anesth Analg. 2001;93(1):7–13. [DOI] [PubMed] [Google Scholar]
- 120.Sacha GL, Bauer SR, Lat I. Vasoactive agent use in septic shock: beyond first-line recommendations. Pharmacotherapy. 2019;39(3):369–381. [DOI] [PubMed] [Google Scholar]
- 121.Gordon AC, Wang N, Walley KR, Ashby D, Russell JA. The cardiopulmonary effects of vasopressin compared with norepinephrine in septic shock. Chest. 2012;142(3):593–605. [DOI] [PubMed] [Google Scholar]
- 122.Dunser MW, Mayr AJ, Ulmer H, et al. Arginine vasopressin in advanced vasodilatory shock: a prospective, randomized, controlled study. Circulation. 2003;107(18):2313–2319. [DOI] [PubMed] [Google Scholar]
- 123.Torgersen C, Dunser MW, Wenzel V, et al. Comparing two different arginine vasopressin doses in advanced vasodilatory shock: a randomized, controlled, open-label trial. Intensive Care Med. 2010;36(1):57–65. [DOI] [PubMed] [Google Scholar]
- 124.Russell JA, Walley KR, Singer J, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358(9):877–887. [DOI] [PubMed] [Google Scholar]
- 125.Harper D, Chandler B. Splanchnic circulation. BJA Education. 2015;16(2):66–71. [Google Scholar]
- 126.Morici N, Sacco A, Oliva F, et al. Epinephrine for acute decompensated heart failure and low output state: friend or foe? Int J Cardiol. 2011;149(3):384–385. [DOI] [PubMed] [Google Scholar]
- 127.Stratton JR, Pfeifer MA, Ritchie JL, Halter JB. Hemodynamic effects of epinephrine: concentration-effect study in humans. J Appl Physiol (1985). 1985;58(4):1199–1206. [DOI] [PubMed] [Google Scholar]
- 128.Levy B, Bollaert PE, Charpentier C, et al. Comparison of norepinephrine and dobutamine to epinephrine for hemodynamics, lactate metabolism, and gastric tonometric variables in septic shock: a prospective, randomized study. Intensive Care Med. 1997;23(3):282–287. [DOI] [PubMed] [Google Scholar]
- 129.Annane D, Vignon P, Renault A, et al. Norepinephrine plus dobutamine versus epinephrine alone for management of septic shock: a randomised trial. Lancet. 2007;370(9588):676–684. [DOI] [PubMed] [Google Scholar]
- 130.Mahmoud KM, Ammar AS. Norepinephrine supplemented with dobutamine or epinephrine for the cardiovascular support of patients with septic shock. Indian J Crit Care Med. 2012; 16(2):75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sato R, Ariyoshi N, Hasegawa D, et al. Effects of inotropes on the mortality in patients with septic shock. J Intensive Care Med. 2019:885066619892218. [DOI] [PubMed] [Google Scholar]
- 132.Myburgh JA, Higgins A, Jovanovska A, et al. A comparison of epinephrine and norepinephrine in critically ill patients. Intensive Care Med. 2008;34(12):2226–2234. [DOI] [PubMed] [Google Scholar]
- 133.Levy B, Perez P, Perny J, Thivilier C, Gerard A. Comparison of norepinephrine-dobutamine to epinephrine for hemodynamics, lactate metabolism, and organ function variables in cardiogenic shock. A prospective, randomized pilot study. Crit Care Med. 2011;39(3):450–455. [DOI] [PubMed] [Google Scholar]
- 134.Nalos M, Leverve X, Huang S, et al. Half-molar sodium lactate infusion improves cardiac performance in acute heart failure: a pilot randomised controlled clinical trial. Crit Care. 2014; 18(2): R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wutrich Y, Barraud D, Conrad M, et al. Early increase in arterial lactate concentration under epinephrine infusion is associated with a better prognosis during shock. Shock. 2010;34(1):4–9. [DOI] [PubMed] [Google Scholar]
- 136.Omar S, Burchard AT, Lundgren AC, Mathivha LR, Dulhunty JM. The relationship between blood lactate and survival following the use of adrenaline in the treatment of septic shock. Anaesth Intensive Care. 2011;39(3):449–455. [DOI] [PubMed] [Google Scholar]
- 137.De Backer D, Aldecoa C, Njimi H, Vincent JL. Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis. Crit Care Med. 2012;40(3):725–730. [DOI] [PubMed] [Google Scholar]
- 138.Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation. 2008;117(5): 686–697. [DOI] [PubMed] [Google Scholar]
- 139.Giamouzis G, Butler J, Starling RC, et al. Impact of dopamine infusion on renal function in hospitalized heart failure patients: results of the dopamine in acute decompensated heart failure (DAD-HF) Trial. J Card Fail. 2010;16(12):922–930. [DOI] [PubMed] [Google Scholar]
- 140.Triposkiadis FK, Butler J, Karayannis G, et al. Efficacy and safety of high dose versus low dose furosemide with or without dopamine infusion: the Dopamine in Acute Decompensated Heart Failure II (DAD-HF II) trial. Int J Cardiol. 2014;172(1): 115–121. [DOI] [PubMed] [Google Scholar]
- 141.Mebazaa A, Motiejunaite J, Gayat E, et al. Long-term safety of intravenous cardiovascular agents in acute heart failure: results from the European Society of Cardiology Heart Failure Long-Term Registry. Eur J Heart Fail. 2018;20(2):332–341. [DOI] [PubMed] [Google Scholar]
- 142.Hiemstra B, Koster G, Wetterslev J, et al. Dopamine in critically ill patients with cardiac dysfunction: a systematic review with meta-analysis and trial sequential analysis. Acta Anaesthesiol Scand. 2019;63(4):424–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.De Backer D, Moraine JJ, Berre J, Kahn RJ, Vincent JL. Effects of dobutamine on oxygen consumption in septic patients. Direct versus indirect determinations. Am J Respir Crit Care Med. 1994;150(1):95–100. [DOI] [PubMed] [Google Scholar]
- 144.Guarracino F, Bertini P, Pinsky MR. Cardiovascular determinants of resuscitation from sepsis and septic shock. Critical Care. 2019;23(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hernandez G, Bruhn A, Luengo C, et al. Effects of dobutamine on systemic, regional and microcirculatory perfusion parameters in septic shock: a randomized, placebo-controlled, double-blind, crossover study. Intensive Care Med. 2013;39(8):1435–1443. [DOI] [PubMed] [Google Scholar]
- 146.Hayes MA, Timmins AC, Yau EH, et al. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. 1994;330(24):1717–1722. [DOI] [PubMed] [Google Scholar]
- 147.Wang XC, Zhu DM, Shan YX. Dobutamine therapy is associated with worse clinical outcomes compared with nesiritide therapy for acute decompensated heart failure: a systematic review and meta-analysis. Am J Cardiovasc Drugs. 2015;15(6):429–437. [DOI] [PubMed] [Google Scholar]
- 148.Cheng L, Yan J, Han S, et al. Comparative efficacy of vasoactive medications in patients with septic shock: a network meta-analysis of randomized controlled trials. Crit Care. 2019; 23(1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163–171. [DOI] [PubMed] [Google Scholar]
- 150.Mbuagbaw L, Rochwerg B, Jaeschke R, et al. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst Rev. 2017;6(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Colucci WS, Wright RF, Jaski BE, Fifer MA, Braunwald E. Milrinone and dobutamine in severe heart failure: differing hemodynamic effects and individual patient responsiveness. Circulation. 1986;73(3 Pt 2):III175–III183. [PubMed] [Google Scholar]
- 152.Mellor A Use of milrinone in non-hyperdynamic sepsis. Critical Care. 1999;2(1):1362. [Google Scholar]
- 153.Nunez S, Maisel A. Comparison between mixed venous oxygen saturation and thermodilution cardiac output in monitoring patients with severe heart failure treated with milrinone and dobutamine. Am Heart J. 1998;135(3):383–388. [DOI] [PubMed] [Google Scholar]
- 154.Cuffe MS, Califf RM, Adams KF Jr, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002;287(12):1541–1547. [DOI] [PubMed] [Google Scholar]
- 155.Schmittinger CA, Dunser MW, Haller M, et al. Combined milrinone and enteral metoprolol therapy in patients with septic myocardial depression. Crit Care. 2008;12(4): R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang Z, Wu Q, Nie X, Guo J, Yang C. Combination therapy with milrinone and esmolol for heart protection in patients with severe sepsis: a prospective, randomized trial. Clin Drug Investig. 2015;35(11):707–716. [DOI] [PubMed] [Google Scholar]
- 157.Lowes BD, Tsvetkova T, Eichhorn EJ, Gilbert EM, Bristow MR. Milrinone versus dobutamine in heart failure subjects treated chronically with carvedilol. Int J Cardiol. 2001;81(2–3): 141–149. [DOI] [PubMed] [Google Scholar]
- 158.Fonarow GC, Hernandez AF, Solomon SD, Yancy CW. Potential mortality reduction with optimal implementation of angiotensin receptor neprilysin inhibitor therapy in heart failure. JAMA Cardiology. 2016;1(6):714–717. [DOI] [PubMed] [Google Scholar]
- 159.Suzuki T, Suzuki Y, Okuda J, et al. Sepsis-induced cardiac dysfunction and beta-adrenergic blockade therapy for sepsis. J Intensive Care. 2017;5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.de Montmollin E, Aboab J, Mansart A, Annane D. Bench-to-bedside review: beta-adrenergic modulation in sepsis. Crit Care. 2009;13(5):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ackland GL, Yao ST, Rudiger A, et al. Cardioprotection, attenuated systemic inflammation, and survival benefit of beta1-adrenoceptor blockade in severe sepsis in rats. Crit Care Med. 2010;38(2):388–394. [DOI] [PubMed] [Google Scholar]
- 162.Hagiwara S, Iwasaka H, Matumoto S, Hidaka S, Noguchi T. Effects of an angiotensin-converting enzyme inhibitor on the inflammatory response in in vivo and in vitro models. Crit Care Med. 2009;37(2):626–633. [DOI] [PubMed] [Google Scholar]
- 163.Haeusler G Pharmacology of beta-blockers: classical aspects and recent developments. J Cardiovasc Pharmacol. 1990; 16(suppl 5):S1–S9. [PubMed] [Google Scholar]
- 164.Gilbert EM, Abraham WT, Olsen S, et al. Comparative hemodynamic, left ventricular functional, and antiadrenergic effects of chronic treatment with metoprolol versus carvedilol in the failing heart. Circulation. 1996;94(11):2817–2825. [DOI] [PubMed] [Google Scholar]
- 165.Gilbert EM, Olsen SL, Renlund DG, Bristow MR. beta-adrenergic receptor regulation and left ventricular function in idiopathic dilated cardiomyopathy. Am J Cardiol. 1993;71(9): 23C–29C. [DOI] [PubMed] [Google Scholar]
- 166.Metra M, Nodari S, D’Aloia A, et al. Beta-blocker therapy influences the hemodynamic response to inotropic agents in patients with heart failure: a randomized comparison of dobutamine and enoximone before and after chronic treatment with metoprolol or carvedilol. J Am Coll Cardiol. 2002;40(7):1248–1258. [DOI] [PubMed] [Google Scholar]
- 167.Asano K, Zisman LS, Yoshikawa T, Headley V, Bristow MR, Port JD. Bucindolol, a nonselective beta 1- and beta 2-adrenergic receptor antagonist, decreases beta-adrenergic receptor density in cultured embryonic chick cardiac myocyte membranes. J Cardiovasc Pharmacol. 2001;37(6):678–691. [DOI] [PubMed] [Google Scholar]
- 168.Tisdale JE, Patel R, Webb CR, Borzak S, Zarowitz BJ. Electro-physiologic and proarrhythmic effects of intravenous inotropic agents. Prog Cardiovasc Dis. 1995;38(2):167–180. [DOI] [PubMed] [Google Scholar]
- 169.Mert KU, Mert GO, Morrad B, Tahmazov S, Mutlu F, Cavusoglu Y. Effects of ivabradine and beta-blocker therapy on dobutamine-induced ventricular arrhythmias. Kardiol Pol. 2017;75(8):786–793. [DOI] [PubMed] [Google Scholar]
- 170.Macchia A, Romero M, Comignani PD, et al. Previous prescription of beta-blockers is associated with reduced mortality among patients hospitalized in intensive care units for sepsis. Crit Care Med. 2012;40(10):2768–2772. [DOI] [PubMed] [Google Scholar]
- 171.Contenti J, Occelli C, Corraze H, Lemoel F, Levraut J. Long-term beta-blocker therapy decreases blood lactate concentration in severely septic patients. Crit Care Med. 2015;43(12): 2616–2622. [DOI] [PubMed] [Google Scholar]
- 172.DeMott JM, Patel G, Lat I. Effects of chronic antihypertensives on vasopressor dosing in septic shock. Ann Pharmacother. 2018; 52(1):40–47. [DOI] [PubMed] [Google Scholar]
- 173.Fuchs C, Wauschkuhn S, Scheer C, et al. Continuing chronic beta-blockade in the acute phase of severe sepsis and septic shock is associated with decreased mortality rates up to 90 days. Br J Anaesth. 2017;119(4):616–625. [DOI] [PubMed] [Google Scholar]
- 174.US National Institutes of Health. ClinicalTrials.gov. 2017. Accessed July 22, 2019. https://ClinicalTrials.gov/show/NCT03190408.
- 175.Prins KW, Neill JM, Tyler JO, Eckman PM, Duval S. Effects of beta-blocker withdrawal in acute decompensated heart failure: a systematic review and meta-analysis. JACC Heart Fail. 2015; 3(8):647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bohm M, Link A, Cai D, et al. Beneficial association of beta-blocker therapy on recovery from severe acute heart failure treatment: data from the survival of patients with acute heart failure in need of intravenous inotropic support trial. Crit Care Med. 2011;39(5):940–944. [DOI] [PubMed] [Google Scholar]
- 177.Jondeau G, Neuder Y, Eicher JC, et al. B-convinced: beta-blocker continuation vs. interruption in patients with congestive heart failure hospitalized for a decompensation episode. Eur Heart J. 2009;30(18):2186–2192. [DOI] [PubMed] [Google Scholar]
- 178.Hsu WT, Galm BP, Schrank G, et al. Effect of renin-angiotensin-aldosterone system inhibitors on short-term mortality after sepsis: a population-based cohort study. Hypertension. 2020;75(2): 483–491. [DOI] [PubMed] [Google Scholar]
- 179.Hsieh MS, How CK, Hsieh VC, Chen PC. Preadmission antihypertensive drug use and sepsis outcome: impact of angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs). Shock. 2020;53(4):407–415. [DOI] [PubMed] [Google Scholar]
- 180.Hessels L, Hoekstra M, Mijzen LJ, et al. The relationship between serum potassium, potassium variability and in-hospital mortality in critically ill patients and a before-after analysis on the impact of computer-assisted potassium control. Crit Care. 2015;19:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jorres A Acute kidney injury in sepsis: transient or intrinsic? Crit Care. 2013;17(6):1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lu B, Gerard NP, Kolakowski LF Jr., et al. Neutral endopeptidase modulation of septic shock. J Exp Med. 1995;181(6): 2271–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Shahreyar M, Fahhoum R, Akinseye O, Bhandari S, Dang G, Khouzam RN. Severe sepsis and cardiac arrhythmias. Ann Transl Med. 2018;6(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Seguin P, Signouret T, Laviolle B, Branger B, Malledant Y. Incidence and risk factors of atrial fibrillation in a surgical intensive care unit. Crit Care Med. 2004;32(3):722–726. [DOI] [PubMed] [Google Scholar]
- 185.Makrygiannis SS, Margariti A, Rizikou D, et al. Incidence and predictors of new-onset atrial fibrillation in noncardiac intensive care unit patients. J Crit Care. 2014;29(4):697. e691–e695. [DOI] [PubMed] [Google Scholar]
- 186.Salman S, Bajwa A, Gajic O, Afessa B. Paroxysmal atrial fibrillation in critically ill patients with sepsis. J Intensive Care Med. 2008;23(3):178–183. [DOI] [PubMed] [Google Scholar]
- 187.Balik M, Kolnikova I, Maly M, Waldauf P, Tavazzi G, Kristof J. Propafenone for supraventricular arrhythmias in septic shock—comparison to amiodarone and metoprolol. J Crit Care. 2017; 41:16–23. [DOI] [PubMed] [Google Scholar]
- 188.Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306(20):2248–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Masarone D, Limongelli G, Rubino M, et al. Management of arrhythmias in heart failure. J Cardiovasc Dev Dis. 2017;4(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1–76. [DOI] [PubMed] [Google Scholar]
- 191.Sibley S, Muscedere J. New-onset atrial fibrillation in critically ill patients. Can Respir J. 2015;22(3):179–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kanji S, Williamson DR, Yaghchi BM, Albert M, McIntyre L Canadian Critical Care Trials Group. Epidemiology and management of atrial fibrillation in medical and noncardiac surgical adult intensive care unit patients. J Crit Care. 2012;27(3):326. e321–e328. [DOI] [PubMed] [Google Scholar]
- 193.Gillinov AM, Bagiella E, Moskowitz AJ, et al. Rate control versus rhythm control for atrial fibrillation after cardiac surgery. N Engl J Med. 2016;374(20):1911–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Liu WC, Lin WY, Lin CS, et al. Prognostic impact of restored sinus rhythm in patients with sepsis and new-onset atrial fibrillation. Crit Care. 2016;20(1):373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Clark DM, Plumb VJ, Epstein AE, Kay GN. Hemodynamic effects of an irregular sequence of ventricular cycle lengths during atrial fibrillation. J Am Coll Cardiol. 1997;30(4):1039–1045. [DOI] [PubMed] [Google Scholar]
- 196.Walkey AJ, Evans SR, Winter MR, Benjamin EJ. Practice patterns and outcomes of treatments for atrial fibrillation during sepsis: a propensity-matched cohort study. Chest. 2016;149(1): 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lee YR, Seth MS, Soney D, Dai H. Benefits of beta-blockade in sepsis and septic shock: a systematic review. Clin Drug Investig. 2019;39(5):429–440. [DOI] [PubMed] [Google Scholar]
- 198.Gore DC, Wolfe RR. Hemodynamic and metabolic effects of selective beta1 adrenergic blockade during sepsis. Surgery. 2006;139(5):686–694. [DOI] [PubMed] [Google Scholar]
- 199.Balik M, Rulisek J, Leden P, et al. Concomitant use of beta-1 adrenoreceptor blocker and norepinephrine in patients with septic shock. Wien Klin Wochenschr. 2012;124(15–16):552–556. [DOI] [PubMed] [Google Scholar]
- 200.Du W, Wang XT, Long Y, Liu DW. Efficacy and safety of esmolol in treatment of patients with septic shock. Chin Med J (Engl). 2016;129(14):1658–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Iskandrian AS, Bemis CE, Hakki AH, et al. Effects of esmolol on patients with left ventricular dysfunction. J Am Coll Cardiol. 1986;8(1):225–231. [DOI] [PubMed] [Google Scholar]
- 202.Morelli A, Ertmer C, Westphal M, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA. 2013;310(16):1683–1691. [DOI] [PubMed] [Google Scholar]
- 203.Morelli A, Singer M, Ranieri VM, et al. Heart rate reduction with esmolol is associated with improved arterial elastance in patients with septic shock: a prospective observational study. Intensive Care Med. 2016;42(10):1528–1534. [DOI] [PubMed] [Google Scholar]
- 204.Schwartz A, Brotfain E, Koyfman L, Klein M. Cardiac arrhythmias in a septic ICU population: a review. J Crit Care Med (Targu Mures). 2015;1(4):140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.American College of Cardiology Foundation, American Heart Association Task Force on Practice Guidelines, Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128(16): e240–327. [DOI] [PubMed] [Google Scholar]
- 206.Lee CC, Lee MG, Lee WC, et al. Preadmission use of calcium channel blocking agents is associated with improved outcomes in patients with sepsis: a population-based propensity score-matched cohort study. Crit Care Med. 2017;45(9): 1500–1508. [DOI] [PubMed] [Google Scholar]
- 207.Balser JR, Martinez EA, Winters BD, et al. Beta-adrenergic blockade accelerates conversion of postoperative supraventricular tachyarrhythmias. Anesthesiology. 1998;89(5):1052–1059. [DOI] [PubMed] [Google Scholar]
- 208.Demircan C, Cikriklar HI, Engindeniz Z, et al. Comparison of the effectiveness of intravenous diltiazem and metoprolol in the management of rapid ventricular rate in atrial fibrillation. Emerg Med J. 2005;22(6):411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Fromm C, Suau SJ, Cohen V, et al. Diltiazem vs. metoprolol in the management of atrial fibrillation or flutter with rapid ventricular rate in the emergency department. J Emerg Med. 2015; 49(2):175–182. [DOI] [PubMed] [Google Scholar]
- 210.Goldenberg IF, Lewis WR, Dias VC, Heywood JT, Pedersen WR. Intravenous diltiazem for the treatment of patients with atrial fibrillation or flutter and moderate to severe congestive heart failure. Am J Cardiol. 1994;74(9):884–889. [DOI] [PubMed] [Google Scholar]
- 211.Hirschy R, Ackerbauer KA, Peksa GD, O’Donnell EP, DeMott JM. Metoprolol vs. diltiazem in the acute management of atrial fibrillation in patients with heart failure with reduced ejection fraction. Am J Emerg Med. 2019;37(1):80–84. [DOI] [PubMed] [Google Scholar]
- 212.Siu CW, Lau CP, Lee WL, Lam KF, Tse HF. Intravenous diltiazem is superior to intravenous amiodarone or digoxin for achieving ventricular rate control in patients with acute uncomplicated atrial fibrillation. Crit Care Med. 2009;37(7):2174–2179; quiz 2180. [DOI] [PubMed] [Google Scholar]
- 213.Delle Karth G, Geppert A, Neunteufl T, et al. Amiodarone versus diltiazem for rate control in critically ill patients with atrial tachyarrhythmias. Crit Care Med. 2001;29(6):1149–1153. [DOI] [PubMed] [Google Scholar]
- 214.Means KN, Gentry AE, Nguyen TT. Intravenous continuous infusion vs. oral immediate-release diltiazem for acute heart rate control. West J Emerg Med. 2018;19(2):417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014; 130(23):2071–2104. [DOI] [PubMed] [Google Scholar]
- 216.Clemo HF, Wood MA, Gilligan DM, Ellenbogen KA. Intravenous amiodarone for acute heart rate control in the critically ill patient with atrial tachyarrhythmias. Am J Cardiol. 1998;81(5): 594–598. [DOI] [PubMed] [Google Scholar]
- 217.Shojaee M, Feizi B, Miri R, Etemadi J, Feizi AH. Intravenous amiodarone versus digoxin in atrial fibrillation rate control: a clinical trial. Emerg (Tehran). 2017;5(1):e29. [PMC free article] [PubMed] [Google Scholar]
- 218.Khan IA, Mehta NJ, Gowda RM. Amiodarone for pharmacological cardioversion of recent-onset atrial fibrillation. Int J Cardiol. 2003;89(2–3):239–248. [DOI] [PubMed] [Google Scholar]
- 219.Scarlatescu E, Tomescu D, Arama SS. Anticoagulant therapy in sepsis. The importance of timing. J Crit Care Med (Targu Mures). 2017;3(2):63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Marti-Carvajal AJ, Sola I, Gluud C, Lathyris D, Cardona AF. Human recombinant protein C for severe sepsis and septic shock in adult and paediatric patients. Cochrane Database Syst Rev. 2012;12:CD004388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Warren BL, Eid A, Singer P, et al. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA. 2001;286(15):1869–1878. [DOI] [PubMed] [Google Scholar]
- 222.Abraham E, Reinhart K, Opal S, et al. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA. 2003;290(2):238–247. [DOI] [PubMed] [Google Scholar]
- 223.Yamakawa K, Umemura Y, Hayakawa M, et al. Benefit profile of anticoagulant therapy in sepsis: a nationwide multicentre registry in Japan. Crit Care. 2016;20(1):229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Walkey AJ, Quinn EK, Winter MR, McManus DD, Benjamin EJ. Practice patterns and outcomes associated with use of anticoagulation among patients with atrial fibrillation during sepsis. JAMA Cardiol. 2016;1(6):682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Nasraway SA, Rackow EC, Astiz ME, Karras G, Weil MH. Inotropic response to digoxin and dopamine in patients with severe sepsis, cardiac failure, and systemic hypoperfusion. Chest. 1989;95(3):612–615. [DOI] [PubMed] [Google Scholar]
- 226.Rathore SS, Curtis JP, Wang Y, Bristow MR, Krumholz HM. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA. 2003;289(7):871–878. [DOI] [PubMed] [Google Scholar]
- 227.Ahmed A, Gambassi G, Weaver MT, Young JB, Wehrmacher WH, Rich MW. Effects of discontinuation of digoxin versus continuation at low serum digoxin concentrations in chronic heart failure. Am J Cardiol. 2007;100(2):280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lopes RD, Rordorf R, De Ferrari GM, et al. Digoxin and mortality in patients with atrial fibrillation. J Am Coll Cardiol. 2018; 71(10):1063–1074. [DOI] [PubMed] [Google Scholar]
- 229.MacLeod-Glover N, Mink M, Yarema M, Chuang R.Digoxin toxicity: case for retiring its use in elderly patients? Can Fam Physician. 2016;62(3):223–228. [PMC free article] [PubMed] [Google Scholar]
- 230.Papadakis MA, Wexman MP, Fraser C, Sedlacek SM. Hyperkalemia complicating digoxin toxicity in a patient with renal failure. Am J Kidney Dis. 1985;5(1):64–66. [DOI] [PubMed] [Google Scholar]
- 231.Gomez H, Kellum JA. Sepsis-induced acute kidney injury. Curr Opin Crit Care. 2016;22(6):546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Eichhorn EJ, Gheorghiade M. Digoxin. Prog Cardiovasc Dis. 2002;44(4):251–266. [DOI] [PubMed] [Google Scholar]
- 233.Hou ZY, Chang MS, Chen CY, et al. Acute treatment of recent-onset atrial fibrillation and flutter with a tailored dosing regimen of intravenous amiodarone. a randomized, digoxin-controlled study. Eur Heart J. 1995;16(4):521–528. [DOI] [PubMed] [Google Scholar]
- 234.Herasevich S, Bennett CE, Schwegman AR, Subat YW, Gajic O, Jayaprakash N. Hemodynamic profiles following digoxin use in patients with sepsis in the ICU. J Crit Care. 2019;54:175–179. [DOI] [PubMed] [Google Scholar]
- 235.Fuller BM, Mohr NM, Graetz TJ, et al. The impact of cardiac dysfunction on acute respiratory distress syndrome and mortality in mechanically ventilated patients with severe sepsis and septic shock: an observational study. J Crit Care. 2015;30(1):65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Alviar CL, Miller PE, McAreavey D, et al. Positive pressure ventilation in the cardiac intensive care unit. J Am Coll Cardiol. 2018;72(13):1532–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Feihl F, Broccard AF. Interactions between respiration and systemic hemodynamics. Part I: basic concepts. Intensive Care Med. 2009;35(1):45–54. [DOI] [PubMed] [Google Scholar]
- 238.Luecke T, Pelosi P. Clinical review: positive end-expiratory pressure and cardiac output. Crit Care. 2005;9(6):607–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wiesen J, Ornstein M, Tonelli AR, Menon V, Ashton RW. State of the evidence: mechanical ventilation with PEEP in patients with cardiogenic shock. Heart. 2013;99(24):1812–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.De Backer D, Vincent JL. Should we measure the central venous pressure to guide fluid management? Ten answers to 10 questions. Crit Care. 2018;22(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Barbar SD, Clere-Jehl R, Bourredjem A, et al. Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med. 2018;379(15):1431–1442. [DOI] [PubMed] [Google Scholar]
- 242.Lin WT, Lai CC, Chang SP, Wang JJ. Effects of early dialysis on the outcomes of critically ill patients with acute kidney injury: a systematic review and meta-analysis of randomized controlled trials. Scientific Reports. 2019;9(1):18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Liu Y, Davari-Farid S, Arora P, Porhomayon J, Nader ND. Early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury after cardiac surgery: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2014;28(3):557–563. [DOI] [PubMed] [Google Scholar]
- 244.Costanzo MR, Guglin ME, Saltzberg MT, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007;49(6): 675–683. [DOI] [PubMed] [Google Scholar]
- 245.Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367(24):2296–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.van der Sande FM, Mulder AW, Hoorntje SJ, et al. The hemodynamic effect of different ultrafiltration rates in patients with cardiac failure and patients without cardiac failure: comparison between isolated ultrafiltration and ultrafiltration with dialysis. Clin Nephrol. 1998;50(5):301–308. [PubMed] [Google Scholar]
- 247.Daugirdas JT. Pathophysiology of dialysis hypotension: an update. Am J Kidney Dis. 2001;38(4 suppl 4):S11–S17. [DOI] [PubMed] [Google Scholar]
- 248.Chou JA, Kalantar-Zadeh K, Mathew AT. A brief review of intradialytic hypotension with a focus on survival. Semin Dial. 2017;30(6):473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]


