Abstract
Objective
Magnetic resonance imaging (MRI) is currently the gold standard to diagnose and monitor osteochondritis dissecans (OCD) of the knee. The purpose of this study was to evaluate for the first time if ultrasound imaging can be used to visualize osteochondritis dissecans of the distal femur.
Design
From May 2008 to December 2013, 44 children (26 boys and 18 girls) presenting with OCD of the knee in our department were examined and evaluated by ultrasound imaging. Mean age at diagnosis was 11.8 ± 2.2 years. Two independent experienced orthopedic surgeons analyzed the localization, stage, and the size of the OCD via ultrasound and compared the results with the MRI findings.
Results
Ultrasonic examination has limitations in assessing the OCD stage I and therefore is not suitable for evaluating this stage of the disease. In stages II to IV, a good correlation to MRI regarding defect localization and size can be found, when the defect is localized in a region that is accessible to ultrasonic examination.
Conclusion
Ultrasonic scan is an appropriate tool for the screening and monitoring of OCDs stages II to IV. It provides an inexpensive and readily available alternative to MRI. In addition, the healing process of higher grade defects as well as the screening of the opposite side can also be performed by ultrasound. Detection of defects being localized close to the intercondylar notch or far posterior on the lateral condyle are limitations for the use of ultrasound.
Keywords: osteochondritis dissecans, subchondral bone, articular cartilage, magnetic resonance imaging, ultrasound imaging
Introduction
Osteochondritis dissecans (OCD) is a pathological condition affecting the subchondral bone, with the risk of destabilization of an osteochondral joint segment and the potential to result in severe function loss, joint blockage, and premature osteoarthritis.1 Children who are active in athletics are more frequently affected.2 Multiple etiological factors like trauma, ischemia, abnormal ossification patterns, genetic disorders, and endocrine aspects may play a role in the development of the disease.3,4 Current findings in a histological study revealed focal accumulation of nonmineralized bone matrix indicating a lack of mineralization in OCD. This correlates with a low level of vitamin D.5 The medial condyle of the distal femur is the most frequently affected location.6 Magnetic resonance imaging (MRI) is currently the modality of choice to diagnose, stage and monitor the progression and/or healing of OCD of the knee.7,8
An inexpensive and more widely available tool for the assessment of a variety of musculoskeletal disorders is the use of ultrasound (US).9,10 The purpose of this study was to evaluate if US imaging is reliable in identifying OCD of the distal femur in comparison with MRI.
Methods
In this prospective study, 44 children who were transferred to our clinic with pain in the knee and evidence of OCD lesion at the distal femur in MRI were analyzed. Investigation period was from May 2008 to December 2013. Eligibility criteria were age <18 years, no previous surgical treatment, informed consent by parents, and proof of OCD in MRI in at least 1 knee joint. All patients with proven OCDs in MRI despite of size and stage were included. Consecutive 26 male (59.1%) and 18 female (40.9%) patients with a mean age of 11.8 ± 2.17 years were included in the study. A total of 24 patients (54.5%) had an OCD of the right knee, 20 of the left knee (45.5%). In all, 68.2% (30 cases) of the OCDs were localized at the medial femoral condyle, 25% (11 cases) at the lateral condyle, and 6.8% (3 cases) had medial and lateral OCDs in unilateral manifestation. There were 2 patients with bilateral manifestations diagnosed in MRI.
Two investigators independently performed and analyzed all US data. Raters were aware of existence but not size and location of the OCDs. Results were compared and measuring differences of 1 mm were rated as equal. In case of differences of more than 1 mm, the arithmetic mean of both measurements was chosen. The same method was applied for the measurements of the MRI scans. Maximum period between MRI and US was 4 weeks. Finally, all results were compared regarding accuracy, size of the OCD, and classification, especially regarding stability. In case of perifocal cysts or fluid margin in MRI, surgery was mandatory. Eighteen patients received surgical treatment, so in these cases intraoperative stability was possible to compare MRI and US valuation.
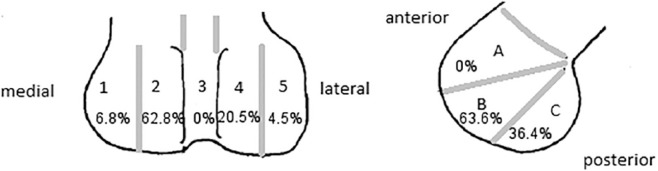
To describe OCD localization, we used a method published by Cahill and Berg11 ( Fig. 1 ).
Figure 1.
Osteochondritis dissecans (OCD) localization modified according to Cahill and Berg (1983).11 In the anteroposterior diagram, the anatomic zones 1 to 5 are used. They are delineated by bisecting the individual condyles. Zone 3 is outlined by the walls of the intercondylar tunnel. In the lateral diagram, zone A is limited by a line projected along the roof of the intercondylar tunnel. Zones B and C are separated by a line projecting distally from and parallel to the posterior femoral cortex.
Magnetic Resonance Imaging of the Knee
MRI with T1- and T2-weighted sequences in axial, coronal, and sagittal planes of the knee were presented by all patients. Sagittal dimension of OCD and radiological classification according to Dipaola et al.12 were determined:
Stage 1: Thickening of articular cartilage and low signal changes.
Stage 2: Articular cartilage breached, low signal rim behind fragment indicating fibrous attachment.
Stage 3: Articular cartilage breached, high signal changes behind fragment indicating synovial fluid between fragment and underlying subchondral bone.
Stage 4: Loose body.
No MR arthrography was performed.
Ultrasound Examination
All children received US examinations of both knees. All investigations were performed with the patient lying supine and the knee flexed to 90°. A broadband linear array transducer 3 to 12 MHz (L12-3, Koninklijke Philips N.V.) was used in all patients. It was placed on the medial and lateral femoral condyles in coronal and sagittal planes ( Fig. 2 ). For the purpose of this study, the size and location of the lesion were determined and an attempt to rate lesion stability was performed. Using US, the normal cartilage of the joint can clearly be distinguished from the underlying bone. If a subchondral lesion was present a fragmented base could be seen. Pathological findings were recorded.
Figure 2.
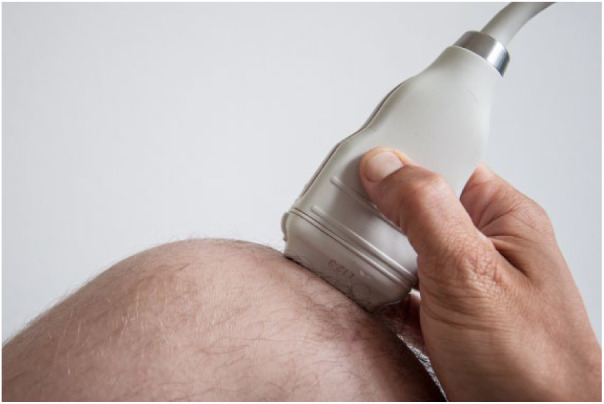
Ultrasonic examination of distal femur in the sagittal plane.
In analogy to MRI, we suggest the following criteria: 1 = no lesion, 2 = continuous cartilage with irregularity articular surface, 3 = hyperechoic subchondral bone surface with moderate irregularity and discontinuity, 4 = complete disruption of echogenic articular surface and a hyperechoic subchondral bone surface with heterogeneously material. Criteria 1 and 2 are considered to be stable and 3 and 4 are considered unstable.
Statistics
Descriptive statistics were used to analyze the basic characteristics of the data. Continuous variables were presented as mean and standard deviation (SD). Differences between groups were calculated using the Student t test. Mann-Whitney U test was used to compare accuracy of MRI and US. A P value <0.05 was considered statistically significant. Statistical analysis was performed using SPSS statistical software (SPSS version 19.0, IBM Corp. Armonk, NY, USA).
To calculate sensitivities, the gold standard needs to be defined. In this study, MRI was defined as the gold standard. For the tests to agree, they also had to agree on location. Sensitivity of US was calculated as the proportion of those with describable OCD if MRI was positive.
Results
Mean stage on MRI of all OCDs was 2.4 ± 0.7 (stage 1 = 6.8%, 2 = 43.2%, 3 = 38.7%, 4 = 11.3%). The average stage of OCDs treated surgically and treated nonoperatively was 2.8 ± 0.7 and 2.1 ± 0.6, respectively.
Only 2 OCDs could not be diagnosed by US examination. One was a stage 1 OCD measuring 1.6 cm in length localized close to the intercondylar notch and the other was a stage 2 OCD measuring 0.6 cm in diameter and located far posterior on the lateral condyle. In total, 95.5% of all lesions could be detected by US (accuracy P = 0.04).
Mean size of OCDs measured on US imaging in the sagittal plane was 2.2 ± 0.7 cm and on MRI scans 2.2 ± 0.8 cm. The difference was not statistically significant (P = 0.29).
MRI showed signs of unstable OCDs in 18 cases (rim of fluid signal intensity, multiple breaks in the subchondral plate, and a second outer rim of low T2-weighted signal intensity). To evaluate instability of OCDs further, lesion width, cystic like lesion size, and patient age are used.13 Because of unclear criteria for stability of OCDs using US examination, one investigator rated 10 OCDs while another investigator rated 12 OCDs to be unstable. In a consensus between both investigators, 12 OCDs were rated as unstable.
Following the classification by Cahill and Berg,11 6.8% of OCDs were localized in zone 1, 68.2% in zone 2, 0% in zone 3, 20.5% in zone 4, and 4.5% in zone 5. In the anteroposterior view, we found 0% in zone A, 63.6% in zone B, and 36.4% in zone C. The most frequent location was zone 2B with 50% of all cases. A majority of these cases were in between zones B and C. No additional OCD at patella was recognized.
All 42 visible OCDs on US examination could be found in exactly the same location as on MRI (Figs. 3 and 4).
Figure 3.
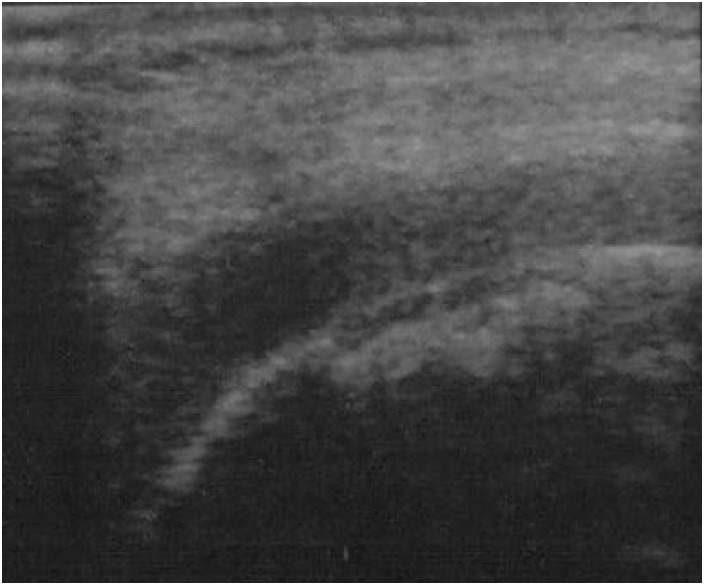
An 11-year-old boy with osteochondritis dissecans, in ultrasound imaging.
Figure 4.
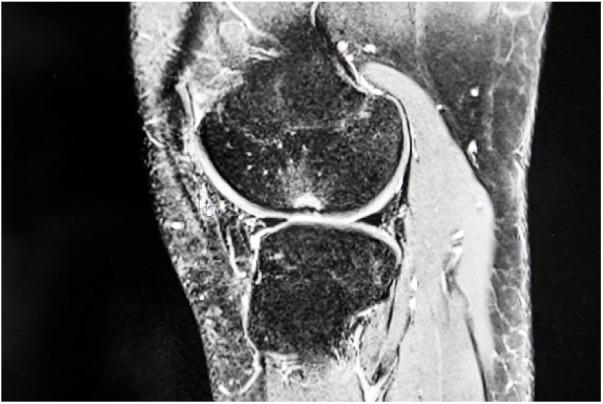
An 11-year-old boy with osteochondritis dissecans, in magnetic resonance imaging.
Eighteen of 44 children underwent surgical treatment because of imminent instability in MRI. Intraoperatively, 6 OCDs were defined unstable and had to be stabilized. These 6 cases were rated incorrectly as stable on US examination by both investigators. Clear criteria for instability on US could not be established even when intraoperative findings were taken into account.
A total of 40.9% of patients underwent surgery during clinical course, 59.1% had non-operative treatment. Time lapse between initial diagnoses and surgery was 15.0 ± 15.1 months. All children had MRI examinations at intervals of 6 months. The indication for surgery was given in case of progress or imminent instability of OCD lesion. In all, 67% received arthroscopic-guided retrograde drilling, 33% refixation with resorbable pins.
Discussion
Traditionally, plain radiographs are used to describe the characteristics of OCDs. MRI is recommended for additional assessment of OCDs and is believed to be superior to other imaging techniques in evaluation of a painful knee.13,14
Various classification systems are used to rate stage and stability of the lesions.15,16
US is an established method to diagnose diseases of musculoskeletal system, for example, shoulder.17 However, to our knowledge, the use of US to analyze OCD of the knee has not been attempted widely in the literature. It is an inexpensive, portable, and widely available instrument without the drawback of radiation.9,18 Furthermore, it is quick and straightforward to use especially in children, while the use of MRI in young children may involve sedation or anesthesia.19
In 1989, the first report on diagnosis of OCDs on US versus conventional radiography was published. All 25 cases seen on radiographs could easily be identified with US imaging.20
Reed et al.21 could show the accuracy of MRI versus arthroscopy in the assessment of cartilage lesions. Good results for MRI were published, but some chondral lesions were still overlooked. To improve the identification of OCD via arthroscopy, Penttilä et al.22 combined this method with intraoperative US imaging and were able to further clarify the grading of the lesion.22 But, to the best of our knowledge, no study has compared MRI and US.
This feasibility study was done to correlate US and MRI findings in OCD of the distal femur. US was found to be capable of accurately measuring both cartilage thickness and the extent and depth of cartilage defects in a cadaver model with a 10 MHz transducer.23 We were able to confirm those findings while using US to delineate OCD of the distal femur in immature patients. However, criteria to identify instability of lesion could not be detected.
The majority of our patients had a stage II OCD on MRI. Only 1 OCD in this stage could not be detected by US. The defect was localized on the posterior aspect of the lateral femoral condyle. This patient had insufficient knee flexion, which made the examination of the posterior aspects of the condyle difficult. This patient could be examined from posterior with knee extended. Three OCDs were classified as stage I, and 1 of them was not identified on US. The defect was localized in zone 3 according to Cahill and Berg.11 Therefore, grade I lesions may not be detected by US, especially when they are located close to the intercondylar notch or close to the patella.20
According to literature, 75% to 80% of OCDs are seen on the posterior-lateral aspect of the medial femoral condyle (1C), fewer than 25% of cases are located at the posterior aspect of the lateral femoral condyle (4/5C), patella, and trochlea.24,25 Our study showed similar results.
To plan treatment of OCDs, an adequate evaluation of the subchondral bone and articular cartilage with regard to the stability of the OCD is required. Stable OCDs in skeletally immature patients can be treated nonsurgically with a success rate of about 90%. Those who fail nonoperative treatment or unstable lesions require surgical intervention.2 Lesion stability is the most important predictor of a successful nonoperative management. Dipaola et al.12 established a commonly used classification system for OCDs consisting of 4 grades: Grades 1 and 2 are rates as stable, while grades 3 and 4 are rated as unstable. An unstable OCD has a T2 signal change behind the fragment suggesting fluid behind the lesion.12 Another frequently used classification system to identify instability by MRI was established by De Smet et al.26 and revised for immature patients by Kijowski et al.,27 because they found a significant lower specificity for detecting unstable juvenile OCD lesions using criteria developed for adults. So they recommend that a high T2 signal intensity rim around an OCD lesion of immature patients represents instability only if it has the same intensity as adjacent joint fluid and is accompanied by a second outer rim of low T2 signal intensity or multiple breaks in the subchondral bone plates.26,27 Considering MRI being the gold standard imaging method to detect OCD, US showed a high sensitivity related to MRI in our study. But regarding grading of OCD, US was not convincing.
It is difficult to stage OCD when applying US. However, in analogy to MRI, the following criteria could be used: 1 = no lesion, 2 = continuous cartilage with irregularity articular surface, 3 = hyperechoic subchondral bone surface with moderate irregularity and discontinuity, 4 = complete disruption of echogenic articular surface and a hyperechoic subchondral bone surface with heterogeneously material. Criteria 1 and 2 are considered to be stable and 3 and 4 are considered unstable. In our study, of all OCDs, 10 OCDs were considered to be unstable on US by 1 investigator and 12 by the other.
Eighteen of 44 children underwent surgical treatment. Intraoperatively, only 6 OCDs were defined as unstable and had to be stabilized. Therefore, we currently do not recommend assessing stability with US. However, it may be used to define location, size progression, and signs of fragment dislocation. It may also be used as a readily available screening method for knee pain and to assess the contralateral knee joint. Bilateral involvement with OCD on the distal femur is reported between 12.6% and 38.9%. Sensitivity of US to detect OCD was 0.9545 in this study. Also, there is the bias of known OCD in our cohort; we believe US examination can easily be used to rule out contralateral disease when one knee is affected.6,11,28
Aigner et al.29 found an accuracy of US, for in vivo measurement of meniscal bearings, between 0.4 and 0.7 mm when using an 8-MHz US device. Therefore, it may be possible to see ruptures of cartilage and judge stability of OCDs with higher resolution in the future.29
Knowledge of OCD in MRI as potential bias and number of patients could be seen as study limitations.
In summary, US is an appropriate tool for the screening and monitoring of OCDs stages II to IV. It provides an inexpensive and readily available alternative to MRI. In addition, the healing process of higher grade defects as well as the screening of the opposite side can also be performed by US. Detection of defects being localized close to the intercondylar notch or far posterior on the lateral condyle are limitations for the use of US.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and with the 1964 Helsinki declaration and its later amendments or comparable ethical standard.
Informed Consent: Written informed consent was obtained from parents of all children before the study.
Trial Registration: Not applicable.
ORCID iD: Josephine Berger-Groch  https://orcid.org/0000-0003-4952-8271
https://orcid.org/0000-0003-4952-8271
References
- 1. Edmonds EW, Shea KG. Osteochondritis dissecans: editorial comment. Clin Orthop. 2013;471:1105-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robertson W, Kelly BT, Green DW. Osteochondritis dissecans of the knee in children. Curr Opin Pediatr. 2003;15:38-44. [DOI] [PubMed] [Google Scholar]
- 3. Abouassaly M, Peterson D, Salci L, Farrokhyar F, D’Souza J, Bhandari M. et al. Surgical management of osteochondritis dissecans of the knee in the paediatric population: a systematic review addressing surgical techniques. Knee Surg Sports Traumatol Arthrosc. 2014;22:1216-24. [DOI] [PubMed] [Google Scholar]
- 4. Weiss JM, Nikizad H, Shea KG, Gyurdzhyan S, Jacobs JC, Cannamela PC. et al. The incidence of surgery in osteochondritis dissecans in children and adolescents. Orthop J Sports Med. 2016;4:2325967116635515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krause M, Lehmann D, Amling M, Rolvien T, Frosch KH, Püschel K. et al. Intact bone vitality and increased accumulation of nonmineralized bone matrix in biopsy specimens of juvenile osteochondritis dissecans: a histological analysis. Am J Sports Med. 2015;43:1337-47. [DOI] [PubMed] [Google Scholar]
- 6. Hefti F, Beguiristain J, Krauspe R, Möller-Madsen B, Riccio V, Tschauner C. et al. Osteochondritis dissecans: a multicenter study of the European Pediatric Orthopedic Society. J Pediatr Orthop B. 1999;8:231-45. [PubMed] [Google Scholar]
- 7. Mestriner LA. Osteochondritis dissecans of the knee: diagnosis and treatment. Rev Bras Ortop. 2012;47:553-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Smet AA, Ilahi OA, Graf BK. Untreated osteochondritis dissecans of the femoral condyles: prediction of patient outcome using radiographic and MR findings. Skeletal Radiol. 1997;26:463-7. [DOI] [PubMed] [Google Scholar]
- 9. Friedman L, Finlay K, Jurriaans E. Ultrasound of the knee. Skeletal Radiol. 2001;30:361-77. [DOI] [PubMed] [Google Scholar]
- 10. Blankstein A, Ganel A, Mirovsky Y, Chechick A, Dudkiewicz I. Early diagnosis of generalized knee pain and osteoarthritis by ultrasound. Aktuelle Traumatol. 2006;36:175-9. [Google Scholar]
- 11. Cahill BR, Berg BC. 99m-Technetium phosphate compound joint scintigraphy in the management of juvenile osteochondritis dissecans of the femoral condyles. Am J Sports Med. 1983;11:329-35. [DOI] [PubMed] [Google Scholar]
- 12. Dipaola JD, Nelson DW, Colville MR. Characterizing osteochondral lesions by magnetic resonance imaging. Arthroscopy. 1991;7:101-4. [DOI] [PubMed] [Google Scholar]
- 13. Krause M, Hapfelmeier A, Möller M, Amling M, Bohndorf K, Meenen NM. Healing predictors of stable juvenile osteochondritis dissecans knee lesions after 6 and 12 months of nonoperative treatment. Am J Sports Med. 2013;41:2384-91. [DOI] [PubMed] [Google Scholar]
- 14. Quatman CE, Quatman-Yates CC, Schmitt LC, Paterno MV. The clinical utility and diagnostic performance of MRI for identification and classification of knee osteochondritis dissecans. J Bone Joint Surg Am. 2012;94:1036-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Uppstrom TJ, Gausden EB, Green DW. Classification and assessment of juvenile osteochondritis dissecans knee lesions. Curr Opin Pediatr. 2016;28:60-7. [DOI] [PubMed] [Google Scholar]
- 16. Heywood CS, Benke MT, Brindle K, Fine KM. Correlation of magnetic resonance imaging to arthroscopic findings of stability in juvenile osteochondritis dissecans. Arthroscopy. 2011;27:194-9. [DOI] [PubMed] [Google Scholar]
- 17. Kurz AZ, Kelly MJ, Hackett L, Murrell GAC. Effect of surgeon-sonographer interaction on ultrasound diagnosis of rotator cuff tears: a five-year cohort study in 775 shoulders. J Shoulder Elbow Surg. 2016;25:1385-94. [DOI] [PubMed] [Google Scholar]
- 18. Court-Payen M. Sonography of the knee: intra-articular pathology. J Clin Ultrasound. 2004;32:481-90. [DOI] [PubMed] [Google Scholar]
- 19. Metterlein T, Haubner F, Knoppke B, Graf B, Zausig Y. An unexpected ferromagnetic foreign body detected during emergency magnetic resonance imaging: a case report. BMC Res Notes. 2014;7:808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gregersen HE, Rasmussen OS. Ultrasonography of osteochondritis dissecans of the knee. A preliminary report. Acta Radiol. 1989;30:552-4. [PubMed] [Google Scholar]
- 21. Reed ME, Villacis DC, Hatch GF, 3rd, Burke WS, Colletti PM, Narvy SJ. et al. 3.0-Tesla MRI and arthroscopy for assessment of knee articular cartilage lesions. Orthopedics. 2013;36:e1060-e1064. [DOI] [PubMed] [Google Scholar]
- 22. Penttilä P, Liukkonen J, Joukainen A, Virén T, Jurvelin JS, Töyräs J. et al. Diagnosis of knee osteochondral lesions with ultrasound imaging. Arthrosc Tech. 2015;4:e429-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mathiesen O, Konradsen L, Torp-Pedersen S, Jørgensen U. Ultrasonography and articular cartilage defects in the knee: an in vitro evaluation of the accuracy of cartilage thickness and defect size assessment. Knee Surg Sports Traumatol Arthrosc. 2004;12:440-3. [DOI] [PubMed] [Google Scholar]
- 24. Louisia S, Beaufils P, Katabi M, Robert H; French Society of Arthroscopy. Transchondral drilling for osteochondritis dissecans of the medial condyle of the knee. Knee Surg Sports Traumatol Arthrosc. 2003;11:33-9. [DOI] [PubMed] [Google Scholar]
- 25. Lasangios NG, Giannoudis PV. Osteochondritis dissecans. In: Lasangios NG, Kanakaris NK, Giannoudis PV, editors. Trauma and orthopaedic classifications: a comprehensive overview. London, England: Springer; 2015. p. 445-8. [Google Scholar]
- 26. De Smet AA, Ilahi OA, Graf BK. Reassessment of the MR criteria for stability of osteochondritis dissecans in the knee and ankle. Skeletal Radiol. 1996;25:159-63. [DOI] [PubMed] [Google Scholar]
- 27. Kijowski R, Blankenbaker DG, Shinki K, Fine JP, Graf BK, De Smet AA. Juvenile versus adult osteochondritis dissecans of the knee: appropriate MR imaging criteria for instability. Radiology. 2008;248:571-8. [DOI] [PubMed] [Google Scholar]
- 28. Mubarak SJ, Carroll NC. Juvenile osteochondritis dissecans of the knee: etiology. Clin Orthop Relat Res. 1981;(157):200-11. [PubMed] [Google Scholar]
- 29. Aigner C, Radl R, Pechmann M, Rehak P, Windhager R. In vivo assessment of mobility of meniscal bearings with ultrasound [in German]. Orthopade. 2003;32:287-91. [DOI] [PubMed] [Google Scholar]