This cohort study evaluates whether anesthesiologist expertise and experience are factors in morbidity and readmission among patients who underwent esophagectomy, pancreatectomy, or hepatectomy for gastrointestinal cancer.
Key Points
Question
Is there an association between anesthesiologist volume and adverse perioperative outcomes?
Findings
In this cohort study of 8096 adults who underwent esophagectomy, pancreatectomy, or hepatectomy for cancer, care provided by anesthesiologists with high procedure volume (≥6 procedures per year) vs care by anesthesiologists with low procedure volume was independently associated with a lower risk of combined major morbidity and readmission over 90 days.
Meaning
Results of this study support organizing perioperative care to increase anesthesiologist volume and decrease the risk of adverse postoperative outcomes.
Abstract
Importance
Intraoperative anesthesiology care is crucial to high-quality surgical care. The clinical expertise and experience of anesthesiologists may decrease the risk of adverse outcomes.
Objective
To examine the association between anesthesiologist volume and short-term postoperative outcomes for complex gastrointestinal (GI) cancer surgery.
Design, Setting, and Participants
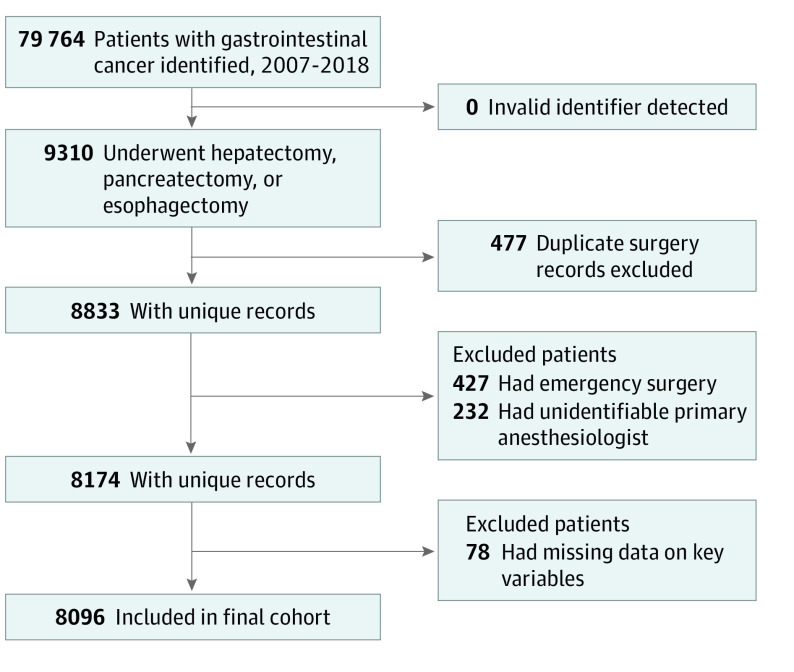
This population-based cohort study used administrative health care data sets from various data sources in Ontario, Canada. Adult patients who underwent esophagectomy, pancreatectomy, or hepatectomy for GI cancer from January 1, 2007, to December 31, 2018, were eligible. Patients with an invalid identification number, a duplicate surgery record, and missing primary anesthesiologist information were excluded.
Exposures
Primary anesthesiologist volume was defined as the annual number of procedures of interest (esophagectomy, pancreatectomy, and hepatectomy) supported by that anesthesiologist in the 2 years before the index surgery. Volume was dichotomized into low-volume and high-volume categories, with 75th percentile or 6 or more procedures per year selected as the cutoff point.
Main Outcome and Measures
The primary outcome was a composite of 90-day major morbidity (with a Clavien-Dindo classification grade 3-5) and readmission. Secondary outcomes were individual components of the primary outcome. The association between exposure and outcomes was examined using multivariable logistic regression models, accounting for potential confounders.
Results
Of the 8096 patients included, 5369 were men (66.3%) and the median (interquartile range [IQR]) age was 65 (57-72) years. Operations were supported by 842 anesthesiologists and performed by 186 surgeons, and the median (IQR) anesthesiologist volume was 3 (1.5-6) procedures per year. A total of 2166 patients (26.7%) received care from high-volume anesthesiologists. Primary outcome occurred in 36.3% of patients in the high-volume group and 45.7% of patients in the low-volume group. After adjustment, care by high-volume anesthesiologists was independently associated with lower odds of the primary outcome (adjusted odds ratio [aOR], 0.85; 95% CI, 0.76-0.94), major morbidity (aOR, 0.83; 95% CI, 0.75-0.91), unplanned intensive care unit admission (aOR, 0.84; 95% CI, 0.76-0.94), but not readmission (aOR, 0.87; 95% CI, 0.73-1.05) or mortality (aOR, 1.05; 95% CI, 0.84-1.31). E-values analysis indicated that an unmeasured variable would unlikely substantively change the observed risk estimates.
Conclusions and Relevance
This study found that, among adults who underwent complex gastrointestinal cancer surgery, those who received care from high-volume anesthesiologists had a lower risk of adverse postoperative outcomes compared with those who received care from low-volume anesthesiologists. These findings support organizing perioperative care to increase anesthesiologist volume to optimize patient outcomes.
Introduction
Higher institution and surgeon case volumes and designated comprehensive centers have been associated with improved short- and long-term outcomes for cancer surgery.1,2,3,4,5,6 These data led to policy changes and regionalization of complex cancer surgery in many jurisdictions.7,8,9,10,11,12 Although the volume-outcome association has been extensively studied for surgeons, data on such an association for other members of the care team remain scarce. Each critical step in the management of surgical patients is important in optimizing patient outcomes.13
Anesthesiologists play a key role in the delivery and safety of surgical care. Anesthesiology practice for cancer surgery has become increasingly complex and subspecialized.14 Intraoperative management, including fluid administration, invasive monitoring, transfusion thresholds, and analgesia strategies, is associated with bleeding, postoperative recovery, and potentially anastomotic healing and therefore plays an important role in optimizing outcomes.15,16,17,18,19 Whether and how anesthesiology case volume is associated with perioperative outcomes is largely unknown.
Complex gastrointestinal (GI) cancer surgery represents a unique opportunity to examine the volume-outcome association for anesthesiologists. In particular, esophagectomy, pancreatectomy, and hepatectomy present a substantial risk of postoperative morbidity (30%-57%) and mortality (2%-4%).20,21,22,23 These operations are targeted by volume-outcome initiatives; the Leapfrog Group initiative recommends surgical annual case volume minimums for institutions (20 cases) and surgeons (7-10 cases) to improve outcomes.23 In addition, these surgical procedures require unique intraoperative management that differs from other procedures with specific fluid and blood management strategies and longer operating times; thus, they may be more susceptible to variation that is associated with experience and expertise.16,17,18,24
Therefore, we conducted a population-based cohort study to examine the association between anesthesiologist volume and short-term postoperative outcomes. We hypothesized that higher procedure volume would be associated with lower 90-day major morbidity and readmission.
Methods
Study Design and Data Sources
Linked administrative health care data sets from the province of Ontario, Canada, obtained through ICES (formerly, the Institute of Evaluative Clinical Sciences) were used to conduct a retrospective, population-based cohort study. Data sets held at ICES include information on all publicly funded administrative health services for the population of Ontario since 1986. These data are collected by the Ministry of Health and Long-term Care and are made available for study through ICES. This study was approved by the Research Ethics Board at Sunnybrook Health Sciences Centre, which waived the informed consent requirement because this was a population-based study of previously collected data. We followed the Reporting of Studies Conducted Using Observational Routinely Collected Health Data (RECORD) statement.
Among the data sources was the Ontario Cancer Registry, a provincial database of all patients with a cancer diagnosis that captures greater than 98% of incident cancers.25,26 Additional data were obtained from the Registered Persons Database, the Canadian Institute for Health Information Discharge Abstract Database, the National Ambulatory Care Reporting System, the Cancer Activity Level Reporting data set, and the Ontario Health Insurance Plan Claims Database.27 Data sets were linked using unique encoded identifiers and were analyzed at ICES. Those data sets have been independently validated through a number of audits and validation studies and have been demonstrated to provide valid diagnostic, procedural, and outcome data.28,29,30,31,32 Details of the data sets are provided in eTable 1 in the Supplement.
Study Population and Exposure
The 13.5 million residents of Ontario receive health services through a government-administered single-payer system.33 Specialized cancer services are regionalized in Ontario. Since 2006, hepatopancreaticobiliary cancer surgery has been concentrated to 10 and esophageal cancer surgery to 15 designated centers of excellence, with minimum requirements of institutional annual case volume, surgeon fellowship, and perioperative care infrastructure. However, the organization of operating room and anesthesiology services is not stipulated within provincial requirements.
We identified patients aged 18 years or older with a diagnosis of GI cancer from January 1, 2007, to December 31, 2018, using International Classification of Diseases for Oncology, Third Revision, codes. Patients who underwent elective esophagectomy, pancreatectomy, or hepatectomy were retained. Patients were excluded if they had an invalid identification number, had a duplicate surgery record (primary record could not be identified reliably), and if their primary anesthesiologist could not be identified. If a patient underwent more than 1 procedure of interest, the first procedure was used for analysis.
The exposure of interest was the primary anesthesiologist volume for esophagectomy, pancreatectomy, and hepatectomy. For each index surgery, the primary anesthesiologist volume was defined as the mean annual number of procedures of interest (esophagectomy, pancreatectomy, and hepatectomy) supported by the anesthesiologist in the 2 years before the index surgery. As such, the anesthesiologist volume could change dynamically over time, which is a more rigorous approach for capturing volume than assigning a fixed volume to each anesthesiologist’s mean case load over the study period.34,35
The primary anesthesiologist was identified as the clinician who billed a claim code with suffix-C (which indicates anesthesiology) that corresponded to the surgeon’s claim code on the day of surgery or, if not identified, on the day before or after surgery (to account for administrative data-entry errors). Only 1 anesthesiologist can claim a surgical procedure code with the anesthesiology suffix-C per day. Handover codes used by any additional anesthesiologists were excluded. Patients were excluded if the primary anesthesiologist could not be identified.
Outcomes and Covariates
The primary outcome was a composite of 90-day major morbidity (including mortality) and readmission to any hospital in the province.36 Major morbidity was defined as Clavien-Dindo classification grade 3 to 5 postoperative complications, which included mortality as a grade 5 complication.37 The 90-day time window was chosen because it provides a better representation of the morbidity burden of surgery.38,39,40 Secondary outcomes were the components of the primary outcomes (90-day major morbidity with Clavien-Dindo grade 3 and 4 complications, 90-day readmission, 90-day mortality with Clavien-Dindo grade 5 complications) and unplanned intensive care unit admission (rather than routine postoperative intensive care unit monitoring). Outcome definitions are provided in eTable 2 in the Supplement.
Patients were followed up until the date of death, date of last clinical contact with the health care system, or end of study date on March 31, 2019. This timeline allowed for the opportunity to carry out 90 days of follow-up for all patients.
Baseline characteristics were measured at the time of surgery. Rural residency was ascertained according to the Rurality Index of Ontario.41 Material deprivation quintile, a multidimensional ecological measure that incorporates socioeconomic factors, such as education and income, was used to assess socioeconomic status.42 The comorbidity burden was measured using the Elixhauser Comorbidity Index, with the number of comorbidities (excluding cancer and metastases diagnoses) summed to create a continuous variable.43 Receipt of neoadjuvant therapy was specified as chemotherapy or chemoradiation therapy received within 180 days prior to surgery.44,45 Surgical approach was divided into minimally invasive or open surgery. Complete intraoperative anesthesiologist handover, which has been associated with inferior outcomes, was identified using a unique billing code (E005C) on the day of index surgery.46 The duration of surgery was computed using anesthesia time unit billing claims as previously validated.47,48 Surgeon and institutional volumes were computed similarly to anesthesiologist volume.
Statistical Analysis
We first explored the linear and nonlinear associations between anesthesiologist volume and the primary outcome using linear regression and restricted cubic splines with 4 knots.49 We examined the restricted cubic spline to identify any inflection point that could be used to establish a clinically meaningful dichotomization of anesthesiologist volume into low- and high-volume categories.50
After establishing a volume cutoff point, we divided patients into groups according to whether they had received care from anesthesiologists with a low volume or a high volume of procedures during the index surgery. Groups were compared using standardized mean differences, with a difference of less than 10% considered to be not significant.51,52 We examined the association between anesthesiologist volume and outcomes using the exposure as both a linear and a dichotomous variable while adjusting for potential confounders. We implemented multivariable logistic regression models with the generalized estimating equations approach to account for clustering at the institutional level. The following covariates were identified a priori as potential confounders for inclusion in the models: age, sex, rural residency, material deprivation, comorbidity burden, neoadjuvant therapy, type of surgery, surgical approach, year of surgery, anesthesiologist handover, surgeon annual volume (log-transformed), and institutional annual volume (log-transformed). Collinearity was assessed and defined as a variance inflation factor of 2.5 or higher. Duration of surgery was considered on the causal pathway and therefore was not included in the primary model; however, we conducted a sensitivity analysis that included this covariate in the multivariable model. Results from the regression models were reported as odds ratios (ORs) with 95% CIs.
We stratified the analysis by type of surgery, whereby the volume of esophagectomy was associated with postesophagectomy outcomes, pancreatectomy was associated with postpancreatectomy outcomes, and hepatectomy was associated with posthepatectomy outcomes. We looked at missing data for key variables. Institutional volume data were missing for 0.4%, rural residency data were missing for 0.1%, and material deprivation data were missing for 0.5% of the cohort. A complete case analysis was performed, but patients with missing data were excluded. All analyses were conducted with SAS Enterprise Guide, version 7.1 (SAS Institute Inc).
Sensitivity Analyses and Unmeasured Confounding
A priori sensitivity analyses were performed. First, we varied the cutoff point for low- vs high-volume anesthesiologists when using the exposure as a dichotomous variable. Second, we constructed a 3-level hierarchical model for the association between exposure and primary outcome to assess the proportion of variation in outcome that was associated with each of the following: (1) patient and treatment characteristics, (2) institutional volume, and (3) anesthesiologist volume. Third, we examined the association between anesthesiologist volume and 30-day outcomes.
We assessed the potential association of unmeasured confounding using the E-value method.53,54,55 This E-value method estimates the minimum strength of the association that an unmeasured confounder would need to have with both the exposure and the outcome, while controlling for other confounders, to explain the observed association between exposure and outcome.
Results
A total of 8096 patients were included, among whom 5369 were men (66.3%) and the median (interquartile range [IQR]) age was 65 (57-72) years (Figure 1, Table). Overall, 842 anesthesiologists and 186 surgeons treated the included patients over the study period (January 1, 2007, to December 31, 2018). The median (IQR) anesthesiologist volume was 3 (1.5-6) procedures per year. The median (IQR) surgeon volume was 27 (15-45) procedures per year, and the median (IQR) institutional volume was 373 (127-712) procedures per year.
Figure 1. Flowchart of Cohort Creation.
Table. Characteristics of Included Patients Stratified by Anesthesiologist Volume.
| Characteristic | No. (%) | Standardized mean difference (%)a | ||
|---|---|---|---|---|
| All patients | Low-volume anesthesiologist group (<6 per year) | High-volume anesthesiologist group (≥6 per year) | ||
| Total | 8096 | 5930 (73.3) | 2166 (26.7) | NA |
| Age, median (IQR), y | 65 (57-72) | 65 (57-72) | 64 (56-72) | 5 |
| Male sex | 5369 (66.3) | 4025 (67.9) | 1344 (62.0) | 12 |
| Female sex | 2727 (33.7) | 1905 (32.1) | 822 (38.0) | 12 |
| Rural residency | 1094 (13.5) | 846 (14.3) | 248 (11.4) | 8 |
| High comorbidity burden, Elixhauser Comorbidity Index >4 | 654 (8.1) | 471 (7.9) | 183 (8.4) | 2 |
| Material deprivation | ||||
| 1st, Least deprived | 1708 (21.1) | 1255 (21.2) | 453 (20.9) | 1 |
| 2nd | 1638 (20.2) | 1160 (19.6) | 478 (22.1) | 6 |
| 3rd | 1639 (20.2) | 1225 (20.7) | 414 (19.1) | 4 |
| 4th | 1630 (20.1) | 1175 (19.8) | 455 (21.0) | 3 |
| 5th, Most deprived | 1481 (18.3) | 1115 (18.8) | 366 (16.9) | 5 |
| Surgical procedure | ||||
| Esophagectomy | 2806 (34.7) | 2470 (41.7) | 336 (15.5) | 60 |
| Hepatectomy | 4207 (52.0) | 2693 (45.4) | 1514 (69.9) | 51 |
| Pancreatectomy | 1083 (13.4) | 767 (12.9) | 316 (14.6) | 5 |
| Minimally invasive surgery | 1951 (24.1) | 1396 (23.5) | 555 (25.6) | 5 |
| Neoadjuvant therapy | 2890 (35.7) | 2217 (37.4) | 673 (31.1) | 13 |
| Duration of surgery, median (IQR) h | 5 (4-7) | 6 (4-7) | 5 (4-7) | 14 |
| Intraoperative anesthesiologist handover | 1525 (18.8) | 769 (13.0) | 756 (34.9) | 53 |
| Surgeon volume, median (IQR), procedures per yb | 27 (15-45) | 23 (13-37) | 44 (27-79) | 87 |
| Institutional volume, median (IQR), procedures per yb | 373 (127-712) | 307 (92-499) | 704 (366-839) | 99 |
Abbreviations: IQR, interquartile range; NA, not applicable.
Standardized difference less than 10% was considered not significant.
Volume is presented at the patient level.
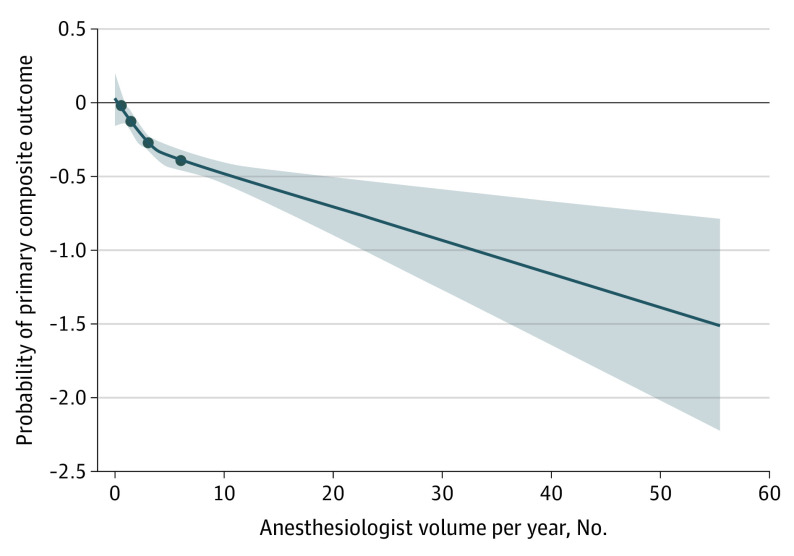
Evidence was found of a nonlinear association between anesthesiologist volume and the primary composite outcome given that both linear and quadratic volume terms were statistically significant. The restricted cubic splines linking anesthesiologist volume and the primary outcome were negatively sloped with an inflection point between the 50th and 75th percentiles of the anesthesiologist volume (Figure 2). The 75th percentile was chosen as the cutoff point (≥6 procedures per year; eTable 3 in the Supplement). The characteristics of patients in the high- and low-volume anesthesiologist groups were compared (Table). A total of 2166 patients (26.7%) received care from high-volume anesthesiologists.
Figure 2. Probability of Primary Composite Outcome After Surgery According to Anesthesiologist Volume Using 4-Knot Restricted Cubic Splines.
The circles indicate the 4 knots of the restricted cubic splines: 5th, 25th, 50th, and 75th percentiles. Shaded areas show 95% CIs.
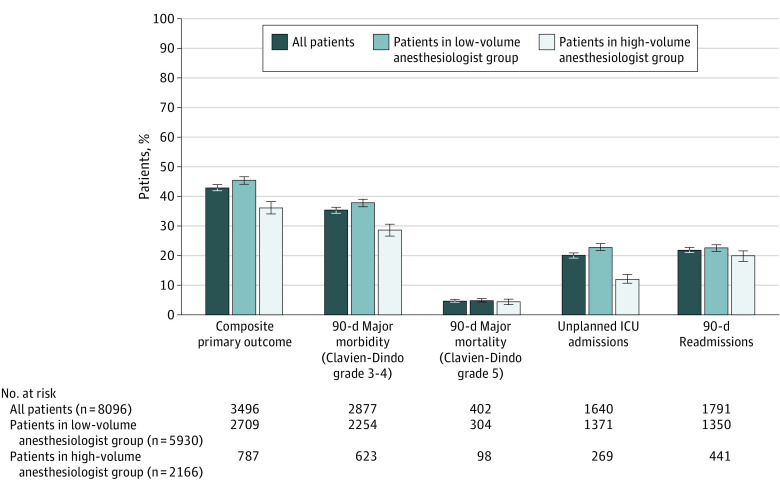
Outcomes stratified by anesthesiologist volume are presented in Figure 3. In bivariable analysis, the unadjusted odds of primary composite outcome were lower in patients in the high-volume anesthesiologist group compared with the low-volume anesthesiologist group (unadjusted OR, 0.81; 95% CI, 0.73-0.90). Primary outcome was observed in 787 of 2166 patients (36.3%) in the high-volume group and in 2709 of 5930 patients in the low-volume group (45.7%) (Figure 3).
Figure 3. Short-term Outcomes Stratified by Anesthesiologist Volume.
Error bars represent 95% CIs. ICU indicates intensive care unit.
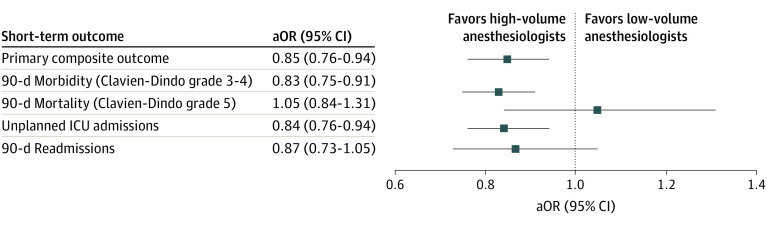
The effect estimates of high- vs low-volume anesthesiologist group for each outcome after adjustments for potential confounders, including surgeon volume and institutional volume, are presented in Figure 4. Care by a high-volume anesthesiologist was independently associated with lower odds of primary composite outcome (adjusted OR [aOR], 0.85; 95% CI, 0.76-0.94). When used as a continuous variable, anesthesiologist volume was also independently associated with the primary composite outcome (aOR, 0.990; 95% CI, 0.980-0.999). For secondary outcomes, care by a high-volume anesthesiologist was independently associated with lower odds of 90-day major morbidity (aOR, 0.83; 95% CI, 0.75-0.91) and unplanned intensive care unit admission (aOR, 0.84; 95% CI, 0.76-0.94) but not mortality, albeit with a smaller number of events (n = 402; aOR, 1.05; 95% CI, 0.84-1.31), or readmissions (aOR, 0.87; 95% CI, 0.73-1.05).
Figure 4. Adjusted Association Between Short-term Outcomes and Anesthesiologist Volume.
Association was adjusted for age, sex, comorbidity burden, rural residency, income quintile, surgical procedure, surgical approach, year of surgery, neoadjuvant therapy, anesthesiologist handover, surgeon annual volume, and institutional annual volume. aOR indicates adjusted odds ratio; ICU, intensive care unit.
Subgroup and Sensitivity Analyses
For each subgroup, cutoff points were chosen at the 75th percentile (eFigure and eTable 3 in the Supplement). Care by a high-volume anesthesiologist was associated with decreased odds of the primary composite outcome for hepatectomy (statistically significant; aOR, 0.83 [95% CI, 0.72-0.97]) and pancreatectomy (not statistically significant; aOR, 0.77 [95% CI, 0.55-1.07]) but not for esophagectomy (aOR, 1.07; 95% CI, 0.91-1.24). The direction of these ORs was in agreement with the interpretation from the spline figures (eFigure in the Supplement).
Results of sensitivity analyses are presented in eTable 4 in the Supplement. Adding operative duration to the multivariable model did not alter the results. The direction of the association remained with alternate cutoff points for high-volume anesthesiology of 4 or more (50th percentile) and 7 or more (80th percentile) procedures per year. The primary model with a cutoff point of 6 or more procedures per year performed the best, with the lowest quasi-likelihood under independence model criterion of 1066.35. Using a hierarchical model, the association was similar for the high-volume anesthesiologist group (OR, 0.83; 95% CI, 0.73-0.95); variation in primary composite outcome was higher for patient and treatment characteristics, including procedure type and surgeon volume (87.5%), compared with institutional characteristics (11.1%) and anesthesiologist volume (1.4%).
The association between outcomes and volume persisted when examining 30-day outcomes, with an OR of 0.81 (95% CI, 0.73-0.88) for care by high-volume vs by low-volume anesthesiologists (eTable 4 in the Supplement).
Unmeasured Confounding
The E-value for the association between anesthesiologist volume and the primary composite outcome was 1.63. This E-value indicated the strength of the unmeasured confounding necessary to invalidate the observed association. An unmeasured confounder would need to be associated with a 1.63-fold or greater increased probability of being in the high-volume anesthesiologist group and a 1.63-fold or greater increased risk of the primary outcome while adjusting for other covariates. None of the covariates presented such a magnitude of association with both the anesthesiologist volume exposure and the primary composite outcome that it was unlikely, although not impossible, that an unmeasured variable would substantively change the study results (eTables 5 and 6 in the Supplement).
Discussion
In this population-based cohort study, we observed better short-term outcomes after esophagectomy, pancreatectomy, and hepatectomy for patients who received care from high-volume anesthesiologists. Care by high-volume anesthesiologists was independently associated with 15% lower odds of combined 90-day major morbidity (including mortality) and readmission, after adjusting for patient case mix, institutional volume, and surgeon volume.
Although the volume-outcome association in cancer surgery has been extensively studied at the surgeon and institutional levels, there are little data regarding other equally important components of care.1,2,3,4,5,6,7,8,10,11,12,13 Evidence has emerged that anesthesiologist expertise and practice choices are associated with postoperative outcomes, but this has often been overlooked.56 Only 1 single-center retrospective cohort study assessed surgeon and anesthesiologist volumes for GI surgery, specifically pancreatectomy.57 Even though much variation in perioperative outcomes appeared to be associated with patient characteristics, that study identified variability in outcomes at the surgeon and anesthesiologist levels.57 Other studies have documented variation in intraoperative management, such as fluid administration, but did not examine the association with outcomes.58,59
The current study is important because it presents a real-world assessment of the association between anesthesiologist volume and perioperative outcomes. All patients were treated at designated centers of excellence with policy-mandated regionalization that met the quality standards set by the Leapfrog Group, which offers standardization of institutional volume, surgeon volume, and institutional resources that contribute to the capacity to rescue patients from complications.23 This structure of care standardizes the care received by patients at the system level as much as possible and minimizes between-institution differences in clinical resources for patients treated by high- and low-volume anesthesiologists, in addition to adjusting analyses for institutional volume. We further adjusted the analyses for surgeon volume and institutional volume to account for potential residual variation in surgical volume despite regionalization. Such a setting and analysis allow for the examination of factors beyond surgeon and institutional volumes and institutional resources that may play a role in outcomes. For instance, many efforts have been made to standardize institutional and surgeon volumes, training, and postoperative recovery pathways1,2,3,4,5,6,60,61; the current work also supports a focus on standardization of anesthesiology care.
Decision-making and physician preferences are recognized factors in unwarranted variation in care and outcomes and should be targeted to improve quality of care.62,63 Unwarranted variation refers to the proportion of variation that cannot be explained by differences in the patient population but by the quality of care.64 Variation in key elements of intraoperative management of complex GI cancer surgery has been documented.58,59 Hierarchical modeling in a sensitivity analysis indicated that patient and institutional characteristics accounted for most of the variation in outcomes. Despite this finding, we identified an independent association between anesthesiologist volume and patient outcomes in a system with standardized institutional and surgical requirements and while accounting for patient and clinical characteristics as well as residual differences in institutional and surgeon volumes.
Volume in itself is not a factor in better outcomes but is often a proxy for other factors such as clinical experience and expertise, processes of care, or team organization.65,66 We adjusted for those factors that could be measured, such as anesthesiologist handover, which was more common in the high-volume group, surgeon volume, and institutional volume. Proposed mechanisms for volume-outcome associations at the physician level include enhanced skills from treating more patients and receipt of more referrals from achieving better outcomes.67 In this study, all anesthesiologists practiced in designated thoracic and hepatopancreaticobiliary surgery centers. Thus, referral bias only pertained to anesthesiology practice through the institutional volume, which was accounted for in the analysis. The volume-outcome association is therefore likely better explained by clinical experience. Identifying which practices and processes of care underpinned the volume-outcome association was outside the scope of this study, but it will be the focus of future work to support changes in care delivery.
Implications of our results included organizing perioperative care to increase anesthesiologist volume. The median anesthesiologist volume was very low despite policy-mandated regionalization of esophagectomy, pancreatectomy, and hepatectomy, which contrasted with high surgeon and institutional volumes. The only other study that examined anesthesiologist volume for pancreatectomy also reported that a small number of surgeons (n = 11) and a large number of anesthesiologists (n = 100) provided care to patients, with a dichotomy between high annual volume for surgeons (18 to 101 procedures per year) and low annual volume for anesthesiologists (1 to 15 procedures per year) within a single high-volume center.57 This finding suggests that redistribution of volume within institutions, such as creating specialized anesthesiology teams, could increase the number of high-volume anesthesiologists. Redistribution of volume may be more challenging in smaller centers in which a smaller pool of anesthesiologists leads to a broader range of case requirements. If the cutoff point of 6 procedures per year cannot be met, any increase in volume could result in improved outcomes, as shown by the linear volume-outcome associations, although the magnitude may vary. In addition, smaller centers could build clinical networks with high-volume anesthesiologists to support knowledge and expertise sharing. Furthermore, identifying optimal behaviors of high-volume anesthesiologists that lead to improved outcomes can be benchmarked through communities of practice at institutions that require low-volume anesthesiologists to perform the same work.
Strengths and Limitations
This study’s strength is its true population-based design. Although the public health system and policy-mandated regionalized care setting may alter the generalizability to some other health systems, it provides an ideal environment to examine the contribution of anesthesiologist volume and in which surgeon and institutional volumes are controlled and access to high-volume care is not compounded by insurance status.
This study also has several limitations. Because of its retrospective design and use of administrative data, the data were not collected specifically to answer the research question, and some information was not available. Consistent staging information was not available to assess the extent of disease; however, we used operating time as a surrogate for the complexity of surgery in a sensitivity analysis, which did not alter the results. The definition of volume was key to this analysis. We had to choose a cutoff point to define high volume; although this threshold may remain arbitrary, we used a rigorous statistical approach to select it. The association between exposure and outcomes persisted when treating the exposure variable as linear, quadratic, and binary, showing a consistent association that was independent of analytical choices. The dichotomous cutoff point of 6 procedures per year per anesthesiologist was dependent on the existing volume in the cohort, which was low. It is possible that a higher cutoff point would be identified in a cohort with a higher volume overall. Even though the absolute cutoff point may differ depending on the health system and practice setting, the observations regarding a high-volume practice concept remain valid. We used a robust measure of volume that was dynamic over the study period rather than assuming a homogeneous distribution of volume for each anesthesiologist. To provide a pragmatic assessment of volume, taking into consideration cross-expertise for complex procedures, we combined the volume for esophagectomy, pancreatectomy, and hepatectomy, which shared similar needs for intraoperative management. Even though confounding bias can never be eliminated, we performed detailed analyses to adjust for measurable potential confounders and assessed whether an unmeasured confounder would likely negate the observed results using the E-value approach.53,54
Conclusions
In this large population-based analysis, we identified an independent association between anesthesiologist volume and short-term outcomes of complex GI procedures. Patients who received care from high-volume anesthesiologists had lower odds of combined 90-day major morbidity and readmission after esophagectomy, pancreatectomy, and hepatectomy, after adjusting for patient case mix, surgeon volume, and institutional volume. We believe that the findings support organizing perioperative care to increase anesthesiologist volume and health care organizations that facilitate care delivery by high-volume anesthesiologists for complex GI cancer surgery.
eTable 1. Data Sources
eTable 2. Definitions and Coding Strategy for Outcomes
eTable 3. Cut-off Points for Anesthesia Provider-Volume by Percentiles, for the Entire Cohort and Stratified by Surgical Procedure
eFigure. Probability of Primary Composite Outcome After Surgery According to Anesthesia Provider-Volume, Stratified by Surgical Procedure for Esophagectomy (A), Pancreatectomy (B), and Hepatectomy (C) (4-Knots Restricted Cubic Spline)
eTable 4. Results of Sensitivity Analyses on Primary Composite Outcome (90-Day Major Morbidity and Re-admission)
eTable 5. Adjusted Association Between Baseline Characteristics and Dichotomous Exposure of Anesthesiology PV (Multivariable Model)
eTable 6. Adjusted Association Between Baseline Characteristics and Primary Composite Outcome (Multivariable Model)
References
- 1.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349(22):2117-2127. doi: 10.1056/NEJMsa035205 [DOI] [PubMed] [Google Scholar]
- 2.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364(22):2128-2137. doi: 10.1056/NEJMsa1010705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilimoria KY, Bentrem DJ, Feinglass JM, et al. Directing surgical quality improvement initiatives: comparison of perioperative mortality and long-term survival for cancer surgery. J Clin Oncol. 2008;26(28):4626-4633. doi: 10.1200/JCO.2007.15.6356 [DOI] [PubMed] [Google Scholar]
- 4.Funk LM, Gawande AA, Semel ME, et al. Esophagectomy outcomes at low-volume hospitals: the association between systems characteristics and mortality. Ann Surg. 2011;253(5):912-917. doi: 10.1097/SLA.0b013e318213862f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnston MJ, Singh P, Pucher PH, et al. Systematic review with meta-analysis of the impact of surgical fellowship training on patient outcomes. Br J Surg. 2015;102(10):1156-1166. doi: 10.1002/bjs.9860 [DOI] [PubMed] [Google Scholar]
- 6.Ely S, Alabaster A, Ashiku SK, Patel A, Velotta JB. Regionalization of thoracic surgery improves short-term cancer esophagectomy outcomes. J Thorac Dis. 2019;11(5):1867-1878. doi: 10.21037/jtd.2019.05.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simunovic M, Urbach D, Major D, et al. Assessing the volume-outcome hypothesis and region-level quality improvement interventions: pancreas cancer surgery in two Canadian Provinces. Ann Surg Oncol. 2010;17(10):2537-2544. doi: 10.1245/s10434-010-1114-0 [DOI] [PubMed] [Google Scholar]
- 8.Gasper WJ, Glidden DV, Jin C, Way LW, Patti MG. Has recognition of the relationship between mortality rates and hospital volume for major cancer surgery in California made a difference?: A follow-up analysis of another decade. Ann Surg. 2009;250(3):472-483. doi: 10.1097/SLA.0b013e3181b47c79 [DOI] [PubMed] [Google Scholar]
- 9.Allgood PC, Bachmann MO. Effects of specialisation on treatment and outcomes in screen-detected breast cancers in Wales: cohort study. Br J Cancer. 2006;94(1):36-42. doi: 10.1038/sj.bjc.6602894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dikken JL, Dassen AE, Lemmens VEP, et al. Effect of hospital volume on postoperative mortality and survival after oesophageal and gastric cancer surgery in the Netherlands between 1989 and 2009. Eur J Cancer. 2012;48(7):1004-1013. doi: 10.1016/j.ejca.2012.02.064 [DOI] [PubMed] [Google Scholar]
- 11.Fischer C, Lingsma H, Klazinga N, et al. Volume-outcome revisited: the effect of hospital and surgeon volumes on multiple outcome measures in oesophago-gastric cancer surgery. PLoS One. 2017;12(10):e0183955. doi: 10.1371/journal.pone.0183955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swan RZ, Niemeyer DJ, Seshadri RM, et al. The impact of regionalization of pancreaticoduodenectomy for pancreatic cancer in North Carolina since 2004. Am Surg. 2014;80(6):561-566. doi: 10.1177/000313481408000619 [DOI] [PubMed] [Google Scholar]
- 13.Institute of Medicine; National Research Council National Cancer Policy Board ; Hewitt M, Petitti D, eds. Interpreting the Volume–Outcome Relationship in the Context of Cancer Care. National Academies Press; 2001. [PubMed] [Google Scholar]
- 14.Wigmore T, Gottumukkala V, Riedel B. Making the case for the subspecialty of onco-anesthesia. Int Anesthesiol Clin. 2016;54(4):19-28. doi: 10.1097/AIA.0000000000000117 [DOI] [PubMed] [Google Scholar]
- 15.Fischer M, Matsuo K, Gonen M, et al. Relationship between intraoperative fluid administration and perioperative outcome after pancreaticoduodenectomy: results of a prospective randomized trial of acute normovolemic hemodilution compared with standard intraoperative management. Ann Surg. 2010;252(6):952-958. doi: 10.1097/SLA.0b013e3181ff36b1 [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann KB, Baar W, Glatz T, et al. Epidural analgesia and avoidance of blood transfusion are associated with reduced mortality in patients with postoperative pulmonary complications following thoracotomic esophagectomy: a retrospective cohort study of 335 patients. BMC Anesthesiol. 2019;19(1):162. doi: 10.1186/s12871-019-0832-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng ESW, Hallet J, Hanna SS, et al. Is central venous pressure still relevant in the contemporary era of liver resection? J Surg Res. 2016;200(1):139-146. doi: 10.1016/j.jss.2015.08.005 [DOI] [PubMed] [Google Scholar]
- 18.Behman R, Hanna S, Coburn N, et al. Impact of fluid resuscitation on major adverse events following pancreaticoduodenectomy. Am J Surg. 2015;210(5):896-903. doi: 10.1016/j.amjsurg.2015.04.020 [DOI] [PubMed] [Google Scholar]
- 19.Zakhaleva J, Tam J, Denoya PI, Bishawi M, Bergamaschi R. The impact of intravenous fluid administration on complication rates in bowel surgery within an enhanced recovery protocol: a randomized controlled trial. Colorectal Dis. 2013;15(7):892-899. doi: 10.1111/codi.12180 [DOI] [PubMed] [Google Scholar]
- 20.Bagante F, Ruzzenente A, Beal EW, et al. Complications after liver surgery: a benchmark analysis. HPB (Oxford). 2019;21(9):1139-1149. doi: 10.1016/j.hpb.2018.12.013 [DOI] [PubMed] [Google Scholar]
- 21.Ceppa EP, Pitt HA, Nakeeb A, et al. Reducing readmissions after pancreatectomy: limiting complications and coordinating the care continuum. J Am Coll Surg. 2015;221(3):708-716. doi: 10.1016/j.jamcollsurg.2015.05.012 [DOI] [PubMed] [Google Scholar]
- 22.Zheng R, Tham EJH, Rios-Diaz AJ, et al. A 10-year ACS-NSQIP analysis of trends in esophagectomy practices. J Surg Res. 2020;256:103-111. doi: 10.1016/j.jss.2020.06.008 [DOI] [PubMed] [Google Scholar]
- 23.Leapfrog Group. Surgical volume . Published March 20, 2018. Accessed July 17, 2020. https://www.leapfroggroup.org/ratings-reports/surgical-volume
- 24.Durkin C, Schisler T, Lohser J. Current trends in anesthesia for esophagectomy. Curr Opin Anaesthesiol. 2017;30(1):30-35. doi: 10.1097/ACO.0000000000000409 [DOI] [PubMed] [Google Scholar]
- 25.Robles SC, Marrett LD, Clarke EA, Risch HA. An application of capture-recapture methods to the estimation of completeness of cancer registration. J Clin Epidemiol. 1988;41(5):495-501. doi: 10.1016/0895-4356(88)90052-2 [DOI] [PubMed] [Google Scholar]
- 26.Clarke EA, Marrett LD, Kreiger N. Cancer registration in Ontario: a computer approach. IARC Sci Publ. 1991;(95):246-257. [PubMed] [Google Scholar]
- 27.Juurlink D, Preyra C, Croxford R, et al. Canadian Institute for Health Information Discharge Abstract Database: A Validation Study. ICES; 2006. [Google Scholar]
- 28.Hux JE, Ivis F, Flintoft V, Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25(3):512-516. doi: 10.2337/diacare.25.3.512 [DOI] [PubMed] [Google Scholar]
- 29.Gershon AS, Wang C, Guan J, Vasilevska-Ristovska J, Cicutto L, To T. Identifying individuals with physician diagnosed COPD in health administrative databases. COPD. 2009;6(5):388-394. doi: 10.1080/15412550903140865 [DOI] [PubMed] [Google Scholar]
- 30.Cochran WG. Some methods for strengthening the common χ2 tests. Biometrics. 1954;10(4):417-451. doi: 10.2307/3001616 [DOI] [Google Scholar]
- 31.Austin PC, Daly PA, Tu JV. A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J. 2002;144(2):290-296. doi: 10.1067/mhj.2002.123839 [DOI] [PubMed] [Google Scholar]
- 32.Altoijry A, Al-Omran M, Lindsay TF, Johnston KW, Melo M, Mamdani M. Validity of vascular trauma codes at major trauma centres. Can J Surg. 2013;56(6):405-408. doi: 10.1503/cjs.013412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Health Canada, Government of Canada . Canada's health care system. Modified September 2019. Accessed March 1, 2020. https://www.canada.ca/en/health-canada/services/health-care-system/reports-publications/health-care-system/canada.html
- 34.Ravi B, Jenkinson R, Austin PC, et al. Relation between surgeon volume and risk of complications after total hip arthroplasty: propensity score matched cohort study. BMJ. 2014;348:g3284. doi: 10.1136/bmj.g3284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bielawska B, Hookey LC, Sutradhar R, et al. Anesthesia assistance in outpatient colonoscopy and risk of aspiration pneumonia, bowel perforation, and splenic injury. Gastroenterology. 2018;154(1):77-85.e3. doi: 10.1053/j.gastro.2017.08.043 [DOI] [PubMed] [Google Scholar]
- 36.Irony TZ. The “utility” in composite outcome measures: measuring what is important to patients. JAMA. 2017;318(18):1820-1821. doi: 10.1001/jama.2017.14001 [DOI] [PubMed] [Google Scholar]
- 37.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.In H, Palis BE, Merkow RP, et al. Doubling of 30-day mortality by 90 days after esophagectomy: a critical measure of outcomes for quality improvement. Ann Surg. 2016;263(2):286-291. doi: 10.1097/SLA.0000000000001215 [DOI] [PubMed] [Google Scholar]
- 39.Swanson RS, Pezzi CM, Mallin K, Loomis AM, Winchester DP. The 90-day mortality after pancreatectomy for cancer is double the 30-day mortality: more than 20,000 resections from the National Cancer Data Base. Ann Surg Oncol. 2014;21(13):4059-4067. doi: 10.1245/s10434-014-4036-4 [DOI] [PubMed] [Google Scholar]
- 40.Mayo SC, Shore AD, Nathan H, et al. Refining the definition of perioperative mortality following hepatectomy using death within 90 days as the standard criterion. HPB (Oxford). 2011;13(7):473-482. doi: 10.1111/j.1477-2574.2011.00326.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kralj B. Measuring “rurality” for purposes of health-care planning: an empirical measure for Ontario. Ont Med Rev. 2000;67:33-52. [Google Scholar]
- 42.Matheson FI, Dunn JR, Smith KLW, Moineddin R, Glazier RH. Development of the Canadian Marginalization Index: a new tool for the study of inequality. Can J Public Health. 2012;103 (8 suppl 2):S12-S16. doi: 10.1007/BF03403823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi: 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 44.Nam RK, Cheung P, Herschorn S, et al. Incidence of complications other than urinary incontinence or erectile dysfunction after radical prostatectomy or radiotherapy for prostate cancer: a population-based cohort study. Lancet Oncol. 2014;15(2):223-231. doi: 10.1016/S1470-2045(13)70606-5 [DOI] [PubMed] [Google Scholar]
- 45.Kagedan DJ, Abraham L, Goyert N, et al. Beyond the dollar: influence of sociodemographic marginalization on surgical resection, adjuvant therapy, and survival in patients with pancreatic cancer. Cancer. 2016;122(20):3175-3182. doi: 10.1002/cncr.30148 [DOI] [PubMed] [Google Scholar]
- 46.Jones PM, Cherry RA, Allen BN, et al. Association between handover of anesthesia care and adverse postoperative outcomes among patients undergoing major surgery. JAMA. 2018;319(2):143-153. doi: 10.1001/jama.2017.20040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dossa F, Simpson AN, Sutradhar R, et al. Sex-based disparities in the hourly earnings of surgeons in the fee-for-service system in Ontario, Canada. JAMA Surg. 2019;154(12):1134-1142. doi: 10.1001/jamasurg.2019.3769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Redelmeier DA, Thiruchelvam D, Daneman N. Introducing a methodology for estimating duration of surgery in health services research. J Clin Epidemiol. 2008;61(9):882-889. doi: 10.1016/j.jclinepi.2007.10.015 [DOI] [PubMed] [Google Scholar]
- 49.Marrie RA, Dawson NV, Garland A. Quantile regression and restricted cubic splines are useful for exploring relationships between continuous variables. J Clin Epidemiol. 2009;62(5):511-517.e1. doi: 10.1016/j.jclinepi.2008.05.015 [DOI] [PubMed] [Google Scholar]
- 50.Kulkarni GS, Laupacis A, Urbach DR, Fleshner NE, Austin PC. Varied definitions of hospital volume did not alter the conclusions of volume-outcome analyses. J Clin Epidemiol. 2009;62(4):400-407. doi: 10.1016/j.jclinepi.2008.07.008 [DOI] [PubMed] [Google Scholar]
- 51.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(6):1228–1234. doi: 10.1080/03610910902859574 [DOI] [Google Scholar]
- 52.Zhang Z, Kim HJ, Lonjon G, Zhu Y; AME Big-Data Clinical Trial Collaborative Group . Balance diagnostics after propensity score matching. Ann Transl Med. 2019;7(1):16. doi: 10.21037/atm.2018.12.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the e-value. Ann Intern Med. 2017;167(4):268-274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 54.Haneuse S, VanderWeele TJ, Arterburn D. Using the e-value to assess the potential effect of unmeasured confounding in observational studies. JAMA. 2019;321(6):602-603. doi: 10.1001/jama.2018.21554 [DOI] [PubMed] [Google Scholar]
- 55.Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Web site and R package for computing e-values. Epidemiology. 2018;29(5):e45-e47. doi: 10.1097/EDE.0000000000000864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glance LG, Neuman M, Martinez EA, Pauker KY, Dutton RP. Performance measurement at a “tipping point.” Anesth Analg. 2011;112(4):958-966. doi: 10.1213/ANE.0b013e31820e778d [DOI] [PubMed] [Google Scholar]
- 57.Gani F, Kim Y, Weiss MJ, et al. Effect of surgeon and anesthesiologist volume on surgical outcomes. J Surg Res. 2016;200(2):427-434. doi: 10.1016/j.jss.2015.08.010 [DOI] [PubMed] [Google Scholar]
- 58.Kim Y, Ejaz A, Gani F, et al. Crystalloid administration among patients undergoing liver surgery: defining patient- and provider-level variation. Surgery. 2016;159(2):389-398. doi: 10.1016/j.surg.2015.06.037 [DOI] [PubMed] [Google Scholar]
- 59.Lilot M, Ehrenfeld JM, Lee C, Harrington B, Cannesson M, Rinehart J. Variability in practice and factors predictive of total crystalloid administration during abdominal surgery: retrospective two-centre analysis. Br J Anaesth. 2015;114(5):767-776. doi: 10.1093/bja/aeu452 [DOI] [PubMed] [Google Scholar]
- 60.Coolsen MME, Wong-Lun-Hing EM, van Dam RM, et al. A systematic review of outcomes in patients undergoing liver surgery in an enhanced recovery after surgery pathways. HPB (Oxford). 2013;15(4):245-251. doi: 10.1111/j.1477-2574.2012.00572.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kennedy EP, Rosato EL, Sauter PK, et al. Initiation of a critical pathway for pancreaticoduodenectomy at an academic institution—the first step in multidisciplinary team building. J Am Coll Surg. 2007;204(5):917-923. doi: 10.1016/j.jamcollsurg.2007.01.057 [DOI] [PubMed] [Google Scholar]
- 62.Mercuri M, Gafni A. Medical practice variations: what the literature tells us (or does not) about what are warranted and unwarranted variations. J Eval Clin Pract. 2011;17(4):671-677. doi: 10.1111/j.1365-2753.2011.01689.x [DOI] [PubMed] [Google Scholar]
- 63.Sepucha K, Ozanne E, Mulley AG Jr. Doing the right thing: systems support for decision quality in cancer care. Ann Behav Med. 2006;32(3):172-178. doi: 10.1207/s15324796abm3203_2 [DOI] [PubMed] [Google Scholar]
- 64.Goodman DC. Unwarranted variation in pediatric medical care. Pediatr Clin North Am. 2009;56(4):745-755. doi: 10.1016/j.pcl.2009.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Halm EA, Anderson LD Jr, Gerber DE. Understanding the relationship between care volume and clinical outcomes in multiple myeloma. J Clin Oncol. 2017;35(6):580-582. doi: 10.1200/JCO.2016.70.4726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thiemann DR, Coresh J, Oetgen WJ, Powe NR. The association between hospital volume and survival after acute myocardial infarction in elderly patients. N Engl J Med. 1999;340(21):1640-1648. doi: 10.1056/NEJM199905273402106 [DOI] [PubMed] [Google Scholar]
- 67.Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137(6):511-520. doi: 10.7326/0003-4819-137-6-200209170-00012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Data Sources
eTable 2. Definitions and Coding Strategy for Outcomes
eTable 3. Cut-off Points for Anesthesia Provider-Volume by Percentiles, for the Entire Cohort and Stratified by Surgical Procedure
eFigure. Probability of Primary Composite Outcome After Surgery According to Anesthesia Provider-Volume, Stratified by Surgical Procedure for Esophagectomy (A), Pancreatectomy (B), and Hepatectomy (C) (4-Knots Restricted Cubic Spline)
eTable 4. Results of Sensitivity Analyses on Primary Composite Outcome (90-Day Major Morbidity and Re-admission)
eTable 5. Adjusted Association Between Baseline Characteristics and Dichotomous Exposure of Anesthesiology PV (Multivariable Model)
eTable 6. Adjusted Association Between Baseline Characteristics and Primary Composite Outcome (Multivariable Model)