Key Points
Question
What are the clinical outcomes after hospitalization for COVID-19?
Findings
Four months after hospitalization, in an uncontrolled cohort study of 478 survivors of COVID-19, at least 1 new-onset symptom was reported by telephone interview by 244 patients (51%), including fatigue in 134 of 431 (31%), cognitive symptoms in 86 of 416 (21%), and dyspnea in 78 of 478 (16%). Computed tomographic lung scan abnormalities were reported in 63% of 171 patients assessed at an ambulatory visit, mainly subtle ground-glass opacities. Fibrotic lesions were observed in 19% of these 171 patients.
Meaning
This study provides clinical status of a cohort of patients 4 months after hospitalization for COVID-19, but further research is needed to understand longer-term outcomes.
Abstract
Importance
Little is known about long-term sequelae of COVID-19.
Objective
To describe the consequences at 4 months in patients hospitalized for COVID-19.
Design, Setting, and Participants
In a prospective uncontrolled cohort study, survivors of COVID-19 who had been hospitalized in a university hospital in France between March 1 and May 29, 2020, underwent a telephone assessment 4 months after discharge, between July 15 and September 18, 2020. Patients with relevant symptoms and all patients hospitalized in an intensive care unit (ICU) were invited for further assessment at an ambulatory care visit.
Exposures
Survival of hospitalization for COVID-19.
Main Outcomes and Measures
Respiratory, cognitive, and functional symptoms were assessed by telephone with the Q3PC cognitive screening questionnaire and a checklist of symptoms. At the ambulatory care visit, patients underwent pulmonary function tests, lung computed tomographic scan, psychometric and cognitive tests (including the 36-Item Short-Form Health Survey and 20-item Multidimensional Fatigue Inventory), and, for patients who had been hospitalized in the ICU or reported ongoing symptoms, echocardiography.
Results
Among 834 eligible patients, 478 were evaluated by telephone (mean age, 61 years [SD, 16 years]; 201 men, 277 women). During the telephone interview, 244 patients (51%) declared at least 1 symptom that did not exist before COVID-19: fatigue in 31%, cognitive symptoms in 21%, and new-onset dyspnea in 16%. There was further evaluation in 177 patients (37%), including 97 of 142 former ICU patients. The median 20-item Multidimensional Fatigue Inventory score (n = 130) was 4.5 (interquartile range, 3.0-5.0) for reduced motivation and 3.7 (interquartile range, 3.0-4.5) for mental fatigue (possible range, 1 [best] to 5 [worst]). The median 36-Item Short-Form Health Survey score (n = 145) was 25 (interquartile range, 25.0-75.0) for the subscale “role limited owing to physical problems” (possible range, 0 [best] to 100 [worst]). Computed tomographic lung-scan abnormalities were found in 108 of 171 patients (63%), mainly subtle ground-glass opacities. Fibrotic lesions were observed in 33 of 171 patients (19%), involving less than 25% of parenchyma in all but 1 patient. Fibrotic lesions were observed in 19 of 49 survivors (39%) with acute respiratory distress syndrome. Among 94 former ICU patients, anxiety, depression, and posttraumatic symptoms were observed in 23%, 18%, and 7%, respectively. The left ventricular ejection fraction was less than 50% in 8 of 83 ICU patients (10%). New-onset chronic kidney disease was observed in 2 ICU patients. Serology was positive in 172 of 177 outpatients (97%).
Conclusions and Relevance
Four months after hospitalization for COVID-19, a cohort of patients frequently reported symptoms not previously present, and lung-scan abnormalities were common among those who were tested. These findings are limited by the absence of a control group and of pre-COVID assessments in this cohort. Further research is needed to understand longer-term outcomes and whether these findings reflect associations with the disease.
This French cohort study describes coronavirus disease sequelae at 4 months in patients hospitalized for COVID-19 between March and May 2020, including assessment of respiratory, cognitive, and functional symptoms and test and imaging findings.
Introduction
The effects of COVID-19 are highly variable, ranging from individuals who are asymptomatic to patients who develop severe acute respiratory distress syndrome, with potential involvement of almost all organs and systems.1 These acute symptoms have been well described since the first cohort studies that were published at the beginning of the pandemic.2
However, the possible long-term sequelae of COVID-19 have become an increasing concern. The inflammatory storm that characterizes severe forms of the disease suggests that serious tissue sequelae may affect various organ systems. Other coronaviruses have been shown to induce long-term effects, especially in the lungs.3
Although the long-term sequelae of individual organ damage have been reported,3,4,5,6,7,8,9 there have been few comprehensive evaluations of long-term consequences of COVID-19.10,11,12 In addition, most of the studies have included patients who actively decided to participate in follow-up.10,12 The aim of the present study was to systematically assess, 4 months after discharge, the clinical status of survivors of COVID-19 disease requiring hospitalization.
Methods
Population
Patients provided written informed consent. The Ethics Committee of the French Intensive Care Society (CE20-56) approved the study. The COMEBAC (Consultation Multi-Expertise de Bicêtre Après COVID-19) uncontrolled cohort study included adult patients admitted to the Bicêtre Hospital (Paris-Saclay University hospitals) in France for COVID-19 from March 1 to May 29, 2020. Inclusion criteria were survival 4 months after hospital discharge or after intensive care unit (ICU) discharge for patients who had been admitted to an ICU (referred to as “ICU patients” hereafter), who were older than 18 years, who had been hospitalized for greater than 24 hours primarily because of COVID-19, and who had received a diagnosis of SARS-CoV-2 infection by reverse transcriptase–polymerase chain reaction (RT-PCR) , by typical computed tomographic (CT) lung scan associated with clinical features, or both.
Exclusion criteria were death within 4 months after discharge, persistent hospitalization, end-stage cancer, dementia, nosocomial COVID-19 infection, and incidental positive SARS-CoV-2 RT-PCR result during a hospital stay for a different medical indication. Medical charts of patients were reviewed to establish that patients met the eligibility criteria.
Description of the Study Hospital
The Bicêtre Hospital is one of the university hospitals in the Paris region (Assistance Publique-Hôpitaux de Paris). It has 907 beds, including 53 in adult ICUs, 30 in adult intermediate severity units, 8 in a pediatric ICU, and 12 in a pediatric intermediate-severity unit. In 2019, 18 678 adult patients were admitted to the hospital. At the peak of the first wave of COVID-19, from March to May 2020, the total number of ICU beds with mechanical ventilation capacity was increased to 101 from 53, with adult patients undergoing invasive ventilation in the adult and pediatric ICUs and intermediate-severity units, the operating rooms, and the recovery room. High-flow nasal canula and noninvasive and invasive mechanical ventilation were used only in these areas.
Initial Hospitalization
Patient medical history, illness evolution, and treatment during hospitalization were retrospectively collected from medical records. Kidney failure was diagnosed according to the KDIGO criteria.13
Telephone Assessment
Three to 4 months after hospital or ICU discharge, patients were contacted by telephone by a medical officer and administered a questionnaire that included general condition and respiratory, cognitive, and neurologic symptoms (with the Q3PC cognitive screening questionnaire).14 The questionnaire and the list of symptoms in question are shown in the Supplement. Patients were asked whether symptoms existed before they developed COVID-19. All symptoms were listed, without any interpretation. No psychological evaluation was performed.
In addition, patients with no history of chronic kidney disease and with high plasma creatinine levels (>1.47 mg/dL [130 μmol/L]) or estimated glomerular filtration rate less than 60 mL/min/1.73 m2 at hospital discharge were requested to have their serum creatinine levels reassessed. Patients were asked whether a lung CT scan had been performed after hospitalization, and if so, the lung CT scan was reviewed.
All ICU patients and those who were symptomatic were invited for further evaluation in the ambulatory setting. Symptomatic patients were defined as those reporting symptoms at the telephone interview (except for anosmia), all patients who had persistent creatinine-level elevation, and all those who had persistent abnormalities on a lung CT scan conducted after hospitalization (including any residual ground-glass opacities, bronchial or bronchioloalveolar abnormalities, lung condensations, or interstitial thickening).
Ambulatory Setting
The following assessments were performed in the outpatient facility between July 15 and September 18, 2020.
General Assessment
In addition to receiving a general clinical examination and a having a blood sample taken, patients were evaluated for their quality of life (36-Item Short-Form Health Survey questionnaire)15 and fatigue (Multidimensional Fatigue Inventory scale)16 (eTable 1 in the Supplement). The 36-Item Short-Form Health Survey questionnaire evaluates 8 domains: physical functioning, role limitations owing to physical health, role limitations owing to emotional problems, energy/fatigue, emotional well-being, social functioning, pain, and general health. Each subscale ranges from 0 to 100, corresponding to the worst and best health state, respectively. The 20-item Multidimensional Fatigue Inventory questionnaire evaluates 4 domains: general fatigue, reduced activity, motivation, and mental fatigue. Each subscale ranges from 1 to 5, corresponding to the best and worst feeling owing to fatigue for each domain, respectively.16
Respiratory Assessment
In addition to its evaluation at the telephone interview, dyspnea was assessed by the modified Medical Research Council scale. A 6-minute walk test was performed according to current recommendations.17 Patients completed pulmonary function tests (see the Supplement). Dysfunctional breathing was assessed with the Nijmegen questionnaire18 (eTable 1 in the Supplement) and a hyperventilation provocation test. Medical records of patients reporting new-onset or increased dyspnea compared with before COVID-19 infection were analyzed by 2 pulmonologists to determine its cause, who reached consensus in case of disagreement.
Lung CT Scan
A high-resolution lung CT scan was performed for all patients (Supplement). Two readers, blinded to clinical evaluation, reviewed the CT scans, reaching consensus regarding any disagreements.
Cardiac Assessment
All ICU patients, those with pulmonary embolism during hospitalization, and those with cardiac symptoms on examination at the outpatient clinic were evaluated with transthoracic echocardiography.
Psychological and Cognitive Assessment
All patients underwent psychometric tests, an interview with a neuropsychologist, and an interview with a psychologist. Global cognitive assessment was performed with the Montreal Cognitive Assessment19 adapted to age and education level20 (eTable 1 in the Supplement). Attention was assessed through the d2-R test.21 Memory complaint was assessed through the McNair self-questionnaire.21 “Cognitive complaint” was defined by an impaired McNair score, reported cognitive symptoms, or both. “Cognitive impairment” was defined by an impaired Montreal Cognitive Assessment or d2-R score.
Anxiety symptoms were evaluated with the anxiety subscale of the Hospital Anxiety and Depression Scale,22 depression symptoms with the 13-item Beck Depression Inventory score,23 insomnia with the Insomnia Severity Index, and posttraumatic symptoms with the Posttraumatic Stress Disorder Checklist (PCL-5 scale)24 (eTable 1 in the Supplement).
Serologic Tests
In all outpatients, anti–SARS-CoV-2 total immunoglobulin level (Elecsys; Roche Diagnostic) and IgM and IgG levels (NG-Biotech) were assessed.25
Statistical Analysis
Study data were collected and managed with Research Electronic Data Capture tools hosted at Assistance Publique-Hôpitaux de Paris.26,27 Data are presented as counts and percentages, means (SD), and medians (interquartile range [IQR]). All participants for whom the variables of interest were available were included in the final analysis and no assumptions were made for missing data. Because of the importance of understanding the effect of intubation on patient outcomes, the outcomes have been categorized as nonintubated vs intubated. Analysis was performed with the R statistical package version 4.0.0 (R Foundation for Statistical Computing).
Results
Patients
Among the 1151 adult patients hospitalized in the Bicêtre Hospital because of COVID-19 from March 1 to May 29, 2020, 834 were eligible for telephone assessment and 478 (57%) consented, including 142 of 172 ICU patients and 336 of 662 non-ICU patients (Figure 1). The characteristics of the 673 patients who did not participate in the study are shown in eTable 2 in the Supplement. The patients who did not consent were similar to those who did.
Figure 1. Flow of Patient Screening and Enrollment.
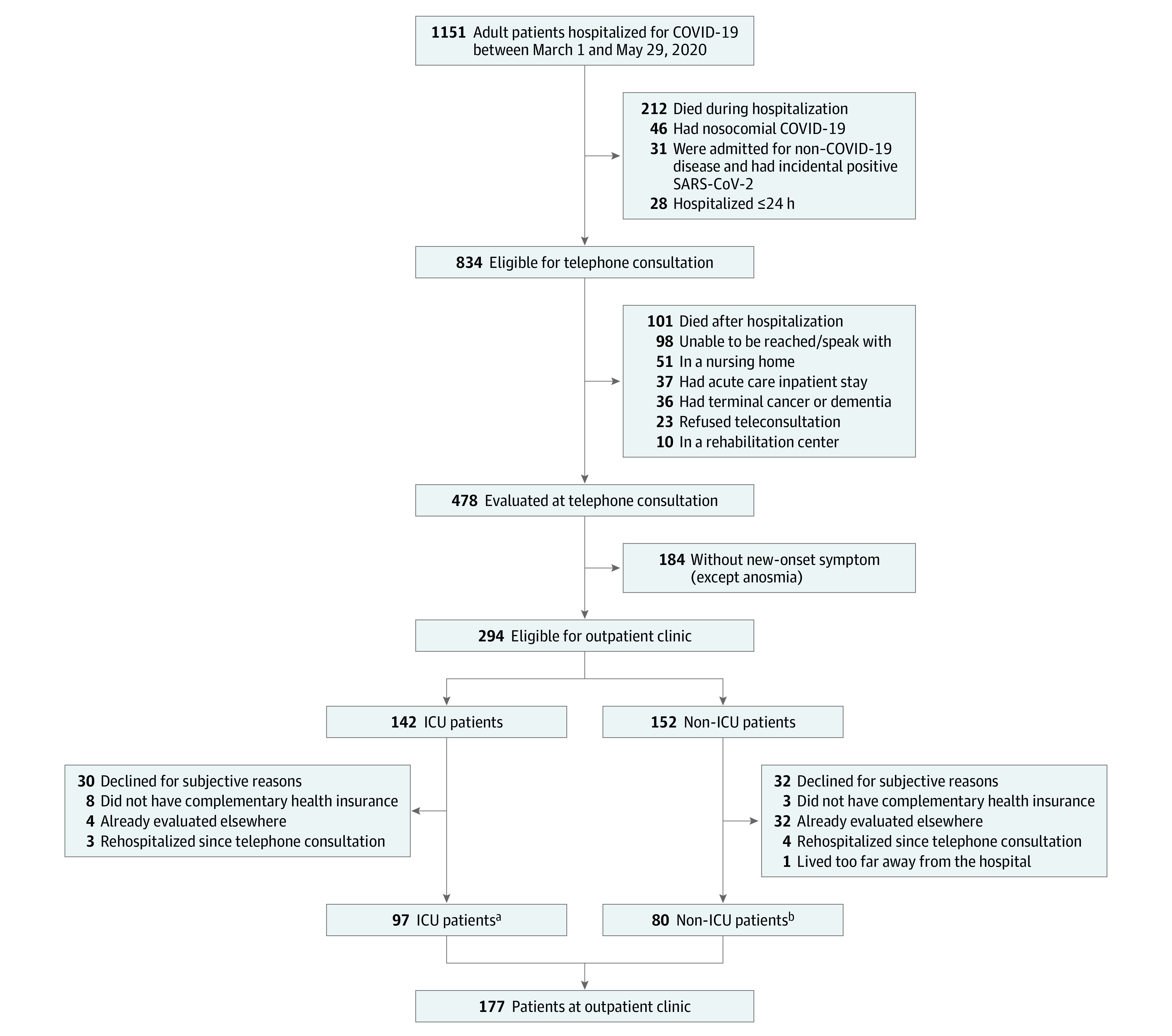
aIncluding 51 of 97 patients (53%) with invasive mechanical ventilation, 51 (53%) receiving vasopressors, and 8 (8%) with extracorporeal membrane oxygenation.
bIncluding 44 of 80 patients (55%) with persistent neurologic symptoms, 27 (34%) with persistent respiratory symptoms, 5 (6%) with abnormal computed tomographic scan results, and 2 (3%) with persistent kidney failure.
COVID-19 had been diagnosed with RT-PCR in 415 patients (86.8%) and by an association of typical clinical signs and CT-scan lung images in 63 patients (13.2%). Of 294 patients who were eligible for an ambulatory assessment (n = 135 ICU patients and 159 non-ICU patients), 177 consented (n = 97 ICU patients and 80 non-ICU patients). The median time to telephone assessment was 113 days after hospital discharge (IQR, 94-128 days) and the median time of the ambulatory assessment was 125 days after discharge (IQR, 107-144 days). The mean age of the 478 patients was 60.9 years (SD, 16 years) and 57.9% were men. The patients who attended the ambulatory visit were younger (mean age, 56.9 years) and generally had more comorbid conditions. The 2 most common treatments for all patients were azithromycin and tocilizumab (Table 1). Of the 142 patients in the ICU, 73 had been intubated (Table 1). Comparisons of the characteristics of patients who attended vs did not attend the ambulatory facility, of those who visited the outpatient facility for neurocognitive vs respiratory symptoms, and by younger and older than 75 years are shown in eTables 3, 4, and 5 in the Supplement, respectively.
Table 1. Baseline and Hospitalization Characteristics of the Patients With Telephone Assessment.
| No./total (%) | |||
|---|---|---|---|
| All patients (n = 478) | No ambulatory visit (n = 301) | Attended the ambulatory visit (n = 177) | |
| Age, mean (SD), y | 60.9 (16.1) | 63.4 (17.2) | 56.9 (13.2) |
| Women | 201 (42.1) | 133 (44.2) | 68 (38.4) |
| Men | 277 (57.9) | 168 (55.8) | 109 (61.6) |
| Body mass index, mean (SD) [No.] | 28.8 (5.6) [351] | 28.5 (5.9) [186] | 29.1 (5.4) [165] |
| Comorbidities | |||
| Hypertension | 225 (47.1) | 150 (49.8) | 75 (42.4) |
| Obesity | 130/351 (37.0) | 63/186 (33.9) | 67/165 (40.6) |
| Diabetes | 128 (26.8) | 76 (25.2) | 52 (29.4) |
| Chronic heart disease | 77 (16.1) | 63 (20.9) | 14 (7.9) |
| Respiratory disease (other than COPD) | 75 (15.7) | 45 (15.0) | 30 (16.9) |
| Chronic kidney disease | 51 (10.7) | 34 (11.3) | 17 (9.6) |
| Declared psychiatric disorder | 42 (8.8) | 30 (10.0) | 12 (6.8) |
| Neurodegenerative disorder | 34 (7.1) | 32 (10.6) | 2 (1.1) |
| Alcohol misuse | 21/450 (4.7) | 12/282 (4.3) | 9/168 (5.4) |
| Active cancer | 18 (3.8) | 15 (5.0) | 3 (1.7) |
| Other immunosuppression | 18 (3.8) | 11 (3.7) | 7 (4.0) |
| COPD | 17 (3.6) | 12 (4.0) | 5 (2.8) |
| Long-term dialysis | 17 (3.6) | 11 (3.7) | 6 (3.4) |
| HIV infection | 12 (2.5) | 9 (3.0) | 3 (1.7) |
| Solid organ transplantation | 9 (1.9) | 5 (1.7) | 4 (2.3) |
| Liver disease | 7 (1.5) | 2 (0.7) | 5 (2.8) |
| Pregnancy | 5 (1.0) | 3 (1.0) | 2 (1.1) |
| Bone marrow transplantation | 2 (0.4) | 2 (0.7) | 0 |
| Smoking | |||
| No (<5 pack-years) | 343/452 (75.9) | 214/283 (75.6) | 129/169 (76.3) |
| Former (≥5 pack-years) | 83/452 (18.4) | 58/283 (20.5) | 25/169 (14.8) |
| Active | 26/452 (5.8) | 11/283 (3.9) | 15/169 (8.9) |
| Specific treatments | |||
| Azithromycin | 120 (25.1) | 67 (22.3) | 53 (29.9) |
| Tocilizumab (anti–IL-6) | 37 (7.7) | 10 (3.3) | 27 (15.3) |
| Hydroxychloroquine | 32 (6.7) | 14 (4.7) | 18 (10.2) |
| Corticosteroids | 24 (5.0) | 17 (5.6) | 7 (4.0) |
| Lopinavir/ritonavir | 16 (3.3) | 8 (2.7) | 8 (4.5) |
| Anakinra (anti–IL-1RA) | 11 (2.3) | 3 (1.0) | 8 (4.5) |
| Remdesivir | 5 (1.0) | 2 (0.7) | 3 (1.7) |
| Vasopressors | 74 (15.5) | 23 (7.6) | 51 (28.8) |
| Active anticoagulation (at full therapeutic dose) | 75 (15.7) | 26 (8.6) | 49 (27.7) |
| Pulmonary embolism during hospitalization | 41 (8.6) | 12 (4.0) | 29 (16.4) |
| Acute kidney injury during hospitalization | 95 (19.9) | 53 (17.6) | 42 (23.7) |
| Hospitalization in ICU | 142 (29.7) | 45 (15.0) | 97 (54.8) |
| Duration of ICU stay, median (IQR), d | 9 (4-19) | 6.5 (4-15) | 9 (4-22) |
| Total duration of hospitalization, median (IQR), d | 9 (4-15) | 8 (4-12) | 13 (6-23) |
Abbreviations: COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; IQR, interquartile range.
Telephone Assessment
Of the 478 patients, 244 (51%) reported at least 1 symptom that did not exist before COVID-19 infection (eFigure 1 in the Supplement; Table 2), including fatigue, 31.1% (134 of 431); memory difficulties, 17.5% (73 of 416); dyspnea, 16.3% (78 of 478); and persistent paresthesia, 12.1% (51 of 421).
Table 2. Results of the Telephone Assessment in Nonintubated and Intubated Patients.
| No./total (%) | |||
|---|---|---|---|
| All patients (n = 478) | Nonintubated (n = 405) | Intubated (n = 73) | |
| Time from hospital discharge to telephone assessment, median (IQR), d [No.] | 113 (94-128) [442] | 121 (104-131) [375] | 93 (77-110) [67] |
| Declared symptomsa | |||
| Dyspnea | 78 (16.3) | 53 (13.1) | 25 (34.2) |
| Cough | 21/420 (5) | 16/358 (4.5) | 5/62 (8.1) |
| Chest discomfort/pain | 34/418 (8.1) | 25/356 (7) | 9/62 (14.5) |
| Fatigue | 134/431 (31.1) | 110/368 (29.9) | 24/63 (38.1) |
| Anorexia | 34/436 (7.8) | 25/370 (6.7) | 9/66 (13.6) |
| Weight loss >5% baseline weight | 31/342 (9.1) | 30/281 (10.7) | 1/61 (1.6) |
| Anosmia | 25/419 (6.0) | 19/357 (5.3) | 6/62 (9.7) |
| Headaches | 23/420 (5.5) | 22/358 (6.2) | 1/62 (1.6) |
| Paresthesia | 51/421 (12.1) | 40/359 (11.1) | 11/62 (17.7) |
| Cognitive testing (Q3PC questionnaire)a,b | |||
| Memory difficulties | 73/416 (17.5) | 63/354 (17.8) | 10/62 (16.1) |
| Mental slowness | 42/415 (10.1) | 38/353 (10.8) | 4/62 (6.5) |
| Concentration problems | 41/412 (10.0) | 35/351 (10.0) | 6/61 (9.8) |
Abbreviation: IQR, interquartile range.
Signs were declared as new onset during or after hospitalization for COVID-19 and persistent at the telephone assessment.
The range, direction, and characteristics of the Q3PC cognitive screening questionnaire are shown in eTable 1 in the Supplement.
Eighty-six of 416 patients (20.7%) reported at least 1 cognitive symptom. Memory difficulties, mental slowness, and concentration problems more than once a week were reported by 73 of 416 patients (17.5%), 42 of 415 (10.1%), and 41 of 412 (10%), respectively (Table 2; eFigure 1 in the Supplement).
The results of the telephone assessment according to age and to the reported symptoms are shown in eTables 5 and 6 in the Supplement, respectively.
Ambulatory Assessment
General Condition
The results of the ambulatory assessment according to the symptoms that triggered the visit to this facility are provided in eTable 7 in the Supplement. The median 36-Item Short-Form Health Survey score, evaluated in 130 patients, was 25 (IQR, 25.0-75.0) for the subscale “role limited owing to physical problems,” 46.9 (IQR, 31.2-68.8) for “vitality,” and 57.5 (IQR, 40.0-75.0) for “general health” (potential best score, 100; worst score, 0). The median score for the 20-item Multidimensional Fatigue Inventory questionnaire, evaluated in 145 patients, was 4.5 (IQR, 3.0-5.0) for reduced motivation and 3.7 (IQR, 3.0-4.5) for mental fatigue (potential best score, 1; worst score, 5) (eFigure 2 in the Supplement).
Psychological and Neurologic Assessment
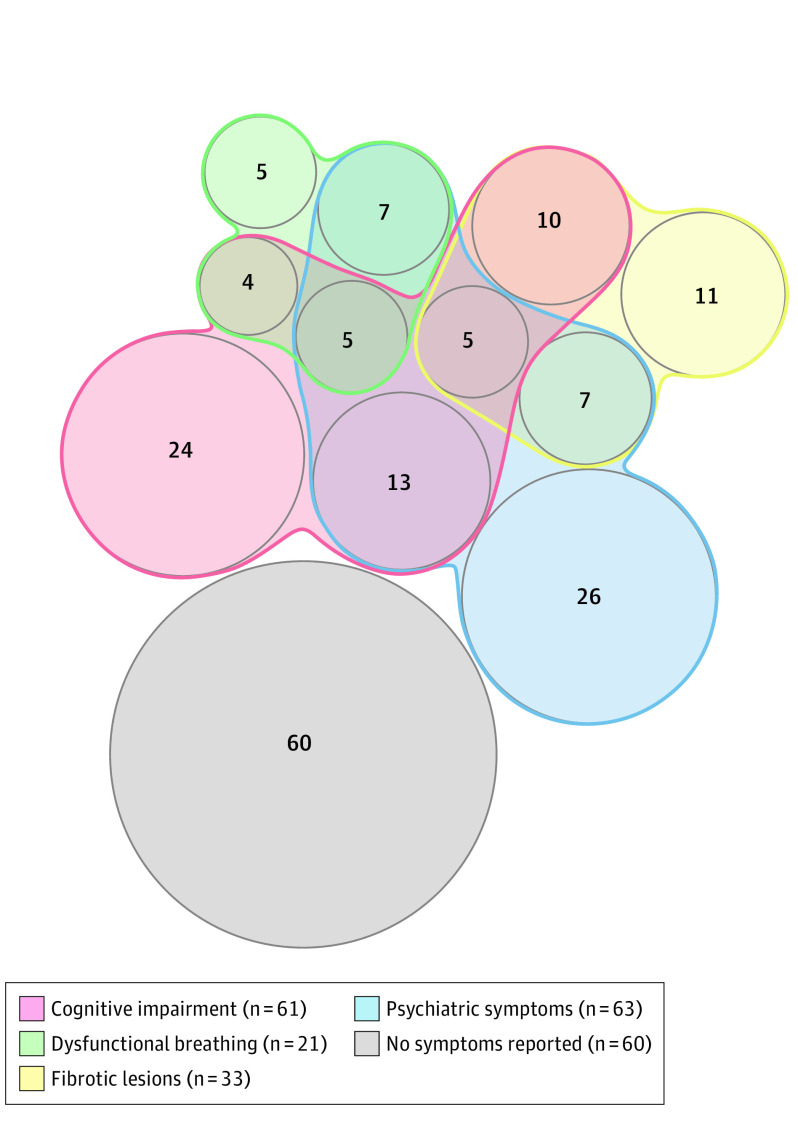
Cognitive impairment was confirmed in 38.4% of patients (61/159) (Table 3, Figure 2), more commonly in patients aged 75 years or older (eTable 5 in the Supplement). In ICU patients, anxiety, depression, and significant posttraumatic symptoms were observed in 22 of 94 (23.4%), 17 of 94 (18.1%), and 7 of 94 (7.4%), respectively.
Table 3. Results of the In-Person Outpatient Clinic Visit in Nonintubated and Intubated Patients.
| No./total (%) | |||
|---|---|---|---|
| All patients (n = 177) | Nonintubated (n = 126) | Intubated (n = 51) | |
| Time from hospital discharge to outpatient clinic, median (IQR), d [No.] | 125 (107-144) [157] | 134 (116-150) [107] | 105 (90.2-119) [50] |
| Respiratory assessment | |||
| mMRC scale score for dyspnea, median (IQR) [No.]a | 1 (1-2) [115] | 1 (1-2) [80] | 1 (0.5-2) [35] |
| Persistent cough | 23/172 (13.4) | 19/123 (15.4) | 4/49 (8.2) |
| 6-Minute walk test, median (IQR), m [No.] | 462 (380-507) [161] | 464 (382-502) [112] | 462 (380-523) [49] |
| Abnormal lung CT scan result | 108/171 (63.2) | 71/122 (58.2) | 37/49 (75.5) |
| Persistent ground-glass opacities | 72/170 (42.4) | 45/121 (37.2) | 27/49 (55.1) |
| Lung fibrotic lesions | 33/170 (19.4) | 15/121 (12.4) | 18/49 (36.7) |
| FEV1 (expressed as % of theory), median (IQR) [No.] | 92 (80-102) [157] | 92 (79-103) [108] | 90 (80-102) [49] |
| FEV1/FVC, median (IQR) [No.] | 83 (79-87) [157] | 81 (78-86) [108] | 84 (82-87) [49] |
| TLC (expressed as % of theory) [No.] | 83 (15) [149] | 86 (15) [104] | 76 (14) [45] |
| DLCO <70% | 33/152 (21.7) | 16/105 (15.2) | 17/47 (36.2) |
| Echocardiography assessment | |||
| RV dilatation on echocardiography | 20/79 (25.3) | 11/35 (31.4) | 9/44 (20.5) |
| LVEF 40%-50% on echocardiographyb | 10/83 (12.0) | 2/38 (5.3) | 8/45 (17.8) |
| Neurologic and psychological assessmenta,c | |||
| Cognitive complaint (impaired McNair score, reported cognitive symptoms, or both) | 79/159 (49.7) | 55/109 (50.5) | 24/50 (48.0) |
| Cognitive impairment (impairment of either MoCA or d2-R score) | 61/159 (38.4) | 40/109 (36.7) | 21/50 (42.0) |
| Symptoms of anxiety (HADS-Anxiety) | 53/169 (31.4) | 40/119 (33.6) | 13/50 (26.0) |
| Symptoms of depression (BDI test) | 35/170 (20.6) | 26/120 (21.7) | 9/50 (18.0) |
| Insomnia (ISI score) | 90/168 (53.6) | 68/118 (57.6) | 22/50 (44.0) |
| Symptoms of PTSD (PCL-5 score) | 24/169 (14.2) | 19/119 (16.0) | 5/50 (10.0) |
Abbreviations: BDI, Beck Depression Inventory; CT, computed tomography; DLCO, diffusing capacity of the lungs for carbon monoxide; FEV1, forced expiratory volume in the first second of expiration; FVC, forced vital capacity; HADS-Anxiety, anxiety subscale of the Hospital Anxiety and Depression Scale; IQR, interquartile range; ISI, Insomnia Severity Index; LVEF, left ventricular ejection fraction; mMRC, modified Medical Research Council; MoCA, Montreal Cognitive Assessment; PTSD, posttraumatic stress disorder; RV, right ventricular; TLC, total lung capacity.
Signs were declared as new onset during or after hospitalization for COVID-19 and persistent at the assessment at the outpatient facility.
No patient had an LVEF less than 40%.
The range, direction, and characteristics of the McNair, MoCA, and d2-R scores; of the anxiety subscale score of the HADS; of the BDI test results; and of the ISI and PCL-5 scores are shown in eTable 1 in the Supplement.
Figure 2. Visualization of Symptoms and Findings That Did Not Exist Before COVID-19 Infection in 177 Patients at the Outpatient Clinic, 4 Months After COVID-19 Hospitalization.
Numbers represent patients with the symptoms/findings or association of symptoms; 60 patients did not report these symptoms or have these findings. Patients could have more than 1; hence, the sum of the groups exceeds 177.
Muscular weakness compatible with ICU-related neuromyopathy was identified in 14 of 51 patients (27.5%) who had been intubated.
Lung CT Scan
Lung CT scan was performed for 171 patients and showed abnormalities in 37 of 49 intubated individuals (75.5%) and 71 of 122 nonintubated ones (58.2%). Abnormalities were most commonly ground-glass opacities (Table 3). Fibrotic lesions were observed in 19.3% of patients (33/171), with subpleural predominance in 30 of 33 individuals (90.9%). Fibrotic lesions affected 4 lobes (IQR, 3-5) and involved less than 25% of lung parenchyma in all but 1 case. In the 49 patients who had received a diagnosis of acute respiratory distress syndrome, fibrotic lesions and reticulations were observed in 38.8% (19/49) and 69.4% (34/49), respectively.
Respiratory Assessment
The Nijmegen score was greater than 22 in 37 of 177 cases (20.9%), indicating dysfunctional breathing. This was confirmed by a positive hyperventilation provocation test result in 21 of 177 patients (11.9%) (Table 3), of whom 4 had a history of asthma, with normal pulmonary function test results and satisfactory control of asthma at the assessment. No fibrotic lesions were reported in these patients with confirmed dysfunctional breathing.
Among the 78 patients attending the ambulatory facility who reported new-onset dyspnea, a cause was identified in 61 cases (78.2%). Dyspnea was attributed to abnormalities on lung CT scan in 44 of 78 patients (56.4%), including fibrotic lesions in 18 of 78 cases (23.1%), and to hyperventilation provocation test-confirmed dysfunctional breathing in 14 of 78 patients (17.9%). Dyspnea was potentially explained by underlying chronic lung diseases in 7 patients (4 with uncontrolled asthma; 2, chronic obstructive pulmonary disease; and 1, obstructive sleep apnea), by left ventricular dysfunction in 3 patients, and by physical deconditioning in 2 patients.
The mean diffusing capacity of the lungs for carbon monoxide was 87% of the predicted value (SD, 23%) in 152 patients. It was 77% (SD, 17%) in the 49 patients with previous acute respiratory distress syndrome. Twenty-three of 33 patients (69.7%) with diffusing capacity of the lungs for carbon monoxide less than 70% had persistent lung CT-scan abnormalities, including 12 of 33 patients (36.4%) with fibrotic lesions.
Cardiovascular Assessment
Echocardiography identified a left ventricular ejection fraction less than 50% in 8 of 83 patients (9.6%), with no value less than 40% (Table 3). All patients with left ventricular ejection fraction less than 50% had been ICU patients. Left ventricular dilatation was identified in only 1 patient, in whom it preexisted.
Kidney Assessment
Among the 95 of 478 patients (19.9%) who had experienced acute kidney injury during hospitalization, 2 displayed a persistent alteration of kidney function at 4 months. Both had required ICU care.
Anti–SARS-CoV-2 Serology
A positive serology result was obtained for 172 of 177 patients (97.2%). Three of these 172 patients never had a positive RT-PCR result for SARS-CoV-2 and had received a diagnosis of COVID-19 according to clinical and lung CT-scan findings. The serologic index (ie, serologic titer of total Ig) was similar between ICU and non-ICU patients and lower in immunocompromised ones (eFigure 3 in the Supplement).
Discussion
In a cohort study of 478 patients who were evaluated 4 months after hospitalization for COVID-19, 51% reported at least 1 symptom that did not exist before the disease. The most common symptoms were fatigue, cognitive problems, and new-onset dyspnea. Among patients who returned for further evaluation, CT scan frequently revealed lung persistent abnormalities, as well as lung fibrotic lesions in a minority of patients. Persistent cardiac dysfunction and kidney failure were uncommon. Almost all patients had positive anti–SARS-CoV-2 serology.
There have been substantial concerns about the respiratory sequelae of COVID-19.1 However, severe pulmonary sequelae were infrequent in the patients in the present study, although all had experienced a severe or very severe form of COVID-19. Results for lung CT scan, performed for all symptomatic and ICU patients, were abnormal in most patients, but the most frequent lesions were subtle ground-glass opacities, confirming previous findings.11 Fibrotic lesions, suspected because of the severe initial inflammation,1 were present in only 19% of patients with CT scans, which is consistent with 2 smaller studies reporting CT scan at 3 months after COVID-19.6,12 In the present cohort, the fibrotic changes affected less than 25% of the lung parenchyma in all but 1 patient and occurred almost exclusively in ICU patients. Although it is possible that patients with mild fibrotic lesions did not report dyspnea at telephone consultation and subsequently did not undergo a CT scan, it appears that this represents a small proportion of the 478 evaluated patients.
Although severe lung sequelae were uncommon, new-onset dyspnea was reported in 16% of patients. If confirmed in other studies and found to be persistent, this could be clinically important, given the large number of seriously ill patients with COVID-19 worldwide. Furthermore, although parenchymal sequelae were the most common finding, dysfunctional breathing was confirmed by hyperventilation provocation test in 12% of patients, a finding that to our knowledge has not previously been described. Dysfunctional breathing is most likely not specific to COVID-19. Nevertheless, it might be the sequela of the dissociation between dyspnea and the severity of hypoxemia described in COVID-19 patients at the acute phase.28,29
In the present cohort, cognitive problems, reported by telephone assessment and confirmed at the outpatient clinic, were frequent: 21% of the patients reported at least 1 cognitive symptom, and cognitive impairment was confirmed in 38% of patients who were subsequently evaluated. The underlying mechanisms are unknown, but these symptoms might be the sequala of central nerve system injury by SARS-CoV-2, as occurs during other viral infections such as glandular fever.30
Psychological testing was conducted only on patients who returned for an ambulatory assessment, making it difficult to determine the true prevalence of these findings. Symptoms of anxiety were found in 31% of patients and symptoms of depression in 21%. Conversely, the prevalence of psychological symptoms in ICU patients, systematically evaluated at the outpatient clinic, was meaningful. These symptoms appear to have been less common in ICU patients than in the overall assessed population. The psychological consequences of an ICU stay have been well described.31
Although echocardiographic evaluation of cardiac function was performed only in ICU and symptomatic patients, systolic left ventricular dysfunction was rare and found exclusively in ICU patients. A critical limitation is that the pre–COVID-19 cardiac function was not known, but these findings suggest that possible heart damage from COVID-19 may not have frequent sequelae. However, patients with severe cardiac injury leading to death within 4 months would have been excluded from this study.
Limitations
This study has several limitations. First, this investigation was an uncontrolled cohort study, which precludes comparison of prevalence of findings with patients not experiencing COVID-19. Second, this study was performed during the first months of the epidemic when corticosteroids, anticoagulation at higher doses, and treatment with other immunomodulators were not systematically used. Third, the absence of a non–COVID-19 control group or even pre–COVID-19 assessments on the same patients limits the ability to conclude that findings after 4 months are temporally related to COVID. Fourth, many patients who were invited to participate declined both the telephone assessments and the subsequent ambulatory assessments. It is possible that patients who did not participate had fewer symptoms than those who did.
Conclusions
Four months after hospitalization for COVID-19, a cohort of patients frequently reported symptoms not previously present, and lung-scan abnormalities were common among those who were tested. These findings are limited by the absence of a control group and of pre–COVID-19 assessments in this cohort; further research is needed to understand longer-term outcomes and whether these findings reflect associations with the disease.
List of symptoms evaluated at telephone consultation
Questionnaire administered during telephone consultation
Pulmonary function tests
Dysfunctional breathing
Nijmegen questionnaire
Lung CT scan
Supplementary table 1. Tests used for psychological, cognitive and respiratory evaluation
Supplementary table 2. Baseline and hospitalization characteristics of patients who did not have the telephone assessment and of patients who had the telephone assessment according to their intubation status during the hospital stay
Supplementary table 3. Baseline and hospitalization characteristics and results of the telephone assessment in the 177 patients who attended the ambulatory visit compared to the 117 patients who did not
Supplementary table 4. Baseline and hospitalization characteristics of the patients who had the telephone assessment classified by symptoms triggering the visit to the outpatient facility
Supplementary table 5. Results of the telephone assessment and of the ambulatory visit depending on age (< 75 vs. ≥ 75 yr)
Supplementary table 6. Results of the telephone assessment classified by symptoms triggering the visit to the outpatient facility
Supplementary table 7. Results of the ambulatory visit classified by symptoms triggering the visit to the outpatient facility
Supplementary figure 1. Visualization of symptoms* and their overlap presented by the 192 patients (out of 478 patients) who presented at least one symptom at teleconsultation
Supplementary figure 2. Results of the 36-item Short-Form Health Survey (SF-36) and Multidimensional Fatigue Inventory (MFI-20 scale) scores of the patients admitted to the outpatient clinic
Supplementary figure 3. Anti-SARS-CoV-2 serological index of the patients admitted to the outpatient clinic
References
Collaborators
References
- 1.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782-793. doi: 10.1001/jama.2020.12839 [DOI] [PubMed] [Google Scholar]
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. doi: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 3.Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(11):1265-1273. doi: 10.1001/jamacardio.2020.3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah AS, Wong AW, Hague CJ, et al. A prospective study of 12-week respiratory outcomes in COVID-19–related hospitalisations. Thorax. 2020;thoraxjnl-2020-216308. doi: 10.1136/thoraxjnl-2020-216308 [DOI] [PubMed] [Google Scholar]
- 5.Wei J, Yang H, Lei P, et al. Analysis of thin-section CT in patients with coronavirus disease (COVID-19) after hospital discharge. J Xray Sci Technol. 2020;28(3):383-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao YM, Shang YM, Song WB, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. doi: 10.1016/j.eclinm.2020.100463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazza MG, De Lorenzo R, Conte C, et al. ; COVID-19 BioB Outpatient Clinic Study Group . Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594-600. doi: 10.1016/j.bbi.2020.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker C, Shalev D, Hsu I, et al. Depression, anxiety, and acute stress disorder among patients hospitalized with coronavirus disease 2019: a prospective cohort study. Psychosomatics. 2020;S0033-3182(20)30262-0. doi: 10.1016/j.psym.2020.1010.1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Gassel RJJ, Bels JLM, Raafs A, et al. High prevalence of pulmonary sequelae at 3 months after hospital discharge in mechanically ventilated survivors of COVID-19. Am J Respir Crit Care Med. 2021;203(3):371-374. doi: 10.1164/rccm.202010-3823LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Lorenzo R, Conte C, Lanzani C, et al. Residual clinical damage after COVID-19: a retrospective and prospective observational cohort study. PLoS One. 2020;15(10):e0239570. doi: 10.1371/journal.pone.0239570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C, Huang L, Wang Y, et al. 6-Month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220-232. doi: 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Borst B, Peters JB, Brink M, et al. . Comprehensive health assessment three months after recovery from acute COVID-19. Clin Infect Dis. 2020:ciaa1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Eckardt KU, Dorman NM, et al. Nomenclature for kidney function and disease: report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2020;97(6):1117-1129. doi: 10.1016/j.kint.2020.02.010 [DOI] [PubMed] [Google Scholar]
- 14.Metral M, Nadin I, Locatelli I, et al. ; Neurocognitive Assessment in the Metabolic and Aging Cohort (NAMACO) Study Group, Swiss HIV Cohort Study . How helpful are the European AIDS Clinical Society cognitive screening questions in predicting cognitive impairment in an aging, well-treated HIV-positive population? HIV Med. 2020;21(5):342-348. doi: 10.1111/hiv.12828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ware JE Jr, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36), I: conceptual framework and item selection. Med Care. 1992;30(6):473-483. doi: 10.1097/00005650-199206000-00002 [DOI] [PubMed] [Google Scholar]
- 16.Gentile S, Delarozière JC, Favre F, Sambuc R, San Marco JL. Validation of the French “Multidimensional Fatigue Inventory” (MFI 20). Eur J Cancer Care (Engl). 2003;12(1):58-64. doi: 10.1046/j.1365-2354.2003.00295.x [DOI] [PubMed] [Google Scholar]
- 17.Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428-1446. doi: 10.1183/09031936.00150314 [DOI] [PubMed] [Google Scholar]
- 18.Boulding R, Stacey R, Niven R, Fowler SJ. Dysfunctional breathing: a review of the literature and proposal for classification. Eur Respir Rev. 2016;25(141):287-294. doi: 10.1183/16000617.0088-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 20.Roussel M, Godefroy O. La batterie GRECOGVASC. Évaluation et diagnostic des troubles neurocognitifs vasculaires avec ou sans contexte d'accident vasculaire cérébral. De Boeck; 2016.
- 21.Brickenkamp R, Schmidt-Atzert L, Liepmann D. Aufmerksamkeits-und Konzentrationstest. Hogrefe; 2010.
- 22.Barczak P, Kane N, Andrews S, Congdon AM, Clay JC, Betts T. Patterns of psychiatric morbidity in a genito-urinary clinic: a validation of the Hospital Anxiety Depression Scale (HAD). Br J Psychiatry. 1988;152:698-700. doi: 10.1192/bjp.152.5.698 [DOI] [PubMed] [Google Scholar]
- 23.Beck AT, Beamesderfer A. Assessment of depression: the Depression Inventory. In: Psychological Measurements in Psychopharmacology. Karger; 1974:151-159. [DOI] [PubMed] [Google Scholar]
- 24.Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28(6):489-498. doi: 10.1002/jts.22059 [DOI] [PubMed] [Google Scholar]
- 25.Dortet L, Emeraud C, Vauloup-Fellous C, et al. Rapid determination of SARS-CoV-2 antibodies using a bedside, point-of-care, serological test. Emerg Microbes Infect. 2020;9(1):2212-2221. doi: 10.1080/22221751.2020.1826892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium . The REDCap Consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Couzin-Frankel J. The mystery of the pandemic’s “happy hypoxia.” Science. 2020;368(6490):455-456. doi: 10.1126/science.368.6490.455 [DOI] [PubMed] [Google Scholar]
- 29.Dhont S, Derom E, Van Braeckel E, Depuydt P, Lambrecht BN. The pathophysiology of “happy” hypoxemia in COVID-19. Respir Res. 2020;21(1):198. doi: 10.1186/s12931-020-01462-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White PD, Dash AR, Thomas JM. Poor concentration and the ability to process information after glandular fever. J Psychosom Res. 1998;44(2):269-278. doi: 10.1016/S0022-3999(97)00186-4 [DOI] [PubMed] [Google Scholar]
- 31.Jackson JC, Pandharipande PP, Girard TD, et al. ; Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors (BRAIN-ICU) Study Investigators . Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2(5):369-379. doi: 10.1016/S2213-2600(14)70051-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of symptoms evaluated at telephone consultation
Questionnaire administered during telephone consultation
Pulmonary function tests
Dysfunctional breathing
Nijmegen questionnaire
Lung CT scan
Supplementary table 1. Tests used for psychological, cognitive and respiratory evaluation
Supplementary table 2. Baseline and hospitalization characteristics of patients who did not have the telephone assessment and of patients who had the telephone assessment according to their intubation status during the hospital stay
Supplementary table 3. Baseline and hospitalization characteristics and results of the telephone assessment in the 177 patients who attended the ambulatory visit compared to the 117 patients who did not
Supplementary table 4. Baseline and hospitalization characteristics of the patients who had the telephone assessment classified by symptoms triggering the visit to the outpatient facility
Supplementary table 5. Results of the telephone assessment and of the ambulatory visit depending on age (< 75 vs. ≥ 75 yr)
Supplementary table 6. Results of the telephone assessment classified by symptoms triggering the visit to the outpatient facility
Supplementary table 7. Results of the ambulatory visit classified by symptoms triggering the visit to the outpatient facility
Supplementary figure 1. Visualization of symptoms* and their overlap presented by the 192 patients (out of 478 patients) who presented at least one symptom at teleconsultation
Supplementary figure 2. Results of the 36-item Short-Form Health Survey (SF-36) and Multidimensional Fatigue Inventory (MFI-20 scale) scores of the patients admitted to the outpatient clinic
Supplementary figure 3. Anti-SARS-CoV-2 serological index of the patients admitted to the outpatient clinic
References
Collaborators