This cohort study examines the metabolic cost of initiating exercise and its functional consequences.
Key Points
Question
What is the metabolic cost of starting exercise in individuals across the spectrum of functional limitation, including heart failure with preserved ejection fraction (HFpEF)?
Findings
In this cohort study of 3047 individuals from the Framingham Heart Study (an observational cohort study) and 184 individuals with HFpEF using comprehensive cardiopulmonary exercise testing, metabolic cost (in watts) of unloaded exercise (internal work) was determined. Obesity was associated with internal work across both the Framingham Heart Study and HFpEF cohorts; in patients with HFpEF, greater internal work was associated with more significant hemodynamic alterations in unloaded exercise.
Meaning
A greater energetic cost of exercise initiation is associated with obesity and greater excursions in filling pressures early in exercise, suggesting that exercise initiation (before imposing an external load on the cardiovascular system) may represent and important locus of functional limitation in HFpEF.
Abstract
Importance
Heart failure with preserved ejection fraction (HFpEF) is a joint metabolic and cardiovascular disorder with significant noncardiac contributions.
Objective
To define and quantify the metabolic cost of initiating exercise in individuals with and without HFpEF and its functional consequences.
Design, Setting, and Participants
This prospective cohort study included individuals with hemodynamically confirmed HFpEF from the Massachusetts General Hospital Exercise Study (MGH-ExS) and community-dwelling participants from the Framingham Heart Study (FHS). Analysis began April 2016 and ended November 2020.
Exposures
Internal work (IW), a measure of work equivalents required to initiate movement.
Main Outcomes and Measures
Using breath-by-breath oxygen uptake (V̇o2) measurements and V̇o2-work rate associations, cost of initiating exercise (IW) in patients with HFpEF (MGH-ExS) and in community-dwelling individuals (FHS) was quantified. Linear regression was used to estimate associations between IW and clinical/hemodynamic measures.
Results
Of 3231 patients, 184 (5.7%) had HFpEF and were from MGH-ExS, and 3047 (94.3%) were community-dwelling individuals from FHS. In the MGH-ExS cohort, 86 (47%) were women, the median (interquartile range) age was 63 (53-72) years, and the median (interquartile range) peak V̇o2 level was 13.33 (11.77-15.62) mL/kg/min. In the FHS cohort, 1620 (53%) were women, the median (interquartile range) age was 54 (48-60) years, and the median (interquartile range) peak V̇o2 level was 22.2 (17.85-27.35) mL/kg/min. IW was higher in patients with HFpEF and accounted for 27% (interquartile range, 21%-39%) of the total work (IW + measured external workload on the cycle), compared with 15% (interquartile range, 12%-20%) of that in FHS participants. Body mass index accounted for greatest explained variance in patients with HFpEF from MGH-ExS and FHS participants (22% and 18%, respectively), while resting cardiac output and biventricular filling pressures were not significantly associated with variance in IW in patients with HFpEF. A higher IW in patients with HFpEF was associated with a greater increase in left- and right-sided cardiac filing pressure during unloaded exercise, despite similar resting hemodynamic measures across IW.
Conclusions and Relevance
This study found that internal work, a new body mass index–related measure reflecting the metabolic cost of initiating movement, is higher in individuals with HFpEF compared with middle-aged adults in the community and is associated with steep, early increases in cardiac filling pressures. These findings highlight the importance of quantifying heterogeneous responses to exercise initiation when evaluating functional intolerance in individuals at risk for or with HFpEF.
Introduction
While studies of exercise limitation in heart failure (HF) traditionally have a cardiocentric focus, functional limitation may reside outside the heart, especially in HF with preserved ejection fraction (HFpEF). In HFpEF, limitations during daily low-level activity are frequent and multifactorial,1,2,3 highlighting the need to precisely phenotype extra-cardiac abnormalities that may contribute to impaired exercise capacity. Here, we define and characterize a measure quantifying the metabolic cost of initiating unloaded exercise (internal work [IW]) in patients with HFpEF compared with community-dwelling individuals in whom we performed a matched cardiopulmonary exercise testing (CPET) protocol to measure IW.
Methods
Cohort Description
Massachusetts General Hospital Exercise Study
We included individuals at our center between April 2006 and April 2019 who underwent CPET with pulmonary arterial catheterization for evaluation of dyspnea with symptomatic HFpEF (New York Heart Association class II-IV) based on Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Heart Failure With Preserved Ejection Fraction (RELAX) HFpEF criteria,4 including maximum effort (peak respiratory exchange ratio >1.05), left ventricular ejection fraction of 0.50 or more, and 1 or more of the following within 12 months prior to CPET1: hospitalization for HF or intravenous loop diuretics2; chronic loop diuretic therapy with diastolic dysfunction (by left atrial enlargement)3; resting pulmonary capillary wedge pressure (PCWP) of 15 mm Hg or more, PCWP/cardiac output slope of 2.0 mm Hg/L/min or more, or upright exercise PCWP more than 20 mm Hg (hemodynamic criterion). The majority of participants fulfilled hemodynamic criteria for HFpEF on day of testing (151 of 184 [82%]). Clinical covariates were obtained via examination (age, sex, body mass index [BMI]) or retrospective medical record review. Massachusetts General Hospital institutional review board approved this study. Participants provided written informed consent.
Framingham Heart Study
Enrollment of the Framingham Heart Study (FHS) cohorts have been described.6,7 Of study participants attending the third FHS examination (April 2016-April 2019), the FHS study sample included 3047 individuals after exclusions for submaximal effort [peak respiratory exchange ratio, ≤1.0; n = 55], inability to calculate IW [n = 14], or missing BMI [n = 1]). Clinical covariates were defined as described.8 Boston University Medical Center institutional review board approved the study protocol. Participants provided written informed consent.
CPET
The methods for each Massachusetts General Hospital Exercise Study (MGH-ExS) and FHS have been published previously by our group.9,10 They are reiterated here.
MGH-ExS
Patients took prescribed medications as usual before CPET and underwent pulmonary arterial catheterization (internal jugular vein) and radial arterial catheterization. Resting mean right atrial pressure, mean pulmonary arterial pressure, and PCWP were measured in the supine and upright positions. Participants performed maximum incremental exercise with upright cycle ergometry after an initial 3- to 5-minute period of rest and a 3-minute period of unloaded exercise (MedGraphics). An individualized ramp protocol was used based on the participant’s estimated fitness level (5-25 watts [W] per minute) to achieve a target of 8 to 12 minutes of exercise. Prior to initiation of loaded exercise, participants pedaled against 0 W of resistance at 60 rpm (unloaded exercise). Hemodynamic measurements were obtained simultaneous with exercise (Royal Phillips Electronics) as previously described.8,11 We quantified mean right atrial pressure, pulmonary arterial pressure, PCWP, and systemic arterial pressure in the upright position at end expiration while patients were seated on the cycle ergometer. Measurements were made at rest and at 1-minute intervals during exercise. Fick cardiac output was quantified each minute throughout exercise by measuring oxygen uptake (V̇o2) and radial arterial and mixed venous O2 saturation at the same time (in conjunction with hemoglobin) to calculate the arteriovenous O2 content difference (CavO2). Arterial O2 content (CaO2) was calculated as (hemoglobin, g/dL × 1.39 × arterial oxygen saturation) + (0.003 × Pao2). Mixed venous O2 content was calculated as (hemoglobin × 1.39 × mixed venous O2 saturation) + (0.003 × PvO2). Peak V̇o2 was defined as highest O2 uptake averaged over 30 seconds during the last 90 seconds of symptom-limited exercise as previously described.10 Percent predicted peak V̇o2 was calculated using the formula derived by Wasserman.12
FHS
FHS participants performed maximum, effort-limited CPET on a cycle ergometer (Lode) with measures of breath-by-breath gas exchange by metabolic cart (MedGraphics) as previously described.8 Heart rate was monitored continuously. Blood pressure was manually measured every 2 minutes via sphygmomanometry. The exercise protocol included 4 stages analogous to the MGH-ExS protocol1: rest (at least 3 minutes)2; unloaded exercise (3 minutes)3; incremental exercise (15 vs 25 W/min based on self-reported physical activity levels)4; and recovery and rest (3 minutes unloaded cycling with 1 final minute of rest). Peak V̇o2 was determined as the highest 30-second median during the final 90 seconds of exercise.
Of note, cycle ergometry was selected as the preferred modality to investigate unloaded vs loaded exercise, as seated exercise provides body weight support such that V̇o2 can be measured purely during leg activity against 0 W of resistance (unlike walking, which requires energy for body support). As a sensitivity analysis, we explored the correlation in IW in 12 individuals (either healthy volunteers or those clinically referred for CPET studies) who performed both unloaded exercise on a cycle ergometer and a standardized low-level exercise initiation on the treadmill (4% grade, 0.8 mph, for 3 minutes, identical to the cycle ergometry unloaded exercise period). Comparison of matched cycle ergometry and treadmill test in individuals who performed both modalities on the same day were correlated (Spearman ρ = 0.75, P = .003).
Metabolic Cost of Exercise Initiation
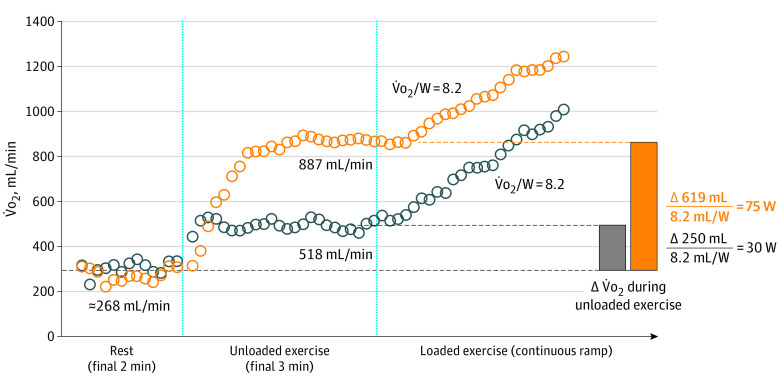
IW quantifies the work equivalents (in watts) expended during unloaded exercise, where an individual performed work against no external resistance (ergometer set to 0 W). A schematic of IW calculation is displayed in Figure 1. IW was calculated by dividing the difference in V̇o2 from rest to the end of unloaded exercise by the V̇o2/W slope (termed aerobic efficiency) calculated during loaded exercise. We calculated aerobic efficiency using V̇o2 and workload starting from 1 minute after initiation of incremental ramp exercise to peak exercise. In cases where the calculated aerobic efficiency was less than 4.0 mL/W (MGH-ExS: n = 12; FHS: n = 2), a value of 4.0 mL/w was imputed for aerobic efficiency (4 SDs below the mean normative value, 10 [1.5] mL/W12). A single outlier value for internal work (>4 SD) was reported as the value approximating 4 SDs above the mean (150 W). The O2 uptake at rest was defined as the median value of the V̇o2 displayed in the final 2 minutes of the resting period of the CPET. The O2 uptake at the end of unloaded exercise was defined as the median value of the V̇o2 displayed in the final 30 seconds of the unloaded exercise period. Measured workload during resistance (external work) was the workload registered by the ergometer at peak exercise, with proportional IW calculated as the ratio of IW to the total work performed (IW + external work).
Figure 1. Internal Work.
Two participants with different values for internal work are shown. Internal work was calculated by dividing the difference in oxygen uptake (V̇o2) from rest to the end of unloaded exercise by the V̇o2/W slope calculated during loaded exercise. W indicates watts.
Statistical Analysis
We assessed how clinical features in both FHS and MGH-ExS explained variance (R2) in IW using linear models for IW (log-transformed, standardized) in nested models successively adjusted for age, sex, BMI, diabetes, hypertension, cardiovascular disease, resting systolic and diastolic blood pressure, resting heart rate, absolute peak V̇o2, and resting cardiac output and filling pressures (in MGH-ExS). We used type 1 sums of squares to assess explanatory variance of each covariate (expressed as sum of squares for a given term divided by model sum of squares). We examined associations between IW and changes in characteristic hemodynamic and gas exchange measures in individuals with HFpEF (MGH-ExS) between rest to end of unloaded exercise (exercise initiation) and in a submaximal loaded exercise period (a 30-W period of work during early submaximal exercise). We constructed linear models for change in each measure (change in right atrial pressure, PCWP, mean pulmonary arterial pressure, heart rate, stroke volume, and CavO2 [O2 extraction]) as a function of IW quartile, adjusted for age, sex, and BMI (log) to estimate association between IW quartile and hemodynamic measures.
R version 4.0.2 (R Foundation) was used, with a 2-tailed P value less than .05 considered statistically significant. Analysis began April 2016 and ended November 2020.
Results
Baseline characteristics and exercise features for the FHS (n = 3047) and the MGH-ExS HFpEF (n = 184) cohorts are reported in Table 1, with greater peak V̇o2 levels in FHS and hemodynamic profile consistent with HFpEF in MGH-ExS. IW and the amount of IW expended in unloaded exercise compared with total work (proportional IW) were numerically higher in individuals with HFpEF (MGH-ExS) compared with those in the community (FHS; Figure 2), with 91 individuals with HFpEF (49%) exhibiting an IW greater than 75th percentile in FHS participants with normal exercise capacity (defined in Figure 2). Clinical measures, BMI, standard vital signs, and peak V̇o2 levels explained less than a third of the total variance in IW in linear models (adjusted R2 = 0.25 in FHS; R2 = 0.31-0.33 in MGH-ExS; Table 2, Figure 2), with BMI accounting for the greatest proportion of variance explained by included clinical features. Resting cardiac output and biventricular filling pressures (MGH-ExS; model 2, Table 2) were not significantly associated with IW. Of note, in the FHS cohort, waist circumference exhibited a similar magnitude of association with log-IW in fully adjusted models (estimated standardized β for log-waist circumfrence = 0.44; P < .001) as log-BMI. Individuals with a greater IW displayed greater change in hemodynamic indices of HFpEF and components of V̇o2 from the resting state to the end of unloaded exercise compared with the change observed over 30 W in early loaded exercise (Figure 3). In general, filling pressures rose in proportion to IW by the end of unloaded exercise with more modest changes during early loaded exercise.
Table 1. Baseline Clinical, Demographic, and Hemodynamic Characterization of the Cohorts.
| Characteristic | HFpEF (MGH-ExS; n = 184) | Community cohort (FHS; n = 3047) | ||
|---|---|---|---|---|
| Median (interquartile range) | No. missing | Median (interquartile range) | No. missing | |
| Age, y | 63.0 (53.0-72.0) | 0 | 54.4 (48.2-60.3) | 0 |
| Female, No. (%) | 86 (46.7) | 0 | 1620 (53.2) | 0 |
| BMI | 32.4 (28.0-36.5) | 0 | 27.5 (24.4-31.4) | 0 |
| Internal work, watt | 32.4 (25.3-48.3) | 0 | 29.1 (24.1-34.9) | 0 |
| Proportional internal work | 0.27 (0.21-0.39) | 0 | 0.15 (0.12-0.20) | 0 |
| Aerobic efficiency, mL/W | 9.1 (7.6-10.0) | 0 | 9.0 (8.4-9.6) | 0 |
| Peak V̇o2, mL/kg/min | 13.3 (11.8-15.6) | 0 | 22.2 (17.9-27.4) | 2 |
| Peak watts | 85.8 (67.8-113.0) | 0 | 159.0 (123.0-213.0) | 0 |
| Peak RER | 1.17 (1.09-1.24) | 0 | 1.21 (1.15-1.28) | 0 |
| Diabetes, No. (%) | 62 (33.7) | 0 | 228 (7.5) | 10 |
| Hypertension, No. (%) | 131 (71.2) | 0 | 1456 (47.8) | 0 |
| Heart rate, beats/min | ||||
| Resting | 75.0 (66.0-85.0) | 0 | 71.0 (64.0-79.0) | 0 |
| Peak | 123.0 (103.1-138.0) | 0 | 153.0 (140.0-165.0) | 1 |
| Systolic blood pressure, mm Hg | ||||
| Resting | 146.0 (132.0-165.0) | 0 | 122.0 (112.0-136.0) | 0 |
| Peak | 186.0 (162.0-209.5) | 1 | 180.0 (164.0-198.0) | 0 |
| Diastolic blood pressure, mm Hg | ||||
| Resting | 75.0 (68.3-84.5) | 1 | 80.0 (74.0-84.0) | 0 |
| Peak | 86.0 (74.5-98.0) | 1 | 84.0 (78.0-90.0) | 0 |
| CavO2, mL/dL | ||||
| Resting | 6.0 (5.4-7.0) | 1 | NA | NA |
| Peak | 11.6 (10.5-13.0) | 6 | NA | NA |
| Upright RAP, mm Hg | ||||
| Resting | 3.0 (2.0-5.0) | 9 | NA | NA |
| Peak | 12.0 (9.0-15.0) | 3 | NA | NA |
| Upright mean PAP, mm Hg | ||||
| Resting | 18.0 (16.0-22.0) | 0 | NA | NA |
| Peak | 40.0 (35.0-47.0) | 4 | NA | NA |
| Upright mean PCWP, mm Hg | ||||
| Resting | 7.0 (6.0-10.0) | 1 | NA | NA |
| Peak | 26.0 (20.0-30.0) | 11 | NA | NA |
| Cardiac output, L/min | ||||
| Resting | 4.7 (4.0-5.6) | 0 | NA | NA |
| Peak | 10.6 (8.2-13.3) | 0 | NA | NA |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CavO2, arteriovenous O2 content difference; FHS, Framingham Heart Study; HFpEF, heart failure with preserved ejection fraction; MGH-ExS, Massachusetts General Hospital Exercise Study; NA, not applicable; PAP, pulmonary arterial pressure; RAP, right atrial pressure; PCWP, pulmonary capillary wedge pressure; RER, respiratory exchange ratio; V̇o2, oxygen uptake.
Figure 2. Internal Work in the FHS and in HFpEF.
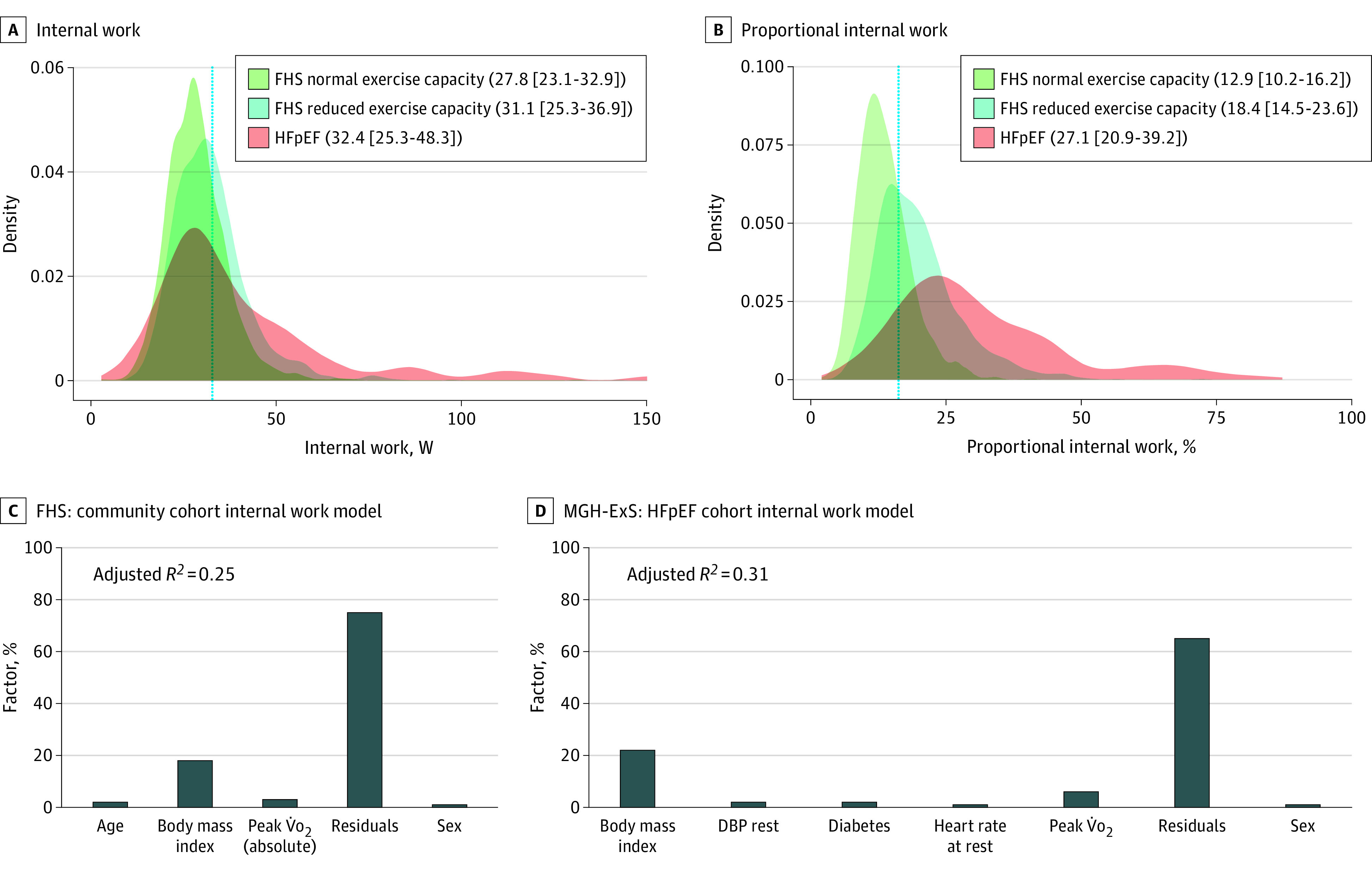
A, Distribution of internal work in Framingham Heart Study (FHS) participants and individuals with heart failure with preserved ejection fraction (HFpEF) displayed as a density function, with normal exercise capacity defined as 90% or more of predicted peak V̇o2. Panel B shows a similar figure for proportional internal work. The numbers in the legend show the median (interquartile range) of each measure in the different subsamples. The dotted vertical lines in panels A and B represent the 75th percentile of internal work (A) or proportional internal work (B) of the FHS normal exercise capacity distribution. Panels C and D show internal work variance explained by each covariate. Covariates without more than 1% variance explained (after rounding) were not included here. Massachusetts General Hospital Exercise Study (MGH-ExS) models used in panel D did not include resting cardiac output or filling pressures. DBP indicates diastolic blood pressure.
Table 2. Correlates of Internal Work in the Community (FHS) and HFpEF (MGH-ExS)a.
| Covariate | Model (adjusted R2 = 0.25; n = 3037) | |||
|---|---|---|---|---|
| Estimated β | P value | |||
| FHS | ||||
| Age | 0.0085 | .65 | ||
| Female | –0.43 | <.001 | ||
| BMI, log | 0.48 | <.001 | ||
| Diabetes | 0.076 | .23 | ||
| Hypertension | –0.014 | .71 | ||
| CVD | –0.096 | .23 | ||
| Resting SBP, log | 0.019 | .39 | ||
| Resting DBP, log | –0.073 | <.001 | ||
| Resting HR, log | 0.0073 | .67 | ||
| Peak V̇o2, log, mL/min | –0.29 | <.001 | ||
| Covariate | Model 1 (adjusted R2 = 0.31; n = 183) | Model 2 (adjusted R2 = 0.33; n = 173) | ||
| Estimated β | P value | Estimated β | P value | |
| HFpEF (MGH-ExS) | ||||
| Age | –0.096 | .22 | –0.073 | .39 |
| Female | –0.17 | .28 | –0.082 | .63 |
| BMI, log | 0.67 | <.001 | 0.70 | <.001 |
| Diabetes | 0.22 | .12 | 0.294 | .05 |
| Hypertension | 0.060 | .69 | 0.066 | .67 |
| Resting SBP, log | 0.127 | .15 | 0.108 | .26 |
| Resting DBP, log | –0.174 | .05 | –0.147 | .13 |
| Resting HR, log | 0.036 | .61 | 0.038 | .61 |
| Peak V̇o2, log, mL/min | –0.411 | <.001 | –0.36 | .002 |
| Resting PCWP, log | NA | NA | 0.16 | .11 |
| Resting RA pressure | NA | NA | –0.128 | .19 |
| Resting CO, log | NA | NA | –0.023 | .80 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CO, cardiac output; CVD, cardiovascular disease; DBP, diastolic blood pressure; FHS, Framingham Heart Study; HFpEF, heart failure with preserved ejection fraction; HR, heart rate; MGH-ExS, Massachusetts General Hospital Exercise Study; NA, not applicable; PCWP, pulmonary capillary wedge pressure; RA, right atrial pressure; SBP, systolic blood pressure; V̇o2, oxygen uptake.
Continuous variables were standardized. Values were log transformed where specified. RA was not log transformed because of some negative values.
Figure 3. Greater Internal Work and Hemodynamic Alterations in Unloaded vs Early-Loaded Exercise.
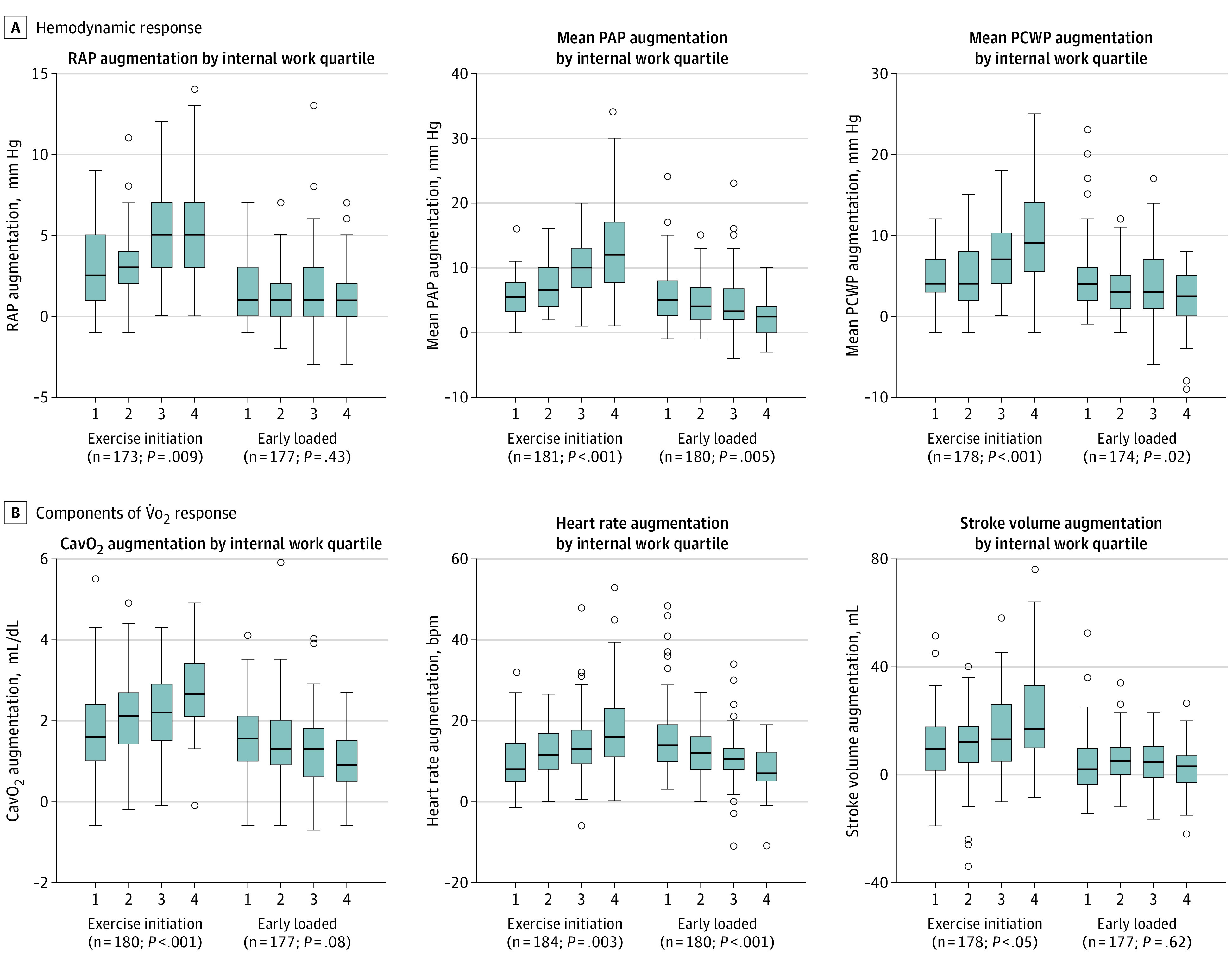
A, Hemodynamic responses that characterize heart failure with preserved ejection fraction across quartiles of internal work in unloaded exercise (ie, exercise initiation) and early-loaded exercise (30 W of loaded exercise occurring early in the incremental ramp portion of exercise). Each box plot displays the change in a hemodynamic measure (augmentation), calculated as the value of the variable at the end of the phase minus its value at the beginning of the phase. B, Analogous analyses for the components of V̇o2. P values represent the P values for each measure across internal work quartiles in adjusted linear models. CavO2 indicates arteriovenous O2 content difference; PAP, pulmonary arterial pressure; PCWP, pulmonary capillary wedge pressure; RAP, right atrial pressure.
Discussion
Limitations in exercise capacity expressed as exertional dyspnea or fatigue command attention in evaluating symptoms and severity of HFpEF but are incompletely understood. Here, we define and characterize a novel exercise-based measurement (IW) as a quantitative measure of the metabolic cost of initiating unloaded exercise. IW is elevated in HFpEF and is on median 27% of total achievable workload (vs 12.9% among individuals with preserved exercise capacity in the community; Figure 2B). A higher IW in patients with HFpEF was associated with greater increase in cardiac filling pressure (eg, PCWP) and higher expenditure of heart rate and stroke volume reserve before application of any external load. Of the covariates assessed, BMI explained the greatest variance in IW, although IW is not a simple surrogate for BMI because the majority of its variance remains unexplained. Finally, we observed substantial overlap in IW between individuals in the community and those with hemodynamic HFpEF, particularly in FHS participants with impaired exercise capacity.
Given the alignment of metabolic risk with HFpEF, the study of IW (a measure associated with BMI) in HFpEF is natural. IW assessment complements cardiocentric assessments of functional intolerance in HFpEF. The same degree of registered workload (external work) may come at vastly different metabolic costs (O2 consumption) because of variable IW performed prior to loaded exercise. Furthermore, these findings dispute the use of hemodynamic response at fixed workloads as an end point in clinical trials,13 as a standardized workload (eg, 30 W) in patients with HFpEF does not represent uniform metabolic stress across individuals. Excessive V̇o2 use appears manifest at the onset of exercise in HFpEF, with attendant HFpEF defining hemodynamic changes during unloaded exercise proportional to IW. These sharp increments in biventricular filling pressures in patients with high IW may help to explain why even light activity often elicits marked symptoms in patients with HFpEF. Accordingly, in individuals with suspected HF with high IW, therapies targeting the efficiency of exercise initiation (eg, weight loss and exercise) may be more likely to offer more clinical improvements over cardiocentric interventions.
In studies of obesity in children, excess adiposity has been associated with greater V̇o2 during unloaded cycle exercise.14 In this study, V̇o2 during unloaded exercise was associated with lean and fat mass, whereas peak V̇o2 was only associated with lean mass, suggesting that further parsing body composition metrics may be helpful in understanding the physiology of IW. Furthermore, additional inputs to increased IW (eg, increased V̇o2 cost of breathing from obese habitus or HF) are likely contributory factors. These observations highlight the importance of additional study in this space to further refine the determinants of IW, including which of these may be targetable in HFpEF.
Limitations
There are several limitations to the current work. While IW may overlap between HFpEF and individuals in the community, the purpose of our study was not to develop a discriminative measure between individuals with HFpEF and without HFpEF. Instead, we sought to define the measurement of IW as part of efforts to parse contributions to exercise limitations in HFpEF and to understand IW’s hemodynamic and clinical correlates. In addition, further work is required to define whether metabolic mechanisms beyond BMI drive IW (eg, skeletal muscle phenotyping) and whether IW itself is mutable with metabolic intervention. Certainly, specific subgroups (such as those with discordant findings of high BMI/low IW or its inverse) merit special interrogation because they may provide an opportunity to investigate unique physiologic implications of IW. Explanatory models for IW may be limited by residual confounding, and future work delineating other explanatory measures will be important to extend this work. Our exercise mode was a cycle ergometer, which does not necessarily mimic diverse daily activities. Nevertheless, we found that IW by cycle and treadmill CPET were associated, relevant to the clinical translatability of this measure as many exercise laboratories perform treadmill CPET (instead of a cycle ergometry).
Conclusions
While inference of causality of IW on hemodynamic derangements in HFpEF is not possible, our findings provide a way to quantify the extent to which a high metabolic cost of exercise initiation contributes to exercise intolerance in patients. Further work to define mechanisms of how IW affects HFpEF physiology (beyond BMI; eg, ventricular-vascular interactions, skeletal muscle efficiency) are needed, specifically whether this physiology is mutable with metabolic intervention. Nevertheless, these findings suggest the importance of accounting for heterogeneity of responses to early, unloaded exercise when evaluating exercise response patterns across the spectrum of cardiovascular disease.
References
- 1.Borlaug BA, Anstrom KJ, Lewis GD, et al. ; National Heart, Lung, and Blood Institute Heart Failure Clinical Research Network . Effect of inorganic nitrite vs placebo on exercise capacity among patients with heart failure with preserved ejection fraction: the INDIE-HFpEF Randomized Clinical Trial. JAMA. 2018;320(17):1764-1773. doi: 10.1001/jama.2018.14852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redfield MM, Velazquez EJ, Braunwald E. Nitrates in heart failure with preserved ejection fraction. N Engl J Med. 2016;374(16):1589. [DOI] [PubMed] [Google Scholar]
- 3.Shah SJ, Voors AA, McMurray JJV, et al. Effect of neladenoson bialanate on exercise capacity among patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2019;321(21):2101-2112. doi: 10.1001/jama.2019.6717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redfield MM, Chen HH, Borlaug BA, et al. ; RELAX Trial . Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309(12):1268-1277. doi: 10.1001/jama.2013.2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis. 1984;129(2 pt 2):S49-S55. doi: 10.1164/arrd.1984.129.2P2.S49 [DOI] [PubMed] [Google Scholar]
- 6.Splansky GL, Corey D, Yang Q, et al. The third generation cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165(11):1328-1335. doi: 10.1093/aje/kwm021 [DOI] [PubMed] [Google Scholar]
- 7.Abraham TM, Massaro JM, Hoffmann U, Yanovski JA, Fox CS. Metabolic characterization of adults with binge eating in the general population: the Framingham Heart Study. Obesity (Silver Spring). 2014;22(11):2441-2449. doi: 10.1002/oby.20867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nayor M, Xanthakis V, Tanguay M, et al. Clinical and hemodynamic associations and prognostic implications of ventilatory efficiency in patients with preserved left ventricular systolic function. Circ Heart Fail. 2020;13(5):e006729. doi: 10.1161/CIRCHEARTFAILURE.119.006729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhakal BP, Malhotra R, Murphy RM, et al. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail. 2015;8(2):286-294. doi: 10.1161/CIRCHEARTFAILURE.114.001825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malhotra R, Bakken K, D’Elia E, Lewis GD. Cardiopulmonary exercise testing in heart failure. JACC Heart Fail. 2016;4(8):607-616. doi: 10.1016/j.jchf.2016.03.022 [DOI] [PubMed] [Google Scholar]
- 11.Ho JE, Zern EK, Lau ES, et al. Exercise pulmonary hypertension predicts clinical outcomes in patients with dyspnea on effort. J Am Coll Cardiol. 2020;75(1):17-26. doi: 10.1016/j.jacc.2019.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wasserman K. Principles of Exercise Testing and Interpretation. Wolters Kluwer Health, 2015. [Google Scholar]
- 13.Feldman T, Mauri L, Kahwash R, et al. ; REDUCE LAP-HF I Investigators and Study Coordinators . Transcatheter interatrial shunt device for the treatment of heart failure with preserved ejection fraction (REDUCE LAP-HF I [Reduce Elevated Left Atrial Pressure in Patients With Heart Failure]): A phase 2, randomized, sham-controlled trial. Circulation. 2018;137(4):364-375. doi: 10.1161/CIRCULATIONAHA.117.032094 [DOI] [PubMed] [Google Scholar]
- 14.Norman AC, Drinkard B, McDuffie JR, Ghorbani S, Yanoff LB, Yanovski JA. Influence of excess adiposity on exercise fitness and performance in overweight children and adolescents. Pediatrics. 2005;115(6):e690-e696. doi: 10.1542/peds.2004-1543 [DOI] [PMC free article] [PubMed] [Google Scholar]