Abstract
Objectives
Multiple RCTs of interleukin-6 (IL-6) inhibitors in COVID-19 have been published, with conflicting conclusions. We performed a meta-analysis to assess the impact of IL-6 inhibition on mortality from COVID-19, utilising meta-regression to explore differences in study results.
Methods
Systematic database searches were performed to identify RCTs comparing IL-6 inhibitors (tocilizumab and sarilumab) to placebo or standard of care in adults with COVID-19. Meta-analysis was used to estimate the relative risk of mortality at 28 days between arms, expressed as a risk ratio. Within-study mortality rates were compared, and meta-regression was used to investigate treatment effect modification.
Results
Data from nine RCTs were included. The combined mortality rate across studies was 19% (95% CI: 18, 20%), ranging from 2% to 31%. The overall risk ratio for 28-day mortality was 0.90 (95% CI: 0.81, 0.99), in favour of benefit for IL-6 inhibition over placebo or standard of care, with low treatment effect heterogeneity: I2 0% (95% CI: 0, 53%). Meta-regression showed no evidence of treatment effect modification by patient characteristics. Trial-specific mortality rates were explained by known patient-level predictors of COVID-19 outcome (male sex, CRP, hypertension), and country-level COVID-19 incidence.
Conclusions
IL-6 inhibition is associated with clinically meaningful improvements in outcomes for patients admitted with COVID-19. Long-term benefits of IL-6 inhibition, its effectiveness across healthcare systems, and implications for differing standards of care are currently unknown.
Keywords: COVID-19, IL-6, Tocilizumab, Sarilumab, Meta-analysis
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has resulted in over 100 million human cases and 2.5 million deaths since its emergence in Wuhan, China, in December 2019.1 Caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), presentations of COVID-19 range from asymptomatic illness to viral pneumonia and severe disease. Symptoms are characterised by respiratory failure, acute respiratory distress syndrome, sepsis and death. Although most cases of COVID-19 are mild, approximately 5% of those infected require admission to intensive care units (ICU), and the case-fatality rate for those admitted to ICU approaches 40%.2 Effective treatments for severe COVID-19 have become a global research priority.
Pathological hyperinflammation triggered by SARS-CoV-2 infection is a major driver of COVID-19 disease severity and death. Interleukin-6 (IL-6) release is a central part of immunocompetence, including control of viral infections;3 however, dysregulated IL-6 release contributes to hyperinflammation, severe disease, and the tissue damage seen in acute lung injury.4 , 5 Elevated levels of IL-6 correlate with blood levels of SARS-CoV-2 RNA, increased disease severity, and a worse prognosis.6 IL-6 is postulated to play a key role in the cytokine storm seen in severe COVID-19, and resultant multi-organ damage.7
Observational studies and randomised controlled trials (RCTs) have evaluated the effect of IL-6 blockade in severe COVID-19.8, 9, 10, 11, 12, 13 Tocilizumab and sarilumab, two licensed IL-6 receptor blockers, have recently been approved in the US and UK for use in patients with severe COVID-19.14, 15, 16 However, controversy remains around published RCTs, with some demonstrating benefit, and others failing to do so.
In this systematic review and meta-analysis of RCTs, we sought to assess the impact of IL-6 inhibition on mortality in the treatment of COVID-19. We utilised meta-regression to explore reasons behind differences in study results, including characteristics of study populations, background incidence and mortality from COVID-19, and human development index in the regions where the studies were conducted.
Methods
Database search strategy
A systematic literature search was conducted using MEDLINE, Embase and MedRxiv to identify studies reporting RCTs of IL-6 inhibitors in COVID-19. Search terms employed included interleukin-6, tocilizumab, sarilumab, COVID-19, SARS-CoV-2, and their respective synonyms. The search was limited to articles published between 1st January 2020 and February 2021. A rerun of the search was performed on 22nd February 2021, prior to the final analysis, to identify further trials that could be incorporated into the review. Further information regarding the search strategy is available in the Supplementary material.
The search was performed in accordance with the preferred reporting system for systematic reviews (PRISMA),17 and registered with the international prospective register of systematic reviews (PROSPERO registration ID: CRD42021235606).
Eligibility criteria and study selection
Eligible studies were RCTs in adult patients with suspected or confirmed COVID-19, comparing an IL-6 inhibitor to placebo, standard of care or an alternative COVID-19 treatment. Conference abstracts, case reports, letters to the editor, review articles, case-control studies and cohort studies were excluded. RCTs at high risk of bias (as detailed below) were excluded from further analysis.
Records were managed in EndNote v9. Study titles and abstracts were screened independently by two investigators (ET, AB), and the full text of relevant studies were retrieved and assessed for eligibility. Disagreements were resolved through involvement of a third reviewer (JRH).
Data collection
Data were extracted independently by two investigators (ET, VP), with involvement of a third reviewer (MDR) to resolve discrepancies, where required. Data extracted included the study source (author, journal, publication date), study characteristics (type of blinding, study exclusion and inclusion criteria, study time window), intervention and comparator arm details (medication, dosage, route), demographics (age, gender, ethnicity), comorbidities (diabetes mellitus, hypertension, body mass index (BMI)), disease severity, C-reactive protein (CRP), ICU admissions, 14- and 28-day mortality. Minority ethnicity was defined as any ethnicity other than the major ethnic group within each individual study.
The primary outcome of interest was 28-day mortality, reported using an intention-to-treat (unmodified) method. When 28-day mortality data were not available, the closest date to day 28 was used, as specified in the text. Secondary outcomes of interest were 14-day mortality and ICU admissions.
Peak 14-day COVID-19 incidence and mortality rates during the recruitment windows for countries included within each study were collected from the European Centre for Disease Prevention and Control, in addition to the Human Development Index (HDI).18 , 19 The HDI is a composite index incorporating life expectancy, education, and per capita income indicators, and is used to rank countries into tiers of human development. For multinational studies, when sufficient data on country breakdown were provided, weighted means of COVID-19 incidence, mortality rate and HDI were calculated according to the proportion of patients recruited from each country; in cases where insufficient information was provided, values for the majority-recruiting country were used.
Risk of bias and study quality were assessed using the Cochrane Risk of Bias 2 (RoB2) tool.20 This was applied to each study independently by two reviewers (ET, AB), with disagreements resolved by involvement of a third reviewer (BDC).
The data utilised in this study are freely available online; no ethical approval was required for the conduct of this study.
Meta-analysis and meta-regression
All statistical analyses were conducted using Stata 16 (StataCorp LLC, USA). The incidence of 28-day mortality was reported for each included study. Two meta-analyses were performed: the first estimated the relative risk of mortality between intervention and control arms, expressed as risk ratios (RR) with 95% confidence intervals. For studies comparing two interventions (either different doses or different IL-6 inhibitors), the intervention arms were included separately, whilst the number of patients contributing to the control arm were divided equally for use as a comparator.21 The second meta-analysis examined the overall mortality rate, with 95% confidence intervals, for the combined intervention and control arms of each individual study. Meta-analyses were performed using the random-effects DerSimonian and Laird method, and compared graphically with forest plots. Heterogeneity between studies was assessed using I² statistics. No study arms had zero events, and so no continuity correction was required.
Random effects meta-regression was used to evaluate the impact of differences in the characteristics of each study on the treatment effect. Characteristics considered included demographics, comorbidities, country-level differences in peak COVID-19 incidence, country-specific COVID-19 mortality rates, and country-specific HDI. Separate models were estimated for each characteristic.
Results
Study characteristics
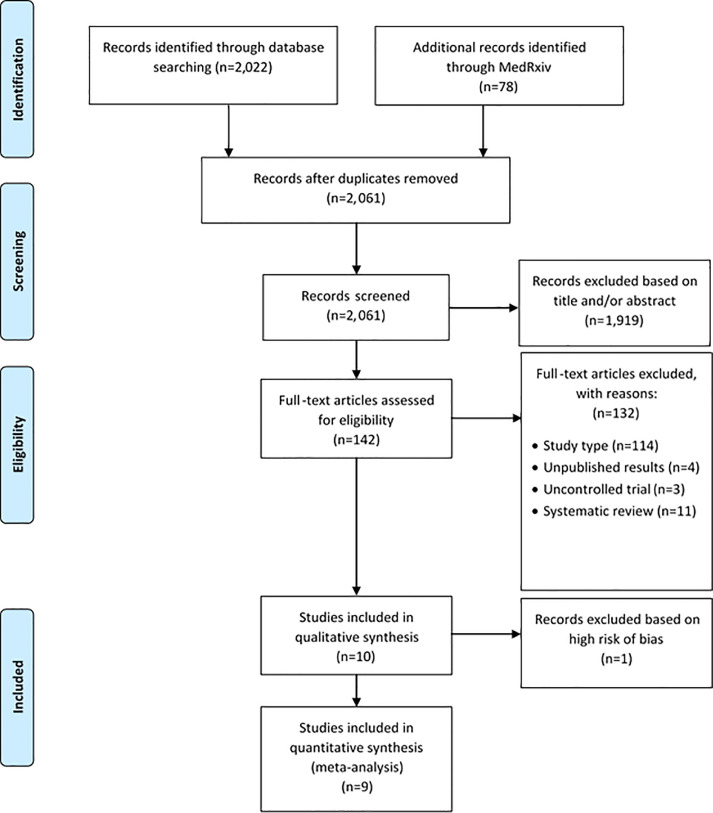
The systematic literature search identified 2,061 articles, of which 10 RCTs met the pre-defined inclusion criteria (Fig. 1 ).11 , 12 , 22, 23, 24, 25, 26, 27, 28, 29 Details of the included studies and baseline characteristics are shown in Tables 1 and 2 . A summary of study bias, assessed using RoB2, is shown in Supplementary Fig. 1. One study was excluded from further analyses due to a high risk of bias, with small participant numbers (total sample size, n = 26).29 Of the nine RCTs included, seven were deemed to have a low risk of bias, 12 , 22, 23, 24, 25, 26, 27 and two were considered to have some concerns for bias.11 , 28 Five of the studies had been published in a peer-reviewed journal,11 , 12 , 24, 25, 26 and four were awaiting publication.22 , 23 , 27 , 28 All studies reported the study process for randomisation; four had a double-blind design;22 , 23 , 25 , 26 five were open label.11 , 12 , 24 , 27 , 28 Five studies deviated from either the intended intervention or intended primary or secondary outcomes.11 , 12 , 22 , 24 , 26 One study (RECOVERY) had significant incomplete data that were not accounted for.28 Three studies had some bias in their selection of reported results.11 , 24 , 26 Tocilizumab was the sole intervention in seven studies.11 , 12 , 23, 24, 25, 26 , 28 Sarilumab was the sole intervention in one study, with two dosing regimens (200 mg and 400 mg subcutaneous injections).22 REMAP-CAP examined tocilizumab 8 mg/kg IV infusion (one to two doses) and sarilumab 400 mg IV infusion (single dose) in separate intervention arms.27 Four studies were conducted in multiple countries globally;22 , 23 , 26 , 27 five studies were conducted in single countries.11 , 12 , 24 , 25 , 28 RECOVERY was the largest study (n = 4,116), and was conducted in the UK.28 There was heterogeneity in study inclusion criteria: eight studies required a specific hypoxia threshold;11 , 12 , 23 – 28 four studies required a CRP threshold;11 , 12 , 25 , 28 seven studies required a pre-defined radiographic change.11 , 12 , 22, 23, 24 , 26 , 28 The EMPACTA study targeted sites with a high proportion of minority ethnic groups deemed at high risk of COVID-19 mortality.26 One study recruited patients solely from critical care, however 86% of these patients were not receiving invasive mechanical ventilation at baseline.27 Three studies excluded mechanically ventilated patients at baseline.12 , 24, 26
Fig. 1.
PRISMA flowchart of studies identified from the systematic literature search.
Adapted from: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(6): e1000097. doi:10.1371/journal.pmed1000097.
Table 1.
Summary of included studies: study characteristics.
| Study | Primary Author, year | Blinding | Intervention drug/dose/route (n) | Comparator (n) | Trial period | Country (no. of sites) | Background COVID-19 Incidence (per 100,000 population) | Background COVID-19 Mortality (per 100,000 population) | Human development index |
|---|---|---|---|---|---|---|---|---|---|
| COVACTA 23 | Rosas, 2020 | Double-blind | Tocilizumab 8 mg/kg, IV (294) | Placebo (144) | 3 April–24 June, 2020 | Canada (3), Denmark (4), France (7), Germany (4), Italy (2), Netherlands (4), Spain (7), UK (7), US (23) | 115.9 | 12.6 | 0.924 |
| RCT-TCZ-COVID-19 12 | Salvarani, 2020 | Open-label | Tocilizumab 8 mg/kg, IV (60) | Standard of care (66) | 31 March–11 June, 2020 | Italy (24) | 115.7 | 17.2 | 0.892 |
| CORIMUNO-TOCI-1 24 | Hermine, 2020 | Open-label | Tocilizumab 8 mg/kg, IV (63) | Standard of care (67) | 31 March–18 April, 2020 | France (9) | 82.4 | 17.6 | 0.901 |
| BACC Bay 25 | Stone, 2020 | Double-blind | Tocilizumab 8 mg/kg, IV (161) | Placebo (82) | 20 April–15 June, 2020 | US (7) | 123.4 | 9.9 | 0.926 |
| EMPACTA 26 | Salama, 2020 | Double-blind | Tocilizumab 8 mg/kg, IV (249) | Placebo (128) | 14 May–30 Sep, 2020 | Brazil, Kenya, Mexico, Peru, South Africa, US (Total n = 69) | 280.7 | 6.6 | 0.926 |
| REMAP-CAP IL-6 | |||||||||
| Tocilizumab 27 | Gordon, 2021 | Open-label | Tocilizumab 8 mg/kg, IV (353) | Standard of care (201) | 19 April–19 Nov, 2020 | UK (98), Netherlands (7), Australia (3), New Zealand (2), Ireland (1), Saudi Arabia (1) | 491.3 | 14.8 | 0.933 |
| REMAP-CAP IL-6 Sarilumab 27 | Gordon, 2021 | Open-label | Sarilumab 400 mg, IV (48) | Standard of care (201) | 19 April–19 Nov, 2020 | UK (98), Netherlands (7), Australia (3), New Zealand (2), Ireland (1), Saudi Arabia (1) | 491.3 | 14.8 | 0.933 |
| TOCIBRAS 11 | Veiga, 2021 | Open-label | Tocilizumab 8 mg/kg, IV (65) | Standard of care (64) | 8 May–17 July, 2020 | Brazil (9) | 244.9 | 6.9 | 0.765 |
| Lescure Sarilumab 200 mg 22 | |||||||||
| Lescure, 2021 | Double-blind | Sarilumab 200 mg, SC (159) | Placebo (42) | 28 March–3 July, 2020 | Argentina, Brazil, Canada, Chile, France, Germany, Israel, Italy, Japan, Russia, and Spain (Total n = 45) | 262.5 | 9.9 | 0.848 | |
| Lescure Sarilumab 400 mg 22 | |||||||||
| Lescure, 2021 | Double-blind | Sarilumab 400 mg, SC (173) | Placebo (42) | 28 March–3 July, 2020 | Argentina, Brazil, Canada, Chile, France, Germany, Israel, Italy, Japan, Russia, and Spain (Total n = 45) | 262.5 | 9.9 | 0.848 | |
| RECOVERY 28 | Horby, 2021 | Open-label | Tocilizumab 400–800 mg IV (2022) | Standard of care (2,094) | 23 April–24 Jan, 2021 | UK (131) | 1114.3 | 24.8 | 0.932 |
| Peking 29 TCZ and Favipiravir | Zhao, 2020 | Open-label | Tocilizumab 4–8 mg/kg, IV with Favipiravir 600–1600 mg, PO (14) | Favipiravir, 600–1600 mg, PO (3.5) | 2 February–15 March, 2020 | China (4) | 3.71 | 0.1 | 0.761 |
| Peking 29 TCZ | Zhao, 2020 | Open-label | Tocilizumab 4–8 mg/kg, IV (5) | Favipiravir 600–1600 mg, PO (3.5) | 2 February–15 March, 2020 | China (4) | 3.71 | 0.1 | 0.761 |
Summary table of studies identified from a systematic search for RCTs in adults with COVID-19, comparing IL-6 inhibitors (tocilizumab or sarilumab) with standard of care, placebo or alternative treatments. Country-level peak 14-day COVID-19 incidence (per 100,000 population) and mortality rates (per 100,000 population) are shown for countries included within each study over the study time window (data from the European Centre for Disease Prevention and Control). TCZ: tocilizumab; IV: intravenous; SC: subcutaneous; PO: oral.
Table 2.
Summary table of the included studies: baseline characteristics.
| Study | Age intervention, Mean (SD) | Age control, Mean (SD) | Male sex intervention, n (%) | Male sex control, n (%) | Minority ethnicity intervention, n (%) | Minority ethnicity control, n (%) | CRP Intervention mg/L, Median (IQR) | CRP Control mg/L, Median (IQR) | Diabetes mellitus intervention, n (%) | Diabetes mellitus control, n (%) | Hypertension intervention, n (%) | Hypertension control, n (%) | Mechanical ventilation intervention, n (%) | Mechanical ventilation control, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COVACTA 23 | 60.9 (14.6) | 60.6 (13.7) | 205 (69.7) | 101 (70.1) | 79 (26.8) | 47 (32.6) | 168.4 (101.4)† | 172.6 (114)† | 105 (35.7) | 62 (43.1) | 178 (60.5) | 94 (65.3) | 111 (37.8) | 54 (37.5) |
| RCT-TCZ-COVID-19 12 | 61.5 (51.5–73.5)* | 60 (54.0–69.0)* | 40 (66.7) | 37 (56.1) | – | – | 105 (50–146) | 65 (32–118) | 10 (16.7) | 9 (13.6) | 27 (45) | 29 (43.9) | 0 (0) | 0 (0) |
| CORIMUNO-TOCI-1 24 | 64 (57.1–74.3)* | 63.3 (57.1–72.3)* | 44 (70) | 44 (66) | – | – | 119.5 (74.5–219.5) | 127 (84–171) | 20 (33) | 23 (34) | – | – | 0 (0) | 0 (0) |
| BACC Bay 25 | 61.6 (46.4–69.7)* | 56.5 (44.7–67.8)* | 96 (60) | 45 (55) | 32 (20) | 19 (23) | 116 (67.1–190.6) | 94.3 (58.4–142) | 45 (28) | 30 (37) | 80 (50) | 38 (46) | 15 (9.3) | 6 (7.3) |
| EMPACTA 26 | 56 (14.3) | 55.6 (14.9) | 150 (60.2) | 73 (57) | 96 (38.6) | 56 (43.7) | 124.5 (2.5–2099) | 143.4 (9–3776) | 105 (42.2) | 48 (37.5) | 119 (47.8) | 63 (49.2) | 0 (0) | 0 (0) |
| REMAP-CAP IL-6 | ||||||||||||||
| Tocilizumab 27 | 61.5 (12.5) | 61.1 (12.8) | 261 (73.9) | 141.5 (70.4) | 55 (24.2) | 30.5 (21.9) | 150 (85–221) | 130 (71–208) | 123 (35.2) | 75 (37.4) | – | – | 104 (29.5) | 60.5 (30.1) |
| REMAP-CAP IL-6 Sarilumab 27 | 63.4 (13.4) | 61.1 (12.8) | 39 (81.3) | 141.5 (70.4) | 9 (23.1) | 30.5 (21.9) | 136 (105–204) | 130 (71–208) | 13 (27.1) | 75 (37.4) | – | – | 8 (16.7) | 60.5 (30.1) |
| TOCIBRAS 11 | 57.4 (15.7) | 57.5 (13.5) | 44 (68) | 44 (69) | – | – | 160 (104)† | 193 (283)† | 22 (34) | 20 (31) | 30 (46) | 34 (53) | 11 (17) | 10 (15.6) |
| Lescure Sarilumab 200 mg 22 | ||||||||||||||
| 58 (51.0–67.0)* | 60 (53.0–69.5)* | 108 (67.9) | 27 (64.3) | 8 (5) | 3.5 (8.3) | 94.1 (44.6–176.8) | 95.5 (55.5–184.4) | 45 (28.3) | 9 (21.4) | 68 (42.8) | 19.5 (46.4) | 17 (10.7) | 4.5 (10.7) | |
| Lescure Sarilumab 400 mg 22 | ||||||||||||||
| 58 (48.0–67.0)* | 60 (53.0–69.5)* | 99 (57.2) | 27 (64.3) | 14 (8.1) | 3.5 (8.3) | 96.1 (48.1–160.6) | 95.5 (55.5–184.4) | 47 (27.2) | 9 (21.4) | 70 (40.5) | 19.5 (46.4) | 24 (13.9) | 4.5 (10.7) | |
| RECOVERY 28 TCZ | 63.3 (13.7) | 63.9 (13.6) | 1335 (66) | 1437 (68.6) | 341 (16.9) | 357 (17) | 143 (107–203) | 144 (106–205) | 569 (28.1) | 600 (28.7) | – | – | 268 (13.3) | 294 (14) |
| Peking 29 TCZ and Favipriavir | 75* | 70* | 6 (42.9) | 2.5 (71.4) | – | – | – | – | 1 (7.1) | 0.5 (14.3) | 6 (42.9) | 1.5 (42.9) | 0 (0) | 0 (0) |
| Peking 29 TCZ | 71* | 70* | 3 (60) | 2.5 (71.4) | – | – | – | – | 1 (20) | 0.5 (14.3) | 2 (40) | 1.5 (42.9) | 0 (0) | 0 (0) |
Summary table of baseline characteristics of studies identified from a systematic search for RCTs in adults with COVID-19, comparing IL-6 inhibitors (tocilizumab or sarilumab) with standard of care, placebo or alternative treatments. Minority ethnicity was defined as any ethnicity other than the major ethnic group within each individual study. CRP: C-reactive protein; HTN: hypertension; BMI: body mass index. *Median (IQR), †Mean (SD).
Across the studies, mean age ranged from 56 to 64 years old; the proportion of male subjects ranged from 58% to 73%. Of the six studies that reported ethnicity, the proportion of minority ethnic groups ranged from 6% to 40%; COVACTA and EMPACTA recruited the highest proportions of minority ethnic groups at 29% and 40%, respectively. The proportion of patients with diabetes mellitus ranged from 15% to 41%. In the studies reporting hypertension and BMI, the proportion of subjects with hypertension ranged from 42% to 62%; median BMI ranged from 28 to 32 kg/m2. Median CRP at baseline ranged from 84 to 176 mg/L.
Peak 14-day COVID-19 incidence during the study recruitment window for countries included within each study ranged from 82/100,000 (CORIMUNO-TOCI-1, France) to 1,114/100,000 (RECOVERY, UK); peak 14-day COVID-19 mortality rate ranged from 6.6/100,000 (EMPACTA) to 24.8/100,000 (RECOVERY). The HDI varied from 0.765 (TOCIBRAS, Brazil) to 0.933 (REMAP-CAP, Global).
Meta-analysis of 28-day mortality, comparing IL-6 inhibition to standard of care or placebo
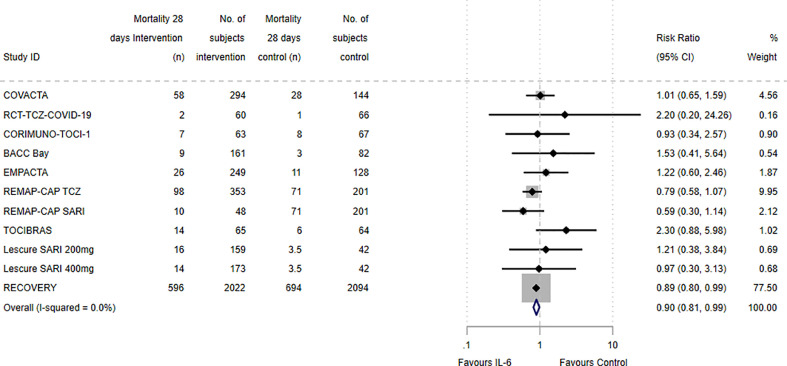
Meta-analysis of the risk ratio for 28-day mortality between intervention and control arms in the nine included RCTs is presented in Fig. 2 . The majority of studies reported mortality at 28 days; RCT-TCZ-COVID-19 reported at day 30, TOCIBRAS and Lescure et al. at day 29, and REMAP-CAP at day 21. The overall risk ratio for mortality across all included studies was 0.90 (95% CI: 0.81, 0.99) in favour of benefit for IL-6 inhibition relative to placebo or standard of care, with an I 2 of 0% (95% CI: 0, 53%). The RECOVERY study demonstrated a significant benefit in 28-day mortality, favouring tocilizumab (400 mg to 800 mg IV infusion, weight-based dosing) combined with standard of care, over standard of care alone: risk ratio, 0.89 (95% CI: 0.80, 0.99). Due to its large participant number relative to other included studies, RECOVERY was assigned a weight of 77.5% in the meta-analysis. The individual tocilizumab and sarilumab arms of REMAP-CAP showed no significant benefit in 21-day mortality in our analyses; this differs from the results of adjusted analyses performed by the REMAP-CAP authors, which did show mortality benefit for the individual treatment arms. The remaining studies demonstrated no significant differences in mortality between IL-6 inhibition or placebo/standard of care; however, as indicated by the low I 2 statistic, the effect estimates were all consistent with the small treatment effect in favour of IL-6 inhibition. Sensitivity analysis excluding RECOVERY demonstrated a similar effect estimate, although with wider confidence intervals (risk ratio, 0.92; 95% CI: 0.75, 1.13). There were insufficient data to meta-analyse secondary outcomes, including 14-day mortality or ICU admissions.
Fig. 2.
Forest plot of the 28-day mortality risk ratios between intervention and control arms.
Meta-analysis of the relative risk of mortality between intervention and control arms of included studies, expressed as risk ratios with 95% confidence intervals (CI), depicted graphically as a forest plot. The majority of studies reported mortality at 28 days; RCT-TCZ-COVID-19 reported at day 30, TOCIBRAS and Lescure et al. at day 29, and REMAP-CAP at day 21. The relative weighting of each study from a random effects model is shown. For studies comparing two interventions (either different doses or different IL-6 inhibitors), the intervention arms are included separately, whilst the number of patients contributing to the control arms were divided equally for use as a comparator. Heterogeneity between studies was assessed using I² statistics. TCZ: Tocilizumab; SARI: Sarilumab.
Meta-analysis of overall mortality rate
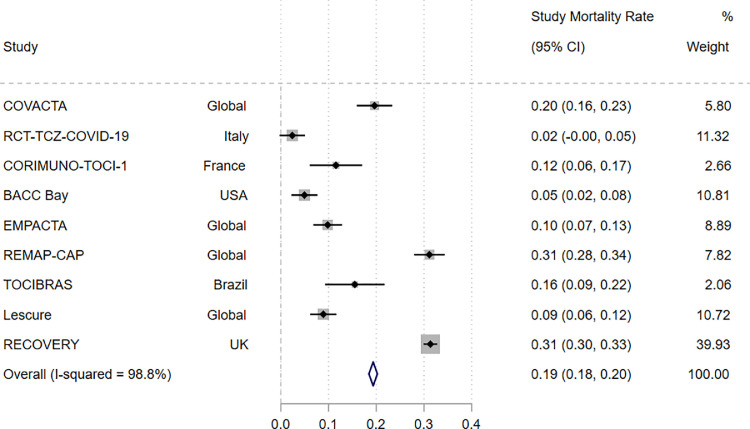
To evaluate differences in mortality rates across trials, we explored population and design heterogeneity as explanatory factors. Meta-analysis of overall mortality rate from the combined intervention and control arms of each study is presented in Fig. 3 . The mortality rate varied widely between studies, from 2% in RCT-TCZ-COVID-19 to 31% in REMAP-CAP and RECOVERY. The combined mortality rate across the studies was 19% (95% CI: 18, 20%), with an I2 of 98.8% (95% CI: 98.5, 99.1%) indicating high statistical heterogeneity across studies.
Fig. 3.
Comparison of incidence rate for mortality at day 28 in the combined intervention and control arms of each study.
Meta-analysis of overall mortality rate, with 95% confidence intervals (CI), for the combined intervention and control arms of each individual study, depicted graphically as a forest plot. The relative weighting of each study is shown. Heterogeneity between studies was assessed using I² statistics.
Meta-regression to explore variation in mortality
Meta-regression was performed to explore the relationship between mortality and individual-level predictors (age, sex, minority ethnicity, diabetes mellitus, hypertension and CRP) or population-level predictors (background COVID-19 incidence, background COVID-19 mortality rate, HDI). Meta-regression for BMI was not included due to a lack of reporting in studies.
In the analyses considering the risk ratios for 28-day mortality between intervention and control arms, meta-regression found no statistically significant associations (Supplementary Table 1 and Supplementary Figs. 2 to 10). This finding is unsurprising given the low statistical heterogeneity between study treatment effect estimates and no expected effect modification by the characteristics considered.
In the analyses considering overall mortality rate, meta-regression revealed that variability in mortality was explained by known patient factors associated with predictors of COVID-19 outcome (Supplementary Table 2 and Supplementary Figs. 11 to 19). Specifically, statistically significant associations were present for male sex (beta coefficient: 0.016; 95% CI: 0.007, 0.03; p = 0.004), median CRP (beta coefficient: 0.002; 95% CI: 0.00006, 0.005; p = 0.045), and hypertension (beta coefficient: 0.007; 95% CI: 0.0001, 0.013; p = 0.047). Background COVID-19 incidence was the only significant country-level predictor explaining variability in mortality rate (beta coefficient: 0.0003; 95% CI: 0.00008, 0.0005; p = 0.010).
Discussion
The headline finding from our primary analysis is that IL-6 inhibitor use is associated with a clinically meaningful improvement in outcomes for patients admitted with severe COVID-19 (pooled risk ratio 0.90; 95% CI: 0.81, 0.99). Our findings extend on previous inconclusive findings of two IL-6 inhibition meta-analyses.9 , 30 Our new analysis is heavily influenced by a single very large study, RECOVERY, which accounted for over three quarters of the overall study weighting in the analysis. Whilst the other studies differ in their point estimates, it is crucial to appreciate that the results are not inconsistent with one another; the confidence intervals of the smaller studies are wide, and all include the point estimate observed in RECOVERY. Sensitivity analysis excluding RECOVERY provided a treatment effect estimate that was very similar to the primary analysis.
The findings highlight the limitations of small study sizes. Even TOCIBRAS, a Brazilian study that was stopped prematurely due to concerns of higher mortality in the tocilizumab arm, had a 95% confidence interval that included the value observed in RECOVERY. This highlights how difficult decisions can be for data monitoring and ethics committees providing trial oversight when faced with interim results on small numbers of participants, and therefore high uncertainty in parameter estimates.
A striking observation from our meta-analysis was that overall mortality varied widely across the studies, ranging from 2% to over 30%. Variation in the overall mortality between studies was considerable but explained by patient-level and country-level factors; these included differences in patient severity at enrolment, as well as country-level COVID-19 incidence.
Subgroup analyses within the RECOVERY trial found no effect modification attributable to age, sex, ethnicity, level of respiratory support, days since symptom onset, or use of systemic corticosteroids.28 We were able to replicate findings in our meta-regressions for age, sex and ethnicity, and extend to CRP and comorbidity. To date, there is no evidence that patient-level factors predict the effectiveness of IL-6 inhibition. The findings must be taken in the context of the study populations considered, which were generally people with severe COVID-19 disease. Importantly, the effects were consistent, irrespective of older age or CRP at study enrolment. We cannot rule out effect modification in people with non-severe (non-hospitalised) disease.
The observation that overall mortality rates in the trials were, in part, explained by country-level COVID-19 peak incidence during the trial window is an important observation. Disease incidence is likely to capture multiple country-specific attributes that contribute to mortality, including hospital bed pressures, staffing ratios, access to standard of care therapies (including high-flow oxygen or non-invasive ventilation), and the extent of virus spread amongst vulnerable populations. It is relevant to consider that the frequency of the primary outcome will also impact upon study power and treatment effect precision. Consistent with this, studies with wider confidence intervals were typically those in which the primary endpoint occurred less frequently.
Strengths and limitations
The findings of this analysis, as with any meta-analysis, must be taken within the context of the methodological heterogeneity between studies. However, despite large heterogeneity in mortality rates across studies, design heterogeneity was relatively low, and treatment effects were not observed to be inconsistent.
We have observed results for two IL-6 inhibition therapies (tocilizumab and sarilumab) and have made no attempt to separate effects. In REMAP-CAP, the effect size was greater for sarilumab, although the number treated with sarilumab was far smaller, and so the accompanying effect estimate was less precise. Given the similar mode of action, and comparable treatment effects and safety profiles in other therapeutic areas,31 we do not consider the differences between the IL-6 inhibitor drugs a priority for analysis, either our own or future studies.
Generalisability of our results to all countries may not be appropriate. As aforementioned, substantial weight was applied to RECOVERY, which took place entirely within the UK. It is possible that the benefits of IL-6 inhibition may not be apparent across all healthcare settings. The potential for the treatment to be offset by harm may be greater in countries with a higher background infection burden. For example, IL-6 inhibition has the potential to reactivate latent tuberculosis or viral hepatitis;32 , 33 in countries with a high background prevalence of these infections, the risk-benefit may differ.
Differences amongst studies in the definition of standard of care also exist. RECOVERY did explore the impact of background corticosteroid use and observed no difference in the benefits of IL-6 inhibition. However, insufficient data were available to examine the impact of co-administration of remdesivir, another treatment that is available as standard of care in some countries.
Conclusions
This updated systematic review and meta-analysis supports the use of IL-6 inhibition for the treatment of severe, hospitalised COVID-19 disease. The findings are heavily weighted by the results of a single large study conducted in the UK, although the results across all studies are consistent with the overall effect estimate. The benefits of IL-6 inhibition over the medium to long term remain unknown, as well as the effectiveness of therapy when instituted in low- or middle-income countries, where standard of care may differ to that delivered in the clinical trials.
Declaration of Competing Interest
JG receives speaker fees from Abbvie, Biovitrum, BMS, Celgene, Chugai, Gilead, Janssen, Lilly, Novartis, Pfizer, Roche, Sanofi, Sobi and UCB. MDR has received honoraria from Pfizer and UCB. BDC has received honoraria from Abbvie. MY has received honoraria from Abbvie and UCB. All other authors have nothing to declare.
JG, MDR, BDC, KB, MY, DN, ADB, JRH were investigators for the RECOVERY trial.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. MDR and VBA receive funding from the National Institute for Health Research. BDC receives funding from Innovate UK. ET, AB, VP, JRH contributed equally to this work.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2021.03.008.
Appendix. Supplementary materials
References
- 1.Aziz M., Haghbin H., Abu Sitta E., Nawras Y., Fatima R., Sharma S. Efficacy of tocilizumab in COVID-19: a systematic review and meta-analysis. J Med Virol. 2021;93(3):1620–1630. doi: 10.1002/jmv.26509. [DOI] [PubMed] [Google Scholar]
- 2.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 3.Rose-John S., Winthrop K., Calabrese L. The role of IL-6 in host defence against infections: immunobiology and clinical implications. Nat Rev Rheumatol. 2017;13(7):399–409. doi: 10.1038/nrrheum.2017.83. [DOI] [PubMed] [Google Scholar]
- 4.Cross L.J.M., Matthay M.A. Biomarkers in acute lung injury: insights into the pathogenesis of acute lung injury. Crit Care Clin. 2011;27(2):355–377. doi: 10.1016/j.ccc.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10) doi: 10.1101/cshperspect.a016295. a016295-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y. Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) Is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin Infect Dis. 2020;71(8):1937–1942. doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vardhana S.A., Wolchok J.D. The many faces of the anti-COVID immune response. J Exp Med. 2020;217(6) doi: 10.1084/jem.20200678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solis-García Del Pozo J., Galindo M.F., Nava E., Jordán J. A systematic review on the efficacy and safety of IL-6 modulatory drugs in the treatment of COVID-19 patients. Eur Rev Med Pharmacol Sci. 2020;24(13):7475–7484. doi: 10.26355/eurrev_202007_21916. [DOI] [PubMed] [Google Scholar]
- 9.Tleyjeh I.M., Kashour Z., Damlaj M., Riaz M., Tlayjeh H., Altannir M. Efficacy and safety of tocilizumab in COVID-19 patients: a living systematic review and meta-analysis. Clin Microbiol Infect. 2021;27(2):215–227. doi: 10.1016/j.cmi.2020.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2(8):e474–ee84. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veiga V.C., Prats J., Farias D.L.C., Rosa R.G., Dourado L.K., Zampieri F.G. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ. 2021;372:n84. doi: 10.1136/bmj.n84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salvarani C., Dolci G., Massari M., Merlo D.F., Cavuto S., Savoldi L. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Della-Torre E., Campochiaro C., Cavalli G., De Luca G., Napolitano A., La Marca S. Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: an open-label cohort study. Ann Rheum Dis. 2020;79(10):1277–1285. doi: 10.1136/annrheumdis-2020-218122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coronavirus » Interim Clinical Commissioning Policy: tocilizumab for hospitalised patients with COVID-19 pneumonia (adults). England.nhs.uk. https://www.england.nhs.uk/coronavirus/publication/interim-clinical-commissioning-policy-tocilizumab-for-hospitalised-patients-patients-with-covid-19-pneumonia-adults/. Accessed February 24, 2021.
- 15.Coronavirus » Interim Clinical Commissioning Policy: sarilumab for critically ill patients with COVID-19 pneumonia (adults). England.nhs.uk. https://www.england.nhs.uk/coronavirus/publication/interim-clinical-commissioning-policy-sarilumab-for-critically-ill-patients-with-covid-19-pneumonia-adults/. Accessed February 24, 2021.
- 16.Statement on Tocilizumab | COVID-19 Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/statement-on-tocilizumab/. Accessed February 24, 2021.
- 17.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Human Development Index (HDI) | Human Development Reports http://hdr.undp.org/en/content/human-development-index-hdi. Accessed February 24, 2021.
- 19.European Centre for Disease Prevention and Control; 2021. COVID-19 situation update for the EU/EEA, as of week 6.https://www.ecdc.europa.eu/en/cases-2019-ncov-eueea Accessed February 24, 2021. Accessed February 24. [Google Scholar]
- 20.Higgins J.P., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A. Cochrane; 2020. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020)www.training.cochrane.org/handbook Available from. Accessed February 24, 2021. [Google Scholar]
- 22.Lescure F.-.X., Honda H., Fowler R.A., Lazar J.S., Shi G., Wung P., et al. Sarilumab treatment of hospitalised patients with severe or critical COVID-19: a multinational, randomised, adaptive, phase 3, double-blind, placebo-controlled trial. medRxiv 2021: 2021.02.01.21250769.
- 23.Rosas I.O., Bräu N., Waters M., Go R., Hunter B.D. , Bhagani S. Tocilizumab in hospitalized patients with COVID-19 pneumonia. medRxiv 2020: 2020.08.27.20183442.
- 24.Hermine O., Mariette X., Tharaux P.L., Resche-Rigon M., Porcher R., Ravaud P. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stone J.H., Frigault M.J., Serling-Boyd N.J., Fernandes A.D., Harvey L., Foulkes A.S. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salama C., Han J., Yau L., Reiss W.G., Kramer B., Neidhart J.D. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2020;384(1):20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon A.C., Mouncey P.R., Al-Beidh F., Rowan K.M., Nichol A.D., Arabi Y.M. Interleukin-6 receptor antagonists in critically ill patients with Covid-19 – preliminary report. medRxiv 2021: 2021.01.07.21249390.
- 28.Horby P.W., Pessoa-Amorim G., Peto L., Brightling C.E., Sarkar R., Thomas K. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial. medRxiv 2021: 2021.02.11.21249258.
- 29.Zhao H., Zhu Q., Zhang C., Li J., Wei M., Qin Y. Tocilizumab combined with favipiravir in the treatment of COVID-19: a multicenter trial in a small sample size. Biomed Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.110825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lan S.H., Lai C.C., Huang H.T., Chang S.P., Lu L.C., Hsueh P.R. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents. 2020;56(3) doi: 10.1016/j.ijantimicag.2020.106103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emery P., Rondon J., Parrino J., Lin Y., Pena-Rossi C., van Hoogstraten H. Safety and tolerability of subcutaneous sarilumab and intravenous tocilizumab in patients with rheumatoid arthritis. Rheumatology. 2019;58(5):849–858. doi: 10.1093/rheumatology/key361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cantini F., Nannini C., Niccoli L., Petrone L., Ippolito G., Goletti D. Risk of tuberculosis reactivation in patients with rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis receiving non-anti-TNF-targeted biologics. Mediators Inflamm. 2017;2017 doi: 10.1155/2017/8909834. 8909834- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen L.F., Mo Y.Q., Jing J., Ma J.D., Zheng D.H., Dai L. Short-course tocilizumab increases risk of hepatitis B virus reactivation in patients with rheumatoid arthritis: a prospective clinical observation. Int J Rheum Dis. 2017;20(7):859–869. doi: 10.1111/1756-185X.13010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.