Abstract
Background
Early antiretroviral therapy (ART) is necessary for HIV epidemic control and depends on early diagnosis and successful linkage to care. Since 2014, annual household-based HIV testing and counselling (HBHTC) and linkage services have been provided through the Chókwè Health and Demographic Surveillance System (CHDSS) for residents testing HIV-positive in this high HIV-burden district.
Methods
District-wide Test and Start (T&S, ART for all people living with HIV [PLHIV]) began in August 2016, supported by systematic interventions to improve linkage to care and treatment. Annual rounds (R) of random household surveys were conducted to assess trends in population prevalence of ART use and viral load suppression (VLS; <1000 viral RNA copies/mL).
Results
Between R1 (April 2014-April 2015) and R5 (April 2018-Mar 2019), 46,090 (67.2%) of 68,620 residents aged 15-59 years were tested for HIV at home at least once, and 3,711 were newly diagnosed with HIV and provided linkage services. Population prevalence of current ART use among PLHIV increased from 65.0% to 87.5% between R1 and R5. ART population prevalence was lowest among men aged 25-34 (67.8%) and women 15-24 (78.0%) years, and highest among women aged 35-44 (93.6%) and 45-59 years (93.7%) in R5. VLS prevalence increased among all PLHIV aged 15-59 years from 52.0% in R1 to 78.3% in R5.
Discussion
Between 2014 and 2019, CHDSS residents surpassed the UNAIDS targets of ≥81% of PLHIV on ART and ≥73% virally suppressed. This achievement supports the combination of efforts from HBHTC, support for linkage to care and treatment, and continued investments in T&S implementation.
Keywords: antiretroviral therapy coverage, population viral suppression, UNAIDS 90 90 90
BACKGROUND
Mozambique is a low-income country in Southern Africa, with one of the highest HIV burdens in the world.1 In 2018, there were an estimated 2.2 million people living with HIV (PLHIV), and HIV prevalence among adults aged 15-49 years was 12.6%. The Southern region bears a disproportionate burden of disease, with the highest adult HIV prevalence in Gaza province (24.5%).2 The largely agricultural Chókwè District in Gaza province is home to the Chókwè Health and Demographic Surveillance System (CHDSS), through which annual household-based HIV testing and counselling (HBHTC), an HIV prevention survey, and linkage to HIV care services have been provided since 2014.
Early antiretroviral therapy (ART) reduces morbidity and mortality among PLHIV, prevents transmission, and when high population-level ART coverage is achieved, reduces individual risk for HIV acquisition.3-6 ART scale-up is therefore critical for the control of the HIV epidemic. In Mozambique, ART coverage over the last decade has improved remarkably, with a 37-fold increase in annual ART enrollment between 2004 and 2013, and 1.2 million PLHIV on treatment by 2018.7,8 Low rates of diagnosis, poor linkage to care, and loss to follow up, however, remain significant barriers to achieving the globally-endorsed Joint United Nations Program on HIV and AIDS (UNAIDS) targets of ≥90% of PLHIV diagnosed (first 90), ≥90% of diagnosed PLHIV on ART (second 90, representing 81% of all PLHIV), and ≥90% of PLHIV on ART with viral load suppression (third 90, representing 73% of all PLHIV) by 2020.2,9 While worldwide, population-level UNAIDS target achievement was 79%, 62% (78% of those diagnosed on ART) and 53% (86% of those on ART with VLS) in 2018;10 Mozambique’s achievement for the first and second 90s were only 72% and 56%, respectively (third 90 not reported).1
Early ART initiation and retention is often dependent on early diagnosis, and the World Health Organization (WHO) recommends, among several HIV testing options, comprehensive HBHTC to increase testing uptake, especially among individuals who may not be easily reached through facility-based services, and provision of follow-up linkage services to those who test HIV positive.11,12 However, in several widely-disseminated studies of HBHTC – even in the context of Test and Start (T&S, ART for all PLHIV), population-level ART coverage and HIV incidence declines remained sub-optimal, specifically due to poor linkage to ART after HIV diagnosis.13-15 Achievement of the UNAIDS 90 90 90 goals thus requires a successful combination of HIV case-finding as well as adoption of streamlined linkage to universal, patient-centered, life-long ART.
While trends in HIV diagnostic coverage will largely be presented elsewhere, here we report trends in population prevalence of current ART use and viral load suppression (VLS, <1000 viral RNA copies/mL) among HIV-positive residents aged 15-59 years who were surveyed through the CHDSS from 2014-2019, in the context of annual HBHTC, T&S (beginning in August 2016), and strengthened support for streamlined linkage to and delivery of HIV care and treatment. To supplement understanding of these trends in the second and third 90’s, we also report trends in receipt of linkage to ART services among PLHIV who recently tested HIV-positive; use of facility-based fast-tracking and community ART support groups for antiretroviral (ARV) refills, self-reported prevalence of ever missing a complete ARV dose among those initiated on ART, and self-reported ART-related knowledge and beliefs among all PLHIV.
METHODS
Intervention
HBHTC
The CHDSS catchment area encompasses approximately half of Chókwè District’s population (almost 100,000 residents within approximately 20,000 households in Chókwè city and seven surrounding villages in Gaza) and, since 2014, has provided annual rounds of home-based, door-to-door HIV testing. The first round of HIV surveillance (R1) was conducted from April 2014-April 2015, followed by R2 from May-December 2015, R3 from May-December 2016, R4 from March-November 2017, and R5 from April 2018-March 2019. During each round, all CHDSS households were visited and consenting residents offered HBHTC and a brief interview; in R1, because 99% of residents who reported a prior HIV diagnosis tested HIV-positive through the CHDSS, those who reported a prior HIV diagnosis in subsequent rounds could opt out of testing.16 HIV counselling and testing was performed by certified lay counselors according to Mozambique’s national rapid HIV testing algorithm.17
Linkage Services
All identified HIV-positive participants who reported not currently being in HIV care or on ART (both newly and self-reported to be previously diagnosed) were counselled and provided with linkage to care services. Lay counsellors helped patients choose a clinic to receive ART services, and assessed potential barriers to enrollment (e.g., low perceived need for ART, fear of stigma and discrimination, transportation costs, etc.) at the first post-test counselling session. Counsellors then conducted up to five home visits in the subsequent six months during which they determined whether patients had enrolled in HIV care, and provided psychosocial support, informational counseling, and encouragement to help overcome new or remaining barriers to care. When T&S was adopted, facility-based peer educators were hired to provide specific community outreach to improve patient navigation, case management, and linkage from community-based testing to facility-based services. A community program officer was also hired to supervise lay staff, and provide oversight and coordination for linkage and retention activities in Chókwè.
Test and Start
In March 2016, ART eligibility in Mozambique was expanded from a CD4 count <350/μL (R1-R2 [2014-2015]) to <500/μL (first half of R3, 2016), and a phased implementation of T&S was planned. Since 2013, lifelong ART was offered to all pregnant women in Mozambique, regardless of CD4 count. On August 29, 2016, the government of Mozambique began T&S in high-burden districts, including most provincial capitals. Chókwè District was the only rural district included in this first phase of T&S, and all ART facilities in CHDSS began T&S before R4 (2017). T&S was supported through healthcare worker trainings, community sensitization and interventions to improve service delivery such as through client-centered, “differentiated” models of care including facility-based fast-track services (where patients could obtain ARV refills without seeing a healthcare provider every time) and community ART support groups (where member patients could obtain ARV refills without having to visit healthcare facilities). Participation in these models was assessed during the brief HBHTC interview for patients reporting a prior HIV diagnosis and ART use.
Data Collection
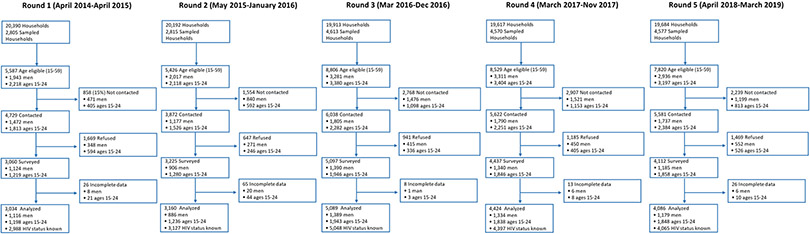
In a random sample of CHDSS households (10% in R1-R2 and 20% in R3-R5, stratified by urban versus rural location and gender), consenting residents aged 15-59 years were asked to participate in a 20-30 minute paper-based (R1) or tablet-based (R2-R5) HIV prevention survey (HPS). HPS included single questions or items on ART-related knowledge (e.g., awareness of prescribed ARVs), beliefs (e.g., perceived efficacy of ARVs for a long life), and behaviors (e.g., receipt of linkage to ART services and ever missed complete ARV dose). Depending on the question or item, responses were measured as “yes/no,” “agree/disagree,” “not applicable,” or “don’t know.” In each round, between 2,805-4,613 households were sampled, and of 5,426-8,806 household members aged 15-59 years identified, 3,872–6,038 (66-85%) were contacted, of whom 3,034–5,089 (64-84%) participated in HPS and were included in analyses (Figure 1).
Figure 1:
CHDSS HIV Prevention Survey (HPS) Enrollment Cascade
For consenting HIV-positive HPS participants, up to 1 mL of whole blood was collected, transported on ice to the CHDSS laboratory within eight hours and processed for CD4 testing using the Becton Dickinson FACS Count. Remnant specimens after CD4 testing were used to make dried blood spot (DBS) cards. DBS cards were stored at −80°C, and specimens from R1-R3 were sent to the U.S. Centers for Disease Control and Prevention (CDC) laboratory in Atlanta for viral load testing. Viral load testing was performed with the Roche (Pleasanton, CA, USA) COBAS® AmpliPrep/COBAS® TaqMan® (CAP/CTM) HIV-1 Test, version 2.0 using the free virus elution protocol.18 Once VL testing capacity was established in-country in 2019, specimens collected from R4-R5 were tested at the Instituto Nacional de Saúde, Marracuene, Mozambique using the same Roche testing methodology as in Atlanta.
Definition of Current ART Use
Participants who reported previously testing HIV positive were asked about prior and current engagement in HIV care and current use of ART. Self-reported ART use was considered verified if a client showed a current ARV pill bottle to the interviewer. Given the known low sensitivity of self-reported ART use (77%),19 and that only 1% of PLHIV not using ART are expected to achieve durable VLS,20 all PLHIV with VLS were defined as using ART.
Data Analysis
Prevalence of HIV infection, ART use, VLS, and other reported outcomes from HPS were estimated with SAS SURVEYFREQ (SAS version 9.3, SAS Institute Inc., Cary, NC, USA). All estimates were weighted to the 2016 census using inverse probability weights for sex, age group, and geographic area. Standard errors were adjusted for the survey design (census-based weights and clustering within households).
Ethics
This study received ethical approval by the CHDSS Community Advisory Board, National AIDS Control Program of the Mozambique Ministry of Health, and the Mozambique National Bioethics Committee. The protocol was also reviewed in accordance with CDC human research protection procedures and was determined to be research, but CDC investigators did not interact with human subjects or have access to identifiable data or specimens for research purposes. Voluntary, written informed consent was obtained from all study participants before conducting any study interviews or biomedical testing.
RESULTS
HIV Diagnosis and Case Finding through HBHTC
During R1-R5, 46,090 (67.2%) of the 68,620 CHDSS residents who were aged 15-59 years at any point during the study tested for HIV at home at least once, and 3,711 (26.4% men and 25.9% persons aged 15-24 years) were newly HIV diagnosed and provided linkage services. An additional 396 previously HIV diagnosed clients who reported not being in HIV care or on ART (23.5% men and 19.4% persons aged 15-24 years), were identified and also provided with linkage to care services.
HPS Participant Characteristics
Across rounds, men (27-37%) and urban residents (37-46%) were less represented in the survey and analysis than women, older adults, and rural populations. Estimated HIV prevalence decreased slightly over time, from 25.1% (95% confidence interval [CI]: 23.3-26.9) in R1 to 22.9% (95% CI 21.3-24.6) by R5.
Of 4,907 HIV-positive participants across all five rounds, 74-84% in all rounds were women, median age ranged between 36 (interquartile range [IQR]: 29-44) in R1 and 38 (IQR: 30-46) in R5, and most people lived in a rural area. The proportion of PLHIV with advanced disease (CD4 <200 cells/μL) ranged from 17.7% in R3 to 9.1% in R5 (Table 1). Awareness of HIV status (prior HIV diagnosis) increased from 73.8% (95% CI: 70.3-77.2) in R1 to 95.4% (95% CI: 93.6-97.1) in R5.
Table 1:
Descriptive characteristics of HIV-positive* HIV Prevention Survey participants, by round
| Round 1 (Apr 2014- Apr 2015) |
Round 2 (May-Dec 2015) |
Round 3 (May-Dec 2016) |
Round 4 (Mar-Nov 2017) |
Round 5 (Apr 2018- Mar 2019) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | N | % | N | % | N | % | N | % | N | % |
| Total | 758 | 807 | 1331 | 1065 | 946 | |||||
| Gender | ||||||||||
| Male | 200 | 26.4 | 155 | 19.2 | 223 | 16.8 | 212 | 19.9 | 150 | 15.9 |
| Female | 558 | 73.6 | 652 | 80.8 | 1108 | 83.2 | 853 | 80.1 | 796 | 84.1 |
| Age | ||||||||||
| 15-24 | 89 | 11.7 | 103 | 12.8 | 155 | 11.6 | 115 | 10.8 | 108 | 11.4 |
| 25-34 | 253 | 33.4 | 259 | 32.1 | 361 | 27.1 | 292 | 27.4 | 266 | 28.1 |
| 35-44 | 244 | 32.2 | 231 | 28.6 | 453 | 34.0 | 356 | 33.4 | 302 | 31.9 |
| 45-59 | 172 | 22.7 | 214 | 26.5 | 362 | 27.2 | 302 | 28.4 | 270 | 28.5 |
| Marital status | ||||||||||
| Single | 61 | 8.0 | 127 | 15.7 | 299 | 22.5 | 145 | 13.6 | 136 | 14.4 |
| Married or living together | 501 | 66.1 | 501 | 62.1 | 780 | 58.6 | 652 | 61.2 | 556 | 58.8 |
| Divorced/separated/widowed | 196 | 25.9 | 179 | 22.2 | 249 | 18.7 | 268 | 25.2 | 253 | 26.7 |
| Urbanicity | ||||||||||
| Rural | 415 | 54.7 | 525 | 65.1 | 907 | 68.1 | 715 | 67.1 | 616 | 65.1 |
| Urban | 344 | 45.3 | 282 | 34.9 | 424 | 31.9 | 350 | 32.9 | 330 | 34.9 |
| CD4 cell count† | ||||||||||
| <200/μL | 81 | 12.1 | 91 | 15.5 | 184 | 17.7 | 85 | 10.8 | 70 | 9.1 |
| 200-<350/μL | 140 | 20.9 | 114 | 19.5 | 218 | 21.0 | 131 | 16.7 | 122 | 15.8 |
| 350-500/μL | 150 | 22.4 | 120 | 20.5 | 222 | 21.4 | 178 | 22.6 | 156 | 20.2 |
| >500/μL | 299 | 44.6 | 261 | 44.6 | 413 | 39.8 | 392 | 49.9 | 423 | 54.9 |
The total number of participants surveyed and analyzed (regardless of HIV status) was 3,034 in R1, 3,160 in R2, 5,089 in R3, 4,424 in R4, and 4,086 in R5 (see Figure 1)
not all HPS participants had a blood sample taken or were successfully tested for CD4
Trends in Receipt of Linkage Services
Of HPS participants who reported first testing HIV positive in the past 12 months, the proportion who reported meeting with someone in Chókwè District who told them it was their job to help with their HIV diagnosis and enrollment in care (receipt of linkage services) increased from 43.0% (95% CI: 31.3-54.7) in R1 to 82.1% (95% CI: 78.1-86.1%) in R5; the greatest increases in this proportion occurred among men and PLHIV aged 35-44 years (Table 2). By R5, 95.8% (95% CI: 92.7-98.9) of newly diagnosed PLHIV who reported receiving this service reported that it helped them cope with their diagnosis and 96.0% (95% CI: 92.9-99.1) reported that it helped them enroll in HIV care.
Table 2:
Self-reported uptake of linkage-to-care services among survey participants who tested HIV positive for the first time in the past 12 months, by Chókwè Health and Demographic Surveillance System round.*
| Round 1 (Apr 2014-Apr 2015) N=86 |
Round 2 (May-Dec 2015) N=250 |
Round 3 (May-Dec 2016) N=398 |
Round 4 (Mar-Nov 2017) N=447 |
Round 5 (Apr 2018-Mar 2019) N=362 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI |
| Total | 43.0 | 31.5-54.5 | 72.0 | 65.8-78.3 | 76.0 | 70.6-81.4 | 85.5 | 81.7-89.4 | 82.1 | 78.1-86.1 |
| Gender | ||||||||||
| Male | 31.8 | 4.7-59.0 | 78.7 | 65.1-92.2 | 75.4 | 61.6-89.3 | 84.6 | 75.9-93.2 | 90.5 | 83.7-97.3 |
| Female | 45.3 | 33.3-57.3 | 69.9 | 63.1-76.7 | 76.2 | 71.0-81.4 | 85.9 | 81.8-90.0 | 79.6 | 75.0-84.2 |
| Age | ||||||||||
| 15-24 | 58.2 | 35.1-81.4 | 72.3 | 54.7-89.8 | 71.9 | 57.8-86.0 | 72.3 | 56.9-87.7 | 72.6 | 58.3-87.0 |
| 25-34 | 49.9 | 32.3-67.4 | 68.6 | 57.8-79.5 | 74.3 | 64.2-84.4 | 83.2 | 75.9-90.5 | 80.1 | 72.3-88.0 |
| 35-44 | 32.1 | 12.6-51.6 | 77.7 | 66.9-88.5 | 80.0 | 71.6-88.4 | 88.7 | 82.6-94.9 | 84.3 | 78.0-90.6 |
| 45-59† | - | - | 69.4 | 56.5-82.3 | 74.4 | 62.3-86.5 | 89.1 | 82.4-95.7 | 84.3 | 77.5-91.2 |
| Urbanicity | ||||||||||
| Rural | 34.8 | 21.1-48.6 | 62.4 | 54.7-70.0 | 74.4 | 68.9-79.9 | 81.7 | 77.0-86.4 | 69.5 | 63.4-75.5 |
| Urban | 50.8 | 33.4-68.2 | 79.3 | 70.3-88.3 | 77.2 | 68.6-85.8 | 88.7 | 82.9-94.5 | 92.5 | 87.8-97.3 |
Patient self-report of meeting with someone in Chókwè who told them it was their job to help them with their HIV diagnosis and enrollment in care after they tested HIV positive within the past 12 months.
In R1 there were only 8 participants in the 45-59 age group; due to the small cell size, we did not estimate a linkage rate for this group
Trends in Prevalence of Current ART Use
Estimated population prevalence of current ART use among all PLHIV increased from 65.0% (95% CI: 61.2-68.7) in R1 to 87.5% (95% CI: 84.7-90.3) in R5 (Figure 2); 85.3% of PLHIV self-reporting current ART use showed their ARV pill bottle to a counsellor. Among previously diagnosed PLHIV aged 15-59 years, prevalence of current ART use increased from 88.1% (95% CI: 85.1-91.1) in R1 to 91.9% (95% CI: 89.5-94.3) in R5. Of participants reported to be on ART by R5, 22.3% (95% CI: 18.8-25.7) were newly diagnosed with HIV at home during prior CHDSS rounds.
Figure 2:
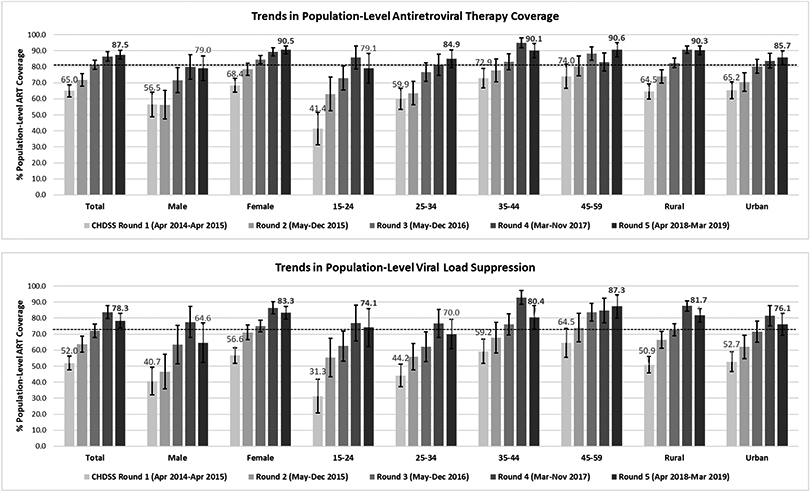
Trends in population prevalence of antiretroviral therapy (ART) coverage and viral load suppression (VLS) by Chókwè Health and Demographic Surveillance System round (bolded figures indicates achievement of the UNAIDS 2nd or 3rd 90 target, denoted by dotted lines)
*ART coverage includes self-reported coverage, verified coverage (if a ARV pill bottle was shown to the interviewing counsellor) and VLS-adjusted coverage (including all PLHIV with VLS (<1000 viral RNA copies/μL), in addition to all those self-reporting ART use
Increases in prevalence of ART use among all PLHIV occurred among both men and women, although by R5 the prevalence of current ART use for men (79.0%, 95% CI: 71.3-86.8) remained lower than that for women (90.5%, 95% CI: 88.0-93.0) (Figure 2). Similar increases occurred among all age groups, with prevalence of ART use by R5 higher than 90% for PLHIV aged 35-59 years. Although PLHIV aged 15-24 years had the lowest prevalence of ART use, prevalence in this group increased the most, from 41.4% (95% CI: 31.2-51.6) in R1 to 79.1% (95% CI: 69.8-88.4) by R5. By R5, prevalence of ART use among PLHIV remained lowest among men aged 25-34 (67.8%, 95% CI:49.9-85.7) years, but exceeded 81% among men aged 15-24 (84.8%, 95% CI 63.9-100) and 35-59 (82.5%, 95% CI: 73.8-91.2) years, and among women aged 25-59 years (92.0%, 95% CI 89.6-94.4).
Trends in Prevalence of Viral Load Suppression
Prevalence of VLS among all PLHIV increased from 52.0% (95% CI: 47.7-56.4) in R1 to 78.3% (95% CI: 73.8-82.8) in R5 (Figure 2). By R5, prevalence of VLS exceeded the UNAIDS 90-90-90 population target of 73% overall, for all sex, age and urbanicity categories except for men overall (64.6%, 95% CI: 52.3-76.9) and PLHIV aged 25-34 years (70.0, 95% CI: 60.8-79.2). Among PLHIV aged 15-59 years using ART, prevalence of VLS increased from 78.3% (95% CI: 74.1-82.6) in R1 to 86.5% (95% CI: 83.0-90.0) in R5. Prevalence of VLS among those who did not report using ART was 28.1% (95% CI:22.5-33.8), 28.4% (95% CI: 21.4-35.3), 30.2% (95% CI:21.6-38.9), 55.8% (95% CI: 41.0-70.6), and 30.6% (95% CI:17.2-44.0) in R1-R5, respectively.
Prevalence of ART-related Knowledge, Beliefs, & Behaviors
Prevalence of ART-related knowledge and beliefs among PLHIV was stable over time, including awareness of ART (88.4%-94.0%) and (among PLHIV with this awareness) endorsement of beliefs that PLHIV can live a long and healthy life if they adhere to ART (94.7%-99.9%), that ART is more effective than traditional medicine in treating HIV/AIDS (95.0%-99.2%), and that ART medications do not need to be hidden from others (76.6-87.3%).
Of PLHIV currently on ART, the prevalence of those reporting ever missing a complete dose was 8.4% (95% CI: 5.2-11.7) in R1, 9.5% (95% CI: 5.6-13.5) in R2, 7.5% (95% CI: 5.1-10.0) in R3, 3.7% (95% CI: 1.6-5.9) in R4, and 4.7% (95% CI: 2.6-6.8) in R5. Participation in community ART support groups (available since 2009) was low ranging from 1.0% (95% CI: 0.1-1.1) in R1 to 4.4% (95% CI: 2.8-6.0) in R5. Participation in fast-tracked healthcare visits increased from a prevalence of 24.7% (95% CI: 20.8-28.6) in R1 to 72.0% (95% CI: 68.2-75.8) in R5.
By R5, lower rates of youth aged 15-24 years reported hearing of ART than older PLHIV (85.5% of youth aged 15-24 years versus 93.0% of persons aged 25-34 years, 93.2% of persons aged 35-44 years, and 92.7% of persons aged 45-59 years). Young adults aged 15-34 years trended towards being more likely to report having ever missed a complete dose of ARVs, but this difference was not significant (Table 3).
Table 3:
Antiretroviral (ARV)-related knowledge, beliefs and behaviors among PLHIV by Chókwè Health and Demographic Surveillance System Round 5, by gender and age band
| Ever heard about ARVs |
If heard about ARVs: | If taking ARVs: |
|||
|---|---|---|---|---|---|
| Agrees that PLHIV can live a long/healthy life if on ARVs |
Disagrees that traditional medicines are as good as ARVs |
Disagrees that PLHIV taking ARVs need to hide them |
Ever missed a complete dose of ARV medicines? |
||
| Variable | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) |
| Total | 90.1 (88.8-91.3) | 97.3 (96.7-98.0) | 94.8 (93.7-95.9) | 77.3 (75.2-79.4) | 4.7 (2.6-6.8) |
| Gender | |||||
| Male | 88.5 (86.2-90.7) | 97.0 (95.8-98.2) | 93.5 (91.4-95.6) | 77.4 (74.1-80.7) | 5.5 (0.0-11.0) |
| Female | 91.0 (89.8-92.3) | 97.5 (96.8-98.2) | 95.6 (94.6-96.6) | 77.2 (75.0-79.5) | 4.5 (2.2-6.7) |
| Age | |||||
| 15-24 | 85.5 (83.6-87.5) | 95.8 (94.6-97.0) | 93.7 (92.1-95.3) | 72.4 (69.2-75.5) | 10.1 (1.9-18.4) |
| 25-34 | 93.0 (90.7-95.3) | 98.5 (97.4-99.5) | 94.4 (92.0-96.8) | 80.3 (76.6-84.0) | 8.0 (3.0-13.0) |
| 35-44 | 93.2 (90.6-95.8) | 98.5 (97.1-99.8) | 96.1 (93.9-98.2) | 78.4 (74.2-82.5) | 2.2 (0.2-4.1) |
| 45-59 | 92.7 (90.2-95.2) | 97.6 (96.3-98.8) | 96.4 (94.4-98.4) | 82.3 (78.5-86.0) | 2.2 (0.0-4.5) |
| Urbanicity | |||||
| Rural | 90.5 (89.1-91.8) | 97.7 (97.0-98.4) | 96.8 (96.0-97.6) | 81.5 (79.3-83.7) | 4.0 (2.0-6.0) |
| Urban | 89.8 (88.1-91.6) | 97.1 (96.2-98.0) | 93.7 (92.1-95.4) | 74.8 (71.7-77.9) | 5.3 (1.8-8.8) |
DISCUSSION
Among CHDSS residents aged 15-59 years in a high HIV-burden district of Mozambique, current ART use among all PLHIV increased from 65% in 2014 to 88% in 2019, surpassing the second UNAIDS “90” population target of 81% Additionally, among previously diagnosed PLHIV aged 15-59 years, ART use increased from 88% to 92%, surpassing the 90% target.19,21,22 Similarly, by R5 (April 2018-March 2019), VLS among all PLHIV reached 78%, surpassing the third UNAIDS “90” population target of 73%. However, among those using ART, prevalence of VLS only increased from 78% to 87% between R1 and R5, remaining just shy of the targeted 90%. This underscores the important contribution of diagnostic and ART coverage in driving overall achievement of the population-level viral load suppression target, as well as the need for continued work to optimize ART adherence and use of newly-recommended ARV regimens (such as integrase inhibitors) to improve viral suppression rates in Mozambique. Overall, CHDSS achievements in Chókwè District far surpass the global average of 62% population-wide ART coverage and 53% population-wide VLS, as well as progress in Mozambique as a whole.1 The substantial increase in ART coverage was facilitated through a combination of annual HBHTC (driving an increase in HIV case finding), T&S implementation beginning in mid-2016 (increasing the number of people eligible for and initiated on ART), and strengthened provision of supportive and streamlined linkage and treatment services.
Comprehensive HBHTC is recommended by WHO to increase HIV testing amongst people not easily reached by traditional, facility-based services, and has been shown to improve engagement in care when integrated with facilitated linkage services.12,23 In Chókwè District, CHDSS-based HBHTC identified over 4,000 newly and previously diagnosed PLHIV in need of ART, and by R5, 22% of HIV-positive participants reported being first diagnosed through a prior CHDSS surveillance round. However, findings from other studies in sub-Saharan Africa indicate that simply increasing HIV case finding is often not enough to drive ART initiation.
A 2016 systematic review of 14 studies from six countries reporting on linkage to care following HBHTC found that less than a third of participants linked in five of six studies where participants were referred without additional interventions, and overall ART initiation among those eligible ranged widely from 14-95%.11 More recently, investigators from another semi-rural district in Mozambique with an HDSS reported high testing yield, but only 35% enrollment in facility-based HIV care from home-based testing (with 25% facility-based ART initiations overall)24 and, in Lesotho, even home-based HIV testing followed by same-day, home-based ART initiation only resulted in 69% linkage to facility-based care by three months.15 These findings support the need for additional linkage interventions, such as the multiple counsellor encounters, barrier identification and resolution, home visits and psychosocial support services provided to CHDSS clients. Indeed, reported receipt of these linkage to care services increased from around 40% to 82% between R1 and R5, and clients responding to the survey questionnaire increasingly reported finding these services very helpful for enrolling in HIV care.
However, even in the context of increasing diagnostic coverage and successful linkage to care interventions, the second and third UNAIDS 90-90-90 targets could not be reached without adoption and implementation of universal T&S. Among more than 3,500 PLHIV surveyed between 2013 and 2015 through the Botswana Combination Prevention Project (BCPP), although 83% knew their HIV status and 95% of those eligible according to HIV guidelines at the time were on ART, ART coverage was only 73% overall before the implementation of T&S;25 in the years following T&S roll out however, they were able to achieve 88% population-level VLS.26 Efforts like these, and those in Chókwè District, which focused on improving community-based diagnosis and linkage to care interventions while also implementing T&S, have been most successful in improving community-wide ART coverage and viral load suppression. In Eswatini, a peer-delivered package of CDC- and WHO-recommended linkage services provided during T&S resulted in near universal ART initiation (96%; 88% on the day of diagnosis) among those testing HIV positive in the community, including for men and young adults, and with 95% of clients returning for at least their first ART refill.27 In the Sustainable East Africa Research in Community Health (SEARCH) study, after three years of a community-based HIV testing and streamlined linkage to ART intervention in the context of T&S, prevalence of VLS in the intervention group reached 79%;28 similar success was noted for those receiving a combination prevention intervention with T&S in the HPTN 071 (PopART) trial in Zambia and South Africa (72% VLS at 24 months).29 Conversely, in a cluster-randomized trial in KwaZulu Natal, South Africa, universal ART (T&S) was not successful in improving ART coverage, due largely to less than a third of newly diagnosed PLHIV linking to facility-based care within six months.14 This exemplifies that success of T&S in facilitating achievement of 90-90-90 targets is critically dependent on successful HIV diagnosis and linkage to ART.
Finally, even with successful diagnosis, linkage, and adoption of T&S, streamlined, patient-centered ART services are critical to HIV program success.30 In the SEARCH study HIV-positive individuals were offered immediate clinic appointments, personal introductions to clinic staff, clinician phone numbers, one-time transport vouchers, and text or telephone-based appointment reminders.31 Similar success in Chókwè District was facilitated by programmatic interventions also implemented elsewhere in the country in the context of national T&S scale up. These included efforts to make facilities more welcoming and improve patient flow, a pilot of multi-month ART scripting, and community sensitization on T&S, which all may have contributed to reduced perceived barriers to ART initiation and retention among diagnosed PLHIV. In contrast, in one randomized study of linkage strategies in rural South Africa and Uganda where linkage to clinics following community-based testing was 93% but ART initiation was only 37%, the authors cited barriers at overburdened clinics as a major obstacle.32 A significant increase in uptake of the fast-tracked ART refill option for differentiated ART delivery between CHDSS rounds (from 25% to 72%) may have reduced some of this burden in Chókwè District. A nested study within PopART evaluated predictors of timely linkage to ART in the context of universal T&S and found that PLHIV least likely to achieve timely linkage to ART were more likely to find clinics overcrowded or hours inconvenient. They were also more likely to have stronger feelings of shame about their HIV-positivity, not feel ready for treatment, or prefer to wait for ART until they felt sick.33 In the CHDSS, questionnaire respondents generally had positive beliefs about HIV, such as that PLHIV can live a long and healthy life on ART, and that they do not need to hide their medications from other people.
Despite overall successes achieved in the CHDSS, reported ART coverage by R5 remained lowest for men aged 25-34 and women aged 15-24 years (67.8% and 78.0%, respectively), similar to observations about men and youth reported in the literature.13,25,31,34,35 However, the most significant improvements across CHDSS rounds were actually seen in these groups, suggesting success of the combined interventions in reaching them with testing, linkage, and treatment interventions. Notably, for youth aged 15-24 years significant improvement in ART coverage was seen between R3 and R4 of the CHDSS (after T&S was rolled out). However, despite impressive gains in terms of HIV knowledge (knowing the existence of ARVs) by R5, they lagged behind other groups in ARV adherence – indicating continued need for strengthened community messaging and treatment support.
This study was subject to several limitations. First, because our study design did not include control communities, we could not estimate the intervention effect of our combination intervention on ART and VLS coverage. While observed increases in ART coverage and VLS were likely related to our HBHTC and linkage interventions, the descriptive nature of our study precludes a definitive conclusion of causality. Second, although we used a combination of self-report and viral load suppression to define ART use, prevalence of current ART use might remain underestimated given low sensitivity of self-report and that not all ART patients are virally suppressed. Notably, we found across survey rounds that 28.1%-55.8% of PLHIV who did not report ART use were virally suppressed. In Rakai, Uganda, although specificity of self-reported ART use was 99%, sensitivity was only 77%.19 Other studies have found variable degrees of ART nondisclosure.21,22 Third, VL testing for R4-R5 occurred in Mozambique with new in-country testing capacity, using the same VL testing platforms and protocols for specimen preparation, storage, and transportation that were used in Atlanta for R1-3. Although specimen ages differed at the time of testing in Atlanta and Mozambique, differential effects on misclassifying patients with VLS is expected to be negligible due to our consistent procedures.36 Fourth, lower proportions of men (who may be less likely to link, initiate ART, and stay in care than women) participated in HPS, despite efforts to improve recruitment through second household visits at times identified as most convenient for working household members, and during holiday seasons when men working in South Africa were more likely to be home. Although results were weighted to 2016 census demographics to account for non-response, residual bias might reduce the validity of estimates for men. Finally, although our interventions were implemented in only one high HIV prevalence, rural district in Mozambique, our findings are consistent with many studies in sub-Saharan Africa that demonstrate the use of similar interventions to make substantial progress towards achieving 90-90-90.27-29
Overall, population diagnostic, ART, and VLS coverage increased significantly among PLHIV in the CHDSS (about half of the population of Chókwè District, Mozambique) between 2014 and 2019, to ultimately surpass the UNAIDS targets of 90%, 81% and 73%, respectively. This success supports the combination of efforts spanning HBHCT, supportive and streamlined linkage to and delivery of HIV care and treatment services, and continued investments for T&S.
ACKNOWLEDGEMENTS
In memory of Dr. Ricardo Thompson, who tragically passed away in June 2020, shortly before this manuscript was submitted for publication. Without his founding of the CHDSS and passionate efforts to improve the health of the people of Chókwè and Mozambique, none of this would have been possible.
The authors would also like to thank the Chókwè Health Research and Training Centre, Jhpiego and the Mozambique National Institutes of Health (INS) for making the CHDSS possible, the Molecular Virology Laboratory Team at INS and International Lab Branch at CDC Atlanta for their work on VL testing, the Elizabeth Glaser Pediatric AIDS Foundation for their work scaling up Test and Start in Chókwè, many people within the Gaza Provincial and Chókwè District Directorates of Health for their support, and the Mozambique Ministry of Health. Finally, we thank the healthcare providers of Chókwè and the CHDSS participants who dedicated their time to this study.
Disclaimer: This study has been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under the terms of cooperative agreement GH000080. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies.
Footnotes
Early results from this study were presented at the 22nd International AIDS Conference in Amsterdam, Netherlands, July 23-27, 2018.
No conflicts of interest declared.
REFERENCES
- 1.Joint United Nations Programme on HIV. AIDS info database. 2018; http://aidsinfo.unaids.org/.
- 2.Ministério da Saúde (MISAU) INdEI, and ICF. Inquérito de Indicadores de Imunização, Malária e HIV/SIDA em Moçambique - IMASIDA, 2015,. 2018; https://dhsprogram.com/publications/publication-ais12-ais-final-reports.cfm.
- 3.Danel C, Moh R, Gabillard D, et al. A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa. The New England journal of medicine. 2015;373(9):808–822. [DOI] [PubMed] [Google Scholar]
- 4.Group ISS, Lundgren JD, Babiker AG, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. The New England journal of medicine. 2015;373(9):795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral Therapy for the Prevention of HIV-1 Transmission. The New England journal of medicine. 2016;375(9):830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339(6122):966–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.UNAIDS. HIV and AIDS Estimates (2016). 2017; www.unaids.org/en/regionscountries/countries/mozambique. Accessed March 21, 2018.
- 8.Auld AF, Shiraishi RW, Couto A, et al. A Decade of Antiretroviral Therapy Scale-up in Mozambique: Evaluation of Outcome Trends and New Models of Service Delivery Among More Than 300,000 Patients Enrolled During 2004-2013. J Acquir Immune Defic Syndr. 2016;73(2):e11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UNAIDS. 90-90-90- An ambitious treatment target to help end the AIDS epidemic. 2014.; http://www.unaids.org/en/resources/documents/2014/90-90-90. Accessed March 19, 2017.
- 10.UNAIDS. Understanding measures of progress towards the 90 90 90. 2017.; http://www.unaids.org/en/resources/infographics/measures-progress-909090. Accessed March 21, 2018.
- 11.Ruzagira E, Baisley K, Kamali A, Biraro S, Grosskurth H, Working Group on Linkage to HIVC. Linkage to HIV care after home-based HIV counselling and testing in sub-Saharan Africa: a systematic review. Trop Med Int Health. 2017;22(7):807–821. [DOI] [PubMed] [Google Scholar]
- 12.WHO. Consolidated guidelines on HIV testing services. 2014.; http://www.who.int/hiv/pub/guidelines/hiv-testing-services/en/. Accessed April 4, 2018.
- 13.Hayes R, Floyd S, Schaap A, et al. A universal testing and treatment intervention to improve HIV control: One-year results from intervention communities in Zambia in the HPTN 071 (PopART) cluster-randomised trial. PLoS Med. 2017;14(5):e1002292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwuji CC, Orne-Gliemann J, Larmarange J, et al. Universal test and treat and the HIV epidemic in rural South Africa: a phase 4, open-label, community cluster randomised trial. Lancet HIV. 2018;5(3):e116–e125. [DOI] [PubMed] [Google Scholar]
- 15.Labhardt ND, Ringera I, Lejone TI, et al. Effect of Offering Same-Day ART vs Usual Health Facility Referral During Home-Based HIV Testing on Linkage to Care and Viral Suppression Among Adults With HIV in Lesotho: The CASCADE Randomized Clinical Trial. JAMA. 2018;319(11):1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shodell D NR, MacKellar D, Thompson R, Casavant I, Mugabe D, et al. Low and decreasing prevalence and rate of false positive HIV diagnosis -- Chókwè District, Mozambique, 2014-2017. Morbid Mortal Wkly Rep. 2018;67:1363–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.República de Moçambique Ministério da Saúde. Directriz Nacional Para a Implementaçao do Aconselhamento e Testagem em Saúde,. 2015; http://www.misau.gov.mz/index.php/guioes?download=142:guiao-de-implementacao-da-abordagem-do-testar-e-iniciar.
- 18.Zhang G BM, Jadczak S, Nguyen S, Diallo K, Beard RS, et al. Stability of Dried Blood Spot Specimens for HIV-1 Viral Load Testing with the Roche Free Virus Elution Protocol. ASLM2016 International Conference; 2016; Johannesburg, South Africa. [Google Scholar]
- 19.Grabowski MK, Reynolds SJ, Kagaayi J, et al. The validity of self-reported antiretroviral use in persons living with HIV: a population-based study. AIDS. 2018;32(3):363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olson AD, Meyer L, Prins M, et al. An evaluation of HIV elite controller definitions within a large seroconverter cohort collaboration. PLoS One. 2014;9(1):e86719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fogel JM, Wang L, Parsons TL, et al. Undisclosed antiretroviral drug use in a multinational clinical trial (HIV Prevention Trials Network 052). J Infect Dis. 2013;208(10):1624–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahle EM, Kashuba A, Baeten JM, et al. Unreported antiretroviral use by HIV-1-infected participants enrolling in a prospective research study. J Acquir Immune Defic Syndr. 2014;65(2):e90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Genberg BL LH, Hogan JW, Some F, Wachira J, Wu XK, et al. Point of diagnosis and patient retention in HIV care in Western Kenya. J Acquir Immune Defic Syndr. 2018;78:383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez-Varela E, Fuente-Soro L, Augusto OJ, et al. Continuum of HIV Care in Rural Mozambique: The Implications of HIV Testing Modality on Linkage and Retention. J Acquir Immune Defic Syndr. 2018;78(5):527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaolathe T, Wirth KE, Holme MP, et al. Botswana's progress toward achieving the 2020 UNAIDS 90-90-90 antiretroviral therapy and virological suppression goals: a population-based survey. Lancet HIV. 2016;3(5):e221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makhema J WK, Holme MP, et al. Universal Testing, Expanded Treatment, and Incidence of HIV Infection in Botswana. The New England journal of medicine. 2019;381:230–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacKellar D WD, Dhlamini M, et al. Overcoming Barriers to HIV Care: Findings from a Peer-Delivered, Community-Based, Linkage Case Management Program (CommLink), Eswatini, 2015-2018. AIDS Behav. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Havlir D BL, Charlebois ED, et al. HIV Testing and Treatment with the Use of a Community Health Approach in Rural Africa. The New England journal of medicine. 2019;381:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayes RJ DD, Floyd S, et al. Effect of Universal Testing and Treatment on HIV Incidence - HPTN 071 (PopART). The New England journal of medicine. 2019;381:207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2016.; http://www.who.int/hiv/pub/arv/arv-2016/en/. Accessed April 4, 2018. [PubMed]
- 31.Petersen M, Balzer L, Kwarsiima D, et al. Association of Implementation of a Universal Testing and Treatment Intervention With HIV Diagnosis, Receipt of Antiretroviral Therapy, and Viral Suppression in East Africa. JAMA. 2017;317(21):2196–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnabas RV, van Rooyen H, Tumwesigye E, et al. Uptake of antiretroviral therapy and male circumcision after community-based HIV testing and strategies for linkage to care versus standard clinic referral: a multisite, open-label, randomised controlled trial in South Africa and Uganda. Lancet HIV. 2016;3(5):e212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabapathy K, Mubekapi-Musadaidzwa C, Mulubwa C, et al. Predictors of timely linkage-to-ART within universal test and treat in the HPTN 071 (PopART) trial in Zambia and South Africa: findings from a nested case-control study. J Int AIDS Soc. 2017;20(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huerga H, Van Cutsem G, Ben Farhat J, et al. Progress towards the UNAIDS 90-90-90 goals by age and gender in a rural area of KwaZulu-Natal, South Africa: a household-based community cross-sectional survey. BMC Public Health. 2018;18(1):303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grobler A, Cawood C, Khanyile D, Puren A, Kharsany ABM. Progress of UNAIDS 90-90-90 targets in a district in KwaZulu-Natal, South Africa, with high HIV burden, in the HIPSS study: a household-based complex multilevel community survey. Lancet HIV. 2017;4(11):e505–e513. [DOI] [PubMed] [Google Scholar]
- 36.Brambilla D JC, Aldrovandi G, et al. Multicenter Evaluation of Use of Dried Blood and Plasma Spot Specimens in Quantitative Assays for Human Immunodeficiency Virus RNA: Measurement, Precision, and RNA Stability. J Clin Microbiol. 2003;41(5):1888–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]