Abstract
Background:
Widespread HCV treatment for hepatocellular carcinoma (HCC) patients remains limited. Our aim was to evaluate the association of HCV treatment with survival and assess barriers to treatment.
Methods:
Patients in the U.S. Safety Net Collaborative with HCV and HCC were included. Primary outcome was overall survival (OS). Secondary outcomes were recurrence-free survival (RFS) and barriers to receiving HCV treatment.
Results:
Of 941 patients, 57% received care at tertiary referral centers (n=533), 74% did not receive HCV treatment (n=696), 6% underwent resection (n=54), 17% liver transplant (n=163), 50% liver-directed therapy (n=473), and 7% chemotherapy (n=60).
HCV treatment was associated with improved OS compared to no HCV treatment (70 vs 21 months, p<0.01), persisting across clinical stages, HCC treatment modalities, and treatment facilities (all p<0.01). Surgical patients who received HCV treatment had improved RFS compared to those who did not (91 vs 80 months, p=0.03). On MVA, HCV treated patients had improved OS and RFS.
On MVA, factors associated with failure to receive HCV treatment included Black race, higher MELD, and advanced clinical stage (all p<0.05).
Conclusion:
HCV treatment for HCC patients portends improved survival, regardless of clinical stage, HCC treatment, or facility type. Efforts must address barriers to HCV treatment.
Background
Hepatocellular carcinoma (HCC) is the most common primary neoplasm of the liver, affecting 6 per 100,000 persons in the United States each year.1 Between 2008 and 2016, the incidence of HCC has steadily increased by 3% annually, with recent projections indicating its continued growth.2 This is likely attributable to a high prevalence of hepatitis C virus (HCV), the most common etiology of HCC in the U.S.1
The management of HCC is largely dictated by the Barcelona Clinical Liver Cancer (BCLC) criteria, which takes into account patient factors such as performance status and Child-Pugh status, as well as oncologic considerations such as the size and number tumors.3 For localized and early stage disease, surgical resection or liver transplantation offers a potentially curative option, with 5-year survival rates exceeding 70%.4 Modalities for unresectable disease include liver-directed therapy and chemotherapy. However, the efficacy of these treatments is limited by the risk of HCC progression, especially in the context of ongoing HCV infection, which creates a persistent inflammatory state and drives treatment resistance.5
Historically, the mainstay of treatment for HCV included interferon-based regimens with or without ribavirin. Given a significant adverse drug reaction profile, this regimen had poor adherence and low overall rates of sustained virologic response (SVR).6 Fortunately, the introduction of direct acting antivirals (DAAs) in 2011 and their widespread dissemination in 2015 offered a more favorable side-effect profile and SVR rates greater than 97%.7 With the successful treatment of HCV, studies have established a marked improvement in HCC patient outcomes.8 Furthermore, a recent retrospective review of 22,500 HCV-infected patients treated with DAAs with SVR resulted in a 76% reduction in risk of developing HCC compared to those who did not achieve SVR.9
While DAAs offer an avenue for improved clinical outcomes for HCC patients, with a median survival of 72 vs 12 months (p < 0.01) when comparing patients who received DAA therapy to those who did not, access remains a major concern.10 Known barriers to treatment include prohibitive costs, as well as patient, provider, and system-level factors which span health insurance status, low socioeconomic status, and referral-associated delays, all of which can be compounded in a safety net hospital setting.11,12 Currently, there is limited data directly comparing barriers and clinical outcomes based on treatment facility in this high-risk patient population. Our aim was to assess the impact of HCV treatment on survival in patients with concurrent HCC treated at safety net hospitals compared to tertiary referral centers. We also sought to determine the associated barriers to receiving HCV treatment in these two populations.
Methods
Data source and cohort selection
In this retrospective cohort study, patients were selected from the United States Safety Net Collaborative, a consortium of five large safety net hospitals and their tertiary referral center counterparts, including Grady Memorial Hospital, Parkland Memorial Hospital, Jackson Memorial Hospital, Bellevue Hospital, Ben Taub Hospital, Emory University, University of Texas Southwestern Medical School, University of Miami Miller School of Medicine, and New York University Medical School. All patients greater than 18 years of age with a diagnosis of HCC due to HCV etiology with known HCV treatment status were included from 2012 to 2014. Patients with extrahepatic disease (stage IVb), a positive macroscopic margin on liver resection (R2), recurrent disease, and non-hepatocellular carcinoma histology were excluded. Institutional Review Board approval was obtained at each site prior to data collection.
Study variables and outcomes
Demographic, pathologic, operative, post-operative, and survival outcomes data were collected via retrospective review of patient electronic medical records. Clinical staging was based on American Joint Committee on Cancer (AJCC) Guidelines 8th edition. HCC treatment was categorized as no treatment, surgery (right hepatectomy, extended right hepatectomy, left hepatectomy, extended left hepatectomy, sectionectomy, and non-anatomic resection), liver transplant, liver-directed therapy (radiofrequency ablation [RFA], microwave ablation [MVA], transarterial chemoembolization [TACE], radioembolization [Y90], and radiation, regardless of repetitive procedures) and chemotherapy. HCV treatment included DAAs, interferon-based regimens, and multiple treatment types. Health insurance included private, government provided, including Medicaid and Medicare, or a hospital card. Analysis was stratified by receipt of HCV treatment and treatment facility.
The primary outcome was overall survival (OS). Secondary outcomes were recurrence-free survival (RFS) and receipt of HCV treatment.
Statistical analysis
Statistical analysis was performed using SPSS 26.0 software (IMB Inc., Armonk, NY). Analyses were conducted specifying a significant level (alpha) of 0.05. Chi-squared or Fisher’s exact tests were used for comparing categorical variables. Student’s t-test or Mann–Whitney tests were used for comparing the means and medians of continuous variables, respectively. Comparative analyses were performed to compare the cohorts that did and did not receive HCV treatment. Kaplan–Meier analyses, log-rank tests, and univariate Cox regression were performed to determine associations between HCV treatment status and OS. Univariate and multivariable binary logistic regression were used to determine the association of clinicopathologic variables and receipt of HCV treatment. Covariates that were deemed clinically relevant and/or statistically significant on univariate analyses were selected for inclusion in multivariable models.
Results
Patient characteristics
Of the 1910 patients in the U.S. Safety Net Collaborative database, 941 met inclusion criteria. The demographic and clinicopathologic characteristics of the study population are outlined in Table 1. Twenty-six percent (n = 245) of patients received HCV treatment. The median age of patients was 60 years (IQR 56–64). Seventy-eight percent (n = 734) were male and 22% (n = 207) were female. The majority of patients were insured (89%, n = 769). Ninety-five percent (n = 887) had cirrhosis with a median MELD score of 10 (IQR 8–15). Most patients had clinical stage I (48%, n = 428) and II (24%, n = 215) disease. Patients who received care at a tertiary referral center comprised 57% (n = 533) of the study population, compared to 43% (n = 408) who received care at a safety net hospital. Among those who received HCV treatment, 76% (n = 186) of patients received care at a tertiary referral center, while 24% (n = 59) received care at a safety net hospital. Conversely, only 35% and 14% of eligible patients received HCV treatment at tertiary referral centers and safety net hospitals, respectively. For the management of HCC, 6% (n = 54) underwent resection, 17% (n = 163) received a liver transplant, 50% (n = 473) received liver-directed therapy, 6% (n = 60) received chemotherapy, and 20% (n = 191) received no treatment. Median follow-up was 18 months (IQR 6–46).
Table 1.
Baseline characteristics of HCC patients with HCV based on HCV treatment status
| Variable | All patients | No HCV treatment | HCV treatment | p-value |
|---|---|---|---|---|
| n (%) 941 | n (%) 696 (74) | n (%) 245 (26) | ||
| Age median (median, IQR) | 60 (56–64) | 59 (55–64) | 62 (58–65) | <0.01 |
| Gender | ||||
| Female | 207 (22) | 154 (22) | 53 (22) | 0.87 |
| Male | 734 (78) | 542 (78) | 192 (78) | |
| Race | ||||
| White | 448 (56) | 296 (51) | 152 (68) | <0.01 |
| Black | 297 (37) | 242 (42) | 55 (24) | |
| Asian | 52 (7) | 36 (6) | 16 (7) | |
| Unknown | 7 (1) | 5 (1) | 2 (1) | |
| Insurance status | ||||
| Uninsured | 95 (11) | 85 (14) | 10 (4) | <0.01 |
| Insured | 769 (89) | 539 (86) | 23 (96) | |
| Mental health diagnosis | ||||
| No | 814 (87) | 589 (85) | 225 (92) | <0.01 |
| Yes | 119 (13) | 99 (14) | 20 (8) | |
| BMI | ||||
| <18.49 | 23 (2) | 20 (3) | 3 (1) | 0.11 |
| 18.5–24.99 | 292 (32) | 227 (34) | 65 (27) | |
| 25–29.99 | 336 (37) | 231 (34) | 105 (43) | |
| 30–34.99 | 175 (19) | 127 (19) | 48 (20) | |
| 35–39.99 | 60 (7) | 46 (7) | 14 (6) | |
| >40 | 30 (3) | 22 (3) | 8 (3) | |
| ASA class | ||||
| I | 2 | 1 (1) | 1 (1) | <0.01 |
| II | 36 | 23 (26) | 13 (15) | |
| III | 57 | 36 (40) | 21 (25) | |
| IV | 78 | 28 (32) | 50 (59) | |
| V | 1 | 1 (1) | 0 (0) | |
| Cirrhosis | ||||
| No | 50 (5.3) | 34 (5) | 16 (7) | 0.34 |
| Yes | 887 (95) | 658 (95) | 229 (94) | |
| MELD (median, IQR) | 10 (8–15) | 11 (8–16) | 10 (7–13) | 0.03 |
| Clinical stage | ||||
| I | 428 (48) | 290 (44) | 138 (58) | <0.01 |
| II | 215 (24) | 162 (25) | 53 (22) | |
| III | 163 (18) | 129 (20) | 34 (14) | |
| IVa | 86 (10) | 73 (11) | 13 (6) | |
| Treatment facility | ||||
| Tertiary referral center | 533 (57) | 347 (50) | 186 (76) | <0.01 |
| Safety-net | 408 (43) | 349 (50) | 59 (24) | |
| HCV treatment | ||||
| No | 696 (74) | 696 (74) | 0 | <0.01 |
| Direct acting antiviral | 46 (5) | 0 | 46 (19) | |
| IFN | 93 (10) | 0 | 93 (38) | |
| Multiple treatment types | 106 (11) | 0 | 106 (43) | |
| HCC treatment | ||||
| No treatment | 191 (20) | 173 (25) | 18 (7) | <0.01 |
| Surgery | 54 (6) | 42 (6) | 12 (5) | |
| Transplant | 163 (17) | 71 (10) | 92 (38) | |
| Liver-directed therapy | 473 (50) | 354 (51) | 119 (49) | |
| Chemotherapy | 60 (6) | 56 (8) | 4 (2) | |
| Median follow-up (IQR) | 18 (6–46) | 14 (5–35) | 39 (14–61) | <0.01 |
Patients who received HCV treatment were older (62 vs 59 years, p < 0.01), more likely to be White (68 vs 51%, p < 0.01), to have insurance coverage (96 vs 86%, p < 0.01), and to have a lower MELD score at diagnosis (10 vs 11, p = 0.03) compared to those who did not receive HCV treatment. These patients were also more likely to have clinical stage I disease (58 vs 44%, p < 0.01), to receive treatment at a tertiary referral center (76 vs 50%, p < 0.01), to receive HCC treatment (93 vs 75%, p < 0.01), and had longer follow-up (39 vs 14 months, p < 0.01). These patients were less likely to have a mental health diagnosis (8 vs 14%, p < 0.01).
Survival analysis
For all patients, HCV treatment was associated with improved median OS compared to no HCV treatment (70 vs 21 months, p < 0.01; Fig. 1a). This association persisted across all clinical stages (all p < 0.01), and all HCC treatment modalities (all p < 0.01). On univariate Cox regression, insurance coverage, HCC treatment (resection, transplant, and liver-directed therapy), and HCV treatment were associated with improved overall survival (Table 2). On multivariable Cox regression, accounting for age, insurance type, MELD, clinical stage, treatment facility type, and HCC treatment, HCV treatment remained independently associated with improved OS (HR: 0.65, 95% CI: 0.51–0.83, p < 0.01). Notably, treatment at a safety net facility was not a predictor for decreased overall survival in the multi-variable model. On subset analysis by treatment facility type, when patients received HCV treatment, the degree of improvement in survival compared to no treatment was similar regardless if treated at a tertiary referral center (5-yr OS: 56 vs 31%, p < 0.01; Fig. 1c) or a safety net hospital (5-yr OS: 51 vs 23%, p < 0.01; Fig. 1d).
Figure 1.
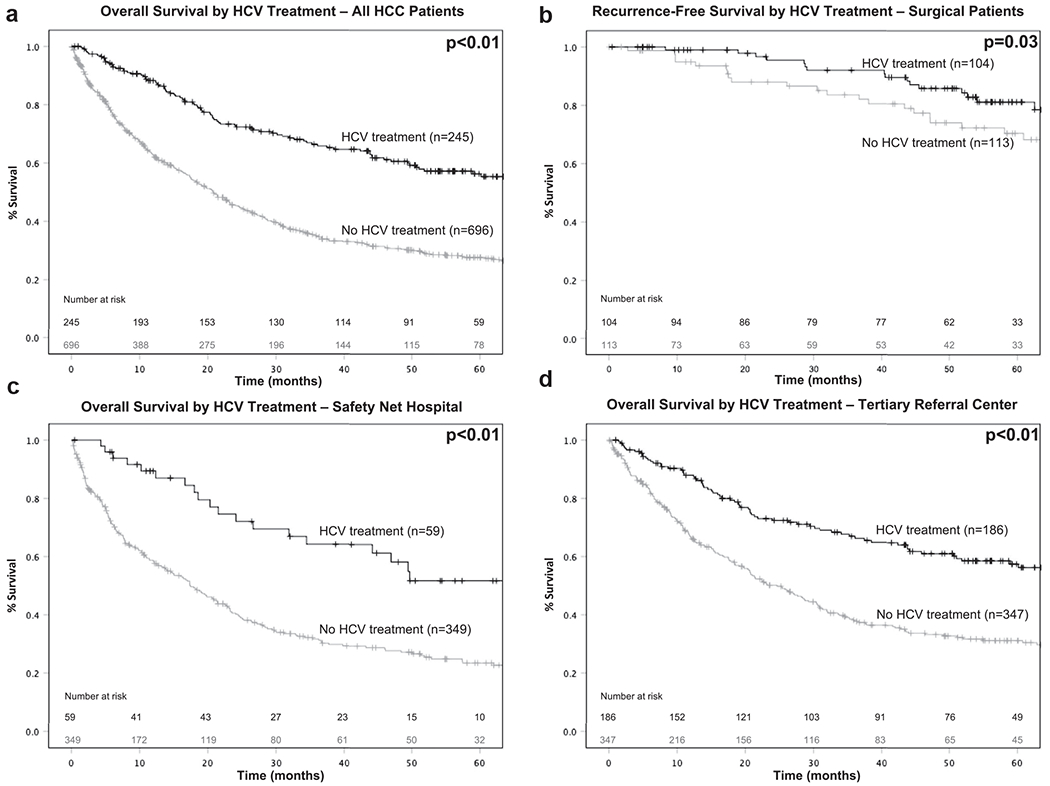
a: overall survival by HCV treatment for all HCC patients, b: recurrence-free survival by HCV treatment for surgical patients, c: overall survival by HCV treatment at safety net hospitals, d: overall survival by HCV treatment at tertiary referral centers
Table 2.
Univariate and multivariable cox regression for overall survival for all HCC patients with HCV
| Variable | Univariate Cox regression | Multivariable Cox regression | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR | p-value | |
| Age (median, IQR) | 0.99 (0.97–0.99) | 0.01 | 1.00 (0.99–1.01) | 0.94 |
| Gender | ||||
| Female | Reference | |||
| Male | 1.16 (0.93–1.44) | 0.19 | ||
| Race | ||||
| White | Reference | |||
| Black | 1.14 (0.94–1.39) | 0.18 | ||
| Asian | 0.71 (0.43–1.18) | 0.18 | ||
| Unknown | 0.76 (0.24–2.37) | 0.64 | ||
| Insurance status | ||||
| Uninsured | Reference | Reference | ||
| Insured | 0.54 (0.41–0.71) | <0.01 | 0.92 (0.66–1.28) | 0.61 |
| Cirrhosis | ||||
| No | Reference | |||
| Yes | 1.42 (0.93–2.16) | 0.10 | ||
| MELD (median, IQR) | 1.03 (1.02–1.03) | <0.01 | 1.02 (1.01–1.02) | <0.01 |
| Clinical stage | ||||
| I | Reference | Reference | ||
| II | 1.54 (1.22–1.95) | <0.01 | 1.59 (1.24–2.07) | <0.01 |
| III | 3.4 (2.7–4.35) | <0.01 | 2.99 (2.29–3.89) | <0.01 |
| IVa | 4.1 (3.11–5.52) | <0.01 | 2.32 (1.69–3.18) | <0.01 |
| Treatment facility | ||||
| Tertiary referral center | Reference | Reference | ||
| Safety-net | 1.44 (1.20–1.71) | <0.01 | 0.94 (0.75–1.16) | 0.55 |
| HCC treatment | ||||
| No treatment | Reference | Reference | ||
| Surgery | 0.08 (0.05–0.13) | <0.01 | 0.11 (0.07–0.19) | <0.01 |
| Transplant | 0.03 (0.02–0.05) | <0.01 | 0.04 (0.02–0.06) | <0.01 |
| Liver-directed therapy | 0.23 (0.19–0.29) | <0.01 | 0.38 (0.21–0.36) | <0.01 |
| Chemotherapy | 0.74 (0.53–1.02) | 0.07 | 0.59 (0.39–0.87) | <0.01 |
| HCV treated | ||||
| No | Reference | Reference | ||
| Yes | 0.41 (0.33–0.52) | <0.01 | 0.65 (0.51–0.83) | <0.01 |
Recurrence-free survival analysis in patients with complete tumor extirpation
On subset analysis for patients who underwent complete tumor extirpation (surgical resection or liver transplant), patients who received HCV treatment had improved RFS compared to those who did not (91 vs 80 months, p = 0.03; Fig. 1b). On univariate Cox regression, the presence of cirrhosis and HCV treatment was associated with improved RFS. Asian race was associated with worse RFS. On multivariable Cox regression, accounting for race, presence of cirrhosis, and treatment facility, HCV treatment remained associated with improved RFS. Treatment at a safety net hospital was not a predictor for worse RFS on univariate or multivariable analysis.
Barriers to receiving HCV treatment
For all patients, factors associated with a decreased odds of receiving HCV treatment on univariate analysis include Black race, higher MELD score, advanced clinical stage, and care at a safety net hospital (all p < 0.05) (Table 4). On multivariable logistic regression, accounting for age, insurance status, and HCC treatment, Black race, higher MELD score, and clinical stage II were associated with a decreased odds of receiving HCV treatment, while receiving a liver transplant or undergoing liver-direct therapy was associated with an increased odds of receiving HCV treatment. When stratifying by treatment facility, no significant barriers to HCV treatment were noted when accounting for the relevant demographic and clinicopathologic factors. At tertiary referral centers, Black race, higher MELD score, and clinical stage II were associated with a decreased odds of receiving HCV treatment in the adjusted model accounting for age, insurance status, and HCC treatment modality. Notably, care at a safety net hospital was not a barrier to receiving HCV treatment in the multivariable model.
Table 4.
Univariate and multivariable binary logistic regression for receipt of HCV treatment for all HCC patients with HCV
| Variable | Univariate logistic regression | Multivariable logistic regression | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |||
| All patients | ||||||
| Age (median, IQR) | 1.04 (1.02–1.06) | <0.01 | 1.02 (0.99–1.05) | 0.07 | ||
| Gender | ||||||
| Female | Reference | |||||
| Male | 1.03 (0.72–1.47) | 0.87 | ||||
| Race | ||||||
| White | Reference | Reference | ||||
| Black | 0.44 (0.31–0.63) | <0.01 | 0.59 (0.39–0.88) | 0.01 | ||
| Asian | 0.87 (0.47–1.61) | 0.65 | 0.87 (0.43–1.75) | 0.69 | ||
| Unknown | 0.78 (0.15–4.06) | 0.77 | 1.08 (0.17–1.75) | 0.94 | ||
| Insurance status | ||||||
| Uninsured | Reference | Reference | ||||
| Insured | 3.63 (1.85–7.11) | <0.01 | 1.69 (0.76–3.78) | 0.20 | ||
| Cirrhosis | ||||||
| No | Reference | |||||
| Yes | 0.74 (0.40–1.37) | 0.34 | ||||
| MELD (median, IQR) | 0.95 (0.92–0.97) | <0.01 | 0.96 (0.93–0.99) | 0.04 | ||
| Clinical stage | ||||||
| I | Reference | Reference | ||||
| II | 0.69 (0.48–0.99) | 0.05 | 0.58 (0.37–0.92) | 0.02 | ||
| III | 0.55 (0.36–0.85) | <0.01 | 1.09 (0.65–1.85) | 0.73 | ||
| IVa | 0.37 (0.20–0.70) | <0.01 | 0.75 (0.37–1.52) | 0.42 | ||
| Treatment facility | ||||||
| Tertiary referral center | Reference | Reference | ||||
| Safety-net | 0.32 (0.23–0.44) | <0.01 | 0.76 (0.50–1.16) | 0.21 | ||
| HCC treatment | ||||||
| No treatment | Reference | Reference | ||||
| Surgery | 2.75 (1.23–6.14) | 0.01 | 1.99 (0.75–5.28) | 0.17 | ||
| Transplant | 12.45 (7.00–22.15) | <0.01 | 12.34 (5.79–26.33) | <0.01 | ||
| Liver-directed therapy | 3.23 (1.91–5.48) | <0.01 | 2.73 (1.36–5.50) | <0.01 | ||
| Chemotherapy | 0.69 (0.22–2.11) | 0.51 | 1.22 (0.36–4.15) | 0.76 | ||
| Patients at safety net hospitals | ||||||
| Age (median, IQR) | 1.02 (0.98–1.05) | 0.32 | ||||
| Gender | ||||||
| Female | Reference | |||||
| Male | 1.11 (0.58–2.15) | 0.75 | ||||
| Race | ||||||
| White | Reference | Reference | ||||
| Black | 0.46 (0.25–0.88) | 0.02 | 0.61 (0.31–1.21) | 0.16 | ||
| Asian | 0.89 (0.29–2.64) | 0.83 | 1.05 (0.33–3.39) | 0.93 | ||
| Unknown | - | - | - | - | ||
| Insurance status | ||||||
| Uninsured | Reference | Reference | ||||
| Insured | 1.70 (0.66–4.38) | 0.27 | 1.23 (0.40–3.79) | 0.72 | ||
| Cirrhosis | ||||||
| No | Reference | |||||
| Yes | 2.99 (0.40–22.29) | 0.28 | ||||
| MELD (median, IQR) | 0.97 (0.92–1.01) | 0.14 | 0.98 (0.92–1.03) | 0.39 | ||
| Clinical stage | ||||||
| I | Reference | Reference | ||||
| II | 0.79 (0.42–1.49) | 0.47 | 0.72 (0.35–1.49) | 0.38 | ||
| III | 0.53 (0.24–1.17) | 0.12 | 0.91 (0.37–2.21) | 0.83 | ||
| IVa | 0.12 (0.02–0.87) | 0.04 | 0.18 (0.02–1.42) | 0.10 | ||
| HCC treatment | ||||||
| No treatment | Reference | Reference | ||||
| Surgery | 4.12 (1.14–14.88) | 0.03 | 1.71 (0.39–7.37) | 0.38 | ||
| Transplant | 11.69 (4.29–31.87) | <0.01 | 7.29 (2.36–22.51) | <0.01 | ||
| Liver-directed therapy | 2.25 (0.84–6.01) | 0.11 | 1.35 (0.45–4.06) | 0.59 | ||
| Chemotherapy | 1.24 (0.23–6.55) | 0.80 | 1.57 (0.28–8.91) | 0.61 | ||
| Patients at tertiary referral centers | ||||||
| Age (median, IQR) | 1.04 (1.02–1.07) | <0.01 | 1.03 (1.01–1.06) | 0.02 | ||
| Gender | ||||||
| Female | Reference | |||||
| Male | 1.00 (0.68–1.48) | 0.98 | ||||
| Race | ||||||
| White | Reference | Reference | ||||
| Black | 0.44 (0.29–0.65) | <0.01 | 0.53 (0.34–0.83) | <0.01 | ||
| Asian | 0.86 (0.43–1.71) | 0.66 | 0.79 (0.37–0.83) | 0.57 | ||
| Unknown | 1.03 (0.19–5.38) | 0.97 | 1.42 (0.23–8.78) | 0.70 | ||
| Insurance status | ||||||
| Uninsured | Reference | Reference | ||||
| Insured | 5.55 (2.22–13.89) | <0.01 | 2.46 (0.89–6.79) | 0.08 | ||
| Cirrhosis | ||||||
| No | ||||||
| Yes | ||||||
| MELD (median, IQR) | 0.59 (0.31–1.11) | 0.10 | 0.95 (0.91–0.99) | 0.02 | ||
| Clinical stage | ||||||
| I | Reference | Reference | ||||
| II | 0.65 (0.43–0.99) | 0.05 | 0.52 (0.31–0.88) | 0.01 | ||
| III | 0.56 (0.35–0.91) | 0.02 | 1.21 (0.67–2.18) | 0.52 | ||
| IVa | 0.46 (0.24–0.88) | 0.02 | 1.07 (0.50–2.27) | 0.87 | ||
| HCC treatment | ||||||
| No treatment | Reference | Reference | ||||
| Surgery | 2.22 (0.83–5.90) | 0.11 | 2.18 (0.66–7.29) | 0.20 | ||
| Transplant | 12.75 (6.62–24.52) | <0.01 | 18.56 (7.37–46.76) | <0.01 | ||
| Liver-directed therapy | 3.61 (1.97–6.62) | <0.01 | 3.89 (1.65–9.23) | <0.01 | ||
| Chemotherapy | 0.48 (0.10–217) | 0.34 | 0.99 (0.19–5.18) | 0.99 | ||
Discussion
In this multi-institutional study, HCV treatment was associated with improved OS in all patients and improved RFS in surgical patients, regardless of clinical stage, HCC treatment modality, or treatment facility type. However, only a small subset of patients seen at safety net hospitals and tertiary referral centers received HCV treatment. Identified barriers to receiving HCV treatment include Black race, higher MELD score, and HCC clinical stage. For all patients, while insurance status and treatment facility were significant on univariate analysis, in the multivariable model, these were no longer predictors of not receiving HCV treatment. When the unique challenges patients at safety net hospitals face are addressed and patients go on to receive HCV treatment, long-term outcomes are similar to those of their peers at tertiary referral centers. Deliberate efforts must be directed towards removing the obstacles that prevent this vulnerable patient population from receiving the standard-of-care treatment.
For patients with HCC, HCV treatment portends improved short and long-term outcomes. While a minority of studies in 2016 report a higher risk of HCC recurrence with DAA therapy, more recent prospective studies and meta-analyses demonstrate HCV treatment with DAAs is associated with lower HCC recurrence risk, especially when DAA initiation is delayed 6–12 months from HCC treatment.13–18 Routinely, participating institutions in the Safety Net Collaborative elected for HCC management to precede HCV therapy. In HCC patients with HCV treated with DAAs, Singal et al. demonstrated recurrence rates range from 0 to 59% within 2 years, with a pooled estimate for recurrence of 25% (95% CI: 19.4–31.2).19 We report 2-year recurrence rates of 5 and 14% for patients who did and did not receive HCV treatment (Fig. 1b, Panel b). With respect to long-term outcomes, Dang et al. reported improved 5-year OS in East Asian HCC patients who received HCV treatment, compared to those who did not receive treatment (88 vs 66%, p < 0.01).20 Similarly, in the U.S., a 2019 retrospective cohort study showed a reduced mortality in HCC patients who received DAA therapy compared to those who did not (HR: 0.54, 95% CI: 0.33–0.90) and a 2-year OS of 88 vs 76%.16 In our study, 5-year survival was of 55 vs 27% for patients who received HCV treatment compared to those who did not (p < 0.01). This association of HCV treatment with improved survival persisted on multivariable analysis regardless of treatment facility. In addition, the HCV treatment variable was comprised of DAA treatment, interferon, and combination DAA and interferon-based regimens. Regardless, the therapeutic benefit of HCV treatment remains clear.
While the impact of HCV treatment on patients with concomitant HCC is apparent, unfortunately, 86% of patients at safety net hospitals and 65% of patients at tertiary referral centers did not receive HCV treatment. Among those who received HCV treatment, only 19% were administered DAAs, suggesting these patients are confronting substantial barriers to accessing these medications. Significant differences in the proportion of patients receiving DAA therapy at tertiary referral centers (6%) and safety net hospitals (3%) were also noted. Reasons for this are multifactorial and likely stem from patient, provider, and system-level factors. At safety net hospitals, these obstacles are compounded, especially in a patient population where inequities in the social determinants of health, which encompass economic stability, educational attainment, and access to health care, are highly prevalent.21
At the individual level, demographic and social factors associated with not receiving DAA therapy are well-documented, which include lack of health insurance, a history of substance abuse, and comorbid disease.22 Our multivariable analysis determined Black race, higher MELD score, and advanced HCC clinical stage to be associated with decreased odds of receiving HCV treatment. These findings highlight the racial/ethnic disparities present in this vulnerable patient population, with race a likely proxy for low socioeconomic status. Higher MELD score and clinical stage are representative of limited engagement with the health care system. Similarly, Mokdad et al. reported a decreased likelihood of patients at safety net hospitals to receive HCC therapy compared to those not at a safety net hospitals (60 vs 40%, p < 0.01), despite matched tumor stages.23 These findings further highlight decreased utilization of health care resources among safety net hospital patients.
Considering provider and system-level drivers, sub-optimal screening and access to definitive HCV treatment contribute to the high prevalence of untreated disease. In the U.S., 45–85% of HCV patients are unaware of their status.24 Prior work at a Grady Memorial Hospital, a high-volume safety net hospital in the Southeastern U.S., revealed 74% of HCC patients were HCV-positive, with only 15% of patients receiving treatment at the time of diagnosis.25 Formalized screening programs are critical for early detection and intervention, especially for at-risk patients.
Once a patient is diagnosed with HCV and is able to seek care, the price of DAA therapy can be prohibitive, with a 12-week course ranging from $40,000–123,000.26,27 Further, though DAA therapy is routinely covered by health insurance in the U.S., reimbursement criteria remains inconsistent, and often does not align with national treatment guidelines, thus hampering DAA distribution. For example, variable Medicaid prior authorization policies have been shown to further restrict the widespread distribution of DAAs. Clinical indications warranting reimbursement vary based on location and coverage policy, which can include advanced cirrhosis, suppressed HIV levels, and negative drug toxicology screens.28 These criteria often preclude patients served by safety net hospitals. The fact that a diagnosis of cirrhosis is a requirement of 75% of prior authorizations for DAAs may account for the association of cirrhosis with improved RFS in our univariate analysis (Table 3).26 While costly upfront, treatment of HCV halts the continuous insult on the liver and prevents further liver decompensation, liver-related complications, and accompanying costly interventions. To ultimately decrease HCV and HCC associated morbidity and mortality, we advocate for unfettered access to these life-saving medications. State Medicaid policies must be updated to ensure equitable access.
Table 3.
Univariate and multivariable Cox regression for recurrence-free survival for all HCC patients with HCV
| Variable | Univariate cox regression | Multivariable cox regression | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age (median, IQR) | 1.01 (0.96–1.05) | 0.80 | ||
| Gender | ||||
| Female | Reference | |||
| Male | 1.08 (0.49–2.35) | 0.85 | ||
| Race | ||||
| White | Reference | Reference | ||
| Black | 1.24 (0.58–2.67) | 0.58 | 1.11 (0.51–2.41) | 0.80 |
| Asian | 3.33 (1.39–7.97) | <0.01 | 2.51 (0.96–0.55) | 0.06 |
| Unknown | - | - | - | |
| Insurance status | ||||
| Uninsured | Reference | |||
| Insured | - | - | ||
| Cirrhosis | ||||
| No | Reference | Reference | ||
| Yes | 0.38 (0.18–0.81) | 0.01 | 0.52 (0.22–1.23) | 0.14 |
| MELD (median, IQR) | 0.99 (0.92–1.05) | 0.64 | ||
| Clinical stage | ||||
| I | Reference | |||
| II | 1.19 (0.57–2.5) | 0.63 | ||
| III | 2.22 (0.95–5.18) | 0.07 | ||
| IVa | - | 0.98 | ||
| Treatment facility | ||||
| Tertiary referral center | Reference | Reference | ||
| Safety-net | 0.89 (0.46–1.70) | 0.72 | 0.73 (0.35–1.54) | 0.42 |
| HCV treated | ||||
| No | Reference | Reference | ||
| Yes | 0.51 (0.27–0.95) | 0.03 | 0.41 (0.20–0.83) | 0.01 |
| Final margin status | ||||
| R0 | Reference | |||
| R1 | 2.05 (0.49–8.54) | 0.32 |
While the introduction of DAAs have transformed the management of HCV, there remains significant obstacles for patients to receive treatment. Fortunately, there are approaches that may improve HCV and HCC cure rates. It is important to recognize once a patient received HCV treatment, safety net hospital designation in and of itself was not an independent predictor for decreased survival or early recurrence, underscoring the importance of health care access and delivery.
In our effort to address modifiable barriers, solutions that target HCV and HCC screening, referral, diagnosis, and treatment delivery are essential. Patient outreach, education, screening, and counseling programs that further integrate existing resources at safety net hospitals, spanning patient navigation services, social work, and substance abuse clinics may improve treatment success.29 In addition, the use of dedicated HCV/HCC treatment clinics and disease management teams help streamline treatment protocols, and have been associated with increased specialist referrals, care delivery, and survival.30 After treatment, post-SVR HCC surveillance programs at a safety net hospital have also been shown to improve long-term outcomes.31 Lastly, by working with community resources and primary care providers, pipelines that promote strong referral patterns ensure continued HCV, HCC, and cirrhotic patient engagement.
Limitations of this study include its retrospective design, though a large multi-institutional collaborative database eliminates single-institution bias and may better allow for generalizability. Second, the study time period was a limitation. With FDA approval of second-generation DAAs in late 2013 and its widespread dissemination in 2014–2015, it is likely we underestimate the extent of DAA use compared to current trends.32 Third, data regarding HCV treatment start or end dates were not collected. As a result, we do not have access to time intervals between definitive HCC treatment and HCV therapy initiation, thus the optimal timing for HCV or DAA therapy is unclear. Though given the timeframe of our study, it is likely patients received DAAs after HCC treatment. In addition, principal investigators of the U.S. Safety Net Collaborative reported the general practice pattern at each center is to manage HCC prior to pursuing HCV treatment. Finally, details with respect to the specific DAA regimen and duration of therapy were not available.
Conclusions
In summary, this multi-institutional study provides real-world evidence to support HCV treatment in patients with concomitant HCC. HCV treatment improves overall and recurrence-free survival in all patients with HCC, regardless of clinical stage, HCC treatment modality or type of treatment facility. While DAAs were a major advance for HCV treatment, offering a promising solution to halt the progression of liver disease, there exist significant challenges in accessing this treatment. In order to optimize the care of these high-risk patients, we must work to remove these modifiable barriers by incorporating existing resources at safety net hospitals with novel, patient-centered solutions to maximize this potential.
Acknowledgements
This study is supported in part by the Katz Foundation and the National Center for Advancing Translational Science, grant/award number: UL1TR002378/TL1TR002382.
Footnotes
Ethical statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of interest
None declared.
References
- 1.Ghouri YA, Mian I, Rowe JH. (2017) Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. J Carcinog 16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. (2017) Incidence of hepatocellular carcinoma in all 50 United States, from 2000 through 2012. Gastroenterology 152:812–820 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Fuster J, Bruix J,Barcelona-Clinic Liver Cancer G. (2004) The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl 10:S115–S120. [DOI] [PubMed] [Google Scholar]
- 4.Ma KW, Cheung TT. (2017) Surgical resection of localized hepatocellular carcinoma: patient selection and special consideration. J Hepatocell Carcinoma 4:1 −9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. (2015) Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg 261:947–955. [DOI] [PubMed] [Google Scholar]
- 6.Younossi ZM, Stepanova M, Henry L, Nader F, Younossi Y, Hunt S. (2016) Adherence to treatment of chronic hepatitis C: from interferon containing regimens to interferon and ribavirin free regimens. Med (Baltimore) 95e4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simmons B, Saleem J, Hill A, Riley RD, Cooke GS. (2016) Risk of late relapse or reinfection with hepatitis C virus after achieving a sustained virological response: a systematic review and meta-analysis. Clin Infect Dis 62:683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waziry R, Hajarizadeh B, Grebely J, Amin J, Law M, Danta M et al. (2017) Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: a systematic review, meta-analyses, and meta-regres-sion. J Hepatol 67:1204–1212. [DOI] [PubMed] [Google Scholar]
- 9.Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. (2017) Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology 153:996–1005 e1. [DOI] [PubMed] [Google Scholar]
- 10.Kamp WM, Sellers CM, Stein S, Lim JK, Kim HS. (2019) Impact of direct acting antivirals on survival in patients with chronic hepatitis C and hepatocellular carcinoma. Sci Rep 9:17081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrill JA, Shrestha M, Grant RW. (2005) Barriers to the treatment of hepatitis C. Patient, provider, and system factors. J Gen Intern Med 20: 754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beck KR, Kim N, Khalili M. (2016) Sofosbuvir-Containing regimens for chronic hepatitis C are successful in the safety-net population: a real-world experience. Dig Dis Sci 61:3602–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolly P, Waidmann O, Vermehren J, Moreno C, Vogeli I, Berg T et al. (2017) Hepatocellular carcinoma recurrence after direct antiviral agent treatment: a European multicentre study. J Hepatol 67:876–878. [DOI] [PubMed] [Google Scholar]
- 14.Minami T, Tateishi R, Nakagomi R, Fujiwara N, Sato M, Enooku K et al. (2016) The impact of direct-acting antivirals on early tumor recurrence after radiofrequency ablation in hepatitis C-related hepatocellular carcinoma. J Hepatol 65:1272–1273. [DOI] [PubMed] [Google Scholar]
- 15.Saraiya N, Yopp AC, Rich NE, Odewole M, Parikh ND, Singal AG. (2018) Systematic review with meta-analysis: recurrence of hepatocellular carcinoma following direct-acting antiviral therapy. Aliment Pharmacol Ther 48:127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singal AG, Rich NE, Mehta N, Branch AD, Pillai A, Hoteit M et al. (2019) Direct-acting antiviral therapy for hepatitis C virus infection is associated with increased survival in patients with a history of hepatocellular carcinoma. Gastroenterology 157:1253–1263.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singal AG, Rich NE, Mehta N, Branch A, Pillai A, Hoteit M et al. (2019) Direct-acting antiviral therapy not associated with recurrence of hepatocellular carcinoma in a multicenter North American cohort study. Gastroenterology 156:1683–16892. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reig M, Marino Z, Perello C, Inarrairaegui M, Ribeiro A, Lens S et al. (2016) Unexpected high rate of early tumor recurrence in patients with HCV-related HOC undergoing interferon-free therapy. J Hepatol 65: 719–726. [DOI] [PubMed] [Google Scholar]
- 19.Singal AG, Lim JK, Kanwal F. (2019) AGA clinical practice update on interaction between oral direct-acting antivirals for chronic hepatitis C infection and hepatocellular carcinoma: expert review. Gastroenterology 156:2149–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dang H, Yeo YH, Yasuda S, Huang C-F, lio E, Landis C et al. (2019) Cure with interferon free DAA is associated with increased survival in patients with HCV related HCC from both East and west. Hepatology (Baltimorei MD). 10.1002/hep.30988. [DOI] [PubMed] [Google Scholar]
- 21.Khalili M, Wong RJ. (2018) Underserved does not mean undeserved: unfurling the HCV care in the safety net. Dig Dis Sci 63:3250–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuckerman A, Douglas A, Nwosu S, Choi L, Chastain C. (2018) Increasing success and evolving barriers in the hepatitis C cascade of care during the direct acting antiviral era. PLoS One 13e0199174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mokdad AA, Murphy CC, Pruitt SL, Mansour JC, Marrero JA, Singal AG et al. (2018) Effect of hospital safety net designation on treatment use and survival in hepatocellular carcinoma. Cancer 124:743–751. [DOI] [PubMed] [Google Scholar]
- 24.Ward JW. (2013) The hidden epidemic of hepatitis C virus infection in the United States: occult transmission and burden of disease. Top Antivir Med 21:15–19. [PMC free article] [PubMed] [Google Scholar]
- 25.Duininck G, Lopez-Aguiar AG, Lee RM, Miller L, Dariushnia S, Wu C et al. (2019) Optimizing cancer care for hepatocellular carcinoma at a safety-net hospital: the value of a multidisciplinary disease management team. J Surg Oncol 120:1365–1370. [DOI] [PubMed] [Google Scholar]
- 26.Park H, Wang W, Henry L, Nelson DR. (2019) Impact of all-oral direct-acting antivirals on clinical and economic outcomes in patients with chronic hepatitis C in the United States. Hepatology 69:1032–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henry B (2018) Drug pricing & challenges to hepatitis c treatment access. J Health Biomed Law 14:265–283. [PMC free article] [PubMed] [Google Scholar]
- 28.Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, Taylor LE. (2015) Restrictions for medicaid reimbursement of sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med 163: 215–223. [DOI] [PubMed] [Google Scholar]
- 29.Marshall MC, Herrera JL. (2018) Lack of patient compliance in real-world practice negatively affects sustained viral response rates to direct acting agent therapy for hepatitis C. Dig Dis Sci 63:3228–3232. [DOI] [PubMed] [Google Scholar]
- 30.Charriere B, Muscari F, Maulat C, Bournet B, Bonnet D, Bureau C et al. (2017) Outcomes of patients with hepatocellular carcinoma are determined in multidisciplinary team meetings. J Surg Oncol 115: 330–336. [DOI] [PubMed] [Google Scholar]
- 31.Kim NJ, Magee C, Cummings C, Park H, Khalili M. (2018) Liver disease monitoring practices after hepatitis C cure in the underserved population. Hepatol Common 2:1274–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spradling PR, Xing J, Rupp LB, Moorman AC, Gordon SC, Lu M et al. (2018) Uptake of and factors associated with direct-acting antiviral therapy among patients in the chronic hepatitis cohort study, 2014 to 2015. J Clin Gastroenterol 52:641 −647. [DOI] [PMC free article] [PubMed] [Google Scholar]


