Abstract
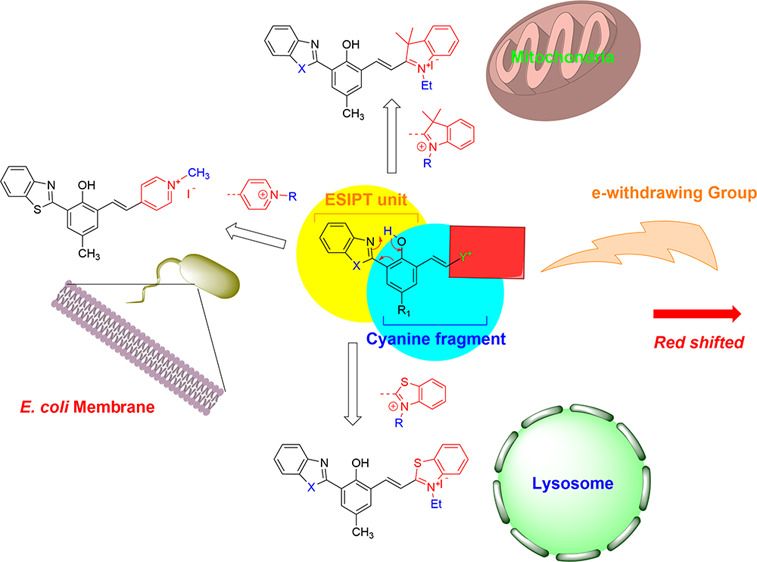
In this review, we will summarize our recent progress in the design and application of novel organic sensors with emission in the near-infrared region (600–900 nm). By coupling different functional groups with excited-state intramolecular proton transfer (ESIPT) segments, new probes are developed to achieve a large Stokes shift, high sensitivity, and selectivity and to tune the emission toward the near-infrared region. The developed probes exhibit attractive optical properties for bioimaging and environmental science applications. In addition, we further discuss the photophysical properties of ESIPT dyes and how their fluorescence could be affected by structural/environmental factors, which should be considered during the development of robust ESIPT-based fluorescence probes. Their potential applications as imaging reagents are illustrated for intracellular membranes, mitochondria, lysosomes, and some biomolecules.
1. Introduction
Fluorescence organic dyes have been widely used in biology, pharmacology, and environmental science as selective sensors for specific analytes for more than a century.1 The novel fluorescent dyes with excellent photostability, high fluorescence quantum yield, high sensitivity, and selectivity are desirable for medical diagnostics, cell biology applications, and drug delivery in recent years, especially after the global pandemic.2 The species to be detected can react with sensors3,4 or interact through host–guest interaction5 and then give a fluorescence signal. Some molecular probes that emit in the near-infrared (NIR) region are ideal to be developed for analytical and biological applications such as in cell imaging. In comparison with commercial dyes that emit in the UV/vis range, NIR dyes have advantages of deep tissue penetration, less tissue damage, and minimal interference caused by tissue absorption and autofluorescence.6−10
Donor–acceptor-involved fluorescence mechanisms for certain dyes have been well-investigated over the past decade. Typically, there are three well-known mechanisms which include PET (photoinduced electron transfer), FRET (Förster resonance energy transfer), and ICT (internal charge transfer).1 Traditional dyes based on these three mechanisms always have a noticeable restriction. Their characteristic small Stokes shift (Δλ < 25 nm) causes self-quenching and impedes their application in species detection, as the detected signals may not accurately reflect the analyte concentrations.11,12
Excited-state intramolecular proton transfer (ESIPT), discovered by Weller in the 1950s,13 involves a four-level photochemical process, which leads to low energy fluorescence with a large Stokes shift and enhanced quantum yield.1,14 The molecules typically contain a hydrogen bond donor (−OH) and exist in an enol form in the ground state. When the enol tautomer 1 is excited upon photon absorption, the hydrogen atom is transferred to the −C=N– group (a proton acceptor), resulting in the excited keto tautomer 2 that gives long-wavelength emission (Scheme 1).15 2-(2′-Hydroxyphenyl)benzoxazole (HBO, X = O in 1) and 2-(2′-hydroxyphenyl)benzothiazole (HBT, X = S in 1) and their derivatives are the most common ESIPT chromophores.1 However, the emission of such designs is often limited to blue, green, and orange-red colors.
Scheme 1. Keto and Enol Tautomerization in ESIPT.
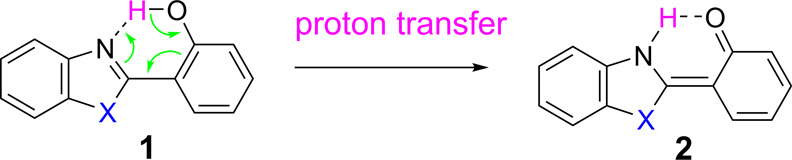
The proton transfer from the phenol to the −C=N– group can be viewed simply as an acid–base reaction, as the phenolic proton is known to become more acidic in the excited state (pKa ∼ 5.7).16 The acidity of the phenolic proton could be influenced by a suitable electron-withdrawing or electron-donating substituent, which could actively perturb the proton transfer process.7−10
2. ESIPT-Based Fluorescent Probes and Their Applications
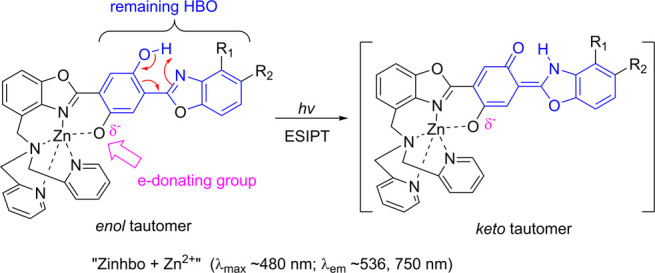
In the effort of seeking ESIPT fluorophores with NIR emission and large Stokes shifts, our group has recently synthesized a series of Zinhbo compounds,5 in which a strong e-donating group (i.e., −OH) is placed at the para-position of the phenol group that is involved for ESIPT (Scheme 2). Interestingly, its zinc complex (i.e., “Zinhbo-Zn2+”) gives NIR emission while retaining the dual emission feature of ESIPT fluorophores (with λem at ∼536 and 750 nm from its enol and keto tautomers, respectively). These compounds have been demonstrated to be useful for intracellular sensing of Zn2+ cations via live cell fluorescence imaging.
Scheme 2. Keto and Enol Tautomerization in Zinhbo-Zn2+.
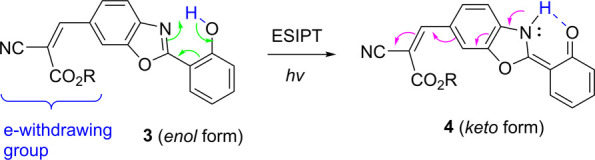
An alternative strategy to tune the optical absorption/emission is attaching an electron-withdrawing group to ESIPT chromophores. Among very limited examples is HBO derivative 3, in which a strong electron-withdrawing group is attached to the benzoxazole segment.17 Compound 3 is reported to exhibit λabs ≈ 370 nm, which is only slightly affected by solvent polarity. Whereas its enol form gives emission at λem ≈ 430 nm, its keto form gives solvent-dependent emission (λem ≈ 525 nm in cyclohexane and ∼600 nm in ether). Interestingly, the ESIPT enables a strong donor–acceptor interaction, as shown by the arrows in 4 (Scheme 3). However, the compound exhibits low fluorescence quantum yield (φfl = 2.6 × 10–3 in cyclohexane) and a radiative lifetime of ∼0.725 ns in nonpolar solvent with the absence of keto emission.
Scheme 3. ESIPT-Induced Tautomerization of 3.
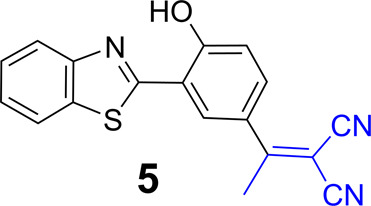
It is assumed that the electronic impact can be achieved by introducing a substituent on the phenol ring of the HBO unit. One example is 5 (Scheme 4), which is reported to exhibit two absorption bands (λabs = 382 and 458 nm) and very weak fluorescence (λem ≈ 510 nm) in DMSO/H2O solution (8:2, v/v) at 25 °C.4 It appears that the classical e-withdrawing groups may play a very limited role in tuning the emission toward longer wavelength.
Scheme 4. Structure of 5.
Another strategy is to couple the traditional cyanine dyes with ESIPT chromophores. As a typical class of dyes, cyanines possess significant advantages including high fluorescence quantum yields and tunable emission,18 which have made them popular in various applications for decades. However, conventional cyanine dyes display small Stokes shifts, e.g., often less than 30 nm.19 It can be assumed that the combination of ESIPT chromophore with cyanine structures could lead to attractive new dyes that exhibit long-wavelength emission (of cyanines), while still giving ESIPT emission with a large Stokes shifts. As illustrated in Scheme 5, a cyanine terminal group (Y+) is attached to the ortho-position of the central phenol ring. In the ground state of 6a, the ESIPT and cyanine fragments share the central phenol ring. However, the benzoxazole (part of the ESIPT unit) has a poor electronic connection with the cyanine terminal group (−CH=CH–Y+), as they are placed at 2.6-position of the central phenol. The electronic connection between the two groups is greatly enhanced in the excited keto state 6b, thereby generating ESIPT emission with a large Stokes shift. The presence of an e-withdrawing group could also enhance the acidity of the phenolic proton, thereby increasing the ESIPT emission.
Scheme 5. ESIPT Probes with Different Acceptors.
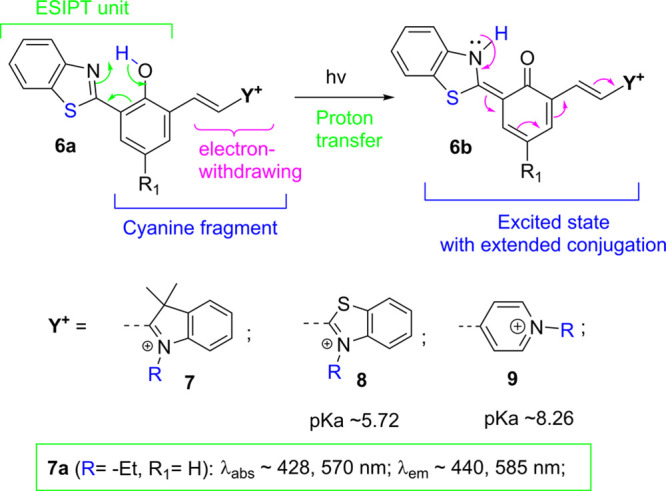
Recently, Paul et al. reported that 7a was used for hydrogen sulfide detection.20 A later study from our group10 suggests that the absorption peaks at ∼428 and 570 nm could be attributed to the neutral form Ar–OH and its anionic form Ar–O–, respectively. By comparison with its model compound (10), the study revealed that the absorption, λabs, of 8 is basically determined by the cyanine fragment (Figure 1). However, the presence of the ESIPT unit in 8 causes a significant bathochromic shift in emission, by about ∼100 nm in comparison to that with 10.8
Figure 1.
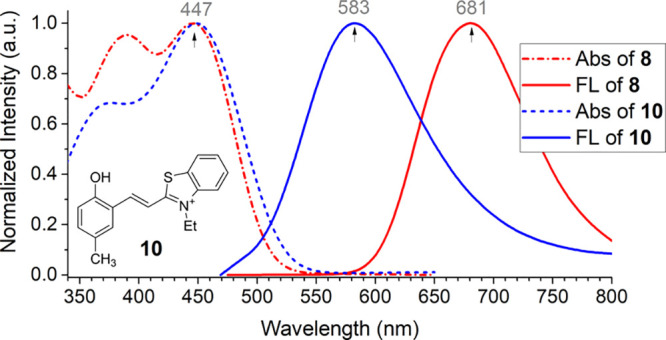
UV–vis absorption and fluorescence spectra of compounds 8 (X = S, red curves) and 10 (blue curve) in CH2Cl2. Replotted with permission from ref (8). Copyright 2017 Royal Society of Chemistry.
Strong emission from only the keto tautomer of 8 is observed (Figure 1), in contrast to traditional ESIPT dyes that give emission from both enol and keto tautomers.21 It appears that the e-withdrawing group increases the acidity of phenolic proton and facilitates the proton transfer. In a collaborated effort, study from Guo et al. further revealed the details of the photophysical process, showing the fluorescence lifetime of 1.75 ns for 8 in CH3CN.14 These findings consistently indicate that the ESIPT is a rapid process. Although this class of new fluorescent dyes have strong donor–acceptor interaction in the excited state, as illustrated in 6b, they exhibit reasonably high fluorescence quantum yields (φfl ≈ 0.20–0.35), which opens the possibility for various applications.
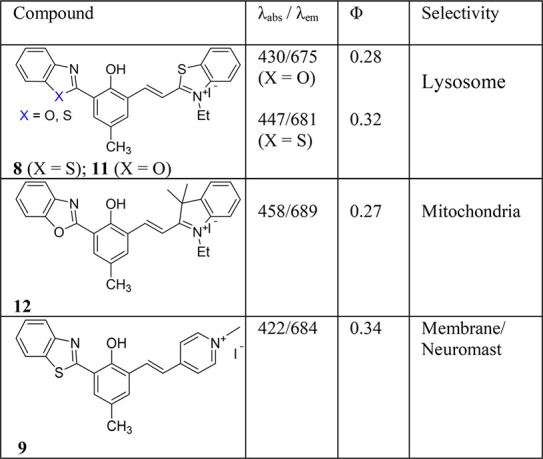
The synthesis of additional examples allows us to further evaluate the properties of ESIPT–cyanine dyes. As shown in Table 1, the absorption λabs values change with the type of the e-withdrawing groups (compare 11, 12, and 9), consistent with the assumption that the dye’s conjugation length is basically defined by the cyanine segment.10
Table 1. Photophysical Properties of ESIPT–Cyanine Dyes in DCM and Their Intracellular Selectivity.
Temperature Effect on Emission
As seen from 6, the ESIPT–cyanine dyes include an ESIPT unit and a cyanine segment. Once the ESIPT occurs in the excited state (upon photon absorption), it enables a strong donor–acceptor interaction (shown in 6b). Low-temperature fluorescence has been used to evaluate the impact of the cyanine segment.7,8 As illustrated in Figure 2a, fluorescence at 712 nm can be observed from the ethanol solution of 8 at room temperature. As the temperature is decreased by cooling the solution with liquid nitrogen, the fluorescence signal is increased significantly, and the emission peak is blue-shifted to 635 nm. The spectral blue shift occurs in a narrow range of temperature from −100 to −125 °C, as the solution is frozen to prevent intramolecular charge transfer.8 It should be noted that the keto emission predominates at low temperature (Figure 2b), as reported from HBO 1 in MeOH solution in a previous study.15 Since ESIPT will not be restricted by low temperature (as observed from spectra of 1), the emission peak at 635 nm, observed from low-temperature fluorescence spectra of 8, is likely from the keto tautomer, and the observed spectral blue shift can be attributed mainly to ICT.
Figure 2.
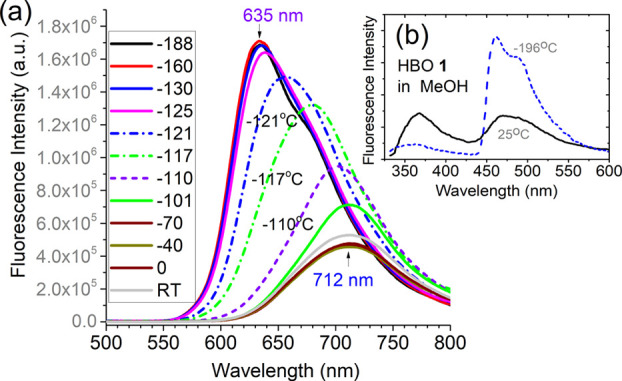
(a) Fluorescence spectra of 8 in EtOH (3 μM) at different temperatures in degrees Celsius (°C). Replotted with permission from ref (8). Copyright 2017 Royal Society of Chemistry. (b) Fluorescence spectra of HBO 1 in MeOH excited at 320 nm at room temperature and at −196 °C. Reproduced from ref (15). Copyright 1994 American Chemical Society.
Effect of Regiochemistry
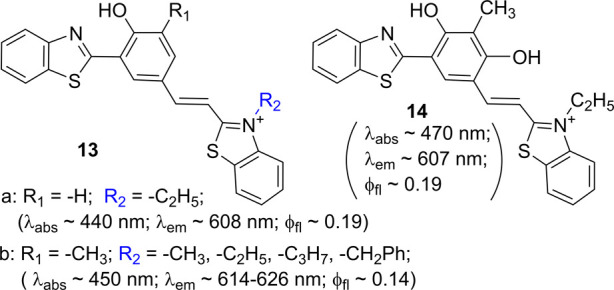
The e-withdrawing group in 6 is at the ortho-position of the phenol ring. Recently, our group examined the possibility of moving the e-withdrawing group to the para-position of the phenol ring by synthesis of 13.22 In CH2Cl2, 13a exhibited the fluorescence λem ≈ 608 nm (φfl ≈ 0.17), which was notably blue-shifted in comparison to that with 8. Introduction of the second hydroxy group, as seen in 14, could generate a notable bathochromic shift in λabs (in CH2Cl2). However, the emission of 14 was slightly blue-shifted, thus decreasing the Stokes shift. The results clearly illustrate that the optical properties of this system are tunable. At a similar time, Rodembusch et al.23 reported the synthesis of 13b (with R2 = −CH3 and −C8H17), which was shown to be a useful pH sensor, although their conclusion is different (in terms of ESIPT event and Stokes shift) (Scheme 6)
Scheme 6. Structure and Photophysical Properties of 13 and 14.
Biological Cell Studies
Despite interests in using ESIPT for molecular probe design in recent year,1 little is known about using ESIPT probes for imaging subcellular organelles until recently (in 2017).8 It should be noted that the phenolic proton becomes acidic, with pKa ≈ 5.7 estimated from 8, as a consequence of attaching the cyanine fragment. The ability of 8 to give a large fluorescence turn on in an acidic environment (pH < 6) leads to its application for fluorescence imaging of intracellular organelles, i.e., lysosomes, which are acidic (pH ≈ 4–5) for optimal function.24
The most attractive features of the reported ESIPT–cyanine dyes are their potential in molecular imaging, due to their unique photophysical properties (large Stokes shift), excellent biocompatibility, and high cell penetration ability.8−10,22 The fluorescence of 13b (R2 = −CH3) was sensitive to its environmental pH, with pKa ≈ 6.01.22 Benefiting from its large fluorescence turn on upon protonation (Ar–O– + H+ → ArOH), probe 13b exhibits selectivity toward intracellular lysosomes similar to that of its regioisomer 8. However, the low pKa is not the sole reason for the observed lysosome selectivity, as both 8 (pKa ∼ 5.72, X = S) and 11 (pKa ∼ 8.72, X = O) are targeting lysosomes.8,10 The intriguing lysosome selectivity of 11 is clearly evidenced by a co-staining experiment with commercial MitoTracker Green and LysoTracker Red (Figure 3), and the probe exhibits excellent colocalization with the known LysoTracker (Figure 3D,H).
Figure 3.
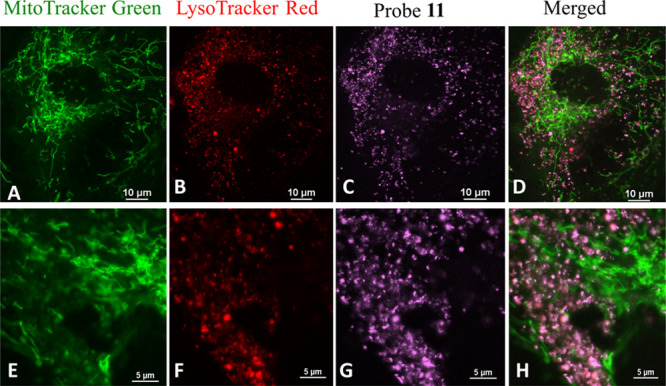
Confocal fluorescence images of normal human lung fibroblast (NHLF) cells co-stained with MitoTracker Green (A), LysoTracker Red (B), probe 11 (C), and their overlap (D). Images E–H are 4 times digitally enhanced for A–D, respectively. Reproduced from ref (10). Copyright 2019 American Chemical Society.
Since ESIPT–cyanine dyes contain two segments, one fundamental question is whether the ESIPT or cyanine segment has a larger impact on the intracellular selectivity. In one experiment, the −OH group in 13b was converted to a −OCH3 group, and the resulting derivative maintained the lysosome selectivity.22 The same lysosome selectivity is also observed from 8 (with HBT) and 11 (with HBO).8,10 The result suggests that the ESIPT unit might have less influence on the cellular selectivity.
When the benzothiazolium terminal group in 11 was replaced by the indolium group, however, the resulting 7 became the mitochondria-targeting probe.10 The drastic switch in intracellular selectivity can be seen clearly by the remarkable colocalization of 12 with MitoTracker Green (Figure 4), in sharp contrast to 11 (Figure 3D). The mitochondrial selectivity of 12 can be attributed to its Coulombic attraction toward a negative potential gradient across the mitochondrial matrix. Interestingly, the ESIPT–cyanine dye 9 with a pyridinium group stained the membrane of E. coli cells and does not show any selectivity toward eukaryotic cells in vivo; however, the dye selectively stained neuromast hair cells and supporting cells in zebrafish.7 Therefore, the cyanine terminal groups play a vital role in tuning the cellular selectivity.
Figure 4.
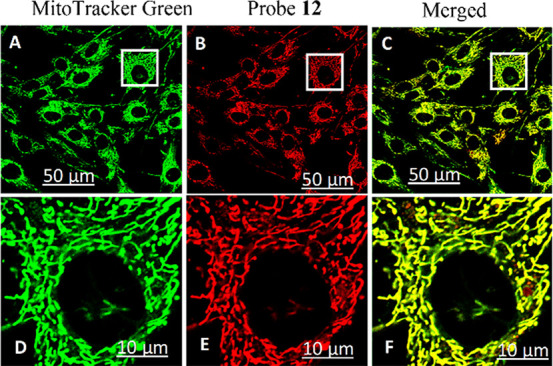
Images of a normal human lung fibroblast (NHLF) stained with (A) LysoTracker Green, (B) probe 12, and (C) their overlap. Images D–F are 4 times digitally enhanced images of the region covered by white rectangular box in A–C, respectively. Reproduced from ref (10). Copyright 2019 American Chemical Society.
Sensor Application Based on Selective Reactions
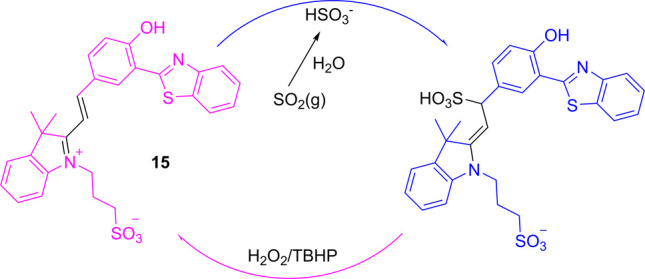
The structure of ESIPT–cyanine dyes is well-suitable for conjugated addition (Michael addition reaction), as a vinyl bond is connected to a strong e-withdrawing group (called a Michael acceptor). Such a structural feature can be used for the detection of various Michael donors, such as biologically important bisulfite and thiols. Recently, 7a has been used for hydrogen sulfide detection,20 and 15 has also been demonstrated as a reversible colorimetric and ratiometric probe for SO2/HSO3– (Scheme 7) in cancer cells (MCF-7).3 Similarly, Chen et al. reported the ratiometric fluorescence detection of ROS (reactive oxygen species) such as HOCl/OCl– in HeLa cells using probe 9.25 During the process, addition of the ROS to the conjugated π-bond breaks the extended π-conjugation, and the emission is given by only the ESIPT chromophore, which is hypsochromically shifted from the original probe.
Scheme 7. Structure of 15 and the Proposed Mechanism for the HSO3–/H2O2 (TBHP)-Induced Redox Cycle.
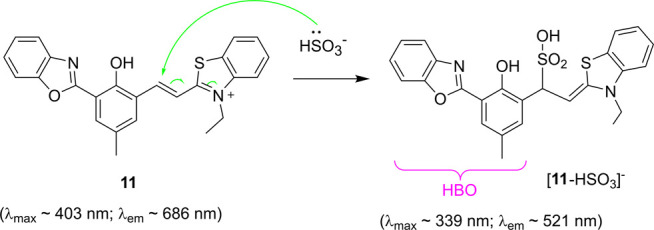
While the previous studies have been focusing on using the probes with HBT as the ESIPT unit, our group examined the possible use of 11 for bisulfite detection in NHLF cells as it became available.10 In 1:1 EtOH/PBS buffer, the absorption (λmax = 410 nm) and fluorescence (λem = 668 nm) of 11 gradually blue-shifted along with the formation of its adduct [11-HSO3–] (Scheme 8), showing a good linear response.
Scheme 8. Reaction of 11 with Bisulfite.
3. Conclusion
Excited-state intramolecular proton transfer (ESIPT) provides a unique photochemical process to generate fluorescence with a large Stokes shift. Continuous efforts in developing the NIR-emitting ESIPT-based probes could lead to valuable molecular probes for specific recognition of targets, including in vivo cellular imagining. In this review, we summarize the recent progress in using various strategies to tune the ESIPT emission toward longer wavelengths. The study using the substituent effect incorporates an ESIPT mechanism with strong electronic donor–donor or donor–acceptor interaction in order to shift the emission into the desirable NIR region (∼700 nm). Coupling the ESIPT and cyanine fragments has led to various new probes. The role of each part in the structure has been discussed. They have shown attractive applications in cellular imaging for recognizing intracellular organelles. As more and more ESIPT–cyanine dyes are synthesized, we anticipate continuous discovery of novel fluorophores that exhibit attractive optical properties to meet the need for molecular imaging applications.
Acknowledgments
We acknowledge support from NIH (Grant No. 1R15GM1264-3801A1) and partial support from Coleman Endowment from The University of Akron.
Glossary
Abbreviations
- ESIPT
excited states intramolecular proton transfer
- NIR
near-infrared
- PET
photoinduced electron transfer
- FRET
Förster resonance energy transfer
- ICT
internal charge transfer
- HBO
2-(2′-hydroxyphenyl)benzoxazole
- HBT
2-(2′-hydroxyphenyl)benzothiazole
- NMR
nuclear magnetic resonance
- DCM
dichloromethane
- NHLF
normal human lung fibroblast
- ROS
reactive oxygen species
Biographies

Yonghao Li is a Ph.D. candidate in Chemistry at the University of Akron, USA. He received his B.Sc. in Chemistry in 2017 from Shandong University, China, and is currently pursuing his Ph.D. degree in Organic Chemistry. His research interest is mainly in the synthesis of ESIPT-based conjugated heterocyclic compounds, especially novel cyanine dyes and their application in bioimaging.

Dr. Dipendra Dahal did his master’s degree in Organic Chemistry from the Tribhuvan University, Nepal, and his M.S. and Ph. D. in Chemistry from The University of Akron, USA. He is currently a postdoctoral research fellow at the Center for Blood Oxygen Transport and Hemostasis (CBOTH) at the University of Maryland Baltimore, with experience in the Organic Chemistry laboratory, synthesis, characterization, and application of molecular probes, fluorescence sensors, medicinal chemistry, and drug discovery and drug delivery. His research interests involve fluorescence probes, synthesis of drugs, and prodrugs for biomedical applications, therapeutics, and drug delivery.

Chathura Abeywickrama received his Ph.D. in 2018 from The University of Akron under the supervision of Professor Yi Pang, focusing on developing small molecule fluorescent probes for bioimaging applications. He is currently working as an Organic Chemist at St. Jude Children’s Research Hospital. His research interests are following developing photostable fluorescent probes for small-molecule FRET imaging, development of hybrid fluorophore systems and organelle-targeted small molecule fluorescent probes, and understanding structure–selectivity relationships of molecular probes.

Dr. Yi Pang received his Ph.D. degree in Organic Chemistry in 1990 from Iowa State University. He worked as postdoctoral fellow in Ames Laboratory from 1991 to 1993. He’s currently a professor in the Department of Chemistry, University of Akron, with a research position in the Maurice Morton Institute of Polymer Science. His current research interests include synthesis of luminescent polymers and fluorescent molecular probes and their potential applications for imaging biologically important species.
Author Present Address
§ Departments of Diagnostic Radiology and Nuclear Medicine and Pediatrics, Center for Blood Oxygen Transport and Hemostasis, University of Maryland Baltimore School of Medicine, Health Sciences Facility III, 670 West Baltimore Street, Baltimore, MD 21201, USA.
Author Present Address
⊥ St. Jude Children’s Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105, USA.
The authors declare no competing financial interest.
References
- Sedgwick A. C.; Wu L.; Han H.-H.; Bull S. D.; He X.-P.; James T. D.; Sessler J. L.; Tang B. Z.; Tian H.; Yoon J. Excited-State Intramolecular Proton-Transfer (ESIPT) Based Fluorescence Sensors and Imaging Agents. Chem. Soc. Rev. 2018, 47 (23), 8842–8880. 10.1039/C8CS00185E. [DOI] [PubMed] [Google Scholar]
- Woo C. H.; Jang S.; Shin G.; Jung G. Y.; Lee J. W. Sensitive Fluorescence Detection of SARS-CoV-2 RNA in Clinical Samples via One-Pot Isothermal Ligation and Transcription. Nat. Biomed. Eng. 2020, 4 (12), 1168–1179. 10.1038/s41551-020-00617-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Guan L.; Yu H.; Yan Y.; Du L.; Liu Y.; Sun M.; Huang D.; Wang S. Reversible Fluorescent Probe for Selective Detection and Cell Imaging of Oxidative Stress Indicator Bisulfite. Anal. Chem. 2016, 88 (8), 4426–4431. 10.1021/acs.analchem.6b00061. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Zhong X.; Qu W.; Shi T.; Liu H.; He H.; Zhang X.; Wang S. A Highly Selective HBT-Based “Turn-on” Fluorescent Probe for Hydrazine Detection and Its Application. Tetrahedron Lett. 2017, 58 (26), 2596–2601. 10.1016/j.tetlet.2017.05.071. [DOI] [Google Scholar]
- Wang J.; Baumann H.; Bi X.; Shriver L. P.; Zhang Z.; Pang Y. Efficient Synthesis of NIR Emitting Bis[2-(2’-Hydroxylphenyl)Benzoxazole] Derivative and Its Potential for Imaging Applications. Bioorg. Chem. 2020, 96, 103585. 10.1016/j.bioorg.2020.103585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangioni J. In Vivo Near-Infrared Fluorescence Imaging. Curr. Opin. Chem. Biol. 2003, 7 (5), 626–634. 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Dahal D.; Ojha K. R.; Alexander N.; Konopka M.; Pang Y. An NIR-Emitting ESIPT Dye with Large Stokes Shift for Plasma Membrane of Prokaryotic (E. Coli) Cells. Sens. Actuators, B 2018, 259, 44–49. 10.1016/j.snb.2017.12.041. [DOI] [Google Scholar]
- Dahal D.; McDonald L.; Bi X.; Abeywickrama C.; Gombedza F.; Konopka M.; Paruchuri S.; Pang Y. An NIR-Emitting Lysosome-Targeting Probe with Large Stokes Shift via Coupling Cyanine and Excited-State Intramolecular Proton Transfer. Chem. Commun. 2017, 53 (26), 3697–3700. 10.1039/C7CC00700K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald L.; Dahal D.; Konopka M.; Liu Q.; Pang Y. An NIR Emitting Styryl Dye with Large Stokes Shift to Enable Co-Staining Study on Zebrafish Neuromast Hair Cells. Bioorg. Chem. 2019, 89, 103040. 10.1016/j.bioorg.2019.103040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahal D.; Pokhrel S.; McDonald L.; Bertman K.; Paruchuri S.; Konopka M.; Pang Y. NIR-Emitting Hemicyanines with Large Stokes’ Shifts for Live Cell Imaging: From Lysosome to Mitochondria Selectivity by Substituent Effect. ACS Appl. Bio Mater. 2019, 2 (9), 4037–4043. 10.1021/acsabm.9b00564. [DOI] [PubMed] [Google Scholar]
- Ren T.-B.; Xu W.; Zhang W.; Zhang X.-X.; Wang Z.-Y.; Xiang Z.; Yuan L.; Zhang X.-B. A General Method To Increase Stokes Shift by Introducing Alternating Vibronic Structures. J. Am. Chem. Soc. 2018, 140 (24), 7716–7722. 10.1021/jacs.8b04404. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Xiao Y.; Qian X. A Ratiometric Fluorescent Probe Based on FRET for Imaging Hg2+ Ions in Living Cells. Angew. Chem., Int. Ed. 2008, 47 (42), 8025–8029. 10.1002/anie.200803246. [DOI] [PubMed] [Google Scholar]
- Weller A. Über Die Fluoreszenz Der Salizylsäure Und Verwandter Verbindungen. Naturwissenschaften 1955, 42 (7), 175–176. 10.1007/BF00595299. [DOI] [Google Scholar]
- Guo Y.; Dahal D.; Kuang Z.; Wang X.; Song H.; Guo Q.; Pang Y.; Xia A. Ultrafast Excited State Intramolecular Proton/Charge Transfers in Novel NIR-Emitting Mole-cules. AIP Adv. 2019, 9 (1), 015229. 10.1063/1.5088674. [DOI] [Google Scholar]
- Das K.; Sarkar N.; Ghosh A. K.; Majumdar D.; Nath D. N.; Bhattacharyya K. Excited-State Intramolecular Proton Transfer in 2-(2-Hydroxyphenyl)Benzimidazole and -Benzoxazole: Effect of Rotamerism and Hydrogen Bonding. J. Phys. Chem. 1994, 98 (37), 9126–9132. 10.1021/j100088a006. [DOI] [Google Scholar]
- Pines E.UV–Visible Spectra and Photoacidity of Phenols, Naphthols and Pyrenols. The Chemistry of Phenols; John Wiley & Sons, Ltd.: New York, 2003; pp 491–527. [Google Scholar]
- Kim C. H.; Park J.; Seo J.; Park S. Y.; Joo T. Excited State Intramolecular Proton Transfer and Charge Transfer Dynamics of a 2-(2’-Hydroxyphenyl)Benzoxazole Derivative in Solution. J. Phys. Chem. A 2010, 114 (18), 5618–5629. 10.1021/jp909438p. [DOI] [PubMed] [Google Scholar]
- Klehs K.; Spahn C.; Endesfelder U.; Lee S. F.; Fürstenberg A.; Heilemann M. Increasing the Brightness of Cyanine Fluorophores for Single-Molecule and Superresolution Imaging. ChemPhysChem 2014, 15 (4), 637–641. 10.1002/cphc.201300874. [DOI] [PubMed] [Google Scholar]
- Lavis L. D.; Raines R. T. Bright Building Blocks for Chemical Biology. ACS Chem. Biol. 2014, 9 (4), 855–866. 10.1021/cb500078u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S.; Goswami S.; Das Mukhopadhyay C. A Remarkable Ratiometric Fluorescent Chemodosimeter for Very Rapid Detection of Hydrogen Sulfide in the Vapour Phase and Living Cells. New J. Chem. 2015, 39 (11), 8940–8947. 10.1039/C5NJ01297J. [DOI] [Google Scholar]
- Bi X.; Liu B.; McDonald L.; Pang Y. Excited-State Intramolecular Proton Transfer (ESIPT) of Fluorescent Flavonoid Dyes: A Close Look by Low Temperature Fluorescence. J. Phys. Chem. B 2017, 121 (19), 4981–4986. 10.1021/acs.jpcb.7b01885. [DOI] [PubMed] [Google Scholar]
- Abeywickrama C. S.; Bertman K. A.; Mcdonald L. J.; Alexander N.; Dahal D.; Baumann H. J.; Salmon C. R.; Wesdemiotis C.; Konopka M.; Tessier C. A.; Pang Y. Synthesis of Highly Selective Lysosomal Markers by Coupling 2-(2’-Hydroxyphenyl)Benzothiazole (HBT) with Benzothiazolium Cyanine (Cy): The Impact of Substituents on Selectivity and Optical Properties. J. Mater. Chem. B 2019, 7 (47), 7502–7514. 10.1039/C9TB01672D. [DOI] [PubMed] [Google Scholar]
- Coelho F. L.; da Costa Duarte R.; de Avila Braga C.; Toldo J. M.; Bruno Goncalves P. F.; da Silveira Santos F.; Rodembusch F. S. Benzothiazole Merocyanine Dyes as Middle PH Optical Sensors. Dyes Pigm. 2020, 176, 108193. 10.1016/j.dyepig.2020.108193. [DOI] [Google Scholar]
- Pillay C. S.; Elliott E.; Dennison C. Endolysosomal Proteolysis and Its Regulation. Biochem. J. 2002, 363, 417–429. 10.1042/bj3630417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.; Wei T.; Zhang Z.; Zhang W.; Lv J.; Chen T.; Chi B.; Wang F.; Chen X. A Mitochondria-Targeted Fluorescent Probe for Ratiometric Detection of Hypochlorite in Living Cells. Chin. Chem. Lett. 2017, 28 (10), 1957–1960. 10.1016/j.cclet.2017.05.010. [DOI] [Google Scholar]


