Abstract
Urbanization contributes to the formation of novel elemental combinations and signatures in terrestrial and aquatic watersheds, also known as ‘chemical cocktails.’ The composition of chemical cocktails evolves across space and time due to: (1) elevated concentrations from anthropogenic sources, (2) accelerated weathering and corrosion of the built environment, (3) increased drainage density and intensification of urban water conveyance systems, and (4) enhanced rates of geochemical transformations due to changes in temperature, ionic strength, pH, and redox potentials. Characterizing chemical cocktails and underlying geochemical processes is necessary for: (1) tracking pollution sources using complex chemical mixtures instead of individual elements or compounds; (2) developing new strategies for co-managing groups of contaminants; (3) identifying proxies for predicting transport of chemical mixtures using continuous sensor data; and (4) determining whether interactive effects of chemical cocktails produce ecosystem-scale impacts greater than the sum of individual chemical stressors. First, we discuss some unique urban geochemical processes which form chemical cocktails, such as urban soil formation, human-accelerated weathering, urban acidification-alkalinization, and freshwater salinization syndrome. Second, we review and synthesize global patterns in concentrations of major ions, carbon and nutrients, and trace elements in urban streams across different world regions and make comparisons with reference conditions. In addition to our global analysis, we highlight examples from some watersheds in the Baltimore-Washington DC region, which show increased transport of major ions, trace metals, and nutrients across streams draining a well-defined land-use gradient. Urbanization increased the concentrations of multiple major and trace elements in streams draining human-dominated watersheds compared to reference conditions. Chemical cocktails of major and trace elements were formed over diurnal cycles coinciding with changes in streamflow, dissolved oxygen, pH, and other variables measured by high-frequency sensors. Some chemical cocktails of major and trace elements were also significantly related to specific conductance (p<0.05), which can be measured by sensors. Concentrations of major and trace elements increased, peaked, or decreased longitudinally along streams as watershed urbanization increased, which is consistent with distinct shifts in chemical mixtures upstream and downstream of other major cities in the world. Our global analysis of urban streams shows that concentrations of multiple elements along the Periodic Table significantly increase when compared with reference conditions. Furthermore, similar biogeochemical patterns and processes can be grouped among distinct mixtures of elements of major ions, dissolved organic matter, nutrients, and trace elements as chemical cocktails. Chemical cocktails form in urban waters over diurnal cycles, decades, and throughout drainage basins. We conclude our global review and synthesis by proposing strategies for monitoring and managing chemical cocktails using source control, ecosystem restoration, and green infrastructure. We discuss future research directions applying the watershed chemical cocktail approach to diagnose and manage environmental problems. Ultimately, a chemical cocktail approach targeting sources, transport, and transformations of different and distinct elemental combinations is necessary to more holistically monitor and manage the emerging impacts of chemical mixtures in the world’s fresh waters.
Keywords: urban evolution, urban karst, urban watershed continuum, freshwater salinization syndrome, human-accelerated weathering
Introduction
Most of the world’s human population lives in urban areas and relies on the structure, function, and services provided by urban ecosystems for survival (Grimm et al. 2008). The built environment and its structure, ecosystem functions, and ecosystem services evolve over time based on human selective pressures and adaptations (Kaushal et al. 2014; Kaushal et al. 2015). Although less considered, urbanization can modify abundances, distributions, and ratios of elements in terrestrial and aquatic environments (Kaye et al. 2006; Lyons and Harmon 2012; Chambers et al. 2016; Kaushal et al. 2019). We recently proposed that atmospheric deposition, land use, geology, and climate enhance the formation and transport of novel combinations or mixtures of elements in watersheds, hereafter referred to as ‘chemical cocktails’ (Kaushal et al. 2018a, 2018b). Chemical cocktails of elements are formed when there is increased probability of biogeochemical interactions, converging transport pathways, and/or novel anthropogenic sources (Kaushal et al. 2018a, 2018b). Watershed chemical cocktails provide distinct signatures of shifting human activities associated with environmental degradation and ecosystem restoration during Earth’s latest geological epoch, the Anthropocene. Distinct and diverse watershed chemical cocktails originate from sources such as sewage, automobiles, weathering of impervious surfaces, synthetic chemicals, and widespread proliferation of mineral resources used in human settlements (Bernhardt, Rosi, and Gessner 2017; Long et al. 2017; Kaushal, Gold, et al. 2018; Kaushal, Likens, et al. 2018; Blaszczak et al. 2019). An understanding of the formation of chemical cocktails in watersheds over time and space is important because it can facilitate approaches to co-manage groups of pollutants with similar fate and transport. A watershed chemical cocktail approach can also enable identification and development of continuous sensor proxies for predicting the simultaneous behavior of multiple urban contaminants and chemical mixtures over time. In addition, a watershed chemical cocktail approach allows characterization of emergent effects of chemical mixtures and quantification of ecosystem impacts beyond a simple sum of effects of individual chemicals.
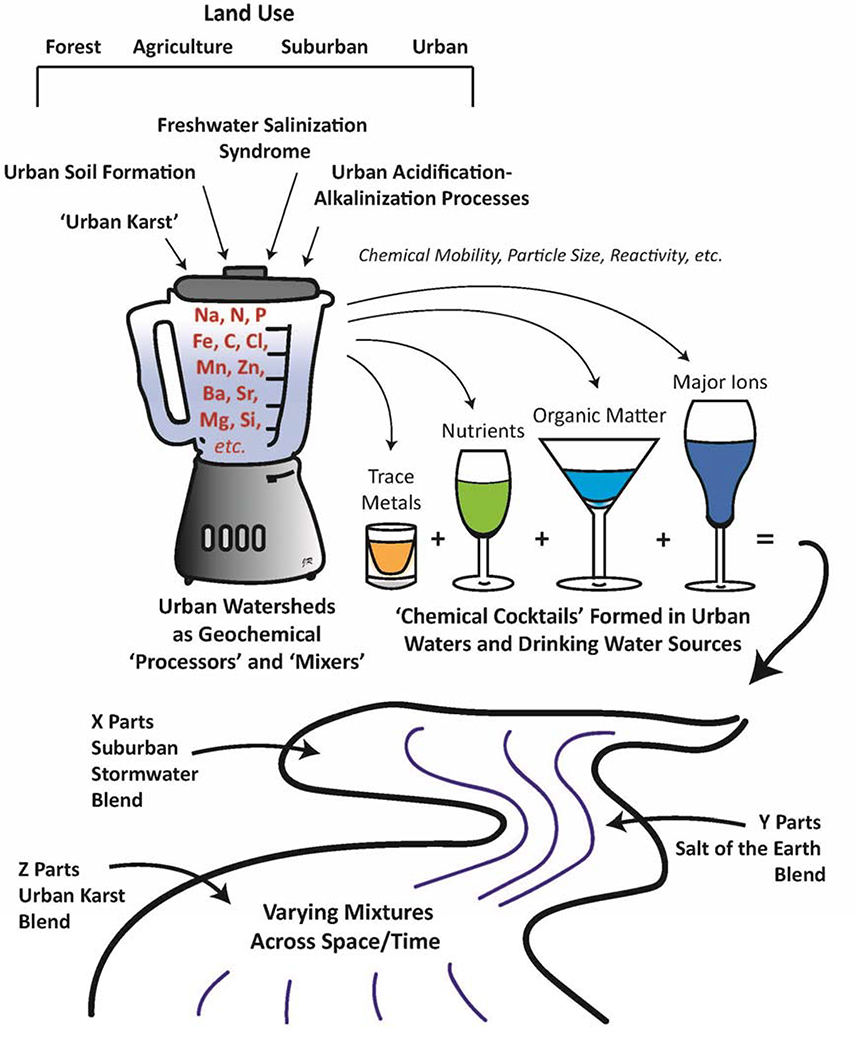
In this paper, we explore how chemical cocktails are made in urban watersheds over space and time. We also illustrate some novel processes contributing to geochemical and biogeochemical patterns in urban infrastructure, soils, and waters. First, we explore novel processes in urban watersheds that influence elemental cycles including urban soil formation, human-accelerated weathering, urban acidification-alkalinization processes, and freshwater salinization syndrome. Second, we review origins and concentrations of distinct chemical cocktails of major ions, carbon, nutrients, and trace metals in different urban waters. We make comparisons between the chemistry of urban waters with minimally disturbed reference watersheds where possible. Thirdly, we conclude with some management implications of chemical cocktails for urban water quality. The watershed chemical cocktail approach allows us to identify and characterize how distinct and diverse chemical mixtures evolve in urban ecosystems (Figure 1).
Figure 1.
Conceptual model illustrating how groups of elements can be grouped as ‘chemical cocktails’ and transported in urban waters. Elemental inputs to the watershed originate from different urban geochemical sources and processes. Chemical cocktails are mixed hydrologically across time and space in watersheds through surface flowpaths (impervious surfaces, construction materials, altered geomorphology, etc.) and subsurface flowpaths (stream burial, urban karst, pipes, soil compaction, etc.). Urban geochemical processes form distinct elemental combinations of nutrients, metals, salt ions, and organics that are transported and transformed along hydrologic flowpaths based on factors such as chemical mobility, particle size, and reactivity. These different chemical cocktails can become mixed together along hydrologic flowpaths and their sources may be traced using diverse analytical approaches.
Part 1. Examples of Urban Processes that Make Chemical Cocktails
Urban Soil Formation: Novel Parent Materials Create Diverse Chemical Cocktails of Major and Trace Elements
Soils remove, transform, and release elements into the hydrosphere and play a major role in influencing many elemental cycles to form chemical cocktails in Anthropocene waters (Lehmann and Stahr 2007). Human activities changing the built environment lead to an “urban evolution” of the structure, function, and ecosystem services of urban areas over time (Kaushal, McDowell, and Wollheim 2014; Kaushal et al. 2015; Hale 2016; Parr et al. 2015; McPhillips and Matsler 2018). It is evident that urbanization influences every state factor of soil formation as humans contaminate, deplete, erode, and move soils around landscapes, which influences the diversity, distribution, and abundance of elements. Urban evolution creates novel parent materials from human-made materials with distinct elemental combinations from which soils are formed. Human-made materials from which soils form, also known as artifacts or technogenic materials, include bricks, crushed stone, concrete, plastic, mine waste, industrial waste, and metal objects (Effland and Pouyat 1997) (Figure 2). The soil classification ‘technosol’ defines a soil containing more than 20% artifacts. It is common for urban soils to contain many artifacts. Technogenic materials in urban soils are spatially heterogeneous due to excavation, transport and deposition (Huot et al. 2015). Urban soils are commonly alkaline due to construction materials but can be acidic due to coal and sulfuric acids (Lehmann and Stahr 2007). Urban soils can be high in organic matter and nutrients due to ongoing organic additions, including refractory combustion byproducts and residues; other areas of urban soils can be very low in organic matter and available nutrients, particularly when they originated as clean fill and have been swept or otherwise cleaned of organic matter over time (Lehmann and Stahr 2007). Artifacts can lead to the formation of soil characteristics not native to the climate in which they form. For example, the accumulation of gypsum (CaSO4-H2O) in lower soil layers, which is usually seen in dry climates, can occur in temperate humid climates due to the presence of gypsum construction materials such as drywall (Zikeli et al. 2005). Urban soils are also characterized by missing B horizons, which is the result of grading, excavation, and filling that can mix soil layers (destroying pedogenic features) or bury them far enough below other mixed materials to substantially influence the vertical distribution of chemical mixtures in these soils, altering their function, morphology, and classification (Herrmann et al. 2018). Leaching of soluble compounds and oxidation of sulfides leads to formation of secondary minerals such as sulfates and Fe (hydr)oxides, which occur in both technogenic materials and natural materials, though rapid initial weathering may occur as some anthropogenic materials are not at equilibrium in the environment in which we place them (Huot et al. 2015). Minerals of unnatural composition and minerals not often found in natural soils can be found in soils created from technogenic materials. Calcite and gypsum are added by concrete. Some industries made clinker, which is common in urban soils and consists of a few minerals including alite, belite, tricalcium aluminate, and brownmillerite. There are many novel minerals and chemical mixtures caused by human activity that have never been found naturally occurring in Earth’s crust (Hazen et al. 2017). Urbanization has accelerated the time in which soil forming processes occur, and as a result urban soils are often young due to frequent anthropedoturbation by construction and other human activities (e.g., mixing brings deeper soil horizons in contact with surficial processes) (Effland and Pouyat 1997). Furthermore, legacy artifacts found in urban soils such as underground storage tanks of petrochemicals and hazardous materials in old construction materials such as asbestos tiles (DeKimpe and Morel 2000) can also contribute to formation of unique elemental combinations during urban soil formation. By adding technogenic materials to urban ecosystems, humans accelerate erosion, chemical weathering and formation of distinct chemical cocktails in urban soils. Removal, transportation, and deposition of technogenic materials can break up soils, breaking apart organizational units like clods and fragmenting solids like bedrock and rock fragments. This can result in increased surface area of the material and exposes reactive surfaces that have yet to be weathered to water, oxygen, and other solutes. All of these processes could result in increased weathering rates similar to some mining sites as analogs (weathering is discussed in more detail below) (Daniels et al. 2016).
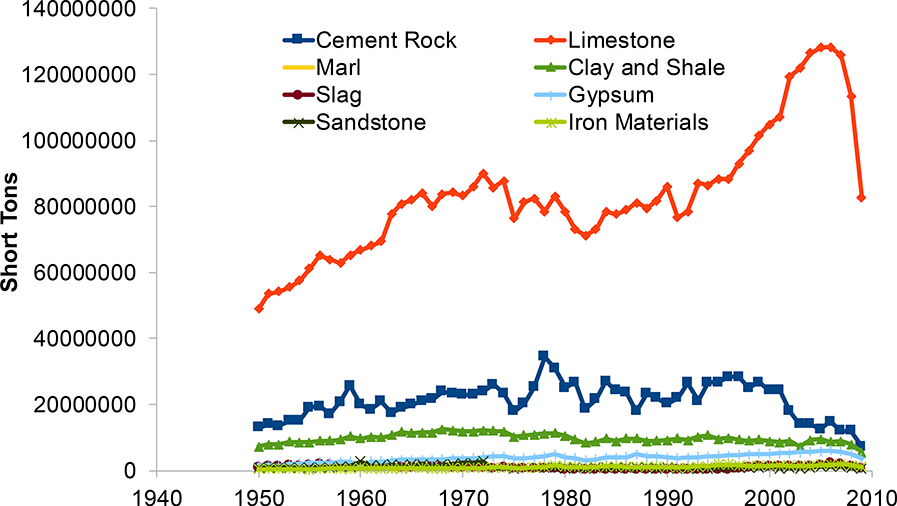
Figure 2.
Long-term changes in geologic materials used to make concrete in the United States. This widespread proliferation of geologic materials used to make concrete can degrade over time through accelerated weathering of impervious surfaces contributing to transport of chemical cocktails of major ions to urban waters. Data are from U.S. Geological Survey Minerals Yearbook.
Weathering of the ‘Urban Karst’ Makes Diverse Chemical Cocktails of Major Elements
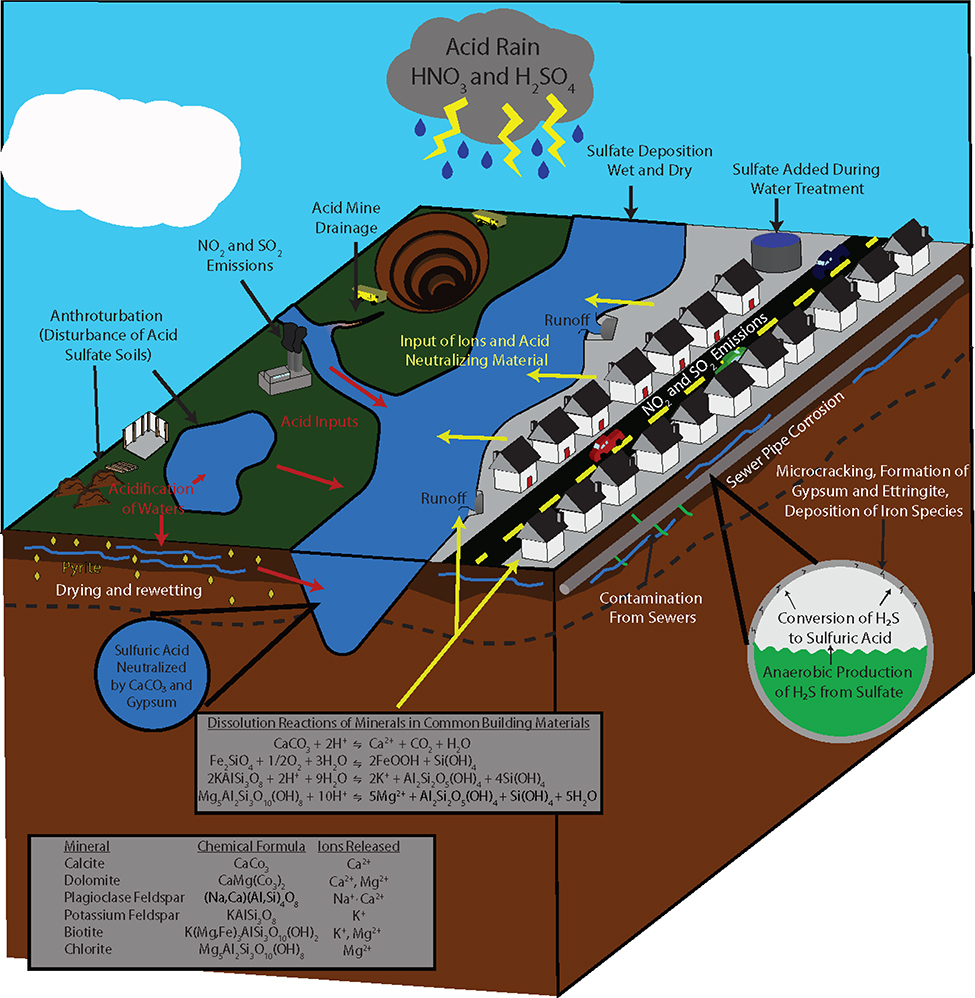
Chemical weathering from acid precipitation is often distinct in urban areas due to their unique, carbonate-rich lithologies, sometimes referred to as “urban karst.” Urban karst refers to the built environment of buildings, bridges, engineered river banks, and impervious surfaces which comprise upward of 75 to 85% of the land surface in industrial, commercial, and densely populated residential environments (Chester Jr and Gibbons 1996; C. Wu and Murray 2003). Accelerated chemical weathering rates occur because many of the minerals found in urban karst are thermodynamically unstable at the temperatures and pressures present at the Earth’s surface (Camuffo 2015). Carbonate minerals, such as calcite and dolomite, are the most soluble minerals commonly found in the built environment. Feldspars, clay minerals, and quartz are also vulnerable to acid rain, though with decreasing solubilities (Camuffo 2015). In general, the solubility of a mineral increases as calcium and magnesium content increases and silica content decreases (Camuffo 2015). Cations (such as Ca2+, Mg2+, Na+ and K+) are released when acidic compounds react with minerals commonly found in building materials such as calcite, dolomite, feldspar biotite and chlorite (Wright et al. 2011; Camuffo 2015; Kaushal et al. 2017) (Figure 3). Calcareous concrete pipes weather at a significant rate, first through a reaction between carbon dioxide and concrete hydrates like calcium hydroxide (Ca(OH)2 or portlandite) and calcium silicate hydrates (CSH) (Davies et al. 2010), producing calcium carbonate (CaCO3) and water (Figure 3). Low pH water from acid precipitation reacts with these alkaline hydration products of cement creating calcium salts and distinct and diverse chemical cocktails rich in base cations and carbonates (Figure 3).
Figure 3.
Conceptual diagram of human-accelerated weathering of impervious surfaces and corrosion of pipes illustrating how human activities contribute major ions and metals to distinct chemical cocktails in urban waters. Human-accelerated weathering and corrosion of infrastructure can be associated with acidic precipitation and anaerobic processes in sewer pipes.
Urban Acidification-Alkalinization Processes Make Iron and Sulfur-Rich Chemical Cocktails
Disturbance of hypersulfidic soil materials during construction contributes to sulfuricization, the process by which sulfidic materials oxidize. Sulfuric acid weathers existing minerals and new mineral phases are formed (Fanning et al. 2017), which can produce acidic soils with pH values less than 2.0–3.5. In response, local waters can become rich in chemical cocktails enriched in dissolved iron and iron oxide flocculate, and the soil surface can become crusted with hydrated sulfate minerals including halotrychite and copiapite group minerals. These elevated levels of iron, aluminum, and acidity also lead to iron and sulfur-rich chemical cocktails (Daniels and Orndorff 2003; Fanning et al. 2004). Sulfuricization can also occur when acid sulfate soils are used as a source for imported fill materials (e.g., to “cap” landfills). In addition to direct disturbance of soil materials, sulfuricization can also be triggered in urban areas by manipulation of the water table. Groundwater levels are altered by drainage, either by ditches, pipes, or uncontrolled incision of urban headwater streams, contributing to urban hydrologic drought and oxic conditions (Groffman et al. 2003). While sulfuricization can cause a decrease in pH and the formation of acidic soils, most urban streams still have a higher pH and are alkaline due to weathering of urban karst and high acid neutralization capacity (Ometo et al. 2000; Zampella 1994). For example, cement has a high acid neutralizing capacity of 955 mg CaCO3 eq./g (Sephton and Webb 2017), which neutralizes sulfuric acid produced by sulfuricization and makes many urban waters alkaline (Lehmann and Stahr 2007) (Figure 3). Because both limestone and concrete are common building materials (coupled with alkaline soils present in urban environments), many acidic waters originating in acid sulfate soils will eventually still become alkaline and result in higher pH (Kaushal et al. 2017).
Sulfuricization is not generally detectable in urban streams due to the vast amount of acid neutralization that occurs. However, sulfuricization still contributes to the localized weathering of “urban karst” and subsurface infrastructure, such as sewer pipes (Figure 3). A major problem in sewer systems is the corrosion of the metal and concrete pipes (Boon 1995; Mori et al. 1991; Haaning Nielsen et al. 2005; Nielsen and Hvitved-Jacobsen 1988; Pomeroy and Bowlus 1946). The primary causes of corrosion are due to the production of hydrogen sulfide (H2S) and the oxidation of H2S to form sulfuric acid (Pomeroy and Bowlus 1946), wherein both processes may be microbially mediated. The production of H2S is initiated by the reduction of sulfate in wastewater when sulfate interacts with microbes living in biofilms on the walls of sewer pipes and in the sediments in sewer pipes (Liu et al. 2015; Nielsen and Hvitved-Jacobsen 1988). This reduction is dependent on anaerobic bacteria and chemical oxygen demand, as well as sulfate and organic matter availability (Nielsen and Hvitved-Jacobsen 1988). Although H2S is corrosive and results in the corrosion of metal pipes (Boon 1995), the oxidation of sulfide into sulfate (sulfuric acid) also contributes to the corrosion of concrete pipes (Nielsen et al. 2005). The corrosion of the concrete pipes occurs along an acid front where the cement is broken down into gypsum and ettringite by the following reactions (Jiang et al. 2014).
The products of these reactions have larger volumes than uncorroded cement, and thus cause expansion and microcracking of the concrete. The weathering of concrete and corrosion of pipes contributes to the release of chemical cocktails enriched in weathering products such as Ca2+, Mg2+, HCO3−, SO42− and/or metals, such as iron, into surface and groundwater (e.g. see equations below) (Figure 3).
Freshwater Salinization Syndrome Makes Diverse Chemical Cocktails
Chronic salinization of surface waters has occurred regionally (Kaushal et al. 2005) and on continental and global scales (Kaushal et al. 2018a, 2018b, 2018c) due to increasing coverage by impervious surfaces and associated salt sources such as road deicers. This phenomenon, known as the Freshwater Salinization Syndrome (FSS); Kaushal et al. 2018a, 2018b, 2018c) influences the transport and transformation of base cations, carbon, nutrients, and trace metals from watersheds to streams. FSS enhances mobilization of various organic carbon compounds, nutrients, metals, and cations due to coupled biotic and abiotic processes such as ion exchange, rapid nitrification, pH changes, increased ionic strength, organic matter dispersal, and chloride complexation. Unanticipated geochemical relationships associated with FSS impact the efficacy of stream restoration strategies, urban water quality, and safe drinking water. At slightly elevated salinity, the linkage may be due to abiotic mechanisms such as cation exchange on the sediment surface or organic-metal ligand dispersal. At significantly elevated salinity (or prolonged salinity), the cation exchange sites could saturate, and the coupling may be due to biotic mechanisms such as a rapid reduction in nutrient processing, a loss of structure and function of lotic ecosystems, and/or microbial lysis. Chemical cocktails made by FSS can be categorized into distinct mixtures (Table 1). Because relatively little is still known regarding the synergistic effects of FSS in the environment, we propose the following categorization of chemical cocktails:
Table 1:
A broad summary of the geochemical affinities of species of nitrogen, phosphorus, carbon, base cations, and trace metals. A major point of this table is to not only list the geochemical affinities, but to give a prediction on how Freshwater Salinization Syndrome can influence the cycling of chemical constituents in urban streams. Based on their affinities, the elements/compounds were demarcated into 1 or more of the 4 categories mentioned in the text (1=organic matter complexes, 2=ion exchangeable, 3=redox sensitive, 4=transition metals), and a directional hypothesis for change in concentration in response to FSS was postulated.
| Elemental Compound | Geochemical Affinities | Major Process affected by salinization | Unknown in geochemical behavior | FSS Cocktail Group and Directional Hypothesis | Response Variable | Other studies |
|---|---|---|---|---|---|---|
| NH4+ | Production in sediments during organic matter decomposition, Strong adsorption onto sediment particles in freshwater (especially clay) | Increase in Na, competition for exchange sites, inhibition of nitrification microbes | Temperature dependency of adsorption, desorption contribution to N mineralization | 2,3, Increase | TDN | Weston et al, 2010, Seitzinger et al, 1991 |
| NO3− | Relatively mobile form on N, Product of nitrification in shallow sediments | Increase in NH4 leading to increases in nitrification, inhibition of microbes | Hydrologic flowpath sources in human-dominated watersheds | 2, Increase | ||
| DON | Protein and amino acids, largely refractory, Intermediate reduced products during N cycle by microbes or humification reactions, Redox sensitive, coupled with DOC | Increases in ionic strength inducing organic matter colloid dispersion, inhibition of ammonification microbes | Measurement and classification of DON is difficult. | 3, Decrease | Burdige and Zheng, 1998 | |
| SiO4 | Sediment surface reactions with alkali complexes to form sodium complexes, Ionic composition increases reactivity and hydrolysis reactions, Stable behavior in soils | Increased ionic strength can increase the dissolution kinetics of Silica from quartz or oxide sediments, Increase Na concentrations | Microscopic role of Na at the oxide mineral-solution interface, stoichiometric relationship with aluminum | 1, Increase | Si | Dove and Elston, 1992 |
| Ortho PO43− | Complexation reactions with metals (Fe, Al, Mn) and base cations to insoluble forms, Flocculation/settling of insoluble forms, Redox reactions, Behavior coupled with S | Increased ionic inducing competing processes of flocculation and colloid dispersal, Changes in pH increasing reduction potential, dissolution desorption, Presence of anions (Cl−, NO3−) increase PO43− sorption due to keeping Fe oxidized | Microbial cycling pathways and buffer system, exchange-able phosphorous content on sediment and soils, anaerobic-aerobic transition in stream sediments | 1,3, Increase or net effects of competing processes | SRP | Baldwin et al, 2006, Duan and Kaushal 2015, House, 2003 |
| Poly/Organic PO43− | Amino and fatty acids bound within organic matter colloids, | Changing ionic composition inhibiting microbial cycling | N/A | |||
| CO2 | Solubility of CO2 decreases with salinity, microbial metabolism and chemical weathering byproducts | Inhibition of microbial respiration and mineralization, pH induced dissolution of carbonate minerals | Temperature kinetics in sediments and rivers | 1, Increase | DIC | Weiss, 1974 |
| HCO3− | Solubility increases with salinity, chemical weathering byproducts | 1, Increase | Meybeck, 2003 | |||
| Humic/Fulvic Acids | Protein-like, Redox sensitive, Generally coupled with polyphosphates and DON, potentially hydrophobic, binds to colloidal, tendency to flocculate, complexation reaction with base and metal cations | Na dispersal could increase solubility of protein-like material, inhibition of microbial process | Lability of specific compounds and influence of hydrologic flowpaths | 1,3, Increase with greater solubility at higher pH or no change | DOC | Green et al, 2009, Duan and Kaushal 2015 |
| Ca | Outer-sphere surface Reactions (hydrated), High Ionic Potential, Sorption affinity controlled by oxidation state, then ionic radius, Potential redox reactions | Increased Na concentrations increases competition for sediment exchange site | Effect of changing ionic composition on sediment and reduction potential | 1,2,3, Increase | Ca | Stumm and Morgan 1996, Honeyman and Santschi, 1988, Vengosh, 2003 |
| Mg | 1,2, Increase | Mg | ||||
| K | 1, 2, Increase or Constant* | K | ||||
| Zn | Redox Reactions, Surface Protolysis Reactions, Electrolyte Surface Reactions, Cation Inner-sphere Surface Reactions, Low Ionic Potential, Sorption affinity controlled by oxidation state, then ionic radius | Increased Na concentrations increases competition for sediment exchange site | Effect of changing ionic composition on sediment and reduction potential | 2,3, Increase | Zn | Stumm and Morgan 1996, Honeyman and Santschi, 1988, Vengosh, 2003, Kaushal et al. (2018 a, b) |
| Sr | 2,3, Increase | Sr | ||||
| Cu | Redox Reactions, Complexation Reactions, Chelation-ligand formation, Surface Protolysis Reactions, Electrolyte Surface Reactions, Cation Inner-sphere Surface Reactions, Low Ionic Potential, Transition metal: sorption affinity depends on electron configuration | Shift in pH affects reduction potential, changes in ionic strength causing colloid dispersal from sediment layer to suspension, increased chloride concentration enabling formation of chloro-organic matter complexes, inhibition of microbial metabolisms | Electrostatic thresholds for sorption affinity, anaerobic-aerobic transition in stream sediments, effect of changing ionic competition on reduction potential | 3,4, Increase | Cu | |
| Mn | 3,4, Increase | Mn |
Although we hypothesize the concentrations of elements in FSS cocktail group 1 and 2 to increase and we place K within these groups by behavior, K has a larger ionic radius than Na and is more biologically reactive, which can affect its concentrations.
FSS Organic Matter Complexed Chemical Cocktails: elements/compounds with affinities to complex with natural organic matter. Salinization increases the chloride concentration, which could increase the solubility of metals associated with colloids. Salinization increases the ionic strength of water, which could disperse colloids from the sediment surface into suspension. Through dissolution reactions, elements on colloids could enter the dissolved phase, and the concentration of these elements be influenced by natural organic matter solubility.
FSS Ion Exchangeable Chemical Cocktails: elements/compounds with outer-sphere electrostatic affinities to sorb onto sediment particles. Through increases in chloride concentrations, salinization increases the ionic strength of water, which could affect sorption properties. More importantly, through increases in sodium concentrations (e.g. road salt), salinization could increase the competition for cation exchange sites on sediment surfaces. Within an isovalent series, the response of these elements is dominated by their ionic radii as larger cations have a greater sorption affinity due to lower ionic potentials than smaller cations. Across oxidation states, the response of these elements to FSS is a function of their location/exposure on the sediment surface, and the concentration of these elements can typically increase after salinization.
FSS Redox-Sensitive Chemical Cocktails: these are redox-sensitive elements/compounds. Due to ion-exchange with hydrogen, salinization could induce abrupt shifts in pH, which could affect the reduction potential at the sediment-water interface. Increases in ionic strength can cause organic matter dispersal off the sediment surface and shifts in microbial processing, or cellular lysis, both of which could expose the deeper sediment to oxygen and affect the reduction potential. The response of these types of cocktails can be strongly coupled with DOC concentrations, which is also highly redox-sensitive.
FSS Synthetic Chemical Cocktails: the types of chemical cocktails that we mainly discuss are anions, cations, organic matter, metals, nutrients, etc., which are not synthetic (Kaushal et al. 2018 a, b, c). However, there has been widespread proliferation of synthetic chemicals in the environment (Bernhardt et al. 2017) that may also be influenced by FSS such as endocrine disrupting chemicals (EDCs), polycyclic aromatic hydrocarbons (PAHs), and others (Brunk et al. 1997; Borrirukwisitsak et al. 2012). Investigating FSS impacts on mobilization and cycling of synthetic chemical cocktails can improve our characterization of urban geochemistry into the future research.
Part 2. Examples of Distinct Chemical Cocktails in Urban Waters
Urbanization Makes Chemical Cocktails of Major Ions
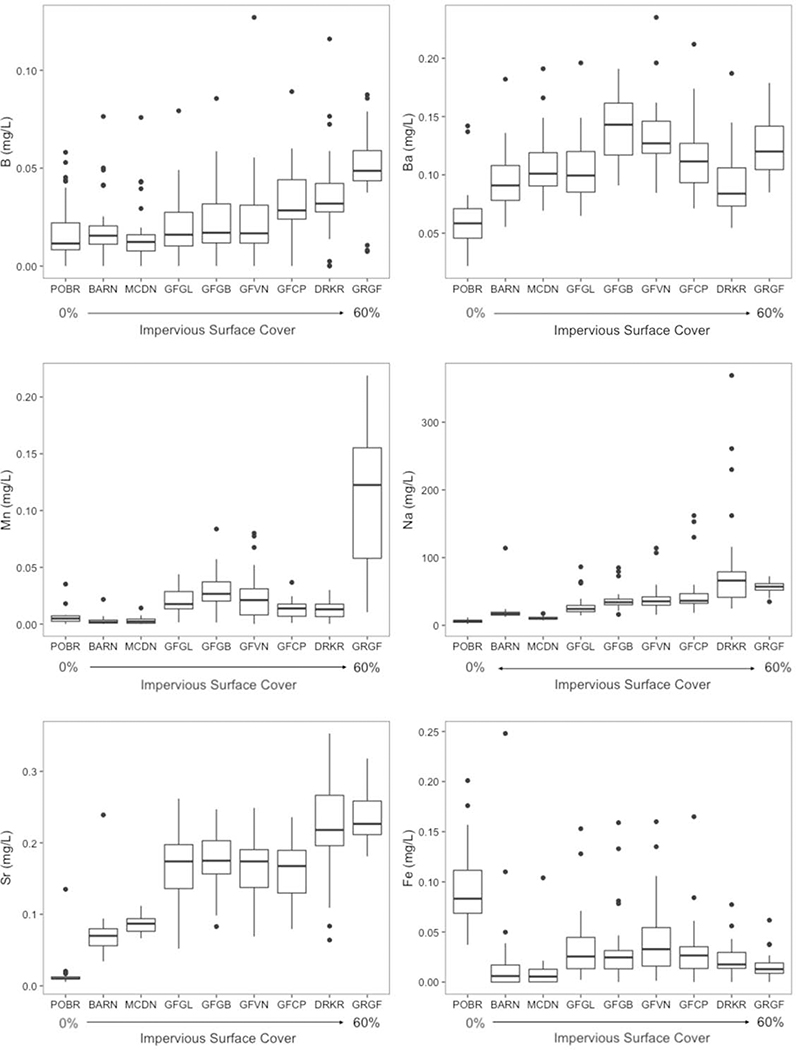
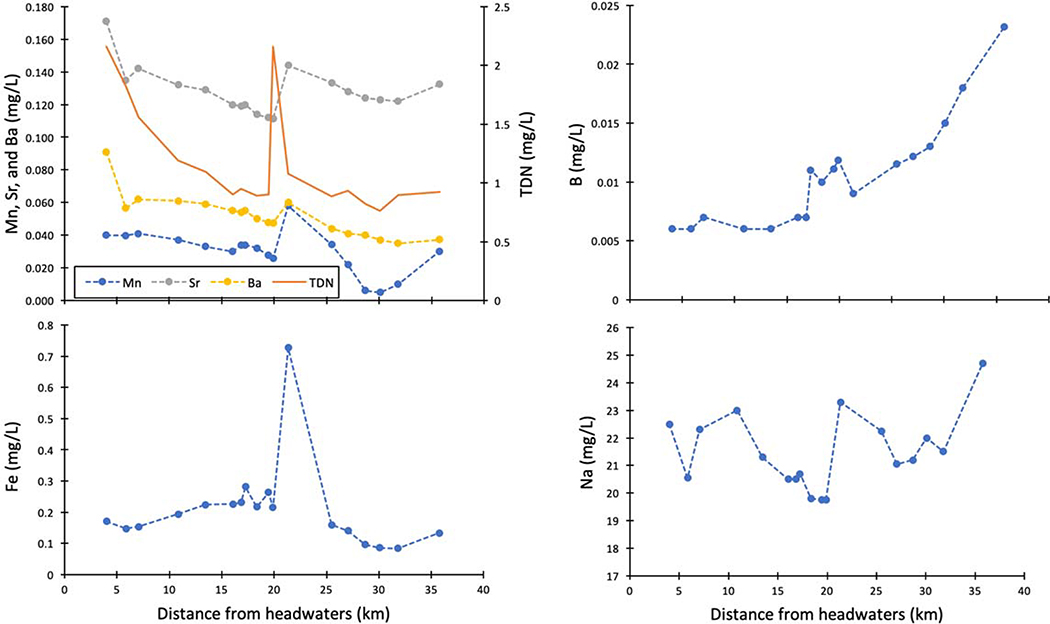
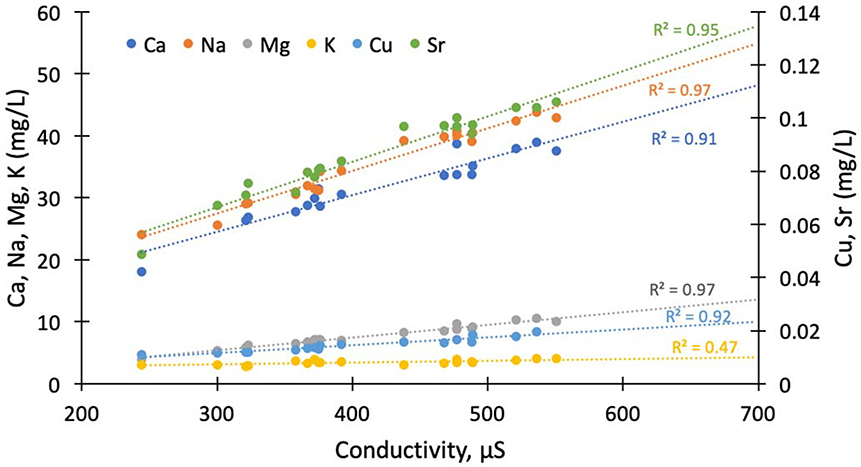
Major ions appear regularly in surface waters at concentrations greater than 1 mg L−1, and some examples are Ca2+, Mg2+, Na+, K+, Cl−, SO42−, HCO3−, and CO32− (Figure 4). Major ion concentrations are consistently elevated in urban streams vs. minimally disturbed reference conditions, but there is regional variability (Figure 4). Chemical cocktails of major ions form due to inputs from wastewater, infrastructure dissolution, evaporative concentration, atmospheric dust, road salt application, and soil acidification (Table 2). Typically, sewage has high concentrations of Na+, Cl−, SO42−, and K+ relative to background concentrations. Ca2+, Mg2+, and HCO3− are also present in high concentrations in sewage and can also reflect weathering sources (Kaushal et al. 2017). K+, which is a limiting nutrient for many plants occurs in relatively low concentrations in natural streams, and is attributed almost solely to human urine in urban streams. Rose (2007) found a strong correlation between Na+, K+, and Cl− concentrations within 50 study basins, which was interpreted to reflect the electrolytes present in the human body. Boron and silica, which are not major ions, are also robust tracers for human activity (Neal et al. 2000; Takagi et al. 2017), as they are not highly concentrated in natural waters, but are concentrated in sewage due to detergents (silica can also be found in some processed foods as clay minerals, where Si can be solubilized). In fact, boron concentrations increase with watershed urbanization along with sodium concentrations from road salt and sewage; strontium concentrations from concrete and weathering sources also increase with Ca2+ and most major ions (Purdy and Wright 2019) (Figure 5). Ba2+ is another trace element that can increase with major ions in response to watershed urbanization due to inputs from sewage, paint, bricks, ceramics, glass, rubber, automobiles, and medical and industrial wastes (Monaci and Bargagli 1997; Purdy and Wright 2019) (Figure 5). Finally, manganese is one more example of a trace element that can increase with major ions in response to watershed urbanization, and this is likely due to stormdrain and groundwater inputs, batteries, pipes, automobiles and other sources (Paul and Meyer 2001; Joselow et al. 1978) (Figure 5). Thus, chemical cocktails of major ions and trace elements can mix, interact, and co-occur together in urban waters.
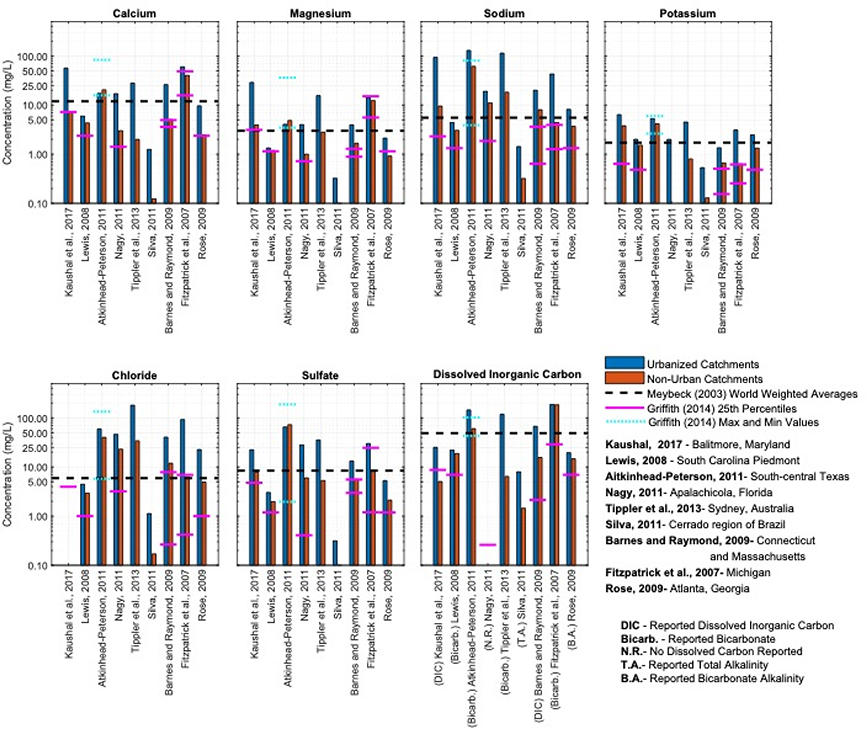
Figure 4.
Mean major ion concentrations in streams for urbanized watersheds and reference watersheds for nine selected studies spanning a range of study areas. Mean dissolved concentrations of major ions are consistently higher within urban watersheds when compared to reference watersheds, despite variability in absolute concentrations among the nine studies. This result is evidence of the ‘overprint’ of urban geochemical processes influencing major ions, which are superimposed onto natural geochemical processes influencing major ion concentrations. Where applicable, the 25th percentile concentration reported by Griffith (2014) is indicated using pink lines for the corresponding US EPA Level III Ecoregion encompassing each study area. Where study areas span Ecoregions, both are indicated. Griffith (2014) only reported maximum and minimum values in some Ecoregions due to limited data availability. These are shown using blue lines for studies without a 25th percentile concentration. Two studies are outside the United States; the world weighted average river concentrations provided by Meybeck (2003) are shown as a dashed horizontal line for reference. The nine studies reported dissolved inorganic carbon (DIC) species in different ways; these ways are listed in the bottom right plot within the figure. Supporting Information includes further details.
Table 2.
Examples of urban geochemical processes and the major ions affected by each process.
| Urban Geochemical Process | Chemical Cocktails of Major Ions | Some Literature Examples |
|---|---|---|
| Wastewater Inputs | K+, Na+, Cl−, SO42− |
Verbanck et al. (1989) Rose (2007) |
| Infrastructure Dissolution | Ca2+, Mg2+, HCO3−, CO32− | Tippler et al. (2014) Davies et al. (2010) |
| Evaporative Concentration | All Major Ions | Grimmond and Oke (1999) |
| Atmospheric Particulates | SO42−, Cl− | (Stumm and Morgan; Honeyman and Santschi 1988; Vengosh 2003) |
| Road Salt Applications | Na+, Ca2+, Mg2+, Cl− | Steele, McDowell, and Aitkenhead-Peterson (2010) |
| Soil Acidification and Weathering | Na+, Mg2+, Ca2+, K+, and HCO3− | Aquilina et al. (2012) |
| Urban storm sewer | F−, Cl−, SO42−, Li+, Na+, NH4+, and K+. | Gardner and Carey (2004) |
Figure 5.
Concentrations of major and trace elements in streams draining a land use gradient at the Baltimore Long-Term Ecological Research (LTER) site over almost 2 years of bi-weekly sampling. Center vertical lines of the box and whiskers indicate medians. Lengths of each whisker show ranges within which the central 50% of the values lie. Edges of the boxes indicate the first and third quartiles. Dark circles represent outside values.
Infrastructure dissolution through urban karst in watersheds can create an anthropogenic signal that overrides natural factors and other land use changes. Impervious surface cover in a watershed is linked to increased base cation loads across a variety of settings because chemical weathering of building material is a steady source of cations (e.g., Ca2+, Mg2+, and HCO3−). Studies investigating the influence of concrete weathering in urban watersheds show that the artificial lithology of concrete is able to overwhelm the natural lithology in determining major ion chemistry. Barnes and Raymond (2009) found that in 19 small watersheds with minimal lithologic differences ion loads in urban streams were significantly higher than in forested or agricultural streams and that human activities contributed 54% to 79% of the dissolved inorganic carbon in streams. Tippler et al. (2014) found an 18-fold increase in HCO3−, a 14-fold increase in Ca2+, and a 6-fold increase in salinity in urbanized watersheds versus reference conditions. Fitzpatrick et al. (2007) evaluated baseflow from 31 streams across a land use gradient and observed that Na+, K+, Cl−, and SO42− concentrations in streams increased with urbanization. Similarly, Kaushal et al. (2017) found statistically significant positive linear relationships between impervious surface cover and cations (Ca2+, Na+, K+) as well as DIC, pH, and silica for streams at the Baltimore LTER site. Other studies in Baltimore streams have shown similar increases in major ions in response to urbanization (e.g., Moore et al. 2017).
Weathering of urban karst also influences sulfate, an abundant major ion in urban waters, which is contained in many building materials (e.g. gypsum, concrete, and roofing tiles) (Cevik et al. 2011). The chemical weathering of these materials contributes to sulfate inputs to urban streams. In urban ecosystems, sulfate-rich chemical cocktails typically increase in surface and groundwaters. Pikaar et al. (2014) identified three major sources of sulfate in sewer systems: source waters, addition of aluminum or iron sulfates during water treatment, and human waste. In South East Queensland, Australia, Pikaar et al. (2014) found that 52% of the sulfate in the sewage system was from aluminum sulfate added during water treatment, 10% from source water, and 38% from human waste; totaling ~ 17 mg SO4-S L-1. Appleyard (1995) concluded that groundwater in non-urban environments had an average of 8 mg/L of SO42− and groundwater in older, urban environments increased to an average of 69 mg/L of SO42− due to the oxidation of sulfide containing soils and use of fertilizers. The influence of anthropogenic sulfur has also been noted in Paris, France, where during major urbanization in the late 1800s, the sulfur content of groundwater doubled in a historical underground aqueduct (an older form of urban karst) (Pons-Branchu et al. 2017). SO42− also contaminates groundwater in China due to air pollution, use of household detergents, industrial runoff, and agricultural inputs (Li et al. 2006).
Evaporation and atmospheric deposition can also enhance formation of watershed chemical cocktails of major ions. In downtown and light industrial locations, evapotranspiration rates can exceed precipitation and require piped water supply (Grimmond and Oke 1986). This results in an evaporative concentration of all major ions in the urban environment. Other direct anthropogenic inputs include atmospheric dust and by atmospheric deposition from road salt applications (Blomqvist and Johansson 1999). Atmospheric dust inputs can represent the underlying lithology, if anthropogenic land denudation exposes minerals to aeolian transport mechanisms. Major ions that are released as dust particles can chemically weather. Sulfate and chloride aerosols may be contributed by through proximity to coal mining and marine air masses (Griffith 2014). Aerosol salt additions also have the potential to increase nitrate leaching and reduce mobility of dissolved organic carbon (Compton and Church 2011), which could form chemical cocktails. In turn, anions like nitrate can also be deposited from the atmosphere and contribute to accelerated concrete weathering through acidification, a phenomenon that is exacerbated by watershed impervious cover (Riha et al. 2014).
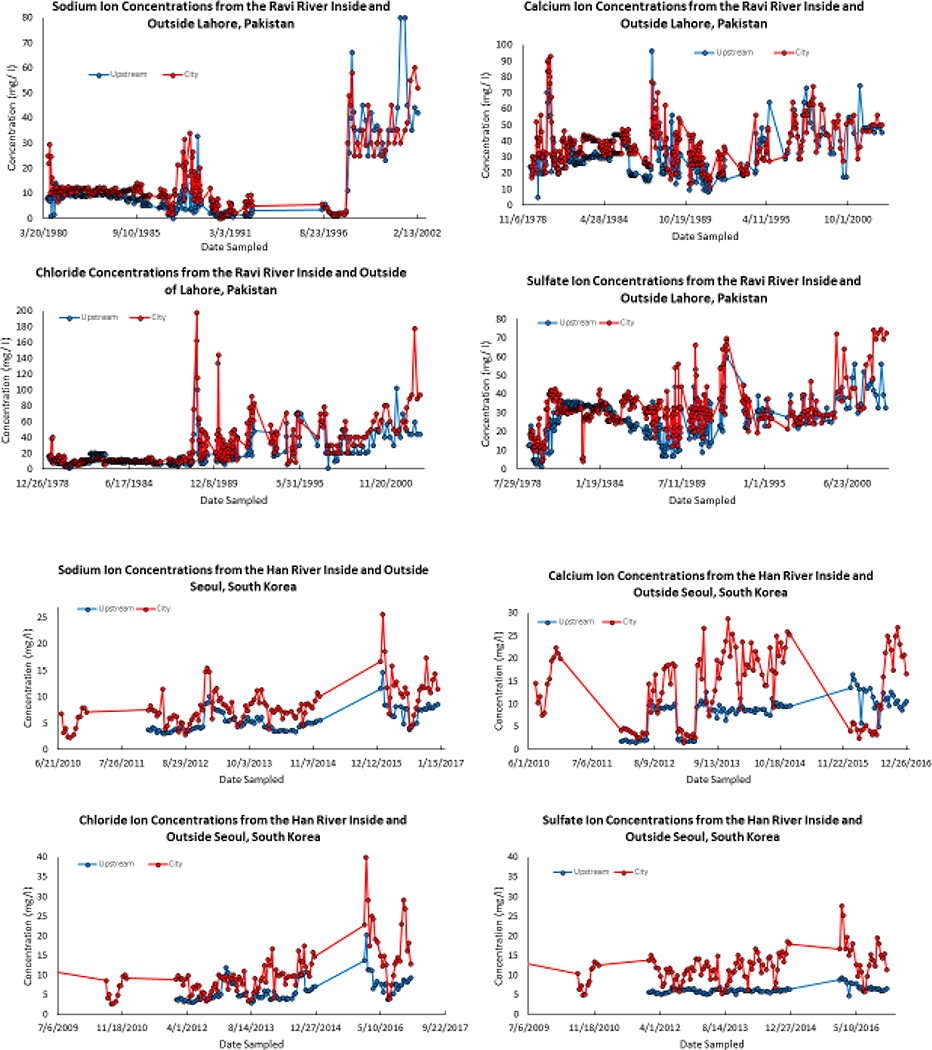
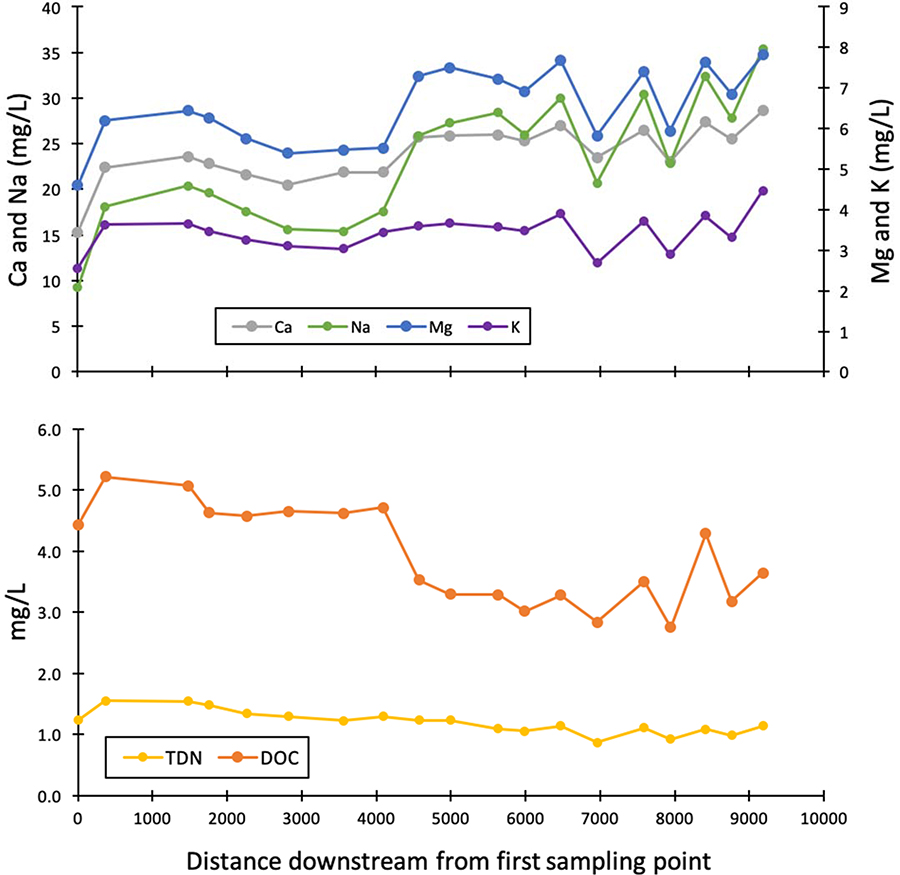
Globally, streams draining other cities can show these patterns of increased major ions compared with streams draining less urbanized land use further upstream. For example, along the Ravi River inside and outside of Lahore, Pakistan concentrations of Na+, Ca2+, Cl−, and SO42 are significantly higher in the city than in the river upstream of the city over 3 decades from 1978 to 2002 with changes over time. Likewise, Na+, Ca2+, Cl−, and SO42 are significantly higher in the Han River downstream vs. upstream of Seoul, South Korea from 2010 to 2016 (Figure 6). Interestingly, Cl− concentrations increase over time in these locations indicating potential impacts of the freshwater salinization syndrome in Asia (Figure 6). Additionally, synoptic data collected along the Anacostia River in Maryland, USA showed increasing concentrations of major ions downstream (Figure 7). Concentrations of major ions increased along the drainage basin of the Anacostia River (as cumulative percent impervious surface area increased downstream from the first sampling point), which either suggests increasing anthropogenic sources with urbanization and/or accumulating inputs from chronic groundwater contamination in urban areas (Figure 7). Conversely, nitrogen and carbon showed an opposite pattern with distance downstream, which was potentially due to dilution and/or biogeochemical transformation. Elevated concentrations of major ions in response to urbanization are related to a variety of processes such as urban soil formation from novel urban parent materials, human-accelerated weathering of urban karst, urban acidification-alkanization processes, and the FSS.
Figure 6.
Major ions can increase in rivers downstream of cities. For example, along the Ravi River inside and outside of Lahore, Pakistan concentrations of Na+, Ca2+, Cl−, and SO42 are significantly higher in the city than in the river upstream of the city over 3 decades from 1978 to 2002. Likewise, Na+, Ca2+, Cl−, and SO42 are significantly higher in the Han River downstream versus upstream of Seoul, South Korea from 2010 to 2016. Data are from GEMStat.
Figure 7.
Concentrations of major ions simultaneously increase with increasing distance downstream (meters) from a fixed sampling point along the Anacostia River near College Park, Maryland. Conversely, concentrations of dissolved organic carbon (DOC) and total dissolved nitrogen (TDN) decrease with increasing distance downstream. The simultaneous changes in concentrations suggest similar hydrogeochemical processes and sources contributing to the formation of different chemical cocktails along urbanized drainage networks.
Urbanization Makes Chemical Cocktails of Dissolved Organic Matter
Urbanization makes chemical cocktails of natural organic matter from road runoff, grass clippings, fertilized lawns and fields, and storm drains (Li et al. 2006; Helmreich et al. 2010; Newcomer et al. 2012). Urbanization shifts dissolved organic matter (DOM) away from humic and protein-like DOM, which is often more common in natural environments. Other studies have suggested that urbanization may increase protein-like DOM, but are in agreement that humic-like DOM decreases and fulvic acid-like DOM increases with urbanization (Hosen et al. 2014; Williams et al. 2016). Urban DOM concentrations vary seasonally due to differences in anthropogenic sources, leaf fall, and/or algal blooms (Pons-Branchu et al. 2017). Principal components analysis shows a clear difference in DOM signatures between forested and urban sites; photolabile and recalcitrant components (linked to terrestrial DOM sources) and percent fluorescence of both components are positively related to watershed impervious cover (Hosen et al. 2014). Aromaticity of DOM can be negatively correlated with impervious cover and can be influenced by microbial activity (Hosen et al. 2014). In general, urban streams appear to be enriched with DOM signatures that have a low molecular weight and aromaticity, similarly to other anthropogenically impaired waterways like those affected by agriculture or wastewater (Hosen et al. 2014).
In addition to natural organic matter, a range of anthropogenic carbon signatures are present in urban waters at trace level concentrations (<1μg/l) such as sterols, pesticides petrochemicals, polycyclic aromatic hydrocarbons (PAHs) and antibiotics (Glassmeyer and Shoemaker 2005, Rosi et al. 2018). Anthropogenic sources of organic matter found in urban waters also encompass: agricultural pesticides such as atrazine, steroidal hormones commonly like 17β-estradiol, antibiotics including tetracycline, and chemicals found in personal care products such as triclosan (Kolpin et al. 2013). Other legacy organic contaminants include polychlorinated biphenyls (PCBs) (Rodenburg and Ralston 2017) and polybrominated diphenyl ethers (PBDEs), a common flame retardant (Rodenburg et al. 2014). While most new production of these chemicals has been phased out in industrialized countries, legacy sources are persistent and continue to contaminate urban waters (Rodenburg et al. 2014; 2010; Praipipat et al. 2017). A survey of chemical and microbial compounds originating from wastewater discharges found that flame retardants common in furniture and bedding are the most common chemical class, being found in ~75% of surveyed rivers (Glassmeyer and Shoemaker 2005). This was followed by plant and animal sterols and non-prescription pharmaceuticals at ~60% and ~ 40% respectively. Wastewater leaks also contribute high levels of estrone, nonylphenol, propylparaben, bisphenol A, triclosan, methyparaben, herbicides, 2-phenylphenol, and other organic contaminants (Peng et al. 2008). Many other organic matter sources can make chemical cocktails such as petrochemicals from leaking underground storage tanks and per-and polyfluoroalkyl substances (PFAS), which have been used in making carpets, plastics, cookware, etc., which we do not review here.
Streamflow, stormwater management, and seasonality all influence chemical cocktails of organic matter in urban waters. For example, chemical cocktails are mixed hydrologically across time and space through transport through altered surface flowpaths (impervious surfaces, construction materials, altered geomorphology etc.) and subsurface flowpaths (stream burial, urban karst, pipes, soil compaction etc.) Firstly, streamflow conditions (e.g., stormflow vs. baseflow) can influence the quantity and quality of organic matter chemical cocktails particularly during the first flush of storm events (Peng et al. 2008; Hook and Yeakley 2005; Kaushal et al. 2014). During the first flush, pulses in DOC concentrations and fluxes are amplified by impervious surfaces and combined sewer system overflows (Sickman et al. 2007); this can occur due to the accumulation of leaves and organic matter on impervious surfaces during dry weather conditions and flushing of organic matter into stormdrains during wet weather conditions contributing to a ‘gutter subsidy’ of organic matter to urban streams (Kaushal and Belt 2012). Secondly, stormwater management influences retention of anthropogenic carbon sources including particulates, polyaromatic cyclic hydrocarbons (PAHs), oil, and grease (Hsieh and Davis 2005; Badin et al. 2008). Sorption to organic matter and filtration of particulate-bound pollutants are the primary removal mechanisms for a range of other compounds associated with organic matter chemical cocktails including phosphorus, oil and grease, PAHs, and pesticides (Badin et al. 2008; Davis et al. 2010). Thirdly, seasonality plays an important role in the quantity, quality, and diversity of organic matter chemical cocktails. During leaf fall, organic C, N, and P chemical cocktails are linked to leaf litter and other organic matter which accumulates on pavement and in pipes and culverts (Duan et al. 2014; Hobbie et al. 2014; Selbig 2016; Smith and Kaushal 2015). During spring and summer months, there can also be increases in concentrations and lability of organic matter chemical cocktails in urban waters due to contributions from algae and bacteria in streams (Kaushal et al. 2014; Arango et al. 2017). Ultimately, watershed chemical cocktails of organic matter vary over space and time based on hydrology, management, and seasonality.
Urbanization Makes Chemical Cocktails of Nutrients
Nitrogen-Rich Chemical Cocktails
Urbanization forms nitrogen-rich chemical cocktails transported in septic systems and sewers. Aging sanitary infrastructure can leak into urban groundwater and transport significant amounts of nitrogen-rich runoff into streams during rainstorms and baseflow. Cities can have increased N inputs to ground water and streams from leaky pipes and sanitary infrastructure (Kaushal et al. 2011; Pennino et al. 2016; Gabor et al. 2017). For example, sewage contributions of dissolved inorganic nitrogen (DIN) ranged from 6 to 14 kg ha-1 yr-1 in an urban watershed in Pittsburgh, Pennsylvania, USA, and significant DIN loading can occur during both stormflow and baseflow conditions (Divers et al. 2013). Septic systems across different regions can also discharge nutrients below the rooting zone and by-pass plant uptake, which also contributes to nitrogen-rich chemical cocktails in urban ground and surface waters (Steffy and Kilham 2004; Kaushal et al. 2006).
Urbanization makes nitrogen-rich chemical cocktails from atmospheric deposition and lawn fertilizers. For example, atmospheric deposition of nitrogen is 47% and 22% higher in urban and suburban areas, respectively, compared to nonurban areas (Bettez and Groffman 2013). In particular, roads are hotspots for nitrogen deposition, and failing to account for the deposition of nitrogen from fossil fuel combustion on roadways underestimates total nitrogen inputs by 13%−25% (Bettez and Groffman 2013). In addition, nitrogen fertilizers applied to lawns can be transported to urban waters via volatilization and atmospheric deposition, surface runoff, or groundwater inputs (Puckett 1994). The application of nitrogen fertilizer to residential lawns can be comparable to the amount applied to agricultural fields and golf courses. For example, homeowners applied 97.6 +/− 88.3 kg N/ha to their lawns in Baltimore, Maryland, USA (Law et al 2004). Higher fertilizer applications were correlated with newer developments, accounting for up to 53% of the total nitrogen inputs to watersheds (Law et al. 2004). For lawns established over a decade, nitrate leaching ranged from 5 mg/l to as high as 20 mg/l in (Frank et al. 2006). Inorganic nitrogen leaching was as high as 30% in areas that used turfgrass (Wang et al. 2014), although urban lawns can have a higher capacity to retain nitrogen than urban forests (Raciti et al. 2008). Ultimately, the amount of nitrogen retained in lawns is dependent on the age of the lawn, the frequency of fertilizer additions, and socioeconomic factors (Raciti et al. 2008).
Finally, nitrogen transformations in urban watersheds influence chemical cocktails in urban waters. In some cases, engineered hydrologic flowpaths and compacted urban soils reduce N transformations; in other cases, N is significantly retained and transformed during baseflow conditions (Groffman et al. 2004; Kaushal and Belt 2012). For example, nitrate can be reduced to N2 in “hot spots” by microbial denitrification where conditions are anoxic, dissolved organic carbon concentrations are sufficient, and flow rate is low enough to allow for adequate mixing of the necessary constituents such as in urban riparian groundwater ecosystems (Kaushal et al. 2008; Mayer et al. 2010). In addition, urban environments create new hotspots for enhanced denitrification in stormwater detention basins, ditches, gutters, lawns and other places where water, nitrate and organic matter accumulate with sufficient hydrologic residence times (Kaye et al. 2006). Finally, organic nitrogen transformations in urban watersheds contribute to chemical cocktails of humic substances, amino acids, amino sugars and tannins, which influence primary production, trigger harmful algal blooms, and alter the solubility and mobility of metals and pesticides (Aitkenhead-Peterson et al. 2009; Kaushal and Lewis 2003; Petrone et al. 2010; Tufford et al. 2003).
Phosphorus-Rich Chemical Cocktails
Urbanization makes phosphorus-rich chemical cocktails from mixing sewage, fertilizers, and runoff from impervious surfaces. The most common forms of P in urban waters are phosphates which can be either organic (bound to plant or animal tissue) or inorganic (ortho-/poly-phosphates). In a study of 54 UK rivers, the main source of soluble reactive phosphorus was urban wastewater effluent, which was most concentrated during the peak growing season, thereby posing a greater risk for eutrophication (Jarvie et al. 2006). Runoff from impervious surfaces also contains P, and lawns and streets were the two largest sources of P found in some waters of the Midwestern U.S. (Waschbusch, 2000). Distribution of P pools have been attributed to human density, developed land use, impervious surface cover, and asphalt (Russell et al. 2008, Duan et al. 2012, Metson et al. 2012). The amount of P loading to streams may not be simply related to impervious surface cover, but also drainage simplification and increased hydraulic efficiency of storm water drainage to urban streams (Walsh et al. 2005). Drainage intensification creates shorter and quicker hydrologic flow paths from the landscape, which can funnel significant amounts of P-rich chemical cocktails from nonpoint sources to urban streams.
Biogeochemical transformation of phosphorus-rich chemical cocktails differs from that of nitrogen-rich chemical cocktails. Unlike nitrogen, P cannot be permanently removed through processes like microbial denitrification. P can only be retained by biological uptake, sorption onto particles that are then buried, or mineral precipitation. For example, a major chemical interaction between sediment and P is the coprecipitation of P with calcite, a process driven by the reduction of CO2 or HCO3− during photosynthesis (House 2003). Phosphate can also bind to iron hydroxides creating a Fe(II) phosphate, and in solutions of high SRP, this is precipitated as the mineral vivianite (House 2003). Because P is not permanently removed, it can be remobilized and released back into the water column of urban aquatic ecosystems (Jarvie et al. 2005). Whether sediments in urban waters act as sinks or sources of P varies spatially and temporally (Duan et al. 2016). For example, sorption of P to sediments is controlled by oxygen levels and temperature. The oxic zone is important in controlling the exchange of P between urban sediments and the water column, and in this zone the equilibrium phosphate concentration depends on temperature (House 2003). Urbanization can influence solubility of mineral P by increasing water temperatures through riparian tree removal, wastewater and industrial discharges, and runoff from impervious surfaces (Barrow 1979). The release of P from urban sediments also depends on pH, the most P being released under alkaline conditions and the least under neutral pH (Wu et al. 2014). Phosphorus release from sediments may increase as some urban waters become more alkaline due to freshwater salinization syndrome and human-accelerated weathering (Kaushal et al. 2013, Kaushal et al. 2017).
Silica-Rich Chemical Cocktails
Relatively little is known regarding how urbanization alters Si-rich chemical cocktails compared to nitrogen and phosphorus. However, urbanization can increase dissolved Si transport in watersheds in multiple ways. Rivers draining areas with higher population density have higher Si concentrations (Takagi et al. 2017). Urbanization leads to the replacement of forests and grasslands with buildings and urban infrastructure, which also result in reduced dissolved Si uptake by vegetation (sensu Fulweiler and Nixon 2005; Carey and Fulweiler 2012; 2013, Takagi et al. 2017). Impervious surfaces prevent infiltration and increase runoff, which increases river discharge and can increase Si transport from landscapes and anthropogenic sources to streams and rivers (Fulweiler and Nixon 2005; Carey and Fulweiler 2012; 2013). Approximately 9% of dissolved Si in some river basins can originate from anthropogenic inputs (Zhang et al. 2016). Sewage and wastewater flow systems also alter the flow paths of groundwater and runoff and influence Si in urban watersheds (Maguire and Fulweiler 2016); for example, there is the potential for groundwater to infiltrate into wastewater pipes (Kaushal and Belt 2012). Finally, urban infrastructure acts as exposed bedrock, which leads to an increase in dissolved Si when chemically weathered (Sferratore et al. 2006; Maguire and Fulweiler 2016). Weathering of impervious surfaces can increase the pH of urban waters, and the solubility of Si appears to increase in waters with pH > 8, due to the additional presence of H3SiO4 (Alexander et al. 1954). A combination of freshwater salinization syndrome, human-accelerated weathering, increased photosynthesis by algal blooms, and increased use of road salts can synergistically increase the pH of some urban waters to pH levels > 8 over time (Kaushal et al. 2018). Ultimately, an increase in Si loading and a decrease in removal processes can result in greater Si transport downstream in urban watersheds. Compared with N and P, more work is necessary to elucidate the effects of urbanization on Si-rich chemical cocktails, however.
Urbanization Makes Chemical Cocktails of Trace Elements
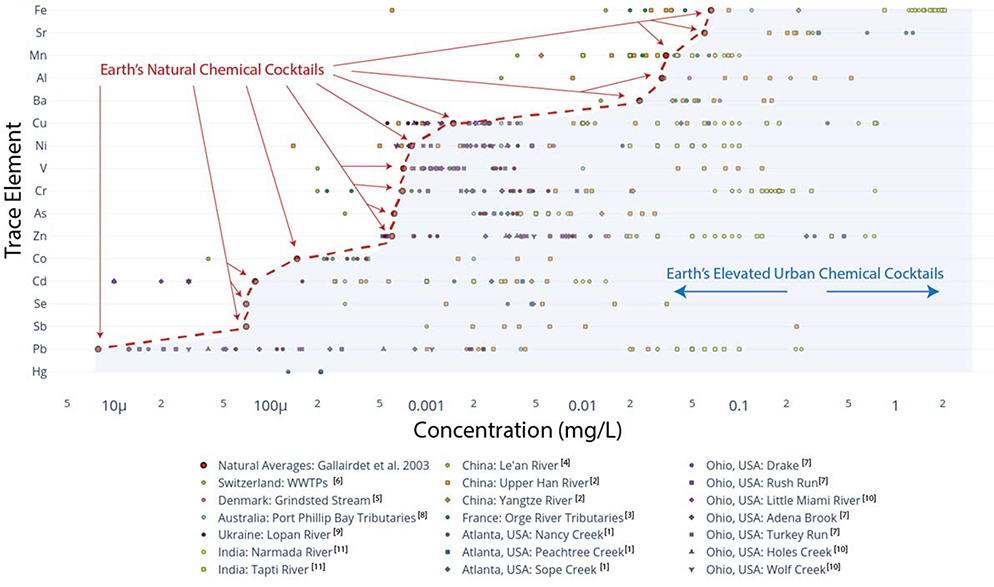
Trace elements in the built environment differ widely from their expected natural concentrations (Figure 8). Here, we define trace elements as those < 1 mg/L in concentration in surface and ground waters. Trace elements are typically significantly elevated in urban streams across land use and as compared to concentrations in major world rivers on a global scale (Figures 5, 8). Zinc, copper, cadmium, and lead are the four major trace element pollutants in rivers worldwide, and lead is regularly used as an indicator species to assess the level of pollution by urban run-off in a water body (Mohiuddin et al. 2010). Trace elements are ubiquitous in fossil fuels and metal ores which form the basis for many pollutants found in urban landscapes (Pacyna and Pacyna 2001) (Table 3). For example, arsenic, lead, selenium, antimony, vanadium, and cobalt all are found in higher concentrations in many urban watersheds at levels exceeding the World Health Organization’s drinking water standards (S. Li and Zhang 2010). Sources of trace elements in urban systems include the combustion of gasoline for automobiles, the combustion of coal and oil in power plant settings, refuse incineration, municipal wastewater treatment, sewage sludge, vehicular and industrial discharge, leakage from decaying service pipes, corrosion of metal objects, and atmospheric deposition from industrial regions adjacent to urban centers (Pacyna and Pacyna 2001; Cereceda-Balic et al. 2012; Mohiuddin et al. 2010; Leung and Jiao 2006; Senesil et al. 1999; Argyraki and Kelepertzis 2014) (Table 3). In some cases, trace elements can increase downstream with watershed urbanization, but in other cases their longitudinal patterns may be more complex due to local hot spots of pollution sources (Figure 9). However, concentrations of trace elements can still show similar patterns with distance downstream suggesting the formation of chemical cocktails (Figure 9).
Figure 8.
Global averages of natural concentrations of trace elements in streams and rivers (shown as red dots) are in order from greatest concentration on the bottom to the least concentration on the top. Examples of elemental concentrations in some different urban streams and rivers are typically higher than the natural global averages (shown as black dots). Further information regarding numbers corresponding to specific data sets from literature references are provided in the Supporting Information.
Table 3:
Selected sources of trace elements ubiquitous in the built environment. Data are from Pacyna & Pacyna, 2001; Leung & Jiao, 2006; Nriagu & Pacyna, 1988; Gardner and Carey 2004; Hjortenkrans 2007.
| Anthropogenic Source | Chemical Cocktails of Trace Elements |
|---|---|
| Gasoline combustion | Pb, Mn |
| Coal* and oil combustion (power plants) | V, Ni, Cr, Pb, Mn, Zn, Cu, As, Mo, Sb, Ti, Hg*, Cd, Se*, Mo* |
| Refuse incineration** | Pb, Zn, Cr, Mn, Cu, As, Ni, Hg, Sb, Sn, V, Se, Cd |
| Municipal wastewater treatment | Mn, Ni, Zn, Cr, Cu, As, Pb, Cd |
| Sewage sludge | B, Cr, Pb, As, Zn, Sb, Sn |
| Motor oil | V, Zn, Cr, Cu, Pb, Ni, and Mo |
| Vehicular and industrial discharge | Zn, Pb, As, Cr, Cu |
| Vehicle brake and tire wear | Cd, Cu, Zn, Pb, Sb, Ni, Zn |
| Leakage from pipes | Sr, Se |
| Corrosion of metal objects | Mn, V, Co, Mo |
| Atmospheric deposition | Pb, Zn, Cu, Ni, As, Mn, Cr, V |
denote trace elements produced by coal combustion, not in significant quantities via oil combustion.
denote refuse incineration is highly variable and dependent on the composition of the original refuse (listed elements are those seen on average).
Figure 9.
An example of synoptic patterns in elemental concentrations along the Gwynns Falls watershed of the Baltimore Long-Term Ecological Research site. Concentrations of major and trace elements increase, peak, or show pulsed patterns with increasing distance downstream based on sources, transport, and transformations. Some elemental mixtures show very similar longitudinal patterns suggesting that they can be transported and transformed as watershed chemical cocktails.
Urban rivers can have higher amounts of bioreactive dissolved organic carbon (DOC) when compared to their natural counterparts (Kaushal et al. 2014). Trace metals in rivers and streams are mainly found adsorbed onto the surface of particulate clay sediments and associated DOC. Both inorganic and organic colloids are important modifiers of trace element mobility and chemical cocktails of metals in urban waters (Kaushal et al. 2018a). As clay and/or organic carbon content in stream sediments increase, the concentration of trace elements in streams increases. Concentrations of rare earth elements (REEs) and other associated trace elements such as iron, aluminum, manganese, and zinc have been demonstrated to correlate to certain pH and DOC concentrations in aquatic environments (Gaillardet et al. 2003). For example, most rare earth elements are not coupled with concentrations of major solutes in rivers, and instead are controlled by pH and dissolved organic carbon concentration in river waters.
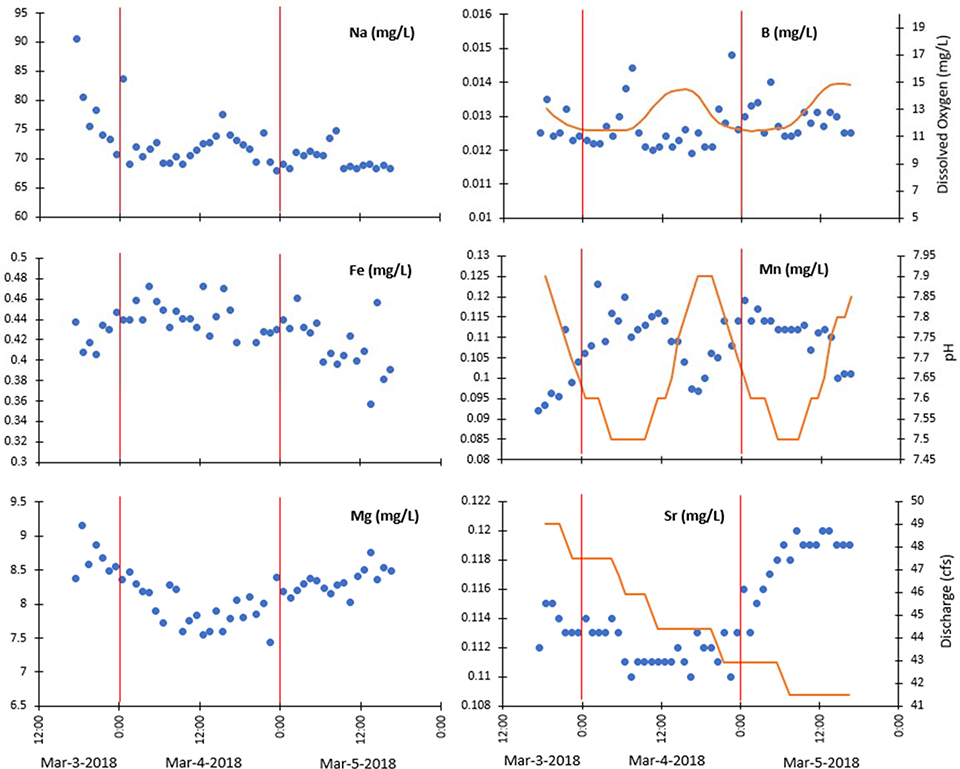
In addition to organic matter and inorganic colloids, other chemical factors in rivers influence mobility of trace metals such as alkalinity and redox conditions, presence of other trace elements, and major elements. There are relationships between trace and major elements and chemical factors, which we now discuss with a few subsequent examples. For example, alkali and alkaline earth trace elements in rivers show a strong correlation with sodium and calcium concentrations. Concentrations of trace elements such as molybdenum, zinc, cadmium, rubidium, strontium, boron, and barium also correlate strongly to major element concentrations; for example molybdenum correlates with sulfate in global rivers (Gardner et al. 2017). Vanadium, copper, arsenic, barium, and uranium concentrations strongly correlate to the pH of water, while copper correlates with silica concentrations, and selenium with sulfate (Gaillardet et al. 2003). Interestingly, concentrations of some trace and major elements show diurnal cycles also tracking changes in pH, dissolved oxygen, streamflow and other environmental variables (Kaushal et al. 2018a) (Figure 10). More research is necessary to go beyond routine grab sampling to better understand underlying patterns and processes related to correlations between diurnal changes in major and trace elements and chemical factors in urban streams, however.
Figure 10.
Chemical cocktails of major and trace elements are formed over diurnal cycles in the Anacostia River near College Park, MD coinciding with changes in streamflow, dissolved oxygen, and pH from in situ sensor data in streams. Orange bars indicate the start of each new day. Elemental concentrations from laboratory measurements are plotted as blue circles on the left axes, while data measured by high-frequency sensors is plotted as orange lines on the right axis. Red vertical bars indicate the start of each new day.
From a global perspective, there are deviations in urban chemical cocktails of trace elements when compared to chemical cocktails of trace elements found in rivers under mostly natural conditions. Concentrations of trace elements in urban streams are typically much higher than natural conditions, and there can be unique chemical cocktails that are formed across different sites and regions (Figure 8). Our analysis of chemical cocktails of trace elements in urban streams and rivers around the world suggest that the chemical cocktail approach can be used to track and distinguish distinct sources of pollution and contaminant mixtures across urban watersheds. Furthermore, watershed management efforts should consider differences in the composition of chemical cocktails across regions and their unique interactive effects on organisms as multiple stressors to protect aquatic life and human health.
Part 3. Managing Urban Chemical Cocktails
Managing water pollution needs to move beyond monitoring individual chemicals in the environment towards identifying and characterizing complex chemical mixtures in order to better understand the toxicological and synergistic effects of multiple chemicals on human and environmental health (Escher et al. 2020). A more holistic approach will require a better understanding of the sources, composition, and behaviors of different and distinct chemical cocktails over time and space. Currently, water quality is typically managed using an individual contaminant approach in most regions of the world. Resource management agencies do not manage complex chemical mixtures but establish regulations for individual stressors rather than multiple stressors (National Research Council et al. 2001). An understanding of the complex interactions between diverse chemical mixtures and stressors and their ecological, toxicological, and human health effects are just now gaining more appreciation (Monosson 2005).
In some cases, managing contaminants one at a time can be more expensive and less effective than managing sources and impacts of chemical cocktails holistically. For example, chemical mixtures impact: drinking water taste problems and harmful trihalomethane formation during drinking water treatment (different organic chemical mixtures interacting with chlorination); eutrophication and hypoxia problems leading to fish kills, harmful algal blooms, and reduced water clarity (nutrient-rich chemical cocktails with N, P, and Si alleviating nutrient limitation); increased toxicity to aquatic life in urban waters containing cocktails of heavy metals and organic contaminants from tires and vehicle emissions (Feist et al. 2017; Peter et al. 2018). By classifying similar and/or shared sources, transport, and/or transformations, the watershed chemical cocktail concept can foster the development of co-management strategies to holistically manage multiple chemicals when those chemicals behave similarly (Kaushal et al. 2018, Kaushal et al. 2019). Below, we discuss potential strategies for managing urban chemical cocktails.
Managing Chemical Cocktails by Reducing Shared Pollutant Sources and Impervious Cover
Managing shared sources, transport, and transformation of chemical cocktails (e.g., air pollution, sewage, weathering of impervious surfaces, stormwater inputs and other sources mentioned throughout this paper) is critical for reducing urban contaminants. For example, source reductions through regulatory mandates (e.g. Total Maximum Daily Loads or TMDL’s) are critical for source control. However, reducing pollutant loads often involves complex land use decisions, human behavioral changes, and timely identification of emerging contaminants of concern. For example, microplastics are now recognized as a ubiquitous ingredient in many organic chemical cocktails (Rochman 2018) and may interact with metals, salt ions, and nutrients as multiple stressors to aquatic life. Reducing sources of microplastics is dependent upon reducing consumption of plastics and disposing of plastics before they are released to the environment. In addition, other activities contribute to microplastic sources and transport such as impervious surface cover and tire wear from vehicles. Rarely is the source of a particular contaminant consolidated, which makes removal difficult. A notable exception is the successful removal of diffuse legacy sediments along streams; removal of legacy sediments has been estimated to be an effective, economical means of reducing chemical cocktails characterized by agricultural nutrients (Fleming et al. 2019). Although there are many challenges, identification of similar sources is critical for reducing chemical cocktails.
Another approach for reducing transport of chemical cocktails is to reduce impervious surface cover and subsurface urban karst (Kaushal and Belt 2012), which both increase transport sediments and solutes (Ca2+, Mg2+, HCO3−, etc.) in drainage waters. Options to reduce impervious surfaces and enhance greenspace include various green infrastructure approaches, which can be incorporated into a comprehensive low impact development (LID) designs including green roofs, bioretention systems, and permeable pavement (Martin-Mikle et al. 2015; Passeport et al. 2013). Vacant lots also represent large infiltration areas in an otherwise impervious landscape that can be collectively managed to improve ecosystem services including stormwater management (Shuster et al. 2017; Green et al. 2016). More work is necessary to characterize transport of chemical cocktails into urban ground water as a result of enhancing infiltration. The greening of cities can represent an ‘urban evolution’ towards a more sustainable form, function, and transformation of chemical cocktails in urban watersheds ( e.g., Kaushal et al. 2014; Kaushal et al. 2015; Kelleher et al. 2020; Hobbie and Grimm 2020).
Managing Chemical Cocktails by Microbiomes and Phytoremediation
Plants, biofilms, and microbes in wetlands assimilate contaminants and/or transform contaminants through metabolic breakdown. Thus, phytoaccumulation of metals in the organic matter of plants can also be used to manage and transform chemical cocktails. For example, the common wetland plant Lemna minor accumulates and transforms Cd, Cr, Cu, and Se in its tissues, removing these elements from urban soils (Zayed et al. 1998). Microbial uptake can further retain and transform trace elements in the built environment, especially alkalinity- and metal-tolerant species in microbiomes (Mora et al. 2005). Thus, integrating soil microbiomes and metal-tolerant plant species is another potential remediation strategy for managing chemical cocktails of trace metals. For biotic remediation to be most effective at reducing trace metals, however, it must be combined with significant reductions in sources, including vehicular and electrical emissions, refuse incineration, and corrosion of aging infrastructure (as discussed earlier). This is because microbiomes and phytoremediation can have thresholds for the amounts and forms of contaminants that can be assimilated and transformed.
Managing Chemical Cocktails by Riparian Buffers and Wetlands
Most sources of chemical cocktails are difficult to identify and/or are diffuse in the environment. Therefore, managing biogeochemical transformations in watersheds in wetlands and riparian buffers is also key. Natural systems and those restored or designed to mimic natural systems like wetlands and riparian buffers have the capacity to transform and reduce chemical cocktails (Jefferson et al. 2017; Costello et al. 2020). Much work has shown that natural and engineered wetlands can be effective at attenuating transport of multiple contaminants. For example, constructed wetlands have frequently been used for wastewater treatment and are highly effective at remediating pesticides (Lv et al. 2017), mixtures of pharmaceuticals (He et al. 2018), and metals (Gill et al. 2017). In addition, planting vegetation in riparian buffers can reduce exposure of soils, erosion, and weathering, which can mobilize chemical cocktails from soils and urban infrastructure. Riparian buffers are also effective at retaining multiple contaminants and sediments by capturing sediments and multiple chemicals bound to those sediments (e.g., phosphorus bound to clay particles) and by enhancing denitrification (Hoffmann et al. 2009). Riparian buffer width and hydrologic flow paths can enhance the effectiveness of nutrient removal and transformation (Mayer et al. 2007). Ultimately, physical, biological, and hydrologic factors influence the efficacy of riparian buffers in reducing transport of chemical cocktails to streams and rivers.
Managing Chemical Cocktails by Green Infrastructure
Green infrastructure shows varying efficacy at retaining and transforming nutrients, metals, and organic contaminants based on approaches such as swales, green roofs, bioretention ponds, rain gardens (Shuster et al. 2017), and rain barrels (Thurston et al. 2010). Treatment of stormwater by filtering through soils to simulate green infrastructure can reduce toxicity of complex chemical mixtures from roadside runoff and prevent salmon mortality (Spromberg et al. 2016). The key to effective functioning of green infrastructure is enhancing the ability to capture and retain water and particles for extended periods and to create and sustain conditions conducive for biogeochemical transformations to occur. This may involve: 1) dilution, 2) increasing microbial activity and plant uptake, 3) providing mineral or organic substrates for adsorption, and/or 4) activating or catalyzing chemo-transformation. For example, salt-rich chemical cocktails may be diluted by precipitation events and/or hydrologic flushing (Cooper et al. 2014) or releases of less salty reservoir water where dams exist (Knowles 2002). However, soils and shallow groundwater can also be a reservoir of salt ions (Kaushal et al. 2005, Cooper et al. 2014) and salt ions can be retained in green infrastructure (Mullins et al. 2020). Finally, enhancing microbial activity in green infrastructure can retain and transform nutrient-rich chemical cocktails, especially those containing nitrogen, where denitrifiers can be enhanced by adding organic carbon and/or inducing optimal dissolved oxygen and redox conditions as is done in wastewater treatment (Oakley et al. 2010).
Managing Chemical Cocktails by Permeable Reactive Barriers and Reactive Beds
Permeable reactive barriers (PRB’s) are engineered subsurface systems that are designed to intersect groundwater plumes before they reach receiving waters or move into drinking water supplies (Passeport et al. 2013). PRB’s are filled with materials such as organic matter or zero valent iron that form substrates for growth of microbes to treat contaminants such as arsenic (He et al. 2008; Ludwig et al. 2009; Wilkin et al. 2009) or process nitrogen (Schipper and Vojvodić-Vuković 2001). For example, chemical cocktails of heavy metals in acid mine drainage can be retained in pervious concrete barriers by raising the pH of drainage water (Shabalala et al. 2017). Construction of such barriers varies by reactive fill material, installation, incorporation into the substrate, and are meant to treat shallow plumes 4–5 m deep (Schipper et al. 2010). Reactive beds function similarly to PRB’s by providing an organic substrate for microbial activity (e.g. denitrification) but are designed along a horizontal plane using containers a few meters deep filled with organic matter that receive discharge from wastewater or agricultural drainage. Conversely, reactive layers of organic material can be mechanically mixed into deeper soils to treat deeper plumes (Passeport et al. 2013). PRB’s and reactive beds are generally small and designed to address point sources of contaminants such as those derived from a spill or in waste water treatment.
Managing Chemical Cocktails by Stream Restoration
Stream restoration is also increasingly being used to manage sediments and transform nutrients in urban watersheds (Newcomer-Johnson et al. 2014; Newcomer Johnson et al. 2016), although less work has investigated the potential for stream restoration to retain and transform chemical cocktails of metals, organic contaminants, and emerging contaminants. In cities with high levels of impervious surface cover, many streams have been buried and contained in pipes or conduits (Elmore and Kaushal 2008), increasing the transport of chemical cocktails. Conversely, certain forms of stream restoration have the potential to transform chemical cocktails derived from multiple sources or diffuse sources in a watershed (Newcomer Johnson et al. 2016; Passeport et al. 2013). Buried streams and those streams in ditches, canals, or encased in concrete, allow rapid transport of chemical cocktails through subsurface hydrologic flowpaths (Elmore and Kaushal 2008). Where encasement can be removed, increased percolation and groundwater-surface water exchange can increase the likelihood of subsurface microbial transformation and/or biological uptake of nutrient-rich chemical cocktails in plants and biofilms. Where buried streams can be daylighted, not only is groundwater-surface water exchange increased thereby increasing microbial transformations of nutrient cocktails (Beaulieu et al. 2014; Pennino et al. 2014), but also, water is exposed to daylight where photolysis can break down chemical cocktails containing pharmaceuticals such as morphine, codeine, and methamphetamine (Lin et al. 2013; Lin et al. 2014; Wang et al. 2014). In agricultural areas with nutrient-rich chemical cocktails, two-stage ditches have also been shown to be effective at enhancing conditions for removal of nitrogen and phosphorus by altering hydrologic flowpaths and retention within streambanks (Hanrahan et al. 2018). However, watershed scale implementation of both nutrient source reductions and stream restoration would be necessary to significantly reduce nutrients (Christopher et al. 2017), which has also been shown for urban drainage networks (Beaulieu et al. 2015).
Managing Chemical Cocktails by Activated Carbon, Zeolites, Nanoparticles, and Mixed Media
As discussed previously, various infiltration systems including basins, chambers, and gutters have been designed to intercept, remove, and transform metals, hydrocarbons, nutrients, PAHs, etc. (Fronczyk et al. 2020). Typically, these systems are designed to treat runoff in small, contained catchments like parking lots or street gutters via percolation through soils and sands. Other mixed media may also be added such as perlite, vermiculite, activated carbon, zero valent iron, wood mulch, limestone, zeolites, peat, etc. Peat-containing, permeable wall treatment systems can be effective at removing PAHs, Zn, Pb, and Cu (Fronczyk et al. 2020), while systems with gravel, vermiculite, and zeolites can significantly reduce chemical cocktails with mineral oil, ammonia, Cu, Zn, and PAHs (Fuerhacker et al. 2011). In addition, gutter systems filled with activated carbon have demonstrated near complete removal of suspended solids, TOC, Cu, and Pb, but have been ineffective at removing salts from road deicers (Hilliges et al. 2013). Infiltration systems and reactive beds with wood mulch or other carbon substrates can be effective at removing nitrogen oxides and ammonium ions via denitrification (Schipper et al. 2010). Mineral media such as zeolite, vermiculite, perlite, spongolite, limestone, sand, silt, and clays have been used to treat road runoff and heavy metals (Fronczyk et al. 2020; Fuerhacker et al. 2011; Reddy and Kumar 2017). Organic media such as peat, compost, wood mulch, and other carbon containing media (e.g. lignite coke, biochar, activated carbon) have been used to retain contaminants including petrochemicals and organic compounds (Fronczyk et al. 2020). Zero valent iron is often added to permeable barriers and infiltration systems to retain organic contaminants, heavy metals, radionuclides, and nitrates (Henderson and Demond 2007; Liu and Wang 2019). In addition, zeolites (positively charged, hydrated aluminosilicate minerals containing alkaline-earth metals) are active ion exchange substrates for removing charged particles like ammonium ions (Fronczyk et al. 2020). Soil media may be simultaneously optimized for retaining water from precipitation and enhancing plant growth (Bollman et al. 2019), while these media matrixes may also be enhanced with additives such as biochar created by pyrolysis of various organic matter stocks at high temperatures (Novak et al. 2014). This creates highly adsorptive material that can be tailored to remove chemical cocktails containing heavy metals (Niazi et al. 2018) and/or antibiotic mixtures (Lu et al. 2018). Finally, engineered nanoparticles (ENP’s), while themselves potential ingredients for chemical cocktails (Hartmann and Baun 2010), can also be engineered to reduce toxicity of chemical cocktails of pesticides and heavy metals through adsorption to the nanoparticles (Hartmann et al. 2010; Knauer et al. 2007).
Managing Chemical Cocktails by Continuous Monitoring Using Water Quality Sensors
Over recent decades, there has been a widespread proliferation of environmental sensor data related to monitoring and managing water quality in streams ( e.g., Spencer et al. 2007; Pellerin et al. 2012; Wollheim et al. 2017; Vaughan et al. 2017). This has been largely driven by agencies such as the U.S. Geological Survey and other monitoring entities. Although multi-parameter sensors can initially be expensive to install and maintain, they offer the potential to obtain high-frequency continuous water quality data for streams and rivers over years. Given that chemical concentrations and loads change rapidly in urban watersheds, continuous monitoring can reveal the presence of chemical cocktails and peaks, persistence, and lag times of multiple contaminants (Haq et al. 2018, Kaushal et al. 2019). For example, specific conductance can be related to concentrations of some base cations and trace metals in urban streams (Figure 11) (Kaushal et al. 2018), and that there can varying relationships between chemical cocktails of trace metals and specific conductance during winter months vs. other seasons (Kaushal et al. 2019). In some streams, seasonal changes in concentrations of base cations and trace metal ions are primarily due to inputs of road salts during winter months and enhanced ion exchange in soils and mobilization of base cations and metals (Kaushal et al. 2019). Stream temperature, dissolved oxygen, pH and other parameters can also be related to elemental concentrations within chemical cocktails (Kaushal et al. 2018), which suggests data from sensors as potential proxies for different elements across the Periodic Table. The ability to more accurately predict peaks and persistence of distinct chemical cocktails allows us to understand the timing, magnitude, and duration of exceedances of different thresholds which: impair aquatic life in streams, increase corrosion potential of source waters, degrade drinking water quality, and pose recreation and public health risks.
Figure 11.
There are significant relationships between specific conductance and some major and trace elements in Paint Branch, an urban stream near Washington D.C.
Managing Chemical Cocktails by Predictive Modeling
Comprehensive data on the spatial and temporal variability of chemical cocktails in the environment is necessary for managing water pollution. However, this level of information is often lacking in addition to a complete understanding of the toxicological effects of different chemical cocktails. Therefore, developing a risk management approach is key to evaluating potential impacts to humans and the environment (Bopp et al. 2019; Posthuma et al. 2018). Methods for estimating toxicity of chemical cocktails have recently been evaluated in Europe (Kienzler et al. 2016; Scientific Committee on Health and Environmental Risks 2012) based on the concepts of response additivity, probability of responses to individual components of a cocktail, concentration additivity, and/or an aggregated toxicity based on the sum of toxicity of individual chemicals (de Zwart et al. 2018). Modeling and forecasting are necessary to predict scenarios of impacts, especially where uncertainty exists due to lack of information on toxicity and/or incomplete or low-resolution empirical survey data (Abdelnour et al. 2013; Barnhart et al. 2018; Beaulieu et al. 2015). Previous researchers have developed a tiered approach to assessing exposure risks based on chemical concentrations in runoff, runoff volume from impervious surfaces in the watershed, and the associated chemical exposure mixture profiles for respective scenarios in urban environments (de Zwart et al. 2018). Such modeling must be done in concert with field level monitoring to provide realistic model outputs. Future work can characterize risks based on land-use scenarios, pollution sources, dilution effects, climate change, and predict ecosystem effects on species losses and other parameters based on chemical compositions of cocktails, concentrations, and temporal variability (sensu Posthuma et al. 2018). Such efforts will require coordination with multiple resource agencies, NGO’s, and researchers to plan, prioritize, and implement watershed-scale management strategies.
Challenges to Managing Chemical Cocktails and Avoiding Unintended Consequences
Seemingly subtle variations in management approaches can have disproportionate effects on ecosystem outcomes and/or unintended consequences. For example, the lability of organic carbon source (e.g. grass vs algae vs leaves) dictates denitrification activity and phosphorous mobilization (Arango et al. 2017; Duan et al. 2019; Newcomer et al. 2012). Unintended mobilization of phosphorous and precipitation of iron from imported rock substrates can also occur in stream restoration and stormwater management features (regenerative stormwater conveyance) when impounded streamwater creates unique anoxic and redox conditions (Duan et al. 2019). It is also important to recognize that some chemical cocktails may be difficult to remove using standard management practices. Common stormwater management has been ineffective for addressing chemical cocktails stemming from road salt contamination (Snodgrass et al. 2017). Other chemical cocktails can be chronically persistent and may not be significantly transformed or assimilated in the urban environment (e.g. plastics, PFAS, dioxins, PCP’s, etc.). Where these chemical cocktails exist, management efforts may need to focus on isolating and containing the chemical cocktails to avoid human exposure, such as at an USEPA Superfund sites (where public access is restricted and consumption of drinking water or food and fish is prohibited or discouraged). Sometimes, extreme and expensive measures such as dredging river sediments (Wasserman et al. 2016), pumping and treating contaminated groundwater (Truex et al. 2017), or capping the sources of chemical cocktails (Taneez et al. 2018) may be the management options of last resort.
Future Research Directions for Monitoring and Managing Chemical Cocktails
Throughout this paper, we have emphasized that research and environmental management communities can potentially infer many processes related to elemental sources, transport, and transformation along hydrologic flowpaths and chemical evolution of major and trace elements. Future research can use data on chemical cocktails to make inferences and new discoveries about urban groundwater systems and groundwater-surface water interactions. For example, information about chemical cocktails can contribute to a better understanding of gaps in our knowledge of the urban hydrologic cycle, if we had more major and trace element data from both shallow groundwater systems and streams. In addition, more research is necessary to identify how the different processes that define each chemical cocktail affect surface and groundwater differently and how groundwater-surface water interactions (and presumably infiltration and inflow of groundwater and storm water into wastewater pipes and sewage overflow from infrastructure) impact the mixtures of different cocktails.
In addition, geochemical mixing model approaches can be developed using chemical cocktails to track pollution sources in watersheds. Different hydrologic flowpaths along urban ecosystems are characterized by mixtures of distinct chemical cocktails we describe throughout this paper. Along hydrologic flowpaths, surface waters in particular acquire a chemical composition that is the result of the mixing of two or more chemical cocktails. More research is necessary to understand how receiving waters are impacted by the aggregation of distinct and different urban chemical cocktails. Future work can develop statistical methods to perform “unmixing” exercises in geochemically complex waters to determine whether we can reconstitute the complex mixture of chemical compositions from distinct ‘endmembers’ of different chemical cocktails associated with sources. Chemical source tracking approaches could be developed, which are analogous to what is done in many modern sediment and nutrient source-tracking studies. Multiple lines of evidence, including laboratory experiments, empirical data collection, and statistical modeling could all be used in attempts to perform chemical cocktail ‘unmixing’ exercises and improve pollution source tracking in urban waters.
More research is also necessary to delineate how the spatial distributions of various chemical cocktails we discuss throughout this paper are related to the spatial distribution of human activities and land uses across broader spatial scales and hydrologic flowpaths along watersheds (including the exurban, suburban, and agricultural areas that are in the headwaters and the more densely urban areas downstream). The spatial distribution of different chemical cocktails is also due to the evolution of chemical cocktails along the rainfall-runoff pathway (some primarily in urban soils, and others in the shallow groundwater system). In addition, some chemical cocktails evolve within sewage infrastructure and leak into both groundwater and surface water. Along the broader urban watershed continuum (Kaushal and Belt 2012), the cumulative and interactive effects of these different chemical cocktails on water quality may be particularly important in receiving waters.
Conclusions
Urban environments make distinct chemical cocktails, and the watershed chemical cocktail approach can be used to trace pollution sources, better inform co-management strategies for multiple contaminants, track multiple chemicals using proxies from continuous sensors, and understand more holistic impacts on ecosystems from an ecotoxicological perspective. Grouping of elements has already been useful in chemistry through the organization of groups and families of elements along the Periodic Table. In addition, geochemists widely use classification of similarities in chemical behavior of elements in the Earth based on their affinities for reactivity with other elements (siderophillic or affinity for Fe, chalcophillic or affinity for S, Se, Te etc.). Our review and synthesis show that chemical cocktails form and evolve in urban waters over diurnal cycles, decades, and throughout drainage basins (Figures 4–10). Contaminant transport and transformation do not occur one element at time in urban watersheds. Multiple approaches may need to be implemented to retain and transform chemical cocktails in watersheds either simultaneously or sequentially along hydrologic flowpaths (Newcomer Johnson et al. 2016) to capture a significant portion of urban runoff (inputs). Ultimately, a watershed chemical cocktail approach targeting sources, transport, and transformations of distinct elemental combinations and contaminants is necessary to more holistically monitor and manage the emerging impacts of chemical mixtures in the world’s fresh waters.
Supplementary Material
Acknowledgements
All authors contributed to writing, concepts, and data analyses in this synthesis paper; Jenna Reimer kindly and significantly helped throughout the revision process. Significant funding for data collection/analyses in this paper was provided by NSF EAR1521224, NSF CBET1058502, NSF Coastal SEES1426844, and NSF DEB-0423476 and DEB-1027188. The information in this document has been subjected to U.S. Environmental Protection Agency (Agency) peer and administrative review, and it has been approved for publication as an Agency document. The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the Agency. Any mention of trade names, products, or services does not imply an endorsement by the U.S. Government or the Agency. Peter Groffman kindly provided logistical support at the Baltimore Long-Term Ecological Research site, and we are grateful for his continued support over years. Bob Shedlock and three other reviewers provided helpful suggestions that improved this project.
Bibliography
- Abdelnour Alex, McKane Robert B., Marc Stieglitz, Feifei Pan, and Yiwei Cheng. 2013. “Effects of Harvest on Carbon and Nitrogen Dynamics in a Pacific Northwest Forest Catchment.” Water Resources Research 49 (3): 1292–1313. 10.1029/2012WR012994. [DOI] [Google Scholar]
- Aitkenhead-Peterson JA, Steele MK, Nahar N, and Santhy K. 2009. “Dissolved Organic Carbon and Nitrogen in Urban and Rural Watersheds of South-Central Texas: Land Use and Land Management Influences.” Biogeochemistry 96 (1): 119–29. 10.1007/s10533-009-9348-2. [DOI] [Google Scholar]
- Aitkenhead-Peterson Jacqueline A., Nahar Nurun, Harclerode Cara L., and Stanley Nina C.. 2011. “Effect of Urbanization on Surface Water Chemistry in South-Central Texas.” Urban Ecosystems 14 (2): 195–210. 10.1007/s11252-010-0147-2. [DOI] [Google Scholar]
- Alexander Go B., Heston WM, and Iler RK. 1954. “The Solubility of Amorphous Silica in Water.” The Journal of Physical Chemistry 58 (6): 453–55. 10.1021/j150516a002. [DOI] [Google Scholar]
- Appleyard S 1995. “The Impact Of Urban Development On Recharge And Groundwater Quality In A Coastal Aquifer Near Perth, Western Australia.” Hydrogeology Journal 3 (2): 65–75. 10.1007/s100400050072. [DOI] [Google Scholar]
- Aquilina Luc, Poszwa Anne, Walter Christian, Vergnaud Virginie, Anne-Catherine Pierson-Wickmann, and Laurent Ruiz. 2012. “Long-Term Effects of High Nitrogen Loads on Cation and Carbon Riverine Export in Agricultural Catchments.” Environmental Science & Technology 46 (17): 9447–55. 10.1021/es301715t. [DOI] [PubMed] [Google Scholar]
- Arango Clay P., Beaulieu Jake J., Fritz Ken M., Hill Brian H., Elonen Colleen M., Pennino Michael J., Mayer Paul M., Kaushal Sujay S., and Balz Adam D.. 2017. “Urban Infrastructure Influences Dissolved Organic Matter Quality and Bacterial Metabolism in an Urban Stream Network.” Freshwater Biology 62 (11): 1917–28. 10.1111/fwb.13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyraki Ariadne, and Kelepertzis Efstratios. 2014. “Urban Soil Geochemistry in Athens, Greece: The Importance of Local Geology in Controlling the Distribution of Potentially Harmful Trace Elements.” Science of The Total Environment 482–483 (June): 366–77. 10.1016/j.scitotenv.2014.02.133. [DOI] [PubMed] [Google Scholar]
- Badin Anne-Laure, Faure Pierre, Bedell Jean-Philippe, and Delolme Cécile. 2008. “Distribution of Organic Pollutants and Natural Organic Matter in Urban Storm Water Sediments as a Function of Grain Size.” Science of The Total Environment 403 (1): 178–87. 10.1016/j.scitotenv.2008.05.022. [DOI] [PubMed] [Google Scholar]
- Barnes Rebecca T., and Raymond Peter A.. 2009. “The Contribution of Agricultural and Urban Activities to Inorganic Carbon Fluxes within Temperate Watersheds.” Chemical Geology 266 (3): 318–27. 10.1016/j.chemgeo.2009.06.018. [DOI] [Google Scholar]
- Barnhart Bradley L., Golden Heather E., Kasprzyk Joseph R., Pauer James J., Jones Chas E., Sawicz Keith A., Hoghooghi Nahal, et al. 2018. “Embedding Co-Production and Addressing Uncertainty in Watershed Modeling Decision-Support Tools: Successes and Challenges.” Environmental Modelling & Software 109 (November): 368–79. 10.1016/j.envsoft.2018.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow NJ 1979. “Three Effects of Temperature on the Reactions Between Inorganic Phoshate and Soil.” Journal of Soil Science 30 (2): 271–79. 10.1111/j.1365-2389.1979.tb00984.x. [DOI] [Google Scholar]
- Beaulieu Jake J., Golden Heather E., Knightes Christopher D., Mayer Paul M., Kaushal Sujay S., Pennino Michael J., Arango Clay P., et al. 2015. “Urban Stream Burial Increases Watershed-Scale Nitrate Export.” PLOS ONE 10 (7): e0132256. 10.1371/journal.pone.0132256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu Jake J., Mayer Paul M., Kaushal Sujay S., Pennino Michael J., Arango Clay P., Balz David A., Canfield Timothy J., et al. 2014. “Effects of Urban Stream Burial on Organic Matter Dynamics and Reach Scale Nitrate Retention.” Biogeochemistry 121 (1): 107–26. 10.1007/s10533-014-9971-4. [DOI] [Google Scholar]
- Bernhardt Emily S., Rosi Emma J., and Gessner Mark O.. 2017. “Synthetic Chemicals as Agents of Global Change.” Frontiers in Ecology and the Environment 15 (2): 84–90. 10.1002/fee.1450. [DOI] [Google Scholar]
- Bettez Neil D., and Groffman Peter M.. 2013. “Nitrogen Deposition in and near an Urban Ecosystem.” Environmental Science & Technology 47 (11): 6047–51. 10.1021/es400664b. [DOI] [PubMed] [Google Scholar]
- Blaszczak Joanna R., Delesantro Joseph M., Zhong Ying, Urban Dean L., and Bernhardt Emily S.. 2019. “Watershed Urban Development Controls on Urban Streamwater Chemistry Variability.” Biogeochemistry 144 (1): 61–84. 10.1007/s10533-019-00572-7. [DOI] [Google Scholar]
- Blomqvist Göran, and Johansson Eva-Lotta. 1999. “Airborne Spreading and Deposition of De-Icing Salt — a Case Study.” Science of The Total Environment 235 (1): 161–68. 10.1016/S0048-9697(99)00209-0. [DOI] [Google Scholar]
- Bollman Michael A., DeSantis Grace E., DuChanois Ryan M., Montana Etten-Bohm, Olszyk David M., Lambrinos John G., and Mayer Paul M.. 2019. “A Framework for Optimizing Hydrologic Performance of Green Roof Media.” Ecological Engineering 140 (December): 105589. 10.1016/j.ecoleng.2019.105589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon Arthur G. 1995. “Septicity in Sewers: Causes, Consequences and Containment.” Water Science and Technology 31 (7): 237–53. 10.2166/wst.1995.0240. [DOI] [Google Scholar]
- Bopp Stephanie K., Kienzler Aude, Richarz Andrea-Nicole, van der Linden Sander C., Alicia Paini, Nikolaos Parissis, and Worth Andrew P.. 2019. “Regulatory Assessment and Risk Management of Chemical Mixtures: Challenges and Ways Forward.” Critical Reviews in Toxicology 49 (2): 174–89. 10.1080/10408444.2019.1579169. [DOI] [PubMed] [Google Scholar]
- Borrirukwisitsak Siriporn, Keenan Helen E., and Caroline Gauchotte-Lindsay. 2012. “Effects of Salinity, PH and Temperature on the Octanol-Water Partition Coefficient of Bisphenol A.” International Journal of Environmental Science and Development, 460–64. 10.7763/IJESD.2012.V3.267. [DOI] [Google Scholar]
- Brunk Brett K., Jirka Gerhard H., and Lion Leonard W.. 1997. “Effects of Salinity Changes and the Formation of Dissolved Organic Matter Coatings on the Sorption of Phenanthrene: Implications for Pollutant Trapping in Estuaries.” Environmental Science & Technology 31 (1): 119–25. 10.1021/es9602051. [DOI] [Google Scholar]
- Camuffo Dario. 2015. “Weathering of Building Materials.” In Urban Pollution and Changes to Materials and Building Surfaces, Volume 5:19–64. Air Pollution Reviews, Volume 5. IMPERIAL COLLEGE PRESS. 10.1142/9781783268863_0002. [DOI] [Google Scholar]
- Carey JC, and Fulweiler RW. 2012. “Human Activities Directly Alter Watershed Dissolved Silica Fluxes.” Biogeochemistry 111 (1): 125–38. 10.1007/s10533-011-9671-2. [DOI] [Google Scholar]
- ———. 2013. “Watershed Land Use Alters Riverine Silica Cycling.” Biogeochemistry 113 (1): 525–44. 10.1007/s10533-012-9784-2. [DOI] [Google Scholar]
- Cereceda-Balic F, Palomo-Marín MR, Bernalte E, Vidal V, Christie J, Fadic X, Guevara JL, Miro C, and Pinilla Gil E. 2012. “Impact of Santiago de Chile Urban Atmospheric Pollution on Anthropogenic Trace Elements Enrichment in Snow Precipitation at Cerro Colorado, Central Andes.” Atmospheric Environment 47 (February): 51–57. 10.1016/j.atmosenv.2011.11.045. [DOI] [Google Scholar]
- Cevik U, Damla N, Van Grieken R, and Vefa Akpınar M. 2011. “Chemical Composition of Building Materials Used in Turkey.” Construction and Building Materials 25 (4): 1546–52. 10.1016/j.conbuildmat.2010.08.011. [DOI] [Google Scholar]
- Chambers Lisa G., Chin Yu-Ping, Filippelli Gabriel M., Gardner Christopher B., Herndon Elizabeth M., Long David T., Lyons W. Berry, et al. 2016. “Developing the Scientific Framework for Urban Geochemistry.” Applied Geochemistry 67 (April): 1–20. 10.1016/j.apgeochem.2016.01.005. [DOI] [Google Scholar]
- Christopher Sheila F., Tank Jennifer L., Mahl Ursula H., Yen Haw, Arnold Jeffrey G., Trentman Matt T., Sowa Scott P., et al. 2017. “Modeling Nutrient Removal Using Watershed-Scale Implementation of the Two-Stage Ditch.” Ecological Engineering, Ecological Engineering of Sustainable Landscapes, 108 (November): 358–69. 10.1016/j.ecoleng.2017.03.015. [DOI] [Google Scholar]
- Compton Jana E., and Church M. Robbins. 2011. “Salt Additions Alter Short-Term Nitrogen and Carbon Mobilization in a Coastal Oregon Andisol.” Journal of Environmental Quality 40 (5): 1601–6. 10.2134/jeq2011.0013. [DOI] [PubMed] [Google Scholar]
- Cooper Curtis A., Mayer Paul M., and Faulkner Barton R.. 2014. “Effects of Road Salts on Groundwater and Surface Water Dynamics of Sodium and Chloride in an Urban Restored Stream.” Biogeochemistry 121 (1): 149–66. 10.1007/s10533-014-9968-z. [DOI] [Google Scholar]
- Costello David M., Hartung Erik W., Stoll Jordyn T., and Jefferson Anne J.. 2020. “Bioretention Cell Age and Construction Style Influence Stormwater Pollutant Dynamics.” Science of The Total Environment 712 (April): 135597. 10.1016/j.scitotenv.2019.135597. [DOI] [PubMed] [Google Scholar]
- Daniels W L, and Orndorff ZW. 2003. “Acid Rock Drainage From Highway and Construction Activities in Virginia, USA,” 10. [Google Scholar]
- Daniels WL, Zipper CE, Orndorff ZW, Skousen J, Barton CD, McDonald LM, and Beck MA. 2016. “Predicting Total Dissolved Solids Release from Central Appalachian Coal Mine Spoils.” Environmental Pollution 216 (September): 371–79. 10.1016/j.envpol.2016.05.044. [DOI] [PubMed] [Google Scholar]
- Davies PJ, Wright IA, Jonasson OJ, and Findlay SJ. 2010. “Impact of Concrete and PVC Pipes on Urban Water Chemistry.” Urban Water Journal 7 (4): 233–41. 10.1080/1573062X.2010.484502. [DOI] [Google Scholar]
- Davis Allen P., Traver Robert G., and Hunt William F.. 2010. “Improving Urban Stormwater Quality: Applying Fundamental Principles.” Journal of Contemporary Water Research & Education 146 (1): 3–10. 10.1111/j.1936-704X.2010.00387.x. [DOI] [Google Scholar]
- DeKimpe Christian, and Morel Jean-Louis. 2000. “URBAN SOIL MANAGEMENT: A GROWING CONCERN.” January 2000. https://oce.ovid.com/article/00010694-200001000-00005/HTML.
- Divers Marion, Elliott Emily, and Bain Daniel. 2013. “Constraining Nitrogen Inputs to Urban Streams from Leaking Sewers Using Inverse Modeling: Implications for Dissolved Inorganic Nitrogen (DIN) Retention in Urban Environments.” Environmental Science Technology 47 (February): 1816–23. 10.1021/es304331m. [DOI] [PubMed] [Google Scholar]
- Duan Shuiwang, Katie Delaney-Newcomb, Kaushal Sujay S., Findlay Stuart E. G., and Belt Kenneth T.. 2014. “Potential Effects of Leaf Litter on Water Quality in Urban Watersheds.” Biogeochemistry 121 (1): 61–80. 10.1007/s10533-014-0016-9. [DOI] [Google Scholar]
- Duan Shuiwang, Mayer Paul M., Kaushal Sujay S., Wessel Barret M., and Johnson Thomas. 2019. “Regenerative Stormwater Conveyance (RSC) for Reducing Nutrients in Urban Stormwater Runoff Depends upon Carbon Quantity and Quality.” Science of The Total Environment 652 (February): 134–46. 10.1016/j.scitotenv.2018.10.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Shuiwang, Tamara Newcomer-Johnson, Paul Mayer, and Kaushal Sujay. 2016. “Phosphorus Retention in Stormwater Control Structures across Streamflow in Urban and Suburban Watersheds.” Water 8 (9): 390. 10.3390/w8090390. [DOI] [Google Scholar]
- Effland William R., and Pouyat Richard V.. 1997. “The Genesis, Classification, and Mapping of Soils in Urban Areas.” Urban Ecosystems 1 (4): 217–28. 10.1023/A:1018535813797. [DOI] [Google Scholar]
- Elmore Andrew J., and Kaushal Sujay S.. 2008. “Disappearing Headwaters: Patterns of Stream Burial Due to Urbanization.” Frontiers in Ecology and the Environment 6 (6): 308–12. 10.1890/070101. [DOI] [Google Scholar]
- Escher Beate I., Stapleton Heather M., and Schymanski Emma L.. 2020. “Tracking Complex Mixtures of Chemicals in Our Changing Environment.” Science 367 (6476): 388–92. 10.1126/science.aay6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning DS, Cary Coppock, Orndorff ZW, Daniels WL, and Rabenhorst MC. 2004. “Upland Active Acid Sulfate Soils from Construction of New Stafford County, Virginia, USA, Airport.” Soil Research 42 (6): 527–36. 10.1071/SR03085. [DOI] [Google Scholar]
- Fanning Delvin S., Rabenhorst Martin C., and Fitzpatrick Robert W.. 2017. “Historical Developments in the Understanding of Acid Sulfate Soils.” Geoderma 308 (December): 191–206. 10.1016/j.geoderma.2017.07.006. [DOI] [Google Scholar]
- Feist Blake E., Buhle Eric R., Baldwin David H., Spromberg Julann A., Damm Steven E., Davis Jay W., and Scholz Nathaniel L.. 2017. “Roads to Ruin: Conservation Threats to a Sentinel Species across an Urban Gradient.” Ecological Applications 27 (8): 2382–96. 10.1002/eap.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick ML, Long DT, and Pijanowski BC. 2007. “Exploring the Effects of Urban and Agricultural Land Use on Surface Water Chemistry, across a Regional Watershed, Using Multivariate Statistics.” Applied Geochemistry, Metal interactions with natural organic matter and Watershed-scale geochemistry, 22 (8): 1825–40. 10.1016/j.apgeochem.2007.03.047. [DOI] [Google Scholar]
- Fleming Patrick M., Merritts Dorothy J., and Walter Robert C.. 2019. “Legacy Sediment Erosion Hot Spots: A Cost-Effective Approach for Targeting Water Quality Improvements.” Journal of Soil and Water Conservation 74 (4): 67A–73A. 10.2489/jswc.74.4.67A. [DOI] [Google Scholar]
- Frank Kevin W., O’Reilly Kevin M., Crum James R., and Calhoun Ronald N.. 2006. “The Fate of Nitrogen Applied to a Mature Kentucky Bluegrass Turf.” Crop Science 46 (1): 209–15. 10.2135/cropsci2005.04-0039. [DOI] [Google Scholar]
- Fronczyk Joanna, Katarzyna Markowska-Lech, and Ayla Bilgin. 2020. “Treatment Assessment of Road Runoff Water in Zones Filled with ZVI, Activated Carbon and Mineral Materials.” Sustainability 12 (3): 873. 10.3390/su12030873. [DOI] [Google Scholar]
- Fuerhacker Maria, Tadele Measho Haile, Bernhard Monai, and Mentler Axel. 2011. “Performance of a Filtration System Equipped with Filter Media for Parking Lot Runoff Treatment.” Desalination 275 (1): 118–25. 10.1016/j.desal.2011.02.041. [DOI] [Google Scholar]
- Fulweiler Robinson W., and Nixon Scott W.. 2005. “Terrestrial Vegetation and the Seasonal Cycleof Dissolved Silica in a Southern New Englandcoastal River.” Biogeochemistry 74 (1): 115–30. 10.1007/s10533-004-2947-z. [DOI] [Google Scholar]
- Gabor Rachel S., Hall Steven J., Eiriksson David P., Jameel Yusuf, Millington Mallory, Stout Trinity, Barnes Michelle L., et al. 2017. “Persistent Urban Influence on Surface Water Quality via Impacted Groundwater.” Environmental Science & Technology 51 (17): 9477–87. 10.1021/acs.est.7b00271. [DOI] [PubMed] [Google Scholar]
- Gaillardet J, Viers J, and Dupré B. 2003. “Trace Elements in River Waters.” Treatise on Geochemistry 5 (December): 605. 10.1016/B0-08-043751-6/05165-3. [DOI] [Google Scholar]
- Gardner Christopher B., and Carey Anne E.. 2004. “Trace Metal and Major Ion Inputs into the Olentangy River from an Urban Storm Sewer.” Environmental Science & Technology 38 (20): 5319–26. 10.1021/es0497835. [DOI] [PubMed] [Google Scholar]
- Gardner Christopher B., Carey Anne E., Lyons W. Berry, Goldsmith Steven T., McAdams Brandon C., and Trierweiler Annette M.. 2017. “Molybdenum, Vanadium, and Uranium Weathering in Small Mountainous Rivers and Rivers Draining High-Standing Islands.” Geochimica et Cosmochimica Acta 219 (December): 22–43. 10.1016/j.gca.2017.09.012. [DOI] [Google Scholar]
- Gill Laurence W., Ring Pamela, Casey Brian, Higgins Neil M. P., and Johnston Paul M.. 2017. “Long Term Heavy Metal Removal by a Constructed Wetland Treating Rainfall Runoff from a Motorway.” Science of The Total Environment 601–602 (December): 32–44. 10.1016/j.scitotenv.2017.05.182. [DOI] [PubMed] [Google Scholar]
- Glassmeyer ST, and Shoemaker JA. 2005. “Effects of Chlorination on the Persistence of Pharmaceuticals in the Environment.” Bulletin of Environmental Contamination and Toxicology 74 (1): 24–31. 10.1007/s00128-004-0543-5. [DOI] [PubMed] [Google Scholar]
- Green Olivia Odom, Garmestani Ahjond S., Albro Sandra, Ban Natalie C., Berland Adam, Burkman Caitlin E., Gardiner Mary M., et al. 2016. “Adaptive Governance to Promote Ecosystem Services in Urban Green Spaces.” Urban Ecosystems 19 (1): 77–93. 10.1007/s11252-015-0476-2. [DOI] [Google Scholar]
- Griffith Michael B. 2014. “Natural Variation and Current Reference for Specific Conductivity and Major Ions in Wadeable Streams of the Conterminous USA.” Freshwater Science 33 (1): 1–17. 10.1086/674704. [DOI] [Google Scholar]
- Grimm Nancy B., Faeth Stanley H., Golubiewski Nancy E., Redman Charles L., Wu Jianguo, Bai Xuemei, and Briggs John M.. 2008. “Global Change and the Ecology of Cities.” Science 319 (5864): 756–60. 10.1126/science.1150195. [DOI] [PubMed] [Google Scholar]
- Grimmond CSB, and Oke TR. 1986. “Urban Water Balance: 2. Results From a Suburb of Vancouver, British Columbia.” Water Resources Research 22 (10): 1404–12. 10.1029/WR022i010p01404. [DOI] [Google Scholar]
- Grimmond CSB, and Oke TR. 1999. “Evapotranspiration Rates in Urban Areas,” 9.
- Groffman Peter M., Bain Daniel J., Band Lawrence E., Belt Kenneth T., Brush Grace S., Grove J. Morgan, Pouyat Richard V., Yesilonis Ian C., and Zipperer Wayne C.. 2003. “Down by the Riverside: Urban Riparian Ecology.” Frontiers in Ecology and the Environment 1 (6): 315–21. 10.1890/1540-9295(2003)001[0315:DBTRUR]2.0.CO;2. [DOI] [Google Scholar]
- Groffman Peter M., Law Neely L., Belt Kenneth T., Band Lawrence E., and Fisher Gary T.. 2004. “Nitrogen Fluxes and Retention in Urban Watershed Ecosystems.” Ecosystems 7 (4): 393–403. 10.1007/s10021-003-0039-x. [DOI] [Google Scholar]
- Nielsen Haaning, Asbjørn Piet Lens, Vollertsen Jes, and Thorkild Hvitved-Jacobsen. 2005. “Sulfide–Iron Interactions in Domestic Wastewater from a Gravity Sewer.” Water Research 39 (12): 2747–55. 10.1016/j.watres.2005.04.048. [DOI] [PubMed] [Google Scholar]
- Hale Rebecca L. 2016. “Spatial and Temporal Variation in Local Stormwater Infrastructure Use and Stormwater Management Paradigms over the 20th Century.” Water 8 (7): 310. 10.3390/w8070310. [DOI] [Google Scholar]
- Hanrahan Brittany R., Tank Jennifer L., Dee Martha M., Trentman Matt T., Berg Elizabeth M., and McMillan Sara K.. 2018. “Restored Floodplains Enhance Denitrification Compared to Naturalized Floodplains in Agricultural Streams.” Biogeochemistry 141 (3): 419–37. 10.1007/s10533-018-0431-4. [DOI] [Google Scholar]
- Hartmann NB, Von der Kammer F, Hofmann T, Baalousha M, Ottofuelling S, and Baun A. 2010. “Algal Testing of Titanium Dioxide Nanoparticles—Testing Considerations, Inhibitory Effects and Modification of Cadmium Bioavailability.” Toxicology, Potential Hazard of Nanoparticles: From Properties to Biological & Environmental Effects, 269 (2): 190–97. 10.1016/j.tox.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Hartmann Nanna B., and Baun Anders. 2010. “The Nano Cocktail: Ecotoxicological Effects of Engineered Nanoparticles in Chemical Mixtures.” Integrated Environmental Assessment and Management 6 (2): 311–13. 10.1002/ieam.39. [DOI] [PubMed] [Google Scholar]
- Hazen Robert M., Grew Edward S., Origlieri Marcus J., and Downs Robert T.. 2017. “On the Mineralogy of the ‘Anthropocene Epoch.’” American Mineralogist 102 (3): 595–611. 10.2138/am-2017-5875. [DOI] [Google Scholar]
- He Y. Thomas, Wilson John T., and Wilkin Richard T.. 2008. “Transformation of Reactive Iron Minerals in a Permeable Reactive Barrier (Biowall) Used to Treat TCE in Groundwater.” Environmental Science & Technology 42 (17): 6690–96. 10.1021/es8010354. [DOI] [PubMed] [Google Scholar]
- He Yujie, Sutton Nora B., Rijnaarts Huub H. M., and Langenhoff Alette A. M.. 2018. “Pharmaceutical Biodegradation under Three Anaerobic Redox Conditions Evaluated by Chemical and Toxicological Analyses.” Science of The Total Environment 618 (March): 658–64. 10.1016/j.scitotenv.2017.07.219. [DOI] [PubMed] [Google Scholar]
- Helmreich Brigitte, Hilliges Rita, Schriewer Alexander, and Horn Harald. 2010. “Runoff Pollutants of a Highly Trafficked Urban Road – Correlation Analysis and Seasonal Influences.” Chemosphere 80 (9): 991–97. 10.1016/j.chemosphere.2010.05.037. [DOI] [PubMed] [Google Scholar]
- Henderson Andrew D., and Demond Avery H.. 2007. “Long-Term Performance of Zero-Valent Iron Permeable Reactive Barriers: A Critical Review.” Environmental Engineering Science 24 (4): 401–23. 10.1089/ees.2006.0071. [DOI] [Google Scholar]
- Herrmann Dustin L., Schifman Laura A., and Shuster William D.. 2018. “Widespread Loss of Intermediate Soil Horizons in Urban Landscapes.” Proceedings of the National Academy of Sciences 115 (26): 6751–55. 10.1073/pnas.1800305115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliges Rita, Schriewer Alexander, and Helmreich Brigitte. 2013. “A Three-Stage Treatment System for Highly Polluted Urban Road Runoff.” Journal of Environmental Management 128 (October): 306–12. 10.1016/j.jenvman.2013.05.024. [DOI] [PubMed] [Google Scholar]
- Hobbie Sarah E., Baker Lawrence A., Buyarski Christopher, Nidzgorski Daniel, and Finlay Jacques C.. 2014. “Decomposition of Tree Leaf Litter on Pavement: Implications for Urban Water Quality.” Urban Ecosystems 17 (2): 369–85. 10.1007/s11252-013-0329-9. [DOI] [Google Scholar]
- Hobbie Sarah E., and Grimm Nancy B.. 2020. “Nature-Based Approaches to Managing Climate Change Impacts in Cities.” Philosophical Transactions of the Royal Society B: Biological Sciences 375 (1794): 20190124. 10.1098/rstb.2019.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann Carl Christian, Kjaergaard Charlotte, Jaana Uusi-Kämppä, Hans Christian Bruun Hansen, and Kronvang Brian. 2009. “Phosphorus Retention in Riparian Buffers: Review of Their Efficiency.” Journal of Environmental Quality 38 (5): 1942–55. 10.2134/jeq2008.0087. [DOI] [PubMed] [Google Scholar]
- Hook Aaron M., and Yeakley J. Alan. 2005. “Stormflow Dynamics of Dissolved Organic Carbon and Total Dissolved Nitrogen in a Small Urban Watershed.” Biogeochemistry 75 (3): 409–31. 10.1007/s10533-005-1860-4. [DOI] [Google Scholar]
- Hosen Jacob D., McDonough Owen T., Febria Catherine M., and Palmer Margaret A.. 2014. “Dissolved Organic Matter Quality and Bioavailability Changes Across an Urbanization Gradient in Headwater Streams.” Environmental Science & Technology 48 (14): 7817–24. 10.1021/es501422z. [DOI] [PubMed] [Google Scholar]
- House William A. 2003. “Geochemical Cycling of Phosphorus in Rivers.” Applied Geochemistry 18 (5): 739–48. 10.1016/S0883-2927(02)00158-0. [DOI] [Google Scholar]
- Hsieh Chi-hsu, and Davis Allen P.. 2005. “Evaluation and Optimization of Bioretention Media for Treatment of Urban Storm Water Runoff.” Journal of Environmental Engineering 131 (11): 1521–31. 10.1061/(ASCE)0733-9372(2005)131:11(1521). [DOI] [Google Scholar]
- Huot Hermine, Simonnot Marie-Odile, and Jean Louis Morel. 2015. “Pedogenetic Trends in Soils Formed in Technogenic Parent Materials:” Soil Science 180 (4/5): 182–92. 10.1097/SS.0000000000000135. [DOI] [Google Scholar]
- Jarvie Helen P., Jürgens Monika D., Williams Richard J., Colin Neal, Davies Jennifer J. L., Cyril Barrett, and John White. 2005. “Role of River Bed Sediments as Sources and Sinks of Phosphorus across Two Major Eutrophic UK River Basins: The Hampshire Avon and Herefordshire Wye.” Journal of Hydrology, Nutirent Mobility within River Basins: A European Perspective, 304 (1): 51–74. 10.1016/j.jhydrol.2004.10.002. [DOI] [Google Scholar]
- Jarvie Helen P., Neal Colin, and Withers Paul J. A.. 2006. “Sewage-Effluent Phosphorus: A Greater Risk to River Eutrophication than Agricultural Phosphorus?” Science of The Total Environment, Urban Environmental Research in the UK: The Urban Regeneration and the Environment (NERC URGENT) Programme and associated studies, 360 (1): 246–53. 10.1016/j.scitotenv.2005.08.038. [DOI] [PubMed] [Google Scholar]
- Jefferson Anne J., Bhaskar Aditi S., Hopkins Kristina G., Fanelli Rosemary, Avellaneda Pedro M., and McMillan Sara K.. 2017. “Stormwater Management Network Effectiveness and Implications for Urban Watershed Function: A Critical Review.” Hydrological Processes 31 (23): 4056–80. 10.1002/hyp.11347. [DOI] [Google Scholar]
- Jiang Guangming, Wightman Elaine, Donose Bogdan C., Yuan Zhiguo, Bond Philip L., and Keller Jurg. 2014. “The Role of Iron in Sulfide Induced Corrosion of Sewer Concrete.” Water Research 49 (February): 166–74. 10.1016/j.watres.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Joselow MM, Tobias E, Koehler R, Coleman S, Bogden J, and Gause D. 1978. “Manganese Pollution in the City Environment and Its Relationship to Traffic Density.” American Journal of Public Health 68 (6): 557–60. 10.2105/AJPH.68.6.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold Chester L. Jr, and Gibbons C. James. 1996. “Impervious Surface Coverage: The Emergence of a Key Environmental Indicator.” Journal of the American Planning Association 62 (2): 243–58. 10.1080/01944369608975688. [DOI] [Google Scholar]
- Kaushal Sujay S., and Belt Kenneth T.. 2012. “The Urban Watershed Continuum: Evolving Spatial and Temporal Dimensions.” Urban Ecosystems 15 (2): 409–35. 10.1007/s11252-012-0226-7. [DOI] [Google Scholar]
- Kaushal Sujay S., Duan Shuiwang, Doody Thomas R., Haq Shahan, Smith Rose M., Johnson Tamara A. Newcomer, Katie Delaney Newcomb, et al. 2017. “Human-Accelerated Weathering Increases Salinization, Major Ions, and Alkalinization in Fresh Water across Land Use.” Applied Geochemistry, Urban Geochemistry, 83 (August): 121–35. 10.1016/j.apgeochem.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal Sujay S., Gold Arthur J., Bernal Susana, Johnson Tammy A. Newcomer, Kelly Addy, Amy Burgin, Burns Douglas A., et al. 2018. “Watershed ‘Chemical Cocktails’: Forming Novel Elemental Combinations in Anthropocene Fresh Waters.” Biogeochemistry 141 (3): 281–305. 10.1007/s10533-018-0502-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal Sujay S., Groffman Peter M., Band Lawrence E., Elliott Emily M., Shields Catherine A., and Kendall Carol. 2011. “Tracking Nonpoint Source Nitrogen Pollution in Human-Impacted Watersheds.” Environmental Science & Technology 45 (19): 8225–32. 10.1021/es200779e. [DOI] [PubMed] [Google Scholar]
- Kaushal Sujay S., Groffman Peter M., Likens Gene E., Belt Kenneth T., Stack William P., Kelly Victoria R., Band Lawrence E., and Fisher Gary T.. 2005. “Increased Salinization of Fresh Water in the Northeastern United States.” PNAS 102 (38): 13517–13520; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal Sujay S., Groffman Peter M., Mayer Paul M., Striz Elise, and Gold Arthur J.. 2008. “Effects of Stream Restoration on Denitrification in an Urbanizing Watershed.” Ecological Applications 18 (3): 789–804. 10.1890/07-1159.1. [DOI] [PubMed] [Google Scholar]
- Kaushal Sujay S., Lewis William M. Jr, and McCutchan James H. Jr. 2006. “Land Use Change And Nitrogen Enrichment Of A Rocky Mountain Watershed.” Ecological Applications 16 (1): 299–312. 10.1890/05-0134. [DOI] [PubMed] [Google Scholar]
- Kaushal Sujay S., and Lewis William M.. 2003. “Patterns in the Chemical Fractionation of Organic Nitrogen in Rocky Mountain Streams.” Ecosystems 6 (5): 483–92. 10.1007/s10021-003-0175-3. [DOI] [Google Scholar]
- Kaushal Sujay S., Likens Gene E., Pace Michael L., Utz Ryan M., Haq Shahan, Gorman Julia, and Grese Melissa. 2018. “Freshwater Salinization Syndrome on a Continental Scale.” Proceedings of the National Academy of Sciences 115 (4): E574–83. 10.1073/pnas.1711234115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal Sujay S., Likens Gene E., Utz Ryan M., Pace Michael L., Grese Melissa, and Yepsen Metthea. 2013. “Increased River Alkalinization in the Eastern U.S.” Environmental Science & Technology 47 (18): 10302–11. 10.1021/es401046s. [DOI] [PubMed] [Google Scholar]
- Kaushal Sujay S., McDowell William H., and Wollheim Wilfred M.. 2014. “Tracking Evolution of Urban Biogeochemical Cycles: Past, Present, and Future.” Biogeochemistry 121 (1): 1–21. 10.1007/s10533-014-0014-y. [DOI] [Google Scholar]
- Kaushal Sujay S., McDowell William H., Wollheim Wilfred M., Johnson Tamara A. Newcomer, Mayer Paul M., Belt Kenneth T., and Pennino Michael J.. 2015. “Urban Evolution: The Role of Water.” Water 7 (8): 4063–87. 10.3390/w7084063. [DOI] [Google Scholar]
- Kaushal Sujay S., Pace Michael L., Haq Shahan, Wood Kelsey L., Galella Joseph G., Morel Carol, Doody Thomas R., et al. 2019. “Novel ‘chemical Cocktails’’ in Inland Waters Are a Consequence of the Freshwater Salinization Syndrome.’” Philosophical Transactions of the Royal Society B: Biological Sciences 374 (1764): 20180017. 10.1098/rstb.2018.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye Jason P., Groffman Peter M., Grimm Nancy B., Baker Lawrence A., and Pouyat Richard V.. 2006. “A Distinct Urban Biogeochemistry?” Trends in Ecology & Evolution 21 (4): 192–99. 10.1016/j.tree.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Kelleher Christa, Golden Heather E., Burkholder Sean, and Shuster William. 2020. “Urban Vacant Lands Impart Hydrological Benefits across City Landscapes.” Nature Communications 11 (1): 1–11. 10.1038/s41467-020-15376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienzler Aude, Bopp Stephanie K., Sander van der Linden, Elisabet Berggren, and Andrew Worth. 2016. “Regulatory Assessment of Chemical Mixtures: Requirements, Current Approaches and Future Perspectives.” Regulatory Toxicology and Pharmacology 80 (October): 321–34. 10.1016/j.yrtph.2016.05.020. [DOI] [PubMed] [Google Scholar]
- Knauer Katja, Sobek Anna, and Bucheli Thomas D.. 2007. “Reduced Toxicity of Diuron to the Freshwater Green Alga Pseudokirchneriella Subcapitata in the Presence of Black Carbon.” Aquatic Toxicology 83 (2): 143–48. 10.1016/j.aquatox.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Knowles Noah. 2002. “Natural and Management Influences on Freshwater Inflows and Salinity in the San Francisco Estuary at Monthly to Interannual Scales.” Water Resources Research 38 (12): 25–1–25–11. 10.1029/2001WR000360. [DOI] [Google Scholar]
- Kolpin Dana W., Blazer Vicki S., Gray James L., Focazio Michael J., Young John A., Alvarez David A., Iwanowicz Luke R., et al. 2013. “Chemical Contaminants in Water and Sediment near Fish Nesting Sites in the Potomac River Basin: Determining Potential Exposures to Smallmouth Bass (Micropterus Dolomieu).” Science of The Total Environment 443 (January): 700–716. 10.1016/j.scitotenv.2012.09.063. [DOI] [PubMed] [Google Scholar]
- Law Neely, Band Lawrence, and Grove Morgan. 2004. “Nitrogen Input from Residential Lawn Care Practices in Suburban Watersheds in Baltimore County, MD.” Journal of Environmental Planning and Management 47 (5): 737–55. 10.1080/0964056042000274452. [DOI] [Google Scholar]
- Lehmann Andreas, and Stahr Karl. 2007. “Nature and Significance of Anthropogenic Urban Soils.” Journal of Soils and Sediments 7 (4): 247–60. 10.1065/jss2007.06.235. [DOI] [Google Scholar]
- Leung Chi-Man, and Jiu Jimmy Jiao. 2006. “Heavy Metal and Trace Element Distributions in Groundwater in Natural Slopes and Highly Urbanized Spaces in Mid-Levels Area, Hong Kong.” Water Research 40 (4): 753–67. 10.1016/j.watres.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Lewis Gregory P., Mitchell Jennifer D., Andersen C. Brannon, Haney Dennis C., Min-Ken Liao, and Sargent Kenneth A.. 2007. “Urban Influences on Stream Chemistry and Biology in the Big Brushy Creek Watershed, South Carolina.” Water, Air, and Soil Pollution 182 (1): 303–23. 10.1007/s11270-007-9340-1. [DOI] [Google Scholar]
- Li Siyue, and Zhang Quanfa. 2010. “Risk Assessment and Seasonal Variations of Dissolved Trace Elements and Heavy Metals in the Upper Han River, China.” Journal of Hazardous Materials 181 (1): 1051–58. 10.1016/j.jhazmat.2010.05.120. [DOI] [PubMed] [Google Scholar]
- Li Xiao-Dong, Masuda Harue, Kusakabe Minoru, Yanagisawa Fumitaka, and Zeng Hai-Ao. 2006. “Degradation of Groundwater Quality Due to Anthropogenic Sulfur and Nitrogen Contamination in the Sichuan Basin, China.” GEOCHEMICAL JOURNAL 40 (4): 309–32. 10.2343/geochemj.40.309. [DOI] [Google Scholar]
- Lin Angela Yu-Chen, Lin Yen-Ching, and Lee Wan-Ning. 2014. “Prevalence and Sunlight Photolysis of Controlled and Chemotherapeutic Drugs in Aqueous Environments.” Environmental Pollution 187 (April): 170–81. 10.1016/j.envpol.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Lin Angela Yu-Chen, Wang Xiao-Huan, and Lee Wan-Ning. 2013. “Phototransformation Determines the Fate of 5-Fluorouracil and Cyclophosphamide in Natural Surface Waters.” Environmental Science & Technology 47 (9): 4104–12. 10.1021/es304976q. [DOI] [PubMed] [Google Scholar]
- Liu Yiwen, Ni Bing-Jie, Ramon Ganigué Ursula Werner, Sharma Keshab R., and Yuan Zhiguo. 2015. “Sulfide and Methane Production in Sewer Sediments.” Water Research 70 (March): 350–59. 10.1016/j.watres.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Liu Yong, and Wang Jianlong. 2019. “Reduction of Nitrate by Zero Valent Iron (ZVI)-Based Materials: A Review.” Science of The Total Environment 671 (June): 388–403. 10.1016/j.scitotenv.2019.03.317. [DOI] [PubMed] [Google Scholar]
- Long David T., Voice Thomas C., Xagaroraki Irene, Chen Ao, Wu Huiyun, Lee Eunsang, Oun Amira, and Xing Fangli. 2017. “Patterns of C-q Hysteresis Loops and within an Integrative Pollutograph for Selected Inorganic and Organic Solutes and E. Coli in an Urban Salted Watershed during Winter-Early Spring Periods.” Applied Geochemistry, Urban Geochemistry, 83 (August): 93–107. 10.1016/j.apgeochem.2017.03.002. [DOI] [Google Scholar]
- Lu Jian, Wu Jun, Zhang Cui, Zhang Yuxuan, Lin Yichen, and Luo Yongming. 2018. “Occurrence, Distribution, and Ecological-Health Risks of Selected Antibiotics in Coastal Waters along the Coastline of China.” Science of The Total Environment 644 (December): 1469–76. 10.1016/j.scitotenv.2018.07.096. [DOI] [PubMed] [Google Scholar]
- Ludwig Ralph D., Smyth David J. A., Blowes David W., Spink Laura E., Wilkin Richard T., Jewett David G., and Weisener Christopher J.. 2009. “Treatment of Arsenic, Heavy Metals, and Acidity Using a Mixed ZVI-Compost PRB.” Environmental Science & Technology 43 (6): 1970–76. 10.1021/es802394p. [DOI] [PubMed] [Google Scholar]
- Lv Tao, Carvalho Pedro N., Zhang Liang, Zhang Yang, Button Mark, Arias Carlos A., Weber Kela P., and Brix Hans. 2017. “Functionality of Microbial Communities in Constructed Wetlands Used for Pesticide Remediation: Influence of System Design and Sampling Strategy.” Water Research 110 (March): 241–51. 10.1016/j.watres.2016.12.021. [DOI] [PubMed] [Google Scholar]
- Lyons W. Berry, and Harmon Russell S.. 2012. “WHY URBAN GEOCHEMISTRY?” Elements 8 (6): 417–22. 10.2113/gselements.8.6.417. [DOI] [Google Scholar]
- Maguire Timothy J., and Fulweiler Robinson W.. 2016. “Urban Dissolved Silica: Quantifying the Role of Groundwater and Runoff in Wastewater Influent.” Environmental Science & Technology 50 (1): 54–61. 10.1021/acs.est.5b03516. [DOI] [PubMed] [Google Scholar]
- Martin-Mikle Chelsea J., de Beurs Kirsten M., Julian Jason P., and Mayer Paul M.. 2015. “Identifying Priority Sites for Low Impact Development (LID) in a Mixed-Use Watershed.” Landscape and Urban Planning 140 (August): 29–41. 10.1016/j.landurbplan.2015.04.002. [DOI] [Google Scholar]
- Mayer Paul M., Groffman Peter M., Striz Elise A., and Kaushal Sujay S.. 2010. “Nitrogen Dynamics at the Groundwater–Surface Water Interface of a Degraded Urban Stream.” Journal of Environmental Quality 39 (3): 810–23. 10.2134/jeq2009.0012. [DOI] [PubMed] [Google Scholar]
- Mayer Paul M., Reynolds Steven K., McCutchen Marshall D., and Canfield Timothy J.. 2007. “Meta-Analysis of Nitrogen Removal in Riparian Buffers.” Journal of Environmental Quality 36 (4): 1172–80. 10.2134/jeq2006.0462. [DOI] [PubMed] [Google Scholar]
- McPhillips Lauren E., and Matsler A. Marissa. 2018. “Temporal Evolution of Green Stormwater Infrastructure Strategies in Three US Cities.” Frontiers in Built Environment 4. 10.3389/fbuil.2018.00026. [DOI] [Google Scholar]
- Metson Geneviève S., Hale Rebecca L., Iwaniec David M., Cook Elizabeth M., Corman Jessica R., Galletti Christopher S., and Childers Daniel L.. 2012. “Phosphorus in Phoenix: A Budget and Spatial Representation of Phosphorus in an Urban Ecosystem.” Ecological Applications 22 (2): 705–21. 10.1890/11-0865.1. [DOI] [PubMed] [Google Scholar]
- Mohiuddin KM, Zakir HM, Otomo K, Sharmin S, and Shikazono N. 2010. “Geochemical Distribution of Trace Metal Pollutants in Water and Sediments of Downstream of an Urban River.” International Journal of Environmental Science & Technology 7 (1): 17–28. 10.1007/BF03326113. [DOI] [Google Scholar]
- Monaci F, and Bargagli R. 1997. “Barium and Other Trace Metals as Indicators of Vehicle Emissions.” Water, Air, and Soil Pollution 100 (1): 89–98. 10.1023/A:1018318427017. [DOI] [Google Scholar]
- Monosson Emily. 2005. “Chemical Mixtures: Considering the Evolution of Toxicology and Chemical Assessment.” Environmental Health Perspectives 113 (4): 383–90. 10.1289/ehp.6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore Joel, Bird Darcy L., Dobbis Seth K., and Woodward Gregory. 2017. “Nonpoint Source Contributions Drive Elevated Major Ion and Dissolved Inorganic Carbon Concentrations in Urban Watersheds.” Environmental Science & Technology Letters 4 (6): 198–204. 10.1021/acs.estlett.7b00096. [DOI] [Google Scholar]
- Mora Alfredo Pérez de, Ortega-Calvo J. Julio, Francisco Cabrera, and Engracia Madejón. 2005. “Changes in Enzyme Activities and Microbial Biomass after ‘in Situ’ Remediation of a Heavy Metal-Contaminated Soil.” Applied Soil Ecology 28 (2): 125–37. 10.1016/j.apsoil.2004.07.006. [DOI] [Google Scholar]
- Mori T, Koga M, Hikosaka Y, Nonaka T, Mishina F, Sakai Y, and Koizumi J. 1991. “Microbial Corrosion of Concrete Sewer Pipes, H2S Production from Sediments and Determination of Corrosion Rate.” Water Science and Technology 23 (7–9): 1275–82. 10.2166/wst.1991.0579. [DOI] [Google Scholar]
- Mullins Angela R., Bain Daniel J., Erin Pfeil-McCullough, Hopkins Kristina G., Lavin Sarah, and Copeland Erin. 2020. “Seasonal Drivers of Chemical and Hydrological Patterns in Roadside Infiltration-Based Green Infrastructure.” Science of The Total Environment 714 (April): 136503. 10.1016/j.scitotenv.2020.136503. [DOI] [PubMed] [Google Scholar]
- Nagy R. Chelsea, Lockaby B. Graeme, Latif Kalin, and Chris Anderson. 2012. “Effects of Urbanization on Stream Hydrology and Water Quality: The Florida Gulf Coast.” Hydrological Processes 26 (13): 2019–30. 10.1002/hyp.8336. [DOI] [Google Scholar]
- National Research Council, Division on Earth and Life Studies, Commission on Geosciences, Environment and Resources, Water Science and Technology Board, and Committee to Assess the Scientific Basis of the Total Maximum Daily Load Approach to Water Pollution Reduction. 2001. Assessing the TMDL Approach to Water Quality Management. National Academies Press. [Google Scholar]
- Neal Colin, Jarvie Helen P., Howarth Sharon M., Whitehead Paul G., Williams Richard J., Neal Margaret, Harrow Martin, and Wickham Heather. 2000. “The Water Quality of the River Kennet: Initial Observations on a Lowland Chalk Stream Impacted by Sewage Inputs and Phosphorus Remediation.” Science of The Total Environment 251–252 (May): 477–95. 10.1016/S0048-9697(00)00400-9. [DOI] [PubMed] [Google Scholar]
- Johnson Newcomer, Tamara A, Kaushal Sujay S., Mayer Paul M., Smith Rose M., and Sivirichi Gwen M.. 2016. “Nutrient Retention in Restored Streams and Rivers: A Global Review and Synthesis.” Water 8 (4): 116. 10.3390/w8040116. [DOI] [Google Scholar]
- Newcomer Tamara A., Kaushal Sujay S., Mayer Paul M., Shields Amy R., Canuel Elizabeth A., Groffman Peter M., and Gold Arthur J.. 2012. “Influence of Natural and Novel Organic Carbon Sources on Denitrification in Forest, Degraded Urban, and Restored Streams.” Ecological Monographs 82 (4): 449–66. 10.1890/12-0458.1. [DOI] [Google Scholar]
- Newcomer-Johnson Tamara A., Kaushal Sujay S., Mayer Paul M., and Grese Melissa M.. 2014. “Effects of Stormwater Management and Stream Restoration on Watershed Nitrogen Retention.” Biogeochemistry 121: 81–106. [Google Scholar]
- Niazi Nabeel Khan, Bibi Irshad, Shahid Muhammad, Yong Sik Ok Edward D. Burton, Wang Hailong, Shaheen Sabry M., Rinklebe Jörg, and Andreas Lüttge. 2018. “Arsenic Removal by Perilla Leaf Biochar in Aqueous Solutions and Groundwater: An Integrated Spectroscopic and Microscopic Examination.” Environmental Pollution 232 (January): 31–41. 10.1016/j.envpol.2017.09.051. [DOI] [PubMed] [Google Scholar]
- Nielsen Per Halkjær, and Thorkild Hvitved-Jacobsen. 1988. “Effect of Sulfate and Organic Matter on the Hydrogen Sulfide Formation in Biofilms of Filled Sanitary Sewers.” Journal (Water Pollution Control Federation) 60 (5): 627–34. [Google Scholar]
- Novak Jeffrey M., Cantrell Keri B., Watts Donald W., Busscher Warren J., and Johnson Mark G.. 2014. “Designing Relevant Biochars as Soil Amendments Using Lignocellulosic-Based and Manure-Based Feedstocks.” Journal of Soils and Sediments 14 (2): 330–43. 10.1007/s11368-013-0680-8. [DOI] [Google Scholar]
- Nriagu Jerome O., and Pacyna Jozef M.. 1988. “Quantitative Assessment of Worldwide Contamination of Air, Water and Soils by Trace Metals.” Nature 333 (6169): 134–39. 10.1038/333134a0. [DOI] [PubMed] [Google Scholar]
- Oakley Stewart M., Gold Arthur J., and Oczkowski Autumn J.. 2010. “Nitrogen Control through Decentralized Wastewater Treatment: Process Performance and Alternative Management Strategies.” Ecological Engineering, Managing Denitrification in Human Dominated Landscapes, 36 (11): 1520–31. 10.1016/j.ecoleng.2010.04.030. [DOI] [Google Scholar]
- Ometo Jean Pierre H. B., Martinelli Luiz A., Ballester Maria Victoria, Alaídes Gessner, Krusche Alex V., Victoria Reynaldo L., and Williams Michael. 2000. “Effects of Land Use on Water Chemistry and Macroinvertebrates in Two Streams of the Piracicaba River Basin, South-East Brazil.” Freshwater Biology 44 (2): 327–37. 10.1046/j.1365-2427.2000.00557.x. [DOI] [Google Scholar]
- Pacyna JM, and Pacyna EG. 2001. “An Assessment of Global and Regional Emissions of Trace Metals to the Atmosphere from Anthropogenic Sources Worldwide.” Environmental Reviews 9 (4): 269–98. 10.1139/a01-012. [DOI] [Google Scholar]
- Parr Thomas B., Smucker Nathan J., Bentsen Catherine N., and Neale Martin W.. 2015. “Potential Roles of Past, Present, and Future Urbanization Characteristics in Producing Varied Stream Responses.” Freshwater Science 35 (1): 436–43. 10.1086/685030. [DOI] [Google Scholar]
- Passeport Elodie, Vidon Philippe, Forshay Kenneth J., Harris Lora, Kaushal Sujay S., Kellogg Dorothy Q., Lazar Julia, Mayer Paul, and Stander Emilie K.. 2013. “Ecological Engineering Practices for the Reduction of Excess Nitrogen in Human-Influenced Landscapes: A Guide for Watershed Managers.” Environmental Management 51 (2): 392–413. 10.1007/s00267-012-9970-y. [DOI] [PubMed] [Google Scholar]
- Paul Michael J., and Meyer Judy L.. 2001. “Streams in the Urban Landscape.” Annual Review of Ecology and Systematics 32 (1): 333–65. 10.1146/annurev.ecolsys.32.081501.114040. [DOI] [Google Scholar]
- Pellerin Brian A., John Franco Saraceno, Shanley James B., Sebestyen Stephen D., Aiken George R., Wollheim Wilfred M., and Bergamaschi Brian A.. 2012. “Taking the Pulse of Snowmelt: In Situ Sensors Reveal Seasonal, Event and Diurnal Patterns of Nitrate and Dissolved Organic Matter Variability in an Upland Forest Stream.” Biogeochemistry 108 (1): 183–98. 10.1007/s10533-011-9589-8. [DOI] [Google Scholar]
- Pellerin Brian A., Wollheim Wilfred M., Hopkinson Charles S., McDowell William H., Williams Michael R., Vörösmarty Charles J., and Daley Michelle L.. 2004. “Role of Wetlands and Developed Land Use on Dissolved Organic Nitrogen Concentrations and DON/TDN in Northeastern U.S. Rivers and Streams.” Limnology and Oceanography 49 (4): 910–18. 10.4319/lo.2004.49.4.0910. [DOI] [Google Scholar]
- Peng Xianzhi, Yu Yiyi, Tang Caiming, Tan Jianhua, Huang Qiuxin, and Wang Zhendi. 2008. “Occurrence of Steroid Estrogens, Endocrine-Disrupting Phenols, and Acid Pharmaceutical Residues in Urban Riverine Water of the Pearl River Delta, South China.” Science of The Total Environment 397 (1): 158–66. 10.1016/j.scitotenv.2008.02.059. [DOI] [PubMed] [Google Scholar]
- Pennino Michael J., Kaushal Sujay S., Beaulieu Jake J., Mayer Paul M., and Arango Clay P.. 2014. “Effects of Urban Stream Burial on Nitrogen Uptake and Ecosystem Metabolism: Implications for Watershed Nitrogen and Carbon Fluxes.” Biogeochemistry 121 (1): 247–69. 10.1007/s10533-014-9958-1. [DOI] [Google Scholar]
- Pennino Michael J., Kaushal Sujay S., Mayer Paul M., Utz Ryan M., and Cooper Curtis A.. 2016. “Stream Restoration and Sewers Impact Sources and Fluxes of Water, Carbon, and Nutrients in Urban Watersheds.” Hydrology and Earth System Sciences 20 (8): 3419–39. 10.5194/hess-20-3419-2016. [DOI] [Google Scholar]
- Peter Katherine T., Tian Zhenyu, Wu Christopher, Lin Peter, White Sarah, Du Bowen, McIntyre Jenifer K., Scholz Nathaniel L., and Kolodziej Edward P.. 2018. “Using High-Resolution Mass Spectrometry to Identify Organic Contaminants Linked to Urban Stormwater Mortality Syndrome in Coho Salmon.” Environmental Science & Technology 52 (18): 10317–27. 10.1021/acs.est.8b03287. [DOI] [PubMed] [Google Scholar]
- Petrone Kevin C., Hughes Justin D., Van Niel Thomas G., and Silberstein Richard P.. 2010. “Streamflow Decline in Southwestern Australia, 1950–2008.” Geophysical Research Letters 37 (11). 10.1029/2010GL043102. [DOI] [Google Scholar]
- Pikaar Ilje, Sharma Keshab R., Hu Shihu, Gernjak Wolfgang, Keller Jürg, and Yuan Zhiguo. 2014. “Reducing Sewer Corrosion through Integrated Urban Water Management.” Science 345 (6198): 812–14. 10.1126/science.1251418. [DOI] [PubMed] [Google Scholar]
- Pomeroy Richard, and Bowlus Fred D.. 1946. “Progress Report on Sulfide Control Research.” Sewage Works Journal 18 (4): 597–640. [PubMed] [Google Scholar]
- Pons-Branchu E, Roy-Barman M, Jean-Soro L, Guillerme A, Branchu P, Fernandez M, Dumont E, Douville E, JL Michelot, and Phillips AM. 2017. “Urbanization Impact on Sulfur Content of Groundwater Revealed by the Study of Urban Speleothem-like Deposits: Case Study in Paris, France.” Science of The Total Environment 579 (February): 124–32. 10.1016/j.scitotenv.2016.10.234. [DOI] [PubMed] [Google Scholar]
- Posthuma Leo, Brown Colin D., de Zwart Dick, Jerome Diamond, Dyer Scott D., Holmes Christopher M., Marshall Stuart, and Burton G. Allen. 2018. “Prospective Mixture Risk Assessment and Management Prioritizations for River Catchments with Diverse Land Uses.” Environmental Toxicology and Chemistry 37 (3): 715–28. 10.1002/etc.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praipipat Pornsawai, Meng Qingyu, Miskewitz Robert J., and Rodenburg Lisa A.. 2017. “Source Apportionment of Atmospheric Polychlorinated Biphenyls in New Jersey 1997–2011.” Environmental Science & Technology 51 (3): 1195–1202. 10.1021/acs.est.6b04572. [DOI] [PubMed] [Google Scholar]
- Puckett Larry J. 1994. “Nonpoint and Point Sources of Nitrogen in Major Watersheds of the United States.” USGS Numbered Series 94–4001. Water-Resources Investigations Report. Geological Survey (U.S.). http://pubs.er.usgs.gov/publication/wri944001. [Google Scholar]
- Purdy K, and Wright IA. 2019. “Impact of Concrete on Riparian Ecosystems.” IOP Conference Series: Earth and Environmental Science 344 (November): 012033. 10.1088/1755-1315/344/1/012033. [DOI] [Google Scholar]
- Raciti SM, Groffman PM, and Fahey TJ. 2008. “Nitrogen Retention in Urban Lawns and Forests.” Ecological Applications 18 (7): 1615–26. 10.1890/07-1062.1. [DOI] [PubMed] [Google Scholar]
- Reddy Krishna R., and Kumar Girish. 2017. “Permeable Reactive Filter Systems for the Treatment of Urban Stormwater Runoff with Mixed Pollutants.” Geotechnical Frontiers 2017, Proceedings,, 508–17. 10.1061/9780784480434.055. [DOI] [Google Scholar]
- Riha Krystin M., Michalski Greg, Gallo Erika L., Lohse Kathleen A., Brooks Paul D., and Meixner Tom. 2014. “High Atmospheric Nitrate Inputs and Nitrogen Turnover in Semi-Arid Urban Catchments.” Ecosystems 17 (8): 1309–25. 10.1007/s10021-014-9797-x. [DOI] [Google Scholar]
- Rochman Chelsea M. 2018. “Microplastics Research—from Sink to Source.” Science 360 (6384): 28–29. 10.1126/science.aar7734. [DOI] [PubMed] [Google Scholar]
- Rodenburg Lisa A., Meng Qingyu, Yee Don, and Greenfield Ben K.. 2014. “Evidence for Photochemical and Microbial Debromination of Polybrominated Diphenyl Ether Flame Retardants in San Francisco Bay Sediment.” Chemosphere 106 (July): 36–43. 10.1016/j.chemosphere.2013.12.083. [DOI] [PubMed] [Google Scholar]
- Rodenburg Lisa A., and Ralston David K.. 2017. “Historical Sources of Polychlorinated Biphenyls to the Sediment of the New York/New Jersey Harbor.” Chemosphere 169 (February): 450–59. 10.1016/j.chemosphere.2016.11.096. [DOI] [PubMed] [Google Scholar]
- Rodenburg Lisa A., Valle Sandra N., Panero Marta A., Muñoz Gabriela R., and Shor Leslie M.. 2010. “Mass Balances on Selected Polycyclic Aromatic Hydrocarbons in the New York–New Jersey Harbor.” Journal of Environmental Quality 39 (2): 642–53. 10.2134/jeq2009.0264. [DOI] [PubMed] [Google Scholar]
- Rose Seth. 2007. “The Effects of Urbanization on the Hydrochemistry of Base Flow within the Chattahoochee River Basin (Georgia, USA).” Journal of Hydrology 341 (1): 42–54. 10.1016/j.jhydrol.2007.04.019. [DOI] [Google Scholar]
- Schipper Louis A., Robertson Will D., Gold Arthur J., Jaynes Dan B., and Cameron Stewart C.. 2010. “Denitrifying Bioreactors—An Approach for Reducing Nitrate Loads to Receiving Waters.” Ecological Engineering, Managing Denitrification in Human Dominated Landscapes, 36 (11): 1532–43. 10.1016/j.ecoleng.2010.04.008. [DOI] [Google Scholar]
- Schipper Louis A, and Maja Vojvodić-Vuković. 2001. “Five Years of Nitrate Removal, Denitrification and Carbon Dynamics in a Denitrification Wall.” Water Research 35 (14): 3473–77. 10.1016/S0043-1354(01)00052-5. [DOI] [PubMed] [Google Scholar]
- Scientific Committee on Health and Environmental Risks. 2012. “Toxicity and Assessment of Chemical Mixtures.” European Commission, Brussels, Belgium. [Google Scholar]
- Selbig William R. 2016. “Evaluation of Leaf Removal as a Means to Reduce Nutrient Concentrations and Loads in Urban Stormwater.” Science of The Total Environment 571 (November): 124–33. 10.1016/j.scitotenv.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Senesil Giorgio S., Baldassarre G, Senesi N, and Radina B. 1999. “Trace Element Inputs into Soils by Anthropogenic Activities and Implications for Human Health.” Chemosphere, Matter and Energy Fluxes in the Anthropocentric Environment, 39 (2): 343–77. 10.1016/S0045-6535(99)00115-0. [DOI] [PubMed] [Google Scholar]
- Sephton Michael G., and Webb John A.. 2017. “Application of Portland Cement to Control Acid Mine Drainage Generation from Waste Rocks.” Applied Geochemistry 81 (June): 143–54. 10.1016/j.apgeochem.2017.03.017. [DOI] [Google Scholar]
- Sferratore Agata, Garnier Josette, Billen Gilles, Conley Daniel J., and Pinault Séverine. 2006. “Diffuse and Point Sources of Silica in the Seine River Watershed.” Environmental Science & Technology 40 (21): 6630–35. 10.1021/es060710q. [DOI] [PubMed] [Google Scholar]
- Shabalala Ayanda N., Ekolu Stephen O., Diop Souleymane, and Solomon Fitsum. 2017. “Pervious Concrete Reactive Barrier for Removal of Heavy Metals from Acid Mine Drainage − Column Study.” Journal of Hazardous Materials 323 (February): 641–53. 10.1016/j.jhazmat.2016.10.027. [DOI] [PubMed] [Google Scholar]
- Shuster William D., Darner Robert A., Schifman Laura A., and Herrmann Dustin L.. 2017. “Factors Contributing to the Hydrologic Effectiveness of a Rain Garden Network (Cincinnati OH USA).” Infrastructures 2 (3): 11. 10.3390/infrastructures2030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sickman JO, Zanoli MJ, and Mann HL. 2007. “Effects of Urbanization on Organic Carbon Loads in the Sacramento River, California: URBANIZATION AND RIVERINE CARBON LOADS.” Water Resources Research 43 (11). 10.1029/2007WR005954. [DOI] [Google Scholar]
- Silva José Salomão Oliveira, da Cunha Bustamante Mercedes Maria, Daniel Markewitz, Krusche Alex Vladimir, and Laerte Guimarães Ferreira. 2011. “Effects of Land Cover on Chemical Characteristics of Streams in the Cerrado Region of Brazil.” Biogeochemistry 105 (1–3): 75–88. 10.1007/s10533-010-9557-8. [DOI] [Google Scholar]
- Smith Rose M., and Kaushal Sujay S.. 2015. “Carbon Cycle of an Urban Watershed: Exports, Sources, and Metabolism.” Biogeochemistry 126 (1): 173–95. 10.1007/s10533-015-0151-y. [DOI] [Google Scholar]
- Snodgrass Joel W., Moore Joel, Lev Steven M., Casey Ryan E., Ownby David R., Flora Robert F., and Izzo Grant. 2017. “Influence of Modern Stormwater Management Practices on Transport of Road Salt to Surface Waters.” Environmental Science & Technology 51 (8): 4165–72. 10.1021/acs.est.6b03107. [DOI] [PubMed] [Google Scholar]
- Spencer Robert G. M., Pellerin Brian A., Bergamaschi Brian A., Downing Bryan D., Kraus Tamara E. C., Smart David R., Dahlgren Randy A., and Hernes Peter J.. 2007. “Diurnal Variability in Riverine Dissolved Organic Matter Composition Determined by in Situ Optical Measurement in the San Joaquin River (California, USA).” Hydrological Processes 21 (23): 3181–89. 10.1002/hyp.6887. [DOI] [Google Scholar]
- Spromberg Julann A., Baldwin David H., Damm Steven E., McIntyre Jenifer K., Michael Huff, Sloan Catherine A., Anulacion Bernadita F., Davis Jay W., and Scholz Nathaniel L.. 2016. “Coho Salmon Spawner Mortality in Western US Urban Watersheds: Bioinfiltration Prevents Lethal Storm Water Impacts.” Journal of Applied Ecology 53 (2): 398–407. 10.1111/1365-2664.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele Meredith K., McDowell William H., and Aitkenhead-Peterson Jacqueline A.. 2010. “Chemistry of Urban, Suburban, and Rural Surface Waters.” Urban Ecosystem Ecology agronomymonogra (urbanecosysteme): 297–339. 10.2134/agronmonogr55.c15. [DOI] [Google Scholar]
- Steffy Luanne Y., and Kilham Susan S.. 2004. “ELEVATED Δ15N IN STREAM BIOTA IN AREAS WITH SEPTIC TANK SYSTEMS IN AN URBAN WATERSHED.” Ecological Applications 14 (3): 637–41. 10.1890/03-5148. [DOI] [Google Scholar]
- Takagi Kimberly K., Hunter Kimberley S., Cai Wei-Jun, and Joye Samantha B.. 2017. “Agents of Change and Temporal Nutrient Dynamics in the Altamaha River Watershed.” Ecosphere 8 (1): e01519. 10.1002/ecs2.1519. [DOI] [Google Scholar]
- Taneez Mehwish, Hurel Charlotte, Mady Franck, and Francour Patrice. 2018. “Capping of Marine Sediments with Valuable Industrial By-Products: Evaluation of Inorganic Pollutants Immobilization.” Environmental Pollution 239 (August): 714–21. 10.1016/j.envpol.2018.04.089. [DOI] [PubMed] [Google Scholar]
- Thurston Hale W., Taylor Michael A., Shuster William D., Roy Allison H., and Morrison Matthew A.. 2010. “Using a Reverse Auction to Promote Household Level Stormwater Control.” Environmental Science & Policy 13 (5): 405–14. 10.1016/j.envsci.2010.03.008. [DOI] [Google Scholar]
- Tippler Carl, Wright Ian A., Davies Peter J., and Hanlon Alison. 2014a. “The Influence of Concrete on the Geochemical Qualities of Urban Streams.” Marine and Freshwater Research 65 (11): 1009. 10.1071/MF13164. [DOI] [Google Scholar]
- Truex Michael, Johnson Chris, Macbeth Tamzen, Becker Dave, Lynch Kira, Giaudrone Dominic, Frantz Aaron, and Lee Hope. 2017. “Performance Assessment of Pump-and-Treat Systems.” Groundwater Monitoring & Remediation 37 (3): 28–44. 10.1111/gwmr.12218. [DOI] [Google Scholar]
- Tufford Daniel L., Samarghitan Carmen L., McKellar Hank N., Porter Dwayne E., and Hussey James R.. 2003. “Impacts of Urbanization on Nutrient Concentrations in Small Southeastern Coastal Streams1.” JAWRA Journal of the American Water Resources Association 39 (2): 301–12. 10.1111/j.1752-1688.2003.tb04385.x. [DOI] [Google Scholar]
- United Nations Environment Programme. 2018. “GEMStat Database of the Global Environment Monitoring System for Freshwater (GEMS/Water) Programme.” International Centre for Water Resources and Global Change, Koblenz. Data available upon request from GEMS/Water Data Centre: gemstat.org [Google Scholar]
- U.S. Geological Survey. 2016. Metals and Minerals: U.S. Geological Survey Minerals Yearbook. Vol. 1. [Google Scholar]
- Vaughan MCH, Bowden WB, Shanley JB, Vermilyea A, Sleeper R, Gold AJ, Pradhanang SM, et al. 2017. “High-Frequency Dissolved Organic Carbon and Nitrate Measurements Reveal Differences in Storm Hysteresis and Loading in Relation to Land Cover and Seasonality.” Water Resources Research 53 (7): 5345–63. 10.1002/2017WR020491. [DOI] [Google Scholar]
- Verbanck Michel, Vanderborght Jean-Pierre, and Wollast Roland. 1989. “Major Ion Content of Urban Wastewater: Assessment of per Capita Loading.” Research Journal of the Water Pollution Control Federation 61 (11/12): 1722–28. [Google Scholar]
- Walsh Christopher J., Roy Allison H., Feminella Jack W., Cottingham Peter D., Groffman Peter M., and Morgan Raymond P.. 2005. “The Urban Stream Syndrome: Current Knowledge and the Search for a Cure.” Journal of the North American Benthological Society 24 (3): 706–23. 10.1899/04-028.1. [DOI] [Google Scholar]
- Wang Y (Chinese Academy of Sciences, C. (North Carolina State Univ Tu C (Shaanxi Normal Univ Li L (North Carolina State Univ Tredway D (Hope Valley Country Club (USA)) Lee M (Treyburn Country Club (USA)) Snell X (Northwest A&F Univ Zhang, and Hu S. 2014. “Turfgrass Management Duration and Intensities Influence Soil Microbial Dynamics and Carbon Sequestration.” International Journal of Agriculture and Biology (Pakistan). http://agris.fao.org/agris-search/search.do?recordID=PK2014001156. [Google Scholar]
- Waschbusch. n.d. National Conference on Tools for Urban Water Resource Management and Protection Proceedings, February 710, 2000, Chicago, IL. DIANE Publishing. [Google Scholar]
- Wasserman Julio Cesar, Maria Angélica V. Wasserman, Paulo Rubens G. Barrocas, and Aline Mansur Almeida. 2016. “Predicting Pollutant Concentrations in the Water Column during Dredging Operations: Implications for Sediment Quality Criteria.” Marine Pollution Bulletin 108 (1): 24–32. 10.1016/j.marpolbul.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Wilkin Richard T., Acree Steven D., Ross Randall R., Beak Douglas G., and Lee Tony R.. 2009. “Performance of a Zerovalent Iron Reactive Barrier for the Treatment of Arsenic in Groundwater: Part 1. Hydrogeochemical Studies.” Journal of Contaminant Hydrology 106 (1): 1–14. 10.1016/j.jconhyd.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Williams Clayton J., Frost Paul C., Morales‐Williams Ana M., Larson James H., Richardson William B., Chiandet Aisha S., and Xenopoulos Marguerite A.. 2016. “Human Activities Cause Distinct Dissolved Organic Matter Composition across Freshwater Ecosystems.” Global Change Biology 22 (2): 613–26. 10.1111/gcb.13094. [DOI] [PubMed] [Google Scholar]
- Wollheim WM, Mulukutla GK, Cook C, and Carey RO. 2017. “Aquatic Nitrate Retention at River Network Scales Across Flow Conditions Determined Using Nested In Situ Sensors.” Water Resources Research 53 (11): 9740–56. 10.1002/2017WR020644. [DOI] [Google Scholar]
- Wright IA, Davies PJ, Findlay SJ, and Jonasson OJ. 2011. “A New Type of Water Pollution: Concrete Drainage Infrastructure and Geochemical Contamination of Urban Waters.” Marine and Freshwater Research 62 (12): 1355–61. 10.1071/MF10296. [DOI] [Google Scholar]
- Wu Changshan, and Murray Alan T.. 2003. “Estimating Impervious Surface Distribution by Spectral Mixture Analysis.” Remote Sensing of Environment 84 (4): 493–505. 10.1016/S0034-4257(02)00136-0. [DOI] [Google Scholar]
- Wu Yunhai, Wen Yajun, Zhou Jianxin, and Wu Yunying. 2014. “Phosphorus Release from Lake Sediments: Effects of PH, Temperature and Dissolved Oxygen.” KSCE Journal of Civil Engineering 18 (1): 323–29. 10.1007/s12205-014-0192-0. [DOI] [Google Scholar]
- Zampella Robert A. 1994. “Characterization of Surface Water Quality Along a Watershed Disturbance Gradient1.” JAWRA Journal of the American Water Resources Association 30 (4): 605–11. 10.1111/j.1752-1688.1994.tb03315.x. [DOI] [Google Scholar]
- Zayed Adel, Gowthaman Suvarnalatha, and Terry Norman. 1998. “Phytoaccumulation of Trace Elements by Wetland Plants: I. Duckweed.” Journal of Environmental Quality 27 (3): 715–21. 10.2134/jeq1998.00472425002700030032x. [DOI] [Google Scholar]
- Zhang Qianzhu, Tao Zhen, Ma Zanwen, Tang Wenkui, Gao Quanzhou, Xu Peng, and Lin Youwen. 2016. “Riverine Hydrochemistry and CO2 Consumption in the Tropic Monsoon Region: A Case Study in a Granite-Hosted Basin, Hainan Island, China.” Environmental Earth Sciences 75 (5): 436. 10.1007/s12665-016-5250-0. [DOI] [Google Scholar]
- Zikeli S, Kastler M, and Jahn R. 2005. “Classification of Anthrosols with Vitric/Andic Properties Derived from Lignite Ash.” Geoderma 124 (3): 253–65. 10.1016/j.geoderma.2004.05.004. [DOI] [Google Scholar]
- Dick de Zwart, William Adams, Malyka Galay Burgos, Juliane Hollender, Marion Junghans, Graham Merrington, Derek Muir, et al. 2018. “Aquatic Exposures of Chemical Mixtures in Urban Environments: Approaches to Impact Assessment.” Environmental Toxicology and Chemistry 37 (3): 703–14. 10.1002/etc.3975. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.