Abstract
Neurological manifestations have been reported in COVID-19; however, the route used by SARS-CoV-2 to enter the brain is still under debate. Recent studies have focused on the olfactory route. SARS-CoV-2 viral proteins were also detected in the glossopharyngeal and vagal nerves originating from the lower brainstem and in isolated cells of the brainstem. Our proof of concept in vivo real-time imaging study of mice using an indocyanine green dye indicated that the neurovascular component of the connective tissue of the respiratory mucosa can also provide an alternate route to the brain.
1. Introduction
SARS-CoV-2 is reported as a neurotropic virus that can target the brain and cause neurological complications.1,2 An axonal route from the periphery to the brain through the olfactory nerve and vagus nerve had been demonstrated.1,3,4 Recently, axonal transport through the trigeminal nerve and the involvement of the nucleus tractus solitarius through the cranial nerves VII, IX, and X also have been hypothesized for silent hypoxemia, cytokine storm, and pulmonary edema in COVID-19.5
Anosmia without rhinitis has been reported in COVID-19, and the involvement of the olfactory nerve and the brain was proposed.3,6 It was reported that only pericytes and non-neuronal cells in the human and mouse olfactory epithelium express both ACE2 and TMPRSS2 genes that are essential for the entry of SARS-CoV-2 into the cells. The mature olfactory sensory neurons and the olfactory bulb neurons in the mouse do not express the genes. Therefore, anosmia was proposed to be due to SARS-CoV-2 infection of the non-neuronal cells. Axonal transport from the olfactory mucosa to the brain was questioned, and a vascular route of transport was proposed. It was also proposed that local SARS-CoV-2 seeding of the olfactory epithelium can result in neuron-independent transfer to the brain by targeting the vascular pericytes of the blood vessels of the olfactory nerve. The central cause for the olfactory dysfunction was proposed to be secondary to inflammation of the local pericytes.7
A haematogenous route from the olfactory mucosa to the brain through the cribriform plate has been proposed.3 However, the blood brain barrier prevents entry of most of the foreign substances and microorganisms from the circulation into the brain. SARS-CoV-2 is a large virus of approximately 70–80 nm in diameter with envelope projections that average 15 ± 2 nm in size.8 It has already been reported that the entry of SARS-CoV-2 into the host cell occurs by the binding of the spike protein to angiotensin-converting enzyme 2 (ACE-2). The priming of the spike protein is further done by transmembrane protease serine 2 (TMPRSS2). It was suggested that SARS-CoV-2 infects the endothelial cells in the brain that express ACE2 receptors.4 However, recent studies have shown that ACE2 is very weakly expressed in endothelial cells and is highly expressed only in the pericytes.9
Recently, Neuropilin-1 (NRP1) has been reported as a co-factor that significantly enhances SARS-CoV-2 infectivity and facilitates the entry of the virus into the host cell when co-expressed with ACE-2 and TMPRSS2.10,11 NRP1 is a transmembrane receptor that primarily acts as a co-receptor for various ligands and plays a role in cell proliferation, angiogenesis, and axon control.12 Human autopsy studies have shown that NRP1 is expressed in the human respiratory epithelium and olfactory epithelium. In the olfactory epithelium, it was expressed in the late olfactory neuronal progenitors and the newly differentiated olfactory neurons.10 Based on RNA sequencing, NRP1 is also highly expressed in the brain and is seen in mature astrocytes and endothelial cells. It is also seen in the olfactory bulb, pons, etc.12
Involvement of the brain was considered to occur in COVID-19 when the virus enters the systemic circulation from the lungs and causes inflammation-mediated damage to the blood brain barrier.13 However, the olfactory route has received considerable attention recently.14 Accordingly, in a recent in vivo study using mice, silver nanoparticles coated with a synthetic spike peptide that were administered nasally were detected in the olfactory epithelium expressing NRP1 and in the neurons and blood vessels of the cortex.10 Therefore, the NRP1-mediated entry of SARS-CoV-2 into the brain through the olfactory epithelium has been suggested.12
The relative olfactory area in humans is very small when compared with mice. Therefore, it will be easier for the virus to access the larger respiratory mucosal area in the human nose. Hence, we hypothesize that the underlying neurovascular bundle (involving the branches of the trigeminal nerve, branches of the maxillary artery, and the accompanying veins) in the connective tissue of the respiratory epithelium can provide an alternate route for the virus to reach the brain.
Drug delivery into the brain has been a challenge for more than 100 years. However, drugs with no specific affinity for neurons have also been delivered into the brain through the nasal route.15 We therefore tested our hypothesis with the indocyanine green (ICG) dye. ICG has been used in medicine to measure cardiac output, to study the anatomy of retinal vessels, and to measure liver functional reserve before surgery and in neurosurgery. When given intravenously, ICG binds to plasma proteins within seconds and does not cross the blood brain barrier. When injected outside the blood vessels, ICG binds to proteins and is transported through the local lymphatic channels to the nearby lymph nodes.16 In preclinical studies, ICG has been used as an anterograde and retrograde axonal tracer for the visual pathway.17
2. Results and Discussion
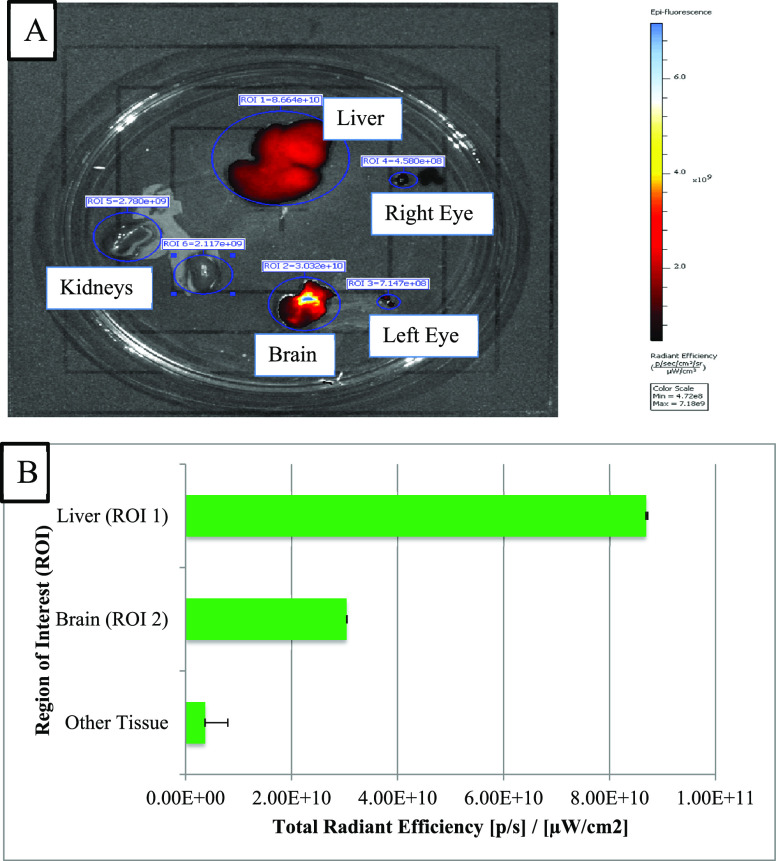
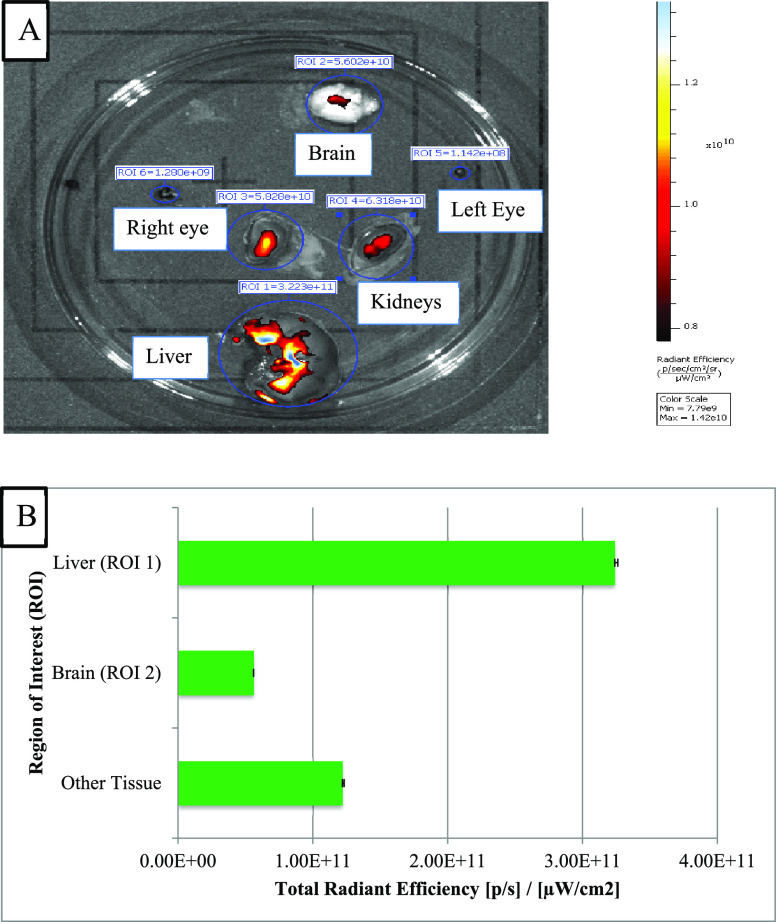
In vivo real-time animal imaging using ICG demonstrated access to the brain through the connective tissue of the respiratory mucosa (Figure 1). As a corroboration, the imaging detected an intense fluorescence signal in the brain dissected from the mouse model in which ICG was injected directly into the connective tissue of the respiratory mucosa. The fluorescence was generalized with the strongest signals toward the midline. Similarly, the generalized fluorescence signal was detected in the liver as it excretes the unchanged dye in the bile (Figure 2A,B). Alternatively, a minimal fluorescence signal was detected in the brain dissected from the mouse model in which ICG was administered topically through the right nostril (Figure 3A,B). The fluorescence was localized toward the midline, and the periphery did not show any fluorescence. A strong and localized fluorescence signal was detected in the liver suggesting faster drainage into the venous compartment and elimination by the liver.
Figure 1.
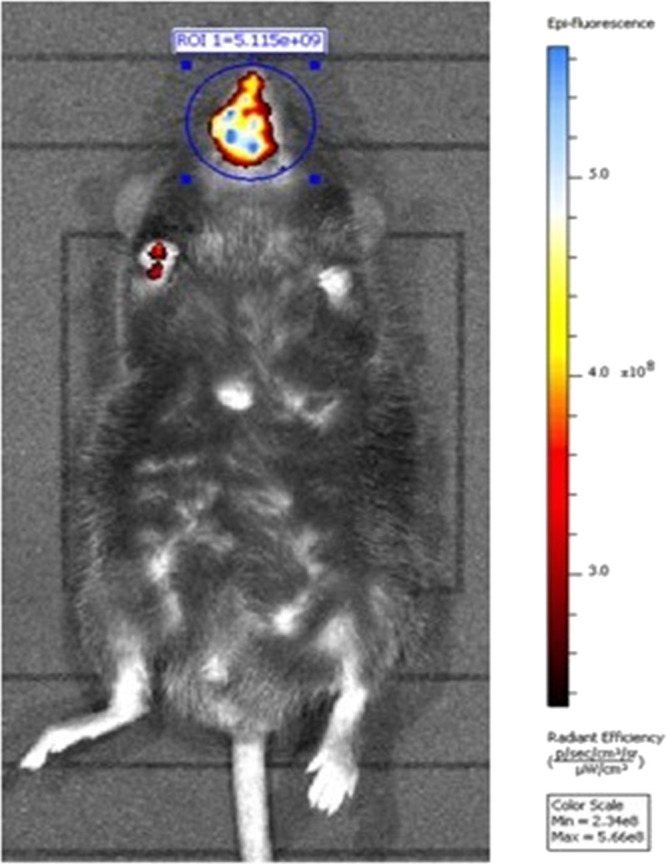
Representative live animal imaging of mice injected with ICG into the connective tissue of the respiratory mucosa in the maxillary sinus region.
Figure 2.
(A) Imaging of different dissected tissues of interest from mice with ICG injected into the connective tissue of the respiratory mucosa. (B) Plot of concentration of fluorescence signals in different dissected tissues of interest.
Figure 3.
(A) Imaging of different dissected tissues of interest from mice with ICG administered as topical drops into the right nostril. (B) Plot of concentration of fluorescence signals in different dissected tissues of interest.
Crossing the blood brain barrier can be a crucial factor in the involvement of the brain by SARS CoV-2. However, the route taken by the virus is still under debate.
Autopsy studies of COVID-19 patients have shown the presence of spike protein in NRP1 positive olfactory epithelial cells, late olfactory neuronal progenitors, and the newly differentiated olfactory neurons.10 Gene expression analysis has also shown an up-regulation of NRP1 and NRP2 in the lung tissue from COVID-19 patients.11
Axonal transport through the trigeminal nerve and the involvement of the nucleus tractus solitarius through the cranial nerves VII, IX, and X also have been hypothesized as probable routes for the central nervous system (CNS) involvement in COVID-19.5 In a recent post-mortem study of COVID-19 patients, SARS-CoV-2 RNA or proteins were detected in the brain tissues of 53% of the cases. In 20% of the cases, both SARS-CoV-2 RNA and protein were detected. SARS-CoV-2 was detected in isolated cells within the medulla oblongata and in the glossopharyngeal and vagal nerves originating from the brainstem.18,19
Intriguingly, autopsies from COVID-19 patients have shown that the lowest level of SARS-CoV-2 viral load is detected in the brain among the organs studied.7 SARS-CoV-2 RNA has been detected only in a very limited number of human cerebrospinal fluid (CSF) samples.14 These findings are not surprising as studies in mice have shown that a small volume of intracerebral injections of influenza virus was confined to the inoculation site and was not detected in the CSF.20
The route taken by the virus to target the brain of the patients is still not confirmed. Therefore, it is possible that SARS-CoV-2 may enter the brain early from the nasal region and probably retained within the neuronal cells expressing the entry receptors.
The trigeminal nerve is the largest cranial nerve and the only sensory afferent in the cerebral circulation. It supplies the nasal respiratory mucosa and the maxillary sinus. It also sends a branch to the olfactory region.
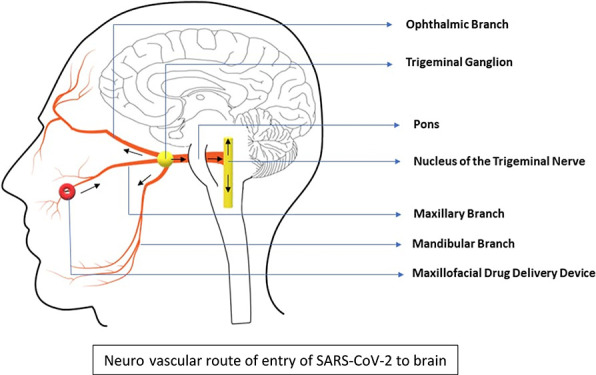
Human autopsy studies have shown that NRP1 is expressed in the human respiratory epithelium and olfactory epithelium.10 The respiratory mucosa also expresses more ACE2 than the olfactory mucosa.7 NRP1 has been reported to enhance the viral entry into the cell in the presence of ACE2.11,12 Neural, vascular, and lymphatic routes from the connective tissue of the respiratory mucosa have also been proposed for delivering drugs into the brain and the retina by bypassing the intact blood brain barrier and blood retinal barrier, respectively.21 It is easier for the virus to access the larger respiratory mucosal area in the human nose. Therefore, if the virus is able to breach the nasal respiratory epithelium and access the underlying connective tissue, then the underlying neurovascular route can be used to target the brain in spite of an intact blood brain barrier (Figure 4).
Figure 4.
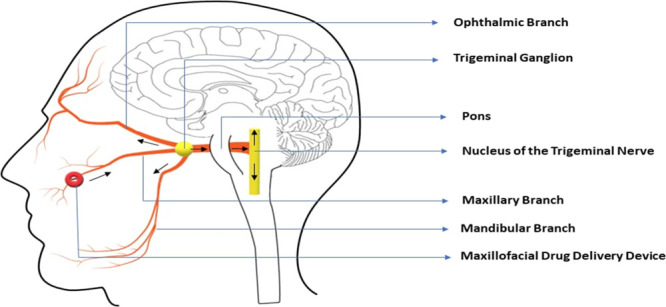
Schematic representation of the neurovascular route of the SARS-CoV-2 entry from the respiratory mucosa in the maxillary sinus region to the brain.
SARS-CoV-2 could be transported to the brain through the perineural and perivascular pathways of the trigeminal nerve from the nasal mucosa. Because of the low titer, the large size, and the shape of the virus, drainage into the CSF through the glymphatic system may be restricted in the presence of an intact blood brain barrier.
To test the hypothesis of a possible alternate route in an in vivo mouse model, ICG was administered directly into the connective tissue of the respiratory mucosa. Though the mice do not fully simulate humans, they have been used as validated experimental models for maxillary sinus research, nasal drug delivery research, and blood brain barrier research. The neurovascular pathways in the connective tissue could transport the dye to the brain, resulting in intense fluorescence in the brain. The fluorescence was significant in the brain and the liver when compared with the nasal route. This difference could be because the nasal respiratory mucosal epithelium acts as a barrier and restricts easy entry of the dye into the connective tissue when delivered as topical nasal drops.
Anosmia and ageusia may be the clue that SARS-CoV-2 is targeting the olfactory region and the taste buds for easy access to the underlying neurovascular pathways of the connective tissue. Thus, early retrograde perineural and perivascular transport of the virus to the brain can occur from the respiratory mucosa also.
The blood brain barrier protects the brain by preventing the entry of pathogens and toxins from the circulating blood into the brain. However, this protection also prevents the entry of useful drugs into the brain.
Out of the drugs under trial for COVID-19, only a few can cross the blood brain barrier. Remdesivir is an antiviral drug, which works at the post-viral entry stage. However, it does not cross the blood brain barrier in sufficient quantities.22 Among quinolone-based antimalarial drugs, hydroxychloroquine has limited blood brain barrier permeability.23 Lopinavir/Ritonavir, Tocilizumab, and Favipiravir show limited brain penetrance. Azithromycin is reported to have good brain penetrance but high tissue binding.24 Artemisinin is an antimalarial drug with better brain penetrance that is being studied for use in COVID-19. Recently, a small study has proposed the use of clonidine, an FDA-approved central sympatholytic drug that can act at the nucleus tractus solitarius, to mitigate SARS-CoV-2-related symptoms.25 Similarly, repurposing of acetylcholine esterase inhibitors and nicotinic compounds developed for Alzheimer’s disease has also been proposed.26
3. Conclusions
The route used by the SARS-CoV-2 to target the brain from the primary site of infection is yet to be well explored. Further to this pilot study, confirmatory studies are required to make a detailed investigation of the pathomechanism behind the early viral entry to the brain. Similarly, drugs with efficient brain penetrance or drug delivery techniques that aid in crossing the blood brain barrier may be needed in the management of COVID-19.
4. Experimental Section
4.1. Animal Experimentation
Male C57BL/6J mice (about 7–8 Months old) weighing 35–40 g were used for the study. The mice were anesthetized using 2:1 Ketamine (80 mg/kg body weight) and Xylazine (7 mg/kg body weight) by subcutaneous injection. The study was approved by the Institutional Animal Ethics Committee, Sri Balaji Vidyapeeth [Deemed to be University] (03/IAEC/MG/11/2018-II).
4.2. In Vivo Imaging
After anesthetization, 50 μg of ICG was either injected directly into the connective tissue of the respiratory mucosa in the maxillary sinus region or delivered as topical drops through the right nostril of the mice. After 10, 20, 30, and 70 min of the injection, the mice were scanned under an IVIS small animal imaging system (Perkin Elmer, Massachusetts, US) to detect the fluorescence from ICG.
The fluorescence specific signal (ratio of the power emitted by a source of radiation to the power consumed by it) was shown as the radiant efficiency (emission light [photons/s/cm2/str]/excitation light [μW/cm2]). Further, the fluorescence intensity indicated how much light (photons) was emitted. It was the extent of emission, and it depended on the concentration of the excited fluorophore. The fluorescence signal for each tissue was calculated by selecting the region of interest (ROI) and measuring as the total radiant efficiency [photons/s]/[μW/cm2]. Total radiant efficiency signified the sums of fluorescent pixels within the ROI. For each region of interest, the mean value and standard deviations were calculated.
After live animal imaging, the mice were sacrificed and major tissues such as the liver, kidney, brain, and eyes were dissected and scanned again to measure the concentration of the fluorescence signal in each tissue of interest.
Acknowledgments
We gratefully acknowledge the Bio-Imaging Facility and technical assistance rendered for conducting the in vivo imaging experiments at the SRM-DBT Platform for Advanced Life Science Technologies, SRM Institute of Science and Technology, Kattankulathur, Chengalpattu District, Tamil Nadu 603203, India. We especially thank Dr. Ranjith Jagadeeshwaran for his help at the Bio-Imaging facility.
Author Contributions
All the authors have contributed equally to the work.
The study is financially supported by the Science and Engineering Research Board (SERB), a statutory body of the Department of Science and Technology (DST), Government of India (EMR/2017/005413).
The authors declare the following competing financial interest(s): The authors, Anoop.U.R and Kavita Verma are stated as the inventors and applicants in the published patent applications US15/177,347 and PCT/IB2016/053899 with National Phase Entry into European Patent Office, Canada, and India. The technique and device are patented in Australia.
Notes
The animal experiments of the study are approved by the Institutional Animal Ethics Committee, Sri Balaji Vidyapeeth [Deemed to be University] (03/IAEC/MG/11/2018-II).
References
- Li Y. C.; Bai W.-Z.; Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020, 92, 552–555. 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice F. G.; Tovar-Moll F.; Moll J.; Munoz D. P.; Ferreira S. T. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and the Central Nervous System. Trends Neurosci. 2020, 43, 355–357. 10.1016/j.tins.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig A. M.; Khaleeq A.; Ali U.; Syeda H. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host–Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem. Neurosci. 2020, 11, 995–998. 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- Toljan K. Letter to the Editor Regarding the Viewpoint Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host–Virus Interaction, and Proposed Neurotropic Mechanism. ACS Chem. Neurosci. 2020, 11, 1192–1194. 10.1021/acschemneuro.0c00174. [DOI] [PubMed] [Google Scholar]
- U.R. A.; Verma K. Happy Hypoxemia in COVID-19-A Neural Hypothesis. ACS Chem. Neurosci. 2020, 11, 1865–1867. 10.1021/acschemneuro.0c00318. [DOI] [PubMed] [Google Scholar]
- Vaira L. A.; Deiana G.; Fois A. G.; Pirina P.; Madeddu G.; De Vito A.; Babudieri S.; Petrocelli M.; Serra A.; Bussu F.; Ligas E.; Salzano G.; De Riu G. Objective evaluation of anosmia and ageusia in COVID -19 patients: Single-center experience on 72 cases. Head Neck 2020, 42, 1252–1258. 10.1002/hed.26204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann D. H.; Tsukahara T.; Weinreb C.; Lipovsek M.; Van den Berge K.; Gong B.; Chance R.; Macaulay I. C.; Chou H.-J.; Fletcher R. B.; Das D.; Street K.; de Bezieux H. R.; Choi Y.-G.; Risso D.; Dudoit S.; Purdom E.; Mill J.; Hachem R. A.; Matsunami H.; Logan D. W.; Goldstein B. J.; Grubb M. S.; Ngai J.; Datta S. R. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv. 2020, 6, eabc5801 10.1126/sciadv.abc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S.; Potdar V.; Cherian S.; Abraham P.; Basu A. Transmission electron microscopy imaging of SARS-CoV-2. Indian J. Med. Res. 2020, 0, 241–243. 10.4103/ijmr.IJMR_577_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardot-Leccia N.; Hubiche T.; Dellamonica J.; Burel-Vandenbos F.; Passeron T. Pericyte alteration sheds light on micro-vasculopathy in COVID-19 infection. Intensive Care Med. 2020, 46, 1777–1778. 10.1007/s00134-020-06147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantuti-Castelvetri L.; Ojha R.; Pedro L. D.; Djannatian M.; Franz J.; Kuivanen S.; van der Meer F.; Kallio K.; Kaya T.; Anastasina M.; Smura T.; Levanov L.; Szirovicza L.; Tobi A.; Kallio-Kokko H.; Österlund P.; Joensuu M.; Meunier F. A.; Butcher S. J.; Winkler M. S.; Mollenhauer B.; Helenius A.; Gokce O.; Teesalu T.; Hepojoki J.; Vapalahti O.; Stadelmann C.; Balistreri G.; Simons M. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J. L.; Simonetti B.; Klein K.; Chen K.-E.; Williamson M. K.; Antón-Plágaro C.; Shoemark D. K.; Simón-Gracia L.; Bauer M.; Hollandi R.; Greber U. F.; Horvath P.; Sessions R. B.; Helenius A.; Hiscox J. A.; Teesalu T.; Matthews D. A.; Davidson A. D.; Collins B. M.; Cullen P. J.; Yamauchi Y. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science 2020, 370, 861–865. 10.1126/science.abd3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J.; Randeva H. S.; Chatha K.; Hall M.; Spandidos D. A.; Karteris E.; Kyrou I. Neuropilin-1 as a new potential SARS-CoV-2 infection mediator implicated in the neurologic features and central nervous system involvement of COVID-19. Mol. Med. Rep. 2020, 22, 4221–4226. 10.3892/mmr.2020.11510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotuhi M.; Mian A.; Meysami S.; Raji C. A. Neurobiology of COVID-19. J. Alzheimer’s Dis. 2020, 76, 3–19. 10.3233/JAD-200581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzini A.; Padovani A. Lifting the mask on neurological manifestations of COVID-19. Nat. Rev. Neurol. 2020, 16, 636–644. 10.1038/s41582-020-0398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge W. M. The Blood-Brain Barrier: Bottleneck in Brain Drug Development. NeuroRX 2005, 2, 3–14. 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni L.; David G.; Mangano A.; Dionigi G.; Rausei S.; Spampatti S.; Cassinotti E.; Fingerhut A. Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg. Endosc. 2015, 29, 2046–2055. 10.1007/s00464-014-3895-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paques M.; Genevois O.; Régnier A.; Tadayoni R.; Sercombe R.; Gaudric A.; Vicaut E. Axon-Tracing Properties of Indocyanine Green. Arch. Ophthalmol. 2003, 121, 367–370. 10.1001/archopht.121.3.367. [DOI] [PubMed] [Google Scholar]
- Matschke J.; Lütgehetmann M.; Hagel C.; Sperhake J. P.; Schröder A. S.; Edler C.; Mushumba H.; Fitzek A.; Allweiss L.; Dandri M.; Dottermusch M.; Heinemann A.; Pfefferle S.; Schwabenland M.; Sumner Magruder D.; Bonn S.; Prinz M.; Gerloff C.; Püschel K.; Krasemann S.; Aepfelbacher M.; Glatzel M. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020, 19, 919–929. 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S. Catch me if you can: SARS-CoV-2 detection in brains of deceased patients with COVID-19. Lancet Neurol. 2020, 19, 883–884. 10.1016/S1474-4422(20)30371-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastorakos P.; McGavern D. The anatomy and immunology of vasculature in the central nervous system. Sci. Immunol. 2019, 4, eaav0492 10.1126/sciimmunol.aav0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anoop U. R.; Verma K. A New Technique and Device for Controlled and Continuous Drug Delivery into the Brain – A Proof of Concept Study. BMJ Innov. 2021, 0, 1–8. 10.1101/746966. [DOI] [Google Scholar]
- Wang M.; Cao R.; Zhang L.; Yang X.; Liu J.; Xu M.; Shi Z.; Hu Z.; Zhong W.; Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S.-W.; Kuo Y.-H.; Liao K.-F. Chronic hydroxychloroquine exposure and the risk of Alzheimer’s disease. Ann. Rheum. Dis. 2019, 10.1136/annrheumdis-2019-216173. [DOI] [PubMed] [Google Scholar]
- Richardson P. J.; Ottaviani S.; Prelle A.; Stebbing J.; Casalini G.; Corbellino M. CNS penetration of potential anti-COVID-19 drugs. J. Neurol. 2020, 267, 1880–1882. 10.1007/s00415-020-09866-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyoju S.; Baral B.; Jha P. Mitigation of Symptomatic SARS-CoV-2 with Clonidine: A Case Series from Nepal. ’A Neuromodulatory Approach to Prevent Complication and Death’. SSRN Electron. J. 2020, 10.2139/ssrn.3743118. [DOI] [Google Scholar]
- Naughton S. X.; Raval U.; Pasinetti G. M. Potential Novel Role of COVID-19 in Alzheimer’s Disease and Preventative Mitigation Strategies. J. Alzheimer’s Dis. 2020, 76, 21–25. 10.3233/JAD-200537. [DOI] [PMC free article] [PubMed] [Google Scholar]