Abstract
Study Objectives:
We aimed (1) to investigate the effect of a 12-week exercise training on sleep quality and heart rate variability in middle-aged and older adults with poor sleep quality and (2) to examine the factors associated with the improvements in sleep quality and heart rate variability parameters.
Methods:
Forty adults aged ≥ 40 years with poor sleep quality (mean age = 62 years; 82.5% female) participated in this study. They were randomized into the exercise group or control group. Each exercise training program consisted of 40 minutes of supervised aerobic exercise training and 10 minutes of stretching class, 3 times a week for 12 weeks. Outcome measures included both subjective (Pittsburgh Sleep Quality Index) and objective (actigraphy recordings) sleep quality assessments, a cardiopulmonary exercise test, and heart rate variability assessment.
Results:
The exercise group showed significant improvements in the global score (P = .003), on all subscales of Pittsburgh Sleep Quality Index (P < .05), and in some heart rate variability parameters compared to the control group. Multiple regression analysis indicated that exercise participation was associated with either the sleep quality (β = −0.617, R2 = .407; F = 6.226, P < .001) or heart rate monitor high frequency normalized units (β = 0.503, R2 = .225; F = 3.200, P = .003) after adjustment for basic characteristics. However, the statistical significance between exercise participation and heart rate monitor high frequency normalized units diminished after controlling for the Pittsburgh Sleep Quality Index.
Conclusions:
Our results indicated that moderate-intensity exercise training had a beneficial effect on sleep quality and cardiac autonomic function. Middle-aged and older adults with poor sleep quality should be encouraged to engage in a moderate-intensity aerobic exercise training to improve their sleep quality and cardiac autonomic function.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Name: The Effects of Exercise Training in Community-dwelling Elderly With Sleep Disturbances With Follow-up; URL: https://clinicaltrials.gov/ct2/show/NCT03005990; Identifier: NCT03005990.
Citation:
Tseng T-H, Chen H-C, Wang L-Y, Chien M-Y. Effects of exercise training on sleep quality and heart rate variability in middle-aged and older adults with poor sleep quality: a randomized controlled trial. J Clin Sleep Med. 2020;16(9):1483–1492.
Keywords: elderly, exercise training, heart rate variability, sleep quality
BRIEF SUMMARY
Current Knowledge/Study Rationale: Exercise training has been suggested as an alternative or complementary approach to existing therapies for middle-aged and older adults with poor sleep quality. However, the effect of exercise training on cardiac autonomic function for this population is yet to be fully elucidated.
Study Impact: Moderate-intensity exercise training significantly improves sleep quality and normalizes cardiac autonomic function. It is suggested that middle-aged and older adults with poor sleep quality should be encouraged to implement moderate-intensity aerobic exercise training to improve their sleep quality and cardiac autonomic function.
INTRODUCTION
Poor sleep quality is one of the most common difficulties facing middle-aged and older adults. The prevalence of poor sleep quality ranges from 3.9% to 40%.1,2 Despite the availability of efficacious pharmacologic3 and nonpharmacologic sleep interventions,4,5 fewer than 15% of patients with chronic insomnia receive treatment or consult a health care provider.6 Poor sleep quality has a significant negative impact on mental and physical health,7 impairs the quality of life, and increases health care costs.8 Inadequate sleep can lead to increased fatigue and excessive daytime sleepiness.9 Among other deleterious effects, it can also impair the metabolic, endocrine, and immune systems.10,11 Previous studies have demonstrated that poor sleep quality has deleterious effects on the incidence of cardiometabolic diseases, quality of life, and mortality.11,12
Sympathetic hyperactivity has been suggested as a factor underpinning the association of older adults with poor sleep quality and the development of cardiovascular disease. However, the pathophysiology of how sustained sympathetic hyperactivity affects cardiovascular function in older adults with poor sleep quality is complex and not completely understood. Heart rate variability (HRV) provides a noninvasive measurement of the cardiac autonomic regulation of the heart.13 Reduced HRV is associated with increased risk of cardiac events and death in healthy individuals.14,15 Individuals with poor sleep quality have lower HRV than those with good sleep.16 Little is known about whether poor sleep quality adversely affects HRV. It is necessary to investigate the possible bidirectional relationship between sleep quality and cardiac autonomic function.
Previous studies found that the effects of cognitive behavioral therapy are sustained better over time than those of pharmacological interventions. However, the main disadvantage of the former approach is the lack of wide availability because it must be done by highly trained specialists.17 Exercise programs that are a safe, low-cost, and effective way to provide short- and long-term physical and psychological benefits are also recommended as an alternative and complementary strategy to prevent and treat sleep disorders.18 A previous meta-analysis demonstrated that increasing regular exercise has a moderately beneficial effect on sleep quality in middle-aged and older adults with sleep complaints.19 However, most studies have measured sleep quality only subjectively using the Pittsburgh Sleep Quality Index (PSQI) questionnaire. In addition, the effects of exercise on autonomic modulation were not established.
Since decreased HRV has been recognized as a cardiovascular risk, improvement of HRV has become important. Although cross-sectional reports have suggested that regular exercise is associated with improved HRV, previous studies examining the effect of exercise on HRV have been inconclusive.20,21 Some studies revealed that moderate-to-vigorous–intensity aerobic exercise training in the elderly improved autonomic modulation.22,23 However, other studies reported no change in HRV in the elderly after an exercise intervention.24,25 In addition, few studies conducted in middle-aged and older adults with poor sleep quality have explored the effects of HRV improvements after exercise intervention.25 Therefore, the aims of this study were: (1) to investigate the effect of a 12-week exercise training intervention on sleep quality and HRV in middle-aged and older adults with poor sleep quality and (2) to examine the factors associated with the improvements in sleep quality and HRV parameters.
METHODS
Study design
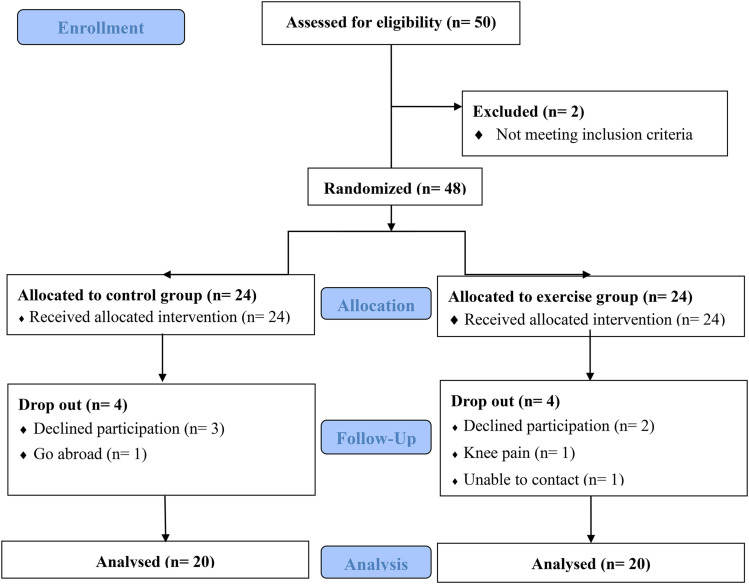
An assessor-blinded, parallel-group, randomized controlled trial was undertaken with participants who complained of poor sleep quality. The study was conducted from January 2017 to October 2017. Participants were randomly allocated to an experimental group or a control group using a concealed allocation schedule (Figure 1). Participants in the experimental group received a 12-week hospital-based exercise program. Participants in the control group received a 1-hour educational session on sleep hygiene. Data were collected 12 weeks later by a tra-ined assessor before randomization.
Figure 1. A flowchart of the patients enrolled in this study.
Participants and setting
Poster campaigns were used to recruit volunteers from communities in several districts of Taipei, Taiwan. We invited middle-aged and older community-dwelling adults (aged ≥ 40 years) with poor sleep quality (PSQI > 5) to participate in this study at Exercise Physiology Laboratory, School and Graduate Institute of Physical Therapy, National Taiwan University, Taipei, Taiwan. Volunteers were ineligible to participate if they had any of the following conditions: (a) uncontrolled cardiovascular diseases or systemic diseases that contraindicate exercise (eg, hypertension, diabetes, unstable angina, myocardial infarction, and cardiac arrhythmia); (b) a clinically diagnosed primary sleep disorder (eg, sleep apnea and restless leg syndrome); (c) a clinically diagnosed psychiatric disorder (eg, depression); (d) any ongoing medication that has a documented effect on the autonomic nervous system (eg, β-blockers); and (e) regular physical exercise (more than twice per week) over the previous 3 months. Participants provided written informed consent before participation. This study was approved by the Taiwan University Institutional Review Board and the institutional ethics committee.
A trained physical therapist collected the data according to standard protocols. All volunteers were interviewed using a structured questionnaire designed to elicit basic information. Baseline exercise testing was performed on all participants to determine their individual exercise capacities and provide them with tailored exercise prescriptions. Assessments included body composition, self-reported and objective sleep quality, exercise capacity, HRV analysis, and physical activity measurements. Participants were randomly assigned to a 12-week aerobic exercise group or the control group. All participants were instructed to not change their dietary intake, daily activity, and medication during the experimental period. The measurements were collected at baseline and after intervention.
Outcome measures
Sleep quality
Sleep quality was measured using the Chinese version of the Pittsburgh Sleep Quality Index (CPSQI) and an actigraph.26 The sum of 7 component scores serves as the global PSQI score (ranging from 0 to 21). Scores > 5 are indicative of poor sleep quality. The PSQI is a widely used self-rated questionnaire for measuring sleep quality with good reliability and validity. The CPSQI had an overall reliability coefficient of 0.82–0.83, and a CPSQI > 5 resulted in a sensitivity and specificity of 98% and 55%, respectively.27
Actigraph is a noninvasive method of monitoring human rest/activity cycles and sleep quality. The participants were instructed to wear the actigraph (Actiwatch-2, Respironics, Inc., Murrysville, PA) for 5 consecutive days, regardless of whether it was during the week or the weekend. Data were analyzed using the Actiware 5.0 software, which evaluates the parameters of the activity/rest cycle. The following parameters were evaluated: total sleep time over each 24-hour period, sleep latency, sleep efficiency, wake after sleep onset, and time to sleep onset. Corresponding sleep diaries, where completed, were used to indicate bedtimes (intraclass correlation = 0.71–0.85).28
Body composition
Body composition was then measured by a bioelectrical impedance analyzer (Maltron BioScan 920, Maltron International, Rayleigh, Essex, UK) using an 800-μA current at a frequency of 50 kHz. The participants were asked to lie in the supine position on a nonconducting surface for 5 minutes with their arms abducted away from their trunk and their legs slightly separated. Four surface electrodes and cables were attached to participant’s right hand and ankle, as shown in the user’s manual. When the measurements had stabilized, the analyzer calculated the percentage of body fat directly from the equation and displayed the value. Previous studies have demonstrated excellent test-retest reliability and validity of measurements obtained by bioelectrical impedance analyzer.29
Cardiac autonomic dysfunction
Cardiac autonomic dysfunction was measured by assessing the resting HRV. Participants were also asked to avoid caffeine, alcohol, smoking, and heavy physical activity for 12 hours prior to testing to control for confounding factors that could alter HRV. Because of diurnal fluctuations in HRV, testing was performed between 14:00 and 17:00 in a comfortable, temperature-controlled (25°C) room with dimmed lighting and absent distraction from noise to minimize interference. After a participant was supine quietly for 20 minutes, a lead I electrocardiogram was taken for 5 minutes while the participant remained supine quietly and breathed normally. An HRV monitor (8Z11, Wegene Technology Inc., Taiwan) acquired and processed electrocardiogram signals in this study. Selected parameters of HRV were standard deviation of all normal to normal intervals (SDNN), power in low frequency (LF; 0.04–0.15 Hz), power in high frequency (HF; 0.15–0.40 Hz), and LF/HF ratio. It is generally accepted that HF is mediated by variations in parasympathetic activity, and the LF power reflects both parasympathetic and sympathetic modulations.30 The raw data of the measures were used in the study. In addition, the LF and HF oscillatory components are also presented in normalized units (nu) (LFnu and HFnu). The normalized unit expresses the power centered in the frequency of interest divided by total power less very-low-frequency power.
Exercise capacity
Each participant performed a symptom-limited maximal exercise test with continuous electrocardiogram monitoring on a cycle ergometer. The criteria for termination included at least 2 of the following: volitional fatigue, heart rate ≥ 90% of the age-predicted maximum, and a respiratory exchange ratio > 1.15. The graded exercise protocol comprised 3-minute stages starting at 0 W, with 25-W increments at each successive stage while maintaining a cadence of 50–60 revolutions per minute. Modified Borg effort scales (0–10) were recorded at the peak exercise intensity. Respiratory gas exchange measurements, including minute ventilation at peak exercise (VEpeak) and peak oxygen consumption (VO2peak), were determined during exercise using a computer-controlled breath-by-breath metabolic measurement system (Vmax29 Metabolic Measurement System, SensorMedics, Anaheim, CA). The ventilation threshold was determined using the V-slope method.31
Depression
Depression was assessed using a validated Chinese version of the short form of the Geriatric Depression Scale (CGDS).32 This tool focuses on nonsomatic symptoms of depression and emphasizes cognitive symptoms. The CGDS includes 15 yes/no self-report questions wherein the participant is expected to circle the appropriate responses and takes approximately 10 minutes to complete. A resulting total score of ≥ 5 is indicative of depression. The overall sensitivity and specificity of GDS are 0.97 and 0.95, respectively, and the intraclass coefficient of test-retest reliability over 2 weeks is 0.83.33
Physical activity
Physical activity was measured by the Chinese version of the 7-day recall physical activity questionnaire. Questions included the number of hours spent sleeping and the level of activity intensity (moderate, hard, and very hard) during the past one week. The time spent in mild activities was obtained by subtracting the sum of the above categories from 168 hours. The metabolic equivalent of task (MET) values used to estimate energy expenditure for different activities were as follows: sleep = 1, mild activities = 1.5, moderate activities = 4, hard activities = 6, and very hard activities = 10. The mean daily energy expenditure was then determined by multiplying the MET values by the hours spent in each of the 5 categories of activity. The test-retest reliability of the Chinese version of 7-day recall physical activity questionnaire was moderate to good in patients with osteopenia and cardiac disease (intraclass correlation = 0.52–0.90).34
Intervention
The program comprised three 50-minute exercise sessions per week over a 12-week period. Participants in the exercise group were required to attend the exercise classes held in our laboratory and conducted by an experienced physical therapist. The presence of the physical therapist allowed for individual monitoring of the progress of the participants. Each exercise session included graded treadmill walking and stretching exercises. The treadmill walking exercise began with a 5-minute warm-up period with approximately 40% intensity of VO2peak; this was followed by a 30-minute training phase. The training intensity was set at about 50–60% of VO2peak and ended with a 5-minute cool-down period at approximately 40% intensity of VO2peak. Participants in the control group did not participate in any supervised exercise and were asked not to change their physical activity habits during the study.
Statistical analysis
Statistical analyses were performed using statistical software for social sciences (SPSS) statistical package, v.19.0 for Windows (SPSS Inc., Chicago, IL). Normal distribution of data was assured using the Shapiro-Wilk test. Otherwise, nonparametric statistics were used. Between-group comparisons at baseline were performed using independent Student’s t test, Mann-Whitney U test, or chi-square test. Two-way repeated-measures analysis of variance was used to analyze the between-group differences of all parameters. Pearson’s correlation coefficient was used to test the correlations between the outcome parameters. Multiple regression analysis was used to test the association among exercise, sleep quality, and HRV. PSQI global score and HFnu were considered as dependent variables, respectively. We used an extended-model approach for covariate adjustment: model 1 = exercise + basic characteristics (age, gender, and the changes of depression score and medication use); then, PSQI and HFnu were subsequently introduced as covariates in model 2. The α level was set at 0.05.
RESULTS
Participant characteristics
Forty-eight eligible volunteers participated in this study and were randomized to exercise and control groups. Twenty participants (83.3%) completed the 12-week training program and 4 dropped out (1 due to other medical complaints unrelated to the exercise regimen and 3 due to personal reasons). Four participants in the control group did not complete the final test due to personal reasons (3 due to family-related factors and 1 relocated out of the area). Consequently, 40 participants (33 women and 7 men; mean age, 61.7 ± 7.0 years) were included, and 90% of them were older than 55 years. The attendance of participants in exercise training was 98.1 ± 4.1%, implying that the participants in our study met the training target.
Table 1 lists the basic characteristics as well as clinical features of all participants. Eleven (27.5%) participants (4 in the control group and 7 in the exercise group) reported using hypnotics (brotizolam, estazolam, lorazepam, alprazolam, zolpidem, and zopiclone). There were no significant differences in any of the basic characteristics between the two groups (P > .05).
Table 1.
Characteristics of all participants.
| Control Group (n = 20) | Exercise Group (n = 20) | P Value | |
|---|---|---|---|
| Age (years) | 62.2 (7.4) | 61.1 (6.8) | .634 |
| <55 years (n, %) | 2, 10% | 2, 10% | 1.000 |
| Men (n, %) | 2, 10% | 5, 25% | .222 |
| Body height (cm) | 157.5 (0.1) | 158.8 (0.1) | .628 |
| Body weight (kg) | 59.1 (9.3) | 61.1 (14.5) | .615 |
| Body mass index (kg/m2) | 23.7 (2.8) | 24.0 (4.0) | .792 |
| Weekly energy expenditure (kcal) | 2025.1 (307.3) | 2058.5 (509.6) | .803 |
| Smoking (never) (n, %) | 100% | 95% | .324 |
| Alcohol consumption (never) (n, %) | 65% | 60% | .628 |
| Use of sleeping medication (n, %) | 4, 20% | 7, 35% | .300 |
| Morbidity (n, %) | |||
| Hypertension | 4, 20% | 3, 15% | .687 |
| Diabetes | 3, 15% | 3, 15% | .442 |
| Hyperlipidemia | 1, 5% | 2, 10% | .561 |
Effects of exercise training on exercise capacity and depressive symptoms
Table 2 shows the results of exercise training on body composition, exercise capacity, and depression. None of the baseline variables showed significant differences between groups. In the exercise group, there were significant increases in VO2peak, predicted values of VO2peak, and O2 pulse, and significant decreases in systolic blood pressure and GDS after exercise training (P < .05), whereas no improvements were found in any of the variables in the control group. The predicted values of VO2peak were significantly decreased in the control group as well (P < .05). In addition, depressive symptoms as assessed by Chinese version of the short form of the Geriatric Depression Scale were also shown to be significantly decreased in the exercise group (P = .04). There were no significant differences between groups in other parameters.
Table 2.
The effect of exercise training on body composition, exercise capacity, and depression symptoms.
| Control Group (n = 20) | Exercise Group (n = 20) | P Value | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Time per Group | Time | Group | |
| Body weight (kg) | 59.1 (9.3) | 59.2 (9.3) | 61.1 (14.5) | 60.9 (14.6) | .703 | .889 | .637 |
| Body mass index (kg/m2) | 23.7 (2.8) | 23.7 (2.8) | 24.0 (4.0) | 24.0 (4.0) | .943 | .705 | .797 |
| Fat (%) | 30.6 (8.0) | 31.2 (8.2) | 27.3 (9.0) | 28.1 (7.3) | .869 | .197 | .210 |
| HR (bpm) | 69.6 (6.2) | 71.6 (8.8) | 70.7 (9.3) | 69.0 (9.7) | .202 | .908 | .735 |
| SBP (mm Hg) | 120.2 (18.5) | 116.9 (12.9) | 126.3 (15.7) | 119.5 (13.6)* | .441 | .029 | .322 |
| DBP (mm Hg) | 74.5 (10.7) | 69.7 (8.8) | 75.7 (12.6) | 70.9 (8.6) | .988 | .008 | .661 |
| Workload (watt) | 87.5 (19.0) | 81.3 (16.0) | 87.5 (15.2) | 88.8 (15.1) | .109 | .281 | .425 |
| Peak HR (bpm) | 138.9 (10.0) | 139.9 (8.9) | 137.6 (9.4) | 138.0 (10.9) | .679 | .278 | .606 |
| Peak VO2 (ml/kg/min) | 19.02 (4.18) | 17.50 (3.39) | 19.24 (4.31) | 21.65 (4.60)* | .001 | .418 | .075 |
| Predicted VO2 (%) | 76.75 (13.88) | 70.40 (14.04)* | 76.35 (19.59) | 85.90 (21.00)* | <.001 | .431 | .149 |
| Peak VE (liters/min) | 41.09 (8.45) | 39.30 (8.17) | 44.50 (10.97) | 47.95 (13.70) | .068 | .556 | .054 |
| O2 pulse (ml/beat) | 8.00 (1.85) | 7.42 (1.77) | 8.71 (2.80) | 9.54 (3.24)* | .009 | .621 | .065 |
| RER (%) | 1.22 (0.11) | 1.25 (0.12) | 1.19 (0.11) | 1.25 (0.13) | .665 | .039 | .679 |
| Energy expenditure (kcal/wk) | 2025.1 (307.3) | 2011.8 (302.5) | 2058.5 (509.6) | 2066.3 (509.5) | .277 | .773 | .742 |
| Geriatric Depression Scale | 2.0 (2.4) | 1.9 (2.3) | 3.7 (4.0) | 2.2 (3.1)* | .090 | .070 | .273 |
Significant difference within group (P < .05). DBP = diastolic blood pressure, HR = heart rate, RER = respiratory exchange ratio, RQ = respiratory quotient, SBP = systolic blood pressure, VO2 = maximal oxygen uptake, VE = minute ventilation.
Effects of exercise training on subjective and objective sleep quality
We assessed sleep quality using both the subjective PSQI questionnaire and the objective actigraphy recording (Table 3). There was no significant difference in the baseline data between the 2 groups (P > .05). In addition, there was no report of long pauses between breaths while asleep, legs twitching or jerking while asleep, episodes of disorientation or confusion during sleep, and other restlessness during sleep in both groups. There were 3 in the control group and 3 in the exercise group who had loud snoring as reported by roommate or bed partner (P = .442).
Table 3.
The effect of exercise training on quality of sleep.
| Control Group (n = 20) | Exercise Group (n = 20) | P Value | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Time per Group | Time | Group | |
| Pittsburgh Sleep Quality Index | |||||||
| Global score | 11.5 (3.1) | 11.0 (3.2) | 13.3 (3.9) | 5.8 (2.9)* | <.001 | <.001 | .065 |
| Global score > 5 (n, %) | 20, 100% | 19, 95% | 20, 100% | 9, 45%* | .003 | <.001 | .003 |
| Sleep duration | 2.6 (0.8) | 2.1 (1.0) | 2.2 (1.2) | 1.0 (0.9)* | <.001 | <.001 | .059 |
| Sleep disturbances | 1.4 (0.5) | 1.3 (0.6) | 1.7 (0.6) | 1.1 (0.2)* | .003 | .001 | .846 |
| Sleep latency | 2.0 (0.8) | 1.9 (0.8) | 2.1 (0.8) | 0.9 (0.7)* | <.001 | <.001 | .050 |
| Daytime dysfunction | 0.90 (0.7) | 0.9 (0.9) | 1.4 (0.9) | 0.6 (0.8)* | .008 | .008 | .733 |
| Sleep efficiency | 2.4 (1.0) | 2.4 (1.2) | 2.4 (1.2) | 0.4 (0.8)* | <.001 | <.001 | .002 |
| Subjective sleep quality | 1.9 (0.6) | 1.9 (0.6) | 2.3 (0.6) | 1.3 (0.6)* | <.001 | <.001 | .053 |
| Use of sleeping medication | 0.9 (1.2) | 0.8 (1.1) | 1.5 (1.4) | 0.7 (1.1)* | .007 | .002 | .002 |
| Self-reported sleep duration (hrs) | 4.8 (1.1) | 5.2 (1.2) | 4.6 (1.6) | 6.1 (1.1)* | .012 | <.001 | <.001 |
| Self-reported sleep latency (mins) | 29.3 (25.5) | 28.0 (17.7) | 29.1 (21.8) | 11.4 (7.8)* | .028 | .012 | .101 |
| Actigraph | |||||||
| Sleep onset latency (mins) | 23.0 (13.7) | 29.5 (17.3) | 24.6 (16.2) | 16.3 (8.2)* | .004 | .694 | .151 |
| Sleep efficiency (%) | 0.74 (0.7) | 0.73 (0.5) | 0.78 (0.12) | 0.82 (0.06)* | .088 | .213 | .009 |
| Total sleep time (mins) | 335.1 (40.3) | 328.9 (39.4) | 338.2 (42.3) | 357.9 (86.6) | .271 | .564 | .261 |
| Wake after sleep onset (mins) | 98.6 (31.2) | 94.5 (29.0) | 72.2 (31.8) | 72.6 (27.6) | .500 | .575 | .012 |
Significant difference within group (P < .05).
The average global index score of PSQI at baseline was around 12.4, indicating that our participants had moderate-to-severe degree of poor sleep quality. The exercise group showed significant improvements in total scores and all subscales of PSQI questionnaire (P < .001). It was worth noting that there were 11 participants (55%) in the exercise group with PSQI global sleep score < 5 (represented as good sleep quality) after exercise training, whereas all participants in the control group were still identified as having poor sleep. In addition, self-reported sleep duration was significantly increased by 1.5 hours, and self-reported sleep-onset latency was significantly reduced by nearly 18 minutes after 12 weeks of training in the exercise group. No significant improvement was found in any scale in the control group.
Sleep quality was also assessed for 5 consecutive nights using objective actigraphy recordings. The results showed that the sleep onset latency decreased significantly from 24.6 ± 16.2 to 16.3 ± 8.2 minutes after exercise training in the exercise group (P = .035), whereas there was no notable reduction in the control group. The exercise group also showed significant post-test differences in sleep efficiency (P = .035), but the control group showed no such differences. However, there were no significant differences noted for total sleep time and waking after sleep onset after 12 weeks of exercise training.
Effects of exercise training on heart rate variability
Table 4 shows the results of exercise training on HRV. There was no significant difference in any HRV parameter at baseline between two groups. Standard deviation of all normal to normal intervals, total power, and raw data of LF and HF (ms2) did not show significant changes after exercise training. The normalized low-frequency (LFnu) in the exercise group was significantly reduced from 62.1 ± 12.2% to 43.3 ± 14.7%, and LF/HF ratio significantly decreased from 2.88 ± 1.84 to 1.06 ± 0.68, which indicated a decrease in sympathetic tone; the normalized high-frequency (HFnu) was significantly increased from 27.7 ± 10.5% to 48.6 ± 13.7%, which indicated an increase in parasympathetic tone. All HRV variables in the control group showed no significant changes.
Table 4.
The effect of exercise training on heart rate variability.
| Control Group (n = 18) | Exercise Group (n = 18) | P Value | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Time per Group | Time | Group | |
| SDNN | 27.7 (14.6) | 26.2 (15.3) | 23.2 (8.6) | 24.4 (8.6) | .370 | .947 | .381 |
| TP (ms2) | 944.4 (960.2) | 877.1 (987.5) | 648.0 (454.5) | 682.9 (404.6) | .493 | .827 | .285 |
| lnTP | 6.4 (1.0) | 6.3 (0.9) | 6.2 (0.8) | 6.3 (0.9) | .493 | .805 | .646 |
| LF (ms2) | 225.2 (367.1) | 247.4 (382.9) | 186.2 (187.0) | 117.8 (109.6) | .141 | .447 | .330 |
| lnLF | 4.8 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.3 (1.0) | .204 | .311 | .387 |
| HF (ms2) | 137.7 (260.6) | 139.1 (258.8) | 78.9 (84.3) | 109.2 (73.7) | .199 | .161 | .462 |
| lnHF | 4.1 (1.2) | 4.2 (1.2) | 3.9 (1.1) | 4.5 (0.8) | .097 | .060 | .937 |
| LFnu (%) | 58.0 (13.3) | 60.5 (11.9) | 62.1 (12.2) | 43.3 (14.7)* | <.001 | .005 | .043 |
| HFnu (%) | 31.6 (11.0) | 33.5 (11.3) | 27.7 (10.5) | 48.6 (13.7)* | .001 | <.001 | .047 |
| LF/HF | 2.35 (1.75) | 1.83 (1.06) | 2.88 (1.84) | 1.06 (0.68)* | .043 | .001 | .707 |
Note: 4 participants (2 for exercise group and 2 for control group) did not complete the post-test evaluation. Missing data had to be accounted for in the analysis through the use of baseline in a last observation carried forward imputation. *Significant difference within group (P < .05). HF = high frequency, LF = low frequency, LF/HF = low frequency/ high frequency, ln = log transformed, nu = normalization, SDNN = standard deviation of all normal to normal intervals, TP = Total power.
The factors associated with sleep quality and HRV improvements
The Pearson’s correlation coefficient was used to examine the changes in PSQI scores and HRV parameters in all participants. The results showed that the changes in LFnu correlated significantly with the PSQI global score and subscales of sleep quality and medicine use (r = .35–.37, P < .005). The changes in HFnu were also shown to be correlated significantly with sleep disturbance (r = −.38, P < .005). The LF/HF correlated significantly with sleep disturbances and daytime dysfunction (r = .37 and .34, respectively, P < .05).
Multiple regression analysis was used to examine the factors associated with sleep quality and HRV (Table 5). Exercise participation was associated with sleep quality (β = −0.617, R2 = 0.407; F = 6.226, P < .001) and HFnu (β = 0.503, R2 = 0.225; F = 3.200, P = .003) after adjustment for basic characteristics (model 1). Furthermore, PSQI and HFnu were subsequently introduced as covariates in model 2. The statistical significance persisted between exercise participation and PSQI (β = −0.522, R2 = 0.402; F = 5.261, P = .002) after controlling for HFnu. However, the statistical significance between exercise participation and HFnu (β = 0.399, R2 = 0.218; F = 2,762, P = .057) diminished after controlling for PSQI, which suggests that the association between HRV and exercise participation is partially explained by the improvement of sleep quality.
Table 5.
Multiple regression testing the association among exercise, sleep quality and HRV.
| Dependent Variable | Independent Variable | Adjusted R2 | Unadjusted R2 | Normalized β | P Value | |
|---|---|---|---|---|---|---|
| β (SD) | 95% CI | |||||
| PSQI global score | Exercise | 41.9% | −5.150 (0.954) | −7.082, −3.218 | −5.396 | <.001 |
| Model 1 | 40.7% | −4.702 (1.064) | −6.866, −2.538 | −0.617 | <.001 | |
| Model 2# | 40.2% | −4.208 (1.218) | −6.690, −1.727 | −0.552 | .002 | |
| HFnu | Exercise | 25.7% | .151 (0.040) | .070, .231 | 0.525 | .001 |
| Model 1 | 22.5% | .146 (0.046) | .052, .240 | 0.503 | .003 | |
| Model 2## | 21.8% | .116 (0.059) | −.004, .235 | 0.399 | .057 | |
Model 1 was controlled for basic characteristics including age, gender, exercise, the change of depression score, and medication use. Model 2# was Model 1 + HFnu; Model 2## was Model 1 + PSQI score.
DISCUSSION
The present study demonstrated that 12 weeks of moderate aerobic exercise training significantly affects sleep quality and normalizes cardiac autonomic function in middle-aged and older adults with poor sleep quality. Exercise training improves HRV by decreasing sympathetic tone and increasing vagal tone; all contribute to the reduction in heart disease. Furthermore, we found that the relationship between exercise and HRV was mediated by improvements of sleep quality. More studies are needed to verify our findings.
Regular exercise is a nonpharmacological treatment for people with poor sleep quality. Our previous meta-analysis summarized several randomized controlled trials and showed that exercise training has a moderate, beneficial effect on subjective sleep quality in middle-aged and older adults with sleep complaints.19 The present study used the PSQI questionnaire to assess subjective sleep quality and showed a significant improvement (drop by 56%) after exercise training. This was higher than the previously reported rates of 39–48%.35,36 The greater effect seen in our study may be a result of the participants having moderate-to-severe degrees of poor sleep quality. In addition to the global PSQI score, both the sleep latency subscore of the PSQI and self-reported sleep latency were significantly improved in our study. Previous studies reported that sleep latency increased with age, which negatively affected sleep quality and quantity.37 The PSQI sleep latency subscore reflects both sleep latency and the frequency of being unable to fall asleep within 30 minutes. Therefore, it is possible that along with the reduction in the time taken to fall asleep, the frequency of experiencing sleep-related complaints was reduced as well in our study. Sleep efficiency in our study showed improvements in either subjective (PSQI) or objective (actigraph) measurement. The total sleep duration of the exercise group also significantly increased in the present study. However, there is not much known about the effects of exercise on sleep duration. Neither King35 nor Elavsky36 found any changes in sleep duration after moderate-intensity aerobic exercise training. Conversely, Reid reported that the self-reported sleep duration significantly increased by 1.25 hours.38 In our study, the self-reported sleep duration showed a mean increase of 1.5 hours in the exercise group. This marked increase in sleep duration may be due primarily to the participants who habitually slept for less than 5 hours a night, which was verified using actigraphy and from participants’ self-reported sleep duration. It was speculated that reduced sleep disturbances and shorter self-reported sleep latency period of PSQI led to an increase in the total sleep duration. However, there are discrepancies in total sleep time between self-reports by questionnaire and actigraphy-measured data. The reason might be due to their different measurement principles. Actigraphy could incorrectly classify those awake but lying motionless as being asleep, and self-reporting by PSQI questionnaire may have recall bias.
Previous studies have reported that regular exercise training can improve cardiac autonomic function in patients with cardiovascular diseases.39 Our findings are in agreement with previous studies that have reported significant improvements in frequency-domain markers of vagal modulation with exercise training, suggesting that positive benefits of regular exercise on the autonomic balance may be an additional mechanism through which exercise provides cardiovascular disease benefit.40,41 A relatively short period of exercise (2 months) at moderate intensity is reportedly sufficient to induce significant changes in HRV in older adults.42 In addition, longer exercise protocols and higher intensities also seem to have some positive effect. Jurca found the effectiveness of aerobic exercise on all vagal-HRV indices during 8 weeks, 3–4 days per weeks at 50% of VO2max in sedentary postmenopausal women.43 Moreover, Shen and Wen showed that longer (10 weeks) and more intense exercise (75–85% VO2max) in sedentary postmenopausal women had a greater effect on their HRV.44 Even though both the weekly time commitment and exercise intensity were well within the consensus recommendation of 150–180 minutes of moderate-intensity exercise per week,39 substantial improvements in HRV were observed. The effect of exercise training on HRV was also moderated by age.45 Kuo reported that parasympathetic regulation declines after 50 years of age, whereas the disappearance of sympathetic dominance is significantly delayed in men.46 Sandercock found that exercise training results in significant increases in the RR interval (the time between heartbeats) and HF, which indicates an increase in the parasympathetic tone.47 However, these changes are influenced by the age of the study population. The effects on HF and the RR interval in our older adults were relatively marginal.
The exact mechanisms underlying the modification of HRV by exercise training remain unknown. Few studies have found that exercise can increase brain-derived neurotrophic factor expression in many brain regions and can also reduce the resting heart rate by increasing parasympathetic activity.48 The adaptation to aerobic training associated with enhanced cardiac vagal activity in middle-aged and older adults with poor sleep quality may be influenced by several metabolic, biochemical, hormonal, and neural changes in the body. An adequate exercise training program may reduce daily stress, lower the resting heart rate, and improve cardiorespiratory capacity, all of which are associated with increases in the cardiac vagal tone. Exercise has a beneficial effect on several risk factors for cardiovascular disease, and this may be associated with improvements in the autonomic balance. Further studies are needed to define the mechanisms that underlie these interactions.
The present study also found a significant correlation between improvements in sleep quality and improvements in HRV (r = .35–.49). Werner et al reported an association between higher resting cardiac vagal tone (HF-HRV) and subjective/objective sleep quality (eg, shorter sleep latency and fewer arousals).49 These findings indicated that a high waking cardiac vagal tone may be a key predictor of healthy sleep. We further used multiple regression analysis to test the relationship between exercise intervention and improvements in sleep quality and HRV. It is interesting that we found the relationship between exercise intervention and improvement in HRV to be moderated by sleep quality improvement, whereas the relationship between exercise intervention and sleep quality improvement was not influenced by improved HRV. It seemed that exercise intervention improved HRV partially by improving the sleep quality. Considering the relatively small sample size, these complex associations remain to be further explored. Future research should use experimental designs or longitudinal assessments to more directly examine the causal direction between sleep quality and HRV.
The present study showed that a moderate-intensity exercise program had beneficial effects on sleep quality, exercise capacity, and the HRV parameters. However, the relationship among these outcomes is complex and not yet completely understood. The sleep-wake (circadian rhythm) pattern coupled with changes in heart rate and blood pressure are induced by declining sympathetic and increasing parasympathetic activity during slow-wave sleep at night. Poor sleep quality and particularly sleeplessness induces a well known constant sympathetic overactivity associated with an increased risk of cardiovascular and metabolic diseases.50 Exercise training was reported to improve sleep quality through its effects on body temperature, sleep debt, and general relaxation.51 Exercise training could also modify sympathovagal balance by stimulating the sympathetic nervous system widely and gradually returning it to pre-exercise values. Our study showed regular exercise improves both sleep quality and sympathovagal function in participants with poor sleep quality. A positive correlation existed between these two outcomes (ie, improvements in sleep quality and sympathovagal function), although the causal relationship could not be assured at this point. The results of this study imply that middle-aged and older adults with poor sleep quality who engage in a moderate-intensity aerobic exercise training could improve their sleep quality and cardiac autonomic function in the short-term. Future large-scale, longitudinal studies are needed to further examine mechanisms that underlie these links.
One strength of our study is that all participants underwent the standard cardiopulmonary exercise testing to obtain their VO2peak, and all exercise training sessions were performed under supervision. The VO2peak derived from cardiopulmonary exercise testing provided the basis for more precise and safe exercise prescriptions. Few previous studies showed real-measured exercise capacity of participants with poor sleep quality; our study provided important basic information for exercise prescription in this population.
We recognize a number of limitations in this study. First, the majority of our participants were postmenopausal women, so to generalize the results of this study to all adults should be undertaken with caution. Despite the effect of gender imbalance on the generalizability of the study, our findings remain relevant to older adults with poor sleep quality because poor sleep is more prevalent among women than men. Second, we excluded patients with possible sleep disorders without the standard test (polysomnography, PSG). Since PSG testing was not scheduled, we could only use medical history inquiry or previous PSG testing as the diagnostic basis. We also used the section in the PSQI questionnaire completed by the person’s bed partner/ roommate to screen for sleep-disordered breathing. In our data, 7 participants who were using sleep aids did not show any symptoms of apnea or restless legs in the past month. Third, the participants were allowed to take sleeping aids during interventions, but all participants were instructed to not change their dietary intake and medication during the experimental period. This minimized the influence of medication on this study. There were no increases of medication dosage required by participants during the study period. In addition, the results of linear regression analysis revealed that exercise was an independent predictor of sleep quality improvement, even though medicine use was used as a covariate. These results indicate that exercise training has an independent effect on sleep quality improvements. Fourth, sleep quality was measured by the PSQI scale without corroboration by clinical measurement (PSG), thus the possibility of recall bias cannot be ruled out completely.
In conclusion, our findings indicate that moderate-intensity exercise training has a significant effect on sleep quality and normalizes cardiac autonomic function. Furthermore, we found that the association between exercise training and HRV may be strongly mediated by sleep quality improvements. Therefore, these findings underscore the need for prospective studies exploring a specific prevention or treatment regimen for middle-aged and older adults with poor sleep quality to reduce their cardiac autonomic dysfunction.
DISCLOSURE STATEMENT
All authors have read and approved the manuscript. The authors declare no conflicts of interest.
ABBREVIATIONS
- HF
power in high frequency
- HRV
heart rate variability
- LF
power in low frequency
- nu
normalized units
- PSQI
Pittsburgh Sleep Quality Index
- SDNN
standard deviation of all normal to normal intervals
- VO2peak
peak oxygen consumption
REFERENCES
- 1.Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3(5 suppl):S7–S10. 10.5664/jcsm.26929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kao CC, Huang CJ, Wang MY, Tsai PS. Insomnia: prevalence and its impact on excessive daytime sleepiness and psychological well-being in the adult Taiwanese population. Qual Life Res. 2008;17(8):1073–1080. 10.1007/s11136-008-9383-9 [DOI] [PubMed] [Google Scholar]
- 3.Glass J, Lanctot KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ. 2005;331(7526):1169. 10.1136/bmj.38623.768588.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montgomery P, Dennis J. Cognitive behavioural interventions for sleep problems in adults aged 60+. Cochrane Database Syst Rev. 2003;1CD003161. [DOI] [PubMed] [Google Scholar]
- 5.Morin CM, Hauri PJ, Espie CA, Spielman AJ, Buysse DJ, Bootzin RR. Nonpharmacologic treatment of chronic insomnia. An American Academy of Sleep Medicine review. Sleep. 1999;22(8):1134–1156. 10.1093/sleep/22.8.1134 [DOI] [PubMed] [Google Scholar]
- 6.Mellinger GD, Balter MB, Uhlenhuth EH. Insomnia and its treatment. Prevalence and correlates. Arch Gen Psychiatry. 1985;42(3):225–232. 10.1001/archpsyc.1985.01790260019002 [DOI] [PubMed] [Google Scholar]
- 7.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59(2):131–136. 10.1001/archpsyc.59.2.131 [DOI] [PubMed] [Google Scholar]
- 8.Simon G, von Korff M. Prevalence, burden and treatment of insomnia in primary care. Am J Psychiatry. 1997;154(10):1417–1423. 10.1176/ajp.154.10.1417 [DOI] [PubMed] [Google Scholar]
- 9.Bliwise DL. Historical change in the report of daytime fatigue. Sleep. 1996;19(6):462–464. 10.1093/sleep/19.6.462 [DOI] [PubMed] [Google Scholar]
- 10.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5(5):253–261. 10.1038/nrendo.2009.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cappuccio FP, Miller MA. Sleep and cardio-metabolic disease. Curr Cardiol Rep. 2017;19(11):110. 10.1007/s11886-017-0916-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sofi F, Cesari F, Casini A, Macchi C, Abbate R, Gensini GF. Insomnia and risk of cardiovascular disease: a meta-analysis. Eur J Prev Cardiol. 2012;21(1):57–64. 10.1177/2047487312460020 [DOI] [PubMed] [Google Scholar]
- 13.Akselrod S, Gordon D, Madwed JB, Snidman NC, Shannon DC, Cohen RJ. Hemodynamic regulation: investigation by spectral analysis. Am J Physiol. 1985;249H867–H875. [DOI] [PubMed] [Google Scholar]
- 14.Tsuji H, Larson MG, Venditti FJJr, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94(11):2850–2855. 10.1161/01.CIR.94.11.2850 [DOI] [PubMed] [Google Scholar]
- 15.Tsuji H, Venditti FJJr, Manders ES, et al. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation. 1994;90(2):878–883. 10.1161/01.CIR.90.2.878 [DOI] [PubMed] [Google Scholar]
- 16.Bonnet MH, Arand DL. Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med. 1998;60(5):610–615. 10.1097/00006842-199809000-00017 [DOI] [PubMed] [Google Scholar]
- 17.van Straten A, Cuijpers P. Self-help therapy for insomnia: a meta-analysis. Sleep Med Rev. 2009;13(1):61–71. 10.1016/j.smrv.2008.04.006 [DOI] [PubMed] [Google Scholar]
- 18.Youngstedt SD. Effects of exercise on sleep. Clin Sports Med. 2005;24(2):355–365xi. 10.1016/j.csm.2004.12.003 [DOI] [PubMed] [Google Scholar]
- 19.Yang PY, Ho KH, Chen HC, Chien MY. Exercise training improves sleep quality in middle-aged and older adults with sleep problems: a systematic review. J Physiother. 2012;58(3):157–163. 10.1016/S1836-9553(12)70106-6 [DOI] [PubMed] [Google Scholar]
- 20.Melanson EL, Freedson PS. The effect of endurance training on resting heart rate variability in sedentary adult males. Eur J Appl Physiol. 2001;85(5):442–449. 10.1007/s004210100479 [DOI] [PubMed] [Google Scholar]
- 21.Amano M, Kanda T, Ue H, Moritani T. Exercise training and autonomic nervous system activity in obese individuals. Med Sci Sports Exerc. 2001;33(8):1287–1291. 10.1097/00005768-200108000-00007 [DOI] [PubMed] [Google Scholar]
- 22.Schuit AJ, van Amelsvoort LG, Verheij TC, et al. Exercise training and heart rate variability in older people. Med Sci Sports Exerc. 1999;31(6):816–821. 10.1097/00005768-199906000-00009 [DOI] [PubMed] [Google Scholar]
- 23.Uusitalo AL, Laitinen T, Vaisanen SB, Länsimies E, Rauramaa R. Effects of endurance training on heart rate and blood pressure variability. Clin Physiol Funct Imaging. 2002;22(3):173–179. 10.1046/j.1475-097X.2002.00414.x [DOI] [PubMed] [Google Scholar]
- 24.Loimaala A, Huikuri H, Oja P, Vuori I. Controlled 5-mo aerobic training improves heart rate but not heart rate variability or baroreflex sensitivity. J Appl Physiol. 2000;89(5):1825–1829. 10.1152/jappl.2000.89.5.1825 [DOI] [PubMed] [Google Scholar]
- 25.Myslivecek PR, Brown CA, Wolfe LA. Effects of physical conditioning on cardiac autonomic function in healthy middle-aged women. Can J Appl Physiol. 2002;27(1):1–18. 10.1139/h02-001 [DOI] [PubMed] [Google Scholar]
- 26.Tsai PS, Wang SY, Wang MY, et al. Psychometric evaluation of the Chinese version of the Pittsburgh Sleep Quality Index (CPSQI) in primary insomnia and control subjects. Qual Life Res. 2005;14(8):1943–1952. 10.1007/s11136-005-4346-x [DOI] [PubMed] [Google Scholar]
- 27.Buysse DJ, Reynolds CF3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 28.Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. 2011;15(4):259–267. 10.1016/j.smrv.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 29.Patil BR, Patkar DP, Mandlik SA, Kuswarkar MM, Jindal GD. Single prediction equation for bioelectrical impedance analysis in adults aged 22-59 years. J Med Eng Technol. 2011;35(2):109–114. 10.3109/03091902.2010.543751 [DOI] [PubMed] [Google Scholar]
- 30.Malik M, Bigger TJ, Camm A, et al. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use.. Eur Heart J. 1996;17(3):354–381. 10.1093/oxfordjournals.eurheartj.a014868 [DOI] [PubMed] [Google Scholar]
- 31.Balady GJ, Arena R, Sietsema K, et al. Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122(2):191–225. 10.1161/CIR.0b013e3181e52e69 [DOI] [PubMed] [Google Scholar]
- 32.D’Ath P, Katona P, Mullan E, Evans S, Katona C. Screening, detection and management of depression in elderly primary care attenders. I: The acceptability and performance of the 15 item Geriatric Depression Scale (GDS15) and the development of short versions. Fam Pract. 1994;11(3):260–266. 10.1093/fampra/11.3.260 [DOI] [PubMed] [Google Scholar]
- 33.Nyunt MS, Fones C, Niti M, Ng TP. Criterion-based validity and reliability of the Geriatric Depression Screening Scale (GDS-15) in a large validation sample of community-living Asian older adults. Aging Ment Health. 2009;13(3):376–382. 10.1080/13607860902861027 [DOI] [PubMed] [Google Scholar]
- 34.Sallis JF, Haskell WL, Wood PD, et al. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121(1):91–106. 10.1093/oxfordjournals.aje.a113987 [DOI] [PubMed] [Google Scholar]
- 35.King AC, Oman RF, Brassington GS, Bliwise DL, Haskell WL. Moderate-intensity exercise and self-rated quality of sleep in older adults. A randomized controlled trial. JAMA. 1997;277(1):32–37. 10.1001/jama.1997.03540250040029 [DOI] [PubMed] [Google Scholar]
- 36.Elavsky S, McAuley E. Lack of perceived sleep improvement after 4-month structured exercise programs. Menopause. 2007;14(3):535–540. 10.1097/01.gme.0000243568.70946.d4 [DOI] [PubMed] [Google Scholar]
- 37.Zhi TF, Sun XM, Li SJ, et al. Associations of sleep duration and sleep quality with life satisfaction in elderly Chinese: The mediating role of depression. Arch Gerontol Geriatr. 2016;65211–217. 10.1016/j.archger.2016.03.023 [DOI] [PubMed] [Google Scholar]
- 38.Reid KJ, Baron KG, Lu B, Naylor E, Wolfe L, Zee PC. Aerobic exercise improves self-reported sleep and quality of life in older adults with insomnia. Sleep Med. 2010;11(9):934–940. 10.1016/j.sleep.2010.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Routledge FS, Campbell TS, McFetridge-Durdle JA, Bacon SL. Improvements in heart rate variability with exercise therapy. Can J Cardiol. 2010;26(6):303–312. 10.1016/S0828-282X(10)70395-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iellamo F, Legramante JM, Massaro M, Raimondi G, Galante A. Effects of a residential exercise training on baroreflex sensitivity and heart rate variability in patients with coronary artery disease: a randomized, controlled study. Circulation. 2000;102(21):2588–2592. 10.1161/01.CIR.102.21.2588 [DOI] [PubMed] [Google Scholar]
- 41.La Rovere MT, Bersano C, Gnemmi M, Specchia G, Schwartz PJ. Exercise-induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infarction. Circulation. 2002;106(8):945–949. 10.1161/01.CIR.0000027565.12764.E1 [DOI] [PubMed] [Google Scholar]
- 42.Ferreira LF, Rodrigues GD, Soares PS. Quantity of aerobic exercise training for the improvement of heart rate variability in older adults. Cardiovasc Sci. 2017;30157–162. [Google Scholar]
- 43.Jurca R, Church TS, Morss GM, et al. Eight weeks of moderate-intensity exercise training increases heart rate variability in sedentary postmenopausal women. Am Heart J. 2004;147(5):e8–e15. 10.1016/j.ahj.2003.10.024 [DOI] [PubMed] [Google Scholar]
- 44.Shen TW, Wen HJ. Aerobic exercise affects T-wave alternans and heart rate variability in postmenopausal women. Int J Sports Med. 2013;34(12):1099–1105. 10.1055/s-0033-1343408 [DOI] [PubMed] [Google Scholar]
- 45.Monahan KD, Dinenno FA, Tanaka H, Clevenger CM, DeSouza CA, Seals DR. Regular aerobic exercise modulates age-associated declines in cardiovagal baroreflex sensitivity in healthy men. J Physiol. 2000;529(1):263–271. 10.1111/j.1469-7793.2000.00263.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuo TB, Lin T, Yang CC, Li CL, Chen CF, Chou P. Effect of aging on gender differences in neural control of heart rate. Am J Physiol. 1999;277H2233–H2239. [DOI] [PubMed] [Google Scholar]
- 47.Sandercock GR, Bromley PD, Brodie DA. Effects of exercise on heart rate variability: inferences from meta-analysis. Med Sci Sports Exerc. 2005;37(3):433–439. 10.1249/01.MSS.0000155388.39002.9D [DOI] [PubMed] [Google Scholar]
- 48.Wu SY, Wang TF, Yu L, et al. Running exercise protects the substantia nigra dopaminergic neurons against inflammation-induced degeneration via the activation of BDNF signaling pathway. Brain Behav Immun. 2011;25(1):135–146. 10.1016/j.bbi.2010.09.006 [DOI] [PubMed] [Google Scholar]
- 49.Werner GG, Ford BQ, Mauss IB, Schabus M, Blechert J, Wilhelm FH. High cardiac vagal control is related to better subjective and objective sleep quality. Biol Psychol. 2015;10679–85. 10.1016/j.biopsycho.2015.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stein PK, Pu Y. Heart rate variability, sleep and sleep disoders. Sleep Med Rev. 2012;16(1):47–66. 10.1016/j.smrv.2011.02.005 [DOI] [PubMed] [Google Scholar]
- 51.Chennaoui M, Arnal P, Sauvet F, Leger D. Slep and exercise: a reciprocal issue?. Sleep Med Rev. 2015;2059–72. 10.1016/j.smrv.2014.06.008 [DOI] [PubMed] [Google Scholar]