Abstract
Study Objectives:
The main aim of this study was to investigate the effects of mandibular advancement appliance (MAA) therapy on jaw-closing muscle activity (JCMA) time-related to respiratory arousals, and on JCMA time-related to nonrespiratory arousals in patients with obstructive sleep apnea.
Methods:
Eighteen patients with OSA (mean ± standard deviation = 49.4 ± 9.8 years) with a mean ± standard deviation apnea-hypopnea index of 22.0 ± 16.0 events/h of sleep participated in a randomized controlled crossover trial in which two ambulatory polysomnographic recordings, 1 with an MAA in situ and another without the MAA in situ, were performed. JCMA was quantified as the sum of rhythmic masticatory muscle activities and other orofacial activities.
Results:
Significant reductions in the apnea-hypopnea index (Z = −2.984; P = .003), in the respiratory arousal index (Z = −2.896; P = .004), and in the JCMA time-related to respiratory arousal index (Z = −3.434; P = .001) were found with MAA in situ. On the nonrespiratory arousal index, and on the JCMA time-related to nonrespiratory arousal index, MAA had no significant effect (T = 2.23; P = .82; and Z = −0.66; P = .51, respectively).
Conclusions:
This study shows that effective mandibular advancement appliance therapy significantly reduces jaw-closing muscle activities time-related to respiratory arousals in OSA patients. Future studies are needed to confirm these findings in obstructive sleep apnea patients with comorbid sleep bruxism.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Name: The Effects of Oral Appliance Therapy on Masseter Muscle Activity in Obstructive Sleep Apnea; Identifier: NCT02011425.
Citation:
Aarab G, Arcache P, Lavigne GJ, Lobbezoo F, Huynh N. The effects of mandibular advancement appliance therapy on jaw-closing muscle activity during sleep in patients with obstructive sleep apnea: a 3–6 months follow-up. J Clin Sleep Med. 2020;16(9): 1545–1553.
Keywords: mandibular, masticatory muscles, obstructive sleep apnea, oral appliance, sleep bruxism, upper airway
BRIEF SUMMARY
Current Knowledge/Study Rationale: A previous study found that the emergence of jaw-closing muscle contractions time-related to respiratory events in patients with obstructive sleep apnea is associated with respiratory arousals rather than with the respiratory events per se. However, this has not been confirmed with obstructive sleep apnea treatment.
Study Impact: This study shows that effective mandibular advancement appliance therapy significantly reduces jaw-closing muscle activities time-related to respiratory arousals in obstructive sleep apnea patients. Future studies are needed to confirm these findings in obstructive sleep apnea patients with comorbid sleep bruxism.
INTRODUCTION
Obstructive sleep apnea (OSA) is a condition characterized by repetitive complete or partial obstruction of the upper airway often related to oxygen desaturations and arousals from sleep.1 The prevalence of OSA varies from 9% to 38% in the general adult population, affecting more men than women, primarily in middle-aged obese individuals.2 Excessive daytime sleepiness and fatigue, lack of concentration, and snoring out loud reported by the patient’s sleep partner are frequently reported complaints.3,4 Furthermore, patients with untreated OSA are at increased risk of hypertension, stroke, heart failure, diabetes, and car accidents.5–9
The gold standard for the diagnosis of OSA is polysomnography (PSG), which requires physiologic measurements of brain activity during sleep and measurements of the amount of airflow reductions and number of oxygen desaturations during sleep.1 Patients with OSA are diagnosed based on an apnea-hypopnea index (AHI) of at least 5 events/h of sleep determined during a PSG recording.1 The severity of OSA is classified as mild (AHI 5 to < 15), moderate (AHI 15 to < 30), and severe (AHI ≥ 30).1 However, the validity and predictive morbidity of AHI is currently debated as a unique biomarker of OSA. Therefore, other parameters that better predict the consequences of OSA can be expected in the near future.10,11
Currently, mandibular advancement appliances (MAAs) are prescribed for the treatment of patients with mild to moderate OSA, and for patients with severe OSA who cannot tolerate continuous positive airway pressure (CPAP).12 The rationale behind the efficacy of MAAs is that advancement of the mandible and tongue improves upper airway patency during sleep by enlarging the upper airway and by decreasing upper airway collapsibility, thereby preventing collapse during sleep.13,14 Furthermore, MAAs are often considered by patients to be a more acceptable treatment modality than CPAP.15,16 Earlier long-term follow-up studies showed no significant differences in the improvement of the AHI and excessive daytime sleepiness between MAA and CPAP in patients with mild and moderate OSA.17,18 Earlier studies also observed that respiratory events (viz, apneas and hypopneas) during sleep in patients with OSA are frequently followed by jaw-closing muscle activity (JCMA).19,20 Therefore, these studies suggested that JCMA occurs to reinstate the mandible and prevent the upper airway from collapsing.19,20 However, there is not enough solid evidence to accept this hypothesis. A recent study analyzed the temporal relationship between respiratory events and JCMA onset based on the reasoning that a cause should precede the effect. In patients with both OSA and sleep bruxism (SB), respiratory events preceded rhythmic JCMA in 55% of events, the opposite was the case in 25% of events, while 20% of events were without any temporal association.21 The authors concluded that in patients with OSA and SB, SB events occurring close to respiratory events represent a secondary form of SB. Further, another study concluded that JCMA is an orofacial manifestation of a general motor reaction to arousal occurring during sleep in patients with OSA. This suggests that jaw-closing muscle contractions after a respiratory event are dependent on the arousal response rather than on the respiratory events per se.22 Based on this finding, we hypothesized that an effective MAA therapy will result in a significant reduction of the JCMA time-related to respiratory arousals, without having an effect on the JCMA time-related to nonrespiratory arousals. We further hypothesized that an effective MAA therapy will result in a significant reduction of the total JCMA. Finally, we hypothesized that MAA therapy will not have an effect on JCMA time-related to respiratory events without arousal. Therefore, the primary aim of the current study was to determine the effects of MAA therapy on JCMA time-related to respiratory arousals and to nonrespiratory arousals in patients with OSA. The secondary aims of this study were: (1) to determine the effects of MAA therapy on the total JCMA; and (2) to determine the effects of MAA therapy on JCMA time-related to respiratory events without arousals.
METHODS
Study design
The study had a randomized controlled crossover design, in which 2 experimental conditions (without MAA in situ versus with MAA in situ) were compared in random order. The data from this study were collected at the Faculté de médicine dentaire, Université de Montréal, Montréal, Québec, Canada. The scientific and ethical aspects of this study’s protocol were approved by the Medical Ethics Committee of the Université de Montréal (13-105-CERES-D). This study is registered at www.clinicaltrials.gov (NCT02011425).
Participants
Patients with OSA referred for an MAA treatment were invited to participate in this study when they met the following inclusion or exclusion criteria. The inclusion criteria for participation were: (1) age between 30 and 65 years, (2) AHI between 15 and 45 events/h of sleep, and (3) an Epworth Sleepiness Score > 10, or at least two of the following symptoms: choking or gasping during sleep, recurrent awakenings from sleep, unrefreshing sleep, daytime fatigue, and impaired concentration. Exclusion criteria were: (1) evidence of respiratory/sleep disorders other than OSA, with the exception of SB, (2) body mass index > 40, (3) medication usage that could influence respiration or sleep, (4) reversible morphological upper airway abnormalities (eg, enlarged tonsils), (5) severe temporomandibular disorders, (6) untreated periodontal problems, (7) dental pain, and (8) lack of retention possibilities for an MAA.
Mandibular advancement appliance
In the present study, a custom-made titratable MAA (SomnoDent Flex; SomnoMed, Ontario, Canada) was used. The MAA was adjusted individually and advancement was titrated in 2 private dental clinics in Montréal using a standardized titration protocol. After assessment of the central relation and maximum protrusion using a construction bite with the George Gauge instrument (Great Lakes Dental Technologies, Tonawanda, NY), the MAA was set at 60% of the maximal advancement at baseline. At each consecutive visit, the MAA was evaluated with respect to OSA symptoms and advanced to approximately 75%, or 90% if OSA symptoms were not relieved or reduced to an acceptable level. On the other hand, if side effects were not acceptable for the participant (eg, signs of temporomandibular dysfunction resulting in difficulty wearing the appliance during sleep), the advancement was adjusted backward to approximately 45%, 60%, or 75%.23 For this study, the mean position after the titration procedure of the MAA was 71% [range, 61–90] of the maximum protrusion. This corresponds to a mean protrusion of 7.5 mm (standard deviation [SD], 1.0; range, 5–9). The patients were instructed to wear the MAA every night following delivery. After an habituation period of 3–6 months, patients underwent 2 follow-up PSG recordings with or without MAA in situ in random order, with an intermission of 1 washout week to control for carryover effects.
Polysomnography
Two ambulatory follow-up recordings were performed at home within 1 week in a random order, using Embla Titanium hardware and RemLogic software (Embla, Ontario, Canada). The following channels were recorded: electroencephalography (F3M2, F4M1, C3M2, C4M1, O1M2, O2M1), electrooculography (right and left), and electromyography (EMG) from the mentalis and submentalis muscles, the right and left masseter and temporalis muscles, and the right and left tibialis muscles. Respiratory parameters were assessed by recording abdominal and thoracic respiratory effort, airflow (oronasal cannula), and oximetry. Body position was determined by a position sensor in the portable recorder. This sensor differentiated between the upright, left side, right side, prone, and supine positions. The mounting was performed by a trained sleep technician. The PSG recordings were scored manually, and standard sleep, respiratory, and arousal outcome variables were obtained by a single experienced and registered polysomnographic technologist from an independent company (Sleep Strategies, Ottawa, Canada), following the criteria of the American Academy of Sleep Medicine.24,25
The follow-up nights within 1 week were used to establish, in a random order, the JCMA and the effects of the MAA on this activity. JCMA (viz, right and left masseter and temporalis muscles) were scored at the Academic Centre for Dentistry Amsterdam (ACTA) by a single, trained investigator (G. Aarab). All data analyses were performed under blind conditions (viz, without MAA in situ vs with MAA in situ). JCMA was scored when the mean EMG amplitude was at least 2 times higher than the EMG baseline signal. A minimum of 3 out of 4 EMG signals (m. masseter left, m. masseter right, m. temporalis left, and m. temporalis right) had to reach this EMG threshold. Bursts were scored, and distinction was made in the type of JCMA following the internationally accepted criteria,26 viz, rhythmic masticatory muscle activity (RMMA; recognized by the type of jaw muscle contraction pattern: phasic [at least 3 rhythmic contractions lasting more than 0.25 seconds], tonic [sustained activity lasting at least 2 seconds], or mixed [phasic and tonic‐sustained types of electromyographic episodes]), orofacial activity (OFA), and other muscle activity (OMA). See Table 1 for a full description of each JCMA variable. OFAs were defined as movements that primarily involved the masticatory muscles (eg, swallowing, lip or tongue movements) but which do not meet the criteria of RMMA episodes (eg, an isolated tonic burst lasting less than 2 seconds). OMAs were defined as muscle activities that are part of major movements (eg, facial, head, neck, and/or body movements).27 All variables were converted into indices, viz, number of events per hour of sleep. JCMA-index was quantified as the sum of RMMAs and orofacial activities per hour of sleep.
Table 1.
Polysomnographic research diagnostic criteria for scoring different types of jaw-closing muscle activity.24,25
| Types of Jaw-Closing Muscle Activitya |
|---|
| • Phasic episode: at least 3 EMG bursts lasting ≥ 0.25 seconds and < 2 seconds |
| • Tonic episode: 1 EMG burst lasting ≥ 2 seconds |
| • Mixed episode: phasic and tonic bursts |
| • RMMA: phasic, tonic, or mixed episodes |
| • OFA: isolated tonic burst < 2 seconds |
| • OMA: jaw-closing muscle activity that is part of major movements (e.g., facial, head, neck, and/or body movements).25 |
| • JCMA: RMMA + OFA |
An EMG burst was considered a different episode if the interval between bursts was > 3 seconds. EMG = electromyography, JCMA = jaw-closing muscle activity, OFA = orofacial activity, OMA = other muscle activity, RMMA = rhythmic masticatory muscle activity.
For the first aim, JCMA was scored in relation to respiratory arousals when JCMA occurred within 5 seconds of the respiratory arousal.28 JCMAs related to arousals (viz, within 5 seconds) but without the appearance of a respiratory event within those 5 seconds were scored as JMCA related to nonrespiratory arousals. For the third aim, only JMCAs in relation to respiratory events within 5 seconds, and without the occurrence of an arousal within those 5 seconds were scored.
Questionnaires
Study data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at the Université de Montréal.29,30 Research Electronic Data Capture is a secure, web-based software platform designed to support data capture for research. Patients filled out several questionnaires at baseline and 2 weeks prior to the 2 follow-up PSG recordings through REDCap. The Epworth Sleepiness Scale (ESS)31 was filled out at the time of inclusion. Two weeks prior to the 2 follow-up recordings, the following questionnaires were filled out: ESS, a questionnaire about adherence, and a questionnaire about side-effects.
ESS
The ESS31 is self-administered and consists of 8 questions. Questions are based on 8 different situations/activities in which patients can doze off or fall asleep. Situations/activities are, for example, watching television, sitting and reading, talking with someone else, following an eating moment, etc. Answers are rated on a 4-point chance scale (0–3), where 0 is no chance of dozing off and 3 is high chance of dozing. The 8 scores are added, which can give a total sum score from 0 to 24. Patients are sleepy when the ESS score is higher than 10. The higher the ESS score, the higher that person’s subjective average daytime sleepiness.
Adherence
The adherence questionnaire consisted of 7 questions. Four questions were about (1) adapting time in days, (2) average, minimum, and maximum wearing time in hours per night, (3) number of nights wearing MAA per week, and (4) number of hours of sleep per night. Patients filled out this information in free text boxes.
The 3 other questions were about comfort level experienced, satisfaction, and treatment efficiency of the MAA. The scores were based on a visual analog scale of 0–100, where 0 was uncomfortable, unsatisfied, and inefficient, and 100 was very comfortable, very satisfied, and very efficient, respectively.
Side-effects
The questionnaire about side-effects included questions about secondary effects during MAA therapy. Patients had to indicate whether and which side-effects occurred, viz, (1) increased salivation during the night, (2) sensation of dry mouth, (3) bad taste, and/or (4) sounds in the temporomandibular joint. Furthermore, they had to indicate whether and which changes in dental occlusion occurred, and how long these changes persisted, viz, (1) no changes in dental occlusion, (2) slight change in dental occlusion that disappeared during the day, (3) changes in dental occlusion and no improvement during the day, and (4) permanent significant changes in dental occlusion. Finally, the overall satisfaction of the treatment was evaluated, where patients could give answers that varied from very satisfied and satisfied to somewhat satisfied and dissatisfied.
Outcome variables
The primary outcome variables for this study were for the primary aim: JCMA time-related to the type of arousal index (respiratory versus nonrespiratory); for the secondary aims: total-JCMA index, and JCMA time-related to respiratory events without arousal index. Secondary outcome variables were the AHI, oxygen desaturation index, snore time, sleep variables, OMA per hour of sleep, excessive daytime sleepiness, self-reported adherence, and side-effects.
Statistical analysis
A minimum sample size of 18 patients was calculated to detect a significant difference between JCMA without MAA in situ and with MAA in situ, with a power of 80% and a significance level of 5% (2-sided).32,33 All primary and secondary outcome variables (ie, sleep, respiratory, EMG, and questionnaire variables) were tested for normality by the Shapiro-Wilk test. The outcome of the Shapiro-Wilk test was confirmed by making histograms for each variable. Based on those interpretations, for pairwise comparisons, paired t tests were used for normally distributed data, and Wilcoxon signed-rank tests for nonnormally distributed data. For normally distributed variables, data are presented in mean ± SD. For nonnormally distributed variables, data are presented in percentiles (25%|median|75%).
For all statistical tests, the means or medians were compared at a 95% confidence interval. A P-value of less than .05 was considered statistically significant. IBM SPSS 26.0 statistics software package (SPSS Inc., Chicago, IL) for Windows was used for statistical analysis.
RESULTS
Patients’ characteristics
In total, 32 patients were recruited, 7 of whom did not fulfill the inclusion criteria. Another 5 patients were excluded from the study: 3 patients withdrew from the study due to lack of time, 1 patient withdrew due to financial reasons, and 1 patient stopped using the MAA treatment due to temporomandibular dysfunction. Two other patients were excluded due to artifacts on the EMG channels. Thus, a total of 18 patients (5 women and 13 men) remained. The mean age of the 18 patients included was 49.4 (SD, 9.8; range, 31–65) years. The mean body mass index of the patients was 28.5 (SD, 3.9; range, 23–38) kg/m2.
Primary outcome variables
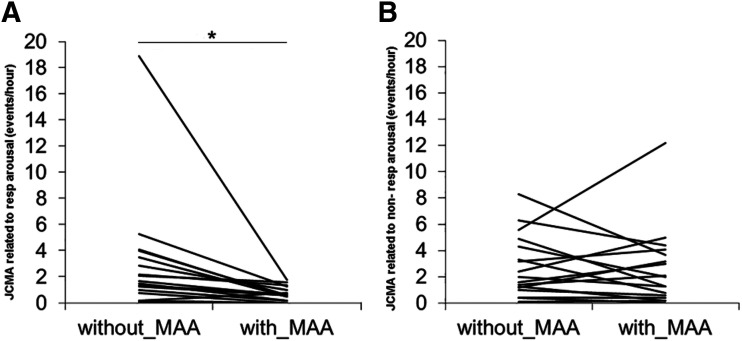
Table 2 shows the 25%|median|75% percentiles of the JCMA indices without MAA in situ and with MAA in situ of the 18 patients with OSA. Significant reductions in the JCMA time-related to respiratory arousal index (Z = −3.434; P = .001) were found with the MAA in situ. However, no significant effect of the MAA was observed on the JCMA time-related to nonrespiratory arousals index (Z= −0.663; P = .507). There was no significant difference in the total-JCMA index between without MAA in situ and with MAA in situ (Z = 1.678; P = .083). Figure 1 shows the individual scores of JCMA indices time-related to respiratory arousals and to nonrespiratory arousals for without MAA in situ and with MAA in situ of the 18 patients. JCMA time-related to respiratory events without arousals was very rare (0.0|0.0|0.0 events/h) and did not change with MAA in situ (P = .864). There was one individual who showed a large reduction in the JCMA time-related to respiratory index from 19 to 2 events/h of sleep with MAA in situ (see Figure 1). The exclusion of this individual from the statistical analyses did not change the outcomes described in this paragraph.
Table 2.
All jaw-closing muscle activity indices of the 18 patients with OSA without MAA in situ and with MAA in situ.
| Without MAAa | With MAAa | Testb | P * | |
|---|---|---|---|---|
| JCMA related to respiratory arousals (events/h) | ||||
| JCMA | 0.7|1.6|3.6 | 0.2|0.6|1.1 | Z = −3.434 | .001* |
| RMMA | 0.0|0.4|1.1 | 0.0|0.2|0.3 | Z = −2.135 | .033* |
| Orofacial activity | 0.4|1.1|2.3 | 0.0|0.3|0.6 | Z = −3.446 | .001* |
| Other muscle activity | 0.0|0.3|0.5 | 0.0|0.1|0.3 | Z = −1.938 | .053 |
| JCMA related to nonrespiratory arousals (events/h) | ||||
| JCMA | 1.2|1.8|4.5 | 0.4|1.7|3.8 | Z = − 0.663 | .507 |
| RMMA | 0.2|0.6|1.4 | 0.0|0.4|0.9 | Z = − 0.341 | .733 |
| Orofacial activity | 0.5|1.3|2.7 | 0.2|1.1|2.6 | Z = − 0.207 | .836 |
| Other muscle activity | 0.3|0.5|0.8 | 0.3|0.7|1.0 | Z = −1.093 | .275 |
| Total JCMA (events/h) | ||||
| JCMA | 2.4|5.5|8.1 | 1.1|2.7|5.1 | Z = −1.678 | .093 |
| RMMA | 0.2|1.2|3.3 | 0.0|0.6|2.2 | Z = −0.994 | .320 |
| Phasic episode | 0.0|0.3|1.3 | 0.0|0.1|0.7 | Z = −1.085 | .278 |
| Tonic episode | 0.0|0.2|0.7 | 0.0|0.2|0.6 | Z = −0.664 | .507 |
| Mixed episode | 0.0|0.0|0.3 | 0.0|0.0|0.3 | Z = −0.564 | .573 |
| Orofacial activity | 1.2|3.1|5.2 | 0.5|1.8|3.9 | Z = −1.656 | .098 |
| Other muscle activity | 0.4|0.7|1.5 | 0.5|1.0|1.3 | Z = 0.000 | 1.000 |
A P-value of < 0.05 is considered statistically significant. aThe 25%|median|75% percentiles are given for not normally distributed variables. bZ = Wilcoxon signed-rank test analysis. MAA = mandibular advancement appliance, OSA = obstructive sleep apnea, RMMA = rhythmic masticatory muscle activity (= phasic episode + tonic episode + mixed episode), JCMA = jaw-closing muscle activity (= RMMA + orofacial activity).
Figure 1. JCMA time-related to respiratory and nonrespiratory arousals indices.
(A) Individual values of the JCMA time-related to respiratory arousals index without MAA in situ and with MAA in situ (n = 18). There was a significant reduction in the JCMA time-related to respiratory arousal index with MAA in situ (*P = .001). (B) Individual values of JCMA time-related to nonrespiratory arousals index (n = 18). No significant effect was observed of the MAA on the JCMA time-related to nonrespiratory arousals index (P = .507).
Secondary outcome variables
Table 3 shows the mean (± SD) or percentile 25%|median|75% values of the sleep and respiratory variables for both PSGs, viz, without and with MAA therapy.
Table 3.
Respiratory and sleep variables of the 18 patients with OSA without MAA in situ and with MAA in situ.
| Outcome Variables | Without MAAa | With MAAa | Testb | P* |
|---|---|---|---|---|
| Sleep | ||||
| TST (h) | 6.0|6.5|7.3 | 5.8|6.5|7.7 | Z = −0.675 | .500 |
| N1 (%) | 11.1 ± 4.8 | 9.8 ± 3.8 | T = 1.743 | .000* |
| N2 (%) | 65.8 ± 5.1 | 62.0 ± 5.8 | T = 2.423 | .234 |
| N3 (%) | 0.4|2.7|7.9 | 2.0|7.2|9.5 | Z = −1.965 | .049* |
| REM (%) | 19.1 ± 3.9 | 21.7 ± 5.2 | T = −2.329 | .050 |
| Sleep efficiency (%) | 88 ± 6.7 | 87.4 ± 6.0 | T = 0.360 | .616 |
| Total AI (events/h) | 18.0 ± 7.5 | 13.9 ± 5.4 | T = 2.693 | .015* |
| Respiratory AI (events/h) | 3.7|6.6|14.2 | 2.3|3.3|7.2 | Z = −2.896 | .004* |
| Non-Respiratory AI (events/h) | 9.0 ± 4.0 | 9.2 ± 4.2 | T = −0.234 | .818 |
| Respiratory | ||||
| AHI (events/h) | 10.0|18.4|30.3 | 4.2|9.6|22.9 | Z = −2.984 | .003*, |
| ODI (events/h) | 20.7 ± 15.27 | 13.5 ± 9.8 | T = 2.338 | .017* |
| Desaturation (min) | 135.8 ± 107.8 | 84.8 ± 66.6 | T = 2.552 | .010*, |
| Snoring time (min) | 30.3 ± 21.1 | 18.0 ± 20.6 | T = 2.992 | .004* |
A P-value of < 0.05 is considered statistically significant. aPercentiles in 25%|median|75% for nonnormally distributed data; mean ± SD for normally distributed data. bT = paired sample t test; Z = Wilcoxon signed-rank test analysis. AHI = apnea-hypopnea index, AI = arousal index, N 1−3 = Non-REM sleep stages, ODI = oxygen desaturation index, REM = rapid eye movement sleep, SD = standard deviation, Total AI = sum of respiratory AI and nonrespiratory AI.
Sleep variables
Sleep stage N1 (%) showed a significant decrease with MAA in situ compared to the condition without MAA in situ (P = .000). Further, sleep stage N3 (%) showed a significant increase with MAA in situ (P = .049). A trend toward a significant change was shown for sleep stage REM with MAA in situ compared to the condition without MAA in situ (P = .050). A significant improvement was also seen in the total arousal index and respiratory arousal index with MAA in situ (see Table 3, P = .015 and P = .004, respectively).
Respiratory variables
The median AHI showed a significant improvement from 10.0|18.4|30.3 events/h of sleep without MAA in situ to 4.2|9.6|22.9 events/h of sleep with MAA in situ (Z= −2.984; P = .003). Furthermore, there was a significant improvement in the oxygen desaturation index (ODI, < 4%) (T = 2.338; P = .017) and snore time (T = 2.992; P = .004) with MAA in situ. All patients showed improvements in their AHI, with 2 exceptions; their AHI was higher on the night with MAA in situ than without MAA in situ. Two out of the 18 patients scored an AHI lower than 5 events/h of sleep without MAA in situ. One of those 2 patients stayed below an AHI of 5 events/h of sleep with MAA in situ and 1 of them showed a small increase in the AHI. Further, 4 other patients scored an AHI lower than 5 events per hour of sleep after MAA insertion. Finally, one patient started with a very high AHI of 68.9 events/h sleep, which improved to 29.9 events/h of sleep after MAA insertion.
Other muscle activities
The occurrence of OMA did not differ between without and with MAA therapy for all defined OMA variables (P > .05; Table 2).
Questionnaires
ESS: There was no significant difference between the ESS without MAA in situ (7.0|11.0|14; n = 17) and with MAA in situ (6|8|14; n = 18) (Z = −1.288; P = .198).
Adherence (Table 4): The patients had a mean adaption time of 15.6 ± 21.3 days (n = 16) for the MAA, with the exception of 2 patients who indicated they were not yet used to the MAA at the end of the habituation period. Patients wore the MAA on average 6.7 ± 1.0 hour per night, 6.1 ± 1.3 nights per week. See Table 4 for other specifications of wearing times. Patients reported on a visual analog scale the level of comfort, satisfaction, and treatment efficiency of their MAA: 60% (range, 3–90], 64% [range, 7–94], and 59% [range, 6–96], respectively.
Table 4.
Outcomes (mean ± SD) of the adherence questionnaire of the 18 patients with OSA.
| Questions | |
|---|---|
| Adapting time MAA (days) | 15.6 ± 21.3a |
| What was the wear time of the device per night? | |
| Average (h/night) | 6.4 ± 1.8 |
| Minimum (h/night) | 4.9 ± 2.2 |
| Maximum (h/night) | 7.5 ± 0.9 |
| How many nights per week did you wear your appliance? | 6.1 ± 1.3 |
| How many hours do you sleep per night? | 7.0 ± 1.4 |
| Level of comfortb | 60.2 ± 27.6 |
| Level of satisfactionc | 63.7 ± 27.6 |
| Treatment efficiencyd | 59.3 ± 24.6 |
n = 16, 2 out of 18 patients were not used to wearing the MAA at the end of the habituation period. bScores are based on a VAS-scale from 0 to 100, where 0 is uncomfortable and 100 is very comfortable. cScores are based on a VAS-scale from 0 to 100, where 0 is unsatisfied and 100 is very satisfied. dScores are based on a VAS-scale from 0 to 100, where 0 is inefficient and 100 is very efficient. MAA = mandibular advancement appliance, SD = standard deviation.
Side-effects: Patients reported the following side-effects of MAA therapy: excessive salivation (n = 8), dry mouth (n = 7), bad taste (n = 6), and temporomandibular joint sounds (n = 4). Dental occlusion changes were not reported (n = 4) or disappeared during the day (n = 14). The satisfaction with MAA therapy varied between dissatisfied (n = 1), a bit satisfied (n = 6), satisfied (n = 7), and very satisfied (n = 4).
DISCUSSION
Our study showed that effective MAA therapy results in a significant decrease of JCMA time-related to respiratory arousals in OSA patients. JCMA time-related to nonrespiratory arousals, however, was not affected by MAA therapy. Further, the total JCMA during sleep was not affected by MAA therapy. Finally, JCMA time-related to respiratory events without arousals was rare and was not significantly affected by MAA therapy.
Primary outcome variables
As expected, MAA therapy improved JCMA time-related to respiratory arousals without having an effect on the JCMA time-related to nonrespiratory arousals. Furthermore, it should be noted that all patients showed a decrease in the JCMA time-related to respiratory arousals index, while the JCMAs time-related to nonrespiratory arousals index response varied between patients after MAA insertion (see Figure 1). A previous study had shown that after experimentally induced arousals, independent of respiratory events, JCMA appears.34 All these outcomes support the findings of Kato et al, that JCMAs are related to respiratory arousals rather than to the respiratory event per se.22 However, this does not preclude that JCMA may still be beneficial for the upper airway obstruction events in patients with OSA.35,36
Further extrapolating our findings, this study suggests that the occurrence of arousals, which are part of the physiology related to OSA, potentially influences the probability of jaw-closing muscle activity, possibly including sleep bruxism. There was a significant reduction of RMMA activity related to respiratory arousals with MAA therapy (P =.033; Table 2). The clinical relevance of this finding is illustrated by the fact that this activity is often associated with breaking or losing teeth, fillings, or implants; musculoskeletal pain (temporomandibular pain); and headaches.33 However, our result should be interpreted with caution since the RMMA activity related to respiratory arousals without MAA in situ was very low (0.0|0.4|1.1 events/h). Therefore, future studies are needed to confirm our current findings in OSA patients with comorbid sleep bruxism, and with other effective OSA treatments, such as continuous positive airway pressure.
MAA therapy had no significant effect on the total-JCMA index. In this study, the respiratory-arousal index represented approximately 50% of the total-arousal index without MAA in situ, which is in line with a previous study.22 As described in the previous paragraph, MAA therapy had only a significant effect on the number of respiratory arousals. Therefore, MAA therapy could only have affected and potentially improved around 50% of the total arousals. Hence, the improvement in the number of JCMAs time-related to respiratory arousals with MAA in situ was too small to significantly improve the total-JCMA index.
Supporting the findings of Kato et al, jaw-closing muscle activities time-related to respiratory events without the occurrence of arousals were rare and were not affected by MAA therapy in our study.22 Kato et al included 19 patients with OSA who had a mean (± SD) AHI of 31.9 (± 19.9) events per hour. In 0% to 6.1% of the respiratory events without the occurrence of an arousal, JCMA occurred.22 In case JCMA time-related to respiratory events without the occurrence of an arousal was significantly affected by MAA therapy, it was also possible that JCMA could be related to the respiratory event itself and not to the respiratory arousal per se. However, this hypothesis was rejected based on current findings.
Secondary outcome variables
The excessive daytime sleepiness of our study group did not improve significantly with MAA therapy. This result is not surprising, because our study group was not sleepy at baseline (ie, ESS was only slightly higher than 10). Therefore, a large significant improvement in excessive daytime sleepiness was not expected. Moreover, only when a study group is very sleepy (ie, ESS higher than 16 out of 24), would it be of clinical relevance to find significant improvements in excessive daytime sleepiness.31
Based on self-report, the patients wore their MAA during 97% of their sleep period. To date, for an objective outcome, adherence chips can be incorporated in the MAA that can detect the exact wearing time of the MAA. In 2 previous studies, this objective tool was compared with subjective outcomes of adherence. Both studies concluded that there is a very high correlation between objective and subjective evaluation of adherence.37,38 According to Dieltjens et al, there is only a mean subjective overestimation of 30 minutes compared to objective tools.38 Therefore, the subjective adherence found in the current study is assumed to be a good approximation of the real adherence.
Side-effects varied from patient to patient. Although one patient was excluded due to temporomandibular dysfunction caused by MAA, all other patients reported only mild, transient side-effects. These findings correspond with outcomes of a previous study on side-effects of MAA therapy.17
Strengths and limitations
Although an inclusion criterion of an AHI between 15 and 45 events/h of sleep was set at the start of the study, a large number of patients, viz, 7 out of 18, scored for the PSG without MAA in situ below the cut-off value of 15 events/h of sleep. This can be explained by the biological fluctuations in the AHI from night to night.39,40 Aarab et al reported that patients with OSA show natural fluctuations in their AHI of more than 10 events/h of sleep from night to night.40 In general, however, this does not result in a different mean AHI of the patient group, because in some patients the AHI increases while in others the AHI decreases from night to night.39–41 Furthermore, the patients included in this study were diagnosed in different hospitals as well as in home settings. Therefore, the observed difference in the AHI between the PSG recording used for the inclusion of patients and the recording without MAA in situ could also be the result of the different PSG recording techniques used in these different settings. To overcome this problem, we decided to perform a new PSG recording without MAA in situ.
In the current study, the mean MAA was set at 71% (range, 61–90) of the maximum protrusion by using a standardized titration protocol. Most previous studies on MAA therapy used a fixed protrusion position of the MAA,42–44 or the MAA was titrated by the patient based on their own evaluation of improvements.45–47 By using a standardized titration protocol that was performed by the dentist, the titration procedure was made comparable between patients. Therefore, bias from an uncontrolled titration procedure was excluded.
Previous studies scored jaw-closing muscle activities in PSGs when the EMG level was ≥ 20% or ≥ 10% of the maximum voluntary contraction (MVC) before sleep.22,48–50 In the current study, a different scoring method was used. Bursts were scored when the EMG level was at least 2 times higher than the baseline EMG before the burst appeared, and only when 3 out of 4 masticatory EMG channels showed this burst.27 In the MVC method, MVCs are defined at the start of the PSG recording, while the impedance of the skin EMG electrodes can fluctuate during the night (eg, due to sweating, temperature shifts, or movements), resulting in more noise in the EMG signal and a shift of the baseline signal. Through these shifts, bursts could easily be missed when the baseline signal increases, or scored when the baseline signal decreases. Consequently, this might result in less accurate scoring of the JCMA. Therefore, we decided to use the 2-times baseline method.
CONCLUSIONS
This study shows that effective mandibular advancement appliance therapy significantly reduces jaw-closing muscle activities time-related to respiratory arousals in OSA patients. Future studies are needed to confirm these findings in OSA patients with comorbid sleep bruxism.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Institution where work was performed: Faculté de médecine dentaire, Université de Montréal, Montréal, Canada. The authors declare no conflict of interest. The SomnoDent appliances (SomnoMed, Ontario, Canada) were provided free of charge. SomnoMed had no input in the data analyses and publication of this article.
ACKNOWLEDGMENTS
The authors thank SomnoMed (Ontario, Canada) for supplying free of charge the SomnoDent appliances used in this study.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- EMG
electromyography
- ESS
Epworth sleepiness scale
- JCMA
jaw-closing muscle activity
- MAA
mandibular advancement appliance
- OMA
other muscle activity
- OSA
obstructive sleep apnea
- PSG
polysomnography
- RMMA
rhythmic masticatory muscle activity
- SB
sleep bruxism
REFERENCES
- 1.American Academy of Sleep Medicine Task Force . Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667–689. 10.1093/sleep/22.5.667 [DOI] [PubMed] [Google Scholar]
- 2.Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med Rev. 2017;34:70–81. 10.1016/j.smrv.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 3.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. 10.1056/NEJM199304293281704 [DOI] [PubMed] [Google Scholar]
- 4.Gottlieb DJ, Whitney CW, Bonekat WH, et al. Relation of sleepiness to respiratory disturbance index: the Sleep Heart Health Study. Am J Respir Crit Care Med. 1999;159(2):502–507. 10.1164/ajrccm.159.2.9804051 [DOI] [PubMed] [Google Scholar]
- 5.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. 10.1056/NEJM200005113421901 [DOI] [PubMed] [Google Scholar]
- 6.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122(4):352–360. 10.1161/CIRCULATIONAHA.109.901801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. 10.1056/NEJMoa043104 [DOI] [PubMed] [Google Scholar]
- 8.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182(2):269–277. 10.1164/rccm.200911-1746OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kendzerska T, Gershon AS, Hawker G, Tomlinson G, Leung RS. Obstructive sleep apnea and incident diabetes. A historical cohort study. Am J Respir Crit Care Med. 2014;190(2):218–225. 10.1164/rccm.201312-2209OC [DOI] [PubMed] [Google Scholar]
- 10.Light M, Owens RL, Schmickl CN, Malhotra A. Precision medicine for obstructive sleep apnea. Sleep Med Clin. 2019;14(3):391–398. 10.1016/j.jsmc.2019.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Chazal P, Sutherland K, Cistulli PA. Advanced polysomnographic analysis for OSA: A pathway to personalized management? Respirology. 2020;25(3):251–258. 10.1111/resp.13564 [DOI] [PubMed] [Google Scholar]
- 12.Ramar K, Dort LC, Katz SG, et al. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. J Clin Sleep Med. 2015;11(7):773–827. 10.5664/jcsm.4858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt-Nowara WW, Meade TE, Hays MB. Treatment of snoring and obstructive sleep apnea with a dental orthosis. Chest. 1991;99(6):1378–1385. 10.1378/chest.99.6.1378 [DOI] [PubMed] [Google Scholar]
- 14.Ng AT, Gotsopoulos H, Qian J, Cistulli PA. Effect of oral appliance therapy on upper airway collapsibility in obstructive sleep apnea. Am J Respir Crit Care Med. 2003;168(2):238–241. 10.1164/rccm.200211-1275OC [DOI] [PubMed] [Google Scholar]
- 15.Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29(2):244–262. 10.1093/sleep/29.2.244 [DOI] [PubMed] [Google Scholar]
- 16.Hamoda MM, Kohzuka Y, Almeida FR. Oral appliances for the management of OSA: an updated review of the literature. Chest. 2018;153(2):544–553. 10.1016/j.chest.2017.06.005 [DOI] [PubMed] [Google Scholar]
- 17.Aarab G, Lobbezoo F, Heymans MW, Hamburger HL, Naeije M. Long-term follow-up of a randomized controlled trial of oral appliance therapy in obstructive sleep apnea. Respiration. 2011;82(2):162–168. 10.1159/000324580 [DOI] [PubMed] [Google Scholar]
- 18.Doff MH, Hoekema A, Wijkstra PJ, et al. Oral appliance versus continuous positive airway pressure in obstructive sleep apnea syndrome: a 2-year follow-up. Sleep. 2013;36(9):1289–1296. 10.5665/sleep.2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollowell DE, Suratt PM. Mandible position and activation of submental and masseter muscles during sleep. J Appl Physiol. 1991;71(6):2267–2273. 10.1152/jappl.1991.71.6.2267 [DOI] [PubMed] [Google Scholar]
- 20.Hollowell DE, Suratt PM. Activation of masseter muscles with inspiratory resistance loading. J Appl Physiol. 1989;67(1):270–275. 10.1152/jappl.1989.67.1.270 [DOI] [PubMed] [Google Scholar]
- 21.Saito M, Yamaguchi T, Mikami S, et al. Temporal association between sleep apnea-hypopnea and sleep bruxism events. J Sleep Res. 2013. 10.1111/jsr.12099 [DOI] [PubMed] [Google Scholar]
- 22.Kato T, Katase T, Yamashita S, et al. Responsiveness of jaw motor activation to arousals during sleep in patients with obstructive sleep apnea syndrome. J Clin Sleep Med. 2013;9(8):759–765. 10.5664/jcsm.2914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aarab G, Lobbezoo F, Hamburger HL, Naeije M. Effects of an oral appliance with different mandibular protrusion positions at a constant vertical dimension on obstructive sleep apnea. Clin Oral Investig. 2010;14(3):339–345. 10.1007/s00784-009-0298-9 [DOI] [PubMed] [Google Scholar]
- 24.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring Systems for Sleep Stages of Human Subjects. Los Angeles: Brain Information Service/Brain Research Institute, UCLA; 1968. [Google Scholar]
- 25.Berry RB, Budhiraja R, Gottlieb DJ, et al. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine . Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J Clin Sleep Med. 2012;8(5):597–619. 10.5664/jcsm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavigne GJ, Rompré PH, Montplaisir JY. Sleep bruxism: validity of clinical research diagnostic criteria in a controlled polysomnographic study. J Dent Res. 1996;75(1):546–552. 10.1177/00220345960750010601 [DOI] [PubMed] [Google Scholar]
- 27.Carra MC, Huynh N, Lavigne GJ. Diagnostic accuracy of sleep bruxism scoring in absence of audio-video recording: a pilot study. Sleep Breath. 2015;19:183–190. 10.1007/s11325-014-0986-9 [DOI] [PubMed] [Google Scholar]
- 28.Kato T, Rompré P, Montplaisir JY, Sessle BJ, Lavigne GJ. Sleep bruxism: an oromotor activity secondary to micro-arousal. J Dent Res. 2001;80(10):1940–1944. 10.1177/00220345010800101501 [DOI] [PubMed] [Google Scholar]
- 29.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris PA, Taylor R, Minor BL, et al.REDCap Consortium . The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 32.Dutra KM, Pereira FJ, Jr., Rompré PH, Huynh N, Fleming N, Lavigne GJ. Oro-facial activities in sleep bruxism patients and in normal subjects: a controlled polygraphic and audio-video study. J Oral Rehabil. 2009;36(2):86–92. 10.1111/j.1365-2842.2008.01912.x [DOI] [PubMed] [Google Scholar]
- 33.Landry-Schönbeck A, de Grandmont P, Rompré PH, Lavigne GJ. Effect of an adjustable mandibular advancement appliance on sleep bruxism: a crossover sleep laboratory study. Int J Prosthodont. 2009;22(3):251–259. [PubMed] [Google Scholar]
- 34.Kato T, Montplaisir JY, Lavigne GJ. Experimentally induced arousals during sleep: a cross-modality matching paradigm. J Sleep Res. 2004;13(3):229–238. 10.1111/j.1365-2869.2004.00409.x [DOI] [PubMed] [Google Scholar]
- 35.Manfredini D, Guarda-Nardini L, Marchese-Ragona R, Lobbezoo F. Theories on possible temporal relationships between sleep bruxism and obstructive sleep apnea events. An expert opinion. Sleep Breath. 2015;19(4):1459–1465. 10.1007/s11325-015-1163-5 [DOI] [PubMed] [Google Scholar]
- 36.Lavigne GJ, Kato T, Kolta A, Sessle BJ. Neurobiological mechanisms involved in sleep bruxism. Crit Rev Oral Biol Med. 2003;14(1):30–46. 10.1177/154411130301400104 [DOI] [PubMed] [Google Scholar]
- 37.Smith YK, Verrett RG. Evaluation of a novel device for measuring patient compliance with oral appliances in the treatment of obstructive sleep apnea. J Prosthodont. 2014;23(1):31–38. 10.1111/jopr.12076 [DOI] [PubMed] [Google Scholar]
- 38.Dieltjens M, Braem MJ, Vroegop AVMT, et al. Objectively measured vs self-reported compliance during oral appliance therapy for sleep-disordered breathing. Chest. 2013;144(5):1495–1502. 10.1378/chest.13-0613 [DOI] [PubMed] [Google Scholar]
- 39.Bittencourt LR, Suchecki D, Tufik S, et al. The variability of the apnoea-hypopnoea index. J Sleep Res. 2001;10(3):245–251. 10.1046/j.1365-2869.2001.00255.x [DOI] [PubMed] [Google Scholar]
- 40.Aarab G, Lobbezoo F, Hamburger HL, Naeije M. Variability in the apnea-hypopnea index and its consequences for diagnosis and therapy evaluation. Respiration. 2009;77(1):32–37. 10.1159/000167790 [DOI] [PubMed] [Google Scholar]
- 41.Chediak AD, Acevedo-Crespo JC, Seiden DJ, Kim HH, Kiel MH. Nightly variability in the indices of sleep-disordered breathing in men being evaluated for impotence with consecutive night polysomnograms. Sleep. 1996;19(7):589–592. 10.1093/sleep/19.7.589 [DOI] [PubMed] [Google Scholar]
- 42.Engleman HM, McDonald JP, Graham D, et al. Randomized crossover trial of two treatments for sleep apnea/hypopnea syndrome: continuous positive airway pressure and mandibular repositioning splint. Am J Respir Crit Care Med. 2002;166(6):855–859. 10.1164/rccm.2109023 [DOI] [PubMed] [Google Scholar]
- 43.Lam B, Sam K, Mok WY, et al. Randomised study of three non-surgical treatments in mild to moderate obstructive sleep apnoea. Thorax. 2007;62(4):354–359. 10.1136/thx.2006.063644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Randerath WJ, Heise M, Hinz R, Ruehle KH. An individually adjustable oral appliance vs continuous positive airway pressure in mild-to-moderate obstructive sleep apnea syndrome. Chest. 2002;122(2):569–575. 10.1378/chest.122.2.569 [DOI] [PubMed] [Google Scholar]
- 45.Barnes M, McEvoy RD, Banks S, et al. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170(6):656–664. 10.1164/rccm.200311-1571OC [DOI] [PubMed] [Google Scholar]
- 46.Hoekema A, Stegenga B, Wijkstra PJ, van der Hoeven JH, Meinesz AF, de Bont LG. Obstructive sleep apnea therapy. J Dent Res. 2008;87(9):882–887. 10.1177/154405910808700917 [DOI] [PubMed] [Google Scholar]
- 47.Tan YK, L’Estrange PR, Luo YM, et al. Mandibular advancement splints and continuous positive airway pressure in patients with obstructive sleep apnoea: a randomized cross-over trial. Eur J Orthod. 2002;24(3):239–249. 10.1093/ejo/24.3.239 [DOI] [PubMed] [Google Scholar]
- 48.Yoshida K. A polysomnographic study on masticatory and tongue muscle activity during obstructive and central sleep apnea. J Oral Rehabil. 1998;25(8):603–609. 10.1046/j.1365-2842.1998.00290.x [DOI] [PubMed] [Google Scholar]
- 49.Sjöholm TT, Lowe AA, Miyamoto K, Fleetham JA, Ryan CF. Sleep bruxism in patients with sleep-disordered breathing. Arch Oral Biol. 2000;45(10):889–896. 10.1016/S0003-9969(00)00044-3 [DOI] [PubMed] [Google Scholar]
- 50.Phillips BA, Okeson J, Paesani D, Gilmore R. Effect of sleep position on sleep apnea and parafunctional activity. Chest. 1986;90(3):424–429. 10.1378/chest.90.3.424 [DOI] [PubMed] [Google Scholar]