Abstract
Study Objectives:
This study aimed to evaluate the effect of weighted chain blankets on insomnia and sleep-related daytime symptoms for patients with major depressive disorder, bipolar disorder, generalized anxiety disorder, and attention deficit hyperactivity disorder.
Methods:
One hundred twenty patients were randomized (1:1) to either a weighted metal chain blanket or a light plastic chain blanket for 4 weeks. The outcome was evaluated using the Insomnia Severity Index as primary outcome measure and day and night diaries, Fatigue Symptom Inventory, and Hospital Anxiety and Depression Scale as secondary outcome measures. Sleep and daytime activity levels were evaluated by wrist actigraphy.
Results:
At 4 weeks, there was a significant advantage in Insomnia Severity Index ratings of the weighted blanket intervention over the light blanket (P < .001) with a large effect size (Cohen’s d 1.90). The intervention by the weighted blanket resulted in a significantly better sleep-maintenance, a higher daytime activity level, and reduced daytime symptoms of fatigue, depression, and anxiety. No serious adverse events occurred. During a 12-month open follow-up phase of the study, participants continuing to use weighted blankets maintained the effect on sleep, while patients switching from a light to a weighted blanket experienced an effect on Insomnia Severity Index ratings similar to that of participants using the weighted blanket from the beginning.
Conclusions:
Weighted chain blankets are an effective and safe intervention for insomnia in patients with major depressive disorder, bipolar disorder, generalized anxiety disorder, or attention deficit hyperactivity disorder, also improving daytime symptoms and levels of activity.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Name: Controlled Study of Chain Blanket for Insomnia; URL: https://clinicaltrials.gov/ct2/show/NCT03546036; Identifier: NCT03546036.
Citation:
Ekholm B, Spulber S, Adler M. A randomized controlled study of weighted chain blankets for insomnia in psychiatric disorders. J Clin Sleep Med. 2020;16(9):1567–1577.
Keywords: insomnia, major depressive disorder, bipolar disorder, ADHD, weighted blankets
BRIEF SUMMARY
Current Knowledge/Study Rationale: Insomnia is common in psychiatric disorders, contributing to the effect of disease. This study aims to evaluate the effect of weighted chain blankets as a treatment for insomnia in patients with psychiatric diagnoses.
Study Impact: In this randomized controlled study, we show that weighted chain blankets, a commonly used empirical treatment for insomnia, significantly reduce the severity of insomnia and improve daytime symptoms in a wide range of psychiatric disorders (major depressive disorder, bipolar disorder, attention deficit hyperactivity disorder, or generalized anxiety disorder). The clinical efficacy was supported by an objective assessment of sleep utilizing wrist actigraphy.
INTRODUCTION
Insomnia is a clinical condition characterized by difficulty of initiating or maintaining sleep, often accompanied by daytime symptoms such as fatigue, anxiety, and depression. Among adults, insomnia is one of the most prevalent psychiatric conditions. Depending on the criteria used, the prevalence in the population varies between 30% and 48% having insomnia symptoms to around 6% fulfilling criteria for the diagnosis.1,2 In studies of patients with comorbid psychiatric diagnoses, the prevalence of insomnia tends to be higher, in average around 40% and reaching 80% in patients with depressive disorder.1,3 Insomnia is often long-lasting. In a study by Mallon et al, 75% of participants with insomnia at baseline showed persistence of insomnia 12 years later.4
Insomnia causes considerable discomfort and decreases the working capacity of the individual. It increases the risk of accidents and mortality, leading to high societal costs.5,6 Many psychiatric disorders are associated with high rates of severe insomnia, associated with substantial functional impairment.7 Up to 70% of bipolar patients and 60% of adults with depression and anxiety suffer from insomnia.8,9 Continued sleep disturbance after successful treatment of affective episodes is a risk factor for relapse in both major depressive and bipolar disorders.9–11 Among patients with attention deficit hyperactivity disorder (ADHD), the prevalence of insomnia has been estimated at 6% to 80%, enhancing the burden of cognitive dysfunction, fatigue, and emotional symptoms.12 Insomnia is a core feature of generalized anxiety disorder, affecting 60% to 70% of patients with this diagnosis.13,14
The primary therapeutic interventions for insomnia are cognitive behavioral therapy and pharmacological treatment. Although sufficient for a majority of patients, about 40% do not respond to those standard treatments.15 There is a need for additional therapies for insomnia.
Two methods commonly used by occupational therapists for patients in psychiatric care is the therapeutic use of compression and weight.16,17 A weighted chain blanket is a blanket in which metal chains have been sewn in. Chain blankets apply pressure to the whole body as a form of deep pressure stimulation and are used for sleeping problems and anxiety.
A suggested explanation for the calming and sleep-promoting effect is the pressure that the chain blanket applies on different points on the body, stimulating the sensation of touch and the sense of muscles and joints, similar to acupressure and massage.18,19 This increases the sense of the body and its limits and provides confidence. There is evidence suggesting that deep pressure stimulation increases parasympathetic arousal of the autonomic nervous system at the same time reducing sympathetic arousal, which is considered to be the cause of the calming effect.20,21 Also, the deep pressure on the body increases levels of oxytocin, which has a central role in relaxation and sleep. Oxytocin may produce anxiolytic-like or sedative effects and an increased pain threshold.22,23 There have also been attempts to improve sleep by other sorts of manipulations of bed clothing. McCall et al randomized 29 healthy adults to either bedsheets impregnated with bioceramic far-infrared technology or standard bedsheets. They found that persons assigned to far-infrared sheets reported fewer insomnia symptoms and less napping.24
Evidence for the clinical effect of weighted blankets for insomnia is scant. In a controlled study of autistic children with sleeping problems, the use of a weighted blanket did not decrease the time to fall asleep or increase the total sleeping time. The weighted blanket was, however, appreciated by children and parents, and blankets were well tolerated.25 In a study of children with ADHD, the authors concluded that the use of ball blankets is a suitable and effective method of treating insomnia.26 In a study of 32 healthy students, the use of weighted blankets was considered safe and calming with a small impact on blood pressure and pulse rate.17 An open study of 33 otherwise healthy participants complaining of chronic insomnia found that the weighted blanket had a positive impact on sleep, by both objective and self-reported measures.27 Evaluations have shown that weighted blankets for adults are a safe method.17,28 Controlled studies of weighted blankets as a treatment for insomnia in adults with co-occurring psychiatric disorders are nonexistent to our best knowledge, and a recent review of the clinical effects of weighted blankets found little evidence that they are helpful for insomnia.29,30
Despite the shortage of scientific evidence, treatment with weighted blankets is an increasingly recommended intervention for relaxation, insomnia, and other sleeping problems.17,26,27 In Stockholm, around 2,700 chain blankets are prescribed to adults in psychiatric care every year.
This project started with an open clinical follow-up study of weighted blankets in 199 psychiatric patients with affective and ADHD diagnoses and co-occurring insomnia.31 Due to the beneficial results, we decided to proceed with a controlled study.
Aims of the study
This study aimed to evaluate the effect of chain blankets on insomnia and sleep-related daytime symptoms for patients with major depressive, bipolar disorder, generalized anxiety disorder or attention deficit hyperactivity disorder in a controlled fashion.
METHODS
Study cohort
The study was performed in accordance with the World Medical Association Declaration of Helsinki after approval by the regional ethical review board in Stockholm (registration number 2015/102-31/2). All study procedures took place at the Affective Disorder Clinic at Psychiatry Southwest in Stockholm, Sweden, after oral information and the signing of a written consent form. Participants were free to withdraw from the study at any time without prejudice. Participants were recruited from affective disorder clinics in the Stockholm county through notification to doctors at the clinics that they could refer patients with psychiatric disorders and co-occurring insomnia to the occupational therapist at the Affective Disorder Outpatient Clinic for possible inclusion in a study of chain blankets. The referring doctors were not part of the study team and made their diagnoses of insomnia before patients consented to the study. The inclusion criteria for the study were clinical insomnia for more than 2 months with a score over 14 points at the Insomnia Severity Index (ISI) and a diagnosis of either major depressive disorder, bipolar disorder, generalized anxiety disorder, or ADHD. Exclusion criteria were active drug abuse, overuse of sleep medication, and illnesses affecting cognitive functions, such as dementia, schizophrenia, severe developmental disorders, Parkinson’s disease and acquired brain injury.
Primary and secondary outcomes
The ISI is the primary outcome measure in this study. ISI is a 7-item, self-report measure for the evaluation of insomnia.32 Items in the ISI concern difficulties falling asleep, problems staying asleep, daytime symptoms connected to the sleeping problem, and worrying about sleeping too little. Each item can be rated from 0 to 4, giving the rating scale a maximum summed score of 28 points. According to the guidelines for interpretation, 0–7 should be interpreted as no clinically significant insomnia, 8–14 as subthreshold insomnia, 15–21 as clinical insomnia of moderate severity, and 22–28 as severe clinical insomnia.32
The impact of sleep problems on quality of life and function during daytime was evaluated using the Fatigue Symptom Inventory, a self-report assessment to measure the intensity and duration of fatigue, and the Hospital Anxiety and Depression Scale (HAD) as a secondary outcome measure.33–35 The HAD is divided into subscales for depressive symptoms (HAD-D) and anxiety symptoms (HAD-A), which are reported separately. Neither the depression nor the anxiety subscale of HAD includes an item related to sleep.
Patients’ sleep symptoms were also evaluated from day and night diaries, a self-rating scale developed by Assistive Technology Stockholm (Stockholm, Sweden) for the evaluation of weighted blankets. In the day and night diaries, patients estimated the time it took to fall asleep (in minutes). They also rated 10 sleep-related symptoms on visual analog scales 65 mm in length, where 1 meant severe problems and 65 no problems. Five items concerned nighttime symptoms (awakenings, relaxed sleep, calm sleep, pain, and anxiety). The rating scale also contained 5 similar visual analog scales for daytime symptoms: alertness, concentration, worrying/relaxed, pain, and anxiety. We implemented objective analyses of nighttime sleep and daytime activity using wrist actigraphy. The study protocol did not include sleep diaries, which prevented us from calculating parameters using time in bed (such as sleep efficiency or latency to fall asleep). However, we could reliably estimate total sleep time, number of awakenings, and total time awake after sleep onset (wake after sleep onset, WASO) as objective measures of nighttime sleep and analyze them in relation with the main outcome measure. For daytime activity, we assessed the activity recorded during the most active 10 hours of the day (circadian peak of activity) by estimating the average hourly activity and the timing of peak of circadian activity.
Inclusion and randomization
From March 2015 to May 2017, 121 patients were referred for evaluation. One patient did not fulfill the criteria and was not included in the study. For a description of the study cohort, see Table 1. The patients were diagnosed clinically by psychiatrists who were not part of the study team or the evaluation of sleep disturbance with the ISI, and who had made the diagnoses before patients consented to be evaluated for the study. Participants were, with few exceptions, in a stable condition relative to their primary diagnoses but were experiencing problematic insomnia. Most of the patients used medication (Table 1). The patients and their doctor were told not to change doses during the study or stop or start using new drugs.
Table 1.
Description of the sample and distribution between the randomized groups.
| Variable | Total n = 120 | Control Blanket n = 56 | Weighted Blanket n = 64 | P-Value | |
|---|---|---|---|---|---|
| Sex | Female | 82 (68.3%) | 34 (60.7%) | 48 (75.0%) | .093 |
| Male | 38 (31.7%) | 22 (39.3%) | 16 (25.0%) | ||
| Age (mean 39.6, range 18–77) | ≤ 40 | 65 (54.2%) | 30 (53.6%) | 35 (54.7%) | .902 |
| >40 | 55 (45.8%) | 26 (46.4%) | 29 (45.3%) | ||
| Diagnoses | Bipolar type 1 | 14 (11.2%) | 8 (14,2%) | 6 (9.4%) | .679 |
| Bipolar type 2 | 25 (20.1%) | 13 (23.2%) | 12 (18.8%) | ||
| Bipolar NOS | 9 (7.5%) | 3 (5.4%) | 6 (9.4%) | ||
| Recurrent depression | 46 (38.3%) | 18 (32.1%) | 28 (43.8%) | ||
| GAD | 13 (10.8%) | 7 (12.5%) | 6 (9.4%) | ||
| ADHD | 13 (10.8%) | 7 (12.5%) | 6 (9.4%) | ||
| Use of hypnotics | Yes | 44 (36.7%) | 21 (37.5%) | 23 (35.9%) | .859 |
| Use of sedatives | Yes | 39 (32.5%) | 14 (25.0%) | 25 (39.1%) | .101 |
| Use of lithium | Yes | 24 (20.0%) | 9 (16.1%) | 15 (23.4%) | .314 |
| Use of anticonvulsants | Yes | 17 (14.2%) | 11 (19.6%) | 6 (9.4%) | .108 |
| Use of antipsychotics | Yes | 27 (22.5%) | 17 (30.4%) | 10 (15.6%) | .054 |
| Use of antidepressants | Yes | 64 (53.3%) | 26 (46.4%) | 38 (59.4%) | .156 |
| Use of stimulants | Yes | 11 (9.2%) | 6 (10.7%) | 5 (7.8%) | .582 |
| Sleep disturbance | Mean duration (years) | 20.2 (SD 15.0) | 19.7 (SD 14.0) | 20.6 (SD 15.9) | .755 |
For the grouping of medications used by the participants of the study, see Table 2. ADHD = attention deficit hyperactivity disorder, Bipolar-NOS = bipolar disorder not otherwise specified, GAD = generalized anxiety disorder, SD = standard deviation.
After inclusion, study participants were evaluated by ISI, the Fatigue Symptom Inventory (FSI), and HAD. During the first week after inclusion, participants were asked to wear an actigraph (GENEActiv, Activinsights Ltd., Cambridgeshire, UK) on the wrist of the nondominant arm in order to record activity. After completing the actigraphy recording, participants were randomly assigned either to a weighted chain blanket or a control blanket in a 1:1 proportion, using a concealed lottery method.
For the weighted chain blanket, a flexible weight protocol was used. Participants assigned to the weighted blanket tried an 8-kg chain blanket at the clinic. If they found it too heavy, a 6-kg weighted chain blanket was offered instead. As a control condition we used blankets into which plastic chains had been sewn of the same shape and size as the metal chains in the weighted blanket. The control blanket weighed 1535 g. When checking the weight of standard blankets for sale in one of the largest stores in Stockholm, we found their weights ranging from 550 to 2389 g (average 1332 g). Study participants were kept blind to treatment allocation by the information that they would be assigned to one of two types of chain blanket, without any information about the difference in weight. Blood pressure and heart rate were registered at randomization, and at the end of 4 weeks’ use of the blankets.
The controlled phase of the study was evaluated with the ISI by a telephone interview at week 1–2 and by visits at 3 and 4 weeks’ use of the blanket. Actigraphy recordings were acquired during the fourth week of the controlled phase of the study. FSI, HAD, and day and night diaries was also assessed at the fourth-week visit. ISI scores at 4 weeks after randomization was the primary endpoint. Response was defined as a decrease of 50% or more in the ISI from baseline to the endpoint. Remission was defined as a score of 7 or less on the ISI scale at the endpoint. All evaluations were performed by a rater blind to the randomization and allocation of blankets.
After completing the controlled phase of the study, participants were invited to enter a 12-month open continuation phase of the study. Participants who had been allocated to the weighted blanket were allowed to continue with weighted blankets, while participants assigned to the control blanket were switched to a weighted blanket. All patients, except 7, accepted the offer. Thus 112 participants continued using weighted blankets in the open part of the study. For the continuation phase, all participants tested 4 different weighted blankets: 2 chain blankets (6- and 8-kg) and two ball blankets (6.5- and 7-kg). After the test, the patients were freely allowed to choose the blanket which suited them best. Follow-up at 8 weeks after randomization with ISI consisted of a visit, and at 6 and 12 months after randomization, a telephone interview. The 7 participants who chose to stop using blankets were included in the follow-up study.
Objective evaluation of sleep
Actigraphic recordings were acquired using GENEActiv Original wrist-worn actigraphs (Activinsights). Briefly, the devices recorded wrist movement using 3-dimensional accelerometers capable of recording up to 8g with a resolution of 3.9mg in either direction at a 30-Hz sampling rate. The raw data was processed in MATLAB (The MathWorks, Natick, MD), using a modified version of the code (https://github.com/DavidRConnell/geneactivReader). The Euclidean norm of change in acceleration vector was first smoothed using a rolling Gauss window spanning 1 second (30 consecutive datapoints), then values below 20mg were set to 0 before computing the sum of changes in acceleration over 1-minute epochs. We defined 2 behavioral states, “sleep” and “wake” based on the recurrence rates calculated using recurrence quantification analysis (RQA), as described by Vanderlei Parro et al.36 This method requires the optimization of 3 parameters: window size (for smoothing the actigraphy data before calculating the recurrence plot), epsilon (the size of the vicinity to define similarity between trajectories in the phase space to derive the binary recurrence plot for RQA), and threshold for binary state classification (“asleep” and “awake”). These parameters are typically optimized by comparing simultaneous recordings of wrist actigraphy and polysomnography, the gold standard for quantitative sleep analysis.37 Polysomnography data were not available for this study, and the psychiatric pathology associated with insomnia in our population precluded the use of parameters optimized on populations of healthy controls available in the literature. Therefore, we used a combination of data-driven parameter optimization and heuristic selection of parameter values. We first smoothed the actigraphy data with a sliding Gauss window (width: 10 minutes). For the binary recurrence plot, we defined trajectories in the phase space to be “similar” if the distance between the points was smaller than the 10th percentile of all distances in the recurrence plot. Last, the threshold used for binary classification of states was determined using Otsu’s method on the recurrence rate. The recurrence rate, calculated as the rate of occurrence of similar trajectories for each datapoint, typically assumes a bimodal distribution, where resting episodes (steady, low-level activity) are characterized by high recurrence rate, while active episodes (variable and relatively intense activity) are characterized by low recurrence rate. Otsu’s method to set the threshold between the 2 states relies on optimization of intraclass variance. Individual thresholds estimated this way were used for binary classification of each datapoint as either “awake” or “asleep.” The calculations for objective parameters of sleep were based on activity recorded between 20:00 and 12:00. The resting period was defined as the timespan between the beginning of the first episode of consolidated sleep longer than 5 minutes (ie, at least 5 consecutive “asleep” datapoints) and the end of the last episode of consolidated sleep longer than 5 minutes. By setting these criteria, we aimed to reduce the bias of including severely fragmented sleep at transitions between consolidated sleep and wake periods. Total sleep time and WASO were estimated as the total number of datapoints labeled as “asleep” or “awake,” respectively, during the resting period.
To evaluate daytime activity, we focused on the circadian peak of activity defined as a continuous 10-hour-long recording segment containing the largest amount of activity. We divided the actigraphy recording into contiguous 24-hour segments (midnight to midnight) and smoothed the data with a 10-hour sliding Gaussian window before detecting the circadian peaks. We recorded the total amount of activity recorded during the circadian peak and the time of occurrence of the circadian peak of activity. All parameters were estimated separately for each 24-hour interval, then averaged per participant before statistical analyses. Participants with fewer than 3 consecutive days recorded were excluded because the within-participant variability is very high when intervals shorter than 4 days are analyzed (unpublished observation).
Statistical method
The analysis was done on an intention-to-treat basis. The randomization procedure was evaluated by a chi-square test for categorical variables (sex, age, diagnosis, and medication [yes/no]), and by t test for independent samples for the duration of sleep disturbance. We analyzed the change over time of the primary outcome variable ISI from preintervention to the end of the controlled phase of the study by the paired t test. Changes in ISI, FSI, and HAD scores during the study were also evaluated by repeated-measures analysis of variance. Response (yes/no) and remission (yes/no) were evaluated by logistic regression models with odds ratios and 95% confidence intervals. We also calculated the effect size by Cohen’s d.
Eight- and 6-kg blankets were treated together as weighted blankets in the analysis. Changes in blood pressure and pulse frequency were tested by repeated-measures analysis of variance. Data for dropouts were treated by the last observation–carried forward method. For the analysis of secondary outcome measures, we used repeated-measures analysis of variance models, followed by contrast analysis. All statistical analyses were performed in Statistica 13 (TIBCO, Palo Alto CA).
RESULTS
There were no significant differences between the randomized groups concerning sex, age, diagnostic composition, medication, or duration of sleep disturbance (Table 1 and for the grouping of medications, see Table 2). Ten participants found the 8-kg chain blanket too heavy and chose to use a 6-kg chain blanket instead. One participant discontinued the study due to feelings of anxiety when using the blanket. Thus, 119 participants completed the controlled phase of the study. Only the participant mentioned above, experiencing anxiety by the blanket, discontinued the controlled phase of the study. Otherwise there were no reports of side-effects. Actigraphy was recorded in 113 patients before treatment (109 recordings included after quality control) and in 84 patients after treatment (79 included after quality control). In the longitudinal analyses we included 77 participants recorded both before and after treatment (35 controls and 42 using weighted blankets, out of which 16 were nonresponders and 36 were responders).
Table 2.
Grouping of medications used by the participants of the study.
| Hypnotics | Nitrazepam |
| Zolpidem | |
| Zopiclone | |
| Propiomazine | |
| Sedatives | Alprazolam |
| Diazepam | |
| Hydroxyzine | |
| Oxazepam | |
| Promethazine | |
| Alimemazine | |
| Lithium | Lithium citrate |
| Lithium carbonate | |
| Anticonvulsants | Lamotrigine |
| Pregabalin | |
| Valproate | |
| Antipsychotics | Aripiprazole |
| Flupentixol | |
| Olanzapine | |
| Perphenazine | |
| Quetiapine | |
| Antidepressants | Amitriptyline |
| Bupropion | |
| Citalopram | |
| Duloxetine | |
| Escitalopram | |
| Fluoxetine | |
| Mirtazapine | |
| Nortriptyline | |
| Paroxetine | |
| Sertraline | |
| Venlafaxine | |
| Vortioxetine | |
| Stimulants | Methylphenidate |
Primary outcome
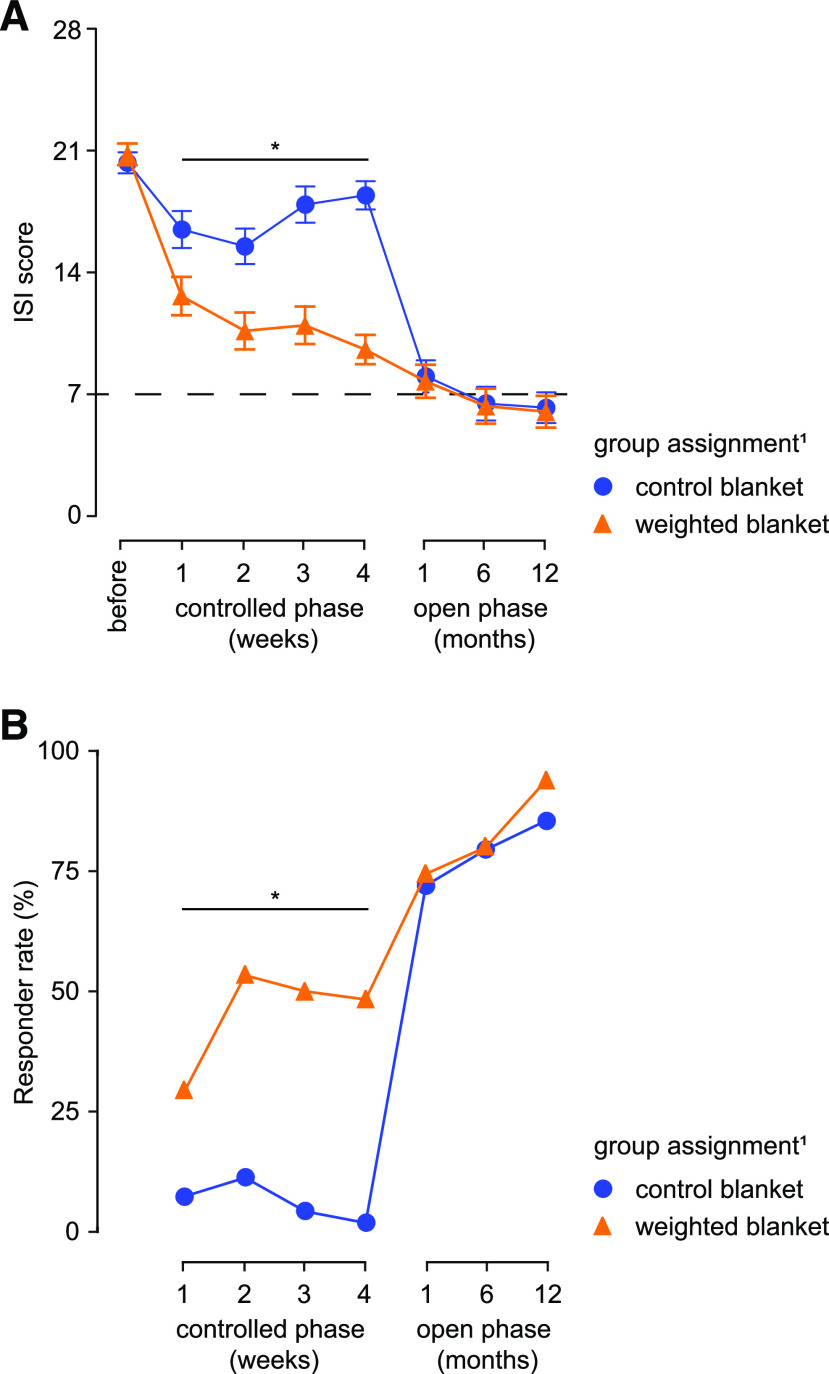
We found a significant effect of the weighted blanket on the primary outcome measure, ISI, compared to the control blanket for all study participants at endpoint (P < .001). In the analysis of all participants, the effect was significant after 1 week of blanket use (Table 3, Figure 1). We found no significant difference in the effect of the weighted blanket for sex or diagnostic subgroups.
Table 3.
Repeated measures analysis of variance.
| Diagnosis | Blanket | PRE | Week 1 | Week 2 | Week 3 | Week 4 | n | P-Value |
|---|---|---|---|---|---|---|---|---|
| ISI | ISI | ISI | ISI | ISI | ||||
| All | Control | 21.2 | 16.9 | 15.5 | 17.5 | 18.8 | 56 | < .001 |
| Weighted | 21.7 | 13.2 | 12.0 | 11.6 | 9.2 | 64 | ||
| Bipolar type 1 | Control | 20.6 | 15.9 | 14.0 | 16.4 | 16.5 | 8 | .026 |
| Weighted | 20.2 | 11.2 | 8.0 | 7.2 | 8.5 | 6 | ||
| Bipolar type 2 | Control | 21.1 | 16.8 | 12.9 | 15.1 | 18.4 | 13 | .001 |
| Weighted | 19.9 | 11.8 | 11.3 | 10.4 | 9.4 | 12 | ||
| Bipolar-NOS | Control | 21.3 | 15.0 | 20.0 | 22.7 | 22.0 | 3 | .001 |
| Weighted | 22.2 | 14.0 | 12.0 | 13.2 | 10.5 | 6 | ||
| Recurrent depression | Control | 21.7 | 17.7 | 17.0 | 17.8 | 18.7 | 18 | < .001 |
| Weighted | 22.7 | 13.5 | 13.2 | 13.0 | 9.6 | 28 | ||
| GAD | Control | 21.6 | 16.9 | 15.7 | 18.6 | 20.0 | 7 | < .001 |
| Weighted | 22.0 | 16.3 | 10.2 | 8.5 | 6.2 | 6 | ||
| ADHD | Control | 20.3 | 17.3 | 16.3 | 19.0 | 20.1 | 7 | .003 |
| Weighted | 21.0 | 13.5 | 13.8 | 14.0 | 9.5 | 6 | ||
| FSI | FSI | |||||||
| All | Control | 84.0 | — | — | — | 74.5 | 56 | < .001 |
| Weighted | 85.1 | — | — | — | 52.9 | 64 | ||
| HAD-D | HAD-D | |||||||
| All | Control | 9.5 | — | — | — | 8.9 | 56 | < .001 |
| Weighted | 10.5 | — | — | — | 6.1 | 64 | ||
| HAD-A | HAD-A | |||||||
| All | Control | 13.6 | — | — | — | 12.9 | 56 | < .001 |
| Weighted | 13.8 | — | — | — | 10.3 | 64 |
Repeated measures analysis of variance between control and weighted blanket on the Insomnia Severity Index (ISI), the primary outcome measure, and the secondary outcomes, FSI, HAD-D, and HAD-A, showing mean values, numbers, and test of significance between the interaction between time and the intervention. ADHD = attention deficit hyperactivity disorder, Bipolar-NOS = bipolar disorder not otherwise specified, FSI = Fatigue Symptom Inventory, GAD = generalized anxiety disorder, HAD-A = Hospital Anxiety and Depression Scale subscale for anxiety symptoms, HAD-D = Hospital Anxiety and Depression Scale subscale for depressive symptoms.
Figure 1. Effects of weighted blankets on insomnia severity.
(A) In the controlled phase, total ISI score decreased significantly in patients using the weighted blankets already after 1 week. In the open phase, the average total ISI score decreased to 7 and lower, indicative of remission (dashed line indicates the ISI score defining remission). (B) The proportion of responders (ie, patients in whom ISI score decreased below 50% of initial value) is significantly higher in patients using weighted blankets compared to patients using control blankets in the controlled phase. In the open phase of the study, where all participants used a weighted blanket, the proportion of responders further increased and was not different between the patients assigned initially to control or weighted blankets. 1Group assignment applies only to the controlled phase (first 4 weeks of the study). *P < .05, interaction effect, repeated measures analysis of variance (A); chi-square test for proportions (B).
The response rate was 59.4% (n = 38) for the weighted blanket group compared to 5.4% (n = 3) for the control blanket group (Figure 1). Remission rate was 42.2% (n = 27) compared to 3.6% (n = 2). The likelihood of responding was almost 26 times greater in the weighted blanket group than in the control blanket group (odds ratio 25.8, 95% CI 6.8–85.7) and the likelihood to remit was nearly 20 times greater (odds ratio 19.7, 95% CI 4.4–87.9). The effect-size was large, with a Cohen’s d of 1.90. Participants who continued to the 12-month open phase of the study numbered 112. Participants who switched from the control blanket to a weighted blanket experienced an effect similar to patients who had used the weighted blanket from the beginning, and improvements increased during the 12-month follow-up period (Figure 1). At 12 months, 92% of all initial participants (n = 119), including those participants who chose not to continue with the blankets, were responders and 78% were in remission.
Analysis of nighttime sleep in relation to primary outcome
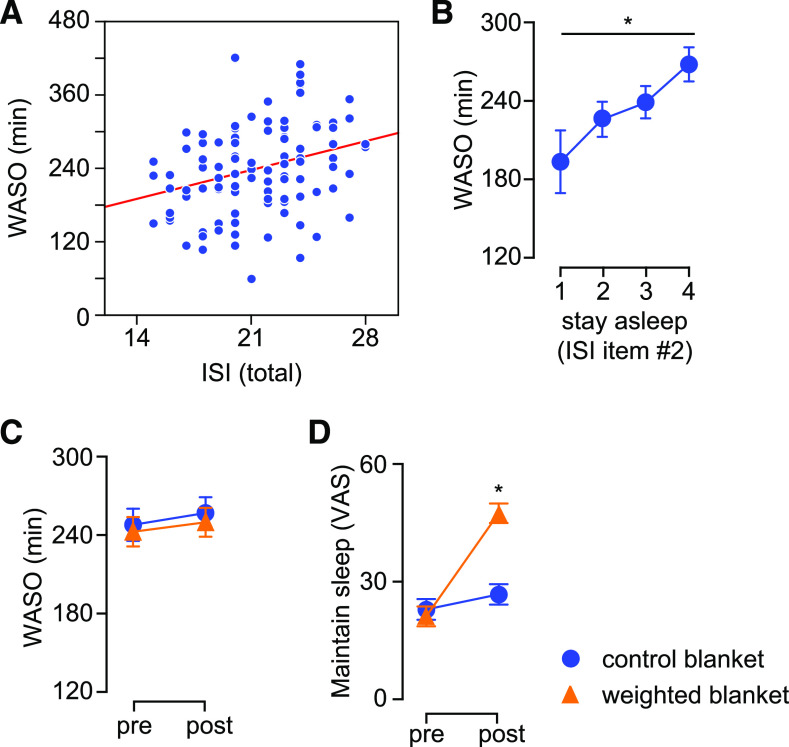
Next, we investigated the effects of weighted blankets on nighttime sleep using objective parameters of sleep derived from actigraphy recordings as well as self-reported scales. First, we validated WASO against the primary outcome measure and found that WASO was positively correlated with the total ISI score before treatment (Figure 2A), and that an increasing score on ISI item no. 2 (difficulty staying asleep) was associated with higher WASO (Figure 2B). Weighted blankets did not have a significant effect on total sleeping time (not shown) or WASO (Figure 2C). However, patients using weighted blankets reported an improvement in the ability to maintain sleep (Figure 2D).
Figure 2. Assessment of nighttime sleep in relation to primary outcome measure.
(A) Total time awake after sleep onset correlated with total ISI score in all patients before treatment (Pearson r = 0.29, P < .05). (B) WASO increased with self-reported difficulty to maintain sleep (item no. 2 on ISI scale) before treatment. (C) Using weighted blankets did not have a significant effect on WASO. (D) Patients using weighted blankets reported improved ability to maintain sleep (assessed by means of visual analog scale, VAS). *P < .05, repeated measures analysis of variance, followed by contrast analysis. VAS = visual analog scale, WASO = wake after sleep onset.
Effects on daytime activity
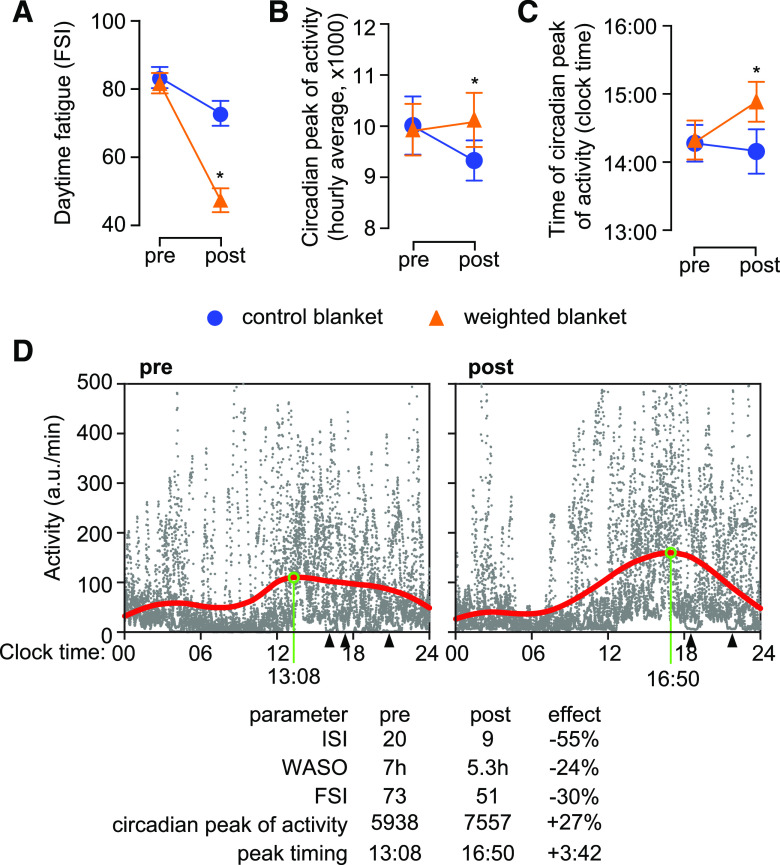
Poor sleep is associated with worse performance in daytime activity.38 When we analyzed the daytime fatigue, as quantified by FSI, we found a significant reduction in FSI in patients using weighted blankets (Table 3, Figure 3A). Next, we asked whether the decrease in FSI was reflected in altered patterns of daytime activity. To this end we focused on the circadian peak of activity, which evaluates the participant’s ability to output sustained activity during the day. We found that patients using weighted blankets displayed a significant increase in circadian peak of activity after treatment compared to controls (Figure 3B). In addition, the circadian peak was delayed in patients using weighted blankets (Figure 3C). This means that the level of activity was increased, and the patients were able to sustain activity for longer time, presumably also with lower number of resting periods required during daytime (illustrated in Figure 3D).
Figure 3. Effects of weighted blankets on fatigue and daytime activity.
(A) Daytime fatigue score decreased significantly after treatment in patients using weighted blankets. (B) Daytime activity increased only in patients using weighted blankets. (C) The time of occurrence of circadian peak of activity was delayed after treatment in patients using weighted blankets, but not in the control group. (D) Illustration of changes in patterns of activity in one patient after using weighted blanket for 4 weeks. Raw data (gray dots) is displayed as the amount of activity (integrated over 1-minute bins) plotted against the time of collection relative to the 24-hour cycle (6 consecutive days on both occasions). The circadian profile (red line) was obtained by smoothing the raw data with a sliding 10-hour-wide Gaussian window. Before treatment, the patient exhibited a rather flat circadian profile, with consistent activity during the night (consolidated sleep episodes between 4:00 and 13:00) and a very short period of consistent activity (between 13:00 and 16:00). In contrast, activity during nighttime was reduced after treatment, and the circadian peak of activity was more robust and occurred later (16:50 vs 13:08). Note that after treatment the patient had only 2 episodes of consolidated sleep (arrowheads) between 12:30 and 23:00 over 6 days, which illustrates the improved ability to sustain activity during daytime. *P < .05, repeated measures analysis of variance, followed by contrast analysis. FSI = Fatigue Symptom Inventory, ISI = Insomnia Severity Index, WASO = wake after sleep onset.
Effect on nighttime sleep and daytime activity in responders
We then analyzed in what way the changes in nighttime sleep may differ in relation to the response to treatment. To this end, we split the group of patients using weighted blankets into responders and nonresponders based on the relative change in ISI score using a threshold value of 50% (responder-relative decrease > 50%; nonresponder-relative decrease < 50%) before assessing the changes induced by the use of weighted blankets. We found that responders displayed a significant decrease in WASO (Figure 4A) and reported a larger improvement in sleep maintenance (Figure 4B) compared to nonresponders. In addition, the increase in the circadian peak of activity was similar in responders and nonresponders (Figure 4C). In contrast, the delay in the timing of the circadian peak of activity was significant only in responders (Figure 4D).
Figure 4. Differences between responders and nonresponders within the group of patients using weighted blankets.
(A) WASO decreased significantly only in responders. (B) Patients responding to treatment reported a more robust increase in sleep maintenance than nonresponders. (C) The increase in activity during the most active part of the day (circadian peak of activity) was similar in responders and nonresponders. (D) The circadian peak of activity was delayed in responders, but not in nonresponders. *P < .05, repeated measures analysis of variance, followed by contrast analysis. VAS = visual analog scale, WASO = wake after sleep onset.
Effect on depression, anxiety, and blood pressure
Also, we found that depressive symptoms and anxiety symptoms decreased significantly for participants allocated to the weighted blanket, compared to participants assigned to the control blanket in the controlled phase (Table 3). The blood pressure decreased significantly from randomization to 4 weeks in both groups, with no significant differences between the weighted and control blanket (Table 3). A few patients with suspected hypertension were referred to primary care.
DISCUSSION
In this randomized study, weighted blankets showed a significant effect on insomnia in patients with major depressive disorder, bipolar disorder, generalized anxiety disorder, or ADHD. The impact on insomnia was clinically meaningful. The mean level of insomnia improved from almost reaching “severe,” to a level of “subthreshold insomnia” on the primary outcome measure, ISI. The likelihood to remit was 26 times larger in the weighted blanket group than in the control blanket group. The 12-month follow-up study supports the long-term effect of weighted blankets. Participants who continued using the weighted blankets maintained the impact on sleep, while patients switching from a light to a weighted blanket got an effect on ISI ratings similar to participants using the weighted blanket from the beginning.
In the controlled phase of the study, 10 participants found the 8-kg chain blanket too heavy and used a 6-kg blanket instead. However, after 4 weeks of use, most of the participants in the open phase selected a heavier blanket. Only 1 participant discontinued the study due to feelings of anxiety when using the blanket. This is a rare occurrence in clinical practice, and patients with claustrophobia are usually able to continue treatment with a lighter blanket.
We found it interesting that there were more than twice as many women as men included in the study. This could be considered as sex bias, but it is consistent with earlier reports on the higher prevalence of insomnia among women.39,40 There is a need for further research to understand why the prevalence of insomnia and other sleeping problems differ so much between women and men. However, no difference between sex in the effect of weighted blankets was found in this study.
In addition to decreasing the severity of insomnia, patients using weighted blankets reported positive effects on daytime fatigue. This was corroborated by objective measures of daytime activity: patients using weighted blankets appeared able to have more sustained active intervals, as demonstrated by the increase in activity level and the delay in the time of occurrence of circadian peak of activity.
The intervention by the weighted blanket also significantly improved symptoms of depression and anxiety compared to the control blanket group. These findings might seem surprising. However, previous studies described similar antidepressant effects of sleep therapy using cognitive behavioral therapy.41,42 One suggested explanation for the antidepressant effect of improved sleep is that depression is more often caused or maintained by insomnia than vice versa.43–45 In addition, reduced daytime fatigue may also facilitate behavioral activation, which is a cornerstone of cognitive behavioral therapy for depression. Other suggested mechanisms are the effect of decreased sleep on the production of brain-derived neurotrophic factor and changes in the cortisol hormonal and immune systems.46,47
We also investigated the differences in the effects of the intervention in patients who responded compared to patients who did not respond. Both self-ratings and objective measures of nighttime sleep indicated that responders experienced significantly better sleep maintenance. Similarly, the effect of weighted blankets on daytime activity point to a stronger effect in responders than in nonresponders.
A limitation of the study was the risk of disclosure of blanket-type to the rater, who was supposed to be blind to treatment allocation. Disclosure of blanket type, however, happened only once during the study when a participant unintentionally revealed the type by relating that the blanket had signaled in a metal detector at an airport security control station. In addition, the study protocol did not include sleep journals or polysomnographic recordings, which restricted the extent of the objective investigation of sleep.
Strengths of the study are the controlled design and the relatively large number of participants. The inclusion of patients with different psychiatric diagnoses improved the generalizability of the study since the reduction in the severity of insomnia was found in all diagnostic subgroups, implying that the effects of weighted blankets are independent of psychiatric diagnosis. The primary outcome measure ISI is an established rating scale for insomnia. In a comparative study of 6 rating scales for insomnia, ISI was found to be the most accurate measure to discriminate cases and non-cases of insomnia.48 Also, the results are supported by objective sleep parameters derived from wrist actigraphy recordings. The long follow up period support the results of the controlled phase of the study. The study has a high ecological validity since the participants were real patients from psychiatric clinics.
In conclusion, we have shown that weighted blankets are an effective, safe, and clinically meaningful treatment for insomnia in patients with co-occurring major depressive disorder, bipolar disorder, ADHD, or generalized anxiety disorder. The intervention by the weighted blanket improved not only nighttime sleep but also daytime functioning, as illustrated by higher activity levels and reduced symptoms of fatigue, depression, and anxiety.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. The study was performed at Psychiatric Clinic Southwest, Karolinska University Hospital Huddinge, Stockholm. The study was supported by grants provided by Region Stockholm (ALF project, application nr. 20140099). The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
Research nurse Inger Römer assisted in conducting the study.
ABBREVIATIONS
- ADHD
attention deficit hyperactivity disorder
- FSI
Fatigue Symptom Inventory
- HAD
Hospital Anxiety and Depression Scale
- ISI
Insomnia Severity Index
- WASO
wake after sleep onset
REFERENCES
- 1.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. 10.1053/smrv.2002.0186 [DOI] [PubMed] [Google Scholar]
- 2.Morin CM, LeBlanc M, Daley M, Gregoire JP, Mérette C. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123–130. 10.1016/j.sleep.2005.08.008 [DOI] [PubMed] [Google Scholar]
- 3.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479–1484. 10.1001/jama.1989.03430110069030 [DOI] [PubMed] [Google Scholar]
- 4.Mallon L, Broman JE, Akerstedt T, Hetta J. Insomnia in sweden: a population-based survey. Sleep Disord. 2014;2014:7 pages. 10.1155/2014/843126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Léger D, Bayon V. Societal costs of insomnia. Sleep Med Rev. 2010;14(6):379–389. 10.1016/j.smrv.2010.01.003 [DOI] [PubMed] [Google Scholar]
- 6.Wickwire EM, Shaya FT, Scharf SM. Health economics of insomnia treatments: The return on investment for a good night’s sleep. Sleep Med Rev. 2016;30:72–82. 10.1016/j.smrv.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 7.Soehner AM, Harvey AG.Prevalence and functional consequences of severe insomnia symptoms in mood and anxiety disorders: results from a nationally representative sample. Sleep. 2012;35(10):1367–1375. 10.5665/sleep.2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harvey AG, Schmidt DA, Scarnà A, Semler CN, Goodwin GM. Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. Am J Psychiatry. 2005;162(1):50–57. 10.1176/appi.ajp.162.1.50 [DOI] [PubMed] [Google Scholar]
- 9.Kraus SS, Rabin LA. Sleep America: managing the crisis of adult chronic insomnia and associated conditions. J Affect Disord. 2012;138(3):192–212. 10.1016/j.jad.2011.05.014 [DOI] [PubMed] [Google Scholar]
- 10.Harvey AG. Sleep and circadian rhythms in bipolar disorder: seeking synchrony, harmony, and regulation. Am J Psychiatry. 2008;165(7):820–829. 10.1176/appi.ajp.2008.08010098 [DOI] [PubMed] [Google Scholar]
- 11.Gershon A, Do D, Satyanarayana S, et al. Abnormal sleep duration associated with hastened depressive recurrence in bipolar disorder. J Affect Disord. 2017;218:374–379. 10.1016/j.jad.2017.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon SY, Jain U, Shapiro C. Sleep in attention-deficit/hyperactivity disorder in children and adults: past, present, and future. Sleep Med Rev. 2012;16(4):371–388. 10.1016/j.smrv.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 13.Anderson DJ, Noyes R, Jr., Crowe RR. A comparison of panic disorder and generalized anxiety disorder. Am J Psychiatry. 1984;141(4):572–575. 10.1176/ajp.141.4.572 [DOI] [PubMed] [Google Scholar]
- 14.Monti JM, Monti D. Sleep disturbance in generalized anxiety disorder and its treatment. Sleep Med Rev. 2000;4(3):263–276. 10.1053/smrv.1999.0096 [DOI] [PubMed] [Google Scholar]
- 15.Morin CM, Vallières A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009;301(19):2005–2015. 10.1001/jama.2009.682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Champagne T, Stromberg N. Sensory approaches in inpatient psychiatric settings: innovative alternatives to seclusion & restraint. J Psychosoc Nurs Ment Health Serv. 2004;42(9):34–44. 10.3928/02793695-20040901-06 [DOI] [PubMed] [Google Scholar]
- 17.Mullen B, Champagne T, Krishnamurty S, Dickson D. Gao RX. Exploring the safety and therapeutic effects of deep pressure stimulation using a weighted blanket. Occup Ther Ment Health. 2008;24(1):65–89. 10.1300/J004v24n01_05 [DOI] [Google Scholar]
- 18.Lee MS, Kim JI, Ernst E. Massage therapy for children with autism spectrum disorders: a systematic review. J Clin Psychiatry. 2011;72(3):406–411. 10.4088/JCP.09r05848whi [DOI] [PubMed] [Google Scholar]
- 19.Waits A, Tang YR, Cheng HM, Tai CJ, Chien LY. Acupressure effect on sleep quality: A systematic review and meta-analysis. Sleep Med Rev. 2018;37:24–34. 10.1016/j.smrv.2016.12.004 [DOI] [PubMed] [Google Scholar]
- 20.Reynolds S, Lane SJ, Mullen B. Effects of deep pressure stimulation on physiological arousal. Am J Occup Ther. 2015;69(3):0010p1-5. [DOI] [PubMed] [Google Scholar]
- 21.Chen HY, Yang H, Meng LF, Chan PS, Yang CY, Chen HM. Effect of deep pressure input on parasympathetic system in patients with wisdom tooth surgery. J Formos Med Assoc. 2016;115(10):853–859. [DOI] [PubMed] [Google Scholar]
- 22.Uvnäs-Moberg K. Antistress pattern induced by oxytocin. News Physiol Sci. 1998;13:22–25. [DOI] [PubMed] [Google Scholar]
- 23.Uvnäs-Moberg K, Arn I, Magnusson D. The psychobiology of emotion: the role of the oxytocinergic system. Int J Behav Med. 2005;12(2):59–65. 10.1207/s15327558ijbm1202_3 [DOI] [PubMed] [Google Scholar]
- 24.McCall William V, Letton A, Lundeen J, Case D, Cidral-Filho Francisco J. The effect of far–infrared emitting sheets on sleep. Res J Text Appear. 2018;22(3):247–259. 10.1108/RJTA-02-2018-0008 [DOI] [Google Scholar]
- 25.Gringras P, Green D, Wright B, et al. Weighted blankets and sleep in autistic children--a randomized controlled trial. Pediatrics. 2014;134(2):298–306. 10.1542/peds.2013-4285 [DOI] [PubMed] [Google Scholar]
- 26.Hvolby A, Bilenberg N. Use of Ball Blanket in attention-deficit/hyperactivity disorder sleeping problems. Nord J Psychiatry. 2011;65(2):89–94. 10.3109/08039488.2010.501868 [DOI] [PubMed] [Google Scholar]
- 27.Ackerley R, Badre G, Olausson H. Positive effects of a weighted blanket on insomnia. J Sleep Med Disord. 2015;2(3):1022. [Google Scholar]
- 28.Champagne T, Mullen B, Dickson D, Krishnamurty S. Evaluating the safety and effectiveness of the weighted blanket with adults during an inpatient mental health hospitalization. Occup Ther Ment Health. 2015;31(3):211–233. 10.1080/0164212X.2015.1066220 [DOI] [Google Scholar]
- 29.SBU. Scientific Knowledge Gap: Weighted Blankets in Sleep Disorders Caused by Motor Agitation or Mental Anxiety (Vetenskaplig kunskapslucka: Tyngdtäcke vid sömnsvårigheter orsakade av motorisk eller psykisk oro). The Swedish Council of Health Technology Assessment (SBU); 2013. https://www.sbu.se/sv/publikationer/kunskapsluckor/tyngdtacke-vid-somnsvarigheter-orsakade-av-motorisk-eller-psykisk-oro-/. Accessed April 20, 2020. [Google Scholar]
- 30.Eron K, Kohnert L, Watters A, Logan C, Weisner-Rose M, Mehler PS. Weighted blanket use: a systematic review. Am J Occup Ther. 2020;74(2):p1–, p14.. [DOI] [PubMed] [Google Scholar]
- 31.Ekholm B, Adler M. Weighted blankets for insomnia in affective disorder and ADHD–a clinical follow up study. PsyArXiv. Preprint posted online November 30, 2018. doi: 10.31234/osf.io/wjsr2. [DOI] [Google Scholar]
- 32.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. 10.1016/S1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- 33.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 34.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77. 10.1016/S0022-3999(01)00296-3 [DOI] [PubMed] [Google Scholar]
- 35.Donovan KA, Jacobsen PB, Small BJ, Munster PN, Andrykowski MA. Identifying clinically meaningful fatigue with the Fatigue Symptom Inventory. J Pain Symptom Manage. 2008;36(5):480–487. 10.1016/j.jpainsymman.2007.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parro VC, Valdo L. Sleep-wake detection using recurrence quantification analysis. Chaos. 2018;28(8):085706. 10.1063/1.5024692 [DOI] [PubMed] [Google Scholar]
- 37.Scott H, Lack L, Lovato N. A systematic review of the accuracy of sleep wearable devices for estimating sleep onset. Sleep Med Rev. 2020;49:101227. 10.1016/j.smrv.2019.101227 [DOI] [PubMed] [Google Scholar]
- 38.Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007. 3, 5, Suppl:S7–S10. [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29(1):85–93. 10.1093/sleep/29.1.85 [DOI] [PubMed] [Google Scholar]
- 40.Green MJ, Espie CA, Benzeval M. Social class and gender patterning of insomnia symptoms and psychiatric distress: a 20-year prospective cohort study. BMC Psychiatry. 2014;14(1):152. 10.1186/1471-244X-14-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blom K, Jernelöv S, Kraepelien M, et al. Internet treatment addressing either insomnia or depression, for patients with both diagnoses: a randomized trial. Sleep. 2015;38(2):267–277. 10.5665/sleep.4412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carney CE, Edinger JD, Kuchibhatla M, et al. Cognitive Behavioral Insomnia Therapy for Those With Insomnia and Depression: A Randomized Controlled Clinical Trial. Sleep. 2017;40(4):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang PP, Ford DE, Mead LA, Cooper-Patrick L, Klag MJ. Insomnia in young men and subsequent depression. The Johns Hopkins Precursors Study. Am J Epidemiol. 1997;146(2):105–114. 10.1093/oxfordjournals.aje.a009241 [DOI] [PubMed] [Google Scholar]
- 44.Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, Rössler W. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31(4):473–480. 10.1093/sleep/31.4.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meerlo P, Havekes R, Steiger A. Chronically restricted or disrupted sleep as a causal factor in the development of depression. Curr Top Behav Neurosci. 2015;25:459–481. 10.1007/7854_2015_367 [DOI] [PubMed] [Google Scholar]
- 46.Rahmani M, Rahmani F, Rezaei N. The Brain-Derived Neurotrophic Factor: Missing Link Between Sleep Deprivation, Insomnia, and Depression. Neurochem Res. 2020;45(2):221–231. 10.1007/s11064-019-02914-1 [DOI] [PubMed] [Google Scholar]
- 47.Kurczewska E, Ferensztajn-Rochowiak E, Rybakowski F, Michalak M, Rybakowski J. Treatment-resistant depression: Neurobiological correlates and the effect of sleep deprivation with sleep phase advance for the augmentation of pharmacotherapy. World J Biol Psychiatry. 2020:1–12. 10.1080/15622975.2020.1755449 [DOI] [PubMed] [Google Scholar]
- 48.Seow LSE, Abdin E, Chang S, Chong SA, Subramaniam M. Identifying the best sleep measure to screen clinical insomnia in a psychiatric population. Sleep Med. 2018;41:86–93. 10.1016/j.sleep.2017.09.015 [DOI] [PubMed] [Google Scholar]