Abstract
Study Objectives:
High-frequency electroencephalographic activity (> 16 Hz activity) is often elevated during nonrapid eye movement sleep among individuals with insomnia, in line with the hyperarousal theory of insomnia. Evidence regarding sleep depth marked by slow-wave activity (< 4 Hz) is more mixed. Distinguishing subcomponents of slow-wave activity (slow-oscillation [< 1 Hz] or delta activity [1–4 Hz)]) may be critical in understanding these discrepancies, given that these oscillations have different neural generators and are functionally distinct. Here we tested the effects of insomnia diagnosis and insomnia treatment on nonrapid eye movement electroencephalography in older adults, distinguishing slow-oscillation and delta power.
Methods:
In 93 older adults with insomnia and 71 good sleeper control participants (mean ages 68 years), effects of insomnia and cognitive behavioral therapy for insomnia (insomnia group only) on electroencephalographic spectral power were analyzed. Main effects and interactions with nonrapid eye movement period were assessed for the following frequency bands: slow-oscillation (0.5–1 Hz), delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), sigma (12–16 Hz), and beta (16–32 Hz).
Results:
Slow-oscillation absolute and relative power were lower in the insomnia group compared with controls. There were no group differences in delta power. Insomnia was also associated with elevated 4–32 Hz absolute and relative power. After cognitive behavioral therapy for insomnia, absolute sigma and beta activity decreased.
Conclusions:
Deficits in slow-wave activity in insomnia are specific to the slow-oscillation. Elevated high frequency activity is reduced for sigma and beta power following cognitive behavioral therapy for insomnia . These findings inform the pathophysiology of insomnia, including the mechanisms underlying cognitive behavioral therapy for insomnia in older adults.
Citation:
Hogan SE, Delgado GM, Hall MH, et al. Slow-oscillation activity is reduced and high frequency activity is elevated in older adults with insomnia. J Clin Sleep Med. 2020;16(9):1445–1454.
Keywords: insomnia, older adults, slow-wave activity, slow-oscillation, hyperarousal
BRIEF SUMMARY
Current Knowledge/Study Rationale: Evidence that low frequency electroencephalographic activity (slow-wave activity) during nonrapid eye movement sleep is diminished in insomnia is mixed. However, the effects of insomnia on slow-wave activity may vary by the range of slow-wave activity (slow-oscillation [0.5–1 Hz] or delta [1–4 Hz]).
Study Impact: Insomnia-control differences were observed for the slow-oscillation but not delta power. Power in frequency bands 4–32 Hz was elevated in insomnia. Following cognitive behavioral therapy for insomnia, absolute sigma and beta activity consistently decreased across nonrapid eye movement periods.
INTRODUCTION
Hyperarousal is a hallmark of insomnia.1,2 Compared with adults without insomnia, adults with insomnia show elevated autonomic activity, including higher cortisol secretion, heart rate, heart rate variability, and increased body temperature.1 Surprisingly, polysomnography, the gold standard of sleep assessment, does not show a consistent pattern of electroencephalographic (EEG) differences between insomnia and controls,3 and the pattern of differences may depend on age.4 Most consistently, high-frequency beta EEG power (ie, > 16 Hz) is elevated in primary insomnia during nonrapid eye movement (NREM) sleep independent of awakenings.1,5–8 However, these differences are not consistently identified.4,9,10 Few studies have examined effects of insomnia treatment on quantitative EEG in insomnia,11,12 but how power in these EEG frequency bands changes with insomnia treatment is also variable across studies. Higher beta power is thought to reflect heightened cortical arousal peri-sleep onset13,14 and early in the night.15 Less consistently, other high-frequency bands, including theta,16 alpha,8 and sigma activity,17 have been shown to differ in insomnia compared with age-matched and sex-matched good sleepers, but the direction of the difference is inconsistent between studies. Moreover, low-frequency activity (slow-wave activity < 4 Hz), which is highest during slow-wave sleep and increases as a function of prior wakefulness duration and sleep depth,18 is reduced in some studies of insomnia,10, 13,16 whereas other studies have found no differences or have found higher slow-wave activity in insomnia.9,10,15,17,19 Thus, it remains unclear whether insomnia is characterized by alterations in sleep-specific features (ie, slow-wave activity)5 in addition to elevated high-frequency EEG activity and whether this underlying EEG physiology is reversed with the dramatic global sleep improvements found with insomnia treatment.
Studies of whole night insomnia NREM EEG have typically treated slow-wave activity as a single frequency range. Alternatively, slow-wave activity can be separated into slow-oscillation (< 1 Hz) and delta (1–4 Hz) components.20,21 Prior studies of whole night NREM EEG in insomnia have often combined slow-oscillation and delta activity9,16,22 or examined only > 1 Hz activity.5 However, these 2 frequency bands have different neural generators,18,21 may interact differently with higher frequency oscillations,23 and are thought to serve distinct cognitive functions.20 For instance, delta activity is driven by thalamocortical oscillations, whereas slow-oscillations are generated cortically, independent of the thalamus.18,21 Furthermore, the slow-oscillation is considered the critical component of slow-wave activity to promote memory consolidation.20 Thus, by examining slow-oscillation and delta components separately, we may more closely capture the EEG phenomenology that underlies physiology and hyperarousal in insomnia. Moreover, by examining specific changes in these 2 frequency bands following treatment for insomnia, we may inform therapeutic targets and better understand the mechanisms underlying improvements in sleep with insomnia treatment.
We assessed NREM EEG differences between older adults with insomnia and good sleeping controls prior to cognitive behavioral therapy for insomnia (CBTI) and, in the insomnia group only, after CBTI. We assessed the following frequency bands: slow-oscillation (0.5–1 Hz), delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), sigma (spindle) (12–16 Hz), and beta (16–32 Hz). Main effects of insomnia and interactions with NREM period were assessed. Assessing older adults has advantages given reported moderating effects of older age on insomnia EEG patterns,4 as well as the prevalence of insomnia in older adults.24
We hypothesized that insomnia and control groups would differ in the slow-oscillation and higher frequencies, particularly beta activity. We hypothesized that differences in slow-oscillation activity would be greatest in earlier NREM periods, suggesting greater sleep drive in control groups, but that high-frequency activity would be elevated across the night in insomnia, in line the 24-hour view of hyperarousal. We further hypothesized that CBTI would lead to a steeper decline in slow-oscillation power as a function of NREM period, suggesting that enhanced sleep drive would be reflected in the slowest component of slow-wave activity.11
METHODS
Overview
Participants were older adults with insomnia and good sleeper controls recruited through the AgeWise program project (AG020677), which assessed polysomnographic sleep in older adults with insomnia before and after an 8-week CBTI treatment. We examined differences in NREM quantitative EEG between control and insomnia groups, as well as differences between the 2 time points (T1 and T2) pre-CBTI to post-CBTI in the insomnia group only.
Participants
Participants over 60 years old were recruited via advertisements, newspaper articles, and community events in the greater Pittsburgh area. No participants were recruited from clinical settings or providers. All participants provided informed consent as approved by the University of Pittsburgh Institutional Review Board. Table 1 describes demographic and clinical characteristics for control and insomnia groups. Participants were included in the insomnia group if they met Diagnostic and Statistical Manual of Mental Disorders, 4th edition/International Classification of Sleep Disorders, 2nd edition diagnostic criteria for primary insomnia. Participants in the insomnia group also had to fulfill severity criteria, including an insomnia severity index score > 10, a combined mean sleep diary-assessed sleep latency + wake after sleep onset > 40 min, and sleep efficiency < 90%. Sleep efficiency was calculated using “lights out” to final awakening as the denominator as opposed to the commonly used denominator of time-in-bed to time-out-of-bed. This stringent definition of sleep efficiency was used because it is shown to have greater sensitivity and specificity than the latter in distinguishing older adults with insomnia from good sleeping controls.25 Participants in the control group had insomnia severity index scores < 7. Participants who met criteria for significant sleep apnea (apnea-hypopnea index [AHI] > 20 events/h based on an in-lab apnea screen night) or restless legs syndrome (based on structured clinical interview) were excluded from the study to rule out sleep problems related to issues other than insomnia. Three participants whose AHI, initially estimated to be < 20 events/h, were excluded from analyses after study participation when their scored record suggested that their AHI was above 20 events/h. AHI scores ranged from 0 to 19.9 events/h. The AHI threshold of 20 events/h was chosen to enhance generalizability, due to the elevated prevalence of sleep apnea among older adults.26 Participants were not eligible for inclusion in either group if they were currently involved in nighttime shift work, had any untreated current severe psychiatric conditions, unstable medical conditions, or neurological disorders (including multiple sclerosis, stroke, Parkinson disease, Alzheimer disease, seizure disorder, delirium, dementia, or previous loss of consciousness > 24 hours). Participants with a history of psychiatric disorders were eligible except in the case of bipolar disorder or psychosis. Participants were not taking over-the-counter sleep aids or other sleep-affecting medications such as hypnotics, antidepressants, antipsychotic medications, anticonvulsants, steroids, or beta blockers. Participants were ineligible if they reported alcohol consumption of > 14 drinks/wk or > 6 drinks at 1 sitting or > 3 caffeine drinks/day (or 400 mg caffeine equivalent), reported in a sleep diary.
Table 1.
Sample characteristics by study group.
| Control (n=71) Mean/n (SD/%) | Insomnia (n=93) Mean/n (SD/%) | |
|---|---|---|
| Age, mean | 67.6 (5.6) | 67.9 (6.9) |
| White/Caucasian, n | 66 (93%) | 84 (90.3%) |
| Women, n | 42 (59.2%) | 67 (72%) |
| Medications, n | 4.8 (2.8) | 6.2 (4.1) |
| Apnea-hypopnea index, mean events/h | 5.3 (4.7) | 5.4 (5.0) |
Standard deviation (SD) or percent of sample in parentheses. Apnea-hypopnea index information was unavailable for 1 participant in the control group.
A total of 170 participants with good sleep and 241 participants with insomnia provided informed consent to participate in the study. Control participants (74) and 102 participants with insomnia passed an in-person physical and apnea screening visit. Of these, 71 control participants and 99 participants with insomnia completed the T1 polysomnography (PSG) assessment and 91 participants with insomnia completed the T2 assessments. Artifact-free PSG data were available for all 71 participants in the control group, 93 participants with insomnia at T1, and 86 participants with insomnia at T2.
Participants are reflected in each group, time point, and NREM period as follows: control: n = 71, 71, 71, 62, 40 for NREM 1–5, respectively; insomnia T1: n = 93, 93, 91, 71, 35 for NREM 1–5, respectively; insomnia T2: n = 86, 86, 85, 70, 29, for NREM 1–5, respectively.
The control group did not undergo any follow-up assessments, as the parent study aims were designed to use CBTI as a probe of a neurobiological model of insomnia.
CBTI
Only participants in the insomnia group underwent CBTI. This intervention included six 45-minute to 50-minute in-person sessions and 2 telephone sessions over 8 consecutive weeks. The sessions were conducted by 2 Master’s level mental health clinicians who guided participants in techniques of sleep restriction, stimulus control, relaxation training, cognitive restructuring, daytime fatigue management, and reduction of nighttime worrying. Participants also kept a sleep diary throughout this 8-week period to monitor sleep parameters.
Assessments and sleep measures
Laboratory-based PSG was recorded on a single postadaptation night at the participants' habitual sleep times as assessed by sleep diary. Methods for power spectral analysis were previously published.27 Briefly, EEG data was recorded from F3, F4, C3, and C4 referenced to A1-A2, band-pass analog filtered from 0.3 to 100 Hz with a 60-Hz notch filter. EEG signals were digitized at a rate of 256 Hz. The raw digitized data were band-limited using a 60-Hz low-pass finite impulse response filter, then decimated to 128 Hz for quantitative analysis. The EEG was segmented into 0.25-Hz bins over 4-s epochs and weighted by a Hamming window. Four-second epochs identified as movement artifact by an automated algorithm28 and visual inspection were excluded from the record. The sleep record was visually scored in 30-s epochs using American Academy of Sleep Medicine criteria.29
Spectral analysis was performed on F and C channels with a 512-point fast Fourier transform using epochs scored as NREM sleep.27 Absolute spectral power was calculated for all of NREM sleep and for individual NREM periods, including only NREM sleep Stage N2 and N3. We excluded epochs scored as wake or Stage N1 as N1 scoring is less reliable than other stages and represents a much lower amount of NREM sleep. Absolute within-band power for each NREM period was calculated for F3, F4, C3, and C4 for the following frequency bands: 0.5–1 Hz (slow-oscillation), 1–4 Hz (delta), 4–8 Hz (theta), 8–12 Hz (alpha), 12–16 Hz (sigma), and 16–32 Hz (beta). Relative power was calculated for each bin by dividing total power within each bin by total power in the 0.5- to 32-Hz range. Results were tested with absolute and relative power to ensure that effects within a given frequency bin were not driven by group differences in overall power. For the present analyses, we calculated an “F3 and F4 channel” variable, which reflected the average of these 2 channels for both absolute power and relative power. For sensitivity analyses, we calculated the average of C3 and C4 for absolute power and relative power. F3 and F4 channels were selected as the primary spectral variable of interest because our analysis focused on slow-wave activity, which is highest over frontal regions.30 Data on relative power from F3 and F4 channels and data from absolute and relative power from C3 and C4 channels are presented in the Supplemental material. The present report focused on absolute power in each frequency band 1) across NREM periods 1–5 and 2) for each NREM period. We tested NREM sleep here because we aimed to focus on dissociations between the slow-oscillation and delta activity components of slow-wave activity for this analysis, and slow-wave activity is highest during NREM sleep.
Statistical analyses
Statistical analyses were performed in Statistical Package for the Social Sciences (SPSS) version 26. Multivariate analyses of variance were used to test effects of group on sleep variables: insomnia severity index, total sleep time, sleep efficiency, wake after sleep onset, and sleep stages N1–N3. Partial correlations controlling for age and sex, performed separately in the control and insomnia groups, tested the association between each frequency band and PSG-assessed sleep efficiency. Change score analyses were additionally run in the insomnia group, only testing the association between change in each frequency band across the whole night and change in PSG-assessed sleep efficiency. Change scores were calculated as T2 minus T1.
Linear mixed model analyses were used for the main analyses to test effects of group and time point, as well as interactions with NREM period, while including all participants’ data regardless of whether they completed both PSG studies and regardless of how many NREM periods they had.
Mixed models tested main effects of group (insomnia vs control), interactions between group and NREM period (1–5), main effects of time point (T1 and T2), and interactions between time point and NREM period. Mixed models testing effects of group included NREM period as a within-subject factor and group as a between-subject factor, with age and sex as covariates, and treating each frequency band separately as the dependent variable. Mixed models testing T1-T2 effects included NREM period and time point as within-subject factors, age and sex as covariates, and each frequency band separately as the dependent variable. Additional sensitivity analyses controlling for AHI were tested for significant main analyses. Significance was assessed before and after Holm-Sidak multiple comparisons correction. Holm-Sidak correction was based on 14 tests for each set of analyses with absolute power (7 frequency bands for both main effects and interactions with NREM period), and 12 tests for each set of analyses with relative power (6 frequency bands for main effects and interactions with NREM period).
RESULTS
Group differences in sleep characteristics
PSG sleep characteristics are displayed in Table 2. At baseline, when both groups were studied, older adults with insomnia showed lower sleep efficiency and less total sleep time and greater wake after sleep onset compared to controls. Groups did not differ significantly in terms of the amount of time spent in Stages N1, N2, N3 or rapid eye movement sleep.
Table 2.
Sleep characteristics.
| Control (n=71) | Insomnia Pre CBTI (n=93) | Insomnia Post CBTI (n=86) | Group Difference F1,164 | Pre Post CBTI Difference t(82) | |
|---|---|---|---|---|---|
| ISI Total | 0.62 (1.1) | 13.7 (4.2) | 5.2 (5.0) | 645.0*** | 14.8*** |
| TST, min | 372.0 (45.9) | 347.4 (63.4) | 335.6 (50.5) | 8.1** | 1.8 |
| SE, % | 82.3 (8.5) | 77.0 (12.1) | 82.7 (11.1) | 9.9** | 4.4*** |
| WASO, min | 67.4 (37.1) | 90.4 (56.5) | 59.3 (44.0) | 9.5** | 6.2*** |
| % N1, min | 8.03 (5.1) | 8.3 (4.7) | 7.9 (5.2) | 0.5 | 1.0 |
| % N2, min | 59.4 (10.2) | 58.6 (9.4) | 57.7 (9.5) | 0.03 | 0.9 |
| % N3, min | 10.9 (9.3) | 10.0 (8.0) | 10.3 (8.9) | 2.1 | 0.5 |
| % REM, min | 21.7 (5.1) | 23.1 (6.3) | 24.1 (5.8) | 2.7 | 1.7 |
Polysomnographic sleep characteristics of controls and the insomnia group. Standard deviation or percent of sample in parentheses. Difference F represents F value based on multivariate analysis of variance for main effects of group controlling for age and sex. Pre Post CBTI t represents paired samples t tests. ***P < 0.001, **P < 0.01, *P < 0.05. ISI = insomnia severity index, REM = rapid eye movement sleep, SE = sleep efficiency, TST = total sleep time, WASO = wake after sleep onset.
Correlations between sleep efficiency and EEG variables
In the control group, there were significant negative associations after controlling for age and sex between sleep efficiency and absolute power for theta, alpha, sigma, and beta (P < .05). In the insomnia group at T1, there was a significant negative association between sleep efficiency and beta power (P < .05). Change score analyses from T1 to T2 in the insomnia group revealed no significant correlations after controlling for age and sex between change in sleep efficiency with absolute power in any frequency bands. There was a marginally significant association between increased sleep efficiency and decreased relative beta power.
Effects of time point on sleep variables
Table 2 displays the changes in standard PSG and spectral variables in the insomnia group from T1 to T2. Significant decreases were found for insomnia severity index and wake after sleep onset. Significant increases were found for sleep efficiency.
Main effects of insomnia
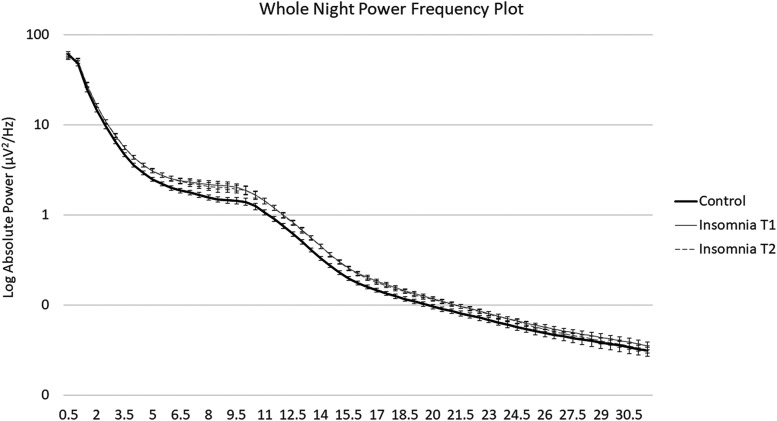
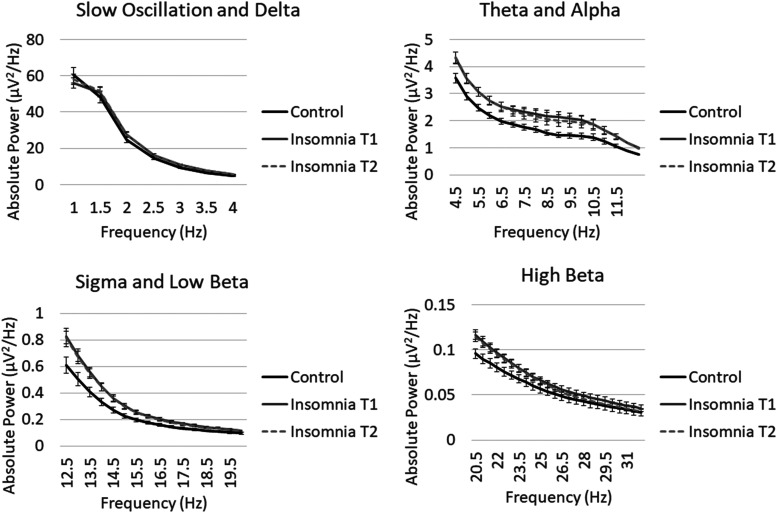
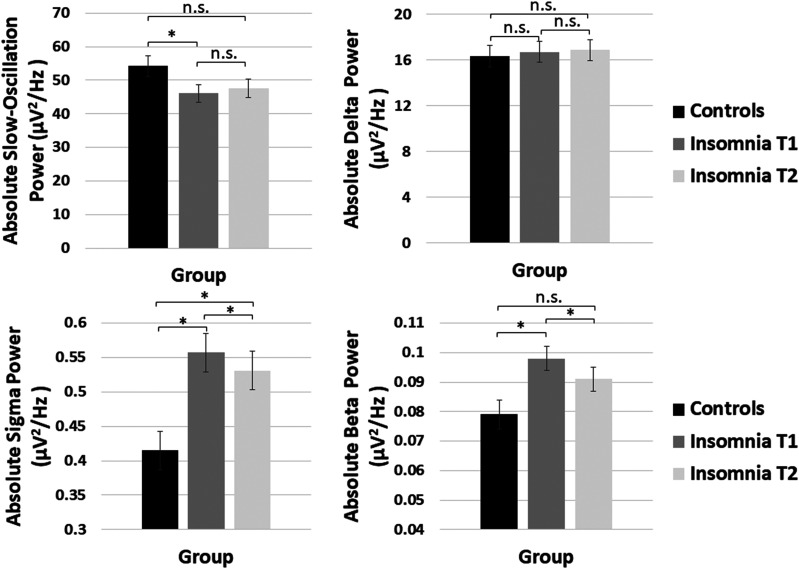
Group differences in control and insomnia participants at T1 are displayed in Figure 1 and Figure 2. Significant main effects of group were observed across NREM periods for slow-oscillation, theta, alpha, sigma, and low and high beta bands (Table 3). Power in the slow-oscillation band was significantly lower in the insomnia group for absolute and relative power (Figure 3), whereas for the other bands showing group differences, power was significantly higher in the insomnia group for absolute and relative power (effects with beta power were only significant for absolute power). Main effects of group on delta power did not approach significance. Differences for relative slow-oscillation power and absolute and relative sigma power survived Holm-Sidak correction, while differences for all other frequency bands did not. Furthermore, controlling for AHI did not alter the significance of these effects. C channels confirmed the same significant main effects of group, with the exception of a marginal effect of group on slow oscillation for absolute power in C channels.
Figure 1. Log-transformed whole night power-spectral plot.
Log transformed absolute power from 0.5 to 32 Hz in control (T1) and insomnia (T1 and T2) groups. Slow-oscillation power was significantly lower in the insomnia group across NREM periods. Theta, alpha, sigma, and beta activity were higher in the insomnia group across NREM periods. Error bars represent standard errors for each half Hz bin. NREM = non-rapid eye movement.
Figure 2. Untransformed whole night power-frequency plots.
Absolute power (not log-transformed) from 0.5 to 32 Hz in control (T1) and insomnia (T1 and T2) groups. Slow-oscillation power was significantly lower in the insomnia group across NREM periods. Theta, alpha, sigma, and beta activity were higher in the insomnia group across NREM periods. Error bars represent standard errors for each half Hz bin. NREM = non-rapid eye movement.
Table 3.
Effects of group and time point by frequency for absolute power in F3 and F4 channels.
| F3,F4 Absolute | 0.5–1 (slow) | 1–4 (delta) | 4–8 (theta) | 8–12 (alpha) | 12–16 (sigma) | 16–32 (beta) | 0.5–32 (total) |
|---|---|---|---|---|---|---|---|
| Group | 5.4, P = 0.021 | 0.01, P = 0.94 | 5.1, P = 0.026 | 6.3, P = 0.013 | 12.0, P = 0.001* | 4.6, P = 0.034 | 0.003, P = 0.953 |
| Group × NREM | 1.1, P = 0.376 | 0.5, P = 0.757 | 2.7, P = 0.031 | 1.7, P = 0.142 | 0.3, P = 0.854 | 2.4, P = 0.048 | 0.4, P = 0.797 |
| Time Point | 0.8, P = 0.364 | 0.1, P = 0.733 | 1.0, P = 0.314 | 0.6, P = 0.436 | 8.6, P = 0.003* | 8.7, P = 0.003* | 0.8, P = 0.778 |
| Time Point × NREM | 0.7, P = 0.619 | 0.5, P = 0.775 | 0.2, P = 0.958 | 0.6, P = 0.643 | 0.03, P = 0.998 | 0.5, P = 0.747 | 0.4, P = 0.800 |
F and P values for all main effects of group and interactions with NREM period, and F and P values for all main effects of time point and interactions with NREM period. Effects of group include control and insomnia groups at T1. Effects of time point include only the insomnia group at T1 and T2. Bold font denotes uncorrected significance. *Denotes significance after Holm-Sidak correction. NREM = non-rapid eye movement.
Figure 3. Mixed model age-adjusted and sex-adjusted means and standard errors for absolute slow-oscillation, delta, sigma, and beta power averaged over NREM periods 1–5 for the control group (black), insomnia T1 (dark gray) and insomnia T2 (light gray).
Control group means reflect group effect models. Insomnia group means reflect time-point effect models. Control T1 and Insomnia T2 significance is based on the post hoc mixed model including only control T1 and insomnia T2 accounting for age and sex. *Denotes significance based on age- and sex-adjusted mixed models. NREM = non-rapid eye movement.
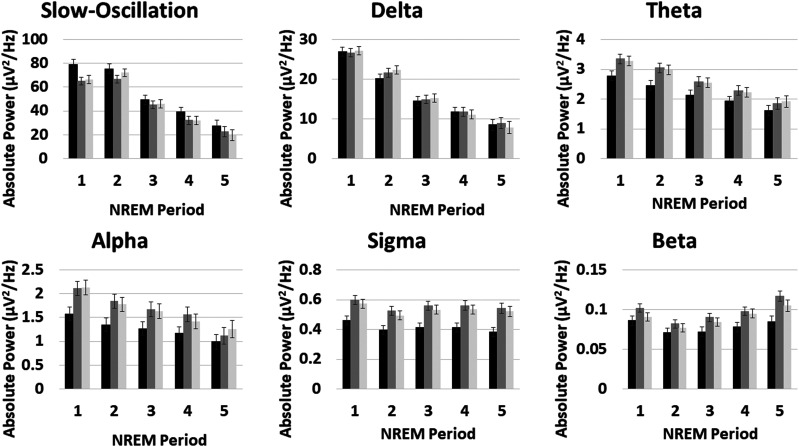
Group × NREM period interactions
Significant group × NREM period interactions were observed for theta and beta absolute power (Table 3). For 4- to 8-Hz theta power, insomnia-control differences diminished across NREM periods. In contrast, insomnia-control differences in beta power were larger in later NREM periods (Figure 4). Both theta and beta effects remained significant after controlling for AHI, but were not significant for relative power and did not reach Holm-Sidak correction. C channels showed no significant interactions between group and NREM period.
Figure 4. Effects of NREM period.
Mixed model age- and sex-adjusted means and standard errors for each frequency band by NREM period 1–5 for the control group T1 (black), insomnia T1 (dark gray), and insomnia T2 (light gray). Control group means reflect group effect models. Insomnia group means reflect time-point effect models. For all plots, the y axis is absolute power, and the x axis is NREM period. Group × NREM period interactions were significant for theta and beta bands. Effects of group were higher early in the night for theta power and later in the night for beta power. Effects of time point did not interact significantly with NREM period. NREM = non-rapid eye movement.
Main effects of time point (insomnia group only)
A marginal increase in relative slow-oscillation power was observed from T1 to T2. A significant main effect of time point was evident for sigma and beta absolute power, showing a small, but consistent, decrease across NREM periods (Figure 3). These effects reached Holm-Sidak correction but were not significant with relative power and were marginally significant when analyses were limited post hoc to NREM 1–4. Controlling for AHI did not alter the significance of these effects. C channel analyses confirmed that sigma and beta time point effects were specific to absolute power and not significant for relative power.
We performed post hoc mixed models controlling for age and sex to test whether control T1 and insomnia T2 power differed significantly from each other (Figure 3). Sigma power in the insomnia group at T2 remained significantly different from control T1, whereas beta power in the insomnia group at T2 was no longer significantly different from beta power in the control group at T1 (F1,154.4 = 1.9, P = .169). As displayed in Figure 3, control T1 and insomnia T2 slow oscillation and delta power were not significantly different from one another.
Time point × NREM period interactions (insomnia group only)
There were no significant time point × NREM period interactions for absolute power. Theta power showed a significant time point × NREM period interaction for relative power (Table 3). This interaction was driven by an increase in power at T2, particularly for NREM 5, and a decrease in theta power in NREM 2 and 3. This effect remained significant after controlling for AHI but was marginally significant when removing NREM period 5. It was not evident for C channels and did not reach Holm-Sidak correction.
DISCUSSION
Slow-wave activity characterized by sleep-specific features (slow oscillation and delta power) is associated positively with arousal threshold and subjective quality of sleep,32,33 and inversely with indices of physiological arousal. These characteristics make slow-wave activity a critical target for insomnia treatment.11,33–35 However, the extant literature is inconsistent with regard to slow-wave activity alterations in insomnia.2 This may be due to standard practice to combine the slow-oscillation and delta frequencies, which have been demonstrated to be functionally distinct.20 By examining these frequencies separately, we demonstrated that the slow oscillation, but not delta power, is lower among individuals with insomnia compared with good sleepers. This deficit in slow-oscillation power, however, was not significantly reversed after CBTI. Relative slow-oscillation power showed only a marginally significant increase. In contrast, high frequency power across theta, alpha, sigma, and beta bands was elevated in insomnia participants compared with good sleepers. Small, but significant decreases in absolute power for sigma and beta power were found consistently across NREM periods following CBTI. Beta power in the insomnia group at T2 was no longer significantly higher than that of the control group, but sigma power remained significantly higher for the insomnia group at T2 compared with the control group.
Group differences in slow-wave activity (< 4 Hz)
Slow-oscillation power, but not delta power, was lower in the insomnia group compared with controls, and this association was unmodulated by NREM period, suggesting consistent group differences in slow-wave activity across the night. The effects of group on slow-oscillation power were consistent across absolute and relative power, with relative power passing Holm-Sidak correction. C channels showed the same pattern with a marginal effect for absolute power and a highly significant effect for relative slow-oscillation power. This suggests that the effect of group on slow-oscillation power was robust to variation in measurement and analysis. The finding that the slow-oscillation was lower in the insomnia group at T1, while delta was not, may explain why prior studies combining < 4 Hz activity found no group differences between insomnia and controls.4,9,17,19 A recent study17 examined slow-oscillation (0.1–1 Hz) and delta activity (1–3.5 Hz), limiting analyses to Stage 2 sleep, and found no effect of insomnia. Limiting analyses to Stage 2 may have missed the majority of slow-oscillation power, which is highest during N3 sleep. Supporting this view, an earlier study16 found evidence that the peak in slow-wave activity, including very slow-wave activity (0.1–3.75 Hz) was reduced in insomnia, particularly within the first NREM period, the period in which slow-wave activity is highest. These findings suggest a role of the slow-oscillation rather than delta power in the pathophysiology of insomnia and may be informative for the field of sleep and cognition, which suggests that the slow oscillation is more functionally relevant for cognitive function.20
While both the slow-oscillation and delta power involve thalamocortical neurons, cortical neurons, and global hyperpolarization, the slow oscillation in particular is capable of grouping other oscillations including delta activity.21 Furthermore, the generators of these 2 oscillations are distinct.18,21 Delta activity is generated by thalamocortical neurons and slow oscillations are generated cortically, independent of the thalamus21,36 Why these 2 components would be differentially affected in insomnia may be difficult to disentangle given their high degree of overlap,21 but future work may benefit from further investigation of the relative benefits of therapeutically targeting the slow oscillation in contrast to delta activity in the context of insomnia.
Group differences in high-frequency activity (> 4 Hz)
Power across theta, alpha, sigma, and beta bands (4–32 Hz) was elevated in insomnia relative to controls, providing further support for hyperarousal in insomnia.16 These effects were consistent across absolute and relative power and C channels (with the exception of beta relative power). Increased high-frequency power in insomnia is most consistently found in the literature for beta power,2,19,37 which is commonly associated with arousal.17 Less consistently, insomnia has been associated with increased alpha5,37 and sigma power,17,37 which are thought to serve as a sleep-protective mechanism.17 The fact that we additionally found elevated theta activity in insomnia was consistent with the hypothesis that lower EEG power in insomnia would be specific to low-frequency slow-wave activity and sleep-specific features. However, Merica et al16 found lower theta power in patients with insomnia. This earlier study had a few notable differences from the current study, including a significantly younger age range (20–47 years of age), inclusion of participants taking benzodiazepines, and a lower frequency definition of theta power (3.75–6.75 Hz). The present study included only older adults who were not prescribed benzodiazepines, which are both associated with lower theta power,10,38,39 and we defined theta power as 4–8 Hz. Direct tests of age effects may help us further understand some of the discrepancies commonly found in the studies of insomnia sleep EEG for midrange frequency bands and their functional correlates.
Group differences in high-frequency activity were modulated by NREM period for theta and beta power. Theta activity differences were greatest earlier in the night, which is consistent with the fact that theta power is a marker of homeostatic sleep drive during wakefulness,40,41 increasing as a function of time awake,42 and correlating positively with sleepiness.41 Thus, these results may further demonstrate differences in sleep drive between older adults with insomnia and controls. Group differences in beta activity were evident across all NREM periods, consistent with many prior studies (as reviewed in Perlis et al.)2, but the significant interaction demonstrated that these differences were greater in later NREM periods, which may suggest that arousal in older adults with insomnia is further exacerbated as initial homeostatic sleep drive diminishes. Both the theta and beta interactions should be interpreted with caution, however, as sensitivity analyses demonstrated that relative power did not show the same interaction by NREM period.
Time point effects on slow-wave activity (< 4 Hz) and high-frequency activity (> 4 Hz)
Changes in slow-oscillation power following CBTI were marginally significant. Future work emphasizing sleep restriction therapy in CBTI to increase homeostatic sleep drive may elucidate whether behavioral enhancement of the slow-oscillation could enhance sleep improvements as well as functions supported by slow-wave activity, such as memory and cognition.20
Consistent with 1 prior study, we found that absolute sigma and beta activity decreased following CBTI.12 These small effects were consistent across F and C channels, but were not significant for relative power and did not pass Holm-Sidak correction. Thus, these results should be interpreted with caution. There may be a trait-like vulnerability to insomnia2 that comes with both low slow-oscillation power and high-frequency sigma and beta power that is challenging to modify behaviorally.
Clinical and theoretical implications
These findings may provide insight regarding quantitative EEG pathophysiological markers of insomnia as well as physiological targets for therapeutic approaches.35 Auditory closed-loop stimulation techniques have been developed to increase slow-wave activity by phase-locking to and enhancing natural slow-waves and thereby “deepening” sleep, primarily tested thus far in healthy controls.43 The current findings showing a deficit in slow-wave activity in insomnia suggest advantages to testing the specific benefits of targeted slow-oscillation enhancement using stimulation techniques to sleep in insomnia. Furthermore, these findings combined with future work on functional outcomes may inform our understanding of the mechanisms of functional deficits associated with insomnia in older adults and potentially help to identify what functional consequences of insomnia are linked to the slow oscillation, such as memory consolidation20 hippocampal neurogenesis,44 and neurotoxin clearance.45
Neurocognitive models suggests that subtle EEG changes such as elevated beta activity could precede subjective sleep complaints.10 If replicable across studies, specific reductions in the slow oscillation, as opposed to delta (1–4 Hz) power or slow-wave sleep, may serve as a similar marker preceding or characterizing subjective sleep complaints in insomnia. This may be particularly useful in older populations where sleep rarely meets the scoring criterion for Stage N3 slow-wave sleep > 10% of the night,46 or in clinical populations, where treatment of comorbid sleep disorders can alleviate clinical symptoms.47
Strengths and limitations
Strengths of the current study included a large sample size relative to prior studies describing insomnia-control differences in quantitative EEG and pre-CBTI to post-CBTI measures in the insomnia group. This study had the advantage of being focused on older adults, a group in which insomnia is highly prevalent and in which slow-wave activity tends to decline. Although recent studies have examined effects of age on the entire EEG power spectrum in insomnia, how age moderates effects of insomnia on the < 1 Hz slow oscillation or its relationship with higher frequency activity remains underexamined.4
A relative weakness of the study is the lack of a control group post-CBTI. This does not allow us to determine how the passage of time or repeated in-lab testing affects the slow-oscillation or change in-high frequency activity. Thus, controlled trials are necessary to determine the robustness of the change in high-frequency activity that we observed in this study. More work examining change in low-frequency and high-frequency activity as a function of response to treatment is needed but is beyond the scope of this paper.
CONCLUSIONS
Older adults with insomnia show lower slow-oscillation power and higher EEG activity, > 4 Hz. Following CBTI, high-frequency EEG power consistently decreased across NREM periods. These findings elucidate the effects of insomnia on distinct slow-wave activity components and strengthen our mechanistic understanding of the consequences of late-life insomnia, which, in turn, could help optimize the precision of pharmacological, psychological, and stimulation treatments.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. This study was funded by the National Institutes of Health Grants MH019986 and AG020677. Infrastructure support was funded by UL1 TR000005 and UL1 RR024153. Investigator effort was funded by National Institutes of Health Grants HL082610 (S.E.H. and G.M.D.) and AG049879 (K.A.W.). Dr. Buysse has served as a paid consultant to Bayer Healthcare, BeHealth Solutions, Cereve/Ebb Therapeutics, Emmi Solutions, and Weight Watchers International. He has also received compensation for educational programs developed for CME Institute, American Academy of Physician Assistants, and Eisai Inc. Dr. Buysse received licensing fees for commercial use of the Pittsburgh Sleep Quality Index, the Daytime Insomnia Symptoms Scale, the Insomnia Symptoms Questionnaire (all copyrights held by University of Pittsburgh), and the Consensus Diary (copyright held by Ryerson University). Dr. Germain is CEO and cofounder and owns equity in Rehat, LLC (d/b/a Noctem). The work presented here has no relationship with any of these potential conflicts. The authors report no other conflicts of interest.
SUPPLEMENTARY MATERIAL
ACKNOWLEDGMENTS
The authors thank Jean Miewald, Mary Fletcher, Erica Pais, Bonnee Wettlaufer, Brian Allison, and Zachary Chakan for assistance with recruitment and data management and collection and Ray Vasko for consultation regarding the EEG data collection and spectral analysis procedures.
Author Contributions: D.J.B., M.H.H., V.L.N., and A.G. designed the study. K.A.W. and D.J.B. defined the scientific investigation. S.E.H., K.A.W., and G.M.D. performed the statistical analyses. K.A.W. and S.E.H. wrote the paper with critical feedback from D.J.B., M.H.H., G.M.D., V.L.N., and A.G.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- CBTI
cognitive behavioral therapy for insomnia
- EEG
electroencephalogram
- NREM
non-rapid eye movement
- PSG
polysomnography
REFERENCES
- 1.Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14(1):9–15. 10.1016/j.smrv.2009.05.002 [DOI] [PubMed] [Google Scholar]
- 2.Perlis ML, Merica H, Smith MT, Giles DE. Beta EEG activity and insomnia. Sleep Med Rev. 2001;5(5):365–376. 10.1053/smrv.2001.0151 [DOI] [PubMed] [Google Scholar]
- 3.Kalmbach DA, Cuamatzi-Castelan AS, Tonnu CV, et al. Hyperarousal and sleep reactivity in insomnia: current insights. Nat Sci Sleep. 2018;10:193–201. 10.2147/NSS.S138823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Svetnik V, Snyder ES, Ma J, Tao P, Lines C, Herring WJ. EEG spectral analysis of NREM sleep in a large sample of patients with insomnia and good sleepers: effects of age, sex and part of the night. J Sleep Res. 2017;26(1):92–104. 10.1111/jsr.12448 [DOI] [PubMed] [Google Scholar]
- 5.Riedner BA, Goldstein MR, Plante DT,et al., Regional patterns of elevated alpha and high-frequency electroencephalographic activity during nonrapid eye movement sleep in chronic insomnia: a pilot study. Sleep 2016; 39(4):801–812. 10.5665/sleep.5632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161(11):2126–2128. 10.1176/appi.ajp.161.11.2126 [DOI] [PubMed] [Google Scholar]
- 7.Nofzinger EA, Price JC, Meltzer CC, et al. et al. Towards a neurobiology of dysfunctional arousal in depression: the relationship between beta EEG power and regional cerebral glucose metabolism during NREM sleep. Psychiatry Res Neuroimaging. 2000;98(2):71–91. 10.1016/S0925-4927(00)00045-7 [DOI] [PubMed] [Google Scholar]
- 8.Freedman RR. EEG power spectra in sleep-onset insomnia. Electroencephalogr Clin Neurophysiol. 1986;63(5):408–413. 10.1016/0013-4694(86)90122-7 [DOI] [PubMed] [Google Scholar]
- 9.Hall MH, Muldoon MF, Jennings JR, Buysse DJ, Flory JD, Manuck SB. Self-reported sleep duration is associated with the metabolic syndrome in midlife adults. Sleep. 2008;31(5):635–643. 10.1093/sleep/31.5.635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bastien CH, LeBlanc ML, Carrier J, Morin CM. Sleep EEG power spectra, insomnia, and chronic use of benzodiazepines. Sleep. 2003;26(3):313–317. 10.1093/sleep/26.3.313 [DOI] [PubMed] [Google Scholar]
- 11.Krystal AD, Edinger JD. Sleep EEG predictors and correlates of the response to cognitive behavioral therapy for insomnia. Sleep. 2010;33(5):669–677. 10.1093/sleep/33.5.669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cervena K, Dauvilliers Y, Espa F, et al. Effect of cognitive behavioural therapy for insomnia on sleep architecture and sleep EEG power spectra in psychophysiological insomnia. J Sleep Res. 2004;13(4):385–393. 10.1111/j.1365-2869.2004.00431.x [DOI] [PubMed] [Google Scholar]
- 13.Lamarche CH, Ogilvie RD. Electrophysiological changes during the sleep onset period of psychophysiological insomniacs. Psychiatric insomniacs, and normal sleepers. Sleep. 1997;20(9):726–733. 10.1093/sleep/20.9.726 [DOI] [PubMed] [Google Scholar]
- 14.Freedman RR, Sattler HL. Physiological and psychological factors in sleep-onset insomnia. J Abnorm Psychol. 1982;91(5):380–389. 10.1037/0021-843X.91.5.380 [DOI] [PubMed] [Google Scholar]
- 15.Perlis ML, Kehr EL, Smith MT, Andrews PJ, Orff H, Giles DE. Temporal and stagewise distribution of high frequency EEG activity in patients with primary and secondary insomnia and in good sleeper controls. J Sleep Res. 2001;10(2):93–104. 10.1046/j.1365-2869.2001.00247.x [DOI] [PubMed] [Google Scholar]
- 16.Merica H, Blois R, Gaillard JM. Spectral characteristics of sleep EEG in chronic insomnia. Eur J Neurosci. 1998;10(5):1826–1834. 10.1046/j.1460-9568.1998.00189.x [DOI] [PubMed] [Google Scholar]
- 17.Spiegelhalder K, Regen W, Feige B, et al. Increased EEG sigma and beta power during NREM sleep in primary insomnia. Biol Psychol. 2012;91(3):329–333. 10.1016/j.biopsycho.2012.08.009 [DOI] [PubMed] [Google Scholar]
- 18.Achermann P, Borbély AA. Low-frequency (<1Hz) oscillations in the human sleep electroencephalogram. Neuroscience. 1997;81(1):213–222. 10.1016/S0306-4522(97)00186-3 [DOI] [PubMed] [Google Scholar]
- 19.Perlis ML, Smith MT, Andrews PJ, Orff H, Giles DE. Beta/gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24(1):110–117. 10.1093/sleep/24.1.110 [DOI] [PubMed] [Google Scholar]
- 20.Staresina BP, Bergmann TO, Bonnefond M, et al. Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat Neurosci. 2015;18(11):1679–1686. 10.1038/nn.4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137(4):1087–1106. 10.1016/j.neuroscience.2005.10.029 [DOI] [PubMed] [Google Scholar]
- 22.Kyle S, Sexton CE, Feige B, et al. Sleep and cognitive performance: cross-sectional associations in the UK Biobank. Sleep Med. 2017;38:85–91. 10.1016/j.sleep.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall L, Helgadóttir H, Mölle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444(7119):610–613. 10.1038/nature05278 [DOI] [PubMed] [Google Scholar]
- 24.Jaussent I, Dauvilliers Y, Ancelin ML, et al. Insomnia symptoms in older adults: associated factors and gender differences. Am J Geriatr Psychiatry. 2011;19(1):88–97. 10.1097/JGP.0b013e3181e049b6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levenson J, Troxel WM, Begley A, et al. A quantitative approach to distinguishing older adults with insomnia from good sleeper controls. J Clin Sleep Med. 2013;9(2):125–131. 10.5664/jcsm.2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14(6):486–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasko RC, Brunner DP, Monahan JP, et al. Power spectral analysis of EEG in a multiple-bedroom, multiple-polygraph sleep laboratory. Int J Med Inform. 1997;46(3):175–184. 10.1016/S1386-5056(97)00064-6 [DOI] [PubMed] [Google Scholar]
- 28.Brunner D, Vasko R, Detka C, Monahan J, Reynolds, C III, Kupfer D. Muscle artifacts in the sleep EEG: Automated detection and effect on all‐night EEG power spectra. J Sleep Res. 1996;5(3):155–164. 10.1046/j.1365-2869.1996.00009.x [DOI] [PubMed] [Google Scholar]
- 29.Iber C, Ancoli-Israel S, Chesson AL Jr, Quan SF; for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 30.Cajochen C, Foy R, Dijk D-J. Frontal predominance of a relative increase in sleep delta and theta EEG activity after sleep loss in humans. Sleep Res Online. 1999;2(3):65–69. [PubMed] [Google Scholar]
- 31.Neckelmann D, Ursin R. Sleep stages and EEG power spectrum in relation to acoustical stimulus arousal threshold in the rat. Sleep. 1993;16(5):467–477. [PubMed] [Google Scholar]
- 32.Rechtschaffen A, Hauri P, Zeitlin M. Auditory awakening thresholds in REM and NREM sleep stages. Percept Mot Skills. 1966;22(3):927–942. 10.2466/pms.1966.22.3.927 [DOI] [PubMed] [Google Scholar]
- 33.Riedel BW, Lichstein KL. Objective sleep measures and subjective sleep satisfaction: How do older adults with insomnia define a good night’s sleep? Psychol Aging. 1998;13(1):159–163. [DOI] [PubMed] [Google Scholar]
- 34.Buysse DJ. Sleep Health: Can We Define It? Does It Matter? Sleep. 2014;37(1):9–17. 10.5665/sleep.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monti JM, Alvariño F, Monti D. Conventional and power spectrum analysis of the effects of zolpidem on sleep EEG in patients with chronic primary insomnia. Sleep. 2000;23(8):1075–1084. 10.1093/sleep/23.8.1g [DOI] [PubMed] [Google Scholar]
- 36.Steriade M, Nunez A, Amzica F. Intracellular analysis of relations between the slow (< 1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci. 1993;13(8):3266–3283. 10.1523/JNEUROSCI.13-08-03266.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krystal AD, Edinger JK, Wohlgemuth WK, Marsh GR. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002;25(6):626–636. [PubMed] [Google Scholar]
- 38.Carrier J, Land S, Buysse DJ, Kupfer DJ, Monk TH. The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20–60 years old). Psychophysiology. 2001;38(2):232–242. 10.1111/1469-8986.3820232 [DOI] [PubMed] [Google Scholar]
- 39.Landolt H-P, Dijk D-J, Achermann P, Borbely AA. Effect of age on the sleep EEG: slow-wave activity and spindle frequency activity in young and middle-aged men. Brain Res. 1996;738(2):205–212. 10.1016/S0006-8993(96)00770-6 [DOI] [PubMed] [Google Scholar]
- 40.Kirov R, Weiss C, Siebner HR, Born J, Marshall L. Slow oscillation electrical brain stimulation during waking promotes EEG theta activity and memory encoding. Proc Natl Acad Sci USA. 2009;106(36):15460–15465. 10.1073/pnas.0904438106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strijkstra AM, Beersma DGM, Drayer B, Halbesma N, Daan S. Subjective sleepiness correlates negatively with global alpha (8–12 Hz) and positively with central frontal theta (4–8 Hz) frequencies in the human resting awake electroencephalogram. Neurosci Lett. 2003;340(1):17–20. 10.1016/S0304-3940(03)00033-8 [DOI] [PubMed] [Google Scholar]
- 42.Finelli L, Baumann H, Borbely AA, Acherman P. Dual electroencephalogram markers of human sleep homeostasis: correlation between theta activity in waking and slow-wave activity in sleep. Neuroscience. 2000;101(3):523–529. 10.1016/S0306-4522(00)00409-7 [DOI] [PubMed] [Google Scholar]
- 43.Papalambros NA, Santostasi G, Malkani RG, et al. Acoustic enhancement of sleep slow oscillations and concomitant memory improvement in older adults. Front Hum Neurosci. 2017;11:109. 10.3389/fnhum.2017.00109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neylan TC, Mueller SG, Wang Z, et al. Insomnia severity is associated with a decreased volume of the CA3/dentate gyrus hippocampal subfield. Biol Psych. 2010;68(5):494–496. 10.1016/j.biopsych.2010.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284(7):861–868. 10.1001/jama.284.7.861 [DOI] [PubMed] [Google Scholar]
- 47.Gebara MA, Siripong N, DiNapoli EA, et al. Effect of insomnia treatments on depression: A systematic review and meta‐analysis. Depress Anxiety. 2018;35(8):717–731. 10.1002/da.22776 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.