Abstract
Study Objectives:
In patients with delayed sleep-wake phase disorder (DSWPD), the circadian clock may be more easily affected by light at night. This creates a potential vulnerability, whereby individuals with irregular schedules may have less stable circadian rhythms. We investigated the stability of circadian timing and regularity of sleep in patients with DSWPD and healthy controls.
Methods:
Participants completed 2 dim-light melatonin onset (DLMO) assessments approximately 2 weeks apart while keeping their habitual sleep/wake schedule. After the second DLMO assessment, light sensitivity was assessed using the phase-resetting response to a 6.5-hour 150-lux stimulus. The change in DLMO timing (DLMO instability) was assessed and related to light sensitivity and the sleep regularity index.
Results:
Relative to healthy controls, patients with DSWPD had later sleep rhythm timing relative to clock time, earlier sleep rhythm timing relative to DLMO, lower sleep regularity index, and greater DLMO instability. Greater DLMO instability was associated with increased light sensitivity across all participants, but not within groups.
Conclusions:
We find that circadian timing is less stable and sleep is less regular in patients with DSWPD, which could contribute to etiology of the disorder. Measures of light sensitivity may be informative in generating DSWPD treatment plans.
Citation:
Watson LA, McGlashan EM, Hosken IT, Anderson C, Phillips AJK, Cain SW. Sleep and circadian instability in delayed sleep-wake phase disorder. J Clin Sleep Med. 2020;16(9):1431–1436.
Keywords: DSWPD, DSPD, sleep regularity, light sensitivity, interindividual differences, melatonin, phase shift
BRIEF SUMMARY
Current Knowledge/Study Rationale: Delayed sleep-wake phase disorder is a persistent sleep disorder that is resistant to treatment. Understanding factors that contribute to the maintenance of delayed sleep-wake phase disorder could help to improve treatment outcomes.
Study Impact: Our findings of greater variability in sleep and circadian timing in patients with delayed sleep-wake phase disorder indicate that behavioral modifications, such as restricting the timing of sleep and light exposure, may reduce circadian phase instability and improve treatment efficacy, especially in those with higher light sensitivity.
INTRODUCTION
Delayed sleep-wake phase disorder (DSWPD) is characterized by a persistent delay in sleep relative to the individual’s desired sleep time.1 Patients with DSWPD often experience a delay in circadian timing, which contributes to their delayed sleep timing. Treatments for DSWPD, such as bright light therapy, typically aim to normalize circadian timing.2 The efficacy of these interventions relies on them being delivered at an appropriate circadian time to achieve a phase advance.3 If timed inappropriately, these interventions may have no effect on circadian phase or even potentially result in further phase delay, perpetuating negative outcomes for those with DSWPD.
An examination of the stability of sleep and circadian rhythms in DSWPD is not currently a part of diagnosis, although there is evidence that instability is a feature of the disorder. For instance, sleep timing has been shown to be more irregular in patients with DSWPD compared with healthy controls, even when asked to sleep within 1 hour of their self-reported average time; that is, even under conditions of restricted variability and when observed sleep timing may not have been typical.4 In patients with DSWPD, greater sleep timing variability has also been associated with greater variability in dim-light melatonin onset (DLMO) times. This has also been seen in patients with DSWPD with unrestricted sleep schedules, where DLMO time shifted in a greater proportion of patients with DSWPD compared with healthy controls.5 A potential mechanism for this instability in patients with DSWPD is light sensitivity, the ease with which light can alter circadian timing. In patients with DSWPD, the circadian system is more responsive to light exposure, as measured by phase resetting,6 pupillary responses,6,7 and melatonin suppression.8
In this study we investigated the stability of circadian timing and regularity of sleep/wake patterns in patients with DSWPD and healthy controls. We included only DWSPD patients with delayed DLMO timing (“circadian DSWPD”9) and using unrestricted sleep schedules to investigate natural variability. We also examined the relationship between these measures and light sensitivity. We hypothesized that patients with DSWPD would have less stable circadian timing and more irregular sleep compared with controls. As secondary analysis, we investigated whether there was a relationship of sleep and circadian stability with light sensitivity, as measured by the phase-shifting response to light at night.
METHODS
The study was approved by the Monash University Human Research Ethics Committee, and all participants gave written informed consent prior to participation. This study was in line with the standards set by the Declaration of Helsinki (revision #7).
Participants
Participants were 19 men: 10 patients with DSWPD (aged 22.4 ± 3.3 years) and 9 healthy controls (aged 22.5 ± 3.3 years). Only male participants were selected to reduce potential variability in light sensitivity and circadian entrainment due to sex.10 As a part of a larger study,6 participants underwent extensive screening, including a medical and psychological assessment. During the medical assessment, participants with DSWPD were diagnosed according to the International Classification of Sleep Disorders (3rd edition), and alternate diagnoses were ruled out. Participants were subsequently deemed eligible if they were of a healthy weight (with body mass index between 18 and 30 kg/m2) and had no current medical or psychological/psychiatric history, were not on any medication, had not recently traveled or engaged in shiftwork, and had sleep-wake behavior consistent with their group (ie, delayed sleep timing in the DSWPD group, regular sleep timing in the control group). Healthy controls had a DLMO time of 22:00 or earlier and a typical phase angle (melatonin onset occurring 1–3 h before habitual bedtime), whereas the patients with DSWPD had a DLMO time of later than 23:00 and occurring between 30 minutes before to any time after their desired bedtime, consistent with the “circadian DSWPD” phenotype.9 Eligibility criteria pertaining to circadian time were evaluated based on the at-home DLMO measurement described below.
Procedures
Participants completed 2 DLMO assessments: 1 at home and 1 in the laboratory. The laboratory DLMO assessment was immediately followed by an assessment of circadian phase shifting in response to light. There were approximately 2 weeks between the in-home and in-laboratory DLMO assessments (mean = 12.1 days, standard deviation = 3.9, range = 7–20 days). Participants completed a sleep diary and actigraphy monitoring between assessments.
At-home DLMO measurement
For the in-home DLMO assessment, participants were seated in a dimly lit room (< 5 lux). This was confirmed by an investigator, and adherence was confirmed by a HOBO pendant light measuring device (Onset Computer Corporation, Bourne, MA) attached to their clothing (on the torso). Participants completed hourly saliva samples using salivettes (Sarstedt, Germany) from 5 hours prior to their habitual bedtime until 2 hours after (a total of 8 samples). Participants were asked to remain seated and have no food or drink from 30 minutes prior to collection of each saliva sample. Participants were also asked to refrain from caffeine, alcohol, and mouthwash on the day of collection. In-home DLMO assessments have been shown to yield comparable measures of circadian timing compared with lab-based assessments, as per Burgess and colleagues11 whose methods we adhered to as closely as possible.
In-home sleep-wake monitoring
At the time of the in-home DLMO assessment, participants were provided with a wrist-worn device (Actiwatch Spectrum Plus, Philips Respironics, Bend, OR) and a sleep diary to record habitual sleep-wake activity patterns up until the in-lab protocol. Activity counts were recorded in 1-minute epochs, and rest intervals were subsequently determined by using a combination of sleep diary data and visual inspection in Actiware (Philips, Bend, OR), with medium sensitivity. Participants were instructed to maintain their typical sleep-wake patterns. Valid sleep-wake recordings ranged 4–24 days (mean = 12.1, standard deviation = 4.6 days). Participants were asked to abstain from caffeine and alcohol during this period, beginning 7 days prior to their admission to the in-laboratory study.
In laboratory DLMO measurement and assessment of light sensitivity
Approximately 14 days after their in-home DLMO assessment (mean = 13.5, standard deviation = 4.7, range = 7–24 days), eligible participants completed a second circadian phase assessment and an assessment of circadian light sensitivity in a 6-day in-laboratory phase-shifting protocol. For the in-lab DLMO measurement, participants were seated in a dimly lit laboratory suite (< 3 lux) and provided hourly saliva samples from 5 hours prior to their habitual bedtime until their habitual bedtime (a total of 6 samples). Participants were seated and food or drinks were not given from 30 minutes prior to collection of each saliva sample. After sleeping, participants underwent the phase-shifting (light sensitivity) assessment. Participants initially underwent a 27-hour constant routine protocol, during which melatonin phase was measured (salivary melatonin) and participants were kept in dim lighting of < 3 lux. Participants were required to remain awake and maintain a constant posture in bed, and were given hourly isocaloric snacks throughout the 27 hours. Following an 8-hour sleep opportunity, participants woke to a 16-hour day during which a 6.5-hour light exposure at 150 lux took place (beginning at the participants’ in-home DLMO time). The remaining hours were spent in lighting of < 3 lux. Following another 8-hour sleep opportunity, a second 27-hour constant routine protocol took place under the same conditions as the first to assess change in circadian phase (see Watson et al6 for a comprehensive phase-shifting protocol description).
Salivary melatonin assays
All samples were analyzed with radioimmunoassay using standards and reagents supplied by Buhlmann Laboratories (RKDSM-2, Buhlmann Laboratories AG, Schönenbuch, Switzerland). Saliva samples were assayed in duplicate, with an intra-assay coefficient of variation of < 6.7%, and interassay coefficients of variation of < 13.2% across assays.
Data analysis
DLMO instability
DLMO was defined in this study as the time at which melatonin concentration exceeded 4 pg/mL12 using linear interpolation. Absolute change in DLMO time between the home DLMO measurement and the first in-lab DLMO measurement was calculated for each individual, and this was used as the measure of DLMO instability.
Phase shifting
Phase-shifting measurements were taken using the method published in Watson et al.6 In short, the change in circadian phase between constant routine protocols was compared with the predicted phase shift for a healthy young adult using the normative data from Khalsa et al13 and Zeitzer et al.14 The ratio of the measured to the expected phase shift was used as the measure of light sensitivity.
Sleep Regularity Index
The Sleep Regularity Index (SRI) is a recently validated measure15–17 for the similarity of sleep/wake patterns on a circadian timescale (ie, between consecutive days). From epoch-by-epoch scoring of sleep vs wake, the metric calculates the probability that epochs 24 hours apart are in the same sleep vs wake state. The metric is scaled to range from 0 to 100, with higher values representing more regular sleep/wake patterns. The SRI was computed for each individual from the scored actigraphy data between DLMO assessments.
Statistical analyses
The average daily sleep rhythm was computed for each individual by averaging percentage time asleep across days in 30-minute clock-time bins or in 30-minute time bins relative to the individual’s DLMO time. A nonlinear mixed effects model was fit to the data for both groups (using the function nlmefit in MATLAB R2017b), assuming a sinusoidal rhythm with a period of 24 hours. Random effects were included at the participant level for the mean, amplitude, and phase. Fixed effects were included for the mean, amplitude, and phase, as well as group-level effects for amplitude and phase, which were used to compare rhythms between the control and DSWPD groups. Cosinor analysis was performed at the individual level to determine individual acrophases. Goodness of fit for these models was assessed using adjusted R2, which ranged from .71 to .86. We also tested an extended cosinor model, using an antilogistic-transformed cosine function18 (6 parameters) to better fit the average shape of the sleep-wake curves. Individual estimates of acrophase were nearly identical using this 6-parameter model compared with the 3-parameter cosinor model, with the largest individual difference being 0.16 hours (mean absolute difference of 0.06 hours). We therefore reported fits using the more parsimonious cosinor model.
DLMO stability (ie, the mean absolute DLMO change) and SRI were compared between groups using Welch’s t tests due to differences in variance between groups. Pearson’s correlation coefficients were calculated to assess the size and direction of the relationships between DLMO variability and phase shifting and DLMO variability and SRI. Correlations were calculated for the entire participant pool and within groups.
RESULTS
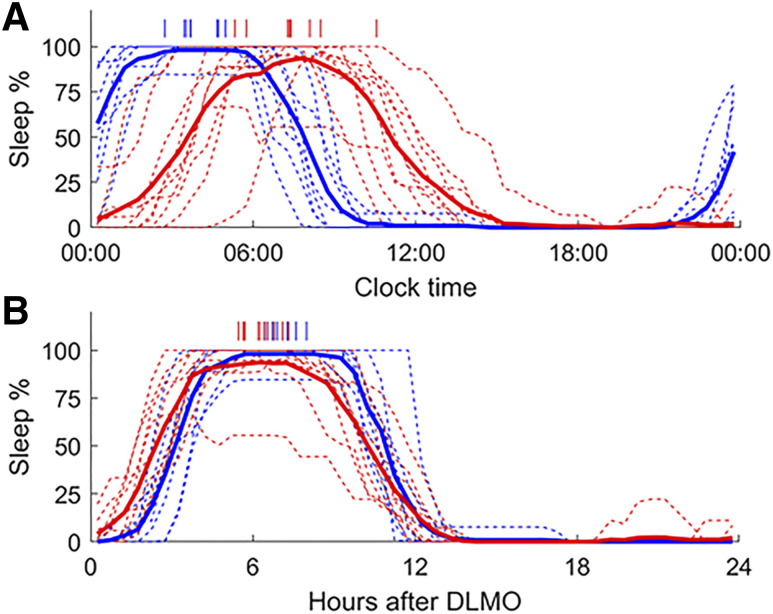
Relative to clock time, there were significant group differences in the average phase of the sleep rhythm between healthy controls and patients with DSWPD, with later sleep rhythms in patients with DSWPD (3:56 ± 0:24 vs 7:28 ± 0:40 h, P < .0001), as shown in Figure 1. Relative to DLMO, there was also a significant difference in acrophase of the sleep rhythm, whereby the sleep acrophase occurred at an earlier circadian phase in patients (7.04 ± 0.19 h vs 6.36 ± 0.32 h, P < .01). Therefore, patients with DSWPD had a later sleep acrophase relative to clock time (by 3.5 h), but at an earlier time relative to DLMO (by 0.7 h) compared with healthy controls. There was no significant difference in amplitude of the sleep rhythm between groups, either with respect to clock time (P = .29) or relative to DLMO time (P = .29).
Figure 1. Daily sleep rhythms relative to clock time and DLMO.
Average daily sleep rhythms relative to clock time (A) and hours (B) after dim-light melatonin onset (DLMO). Data are shown for healthy controls (blue) and patients with Delayed Sleep Wake Phase Disorder (red). Individual sleep rhythms are shown as thin dashed lines, while group averages are shown as thick lines. Individual acrophases, based on cosinor fits, are indicated by vertical dashes at the top of each panel.
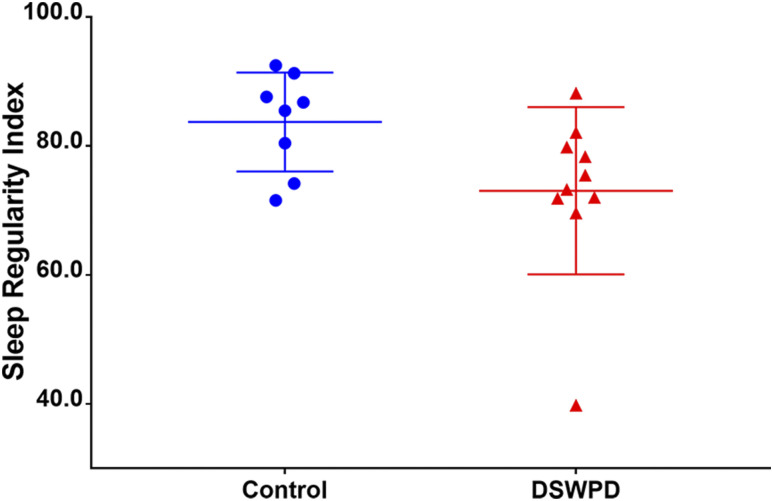
The DSWPD group had a mean SRI score 10.7 points lower than the healthy controls (73.1 ± 13.0 vs 83.7 ± 7.7, P < .05, Cohen’s d = 1.00), indicating that patients with DSWPD had significantly less regular sleep than healthy controls (see Figure 2).
Figure 2. Sleep Regularity Index scores.
Sleep Regularity Index (SRI) scores for healthy controls (blue) and patients with Delayed Sleep Wake Phase Disorder (DSWPD) (red).
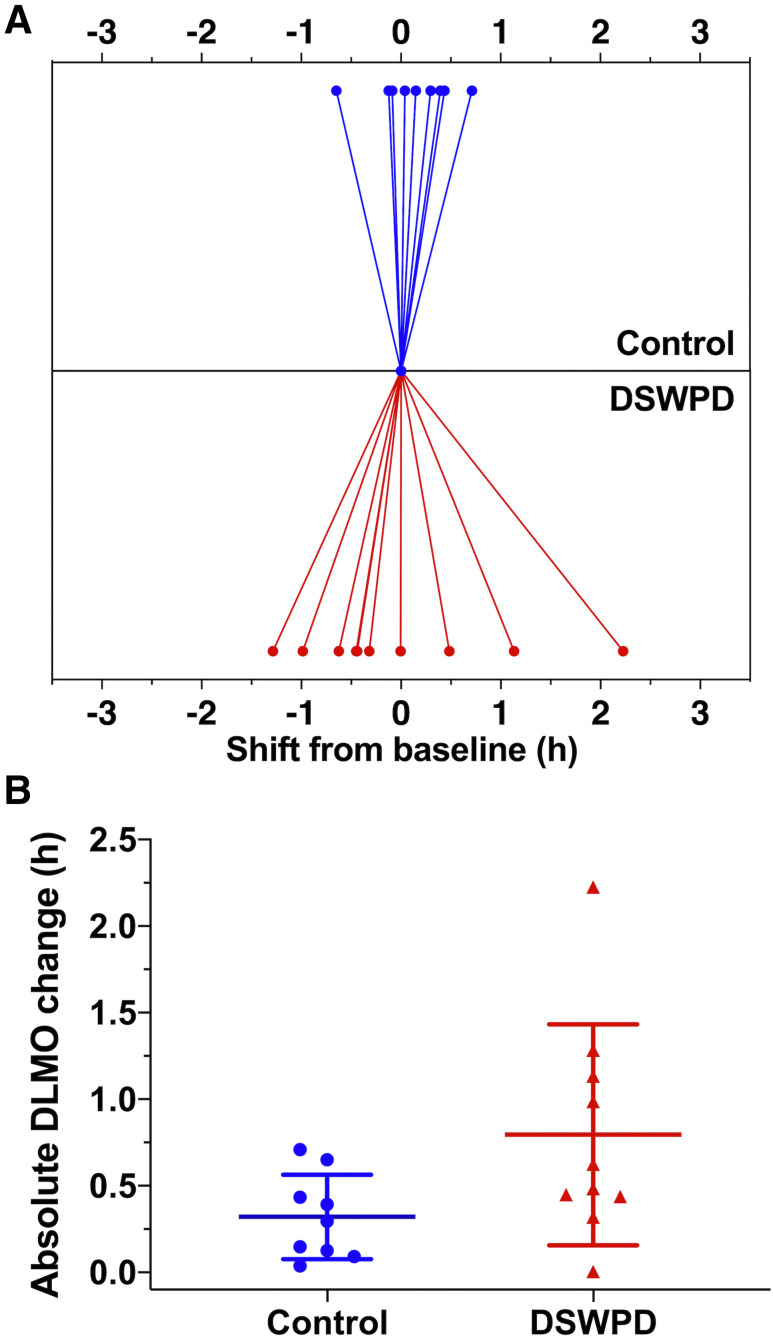
The DSWPD group had on average 28.2 minutes or 147% more variability between at-home and in-lab DLMOs than the healthy controls (0.79 ± 0.64 vs 0.32 ± 0.24 h, P = .05, Cohen’s d = 0.97), indicating a trend toward less DLMO stability approaching significance (P = .05, see Figure 3). Three patients with DSWPD (30%) and zero controls (0%) had variability over 1 hour; this difference was not significant (P = .21, Fisher’s exact test). These group differences were not due to differences in the day-of-week of assessments. Both groups had similar proportion of weekday-to-weekend comparisons (5 out of 9 for healthy controls, 5 out of 10 for DSWPD). There was also no significant difference in DLMO stability when comparing weekday-to-weekend vs within weekday/weekend (1.16 vs. 1.03 h, P = .36).
Figure 3. Circadian variability.
(A) Changes in dim-light melatonin onset (DLMO) time observed for patients with Delayed Sleep Wake Phase Disorder (DSWPD) and healthy controls within habitual light environments. (B) The absolute difference in timing of DLMO between groups.
There was a significant positive association between DLMO instability and sensitivity to light (measured with phase shifting) (r = .46, P < .05) and a nonsignificant trend toward a negative relationship between DLMO instability and SRI (r = −.40, P = .10) for patients with DSWPD and healthy controls, such that less DLMO stability was associated with greater light sensitivity and worse sleep regularity. However, these relationships were not significant within groups (P > .05).
DISCUSSION
Our results show that patients with DSWPD who have delayed melatonin onset timing (ie, patients with “circadian DSWPD”9) experience larger changes in circadian phase (greater DLMO instability) and have more irregular sleep/wake patterns than healthy controls. Additionally, we found that greater light sensitivity is associated with increased variability in circadian phase across groups, but not within groups. These findings indicate that reduced circadian and sleep stability are features of DSWPD and that increased light sensitivity may contribute to instability.
Our finding of DLMO instability is consistent with previous evidence of increased variability in circadian phase in patients with DSWPD. When participants were allowed to keep habitual sleep/wake patterns, 6 of 8 patients with delayed sleep (formerly referred to as delayed sleep phase syndrome) showed a week-to-week shift in DLMO time of > 1 hour compared with 3 of 8 healthy controls.5 More recently, when participants were required to keep stable sleep times, 6 of 22 patients with DSWPD shifted by > 1 hour compared with 2 of 18 healthy controls.4 If our inclusion criteria were applied to these data retrospectively (ie, excluding delayed healthy controls and non-delayed DSWPD), these proportions would remain elevated for 5 of 15 patients with DSWPD and 1 of 15 healthy controls.
Our study is the first to have compared sleep regularity between patients with DSWPD and healthy controls while participants maintained their self-selected (unstructured) sleep/wake patterns. While Burgess and colleagues4 examined sleep regularity in DSWPD, they did so under structured sleep schedules where participants were instructed to sleep within 1 hour of their average time. Due to the structured sleep schedules, their finding of less regular sleep in patients with DSWPD could be interpreted either as a habitual tendency to sleep irregularly or as weaker adherence to the study instructions. Both interpretations are plausible, given lower self-control has been observed in individuals with delayed sleep19 or evening preference.20 The former interpretation is supported by our finding that patients with DSWPD keep habitually less regular sleep/wake patterns than healthy controls. Mechanistically, this may suggest weaker internal coupling of circadian and behavioral rhythms in patients with DSWPD, meaning sleep/wake schedules are more easily displaced on a day-to-day basis relative to circadian time.
Irregular sleep/wake patterns are likely to contribute to less stable circadian rhythms, due to less regular patterns of light exposure.21 Higher light sensitivity could potentially amplify the effects of irregular light exposure on circadian timing. In support of this, we found an association between light sensitivity and stability of circadian timing, with more sensitive individuals exhibiting less stable circadian timing. However, it should be noted that we were unable to assess the regularity or nature of light exposure patterns in this sample. Irregular light exposure patterns likely contribute to unstable circadian phase and could mediate the negative effect of increased light sensitivity on circadian phase. Further research will be needed to determine the relative contribution of light exposure and light sensitivity to stability of circadian timing. Previous research has found that sleep regularity, as quantified by the SRI, mediates functional outcomes in patients with DSWPD.17 Moreover, emerging research indicates that irregular sleep/wake patterns are associated with poor mood and health outcomes.15,16,22 These findings together suggest that targeting regularity of sleep/wake patterns may be an effective adjunct to existing treatments for DSWPD.
We found that patients with DSWPD had an earlier sleep acrophase relative to DLMO than controls. That is, there was less time between DLMO and sleep acrophase in patients with DSWPD. This finding is consistent with the fact that we included only patients with circadian DSWPD, as they tend to have a smaller phase angle between DLMO and habitual bedtime,9 whereas other studies that have included all forms of delayed sleep have found no significant difference in phase angle between patients and controls.23,24 Receiving more light in the delay region of the phase response curve would be a plausible explanation for the initial development of delayed circadian phase. However, the smaller phase angle observed in our sample and others indicates that, on average, patients with circadian DSWPD may actually spend less time awake and therefore get less light exposure in the delay region of the phase response curve to light compared with controls.13 Individuals with DSWPD appear to have a larger phase delaying response to light6 and a longer intrinsic circadian period.25 Both of these physiological differences imply that, for an individual with DSWPD to remain entrained to the 24-hour day, they must on average receive less phase delaying light exposure and/or more phase advancing light exposure than equivalent healthy controls. Although we could not assess light-exposure patterns directly in our sample, this is consistent with the circadian timing of sleep we observed in DSWPD patients compared with controls.
There are some notable limitations to our study. First, our study examined male patients with DSWPD. Given large interindividual variability and potential sex differences in both light sensitivity26 and phase of entrainment,10 further studies are needed to determine whether the same relationships exist in female patients. Second, we could not determine the impact of light history on circadian timing because of a high percentage of missing data due to sensor coverage. Future studies could examine light history using devices that better capture light at eye level. Light exposure patterns likely contribute to instability in circadian timing and determining the relative contribution of behavioral and physiological factors (eg, light sensitivity) would have clinical utility. Finally, although we found a significant association between light sensitivity and circadian stability across all participants, this relationship was not significant within groups. This may be due to our relatively small sample size. Larger samples will be needed to determine whether this relationship differs between patients with DSWPD and healthy controls.
Overall, the present study showed patients with DSWPD experience larger changes in circadian phase and significantly less regular sleep than healthy controls. We also showed that greater light sensitivity was associated with increased variability of circadian phase. These findings indicate that behavioral modifications, such as restricting timing of sleep and/or light exposure may reduce circadian phase instability and improve treatment efficacy, especially in those with higher light sensitivity.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at Monash University. This study was funded by a National Health and Medical Research Council Project Grant to SWC (1087665). LAW and EMM received financial support from the Australian Government through Research Training Program (RTP) Scholarships. LAW, EMM, ITH, and CA report no conflicts of interest related to the results reported in this paper. CA received contract research support from VicRoads, Rio Tinto Coal Australia, and BHP Mining. She received lecturing fees from Ausmed, Healthmed, TEVA, and Philips Healthcare. She is a Theme Leader in the Alertness CRC. SWC received lecturing fees from TEVA and Beacon Lighting, consulting fees from Dyson, and research support from Versalux and Delos. AJKP and SWC are investigators in projects supported by Alertness CRC and received consulting support from Beacon Lighting and research support from Versalux and Delos.
ACKNOWLEDGMENTS
We acknowledge the contribution of the participants, research assistants, honors and PhD students, interns, and medical staff (psychologists and physicians) to the research.
ABBREVIATIONS
- DLMO
dim-light melatonin onset
- DSWPD
delayed sleep-wake phase disorder
- SRI
Sleep Regularity Index
REFERENCES
- 1.American Academy of Sleep Medicine . International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 2.Gradisar M, Smits MG, Bjorvatn B. Assessment and treatment of delayed sleep phase disorder in adolescents. Sleep Med Clin. 2014;9(2):199–210. 10.1016/j.jsmc.2014.02.005 [DOI] [Google Scholar]
- 3.Auger RR, Burgess HJ, Emens JS, Deriy LV, Thomas SM, Sharkey KM. Clinical Practice Guideline for the Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders: Advanced Sleep-Wake Phase Disorder (ASWPD), Delayed Sleep-Wake Phase Disorder (DSWPD), Non-24-Hour Sleep-Wake Rhythm Disorder (N24SWD), and Irregular Sleep-Wake Rhythm Disorder (ISWRD). An Update for 2015. J Clin Sleep Med. 2015;11(10):1199–1236. 10.5664/jcsm.5100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgess HJ, Park M, Wyatt JK, Rizvydeen M, Fogg LF. Sleep and circadian variability in people with delayed sleep-wake phase disorder versus healthy controls. Sleep Med. 2017;3433–39. 10.1016/j.sleep.2017.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wyatt JK, Stepanski EJ, Kirkby J. Circadian phase in delayed sleep phase syndrome: predictors and temporal stability across multiple assessments. Sleep. 2006;29(8):1075–1080. 10.1093/sleep/29.8.1075 [DOI] [PubMed] [Google Scholar]
- 6.Watson LA, Phillips AJK, Hosken IT, et al. Increased sensitivity of the circadian system to light in delayed sleep-wake phase disorder. J Physiol. 2018;596(24):6249–6261. 10.1113/JP275917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGlashan EM, Burns AC, Murray JM, et al. The pupillary light reflex distinguishes between circadian and non-circadian delayed sleep phase disorder (DSPD) phenotypes in young adults. PLoS One. 2018;13(9):e0204621. 10.1371/journal.pone.0204621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aoki H, Ozeki Y, Yamada N. Hypersensitivity of melatonin suppression in response to light in patients with delayed sleep phase syndrome. Chronobiol Int. 2001;18(2):263–271. 10.1081/CBI-100103190 [DOI] [PubMed] [Google Scholar]
- 9.Murray JM, Sletten TL, Magee M, et al. Prevalence of circadian misalignment and its association with depressive symptoms in delayed sleep phase disorder. Sleep. 2017;40(1):1–10. [DOI] [PubMed] [Google Scholar]
- 10.Cain SW, Dennison CF, Zeitzer JM, et al. Sex differences in phase angle of entrainment and melatonin amplitude in humans. J Biol Rhythms. 2010;25(4):288–296. 10.1177/0748730410374943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgess HJ, Wyatt JK, Park M, Fogg LF. Home circadian phase assessments with measures of compliance yield accurate dim light melatonin onsets. Sleep. 2015;38(6):889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagtegaal E, Peeters T, Swart W, Smits M, Kerkhof G, van der Meer G. Correlation between concentrations of melatonin in saliva and serum in patients with delayed sleep phase syndrome. Ther Drug Monit. 1998;20(2):181–183. 10.1097/00007691-199804000-00008 [DOI] [PubMed] [Google Scholar]
- 13.Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549(Pt 3):945–952. 10.1113/jphysiol.2003.040477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeitzer JM, Dijk D-J, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526(3):695–702. 10.1111/j.1469-7793.2000.00695.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips AJK, Clerx WM, O’Brien CS, et al. Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing. Sci Rep. 2017;7(1):3216. 10.1038/s41598-017-03171-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lunsford-Avery JR, Engelhard MM, Navar AM, Kollins SH. Validation of the Sleep Regularity Index in older adults and associations with cardiometabolic risk. Sci Rep. 2018;8(1):14158. 10.1038/s41598-018-32402-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray JM, Phillips AJK, Magee M, et al. Sleep regularity is associated with sleep-wake and circadian timing, and mediates daytime function in delayed sleep-wake phase disorder. Sleep Med. 2019;5893–101. 10.1016/j.sleep.2019.03.009 [DOI] [PubMed] [Google Scholar]
- 18.Marler MR, Gehrman P, Martin JL, Ancoli-Israel S. The sigmoidally transformed cosine curve: a mathematical model for circadian rhythms with symmetric non-sinusoidal shapes. Stat Med. 2006;25(22):3893–3904. 10.1002/sim.2466 [DOI] [PubMed] [Google Scholar]
- 19.Saxvig IW, Pallesen S, Wilhelmsen-Langeland A, Molde H, Bjorvatn B. Prevalence and correlates of delayed sleep phase in high school students. Sleep Med. 2012;13(2):193–199. 10.1016/j.sleep.2011.10.024 [DOI] [PubMed] [Google Scholar]
- 20.Digdon N, Howell A. College students who have an eveningness preference report lower self-control and greater procrastination. Chronobiol Int. 2008;25(6):1029–1046. 10.1080/07420520802553671 [DOI] [PubMed] [Google Scholar]
- 21.Swaminathan K, Klerman EB, Phillips AJK. Are individual differences in sleep and circadian timing amplified by use of artificial light sources?. J Biol Rhythms. 2017;32(2):165–176. 10.1177/0748730417699310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bei B, Wiley JF, Trinder J, Manber R. Beyond the mean: A systematic review on the correlates of daily intraindividual variability of sleep/wake patterns. Sleep Med Rev. 2016;28108–124. 10.1016/j.smrv.2015.06.003 [DOI] [PubMed] [Google Scholar]
- 23.Chang AM, Reid KJ, Gourineni R, Zee PC. Sleep timing and circadian phase in Delayed Sleep Phase Syndrome. J Biol Rhythms. 2009;24(4):313–321. 10.1177/0748730409339611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibui K, Uchiyama M, Okawa M. Melatonin rhythms in Delayed Sleep Phase Syndrome. J Biol Rhythms. 1999;14(1):72–76. 10.1177/074873099129000371 [DOI] [PubMed] [Google Scholar]
- 25.Micic G, de Bruyn A, Lovato N, et al. The endogenous circadian temperature period length (tau) in delayed sleep phase disorder compared to good sleepers. J Sleep Res. 2013;22(6):617–624. 10.1111/jsr.12072 [DOI] [PubMed] [Google Scholar]
- 26.Monteleone P, Esposito G, La Rocca A, Maj M. Does bright light suppress nocturnal melatonin secretion more in women than men?. J Neural Transm (Vienna). 1995;102(1):75–80. 10.1007/BF01276567 [DOI] [PubMed] [Google Scholar]