Abstract
The poly-γ-glutamic acid (PGA) capsule produced by Bacillus anthracis is composed entirely of d-isomer glutamic acid, whereas nonpathogenic Bacillus species produce mixed d-, l-isomer PGAs. To determine if B. anthracis PGA confers a pathogenic advantage over other PGAs, we compared the responses of human innate immune cells to B. anthracis PGA and PGAs from nonpathogenic B. subtilis subsp. chungkookjang and B. licheniformis. Monocytes and immature dendritic cells (iDCs) responded differentially to the PGAs, with B. anthracis PGA being least stimulatory and B. licheniformis PGA most stimulatory. All three elicited IL-8 and IL-6 from monocytes, but B. subtilis PGA also elicited IL-10 and TNF-α, whereas B. licheniformis PGA elicited all those plus IL-1β. Similarly, all three PGAs elicited IL-8 from iDCs, but B. subtilis PGA also elicited IL-6, and B. licheniformis PGA elicited those plus IL-12p70, IL-10, IL-1β, and TNF-α. Only B. licheniformis PGA induced dendritic cell maturation. TLR assays also yielded differential results. B. subtilis PGA and B. licheniformis PGA both elicited more TLR2 signal than B. anthracis PGA, but only responses to B. subtilis PGA were affected by a TLR6 neutralizing Ab. B. licheniformis PGA elicited more TLR4 signal than B. anthracis PGA, whereas B. subtilis PGA elicited none. B. anthracis PGA persisted longer in high m.w. form in monocyte and iDC cultures than the other PGAs. Reducing the m.w. of B. anthracis PGA reduced monocytes’ cytokine responses. We conclude that B. anthracis PGA is recognized less effectively by innate immune cells than PGAs from nonpathogenic Bacillus species, resulting in failure to induce a robust host response, which may contribute to anthrax pathogenesis.
Bacillus anthracis is a highly pathogenic organism and the causative agent of anthrax. Its virulence derives primarily from the products of its two plasmids, pXO1 and pXO2, which carry the genes for the production of toxins (1) and capsule (2, 3), respectively. B. anthracis capsule consists of poly-γ-glutamic acid (PGA), which contributes to virulence by protecting the bacilli from phagocytosis by immune cells (4–6). However, this is not the only way that the PGA capsule may promote virulence. Shed B. anthracis PGA can accumulate in the blood of infected hosts (7), reaching concentrations of >500 μg/ml in mice (8) and >1 mg/ml in rhesus macaques (9). Previously, we reported that shed B. anthracis PGA can impair maturation of human dendritic cells (DCs) in vitro (10) and low m.w. capsule has been reported to enhance the virulence of an attenuated B. anthracis strain (11). Thus, both bacillus-bound and shed B. anthracis capsule can enhance pathogenesis.
Most bacterial capsules are polysaccharides, but B. anthracis capsule is a homopolymer of d-isomer glutamic acid residues connected by γ linkages (7, 12). B. anthracis PGA is fairly resistant to degradation by mammalian proteases because of the γ linkages and is a poorly immunogenic thymus-independent type 2 Ag (13, 14). B. anthracis PGA is covalently linked to the peptidoglycan of the cell wall of the bacillus to form the capsule by capsule depolymerase (CapD), the same enzyme that releases fragments of B. anthracis PGA from the bacillus (11, 15–17). Other nonpathogenic Bacillus species produce similar γ-linked PGA polymers, but these are composed of a mixture of d- and l-isomer glutamic acid residues in contrast to the pure d-isomer polymer produced by B. anthracis (18). PGA produced by B. licheniformis (B. licheniformis, formerly designated B. subtilis American Type Culture Collection 9945a) can have high or low d-isomer content, depending upon culture conditions, especially the concentration of Mn2+ ions (18–21). In contrast, even with the loss of either of its glutamate racemases, B. anthracis continues to produce a PGA composed solely of d-isomer glutamic acid (22). The pure d-isomer content is often regarded as a hallmark feature of B. anthracis PGA. We hypothesized that, because B. anthracis PGA is distinctive from other Bacillus species’ PGAs, it might confer pathogenic advantages.
To explore B. anthracis PGA’s potential to enhance pathogenicity, we compared the responses of human innate immune cells to B. anthracis PGA to their responses to PGAs from nonpathogenic Bacillus species, B. licheniformis and B. subtilis subsp. chungkookjang (B. subtilis). B. licheniformis is soil bacterium like B. anthracis, whereas B. subtilis was isolated from Korean fermented soybean paste, Chung-Kook-Jang (23). Other strains of B. subtilis give fermented soybean foods, such as Japanese natto (24) and Indian kinema (25), their characteristic mucilaginous texture. Strains of B. licheniformis and B. subtilis are widely used in industry to produce PGA for various applications, including food, medicine, cosmetics, and wastewater treatment (18). B. licheniformis and B. subtilis are very rarely associated with human infection and usually only when introduced by trauma or surgery (26). We found that B. licheniformis PGA and B. subtilis PGA were both more stimulatory to human innate immune cells. Compared with exposure to B. anthracis PGA, exposure to B. licheniformis PGA and B. subtilis PGA resulted in increases in the magnitude and variety of cytokines released from both human monocytes and immature DCs (iDCs). We also found that B. licheniformis PGA could induce maturation of iDCs. Seeking to find a mechanism behind the varied responses of the monocytes and iDCs to the three PGAs, we tested them in human TLR2 and TLR4 assays. We found that the PGAs from the nonpathogenic B. licheniformis and B. subtilis species were again more stimulatory than B. anthracis PGA and, in the case of B. subtilis PGA, had a TLR recognition pattern distinct from B. anthracis PGA’s. We also found that B. anthracis PGA persists longer in high m.w. form in the media of human monocyte and iDC cultures than does B. licheniformis PGA or B. subtilis PGA. Whereas the increases in cytokine responses were not directly correlated with the percentage of l-isomer glutamic acid present in B. licheniformis PGA and B. subtilis PGA, the extent of degradation was, suggesting that the inclusion of d-isomer further increases resistance to human proteases above the resistance provided by the γ linkages. To examine if low m.w. PGA, such as occurs in cultures with B. licheniformis PGA or B. subtilis PGA, might be more stimulatory to monocytes, we used sonication to reduce the m.w. of B. anthracis PGA and exposed it to monocytes. We found that a reduction in B. anthracis PGA’s m.w. led to a reduction in the monocytes’ cytokine responses, indicating that the cells recognize high m.w. and perhaps secondary structure of B. anthracis PGA. Taken together, these results indicate that innate immune cells can distinguish between different PGAs and respond differentially. PGAs from nonpathogenic B. licheniformis and B. subtilis are recognized more effectively by the host and stimulate a broader immune response. B. anthracis PGA, which is poorly recognized and far less stimulatory, may contribute to pathogenesis by failing to induce a vigorous innate immune response.
Materials and Methods
PGAs and reagents
B. anthracis Ames was from the United States Army Medical Research Institute of Infectious Diseases collection. Shed B. anthracis PGA was harvested from broth culture supernatants as described previously (10), with some modifications. First, the sterile filtered supernatants were precipitated with trichloroacetic acid before and after Benzonase (EMD Chemicals, Gibbstown, NJ) treatment, which included a 1-h incubation at 37°C before overnight incubation at 4°C. Second, the final pellets were resuspended in sterile water for injection (Life Technologies, Grand Island, NY). Third, copurifying contaminants were separated from the B. anthracis PGA by fast protein liquid chromatography using an AKTA Purifier with an UV 900 detector (GE Healthcare, Chicago, IL), fractionating it over a ceramic hydroxyapatite type II 40-μm column (Bio-Rad Laboratories, Hercules, CA). The B. anthracis PGA pool was then run over a Superose 6 size exclusion chromatography column (Amersham Pharmacia Biotech, Piscataway, NJ) to assess its size and purity by monitoring absorbance at 214, 254, and 280 nm (27). For experiments assessing the effects of reduced m.w., aliquots of purified B. anthracis PGA were placed in sterile polystyrene tubes and sonicated in a Misonix Sonicator 3000 Cup Horn Sonicator (Qsonica, Newtown, CT) for 10 min.
B. subtilis PGA (m.w. 1,500,000–2,000,000) was purchased from Wako Pure Chemical Industries (Osaka, Japan) and dissolved in endotoxin-free 100 mM sodium phosphate (NaP) buffer. B. licheniformis PGA (m.w. ≥ 1,000,000) was purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in water for injection. d- and l-glutamic acid were purchased from Sigma-Aldrich and dissolved in NaP buffer. Synthetic poly-α-d-glutamic acid (m.w. 15,000–50,000) and poly-α-l-glutamic acid (m.w. 50,000–100,000) were purchased from Sigma-Aldrich and dissolved in NaP buffer. All PGAs and reagents were tested for endotoxin contamination using a Limulus amebocyte lysate end point chromogenic assay (Lonza, Walkersville, MD) before use. Triton X-114 (Sigma-Aldrich) was used to remove endotoxin when necessary. Residual Triton X-114 was removed using Pierce Detergent Removal Columns (Thermo Fisher Scientific, Waltham, MA).
Amino acid and chiral analysis
Amino acid analysis of the PGAs was performed by Biosynthesis (Lewisville, TX). For chiral analysis, PGA samples were diluted in water and subjected to vapor-phase acid hydrolysis using 6N HCl at 150°C for varying times of 20–120 min using an Eldex Hydrolysis/Derivatization Workstation (Eldex Laboratories, Napa, CA). Hydrolysates were reconstituted with aqueous 2,3,3,4,4-d5–labeled l-Glu and d-Glu internal standards (C/D/N Isotopes, Pointe-Claire, QC, Canada) and analyzed by quantitative isotope dilution liquid chromatography with tandem mass spectrometry. Separation of isomers was achieved by isocratic elution on an Astec Chirobiotic T 4.6 × 250 mm Column (Sigma-Aldrich) with 30:70 (v/v) 0.025% formic acid in water and methanol at 1 ml/min. Retention times were around 5 and 6 min for l-Glu and d-Glu, respectively. Tandem mass spectrometry data were acquired in positive ion electrospray mode (MH+) on a Thermo Vantage instrument (Thermo Fisher Scientific) using multiple reaction monitoring on native (mass-to-charge ratio [m/z] 148, MH+) and internal standard (m/z 153, MH+) materials with four fragment ions for each. Quantitation was achieved based on native/internal standard area ratios, using standard curves and quality controls comprising pure l-Glu and d-Glu amino acids spanning the range 0.0032–50 ng/μl. Percentages of d-Glu in the PGA samples in the absence of amino acid racemization (which occurs during hydrolysis) were estimated by extrapolation of linear regression fits for typically four hydrolysis time points back to time zero.
Human cells
Leukopacks were obtained from the Department of Transfusion Medicine (Clinical Center, National Institutes of Health, Bethesda, MD). Human monocytes were purified and cultivated as before (10). Experimental monocyte cultures included 500 μg/ml B. anthracis PGA, B. subtilis PGA, or B. licheniformis PGA. On day 3, media samples were removed for cytokine analysis or electrophoresis. Untreated cultures were maintained for 5 d to allow for differentiation into iDCs. On day 5, iDCs were stimulated with 100 ng/ml LPS (from Escherichia coli 055:B5; Sigma-Aldrich), 500 μg/ml B. anthracis PGA, B. subtilis PGA, or B. licheniformis PGA or were maintained in medium alone. On day 7, media samples were removed for cytokine analysis or electrophoresis, and the cells were used for flow cytometry or a CCR7-dependent chemotactic activity assay.
Research involving human subjects adhered to the principles identified in the Belmont Report (1979) and, unless certified as exempt, was conducted in accordance with an Institutional Review Board–approved protocol and in compliance with Department of Defense, federal, and state statutes and regulations relating to the protection of human subjects. Research involving human subjects certified as exempt was conducted in accordance with the conditions specified in connection with an exemption certificate.
Cytokine measurement
Duplicate wells of media samples were assayed for human IL-1β, IL-6, IL-8, IL-10, IL-12p70, IL-2, IFN-γ, GM-CSF, and TNF-α using Human ProInflammatory 9-Plex Platform Ultra-Sensitive plates (Meso Scale Discovery, Gaithersburg, MD). The plates were analyzed using a SECTOR Imager 2400 (Meso Scale Discovery) according to the manufacturer’s protocol.
Flow cytometry
Cells were stained with fluorescently labeled Abs specific for human MHC class I (MHC I), MHC II, CD83, or CCR7 or with isotype control Abs purchased from BD Pharmingen (San Jose, CA). Flow cytometry experiments were run on an FACSCalibur (BD Biosciences, Billerica, MA), and the data were analyzed using CellQuest Pro software (BD Biosciences). Statistical significance of the data were determined by two-tailed t tests comparing average mean fluorescence intensities for the various treatments to the untreated control for six experiments conducted with cells from six donors (GraphPad Software, La Jolla, CA).
Chemotactic activity assay
DCs were resuspended in RPMI 1640 medium supplemented with 1% BSA at 6 × 105 live cells/ml and run in a CCR7-dependent chemotactic activity assay as described previously (10). Fluorescence was read at 480/520 nm in a SpectraMax M5 fluorescence plate reader (Molecular Devices, Sunnyvale, CA). Chemotactic index was calculated by dividing the relative fluorescence units observed in the presence of the CCR7 ligands by the relative fluorescence units observed in the absence of ligand. Statistical significance was determined by comparing the means of the chemotactic indices for the cells exposed to LPS, B. licheniformis PGA, or B. subtilis PGA to the mean chemotactic index for the naive iDC control (GraphPad Software).
Human TLR2 and TLR4 assays
Human embryonic kidney (HEK) cells expressing human TLR2 and a secreted embryonic alkaline phosphatase (SEAP) reporter gene (HEK-Blue hTLR2) were obtained from InvivoGen (San Diego, CA). HEK-Blue hTLR2 cells were plated in 96-well plates in HEK-Blue Detection medium (InvivoGen), which contains a colorimetric substrate for SEAP. Pam3CSK4 and FSL-1 (InvivoGen) were used as positive controls. HEK-Blue hTLR2 cells were also plated in the absence of ligands or vehicles to control for background alkaline phosphatase activity. Twenty-four hours after plating OD650 was read in a SpectraMax M5 plate reader (Molecular Devices). Statistical significance was determined by t tests comparing the mean treatment/background ratio (i.e., OD650 for a treatment divided by the OD650 for cells alone) for each ligand to the mean treatment/background ratio for its vehicle (GraphPad Software). Responses to the different PGAs determined as mean ligand/vehicle ratios (i.e., OD650 for a treatment divided by the OD650 for its vehicle) were compared in t tests (GraphPad Software). Neutralizing polyclonal Abs for human TLR1 and human TLR6 and control normal rat IgG were also obtained from InvivoGen. For assays with neutralizing Abs, the HEK-Blue hTLR2 cells were preincubated with 5 μg/ml neutralizing Ab or normal rat IgG at 37°C/5% CO2 for 10 min prior to plating alone with ligand or with vehicle as per InvivoGen’s protocol.
TLR4 assays were conducted as described for TLR2 using HEK cells expressing human TLR4 and an SEAP reporter gene (HEK-Blue hTLR4; InvivoGen) in place of the HEK-Blue hTLR2 cells and E. coli LPS (Sigma-Aldrich) as the positive control.
Electrophoresis and staining
PGAs were separated by electrophoresis in 1% agarose gels and visualized by staining with 0.1% methylene blue (Sigma-Aldrich) as described previously (28). Monocyte and DC culture media samples were mixed with lithium dodecyl sulfate sample buffer (Invitrogen) and 100 mM DTT (Pierce) and heated at 70°C for 10 min. The prepared samples were separated on 4–12% Bis-Tris polyacrylamide gradient gels run in MOPS-SDS buffer (Invitrogen). PGA was visualized by staining with 0.2 mg/ml Stains-All (Sigma-Aldrich) as described previously (6).
Results
PGAs from nonpathogenic Bacillus species elicit higher levels of cytokine release by human monocytes than PGA from B. anthracis
We hypothesized that B. anthracis PGA confers a pathogenic advantage against the host innate immune system compared with PGAs from nonpathogenic Bacillus species. To test this, we obtained PGAs from nonpathogenic B. subtilis and B. licheniformis to compare with B. anthracis PGA. All three PGAs were composed of >99% glutamic acid by amino acid analysis. All three PGAs yielded single absorption peaks at 214 nm and had no absorbance at 280 or 254 nm when assessed by fast protein liquid chromatography. All three PGAs tested very low in endotoxin (<0.2 endotoxin units/ml). Chiral analysis by quantitative isotope dilution liquid chromatography with tandem mass spectrometry showed that the B. subtilis PGA contained 52% d-isomer glutamic acid, and the B. licheniformis PGA contained 87% d-isomer glutamic acid. B. anthracis PGA, B. subtilis PGA, and B. licheniformis PGA are all high m.w. polymers (Fig. 1), so the primary difference among them appears to be the isomeric composition. We showed previously that purified shed B. anthracis PGA elicits a cytokine response from human monocytes (10) and, in the current study, determined whether the PGAs from nonpathogenic Bacillus species elicit the same response. Media samples from human monocytes cultured with 500 μg/ml B. anthracis PGA, B. subtilis PGA, or B. licheniformis PGA were assayed for IL-8, IL-6, IL-10, IL-1β, IL-12p70, TNF-α, IL-2, and IFN-γ. Five experiments were run with cells from five donors. An increase of ≥50% over the unstimulated control was considered a response. Fig. 2 shows the ranges of the individual cytokine responses in picograms per milliliter in tabular form, and the mean cytokine results in graphic form. Consistent with our previous study, B. anthracis PGA elicited IL-8 and IL-6 responses from all donors, and the mean IL-8 and IL-6 responses were both significant (p < 0.01 and p < 0.05, respectively). Also consistent with our previous results, B. anthracis PGA elicited small TNF-α responses from three out of five donors. However, the mean TNF-α response was NS. None of the other cytokines tested were elicited by B. anthracis PGA. In contrast, B. subtilis PGA elicited a broader variety of cytokines in larger amounts. B. subtilis PGA elicited greater mean amounts of IL-8 and IL-6 than did B. anthracis PGA (p < 0.01 for both). Furthermore, unlike B. anthracis PGA, B. subtilis PGA elicited significant mean TNF-α (p < 0.01) and IL-10 (p < 0.05) responses and elicited IL-β responses from 3/5 donors, although the mean IL-1β response was NS. B. licheniformis PGA elicited cytokines in even larger amounts. Like B. anthracis PGA and B. subtilis PGA, B. licheniformis PGA elicited IL-8 and IL-6 responses from all five donors, and the mean IL-8 and IL-6 responses elicited by B. licheniformis PGA were greater than those elicited by B. subtilis PGA (p < 0.05 and p < 0.001, respectively). B. licheniformis PGA also elicited larger mean TNF-α (p < 0.001) and IL-10 (p < 0.05) responses than B. subtilis PGA. Only B. licheniformis PGA elicited a significant mean IL-1β response (p < 0.01). The amounts of IL-8, IL-6, and TNF-α elicited by B. anthracis PGA were dramatically less than those observed with B. subtilis PGA or B. licheniformis PGA, and as noted above, none of the donors released any IL-10 or IL-1β in response to B. anthracis PGA. Thus, human monocytes responded with a broader variety of cytokines in much larger amounts to the PGAs from nonpathogenic Bacillus species than to B. anthracis PGA. There was no direct correlation between the l-isomer content of the PGAs and their ability to stimulate cytokine responses from monocytes.
FIGURE 1.
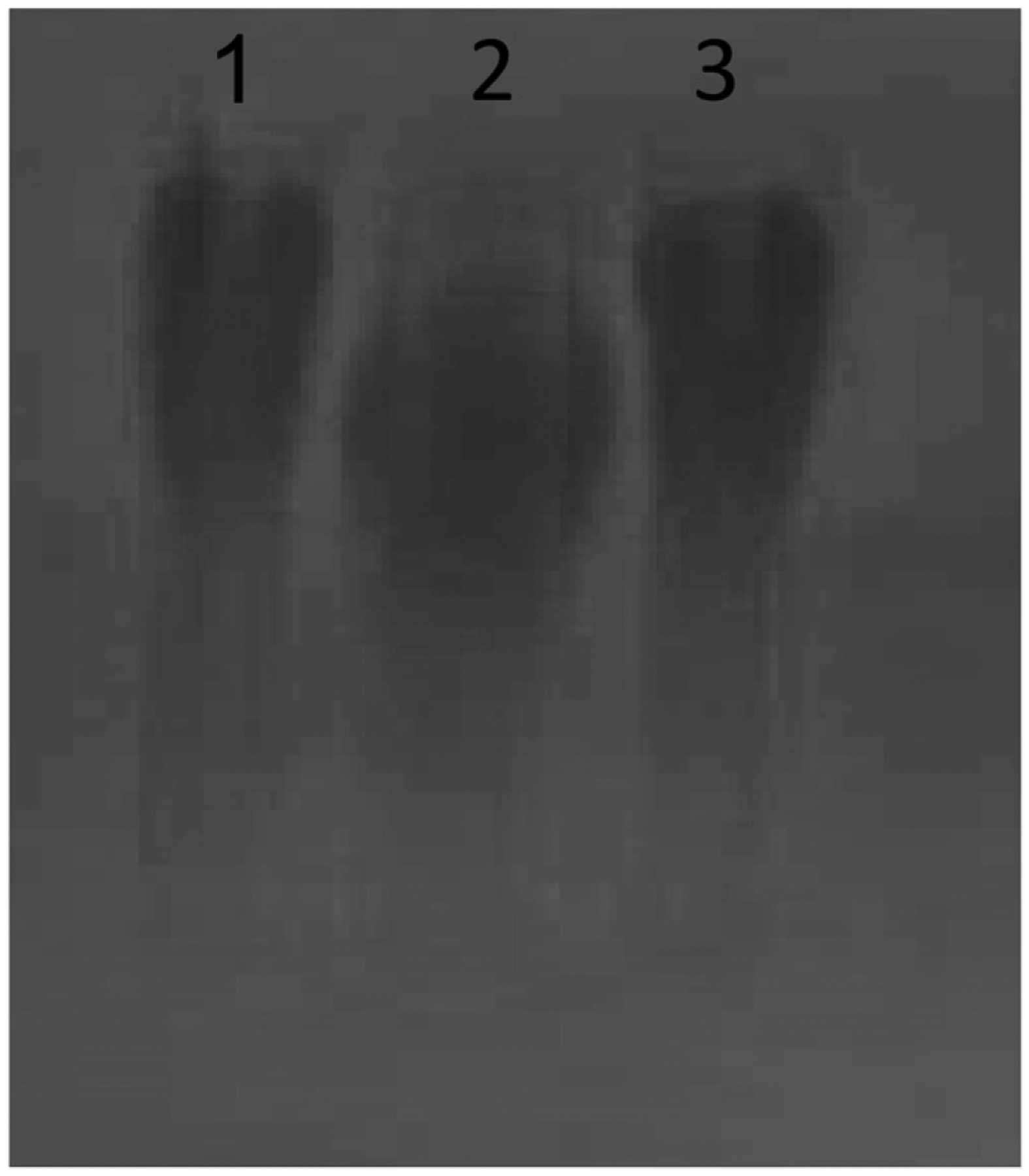
PGAs from B. anthracis, B. subtilis subsp. chungkookjang, and B. licheniformis are high m.w. polymers. 40 μg/ml per lane of B. anthracis PGA (lane 1), B. subtilis PGA (lane 2), and B. licheniformis PGA (lane 3) were electrophoresed on a 1% agarose gel and visualized by 0.1% methylene blue staining. A representative gel is shown (n = 3).
FIGURE 2.
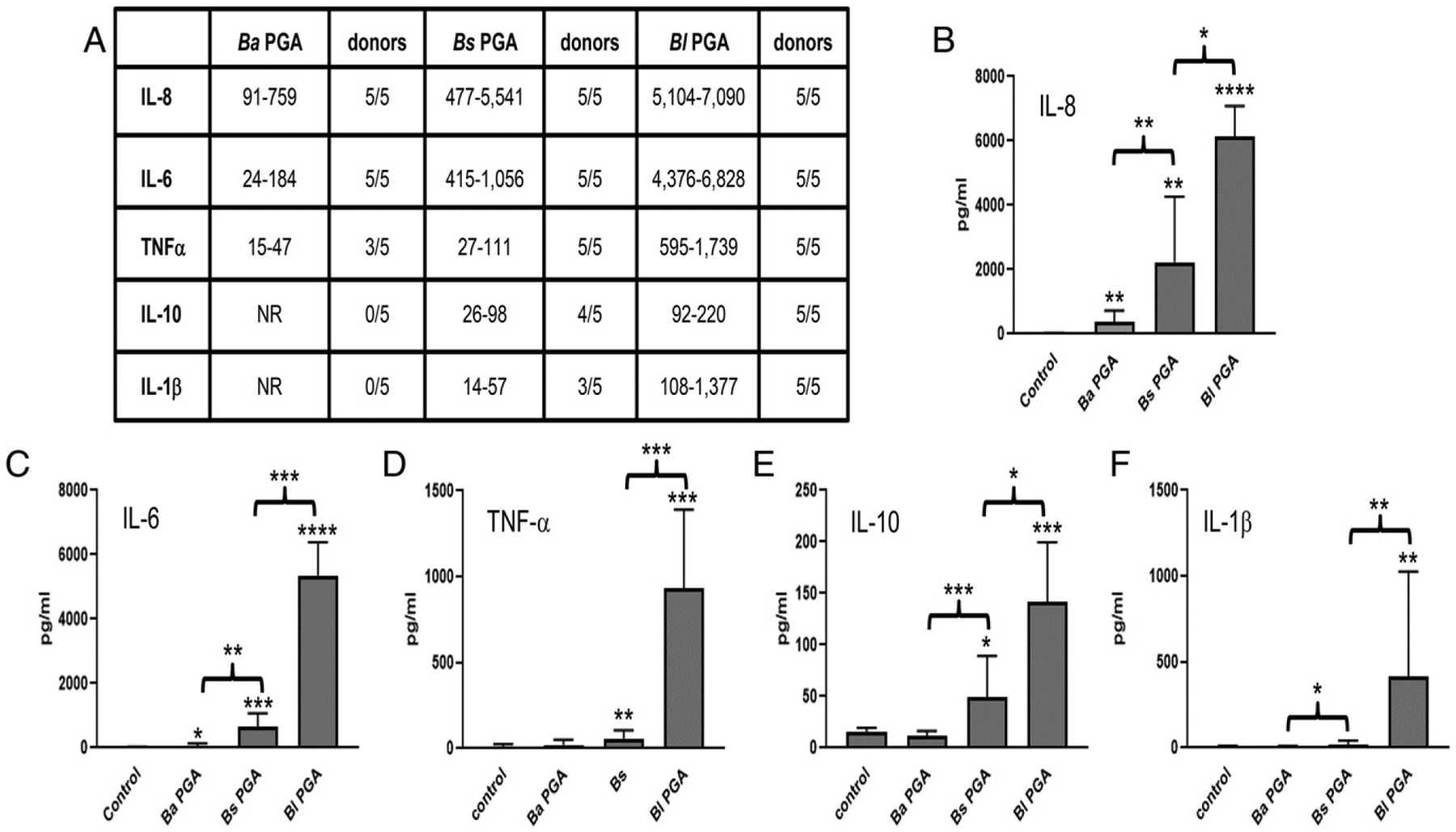
PGAs from nonpathogenic B. subtilis and B. licheniformis elicit larger and broader cytokine responses than B. anthracis PGA from human monocytes. Monocytes were exposed to 500 μg/ml B. anthracis PGA, B. subtilis PGA, or B. licheniformis PGA or to medium alone (control). Media samples were analyzed for cytokines. An increase of ≥50% over the unstimulated control was considered a response. Five experiments were run using cells from five donors (n = 5). (A) The range of individual responses for each cytokine is presented in picograms per milliliter plus the number of donors that had a response. NR, no response. (B–F) Data are presented as geometric means in picograms per milliliter ± geometric SD (n = 5) for IL-8 (B), IL-6 (C), TNF-α (D), IL-10 (E), and IL-1β (F). Statistical significance of the responses to each PGA compared with control and of the differences in responses to the PGAs were determined by paired t tests of the log transformed data. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Ba, B. anthracis; Bl, B. licheniformis; Bs, B. subtilis.
PGAs from nonpathogenic Bacillus species elicit higher levels of cytokine release by human iDCs than PGA from B. anthracis
We showed, in a previous study, that B. anthracis PGA elicits a very modest cytokine response from human iDCs (10) and next determined whether PGAs from nonpathogenic Bacillus species differed from B. anthracis PGA in this response as well. Human monocytes were cultured with IL-4 and GM-CSF to promote differentiation into iDCs and, subsequently, exposed to 500 μg/ml B. anthracis PGA, B. subtilis PGA, or B. licheniformis PGA. Media samples were assayed for cytokines as before. Five experiments were run with cells from five donors. An increase of ≥50% over the unstimulated control was considered a response. Fig. 3 shows the ranges of the individual cytokine responses in picograms per milliliter in tabular form, and the mean cytokine results in graphic form. Consistent with our previous study, B. anthracis PGA elicited very modest IL-8 responses from all five donors. Also consistent with our previous study, not all donors (2/5) released IL-6. In contrast to our previous study, no TNF-α responses were observed. Only the mean IL-8 response was significant (p < 0.01). B. subtilis PGA elicited larger amounts of cytokines and elicited them from more donors than B. anthracis PGA. B. subtilis PGA elicited larger mean amounts of IL-8 and IL-6 than B. anthracis PGA (p < 0.01 for both). Three out of five donors released TNF-α in response to B. subtilis PGA, although the mean TNF-α response was NS. B. licheniformis PGA elicited an even broader variety of cytokines in much larger amounts. Like B. anthracis PGA and B. subtilis PGA, B. licheniformis PGA elicited IL-8 from all five donors. B. licheniformis PGA elicited a larger mean amount of IL-6 than B. subtilis PGA (p < 0.001). B. licheniformis PGA also had the only significant mean TNF-α (p < 0.001), IL-10 (p < 0.001), IL-1β (p < 0.05), and IL-12p70 (p < 0.01) responses. Similar to the monocytes, the iDCs yielded larger and more varied cytokine responses to the PGAs from nonpathogenic Bacillus species with no direct correlation to l-isomer content.
FIGURE 3.
PGAs from nonpathogenic B. subtilis and B. licheniformis elicit larger cytokine responses than B. anthracis PGA from human iDCs. iDCs were exposed to 500 μg/ml B. anthracis PGA, B. subtilis PGA, or B. licheniformis PGA or to medium alone (control). Media samples were analyzed for cytokines. An increase of ≥50% over the unstimulated control was considered a response. Five experiments were run using cells from five donors (n = 5). (A) The range of individual responses for each cytokine are presented in picograms per milliliter plus the number of donors that had a response. NR, no response. (B–G) Data are presented as geometric means in picograms per milliliter ± geometric SD (n = 5) for IL-8 (B), IL-6 (C), TNF-α (D), IL-10 (E), IL-1β (F), and IL-12p70 (G). Statistical significance of the responses to each PGA compared with control and of the differences in responses to the PGAs were determined by paired t tests of the log transformed data. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Ba, B. anthracis; Bl, B. licheniformis; Bs, B. subtilis.
B. licheniformis PGA induces maturation of human iDCs
B. licheniformis PGA elicited IL-8, IL-6, IL-10, IL-12p70, and TNF-α from the iDCs of all five donors plus IL-1β from four out of five donors (Fig. 3A). Because a mixture of TNF-α, IL-6, IL-1β, and PGE2 can be used to mature human iDCs in vitro (29), we hypothesized that B. licheniformis PGA could induce maturation of human iDCs. Human monocytes were differentiated into iDCs as before. B. anthracis PGA, B. subtilis PGA, or B. licheniformis PGA was added to the experimental cultures at 500 μg/ml. Negative control cultures were maintained in medium alone so that the cells would retain the iDC phenotype (Fig. 4A). Positive control cultures were stimulated with LPS. Two days after stimulation with LPS or the PGAs, the cells were analyzed by flow cytometry for expression of MHCs I and II, plus CD83 and CCR7, cell surface markers associated with the mature DC (mDC) phenotype (30, 31). As expected, cells treated with LPS demonstrated increased expression of MHC I (p < 0.001), MHC II (p < 0.01), CD83 (p < 0.0001), and CCR7 (p < 0.0001, n = 6 donors; Fig. 4B). Treatment with B. licheniformis PGA also increased expression of MHC I (p < 0.05), MHC II (p < 0.05), CD83 (p < 0.001), and CCR7 (p < 0.01, n = 6 donors; Fig. 4C). Whereas both LPS and B. licheniformis PGA induced conversion to the mDC phenotype, B. licheniformis PGA stimulated smaller increases in MHC I, CD83, and CCR7 expression than LPS (p < 0.05, p < 0.05, p < 0.01, respectively, n = 6 donors). B. subtilis PGA did not induce conversion to the mDC phenotype (Fig. 4D) and, consistent with our previous study (10), neither did B. anthracis PGA (Fig. 4E).
FIGURE 4.
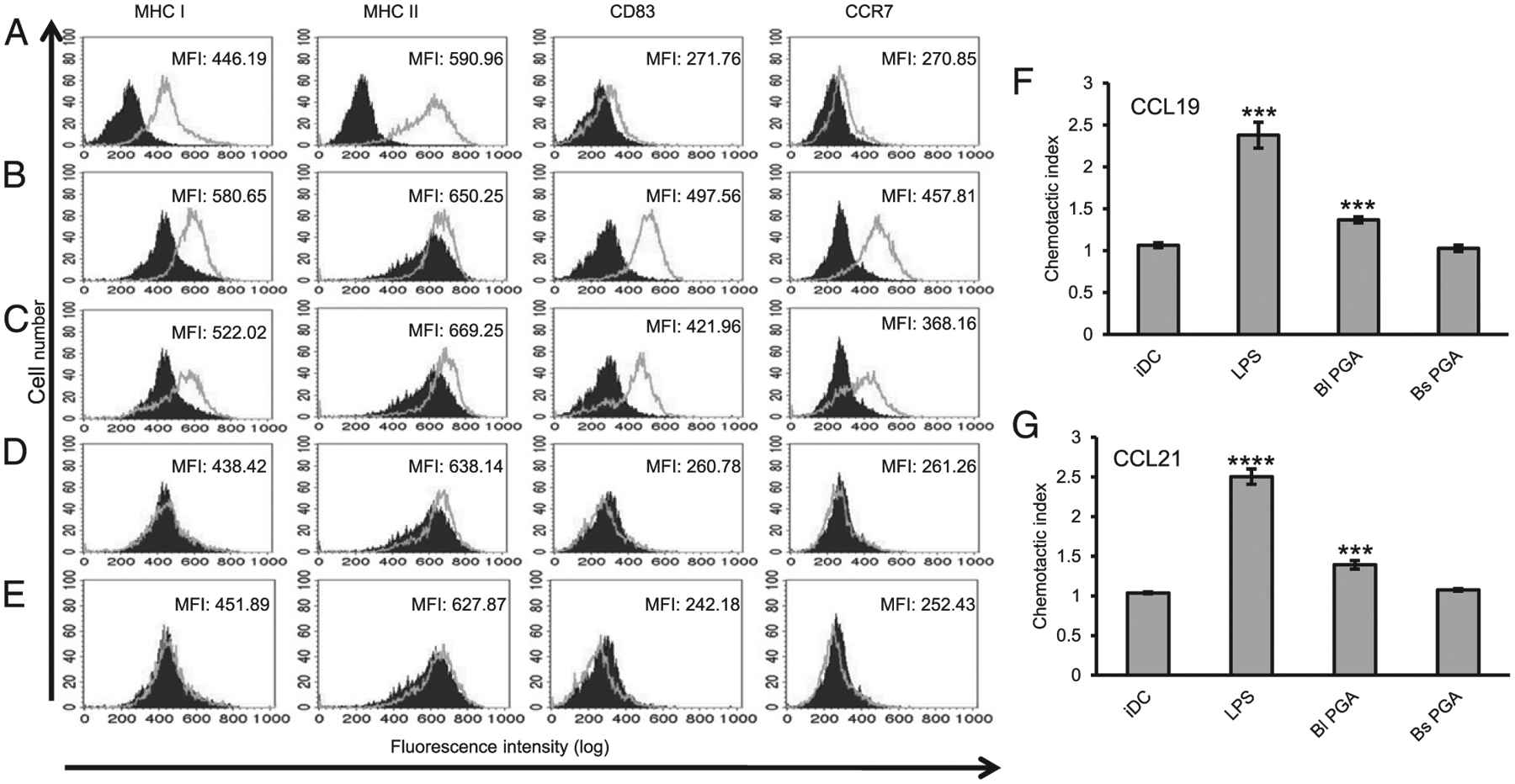
B. licheniformis PGA induces DC maturation. Human iDCs were stimulated with 100 ng/ml LPS, 500 μg/ml B. licheniformis PGA, or 500 μg/ml B. subtilis PGA or were maintained in medium alone. Two days later, the cells were assessed for expression of MHC I, MHC II, CD83, and CCR7 by flow cytometry. Six experiments were run using cells from six donors. Histograms and mean fluorescence intensities (MFI) shown are from a single representative experiment. (A) The filled histograms represent the isotype control and the outlined histograms represent the marker staining for the control iDCs. (B–E) The filled histograms represent the marker staining for the control iDCs. The outlined histograms represent the marker staining for the cells treated with LPS (B), B. licheniformis PGA (C), B. subtilis PGA (D), or B. anthracis PGA (E). (F and G) The cells were run in CCR7-dependent chemotactic activity assays using CCL19 and CCL21 as chemoattractants. Mean data are presented as chemotactic indices ± SEM (n = 4 experiments). Statistical significance was determined by t tests comparing the mean chemotactic index for each of the treatments to the iDC control. ***p < 0.001, ****p < 0.0001. Bl, B. licheniformis; Bs, B. subtilis.
CCR7 expression by mDCs allows them both to migrate to and enter into lymph nodes (32). As functional proof of maturation, we tested DCs exposed to 500 μg/ml B. licheniformis PGA or B. subtilis PGA for CCR7-dependent chemotaxis in a chemotactic activity assay using CCL19 and CCL21 (26) as chemoattractants. Untreated iDCs and LPS-stimulated DCs served as negative and positive controls, respectively. The LPS- and B. licheniformis PGA–treated cells demonstrated chemotaxis toward both CCR7 ligands (Fig. 4F, 4G), indicating functional maturation. Chemotaxis toward CCL19 and CCL21 for the B. licheniformis PGA–treated cells was less than that of the LPS-treated cells (p < 0.001 and p < 0.0001, respectively), consistent with the smaller increase in CCR7 expression we observed. The B. subtilis PGA–treated cells did not demonstrate CCR7-dependent chemotaxis (Fig. 4F, 4G), consistent with retention of the iDC phenotype.
B. anthracis PGA elicits lower signals than B. licheniformis PGA and B. subtilis PGA in a human TLR2 assay
Many bacterial products are recognized by TLRs. A hamster study by Weiss et al. (33) suggested that crude B. anthracis capsule is a TLR2 agonist. PGA from B. licheniformis used as a B. anthracis capsule surrogate has also been shown to be a TLR2 ligand (34, 35). Therefore, we decided to compare B. anthracis PGA with B. licheniformis PGA and B. subtilis PGA in a human TLR2 assay. HEK cells expressing human TLR2 and a reporter gene (HEK-Blue hTLR2 cells) were cultured with 50, 250, or 500 μg/ml B. anthracis PGA, B. licheniformis PGA, or B. subtilis PGA or with equivalent volumes of vehicle. HEK-Blue hTLR2 cells were also cultured without ligand or vehicle to determine the background. The TLR2 coreceptors, TLR1 and TLR6 (36), are endogenously expressed by HEK cells. Pam3CSK4 and FSL-1 served as TLR1/2- and TLR2/6-positive control ligands, respectively. Both positive controls elicited robust signal, confirming that the HEK-Blue hTLR2 cells were capable of recognizing TLR1/2 and TLR2/6 ligands (data not shown). Mean data from four experiments are presented as ligand/vehicle ratios in Fig. 5. B. anthracis PGA, B. licheniformis PGA, and B. subtilis PGA all elicited TLR2 signal at 250 and 500 μg/ml, but none did so at 50 μg/ml (data not shown). Both B. licheniformis PGA and B. subtilis PGA elicited more TLR2 signal than B. anthracis PGA, which had minimal ligand/vehicle ratios of 1.1 and 1.2 at 250 and 500 μg/ml, respectively. TLR2 signals elicited by B. licheniformis PGA and B. subtilis PGA did not differ significantly from each other.
FIGURE 5.
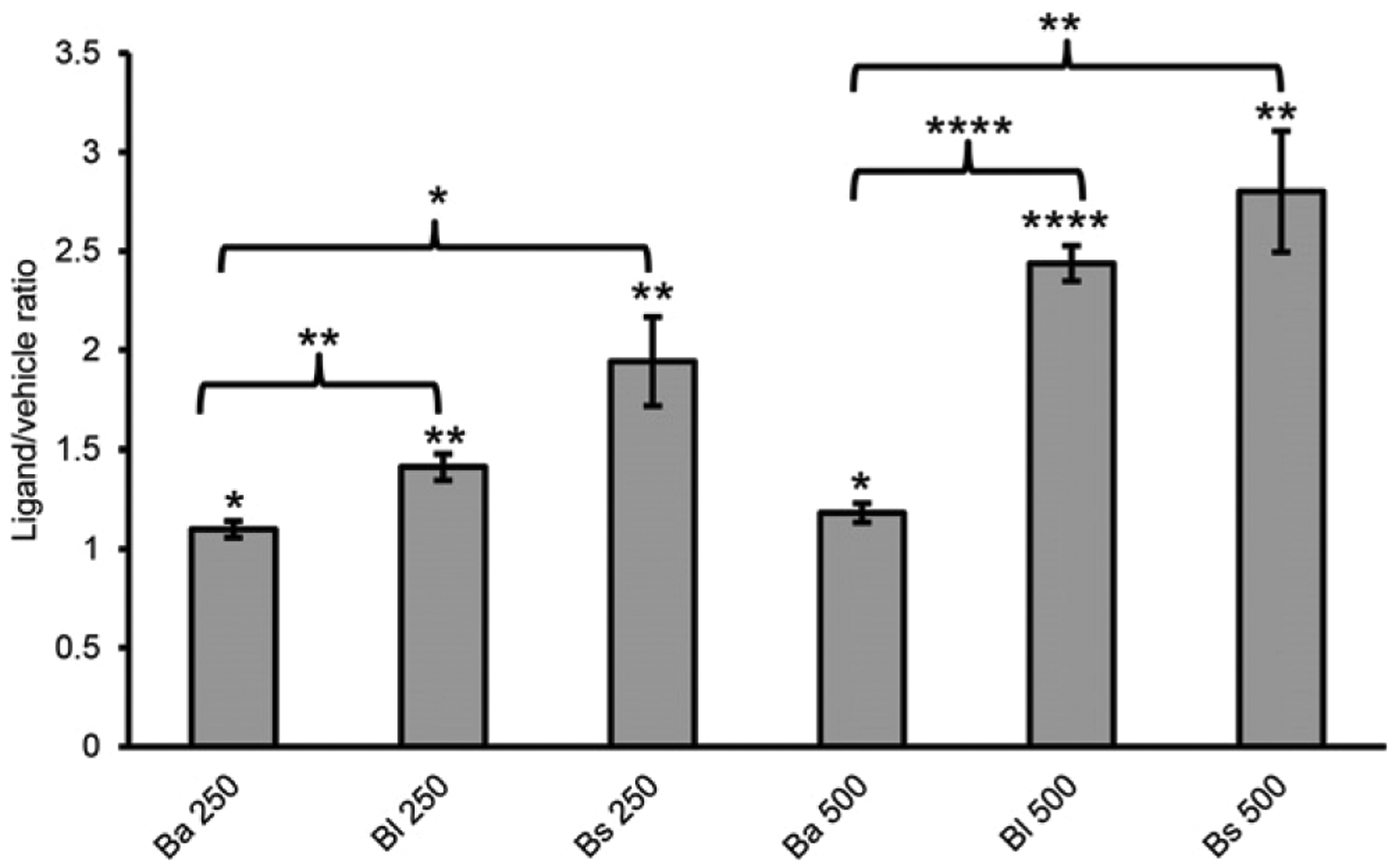
B. anthracis PGA elicits lower signals than B. subtilis PGA and B. licheniformis PGA in a human TLR2 assay. 50, 250, and 500 μg/ml B. anthracis PGA, B. licheniformis PGA, or B. subtilis PGA were tested in a TLR2 assay. Mean data for four experiments are presented as ligand/vehicle ratios ± SEM (n = 4). Statistical significance of signal for each dose of PGA above its vehicle control was determined by t tests comparing the mean treatment/background ratios. Statistical significance of differences between the PGAs were determined by t tests comparing mean ligand/vehicle ratios. No significant responses were observed at 50 μg/ml for any of the PGAs (data not shown). *p < 0.05, **p < 0.01, ***p < 0.0001. Ba, B. anthracis; Bl, B. licheniformis; Bs, B. subtilis.
We next tested 500 μg/ml d–glutamic acid and 500 μg/ml l–glutamic acid to determine if human TLR2 could recognize monomeric glutamic acid. Both failed to elicit TLR2 signal (n = 6 experiments; data not shown). We also tested 500 μg/ml synthetic poly-d-α-glutamic acid and 500 μg/ml synthetic poly-l-α-glutamic acid polymers to see if human TLR2 could recognize α-linked polymers. The α-linked glutamic acid polymers also failed to elicit TLR2 signal (n = 4 experiments; data not shown). These data suggest that human TLR2 specifically recognizes glutamic acid polymers with γ linkages.
B. subtilis PGA is a TLR2/6 agonist
TLR2 can interact with multiple coreceptors (36). The most common coreceptors are TLR1 and TLR6. Therefore, we used neutralizing polyclonal Abs for TLR1 and TLR6 in our human TLR2 assay to assess whether the PGAs were TLR1/2 or TLR2/6 ligands. HEK-Blue hTLR2 cells were preincubated with TLR1 neutralizing Ab, TLR6 neutralizing Ab, or normal rat IgG before being cultured with 500 μg/ml B. anthracis PGA, B. licheniformis PGA, or B. subtilis PGA or with equivalent volumes of vehicle. A total of 0.1 ng/ml Pam3CSK4 and 0.01 ng/ml FSL-1 served as TLR1/2-and TLR2/6-positive controls, respectively. HEK-Blue hTLR2 cells were also cultured without ligand or vehicle to determine the background. Mean data from three experiments are presented as ligand/vehicle ratios in Fig. 6. Normal rat IgG did not prevent Pam3CSK4, FSL-1, or the PGAs from eliciting significant TLR2 signal, as in the previous TLR2 assays, except for the very-low signal induced by B. anthracis PGA. Signal in response to Pam3CSK4 was specifically and significantly reduced by the TLR1 neutralizing Ab, whereas signal in response to FSL-1 was specifically, although not significantly, reduced by the TLR6 neutralizing Ab. Neither the TLR1 nor TLR6 neutralizing Ab had an effect on the TLR2 signal elicited by B. licheniformis PGA or B. anthracis PGA compared with normal rat IgG. However, TLR6 neutralizing Ab specifically and significantly reduced TLR2 signal in response to B. subtilis PGA. These data indicate that B. subtilis PGA signals through a TLR2/6R complex, but B. anthracis PGA and B. licheniformis PGA do not.
FIGURE 6.
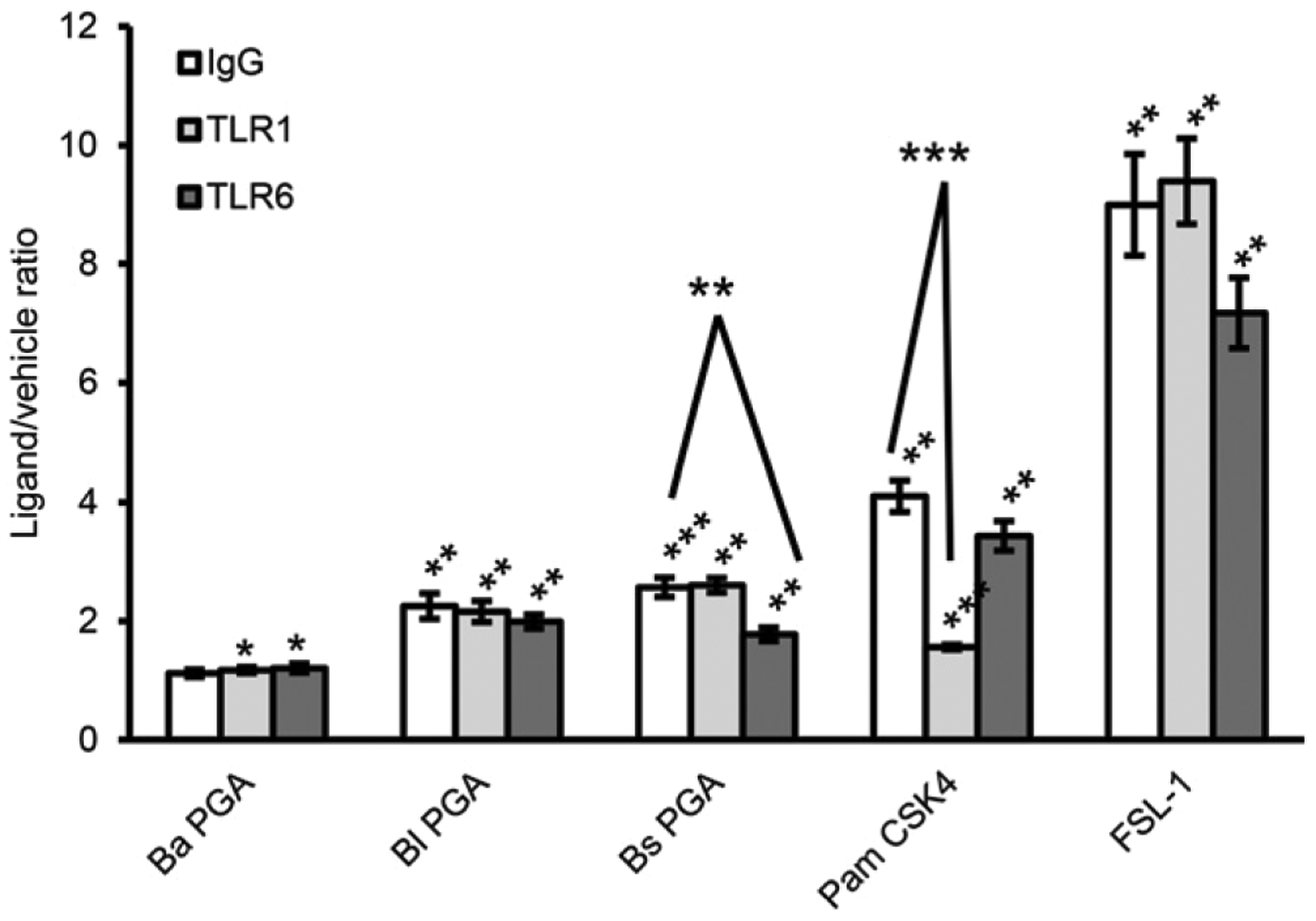
B. subtilis PGA is a human TLR2/6 agonist. 500 μg/ml B. anthracis PGA, B. licheniformis PGA, or B. subtilis PGA was tested in a TLR2 assay with neutralizing polyclonal Abs against TLR1 or TLR6 or normal rat IgG. Pam3CSK4 and FSL-1 were run as TLR1/2-and TLR2/6-positive controls, respectively. Mean data for three experiments are presented as ligand/vehicle ratios ± SEM (n = 3). Statistical significance of signal for each PGA and positive control ligand above its vehicle was determined by t tests comparing the mean treatment/background ratios. Statistical significance of inhibition by the Abs was determined by t tests comparing the mean ligand/vehicle ratios for normal rat IgG treatment versus anti-TLR1 or anti-TLR6 Ab treatment. *p < 0.05, **p < 0.01, ***p < 0.001. Ba, B. anthracis; Bl, B. licheniformis; Bs, B. subtilis.
B. anthracis PGA elicits lower signals than B. licheniformis PGA in a human TLR4 assay
Because B. licheniformis PGA elicits signal comparable to B. subtilis PGA in the TLR2 assay, but elicits significantly larger amounts of cytokines from human monocytes and DCs, it seemed likely that other receptors besides TLR2 are involved in PGA recognition. It has been reported that B. subtilis PGA can signal through TLR4 in murine innate immune cells (37). Therefore, we compared B. anthracis PGA with B. licheniformis PGA and B. subtilis PGA in a human TLR4 assay. HEK cells expressing human TLR4 and a reporter gene (HEK-Blue hTLR4 cells) were cultured with 50, 250, or 500 μg/ml B. anthracis PGA, B. licheniformis PGA, or B. subtilis PGA or with equivalent volumes of vehicle. HEK-Blue hTLR4 cells were also cultured without ligand or vehicle to determine the background. LPS served as a positive control ligand and yielded robust TLR4 signal as expected (data not shown). Mean data from four experiments are presented as ligand/vehicle ratios in Fig. 7A. B. subtilis PGA did not elicit significant TLR4 signal at any concentration (data not shown), indicating that it is not a TLR4 agonist for humans, unlike mice (37). However, human and murine TLR4 differ in their recognition of certain ligands (38), so this may be a species difference. In contrast, B. anthracis PGA elicited very low TLR4 signal at 500 μg/ml, and B. licheniformis PGA elicited TLR4 signal at 250 and 500 μg/ml. As with TLR2, B. licheniformis PGA was a more effective TLR4 ligand than B. anthracis PGA. The mean ligand/vehicle ratios for the PGAs for TLR2 and TLR4 are summarized in tabular form in Fig. 7B. Comparing mean ligand/vehicle ratios, it is clear that B. licheniformis PGA is much more effective as a TLR2 agonist than as a TLR4 agonist at 250 μg/ml (p < 0.05) and at 500 μg/ml (p < 0.001). There was no difference between the mean ligand/vehicle ratios for TLR2 and TLR4 for B. anthracis PGA at 500 μg/ml, but because 250 μg/ml B. anthracis PGA only elicits TLR2 signal, B. anthracis PGA is also more effective as a TLR2 ligand. However, all the PGAs were weak ligands. The highest mean ligand/vehicle ratio was 2.8 for 500 μg/ml B. subtilis PGA for TLR2. In contrast, 0.1 ng/ml Pam3CSK4, 0.01 ng/ml FSL-1, and 1 ng/ml LPS had mean ligand vehicle ratios of 4, 9, and 3.4, respectively. Based upon these responses, we can estimate that the TLR2 ligands Pam3CSK4 and FSL-1 are ~2 × 107 and ~4.5 × 107 times as potent as B. anthracis PGA, and the TLR4 ligand LPS is ~1.7 × 109 times as potent.
FIGURE 7.
B. anthracis PGA and B. licheniformis PGA elicit signal in a human TLR4 assay. (A) 50, 250, and 500 μg/ml B. anthracis PGA, B. licheniformis PGA, or B. subtilis PGA were tested in a TLR4 assay. Mean data for four experiments are presented as ligand/vehicle ratios ± SEM (n = 4). Statistical significance of signal for each dose of PGA above its vehicle was determined by t test comparing the mean treatment/background ratios. Statistical significance of the difference between the responses to 500 μg/ml B. anthracis PGA and μg/ml B. licheniformis PGA was determined by t test. No significant responses to B. subtilis PGA were observed (data not shown). *p < 0.05, ***p < 0.001, ****p < 0.0001. (B) Mean ligand/vehicle ratios for TLR2 (n = 4 experiments) and TLR4 (n = 4 experiments) and their p values are shown. Ba, B. anthracis; Bl, B. licheniformis; Bs, B. subtilis; NR, no response.
As with TLR2, we tested d–glutamic acid, l–glutamic acid, poly-d-α-glutamic acid, and poly-l-α-glutamic acid. Both the monomeric glutamic acids and both the α-linked polymers failed to elicit TLR4 signal (n = 3 experiments; data not shown). These data suggest that, like human TLR2, human TLR4 specifically recognizes glutamic acid polymers with γ linkages.
To see if other TLRs were involved, we submitted B. anthracis PGA to InvivoGen for a human TLR/NOD ligand screen. B. anthracis PGA was not a ligand for TLR3, TLR5, TLR7, TLR8, or TLR9 or for NOD1 or NOD2 (data not shown).
B. anthracis PGA persists longer in high m.w. form in human monocyte and iDC culture medium than does B. subtilis PGA or B. licheniformis PGA
Work by Scorpio et al. (6) has shown that B. anthracis PGA is degraded by the B. anthracis enzyme CapD but is resistant to the B. subtilis bacteriophage enzyme poly-γ-glutamate hydrolase P (PghP), whereas B. subtilis PGA is readily degraded by both enzymes. These data indicate that inclusion of l-isomer glutamic acid increases a PGA’s susceptibility to degradation by PghP. It is possible then that inclusion of l-isomer glutamic acid in PGA may likewise increase its susceptibility to degradation by human enzymes. We therefore compared the stability of B. anthracis PGA, B. subtilis PGA, and B. licheniformis PGA in the medium of human innate immune cell cultures. First, we examined the possibility that the culture conditions (37°C/5% CO2) or some component of the culture medium could cause B. anthracis PGA, B. subtilis PGA, or B. licheniformis PGA to degrade. Each PGA was mixed in culture medium at 125 μg/ml and incubated at 37°C/5% CO2 for 7 d. Gel electrophoresis revealed that all three PGAs are stable under culture conditions (n = 4 experiments; Fig. 8A). Next, we sought to determine whether human monocytes or iDCs were capable of degrading the PGAs. Human monocytes or iDCs were cultured in the presence or absence of 125 μg/ml of B. anthracis PGA, B. subtilis PGA, or B. licheniformis PGA. Media samples were collected after 3 d from monocyte cultures and after 2 d from iDC cultures. Gel electrophoresis results from a single representative experiment (n = 4) are shown in Fig. 8B. B. subtilis PGA is largely degraded in the culture medium of monocytes and iDCs (lanes 8 and 9), and B. licheniformis PGA is also substantially degraded (lanes 11 and 12). In contrast, B. anthracis PGA is only minimally degraded (lanes 5 and 6). These data indicate that PGAs from nonpathogenic Bacillus species containing l-isomer glutamic acid are less resistant to degradation by human enzymes. Furthermore, B. subtilis PGA, which had the highest percentage of l-isomer glutamic acid (48%), was the least resistant, demonstrating a correlation between l-isomer percentage and susceptibility to degradation.
FIGURE 8.
B. anthracis PGA persists longer in high m.w. form in monocyte and iDC culture media than B. subtilis PGA or B. licheniformis PGA. (A) 125 μg/ml B. anthracis PGA, B. licheniformis PGA, or B. subtilis PGA was incubated in culture medium without cells for 7 d at 37°C/5%CO2. Gel order is as follows: 1) culture medium, 2) day 0 B. anthracis PGA, 3) day 7 B. anthracis PGA, 4) day 0 B. subtilis PGA, 5) day 7 B. subtilis PGA, 6) day 0 B. licheniformis PGA, and 7) day 7 B. licheniformis PGA. (B) 125 μg/ml B. anthracis PGA, B. licheniformis PGA, or B. subtilis PGA was added to cultures of human monocytes or iDCs. Samples of the culture media were collected after three (monocytes) or two (iDCs) days of incubation. Gel order is as follows: 1) culture medium, 2) spent monocyte culture medium, 3) spent iDC culture medium, 4) B. anthracis PGA alone, 5) B. anthracis PGA with monocytes, 6) B. anthracis PGA with iDCs, 7) B. subtilis PGA alone, 8) B. subtilis PGA with monocytes, 9) B. subtilis PGA with iDCs, 10) B. licheniformis PGA alone, 11) B. licheniformis PGA with monocytes, and 12) B. licheniformis PGA with iDCs. Media samples were electrophoresed on gradient gels and stained with Stains-All. Four experiments were conducted with cells from four donors. Data from a single representative experiment are shown. Ba, B. anthracis; Bl, B. licheniformis; Bs, B. subtilis.
Reducing the m.w. of B. anthracis PGA by sonication reduces the cytokine response of human monocytes
Because the B. subtilis and B. licheniformis PGAs elicited larger cytokine responses than B. anthracis PGA from both monocytes and iDCs, we thought it possible that the lower m.w. PGA fragments released during degradation were more stimulatory than high m.w. PGA. To test this hypothesis, we used sonication to reduce the m.w. of B. anthracis PGA to sizes comparable to B. subtilis– and B. licheniformis–degradation products (Fig. 9A), exposed monocytes to 500 μg/ml untreated or sonicated B. anthracis PGA in parallel, and assayed for cytokines as before. We chose monocytes for this experiment, because they were more responsive to B. anthracis PGA than iDCs. Five experiments were run with cells from five donors. An increase of ≥50% over the unstimulated control was considered a response. Fig. 9B shows the ranges of the individual cytokine responses in picograms per milliliter. Mean IL-8 and IL-6 released in response to sonicated B. anthracis PGA were reduced compared with untreated B. anthracis PGA (Fig. 9C, p < 0.05, and Fig. 9D, p < 0.01). None of the other cytokines tested were elicited in significant amounts by untreated or sonicated B. anthracis PGA. The reduced responses to the lower m.w. sonicated B. anthracis PGA is the opposite of the enhancement and diversification of the monocyte’s cytokine responses to B. subtilis PGA and B. licheniformis PGA. Therefore, the decreased cytokine responses seen with B. anthracis PGA compared with the nonpathogenic Bacillus PGAs are not due to B. anthracis PGA’s persistence in high m.w. form.
FIGURE 9.
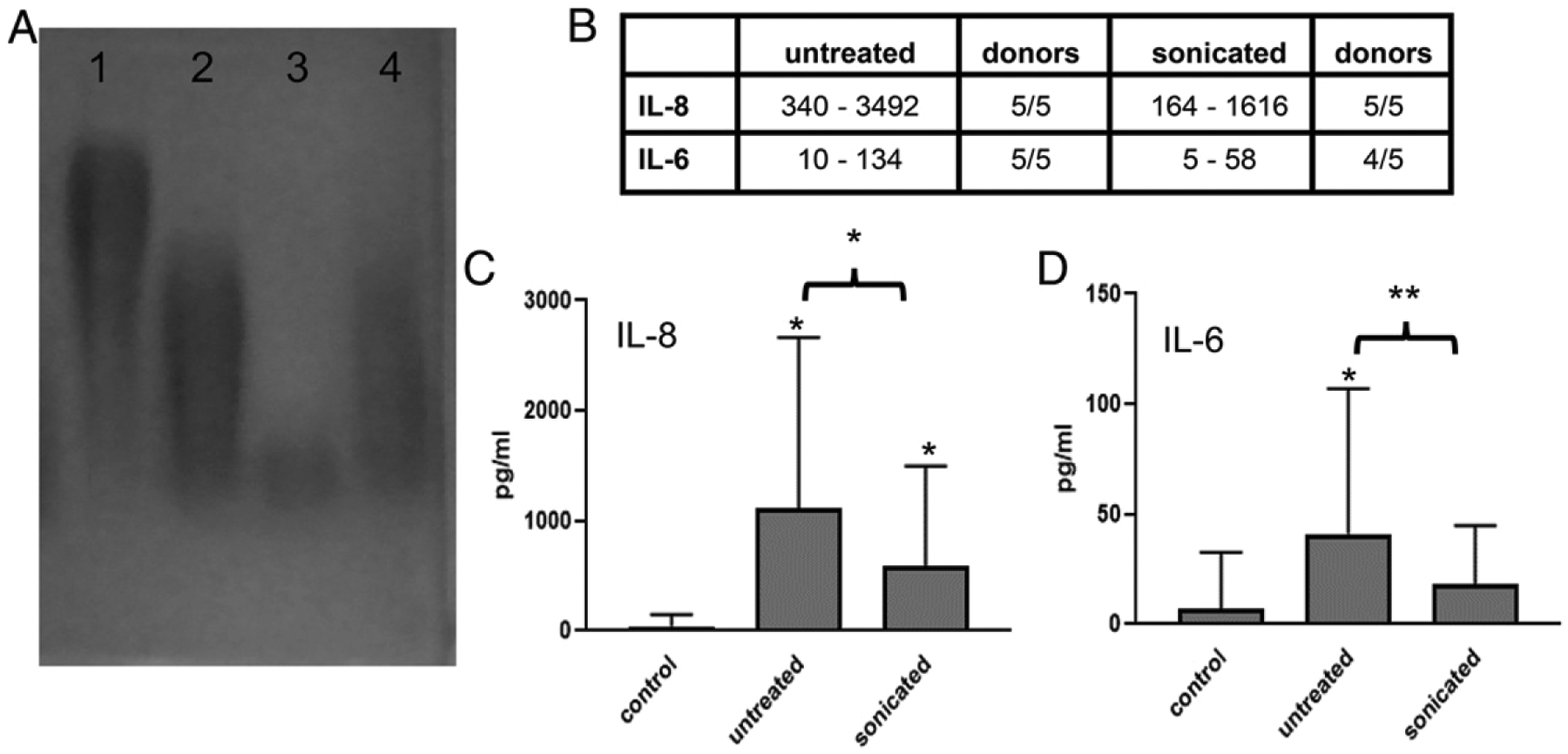
Reducing the m.w. of B. anthracis PGA results in reduced cytokine responses from human monocytes. (A) B. anthracis PGA was sonicated to reduce its m.w., electrophoresed on a 1% agarose gel, and visualized by 0.1% methylene blue staining. Gel order is as follows: 1) untreated B. anthracis PGA, 2) sonicated B. anthracis PGA, 3) B. subtilis PGA exposed to monocytes, and 4) B. licheniformis PGA exposed to monocytes. A representative gel is shown (n = 3). (B) Human monocytes were exposed to 500 μg/ml untreated or sonicated B. anthracis PGA. Media samples were analyzed for cytokines. An increase of ≥50% over the unstimulated control was considered a response. Five experiments were run using cells from five donors (n = 5). The range of individual responses for each cytokine are presented in picograms per milliliter plus the number of donors that had a response. (C and D) Data are presented as geometric means in picograms per milliliter ± geometric SD (n = 5) for IL-8 (C) and IL-6 (D), which were the only significant cytokines. Statistical significance of responses to untreated and sonicated B. anthracis PGA compared with the unstimulated control and the differences in responses to untreated versus sonicated B. anthracis PGA were determined by paired t tests of the log transformed data. *p < 0.05, **p < 0.01.
Discussion
PGAs are produced primarily by Bacillus species, but also by other bacterial species and by archaea and eukaryotes as well (18, 39). It is thought that most organisms that produce PGA secrete it into the environment, where it sequesters heavy metals or decreases local salt concentrations, making adverse environments more habitable (18, 39). Anchoring PGA to the cell surface, as B. anthracis does to form an extracellular capsule covalently attached to the cell wall (15–17), is less common. The opportunistic human pathogen Staphylococcus epidermidis produces a 50% d-, 50% l-isomer PGA, which it attaches to its cell surface, enabling it to survive the high salt concentrations present in human skin and to resist phagocytosis by neutrophils (40). It is possible that B. anthracis PGA may similarly protect B. anthracis from high salt concentrations in the skin in cases of cutaneous anthrax.
B. anthracis differs from nonpathogenic PGA–producing Bacillus species not only in using its PGA to form an extracellular capsule, but also in producing a 100% d-isomer polymer even with the loss of either of its racemases (22). We compared B. anthracis PGA to mixed d-, l-isomer PGAs from two nonpathogens, B. subtilis and B. licheniformis. Although the mixed isomer PGAs elicited larger and more varied cytokine responses, there was no direct relationship between d-isomer content and reduced cytokine responses. Indeed, B. licheniformis PGA, which elicited the largest and broadest cytokine responses, has a greater d-isomer content than B. subtilis PGA (87 versus 52%). Clearly, there are other factors involved. One possibility is that isomeric content has an effect on secondary structure. Evidence for secondary structure of B. anthracis PGA was provided by Joyce et al. (27), who found that reduction of the m.w. of purified B. anthracis PGA resulted in decreased binding by capsule-specific mAbs, and other work with Abs has also indicated that B. anthracis PGA has a defined structure (39, 41). Thus, the reduction in cytokine responses to sonicated B. anthracis PGA we observed may be due to loss of secondary structure. A possible effect of isomeric content on secondary structure could impact receptor recognition. Consistent with this hypothesis, B. licheniformis PGA, which contains 13% l-isomer, signals through TLR2 and TLR4, as does B. anthracis PGA, but more effectively. B. subtilis PGA, which has the largest amount of l-isomer glutamic acid (48%) and, therefore, might be expected to have the most different secondary structure from B. anthracis PGA, does not signal through TLR4 and is the only one to signal through TLR6. Our results are highly suggestive that isomeric composition affects TLR recognition. Jeon et al. (34) reported that B. licheniformis PGA, used as a B. anthracis PGA surrogate, signals through TLR2/6. We saw a small inhibition of TLR2 signal in response to B. licheniformis PGA with TLR6 neutralizing Ab, but it was NS. Although Jeon et al. (34) took great care in assaying their B. licheniformis PGA for contaminants, they did not assess the isomeric composition.
B. subtilis PGA elicited noticeably fewer and smaller cytokine responses from iDCs than from monocytes, whereas B. licheniformis PGA elicited robust and varied cytokine responses from both. Because the monocytes and iDCs were from the same five donors, these differences are not due to genetic variation, but likely to differential receptor expression by monocytes versus iDCs. It has been shown that human iDCs express lower levels of TLR6 than monocytes do (42). Thus, the reduced cytokine responses to B. subtilis PGA from iDCs compared with monocytes is consistent with B. subtilis PGA being primarily a TLR2/6 ligand.
Although TLR1 and TLR6 are the most common partners for TLR2, it can interact with multiple other partners, including scavenger receptor expressed by endothelial cell-1 (SREC-I) (43), TLR10 (44), and TLR4 (45, 46). We found that B. anthracis PGA was a very poor TLR2 ligand and an even less effective TLR4 ligand, which is consistent with the weak cytokine responses we observed. B. licheniformis PGA was a more effective TLR2 ligand than B. anthracis PGA but was not significantly different from B. subtilis PGA. However, B. licheniformis PGA was also a ligand for human TLR4, whereas B. subtilis PGA was not. B. licheniformis PGA’s capability to signal more effectively through both TLR2 and TLR4 may explain why it elicits so much more cytokines and induces maturation of iDCs.
The PGAs’ γ linkages likely contribute to their recognition by TLRs, because neither the poly-d- nor the poly-l-α–linked PGA elicited TLR2 or TLR4 signal. Whereas the synthetic poly-d- and poly-l-α–linked PGAs are large molecules (m.w., 15,000–100,000), they are considerably smaller than the Bacillus PGAs (m.w. ≥ 1,000,000). We did notice a reduction in the monocytes’ cytokine response to B. anthracis PGA after its m.w. had been reduced by sonication. However, although the monocytes’ cytokine responses were greatly reduced, they were not eliminated. Furthermore, Jeon et al. (34) detected TLR2-dependent responses using B. licheniformis PGA that was fragmented to an m.w. of <50,000. This suggests that the α linkages, rather than the reduced m.w., are more likely to be responsible for the absence of TLR signal.
In our previous study, we also exposed human monocytes and iDCs to B. anthracis PGA and assessed cytokine responses (10). The results obtained were very similar to those obtained in the current study except that we did not detect TNF-α in response to B. anthracis PGA by the iDCs in the current study. In our previous study, B. anthracis PGA was compared with a control preparation from a capsule-deficient strain (capA−) to account for the effects of unknown copurifying materials (10). Since that study, we have refined our B. anthracis PGA–purification process and added a hydroxyapatite polishing step to remove those materials. Because TNF-α was observed in response to the capA− preparation (10), the difference in the TNF-α result may be due to the introduction of this additional purification step.
Because B. anthracis PGA was reported to be resistant to degradation by the bacteriophage enzyme PghP compared with B. subtilis PGA (6), we hypothesized that B. anthracis PGA would likewise be more resistant than B. subtilis PGA and B. licheniformis PGA to degradation by human enzymes. This, indeed, was the case. Furthermore B. subtilis PGA, which has a higher percentage of l-isomer (48%) than does B. licheniformis PGA (13%), was more efficiently degraded, suggesting a correlation between increasing l-isomer and susceptibility to degradation. Consistent with these results, increased resistance of B. anthracis PGA to degradation compared with l-isomer–containing PGAs from two other Bacillus species was previously noted by Torii (47) using an extract from dog liver.
In the limited reports of infected animals, B. anthracis PGA of both high and decreasing m.w. are present (8, 48), and B. anthracis PGA has been shown to be associated with anthrax lethal toxin (LTx) in plasma (48). There are conflicting data regarding the significance of this association. It has been reported that low m.w. B. licheniformis PGA (<30,000), used as a surrogate for B. anthracis PGA, enhances LTx killing of J774A.1 cells and increases death in mice when coinjected with LTx (49). However, a more recent study using high m.w. B. licheniformis PGA as a surrogate for B. anthracis PGA indicates that it inhibits, rather than enhances, LTx killing of J774A.1 cells, likely by disrupting proteolytic activation of protective Ag, the cell-binding component of the anthrax toxins, and blocking protective Ag channel formation (50). In this study, inhibition of LTx was not seen when the m.w. was reduced to 15,000–50,000 by CapD treatment or acid hydrolysis. Thus, the effect of B. anthracis PGA on LTx activity remains unclear.
In the current study, we have shown that the responses of human innate immune cells to PGAs from B. anthracis and nonpathogenic B. licheniformis and B. subtilis vary greatly, with B. anthracis PGA consistently eliciting notably weaker responses. These poor responses to B. anthracis PGA are consistent with observations made years ago by Zwartouw and Smith (7) that extracellular B. anthracis PGA purified from plasma exudate of guinea pigs moribund with anthrax did not produce tissue damage when injected intradermally into guinea pigs. B. anthracis PGA’s decreased effectiveness as a ligand for TLR2 and TLR4 likely underlies these reduced responses. The decreased recognition by and reduced responses of human innate immune cells to B. anthracis PGA may further explain how B. anthracis PGA contributes to anthrax pathogenesis.
Acknowledgments
We thank Anna Trivett for purifying the monocytes and Sherry Mou for running cytokine assays.
This work was supported by Defense Threat Reduction Agency Grant CBM.VAXBT. 03.10.RD.015, plan number 921175.
Abbreviations used in this article:
- CapD
capsule depolymerase
- DC
dendritic cell
- HEK
human embryonic kidney
- iDC
immature DC
- LTx
lethal toxin
- mDC
mature DC
- MH+
positive ion electrospray mode
- MHC I
MHC class I
- PGA
poly-γ-glutamic acid
- PghP
poly-γ-glutamate hydrolase P
- SEAP
secreted embryonic alkaline phosphatase
Footnotes
The opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the United States Army.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Mikesell P, Ivins BE, Ristroph JD, and Dreier TM. 1983. Evidence for plasmid-mediated toxin production in Bacillus anthracis. Infect. Immun 39: 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green BD, Battisti L, Koehler TM, Thorne CB, and Ivins BE. 1985. Demonstration of a capsule plasmid in Bacillus anthracis. Infect. Immun 49: 291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uchida I, Sekizaki T, Hashimoto K, and Terakado N. 1985. Association of the encapsulation of Bacillus anthracis with a 60 megadalton plasmid. J. Gen. Microbiol 131: 363–367. [DOI] [PubMed] [Google Scholar]
- 4.Keppie J, Harris-Smith PW, and Smith H. 1963. The chemical basis of the virulence of Bacillus anthracis. IX. Its aggressins and their mode of action. Br. J. Exp. Pathol 44: 446–453. [PMC free article] [PubMed] [Google Scholar]
- 5.Makino S, Uchida I, Terakado N, Sasakawa C, and Yoshikawa M. 1989. Molecular characterization and protein analysis of the cap region, which is essential for encapsulation in Bacillus anthracis. J. Bacteriol 171: 722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scorpio A, Chabot DJ, Day WA, O’brien DK, Vietri NJ, Itoh Y, Mohamadzadeh M, and Friedlander AM. 2007. Poly-gamma-glutamate capsule-degrading enzyme treatment enhances phagocytosis and killing of encapsulated Bacillus anthracis. Antimicrob. Agents Chemother 51: 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zwartouw HT, and Smith H. 1956. Polyglutamic acid from Bacillus anthracis grown in vivo; structure and aggressin activity. Biochem. J 63: 437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kozel TR, Murphy WJ, Brandt S, Blazar BR, Lovchik JA, Thorkildson P, Percival A, and Lyons CR. 2004. mAbs to Bacillus anthracis capsular antigen for immunoprotection in anthrax and detection of antigenemia. Proc. Natl. Acad. Sci. USA 101: 5042–5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyer AE, Quinn CP, Hoffmaster AR, Kozel TR, Saile E, Marston CK, Percival A, Plikaytis BD, Woolfitt AR, Gallegos M, et al. 2009. Kinetics of lethal factor and poly-D-glutamic acid antigenemia during inhalation anthrax in rhesus macaques. Infect. Immun 77: 3432–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jelacic TM, Chabot DJ, Bozue JA, Tobery SA, West MW, Moody K, Yang D, Oppenheim JJ, and Friedlander AM. 2014. Exposure to Bacillus anthracis capsule results in suppression of human monocyte-derived dendritic cells. Infect. Immun 82: 3405–3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makino S, Watarai M, Cheun HI, Shirahata T, and Uchida I. 2002. Effect of the lower molecular capsule released from the cell surface of Bacillus anthracis on the pathogenesis of anthrax. J. Infect. Dis 186: 227–233. [DOI] [PubMed] [Google Scholar]
- 12.Hanby WE, and Rydon HN. 1946. The capsular substance of Bacillus anthracis. Biochem. J 40: 297–309. [PubMed] [Google Scholar]
- 13.Goodman JW, and Nitecki DE. 1967. Studies on the relation of a prior immune response to immunogenicity. Immunology 13: 577–583. [PMC free article] [PubMed] [Google Scholar]
- 14.Wang TT, and Lucas AH. 2004. The capsule of Bacillus anthracis behaves as a thymus-independent type 2 antigen. Infect. Immun 72: 5460–5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Candela T, and Fouet A. 2005. Bacillus anthracis CapD, belonging to the γ-glutamyltranspeptidase family, is required for the covalent anchoring of capsule to peptidoglycan. Mol. Microbiol 57: 717–726. [DOI] [PubMed] [Google Scholar]
- 16.Richter S, Anderson VJ, Garufi G, Lu L, Budzik JM, Joachimiak A, He C, Schneewind O, and Missiakas D. 2009. Capsule anchoring in Bacillus anthracis occurs by a transpeptidation reaction that is inhibited by capsidin. Mol. Microbiol 71: 404–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Candela T, Balomenou S, Aucher W, Bouriotis V, Simore JP, Fouet A, and Boneca IG. 2014. N-acetylglucosamine deacetylases modulate the anchoring of the gamma-glutamyl capsule to the cell wall of Bacillus anthracis. Microb. Drug Resist 20: 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogunleye A, Bhat A, Irorere VU, Hill D, Williams C, and Radecka I. 2015. Poly-γ-glutamic acid: production, properties and applications. Microbiology 161: 1–17. [DOI] [PubMed] [Google Scholar]
- 19.Leonard CG, Housewright RD, and Thorne CB. 1958. Effects of some metallic ions on glutamyl polypeptide synthesis by Bacillus subtilis. J. Bacteriol 76: 499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thorne CB, and Leonard CG. 1958. Isolation of d- and l-glutamyl polypeptides from culture filtrates of Bacillus subtilis. J. Biol. Chem 233: 1109–1112. [PubMed] [Google Scholar]
- 21.Cromwick AM, and Gross RA. 1995. Effects of manganese (II) on Bacillus licheniformis ATCC 9945A physiology and γ-poly(glutamic acid) formation. Int. J. Biol. Macromol 17: 259–267. [DOI] [PubMed] [Google Scholar]
- 22.Oh SY, Richter SG, Missiakas DM, and Schneewind O. 2015. Glutamate racemase mutants of Bacillus anthracis. J. Bacteriol 197: 1854–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashiuchi M, Kamei T, Baek D-H, Shin S-Y, Sung M-H, Soda K, Yagi T, and Misono H. 2001. Isolation of Bacillus subtilis (chungkookjang), a poly-γ-glutamate producer with high genetic competence. Appl. Microbiol. Biotechnol 57: 764–769. [DOI] [PubMed] [Google Scholar]
- 24.Sung MH, Park C, Kim CJ, Poo H, Soda K, and Ashiuchi M. 2005. Natural and edible biopolymer poly-γ-glutamic acid: synthesis, production, and applications. Chem. Rec 5: 352–366. [DOI] [PubMed] [Google Scholar]
- 25.Chettri R, Bhutia MO, and Tamang JP. 2016. Poly-γ-glutamic acid (PGA)-producing Bacillus species isolated from Kinema, Indian fermented soybean food. Front. Microbiol 7: 971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fekete T 2010. Bacillus species and related genera other than Bacillus anthracis. In Principles and Practice of Infectious Diseases, 7th Ed. Mandell GL, Bennett JE, and Dolin R, eds. Churchill Livingstone Elsevier, Philadelphia, PA, p. 2727–2731. [Google Scholar]
- 27.Joyce J, Cook J, Chabot D, Hepler R, Shoop W, Xu Q, Stambaugh T, Aste-Amezaga M, Wang S, Indrawati L, et al. 2006. Immunogenicity and protective efficacy of Bacillus anthracis poly-gamma-D-glutamic acid capsule covalently coupled to a protein carrier using a novel triazine-based conjugation strategy. J. Biol. Chem 281: 4831–4843. [DOI] [PubMed] [Google Scholar]
- 28.Scorpio A, Chabot DJ, Day WA, Hoover TA, and Friedlander AM. 2010. Capsule depolymerase overexpression reduces Bacillus anthracis virulence. Microbiology 156: 1459–1467. [DOI] [PubMed] [Google Scholar]
- 29.Castiello L, Sabatino M, Jin P, Clayberger C, Marincola FM, Krensky AM, and Stroncek DF. 2011. Monocyte-derived DC maturation strategies and related pathways: a transcriptional view. Cancer Immunol. Immunother 60: 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banchereau J, and Steinman RM. 1998. Dendritic cells and the control of immunity. Nature 392: 245–252. [DOI] [PubMed] [Google Scholar]
- 31.Yanagihara S, Komura E, Nagafune J, Watarai H, and Yamaguchi Y. 1998. EBI1/CCR7 is a new member of dendritic cell chemokine receptor that is up-regulated upon maturation. J. Immunol 161: 3096–3102. [PubMed] [Google Scholar]
- 32.Müller G, and Lipp M. 2003. Shaping up adaptive immunity: the impact of CCR7 and CXCR5 on lymphocyte trafficking. Microcirculation 10: 325–334. [DOI] [PubMed] [Google Scholar]
- 33.Weiss S, Levy H, Fisher M, Kobiler D, and Altboum Z. 2009. Involvement of TLR2 in innate response to Bacillus anthracis infection. Innate Immun. 15: 43–51. [DOI] [PubMed] [Google Scholar]
- 34.Jeon JH, Lee HR, Cho MH, Park OK, Park J, and Rhie GE. 2015. The poly-γ-D-glutamic acid capsule surrogate of the Bacillus anthracis capsule is a novel toll-like receptor 2 agonist. Infect. Immun 83: 3847–3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeon JH, Park DB, Woo SJ, Lee HR, Park OK, Park J, and Rhie GE. 2018. Muramyl dipeptide potentiates a Bacillus anthracis poly-γ-d-glutamic acid capsule surrogate that induces maturation and activation of mouse dendritic cells. Cytokine 110: 350–356. [DOI] [PubMed] [Google Scholar]
- 36.van Bergenhenegouwen J, Plantinga TS, Joosten LAB, Netea MG, Folkerts G, Kraneveld AD, Garssen J, and Vos AP. 2013. TLR2 & Co: a critical analysis of the complex interactions between TLR2 and coreceptors. J. Leukoc. Biol 94: 885–902. [DOI] [PubMed] [Google Scholar]
- 37.Lee TY, Kim YH, Yoon SW, Choi JC, Yang JM, Kim CJ, Schiller JT, Sung MH, and Poo H. 2009. Oral administration of poly-gamma-glutamate induces TLR4- and dendritic cell-dependent antitumor effect. Cancer Immunol. Immunother 58: 1781–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaure C, and Liu Y. 2014. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front. Immunol 5: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Candela T, and Fouet A. 2006. Poly-gamma-glutamate in bacteria. Mol. Microbiol 60: 1091–1098. [DOI] [PubMed] [Google Scholar]
- 40.Kocianova S, Vuong C, Yao Y, Voyich JM, Fischer ER, DeLeo FR, and Otto M. 2005. Key role of poly-γ-DL-glutamic acid in immune evasion and virulence of Staphylococcus epidermidis. J. Clin. Invest 115: 688–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kozel TR, Thorkildson P, Brandt S, Welch WH, Lovchik JA, AuCoin DP, Vilai J, and Lyons CR. 2007. Protective and immunochemical activities of monoclonal antibodies reactive with the Bacillus anthracis polypeptide capsule. Infect. Immun 75: 152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakao Y, Funami K, Kikkawa S, Taniguchi M, Nishiguchi M, Fukumori Y, Seya T, and Matsumoto M. 2005. Surface-expressed TLR6 participates in the recognition of diacylated lipopeptide and peptidoglycan in human cells. J. Immunol 174: 1566–1573. [DOI] [PubMed] [Google Scholar]
- 43.Murshid A, Borges TJ, Lang BJ, and Calderwood SK. 2016. The scavenger receptor SREC-I cooperates with toll-like receptors to trigger inflammatory innate immune responses. Front. Immunol 7: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oosting M, Cheng SC, Bolscher JM, Vestering-Stenger R, Plantinga TS, Verschueren IC, Arts P, Garritsen A, van Eenennaam H, Sturm P, et al. 2014. Human TLR10 is an anti-inflammatory pattern-recognition receptor. Proc. Natl. Acad. Sci. USA 111: E4478–E4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernandez-Lizarbe S, Montesinos J, and Guerri C. 2013. Ethanol induces TLR4/TLR2 association, triggering an inflammatory response in microglial cells. J. Neurochem 126: 261–273. [DOI] [PubMed] [Google Scholar]
- 46.Li JY, Liu Y, Gao XX, Gao X, and Cai H. 2014. TLR2 and TLR4 signaling pathways are required for recombinant Brucella abortus BCSP31-induced cytokine production, functional upregulation of mouse macrophages, and the Th1 immune response in vivo and in vitro. Cell. Mol. Immunol 11: 477–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torii M 1955. Decapsulation of Bacillus anthracis. Med. J. Osaka Univ 6: 725–737. [Google Scholar]
- 48.Ezzell JW, Abshire TG, Panchal R, Chabot D, Bavari S, Leffel EK, Purcell B, Friedlander AM, and Ribot WJ. 2009. Association of Bacillus anthracis capsule with lethal toxin during experimental infection. Infect. Immun 77: 749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jang J, Cho M, Chun JH, Cho MH, Park J, Oh HB, Yoo CK, and Rhie GE. 2011. The poly-γ-D-glutamic acid capsule of Bacillus anthracis enhances lethal toxin activity. Infect. Immun 79: 3846–3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kintzer AF, Tang II, Schawel AK, Brown MJ, and Krantz BA. 2012. Anthrax toxin protective antigen integrates poly-γ-D-glutamate and pH signals to sense the optimal environment for channel formation. Proc. Natl. Acad. Sci. USA 109: 18378–18383. [DOI] [PMC free article] [PubMed] [Google Scholar]


