Abstract
Background:
TGF-β1 is a key cytokine involved in both airway inflammation and airway remodeling in asthma because of its anti-inflammatory and profibrotic effect. In our previous study, we found that knockdown of cytosolic β-catenin alleviated the profibrogenic effect of TGF-β1 without influencing its anti-inflammatory effect. However, the exact role of targeting β-catenin in asthma is not yet fully demonstrated. In the present study, we investigated the effect and mechanism of targeting β-catenin in OVA-challenged asthmatic rats with airway inflammation and remodeling features.
Methods:
We integrated experimental asthma model and asthma related cell model to explore the effect of targeting β-catenin on airway inflammation and remodeling of asthma.
Results:
Blocking β-catenin with ICG001, a small molecule inhibitor of β-catenin/TCF via binding to cAMP-response elementbinding protein, attenuated airway inflammation by increasing levels of anti-inflammation cytokines IL-10, IL-35 and decreasing levels of T helper (Th)2 cells and Th17 cytokine. Suppressing β-catenin by ICG001 inhibited airway remodeling via reducing the level of TGF-β1 and the expressions of Snail, MMP-7, MMP-9 and, up-regulating expression of E-cadherin, down-regulating expressions of α-SMA and Fn. Inhibition of β-catenin with ICG001 suppressed TGF-β1 induced proliferation and activation of CCC-REPF-1, blocked TGF-β1 induced epithelial–mesenchymal transition (EMT) of RLE-6TN.
Conclusion:
Blockade of β-catenin/TCF not only prevents TGF-β1 induced EMT and profibrogenic effects involved in pathological remodeling of airway, but also alleviates airway inflammation in asthma by balancing pro-inflammatory and anti-inflammatory cytokine. In conclusion, targeting β-catenin specifically via inhibition of β-catenin/TCF might be a new therapeutic strategy for asthma.
The reviews of this paper are available via the supplemental material section.
Keywords: β-catenin, airway inflammation, airway remodeling, asthma, transforming growth factor-β1
Introduction
Asthma is a very common chronic disease that affects at least 235–334 million individuals worldwide.1 In recent decades, the incidence and mortality of asthma have had a gradual upward trend. It has become a major public health concern.2 The main characteristics of asthma are chronic airway inflammation, remodeling, and hyperresponsiveness.3 The irreversible obstruction and deterioration of lung function caused by airway remodeling results in frequent asthma attack episodes.4 Also, efficacy of pharmacotherapies, including inhaled corticosteroids (ICS) and leukotriene receptor antagonist (LTRA), in preventing airway remodeling in patients with asthma is limited.5 Therefore, an urgent need exists to understand the mechanisms of airway inflammation and airway remodeling in asthma and to find an agent that can alleviate airway inflammation and reverse airway remodeling.
Transforming growth factor-β1 (TGF-β1) is a pleiotropic cytokine and contributes to numerous critical processes such as cellular maturation and differentiation, embryonal development, inflammation, immune regulation, wound healing, and tissue remodeling and repair.6,7 It participates in airway inflammatory responses and fibrotic tissue remodeling in the pathological process of asthma.8 On one hand, TGF-β1 is involved in inflammation by inducing the proliferation of inflammatory cells such as Th17 cells, and is involved in anti-inflammatory response by inducing the proliferation of inducing regulatory T cells (Tregs) and inhibiting the differentiation of T helper (Th) cells.9 On the other hand, TGF-β1 also has a crucial role in airway remodeling by inducing fibroblast proliferation and differentiation into myofibroblasts, thereby contributing to epithelial–mesenchymal transition (EMT).9
TGF-β1 exerts its actions through multiple distinct signal pathways including Smad, non-Smad, and β-catenin pathways.10–12 The Smad-dependent pathway is the canonical signaling pathway for TGF-β1, through the activation of the TGF-β1 type I and II receptors. TGF-β1 can also activate the Smad-independent pathway in a non-canonical fashion through the activation of all three known mitogen-activated protein kinase pathways.9 Additionally, there is a positive link between TGF-β1 and the Wnt/β-catenin pathway. Activation of TGF-β1 enhances Wnt/β-catenin pathway stimulation, which in turn increases the expression of TGF-β1.10 The Wnt/β-catenin pathway is involved in many distinct human pathologies, including cancers and metabolic, inflammatory, and fibrotic diseases.13 β-catenin is a dual-function protein involved both in intercellular adhesion and in regulation of transcription. Recently, β-catenin has been regarded as a regulator and therapeutic target for asthmatic airway remodeling.14 Significant advances have recently been made in the generation of small-molecule inhibitors that target β-catenin directly or indirectly.14 ICG001 is a unique small molecule which selectively inhibits TCF/β-catenin transcription through interacting with cAMP-response elementbinding protein (CBP) and blocking the β-catenin/CBP interaction.15 In our previous study of renal fibrosis,16 we discovered that inhibiting β-catenin with ICG001 can separate the fibrogenic and anti-inflammatory actions of TGF-β1, and thereby mitigate the profibrogenic effect of TGF-β1 without affecting its anti-inflammatory effect. Moreover, our recent study17 has indicated that inhibiting β-catenin/TCF transcription with ICG001 could help reduce the profibrotic effects of TGF-β1 mediated by β-catenin/TCF, and thereby enhance the anti-inflammatory effects mediated by β-catenin/Foxo. Therefore, targeting β-catenin with ICG001 may have the therapeutic effect of alleviating airway inflammation and remodeling in asthma.
In the current study, ICG001 was used to examine the mechanism of targeting β-catenin on airway inflammation and airway remodeling in a rat model of asthma. Alveolar epithelial cells and lung fibroblasts were used in in vitro models to explore the protective effects of targeting β-catenin against TGF-β1-induced profibrogenic and EMT.
Materials and methods
Treatment of the animals
Fifty-six male Wistar rats, aged 2–3 months and weighing 160–180 g, were provided by Shanxi Medical University Experimental Animal Center (Shanxi, China). The rats were maintained in ventilated cages with free access to standard chow and water. All experiments were granted approval by the Animal Ethics Committee of Shanxi Medical University. The experiments were performed in strict accordance with national and international guidelines of laboratory animal care. The rats were divided at random into seven groups: the control group; 4 weeks ovalbumin (OVA) treatment group (model, ICG001, and budesonide); and 8 weeks OVA group (model, ICG001, and budesonide). Except for the control group, all rats were sensitized by intraperitoneal (i.p.) injection of 10% OVA suspension containing l00 mg OVA (Sigma Corporation, USA) and 100 mg aluminum hydroxide powder (Chemical Reagent Factory, Tianjin, China) on day 1 and day 8. Beginning on day 15, the rats were challenged by inhaling 1% OVA daily (20 min each time) for 2 successive weeks (i.e. 4 weeks group) or 6 successive weeks (i.e. 8 weeks group). One-half hour before each OVA exposure, the model group rats were given distilled water (0.16 mL) by i.p. injection, the budesonide group rats were administered budesonide (0.5 mg; AstraZeneca Company, Australia) by aerosol inhalation twice daily (10 min each time), and the rats in the ICG001 group were subcutaneously administered ICG00115 (5 mg/kg; Selleck Chemicals, USA). Control animals were sensitized and challenged by the same procedures with normal saline instead of OVA. All rats were anesthetized by an i.p. injection of 25% urethane (4 mL/kg, i.p.) and euthanized within 24 h after the last challenge. The experiments were conducted with single-blinded analysis.
Histological study of lung tissue
The lower right lobe of the lung from each rat was removed for histological analysis. Paraffin-embedded tissue sections underwent hematoxylin and eosin staining and Masson’s trichrome staining. The sections were observed under light microscopy (100× magnification) to evaluate changes in airway inflammation and airway remodeling and to evaluate fibrosis. On each slide, a minimum of 10 bronchioles with an inner diameter of 150–200 μm were chosen. The thicknesses of the airway wall (Wat) and airway smooth muscle (Wam) were determined by morphometric analysis of transverse sections. The BI2000 medical image analysis system was used to evaluate airway remodeling.
Bronchoalveolar lavage fluid and cell counts
After euthanizing the animals, the left lung of each rat was lavaged three times with 1 mL of sterile saline, at a recovery rate of 80%. The recovered saline was centrifuged at 670 g for 10 min. The supernatant was refrigerated at −80°C to measure the cytokines. Wright stain was used for counting the total cells and eosinophil (EOS) cells in bronchoalveolar lavage fluid (BALF) with the assistance of a light microscope at 400× magnification and a hemocytometer.
Enzyme-linked immunosorbent assay
In addition to collecting BALF, approximately 5 mL of whole blood was collected by cardiac puncture. The blood samples were then centrifuged (at 2000 rev/min for 10 min at 4°C). Sera were obtained and stored at −80°C for enzyme-linked immunosorbent assay (ELISA) testing. TGF-β1, OVA-IgE, interleukin (IL)-4, IL-5, IL-17, IL-10, and IL-35 secretion in BALF and TGF-β1, IL-17, IL-10, IL-35 in sera were measured with an ELISA-kit (eBioscience, USA), based on the manufacturer’s instructions.
Immunohistochemical analysis
Immunohistochemistry was performed using strept avidin–biotin complex (SABC) anti-mouse reagent (Boster Biological Company, Wuhan, China). The primary antibodies were mouse anti-rat E-cadherin (sc-59778), α-smooth muscle actin (α-SMA) (sc-53142), and fibronectin (Fn) antibody (sc18825) (1:100, SantaCruz Company, USA). The secondary antibody was biotinylated goat anti-mouse immunoglobulin G (IgG) antibody (sc-2039; Abcam, USA). All sections were added to the SABC reagent to amplify the reaction, and then stained with diaminobenzidine at room temperature. A light microscope was employed to observe the slices at 400× magnification. Positive areas were brown. For all slices, the mean optical density was detected by using the BI2000 medical image analysis system.
Cell culture and treatment
Alveolar type II epithelial cells (RLE-6TN) and lung fibroblast cells (CCC-REPF-1) were maintained in Dulbecco’s Modified Eagle’s Medium/Ham’s F12 medium (Invitrogen, USA) supplemented with 100 U/mL penicillin, 100 μg/mL gentamicin, and 10% fetal calf serum (Invitrogen, USA) at 37°C in 5% CO2.
In the TGF-β1 treatment experiments, subconfluent cultures of RLE-6TN and CCC-REPF-1 cells were rinsed with phosphate-buffered saline (PBS) (Invitrogen) and treated with 5 μg/L TGF-β1 (Biosource, USA) in the presence or absence of ICG-001 5 μM.16 After 24 h had elapsed, the cells were collected for experimentation.
Cell counting kit-8 assay
Cultured CCC-REPF-1 cells were seeded into 96-well plates (5 × 103 cells/well) and 10 μL cell counting kit-8 (CCK-8) was added to each well. After 1 h of incubation, the absorbance of each well was measured at 450 nm by using a spectrophotometer. Each group had six duplicates.
Western blot analysis
Cultured RLE-6TN cells were washed and then pelleted in PBS at 670 g for 5 min. Each cell pellet contained 2 × 105 cells. The cell pellet was subjected to protein extraction by using a cell lysis buffer. The lung tissues were homogenized, incubated in the RIPA lysis buffer (Elabscience, China), added to a protease inhibitor cocktail, and then centrifuged to obtain extracts of lung proteins. A BCA protein assay kit (ThermoFisher Scientific, USA) was used to measure protein concentration. The samples were loaded with sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene fluoride membrane (Millipore Company, USA). The membranes were blocked with 5% skimmed milk in PBS at 37°C for 2 h, and then incubated with the appropriate primary antibodies overnight at 4°C to assess the protein levels of E-cadherin, Fn, and α-SMA (1:1500; SantaCruz Biotechnologies, USA). The membranes were washed and exposed to their respective horseradish peroxidase conjugated goat anti-mouse IgG (1:1000; sc2031; SantaCruz Company, USA) for 2.5 h at room temperature. The labeled band was detected using an enhanced chemiluminescence detection kit (Invitrogen, USA). The loading control was β-actin. The experiment was repeated three times.
Real time-polymerase chain reaction analysis
Total RNA was isolated and purified from cells by using TRIzol Reagent (Invitrogen, USA). Complementary deoxyribonucleic acid was synthesized by using GoScript™ Reverse Transcription System (Promega, USA). The primers used were as follows:
(1) Snail:
Forward: 5’-CTTGTGTCTGCACGACCTGT-3’;
Reverse: 5’-CTTCACATCCGAGTGGGTTT-3’;
(2) MMP-7:
Forward: 5’-CGGAGATGTTCACTTTGACA-3’;
Reverse: 5’-AAGAGTGACCCAGACCCAGA-3’;
(3) MMP-9:
Forward: 5’-TTCGACTCCAGTAGACAATCC-3’;
Reverse: 5’-CAGAGAACTCGTTATCCAAGCG-3’;
(4) Slug:
Forward: 5’-ATGCATATTCGGACCCACC-3’;
Reverse: 5’-AGATTTGACCTGTCTGCAGCTC-3’;
(5) α-SMA:
Forward: 5’- AGCCAGTCGCCATCAGGAAC-3’;
Reverse: 5’- GGGAGCATCATCCCAGCAA -3’;
(6) Collagen-I:
Forward: 5’- GACATGTTCAGCTTTGTGTACCTC-3’;
Reverse: 5’-GGGACCCTTAGGCCATTGTGA -3’;
(7) Collagen-III:
Forward: 5’- TTTGGCACAGCAGTCCAATGTA-3’;
Reverse: 5’- GACAGATCCCGAGTTCGCAGA-3’;
(8) β-Actin:
Forward: 5’-GATTACTGCTCTGGC TCCTAGCA-3’;
Reverse: 5’-GCCACCGATCCACACAGAGT−3’;
(9) Glyceraldehyde 3-phosphate dehydrogenase:
Forward: 5’- GGTGCTGAGTATGTCGTGGAGT-3’;
Reverse: 5’-CAGTCTTCTGAGTGGCAGTGAT-3’;
(10) RN-ACTB:
Forward: 5’- TGTCACCAACTGGGACGATA-3’;
Reverse: 5’-GGGGTGTTGAAGGTCTCAAA-3’.
The polymerase chain reaction (PCR) conditions were as follows: initial denaturation at 95°C for 10 min, followed by 40 cycles consisting of denaturation at 95°C for 10 s, primer annealing at 50°C for 20 s, and extension at 72°C for 20 s.
Statistical methods
The data are expressed as the mean ± the standard deviation with n representing the number of independent experiments. Statistical analysis was conducted using SPSS19.0 software. All data were tested for normality with the K-S test and tested for homogeneity of variance with the Levene test. Groups were statistically compared by using one-way analysis of variance (ANOVA), followed by least significant difference, or compared by using Kruskal–Wallis testing, followed by Tamhane’s T2 test. Values of p < 0.05 were statistically significant.
Results
Blockade of β-catenin with ICG001 alleviated symptoms
After the OVA challenge, rats in the model groups manifested different degrees of symptoms such as dysphoria, polypnea, abdominal muscle twitching, fecal and urinary incontinence, emaciation, lackluster hair, dilatory movement, lack of motion, and low breathing rate in some severe cases. However, these symptoms were less prominent in the ICG001 or budesonide treatment group rats and no symptoms were observed in the control group rats.
Glucocorticoids (GCs), especially inhaled GCs, are accepted as the most effective therapy for patients because GCs can exert an inhibitory effect on multiple processes in the pathogenesis of asthma.18 Glucocorticoids activate many anti-inflammatory genes and repress many pro-inflammatory genes that have been activated in asthmatic inflammation. GCs also reduce the numbers of inflammatory cells in the airways, including EOSs, T-lymphocytes, mast cells, and dendritic cells which are involved in the pathophysiology in asthma.18 Budesonide is most commonly used as an inhaled GC. In this study, the budesonide group was set as the positive control to assess the effect of ICG001 on the pathological process of asthma. The results demonstrated that the therapeutic effect of ICG001 is similar to budesonide in alleviating the symptoms exhibited by rats.
Blockade of β-catenin with ICG001 alleviated airway inflammation in OVA-induced asthma
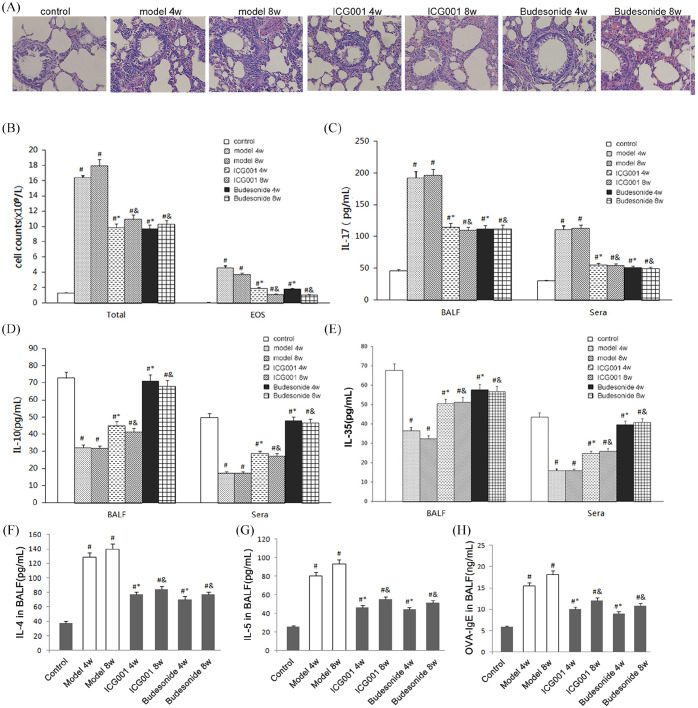
Airway inflammation in the OVA-induced model rats was demonstrated by the infiltration of peribronchial and perivascular inflammatory cells (Figure 1A), and by an increase of the total cells and EOS cells in the BALF (Figure 1B). TGF-β1 can induce Th17 and exert a proinflammatory effect. It also can influence Tregs function, inhibit Th cells differentiation and exert anti-inflammatory effects. The related cytokines and antibody were chosen to evaluate the proinflammatory and anti-inflammatory effects of TGF-β1. Analysis using ELISA revealed an increase in the level of the proinflammatory factor IL-17, which can be secreted by Th17 cells (Figure 1C), and a decrease in the levels of anti-inflammatory factors IL-10 and IL-35 (Figure 1D and E), which can be secreted by Tregs in rats with asthma. Besides, the levels of Th2 cells cytokines IL-4, IL-5, and OVA-specific immunoglobulin (OVA-IgE) were also increased in rats with asthma (Figure 1F, G and H). Inflammatory cell infiltration and the total cell and EOS cell counts were significantly inhibited in rats treated with ICG001 or budesonide, compared with the model groups (Figure 1A and B). In addition, treatment with ICG001 or budesonide attenuated the OVA-induced increase in OVA-IgE, Th2 and Th17 cytokines, and decrease in anti-inflammatory cytokines in the sera and BALF (Figure 1C–E). No significant differences were noted between the ICG001 and budesonide groups, which indicated that ICG001 inhibited airway inflammation in OVA-challenged asthmatic rats by downregulating proinflammatory factors and upregulating anti-inflammatory factors. Moreover, the anti-inflammatory action of ICG001 is similar to that of budesonide.
Figure 1.
ICG001 attenuates airway inflammation in a rat model of asthma. (A) Histopathologic change in the lung of the control group (Control), the 4 weeks or 8 weeks model group (model 4w or model 8w, respectively), the 4 weeks or 8 weeks ICG001 treatment group (ICG 4w or ICG 8w, respectively), and the 4 weeks or 8 weeks budesonide treatment group (budesonide 4w or budesonide 8w, respectively). The lungs were analyzed by using hematoxylin and eosin staining (magnification, ×100). (B) The total and eosinophil cell counts in the bronchoalveolar lavage fluid (BALF) of all seven groups. (C) The levels of interleukin (IL)-17 in BALF and sera of all seven groups. (D) The levels of IL-10 in the BALF and sera of all seven groups. (E) The levels of IL-35 in the BALF and sera of all seven groups. (F) The levels of IL-4 in the BALF of all seven groups. (G) The levels of IL-5 in the BALF of all seven groups. (H) The levels of ovalbumin (OVA)-IgE in the BALF of all seven groups.
The values are expressed as mean ± standard deviation (n = 8).
#p < 0.01 versus control group.
*p < 0.01 versus 4 weeks model group.
&p < 0.01 versus 8 weeks model group.
Blockade of β-catenin with ICG001 alleviated airway remodeling in OVA-induced asthma
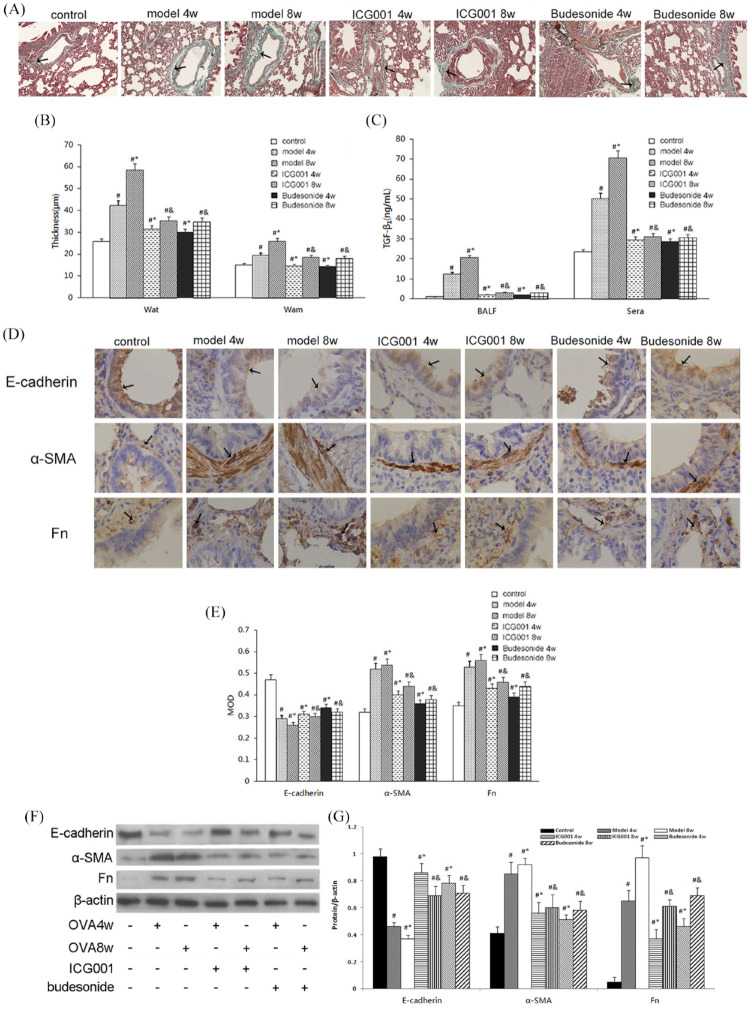
Histopathologic staining by hematoxylin and eosin, to test Wat and Wam, and Masson’s trichrome staining were used to evaluate the degree of airway remodeling, as observed in classic rat models of asthma. The lung tissue from the model rats exhibited airway remodeling such as epithelium damage, bronchial wall thickening (Figure 1A), and a significant increase in collagen deposition in the subepithelial fibrotic tissues (Figure 2A). Moreover, the Wat and Wam of rats in the model group were considerably increased compared with those of the control group (Figure 2B). The administration of ICG001 or budesonide inhibited the increase in Wat and Wam while reducing histological changes in airway remodeling. No significant differences were noted between the ICG001 and budesonide groups.
Figure 2.
ICG001 attenuated airway remodeling and inhibited transforming growth factor (TGF)-β1-induced epithelial mesenchymal transition(EMT) in a rat model of asthma. (A) Collagen deposition in the subepithelial fibrotic tissues of all seven groups, analyzed using Masson’s trichrome stain (magnification, ×100). Black arrows indicate collagen deposition in airways. (B) The thickness of the airway wall (Wat) and the airway smooth muscle (Wam) in all seven groups. (C) The levels of TGF-β1 in the bronchoalveolar lavage fluid (BALF) and sera of all seven groups. (D) Immunohistochemistry images of E-cadherin, alpha-smooth muscle actin (α-SMA), and fibronectin staining in pulmonary tissue slices of all seven groups (magnification, ×400). Black arrows indicate E-cadherin, α-SMA, and fibronectin (Fn) expressions in airways. (E) Quantitative analysis of the immunohistochemistry results of E-cadherin, α-SMA, and Fn, based on relative densitometry intensity. (F) Western blot analysis for E-cadherin, α-SMA, and Fn in the pulmonary tissue of all seven groups. (G) Quantitative analysis of the Western blot results of E-cadherin, α-SMA, and Fn, based on relative densitometry intensity.
MOD: mean optical density.
The values are expressed as mean ± standard deviation (n = 8).
#p < 0.01 versus control group.
*p < 0.01 versus 4 weeks (4w) model group.
&p < 0.01 versus 8 weeks (8w) model group.
TGF-β1 is a significant cytokine involved in airway remodeling, and TGF-β1-induced EMT is an important process in asthmatic airway remodeling. Levels of TGF-β1 in the BALF and sera were detected with ELISA. The model group had significantly elevated levels of TGF-β1 in the sera and BALF (Figure 2C). After administering ICG001 or budesonide, the level of TGF-β1 was significantly reduced compared with that of the model rats (Figure 2C). To determine the effects of ICG001 on EMT in OVA-challenged asthmatic rats, the expression of the epithelial marker E-cadherin and the mesenchymal markers α-SMA and Fn was detected by conducting immunohistochemical analysis. Rats challenged with OVA exhibited reduced expression of E-cadherin and increased expressions of Fn and α-SMA. After the daily administration of ICG001 or budesonide treatment, the transition from epithelial to mesenchymal markers was significantly reduced (Figure 2D and E). Western blot data revealed the same findings (Figure 2F and G), which suggested that ICG001 may attenuate airway remodeling by reducing the secretion of TGF-β1 and inhibiting TGF-β1-induced EMT. Budesonide treatment produced similar results.
Moreover, real-time PCR (RT-PCR) was used to test the messenger ribonucleic acid (mRNA) expression of β-catenin target genes (i.e. Snail, MMP-7, MMP-9), which were associated with TGF-β1-induced EMT. Our results demonstrated that the expression of Snail and MMP-7 was inhibited by ICG001 or budesonide administration (Figure 3).
Figure 3.
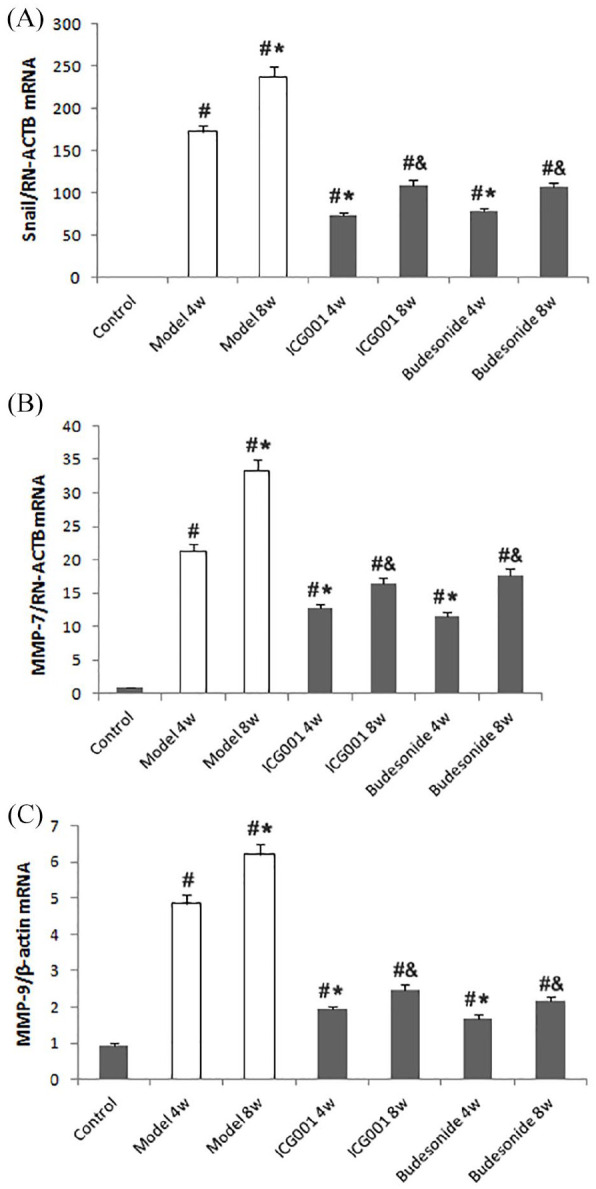
ICG001 inhibits β-catenin target genes in a rat model of asthma. (A) The real-time polymerase chain reaction (RT-PCR) analysis of Snail expression in all seven groups. (B) RT-PCR analysis of matrix metalloproteinase-7 (MMP-7) expression in all seven groups. (C) RT-PCR analysis of MMP-9 expression in all seven groups.
The values are expressed as mean ± standard deviation (SD) (n = 8).
#p < 0.01 versus control group.
*p < 0.01 versus 4 weeks (4w) model group.
&p < 0.01 versus 8 weeks (8w) model group.
The results reported in sections 3.2 and 3.3 indicate that budesonide and ICG001 have a similar effect on attenuating airway inflammation and airway remodeling. The TGF-β1/β-catenin pathway has a significant role in the pathogenesis of asthma. In addition, the vital mechanism of budesonide in treating asthma may be effective in regulating the TGF-β1/β-catenin pathway.
Blockade of β-catenin with ICG001 inhibited TGF-β1 profibrotic action in vitro
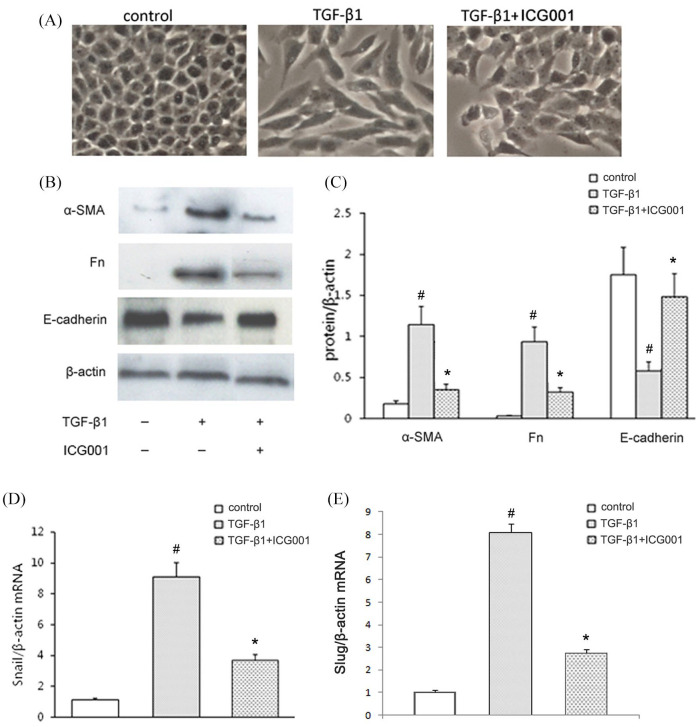
RLE-6TN cells exhibited a cobblestone-like morphology. After being cultured with 5 μg/L TGF-β1 for 24 h, these cells had a spindle-shaped morphology (Figure 4A), reduced expression of the epithelial marker E-cadherin, and increased expression of the mesenchymal markers α-SMA and Fn (Figure 4B and C). A large proportion of the RLE-6TN cells that had undergone ICG001 and TGF-β1 treatment maintained the cobblestone morphology and attenuated the transition from epithelial to mesenchymal markers (Figure 4B and C).
Figure 4.
ICG001 inhibits TGF-β1-induced epithelial mesenchymal transition in RLE-6TN cells. (A) Phase-contrast images of subconfluent RLE-6TN cells (magnification, ×400). (B) Representative Western blots of E-cadherin, alpha-smooth muscle actin (α-SMA) and fibronectin (Fn), compared with the β-actin control in the lysate of untreated or treated RLE-6TN cells. (C) Quantitative analysis results of (B), based on relative densitometry intensity. (D) The expression of Snail in RLE-6TN cells with corresponding treatments was analyzed using real-time polymerase chain reaction (RT-PCR). (E) The expression of Slug in RLE-6TN cells with corresponding treatments was analyzed using RT-PCR.
The values are expressed as the mean ± standard deviation (SD) (n = 6).
#p < 0.01, versus the control group.
*p < 0.01, versus the TGF-β1 group.
TGF-β1, transforming growth factor β1.
In our previous study, we found that TGF-β1 increased the cytosolic β-catenin. Target inhibiting β-catenin can reduce the increase of cytosolic β-catenin in TGF-β1-treated cells.16 In the present study, a transcription target of β-catenin that drives EMT was then examined. As demonstrated by RT-PCR, the expression of Snail and Slug after TGF-β1 stimulation was inhibited by ICG001 (Figure 4D and E). This finding indicated that the blockade of β-catenin suppressed TGF-β1-induced EMT in RLE-6TN cells.
Cultured CCC-REPF-1 cells had a fusiform shape. After incubating them with 5 μg/L TGF-β1 for 24 h, the size and number of these cells were increased. This morphological change was reversed when the cells were treated with a combination of ICG001 and TGF-β1 (Figure 5A). Moreover, the CCK-8 assay revealed the inhibitory effect of ICG001 on TGF-β1-induced fibroblast proliferation (Figure 5B). Furthermore, extracellular matrix (i.e. collagen I and collagen III) and α-SMA (a specific marker for smooth muscle cells) were tested to investigate the impact of ICG001 on TGF-β1-induced fibroblast to myofibroblast transition. The findings of RT-PCR revealed that ICG001 significantly attenuated the TGF-β1-induced increase in the mRNA expression of α-SMA, collagen I, and collagen III (Figure 5C). These results demonstrated that the blockade of β-catenin suppressed TGF-β1-induced fibroblast proliferation and the transition to myofibroblast.
Figure 5.
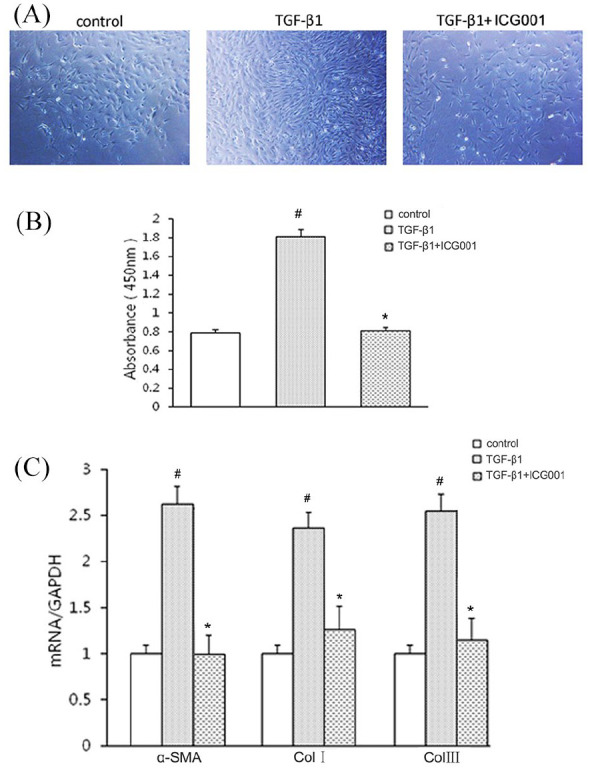
ICG001 inhibits transforming growth factor β1 (TGF-β1)-induced fibroblast proliferation and transition in CCC-REPF-1 cells. (A) Phase-contrast images of subconfluent CCC-REPF-1 cells (magnification, ×100). (B) Proliferation of CCC-REPF-1 cells, detected using the cell counting kit-8 assay (n = 6). (C) The expression of α-SMA, collagen I (Col I), and collagen III (Col III) in CCC-REPF-1 cells with their corresponding treatments was analyzed by using real-time polymerase chain reaction (n = 6).
The values are expressed as the mean ± standard deviation (SD).
#p < 0.01, versus the control group.
*p < 0.01, versus the TGF-β1 group.
TGF-β1, transforming growth factor β1.
Discussion
Asthma is a chronic inflammatory disease that occurs in the airways and is characterized by airway hyperresponsiveness, inflammation, and airway remodeling.19 TGF-β1 may have a role in airway inflammation and remodeling. It can function as a proinflammatory or anti-inflammatory cytokine on inflammatory cells and can participate in inflammatory and immune responses in the airway.9 In addition, it is involved in the process of airway remodeling, which is characterized by epithelial changes, subepithelial fibrosis, airway smooth muscle hyperplasia and hypertrophy, and microvascular changes.9 Therefore, finding a therapeutic target to suppress the proinflammatory and profibrotic effects of TGF-β1 will provide a new approach in the treatment of asthmatic airway inflammation and airway remodeling.
In our previous study of renal fibrosis,16 we found that inhibiting β-catenin with ICG001 can separate the profibrotic and anti-inflammatory actions of TGF-β1. Therefore, the profibrotic effect of TGF-β1 is prevented without affecting its anti-inflammatory actions. Our current study demonstrated that targeting β-catenin with ICG001 could alleviate airway inflammation and airway remodeling in an OVA-challenged rat model of asthma. The effects of inhibiting airway inflammation and remodeling by ICG001 were associated with a significant decrease in Th2 and Th17 cytokines, an increase in anti-inflammatory cytokines, and the amelioration of EMT. Moreover, the inhibition of β-catenin prevented TGF-β1-induced EMT and the fibroblast-to-myofibroblast transition (FMT) in vitro.
The pathogenesis of asthma includes a complex process of interaction between airway inflammation and remodeling. Infiltrating cells such as Th cells, EOSs, neutrophils, and mast cells interact with resident cells of the airways such as fibroblasts, smooth muscle cells, neuronal cells, epithelial cells, and endothelial cells by the release of a plethora of cytokines, enzymes, metabolites, and growth factors, creating a signaling environment that—under chronic conditions—results in airway remodeling. Chronic inflammation resulting in persistently altered airway wall structures and function is regarded as pathological airway remodeling, which decreases lung compliance and increases airway resistance.20 Airway remodeling is characterized by hyperplasia and hypertrophy of airway smooth muscle cells, alveolar and vascular damage, goblet cell metaplasia, and deposition of the extracellular matrix.21 Kwak et al.13 applied OVA to establish a mouse model of asthma and observed structural changes in the airway of asthmatic mice such as smooth muscle hyperplasia, mucous metaplasia, subepithelial fibrosis, and a significant increase in collagen deposition in subepithelial fibrotic tissues. These findings indicated that OVA-sensitized and -challenged rats had airway remodeling. Therefore, in our study, Wistar rats with OVA were challenged to construct a chronic asthma model, based on the experimental method. The signaling protein β-catenin has an important role in lung development, injury, and repair whereas the aberrant expression of Wnt/β-catenin signaling leads to asthmatic airway remodeling.20,21 Thus, β-catenin is a therapeutic target in airway remodeling of asthma. However, few experimental studies have focused on the exact role that targeting β-catenin has on asthmatic airway remodeling.13
Some researchers have demonstrated with chronic asthma models that using ICG001 to target β-catenin prevents β-catenin gene expression, reduces smooth muscle hyperplasia, and alleviates airway goblet cell metaplasia and collagen deposition.22,23 The present study demonstrated that the blockade of β-catenin with ICG001 significantly reversed airway remodeling characteristics such as epithelium damage, bronchial wall thickening, and subepithelial fibrosis, which confirmed the anti-remodeling effect of targeting β-catenin on asthma.
β-catenin signaling may be involved in airway remodeling, although the potential mechanisms remain unclear. β-catenin has a role in asthmatic remodeling in Wnt-dependent and Wnt-independent mechanisms, which include tissue repair, cell proliferation and differentiation, and extracellular matrix production.15 Among Wnt-independent mechanisms, TGF-β1 is a growth factor that is capable of stabilizing β-catenin and activating β-catenin-dependent processes.15 As indicated by recent studies,24 TGF-β1-induced EMT is the mechanism responsible for the pathologic features of asthmatic airway remodeling.25 The activation of β-catenin signals may be involved in the pathological process of EMT triggered by TGF-β1.25 However, no evidence exists that indicates the impact of β-catenin blockade on TGF-β1-induced EMT through the pathological process of asthma in in vivo experiments. In our study, we demonstrated that β-catenin blockade significantly alleviated TGF-β1-induced EMT in the process of OVA-triggered airway remodeling, as manifested in the upregulated expression of E-cadherin, the downregulated expression of α-SMA and Fn, and the reduced expression of MMP-7, MMP-9, and Snail.
In addition to remodeling, another critical feature of asthma is airway inflammation. Reports indicate that Th2 cells, Th17 cells and Tregs have a significant role in asthma.26 Asthma is classically recognized as a typical Th2 cells disease, in which the inflammatory process is dominated by Th2 cells that release the cytokines IL-4, IL-5, and IL-13. These cytokines modulate airway inflammation by activating EOSs and IgE production.27 The Th17 cells secrete IL-17 and mediate the occurrence and progression of inflammatory reactions. The major biological function of IL-17 is to promote inflammatory reactions.25 By comparison, Tregs are involved in the anti-inflammatory process by producing anti-inflammatory cytokines such as IL-10 and IL-35.28,29
Aside from these cytokines, TGF-β1 may have a role in inflammation by inducing the proliferation of Th17 cells, and resistance to inflammation by inducing the proliferation of Tregs.30,31 As mentioned before, Wnt/β-catenin signal takes part in the pathological process of TGF-β1-induced EMT though airway remodeling. However, the role of Wnt/β-catenin in airway inflammation is still controversial. Some studies indicated that activation of the canonical Wnt-1/β-catenin pathway ameliorates the development of allergic airway disease by regulating the immune response and inducting appropriate T cell responses.32,33 On the contrary, other researches showed that Wnt/β-catenin signaling has roles in Th2 cells inflammation and the decline in lung function. Blocking β-catenin could attenuate airway inflammation.13,14 Our previous study16 demonstrated that the degradation of β-catenin had no effect on the TGF-β1-mediated inhibition of macrophage activation. Moreover, targeting β-catenin with ICG001 diverts β-catenin from TCF-mediated transcription to Foxo-mediated transcription and enhances the interaction of β-catenin with Foxo, a transcription factor that regulates the differentiation of TGF-β1-induced Tregs, and thereby enhances the anti-inflammatory effects of TGF-β1.17 The findings of these studies suggested that targeting β-catenin may have an anti-inflammatory effect in asthma. In the present study, we found that the blockade of β-catenin/TCF with ICG001 significantly inhibited inflammatory cell infiltration and inhibited total inflammatory cell and EOS cell counts. These findings confirmed the anti-inflammatory effect of ICG001. In addition, the evidence indicated that ICG001 can alleviate OVA-induced airway inflammation by downregulating the expression of the OVA-IgE, Th2, and Th17 cytokines and upregulating the expression of Tregs-associated anti-inflammatory cytokines IL-10 and IL-35.
Fibrosis is a pivotal feature of the pathological process of airway remodeling in asthma. Fibrosis is essential because it can occur early in the pathogenesis of asthma and is associated with the severity and resistance to therapy.34 Airway epithelial cells and lung fibroblasts are involved in the fibrotic process of airway remodeling in asthma.35 New evidence suggests that the airway epithelium can contribute to airway remodeling through the process of EMT.36 The hallmark trait of EMT is the downregulation of the epithelial marker E-cadherin, the upregulation of the mesenchymal markers α-SMA and Fn, and the increased expression of EMT-activating transcription factors such as Snail, MMP-7, MMP-9, and Slug.24,36,37 By comparison, fibroblasts can contribute to airway remodeling via accumulating in subepithelial regions, differentiating into myofibroblasts, and secreting ECM components.38 As demonstrated by research, TGF-β1 can trigger EMT by affecting the release, stabilization, and activation of β-catenin, and promoting the transcription of its target gene.22 Moreover, TGF-β1 can stimulate fibroblast proliferation and differentiation into myofibroblasts, which have secretory and contractile phenotypes and express α-SMA.39 This process is called “fibroblast-to-myofibroblast transition”.39 Research studies conducted by our team and other investigators have demonstrated that targeting β-catenin may have an antifibrotic effect on renal fibrosis.16,40 However, limited evidence exists regarding targeting β-catenin in asthma-related cells. The current study demonstrated that the selective blockade of β-catenin inhibited TGF-β1-induced EMT in alveolar epithelial cells and suppressed TGF-β1-mediated fibroblast proliferation and FMT in lung fibroblasts.
GCs, especially inhaled GCs, remain the cornerstone of asthma management because GCs can inhibit multiple aspects of the pathogenesis of asthma.18 Budesonide is the most commonly used inhaled GC. Several studies have indicated that atomized budesonide can inhibit the increase in EOSs, TNF-α, IL-4, IL-5, IL-13, and TGF-β1 in the BALF of OVA-sensitized rats or mice, and thereby further alleviate airway inflammation and reduce airway remodeling.41,42
Inhaled budesonide can also repress the expression of TGF-β1, PDGF-A, Smad4, and PAI-1 in lung tissue and improve pulmonary fibrosis.43 Thus, inhaled budesonide has an anti-inflammatory effect and an antifibrotic effect by regulating multiple immune cells and cytokines. Our current study revealed that budesonide can inhibit inflammatory cell infiltration and inhibit total inflammatory cell and EOS cell counts while reversing the airway remodeling characteristics. This phenomenon may be caused by regulating the TGF-β1/β-catenin pathway and further by reducing the levels of the Th2 and Th17 cytokines, increasing the levels of the anti-inflammatory cytokines IL-10 and IL-35, and suppressing TGF-β1-induced EMT.
Conclusion
In conclusion, our findings demonstrated that the blockade of β-catenin/TCF prevents TGF-β1-induced EMT and FMT, which are involved in the pathological remodeling of the airway, and it alleviates airway inflammation in OVA-sensitized rats after OVA-challenge by balancing proinflammatory and anti-inflammatory cytokines. In this sense, targeting β-catenin may have a therapeutic effect on asthmatic airway inflammation and remodeling. These findings provide an experimental basis for targeting β-catenin in the treatment of asthma. However, additional clinical studies are needed to clarify the curative effect and the role of these pathogenetic pathways in patients with asthma.
Supplemental Material
Supplemental material, sj-pdf-1-tar-10.1177_1753466620981858 for Targeted inhibition of β-catenin alleviates airway inflammation and remodeling in asthma via modulating the profibrotic and anti-inflammatory actions of transforming growth factor-β1 by Rujie Huo, Xinli Tian, Qin Chang, Dai Liu, Chen Wang, Jingcui Bai, Runjuan Wang, Guoping Zheng and Xinrui Tian in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-2-tar-10.1177_1753466620981858 for Targeted inhibition of β-catenin alleviates airway inflammation and remodeling in asthma via modulating the profibrotic and anti-inflammatory actions of transforming growth factor-β1 by Rujie Huo, Xinli Tian, Qin Chang, Dai Liu, Chen Wang, Jingcui Bai, Runjuan Wang, Guoping Zheng and Xinrui Tian in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-3-tar-10.1177_1753466620981858 for Targeted inhibition of β-catenin alleviates airway inflammation and remodeling in asthma via modulating the profibrotic and anti-inflammatory actions of transforming growth factor-β1 by Rujie Huo, Xinli Tian, Qin Chang, Dai Liu, Chen Wang, Jingcui Bai, Runjuan Wang, Guoping Zheng and Xinrui Tian in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-4-tar-10.1177_1753466620981858 for Targeted inhibition of β-catenin alleviates airway inflammation and remodeling in asthma via modulating the profibrotic and anti-inflammatory actions of transforming growth factor-β1 by Rujie Huo, Xinli Tian, Qin Chang, Dai Liu, Chen Wang, Jingcui Bai, Runjuan Wang, Guoping Zheng and Xinrui Tian in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-5-tar-10.1177_1753466620981858 for Targeted inhibition of β-catenin alleviates airway inflammation and remodeling in asthma via modulating the profibrotic and anti-inflammatory actions of transforming growth factor-β1 by Rujie Huo, Xinli Tian, Qin Chang, Dai Liu, Chen Wang, Jingcui Bai, Runjuan Wang, Guoping Zheng and Xinrui Tian in Therapeutic Advances in Respiratory Disease
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Footnotes
Availability of data and materials: The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Natural Science Foundation of Shanxi Province of China [2013011055-1]; Fund Program for the Scientific Activities of Selected Returned Overseas Professionals in China {2016[366]}; the Fund Program for the Scientific Activities of Selected Returned Overseas Professionals in Shanxi Province {[2017]144}; and Key Research and Development Program (International Scientific and Technological cooperation) of Shanxi Province [201903D421066].
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Rujie Huo  https://orcid.org/0000-0001-5232-5710
https://orcid.org/0000-0001-5232-5710
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Rujie Huo, Department of Respiratory and Critical Care Medicine, Second Hospital of Shanxi Medical University, Taiyuan, China.
Xinli Tian, Cardiopulmonary Center, General Hospital of PLA Army, Beijing, China.
Qin Chang, Department of Respiratory Medicine, Linfen Central Hospital, Linfen, China.
Dai Liu, Department of Respiratory and Critical Care Medicine, Second Hospital of Shanxi Medical University, Taiyuan, China.
Chen Wang, Pathology Department, Second Hospital of Shanxi Medical University, Taiyuan, China.
Jingcui Bai, Department of Respiratory and Critical Care Medicine, Second Hospital of Shanxi Medical University, Taiyuan, China.
Runjuan Wang, Emergency Department, Central Hospital of China Railway No.3 Engineering Group, Taiyuan, China.
Guoping Zheng, Centre for Transplant and Renal Research, Westmead Institute for Medical Research, University of Sydney, Sydney, NSW, Australia.
Xinrui Tian, Department of Respiratory and Critical Care Medicine, Second Hospital of Shanxi Medical University, 382 Wuyi Road, Xinghualing Area, Taiyuan, China.
References
- 1. Carr TF, Bleecker E. Asthma heterogeneity and severity. World Allergy Organ J 2016; 9: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nunes C, Pereira AM, Morais-Almeida M. Asthma costs and social impact. Asthma Res Pract 2017; 3: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maslan J, Mims JW. What is asthma? Pathophysiology, demographics, and health care costs. Otolaryngol Clin North Am 2014; 47: 13–22. [DOI] [PubMed] [Google Scholar]
- 4. Huang C, Zhang Z, Wang L, et al. ML-7 attenuates airway inflammation and remodeling via inhibiting the secretion of T helper (Th) cells cytokines in mice model of asthma. Mol Med Rep 2018; 17: 6293–6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Montuschi P, Barnes PJ. New perspectives in pharmacological treatment of mild persistent asthma. Drug Discov Today 2011; 16: 1084–1091. [DOI] [PubMed] [Google Scholar]
- 6. Mantel PY, Schmidt-Weber CB. Transforming growth factor-beta: recent advances on its role in immune tolerance. Methods Mol Biol 2011; 677: 303–338. [DOI] [PubMed] [Google Scholar]
- 7. Lee C-M, Park JW, Cho W-K, et al. Modifiers of TGF-β1 effector function as novel therapeutic targets of pulmonary fibrosis. Korean J Intern Med 2014; 29: 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Al-Alawi M, Hassan T, Chotirmall SH. Transforming growth factor β and severe asthma: a perfect storm. Respir Med 2014; 108: 1409–1423. [DOI] [PubMed] [Google Scholar]
- 9. Yang YC, Zhang N, Van Crombruggen K, et al. Transforming growth factor-beta1 in inflammatory airway disease: a key for understanding inflammation and remodeling. Allergy 2012; 67: 1193–1202. [DOI] [PubMed] [Google Scholar]
- 10. Forte A, Galderisi U, Cipollaro M, et al. Epigenetic regulation of TGF-β1 signalling indilative aortopathy of the thoracic ascending aorta. Clin Sci (Lond) 2016; 130: 1389–1405. [DOI] [PubMed] [Google Scholar]
- 11. Vallée A, Lecarpentier Y, Guillevin R, et al. Interactions between TGF-β1, canonical WNT/β-catenin pathway and PPAR γ in radiation-induced fibrosis. Oncotarget 2017; 8: 90579–90604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vallée A, Lecarpentier Y. TGF-β in fibrosis by acting as a conductor for contractile properties of myofibroblasts. Cell Biosci 2019; 9: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kwak HJ, Park DW, Seo JY, et al. The Wnt/β-catenin signaling pathway regulates the development of airway remodeling in patients with asthma. Exp Mol Med 2015; 47: e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kumawat K, Koopmans T, Gosens R. β-catenin as a regulator and therapeutic target for asthmatic airway remodeling. Expert Opin Ther Targets 2014; 18: 1023–1034. [DOI] [PubMed] [Google Scholar]
- 15. Guo Y, Xiao L, Sun L, et al. Wnt/beta-catenin signaling: a promising new target for fibrosis diseases. Physiol Res 2012; 61: 337–346. [DOI] [PubMed] [Google Scholar]
- 16. Tian X, Zhang J, Tan TK, et al. Association of β-catenin with P-Smad3 but not LEF-1 dissociates in vitro profibrotic from anti inflammatory effects of TGF-β1. J Cell Sci 2013; 126: 67–76. [DOI] [PubMed] [Google Scholar]
- 17. Qiao X, Rao P, Zhang Y, et al. Redirecting TGF-β signaling through the β-Catenin/Foxo complex prevents kidney fibrosis. J Am Soc Nephrol 2017; 29: 557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol 2011; 163: 29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mims JW. Asthma: definitions and pathophysiology. Int Forum Allergy Rhinol 2015; 5(Suppl. 1): S2–S6. [DOI] [PubMed] [Google Scholar]
- 20. Fehrenbach H, Wagner C, Wegmann M. Airway remodeling in asthma what really matters. Cell Tissue Res 2017; 367: 551–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hussain M, Xu C, Lu M, et al. Wntβ-catenin signaling links embryonic lung development and asthmatic airway remodeling. Biochim Biophys Acta Mol Basis Dis 2017; 1863: 3226–3242. [DOI] [PubMed] [Google Scholar]
- 22. Koopmans T, Crutzen S, Menzen MH, et al. Selective targeting of CREB-binding protein/β-catenin inhibits growth of and extracellular matrix remodelling by airway smooth muscle. Br J Pharmacol 2016; 173: 3327–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yao L, Zhao H, Tang H, et al. Blockade of β-catenin signaling attenuates toluene diisocyanate-induced experimental asthma. Allergy 2017; 72: 579–589. [DOI] [PubMed] [Google Scholar]
- 24. Hackett TL. Epithelial–mesenchymal transition in the pathophysiology of airway remodelling in asthma. Curr Opin Allergy Clin Immunol 2012; 12: 53–59. [DOI] [PubMed] [Google Scholar]
- 25. Tan RJ, Zhou D, Zhou L, et al. Wnt/β-catenin signaling and kidney fibrosis. Kidney Int Suppl (2011) 2014; 4: 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ding F, Fu Z, Liu B. Lipopolysaccharide exposure alleviates asthma in mice by regulating Th1/T helper (Th) cells and Treg/Th17 balance. Med Sci Monit 2018; 24: 3220–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ji P, Hu H, Yang X, et al. AcCystatin, an immunoregulatory molecule from angiostrongylus cantonensis, ameliorates the asthmatic response in an aluminium hydroxide/ovalbumin-induced rat model of asthma. Parasitol Res 2015; 114: 613–624. [DOI] [PubMed] [Google Scholar]
- 28. Zhu J, Liu X, Wang W, et al. Altered expression of regulatory T and Th17 cells in murine bronchial asthma. Exp Ther Med 2017; 14: 714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ruan G, Tao B, Wang D, et al. Chinese herbal medicine formula Gu-Ben-Fang-Xiao-Tang attenuates airway inflammation by modulating Th17/Treg balance in an ovalbumin-induced murine asthma model. Exp Ther Med 2016; 12: 1428–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Noval Rivas M, Chatila TA. Regulatory T cells in allergic diseases. J Allergy Clin Immunol 2016; 138: 639–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen L, Yang T, Lu D-W, et al. Central role of dysregulation of TGF-β/Smad in CKD progression and potential targets of its treatment. Biomed Pharmacother 2018; 101: 670–681. [DOI] [PubMed] [Google Scholar]
- 32. Reuter S, Martin H, Beckert H, et al. The Wnt/β-catenin pathway attenuates experimental allergic airway disease. J Immunol 2014; 193: 485–495. [DOI] [PubMed] [Google Scholar]
- 33. Trischler J, Shiomi T, Turner DL, et al. Immune modulation of the T cell response in asthma through Wnt10b. Am J Respir Cell Mol Biol 2016; 54: 584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nayak AP, Deshpande DA, Penn RB. New targets for resolution of airway remodeling in obstructive lung diseases. F1000Res 2018; 7: F1000 Faculty Rev-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gon Y, Hashimoto S. Role of airway epithelial barrier dysfunction in pathogenesis of asthma. Allergol Int 2018; 67: 12–17. [DOI] [PubMed] [Google Scholar]
- 36. Rout-Pitt N, Farrow N, Parsons D, et al. Epithelial mesenchymal transition (EMT): a universal process in lung diseases with implications for cystic fibrosis pathophysiology. Respir Res 2018; 19: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Singh R, Mandhani A, Agrawal V, et al. Positive correlation between matrix metalloproteinases and epithelial-to-mesenchymal transition and its association with clinical outcome in bladder cancer patients. Cancer Microenviron 2018; 11: 23–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Royce SG, Cheng V, Samuel CS, et al. The regulation of fibrosis in airway remodeling in asthma. Mol Cell Endocrinol 2012; 351: 167–175. [DOI] [PubMed] [Google Scholar]
- 39. Michalik M, Wójcik-Pszczoła K, Paw M, et al. Fibroblast-to-myofibroblast transition in bronchial asthma. Cell Mol Life Sci 2018; 75: 3943–3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gong Y, Qin Z, Zhou B, et al. MicroRNA-200a inhibits microRNA-200a inhibits transforming growth factor β1-induced proximal tubular epithelial-mesenchymal transition by targeting β-catenin. Nephron 2017; 137: 237–249. [DOI] [PubMed] [Google Scholar]
- 41. Li K, Chen W, Yu B, et al. Effects of fastigial nucleus electrostimulation on airway inflammation and remodeling in an experimental rat model of asthma. Asian Pac J Allergy Immunol 2016; 34: 223–228. [DOI] [PubMed] [Google Scholar]
- 42. Tang X, Nian H, Li X, et al. Effects of the combined extracts of herba epimedii and fructus ligustrilucidi on airway remodeling in the asthmatic rats with the treatment of budesonide. BMC Complement Altern Med 2017; 17: 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen JP, Fan XM, Zhan XQ, et al. Effect of budesonide on Smad4, PDGF-A and PAI-1 in a rat model of pulmonary fibrosis. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2012; 28: 478–480. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tar-10.1177_1753466620981858 for Targeted inhibition of β-catenin alleviates airway inflammation and remodeling in asthma via modulating the profibrotic and anti-inflammatory actions of transforming growth factor-β1 by Rujie Huo, Xinli Tian, Qin Chang, Dai Liu, Chen Wang, Jingcui Bai, Runjuan Wang, Guoping Zheng and Xinrui Tian in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-2-tar-10.1177_1753466620981858 for Targeted inhibition of β-catenin alleviates airway inflammation and remodeling in asthma via modulating the profibrotic and anti-inflammatory actions of transforming growth factor-β1 by Rujie Huo, Xinli Tian, Qin Chang, Dai Liu, Chen Wang, Jingcui Bai, Runjuan Wang, Guoping Zheng and Xinrui Tian in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-3-tar-10.1177_1753466620981858 for Targeted inhibition of β-catenin alleviates airway inflammation and remodeling in asthma via modulating the profibrotic and anti-inflammatory actions of transforming growth factor-β1 by Rujie Huo, Xinli Tian, Qin Chang, Dai Liu, Chen Wang, Jingcui Bai, Runjuan Wang, Guoping Zheng and Xinrui Tian in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-4-tar-10.1177_1753466620981858 for Targeted inhibition of β-catenin alleviates airway inflammation and remodeling in asthma via modulating the profibrotic and anti-inflammatory actions of transforming growth factor-β1 by Rujie Huo, Xinli Tian, Qin Chang, Dai Liu, Chen Wang, Jingcui Bai, Runjuan Wang, Guoping Zheng and Xinrui Tian in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-5-tar-10.1177_1753466620981858 for Targeted inhibition of β-catenin alleviates airway inflammation and remodeling in asthma via modulating the profibrotic and anti-inflammatory actions of transforming growth factor-β1 by Rujie Huo, Xinli Tian, Qin Chang, Dai Liu, Chen Wang, Jingcui Bai, Runjuan Wang, Guoping Zheng and Xinrui Tian in Therapeutic Advances in Respiratory Disease