Abstract
Aims:
Long-term data on TNFi treatment in patients with axSpA is scarce. The objective of this analysis was to assess long-term clinical efficacy of etanercept in early axSpA [including both non-radiographic and radiographic axSpA forms], who participated in the long-term (until year 10) extension of the ESTHER-trial.
Methods:
In the previously reported ESTHER-trial, patients with early active axSpA were randomized to treatment with etanercept (n = 40) or sulfasalazine (n = 36) during the first year. Patients in remission discontinued their therapy and were followed up until the end of year 2; in case of remission-loss, etanercept was (re)-introduced and continued until the end of year 10. If remission was not achieved at year 1, patients continued receiving (or were switched to) etanercept for up to 10 years.
Results:
A total of 19 patients (12 with r-axSpA and 7 with nr-axSpA at baseline) out of the initial 76 patients (= 25%) completed year 10 of the study. In the entire group, a sustained clinical response was seen over 10 years of follow up in the as-observed analysis. Completers were significantly more often male and showed lower values of patient and physician global assessments of disease activity, Ankylosing Spondylitis Disease Activity Score (ASDAS), and Ankylosing Spondylitis Quality of Life questionnaire (ASQoL) scores at baseline as compared with non-completers. When analyzing clinical data of the completers, mean Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and Bath Ankylosing Spondylitis Functional Index (BASFI) values were constantly below 2 and mean ASDAS below 2.1 during follow up with no statistically significant differences between the r-axSpA and nr-axSpA subgroups. A total of 39 serious adverse events were documented over the 10 years, while six of them were seen as possibly associated with the etanercept treatment, which led in five patients to treatment discontinuation.
Conclusion:
A sustained clinical response was observed over the 10 years of the study with comparable response and drop-out rates between r-axSpA and nr-axSpA. Etanercept was well tolerated across the entire treatment period and showed a good safety profile with no new safety signals.
Keywords: adherence, ankylosing spondylitis, axial spondyloarthritis, etanercept, non-radiographic axial spondyloarthritis, remission, sulfasalazine, TNF-inhibitors, treatment
Plain language summary
Long-term clinical efficacy of etanercept in early axial spondyloarthritis
This is the first trial investigating the long-term effect of a TNFi therapy with etanercept in the whole group of axSpA (including both nr- and r-axSpA) over 10 years
Sustained and comparable clinical response to TNFi etanercept in nr-axSpA and r-axSpA was shown over 10 years
No new safety signals for etanercept in this patient group
Introduction
Axial spondyloarthritis (axSpA) is a chronic inflammatory condition that mainly affects the axial skeleton [spine and sacroiliac joints (SIJs)]. Furthermore, the disease can manifest with peripheral arthritis and enthesitis, as well as extra-musculoskeletal involvement such as uveitis, inflammatory bowel disease (IBD), and psoriasis.1 The umbrella term of axSpA covers both patients with non-radiographic (nr-axSpA) and radiographic axSpA [r-axSpA, also called ankylosing spondylitis (AS)]. Both forms of axSpA are covered by the Assessment of SpondyloArthritis International Society (ASAS) classification criteria.2 The terms AS or radiographic axSpA are interchangeable and can be classified based on the presence of structural damage according to the modified New York criteria.3,4
For patients with axSpA who do not respond to treatment with nonsteroidal anti-inflammatory drugs (NSAIDs), tumour necrosis factor alpha (TNF-α) and, more recently, interleukin (IL)-17A inhibitors are possible therapeutic options according to current international management recommendations.5,6
TNF inhibitors (TNFi) have shown good short-term efficacy in controlled trials in patients with early axSpA.7–11 However, data on the long-term clinical efficacy and safety of TNFi in early axSpA is scarce. Studies evaluating long-term efficacy mostly involved longstanding established r-axSpA patients only,12–15 and published data on nr-axSpA is available only for a shorter duration of follow up (up to a maximum of 4 years).16,17
The Etanercept versus Sulfasalazine in Early Axial Spondyloarthritis (ESTHER) trial with etanercept – a recombinant TNF receptor fusion protein – included patients with active early axSpA with a disease duration of <5 years at baseline [ClinicalTrials.gov identifier: NCT00844142], thus allowing the evaluation of long-term treatment responses including both nr-axSpA and r-axSpA subgroups.11 Summarizing what has been previously reported about the ESTHER trial are: clinical and imaging data of year one,11,16,18,19 clinical results from year two and year three as well as the data on radiographic progression until year six.16,20,21
In the current report, we report the clinical 10-year results of treatment with etanercept in patients with axSpA focusing on the comparison of treatment response in nr-axSpA and r-axSpA subgroups.
Methods
Study design and patients
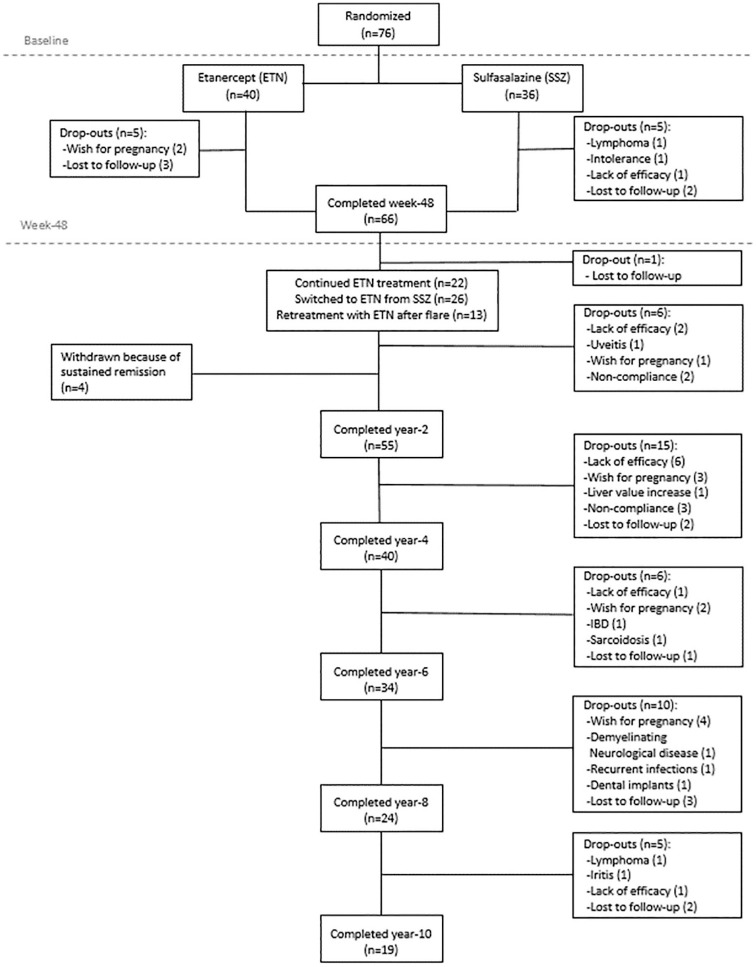
The design of the ESTHER trial, inclusion criteria as well as the comprehensive clinical, radiographic and magnetic resonance imaging (MRI) outcome data up to year six were reported elsewhere.11,16,18,20,22,23 Briefly, 76 patients with early axSpA (with a disease duration of ⩽5 years), who retrospectively fulfilled the ASAS classification criteria for axSpA and had evidence of active inflammation on the baseline MRI in the sacroiliac joints and/or the spine,2 were randomized either to etanercept (n = 40) or sulfasalazine (n = 36) for 48 weeks.11 At the end of this period, patients who were in remission discontinued their therapy and were closely followed up every 6 weeks without active treatment until the end of year two. In the case of a flare [defined as a Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) increase of 2 points or more as compared with the week 48 value], patients started/restarted etanercept and continued it for up to 9 years when coming from the sulfasalazine group and up to 10 years when being treated with etanercept during year one. Patients receiving sulfasalazine who were not in remission at week 48 were switched to etanercept and continued this treatment for up to 9 years; patients already receiving etanercept who were not in remission at week 48 continued the treatment for up to 10 years.20 In total, 17 patients (13 under etanercept treatment and 4 from the sulfasalazine group) achieved remission at Week 48 and therefore had their treatment discontinued. In these patients, etanercept was later restarted in 13 cases due to a flare, while four patients finished the study because of continuous drug-free remission at the end of year two.16 Overall, 19 patients completed the 10-year study period (Figure 1).
Figure 1.
Patient disposition during the whole study period of 10 years.
ETN, etanercept; IBD, inflammatory bowel disease; SSZ sulfasalazine.
Outcome measure assessments
The disease status assessments were performed using established parameters for disease activity [Ankylosing Spondylitis Disease Activity Score (ASDAS)24 and BASDAI25], spinal mobility [Bath ankylosing spondylitis metrology index (BASMI) (based on the linear definition)26], and function [Bath Ankylosing Spondylitis Functional Index (BASFI)27]. Furthermore, the patient (PGA) and physician (PhGA) global assessments were also collected, both were evaluated using a numeric rating scale (NRS) varying from 0 to 10. A modified Maastricht ankylosing spondylitis enthesitis score (MASES) with an additional two sites (medial and lateral condyles of the femur) at each knee (resulting in 17 enthesitis points) and a swollen joint count with 64 joints were performed to evaluate enthesitis and arthritis, respectively.28 Laboratory parameters such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were measured for the assessment of inflammatory activity. Euro Quality of Life Index (EQ-5D) and the disease-specific AS Quality of Life (ASQoL) questionnaires were used to assess the health- and disease-related quality of life.29,30
Treatment response was evaluated by applying the core set criteria for symptomatic improvement in r-axSpA (as suggested by the ASAS) with ASAS20- and ASAS40-response, ASAS 5/6 and ASAS partial remission (PR).31–33 Other assessment tools for treatment response were low disease activity defined as BASDAI <3,14 a 50% improvement in the BASDAI (BASDAI50),32 ASDAS improvement criteria (clinically important improvement and major improvement),24 and intake of nonsteroidal anti-inflammatory drugs (NSAID) using the Assessment of SpondyloArthritis International Society (ASAS) NSAIDs intake score.34 Disease activity categories were also defined with categorical variables as described in the ASDAS cut-off values for disease activity states.24
In addition, we analyzed both the changes in BASDAI, BASFI, ASDAS, and BASMI and the response rates (based on different ASAS criterions (ASAS 20, 40, 5/6, and PR) and BASDAI50 improvement) for the completer group who finished the study at the end of year 10 and for the remaining patients at each time point in the as-observed set separately.
Statistical analysis
The data were analyzed for the group of patients who completed 10 years of the study according to the protocol (completers) and as the as-observed set at the pre-defined time-points. Outcome parameters over time are presented descriptively showing mean and standard deviation (SD) or frequencies and percentages, whichever appropriate. The last observation carried forward (LOCF) method was applied to impute missing data, if the data from the time-point of the visit included in this analysis was missing but available for the previous visit according to the protocol.
Baseline characteristic of patients who completed the study and who dropped out (non-completers) were compared using Mann–Whitney U test for categorical variables, Fischer’s exact test for dichotomous variables and student t test for continuous variables. p values <0.05 were considered statistically significant.
Ethics committee approval
The study was approved by the central ethics committee of the federal state of Berlin (Landesamt für Gesundheit und Soziales, Ethikkommission Berlin; approval number ZS EK 14 EA4/100/05), and written informed consent was obtained in all patients.
[ClinicalTrials.gov identifier: NCT00844142], EudraCT number: 2005-002320-34.
Results
Baseline characteristics
Out of the 76 patients who were included in the original study, 66 (86.8%) completed Year one (35 under etanercept treatment and 31 under sulfasalazine treatment), while 55 patients (72.4%) finished Year two, 40 (52.6%) Year four, 34 (44.7%) Year six, 24 (31.6%) Year eight, and 19 patients (25%) completed the 10-year treatment period (Figure 1). Of these 19 patients, 12 (63.2%) were classified as r-axSpA at baseline and 7 (36.8%) as nr-axSpA at baseline. The reasons for dropouts are described in detail below.
When comparing the baseline characteristics of the two subgroups (completers versus non-completers), there was no difference in mean age, disease duration, the fulfillment of modified New York criteria, NSAID intake, and treatment with etanercept during the first year. However, completers were more often males (78.9% versus 50.9%, respectively, p = 0.034), and had lower patient global, physician global, ASDAS, and ASQoL scores at baseline as compared with non-completers (all p values <0.05). The baseline characteristics of all completers are presented in Table 1 and compared with “non-completers” who dropped out of the study before reaching the final visit at the end of Year 10.
Table 1.
Baseline characteristics of patients with axial spondyloarthritis who completed the 10 years of the trial (completers) and of those who dropped out before the end of Year 10 (non-completers).
| Completers (n = 19) | Non-completers (n = 57) | p value | |
|---|---|---|---|
| Age, years, mean (SD) | 32.5 (7.4) | 32.8 (8.9) | 0.91 |
| Male sex, n (%) | 15 (78.9) | 29 (50.9) | 0.034* |
| Fulfilment of the modified New York criteria for AS, n (%) | 12 (63.2) | 27 (47.4) | 0.24 |
| Symptom duration, years, mean (SD) | 1.1 (1.2) | 1 (1.7) | 0.81 |
| HLA-B27 positivity, n (%) | 18 (94.7) | 44 (77.2) | 0.09 |
| CRP, mg/l, mean (SD) | 7.5 (10.5) | 12 (15.1) | 0.06 |
| Elevated CRP (CRP >5 mg/l), n (%) | 7 (38.9) | 33 (62.3) | 0.09 |
| ESR, mm/h, mean (SD) | 16.8 (15.7) | 23.3 (19.5) | 0.21 |
| Clinical arthritis, n (%) | 4 (21.1) | 15 (26.3) | 0.65 |
| Arthritis joint count, 0–64, mean (SD) | 1.9 (4.4) | 1.8 (4.4) | 0.92 |
| Clinical enthesitis, n (%) | 9 (47.4) | 35 (61.4) | 0.40 |
| Enthesitis score, 0–17, mean (SD) | 2.8 (2.9) | 4.3 (4.4) | 0.20 |
| PGA, 0–10, mean (SD) | 6.1 (1.9) | 7.2 (1.7) | 0.025* |
| PhGA, 0–10, mean (SD) | 5.5 (1.5) | 6.5 (1.2) | 0.007* |
| ASDAS, units, mean (SD) | 3 (0.7) | 3.5 (0.8) | 0.042* |
| BASDAI, 0–10, mean (SD) | 5.4 (1.1) | 5.8 (1.3) | 0.27 |
| BASFI, 0–10, mean (SD) | 4 (2.1) | 4.4 (2) | 0.41 |
| BASMI linear, 0–10, mean (SD) | 2.4 (0.9) | 2.9 (1.3) | 0.08 |
| AS-QoL, 0–18, mean (SD) | 7.6 (3.9) | 10.1 (3.9) | 0.019* |
| EQ-5D, 0–1, mean (SD) | 0.7 (0.3) | 0.6 (0.3) | 0.13 |
| NSAID intake score, 0–100, mean (SD) | 86.5 (22.1) | 86.1 (47.2) | 0.97 |
| Treatment with ETN during 1st year, n (%) | 12 (63.2) | 28 (49.1) | 0.30 |
Significant at p < 0.05.
AS, ankylosing spondylitis; ASQoL, Ankylosing Spondylitis Quality of Life questionnaire; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Disease Functional Index; BASMI, Bath Ankylosing Spondylitis Metrology Index; CRP, C-reactive protein; EQ-5D, Euro Quality of Life Index; ESR, erythrocyte sedimentation rate; HLA, human leukocyte antigen; NSAID, nonsteroidal anti-inflammatory drugs; PhGA, physician’s global assessment; PGA, patient’s global assessment; SD, standard deviation.
Clinical response to etanercept in the whole group of axSpA
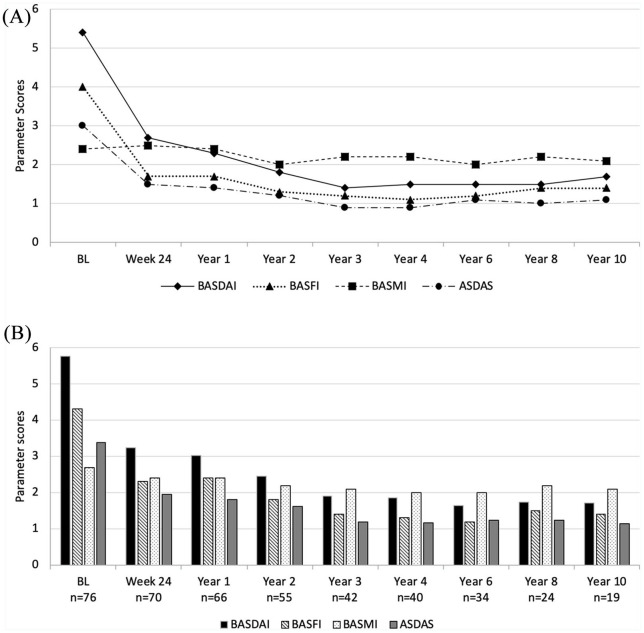
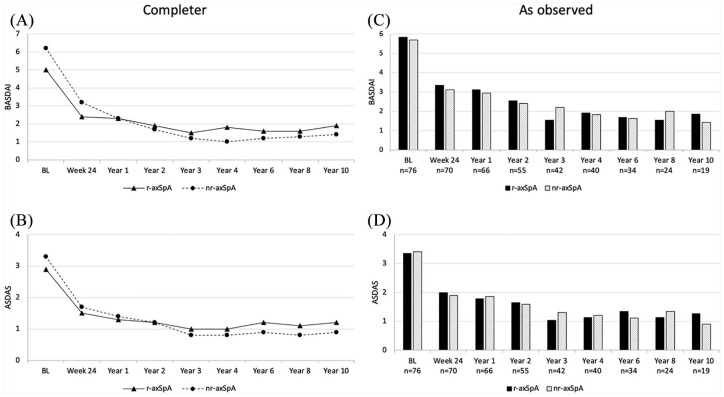
Mean BASDAI, BASFI, and ASDAS showed rapid improvement and then persisted stably low over the whole study period, whereas the mean BASMI remained nearly unchanged over 10 years. Figure 2A shows the changes in these parameters for the completer group as observed at each time-point. After 10 years, the mean BASDAI decreased from 5.4 ± 1.1 at baseline to 1.7 ± 1.6, the mean BASFI decreased from 4.0 ± 2.1 at baseline to 1.4 ± 1.6, while the mean ASDAS decreased from 3.0 ± 0.7 units at baseline to 1.1 ± 0.7 units in the completer group. A state of low disease activity as defined with a BASDAI <3 was reached in 78.9% of the patients at Year 10. Concurrently, the mean CRP level decreased rapidly and then remained stably low (from 7.2 ± 10.3 at baseline to 1.9 ± 2.2 at Year 10).
Figure 2.
Course of disease-related clinical parameters during the 10 years of the study for BASDAI (0–10), BASFI (0–10), ASDAS (0-infinity) and BASMI (0–10). (A) Completer analysis of the 19 patients who reached Year 10 of the study. (B) As-observed analysis for the remaining patients at each time point.
ASDAS, Ankylosing spondylitis Disease Activity Score; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Function Index; BASMI, Bath Ankylosing Spondylitis Metrology Index; BL, baseline.
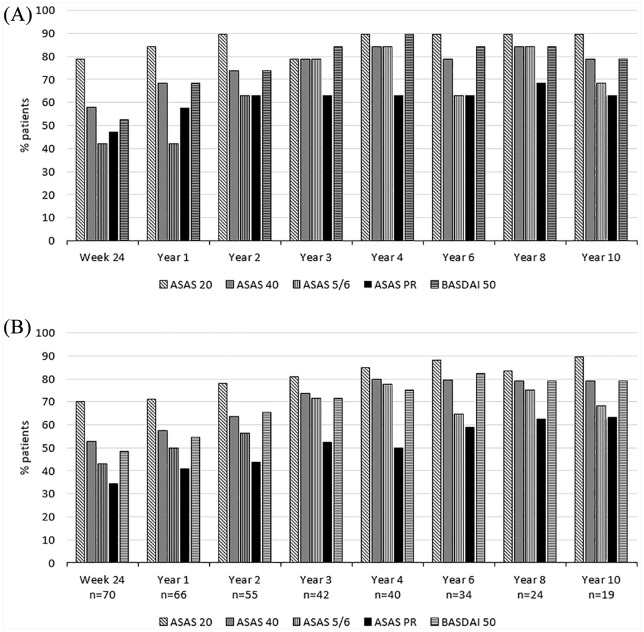
ASAS partial remission was achieved in 12 out of 19 patients (63.2%) at Year 10, which was the same as after 2 years of treatment. ASAS40 and BASDAI50 responses were achieved in 14 patients (73.7%) after 2 years and in 15 patients (78.9%) after 10 years. The response rates based on the different ASAS criteria and BASDAI50 improvement were consistent across completer and as-observed analyses (Figure 3A and B).
Figure 3.
Treatment outcome in percentages for each study time point. (A) Completer analysis of the response data for the 19 patients who reached Year 10 of the study, (B) As-observed analysis for the remaining patients at each time point. Shown are the response data for the 19 patients who reached Year 10 with different time points.
ASAS, Assessment of SpondyloArthritis International Society; ASAS20, ASAS 20% response; ASAS40, ASAS 40% response; ASAS 5/6, ASAS 5/6 response; ASAS PR, ASAS partial remission; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASDAI50, BASDAI 50% response.
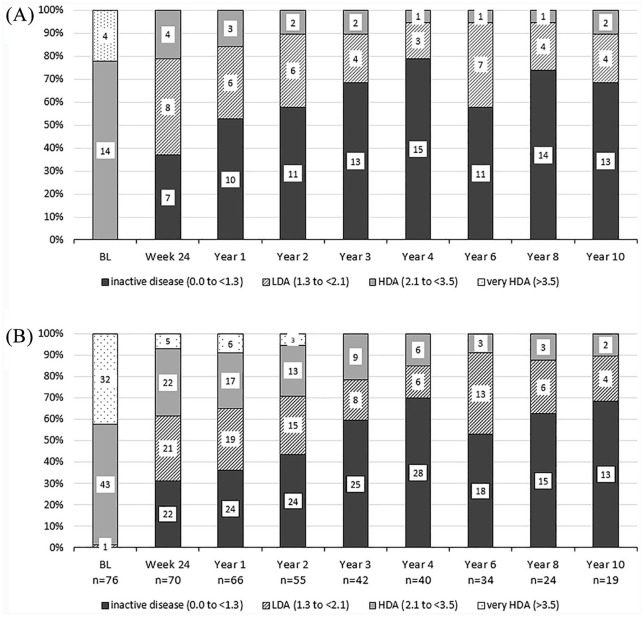
In completer analyses, overall, 11/19 (57.9 %) and 13/18 (72.2%) patients were achieved ASDAS inactive disease status by the end of Years 2 and 10, respectively (Figure 4A). Similar inactive disease status levels were reached in the ‘as-observed’ analysis for the remaining patients at each time point (Figure 4B) while NSAID intake decreased subsequently over the years until year 6 and then remained stable until the end of the study (Supplemental Figure S3) In addition, ASDAS clinically important improvement was observed by over 50% of patients both in completer and observed analyses (Supplemental Figure S1A and B). Results of the analyses using the last observation carried forward method are shown in Supplemental Figure S4.
Figure 4.
ASDAS status of the whole group of axSpA patients. (A) Completer analysis for the 19 patients who reached Year 10 of the study, (B) As-observed analysis for the remaining patients at each timepoint. Numbers in the columns show the number of patients achieving the outcome.
ASDAS, Ankylosing Spondylitis Disease Activity Score; BL, baseline; HDA, high disease activity; LDA, low disease activity.
Clinical response to etanercept in patients with r-axSpA versus nr-axSpA
When data of completers were analyzed within the subgroups of r-axSpA (n = 12) and nr-axSpA (n = 7) separately, disease activity parameters decreased by Week 24 and remained stable until the end of Year 10. Treatment responses were comparable and the highest response rates were observed in both groups between year-2 and -10 (Table 2). In the completer analyses of 19 patients, the mean BASDAI decreased from 5.0 ± 0.7 at baseline to 1.9 ± 1.9 at Year 10 in the r-axSpA group and from 6.2 ± 1.4 to 1.4 ± 1.1 in the nr-axSpA group. Similarly, the mean ASDAS decreased from 2.9 ± 0.6 to 1.2 ± 0.9 and 3.3 ± 0.7 to 0.9 ± 0.3 in the r-axSpA and nr-axSpA group, respectively (Table 2, Figure 5A and B). Comparable decreases in BASDAI and ASDAS were detected in the as-observed analysis for the remaining patients at each timepoint (Figure 5C and D). Similar to disease activity parameters, the mean BASFI levels decreased by Week 24 and remained stable during the follow-up period in patients with both r-axSpA and nr-axSpA (Supplemental Figure S2A and C). Interestingly, the patients with nr-axSpA had lower BASDAI, BASFI, and ASDAS values at Year 10 as compared with patients with r-axSpA, opposite to the baseline. Mean BASMI levels were stable throughout the study both in the completer and in the as-observed analyses (Supplemental Figure S2B and D). Besides, slightly better clinical response rates were observed in patients with nr-axSpA in comparison with patients with r-axSpA at the end of 10-year follow up. Improvements in PGA, PhGA, arthritis count, and enthesitis score were observed in both subgroups and maintained to Year 10 (Table 2).
Table 2.
Long-term efficacy of etanercept in patients with r-axSpA (n = 12) and nr-axSpA (n = 7), who completed 10 years of the ESTHER trial.
| Outcomes | Baseline | Week 24 | Year 1 | Year 2 | Year 3 | Year 4 | Year 6 | Year 8 | Year 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| BASDAI, 0–10, mean (SD) | r-axSpA | 5.0 (0.7) | 2.4 (1.6) | 2.3 (2.1) | 1.9 (1.8) | 1.5 (1.5) | 1.8 (1.7) | 1.6 (1.2) | 1.6 (1.7) | 1.9 (1.9) |
| nr-axSpA | 6.2 (1.4) | 3.2 (2.4) | 2.3 (1.8) | 1.7 (1.8) | 1.2 (1.0) | 1.0 (0.8) | 1.2 (0.8) | 1.3 (0.8) | 1.4 (1.1) | |
| BASFI, 0–10, mean (SD) | r-axSpA | 3.5 (1.8) | 1.4 (1.2) | 1.8 (1.5) | 1.5 (1.8) | 1.4 (1.5) | 1.4 (1.6) | 1.5 (1.5) | 1.6 (1.9) | 1.6 (1.9) |
| nr-axSpA | 4.7 (2.6) | 2.2 (2.0) | 1.6 (1.5) | 0.9 (0.8) | 0.8 (0.6) | 0.6 (0.5) | 0.7 (0.5) | 0.9 (0.7) | 0.9 (0.9) | |
| BASMI linear, 0–10, mean (SD) | r-axSpA | 2.3 (0.9) | 2.4 (1.2) | 2.4 (1.2) | 2.2 (1.3) | 2.4 (0.9) | 2.6 (1.1) | 2.2 (1.2) | 2.3 (1.2) | 2.2 (1.3) |
| nr-axSpA | 2.5 (0.8) | 2.7 (1.0) | 2.4 (0.9) | 1.7 (0.5) | 1.8 (0.8) | 1.6 (0.7) | 1.7 (0.7) | 2.1 (1.0) | 2.1 (0.9) | |
| ASDAS, mean (SD) | r-axSpA | 2.9 (0.6) | 1.5 (0.6) | 1.3 (0.7) | 1.2 (0.6) | 1.0 (0.7) | 1.0 (0.6) | 1.2 (0.7) | 1.1 (0.8) | 1.2 (0.9) |
| nr-axSpA | 3.3 (0.7) | 1.7 (0.7) | 1.4 (0.7) | 1.2 (0.9) | 0.8 (0.5) | 0.8 (0.5) | 0.9 (0.6) | 0.8 (0.5) | 0.9 (0.3) | |
| PtGA, 0–10, mean (SD) | r-axSpA | 5.8 (1.4) | 2.5 (2.2) | 2.1 (1.5) | 1.9 (1.4) | 1.7 (1.7) | 1.7 (1.4) | 1.9 (1.6) | 1.7 (1.5) | 1.9 (2.0) |
| nr-axSpA | 6.7 (2.6) | 2.6 (2.2) | 2 (2.1) | 1.7 (2.4) | 0.9 (1.1) | 1.1 (1.1) | 1 (0.8) | 0.9 (0.7) | 1.0 (1.0) | |
| PhyGA, 0–10, mean (SD) | r-axSpA | 5.4 (1.4) | 1.5 (1.7) | 1.9 (2.2) | 1.4 (1.0) | 0.8 (1.0) | 1.3 (1.8) | 1.2 (1.8) | 1.0 (1.2) | 1.0 (1.3) |
| nr-axSpA | 5.7 (1.7) | 2.1 (2.4) | 1.6 (1.6) | 1.4 (1.3) | 0.4 (0.8) | 0.6 (1.1) | 0.6 (0.5) | 0.6 (0.8) | 0.7 (0.8) | |
| Arthritis count, 0–64, mean (SD) | r-axSpA | 1.0 (1.9) | 0.2 (0.6) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.1 (0.3) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| nr-axSpA | 3.6 (6.9) | 0.0 (0.0) | 0.1 (0.4) | 0.0 (0.0) | 0.6 (1.5) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.1 (0.4) | |
| Enthesitis score, 0–17, mean (SD) | r-axSpA | 3.0 (3.0) | 1.4 (4.1) | 1.3 (3.2) | 0.7 (1.8) | 0.5 (1.7) | 1.1 (3.8) | 0.3 (1.2) | 0.8 (2.6) | 0.8 (1.8) |
| nr-axSpA | 2.4 (2.7) | 1.0 (2.2) | 0.6 (1.1) | 0.0 (0.0) | 0.0 (0.0) | 0.6 (1.5) | 0.3 (0.5) | 0.3 (0.5) | 0.3 (0.5) | |
| CRP, mg/L, mean (SD) | r-axSpA | 8.4 (12.5) | 1.6 (0.5) | 1.5 (0) | 2.2 (3.5) | 1.5 (2.3) | 1.2 (1.2) | 2.1 (2.7) | 2.9 (4.7) | 2.1 (2.4) |
| nr-axSpA | 5.0 (4.6) | 2.8 (3.4) | 2.4 (2.3) | 2.2 (2.6) | 2.7 (5.8) | 2.4 (5.1) | 2.4 (4.8) | 1.8 (2.7) | 1.5 (1.7) | |
| ASAS 20, % | r-axSpA | NA | 75 | 75 | 83.3 | 75 | 83.3 | 83.3 | 83.3 | 83.3 |
| nr-axSpA | NA | 85.7 | 100 | 100 | 85.7 | 100 | 100 | 100 | 100 | |
| ASAS 40, % | r-axSpA | NA | 58.3 | 66.7 | 66.7 | 75 | 83.3 | 66.7 | 83.3 | 66.7 |
| nr-axSpA | NA | 57.1 | 71.4 | 85.7 | 85.7 | 85.7 | 100 | 85.7 | 100 | |
| ASAS 5/6, % | r-axSpA | NA | 41.7 | 33.3 | 58.3 | 75 | 83.3 | 58.3 | 83.3 | 66.7 |
| nr-axSpA | NA | 42.9 | 57.1 | 71.4 | 85.7 | 85.7 | 71.4 | 85.7 | 71.4 | |
| ASAS Partial remission, % | r-axSpA | NA | 41.7 | 50 | 58.3 | 58.3 | 66.7 | 50 | 66.7 | 66.7 |
| nr-axSpA | NA | 57.1 | 71.4 | 71.4 | 71.4 | 57.1 | 85.7 | 71.4 | 57.1 | |
| BASDAI50, % | r-axSpA | NA | 58.3 | 58.3 | 66.7 | 75 | 83.3 | 75 | 75 | 66.7 |
| nr-axSpA | NA | 42.9 | 85.7 | 85.7 | 100 | 100 | 100 | 100 | 100 | |
| BASDAI <3, % | r-axSpA | 0 | 58.3 | 58.3 | 75 | 83.3 | 83.3 | 83.3 | 83.3 | 66.7 |
| nr-axSpA | 0 | 42.9 | 71.4 | 85.7 | 100 | 100 | 100 | 100 | 100 | |
| ASDAS inactive disease, % | r-axSpA | NA | 41.7 | 50.0 | 58.3 | 66.7 | 75.0 | 50 | 75 | 58.3 |
| nr-axSpA | NA | 28.6 | 57.1 | 57.1 | 71.4 | 85.7 | 71.4 | 71.4 | 85.7 | |
| ASDAS major improvement, % | r-axSpA | NA | 33.3 | 41.7 | 33.3 | 33.3 | 33.3 | 25 | 33.3 | 41.7 |
| nr-axSpA | NA | 14.3 | 14.3 | 14.3 | 28.6 | 28.6 | 14.3 | 28.6 | 42.9 |
ASAS, Assessment of SpondyloArthritis international Society; ASDAS, Ankylosing Spondylitis Disease Activity Score; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; BASMI, Bath Ankylosing Spondylitis Mobility Index; CRP, C-reactive protein; NA, not applicable; r-axSpA, radiographic axial spondyloarthritis; nr-axSpA, non-radiographic axial spondyloarthritis; PhGA, physician’s global assessment; PGA, patient’s global assessment; SD, standard deviation.
Figure 5.
BASDAI and ASDAS values in the study population in the two subgroups of patients with r-axSpA and nr-axSpA. (A and B) Completer analysis for the 19 patients who reached Year 10 of the study, (C and D) As-observed analysis for the remaining patients at each timepoint.
ASDAS, Ankylosing spondylitis Disease Activity Score; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Disease Functional Index; BASMI, Bath Ankylosing Spondylitis Disease Metrology Index; nr-axSpA, non-radiographic axial spondyloarthritis; r-axSpA, radiographic axial spondyloarthritis.
Changes in health-related quality of life over 10 years of follow up
Improvements in EQ-5D and ASQoL were observed with etanercept treatment. In the completer analysis, the mean EQ-5D increased from 0.7 ± 0.3 at baseline to 0.9 ± 0.1 at Year two and was maintained up to Year 10 in both patient groups with nr- and r-axSpA. The mean ASQoL score decreased from 7.6 ± 4.0 at baseline to 2.9 ± 3.9 at Year 10 in the completer population, and these improvements were similar in both r-axSpA and nr-axSpA groups. Similar improvements were detected in as-observed analysis for the remaining patients at each time point (data not presented).
Sustained remission under continuous etanercept therapy
In total, 38 patients (19 r-axSpA and 19 nr-axSpA) were under continuous treatment with etanercept for 12 consecutive months or more. From these 38 patients, 86% (n = 32 with 19 r-axSpA and 13 nr-axSpA) reached a status of “remission” defined as the disease category of ASDAS-ID (ASDAS <1.3), and maintained this status for 12 months or longer. The mean duration of sustained remission using this definition was 62.7 ± 37.2 months.
Dropouts and safety data
Of the initial 76 patients, 57 (75%) dropped out due to different reasons (Figure 1). The four patients who reached the end of Year two in ongoing remission dropped out according to the study protocol because of a sustained remission without a disease flare in the 2nd year of the study. The most common reasons for dropouts were loss to follow up (n = 14), lack of efficacy (n = 13), wish for pregnancy (n = 12), and non-compliance (n = 5). The other reasons for discontinuation were intolerance to sulfasalazine, lymphoma, uveitis, elevated liver enzymes, inflammatory bowel disease (IBD), sarcoidosis, demyelinating neurological disease, and recurrent minor infections, whereas uveitis and IBD were counted as “lack of efficacy”.
During the 10 years of follow up, a total of 39 serious adverse events (SAEs) in 20 patients were observed; 30 of these required hospitalization for diagnostic work-up. Among all SAEs, only seven were regarded as possibly or probably treatment-related and three resulted in a discontinuation of etanercept. No cases of opportunistic infections, tuberculosis, or solid tumors were observed.
Discussion
Long-term data on anti-TNF treatment in patients with axSpA is scarce,12–15 especially in patients with early axSpA.16,17 Here, we present the first data of a prospective trial evaluating the effect of etanercept in early axSpA over 10 years of treatment. Our data are the first to investigate such a long-term effect of anti-TNF therapy in the whole group of axSpA (including both radiographic as well as non-radiographic patients).
Given the chronic and progressive course of the disease, treatment options for patients with axSpA should aim for long-term efficacy and tolerability in addition to short-term outcomes. The importance of low disease activity levels, which could be assessed by BASDAI and ASDAS, and the clinical significance of low BASFI and BASMI levels, have been well established for the management of patients with axSpA.5,35 In our study population, mean BASDAI and ASDAS values had demonstrated significant improvement at Week 24 of the trial and continued to be stable on a low level over the 10 years of the study.11 This adds relevant information to previous data evaluating r-axSpA patients receiving infliximab for 8 years, which reported good clinical efficacy in these patients with the radiographic form of axSpA.14 More importantly, the reported improvements in our study concerning the disease activity scores were similar between patients with r-axSpA and nr-axSpA and remained at similar levels over the whole study duration, which indicated no relevant differences in the treatment response between these two groups.
Not only disease activity parameters but also function, as measured with BASFI, showed good improvement and maintained at low levels over the 10 years of the study. In addition, spinal mobility as measured with BASMI was maintained over time, with no differences between r-axSpA and nr-axSpA patients. This is worth mentioning, as it was shown in previous studies that function and mobility have deteriorated over time in r-axSpA patients not being treated with TNFi (35) and physical function and spinal mobility having a strong influence on the quality of the patient’s life.
In this analysis, clinically significant reductions in the combined response criteria, taking signs and symptoms of axSpA into account, as measured by various response parameters such as ASAS20, ASAS40, ASAS5/6, BASDAI50 response rates, and ASAS-PR, were sustained up to year 10 in those patients reaching this time point. Thereby, our results demonstrated that the efficacy formerly reported in short-term trials of etanercept could be maintained.36,37 In addition, during the follow up from Week 48 onwards, low disease activity, as defined with a BASDAI <3, was reached by the vast majority of patients, as described and found in a previous study.14 Interestingly, this was slightly more often the case in the nr-axSpA subgroup. Overall, the response rates were similar in nr-axSpA and r-axSpA patients, supporting the concept of nr-axSpA and r-axSpA both being parts of the same axSpA spectrum with similar disease burden and medical need for effective treatments.
Finally, significant improvements in patient-reported outcomes, such as EQ-5D and ASQoL, were observed with etanercept treatment in patients with r-axSpA and nr-axSpA throughout the whole study period. Notably, 86% of patients under continuous etanercept treatment for at least 12 months were in sustained remission for more than 12 months. Therefore, it could be discussed if these patients would have been candidates for de-escalation of the therapy, as it has been recently shown that spacing bDMARD application intervals might be an alternative in patients with early axSpA.38 As this was not included in our study protocol, this was not performed in the study presented here but would be of high interest in future research projects.
Long-term treatment of axSpA patients with etanercept was comparatively safe, and the safety profile was mostly favorable, as previously noted in various long-term studies with TNFi.13,14,39 Similar safety outcomes were also observed for etanercept in patients with psoriasis, psoriatic arthritis, and rheumatoid arthritis, with a follow-up of 5-, 6-, and up to 10-years, respectively.40–42
Our study has some limitations. Firstly, a relatively high number of patients dropped out over the 10 years of the study, and this may have caused a selection bias due to the inclusion of patients who showed efficacy without significant adverse events. However, we observed similar BASDAI, BASFI, and ASDAS decreases for the remaining patients at each time point in indirect comparison with patients who completed the whole study period. Secondly, disease activity, function, and spinal mobility in patients with axSpA are affected not only by TNFi treatment, but also by NSAIDs and physical therapy. Therefore, we cannot exclude whether there was an effect of these treatment modalities on these outcomes because of the incomplete information about NSAID intake and physical therapy during long-term follow up. Finally, due to the lack of a control group, efficacy outcomes were reported using observed case data without statistical comparisons.
Summarizing the 10 years of this prospective trial: one-fourth of patients who were initially included in this study remained on continuous treatment with etanercept over the 10 years of follow up. Furthermore, lack of efficacy of etanercept was documented in nearly one-fifth of patients as the reasons for study termination, and side effects were seen in approximately 7% of the patients. The most common reason for patient drop out was lack of compliance or loss to follow up, which were documented in another one-fourth of patients, while the wish to have a child was the reason of study discontinuation in one-sixth of patients, reflecting the relatively young age of the study population.
These data will give us important guidance for shared decision making with our patients in the future, knowing that one-quarter of patients will still be under therapy and are showing well-controlled disease activity after one decade, while almost one-fifth showed primary or secondary treatment failure over time and less than 10% had to stop therapy because of side-effects.
In conclusion, etanercept showed long-term clinical response and demonstrated consistent safety when administered in patients with axSpA over 10 years. Moreover, a similar clinical response shows that the presence or absence of radiographic sacroiliitis at baseline does not affect the clinical response to TNFi therapy over time, supporting the concept of axial SpA as one disease.
Supplemental Material
Supplemental material, sj-docx-1-tab-10.1177_1759720X20987700 for Sustained clinical response and safety of etanercept in patients with early axial spondyloarthritis: 10-year results of the ESTHER trial by Fabian Proft, Anja Weiß, Murat Torgutalp, Mikhail Protopopov, Valeria Rios Rodriguez, Hildrun Haibel, Olaf Behmer, Joachim Sieper and Denis Poddubnyy in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
The authors would like to thank ESTHER investigators R. Alten, M. Bohl-Bühler, G-R. Burmester, T. Klopsch, A. Krause, F. Mielke, U. Prothmann, and S. Zinke, and former study physician In-Ho Song who did essential practical work in establishing the trial, our colleagues of the radiology department K.-G. Hermann and C.E. Althoff, who contributed substantially to the study, as well as all patients who participated in the study for their contribution. We also thank J. Listing for his support in the statistical analysis and we are deeply thankful to B. Mandt, B. Buß, and P. Tietz for their support monitoring and coordinating the study over the years. The work of M.T. at Charité - Universitätsmedizin was supported by an award from the Scientific and Technological Research Council of Turkey (TUBITAK).
Footnotes
Conflict of interest statement: FP: research grants from Novartis; speaker and consulting fees from AbbVie, Amgen, Bristol-Myers Squibb, MSD, Novartis, Pfizer, Roche, and UCB Pharma.
AW: has nothing to disclose.
MT: has nothing to disclose.
MP: speaker and consulting fees from AbbVie and Novartis.
VRR: speaker and consulting fees from AbbVie and Novartis.
HH: speaker and consulting fees from Janssen, MSD, Novartis, Pfizer, and Roche.
OB: employee of Pfizer.
JS: research grants from AbbVie, Janssen, MSD, Pfizer, Roche; speaker and consulting fees from AbbVie, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, and UCB Pharma.
DP: research grants from AbbVie, MSD, Novartis, and Pfizer; speaker and/or consulting fees from AbbVie, Biocad, Bristol-Myers Squibb, Gilead, Eli Lilly and Company, GlaxoSmithKline, MSD, Novartis, Pfizer, Roche, and UCB Pharma.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by an unrestricted Grant from Pfizer who also provided study medication.
ORCID iDs: Fabian Proft  https://orcid.org/0000-0003-4306-033X
https://orcid.org/0000-0003-4306-033X
Denis Poddubnyy  https://orcid.org/0000-0002-4537-6015
https://orcid.org/0000-0002-4537-6015
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Fabian Proft, Division of Gastroenterology, Infectious Diseases and Rheumatology, Campus Benjamin Franklin, Charité - Universitätsmedizin Berlin, Hindenburgdamm 30, Berlin, 12203, Germany.
Anja Weiß, Epidemiology Unit, German Rheumatism Research Centre, Berlin, Germany.
Murat Torgutalp, Division of Gastroenterology, Infectious Diseases and Rheumatology, Campus Benjamin Franklin, Charité - Universitätsmedizin Berlin, Berlin, Germany.
Mikhail Protopopov, Division of Gastroenterology, Infectious Diseases and Rheumatology, Campus Benjamin Franklin, Charité - Universitätsmedizin Berlin, Berlin, Germany.
Valeria Rios Rodriguez, Division of Gastroenterology, Infectious Diseases and Rheumatology, Campus Benjamin Franklin, Charité - Universitätsmedizin Berlin, Berlin, Germany.
Hildrun Haibel, Division of Gastroenterology, Infectious Diseases and Rheumatology, Campus Benjamin Franklin, Charité - Universitätsmedizin Berlin, Berlin, Germany.
Olaf Behmer, Pfizer Pharma GmbH, Berlin, Germany.
Joachim Sieper, Division of Gastroenterology, Infectious Diseases and Rheumatology, Campus Benjamin Franklin, Charité - Universitätsmedizin Berlin, Berlin, Germany.
Denis Poddubnyy, Division of Gastroenterology, Infectious Diseases and Rheumatology, Campus Benjamin Franklin, Charité - Universitätsmedizin Berlin, Berlin, Germany; Epidemiology Unit, German Rheumatism Research Centre, Berlin, Germany.
References
- 1. Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet 2017; 390: 73–84. [DOI] [PubMed] [Google Scholar]
- 2. Rudwaleit M, van der Heijde D, Landewe R, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009; 68: 777–783. [DOI] [PubMed] [Google Scholar]
- 3. Boel A, Molto A, van der Heijde D, et al. Do patients with axial spondyloarthritis with radiographic sacroiliitis fulfil both the modified New York criteria and the ASAS axial spondyloarthritis criteria? Results from eight cohorts. Ann Rheum Dis 2019; 78: 1545–1549. [DOI] [PubMed] [Google Scholar]
- 4. van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984; 27: 361–368. [DOI] [PubMed] [Google Scholar]
- 5. van der Heijde D, Ramiro S, Landewe R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 2017; 76: 978–991. [DOI] [PubMed] [Google Scholar]
- 6. Ward MM, Deodhar A, Gensler LS, et al. 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2019; 71: 1599–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sieper J, van der Heijde D, Dougados M, et al. Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomised placebo-controlled trial (ABILITY-1). Ann Rheum Dis 2013; 72: 815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dougados M, van der Heijde D, Sieper J, et al. Symptomatic efficacy of etanercept and its effects on objective signs of inflammation in early nonradiographic axial spondyloarthritis: a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol 2014; 66: 2091–2102. [DOI] [PubMed] [Google Scholar]
- 9. Landewe R, Braun J, Deodhar A, et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled phase 3 study. Ann Rheum Dis 2014; 73: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sieper J, van der Heijde D, Dougados M, et al. A randomized, double-blind, placebo-controlled, sixteen-week study of subcutaneous golimumab in patients with active nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2015; 67: 2702–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Song IH, Hermann K, Haibel H, et al. Effects of etanercept versus sulfasalazine in early axial spondyloarthritis on active inflammatory lesions as detected by whole-body MRI (ESTHER): a 48-week randomised controlled trial. Ann Rheum Dis 2011; 70: 590–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van der Heijde D, Breban M, Halter D, et al. Maintenance of improvement in spinal mobility, physical function and quality of life in patients with ankylosing spondylitis after 5 years in a clinical trial of adalimumab. Rheumatology (Oxford) 2015; 54: 1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baraliakos X, Haibel H, Fritz C, et al. Long-term outcome of patients with active ankylosing spondylitis with etanercept-sustained efficacy and safety after seven years. Arthritis Res Ther 2013; 15: R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baraliakos X, Listing J, Fritz C, et al. Persistent clinical efficacy and safety of infliximab in ankylosing spondylitis after 8 years—early clinical response predicts long-term outcome. Rheumatology (Oxford, England) 2011; 50: 1690–1699. [DOI] [PubMed] [Google Scholar]
- 15. Poddubnyy D, Fedorova A, Listing J, et al. Physical function and spinal mobility remain stable despite radiographic spinal progression in patients with ankylosing spondylitis treated with TNF-alpha inhibitors for up to 10 years. J Rheumatol 2016; 43: 2142–2148. [DOI] [PubMed] [Google Scholar]
- 16. Song IH, Hermann KG, Haibel H, et al. Consistently good clinical response in patients with early axial spondyloarthritis after 3 years of continuous treatment with etanercept: longterm data of the ESTHER trial. J Rheumatol 2014; 41: 2034–2040. [DOI] [PubMed] [Google Scholar]
- 17. van der Heijde D, Dougados M, Landewe R, et al. Sustained efficacy, safety and patient-reported outcomes of certolizumab pegol in axial spondyloarthritis: 4-year outcomes from RAPID-axSpA. Rheumatology (Oxford) 2017; 56: 1498–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Song IH, Hermann KG, Haibel H, et al. Relationship between active inflammatory lesions in the spine and sacroiliac joints and new development of chronic lesions on whole-body MRI in early axial spondyloarthritis: results of the ESTHER trial at week 48. Ann Rheum Dis 2011; 70: 1257–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Song IH, Weiss A, Hermann KG, et al. Similar response rates in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis after 1 year of treatment with etanercept: results from the ESTHER trial. Ann Rheum Dis 2013; 72: 823–825. [DOI] [PubMed] [Google Scholar]
- 20. Song IH, Althoff CE, Haibel H, et al. Frequency and duration of drug-free remission after 1 year of treatment with etanercept versus sulfasalazine in early axial spondyloarthritis: 2 year data of the ESTHER trial. Ann Rheum Dis 2012; 71: 1212–1215. [DOI] [PubMed] [Google Scholar]
- 21. Rios Rodriguez V, Hermann KG, Weiss A, et al. Progression of the structural damage in the sacroiliac joints in patients with early axial spondyloarthritis during a long-term anti-TNF treatment: six-year results of the ESTHER trial. Arthritis Rheumatol 2019. [DOI] [PubMed] [Google Scholar]
- 22. Song IH, Hermann KG, Haibel H, et al. Inflammatory and fatty lesions in the spine and sacroiliac joints on whole-body MRI in early axial spondyloarthritis—3-year data of the ESTHER trial. Semin Arthritis Rheum 2016; 45: 404–410. [DOI] [PubMed] [Google Scholar]
- 23. Rios Rodriguez V, Hermann KG, Weiss A, et al. Progression of structural damage in the sacroiliac joints in patients with early axial spondyloarthritis during long-term anti-tumor necrosis factor treatment: six-year results of continuous treatment with etanercept. Arthritis Rheumatol 2019; 71: 722–728. [DOI] [PubMed] [Google Scholar]
- 24. Machado P, Landewe R, Lie E, et al. Ankylosing Spondylitis Disease Activity Score (ASDAS): defining cut-off values for disease activity states and improvement scores. Ann Rheum Dis 2011; 70: 47–53. [DOI] [PubMed] [Google Scholar]
- 25. Garrett S, Jenkinson T, Kennedy LG, et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994; 21: 2286–2291. [PubMed] [Google Scholar]
- 26. van der Heijde D, Landewé R, Feldtkeller E. Proposal of a linear definition of the Bath Ankylosing Spondylitis Metrology Index (BASMI) and comparison with the 2-step and 10-step definitions. Ann Rheum Dis 2008; 67: 489–493. [DOI] [PubMed] [Google Scholar]
- 27. Calin A, Garrett S, Whitelock H, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol 1994; 21: 2281–2285. [PubMed] [Google Scholar]
- 28. Heuft-Dorenbosch L, Spoorenberg A, van Tubergen A, et al. Assessment of enthesitis in ankylosing spondylitis. Ann Rheum Dis 2003; 62: 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hurst NP, Kind P, Ruta D, et al. Measuring health-related quality of life in rheumatoid arthritis: validity, responsiveness and reliability of EuroQol (EQ-5D). Br J Rheumatol 1997; 36: 551–559. [DOI] [PubMed] [Google Scholar]
- 30. Doward LC, Spoorenberg A, Cook SA, et al. Development of the ASQoL: a quality of life instrument specific to ankylosing spondylitis. Ann Rheum Dis 2003; 62: 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anderson JJ, Baron G, van der Heijde D, et al. Ankylosing spondylitis assessment group preliminary definition of short-term improvement in ankylosing spondylitis. Arthritis Rheum 2001; 44: 1876–1886. [DOI] [PubMed] [Google Scholar]
- 32. Brandt J, Listing J, Sieper J, et al. Development and preselection of criteria for short term improvement after anti-TNF alpha treatment in ankylosing spondylitis. Ann Rheum Dis 2004; 63: 1438–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sieper J, Rudwaleit M, Baraliakos X, et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis 2009; 68(Suppl. 2): ii1–44. [DOI] [PubMed] [Google Scholar]
- 34. Dougados M, Simon P, Braun J, et al. ASAS recommendations for collecting, analysing and reporting NSAID intake in clinical trials/epidemiological studies in axial spondyloarthritis. Ann Rheum Dis 2011; 70: 249–251. [DOI] [PubMed] [Google Scholar]
- 35. Braun J, Sieper J. What is the most important outcome parameter in ankylosing spondylitis? Rheumatology (Oxford, England) 2008; 47: 1738–1740. [DOI] [PubMed] [Google Scholar]
- 36. Rios Rodriguez V, Poddubnyy D. Etanercept for the treatment of non-radiographic axial spondyloarthritis. Expert Rev Clin Immunol 2016; 12: 493–500. [DOI] [PubMed] [Google Scholar]
- 37. Guillot X, Prati C, Sondag M, et al. Etanercept for treating axial spondyloarthritis. Expert Opin Biol Ther 2017; 17: 1173–1181. [DOI] [PubMed] [Google Scholar]
- 38. Landewe RB, van der Heijde D, Dougados M, et al. Maintenance of clinical remission in early axial spondyloarthritis following certolizumab pegol dose reduction. Ann Rheum Dis 2020; 79: 920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Heldmann F, Baraliakos X, Kiltz U, et al. Clinical experience with the European Ankylosing Spondylitis Infliximab Cohort (EASIC): long-term extension over 7 years with focus on clinical efficacy and safety. Clin Exp Rheumatol 2016; 34: 184–190. [PubMed] [Google Scholar]
- 40. Kimball AB, Pariser D, Yamauchi PS, et al. OBSERVE-5 interim analysis: an observational postmarketing safety registry of etanercept for the treatment of psoriasis. J Am Acad Dermatol 2013; 68: 756–764. [DOI] [PubMed] [Google Scholar]
- 41. de Vlam K, Boone C; A The Prove Study Group. Treatment adherence, efficacy, and safety of etanercept in patients with active psoriatic arthritis and peripheral involvement in Belgium for 66 months (PROVE study). Clin Exp Rheumatol 2015; 33: 624–631. [PubMed] [Google Scholar]
- 42. Morgan CL, Emery P, Porter D, et al. Treatment of rheumatoid arthritis with etanercept with reference to disease-modifying anti-rheumatic drugs: long-term safety and survival using prospective, observational data. Rheumatology (Oxford, England) 2014; 53: 186–194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tab-10.1177_1759720X20987700 for Sustained clinical response and safety of etanercept in patients with early axial spondyloarthritis: 10-year results of the ESTHER trial by Fabian Proft, Anja Weiß, Murat Torgutalp, Mikhail Protopopov, Valeria Rios Rodriguez, Hildrun Haibel, Olaf Behmer, Joachim Sieper and Denis Poddubnyy in Therapeutic Advances in Musculoskeletal Disease