Abstract
Background
There are numerous challenges to treating co-occurring symptoms in multiple sclerosis (MS).
Objective
To pilot the feasibility of a novel symptom management platform, CoachMS, to monitor MS symptoms (bladder function, ambulation, and mood: BAM) and respond to changes in real-time.
Methods
In this 12-week randomized controlled pilot trial, participants’ symptoms were monitored using weekly questionnaires and remote ambulatory monitoring (Fitbit Flex2®). Behavioral change principles used included shared goal setting at 2 weeks. Between weeks 2-12, the CoachMS group received targeted contact and interventions if symptoms worsened; the control group were treated through usual clinic practice. Our outcomes were feasibility (retention, adherence and acceptability; primary) and proportion of recommended treatments pursued (secondary); efficacy was explored.
Results
Of 21 participants enrolled, 13 (62%) completed the study; protocol adherence was excellent. CoachMS participants demonstrated greater follow-through with clinical recommendations than controls (OR 9.3, 95% CI (0.9, 97.6)). As a cohort, each BAM symptom tended to improve. Suicidality was detected in one control participant, resulting in urgent evaluation and hospitalization.
Conclusions
The innovative CoachMS platform was feasible and acceptable in this cohort with baseline BAM symptoms. It could represent an accessible, cost-effective tool to monitor MS symptoms in real-time; a larger trial is planned.
Keywords: Multiple sclerosis, quality of life, rehabilitation, digital health, clinical trial, symptomatic treatment
Introduction
Multiple sclerosis (MS) is characterized by a marked variability in symptoms.1 Disease modifying treatments reduce inflammatory exacerbations and slow disability progression,2 yet existing symptoms and disease progression still have a destructive impact on quality of life (QOL).1,3 There are several limitations to current symptom treatment approaches. Symptoms are frequently treated individually, however, they often exacerbate one another 4–6 (e.g. ambulatory limitations and urinary leakages can lead to reduced participation in group activities, social isolation, and exacerbated depression and anxiety).7 Semi-annual evaluations in the neurologist’s clinic may not permit as granular an assessment of often-fluctuating MS symptoms as real-time monitoring.8 Further, the time and cost burdens of the many symptom treatment types (e.g. specialized rehabilitation, safety laboratory testing, medication refills) represent additional activation barriers for patients with MS.9–11
For these reasons, we hypothesized that symptoms are best improved when tackled synergistically, in real-time, and when using principles of behavioral change. We developed CoachMS, a symptom management platform that comprises of 4 innovative features: (1) continuous, closed-loop, symptom monitoring that enables us to evaluate and proactively respond to changes as they occur (real-time) rather than waiting for semi-annual in-clinic assessments; (2) behavioral health principles promoting capability, motivation and opportunity,12,13 (3) evidence-based, comprehensive care (behavioral, rehabilitative and pharmacological) tailored to a patient’s given function, situation and preferences and (4) synergistically treating three prevalent, debilitating, frequently co-occurring14,15 and often undermanaged symptoms affecting people with MS (PwMS): Bladder,16 Ambulation17 and Mood18 (BAM). According to NARCOMS data for example, by 15 years MS duration, 52% PwMS report at least mild bladder dysfunction, 68% at least mild ambulatory impairment, and 29% at least mild depression19 – and thus these symptoms are likely to co-occur in some PwMS. BAM symptoms independently worsen QOL, and have an even greater impact in combination.20,21 Further, recommended therapeutic modalities for each of these symptoms are partially non-overlapping (e.g. antidepressants for mood but pelvic floor therapy for bladder). The burdensome multiple-modality care needs add relevance to both our comprehensive approach and the need to support patients in adopting behaviors (scheduling and attending appointments, collecting medications) likely to improve their symptoms.
Here, we evaluated the feasibility (retention, adherence and acceptability) of the novel CoachMS symptom management platform in a cohort of PwMS. We secondarily evaluated whether it might increase patients’ pursuit of evidence-based pharmacologic, behavioral and rehabilitative strategies targeted to their symptom load.
Methods
Participants
Adults (>18 years) with a diagnosis of MS22 were referred by their MS specialist neurologist at the University of California San Francisco, Multiple Sclerosis and Neuroinflammation Center if they were ambulatory and reported at least 2 BAM symptoms.
Study procedures
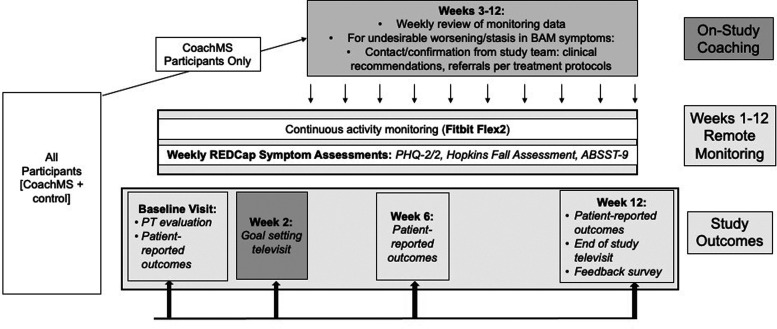
This 12-week randomized, single-blind, controlled clinical pilot study of a symptom management platform for MS (Figure 1) included PwMS if: Expanded Disability Status Scale (EDSS)23 was 1.5-6.5; Bladder Control Scale [BLCS]24 score of >2; Neurostatus Ambulation score of >1; Center for Epidemiological Studies Depression Scale [CES-D]25 score of mild depression or worse. Participants were included if they presented with at least 2 of the 3 BAM symptoms. Exclusion criteria were: no access to a smartphone/personal computer or internet connectivity; cognitive impairment severe enough to preclude participation; or an inability to understand the study protocol and/or consent autonomously.
Figure 1.
Study design.
Baseline visit and physical examination
After providing written informed consent, participants’ neurological history and medications were reviewed by the study neurologist. Then, the study physical therapist (PT) performed a standard PT evaluation (details in Table S1 and Supplementary Appendix 1). These evaluations gauged the behavioral health “COM-B” constructs, i.e. patients’ capability, motivation and opportunity to adopt recommended symptom-management behaviors (Table 1).12,13
Table 1.
Behavioral health principles supporting components of the CoachMS platform.
|
Behavioral health principles adopted |
||||
|---|---|---|---|---|
| Study schedule | Components | Construct or intervention function | Definition, examples | |
| Baseline evaluation | ||||
| Assess baseline function and needs |
• Neurological evaluation and history • Plan 2 weeks of baseline observation (see on-study monitoring for details) |
|||
| • Physical therapy evaluation: | Evaluate Capability, Motivation, Opportunity through individualized interview (Table S1/Supplementary Appendix 1; e.g., for opportunity: “Barriers to activity?” or “Recreational facility availability?”) | |||
| 2-Week check in tele-video visit | ||||
| Review function |
• Review STEPS • Review symptom scores |
|||
| Revise capability, motivation and opportunity | • Reassess capability, motivation and opportunity in light of symptom and activity monitoring | Capability | Capacity to engage in the concerned activity, e.g. hand function strong enough for self-catheterization | |
| Motivation | Processes energizing people to engage in the concerned behavior, e.g. confidence they can achieve STEPS goal with CoachMS-team advice (self-efficacy) | |||
| Opportunity | All factors outside their control that could influence engagement in the concerned behavior, e.g. availability of an accessible swimming pool nearby; friends available for weekly walk; safety of the neighborhood for walking | |||
| Personalized goal setting |
Examples • Bladder: reduce nocturia from 3 events to <1 • Ambulation: increase STEPS by 2,000 daily • Mood: reduce depression to mild category |
Modeling | Providing a STEPS goals based on active patients in that EDSS category | |
| Comprehensive care plan |
• Create action plan • Communicate recommendations with primary clinician |
Care plan derived from evidence-based approaches described in Supplementary Appendix 2, personalized for patient based on Capacity, Motivation, Opportunity. | ||
| Symptom | Behavioral Example | Education | Educate on the importance of setting attainable physical activity and symptom management goals with the intention of modulating health behaviors | |
|
• Bladder • Ambulation • Mood |
• Education on diet and reducing fluid intake late in the day • Plan to park further from stores; drive to a reservoir to walk on flat surface on weekends • Contact treating clinician for an antidepressant prescription |
|||
| Incentivization | Recommend they set the Fitbit to vibrate when average step count (STEPS) goals reached | |||
| Coercion | Discuss the negative effects of sedentarism, especially in people with MS | |||
| Environmental restructuring | Recommend that patient join a gym closer to home or reorganize their living room for safe activity e.g. make space for a wide area with a mat and sturdy furniture for support | |||
| Enablement | Communicate recommendations with primary neurologist to reduce barriers to asking for a prescription change Provide web-links to adapted, specific material to assist with exercising | |||
| On-study symptom self-monitoring (weeks 2–12) | ||||
| Patient self-reports symptoms and tracks activity |
Weekly REDCap Surveys • Bladder: Actionable Bladder Symptom Screening Tool ABSST-9 • Ambulation: Hopkins Falling Scale (falls); Continuous ambulatory monitoring (Fitbit Flex) • Mood: PHQ-4; depression and anxiety. |
Education | Patient becomes more aware of their symptoms and how they interact | |
| CoachMS group only: on-study closed-loop symptom monitoring and response (weeks 2–12) | ||||
| Study team monitors symptoms Targeted contact for symptom worsening/stasis |
Definitions of symptom worsening • ABSST-9: a first-time occurrence of a score 16, or increase 2 • PHQ-4: a first-time occurrence of score 10, or increase 4 • Hopkins Fall Scale: 1 fall Or >800 Fitbit STEPS decrease averaged over a valid week. |
Persuasion | Repeated contact by study team stimulates action | |
| Incentivization | Achieving goals will be noted by study team, who will in turn call less often | |||
| Coercion | Repeated contact until reach goals | |||
| Environmental restructuring | Continue to troubleshoot barrier posed by physical or social context | |||
| Modeling | Continue to encourage a STEPS goals based on active patients in that EDSS category | |||
| Enablement | Continue to provide feedback to the treating clinician to provide a new prescription. | |||
EDSS: Expanded Disability Status Scale; ABSST-9: Actionable Bladder Symptom Screening Tool; PHQ-4: 4-item Patient Health Questionnaire; STEPS: average daily step count.
On-study monitoring
Remote activity: Each participant was asked to don a Fitbit Flex2® activity tracker, on their non-dominant wrist, for 12 weeks. Step count was downloaded weekly from participants’ Fitbit.com account by the study coordinator, who (for the CoachMS group) visually verified that average daily step count (STEPS) was stable or increasing. A valid day was defined as greater than 128 steps/day, and a valid week was one with at least 3 days of valid data.26
Weekly patient-reported BAM symptoms: Patients were prompted weekly via email to complete a short REDCap (Research Electronic Data Capture)27survey for each BAM symptom: Actionable Bladder Symptom Screening Tool (ABSST-9),28 Hopkins Falling Scale,29 and 4-Item Patient Health Questionnaire (PHQ-4, depression and anxiety).30
Two-week goal setting televisit (shared goal setting and recommendations)
As detailed in Table 1, two weeks after study entry, all participants met with the study neurologist and PT via televideo to review the 2-week monitoring data and baseline evaluations, and jointly establish BAM symptom target goals to accomplish by the study completion (e.g. decrease nocturia from 3 events to 1). A series of personalized, evidence-based, comprehensive recommendations (Supplementary Appendix 2) was then generated (e.g. schedule a visit with pelvic floor PT, initiate an antidepressant). Ambulation goals were determined based on “normative” STEPS for the participant’s MS-related disability level:26 a 10-20% STEPS goal increase was initially proposed. These were further tailored to incorporate biomechanical or functional barriers determined from the PT evaluation (e.g. consulting a PT for orthotics, exercising on weekends when less busy) as well as personal health goals (e.g. weight loss or trying yoga). All recommendations were discussed with the patient’s referring/treating neurologist and any referrals or prescriptions were coordinated by them to ensure consistency of care. Altogether, a number of behavioral health intervention functions were introduced to promote participants’ ability to achieve their goals - 12,13 these included education,31 incentivization, training, environmental restructuring, modeling, and enablement (Table 1).
Randomization and blinding
At the conclusion of the goal setting visit, one unblinded research coordinator allocated participants to either the CoachMS or control condition using a simple 1:1 randomization scheme generated using Microsoft Excel. Participants and the other study team members (neurologist and PT) remained blinded to group assignments throughout the study, unless a participant’s weekly surveys or Fitbit data warranted intervention per CoachMS group protocol, or for suicidality (all: see below). Study outcomes included patient-reported outcomes (PROs) collected from patients, and clinical recommendations collected by a different study member (AG) who was blinded to group assignment.
Intervention/coaching between weeks 2 and 12
While participants in both study arms set shared goals (“persuasion”) and were able to self-monitor through the Fitbit and weekly symptom questionnaires (“education”, “incentivization”), between weeks 2 and 12 the CoachMS intervention further involved a “contact and treat to target” approach. This targeted clinical contact was designed to optimize motivation, incentivization and enablement12,13 towards adoption of symptomatic therapies (Table 1).
During these weeks, the study coordinator monitored the surveys and Fitbit data for the CoachMS group to identify any symptom worsening (definitions in Table 1), or symptom remaining worse than set goals. These situations prompted communication from the study coordinator with the patient within 24 hours, to evaluate reasons for changes (e.g. needing new Fitbit charger, not filling antidepressant prescription). New or worsening symptoms triggered contact from the study neurologist, who communicated changes and recommendations with the participants’ treating physician to ensure continuity of care.
In contrast, the control group received conventional, open-loop care (Figure 1) with the exception of situations of threats to self or others. Here, if any participant in either group indicated a positive response to the PHQ-9, a PHQ-9 increase by 1 standard deviation sustained over 2 weeks,30 or any verbal feedback through any contact with the study team, then the treating clinician was immediately notified, and prompt evaluation and intervention taken.
Patient-reported study outcomes
To capture more global measures of MS-related symptoms and function, at baseline, 6, and 12 weeks, participants completed a series of questionnaires through REDCap (Table S1).
End of study televisit
All study completers were interviewed at week 12 (+/– 1 week) by the study neurologist using open-ended questions assessing subjective review of BAM symptoms, as well as feedback on study procedures and impact on QOL. Additionally, participants were asked about competing time demands, such as employment and caregiving duties.
Study outcomes
Feasibility. Following published guidelines, this was assessed by (1) recruitment rates (2) participant retention rates (completion of any week 12 surveys) (3) participant-stated reasons for dropouts,32 (4) study protocol adherence rate (50% of completed weekly questionnaires and 10 weeks of valid STEPS data), and (5) study acceptability: participant qualitative feedback on study procedures and impact.33–35 We also recorded adverse events, including mood as described above.
Clinical recommendation follow-through. Completion of recommendations provided by the study team was taken as the proxy for the coaching intervention as these evidence-based treatments could eventually, based on prior studies, result in the platform’s ultimate efficacy goal, symptom improvement. This was recorded at each study visit, and medical record review after study completion was performed. Medical records were initially reviewed for actions completed during the 12-week study duration, but this timeframe was subsequently expanded to 12 months since many participants had not been able to identify, schedule, and visit recommended providers within the 3-month study duration.
Clinical recommendations were categorized as internal - those that could be completed independently by the participant (e.g. purchasing new footwear), or external - those that required a third party (e.g. seeing a PT). The rates of total completed internal and external recommendations were calculated per patient.
The amount of “competing time demands” for each group was explored, as this could affect time available to follow through on recommendations.
Preliminary efficacy. Changes in BAM symptoms between baseline and 12-week questionnaires, and in STEPS, were compared.
This study was approved by the UCSF Institutional Ethics Review Board (#16-20505) and registered at NCT03335618. All participants provided written informed consent.
Statistical analysis
The primary outcome feasibility measures were assessed quantitatively (descriptive statistics for retention and adherence rates) and qualitatively (stated reasons for drop out, and acceptability via unstructured feedback). The percentage of recommended interventions that comprised the secondary outcome were completed were compared between the two groups using odds ratio chi-square test. The exploratory changes in BAM symptoms (PROs and STEPS) over time were analyzed using either paired Student's t-test or Wilcoxon signed-rank test. Pearson’s correlation coefficient was used to assess relationships between continuous variables. The pre-post STEPS difference was calculated using the baseline week compared to week 12 (or the last week of available data for that participant); missing data for STEPS goals were estimated using median STEPS per EDSS block.36 Statistical analysis of the results and figure generation was conducted using R Version 3.6.0.
Results
Study participants
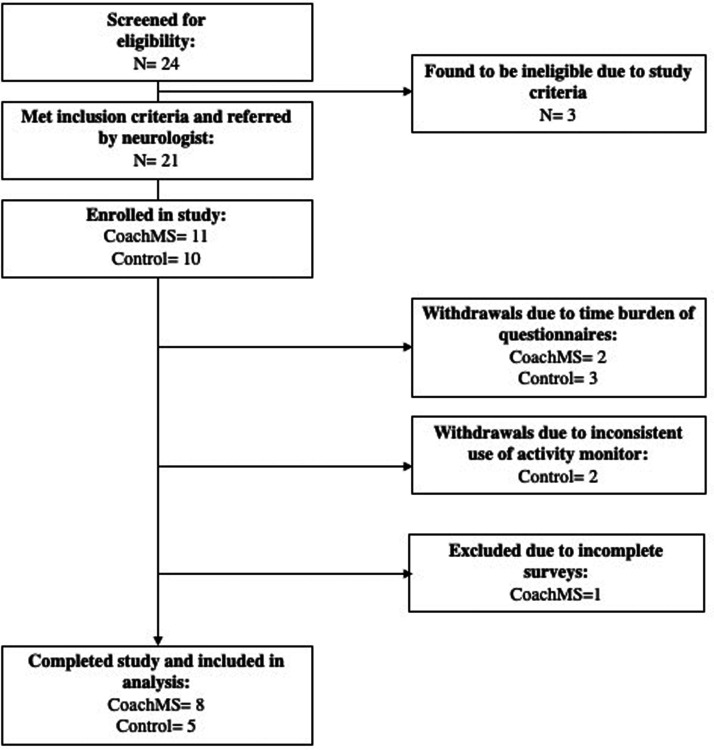
Among 24 patients interested in participating or approached by the study team, 21 patients met eligibility criteria and were enrolled; 10 were randomized to the control group and 11 to the CoachMS group (Figure 2). Given the small sample size, we compared baseline characteristics of both groups to ensure adequate randomization; there was no significant difference in age, sex, disease type, or disease duration (Table 2).
Figure 2.
CONSORT flow diagram.
Table 2.
Baseline demographic and clinical characteristics.
| CoachMS | Control | Total | p value | |||
|---|---|---|---|---|---|---|
| Demographics | Sex | Female | 11 (91.7) | 7 (70.0) | 18 (81.8) | 0.45 |
| (n, %) | Male | 1 (8.3) | 3 (30.0) | 4 (18.2) | ||
| Age Mean (SD) | 47.6 (11.5) | 47.1 (8.9) | 47.4 (10.2) | 0.91 | ||
| Race (n, %) | Asian | 0 (0.0) | 1 (11.1) | 1 (4.8) | 0.13 | |
| Black or African American | 2 (16.7) | 3 (33.3) | 5 (23.8) | |||
| Declined | 0 (0.0) | 2 (22.2) | 2 (9.5) | |||
| Other | 2 (16.7) | 0 (0.0) | 2 (9.5) | |||
| White | 8 (66.7) | 3 (33.3) | 11 (52.4) | |||
| Ethnicity (n, %) | Hispanic or Latino | 2 (16.7) | 0 (0.0) | 2 (9.5) | 0.60 | |
| Not Hispanic or Latino | 10 (83.3) | 9 (100.0) | 19 (90.5) | |||
| Disease characteristics | MS type (n, %) | PPMS | 6 (50.0) | 3 (37.5) | 9 (45.0) | 0.43 |
| RRMS | 6 (50.0) | 4 (50.0) | 10 (50.0) | |||
| SPMS | 0 (0.0) | 1 (12.5) | 1 (5.0) | |||
| Baseline EDSS Median [IQR] | 3.0 [2.5, 5.0] | 3.5 [2.0, 4.0] | 3.5 [2.5, 5.0] | 0.60 | ||
| Disease duration (years) Mean (SD) | 12.7 (8.3) | 11 (3.9) | 12.1 (7) | 0.67 | ||
| Bladder | BLCS Mean (SD) | 10.2 (5.7) | 10.4 (5.1) | 9.7 (4.7) | 0.93 | |
| Bladder (N reporting symptoms) | 10 | 7 | 17 | |||
| Ambulation | MSWS12Mean (SD) | 35.1 (9) | 27.8 (12.4) | 31.8 (11) | 0.12 | |
| T25FW (s)Mean (SD) | 5.5 (2.4) | 7.3 (5.1) | 6.4 (4) | 0.31 | ||
| TUG (s)Mean (SD) | 7.8 (2.5) | 9.2 (4.2) | 8.5 (3.4) | 0.34 | ||
| Balance | MiniBEST Mean (SD) | 24 (2.8) | 23 (5) | 23.5 (4) | 0.67 | |
| BBS Mean (SD) | 42.8 (6.4) | 45 (8.5) | 43.6 (6.7) | 0.67 | ||
| STEPS | Median [IQR] | 6005 [3,14,67,509] | 2813 [2,49,17,416] | 3698 [2,63,57,570] | 0.32 | |
| Ambulation (N reporting symptoms) | 8 | 9 | 17 | |||
| Mood | CES-DMean (SD) | 37.5 (6.8) | 41.1 (9.9) | 39.2 (8.4) | 0.32 | |
| Mood (N reporting symptoms) | 6 | 5 | 11 |
PPMS: primary progressive multiple sclerosis; RRMS: relapsing remitting multiple sclerosis; SPMS: secondary progressive multiple sclerosis; EDSS: Expanded Disability Status Scale; BLCS: Bladder Control Scale; MSWS12: Multiple Sclerosis Walking Scale; T25FW: timed 25 foot walk; TUG: timed up and go; MiniBEST: Mini Balance Evaluations Systems Test; BBS: Berg Balance Scale; STEPS: average daily step count; CES-D: Center for Epidemiological Studies Depression.
Feasibility metrics
Retention
Study retention was 62%: 8/11 participants from the CoachMS group and 5/10 participants from the control group completed a 12-week evaluation.
Adherence
On average, both groups provided over 12 weeks of valid STEPS (CoachMS group: 13.4; control group: 15.5 weeks). Out of the 12 weekly surveys, the CoachMS and control groups completed an average of 10 and 11, respectively. Both groups were sent a median of two REDCap questionnaire reminders.
Participant qualitative feedback
End of study feedback was completed by 10 participants (5 CoachMS, 5 control).
Study protocol. Participants in both groups encountered no difficulties understanding the study protocol and had no major technical difficulties.
Study discontinuation. This was primarily attributed to time burden of completing the detailed study questionnaires (weeks 6 and 12), or difficulties complying with the study protocol, such as syncing or charging the Fitbit (2 CoachMS, 1 control) or wearing it regularly.
Behavioral health strategies. Both groups reported high satisfaction with their involvement in the study and noted the motivational and competitive aspect of the Fitbit to set daily ambulation goals as beneficial. “I'm addicted to the Fitbit. I would try to push myself to beat my goals” stated one CoachMS participant. Both groups reported that the goal setting and/or coaching provided was valuable in managing and increasing activity and improving mood, and that self-reflection on symptoms was a benefit of the weekly questionnaires, reporting that it, “prompted [them] to notice subtle changes from week to week”. Motivational and emotional support from participation in the trial was cited by CoachMS participants as: “encouragement to feel my best and [to keep] myself accountable by meeting my step count every day. The biggest change is in my attitude. I genuinely feel lucky and happy.”
Adverse events. Beyond some discussions of survey burden, no participants reported emotional or physical discomfort caused by the surveys or the Fitbit, or other procedure-related adverse effects. One participant broke his leg directly after baseline assessment (unrelated to study) and completed the study after a hiatus. One control group participant reported suicidal ideation on the weekly PHQ-9; per protocol, the study team immediately notified the medical team, and the participant was admitted to an inpatient psychiatric unit, resulting in symptom stabilization and improvement. During a follow up clinic visit, she reported to the study neurologist, “your study saved my life.”
Study team access. CoachMS participants consistently noted that access to the study clinicians offered an additional source of support to facilitate increase daily activity.
Clinical recommendation follow-through
The CoachMS group had a higher completion rate for external recommendations than the control group [56% vs. 20%, OR 9.33, 95% CI (0.89, 97.62) p = 0.05]. For example, among the 9 participants who were encouraged to seek PT for ambulatory impairments, 4/6 CoachMS participants scheduled an initial evaluation in comparison to 1/3 control participants. Both groups achieved a 25% completion rate for internal recommendations. Most (59%) of these recommendations were completed by study participants within 3 months; another 23% were completed within 6 months and 18% within 12 months.
Exploratory analyses. We captured participants’ competing time demands, to understand how these might impact time to pursue recommended treatments. The CoachMS group worked between 35–50 hours a week; 4/11 participants had children (mean age 17.5, SD 11.6 years). The control group worked between 2–15 hours a week; 3/10 participants had children (mean age 22, SD 8.5 years). Larger studies may evaluate the influence of time allocation on participants’ ability to follow-through on internal recommendations.
Exploratory outcome: Changes in BAM symptoms
All BAM symptoms showed non-significant improvements over the course of the study.
Bladder. Among study completers, 7/8 CoachMS and 5/5 control group participants reported bladder symptoms. Overall, bladder PROs (BLCS, ABSST-9) slightly improved (Table 3) by study completion.
Table 3.
Patient-reported outcomes at baseline and week 12 (for n = 13 study completers).
| Outcome | Timepoint | Mean | SD | N | p value | 95% CI | |
|---|---|---|---|---|---|---|---|
| BLCS | Baseline | 9.40 | 5.27 | 5 | 0.38 | -5.02 | 10.62 |
| Week 12 | 6.60 | 3.05 | 5 | ||||
| ABSST-9 | Baseline | 17.5 | 5.66 | 8 | 0.37 | −4.13 | 10.13 |
| Week 12 | 14.5 | 4.04 | 4 | ||||
| MSWS-12 | Baseline | 34.38 | 11.06 | 8 | 0.94 | −7.46 | 7.96 |
| Week 12 | 34.13 | 13.89 | 8 | ||||
| STEPS | Baseline | 5778 | 3231 | 19 | 0.44 | 4490 | 8030 |
| Week 12 | 6263 | 3943 | 19 | ||||
| CES-D | Baseline | 39.13 | 6.31 | 8 | 0.07 | −0.87 | 15.12 |
| Week 12 | 32.00 | 12.44 | 8 | ||||
BLCS: Bladder Control Scale; ABSST-9: Actionable Bladder Symptoms Screening Tool; MSWS12: Multiple Sclerosis Walking Scale; STEPS: average daily step count; CES-D: Center for Epidemiological Studies Depression.
Ambulation. Overall, median STEPS increased from baseline 3,698 (IQR 2,635–7,570), to 4,801 (IQR 2,237–7,598) (p = 0.63, 95% CI (4.29, 7.71)); while non-significant, the absolute median difference was 1,103 STEPS, which surpasses the purported minimal clinically important difference (MCID; +/– 800 steps).37 STEPS showed a slight decrease in CoachMS participants (median –594) and increase among control participants (+1,629, above the MCID). Of individual STEPS goals established at week 2, CoachMS participants had a mean goal attainment percentage of 43%, compared with 47% in the control group (p = 0.41) (Table S2). No difference in self-reported ambulation ability (MSWS-12) was noted between baseline and week 12, or in the change between groups. Interestingly, participants who met their STEPS goals in the 3 weeks after the 2-week goal setting televisit were more likely to also maintain their goals for the remainder of the study (OR 63, 95% CI (3.3, 1194.8), p = 0.006). MSWS-12 showed a higher correlation with free-living activity [STEPS, r = –0.697, p = 0.002, 95% CI (–0.882, –0.325)] than with walking ability in a supervised clinical setting [T25FW (r = 0.523, p = 0.038, 95% CI (0.037 – 0.809)].
Mood. At baseline, 4/11 of the CoachMS group and 5/10 control group CES-D scores indicated depression (score >16), but by week 12, 0/8 CoachMS and 2/5 control group completers were above this threshold. Pooled depression scores improved between baseline and study end; mean CES-D reduction was -8.1 points (p = 0.073, 95% CI (–0.87, 15.12)), just shy of the 9-point MCID25 (Table 3).
Discussion
The CoachMS pilot platform aimed to evaluate the feasibility of a proactive, interdisciplinary, closed-loop model to decrease the burden of BAM symptoms. We secondarily sought to evaluate whether it might enhance patients’ follow-through with evidence-based clinical recommendations to treat their symptoms.
This study integrated and tested several concurrent, innovative elements, which is atypical for a study of this size and duration. As detailed, the mHealth platform (digital surveys, biosensors and televideo) was used to not only monitor but treat three prevalent, discrete symptoms and interventions in real-time, utilizing a multi-disciplinary medical and rehabilitation team. While still embedding these within the existing clinical care structure. Notably, the study team appropriately triaged a participant with suicidal ideation, speaking to the value of frequent and holistic symptom assessment in a chronic and heterogeneous disease like MS.
Study retention and participant satisfaction. Overall, 62% of participants completed the study and reported overwhelmingly positive feedback at the end-of-study televisit interview. Time burden of the detailed questionnaires (6 and 12 weeks) was cited as the primary reason for withdrawal and may have been magnified by the cohort’s low disability, high proportion of working professionals with caretaking responsibilities, and baseline depression in 9/21 participants. Similar studies26 administering surveys every 3 months observed higher participant adherence, suggesting future studies could improve adherence by tailoring survey frequency and time burden. Notably, reducing questionnaire burden should not come at the cost of missing real-time changes in BAM symptoms.
Participant goal attainment and behavioral change. Early attainment of STEPS goals after the 2-week goal-setting visit translated into a greater likelihood of meeting STEPS goals for the remainder of the study. The vibration notification when the STEPS goal was reached could also have been motivational. More precisely distinguishing between the personal and environmental factors promoting healthy behavior should be included in future studies.
Shared decision-making and follow-through. While internal recommendation follow-through was similar in both groups, the group receiving individualized coaching (CoachMS) had higher external recommendation follow-through at study completion (e.g. consulting a specialized PT for balance). Coaching was designed to facilitate engagement in multiple health behaviors with educational guidance, as PwMS prefer shared decision making (positive outlook, setting goals, and planning ahead).38,39 CoachMS participants were supported based on individual circumstances, and received up to 4 reminders followed by active engagement. This theoretically contributed to increased attainment of external recommendations. Nonetheless, anxiety and depression, or insufficient time or knowledge of available resources, could still represent barriers to care.
There was a trend for BAM symptoms to improve in both groups. Both groups received activity monitoring and goal setting, which are likely factors in facilitating behavioral change.12,13 However, increased external recommendation follow-through in the CoachMS group could reflect improved self-efficacy due to the coaching intervention. These factors are also believed to interact with physical activity goal attainment in MS,40 and hence a key mechanistic outcome in future, larger symptom management studies. A delayed start study design might be considered in the future to reduce reactivity or other confounders, and uncover a true effect of CoachMS. Further, when planning efficacy studies, it will be important to consider the timeframe for improvement. Since bladder and mood symptoms are often difficult to assess and treat, and access to rehabilitation or specialty services limited either geographically or due to insurance coverage, the time required for stabilization or improvement may be longer than 12 weeks.
In conclusion, this pilot enabled us to evaluate the feasibility of a comprehensive intervention, as well as one proximate mechanism (recommendation follow-through) by which such an intervention might improve symptoms over time. The CoachMS pilot study represents a promising foundation for moving digital health research from monitoring to intervening on symptoms in close to real time. A longer trial will enable us to assess the impact of this closed-loop, proactive, integrated symptom management digital platform on clinical improvement.
Supplemental Material
Supplemental material, sj-pdf-1-mso-10.1177_2055217321988937 for CoachMS, an innovative closed-loop, interdisciplinary platform to monitor and proactively treat MS symptoms: A pilot study by Valerie J Block, Arpita Gopal, William Rowles, Chu -Yueh, Jeffrey M Gelfand and Riley Bove in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Supplemental material, sj-pdf-2-mso-10.1177_2055217321988937 for CoachMS, an innovative closed-loop, interdisciplinary platform to monitor and proactively treat MS symptoms: A pilot study by Valerie J Block, Arpita Gopal, William Rowles, Chu -Yueh, Jeffrey M Gelfand and Riley Bove in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Supplemental material, sj-pdf-3-mso-10.1177_2055217321988937 for CoachMS, an innovative closed-loop, interdisciplinary platform to monitor and proactively treat MS symptoms: A pilot study by Valerie J Block, Arpita Gopal, William Rowles, Chu -Yueh, Jeffrey M Gelfand and Riley Bove in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Supplemental material, sj-pdf-4-mso-10.1177_2055217321988937 for CoachMS, an innovative closed-loop, interdisciplinary platform to monitor and proactively treat MS symptoms: A pilot study by Valerie J Block, Arpita Gopal, William Rowles, Chu -Yueh, Jeffrey M Gelfand and Riley Bove in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Acknowledgments
Dr. Bove is a Harry Weaver Scholar of the National Multiple Sclerosis Society.
Footnotes
Conflict of Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: VB, AG, WR, and C-YG have no relevant disclosures. JMG received research support to UCSF from Genentech. Consulting for Biogen and Alexion. RMB receives research support to UCSF from Biogen and Roche Genentech, as well as personal fees for consulting from Alexion, Biogen, EMD Serono, Genzyme Sanofi, Novartis, and Roche Genentech.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Valerie J Block https://orcid.org/0000-0001-6199-5484
Riley Bove https://orcid.org/0000-0002-2034-8800
Supplemental material: Supplemental material for this article is available online.
References
- 1.Hauser SL, Chan JR, Oksenberg JR. Multiple sclerosis: prospects and promise. Ann Neurol 2013; 74: 317–327. [DOI] [PubMed] [Google Scholar]
- 2.Giovannoni G. Disease-modifying treatments for early and advanced multiple sclerosis: a new treatment paradigm. Curr Opin Neurol 2018; 31: 233–243. [DOI] [PubMed] [Google Scholar]
- 3.Biernacki T, Sandi D, Kincses ZT, et al. Contributing factors to health-related quality of life in multiple sclerosis. Brain Behav 2019; 9: e01466-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunn H, Creanor S, Haas B, et al. Risk factors for falls in multiple sclerosis: an observational study. Mult Scler 2013; 19: 1913–1922. [DOI] [PubMed] [Google Scholar]
- 5.Block V, Cohen E, Marmarou T, et al. Bladder dysfunction is associated with cognitive symptoms and walking speed in multiple sclerosis. (10-11th Dec, 2014) In: 4th international symposium on gait and balance in multiple sclerosis: the role of cognition, Cleveland, USA.
- 6.Sung J, Shen S, Motl RW, et al. Bladder function and falls in individuals with multiple sclerosis. Disabil Rehabil 2016; 38: 2193–2197. [DOI] [PubMed] [Google Scholar]
- 7.Peterson EW, Cho CC, Finlayson ML. Fear of falling and associated activity curtailment among middle aged and older adults with multiple sclerosis. Mult Scler 2007; 13: 1168–1175. [DOI] [PubMed] [Google Scholar]
- 8.Bove R, White CC, Giovannoni G, et al. Evaluating more naturalistic outcome measures: a 1-year smartphone study in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2015; 2: e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell JD, Ghushchyan V, Brett McQueen R, et al. Burden of multiple sclerosis on direct, indirect costs and quality of life: national US estimates. Mult Scler Relat Disord 2014; 3: 227–236. [DOI] [PubMed] [Google Scholar]
- 10.Marrie RA, Horwitz R, Cutter G, et al. The burden of mental comorbidity in multiple sclerosis: frequent, underdiagnosed, and undertreated. Mult Scler 2009; 15: 385–392. [DOI] [PubMed] [Google Scholar]
- 11.Smrtka J, Brown T, Bjorklund G. Loss of mobility and the patient burden of multiple sclerosis: expert opinion on relevance to daily clinical practice. Postgrad Med 2016; 128: 145–151. [DOI] [PubMed] [Google Scholar]
- 12.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011; 6: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plow M, Finlayson M. Beyond supervised therapy: promoting behavioral changes in people with MS. Mult Scler 2019; 25: 1379–1386. [DOI] [PubMed] [Google Scholar]
- 14.Alschuler KN, Ehde DM, Jensen MP. The co-occurrence of pain and depression in adults with multiple sclerosis. Rehabil Psychol 2013; 58: 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newland PK, Flick LH, Thomas FP, et al. Identifying symptom co-occurrence in persons with multiple sclerosis. Clin Nurs Res 2014; 23: 529–543. [DOI] [PubMed] [Google Scholar]
- 16.Al Dandan HB, Coote S, McClurg D. Prevalence of lower urinary tract symptoms in people with multiple sclerosis: a systematic review and meta-analysis. Int J MS Care 2020; 22: 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bethoux F. Gait disorders in multiple sclerosis. Continuum 2013; 19: 1007–1022. [DOI] [PubMed] [Google Scholar]
- 18.Fiest KM, Walker JR, Bernstein CN, et al.; CIHR Team Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease. Systematic review and meta-analysis of interventions for depression and anxiety in persons with multiple sclerosis. Mult Scler Relat Disord 2016; 5: 12–26. [DOI] [PubMed] [Google Scholar]
- 19.Kister I, Bacon TE, Chamot E, et al. Natural history of multiple sclerosis symptoms. Int J MS Care 2013; 15: 146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motl RW. Ambulation and multiple sclerosis. Phys Med Rehabil Clin N Am 2013; 24: 325–336. [DOI] [PubMed] [Google Scholar]
- 21.Frohman TC, Castro W, Shah A, et al. Symptomatic therapy in multiple sclerosis. Ther Adv Neurol Disord 2011; 4: 83–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 2014; 83: 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 24.Hoare C, Turnbull GK, Ritvo P, et al. Bowel and bladder dysfunction in multiple-sclerosis patients. Gastroenterology 1993; 104: A11–A. [Google Scholar]
- 25.Carleton RN, Thibodeau MA, Teale MJ, et al. The center for epidemiologic studies depression scale: a review with a theoretical and empirical examination of item content and factor structure. PLoS One 2013; 8: e58067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Block VJ, Lizée A, Crabtree-Hartman E, et al. Continuous daily assessment of multiple sclerosis disability using remote step count monitoring. J Neurol 2017; 264: 316–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jongen PJ, Blok BF, Heesakkers JP, et al. Simplified scoring of the actionable 8-item screening questionnaire for neurogenic bladder overactivity in multiple sclerosis: a comparative analysis of test performance at different cut-off points. BMC Urol 2015; 15: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davalos-Bichara M, Lin FR, Carey JP, et al. Development and validation of a falls-grading scale. J Geriatr Phys Ther 2013; 36: 63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kroenke K, Wu J, Yu Z, et al. Patient health questionnaire anxiety and depression scale: initial validation in three clinical trials. Psychosom Med 2016; 78: 716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plow M, Bethoux F, McDaniel C, et al. Randomized controlled pilot study of customized pamphlets to promote physical activity and symptom self-management in women with multiple sclerosis. Clin Rehabil 2014; 28: 139–148. [DOI] [PubMed] [Google Scholar]
- 32.NIH NIoH. Pilot studies: common uses and misuses. National Center for Complementary and Integrative Health, one of the National Institutes of Health, Access date: 10-03-2020. https://www.nccih.nih.gov/grants/pilot-studies-common-uses-and-misuses
- 33.Eldridge S, Bond C, Campbell M, et al. Definition and reporting of pilot and feasibility studies. Trials 2013; 14: O18-O. [Google Scholar]
- 34.Tickle-Degnen L. Nuts and bolts of conducting feasibility studies. Am J Occup Ther 2013; 67: 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Cathain A, Hoddinott P, Lewin S, et al. Maximising the impact of qualitative research in feasibility studies for randomised controlled trials: guidance for researchers. Pilot Feasibility Stud 2015; 1: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Block VJ, Bove R, Zhao C, et al. Association of continuous assessment of step count by remote monitoring with disability progression among adults with multiple sclerosis. JAMA Netw Open 2019; 2: e190570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Motl RW, Pilutti LA, Learmonth YC, et al. Clinical importance of steps taken per day among persons with multiple sclerosis. PloS One 2013; 8: e73247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamann J, Neuner B, Kasper J, et al. Participation preferences of patients with acute and chronic conditions. Health Expect 2007; 10: 358–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plow MA, Golding MA. Qualitative study of multiple health behaviors in adults with multiple sclerosis. Int J MS Care 2016; 18: 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Motl RW, McAuley E, Doerksen S, et al. Preliminary evidence that self-efficacy predicts physical activity in multiple sclerosis. Int J Rehabil Res 2009; 32: 260–263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-mso-10.1177_2055217321988937 for CoachMS, an innovative closed-loop, interdisciplinary platform to monitor and proactively treat MS symptoms: A pilot study by Valerie J Block, Arpita Gopal, William Rowles, Chu -Yueh, Jeffrey M Gelfand and Riley Bove in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Supplemental material, sj-pdf-2-mso-10.1177_2055217321988937 for CoachMS, an innovative closed-loop, interdisciplinary platform to monitor and proactively treat MS symptoms: A pilot study by Valerie J Block, Arpita Gopal, William Rowles, Chu -Yueh, Jeffrey M Gelfand and Riley Bove in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Supplemental material, sj-pdf-3-mso-10.1177_2055217321988937 for CoachMS, an innovative closed-loop, interdisciplinary platform to monitor and proactively treat MS symptoms: A pilot study by Valerie J Block, Arpita Gopal, William Rowles, Chu -Yueh, Jeffrey M Gelfand and Riley Bove in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Supplemental material, sj-pdf-4-mso-10.1177_2055217321988937 for CoachMS, an innovative closed-loop, interdisciplinary platform to monitor and proactively treat MS symptoms: A pilot study by Valerie J Block, Arpita Gopal, William Rowles, Chu -Yueh, Jeffrey M Gelfand and Riley Bove in Multiple Sclerosis Journal – Experimental, Translational and Clinical