Abstract
Daratumumab is a humanized anti-CD38 IgG1 monoclonal antibody which could be used for multiple myeloma (MM). MM with plasma-cell leukemia (PCL) transformation is highly aggressive and is resistant to conventional therapy. Novel therapeutics are needed for PCL, and daratumumab may play role. We report a case of relapsed/refractory multiple myeloma (RRMM)-transformed PCL successfully treated with daratumumab. The case was a 42-year-old man who was diagnosed with MM 2 years ago and relapsed after six cycles of bortezomib-based chemotherapy. The patient rapidly developed hyperleukocytosis and disseminated intravascular coagulation, and was diagnosed with PCL. Daratumumab-based therapy was tried and the case miraculously obtained complete remission (CR) after four doses of a weekly infusion of daratumumab. Finally the patient received autologous hematopoietic stem-cell transplantation (auto-HSCT) and maintained CR. Moreover, we monitored the immune cell dynamics by flow cytometry (FCM) during daratumumab-based treatment. The immune cell subset analysis revealed significant down-regulation of CD38+ natural killer (NK) cells, regulatory T cells (Tregs) and regulatory B cells (Bregs). Meanwhile cytotoxic T-lymphocyte expansion was observed. In conclusion, daratumumab could rapidly decrease tumor burden, improve the condition of the PCL patient, and serve as a bridging salvage chemotherapy for further chimeric antigen recptor T cell therapy (Car-T) or HSCT, which could potentially improve patient survival. The immune cell dynamic findings in this case suggest that the immunomodulatory mechanism may contribute to the antimyeloma effect of daratumumab.
Keywords: plasma-cell leukemia, daratumumab, immunomodulatory effect
Introduction
Multiple myeloma (MM) with plasma-cell leukemia (PCL) transformation is highly aggressive, has a poor prognosis, and is difficult to treat. We report a case of relapsed/refractory multiple myeloma (RRMM)-transformed PCL successfully treated with daratumumab. Complete remission (CR) was obtained, and the patient received autologous hematopoietic stem cell transplantation (auto-HSCT).
Case presentation
A 42-year-old man was diagnosed with MM following a right humeral fracture. Postoperative pathology revealed extramedullary myeloma involvement, whereas the complete blood count, serum calcium, albumin and globulin levels and renal function test results were normal. Urine and serum immunofixation electrophoresis revealed a concentrated band in the κ light chain lane, with no monoclonal heavy chain. His κ free light chain (FLC) concentration was 1030 mg/l (normal 6.7–22.4 mg/l), and λ FLC concentration was 5.59 mg/l (normal 8.3–27.0 mg/l). The thrombin time was 52.4 s (normal 14–22 s). The β2 microglobulin level was 4.56 mg/l (normal 0.70–1.3 mg/l). Bone marrow (BM) smear revealed 75.5% plasma cells, and flow cytometry (FCM) showed 23.9% abnormal plasma cells with restrictive κ light chain expression. Chromosomal karyotyping revealed 50,XY,t(8;14) (q24;q32), +9, +11, −14, +der(14)t(8;14), +18, +mar[3]/46, XY[17]. Accordingly, the patient was diagnosed with κ light chain MM (Durie Salmon stage IIA, Internatinoal Staging System stage II) having a complex karyotype.
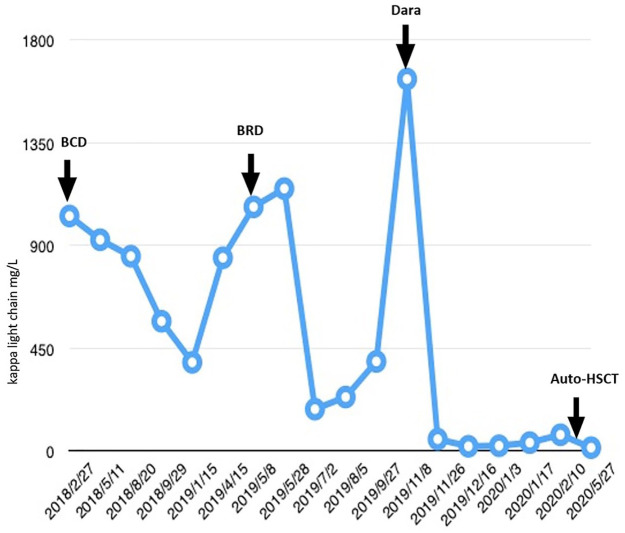
This high-risk patient was initially treated with a BCD regimen (bortezomib 2.4 mg days 1, 8, 15 and 22; cyclophosphamide 450 mg days 1, 8, 15 and 22; and dexamethasone 20 mg days 1–2, 8–9, 15–16 and 22–23). Even though his cycle intervals were prolonged due to amenability issue and infection events, partial response was achieved after four BCD cycles, which improved to very good partial response after six cycles. Before peripheral stem cell harvesting, the κ FLC concentration recovered, indicating MM progression. The patient was switched to the BRD regimen (bortezomib, dexamethasone and lenalidomide), which stabilized the disease. However, soon after, he developed petechiae and ecchymosis and was admitted to the emergency department. His hemoglobin level was 72 g/l, platelet count 31 × 109/l and white blood cell (WBC) count 122.68 × 109/l with 78.0% unclassifiable nucleated cells, the morphology of which resembled plasma cells. The thrombin time was >120 s, and κ FLC increased to 1630 mg/l. The alanine aminotransferase level was high at 534 µ/l (normal <50 µ/l) and the lactate dehydrogenase level was 1788 µ/l (normal <182 µ/l). Peripheral blood (PB) FCM confirmed the presence of numerous clonal plasma cells. It appeared that MM had progressed to PCL. He was in a critical condition with disseminated intravascular coagulation (DIC) and hepatic dysfunction as a result of high tumor burden and liver infiltration.
Considering the nature of PCL and its resistance to conventional therapy, we initiated daratumumab in combination with lenalidomide and dexamethasone (DRd). The first dose of daratumumab (16 mg/kg) was divided to 3 days in week 1 to alleviate tumor lysis syndrome. Lenalidomide (10 mg daily) was administered orally. Remarkably, PB plasma cells were reduced to only 0.2% with negative CD38 expression on FCM analysis after the first week of infusion. In week 2, 1000 mg daratumumab was infused in a single day, and his tumor burden continued reducing, with κ FLC concentration decreasing to 52.4 mg/l, β2 microglobulin level of 3.6 mg/l and no detectable clonal plasma cells in the PB using FCM. However, after week 2 of treatment, the patient developed neutropenia with intermittent fever and WBC count decreasing to 0.31 × 109/l. Lenalidomide was discontinued, and daratumumab infusion was postponed. Following antibiotic therapy and granulocyte colony-stimulating factor (G-CSF) support for 1 week, the third and fourth daratumumab (1000 mg) infusions were re-initiated. After the 4-week regimen, BM smears revealed no plasma cells. Minimal residual disease (MRD) was negative on BM FCM, and the serum FLC ratio returned to normal (Figure 1). Considering the continued CR, absence of MRD and the aggressive nature of his disease, we proposed allogenic HSCT. However, no human leukocyte antigen (HLA)-matching donor was available after checking his siblings and the Chinese Bone Marrow Donor Program. Therefore, stem cells were collected after the first course of DRd therapy using the hematopoietic stem cell mobilizing agents G-CSF and plerixafor. Auto-HSCT was performed after another two cycles of DRd. He received auto-HSCT on 2 April 2020, with melphalan 200 mg/m2 used as preconditioning. The transplant was successful, and at 3 months after transplantation, the patient remained in CR. Lenalidomide was used as the maintenance therapy after transplantation.
Figure 1.
Free light chain concentration of the patient in different treatment stages.
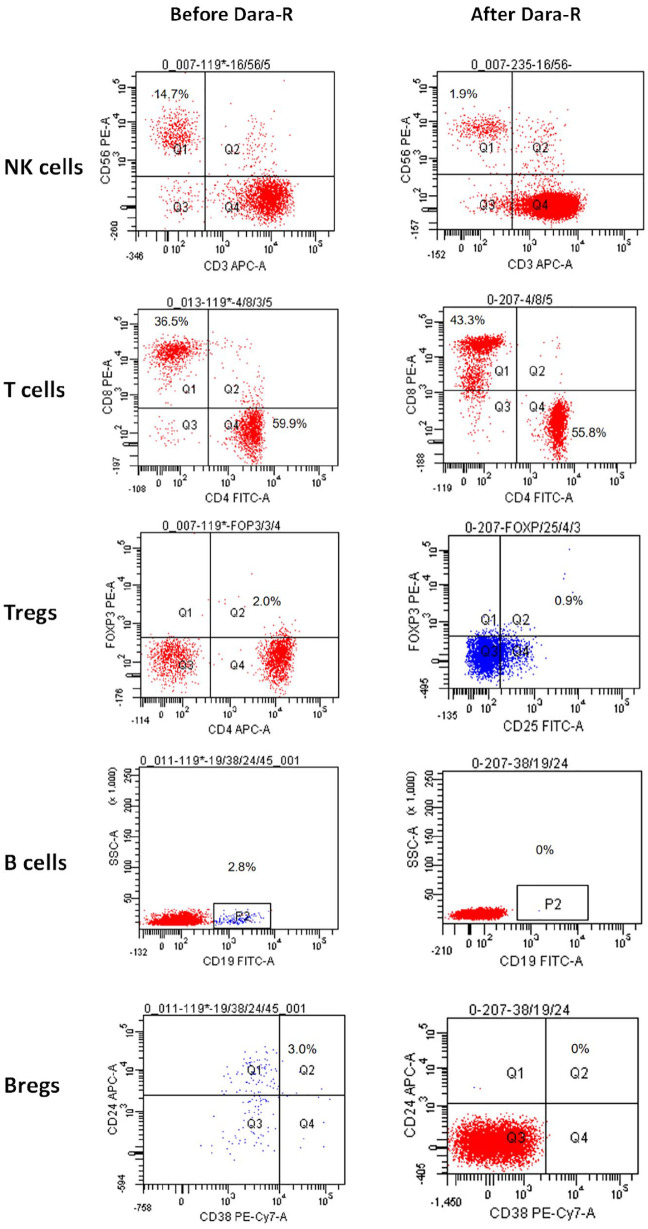
We used FCM to monitor immune cell dynamics weekly during DRd therapy after the first dose of daratumumab. Besides the rapid clearance of plasma cells, both natural killer (NK) cells, B cells and CD38+ regulatory T cells (Tregs) were significantly downregulated, and the proportion of T cells quickly increased, with a higher proportion of CD8+ than CD4+ T cells (Figure 2). NK cell subgroup analysis revealed that CD38high NK cells showed a more significant decrease compared with CD38low NK cells. CD38high NK cells decreased from 13.7% to 1.2%; CD38low NK cells showed no significant decrease (2.7% before treatment, 0.8% after).
Figure 2.
Immune cell dynamics using flow cytometry before and after daratumumab-based therapy.
Rapid downregulation of NK cells, B cells, Bregs, and Tregs can be noted after 4 weeks of daratumumab-based therapy. Meanwhile, the proportion of T cells is increased, with a higher increase of CD8+ compared with CD4+ T cells.
Bregs, regulatory B cells; Tregs, regulatory T cells; NK, natural killer.
Discussion
This high-risk patient with MM showed rapid disease progression to PCL during proteasome inhibitor and immunomodulator-based treatment and was salvaged by daratumumab-based novel therapy. Daratumumab is a humanized anti-CD38 IgG1 monoclonal antibody (mAb), the safety and efficacy of which for MM treatment is well documented. Daratumumab may be effective in approximately 20% of patients with RRMM. However, documentation on the use of daratumumab in primary PCL is scarce.1,2 To the best of our knowledge, our case is the first report of daratumumab treatment of RRMM-transformed PCL resulting in CR and followed by auto-HSCT.
Daratumumab was well tolerated in this patient whose condition was critical when the disease progressed to PCL with DIC. Abnormal coagulation function improved after only 1 week of daratumumab treatment. Deep remission was achieved only after the first DRd cycle, as shown by the normalized FLC ratio and undetectable clonal plasma cells in the BM. No tumor lysis syndrome was observed, indicating that daratumumab has a rapid action and appears suitable for patients with heavy tumor burden. The risk of BM suppression should be considered, particularly when lenalidomide is added.
The immune cell subset analysis in our study revealed significant CD38+ NK cell, Treg, and Breg downregulation along with augmented CTL activity, which is consistent with the outcome of GEN501 and SIRIUS studies.3 In our previous study, we found that regulatory B cells (Bregs) in MM can abrogate NK cell-mediated antibody-dependent cell-mediated cytotoxicity (ADCC) against MM cells. Therefore, decreasing Bregs may enhance the ADCC activity and cytotoxicity against MM triggered by anti-SLAMF7, anti-CD38 and mAbs against other MM-specific antigens.4 Although a recent study using immune profiling has shown that daratumumab may further reduce CD38+ NK cells as well as cause CD38 downregulation in target cells, a subgroup analysis of NK cells in the same study revealed that CD38high NK cells showed a more significant decrease compared with CD38low NK cells.5 Another study further indicated that the remaining CD38low PB NK cells could survive the daratumumab fratricide and retain their anti-MM potential.6 CD38 is expressed at higher levels on Tregs than on conventional T cells (Tcons) in patients with MM. Therefore, anti-CD38 mAb results in the preferential depletion of Tregs, relieving Tcons from Treg suppression, which elicits CD8+ T cell and NK cell-induced MM cell killing. Moreover, these immune effects induced by anti-CD38 mAbs were reportedly further enhanced by immunomodulatory drugs (IMiDs), which favor the combination use of anti-CD38 mAb with IMiDs in patients with RRMM.3 Moreover, it has been proposed that anti-CD38 targeting induces the activation and degranulation of NK cell and leads to an increased expression of CD80/CD86 T cell costimulatory molecules on monocytes, which induces monocyte polarization and activation and stimulates T cell expansion via CD28 binding.7
In some phase I and II clinical trials on MM, NK cell decline following daratumumab exposure did not show significant impact on the efficacy and toxicity outcomes. However, immune cell dynamics other than NK cell fratricide could be employed as a potential efficacy predictor for anti-CD38 mAb therapy, among which Tregs, Bregs and CTLs may be promising candidates. Nonetheless, these immune markers should be validated in clinical cohorts.
In conclusion, this case suggests that daratumumab can be used in RRMM-transformed PCL by rapidly decreasing tumor burden and stabilizing patient condition. Daratumumab may serve as an ideal bridging salvage chemotherapy for further treatment, such as chimeric antigen recptor T cell therapy (Car-T) or HSCT.
Footnotes
Author contributions: Yu Wu initiated this work and is responsible for the final approval of the version to be published. Chen-lu Yang and Kai Shen are responsible for clinical data collection, analysis, and interpretation and manuscript drafting. Neng-gang Jiang and Li Zhang have contributed substantially to the FCM analysis.
Conflict of interest: The author(s) declare that there is no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Innovation Supporting Program of Sichuan Province, China (2018RZ0137) and the Innovation Development Program of Chengdu (2019-YF05-01242-SN).
Informed consent and ethics: Written informed consent was obtained from the patient, and the study was approved by the West China Hospital of Sichuan University Biomedical Research Ethics Committee (#2019-114).
ORCID iD: Yu Wu  https://orcid.org/0000-0001-8708-9711
https://orcid.org/0000-0001-8708-9711
Contributor Information
Chen-lu Yang, Department of Hematology and Hematology Research Laboratory, West China Hospital, Sichuan University, Chengdu, China.
Neng-gang Jiang, Department of Laboratory Medicine, West China Hospital, Sichuan University Chengdu, China.
Li Zhang, Department of Hematology and Hematology Research Laboratory, West China Hospital, Sichuan University, Chengdu, China.
Kai Shen, Department of Hematology and Hematology Research Laboratory, West China Hospital, Sichuan University, Chengdu, China.
Yu Wu, Department of Hematology and Hematology Research Laboratory, West China Hospital, Sichuan University, Guoxue Alley 37, Chengdu 610041, China.
References
- 1. Nalghranyan S, Singh AP, Schinke C. The combination of venetoclax, daratumumab and dexamethasone for the treatment of refractory primary plasma cell leukemia. Am J Hematol 2020; 95: E34–E35. [DOI] [PubMed] [Google Scholar]
- 2. Horisawa Y, Kondo T, Hishizawa M, et al. A case of allogeneic hematopoietic stem cell transplantation for primary plasma cell leukemia after treatment with daratumumab. Ann Hematol 2020; 99: 2699–2701. [DOI] [PubMed] [Google Scholar]
- 3. Zambello R, Barilà G, Manni S, et al. NK cells and CD38: implication for (immuno)therapy in plasma cell dyscrasias. Cells 2020; 9: 768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang L, Tai YT, Ho M, et al. Regulatory B cell–myeloma cell interaction confers immunosuppression and promotes their survival in the bone marrow milieu. Blood Cancer J 2017; 7: e547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Casneuf T, Adams HC, III, van de Donk NWCJ, et al. Deep immune profiling of patients treated with lenalidomide and dexamethasone with or without daratumumab. Leukemia. Epub ahead of print 26 May 2020. DOI: 10.1038/s41375-020-0855-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sarkar S, Chauhan SKS, Daly J, et al. The CD38low natural killer cell line KHYG1 transiently expressing CD16F158V in combination with daratumumab targets multiple myeloma cells with minimal effector NK cell fratricide. Cancer Immunol Immunother 2020; 69: 421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Viola D, Dona A, Caserta E, et al. Daratumumab induces mechanisms of immune activation through CD38+ NK cell targeting. Leukemia. Epub ahead of print 16 April 2020. DOI: 10.1038/s41375-020-0810-4 [DOI] [PMC free article] [PubMed] [Google Scholar]