Abstract
Susac syndrome (SuS) is a rare autoimmune endotheliopathy leading to hearing loss, branch retinal artery occlusions and encephalopathy. Young females are more frequently affected than males, making counselling for family planning an important issue. We reviewed published cases on SuS during pregnancy or in the postpartum period, and selected 27 reports describing the details of 33 patients with SuS. Treatment options and implications for pregnancy and breastfeeding are discussed. We propose new areas for research and suggest a management strategy.
Keywords: pregnancy, Susac syndrome
Introduction
Susac syndrome (SuS) is named after John Susac, who was the first to describe the syndrome of encephalopathy, hearing loss and branch retinal artery occlusions (BRAO).1,2 It is a rare disease, with just over 500 cases described worldwide.3 Diagnostic criteria were proposed by the European Susac Consortium in 2016.4 The pathophysiology of this neuroinflammatory disease, which affects the endothelial cells of microvessels in the brain, cochlea and retina, remains poorly understood. Activated cytotoxic CD8+ T-cells contribute to inflammatory damage of the endothelium. Anti-endothelial cell antibodies are present in 25% of patients, but their role in SuS pathogenesis is not clear.5–7 Treatment is based on expert opinion and case-series as clinical trials are non-existent in this rare disease. A practical treatment guideline for SuS based on a single expert opinion has been proposed recently, offering different therapeutic regimens for milder to more severe forms of the disease.8 Less aggressive treatment recommendations have been made by others.9
SuS affects young women more frequently than men, with a female:male ratio of 3.5:1.10 It is not surprising that, in the age category affected, family planning is often not completed, making counselling necessary. Moreover, SuS can present for the first time, or relapse after a period of disease remission, during pregnancy or in the postpartum period.10
In this article, we review published cases of SuS during pregnancy and the postpartum period, discuss issues in family planning in SuS patients, suggest areas for further research and propose a management strategy.
Review of published cases
We searched the literature (Pubmed) and internet (Google) for published case reports, case series and review articles for descriptions of SuS patients during pregnancy, postpartum or after termination of pregnancy (search terms: Susac, pregnancy, postpartum; search until August 2020) and selected 27 reports describing a total of 33 SuS patients.11–37 All cases are listed in Table 1. The mean age at pregnancy was 28.6 years. In 21 patients, the disease was diagnosed during pregnancy (five in the first trimester, seven in the second trimester, eight in the third trimester and one not reported) and in eight patients there were relapses during pregnancy or in the postpartum period. In two patients, the first symptoms of SuS presented shortly after abortion (one spontaneous, one induced). Only two patients with SuS completed a pregnancy without relapses. In one patient, pregnancy was discovered when she was treated with cyclophosphamide (CYC) and the pregnancy was terminated for this reason. Pregnancy was terminated in six cases to allow treatment with potential foetotoxic drugs like CYC. Delivery of a healthy baby (at term or preterm) was described in 22 cases. One stillbirth and one spontaneous abortion were reported. Notably, six patients had one or more pregnancies, without symptoms of SuS, before the index pregnancy when SuS was diagnosed. Treatment during pregnancy consisted most frequently of steroids, anticoagulant or antiplatelet therapy, with add-on intravenous immunoglobulins (IVIG) in six cases and plasma exchange (PLEX) in two cases. One patient started CYC at 28 weeks gestational age due to ongoing relapses and she delivered a healthy baby.30 Most patients improved on therapy, but residual cognitive, visual and/or hearing impairments were present in most patients. Complete recovery was rare. A few patients had a history of symptoms, compatible with SuS.
Table 1.
Case reports, case series and review articles for descriptions of SuS patients during pregnancy, postpartum or after termination of pregnancy.
| Case | SuS diagnosis before pregnancy | Age at diagnosis of SuS | Age at pregnancy | Gestational age or postpartum | Presenting symptoms | Audiometry | Ophtalmology | MRI | CSF | Treatment | Outcome | Pregnancy outcome | Reference | Prior symptoms and/or pregnancies |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | N | 22 | 21 | postpartum | headache and hearing impairment, confusion, personality change, unsteady gait | bilateral hearing loss | Fundoscopy: cotton-wool spots FA: multiple BRAO bilateral | increased protein (187 mg/dl) | steroids | remarkable improvement | stillbirth at term (anencephalic child) | Coppeto 1984 | delivery of a healthy child 6 years prior; first symptoms started the month before pregancy, with personality change and high protein levels in CSF | |
| 2 | N | 31 | 31 | first trimester | numbness in the extremities, segmental visual loss, diplopia, lethargy, memory loss, change in personality, dysarthria, gait unsteadiness, tinnitus, and hearing loss | bilateral low frequency SNHL, left more than right | Fundoscopy: retinal arteriolar occlusions | 0 wbc/µl increased protein (252 mg/dl) OCB absent | no treatment | speech, memory, gait, personality improved | healthy baby | MacFadyen 1987 | 3 prior pregnancies | |
| 2 | postpartum | sudden deterioration left hearing, fatigue, dysarthria, incoordination in writing and gait, memory problems | Fundoscopy: small intraretinal hemorrhages, adjacent artery narrowing, perivascular sheathing and artery narrowing | 0 wbc/µl increased protein (116mg/dl) | steroids | improvement speech, dizziness and memory, further visual loss | N/A | MacFadyen 1987 | ||||||
| 3 | N | 28 | 28 | 28 | sudden, painless visual loss right eye, periodic imbalance; deterioration over the next month with severe encephalopathy | mild bilateral SNHL | Fundoscopy: retinal arteriolar occlusion, cotton-wool spot | multifocal T2 hyperintensitiesintensity in the deep white matter, anterior corpus callosum, and brain stem | 2 wbc/µl increased protein (207 mg/dl) OCB absent | heparin, warfarin followed by aspirin | gradual improvement after delivery in mental status and walking, persistent visual field deficits | pre-term (33 weeks gestation) healthy boy | Gordon 1991 | no prior symptoms or pregnancies |
| 4 | N | 36 | 36 | immediately postpartum | visual loss, tetrapyramidal signs, confusion | right-sided hearing loss | Fundoscopy: normal | T2 hyperintensities in the supratentorial white matter and basal ganglia | 6 wbc/µl increased protein (264 mg/dl) | during pregnancy: aspirin monotherapy until 26 weeks GA, thereafter low molecular weight heparin; after delivery oral anticoagulants | psychological sequelae and hearing loss despite treatment with hyperbaric oxygen | induction of delivery with prostaglandin gel a terme; urgent caesar section, delivery of healthy girl | Cador-Rousseau 2002 | 3 previous pregancies, of which 1 voluntary abortion, 1 spontaneous abortion and 1 at term pregnancy prior symptoms: 6 years prior sudden left, hearing loss, 2 years prior thrombosis of a branch of the right central retinal artery, 3 months prior to pregnancy thrombosis of a branch of the left central retinal artery |
| 5 | Y | 35 | 35 | pregnancy discovered during cyclophosphamide treatment (before 10 weeks GA) | steroids, anticoagulation,cyclophosphamide,aspirin | therapeutic abortion | Aubart-Cohen 2007 | |||||||
| 6 | N | 25 | 25 | behavioral disturbances | new retinal occlusions | steroids, anticoagulation | therapeutic abortion | Aubart-Cohen 2007 | ||||||
| 6 | Y | 25 | 29 | no relapse during pregnancy | aspirin | healthy baby at term | Aubart-Cohen 2007 | |||||||
| 7 | Y | 30 | 33 | postpartum | confusion, vertigo, and hearing loss | aspirin | healthy baby at term | Aubart-Cohen 2007 | ||||||
| 8 | N | 28 | 28 | 37 | confusion, forgetfulness, hypersomnolence, headaches, hearing difficulties, and episodic visual loss | low frequency SNHL | Fundoscopy: bilateral BRAO with retinal infarcts.FA: bilateral retinal infarcts, BRAO, and arteriolar hyperfluorescence | multiple T2 hyperintensities in the cerebellum and cerebral white matter, including corpus callosum. Many lesions were hypointense on T1-weighted imaging and some demonstrated restricted diffusion | 3 wbc/µl increased protein (121 mg/dl) | aspirin, steroids (IV pulse and oral taper), IVIg, mycophenolate mofetil | Seven months postpartum: short-term memory problems,right eye visual problems, hearing loss in left ear, easy fatiguability | healthy baby girl | Grinspan 2009 | 4 previous pregnancies: 2 healthy children, 1 abruption at 23 weeks, and 1 electiveabortion |
| 9 | N | 23 | 23 | 10 days after voluntary abortion | retro-bulbar headache, photophobia, vomiting andlethargy | bilateral low frequency SNHL | Fundoscopy and FAA: sporadic segmental retinalarterial occlusions in both eyes | multiple punctate foci of restricted diffusion and T2 hyperintensities in the deep white matter of both frontal lobes, a larger lesion in the splenium of the right corpus callosum | 8 wbc/µl increased protein 183 mg/dl | steroids (IV pulse and oral taper), single dose of infliximab, IVIg, cyclophosphamide, aspirin, nifedipine; later cyclophosphamide stopped and switch to azathioprin | After twelve months: tinnitus and hearing loss persisted, cognition continued to improve, ongoing deficits in spontaneous recall, working memory and verbal fluency | voluntary termination of pregnancy at GA of 7 weeks | Hardy 2011 | |
| 10 | N | 25 | 25 | 20 | confusion, difficulty walking, and vision and hearing loss, intermittent headaches | left-sided SNHL | Fundoscopy: left-sided BRAO and cotton wool spots FA not done | T2 hyperintensities in the deep and periventricular white matter, corpuscallosum, pons, and cerebellar peduncles; a 2-3 mm hypointense ‘hole’ in the midportion of the corpus callosum | 16 wbc/µl increased protein (63 mg/dL) | steroids (IV pulse and oral taper), IVIg, aspirin | Improvement of mental status, gait, hearing and visual loss persisted | Deane 2011 | ||
| 10 | 33 | abrupt confusion and worsening gait, new bilateralhearing loss, and new right vision changes | Fundoscopy: retinal ischemia on the right | several new lesions | repeat IV steroids, IVIg, ;postpartum cyclophosphamide and rituximab added; after 3 doses of cyclophosphamide oral azathioprin | Significant improvement; development of livedo reticularis | induction of premature delivery at 35 weeks gestation; delivery of healthy baby girl | Deane 2011 | ||||||
| 11 | N | 35 | 35 | 31 | bilateral visual loss and right hearing loss, cognitive symptoms | SNHL right ear, left side normal | Fundoscopy: bilateral narrowing of arterioles punctiform hemorrhage FA: leakage in multiple arterioles in both eyes | supra- and infra-tentorial T2 hyperintensities in white matter | lymphocytic pleiocytosis increased protein OCB absent | steroid pulse; repeated ; plasma exchange; postpartum cyclophosphamide, followed by mycophenolate; due to ongoing disease activity changed to methotrexate and etanercept | Bilateral hearing loss, visual field defects; ongoing disease activity (retinal vasculitis) after pregnancy for 5 years | caesarean section, premature delivery of health baby boy | Finis 2011 | |
| 12 | N | 30 | 30 | 3 weeks postpartum | headache, hearing loss, attention deficit, personality and mental changes, impaired cognition and memory | SNHL, low and middle tones, right more than left side | Fundoscopy and FA: bilateral BRAO with retinal ischemia, arteriolar shunts, and small vascular dilatations | small T2 hyperintensities, atrophic corpus callosum | normal OCB absent | steroids (IV pulse and oral taper), aspirin, nimodipine | no recurrence after 6 months and 1 year; no improvement in hearing | normal baby | Karelle 2012 | Three episodes of aseptic meningitis (age of 5, 14, and 27 years). Between these events,the patient suffered from migraine-like headache and atypical polyarthritis. |
| 13 | N | 34 | 34 | third trimester | Headaches,numbness andtingling ofhands and face,visual fielddefect, hearingloss, tinnitus | T2 hyperintensities and corpus callosum involvement | 2 wbc/µl increased protein (101 mg/dl) | steroids (oral) , aspirin, plasmapheresis | postpartum symptoms stabilised, recurrence after steroid taper | not reported | Mateen 2012 | |||
| 14 | N | 32 | 32 | postpartum | Vertigo, diplopia,visual loss,tingling ofhands and feet,and amnesticepisodes | Low- to mid-frequencySNHL | T2 hyperintensities and corpus callosum involvement , gadolinium enhancement | 11 wbc/µl increased protein (161 mg/dl) OCB absent | steroids (IV and oral), aspirin, plasmapheresis | improved, later episodes of visual and hearing loss | Mateen 2012 | |||
| 15 | N | 32 | 32 | 32 | change in personality, unsteadiness of gait, slurred speech, evolving to severe disorientation and confusion | multiple small T2 hyperintensities in both supra- and infratentorial locations, some of which exhibited diffusion restriction, several of which in corpus callosum | 13 wbc/µl increased protein (180 mg/dl) OCB absent | steroids (IV and oral), IVIG and mycophenolate and methotrexate | 1 year after the diagnosis the patient was well with markedly improved gait and cognition | emergency caesarean section | Engeholm 2013 | |||
| 16 | N | 28 | 28 | 9 | lower limb weakness, drowsiness and dysarthria | T2 hyperintensities with an unusual pattern of meningeal enhancement after Gadolinium administration; serialMRI showed progressive lesions in the deep white matter, including the basal ganglia and cerebellar peduncles withenhancing lesion in the corpus callosum that progressed to volume loss | 9 wbc/µl increased protein (200 mg/dl) OCB absent | steroids (IV pulse and oral taper), plasma exchange, IVIg | cognitive deficits persisted, hearing and vision remain impaired | at 13 weeks GA 1 viable fetus; therapeutic abortion at 15 weeks GA | Ioannides 2013 | |||
| 17 | N | 21 | 21 | 35 | walking impairment and evolving hearing loss, lack of concentration and disorientation | bilateral moderate low frequency SNHL in the low frequency range, leftmore than right side | FA: normal | multiple small T2 hyperintensitiesin the corpus callosum, periventricular white matter, centrum semiovale, posterior arm of the left internal capsule, pons and cerebral peduncles; some lesions demonstrated restricted diffusion on DWI, as well as hypointensity on T1-weighted imaging | 4 wbc/µl increased protein (109 mg/dl) | low-molecular-weight heparin, IVIg; after delivery start of oral azathioprine and warfarin | After two months: hearing loss persisted, discrete activity on FAAwithout functional visual impairment; no new symptoms; MRI showed new lesions | induction of labour at 37 weeks | Antulov 2014 | |
| 18 | N | 35 | 35 | 37 | hearing loss and tinnitus in the left ear, attacks of vertigo and slight difficulty in finding words | mild hearing loss in theleft ear | normal OCB absent | postpartum: aspirin, steroids (IV and oral taper), cyclophosphamide | BRAO in the right eye 2.5 months after having given birth | Feresiadou 2014 | At the age of 12: encephalopathy, sudden deafness of the right ear and visual field defects in the left eye at the age of 12, followed by permanent hearing and visual defects. Second pregnancy. | |||
| 19 | N | 25 | 25 | 14 | acute onset of right leg shooting pain, followed by complaints of vertigo, blurry vision, headache and gait instability; severe encephalopathy | multiple T2 hyperintensities in the bilatera lwhite matter, deep gray matter,corpus callosum and posterior fossa with corresponding restricted diffusion and T1 hypointensities for the observed corpus callosum lesions | 6 wbc/µl increased protein ( 95 mg/dl)OCB absent | steroids (IV pulse) repeated approx.2 weeks later (oral) when symptoms reoccurred | One month postpartum: hearing difficulty (right sensorineural hearing loss), two months later cognitive difficulties. 1,5 years after initial presentation residual cognitive deficits consisting of visual spatial deficits and difficulty with word recall and vocabulary | healthy baby | Hua 2014 | |||
| 20 | N | 25 | 11 | confusion, short term memory loss, headache and uncoordinated gait | multiple periventricular and deep white matter T2 hyperintense lesions in a perpendicular distribution to the ventricles | steroids (pulse) | healthy baby | Tashman 2014 | ||||||
| 20 | 24 | repeated symptoms | steroids (pulse) | Tashman 2014 | ||||||||||
| 20 | 3 months postpartum | confusion, headache, and lethargy | bilateral low frequency hearing loss, rising to normal at higher frequencies | bilateral BRAOs with retinal infarcts | small, multifocal T2 hyperintensities in the white matter involving the corpus callosum | increased protein | steroids (pulse), mycophenylate | Tashman 2014 | ||||||
| 21 | N | 18 | 24 | visual loss right eye, followed by severe headache | normal | No FA, central retinal artery occlusion | small T2 hyperintensities | steroids (pulse and oral taper), LMWH | symptom free in 4 days, except vision right eye; recurrence of disease activity 1 year after starting estrogen replacement therapy at the age of 50 years (Petty 2001) | Khan 2014 | ||||
| 22 | Y | 37 | 6 weeks postpartum | mild hearing loss right ear, visual aura | FA: BRAO with leakage | steroids (oral), azathioprine ( azathioprine discontinued during pregnancy due to anemia) | full recovery | healthy baby | van der Kooij 2015 | |||||
| 23 | N | 29 | 8 | right hearing loss, vertigo, and mild headache | steroids (oral, pulse) | London 2016 | ||||||||
| 23 | 19 | left visual field deficit, headache | FA: bilateral multiple BRAO | multiple T2 hyperintensities in the deep white matter including the splenium of the corpus callosum and the left cerebellum | mildly elevated protein OCB absent | steroids (pulse and oral taper), antiplatelet therapy; cyclophosphamide 1 g every 4 weeks (initiated at 28 weeks gestational age, due to relapses) | persistent bilateral hypoacousia requiring hearing aid | healthy baby | London 2016 | |||||
| 23 | postpartum | dizziness and visual loss | gadolinium-enhancing lesions | steroids (pulse) | London 2016 | |||||||||
| 24 | N | 21 | 3 months | visual and hearing loss; after currettage rapid onset of encephalopathy | no SNHL | Fundoscopy: retinal edema FA: leakage, no BRAO | increased protein | steroids (oral taper) | complete recovery 2 weeks later | missed abortion | Bhattu 2017 | |||
| 25 | N | 25 | 7 months | visual loss left eye, hearing loss and tinnitus, mild headache | SNHL right ear | Fundoscopy: ischemic retinal edema infero-temporal and cherry-red spot | periventricular and callosal T2 hyperintensities | not reported | steroids (pulse and oral taper) | improvement in headache, some recovery of vision | Manik 2018 | |||
| 26 | N | 19 | 14 | headache, somniloquy | SNHL low frequencies | FA: multiple BRAO | multiple diffusion restrictive T2 hyperintensities, also in corpus callosum | increased protein OCB absent | steroids (pulse) and aspirin | no fetal anomaly | Can Usta 2018 | |||
| 27 | N | 34 | 15 | apathy and behavioral changes; vertigo 6 months prior and an episode of right ear tinnitus 2 months prior | bilateral SNHL | retinal vasculitiscorroborated by FA | hyperintense periventricular white matter lesions in T2 andFLAIR sequences also involving bilateral basal ganglia and with pre-dominant affection of the corpus callosum, in addition to infratentorialcerebellar lesions. Lesional restriction of diffusion but no contrast en-hancement was observed. T1 weighted images showed hypointenselesions in the same topography | CSF values showed proteins of 77 mg/dl,glucose of 52 mg/dl (serum glucose of 89 mg/dl), and no cells. | 5 pulses of methylprednisolone were administeredwithout obvious clinical improvement. Immunomodulatory treatmentwas escalated to intravenous immunoglobulin (IVIg) at 0.4 g/kg/dayfor 5 days; prednisone orally and CCF after abortion | partial remission | therapeutic abortion | Gomez-Figueroa 2018 | ||
| 28 | Y | 23 | 45 | no relapse during pregnancy or postpartum | no treatment since 8 years, 2 years relapse free after delivery | IVF four cycles of GnRH antagonist, ganirelix | healthy twins at 35 weeks GA | Qiu 2020 | ||||||
| 29 | N | 24 | 23 | 11 months postpartum | ataxia, vomiting, minor cognitive impairment and blurred vision in theright eye | T2 hyperintensities inthe deep and subcortical white matter, brainstem and cerebellumassociated with restricted diffusion, callosal snowball lesions | 9wbc/µl increased protein 120 mg/dl | aspirin, steroids (pulse and oral taper), IVIg | After 1 month symptoms resolved, patient fell pregnant, resulting in a spontaneous miscarriage two months later. | Qiu 2020 | ||||
| 30 | N | 24 | 1 month post- spontaneous abortion | subacute severebilateral hearing impairment requiring hearing aids, and partial visualloss in the left eye | mild bilateral low frequency SNHL | FA: bilateral BRAOs | MRI six months post-rituximab was stable | Steroids (oral), rituximab | Qiu 2020 | |||||
| 30 | Y | 25 | 22 months after initial presentation | 11 months after last rituximab dose | healthy baby at 38 weeks | Qiu 2020 | ||||||||
| 31 | N | 34 | 34 | 7 | moderate encephalopathy, vertigo | unilateral SNHL | bilateral | T2 hyperintensities in the supratentorial white and gray matter areas | 14 wbc/µl increased protein (125mg/dl) | aspirin, IV steroids, cyclophosphamide, mycophenolate, | SNHL, visual field deficits, no residual central nervous system symptoms | therapeutic abortion | Wilf-Yarkoni 2020 | |
| 32 | N | 40 | 20 | migraine, bradypsychia, disorientation and behavioral changes | SNHL | bilateral papillitis and ischemic retinal areas | T2 hyperintensities in the supratentorial white matter and corpus callosum with diffusion restriction | IVIG and oral prednisone; after pregnancy add-on of azathioprin | resolution of symptoms | healthy baby at 36-weeks GA after premature rupture of membranes and caesarean section | Ramos-Ruperto 2020 | 1 previous pregnancy without complications | ||
| 32 | 6 months postpartum | bilateral scotomas | retinal infarctions | steroids (pulse), IVIG and cyclophosphamide | improvement | Ramos-Ruperto 2020 | ||||||||
| 33 | N | 37 | puerperium | scotoma | SNHL | branch arterial retinal infarctions | T2 hyperintensities in supratentorial white matter, right internal capsule and splenium of the corpus callosum | steroids (pulse), oral prednisone and azathioprine | no relapses | healthy baby | Ramos-Ruperto 2020 | 2 previous pregancies; self-limited dysarthria and tinnitus during first pregnancy , as well as episodes of headache preceding the scotoma |
AZA, azathioprin; BRAO, branch retinal artery occlusions; CSF, cerebrospinal fluid; CYC, cyclophosphamide; FA, fluorescein angiography; GA, gestational age; IVIG, intravenous immunoglobulins; MMF, mycophenolate mofetil; MRI, magnetic resonance imaging; OCB, oligoclonal bands; SNHL, sensorineural hearing loss; SuS, Susac syndrome; wbc, white blood cells.
Pregnancy planning: general
Fertility
While no specific reports have been published on fertility in patients with SuS, this topic is of importance. Indeed, treatment with CYC may induce infertility in young female patients who have not yet completed their family. The risk depends on the patient’s age at treatment and the cumulative dose.38 Consulting a fertility specialist before the start of this treatment is recommended. As SuS is more frequent in females than in males, a role for hormones in pathophysiology may be suspected. A case of a SuS relapse after starting oestrogen replacement therapy, in a patient who had been in remission for 18 years, has been reported, suggesting the possibility of a role for hormones in triggering late relapse.39 However, recently a female-to-male transgender patient developing SuS under treatment with testosterone was described, challenging the hypothesis of (female) sex hormones as important players.40 Alternatively, this coincidence may not be associated with hormonal treatment at all, as men and women both can be affected. Whether oral contraceptive pills and hormonal treatments used during in vitro fertilisation (IVF) procedures may increase the risk of SuS or SuS relapse remains to be elucidated. A first case of SuS remaining in remission after a successful IVF procedure was published recently .35 Usually patients with SuS are advised to change their systemic contraception to a local method because of the unknown impact of hormonal treatment,41 but the evidence to support this strategy is scarce and is in part taken from the concept that hormonal treatments are considered prothrombogenic.
Genetics and heritability
To our knowledge, no cases of familial SuS have been published. No studies on (immuno)genetics have been reported to date. In a study with 14 patients, all but one SuS patient who was homozygous for HLA C*04, expressed HLA-C*06 and/or HLA-C*07. Comparing the peptide binding motifs of these HLA-C allotypes revealed that the binding motifs of HLA-C*06:02 and HLA-C*07:02 are almost identical.5 SuS is considered to be a non-hereditary disease. However, as in other autoimmune diseases, such as multiple sclerosis (MS), genetic risk factors likely play a role in the development of the disease. This field is still open to research.
Disease activity monitoring
For patients who have a known diagnosis of SuS, regular clinical monitoring during pregnancy and the postpartum period is advisable, to detect disease relapse or recurrence at an early stage. Indeed, the reported cases demonstrate that there is a risk of disease onset and recurrence in these periods. During pregnancy, brain magnetic resonance imaging (MRI) is generally considered to be safe, especially when benefits outweigh potential risks. Gadolinium contrast is not administered during pregnancy, due to slightly increased risk of neonatal death. Moreover, T2 hyperintensities and diffusion weighted imaging, which can show the typical callosal lesions, may be a worthwhile alternative to gadolinium-enhanced MRI.42 For patients who are diagnosed during pregnancy, a fluorescein angiogram may add important diagnostic information. However, fluorescein may cross the placenta and enter the amniotic fluid. There are no teratogenic risks in animals. Safety information in humans is limited and therefore the decision to perform a fluorescein angiography should be made on a case-by-case basis and be performed only when the benefits outweigh the potential risks.43
Timing of pregnancy
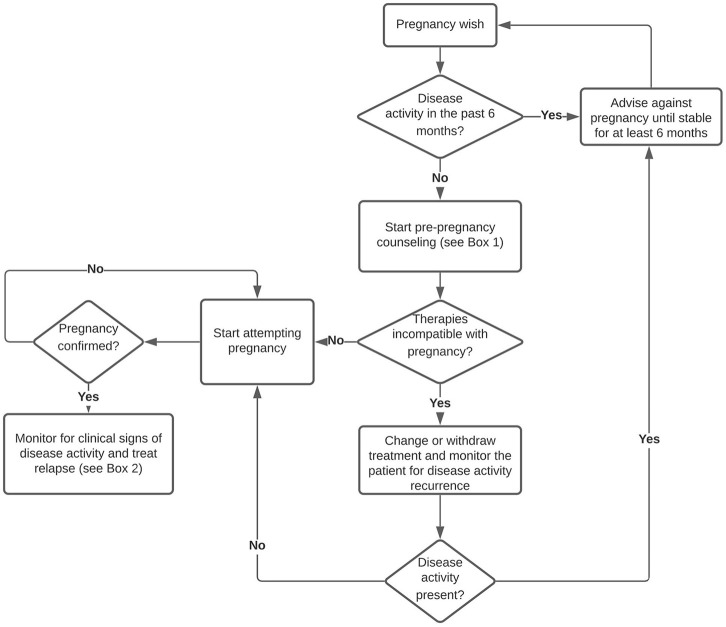
SuS patients attempting pregnancy should preferably be free from disease activity and stable without therapy or stable on a treatment that is compatible with pregnancy. Advance pregnancy planning and counselling is therefore highly recommended in this patient group. It is generally accepted now that women with autoimmune diseases like systemic lupus erythematosus (SLE) and vasculitis may attempt pregnancy during quiescent periods of their disease, maintaining a compatible therapy during the preconception, pregnancy and postpartum periods.44,45 In our opinion, the same advice may be applied to SuS patients. Attempting pregnancy when the disease is not (temporarily) in remission should be advised against, because of the risks to the mother when SuS flares up. Disease remission for a duration of at least 6 months seems prudent before attempting pregnancy. This advice is in accordance with recommendations for patients with SLE who wish to become pregnant.44 However, disease remission for 6 months is no guarantee of no relapse during pregnancy, as disease recurrence has been described 23 years after initial symptoms, potentially elicited by pregnancy.25 In conclusion, timing of a pregnancy should be a shared decision between patient and clinician, and patients should be informed of the risk of disease relapse during or after pregnancy.
Compatibility of commonly used treatments for SuS with pregnancy and breastfeeding
Recommendations on treatment of SuS have been published recently and are based on expert opinion.8 There are no guidelines on treatment of SuS during pregnancy, where potential foetal toxicity of treatments needs to be taken into consideration. In the reported cases from patients with SuS during pregnancy, mainly steroids, IVIG and PLEX have been utilized during pregnancy, whereas cyclophosphamide and rituximab were kept for severe and refractory cases, after delivery (see Table 1). However, in one severe case, CYC was started in the 28th week of pregnancy because of ongoing relapses, without foetal toxicity.30 It is important to note that all treatments described for SuS are off-label use.
Corticosteroids
Corticosteroids are used to treat disease flares, both intravenously in a high-dose pulse and orally in tapering schedules. Risk monitoring during pregnancy consists of following glycemia and blood pressure. Corticosteroids should be avoided in the first trimester, if possible, especially between 8th and 11th gestational week to reduce the slightly elevated risk of cleft lip and palate, but data are scarce.46 One single pulse seems to be safe, while repeated or continued administration of corticosteroids may lead to growth retardation or preterm birth. Others state that prednisolone and methylprednisolone use is safe even in the first trimester.47 Methylprednisolone and prednisolone should be preferred over dexamethasone, because penetration of the placental barrier is only 10%.
Intravenous immunoglobulins
It is important to assess the serostatus of the patient before starting IVIG, as administration of IVIG may lead to false positive serologic results. Indeed, serologic testing will detect endogenous IgG, produced by the patient, as well as administered IgG.48 IVIG will cross the placenta. IVIG are used widely in the treatment of SuS: many case series and case reports describe amelioration of symptoms, and expert opinion recommends IVIG or subcutaneous IG (scIG). IVIG are safe in pregnancy and breastfeeding.47,49
Plasma exchange
PLEX seems to be safe in pregnancy and has been used as a rescue therapy in different neuroimmunological diseases, such as MS, antiphospholipid syndrome, thrombotic thrombocytopenic purpura, neuromyelitis optica spectrum disorders (NMOSD) or myasthenia gravis (MG).50,51 In SuS, PLEX seems to be useful in acute episodes.21 There are no reports of immunoadsorption in SuS.
Mycophenolate mofetil
Mycophenolate mofetil (MMF) is teratotoxic (pregnancy loss, congenital malformations) and should be avoided in pregnancy. Men and women should use effective contraceptives strictly during the treatment period, and women additionally for at least another 6 weeks. No information is available on the excretion and effects of MMF in breast milk; expert recommendation is to avoid breastfeeding with MMF [United States Food and Drug Administration (FDA)].47
Azathioprin
Data on azathioprin (AZA) in other immunological diseases do not show any teratogenic effect, but there are hints of premature births and low birth weight. Whether this is due to the underlying disease, to the drug itself or other drugs used in combination, needs to be resolved. Cases of infants with bone marrow depression after maternal AZA use have been described. These side effects seem to be rare and should be weighed against potential relapses when discontinuing the drug if the mother is stable.47,50 Thus, in treatment-naive pregnant women with SuS onset, AZA should not be the first line treatment. However, AZA can be continued during pregnancy after risk/benefit evaluation. Regular monitoring of leucocytes and thrombocytes is advisable. During lactation, AZA is probably safe, as drug levels in breastmilk remain very low, especially 4 h after intake.52
Methotrexate
Methotrexate (MTX) is contraindicated in pregnant women because of the teratogenic effects. It should be stopped at least 3 months before attempting conception.47 Data on excretion in breastmilk are scarce and lactation should therefore be avoided during MTX use.53,54
Cyclophosphamide
CYC is contraindicated in pregnant women because of the teratogenic effects. However, there is some preliminary evidence in the field of cancer treatment that chemotherapy could be administered during the second and third trimester, with low risk of severe problems for the foetus.47,55,56 In selected cases, treatment with CYC during pregnancy after the first trimester can be considered, in a centre that has experience with management of complicated pregnancies with a multidisciplinary team of at least a gynaecologist, a neurologist and a neonatologist. CYC is excreted in breastmilk, may suppress the infants bone marrow and should be avoided during lactation.57,58
Tumour necrosis factor alpha inhibitors
Tumour necrosis factor alpha (TNF-α) inhibitors are contraindicated in patients with demyelinating disease as these therapies may increase inflammation and induce relapses, underlining the importance of an optimal differential diagnosis. In patients with SuS, TNF-α inhibitors seem to be helpful in case reports and case series in patients with relapses with classic immunotherapies.59 Based on sparse data from case series and case reports, TNF-α inhibitors do not appear to be associated with a high risk of teratogenicity, but a harmful effect cannot be ruled out definitively. In rheumatological diseases, TNF-α inhibitor use may be associated with a higher rate of preterm delivery, but this may be due to disease activity. TNF-αa inhibitor should be discontinued around the third trimester when transfer across the placenta is greatest.47,60 The decision to use TNF-α inhibitors as an off-label medication in pregnant women with SuS should be reserved for very severe or life-threatening disease. Breastfeeding is compatible with TNF-α inhibitors.61
Rituximab
Information on rituximab (RTX) in pregnancy is based on case reports of women with immunological and malignant diseases. The monoclonal antibody can pass the blood–placenta barrier. The average half-life of RTX is 20–31 days. RTX seems to be associated with a higher risk of premature births, with consideration of the potential harmful effect of the underlying disease as a concurrent cause. B cells will be depleted in newborns; thus, measuring B lymphocytes in foetuses is recommended if RTX has been administered after the 20th week of pregnancy. In NMOSD, RTX administration is recommended close to the time of conception to have a long-term protective effect during pregnancy.47,50 RTX is transferred to breast milk in minimal amounts.62,63 Moreover, in breastmilk-fed infants from mothers treated with anti-CD20 therapies, no negative impact on health of the infants up to the age of 1 year was detected.64 To summarise, careful evaluation of the risks and benefits of stopping or the continuation of RTX treatment is necessary. In patients with severe autoimmune disease, it is acceptable to attempt pregnancy closely after the last RTX dose and to consider redosing of RTX if relapses occur during pregnancy.65
Natalizumab
Natalizumab (NAT) is registered as treatment for relapsing remitting MS (RRMS). Its mechanism of action is interesting, because it inhibits lymphocyte adhesion and thus migration through the blood–brain barrier, by blocking alpha4-integrin. In one case, NAT was reported to exacerbate SuS.66 However, in an animal model and in four SuS patients, disease improvement was seen.5 One advantage of NAT is that it has been used during pregnancy in RRMS patients and seems relatively safe in clinical practice. However, insufficient data are available to draw firm conclusions.67–69 Another issue is the risk of progressive multifocal leukoencephalopathy in patients who are likely immunosuppressed by other treatments received prior or concomitantly. From MS, it is known that a rebound of disease activity may occur after cessation of treatment with NAT. When used only in patients with high disease activity, or when alternative treatment options are lacking, treatment with NAT might be continued under careful and frequent control and consideration of all the risks and benefits in pregnant women with RRMS.64,69 The last dose should be administered before the 30–34th week. During lactation, current data for administration of NAT are limited, but reassuring.63,67,69 In conclusion, the mechanism of action of NAT and clinical experience suggest that this agent may be of interest in SuS patients.
Calcineurin inhibitors
Cyclosporin A (CSA) and tacrolimus (TAC) are used in NMOSD, MG and SLE, and sometimes in SuS.51,61,70–72 TAC and CSA should not be started, but can be continued relatively safe in pregnancy. However, strict drug level monitoring is required to limit toxicities. Metabolites of CSA and TAC pass the placental barrier. No major malformations have been reported with CSA or TAC. Premature birth and low birth weight have been reported in humans (FDA). Most data have been derived from patients receiving organ transplantation. Caution in the use of these therapies during pregnancy in SuS is therefore warranted. Limited data suggest that the excreted levels of TAC and CSA in breastmilk are low and unlikely to negatively affect the infant. TAC and CSA are considered probably safe during breastfeeding.73–75 However, caution is warranted and monitoring of drug levels in the infants blood may be necessary, as even with low amounts of CSA excreted in breastmilk, infant levels may have therapeutic concentrations in the blood.76
Acetyl salicylic acid
High-dose acetyl salicylic acid (ASA) should be used with caution in pregnancy. Low dose ASA (81mg) preconception has not been associated with increased risk of major adverse events when used throughout pregnancy.77 Epidemiologic studies describe increased risk of miscarriage, cardiac malformations, and gastroschisis under ASA in early pregnancy; the absolute risk of cardiovascular malformations increased from less than 1% to up to approximately 1.5%. The risk is believed to increase with dose and duration of therapy (FDA). For secondary stroke prevention, low dose ASA during pregnancy is reasonable, and breastfeeding can be considered during intake of low dose ASA.78 In most patients with SuS, ASA is added to reduce the risk of vessel occlusion based on expert opinions; however, evidence is lacking. Luminal occlusion in SuS is caused by hypertrophied and reactive endothelial cells.79,80 Whether ASA effectively reduces endothelial inflammation in SuS remains to be proven.
Nimodipine
Nimodipine is a calcium antagonist that leads to vasodilatation. It is lipophilic and can pass the blood–brain barrier. It has been used in SuS in the past, but the immunopathogenesis does not support the use of nimodipine.
Discussion
We have summarised more than 30 cases of SuS, with description of disease course and treatment during pregnancy or postpartum period. Strikingly, approximately two out of three patients of these cases were diagnosed during pregnancy. One likely explanation is that there is a publication bias towards new diagnosed cases in pregnancy, while pregnancies in SuS patients who are in remission and have a normal course are not reported. A prospective, international registry for patients with SuS, containing specific pregnancy forms, could be a solution to solve this potential reporting bias. Patients who are in remission and have pregnancies without relapse or complications, as well as their treating physicians, should be encouraged to share their data and participate in these registries. Patient-driven or active patient-participation in these registries may help to collect the necessary data. Another potential explanation of SuS relapse during pregnancy is the role of hormones and changes in the immune system. It is well-known that the course of several autoimmune diseases changes during pregnancy. Th1-related diseases such as rheumatoid arthritis or MS tend to stabilise, while Th2-related diseases like SLE or vasculitis carry a risk of exacerbation during pregnancy.45 Systematically studying the immunology of SuS before, during and after pregnancy may lead to better knowledge on pathophysiological mechanisms involved in disease relapse and remission.
Due to the rarity of this disease, there are no randomized controlled trials to guide treatment, and therapy is based on expert opinions and is, in part, based on knowledge of other immunological diseases. We propose a period of at least 6 months disease remission before attempting pregnancy (see Figure 1). This seems a reasonable approach in SuS and is in accordance with recommendations for patients with SLE.44 In our opinion, and based on the risk profile of the drugs, for maintenance treatment during pregnancy, first choices are low dose oral (methyl)prednisolone and monthly IVIG. AZA, CSA and TAC may be considered as maintenance treatment during pregnancy, in patients who are known with SuS, but are not a first choice to start during pregnancy. MMF and MTX should be stopped before attempting conception and should not be started during pregnancy or lactation. RTX, with a last dose not too long before conception, may be a treatment option in patients who had severe disease and who wish to lower the risk of disease exacerbation during pregnancy as much as possible, in analogy with NMOSD management.65 To treat SuS exacerbations during pregnancy and lactation, high-dose IV methylprednisolone can be considered, either alone or in combination with IVIG and/or PLEX. Adding ASA can be considered safe. In severe cases, RTX might be started and NAT might be continued during pregnancy, in analogy with treatment of severe SLE or RRMS. When treatment-refractory, very severe relapses occur, in the second or third trimester of pregnancy, after careful consideration, CYC can be regarded as a rescue therapy option, in analogy to other life-threatening autoimmune disease (see Box 1). During lactation, only small amounts of monoclonal antibodies are excreted into breastmilk. Therefore, TNF-α inhibitors, RTX and NAT may be relatively safe and considered to administer while breastfeeding.62,64,69,81 Also, AZA, TAC and CSA may be safe during lactation. Finally, these pregnancies should be considered as high-risk pregnancies and follow up by or consultation with experts in the field of neuroimmunology is a prerequisite.
Figure 1.
Management of pregnancy in SuS.
SuS, Susac syndrome.
Box 1.
Recommendations on management of SuS patients before, during and after pregnancy.
| Pre-pregnancy |
| • Information provision: Pregnancy in SuS should be considered as high-risk and it should be planned Discuss risk of relapse during pregnancy and post-partum and necessity of monitoring Discuss risks and benefits of immunosuppressive therapies Discuss limitations of current knowledge Refer or discuss case with expert in neuroimmunology • Review disease status: stable for 6 months? • Review treatment compatibility with pregnancy and adjust or withdraw treatments: Stop MMF, MTX, CYC Continue steroids in the lowest possible dose Continue IVIG Consider switch to IVIG alone or IVIG plus AZA or RTX |
| DURING PREGNANCY Mother • Include the patient in a registry if possible • Monitor patients for occurence of clinical symptoms • Perform brain MRI without gadolinium and ohtalmological examination without fluorescein in case of suspected relapse • In case of relapse or first symptoms: First line treatment includes IV and oral (methyl)prednisolone, IVIG, PLEX and ASA Second line treatment includes RTX, NAT Rescue treatment is CYC • In case of unexpected pregnancy and accidental exposure of the fetus to MMF, MTX or CYC: advise ultrasound and provide counselling about the risk of malformations Fetus • Perform structural ultrasound • Monitor fetal growth |
| POST-PARTUM Mother • Perform baseline examinations with neurological examination, fluorescein angiogram, tone-audiometry and brain MRI in the month after delivery. • Decision to breastfeed is dependent on personal risk-benefit evaluation Baby • Check B cell counts in the newborn in case of RTX use closely before conception or during pregnancy. Plan vaccinations accordingly. • Evaluate the newborn for signs or symptoms potentially related to transferred antibodies and/or medication used during the pregnancy. |
| LACTATION |
| • IVIG is safe during lactation • AZA, CSA, TAC, RTX, NAT or TNF-α inhibitors could be considered after risk/benefit evaluation • (methyl)prednisolone (wait 1–4 h after dosing) or PLEX are safe in case of relapse |
AZA, azathioprin; CSA, cyclosporin A; CYC, cyclophosphamide; IVIG, intravenous immunoglobulins; MMF, mycophenolate mofetil; MRI, magnetic resonance imaging; MTX, methotrexate; NAT, natalizumab; PLEX, plasma exchange; RTX, rituximab; TAC, tacrolimus; TNF-α, tumor necrosis factor alpha; SuS, Susac syndrome.
Footnotes
Funding: BW is supported by a clinical PhD fellowship 1701919N and travel grant K219819N from the Research Foundation Flanders
Conflict of interest statement: The institution of BW received travel support to attend meetings, fees for participation in advisory boards, speaker honoraria and grants for research and/or patient support and/or education from Biogen, Roche, Merck, Novartis, Genzyme, Celgene. IK received travel expenses for attending meetings from Pfizer and CSL Behring. IK received speaker honoraria from Daiichi Sankyo.
ORCID iD: Barbara Willekens  https://orcid.org/0000-0002-5212-8837
https://orcid.org/0000-0002-5212-8837
Contributor Information
Barbara Willekens, Department of Neurology, Antwerp University Hospital, Drie Eikenstraat 655, Edegem, 2650, Belgium; Laboratory of Experimental Hematology, Vaccine & Infectious Disease Institute (VAXINFECTIO), Faculty of Medicine and Health Sciences, University of Antwerp, Wilrijk, 2610, Belgium; Translational Neurosciences, Faculty of Medicine and Health Sciences, University of Antwerp, Wilrijk, 2610, Belgium.
Ilka Kleffner, University Hospital Knappschaftskrankenhaus Bochum, Ruhr University Bochum, Bochum, Germany.
References
- 1. Susac JO, Hardman JM, Selhorst JB. Microangiopathy of the brain and retina. Neurology 1979; 29: 313–316. [DOI] [PubMed] [Google Scholar]
- 2. Susac JO. Susac’s syndrome: the triad of microangiopathy of the brain and retina with hearing loss in young women. Neurology 1994; 44: 591–593. [DOI] [PubMed] [Google Scholar]
- 3. Susac syndrome, https://www.orpha.net/consor4.01/www/cgi-bin/OC_Exp.php?lng=EN&Expert=838 (accessed 9 August 2020).
- 4. Kleffner I, Dörr J, Ringelstein M, et al. Diagnostic criteria for Susac syndrome. J Neurol Neurosurg Psychiatry 2016; 87: 1287–1295. [DOI] [PubMed] [Google Scholar]
- 5. Gross CC, Meyer C, Bhatia U, et al. CD8(+) T cell-mediated endotheliopathy is a targetable mechanism of neuro-inflammation in Susac syndrome. Nat Commun 2019; 10: 5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Magro CM, Poe JC, Lubow M, et al. Susac syndrome: an organ-specific autoimmune endotheliopathy syndrome associated with anti-endothelial cell antibodies. Am J Clin Pathol 2011; 136: 903–912. [DOI] [PubMed] [Google Scholar]
- 7. Jarius S, Neumayer B, Wandinger KP, et al. Anti-endothelial serum antibodies in a patient with Susac’s syndrome. J Neurol Sci 2009; 285: 259–261. [DOI] [PubMed] [Google Scholar]
- 8. Rennebohm RM, Asdaghi N, Srivastava S, et al. Guidelines for treatment of Susac syndrome – An update. Int J Stroke 2020; 15: 484–494. [DOI] [PubMed] [Google Scholar]
- 9. Egan RA. Diagnostic criteria and treatment algorithm for Susac syndrome. J Neuroophthalmol 2019; 39: 60–67. [DOI] [PubMed] [Google Scholar]
- 10. Dörr J, Krautwald S, Wildemann B, et al. Characteristics of Susac syndrome: a review of all reported cases. Nat Rev Neurol 2013; 9: 307–316. [DOI] [PubMed] [Google Scholar]
- 11. Coppeto JR, Monteiro MLR, Currie JN, et al. A syndrome of arterial-occlusive retinopathy and encephalopathy. Am J Ophthalmol 1984; 98: 189–202. [DOI] [PubMed] [Google Scholar]
- 12. MacFadyen DJ, Schneider RJ, Chisholm IA. A syndrome of brain, inner ear and retinal microangiopathy. Can J Neurol Sci 1987; 14: 315–318. [DOI] [PubMed] [Google Scholar]
- 13. Gordon DL, Hayreh SS, Adams HP., Jr. Microangiopathy of the brain, retina, and ear: improvement without immunosuppressive therapy. Stroke 1991; 22: 933–937. [DOI] [PubMed] [Google Scholar]
- 14. Cador-Rousseau B, Cazalets C, Decaux O, et al. [Susac’s syndrome in post-partum]. La Rev Med Interne 2002; 23: 667–668. [DOI] [PubMed] [Google Scholar]
- 15. Aubart-Cohen F, Klein I, Alexandra JF, et al. Long-term outcome in Susac syndrome. Medicine 2007; 86: 93–102. [DOI] [PubMed] [Google Scholar]
- 16. Grinspan ZM, Willey JZ, Tullman MJ, et al. Clinical reasoning: a 28-year-old pregnant woman with encephalopathy. Neurology 2009; 73: e74–e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hardy TA, Garsia RJ, Halmagyi GM, et al. Tumour necrosis factor (TNF) inhibitor therapy in Susac’s syndrome. J Neurol Sci 2011; 302: 126–128. [DOI] [PubMed] [Google Scholar]
- 18. Deane KD, Tyler KN, Johnson DW, et al. Susac syndrome and pregnancy: disease management. J Clin Rheumatol 2011; 17: 83–88. [DOI] [PubMed] [Google Scholar]
- 19. Finis D, Stammen J, Gonnermann J. [Retinal arteritis in pregnancy]. Der Ophthalmologe 2011; 108: 676–682. [DOI] [PubMed] [Google Scholar]
- 20. Karelle S, Demanez L, Zangerle PF, et al. Sudden sensorineural hearing loss: when ophthalmology meets otolaryngology. B-ENT 2012; 8: 135–139. [PubMed] [Google Scholar]
- 21. Mateen FJ, Zubkov AY, Muralidharan R, et al. Susac syndrome: clinical characteristics and treatment in 29 new cases. Eur J Neurol 2012; 19: 800–811. [DOI] [PubMed] [Google Scholar]
- 22. Engeholm M, Leo-Kottler B, Rempp H, et al. Encephalopathic Susac’s Syndrome associated with livedo racemosa in a young woman before the completion of family planning. BMC Neurol 2013; 13: 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ioannides ZA, Airey C, Fagermo N, et al. Susac syndrome and multifocal motor neuropathy first manifesting in pregnancy. Aust N Z J Obstet Gynaecol 2013; 53: 314–317. [DOI] [PubMed] [Google Scholar]
- 24. Antulov R, Holjar Erlic I, Perkovic O, et al. Susac’s syndrome during pregnancy – the first Croatian case. J Neurol Sci 2014; 341: 162–164. [DOI] [PubMed] [Google Scholar]
- 25. Feresiadou A, Eriksson U, Larsen HC, et al. Recurrence of Susac Syndrome following 23 Years of Remission. Case Rep Neurol 2014; 6: 171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hua le H, Donlon SL, Okuda DT. A case of Susac syndrome with cervical spinal cord involvement on MRI. J Neurol Sci 2014; 337: 228–231. [DOI] [PubMed] [Google Scholar]
- 27. Tashman Y. Susac’s syndrome in a 25 year old pregnant woman (P5.161). Neurology 2014; 82: P5.161. [Google Scholar]
- 28. Shabbir Khan M, Vaniyan R, Al Khalifa SA. Central retinal artery occlusion in a healthy pregnant woman: a suspected case of Susac syndrome. Bahrain Med Bull 2014; 36: 255–257. [Google Scholar]
- 29. van der Kooij SM, van Buchem MA, Overbeek OM, et al. Susac syndrome: a report of four cases and a review of the literature. Netherlands J Med 2015; 73: 10–16. [PubMed] [Google Scholar]
- 30. London F, Pothalil D, Duprez TP, et al. Potential benefits of early aggressive immunotherapy in Susac syndrome. Acta Neurol Belg 2016; 116: 451–460. [DOI] [PubMed] [Google Scholar]
- 31. Bhattu SR, Talib SH, Anand S, et al. Susac syndrome in a Primigravida: a rare case report. IOSR J Dent Med Sci (IOSR-JDMS) 2017; 16: 10–13. [Google Scholar]
- 32. Geetesh Manik RP, Chinky Sharma. A classic representation of rare Susac syndrome in young pregnant female. J Case Rep 2018; 8: 129–131. [Google Scholar]
- 33. Can Usta N, Boz C, Özmenoğlu M. Pregnancy-induced Susac syndrome: a case report. Turk J Neurol 2018; 24: 70–71. [Google Scholar]
- 34. Gomez-Figueroa E, Garcia-Trejo S, Garcia-Santos RA, et al. Exquisite response to intravenous immunoglobulin in Susac syndrome during pregnancy. eNeurologicalSci 2018; 10: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Qiu J, Riminton DS, Reddel SW, et al. Pregnancy without relapse following treated Susac syndrome. Mult Scler Relat Disord 2020; 45: 102357. [DOI] [PubMed] [Google Scholar]
- 36. Wilf-Yarkoni A, Elkayam O, Aizenstein O, et al. Increased incidence of Susac syndrome: a case series study. BMC Neurol 2020; 20: 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ramos-Ruperto L, Martínez-Sánchez N, Bartha-Rasero JL, et al. Susac syndrome and pregnancy: a relationship to clarify. About two cases and review of the literature. J Matern Fetal Neonatal Med 2020: 1–6. [DOI] [PubMed] [Google Scholar]
- 38. Dooley MA, Nair R. Therapy Insight: preserving fertility in cyclophosphamide-treated patients with rheumatic disease. Nat Clin Pract Rheumatol 2008; 4: 250–257. [DOI] [PubMed] [Google Scholar]
- 39. Petty GW, Matteson EL, Younge BR, et al. Recurrence of Susac syndrome (retinocochleocerebral vasculopathy) after remission of 18 years. Mayo Clin Proc 2001; 76: 958–960. [DOI] [PubMed] [Google Scholar]
- 40. Alungulese AL, García Soldevilla MÁ, Barragán Martínez D, et al. Sex hormones secondary players in Susac’s syndrome. Mult Scler Relat Disord 2020; 44: 102373. [DOI] [PubMed] [Google Scholar]
- 41. Kleffner I, Duning T, Lohmann H, et al. A brief review of Susac syndrome. J Neurol Sci 2012; 322: 35–40. [DOI] [PubMed] [Google Scholar]
- 42. Lum M, Tsiouris AJ. MRI safety considerations during pregnancy. Clin Imaging 2020; 62: 69–75. [DOI] [PubMed] [Google Scholar]
- 43. Halperin LS, Olk RJ, Soubrane G, et al. Safety of fluorescein angiography during pregnancy. Am J Ophthalmol 1990; 109: 563–566. [DOI] [PubMed] [Google Scholar]
- 44. Knight CL, Nelson-Piercy C. Management of systemic lupus erythematosus during pregnancy: challenges and solutions. Open Access Rheumatol 2017; 9: 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Somers EC. Pregnancy and autoimmune diseases. Best Pract Res Clin Obstet Gynaecol 2020; 64: 3–10. [DOI] [PubMed] [Google Scholar]
- 46. Bandoli G, Palmsten K, Forbess Smith CJ, et al. A review of systemic corticosteroid use in pregnancy and the risk of select pregnancy and birth outcomes. Rheumat Dis Clin North Am 2017; 43: 489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Flint J, Panchal S, Hurrell A, et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding—Part I: standard and biologic disease modifying anti-rheumatic drugs and corticosteroids. Rheumatology 2016; 55: 1693–1697. [DOI] [PubMed] [Google Scholar]
- 48. Lichtiger B, Rogge K. Spurious serologic test results in patients receiving infusions of intravenous immune gammaglobulin. Arch Pathol Lab Med 1991; 115: 467–469. [PubMed] [Google Scholar]
- 49. Bewertung der Expertengruppe Off-Label - Fachbereich Neurologie/Psychiatrie - nach § 35c Abs. 1 SGB V zur Anwendung von Intravenösen Immunglobulinen (IVIG) bei der Multiplen Sklerose Addendum 1 (Stand 06.12.2018), https://www.bfarm.de/SharedDocs/Downloads/DE/Arzneimittel/Zulassung/BereitsZugelAM/offlabel/Bewertungen/IVIG_MS_Addendum_I_nebst_Anlage.pdf;jsessionid=096FBCF5C605F0D98A4A652D157A8B1A.2_cid507?__blob=publicationFile&v=4 (2018, accessed 14 September 2020).
- 50. Borisow N, Mori M, Kuwabara S, et al. Diagnosis and Treatment of NMO Spectrum Disorder and MOG-Encephalomyelitis. Front Neurol 2018; 9: 888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sanders DB, Wolfe GI, Benatar M, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology 2016; 87: 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Christensen LA, Dahlerup JF, Nielsen MJ, et al. Azathioprine treatment during lactation. Aliment Pharmacol Ther 2008; 28: 1209–1213. [DOI] [PubMed] [Google Scholar]
- 53. Thorne JC, Nadarajah T, Moretti M, et al. Methotrexate use in a breastfeeding patient with rheumatoid arthritis. J Rheumatol 2014; 41: 2332. [DOI] [PubMed] [Google Scholar]
- 54. Johns DG, Rutherford LD, Leighton PC, et al. Secretion of methotrexate into human milk. Am J Obstet Gynecol 1972; 112: 978–980. [DOI] [PubMed] [Google Scholar]
- 55. Esposito S, Tenconi R, Preti V, et al. Chemotherapy against cancer during pregnancy: A systematic review on neonatal outcomes. Medicine 2016; 95: e4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Maggen C, Dierickx D, Cardonick E, et al. Maternal and neonatal outcomes in 80 patients diagnosed with non-Hodgkin lymphoma during pregnancy: results from the International Network of Cancer, Infertility and Pregnancy. Br J Haematol 2020. [DOI] [PubMed] [Google Scholar]
- 57. Fierro ME, Datta P, Rewers-Felkins K, et al. Cyclophosphamide use in multiple sclerosis: levels detected in human milk. Breastfeeding Med 2019; 14: 128–130. [DOI] [PubMed] [Google Scholar]
- 58. Durodola JI. Administration of cyclophosphamide during late pregnancy and early lactation: a case report. J Natl Med Assoc 1979; 71: 165–166. [PMC free article] [PubMed] [Google Scholar]
- 59. Buelens T, Ossewaarde-van Norel J, de Boer JH, et al. Evaluation of tumor necrosis factor inhibitor therapy in Susac syndrome. Retina (Philadelphia, Pa) 2020; 40: 581–590. [DOI] [PubMed] [Google Scholar]
- 60. Raja H, Matteson EL, Michet CJ, et al. Safety of tumor necrosis factor inhibitors during pregnancy and breastfeeding. Transl Vis Sci Technol 2012; 1: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Grover KM, Sripathi N. Myasthenia gravis and pregnancy. Muscle Nerve 2020; 62: 664–672. [DOI] [PubMed] [Google Scholar]
- 62. Krysko KM, LaHue SC, Anderson A, et al. Minimal breast milk transfer of rituximab, a monoclonal antibody used in neurological conditions. Neurol Neuroimmunol Neuroinflamm 2020; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. LaHue SC, Anderson A, Krysko KM, et al. Transfer of monoclonal antibodies into breastmilk in neurologic and non-neurologic diseases. Neurol Neuroimmunol Neuroinflamm 2020; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ciplea AI, Langer-Gould A, de Vries A, et al. Monoclonal antibody treatment during pregnancy and/or lactation in women with MS or neuromyelitis optica spectrum disorder. Neurol Neuroimmunol Neuroinflamm 2020; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mao-Draayer Y, Thiel S, Mills EA, et al. Neuromyelitis optica spectrum disorders and pregnancy: therapeutic considerations. Nat Rev Neurol 2020; 16: 154–170. [DOI] [PubMed] [Google Scholar]
- 66. Zhovtis Ryerson L, Kister I, Snuderl M, et al. Incomplete Susac syndrome exacerbated after natalizumab. Neurol Neuroimmunol Neuroinflamm 2015; 2: e151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Landi D, Marfia GA. Exposure to natalizumab during pregnancy and lactation is safe – Yes. Mult Scler 2020; 26: 887–889. [DOI] [PubMed] [Google Scholar]
- 68. Airas L. Exposure to natalizumab during pregnancy and lactation is safe – No. Mult Scler 2020; 26: 889–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ciplea AI, Hellwig K. Exposure to natalizumab during pregnancy and lactation is safe – Commentary. Mult Scler 2020; 26: 892–893. [DOI] [PubMed] [Google Scholar]
- 70. Huang W, Wang L, Zhang B, et al. Effectiveness and tolerability of immunosuppressants and monoclonal antibodies in preventive treatment of neuromyelitis optica spectrum disorders: a systematic review and network meta-analysis. Mult Scler Relat Disord 2019; 35: 246–252. [DOI] [PubMed] [Google Scholar]
- 71. Mok CC. Calcineurin inhibitors in systemic lupus erythematosus. Best Pract Res Clin Rheumatol 2017; 31: 429–438. [DOI] [PubMed] [Google Scholar]
- 72. Gruhn N, Pedersen LK, Nielsen NV. Susac’s syndrome: the first case report in a Nordic country, with an 8-year follow-up. Acta Ophthalmol Scand 2005; 83: 757–758. [DOI] [PubMed] [Google Scholar]
- 73. Tacrolimus. Drugs and lactation database (LactMed). Bethesda (MD): National Library of Medicine (US), 2006. [Google Scholar]
- 74. French AE, Soldin SJ, Soldin OP, et al. Milk transfer and neonatal safety of tacrolimus. Ann Pharmacother 2003; 37: 815–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cyclosporine. Drugs and lactation database (LactMed). Bethesda (MD): National Library of Medicine (US), 2006. [Google Scholar]
- 76. Moretti ME, Sgro M, Johnson DW, et al. Cyclosporine excretion into breast milk. Transplantation 2003; 75: 2144–2146. [DOI] [PubMed] [Google Scholar]
- 77. Schisterman EF, Silver RM, Lesher LL, et al. Preconception low-dose aspirin and pregnancy outcomes: results from the EAGeR randomised trial. Lancet 2014; 384: 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Swartz RH, Ladhani NNN, Foley N, et al. Canadian stroke best practice consensus statement: Secondary stroke prevention during pregnancy. Int J Cerebrovasc Dis Stroke 2018; 13: 406–419. [DOI] [PubMed] [Google Scholar]
- 79. Hardy TA, O’Brien B, Gerbis N, et al. Brain histopathology in three cases of Susac’s syndrome: implications for lesion pathogenesis and treatment. J Neurol Neurosurg Psychiatry 2015; 86: 582–584. [DOI] [PubMed] [Google Scholar]
- 80. Agamanolis DP, Klonk C, Bigley K, et al. Neuropathological findings in Susac syndrome: an autopsy report. J Neuropathol Exp Neurol 2019; 78: 515–519. [DOI] [PubMed] [Google Scholar]
- 81. Gerosa M, Argolini LM, Artusi C, et al. The use of biologics and small molecules in pregnant patients with rheumatic diseases. Expert Rev Clin Pharmacol 2018; 11: 987–998. [DOI] [PubMed] [Google Scholar]