Abstract
STUDY QUESTION
Can the density of endometrial glandular openings (DEGO) be a reliable and simple new variable in the prediction of live birth after hysteroscopic adhesiolysis?
SUMMARY ANSWER
The DEGO grade at follow-up hysteroscopy outperforms American Fertility Society (AFS) score in predicting the live birth rate after hysteroscopic adhesiolysis for patients with intrauterine adhesions (IUAs).
WHAT IS KNOWN ALREADY
Several methods, such as endometrial thickness and AFS score, have been proposed for predicting the live birth rate in patients with IUAs who undergo hysteroscopic adhesiolysis.
STUDY DESIGN, SIZE, DURATION
A test cohort of 457 patients with IUAs who underwent hysteroscopic adhesiolysis and had satisfactory follow-up hysteroscopy videos were retrospectively enrolled between January 2016 and January 2017. A validation cohort comprising 285 IUA patients was prospectively enrolled from March 2018 to August 2018.
PARTICIPANTS/MATERIALS, SETTING, METHODS
An automated counting software tested the follow-up hysteroscopy videos to calculate the DEGO grade of all the 742 patients with IUAs after hysteroscopic adhesiolysis. The AFS score for each patient was also calculated at the same follow-up hysteroscopy. Logistic regression analysis was performed to develop prediction models to predict the live birth rate following hysteroscopic adhesiolysis. The performance of each of these prediction models was compared by calculating the AUC.
MAIN RESULTS AND THE ROLE OF CHANCE
In the test cohort (n = 457), 231 patients had a live birth, but 226 patients failed. In the validation cohort (n = 285), 117 patients had a live birth, while 168 patients did not. The logistic regression analysis revealed that both the DEGO grade and AFS score at follow-up hysteroscopy were closely correlated with the live birth rate in patients with IUAs (P = 0). The AUCs of AFS score and DEGO grade in the test cohort were 0.7112 and 0.8498, respectively (P < 0.0001). The AUCs of AFS score and DEGO grade in the prospective external validation cohort were 0.6937 and 0.8248, respectively (P < 0.0001).
LIMITATIONS, REASONS FOR CAUTION
Further well-designed prospective clinical studies with a multicentric larger sample size should be needed to confirm the feasibility and efficacy of DEGO.
WIDER IMPLICATIONS OF THE FINDINGS
The DEGO grade is an accurate predictor factor of live birth rate in patients with IUAs following hysteroscopic adhesiolysis and can represent in the future an important and promising tool for assessing obstetric outcomes in IUAs.
STUDY FUNDING/COMPETING INTEREST(S)
This study is supported by National Key Research and Development Program of China (Grant No. 2018YFC1004800), Natural Science Foundation of China (Grant No. 81671492), Natural Science Foundation of Hunan (Grant No. 2020JJ5859). B.G. is supported by Chinese Scholarship Council (File number. 201806370178). The authors have no conflicts of interest to declare.
TRIAL REGISTRATION NUMBER
N/A
Keywords: density of endometrial glandular openings, AFS score, intrauterine adhesions, hysteroscopic adhesiolysis, live birth rate
Introduction
Intrauterine adhesions (IUAs) is an acquired uterine condition that occurs when scar tissue forms inside the uterine cavity or cervical canal, causing damage to the basal layer of the endometrium (Asherman, 1948; Sugimoto, 1978). IUAs can cause partial or total obliteration of the uterine cavity, reduce the implantation area, and diminish endometrial receptivity by impairing the blood supply (Schenker and Margalioth, 1982; Hooker et al., 2014). Diagnostic hysteroscopy is considered the gold standard among studies on IUAs, as it has higher accuracy in confirming the presence, extent, and morphological characteristics of adhesions, as well as the quality of the endometrium, than ultrasound and hysterosalpingography, and helps in the classification of IUAs (Valle and Sciarra, 1988; Capella-Allouc et al., 1999). Hysteroscopic adhesiolysis is a recommended and effective method to treat IUAs (Chen et al., 2017), which involves releasing the adhesions and restoring the shape of the cavity.
Our understanding of the complex physiology of the endometrium and implantation of the developing embryo is incomplete, although we know that steroid hormones, peptides, growth factors, and cytokines all play a part (Damario et al., 2001; Boomsma and Macklon, 2006; Edwards, 2006). Several methods have been proposed for predicting the live birth rate in patients with IUAs who undergo hysteroscopic adhesiolysis. These methods include using transvaginal ultrasound to evaluate endometrial thickness, as described by Baradwan et al. (2018), and utilizing the American Fertility Society (AFS) score (1988), which takes into account the adhesion area, the nature of the adhesions, and the patient’s menstrual status.
An emerging area of investigation is the physiological importance of the expression of endometrial glandular openings (Masamoto et al., 2000). Hanada et al. (2012) showed that equine glandular density is strongly associated with endometrial function. Synthesis and release of Mucin 1 (MUC-1) and glycodelin-A by endometrial glands, are essential for the fetus in the early stages of pregnancy (Burton et al., 2002; Spencer, 2014). Software for automatic counting of endometrial glandular openings in image frames acquired during diagnostic hysteroscopy has been reported previously (Cunha-Filho et al., 2008). Endometrium can be evaluated by this software objectively in several situations (Hanada et al., 2012). In this study, we used this software to count and classify the endometrium of IUAs into four grades according to the density of endometrial glandular openings (DEGO) on follow-up hysteroscopy video. We then correlated the DEGO grade with the live birth rate following hysteroscopic adhesiolysis, as this relationship has yet to be reported in humans. From this evaluation, we propose using DEGO as a novel variable that is superior to existing methods as a predictor of live birth (Cunha-Filho et al., 2008).
Materials and methods
Patients
Four hundred fifty-seven patients with IUAs who underwent hysteroscopic adhesiolysis between January 2016 and January 2017 and had satisfactory follow-up hysteroscopy videos were retrospectively enrolled as the test cohort. From March 2018 to August 2018, 285 IUA patients were also prospectively enrolled for external validation of the prediction model. The ethics committee of the Third Xiangya Hospital of Central South University approved the study (IRB No. 2019-S465). The procedure was performed following the relevant guidelines and regulations. Informed consent was received after the procedure was fully explained to all participants. The surgeon scored IUAs according to the AFS classification scoring system as follows (1988): 1–4 (mild); 5–8 (moderate); and 9–12 (severe).
The inclusion criteria were: (i) IUAs confirmed by hysteroscopy; (ii) a desire for fertility; and (iii) normal hormone levels and ovulation. The exclusion criteria included: (i) Male infertility; (ii) primary infertility; (iii) tubercular IUAs; (iv) tubal factor infertility; (v) other lesions including endometrial polyps, atypical hyperplasia, or endometrial malignancy; and (vi) loss to follow-up.
After hysteroscopic adhesiolysis, patients were followed up by telephone for over 1 year. Pregnancy outcomes were tracked, including live birth or no live birth (abortion or infertility). The medical records, operative reports, and hysteroscopy videos of these patients were reviewed.
Surgical procedure and postoperative follow-up hysteroscopy
Hysteroscopic adhesiolysis and postoperative follow-up hysteroscopy were performed within 3–7 days following menstruation. Hysteroscopy was carried out using an operative hysteroscope with an outer sheath diameter of 5.4 mm and a working channel diameter of 1.67 mm (KARL STORZ SE & Co. KG–Tuttlingen, Baden-Württemberg, Germany).
When performing the hysteroscopic adhesiolysis, the hysteroscope was introduced into the cervical canal through the cervix with the aim of reaching the intrauterine cavity. The adhesions located in the central part of the uterine cavity were usually dissected first and then the lateral adhesions were cut out. First, the tubal ostia, which is the anatomical landmark during hysteroscopic adhesiolysis, needed to be retrieved. Then, upon its visualization, the adhesions could be easily dissected. In cases of intrauterine anatomy distortion caused by adhesions, 5-Fr double action forceps were used under transabdominal ultrasound monitoring, using a blunt spreading dissection technique (Zhang et al., 2015) to separate the adhesions and anatomically reveal the uterine cavity. If the intrauterine anatomy was clear, the IUAs were separated with 5-Fr single action sharp scissors and the scar tissue covering the intrauterine wall was treated with a ‘cold scissors ploughing technique’ (Zhao et al., 2020b) until the entire uterine cavity had been opened successfully with a clearly visible bilateral fallopian tube ostia.
After hysteroscopic adhesiolysis, a uterine-shaped stainless-steel intrauterine device (IUD) was inserted into the uterine cavity, and a double-channel, 12-Fr Foley catheter balloon, with the top catheter portion beyond the balloon removed, was inserted into the uterine cavity and distended using 2.5 ml of sterile saline, with the balloon in the center of the uterine-shaped IUD (Fig. 1). Hyaluronic acid gel (Pabuçcu et al., 2019) (3 ml) was then injected into the uterine cavity through the catheter.
Figure 1.
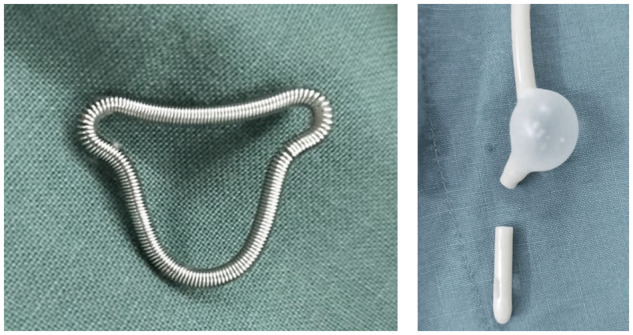
Uterine-shaped stainless-steel IUD and a double-channel, 12-Fr Foley catheter balloon. A uterine-shaped stainless-steel intrauterine device (IUD) was inserted into the uterine cavity, and a double-channel, 12-Fr Foley catheter balloon, with the top catheter portion beyond the balloon removed, was inserted into the uterine cavity and distended using 2.5 ml of sterile saline, with the balloon in the center of the uterine-shaped IUD after hysteroscopic adhesiolysis.
Postoperatively, a hysteroscopic follow-up strategy was conducted (3 months after the first surgery). During the follow-up procedure, the patients’ hysteroscopy videos were recorded.
Method for calculating the DEGO
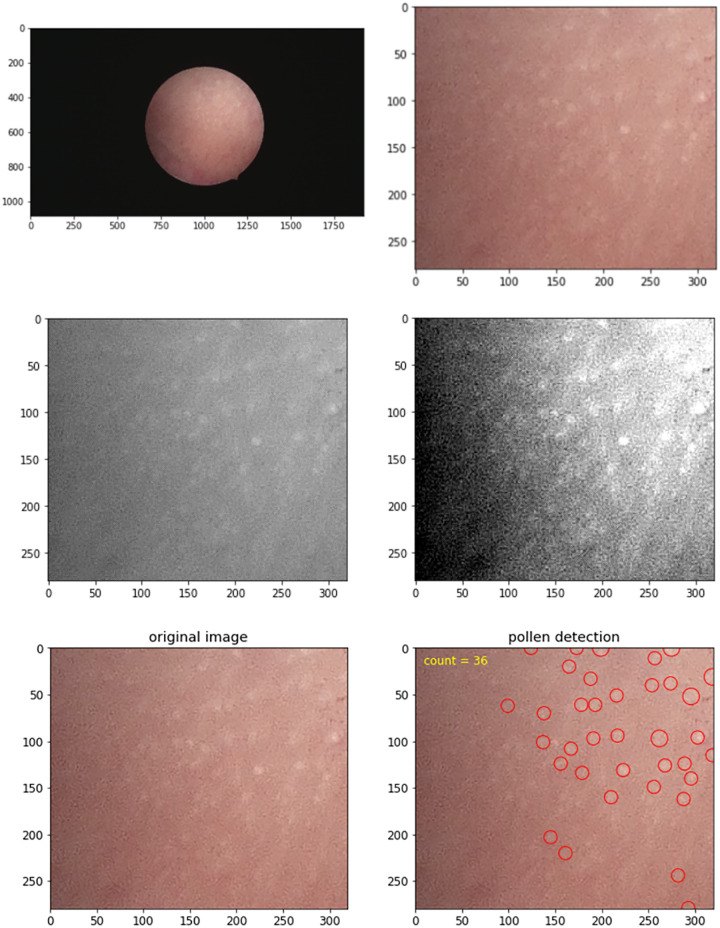
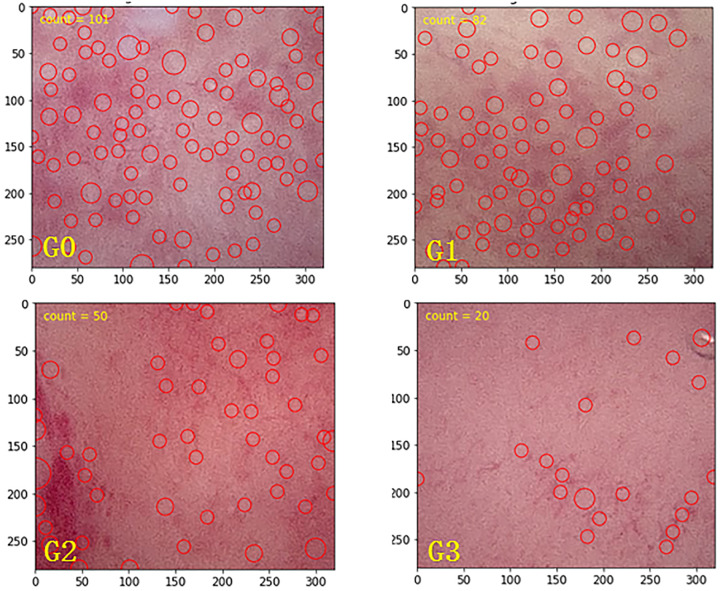
The software program for determining the DEGO in the follow-up hysteroscopy videos that is presented here comprises four steps. Step 1 is key frame selection; key frames are extracted from each video segment using a methodology from the singular value decomposition (Cunha-Filho et al., 2008). This technique is used to retrieve a refined feature space in which visually similar feature vectors (i.e. frames) are more easily clustered, and their relative positions show their correlation and redundancy (Hanada et al., 2012). Step 2 is grayscale imaging; after capturing the key frames in the original images, grayscale transformation is used to enhance the images. Step 3 is local histogram equalization; histogram equalization is a method in image processing that uses contrast adjustment from the image’s histogram. In this study, images with low contrast were enhanced using a method called local histogram equalization, which spreads out the most frequent intensity values in an image. Step 4 is blob detection; in computer images, blobs are bright on dark or bright regions in an image. Laplacian of Gaussian is an operation in computer analysis of images that is used to smooth an image before processing. The program then computes the Laplacian of Gaussian for images with successive SDs and stacks them up in a cube. Blobs are the local maximum in this cube (Fig. 2). The method of classification for DEGO is as follows (see Fig. 3): G0 DEGO: The glands are abundant, and experts judge them to be normal DEGO, the mean ± SD of normal DEGO is 118 ± 25 (range, 92–146); G1 DEGO: less than G0 but over 2/3 G0; G2 DEGO: less than 2/3 G0 but over 1/3 G0; G3 DEGO: less than 1/3 G0.
Figure 2.
The software for determining the DEGO in the follow-up hysteroscopy videos. Step 1: Key frames were extracted from each video segment using a methodology based on the singular value decomposition. Step 2: Grayscale transformation is used to enhance the images. Step 3: The images with low contrast are enhanced using a method called local histogram equalization, which spreads out the most frequent intensity values in an image. Step 4: The program computes the Laplacian of Gaussian for images with successive SD and stacks them up in a cube. Finally, the DEGO in respond image were marked with small circles.
Figure 3.
The method of classification for DEGO: G0 DEGO. The glands are abundant and experts judge them to be normal DEGO, the mean ± SD of normal DEGO was 118 ± 25 (range, 92–146); G1 DEGO: less than G0 but more than 2/3 G0; G2 DEGO: less than 2/3 G0 but more than 1/3 G0; G3 DEGO: less than 1/3 G0.
Other parameters, including the length of the uterine cavity, the number of visible uterine horns, and tubal ostia, were also measured and recorded. The AFS score of each patient was calculated. Two gynecologists confirmed all data of the above parameters.
Statistics
Statistical analysis was performed with the Statistical Analysis System 9.4 (SAS, USA). Differences between the live birth group and the no live birth group were tested using a χ2 test or Fisher’s exact test as appropriate. Logistic regression analysis was applied to decide which were the dominant variables as covariates combined with the AFS score or DEGO grade to establish the live birth rate prediction models. The AUCs of the models were compared to verify their prediction accuracy. A value of P < 0.05 was considered statistically significant.
Results
Telephone follow-up for 1-year post-hysteroscopic adhesiolysis in the test cohort showed that 231 patients had a live birth, and 226 patients did not have a live birth; in the validation cohort, 117 patients had a live birth, and 168 patients did not have a live birth. The automatic counting software showed the mean ± SD DEGO to be 61.7 ± 30 (range, 5–146), and the mean ± SD of normal DEGO to be 118 ± 25 (range, 92–146). Variables including age, recurrent IUAs, menstrual flow, uterine cavity length, number of visible cornu and tubal ostia, and especially AFS score and the DEGO were significantly related to the live birth rate post-hysteroscopic adhesiolysis (P < 0.05). Other variables, including gravidity history and IUA course, did not have any statistical significance in relation to the live birth rate after hysteroscopic adhesiolysis (P > 0.05) (Table I).
Table I.
Clinical characteristics of the patients with intrauterine adhesions in the test cohort and validation cohort.
| Test cohort |
Validation cohort |
|||||||
|---|---|---|---|---|---|---|---|---|
| Clinical characteristic | Category | Live birth group | No live birth group | P1 | Live birth group | No live birth group | P2 | Total |
| Age | N (N missing) | 231 (0) | 226 (0) | P = 0.0003 | 117 (0) | 168 (0) | P = 0.0061 | 742 (0) |
| Mean (SD) | 30.3 (4.30) | 31.9 (4.94) | 30.5 (4.48) | 32.1 (4.84) | 31.2 (4.71) | |||
| Gravidity | 1 | 47 (20.3%) | 53 (23.5%) | P = 0.0038 | 28 (23.9%) | 40 (23.8%) | P = 0.4333 | 168 (22.6%) |
| 2 | 73 (31.6%) | 41 (18.1%) | 39 (33.3%) | 45 (26.8%) | 198 (26.7%) | |||
| ≥3 | 111 (48.1%) | 132 (58.4%) | 50 (42.7%) | 83 (49.4%) | 376 (50.7%) | |||
| Total | 231 (100.0%) | 226 (100.0%) | 117 (100.0%) | 168 (100.0%) | 742 (100.0%) | |||
| Parity | 1 | 228 (98.7%) | 218 (96.5%) | P = 0.1390 | 113 (96.6%) | 161 (95.8%) | P = 0.8744 | 720 (97.0%) |
| 2 | 2 (0.9%) | 7 (3.1%) | 3 (2.6%) | 6 (3.6%) | 18 (2.4%) | |||
| ≥3 | 1 (0.4%) | 1 (0.4%) | 1 (0.9%) | 1 (0.6%) | 4 (0.5%) | |||
| Total | 231 (100.0%) | 226 (100.0%) | 117 (100.0%) | 168 (100.0%) | 742 (100.0%) | |||
| Abortion | 1 | 75 (32.5%) | 75 (33.2%) | P = 0.0692 | 48 (41.0%) | 62 (36.9%) | P = 0.7866 | 260 (35.0%) |
| 2 | 75 (32.5%) | 53 (23.5%) | 34 (29.1%) | 52 (31.0%) | 214 (28.8%) | |||
| ≥3 | 81 (35.1%) | 98 (43.4%) | 35 (29.9%) | 54 (32.1%) | 268 (36.1%) | |||
| Total | 231 (100.0%) | 226 (100.0%) | 117 (100.0%) | 168 (100.0%) | 742 (100.0%) | |||
| Recurrent IUA | Yes | 42 (18.2%) | 75 (33.2%) | P = 0.0003 | 27 (23.1%) | 56 (33.3%) | P = 0.0648 | 200 (27.0%) |
| No | 189 (81.8%) | 151 (66.8%) | 90 (76.9%) | 112 (66.7%) | 542 (73.0%) | |||
| Total | 231 (100.0%) | 226 (100.0%) | 117 (100.0%) | 168 (100.0%) | 742 (100.0%) | |||
| Menstruation | NA | 1 (0.4%) | 1 (0.4%) | P = 0.0168 | 0 (0.0%) | 0 (0.0%) | P = 0.0445 | 2 (0.3%) |
| Eumenorrhea | 29 (12.6%) | 26 (11.5%) | 3 (2.6%) | 0 (0.0%) | 58 (7.8%) | |||
| Oligomenorrhea | 189 (81.8%) | 170 (75.2%) | 110 (94.0%) | 156 (92.9%) | 625 (84.2%) | |||
| Amenorrhea | 12 (5.2%) | 29 (12.8%) | 4 (3.4%) | 12 (7.1%) | 57 (7.7%) | |||
| Total | 231 (100.0%) | 226 (100.0%) | 117 (100.0%) | 168 (100.0%) | 742 (100.0%) | |||
| IUA course | NA | 36 (15.6%) | 43 (19.0%) | P = 1.0000 | 0 (0.0%) | 0 (0.0%) | P = 1.0000 | 79 (10.6%) |
| ≤6 months | 52 (22.5%) | 49 (21.7%) | 46 (39.3%) | 65 (38.7%) | 212 (28.6%) | |||
| >6 months | 143 (61.9%) | 134 (59.3%) | 71 (60.7%) | 103 (61.3%) | 451 (60.8%) | |||
| Total | 231 (100.0%) | 226 (100.0%) | 117 (100.0%) | 168 (100.0%) | 742 (100.0%) | |||
| Uterine cavity length | N (N miss) | 227 (4) | 221 (5) | P = 0.0114 | 117 (0) | 168 (0) | P = 0.1502 | 733 (9) |
| Mean (SD) | 7.2 (0.52) | 7.0 (0.68) | 7.3 (0.61) | 7.2 (0.74) | 7.1 (0.64) | |||
| Uterine horn adhesion | Bilateral adhesion | 4 (1.7%) | 10 (4.4%) | P = 0.0869 | 4 (3.4%) | 18 (10.7%) | P = 0.0446 | 36 (4.9%) |
| Unilateral adhesion | 2 (0.9%) | 6 (2.7%) | 2 (1.7%) | 6 (3.6%) | 16 (2.2%) | |||
| None adhesion | 225 (97.4%) | 210 (92.9%) | 111 (94.9%) | 144 (85.7%) | 690 (93.0%) | |||
| Total | 231 (100.0%) | 226 (100.0%) | 117 (100.0%) | 168 (100.0%) | 742 (100.0%) | |||
| Visibility of fallopian tube ostia | Bilateral invisible | 5 (2.2%) | 22 (9.7%) | P = 0.0000 | 7 (6.0%) | 24 (14.3%) | P = 0.0104 | 58 (7.8%) |
| Unilateral invisible | 5 (2.2%) | 23 (10.2%) | 5 (4.3%) | 17 (10.1%) | 50 (6.7%) | |||
| Bilateral visible | 221 (95.7%) | 181 (80.1%) | 105 (89.7%) | 127 (75.6%) | 634 (85.4%) | |||
| Total | 231 (100.0%) | 226 (100.0%) | 117 (100.0%) | 168 (100.0%) | 742 (100.0%) | |||
| DEGO degree | NA | 1 (0.4%) | 14 (6.2%) | P = 0.0000 | 0 (0.0%) | 0 (0.0%) | P = 0.0000 | 15 (2.0%) |
| G0 | 39 (16.9%) | 3 (1.3%) | 24 (20.5%) | 3 (1.8%) | 69 (9.3%) | |||
| G1 | 152 (65.8%) | 56 (24.8%) | 70 (59.8%) | 38 (22.6%) | 316 (42.6%) | |||
| G2 | 35 (15.2%) | 109 (48.2%) | 19 (16.2%) | 86 (51.2%) | 249 (33.6%) | |||
| G3 | 4 (1.7%) | 44 (19.5%) | 4 (3.4%) | 41 (24.4%) | 93 (12.5%) | |||
| Total | 231 (100.0%) | 226 (100.0%) | 117 (100.0%) | 168 (100.0%) | 742 (100.0%) | |||
| AFS score | N (N miss) | 230 (1) | 226 (0) | P = 0.0000 | 117 (0) | 168 (0) | P = 0.0000 | 741 (1) |
| Mean (SD) | 2.7 (1.01) | 3.5 (2.03) | 2.8 (1.52) | 3.8 (2.28) | 3.2 (1.83) | |||
IUA, Intrauterine adhesion; AFS, American Fertility Society; DEGO, the density of endometrial glandular openings; P1, P-value for Test cohort; P2, P-value for Validation cohort; NA, no answer; G1, grade 1 of DEGO; G2, grade 2 of DEGO; G3, grade 3 of DEGO.
The risk factors for live birth rate were evaluated in univariate analysis. A multivariate logistical regression analysis was conducted for the meaningful variables (P < 0.05) identified by univariate logistical regression analysis. Compared with the live birth group, women in the no live birth group were older (P = 0.0004) and were more likely to have a higher AFS score (P < 0.0001). Women in the no live birth group were also significantly more likely to have DEGO grade of G1 (P = 0.0114), G2 (P < 0.0001) and G3 (P < 0.0001) (Table II).
Table II.
Univariate analysis of the risk factors for live birth rate.
| Variables | Category | Estimate | SE | χ2* | P-value | Odds ratio | 95% confidence interval |
|---|---|---|---|---|---|---|---|
| Age | −0.0733 | 0.0206 | 12.6355 | 0.0004 | 0.929 | 0.893–0.968 | |
| Gravidity | 1 | Reference | |||||
| 2 | 0.697 | 0.2797 | 6.2097 | 0.0127 | 2.008 | 1.16–3.474 | |
| ≥3 | −0.0531 | 0.2382 | 0.0498 | 0.8235 | 0.948 | 0.595–1.512 | |
| Parity | 1 | Reference | |||||
| 2 | −1.2976 | 0.8074 | 2.5832 | 0.108 | 0.273 | 0.056–1.329 | |
| ≥3 | −0.0449 | 1.4174 | 0.001 | 0.9748 | 0.956 | 0.059–15.382 | |
| Abortion | 1 | Reference | |||||
| 2 | 0.3472 | 0.2426 | 2.0477 | 0.1524 | 1.415 | 0.88–2.277 | |
| ≥3 | −0.1905 | 0.2218 | 0.7375 | 0.3905 | 0.827 | 0.535–1.277 | |
| Recurrent IUA | Yes | Reference | |||||
| No | 0.8043 | 0.2215 | 13.1854 | 0.0003 | 2.235 | 1.448–3.45 | |
| Menstruation | Eumenorrhea | Reference | |||||
| Oligomenorrhea | −0.00325 | 0.29 | 0.0001 | 0.9911 | 0.997 | 0.565–1.76 | |
| Amenorrhea | −0.991 | 0.4367 | 5.1493 | 0.0233 | 0.371 | 0.158–0.874 | |
| IUA course | ≤6 months | Reference | |||||
| >6 months | 0.00558 | 0.2326 | 0.0006 | 0.9809 | 1.006 | 0.637–1.586 | |
| Uterine cavity length | 0.4033 | 0.1609 | 6.2813 | 0.0122 | 1.497 | 1.092–2.052 | |
| Uterine horn adhesion | Bilateral adhesion | reference | |||||
| unilateral adhesion | −0.1823 | 1.0083 | 0.0327 | 0.8565 | 0.833 | 0.115–6.013 | |
| None adhesion | 0.9853 | 0.5993 | 2.7026 | 0.1002 | 2.679 | 0.827–8.671 | |
| Visibility of fallopian tube ostia | Bilateral invisible | reference | |||||
| Unilateral invisible | −0.0444 | 0.6992 | 0.004 | 0.9493 | 0.957 | 0.243–3.766 | |
| Bilateral visible | 1.6812 | 0.5055 | 11.0627 | 0.0009 | 5.372 | 1.995–14.467 | |
| DEGO degree | G0 | reference | |||||
| G1 | −1.5663 | 0.6192 | 6.3991 | 0.0114 | 0.209 | 0.062–0.703 | |
| G2 | −3.7008 | 0.6298 | 34.527 | <0.0001 | 0.025 | 0.007–0.085 | |
| G3 | −4.9626 | 0.7948 | 38.9901 | <0.0001 | 0.007 | 0.001–0.033 | |
| AFS scores | −0.3684 | 0.0752 | 23.997 | <0.0001 | 0.692 | 0.597–0.802 |
χ2 test for entire group.
Bivariate and binary logistic regression analysis revealed that AFS score and the DEGO grade were strictly related to the live birth rates in patients with IUAs (Tables III and IV). In the test cohort, the AUC of the prediction model of AFS score with other covariates (excluding the DEGO grade) was 0.7112. The AUC of the prediction model of DEGO grade with other covariates (excluding AFS score) was 0.8498 (Fig. 4). There was a significant statistical difference in the AUCs for DEGO grade and AFS score in the prediction of live birth rate in patients with IUAs (P < 0.0001) (Table V).
Table III.
Multivariate logistical regression analysis of DEGO degree and other covariates (excluding AFS score).
| Variables | Category | Estimate | SE | χ2* | P-value | Odds ratio | 95% confidence interval |
|---|---|---|---|---|---|---|---|
| Test cohort | |||||||
| Intercept | 3.3705 | 1.2008 | 7.8786 | 0.005 | / | / | |
| Age | −0.0849 | 0.0278 | 9.3622 | 0.0022 | 0.919 | 0.87–0.97 | |
| Gravidity | 2 | 0.7324 | 0.354 | 4.2788 | 0.0386 | 2.08 | 1.039–4.163 |
| ≥3 | 0.6298 | 0.3135 | 4.0345 | 0.0446 | 1.877 | 1.015–3.471 | |
| Visibility of fallopian tube ostia | Unilateral invisible | 0.2791 | 0.86 | 0.1053 | 0.7456 | 1.322 | 0.245–7.133 |
| Bilateral visible | 1.3508 | 0.654 | 4.266 | 0.0389 | 3.86 | 1.071–13.909 | |
| DEGO | G1 | −1.4932 | 0.6273 | 5.6653 | 0.0173 | 0.225 | 0.066–0.768 |
| G2 | −3.5891 | 0.6403 | 31.4194 | <0.0001 | 0.028 | 0.008–0.097 | |
| G3 | −4.7467 | 0.8143 | 33.9816 | <0.0001 | 0.009 | 0.002–0.043 | |
| Validation cohort | |||||||
| Intercept | 2.7356 | 1.3021 | 4.4141 | 0.0356 | / | / | |
| Age | −0.0441 | 0.0339 | 1.695 | 0.1929 | 0.957 | 0.895–1.023 | |
| Gravidity | 2 | 0.4683 | 0.4219 | 1.2319 | 0.267 | 1.597 | 0.699–3.652 |
| ≥3 | 0.2055 | 0.394 | 0.272 | 0.602 | 1.228 | 0.567–2.658 | |
| Visibility of fallopian tube ostia | Unilateral invisible | 0.5001 | 0.808 | 0.3831 | 0.5359 | 1.649 | 0.338–8.034 |
| Bilateral visible | 0.401 | 0.5634 | 0.5066 | 0.4766 | 1.493 | 0.495–4.506 | |
| DEGO degree | G1 | −1.3449 | 0.6652 | 4.0872 | 0.0432 | 0.261 | 0.071–0.96 |
| G2 | −3.441 | 0.6819 | 25.4664 | <0.0001 | 0.032 | 0.008–0.122 | |
| G3 | −4.3012 | 0.8331 | 26.6538 | <0.0001 | 0.014 | 0.003–0.069 |
χ2 test for entire group.
Table IV.
Multivariate logistical regression analysis of AFS score and other covariates (excluding DEGO degree).
| Variables | Category | Estimate | SE | χ2* | P-value | Odds ratio | 95% confidence interval |
|---|---|---|---|---|---|---|---|
| Test cohort | |||||||
| Intercept | −0.14 | 1.7925 | 0.0061 | 0.9377 | . | ||
| Age | −0.0904 | 0.0226 | 15.9919 | <0.0001 | 0.914 | 0.874–0.955 | |
| Recurrent IUA | No | 0.6711 | 0.2459 | 7.4449 | 0.0064 | 1.956 | 1.208–3.168 |
| Uterine cavity length | 0.1093 | 0.1831 | 0.3562 | 0.5506 | 1.115 | 0.779–1.597 | |
| Visibility of fallopian tube ostia | Unilateral invisible | 1.3826 | 1.1928 | 1.3435 | 0.2464 | 3.985 | 0.385–41.281 |
| Bilateral visible | 2.6334 | 1.087 | 5.8689 | 0.0154 | 13.92 | 1.653–117.196 | |
| AFS score | −0.275 | 0.0946 | 8.4571 | 0.0036 | 0.76 | 0.631–0.914 | |
| Validation cohort | |||||||
| Intercept | 0.9246 | 1.6803 | 0.3028 | 0.5821 | / | ||
| Age | −0.0739 | 0.028 | 6.9504 | 0.0084 | 0.929 | 0.879–0.981 | |
| Recurrent IUA | No | 0.3485 | 0.291 | 1.4342 | 0.2311 | 1.417 | 0.801–2.507 |
| Uterine cavity length | 0.1499 | 0.1934 | 0.6012 | 0.4381 | 1.162 | 0.795–1.697 | |
| Visibility of fallopian tube ostia | Unilateral invisible | 0.0642 | 0.6983 | 0.0084 | 0.9268 | 1.066 | 0.271–4.191 |
| Bilateral visible | 0.5908 | 0.4834 | 1.4941 | 0.2216 | 1.805 | 0.7–4.656 | |
| AFS score | −0.2482 | 0.0832 | 8.8974 | 0.0029 | 0.78 | 0.663–0.918 |
χ2 test for entire group.
Figure 4.
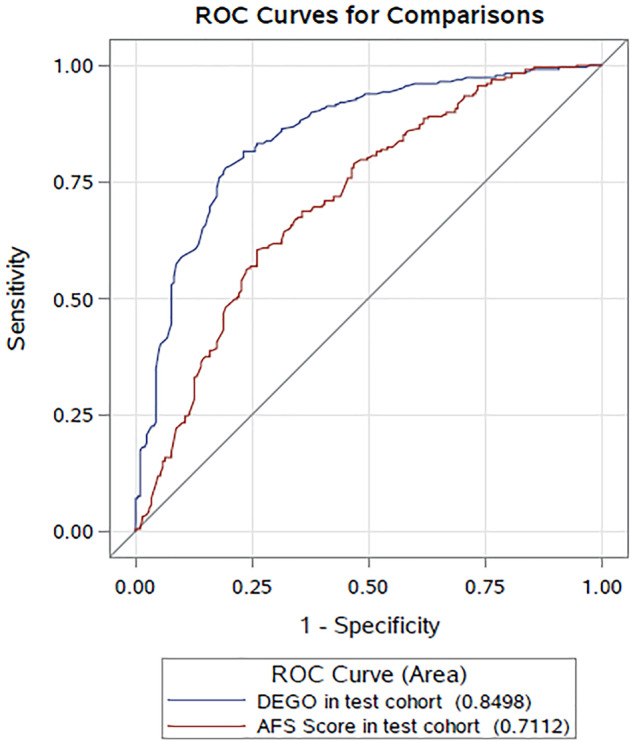
The ROCs (receiver operating characteristic curves) of the prediction models in the test cohort. The AUCs of the prediction models of AFS score with other covariates (excluding DEGO degree) and DEGO degree with other covariates (excluding AFS score).
Table V.
Comparison of the area under the curves of the prediction models in the test cohort.
| Comparison of AUCs | Estimate | SE | 95% CI | χ2* | Pr > χ2* | |
|---|---|---|---|---|---|---|
| DEGO degree—AFS score | 0.1386 | 0.0246 | 0.0905–0.1868 | 31.8172 | <0.0001 | |
χ2 test for entire group; Pr, P-value.
Bivariate and binary logistic regression analyses in the prospective external validation cohort revealed that DEGO grade performed well in predicting the live birth rate in patients with IUAs (Table VI). The AUCs for AFS score and DEGO grade in the validation cohort were 0.6937 and 0.8248, respectively (Fig. 5).
Table VI.
Comparison of the area under the curves of the prediction models in the different cohorts.
| Model | Cohort | AUC | SE | 95% CI | P |
|---|---|---|---|---|---|
| AFS score | Validation cohort | 0.6937 | 0.0317 | 0.6317–0.7557 | P < 0.0001 |
| Test cohort | 0.7112 | 0.0248 | 0.6626–0.7599 | ||
| DEGO degree | Validation cohort | 0.8248 | 0.0253 | 0.7753–0.8743 | P < 0.0001 |
| Test cohort | 0.8498 | 0.0187 | 0.8132–0.8865 |
Figure 5.
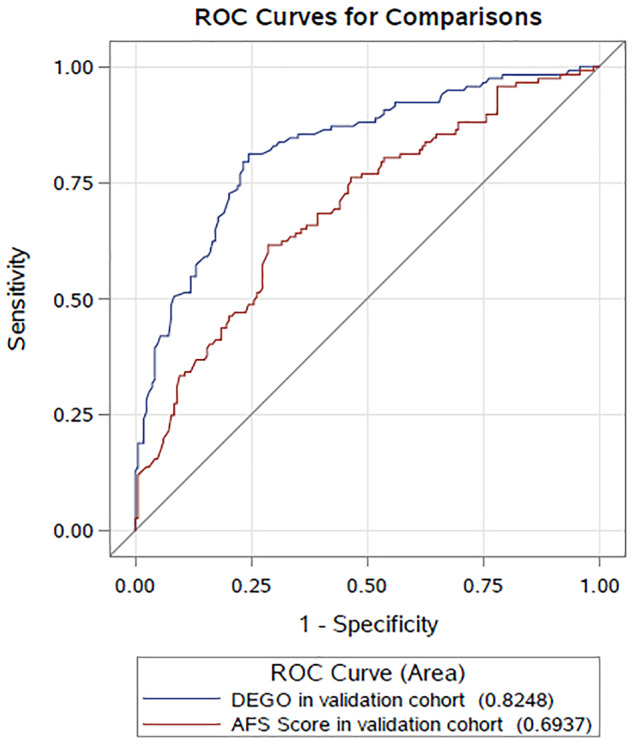
The ROCs of the prediction models in the validation cohort: The AUCs of the prediction models of AFS score with other covariates (excluding DEGO degree) and DEGO degree with other covariates (excluding AFS score).
Discussion
Since hysteroscopy was introduced, the reported incidence and diagnosis of IUAs have increased considerably, especially in patients of childbearing age (Yu et al., 2008a,b). The most common risk factor for IUAs is pregnancy-associated dilation and curettage (March, 2011; Takai et al., 2015). As earlier studies have shown, dilation and curettage after abortion accounts for over 80% of IUAs (Deans and Abbott, 2010; Xiao et al., 2014). In our study, 77.9% of patients had a history of dilation and curettage in early pregnancy, and 5.0% had a history of hysteroscopic diagnostic curettage. This finding was consistent with those of earlier studies (Xiao et al., 2014).
The primary concern for patients with IUAs is their chance to have a live birth following hysteroscopic adhesiolysis. The AFS scoring system was developed for the clinical evaluation of the severity of IUAs and to help predict pregnancy outcomes. The variables of the AFS scoring system include the adhesion area, the nature of the adhesions, and the patient’s menstrual status. Menstrual status is used to test endometrial function; however, this variable is extremely subjective and may not accurately reflect the patient’s endometrial function.
Endometrial biopsy is another commonly used method for assessing endometrial status. However, the utility of endometrial biopsy for evaluating the endometrium has been the primary question of some well-designed studies (Coutifaris et al., 2004). One reason for this is that the prevalence of out-of-phase endometrial biopsies was not found to be different between fertile or infertile populations (Coutifaris et al., 2004). The accuracy of histologic endometrial dating for the detection of endometrial defects is uncertain (Young, 2017). Another reason is that a single biopsy is not necessarily representative of the entire endometrium (Schlafer, 2007). Thus, the assessment of the DEGO from a single biopsy could lead to an inaccurate diagnosis and prognosis (Hanada et al., 2012). Measurement of endometrial thickness by ultrasound is also suboptimal, as it varies along with the menstrual cycle and cannot determine the status of endometrial glands. DEGO is a more reliable marker of endometrial function as the number of glandular openings is constant throughout the menstrual cycle (Lüdicke et al., 2001). However, the appearance of the glandular openings does change during the menstrual cycle (from dot and punctate-type to ring-type) and is related to reproductive status and live birth rate (Li et al., 2010). More awareness of these changes and further study will yield more information for the future assessment of endometrial health.
In our study, the AUC of the prediction model of DEGO grade with other covariates (excluding AFS score) was 0.8498, which was significantly higher than the AUC of the prediction model of AFS score with other covariates (excluding DEGO grade) (AUC = 0.7112). The same performance was observed in the prospectively enrolled external validation cohort. Therefore, DEGO grade can be used to predict the live birth rate of IUA patients with more accuracy than the AFS scoring system.
Additionally, several other variables were found to be related to endometrial regeneration and, consequently, the implantation rates of IUA patients, including age (Maheshwari et al., 2008), pre-hysteroscopic adhesiolysis history (Zhao et al., 2020a), and the length of the uterine cavity (from the fundus to the external cervical os) (Revelli et al., 2016; Gao et al., 2019). These variables were included in our prediction models as covariates. Out of all these variables, however, the DEGO was the most relevant to the endometrium condition and was most closely related to the live birth rate post-hysteroscopic adhesiolysis. With this in mind, surgeons need to do their best to protect the endometrium during hysteroscopic adhesiolysis; for instance, they should use regular scissors instead of electrical instruments, which may cause thermal injury to the endometrium (Malhotra et al., 2012, 2017), applying a ploughing technique to provide a surface with a fresh, rich blood supply on which the endometrium may grow and cover post-hysteroscopic adhesiolysis (Zhang et al., 2015; Zhao et al., 2020b).
An important innovation of this study is to highlight the utility of DEGO grade as a variable in predicting live birth rates for patients with IUAs who undergo hysteroscopic adhesiolysis and have hopes for future fertility. To our knowledge, this is the first study to test DEGO measured by hysteroscopy as a potential predictor of the live birth rate post-hysteroscopic adhesiolysis. Our study revealed that the DEGO grade in follow-up hysteroscopy videos was significantly correlated with the live birth rate. When the AUCs were compared to verify prediction accuracy, DEGO showed a closer correlation with the live birth rate post-hysteroscopic adhesiolysis than other traditional methods, and the strengths of the study lie in its statistical design with external validation of the results.
As with any study, our investigation has limitations. The discussion of DEGO as new technology is limited to what we observed and is germane to the general obstetrics and gynecology. Additionally, endometrial thickness, as monitored by transvaginal ultrasound, was not reported. However, we believe our findings could supply some valuable information for the prediction of live birth rates in IUA patients. Further well-designed prospective clinical studies with a multicentric larger sample size will be needed to confirm the feasibility and efficacy of DEGO.
In conclusion, with DEGO, we propose a new and accurate method for the hysteroscopic evaluation of the endometrium in IUA patients. This alternative method provides a more accurate prediction of live birth rate for patients with IUAs following hysteroscopic adhesiolysis.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Authors’ roles
(i) Conception and design: D.X.; (ii) Collection and assembly of data: X.Z.; (iii) Data analysis and interpretation: B.G., X.Y., and A.Z.; (iv) Manuscript writing: All authors; (v) Final approval of manuscript: all authors.
Funding
This study is supported by National Key Research and Development Program of China (Grant No. 2018YFC1004800), Natural Science Foundation of China (Grant No. 81671492), and Natural Science Foundation of Hunan (Grant No. 2020JJ5859). B.G. is supported by Chinese Scholarship Council (File No. 201806370178).
Conflict of interest
The authors have no conflicts of interest to declare.
References
- AAGL practice report: practice guidelines for management of intrauterine synechiae. J Minim Invasive Gynecol 17:1–7. [DOI] [PubMed] [Google Scholar]
- The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, müllerian anomalies and intrauterine adhesions. Fertil Steril 1988;49:944–955. [DOI] [PubMed] [Google Scholar]
- AAGL practice report: practice guidelines on intrauterine adhesions developed in collaboration with the European Society of Gynaecological Endoscopy (ESGE). Gynecol Surg 2017;14:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asherman JG. Amenorrhoea traumatica (atretica). J Obstet Gynaecol Br Emp 1948;55:23–30. [DOI] [PubMed] [Google Scholar]
- Baradwan S, Shafi D, Baradwan A, Bashir MS, Al-Jaroudi D.. The effect of endometrial thickness on pregnancy outcome in patients with Asherman’s syndrome post-hysteroscopic adhesiolysis. Int J Women’s Health 2018;10:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomsma CM, Macklon NS.. What can the clinician do to improve implantation? Reprod Biomed Online 2006;13:845–855. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E.. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J Clin Endocrinol Metab 2002;87:2954–2959. [DOI] [PubMed] [Google Scholar]
- Capella-Allouc S, Morsad F, Rongières-Bertrand C, Taylor S, Fernandez H.. Hysteroscopic treatment of severe Asherman’s syndrome and subsequent fertility. Hum Reprod 1999;14:1230–1233. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhang H, Wang Q, Xie F, Gao S, Song Y, Dong J, Feng H, Xie K, Sui L.. Reproductive outcomes in patients with intrauterine adhesions following hysteroscopic adhesiolysis: experience from the largest women’s hospital in China. J Minim Invasive Gynecol 2017;24:299–304. [DOI] [PubMed] [Google Scholar]
- Coutifaris C, Myers ER, Guzick DS, Diamond MP, Carson SA, Legro RS, McGovern PG, Schlaff WD, Carr BR, Steinkampf MP. et al. Histological dating of timed endometrial biopsy tissue is not related to fertility status. Fertil Steril 2004;82:1264–1272. [DOI] [PubMed] [Google Scholar]
- Cunha-Filho JS, Arbo E, Rosa V, Sloczinski CR, Berton G, Gavião Neto WP, Genro VK.. Variability of endometrial glandular opening count in infertile patients prior to first IVF treatment. Reprod Biomed Online 2008;17:564–568. [DOI] [PubMed] [Google Scholar]
- Damario MA, Lesnick TG, Lessey BA, Kowalik A, Mandelin E, Seppälä M, Rosenwaks Z, Endometrial markers of uterine receptivity utilizing the donor oocyte model. Hum Reprod 2001;16:1893–1899. [DOI] [PubMed] [Google Scholar]
- Deans R, Abbott J.. Review of intrauterine adhesions. J Minim Invasive Gynecol 2010;17:555–569. [DOI] [PubMed] [Google Scholar]
- Edwards RG. Human implantation: the last barrier in assisted reproduction technologies? Reprod Biomed Online 2006;13:887–904. [DOI] [PubMed] [Google Scholar]
- Gao H, Liu DE, Li Y, Tang J, Hu S, Wu X, Tian Z, Tan H.. Uterine size and volume are associated with a higher clinical pregnancy rate in patients undergoing assisted reproduction technology: A longitudinal study (A STROBE-compliant article). Medicine 2019;98:e14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada M, Maeda Y, Oikawa MA.. Equine endometrial gland density and endometrial thickness vary among sampling sites in thoroughbred mares. J Equine Sci 2012;23:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker AB, Lemmers M, Thurkow AL, Heymans MW, Opmeer BC, Brölmann HA, Mol BW, Huirne JA.. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long-term reproductive outcome. Hum Reprod Update 2014;20:262–278. [DOI] [PubMed] [Google Scholar]
- Lüdicke F, Johannisson E, Helmerhorst FM, Campana A, Foidart J, Heithecker R.. Effect of a combined oral contraceptive containing 3 mg of drospirenone and 30 microg of ethinyl estradiol on the human endometrium. Fertil Steril 2001;76:102–107. [DOI] [PubMed] [Google Scholar]
- Li SC, Feng M, Nie QY, Nie QY, Pan P, Wu SM, Wu JH, Cheng SX, Kang JL, Guo ZW.. [Predictive value of endometrial receptivity and pregnancy outcome by hysteroscopy examination at the phase of implantation window in unexplained infertile women]. Zhonghua Fu Chan Ke Za Zhi 2010;45:184–190. [PubMed] [Google Scholar]
- Maheshwari A, Hamilton M, Bhattacharya S.. Effect of female age on the diagnostic categories of infertility. Hum Reprod 2008;23:538–542. [DOI] [PubMed] [Google Scholar]
- Malhotra N, Bahadur A, Kalaivani M, Mittal S.. Changes in endometrial receptivity in women with Asherman’s syndrome undergoing hysteroscopic adhesiolysis. Arch Gynecol Obstet 2012;286:525–530. [DOI] [PubMed] [Google Scholar]
- March CM. Management of Asherman’s syndrome. Reprod Biomed Online 2011;23:63–76. [DOI] [PubMed] [Google Scholar]
- Masamoto H, Nakama K, Kanazawa K.. Hysteroscopic appearance of the mid-secretory endometrium: relationship to early phase pregnancy outcome after implantation. Hum Reprod 2000;15:2112–2118. [DOI] [PubMed] [Google Scholar]
- Pabuçcu EG, Kovanci E, Şahin Ö, Arslanoğlu E, Yıldız Y, Pabuçcu R.. New crosslinked Hyaluronan gel, intrauterine device, or both for the prevention of intrauterine adhesions. J Soc Laparoendosc Surg 2019;23:e2018.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revelli A, Rovei V, Dalmasso P, Gennarelli G, Racca C, Evangelista F, Benedetto C.. Large randomized trial comparing transabdominal ultrasound-guided embryo transfer with a technique based on uterine length measurement before embryo transfer. Ultrasound Obstet Gynecol 2016;48:289–295. [DOI] [PubMed] [Google Scholar]
- Schenker JG, Margalioth EJ.. Intrauterine adhesions: an updated appraisal. Fertil Steril 1982;37:593–610. [DOI] [PubMed] [Google Scholar]
- Schlafer DH. Equine endometrial biopsy: enhancement of clinical value by more extensive histopathology and application of new diagnostic techniques? Theriogenology 2007;68:413–422. [DOI] [PubMed] [Google Scholar]
- Spencer TE. Biological roles of uterine glands in pregnancy. Semin Reprod Med 2014;32:346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto O. Diagnostic and therapeutic hysteroscopy for traumatic intrauterine adhesions. Am J Obstet Gynecol 1978;131:539–547. [DOI] [PubMed] [Google Scholar]
- Takai IU, Kwayabura AS, Ugwa EA, Idrissa A, Obed JY, Bukar M.. A 10-year review of the clinical presentation and treatment outcome of Asherman’s syndrome at a center with limited resources. Ann Med Health Sci Res 2015;5:442–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle RF, Sciarra JJ.. Intrauterine adhesions: hysteroscopic diagnosis, classification, treatment, and reproductive outcome. Am J Obstet Gynecol 1988;158:1459–1470. [DOI] [PubMed] [Google Scholar]
- Xiao S, Wan Y, Xue M, Zeng X, Xiao F, Xu D, Yang X, Zhang P, Sheng W, Xu J. et al. Etiology, treatment, and reproductive prognosis of women with moderate-to-severe intrauterine adhesions. Int J Gynaecol Obstet 2014;125:121–124. [DOI] [PubMed] [Google Scholar]
- Young SL. Evaluation of endometrial function: a Heraclean or Sisyphean task? Fertil Steril 2017;108:604–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Li TC, Xia E, Huang X, Liu Y, Peng X.. Factors affecting reproductive outcome of hysteroscopic adhesiolysis for Asherman’s syndrome. Fertil Steril 2008a;89:715–722. [DOI] [PubMed] [Google Scholar]
- Yu D, Wong YM, Cheong Y, Xia E, Li TC.. Asherman syndrome – one century later. Fertil Steril 2008a;89:759–779. [DOI] [PubMed] [Google Scholar]
- Zhang A, Jamail G, Xue M, Guan X, Xiao S, Xu D.. Hysteroscopic intrauterine adhesiolysis using the ploughing technique with cold scissors. J Minim Invasive Gynecol 2015;22:934–935. [DOI] [PubMed] [Google Scholar]
- Zhao X, Liu Y, Zhang A, Gao B, Feng Q, Huang H, Zhu X, Sun X, Xu D.. Logistic regression analyses of factors affecting fertility of intrauterine adhesions patients. Ann Transl Med 2020a;8:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Zhang A, Gao B, Burjoo A, Huang H, Xu D.. Cold scissors ploughing technique in hysteroscopic adhesiolysis: a comparative study. Ann Transl Med 2020b;8:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.