Abstract
STUDY QUESTION
What is the current burden of polycystic ovary syndrome (PCOS) at the global, regional, and country-specific levels in 194 countries and territories according to age and socio-demographic index (SDI)?
SUMMARY ANSWER
Slight increases in age-standardized incidence of PCOS and associated disability-adjusted life-years (DALYs) were evidenced among women of reproductive age (15–49 years) from 2007 to 2017 at the global level, and in most regions and countries.
WHAT IS KNOWN ALREADY
No detailed quantitative estimates of the PCOS incidence and DALYs by age and SDI in these 194 countries and territories have been published previously.
STUDY DESIGN, SIZE, DURATION
An age- and SDI-stratified systematic analysis of the PCOS incidence and DALYs across 194 countries and territories has been performed.
PARTICIPANTS/MATERIALS, SETTING, METHODS
We used data from the Global Burden of Diseases, Injuries and Risk Factors Study (GBD) 2017 to estimate the total and age-standard PCOS incidence rates and DALYs rates among women of reproductive age in both 2007 and 2017, and the trends in these parameters from 2007 to 2017.
MAIN RESULTS AND THE ROLE OF CHANCE
Globally, women of reproductive age accounted for 1.55 million (95% uncertainty intervals (UIs): 1.19–2.08) incident cases of PCOS and 0.43 million (0.19–0.82) associated DALYs. The global age-standardized PCOS incidence rate among women of reproductive age increased to 82.44 (64.65–100.24) per 100 000 population in 2017, representing an increase of 1.45% (1.43–1.47%) from 2007 to 2017. The rate of age-standardized DALYs increased to 21.96 (12.78–31.15) per 100 000 population in 2017, representing an increase of 1.91% (1.89–1.93%) from 2007 to 2017. Over the study period, the greatest increase in the age-standardized PCOS incidence and DALYs rates were observed in the middle-SDI and high-middle SDI regions, respectively. At the GBD regional level, the highest age-standardized incidence and DALY rates in 2017 were observed in Andean Latin America, whereas the largest percentage increases in both rates from 2007 to 2017 were observed in Tropical Latin America. At the national level, Ecuador, Peru, Bolivia, Japan, and Bermuda had the highest age-standardized incidence rates and DALYs rates in both 2007 and 2017. The highest increases in both the age-standardized incidence rates and DALYs rates from 2007 to 2017 were observed in Ethiopia, Brazil, and China.
LIMITATIONS, REASONS FOR CAUTION
Although the GBD (2017) study aimed to gather all published and unpublished data, the limited availability of data in some regions might have led to the estimation of wide UIs. Additionally, the PCOS phenotype is complicated and the diagnostic criteria are constantly changing. Consequently, the incidence of PCOS might have been underestimated.
WIDER IMPLICATIONS OF THE FINDINGS
Knowledge about the differences in the PCOS burden across various locations will be valuable for the allocation of resources and formulation of effective preventive strategies.
STUDY FUNDING/COMPETING INTEREST(S)
The study was supported by grants from the Innovative Talent Support Plan of the Medical and Health Technology Project in Zhejiang Province (2021422878), Ningbo Science and Technology Project (202002N3152), Ningbo Health Branding Subject Fund (PPXK2018-02), Sanming Project of Medicine in Shen-zhen (SZSM201803080), and National Social Science Foundation (19AZD013). No potential conflicts of interest relevant to this article were reported.
TRIAL REGISTRATION NUMBER
N/A
Keywords: global burden of disease, polycystic ovary syndrome, incidence, disability-adjusted life-years, socio-demographic index
Introduction
Polycystic ovary syndrome (PCOS) is among the most common endocrine disorders and a major cause of anovulatory infertility in women of reproductive age (15–49 years) (Szilágyi and Szabó, 2003; Balen et al., 2016). Globally, the estimated prevalence of PCOS ranges between 5% and 15% (Azziz, 2016). Compelling evidence suggests that women with PCOS have significantly higher risks of obesity, dyslipidemia, impaired glucose tolerance, and long-term complications such as diabetes, endometrial cancer, and cardiovascular disease (Lim et al., 2012; Wild 2012; Peigné and Dewailly, 2014).
Previous efforts to monitor the PCOS epidemic have focused mainly on reporting the prevalence of disease (Yildiz et al., 2012; Ding et al., 2017; Wolf et al., 2018). However, the annual incidence of PCOS, defined as the rate of new cases per year, provides a better reflection of the epidemiological changes associated with this disease (Guang, 2009). The disability-adjusted life-years (DALYs), a comprehensive measurement of premature mortality and disability, are an advantageous measure that can be compared directly across geographical areas (Capone, 2019).
Globally, the age-standardized prevalence of infertility and associated DALYs among women increased by 0.370% and 0.396% per year, respectively, from 1990 to 2017 (Sun et al., 2019). As PCOS is the most common cause of anovulatory infertility in women (Balen et al., 2016), a better understanding of the current burden of PCOS is essential for the primary prevention of infertility.
To our knowledge, no detailed quantitative estimates of the PCOS incidence and associated DALYs by age and socio-demographic index (SDI) across countries and territories has been published. Therefore, we aimed to provide a comprehensive estimate of the age- and SDI-stratified PCOS incidence at the global, regional, and national levels using data collected from 194 countries and territories during the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD, 2017). Here, we present: the estimated PCOS incidence and DALYs for women of reproductive age; the age-standardized PCOS incidence and DALYs in this population in both 2007 and 2017; and the trends in both variables from 2007 to 2017.
Materials and methods
Overview
GBD (2017) used all of the most recent and available sources of epidemiological survey data and optimized, standardized methods for a comparative assessment of health losses and associated risk factors for 282 causes of death, 354 causes of years lived with disability (YLDs), and 359 causes of DALYs in 194 countries and territories from 1990 to 2017. Details of the methodology of GBD (2017) have been published elsewhere (GBD, 2017; GBD Disease and Injury Incidence and Prevalence Collaborators, 2018; Yadgir et al., 2020). GBD (2017) was divided into 21 regions and 7 GBD super-regions based on the geographic distribution of the 194 included countries and territories.
Data sources
The GBD study used data from both literature reports and epidemiological surveillance. In addition to data sources derived from the scientific literature, surveys, and monitoring, the GBD (2017) also included new sources of data such as hospital discharge records, outpatient visits, and health insurance claims records.
Estimation of incidence and DALYs
For this study, the parameters of PCOS incidence and DALYs were used. The age-standardized rates and the estimated annual percentage changes in the PCOS incidence and DALYs among women of reproductive age were considered quantitative trends representative of the two parameters of PCOS. The age-standardized incidence is defined as the number of cases per 100 000 persons, and the age-standardized DALYs is defined as the number of YLDs and the years of life lost (YLLs) per 100 000 persons after age standardization. The DALYs were derived by summing the YLLs and YLDs thereby incorporating both the fatal and nonfatal burdens (GBD, 2017; DALYs and HALE Collaborators, 2018).
Socio-demographic index
In this study, the results were aggregated by the SDI, which is an aggregative index of development estimated for each geographic entity. This index is computed using the mean of the scaled values of total population fertility, the educational level of residents older than 15 years and the per capita personal income (United Nations Development Programme, 2015). The 194 countries and territories were segmented into five SDI quintiles (low, low-middle, middle, high-middle, and high) according to the SDI of each country in 2017.
Statistical analysis
The GBD used the Bayesian meta-regression tool disMOd-MR 2.1 to determine the causes and sequelae of the YLD results. Cause of death ensemble modeling was the principal method used to estimate the mortality and YLLs. We used a global standard (World Health Organization (WHO) 2000–2025) to calculate the age-standardized rate in women of reproductive age (Ahmad et al., 2001). The data are reported as estimates with 95% uncertainty intervals (UIs) where relevant. The findings are also presented according to the SDIs of various regions, as calculated by GBD (2017). We used R version 3.3.1(R Foundation, Vienna, Austria, https://www.r-project.org/) and Microsoft Excel version 1803 (Microsoft Corporation, Redmond, Washington, https://www.microsoft.com/) to draw the figures.
Results
The PCOS burden at the global level
In 2017, 1.55 million (95% UI: 1.19–2.08) incident cases of PCOS among women of reproductive age (15–49 years) were reported globally, representing an increase of 4.47% (2.86–6.37%) from 2007 to 2017. The global age-standardized incidence rate of PCOS among women of reproductive age was 82.44 (64.65–100.24) per 100 000 population in 2017, which represents an increase of 1.45% (1.43–1.47%) from 2007 to 2017 (Table I;Fig. 1A and C).
Table I.
Incidence and disability-adjusted life-years (DALYs) counts in 2007 and 2017, percentage change in incidence and DALYs counts during 2007–2017, and their age-standardized rate (per 100 000) for polycystic ovary syndrome by socio-demographic index (SDI) and world regions among women of reproductive age (15–49 years).
| Location | Numbers (95% UI) |
Age-standardized rate (per 100 000) (95% UI) |
||||
|---|---|---|---|---|---|---|
| 2007 | 2017 | Percentage change 2007–2017 | 2007 | 2017 | Percentage change, 2007–2017 | |
| Global | ||||||
| Incidence | 1 487 950.25 (1 135 872.28, 1 998 276.87) | 1 554 508.65 (1 190 519.23, 2 081 418.38) | 4.47 (2.86, 6.37) | 81.27 (63.60, 98.93) | 82.44 (64.65, 100.24) | 1.45 (1.43, 1.47) |
| DALYs | 380 399.74 (169 627.31, 738 136.40) | 426 360.81 (189 150.84, 820 947.93) | 12.08 (11.10, 13.07) | 21.55 (12.45, 30.65) | 21.96 (12.78, 31.15) | 1.91 (1.89, 1.93) |
| Low SDI | ||||||
| Incidence | 199 632.20 (150 238.02, 273 541.87) | 263 878.44 (198 563.50, 362 006.64) | 32.18 (29.33, 35.78) | 70.30 (53.86, 86.73) | 71.36 (54.81, 87.92) | 1.52 (1.47, 1.57) |
| DALYs | 43 555.26 (19 146.81, 83 925.86) | 57 774.72 (25 561.48, 111 062.24) | 32.65 (29.26, 36.39) | 18.26 (9.89, 26.64) | 18.63 (10.17, 27.09) | 1.99 (1.94, 2.04) |
| Low-middle SDI | ||||||
| Incidence | 327 978.23 (246 644.92, 448 294.82) | 383 031.55 (290 394.44, 520 223.34) | 16.79 (14.80, 18.75) | 79.99 (62.46, 97.52) | 80.08 (62.54, 97.62) | 0.11 (0.07, 0.15) |
| DALYs | 76 738.41 (33 920.71, 148 125.42) | 94 287.33 (42 151.22, 182 743.15) | 22.87 (20.53, 25.23) | 21.31 (12.26, 30.36) | 21.58 (12.47, 30.68) | 1.27 (1.23, 1.31) |
| Middle SDI | ||||||
| Incidence | 470 180.16 (358 949.44, 631 058.61) | 464 654.32 (358 192.14, 621137.52) | 0.66 (−2.55, 4.48) | 87.28 (68.97, 105.59) | 91.31 (72.58, 110.03) | 4.61 (4.57, 4.65) |
| DALYs | 120 532.95 (53889.93, 232 385.38) | 132 342.09 (58 811.72, 254 410.64) | 9.80 (8.19, 11.49) | 22.92 (13.54, 32.30) | 23.73 (14.18, 33.28) | 3.55 (3.51, 3.59) |
| High-middle SDI | ||||||
| Incidence | 276 668.44 (212 573.07, 373 036.24) | 237 616.88 (186 054.64, 315 908.95) | −14.11 (−17.53, −10.30) | 78.72 (61.33, 96.10) | 82.17 (64.40, 99.93) | 4.39 (4.34, 4.44) |
| DALYs | 75 336.74 (33276.57, 144 988.74) | 78 116.45 (34 608.27, 150 496.72) | 3.69 (1.84, 5.71) | 20.64 (11.74, 29.54) | 21.43 (12.36, 30.50) | 3.82 (3.77, 3.87) |
| High SDI | ||||||
| Incidence | 207 750.78 (163 211.08,273597.69) | 200 422.81 (157 764.15, 263 724.28) | −3.53 (−5.27, −1.73) | 89.52 (70.97, 108.06) | 91.54 (72.79, 110.30) | 2.26 (2.20,2.32) |
| DALYs | 62 705.24 (28224.46, 119 797.35) | 62 307.20 (27 976.30, 118 446.98) | −0.63 (−2.19, 1.05) | 23.60 (14.08, 33.12) | 23.72 (14.18, 33.27) | 0.51 (0.46, 0.56) |
| Southeast Asia, East Asia, and Oceania | ||||||
| Incidence | 428 433.76 (328 285.51, 579 082.03) | 383 967.68 (298 783.75, 511 655.00) | −10.38 (−14.32, −5.67) | 76.03 (58.94, 93.12) | 79.55 (62.07, 97.03) | 4.63 (4.59, 4.67) |
| DALYs | 113 564.29 (49 952.57, 219 430.27) | 116 975.66 (51 486.14, 224 242.44) | 3.00 (1.14, 4.84) | 19.70 (11.00, 28.40) | 20.43 (11.57, 29.28) | 3.68 (3.64, 3.72) |
| East Asia | ||||||
| Incidence | 278 948.79 (213 985.06, 377 047.83) | 229 532.49 (179 381.08, 306 404.54) | −17.72 (−23.64, −11.00) | 71.15 (54.62, 87.68) | 73.78 (56.94, 90.61) | 3.7 (3.65, 3.75) |
| DALYs | 74 851.25 (32 621.80, 144 474.15) | 74 505.80 (32 718.76, 143 308.71) | −0.46 (−2.79, 1.87) | 18.22 (9.85, 26.59) | 18.88 (10.36, 27.40) | 3.62 (3.58, 3.66) |
| Southeast Asia | ||||||
| Incidence | 147 258.67 (112 187.28, 197 523.46) | 151 685.88 (115 471.09, 204 958.95) | 3.01 (0.05, 5.75) | 86.88 (68.61, 105.15) | 87.93 (69.55, 106.31) | 1.21 (1.14, 1.28) |
| DALYs | 38 183.23 (16 982.06, 73 712.78) | 41 781.10 (18 437.92, 80 492.58) | 9.42 (6.58, 12.47) | 23.30 (13.84, 32.76) | 23.62 (14.09, 33.15) | 1.39 (1.32, 1.46) |
| Oceania | ||||||
| Incidence | 2226.29 (1671.31, 3037.14) | 2749.30 (2059.61, 3787.93) | 23.49 (14.44, 31.22) | 79.85 (62.34, 97.37) | 79.92 (62.40, 97.44) | 0.09 (−0.41, 0.59) |
| DALYs | 529.81 (238.26, 1011.61) | 688.76 (307.61, 1334.54) | 30.00 (19.89, 40.24) | 21.53 (12.44, 30.63) | 21.74 (12.60, 30.88) | 0.98 (0.45, 1.51) |
| Central Europe, Eastern Europe, and Central Asia | ||||||
| Incidence | 81 082.63 (62 799.19, 108537.28) | 63 426.50 (49 474.50, 84 449.89) | −21.78 (−23.89, −19.32) | 75.35 (58.34, 92.37) | 77.23 (60.00, 94.45) | 2.49 (2.40, 2.58) |
| DALYs | 21 176.33 (9332.38, 40 795.12) | 20 388.68 (9026.24, 39 393.69) | −3.72 (−5.74, −1.64) | 19.25 (10.65, 27.86) | 19.77 (11.06, 28.49) | 2.7 (2.62, 2.78) |
| Central Asia | ||||||
| Incidence | 20 449.52 (15 533.62, 27 821.62) | 18 258.94 (13 954.53, 24 727.46) | −10.71 (−14.77, −6.61) | 81.65 (63.94, 99.36) | 83.03 (65.17, 100.89) | 1.69 (1.51, 1.87) |
| DALYs | 4685.41 (2066.08, 9112.86) | 5249.38 (2321.00, 10 176.88) | 12.04 (7.61, 16.81) | 21.25 (12.22, 30.29) | 21.65 (12.53, 30.76) | 1.84 (1.66, 2.02) |
| Central Europe | ||||||
| Incidence | 20 043.90 (15 535.40, 26 912.27) | 16 279.80 (12 686.24, 21781.54) | −18.78 (−22.36, −14.71) | 73.42 (56.62,90.21) | 74.45 (57.54,91.37) | 1.41 (1.23,1.59) |
| DALYs | 5544.35 (2439.22, 10 728.02) | 5167.68 (2266.17, 9996.02) | −6.79 (−10.77, −2.89) | 18.64 (10.17, 27.10) | 18.98 (10.44, 27.52) | 1.86 (1.70, 2.02) |
| Eastern Europe | ||||||
| Incidence | 40 589.22 (31 758.37, 54 136.04) | 28 887.76 (22 700.83, 38 166.54) | −28.83 (−31.19, −26.12) | 72.93 (56.19, 89.66) | 74.63 (57.70, 91.56) | 2.33 (2.20, 2.46) |
| DALYs | 10 946.57 (4902.75, 21 203.04) | 9971.62 (4411.37, 19 160.29) | −8.91 (−11.24, −6.68) | 18.81 (10.31, 27.30) | 19.26 (10.66, 27.86) | 2.43 (2.31, 2.55) |
| High income | ||||||
| Incidence | 194 561.32 (152 440.62, 256 251.01) | 192 055.20 (150 868.72, 253 339.33) | −1.29 (−2.83, 0.30) | 88.74 (70.28, 107.21) | 89.60 (71.05, 108.16) | 0.97 (0.91, 1.03) |
| DALYs | 58 613.24 (26 276.15, 112 177.94) | 57 976.79 (26 066.16, 111 051.88) | −1.09 (−2.66, 0.61) | 23.68 (14.14, 33.22) | 23.70 (14.16, 33.24) | 0.07 (0.01, 0.13) |
| High-income Asia Pacific | ||||||
| Incidence | 49 266.02 (37 617.76, 65 602.91) | 48 438.27 (37 232.52, 64 531.60) | −1.68 (−4.64, 2.38) | 149.31 (125.36, 173.26) | 151.10 (127.00, 175.19) | 1.2 (1.05, 1.35) |
| DALYs | 16 171.42 (7171.58, 30 700.23) | 15 273.64 (6749.92, 29 242.97) | −5.55 (−7.93, −2.67) | 36.48 (24.64, 48.32) | 36.57 (24.71, 48.42) | 0.24 (0.11, 0.37) |
| Australasia | ||||||
| Incidence | 6155.36 (4618.69, 8424.21) | 6452.47 (4845.83, 8756.70) | 4.83 (−3.69, 14.20) | 110.88 (90.24, 131.52) | 113.04 (92.20, 133.88) | 1.95 (1.58, 2.32) |
| DALYs | 1741.75 (763.00, 3363.95) | 1946.65 (866.68, 3785.98) | 11.76 (2.84, 21.11) | 27.98 (17.61, 38.35) | 28.53 (18.06, 39.00) | 1.96 (1.62, 2.30) |
| Western Europe | ||||||
| Incidence | 69 750.01 (53 555.19, 94 647.15) | 68 405.03 (52 393.42, 93 926.28) | −1.93 (−4.34, 0.72) | 82.83 (64.99, 100.67) | 83.67 (65.74, 101.60) | 1.01 (0.91, 1.11) |
| DALYs | 24 499.55 (10 829.05, 47 935.59) | 23 614.45 (10 417.48, 46 218.85) | −3.61 (−6.10, −0.90) | 24.31 (14.65, 33.98) | 24.42 (14.73, 34.10) | 0.42 (0.33, 0.51) |
| Southern Latin America | ||||||
| Incidence | 8336.37 (6471.27, 11186.20) | 8570.98 (6587.18, 11 604.68) | 2.81 (−3.21, 8.62) | 53.02 (38.75, 67.30) | 52.91 (38.65, 67.17) | −0.22 (−0.44, 0.00) |
| DALYs | 2031.75 (908.71, 3912.24) | 2263.61 (1015.86, 4317.20) | 11.41 (4.02, 19.74) | 13.28 (6.14, 20.42) | 13.33 (6.17, 20.48) | 0.36 (0.14, 0.58) |
| High-income North America | ||||||
| Incidence | 61 053.57 (47 527.68, 77 033.94) | 60 188.45 (46 910.09, 75 586.52) | −1.42 (−3.91, 1.20) | 75.36 (58.34, 92.37) | 77.26 (60.03, 94.49) | 2.52 (2.42, 2.62) |
| DALYs | 14 168.77 (6383.82, 27 012.26) | 14 878.45 (6653.55, 28 311.35) | 5.01 (1.91, 8.66) | 17.78 (9.52, 26.05) | 18.19 (9.83, 26.55) | 2.31 (2.21, 2.41) |
| Latin America and Caribbean | ||||||
| Incidence | 199 764.41 (151 393.75, 269 122.82) | 211 874.26 (162 170.23, 284 331.72) | 6.06 (3.97, 8.13) | 134.77 (112.02, 157.53) | 138.77 (115.69, 161.86) | 2.97 (2.90, 3.04) |
| DALYs | 49 848.31 (22 341.51, 95 973.70) | 58 157.78 (26 094.93, 111 671.78) | 16.67 (14.36, 18.75) | 35.88 (24.14, 47.62) | 37.14 (25.20, 49.09) | 3.51 (3.44, 3.58) |
| Caribbean | ||||||
| Incidence | 16 582.68 (12 327.10, 22 521.73) | 16 700.09 (12 541.18, 22 903.84) | 0.71 (−4.16, 5.21) | 137.93 (114.91, 160.95) | 140.15 (116.94, 163.35) | 1.61 (1.36, 1.86) |
| DALYs | 4316.37 (1941.55, 8364.84) | 4639.19 (2068.30, 9024.69) | 7.48 (3.08, 12.06) | 37.86 (25.80, 49.92) | 38.53 (26.37, 50.70) | 1.77 (1.51, 2.03) |
| Andean Latin America | ||||||
| Incidence | 33 277.48 (24 509.89, 44829.50) | 35 911.16 (26 913.38, 47 712.18) | 7.91 (1.92, 14.50) | 215.57 (186.79, 244.35) | 220.50 (191.39, 249.60) | 2.29 (2.07, 2.51) |
| DALYs | 7511.31 (3331.24, 14 516.32) | 9144.26 (4098.92, 17 503.49) | 21.74 (15.51, 27.94) | 56.32 (41.61, 71.03) | 57.66 (42.77, 72.54) | 2.37 (2.14, 2.60) |
| Central Latin America | ||||||
| Incidence | 79 369.75 (59 975.29, 10 7841.32) | 86 131.86 (65 590.64, 117 089.86) | 8.52 (5.47, 11.54) | 121.79 (100.16, 143.42) | 124.23 (102.38, 146.07) | 2 (1.89, 2.11) |
| DALYs | 19 155.96 (8535.30, 36 924.45) | 22 626.49 (10 167.91, 43 664.24) | 18.12 (14.89, 21.35) | 32.22 (21.09, 43.34) | 33.08 (21.80, 44.35) | 2.68 (2.57, 2.79) |
| Tropical Latin America | ||||||
| Incidence | 70 534.48 (53 437.16, 95 529.21) | 73 131.15 (55 814.91, 98 279.57) | 3.68 (0.71, 7.01) | 126.77 (104.70, 148.84) | 132.20 (109.66, 154.74) | 4.29 (4.17, 4.41) |
| DALYs | 18 864.67 (8441.90, 36 468.85) | 21 747.84 (9704.51, 42 492.11) | 15.28 (12.03, 19.02) | 34.50 (22.99, 46.01) | 36.08 (24.31, 47.85) | 4.58 (4.46, 4.70) |
| North Africa and Middle East | ||||||
| Incidence | 137 417.97 (103 442.50, 187 454.13) | 146 841.88 (111 240.49, 201 218.05) | 6.86 (3.79, 10.37) | 96.52 (77.26, 115.78) | 95.93 (76.74, 115.13) | −0.61 (−0.68, −0.54) |
| DALYs | 34 806.84 (15 589.09, 67 473.78) | 41 679.47 (18 629.58, 80 951.06) | 19.75 (16.37, 22.98) | 27.19 (16.97, 37.41) | 26.91 (16.74, 37.07) | −1.05 (−1.12, −0.98) |
| South Asia | ||||||
| Incidence | 310 238.18 (235 047.86, 422 489.91) | 372 249.85 (282 540.84, 505 083.95) | 19.99 (17.60, 22.66) | 73.59 (56.78, 90.40) | 74.99 (58.02, 91.97) | 1.91 (1.87, 1.95) |
| DALYs | 74 553.26 (33 077.61, 143 605.11) | 93 021.93 (41 396.20, 177 805.34) | 24.77 (22.15, 27.69) | 19.52 (10.86, 28.18) | 19.99 (11.23, 28.76) | 2.43 (2.39, 2.47) |
| Sub-Saharan Africa | ||||||
| Incidence | 136 451.98 (102 873.39, 186 441.22) | 184 093.27 (139 492.92, 251 768.60) | 34.91 (32.15, 37.80) | 62.29 (46.82, 77.76) | 63.53 (47.91, 79.15) | 1.99 (1.93, 2.05) |
| DALYs | 27 837.46 (12 048.79, 53 659.27) | 38 160.50 (16 599.65, 73 886.42) | 37.08 (33.57, 40.32) | 15.38 (7.69, 23.07) | 15.71 (7.94, 23.48) | 2.13 (2.07, 2.19) |
| Central Sub-Saharan Africa | ||||||
| Incidence | 15 826.41 (11 897.94, 21 548.13) | 21 291.62 (16 129.03, 29 465.92) | 34.53 (28.81, 40.89) | 62.95 (47.40, 78.50) | 63.96 (48.29, 79.64) | 1.6 (1.44, 1.76) |
| DALYs | 3175.14 (1380.99, 6067.53) | 4403.28 (1944.75, 8449.43) | 38.68 (29.74, 47.63) | 15.49 (7.78, 23.21) | 15.80 (8.01, 23.59) | 1.95 (1.77, 2.13) |
| Eastern Sub-Saharan Africa | ||||||
| Incidence | 52 224.72 (39 156.24, 71 776.27) | 72 008.54 (53 868.67, 98 443.47) | 37.88 (35.03,41.20) | 61.86 (46.45,77.28) | 63.57 (47.94,79.20) | 2.76 (2.67,2.85) |
| DALYs | 10 306.15 (4496.43, 19 749.89) | 14 506.41 (6335.20, 27 902.80) | 40.75 (37.05, 44.87) | 15.27 (7.61, 22.93) | 15.70 (7.93, 23.46) | 2.77 (2.67, 2.87) |
| Southern Sub-Saharan Africa | ||||||
| Incidence | 13 083.91 (10 008.68, 17 725.55) | 12 868.66 (9966.94, 17 150.83) | −1.65 (−5.37, 2.28) | 61.71 (46.31, 77.10) | 62.89 (47.35, 78.43) | 1.92 (1.73, 2.11) |
| DALYs | 2827.47 (1220.24, 5457.44) | 3217.11 (1408.95, 6176.36) | 13.78 (9.47, 18.55) | 15.10 (7.48, 22.72) | 15.42 (7.72, 23.11) | 2.1 (1.90, 2.30) |
| Western Sub-Saharan Africa | ||||||
| Incidence | 55 316.94 (41 625.92, 75 533.59) | 77 924.45 (58 795.17, 107 092.76) | 40.87 (35.06, 46.38) | 62.66 (47.14, 78.17) | 63.45 (47.84, 79.06) | 1.26 (1.17, 1.35) |
| DALYs | 11 528.70 (5059.38, 22 207.71) | 16 033.70 (6979.91, 31 395.51) | 39.08 (31.83, 46.18) | 15.52 (7.80, 23.25) | 15.75 (7.97, 23.53) | 1.49 (1.40, 1.58) |
DLAYs, disability-adjusted life-years; SDI, socio-demographic index.
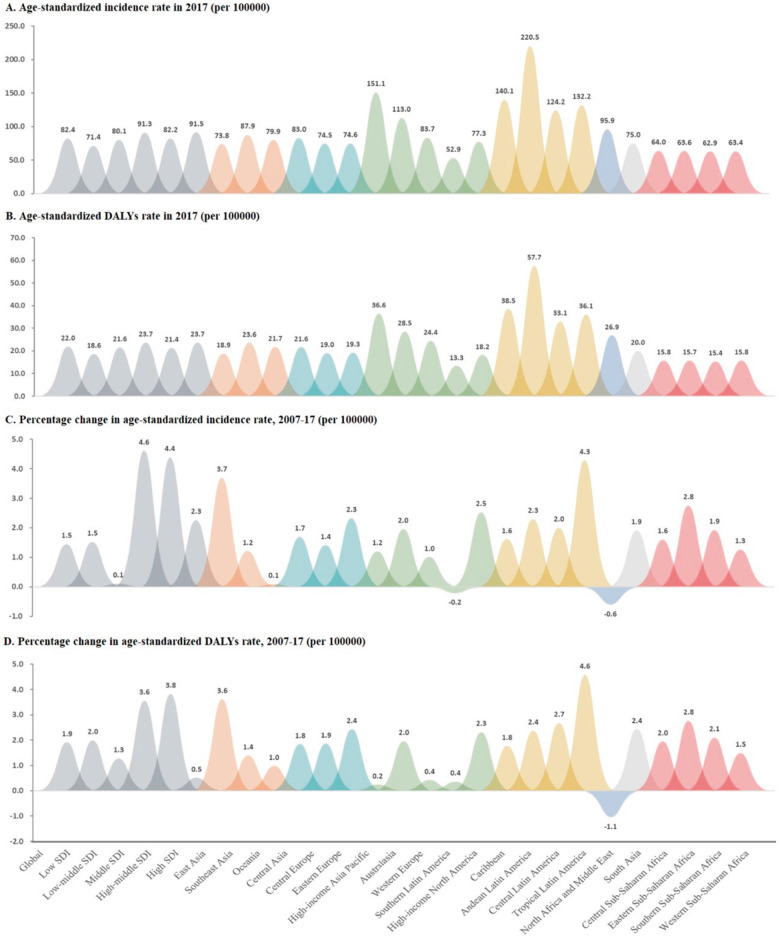
Figure 1.
Age-standardized rates of incidence and disability-adjusted life-years (DALYs) in 2017, percentage change in age-standardized incidence rate and DALYs rate among women in reproductive age (15–49 years) for polycystic ovary syndrome during 2007–2017 by socio-demographic index (SDI) and world regions. (A) Age-standardized incidence rate among women in reproductive age for polycystic ovary syndrome (PCOS) in 2017 by SDI and world regions. (B) Age-standardized DALYs rate among women in reproductive age for PCOS in 2017 by SDI and world regions. (C) Percentage change in age-standardized incidence rate among women in reproductive age for PCOS during 2007–2017 by SDI and world regions. (D) Percentage change in age-standardized DALYs rate among women in reproductive age for PCOS during 2007–2017 by SDI and world regions.
In 2017, the total number of DALYs due to PCOS among women of reproductive age was 0.43 (95% UI: 0.19–0.82) million worldwide, representing a 12.08% (11.10–13.07%) increase from 2007 to 2017. The global age-standardized DALY rate due to PCOS among women of reproductive years was 21.96 (12.78–31.15) per 100 000 population in 2017, representing an increase of 1.91% (1.89–1.93%) from 2007 to 2017 (Table I;Fig. 1B and D).
The PCOS burden at the SDI quintile level
The PCOS incidence varied by SDI quintile in our analysis. In 2017, the highest age-standardized incidence rate was observed in the high-SDI quintile (91.54 (95% UI: 72.79–110.30) per 100 000 population) across all seven super-regions, whereas the lowest age-standardized incidence rate was observed across the low-SDI quintiles (71.36 (54.81–87.92) per 100 000 population). The percentage change in the age-standardized incidence rate among women of reproductive age increased in all of the SDI quintiles over the study period. The highest increase in the age-standardized incidence rate was observed in the middle-SDI quintiles, which exhibited an increase of 4.61% (4.57–4.65%) from 2007 to 2017 (Table I;Fig. 1A and C).
Similarly, the highest rate of age-standardized DALYs in 2017 was observed in the middle-SDI quintiles (23.73 (95% UI: 14.18–33.28) per 100 000 population), and the lowest was observed in the low-SDI quintiles (18.63 (10.17–27.09) per 100 000 population). The percentage change in the age-standardized DALYs rates over the study period also increased in all of the SDI quintiles, although the steepest increase from 2007 to 2017 was observed in the high-middle SDI quintiles (3.82% (3.77–3.87%)) (Table I;Fig. 1B and D).
The PCOS burden at the regional level
In 2017, the highest age-standardized PCOS incidence rates were observed in the geographic regions of Andean Latin America (220.50 (95% UI: 191.39–249.60) per 100 000 population), high-income Asia Pacific (151.10 (127.00–175.19) per 100 000 population) and Caribbean (140.15 (116.94–163.35) per 100 000 population). The lowest age-standardized incidence rate was observed in Southern Latin America (52.91 (38.65–67.17)) per 100 000 population) (Table I;Fig. 1A). The largest increases in the age-standardized incidence rates from 2007 to 2017 were observed in Tropical Latin America (4.29% (4.17–4.41%)), East Asia (3.70% (3.65–3.75%)), and Eastern Sub-Saharan Africa (2.76% (2.67–2.85%)). In contrast, only North Africa and the Middle East (−0.61% (−0.68% to −0.54%)) and southern Latin America (−0.22% (−0.44–0.00%)) exhibited downward trends in the age-standardized PCOS incidence rates from 2007 to 2017 (Table I;Fig. 1C).
The regions of Andean Latin America with (57.66 (95% UI: 42.77–72.54) per 100 000 population), Caribbean (38.53 (26.37–50.70) per 100 000 population) and high-income Asia Pacific (36.57 (24.71–48.42) per 100 000 population) had the highest age-standardized DALYs rates in 2017, whereas Southern Latin America had the lowest age-standardized DALYs rate (13.33 (6.17–20.48) per 100 000 population) (Table I;Fig. 1B). Tropical Latin America (4.58% (4.46–4.70%)), East Asia (3.62% (3.58–3.66%)), and Eastern Sub-Saharan Africa (2.77% (2.67–2.87%)) had the steepest increases in the age-standardized DALYs rates from 2007 to 2017, whereas only North Africa and the Middle East exhibited a downward trend during this period (−1.05% (−1.12% to −0.98%)) (Table I;Fig. 1D).
Countries with the highest PCOS burdens in 2017
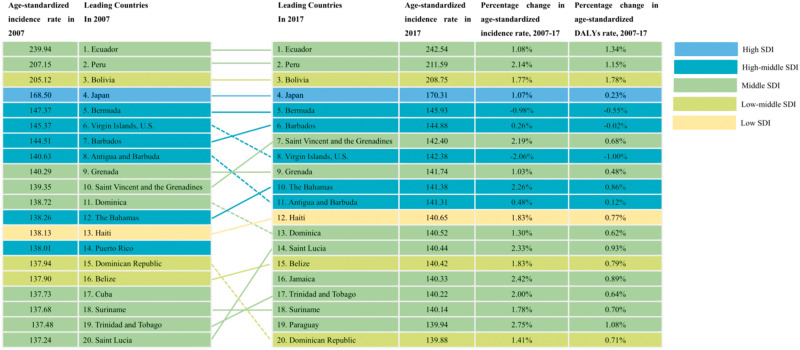
At the national level, the top five countries in terms of the age-standardized incidence rates of PCOS in 2017 were Ecuador (242.54 (95% UI: 212.01–273.06) per 100 000 population), Peru (211.59 (183.08–240.10) per 100 000 population), Bolivia (208.75 (180.43–237.07) per 100 000 population), Japan (170.13 (144.74–195.89) per 100 000 population) and Bermuda (145.93 (122.26–169.61)). In contrast, Argentina (53.75 (39.38–68.12) per 100 000 population), Uruguay (53.26 (38.95–67.56) per 100 000 population) and Chile (50.69 (36.73–64.64) per 100 000 population) had the lowest age-standardized incidence rates in 2017 (Supplementary Table SI, Fig. 2 and Supplementary Fig. S1A). The percentage changes in the age-standardized PCOS incidence rates from 2007 to 2017 varied greatly between countries, with the steepest increases in Brazil (4.33% (4.09–4.57%)), Ethiopia (3.80% (3.08–4.52%)) and China (3.63% (3.45–3.81%)), and the steepest decreases in Afghanistan (−2.91% (−3.72% to −2.11%)) and Austria (−5.68% (−7.08% to −4.27%)) (Supplementary Table SI and Supplementary Fig. S1C).
Figure 2.
Top 20 countries of age-standardized rate and percentage change in age-standardized by incidence and disability-adjusted life-years among women of reproductive age (15–49 years) for polycystic ovary syndrome, 2007–2017.
The top five countries in terms of the PCOS-associated age-standardized DALYs rates in 2017 were Ecuador (63.45 (95% UI: 47.84–79.07) per 100 000 population), Peru (55.65 (41.03–70.27) per 100 000 population), Bolivia (54.85 (40.33–69.36) per 100 000 population), Japan (41.33 (28.73–53.93) per 100 000 population), and Barbados (40.66 (28.16–53.16) per 100 000 population). In contrast, the lowest age-standardized DALYs rates were in Argentina (13.50 (6.30–20.71) per 100 000 population), and Chile (12.87 (5.84–19.91) per 100 000 population) (Supplementary Table SI, Fig. 2 and Supplementary Fig. S1B). Brazil (4.61% (3.71–5.51%)), China (3.68% (3.02–4.33)) and Ethiopia (3.58% (0.19–6.97%)) exhibited the steepest increases in the age-standardized DALYs from 2007 to 2017, whereas Egypt (−4.23% (−5.91% to −2.55%)) and Austria (−5.43% (−9.77% to −1.09%)) exhibited the greatest decreases during the study period (Supplementary Table SI and Supplementary Fig. S1D).
The SDI-specific PCOS burdens across different age groups
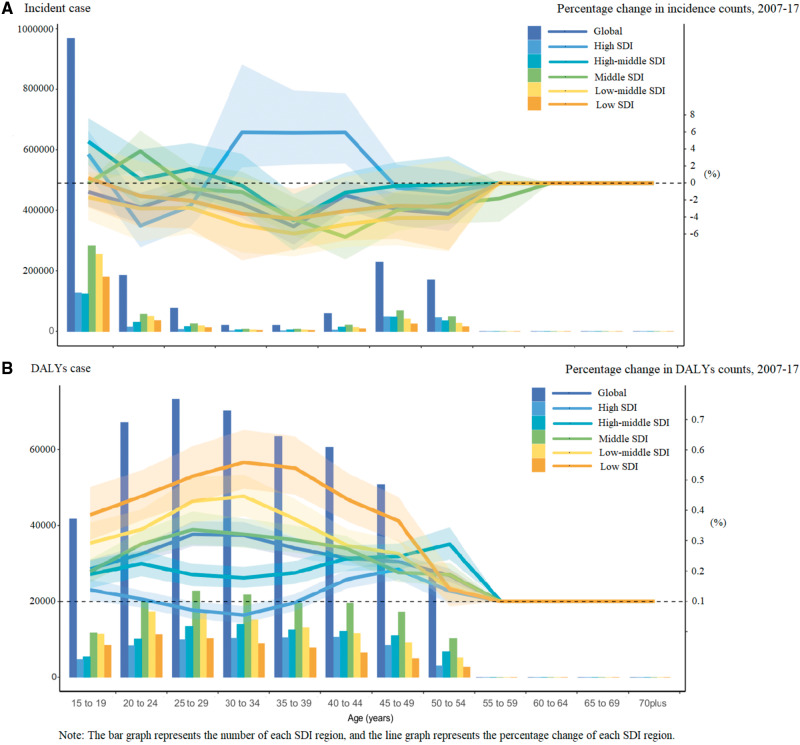
Figure 3 shows the SDI-specific PCOS burdens across different age groups, ranging from 15 to >70 years, in 2017 and the percentage changes from 2007 to 2017 across these age groups. Globally, the incident cases peaked in the 15–19-year-old age group in all SDI quintiles. The DALY cases peaked in the 20–24-year-old age group in the low-SDI and low-middle-SDI quintiles, in the 25–29-year-old age group in the middle-SDI quintiles and in the 30–34-year-old age group in the high-middle-SDI quintiles.
Figure 3.
Age-socio-demographic index (SDI)-specific counts in 2017 and percentage change counts of polycystic ovary syndrome (PCOS), 2007–2017 of incidence and disability-adjusted life-years (DALYs) across different age groups from 15 to over 70 years old. (A) Age-SDI-specific incidence counts in 2017 and their percentage change counts of PCOS during 2007–2017. (B) Age-SDI-specific DALYs counts in 2017 and their percentage change counts of PCOS during 2007–2017.
Regional-specific trends based on the SDI
Supplementary Fig. S2 demonstrates the observed age-standardized incidence and DALY rates at the global and regional levels from 2007 to 2017 and the prospective rates according to the SDI values of the global regions. The expected pattern is essentially nonlinear, with a peak at approximately 0.66 of the SDI value and a subsequent decrease as the SDI value increases.
Discussion
In this study, we comprehensively reported the overall and age-standardized PCOS incidence rates and associated DALYs rates among women of reproductive age at the global, regional, and national levels between 2007 and 2017. In 2017, there were 1.55 million incident PCOS cases, and 0.43 million associated DALYs. Globally, the age-standardized incidence rate and DALY increased slightly (1.45% and 1.91%, respectively) from 2007 to 2017.
To date, no study has investigated the incidence of PCOS comprehensively. Epidemiological studies have reported conflicting results regarding the prevalence of this condition, likely due to the use of different inclusion criteria, sample sizes, ethnicities, study designs, and defined regions (Ding et al., 2017). Heterogeneity in the clinical manifestations of PCOS associated with different ethnicities, the application of inconsistent diagnostic criteria across ethnic groups, and variability in the knowledge of health providers also contributed to the variations in prevalence (Witchel et al., 2020). Thus, consistent and convenient diagnostic criteria are needed for an accurate estimation of the PCOS incidence and prevalence across different ethnicities.
The slight increase in the PCOS incidence may be related to increases in both population growth and aging. Additionally, changes in the diagnostic criteria of PCOS over the past decades might partially account for the slight increase. For example, the incidence of PCOS was estimated to increase significantly when using the Rotterdam criteria, which remain the most widely used PCOS diagnostic criteria worldwide (Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group, 2004). The recent increases in obesity rates might also contribute to the increased incidence of PCOS, as previous research confirmed that the prevalence of PCOS was 2–3 times higher among obese women relative to their non-obese counterparts (Yildiz et al., 2012; Mu et al., 2019). Notably, 68–75% of patients with PCOS remain undiagnosed even after visiting many medical institutions (Futterweit, 1999; March et al., 2010; Wolf et al., 2018), indicating that the incidence of this condition is probably underestimated (Ding et al., 2017).
We observed that the PCOS burden also varied among the SDI quintiles. The greatest increases in the PCOS incidence and DALYs were observed in the middle-SDI and high-middle-SDI quintiles, respectively. These results reflect the higher detection rates, advanced healthcare infrastructure and primary healthcare in these regions. In contrast, the lowest age-standardized PCOS incidence rates and DALYs were observed in the low-SDI quintiles and are indicative of the lower detection rates in this region. For example, ultrasonography, which is commonly used to diagnose PCOS, is limited in Africa in terms of both availability and affordability (Chima and Mamdoo, 2015). In summary, health resource allocation to resource-limited regions needs to be improved.
Regionally, Andean Latin America had both the highest age-standardized PCOS incidence and the highest DALYs, and this outcome was mainly attributable to Ecuador, Peru, and Bolivia. The Ministries of Health in these countries and Colombia have initiated national plans targeted at improving women’s health awareness and health-seeking behavior related to gynecology (Johnson et al., 2018). In Ecuador, the ‘Health on the Go’ project within the 10-Year Health Plan (2015–2025) facilitated the identification of the clinical characteristics of PCOS and thus contributed to the increased incidence rates and DALYs (Roldós et al., 2017). Japan was the only developed country among the leading countries in terms of the age-standardized incidence and DALYs in both 2007 and 2017. This result is probably due to the complete health service system in Japan, the implementation of new revised diagnostic criteria for women of Japanese ethnicity, the extensive use of psychotropic drugs and the high prevalence of female-to-male (FTM) trans-sexuality (Baba et al., 2007, 2011; Watanabe and Kikuchi, 2014).
Tropical Latin America reported the highest increases in both the age-standardized PCOS incidence and DALYs during the study period. These increases were mostly attributable to Brazil, where urban industrialization has led to increases in obesity in all age groups (Canella et al., 2014, 2015). The Brazilian public health system (SUS) provides comprehensive resources and technical guarantees for PCOS detection, and devotes attention to women’s health by implementing interventions such as the maternal and child program (Thumé et al., 2011; Prates et al., 2017).
In contrast, decreases in the PCOS incidence and DALYs were observed in southern Latin America and in North Africa and the Middle East. Southern Latin America also reported the lowest age-standardized incidence and DALYs, which might be due to the fragile and fragmented health systems, poor health awareness, and absolute population decreases in this region (James et al., 2019). Decreases in the PCOS incidence and DALYs in North Africa and the Middle East over the study period were mainly due to lower detection rates, which were a consequence of decreased resources in low-to middle-income countries in this region (Naal et al., 2020). The decreases were exacerbated by international conflicts and the emigration of many experienced health workers (Miseda et al., 2017; Naal et al., 2020).
At the national level, Ethiopia and China exhibited the steepest increases in the PCOS incidence. Ethiopia has the fastest growing economy in Africa and a rapidly growing population. This country is experiencing simultaneous increases in factors associated with PCOS, including obesity, the exposure of farmers to chemical pesticides and a high prevalence of epilepsy (Fix et al., 2020). The efforts of Ethiopia’s Health Development Army, a women-centered community movement aimed at improving healthcare behaviors and practices among women, have improved the early detection of PCOS (Damtew et al., 2018; Rieger et al., 2019). In China, two programs have been introduced to protect women’s health rights: the Program for China’s Women Development (2001–2010) and the Program for China’s Women Development (2011–2020) (Wang, 2001; House, 2011). The ‘Healthy China 2030’ initiative also aims to accelerate the prevention, treatment, and management of fertility-related diseases (Chen et al., 2019). In contrast, the largest decrease in the PCOS incidence was observed in Afghanistan, and this finding was probably due to a low detection rate in a turbulent social environment. Taken together, the data suggest that PCOS is mainly concentrated in low- and middle-income countries, and therefore the increased screening and management of PCOS in these countries should be prioritized.
In addition, we observed the highest PCOS incidence among women aged 15–19 years, consistent with the observation that PCOS usually begins during puberty (Ehrmann et al.,1995; Apter,1998). However, it is difficult to diagnose PCOS during adolescence (Legro et al., 2013), as the manifestations overlap with the physiological changes of puberty (Witchel et al., 2019). Currently, there is no universal standard for the diagnosis of PCOS in adolescents (Witchel et al., 2015). The DALYs peaked in different age groups across the SDI quintiles, suggesting that effective interventions and treatments should target different age groups according to the SDI quintile.
The stability of the global age-standardized PCOS incidence and DALYs over time suggests that the epidemiology of PCOS has not changed. However, the high degrees of variability and inconsistency between the different diagnostic criteria present great challenges to an accurate estimation of the PCOS incidence. Currently, the etiology of PCOS remains unclear. As the clinical manifestations of PCOS vary greatly between women of different ethnic backgrounds, ethnicity-specific guidelines should be established that emphasize the racial differences in screening and diagnostic outcomes, management priorities, and responses to treatment (Ding et al., 2017).
PCOS is a well-documented lifespan disorder. Accordingly, a personalized diagnostic approach and treatment should be promoted during different life stages (Teede et al., 2018a). The recent International PCOS Guidelines promote the prevention, screening and treatment of PCOS during a woman’s reproductive life (Teede et al., 2018b; Peña et al., 2020). The primary interventions comprise healthy lifestyle behaviors and avoidance of excessive weight gain, whereas secondary prevention relies on the early screening of girls at higher risk for PCOS. This guideline also promotes uniform diagnostic criteria, a timely and accurate diagnosis, enhanced education for health professionals and patients, improved screening and diagnosis protocols and the earliest treatment for PCOS-related complications (Conway et al., 2014; Teede et al., 2018a).
To the best of our knowledge, this is the first study to comprehensively assess the PCOS incidence and DALYs at the global, regional and national levels based on data from GBD (2017), which was the first well-rounded study to incorporate several data sources on the incidence of PCOS. We first used DALYs to estimate the PCOS burden, as this measure allows direct comparisons across different regions and countries (Capone, 2019). We also estimated the PCOS incidence and DALYs based on the SDI, which was reconstructed to better reflect the development of each country in GBD 2017 (GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018). Our findings will be valuable for resource allocation and priority settings at the global, regional, and national levels.
The following limitations should be acknowledged. First, although the GBD (2017) study aimed to gather all available data, some regions have limited available data. This may have led to widely estimated UIs. Second, the GBD (2017) study used increased hospital medical records (GBD 2017 DALYs and HALE Collaborators, 2018, 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018). As previously reported, this may result in selection bias that favors people who access health care services (GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018). Furthermore, it is complicated to evaluate the PCOS phenotype, and multiple procedures are required, including clinical and laboratory assessments, pelvic ultrasonography, and multiple clinic visits (Escobar-Morreale, 2018). Therefore, the PCOS incidence might be underestimated. However, detection bias would lead to a higher estimated PCOS incidence in areas with better healthcare access and health awareness, thus there should be caution over resource allocation in these areas of ‘high risk’. In addition, the diagnostic criteria are constantly changing, which contributes to inconsistencies when determining the PCOS incidence.
In conclusion, the global age-standardized PCOS incidence and DALYs rates increased slightly from 2007 to 2017, and these increases were probably related to population growth, resource availability, healthcare access, health awareness, and obesity. The highest increases in the PCOS age-standard incidence and DALYs rates from 2007 to 2017 were observed in the middle-SDI quintile, high-middle-SDI quintiles, tropical Latin America and countries such as Ethiopia, Brazil, and China. Effective interventions and strategies should be established accordingly.
Data availability
The data underlying this article are available in the Global Health Data Exchange at http://ghdx.healthdata.org/ihme data.
Authors’ roles
J.J.L, M.L.J, Y.H.H, Q.H.W, and L.Y.H participated in the study design, analysis, and in the interpretation of data. J.J.L, X.W, S.C.J and L.Y.H reviewed relevant medical literature. J.J.L and L.Y.H prepared the first draft of this manuscript. All coauthors interpreted the data and contributed to subsequent drafts of the manuscript, and all authors have seen and approved the final version.
Funding
The study was supported by grants from the Innovative Talent Support Plan of the Medical and Health Technology Project in Zhejiang Province (2021422878), Ningbo Science and Technology Project (202002N3152), Ningbo Health Branding Subject Fund (PPXK2018-02), Sanming Project of Medicine in Shen-zhen (SZSM201803080) and National Social Science Foundation (19AZD013).
Conflict of interest
No potential conflicts of interest relevant to this article were reported.
Supplementary Material
References
- Ahmad OB, Boschi-Pinto C, Lopez AD, Murray CJ, Inoue M. Age standardization of rates: a new WHO standard. Geneva: World Health Organization; 2001. https://www.who.int/ healthinfo/paper31.pdf.
- Apter D. Endocrine and metabolic abnormalities in adolescents with a PCOS-like condition: consequences for adult reproduction. Trends Endocrinol Metab 1998;9:58–61. [DOI] [PubMed] [Google Scholar]
- Azziz R. Introduction: determinants of polycystic ovary syndrome. Fertil Steril 2016;106:4–5. [DOI] [PubMed] [Google Scholar]
- Baba T, Endo T, Honnma H, Kitajima Y, Hayashi T, Ikeda H, Masumori N, Kamiya H, Moriwaka O, Saito T. et al. Association between polycystic ovary syndrome and female-to-male transsexuality. Hum Reprod 2007;22:1011–1016. [DOI] [PubMed] [Google Scholar]
- Baba T, Endo T, Ikeda K, Shimizu A, Honnma H, Ikeda H, Masumori N, Ohmura T, Kiya T, Fujimoto T. et al. Distinctive features of female-to-male transsexualism and prevalence of gender identity disorder in Japan. J Sex Med 2011;8:1686–1693. [DOI] [PubMed] [Google Scholar]
- Balen AH, Morley LC, Misso M, Franks S, Legro RS, Wijeyaratne CN, Stener-Victorin E, Fauser BCJM, Norman RJ, Teede H. et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update 2016;22:687–708. [DOI] [PubMed] [Google Scholar]
- Canella DS, Levy RB, Martins APB, Claro RM, Moubarac J-C, Baraldi LG, Cannon G, Monteiro CA.. Ultra-processed food products and obesity in Brazilian households (2008-2009). PLoS One 2014;9:e92752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canella DS, Novaes HM, Levy RB.. The influence of excess weight and obesity on health spending in Brazilian households. Cad Saúde Pública 2015;31:2331–2341. [DOI] [PubMed] [Google Scholar]
- Capone A. Towards an effective assessment of the sustainability of health interventions. Ig Sanita Pubbl 2019;75:189–199. [PubMed] [Google Scholar]
- Chen P, Li F, Harmer P.. Healthy China 2030: moving from blueprint to action with a new focus on public health. Lancet Public Health 2019;4:e447. [DOI] [PubMed] [Google Scholar]
- Chima SC, Mamdoo F.. Ethical and legal dilemmas around termination of pregnancy for severe fetal anomalies: a review of two African neonates presenting with ventriculomegaly and holoprosencephaly. Niger J Clin Pract 2015;18:31–39. [DOI] [PubMed] [Google Scholar]
- Conway G, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Franks S, Gambineri A, Kelestimur F, Macut D, Micic D, Pasquali R. et al. European survey of diagnosis and management of the polycystic ovary syndrome: results of the ESE PCOS Special Interest Group's Questionnaire. Eur J Endocrinol 2014;171:489–498. [DOI] [PubMed] [Google Scholar]
- Damtew ZA, Karim AM, Chekagn CT, Fesseha Zemichael N, Yihun B, Willey BA, Betemariam W.. Correlates of the women's development army strategy implementation strength with household reproductive, maternal, newborn and child healthcare practices: a cross-sectional study in four regions of Ethiopia. BMC Pregnancy Childbirth 2018;18:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding T, Hardiman PJ, Petersen I, Wang FF, Qu F, Baio G.. The prevalence of polycystic ovary syndrome in reproductive-aged women of different ethnicity: a systematic review and meta-analysis. Oncotarget 2017;8:96351–96358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrmann DA, Barnes RB, Rosenfield RL.. Polycystic ovary syndrome as a form of functional ovarian hyperandrogenism due to dysregulation of androgen secretion. Endocr Rev 1995;16:322–353. [DOI] [PubMed] [Google Scholar]
- Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol 2018;14:270–284. [DOI] [PubMed] [Google Scholar]
- Fix J, Annesi-Maesano I, Baldi I. et al. Gender differences in respiratory health outcomes among farming cohorts around the globe: findings from the AGRICOH consortium. J Agromed 2020;1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futterweit W. Polycystic ovary syndrome: clinical perspectives and management. Obstet Gynecol Surv 1999;54:403–413. [DOI] [PubMed] [Google Scholar]
- GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1859–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2017;392:1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guang Z. Principles of Epidemiology: Application in Public Health Practice. China: Peking Union Medical College Press, 2009. [Google Scholar]
- House PP. Program for China's Women Development (2011-2020). China: People's Publishing House, 2011. [Google Scholar]
- James SL, Lucchesi LR, Bisignano C. et al. Epidemiology of injuries from fire, heat and hot substances: global, regional and national morbidity and mortality estimates from the Global Burden of Disease 2017 study. Inj Prev 2019;26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CM, Molina Y, Blas M, Erickson M, Bayer A, Gutierrez MC, Nevin PE, Alva I, Rao D.. "The disease is mine, the body is mine, I decide": individual, interpersonal, and institutional barriers and facilitators among survivors of women's cancers in Andean countries. Health Care Women Int 2018;39:522–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, Welt CK.. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2013;98:4565–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SS, Davies MJ, Norman RJ, Moran LJ.. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 2012;18:618–637. [DOI] [PubMed] [Google Scholar]
- March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ.. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod 2010;25:544–551. [DOI] [PubMed] [Google Scholar]
- Miseda MH, Were SO, Murianki CA, Mutuku MP, Mutwiwa SN.. The implication of the shortage of health workforce specialist on universal health coverage in Kenya. Hum Resour Health 2017;15:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu L, Zhao Y, Li R, Lai Y, Chang HM, Qiao J.. Prevalence of polycystic ovary syndrome in a metabolically healthy obese population. Int J Gynecol Obstet 2019;146:164–169. [DOI] [PubMed] [Google Scholar]
- Naal H, El Koussa M, El Hamouch M, Hneiny L, Saleh S.. A systematic review of global health capacity building initiatives in low-to middle-income countries in the Middle East and North Africa region. Global Health 2020;16:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peigné M, Dewailly D.. Long term complications of polycystic ovary syndrome (PCOS). Ann Endocrinol (Paris) 2014;75:194–199. [DOI] [PubMed] [Google Scholar]
- Peña AS, Witchel SF, Hoeger KM, Oberfield SE, Vogiatzi MG, Misso M, Garad R, Dabadghao P, Teede H.. Adolescent polycystic ovary syndrome according to the international evidence-based guideline. BMC Med 2020;18:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prates ML, Machado JC, Silva LSD, Avelar PS, Prates LL, Mendonça ETD, Costa GDD, Cotta RMM.. Performance of primary health care according to PCATool instrument: a systematic review. Ciênc Saúde Coletiva 2017;22:1881–1893. [DOI] [PubMed] [Google Scholar]
- Rieger M, Wagner N, Mebratie A, Alemu G, Bedi A.. The impact of the Ethiopian health extension program and health development army on maternal mortality: a synthetic control approach. Soc Sci Med 2019;232:374–381. [DOI] [PubMed] [Google Scholar]
- Roldós MI, Hopenhayn C, Sacoto F, Bustamante K.. Developing local health policy: profiling needs and opportunities in the Municipality of Quito, Ecuador. J Public Health Pol 2017;38:221–233. [DOI] [PubMed] [Google Scholar]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;81:19–25. [DOI] [PubMed] [Google Scholar]
- Sun H, Gong TT, Jiang YT, Zhang S, Zhao YH, Wu QJ.. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990-2017: results from a global burden of disease study, 2017. Aging (Albany NY) 2019;11:10952–10991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilágyi A, Szabó I.. Endocrine characteristics of polycystic ovary syndrome (PCOS). Indian J Exp Biol 2003;41:694–700. [PubMed] [Google Scholar]
- Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ, Andersen M, Azziz R, International PCOS Network et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod 2018a;33:1602–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ, the International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Clin Endocrinol 2018b;89:251–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thumé E, Facchini LA, Wyshak G, Campbell P.. The utilization of home care by the elderly in Brazil's primary health care system. Am J Public Health 2011;101:868–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Development Programme (UNDP). Human Development Report 2015: Work for Human Development. 2015. https://www.undp.org/content/undp/en/home/librarypage/hdr/2015-human-development-report/. [Google Scholar]
- Wang M. Program for the Development of Chinese Women (2001-2010) brief introduction. China Primary Health Care 2001;15:9–10. [Google Scholar]
- Watanabe K, Kikuchi T.. Adverse events of psychotropic drugs. Seishin Shinkeigaku Zasshi 2014;116:323–331. [PubMed] [Google Scholar]
- Wild RA. Dyslipidemia in PCOS. Steroids 2012;77:295–299. [DOI] [PubMed] [Google Scholar]
- Witchel SF, Burghard AC, Tao RH, Oberfield SE.. The diagnosis and treatment of PCOS in adolescents: an update. Curr Opin Pediatr 2019;31:562–569. [DOI] [PubMed] [Google Scholar]
- Witchel SF, Oberfield S, Rosenfield RL, Codner E, Bonny A, Ibáñez L, Pena A, Horikawa R, Gomez-Lobo V, Joel D. et al. The diagnosis of polycystic ovary syndrome during adolescence. Horm Res Paediatr 2015;83:376–389. [DOI] [PubMed] [Google Scholar]
- Witchel SF, Teede HJ, Peña AS.. Curtailing PCOS. Pediatr Res 2020;87:353–361. [DOI] [PubMed] [Google Scholar]
- Wolf WM, Wattick RA, Kinkade ON, Olfert MD.. Geographical prevalence of polycystic ovary syndrome as determined by region and race/ethnicity. Int J Environ Res Public Health 2018; 15:2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadgir S, Johnson CO, Aboyans V, Adebayo OM, Adedoyin RA, Afarideh M, Alahdab F, Alashi A, Alipour V, Arabloo J, Global Burden of Disease Study 2017 Nonrheumatic Valve Disease Collaborators et al. Global, Regional, and National Burden of calcific aortic valve and degenerative mitral valve diseases, 1990-2017. Circulation 2020;141:1670–1680. [DOI] [PubMed] [Google Scholar]
- Yildiz BO, Bozdag G, Yapici Z, Esinler I, Yarali H.. Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum Reprod 2012;27:3067–3073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the Global Health Data Exchange at http://ghdx.healthdata.org/ihme data.