Abstract
STUDY QUESTION
Do plastic laboratory consumables and cell culture media used in ART contain bisphenols?
SUMMARY ANSWER
The majority of human embryo culture media assessed contained bisphenol S close to the nanomolar concentration range, while no release of bisphenols by plastic consumables was detected under routine conditions.
WHAT IS KNOWN ALREADY
The deleterious effect of the endocrine disruptor bisphenol A (BPA) on female fertility raised concerns regarding ART outcome. BPA was detected neither in media nor in the majority of plastic consumables used in ART; however, it might have already been replaced by its structural analogs, including bisphenol S (BPS).
STUDY DESIGN, SIZE, DURATION
Seventeen plastic consumables and 18 cell culture and ART media were assessed for the presence of bisphenols.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Ten different bisphenols (bisphenol A, S, AF, AP, B, C, E, F, P and Z) were measured using an isotopic dilution according to an on-line solid phase extraction/liquid chromatography/mass spectrometry method.
MAIN RESULTS AND THE ROLE OF CHANCE
While the plastic consumables did not release bisphenols under routine conditions, 16 of the 18 cell culture and ART media assessed contained BPS. Six media exhibited BPS concentrations higher than 1 nM and reached up to 6.7 nM (1693 ng/l).
LARGE SCALE DATA
N/A.
LIMITATIONS, REASONS FOR CAUTION
Further studies are required to investigate a greater number of ART media to identify less potentially harmful ones, in terms of bisphenol content.
WIDER IMPLICATIONS OF THE FINDINGS
As BPS has already been reported to impair oocyte quality at nanomolar concentrations, its presence in ART media, at a similar concentration range, could contribute to a decrease in the ART success rate. Thus far, there has been no regulation of these compounds in the ART context.
STUDY FUNDING/COMPETING INTERESTS
This study was financially supported by the ‘Centre-Val de Loire’ Region (Bemol project, APR IR 2017), INRAE, BRGM, the French National Research Agency (project ANR-18-CE34-0011-01 MAMBO) and the BioMedicine Agency (Project 18AMP006 FertiPhenol). The authors declare that they have no conflict of interest that could be perceived as prejudicing the impartiality of the reported research.
Keywords: assisted reproduction, female infertility, oocyte quality, endocrine disruptors, cell culture / plastic consumables / bisphenols / culture media
Introduction
Researchers have questioned the relation between the increase in human infertility observed in western countries and the impact of environmental factors, especially endocrine disruptors. Previous studies reported positive correlations between the outcome of IVF and embryo transfer and the levels of pollutants in female follicular fluids (Al-Saleh et al., 2010; Jirsova et al., 2010). Bisphenol A (BPA) is a widespread plasticizer, mainly used in polycarbonate monomers, epoxy resins and thermal papers, owing to its heat resistance and elasticity properties (reviewed in Abraham and Chakraborty, 2020). BPA has been extensively used for several decades in the plastic industry, particularly to produce food containers, baby bottles and metal cans. However, it has also been used in medical devices, soaps, lotions, shampoo, nail polish, sunscreen and toys (Eladak et al., 2015; Giulivo et al., 2016; Andaluri et al., 2018; Kirchnawy et al., 2020). Humans are primarily exposed to BPA through diet, as a result of container-content transfer (Kang et al., 2006; Kubwabo et al., 2009; Andra et al., 2015), but also through indoor dust inhalation (Liao et al., 2012b) and the transcutaneous route (Thayer et al., 2016). Widespread BPA use has led to its detection in 95% of patient urine samples in the USA, at concentrations ≥0.1 ng/ml (0.44 nM; Calafat et al., 2005; Calafat et al., 2008), with an average urine and blood concentration of 1–3 ng/ml (4–13 nM; Eladak et al., 2015).
The deleterious effects of BPA on health have previously been reported (Richter et al., 2007; Wetherill et al., 2007; Rochester, 2013). Low BPA concentrations (in the nanomolar range) are associated with obesity, cardiovascular diseases (Lang et al., 2008; Rochester, 2013), type 2 diabetes (Grun and Blumberg, 2007; Lang et al., 2008) and alterations in reproductive function (Peretz et al., 2014). BPA is an endocrine disruptor. It indeed exhibits a weak oestrogenic activity (Nadal et al., 2018). Moreover, the highest urinary BPA concentrations in women undergoing ART are associated with decreased oocyte number and quality, and reduced oestradiol levels (Mok-Lin et al., 2010; Fujimoto et al., 2011; Ehrlich et al., 2012). BPA reportedly also disrupts steroid production in rat, ovine, porcine and human granulosa cells (Mlynarcikova et al., 2005; Zhou et al., 2008; Grasselli et al., 2010; Mansur et al., 2016; Banerjee et al., 2018; Bujnakova Mlynarcikova and Scsukova, 2018; Samardzija et al., 2018; Teteau et al., 2020).
The data suggesting a deleterious effect of BPA on female fertility raised concerns regarding the ART outcome. BPA was not detected in ART media and its presence in plastic consumables used in ART did not lead to a significant leaching into the media (Gatimel et al., 2016). Nevertheless, BPA might not have been detected because it has already been replaced by its structural analogs. Several studies demonstrated that BPA restriction in some countries [EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processings Aids (CEF), 2015; Usman and Ahmad, 2016] led to an increased human exposure to bisphenol S (BPS), an unregulated BPA structural analog (Ye et al., 2015). Therefore, BPS is now being derespectivetected in urine at the same concentration range as BPA [0.02–21 ng/ml or 0.09–91 nM; (Liao et al., 2012a)]. BPS and BPA shared a disruptive effect on ovine granulosa cell steroidogenesis (Teteau et al., 2020). Their structural analogy suggests that BPS and BPA might exhibit similar properties and adverse health effects (Eladak et al., 2015; Ahsan et al., 2018; Campen et al., 2018; Ijaz et al., 2020). BPS also disrupts steroid secretion in human granulosa cells and negatively affects ewe oocyte quality in vitro, even at nanomalar concentrations (Amar et al., 2020; Desmarchais et al., 2020).
There is a need for experimental data to support the hypothesis of the endocrine disrupting properties of bisphenols, but their ubiquitous use, even in research laboratories, makes it more difficult to perform robust assays. In Europe, bisphenols are regulated in food-grade plastic, in which only BPA and BPS are authorized (European Union, 2019). In contrast, plastic-containing devices used for biological assays and for oocyte and embryo handling (for both research applications and ART) could contain other bisphenols that are poorly studied regarding their endocrine disrupting properties. The aim of the present study was, therefore, to investigate whether plastic consumables contained bisphenols, determine if they leach into media under conditions close to those used in routine practice and assess bisphenol presence in ART and cell culture media. Because several bisphenols that are structural analogs of BPA are already used in the industry, we decided to assess 10 different bisphenols: BPA, BPS, bisphenol AF (BPAF), bisphenol AP (BPAP), bisphenol B (BPB), bisphenol C (BPC), bisphenol E (BPE), bisphenol F (BPF), bisphenol P (BPP) and bisphenol Z (BPZ) (Table I).
Table I.
List and formula of the 10 bisphenols assessed in plastic consumables and ART/culture media.
| Abbreviation and structural name | Structural formula | CAS number | MW g/mol |
|---|---|---|---|
|
BPA—Bisphenol A 4,4′-Isopropylidenediphenol |
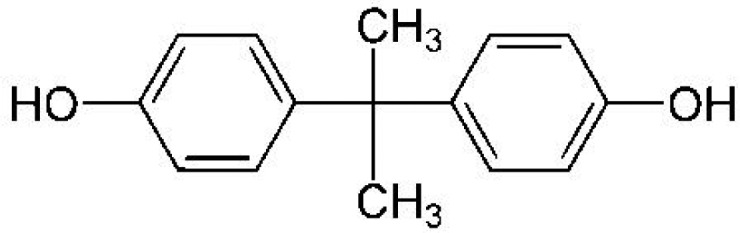
|
80-05-7 | 228.3 |
|
BPAF—Bisphenol AF 4-[1,1,1,3,3,3-Hexafluoro-2-(4-hydroxyphenyl)propan-2-yl]phenol |
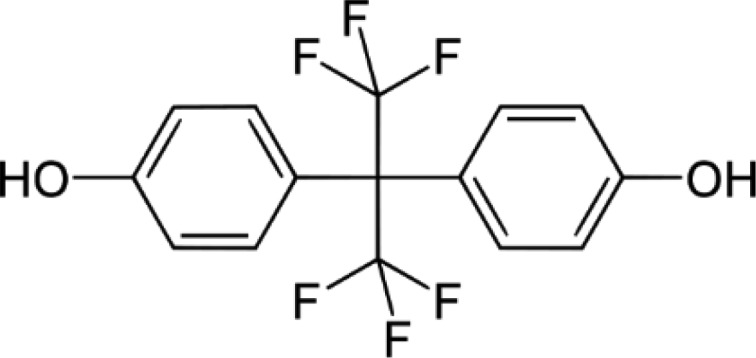
|
1478-61-1 | 336.2 |
|
BPAP—Bisphenol AP 4,4′-(1-Phenylethylidene)bisphenol |
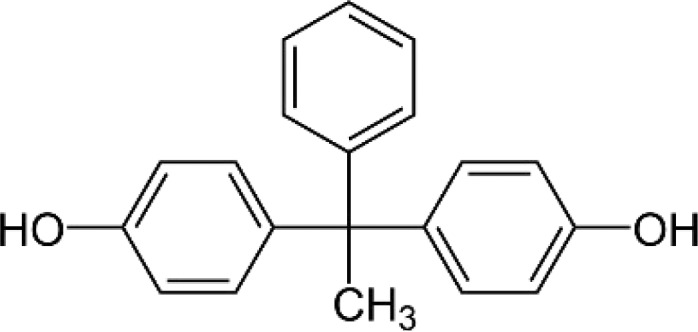
|
1571-75-1 | 290.4 |
|
BPB—Bisphenol B 2,2-Bis(4-hydroxyphenyl)butane |
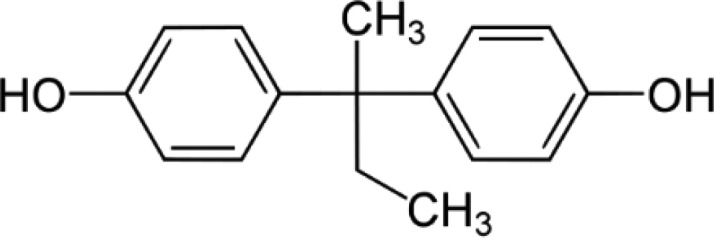
|
77-40-7 | 242.3 |
|
BPC—Bisphenol C 2,2-Bis(4-hydroxy-3-methylphenyl)propane |
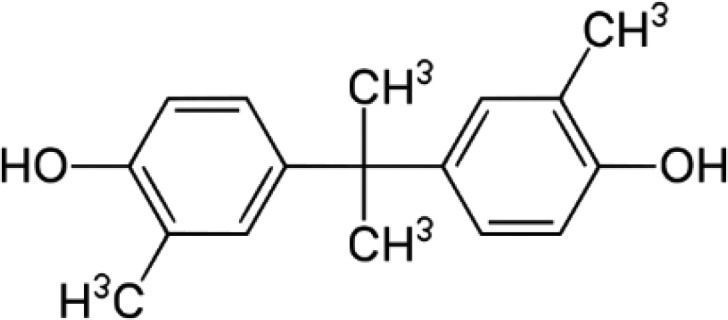
|
79-97-0 | 256.3 |
|
BPE—Bisphenol E 1,1-Bis(4-hydroxyphenyl)ethane |
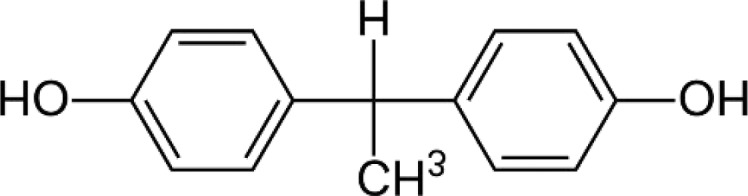
|
2081-08-5 | 214.3 |
|
BPF—Bisphenol F Bis(4-hydroxyphenyl)methane |
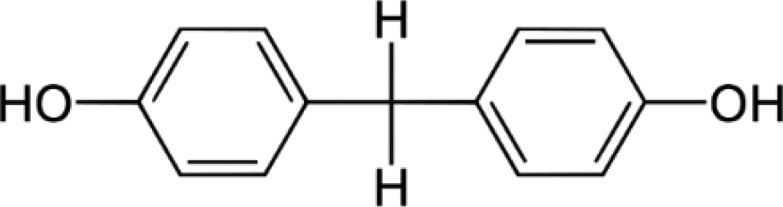
|
620-92-8 | 200.2 |
|
BPP—Bisphenol P 4,4′-(1,4-Phenylenediisopropylidene)bisphenol |
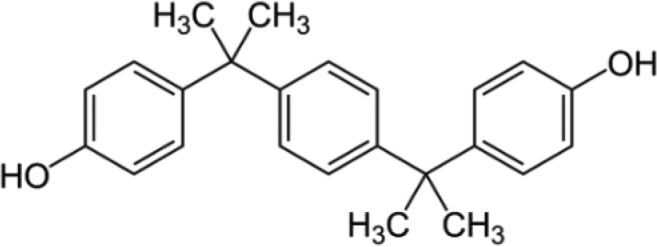
|
2167-51-3 | 346.5 |
|
BPS—Bisphenol S 4,4'-Sulfonyldiphenol |
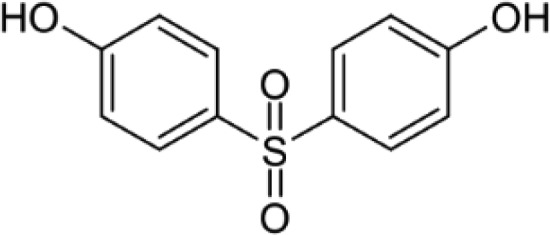
|
80-09-1 | 250.3 |
|
BPZ—Bisphenol Z 4,4′-Cyclohexylidenebisphenol |
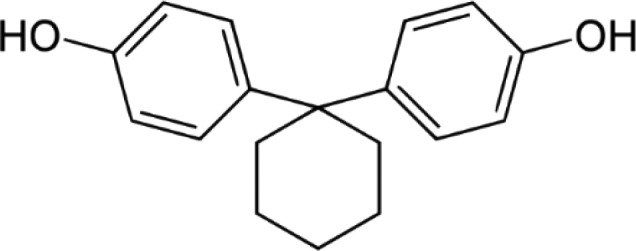
|
843-55-0 | 268.4 |
CAS, Chemical Abstracts Service; MW, molecular weight.
Materials and methods
The aim of this study was to assess the bisphenols released by plastic consumables or that are present in cell culture and ART media. Therefore, no biological materials were needed, and ethics committee approval was irrelevant.
Plastic consumables
The 17 plastic consumables tested were those used in either a research center focused on granulosa cell culture and embryo production (INRAE Centre Val de Loire, UMR Physiology of Reproduction, Nouzilly, France) or in an ART center (Service de Médecine et Biologie de la Reproduction, CHRU de Tours, France). The list of plastic consumables assessed is defined in Table II. Both polystyrene- and polypropylene-based plastics were assessed. The consumables used to collect samples (oocyte or embryo) (tubes, tips), select samples (plastic dishes), cultivate them (cell culture plates or flasks, 4-well petri dishes) or store them (cryopreservation tubes) were evaluated. We also tested media plastic bottles for potential leaching of bisphenols.
Table II.
List of plastic consumables assessed for the presence of bisphenols.
| Sample number | Product type | Brand | Origin | Name | Plastic | Use | Product reference |
|---|---|---|---|---|---|---|---|
| 1 | 50 ml tubes | Clearline, Dominique Dutscher | France | Polystyrene centrifuge tube 50 ml sterile | PS | RL | 380502 |
| 2 | 15 ml tubes | Falcon BD Biosciences | USA | Tube with conical bottom 15 ml (on base) Falcon® | PP | RL | 352097 |
| 3 | 14 ml tubes | Vitrolife | Sweden | Oocyte collection tube 14 ml | PS | ART | 16101 |
| 4 | 1.5 ml tubes | Eppendorf | Germany | Eppendorf Safe-Lock Tube 1.5 ml, Biopur, individually sealed | PP | ART | 0030 121-589 |
| 5 | Tips for oocyte holding and medium preparation | Fisher scientific | France | Fisherbrand™ SureOne™ 1000 µl Filter Tip | PP | RL | 11977724 |
| 6 | VWR | USA | Sterile Aerosol Pipet Tips | PP | ART | 732-0560 | |
| 7 | Gilson | Germany | D1000ST Diamond Tipack | PP | ART | F171501 | |
| 8 | Plates for cell culture | Thermo scientific | Korea | BioLite 96 Microwell Plate | PS | RL | 130188 |
| 9 | Flask for cell culture | Falcon BD Biosciences | USA | Tissue Culture Flask 50 ml | PS | RL | 353014 |
| 10 | Culture medium bottles | Sigma-Aldrich | UK | plastic bottles of Medium 199 | PP | RL | M4530 |
| 11 | Plastic dishes for oocyte collection | VWR | Italy | Petri dish 90 mm | PS | ART | 391-0556 |
| 12 | Thermo scientific | Denmark | Nunc IVF Petri Dish 35 × 10mm Non treated | PS | ART | 150255 | |
| 13 | Plastic dishes for oocyte maturation and fertilization/embryo culture | Falcon BD Biosciences | USA | EASY GRIP Petri dish—35 × 10 mm | PS | RL | 353001 |
| 14 | Thermo scientific | Denmark | Nunclon Delta surface treated 4-well dish | PS | ART | 176740 | |
| 15 | Thermo scientific | USA | IVF ICSI Dish | PS | ART | 150265 | |
| 16 | Vitrolife | Sweden | 5 Well Culture Dish | PS | ART | 16004 | |
| 17 | Tubes for gonadal tissue cryopreservation | Thermo scientific | USA | Nunc Cryotube vials | ART | 375353 |
ART, consumables used in ART labs; RL, consumables used in research labs; PP, polypropylene; PS: polystyrene.
First, plastic consumables were either filled with methanol or cut into pieces and embedded in methanol for 24 h at 40 °C (Table III). These experiments are based on migration tests required for plastic food as stipulated in the Commission Regulation EU 10/2011. Ten bisphenols were then measured (BPA, BPS, BPAF, BPAP, BPB, BPC, BPE, BPF, BPP and BPZ, see below). Each measurement was performed in triplicate.
Table III.
Bisphenols detected after solvent leaching of the plastic consumables.
| Bisphenols (LOQ in ng/l) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample number | Product type | Solvent volume (ml) | Exposure frame | BPA (10) | BPS (2) | BPAF (5) | BPAP (10) | BPB (5) | BPC (5) | BPE (2) | BPF (5) | BPP (10) | BPZ (10) |
| 1 | 50 ml tubes | 20 | Direct filling in the vial | 103 ± 79 | 13 ± 6 | 96 ± 64 | NQ | NQ | NQ | NQ | NQ | NQ | NQ |
| 2 | 15 ml tubes | 10 | 108 ± 101 | 4 ± 1 | 45 ± 14 | NQ | NQ | NQ | NQ | NQ | NQ | NQ | |
| 3 | 14 ml tubes | 2 | 32 ± 15 | 2 ± 0 | 8 ± 4 | NQ | NQ | NQ | NQ | NQ | NQ | NQ | |
| 4 | 1.5 ml tubes | 1.5 | 65 ± 8 | 8 ± 4 | 7 ± 2 | NQ | NQ | NQ | NQ | NQ | NQ | NQ | |
| 5 | Tips | 10 | Cutting into pieces and transferred in glass vial | 308 ± 62 | 30 ± 7 | 30 ± 3 | NQ | NQ | NQ | NQ | NQ | NQ | NQ |
| 6 | 4 | 118 ± 83 | 19 ± 9 | 55 ± 8 | NQ | NQ | NQ | NQ | NQ | NQ | NQ | ||
| 7 | 8 | 736 ± 303 | 85 ± 5 | 52 ± 19 | NQ | NQ | NQ | NQ | NQ | NQ | NQ | ||
| 8 | Plates for cell culture | 5 | 5 ml divided into 16 wells for each replicate | 31(1) | 4 ± 2 | 54 ± 16 | NQ | NQ | NQ | NQ | NQ | NQ | NQ |
| 9 | Flask for cell culture | 10 | Direct filling in the vial | 57 ± 36 | 5 ± 2 | 195 ± 108 | NQ | NQ | NQ | NQ | NQ | NQ | NQ |
| 10 | Culture medium bottles | 25 | 32 ± 24 | 712 ± 1205 | 229 ± 35 | NQ | NQ | NQ | NQ | NQ | NQ | NQ | |
| 11 | Plastic dishes for oocyte collection | 5 | 93 ± 80 | 7 ± 1 | 16 ± 11 | NQ | NQ | NQ | NQ | NQ | NQ | NQ | |
| 12 | 2 | 16 ± 5 | 3 ± 1 | 16 ± 10 | NQ | NQ | NQ | NQ | NQ | NQ | NQ | ||
| 13 | Plastic dishes for oocyte maturation/embryo culture | 2 | 34 ± 15 | 4 ± 2 | 11 ± 2 | NQ | NQ | NQ | NQ | NQ | NQ | NQ | |
| 14 | 1.5 | 1.5 ml divided in 3 wells for each replicate | 13 ± 3 | 2 ± 0 | 12 ± 1 | NQ | NQ | NQ | NQ | NQ | NQ | NQ | |
| 15 | 2 | Direct filling in the vial | 48 ± 4 | 2 ± 1 | 5 ± 1 | NQ | NQ | NQ | NQ | NQ | NQ | NQ | |
| 16 | 3 | 3 ml divided in 3 wells for each replicate | 29 ± 4 | 3 ± 1 | 8 ± 1 | NQ | NQ | NQ | NQ | NQ | NQ | NQ | |
| 17 | Tubes for embryo cryopreservation | 1 | Direct filling in the vial | 87 ± 67 | 4 ± 2 | 9 ± 2 | NQ | NQ | NQ | NQ | NQ | NQ | NQ |
| ref1-2ml | 2 | 71 ± 26 | 3 ± 1 | 8(1) | NQ | NQ | NQ | NQ | NQ | NQ | NQ | ||
| ref2-10ml | 10 | 54 ± 16 | 4 ± 1 | 29 ± 3 | NQ | NQ | NQ | NQ | NQ | NQ | NQ | ||
| ref3-10ml | 10 | Direct filling in the vial for 24 h at 40°C | 25 ± 14 | 8 ± 1 | 17 ± 6 | NQ | NQ | NQ | NQ | NQ | NQ | NQ | |
LOQ, limit of quantification, mentioned below each bisphenols in parentheses; NQ: not quantifiable; bold text: significant difference with the controls P < 0.0001, non-parametric one-way ANOVA with the Tukey post hoc test were performed.
A second leaching test with pure water was implemented on materials having strong positive results in the leaching test with methanol. For each of these products, a second experiment mimicking the conditions close to routine practice, regarding duration of the experiment, were performed (Table IV). For tips, water was pumped through them 10 times before transfer into a glass vial. Controls were realized in parallel in glass vials, using the same water storage conditions used for the leaching tests. All 10 bisphenols were then measured (see below). Each measurement was performed in triplicate.
Table IV.
Routine conditions of our experiments applied to plastic consumables containing bisphenols.
| Sample number | Brand | Name | Temperature | Duration | Volume of water |
|---|---|---|---|---|---|
| 1 | Clearline, Dominique Dutscher | 50 ml Centrifuge tube | 4°C | 2 weeks | 50 ml |
| 5 | Fisher scientific | Fisherbrand™ SureOne™ 1000 µl Filter Tip | Room temperature | 30 s | 1 ml |
| 7 | Gilson | D1000ST Diamond Tipack | Room temperature | 30 s | 1 ml |
| 9 | Falcon BD Biosciences | Tissue Culture Flask 50 ml | 37°C | 48 h | 10 ml |
| 10 | Sigma-Aldrich | medium bottles | 4°C | 1 month | 100 ml |
Cell culture and ART media
Eighteen cell culture media that are usually used for oocyte collection (Medium 199 with Hepes, BO-HEPES-IVM), maturation (Medium 199, BO-IVM), fertilization (BO-IVF) and embryo development (BO-IVC) in ruminants for granulosa cell culture (McCoy’s 5A medium) and for ART in women (SAGE 1-step, Global, Sequential Fert, Sequential Cleav, Sequential Blast, etc.) were assessed for 10 bisphenols (Table V). For media assessment, each measurement (therefore each sample number) corresponded to sampling in separate bottles. In one case (Medium 199), because the BPS level was quite high, several measurements of the same bottle (sample number 19) and of separate bottles of the same batch (sample number 19-21) were assessed. Triplicate measurements were performed when possible and the complete dataset including all replicates is provided in Supplementary Tables SI and SII (in ng/l and nM, respectively). Each sample was analysed directly and after a 10-fold dilution in pure water to avoid a potential matrix effect caused by salts in the media. Two types of controls were associated with this measurement: controls with ultrapure water and each media spiked with a solution of bisphenols (Table VI).
Table V.
List of culture media assessed for the presence of bisphenols.
| Sample number | Product type | Brand | Origin | Name | Vial | Use | Product reference |
|---|---|---|---|---|---|---|---|
| 18-19 | Cell culture medium | Sigma-Aldrich | UK | McCoy 5A Medium | Pl | RL | M8403 |
| 20-21 | Oocyte retrieval, holding and washing media | Origio, Cooper surgical | Denmark | Synvitroflush | Pl | ART | 15840125A |
| 22-23 | Origio, Cooper surgical | Denmark | Flushing medium | Pl | ART | 10840060A | |
| 24 | Ivf Bioscience | UK | BO-WASH | Gl | BEP | 61008 | |
| 25 | Sigma-Aldrich | UK | Medium 199 with Hepes | Pl | BEP | M7528 | |
| 26 | IVM media | Ivf Bioscience | UK | BO-IVM | Gl | BEP | 61002 |
| 27 | Ivf Bioscience | UK | BO-HEPES-IVM | Gl | BEP | 61009 | |
| 28 | Sperm preparation and IVF media | Ivf Bioscience | UK | BO-IVF | Gl | BEP | 61003 |
| 29–30 | Origio, Cooper surgical | Denmark | Gradient 40/80 | Pl | ART | 84022060A | |
| 31–32 | Origio, Cooper surgical | Denmark | Sequential Fert | Pl | ART | 83010060A | |
| 33 | Origio, Cooper surgical | Denmark | Universal IVF medium | Pl | ART | 10310060A | |
| 34–36 | In vitro development media | Origio, Cooper surgical | Denmark | SAGE 1-Step™ | Pl | ART | 67010010A |
| 37 | Origio, Cooper surgical | Denmark | Sequential Cleav | Pl | ART | 83040010A | |
| 38 | Origio, Cooper surgical | Denmark | Sequential Blast | Pl | ART | 83060010A | |
| 39 | LifeGlobal | USA | Global | Pl | ART | LGGG-020 | |
| 40 | Ivf Bioscience | UK | BO-IVC | Gl | BEP | 61001 | |
| 41–45 | Sigma-Aldrich | UK | Medium 199 | Pl | BEP | M4530 | |
| 46 | Embryo washing and handling media | LifeGlobal | USA | Global with HEPES | Pl | ART | LGGH-050 |
BEP, bovine embryo production; Gl, glass vial; Pl, plastic vial; RL, research laboratory.
Table VI.
Bisphenol assessment in cell culture and ART media (in ng/l).
| Bisphenols (LOQ in ng/l) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample number | Product type | Name | Reference number | Batch number | Use | BPA (10) | BPS (2) | BPE (10) | BPF (5) | BPAF (5) | BPAP (10) | BPB (5) | BPC (15) | BPP (10) | BPZ (10) |
| 18 | Cell culture medium | McCoy's 5A Medium | M8403 | SLCB0586 | RL | NQ | 74 | NQ | NQ | NQ | NQ | NQ | NQ | NQ | NQ |
| 19 | SLCB7211 | NQ | 395 | NQ | NQ | 5 | NQ | NQ | NQ | NQ | NQ | ||||
| 20 | Oocyte retrieval, holding and washing media | Synvitroflush | 15840125A | 20250050 | ART | NQ | NQ | NQ | NQ | NQ | NQ | NQ | NQ | NQ | NQ |
| 21 | 20150031 | NQ | NQ | NQ | NQ | NQ | NQ | NQ | NQ | NQ | NQ | ||||
| 22 | Flushing medium | 10840060A | 20280036 | ART | NQ | 456 ± 3 | 12 ± 0.3 | 15 ± 0.6 | NQ | NQ | NQ | NQ | NQ | NQ | |
| 23 | 20040044 | 5 ± 0.4 | 436 ± 3 | 28 ± 0.3 | NQ | NQ | NQ | NQ | NQ | NQ | NQ | ||||
| 24 | BO-WASH | 61008 | WASH1704 | BEP | NQ | 35 ± 0.2 | NQ | 6 ± 0.1 | NQ | NQ | NQ | NQ | NQ | NQ | |
| 25 | Medium 199 with Hepes | M7528 | RNBG6724 | BEP | 28 | 308 | 15 | NQ | NQ | 14 | NQ | NQ | NQ | NQ | |
| 26 | In vitro maturation media | BO-IVM | 61002 | IVM1701N | BEP | 28 ± 1.4 | 53 ± 0.9 | NQ | 17 ± 1.4 | NQ | 16 ± 0.5 | NQ | NQ | NQ | NQ |
| 27 | BO-HEPES-IVM | 61009 | IVMH1602N | BEP | 155 ± 2 | 23 ± 0.6 | NQ | NQ | NQ | NQ | NQ | NQ | NQ | NQ | |
| 28 | Sperm preparation and IVF media | BO-IVF | 61003 | IVF1702N | BEP | 62 ± 1 | 112 ± 2 | 64 ± 1 | NQ | NQ | 38 ± 1.7 | NQ | NQ | NQ | NQ |
| 29 | Gradient 40/80 | 84022060 A | 20110060 | ART | NQ | 67 ± 1 | NQ | NQ | NQ | NQ | NQ | NQ | NQ | NQ | |
| 30 | 20180042 | NQ | 21 ± 1 | NQ | NQ | NQ | NQ | NQ | NQ | NQ | NQ | ||||
| 31 | Sequential Fert | 83010060A | 20190042 | ART | NQ | NQ | NQ | NQ | NQ | NQ | NQ | NQ | NQ | NQ | |
| 32 | 20220035 | NQ | NQ | NQ | NQ | NQ | NQ | NQ | NQ | NQ | NQ | ||||
| 33 | Universal IVF medium | 10310060 A | 20270017 | ART | NQ | 750 ± 24 | 18 ± 0.6 | 20 ± 1.5 | 10 ± 0.4 | NQ | NQ | NQ | NQ | NQ | |
| 34 | In vitro development media | SAGE 1-step | 67010010A | 19370063 | ART | NQ | 283 | 16 | NQ | NQ | NQ | NQ | NQ | NQ | NQ |
| 35 | 19290061 | 12 | 394 | 18 | NQ | 11 | NQ | NQ | NQ | NQ | NQ | ||||
| 36 | 20270046 | NQ | 337 ± 2 | NQ | 9 ± 1.3 | NQ | NQ | NQ | NQ | NQ | NQ | ||||
| 37 | Sequential Cleav | 83040010A | 20320064 | ART | 18 ± 0.7 | 179 ± 4 | 7 ± 1.3 | 8 ± 1.2 | 7 ± 0.5 | NQ | NQ | NQ | NQ | NQ | |
| 38 | Sequential Blast | 83060010A | 20330063 | ART | 4 ± 0.6 | 187 ± 20 | NQ | 3 ± 0.3 | 5 ± 0.1 | NQ | NQ | NQ | NQ | NQ | |
| 39 | Global | LGGG-020 | LGGG-200824U | ART | NQ | 10 ± 0.1 | NQ | NQ | NQ | NQ | NQ | NQ | NQ | NQ | |
| 40 | BO-IVC | 61001 | IVC1703N | BEP | 34 ± 3.8 | 322 ± 4 | 119 ± 6 | 25 ± 2.6 | NQ | 16 ± 0.6 | NQ | NQ | NQ | NQ | |
| 41 | Medium 199—100 ml bottle | M4530 | RNBH8521 | BEP | NQ | 278 | 16 | 5 | NQ | NQ | NQ | NQ | NQ | NQ | |
| 42 | RNBG5443 | NQ | 1188 ± 23 | 26 ± 4.6 | 18 ± 2.3 | NQ | NQ | NQ | NQ | NQ | NQ | ||||
| 43 | RNBG5443 | NQ | 1693 | 33 | 16 | NQ | NQ | NQ | NQ | NQ | NQ | ||||
| 44 | RNBG5443 | NQ | 1220 | 21 | 18 | NQ | NQ | NQ | NQ | NQ | NQ | ||||
| 45 | RNBH6994 | NQ | 233 | NQ | NQ | NQ | NQ | NQ | NQ | NQ | NQ | ||||
| 46 | Embryo washing and handling media | Global with HEPES | LGGH-050 | LGGH-200820C | ART | NQ | 5 ± 0.1 | NQ | NQ | NQ | NQ | NQ | NQ | NQ | NQ |
NQ, not quantifiable; LOQ, mentioned below each bisphenols between brackets; each sample number corresponds to a separate vial; bold text: significant difference with the controls P < 0.0001, non-parametric one-way ANOVA with the Tukey post hoc test were performed; sample number 18, 19, 25, 34, 35, 41, 43, 44 and 45 were not analysed in triplicate.
Bisphenol measurements
HPLC-grade methanol, acetic acid and water were purchased from Fisher Scientific (Illkirch, France). Analytical standards of bisphenols were purchased from Dr. Ehrenstorfer (VWR International, Fontenay sous Bois, France), CIL-Cluzeau (Sainte-Foy-La Grande, France), Sigma-Aldrich (Saint Quentin Fallavier, France) and Techlab (Saint-Julien-lès-Metz, France) as powder (purity> 95%).
Bisphenols were measured using an isotopic dilution, according to an on-line solid phase extraction/liquid chromatography/mass spectrometry (LC/MS) method. One milliliter of sample was spiked with 100 µl of solution containing internal standards (2 µg/l). Of this, 500 µl was injected into the on-line extraction cartridge (Xbridge C18 Direct Connect HP 10 µm, 2.1 mm × 30 mm, 1.8 µm; Waters) coupled with UPLC/MS-MS equipped with an HSS T3 analytical column (2.1 mm × 100 mm, 1.8 µm; Waters). Detection was carried out using a triple quadrupole mass spectrometer (XEVO-TQXS; Waters), fitted with an ESI interface (negative mode) and controlled by MassLynx software (Waters Corporation, Milford MA, USA).
Specific and intense product ions of each target analyte were used for quantification, and a secondary product ion was used as a qualifier ion for confirmatory purposes. Method quantification limits are indicated in Tables III and VI.
The method has been validated on a linear range from the limit of quantification (LOQ) to 1000 ng/l (except for BPP limited to 300 ng/l). Recoveries ranged on water samples between 80% (BPP) and 100% with a high level of repeatability from 3% to 10% (for BPP). Absolute recoveries of bisphenols from media were lower (from 40% to 85%) but totally outweighted by the internal standards that confirmed the need for using isotopic dilution for measurements. Owing to high specificity of each media regarding the matrix, controls concerning potential matrix interference effects have been systematically assessed and results confirmed by spiking or diluting samples when needed. All solvents and steps for the analytical procedure were checked separately to avoid systematic contamination: no pollution from the solvents has been highlighted.
Statistical analyses
The levels of each bisphenol, found in either plastic consumables or cell culture media, were compared among the groups in Rcmdr [R package Rcmdr (Fox, 2005)], R version 4.0.0 (R Core Team, 2015), using non parametric one-way ANOVA [R package lmPerm (Wheeler and Torchiano, 2010)], with the Tukey post hoc test (R package nparcomp; Konietschke et al., 2015). A difference of P ≤ 0.05 was considered significant.
Results
Plastic consumables
After 24 h of methanol action, the 17 plastic consumables listed in Table II were assessed for the presence of 10 bisphenols. BPA, BPS and BPAF were detected in all 17 plastic consumables (Table III). In contrast, BPAP, BPB, BPC, BPE, BPF, BPP and BPZ were detected in none of the consumables. Regarding the level of bisphenols detected compared to the LOQ, five consumables showed a systematic high level of bisphenols (at least 15 times more than the LOQ). BPAF was detected at a high level in sample number 1 (50 ml centrifuge tube) and 9 (cell culture flask). Both BPA and BPS were detected in tips (sample number 5 and 7). Both BPS and BPAF were detected in bottles of cell culture media (sample number 10). This experiment was based on plastic dissolution in methanol: the bisphenol level in and of itself is only a proxy of the plastic composition and has no real meaning on the potential leaching in culture media.
We therefore performed a leaching test on these five plastic consumables (sample numbers 1, 5, 7, 9 and 10) under conditions close to those used in routine practice (Table IV). No quantification of bisphenols, neither in leaching water nor in controls, was reported.
Cell culture and ART media
We provided the level of bisphenols detected in both ng/l (Table VI) and in nM (Supplementary Tables SI and SII) to be able to compare our data to the literature. BPS was the main bisphenol detected in the cell culture media assessed (Table VI). Nevertheless, six bisphenols (BPA, BPS, BPAF, BPAP, BPE and BPF) were found among the 18 culture media assessed, while BPB, BPC, BPP and BPZ were never detected. BPAP was detected in four media and reached 38 ng/l (0.13 nM) in BO-IVF, BPAF was detected in five media and reached 10 ng/l (0.03 nM) in Universal IVF Medium, BPF was detected in nine media and reached 25 ng/l (0.12 nM) in BO-IVC, BPE was detected in eight media and reached 119 ng/l (0.55 nM) in BO-IVC, BPA was detected in nine media and reached 155 ng/l (0.68 nM) in BO-HEPES-IVM and BPS was detected in 16 of the 18 media assessed. BPS was the bisphenol detected at the highest level in all cases: up to 1693 ng/l (6.8 nM) in Medium 199. BPS exceeded 1 nM in six media: 308 ng/l in Medium 199 with Hepes (1.23 nM), 322 ng/l in BO-IVC (1.28 nM), 338 ng/l in SAGE-1 Step (1.35 nM), 446 ng/l in Flushing Medium (1.78 nM), 750 ng/l in Universal IVF Medium (3.0 nM) and 922 ng/l in Medium 199 (3.69 nM).
Discussion
This study aimed to evaluate the presence and/or leaching of 10 bisphenols in plastic consumables and in cell culture and ART media. For the first time, we reported that cell culture media contained bisphenols, notably BPS in nanomolar range concentrations. Moreover, while plastic consumables contain bisphenols, they do not leach detectable levels of bisphenols in conditions close to those used in routine practice. This study highlighted the need for assessing more ART media for the presence of endocrine disruptors.
The finding that raised the most concerns in the present study is the level of BPS detected in cell culture and ART media. Indeed, the BPS level was above 1 nM (0.25 ng/ml) in six media among 18 tested and reached up to 6.8 nM (1.7 ng/ml). BPA was also detected in nine media and reached up to 0.68 nM (0.15 ng/ml). Comparatively, undetectable BPA levels were reported for three and four ART media, respectively (Mahalingaiah et al., 2012; Gatimel et al., 2016). Undetectable meant below the respective LOQ of these studies, 0.27 (Mahalingaiah et al., 2012) or 0.5 ng/ml (Gatimel et al., 2016). To our knowledge, there are no data in the literature reporting BPS presence in cell culture media thus far. Regarding BPS exposure, the level measured in culture media in this study (up to 1.7 ng/ml) is not above the level found in some human fluids (up to 21 ng/ml in urine) (Liao et al., 2012a). Nevertheless, in this study, the level of BPS was measured after glucuronidase treatment of the sample and, therefore, included both BPS (native form) and BPS glucuronide (metabolized form). Moreover, a recent study reported a deleterious effect of 10 nM BPS during 24 h oocyte maturation on ovine oocyte quality, measured in terms of blastocyst rate after in vitro embryo production (Desmarchais et al., 2020). Even a lower concentration of BPS (3 nM) during 48 h oocyte maturation in porcine was reported to significantly reduce the rate of oocytes reaching metaphase II (Zalmanova et al., 2017). In the present study, the BPS level measured in the media is in the same range of concentrations (nanomolar range) as the ones affecting oocyte quality in ovine and porcine. Moreover, ovine and porcine oocytes were exposed to BPS for 24 or 48 h, respectively, only during oocyte maturation. It is still possible that the oocyte maturation stage is more sensitive to the impact of BPS compared to the fertilization and/or early embryo development stages. Nevertheless, during ART, the embryo stayed up to 6 days in the culture medium and, therefore, can be affected by the BPS present in the medium. In addition, BPS was not in the glucuronide form in the medium. The cumulus-oocyte complex likely does not possess the required cellular machinery to transform BPS into its inactive form, BPS glucuronide, meaning that the effect of BPS will last for the duration of the culture. This is different from what happens in vivo, as the glucuronidation of bisphenols occurs rapidly after exposure. Therefore, even if the duration of exposure is short (up to 6 days), the effects might not be negligible compared to a similar in vivo exposure. Moreover, a BPA exposure during early embryo development in bovine (Choi et al., 2016) and murine (Pan et al., 2015) decreased the blastocyst rate and damaged blastocyst development, suggesting an effect not only on oocyte quality but also on early embryo development. These findings raise concerns about the effect of BPS contained in the media on the outcome of ART and suggested the importance of investigating a wider diversity of ART media. Moreover, the presence of bisphenols in cell culture media also raises concerns on results reported in the literature regarding bisphenol effects on cells, oocytes or embryos. These results should be analysed with caution, especially when dealing with nanomolar concentrations of bisphenols, or even lower.
Regarding a potential cocktail effect, even if other bisphenols are less abundant than BPS, the culture media still contained five other bisphenols (BPA, BPAF, BPAP, BPE and BPF). Their cumulative or potentially synergetic effects have not yet been studied on the oocyte. Such accumulation of exogenous molecules can strengthen their deleterious effects on oocyte quality. It is also important to keep in mind that the present study only focused on the bisphenol family. Therefore, only bisphenols have been detected and measured in the culture medium. It is likely that other exogenous molecules could be found in the ART medium, such as phthalates, as is the case in food containers (Gonzalez-Castro et al., 2011) and in IVF media (Takatori et al., 2012). Such combinations of compounds and their cumulative effects on oocyte quality are still poorly studied. Nevertheless, mixtures of endocrine disruptors, including BPA, have already been shown to have cumulative estrogenic effects on human endometrial cells (Aichinger et al., 2020). Other mixtures of compounds even showed synergistic endocrine disruption effects in human adrenocortical cells (Ahmed et al., 2019) or during embryo development in fish (Wu et al., 2018). More studies are required to investigate the effects of mixtures of endocrine disruptors on oocyte quality.
In this study, bisphenols were detected in all the consumables assessed. Indeed, the addition of bisphenols to plastic consumables renders them hard to break, resistant to heat and easy to sterilize. These advantages can explain their widespread use in polypropylene or polystyrene-based plastic consumables. Here, we reported no leaching of the 10 bisphenols assessed from plastic consumables under routine practice conditions. This result is in line with previous studies that focused on BPA (Mahalingaiah et al., 2012; Gatimel et al., 2016). These data are reassuring. Despite the presence of BPA, BPS and BPAF in the plastic consumables used in ART, no additional bisphenol, over and above that detected in ART media, is expected to leach from the consumables. Nevertheless, the present study did not analyze all possible plastic consumables and cannot rule out the possibility that leaching may be observed at higher temperatures than in our conditions. Furthermore, the study from Gatimel et al. (2016) found BPA in the strippers used to remove the cumulus from the oocyte. It would be interesting to analyze these strippers for the 10 bisphenols measured in this study.
The source of the bisphenols measured in culture media is still unknown. Indeed, we still do not know whether the presence of bisphenols came from leaching in the medium’s plastic bottle or from the media production process. Moreover, even media supplied in glass vials exhibited BPS, BPA and/or BPE levels. Our results did not demonstrate a leaching effect from the plastic bottle under routine practice conditions. It is nevertheless possible that leaching had already reached a plateau with the culture media originally present in the bottle and that the leaching experiment performed by replacing these media with water did not allow further leaching from the plastic. To answer this question, close collaboration with the companies producing ART medium would be required, so that media at different steps of the production process could be analyzed.
Regulation for plastic intended to come into contact with food is constantly evolving and integrating new knowledge on the endocrine properties of bisphenols. In parallel, water regulations have been implemented, through the European Union Water framework Directive (BPA and BPS) and European Union Drinking water Directive (BPA). To the author’s knowledge, thus far no considerations are made for these compounds in the ART context.
In this study, analytical methods for bisphenol measurement were developed with many endpoints for quality control, considering the ubiquity and risks of sample contamination. Despite these precautions, some contamination can occur. This is why we chose to focus only on samples exhibiting bisphenol levels higher than 15 times the LOQ. Owing to the limited set of available samples, there is a need to replicate assays on a higher number of samples (both in terms of references and batch numbers assessed) to be more representative and to investigate whether some ART media could be less potentially harmful in terms of bisphenol content.
Conclusion
In conclusion, we showed that the plastic consumables assessed in this paper and used in ART do not release bisphenols under routine conditions. Conversely, cell culture media, as well as media used in ART, exhibited BPS in 16 of the 18 types of media assessed, six of them containing BPS in the nanomolar concentration range. As BPS was already reported to impair oocyte quality, its presence in ART media could contribute to a decrease in the ART success rate. Further studies are required to investigate a greater number of ART media to identify the less deleterious ones, in terms of bisphenol abundance.
Supplementary data
Supplementary data are available at Human Reproduction.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Authors’ roles
A.T. and S.E. participated in the study design and the analyses and drafted the manuscript. A.D., O.T., C.B., C.V., V.M., F.G. and A.B. helped drafted the manuscript and participated in the critical discussion. S.B. participated in the execution of the experimental design, its analyses and the critical discussion.
Funding
This study was financially supported by the ‘Centre-Val de Loire’ Region (Bemol project, APR IR 2017), INRAE, BRGM, the French National Research Agency (project ANR-18-CE34-0011-01 MAMBO) and the BioMedicine Agency (Project 18AMP006 FertiPhenol).
Conflict of interest
The authors declare that they have no conflict of interest that could be perceived as prejudicing the impartiality of the reported research.
Supplementary Material
References
- Abraham A, Chakraborty P. A review on sources and health impacts of bisphenol A. Rev Environ Health 2020;35:201–210. [DOI] [PubMed] [Google Scholar]
- Ahmed KEM, Frøysa HG, Karlsen OA, Blaser N, Zimmer KE, Berntsen HF, Verhaegen S, Ropstad E, Kellmann R, Goksøyr A. Effects of defined mixtures of POPs and endocrine disruptors on the steroid metabolome of the human H295R adrenocortical cell line. Chemosphere 2019;218:328–339. [DOI] [PubMed] [Google Scholar]
- Ahsan N, Ullah H, Ullah W, Jahan S. Comparative effects of Bisphenol S and Bisphenol A on the development of female reproductive system in rats; a neonatal exposure study. Chemosphere 2018;197:336–343. [DOI] [PubMed] [Google Scholar]
- Aichinger G, Pantazi F, Marko D. Combinatory estrogenic effects of bisphenol A in mixtures with alternariol and zearalenone in human endometrial cells. Toxicol Lett 2020;319:242–249. [DOI] [PubMed] [Google Scholar]
- Al-Saleh I, El-Doush I, Grisellhi B, Coskun S. The effect of caffeine consumption on the success rate of pregnancy as well various performance parameters of in-vitro fertilization treatment. Med Sci Monit 2010;16:CR598–605. [PubMed] [Google Scholar]
- Amar S, Binet A, Teteau O, Desmarchais A, Papillier P, Lacroix MZ, Maillard V, Guerif F, Elis S. Bisphenol S Impaired Human Granulosa Cell Steroidogenesis in Vitro. IJMS 2020;21:1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andaluri G, Manickavachagam M, Suri R. Plastic toys as a source of exposure to bisphenol-A and phthalates at childcare facilities. Environ Monit Assess 2018;190:65. [DOI] [PubMed] [Google Scholar]
- Andra SS, Charisiadis P, Arora M, van Vliet-Ostaptchouk JV, Makris KC. Biomonitoring of human exposures to chlorinated derivatives and structural analogs of bisphenol A. Environ Int 2015;85:352–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee O, Singh S, Prasad SK, Bhattacharjee A, Banerjee A, Banerjee A, Saha A, Maji BK, Mukherjee S. Inhibition of catalase activity with 3-amino-1,2,4-triazole intensifies bisphenol A (BPA)-induced toxicity in granulosa cells of female albino rats. Toxicol Ind Health 2018;34:787–797. [DOI] [PubMed] [Google Scholar]
- Bujnakova Mlynarcikova A, Scsukova S. Simultaneous effects of endocrine disruptor bisphenol A and flavonoid fisetin on progesterone production by granulosa cells. Environ Toxicol Pharmacol 2018;59:66–73. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect 2005;113:391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong L-Y, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect 2008;116:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campen KA, Kucharczyk KM, Bogin B, Ehrlich JM, Combelles CMH. Spindle abnormalities and chromosome misalignment in bovine oocytes after exposure to low doses of bisphenol A or bisphenol S. Hum Reprod 2018;33:895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BI, Harvey AJ, Green MP. Bisphenol A affects early bovine embryo development and metabolism that is negated by an oestrogen receptor inhibitor. Sci Rep 2016;6:29318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmarchais A, Téteau O, Papillier P, Jaubert M, Druart X, Binet A, Maillard V, Elis S. Bisphenol S impaired in vitro ovine early developmental oocyte competence. Int J Mol Sci 2020;21:1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S, Williams PL, Missmer SA, Flaws JA, Ye X, Calafat AM, Petrozza JC, Wright D, Hauser R. Urinary bisphenol A concentrations and early reproductive health outcomes among women undergoing IVF. Hum Reprod 2012;27:3583–3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eladak S, Grisin T, Moison D, Guerquin MJ, N'Tumba-Byn T, Pozzi-Gaudin S, Benachi A, Livera G, Rouiller-Fabre V, Habert R. A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil Steril 2015;103:11–21. [DOI] [PubMed] [Google Scholar]
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processings Aids (CEF). Scientific opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J 2015;13:3978. [Google Scholar]
- European Union. Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food. Off J Eur Union, 2019.
- Fox J. Getting started with the R commander: a basic-statistics graphical user interface to R. J Stat Softw 2005;14:1–42. [Google Scholar]
- Fujimoto VY, Kim D, Vom Saal FS, Lamb JD, Taylor JA, Bloom MS. Serum unconjugated bisphenol A concentrations in women may adversely influence oocyte quality during in vitro fertilization. Fertil Steril 2011;95:1816–1819. [DOI] [PubMed] [Google Scholar]
- Gatimel N, Lacroix MZ, Chanthavisouk S, Picard-Hagen N, Gayrard V, Parinaud J, Léandri RD. Bisphenol A in culture media and plastic consumables used for ART. Hum Reprod 2016;31:1436–1444. [DOI] [PubMed] [Google Scholar]
- Giulivo M, Lopez de Alda M, Capri E, Barceló D. Human exposure to endocrine disrupting compounds: Their role in reproductive systems, metabolic syndrome and breast cancer. A review. Environ Res 2016;151:251–264. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Castro MI, Olea-Serrano MF, Rivas-Velasco AM, Medina-Rivero E, Ordonez-Acevedo LG, De Leon-Rodriguez A. Phthalates and bisphenols migration in Mexican food cans and plastic food containers. Bull Environ Contam Toxicol 2011;86:627–631. [DOI] [PubMed] [Google Scholar]
- Grasselli F, Baratta L, Baioni L, Bussolati S, Ramoni R, Grolli S, Basini G. Bisphenol A disrupts granulosa cell function. Domest Anim Endocrinol 2010;39:34–39. [DOI] [PubMed] [Google Scholar]
- Grun F, Blumberg B. Perturbed nuclear receptor signaling by environmental obesogens as emerging factors in the obesity crisis. Rev Endocr Metab Disord 2007;8:161–171. [DOI] [PubMed] [Google Scholar]
- Ijaz S, Ullah A, Shaheen G, Jahan S. Exposure of BPA and its alternatives like BPB, BPF, and BPS impair subsequent reproductive potentials in adult female Sprague Dawley rats. Toxicol Mech Methods 2020;30:60–72. [DOI] [PubMed] [Google Scholar]
- Jirsova S, Masata J, Jech L, Zvarova J. Effect of polychlorinated biphenyls (PCBs) and 1,1,1-trichloro-2,2,-bis (4-chlorophenyl)-ethane (DDT) in follicular fluid on the results of in vitro fertilization-embryo transfer (IVF-ET) programs. Fertil Steril 2010;93:1831–1836. [DOI] [PubMed] [Google Scholar]
- Kang JH, Kondo F, Katayama Y. Human exposure to bisphenol A. Toxicology 2006;226:79–89. [DOI] [PubMed] [Google Scholar]
- Kirchnawy C, Hager F, Osorio Piniella V, Jeschko M, Washüttl M, Mertl J, Mathieu-Huart A, Rousselle C. Potential endocrine disrupting properties of toys for babies and infants. PLoS One 2020;15:e0231171–e0231171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konietschke F, Placzek M, Schaarschmidt F, Hothorn LA. Nparcomp: An R Software Package for Nonparametric Multiple Comparisons and Simultaneous Confidence Intervals. J Stat Soft 2015;64: [Google Scholar]
- Kubwabo C, Kosarac I, Stewart B, Gauthier BR, Lalonde K, Lalonde PJ. Migration of bisphenol A from plastic baby bottles, baby bottle liners and reusable polycarbonate drinking bottles. Food Addit Contam A: Chem Anal Control Expo Risk Assess 2009;26:928–937. [DOI] [PubMed] [Google Scholar]
- Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 2008;300:1303–1310. [DOI] [PubMed] [Google Scholar]
- Liao C, Liu F, Alomirah H, Loi VD, Mohd MA, Moon H-B, Nakata H, Kannan K. Bisphenol S in urine from the United States and seven Asian countries: occurrence and human exposures. Environ Sci Technol 2012a;46:6860–6866. [DOI] [PubMed] [Google Scholar]
- Liao C, Liu F, Guo Y, Moon H-B, Nakata H, Wu Q, Kannan K. Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: implications for human exposure. Environ Sci Technol 2012b;46:9138–9145. [DOI] [PubMed] [Google Scholar]
- Mahalingaiah S, Hauser R, Patterson DG, Woudneh M, Racowsky C. Bisphenol A is not detectable in media or selected contact materials used in IVF. Reprod Biomed Online 2012;25:608–611. [DOI] [PubMed] [Google Scholar]
- Mansur A, Adir M, Yerushalmi G, Hourvitz A, Gitman H, Yung Y, Orvieto R, Machtinger R. Does BPA alter steroid hormone synthesis in human granulosa cells in vitro? Hum Reprod 2016;31:1562–1569. [DOI] [PubMed] [Google Scholar]
- Mlynarcikova A, Kolena J, Fickova M, Scsukova S. Alterations in steroid hormone production by porcine ovarian granulosa cells caused by bisphenol A and bisphenol A dimethacrylate. Mol Cell Endocrinol 2005;244:57–62. [DOI] [PubMed] [Google Scholar]
- Mok-Lin E, Ehrlich S, Williams PL, Petrozza J, Wright DL, Calafat AM, Ye X, Hauser R. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int J Androl 2010;33:385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal A, Fuentes E, Ripoll C, Villar-Pazos S, Castellano-Munoz M, Soriano S, Martinez-Pinna J, Quesada I, Alonso-Magdalena P. Extranuclear-initiated estrogenic actions of endocrine disrupting chemicals: Is there toxicology beyond paracelsus? J Steroid Biochem Mol Biol 2018;176:16–22. [DOI] [PubMed] [Google Scholar]
- Pan X, Wang X, Sun Y, Dou Z, Li Z. Inhibitory effects of preimplantation exposure to bisphenol-A on blastocyst development and implantation. Int J Clin Exp Med 2015;8:8720–8729. [PMC free article] [PubMed] [Google Scholar]
- Peretz J, Vrooman L, Ricke WA, Hunt PA, Ehrlich S, Hauser R, Padmanabhan V, Taylor HS, Swan SH, VandeVoort CA et al. Bisphenol a and reproductive health: update of experimental and human evidence, 2007-2013. Environ Health Perspect 2014;122:775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing (Version 0.97.316). Vienna, Austria: R Foundation for Statistical Computing, 2015. [Google Scholar]
- Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, Vom Saal FS. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol 2007;24:199–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester JR. Bisphenol A and human health: a review of the literature. Reprod Toxicol 2013;42:132–155. [DOI] [PubMed] [Google Scholar]
- Samardzija D, Pogrmic-Majkic K, Fa S, Stanic B, Jasnic J, Andric N. Bisphenol A decreases progesterone synthesis by disrupting cholesterol homeostasis in rat granulosa cells. Mol Cell Endocrinol 2018;461:55–63. [DOI] [PubMed] [Google Scholar]
- Takatori S, Akutsu K, Kondo F, Ishii R, Nakazawa H, Makino T. Di(2-ethylhexyl)phthalate and mono(2-ethylhexyl)phthalate in media for in vitro fertilization. Chemosphere 2012;86:454–459. [DOI] [PubMed] [Google Scholar]
- Teteau O, Jaubert M, Desmarchais A, Papillier P, Binet A, Maillard V, Elis S. Bisphenol A and S impaired ovine granulosa cell steroidogenesis. Reproduction 2020;159:571–583. [DOI] [PubMed] [Google Scholar]
- Thayer KA, Taylor KW, Garantziotis S, Schurman SH, Kissling GE, Hunt D, Herbert B, Church R, Jankowich R, Churchwell MI et al. Bisphenol A, bisphenol S, and 4-hydroxyphenyl 4-isoprooxyphenylsulfone (BPSIP) in urine and blood of cashiers. Environ Health Perspect 2016;124:437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usman A, Ahmad M. From BPA to its analogues: is it a safe journey? Chemosphere 2016;158:131–142. [DOI] [PubMed] [Google Scholar]
- Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, Watson CS, Zoeller RT, Belcher SM. In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol 2007;24:178–198. [DOI] [PubMed] [Google Scholar]
- Wheeler B, Torchiano M. lmPerm: permutation tests for linear models. R Package Version 2010;1: [Google Scholar]
- Wu S, Hu G, Zhao X, Wang Q, Jiang J. Synergistic potential of fenvalerate and triadimefon on endocrine disruption and oxidative stress during rare minnow embryo development. Environ Toxicol 2018;33:759–769. [DOI] [PubMed] [Google Scholar]
- Ye X, Wong L-Y, Kramer J, Zhou X, Jia T, Calafat AM. Urinary concentrations of bisphenol A and three other bisphenols in convenience samples of U.S. adults during 2000–2014. Environ Sci Technol 2015;49:11834–11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalmanova T, Hoskova K, Nevoral J, Adamkova K, Kott T, Sulc M, Kotikova Z, Prokesova S, Jilek F, Kralickova M et al. Bisphenol S negatively affects the meotic maturation of pig oocytes. Sci Rep 2017;7:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Liu J, Liao L, Han S, Liu J. Effect of bisphenol A on steroid hormone production in rat ovarian theca-interstitial and granulosa cells. Mol Cell Endocrinol 2008;283:12–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.


