Abstract
Introduction:
Non-pharmacological treatments (NPTs) have the potential to improve meaningful outcomes for older people at risk of, or living with dementia, but research often lacks methodological rigor and continues to produce mixed results.
Methods:
In the current position paper, experts in NPT research have specified treatment targets, aims, and ingredients using an umbrella framework, the Rehabilitation Treatment Specification System.
Results:
Experts provided a snapshot and an authoritative summary of the evidence for different NPTs based on the best synthesis efforts, identified main gaps in knowledge and relevant barriers, and provided directions for future research. Experts in trial methodology provide best practice principles and recommendations for those working in this area, underscoring the importance of prespecified protocols.
Discussion:
We conclude that the evidence strongly supports various NPTs in relation to their primary targets, and discuss opportunities and challenges associated with a unifying theoretical framework to guide future efforts in this area.
Keywords: cognitive rehabilitation, cognitive stimulation therapy, cognitive training, cognitive-behavioral therapy for insomnia, communication treatments, framework, meditation, mild cognitive impairment, multisensory treatments, music-based treatments, neuromodulation, neuropsychiatric, non-pharmacological, nutritional interventions, occupational therapy, physical exercise training, reminiscence therapy, subjective cognitive decline
1 |. INTRODUCTION
Dementia is currently one of the most pressing health-care issues, with a worldwide rising prevalence due to the aging of the population and the absence of a curative therapy.1,2 The estimated global total costs of dementia were US$ 818 billion in 2015, of which the majority was related to nursing home care and the informal care of caregivers. Dementia also has a considerable disease impact, affecting the quality of life of both people with dementia and their caregivers. Several diseases of the brain might underlie dementia, with Alzheimer’s disease (AD) being the most common underlying cause.
Non-pharmacological treatments (NPTs) can be effective in the management of clinical symptoms and are likely to play an important role in the primary and secondary prevention of dementia. Advantages of NPTs are that they are generally well accepted, have minimal adverse side effects, and can be combined with other NPTs both serially and simultaneously, and with pharmacological treatments without major concerns around interference. NPTs are also applicable to different clinical stages of disease, from dementia3 to mild cognitive impairment (MCI)4 and even cognitively unimpaired adults at risk for dementia.5 As such, NPTs have the potential of having a meaningful impact on cognition, well being, and quality of life throughout the course of age-related neurodegenerative diseases.
NPTs cover a diverse and broad range of intervention categories, including cognitive training, physical exercise, dietary treatments, art-oriented therapy, and reminiscence therapy.6 An influential previous meta-analysis defined NPTs as “any theoretically based, nonchemical, focused, and replicable intervention, conducted with the patient or the caregiver, which potentially provided some relevant benefit.”7 This and many other systematic reviews of NPTs in aging and dementia (eg, Bahar-Fuchs et al.8) have repeatedly pointed out the low quality of much of the evidence behind several NPTs. To improve the quality of evidence for NPTs, more coherent, targeted, and well-reported trials of higher methodological quality are needed, which will in turn lead to firmer conclusions regarding the extent and limits of gains associated with different types of NPT.
In 2015, the Non-Pharmacological Interventions Professional Interest Area (NPI-PIA) group was formed as a successor to the “psychosocial understanding and intervention” PIA within the Alzheimer’s Association International Society to Advance Alzheimer’s Research and Treatment (ISTAART). The NPI-PIA aims to address issues related to the design, methodology, and reporting of studies of NPT in the context of aging and dementia and to stimulate research in this area. In 2017, at the NPI-PIA Annual Meeting at the Alzheimer’s Association International Conference (AAIC) in London, the Executive Committee and members agreed that there are several issues that affect progress in this broad field of research and deemed a position paper necessary. The main issues discussed included the need to (1) identify and comment on key conceptual issues and challenges in response to rapid changes in thinking about dementia and underlying neurodegenerative diseases and their treatment, and (2) advance a more coherent and theoretically driven approach to the classification and description of NPI treatments, which would lead to (3) improved methodological and reporting standards in this area of research.
In this paper, we offer a conceptual framework that may be useful for organizing and classifying research in NPT. We briefly summarize the body of work available in relation to key types of NPT in the context of people with dementia or at risk of dementia due to MCI, subjective cognitive decline, or biomarker profiling, and identify key challenges. Finally, we provide methodological guidelines for the design of trials of NPTs, and conclude with several directions for future research. An overview of the project timeline and main milestones is shown in Figure 1.
FIGURE 1.
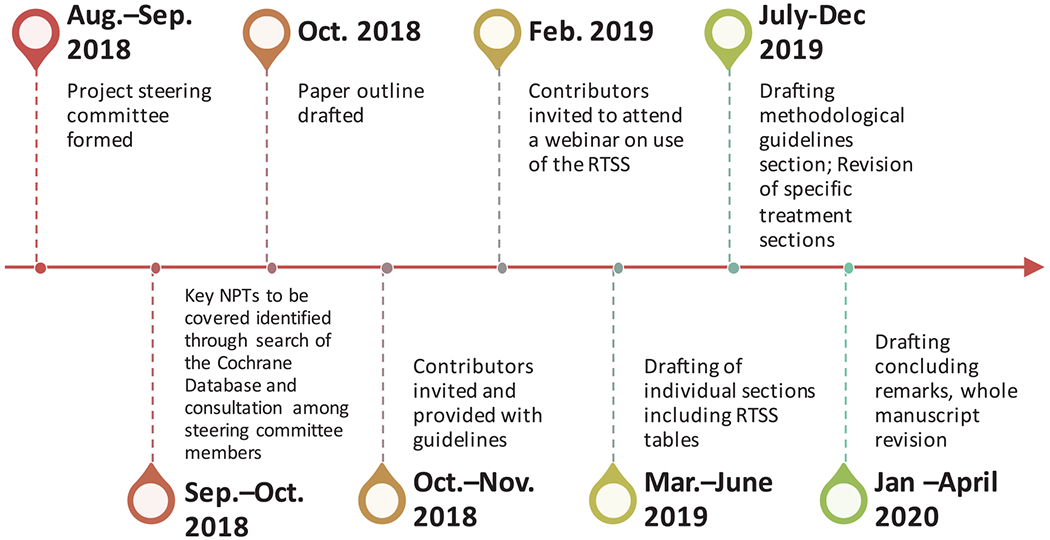
Timeline of the Non-Pharmacological Interventions Professional Interest Area Position Paper Project
1.1 |. Key conceptual issues
Here we briefly highlight some of the conceptual issues that were identified when considering and choosing the appropriate treatments and target groups for the current article.
1.1.1 |. Literature review
We performed a search of the Cochrane Database of Systematic Reviews (September 2019), which yielded 122 systematic reviews on a variety of NPT categories, ranging from aromatherapy to vitamin D3 supplements. The majority of NPTs focused on persons with dementia. Figure 2 depicts the number of studies included in these Cochrane studies, with most studies performed for cognitive training and Chinese herbal medicine.8–10 The quality grading for the level of evidence was modest at best.
FIGURE 2.
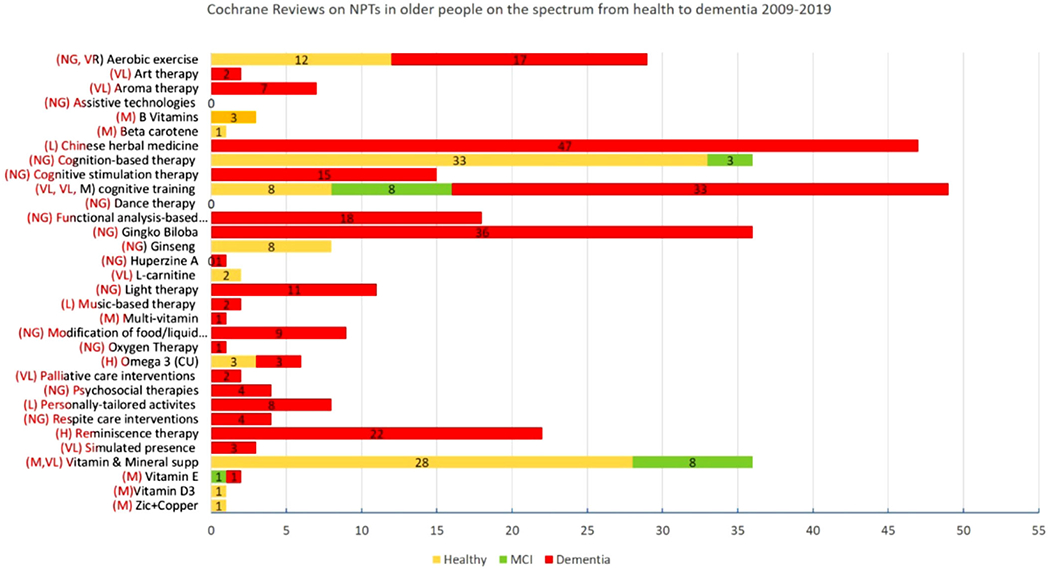
Overview of Cochrane reviews on non-pharmacological treatments (NPTs)
1.1.2 |. Target populations
The current overview focuses on individuals with dementia and those in pre-dementia at risk stages. Treatments in which the target is someone other than the person with or at risk of cognitive decline (eg, family members, caregivers, general practitioners [GPs]), and treatments focusing on training others (eg, caregivers) in the delivery of a treatment are not included in the present paper. In recent years, non-pharmacological treatment studies have broadened their focus by targeting individuals in the pre-dementia stages, based on evidence on dementia risk and modifiable lifestyle risk factors (eg, Livingston et al.11), as well as an increased focus on the biological definition of AD in pre-dementia stages.12 The pre-dementia at risk stages as discussed in the current study include those with memory complaints and biomarker evidence for AD as well as those with MCI. Hence, the NPTs covered in this paper can be relevant in the context of primary, secondary, or tertiary prevention of (objective) cognitive decline and dementia.
1.1.3 |. Treatments
In this paper, we use the term “treatments” or “therapy” rather than “intervention,” in reflection of several principles: (1) While the term “intervention” is sometimes used in the context of both the absence and the presence of a clinical condition, treatment may be more appropriate when a clinical condition is present. (2) The term “intervention” is sometimes used outside of the context of health care (eg, “financial intervention,” “educational intervention”), whereas “treatment” tends to be used more in health-related contexts, such as the context of the current paper. (3) The term intervention may be more appropriate when a wider set of activities, including one or more treatments, are delivered together, and in this sense could be seen as being of a higher order. It is not uncommon to talk about an intervention that includes treatment x or treatment y, but it is less common to talk about a treatment that includes “interventions x or y.” (4) Although “treatments” are more common in the context of pharmacological interventions, drugs are referred to as treatments irrespective of being a “cure.” Likewise, if the basis for this preference is that pharmacological agents change relevant aspects of underlying pathophysiology, a similar argument can be made in relation to some non-drug treatments (eg, the anti-inflammatory effects of exercise).
1.1.4 |. Non-pharmacological
To be included in the current study, the treatment had to explicitly target either cognitive processes or cognition in an everyday context as a primary or secondary aim, and the link to aging needed to be clear. Additional outcomes may or may have not been addressed as treatment targets. The term “non-pharmacological” suggests that every treatment that is not a pharmaceutical drug13 could be considered appropriate. Importantly, the term NPT only defines what treatments are not covered by this title and says nothing about the treatments that are covered by this title. It does not seem to be an ideal place to start an effort to define what it is that this paper is concerned with. However, the main feature that could be said to be common to the treatments covered in this paper is that they are non-drug treatments. Alternative terms sometimes used, such as “psychosocial” or “behavioral” treatments, are also unsatisfactory as several candidate treatments that might be appropriate in this paper do not seem to be well captured by these terms (eg, physical activity, dietary treatments, light therapy, brain stimulation). The class of treatments that could be considered appropriate in our context includes a very wide range of treatments, of various degrees of specificity in terms of features or ingredients, action mechanisms, and targets. The term NPTs/NPIs is also broad enough to encompass treatments that are often referred to as complementary and alternative medicines (eg, Reiki, tai chi), as well as treatments in which the target is someone other than the person with or at risk of cognitive decline (eg, family members, caregivers, GPs), and treatments focusing on training others (eg, caregivers) in the delivery of a treatment. Non-drug treatments are typically complex, and several approaches could be used to try and meaningfully group such treatments, including by professional discipline (eg, psychological treatment, occupational therapy treatment), World Health Organization classification of functioning (ie, impairment, disability, classification), or by the target symptoms (eg, wandering, behavioral and psychological symptoms in dementia [BPSD], cognitive impairment). However, it seems impossible to propose a grouping/classification framework for NPTs that would be entirely satisfactory or mutually exclusive.14–17
1.2 |. A systematic approach to non-pharmacological treatment specification in older people: The Rehabilitation Treatment Specification System (RTSS)
To address the challenges and difficulties associated with the consistent labelling of NPTs, we adopted an approach that was developed to specifying and classifying rehabilitation treatments: The Rehabilitation Treatment Specification System (RTSS).18 Although the RTSS was developed within the framework of rehabilitation, we take the view that “rehabilitation” is appropriate in the current context of treatments, including preventative treatments, to support individuals in minimizing disability and maximizing participation in meaningful life roles. We propose that the field of NPTs in older adults could advance in several ways by drawing on the RTSS framework, including improving treatment development at the protocol stage, treatment reporting, and replication and clinical translation, as well as by improving evidence synthesis through identifying common ingredients and targets across treatments.
According to the RTSS, rehabilitation treatments have a tripartite structure (Figure 3): (1) treatment targets, defined as aspects of function the clinician is attempting to change (eg, improved semantic recall, increased adherence to an exercise regimen); (2) ingredients, defined as clinician actions or objects hypothesized to effect that change (eg, instructions, modeling, coaching); and (3) mechanisms of action, the underlying biological or psychosocial mechanisms by which change occurs. Treatment targets are differentiated from aims, which are aspects of change that require multiple targets (eg, improving sleep, increasing social engagement). All targets can be classified into three categories: Organ Functions, Skills and Habits, and Representations (knowledge, beliefs, and attitudes). We grouped NPTs by target category and reported ingredients and ingredient dose where that information was available. As mechanisms of action of NPTs are mostly invisible and incompletely understood (eg, changes in some aspect of brain function), that element of the RTSS was excluded from this review.
FIGURE 3.
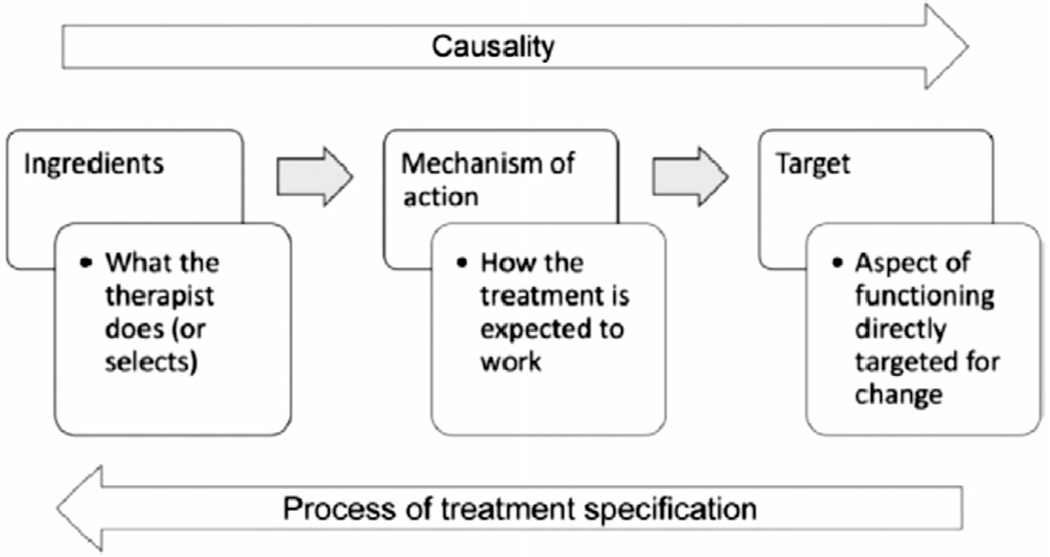
The tripartite structure of treatment theory. Source: Hart et al.18
2 |. OVERVIEW TREATMENT SPECIFICATIONS
The purpose of this paper is to advance the field of NPTs in aging and dementia by promoting a theoretical framework that can be applied to all NPT research. We did not intend to describe and summarize the evidence for every type of NPT available in the context of aging. The treatments that were included in the current paper were selected on the basis of them being reasonably well recognized (as reflected in the availability of at least one Cochrane Review covering that treatment), and on the identification of a suitable expert contributor available to support this undertaking. In this paper, we did not include treatments that are exclusively about environmental modification or that primarily target care partners. These are vast areas of research that will be covered in a separate paper. In relation to each specific treatment area covered in this paper, each section is organized as follows:
-
An executive summary, containing the treatment definition, a statement regarding the amount and quality of the scientific evidence and the main methodological challenges.
In the appendices in supporting information, the executive summary is supplemented by:
A treatment definition and specification using the RTSS framework as a guide.
A summary statement regarding the amount and quality of the evidence in relation to key outcomes in one or more populations covered by the current review based on the most authoritative source.
The main methodological challenges and pressing questions specific to that class of treatment.
A worked-out treatment specification table for that specific treatment.
The treatment specification tables describe the general structure of each treatment, as a tool for comparing typical targets and ingredients across treatments. They are not intended to be prescriptive, as with the exception of a few manualized treatments there was wide variation across studies in both the degree to which treatments were specified, if at all, and implementation methods.
An example covering all relevant components above has been included below in relation to cognitive training. For the remainder of the treatments covered, only the executive summaries are included in the paper, with the complete contribution being included in the supporting information Appendix.
2.1 |. Cognitive training
2.1.1 |. Executive summary
Cognitive training (CT) involves the formal training of global cognition or specific abilities using standardized tasks.19 The assumption behind CT is that underlying cognitive processes can be improved or maintained through training,8 and that training-related cognitive gains may prevent, delay, or slow down cognitive and functional decline in older age.20 High-quality reviews, including two systematic reviews and a systematic overview established that CT leads to moderate improvements in global cognition in people with MCI and dementia.21–24 Evidence for sustained gains, or that go beyond global cognition, is of generally low quality due to high risk of bias in primary trials. Improved implementation of best practice standards in CT research in older age,25 and a better understanding of underlying mechanisms and predictors of gain in individual recipients, are critical research priorities.
2.1.2 |. Treatment specification
CT refers to the formal training of cognitive abilities and processes, usually through repeated practice on standardized tasks designed to reflect specific cognitive domains.8,19 Practice may focus on a single or on multiple cognitive domains; it may include advice on the use of, as well as practice with, internal cognitive strategies (eg, mnemonics), and may be conducted individually or in small groups; and be supervised or unsupervised. A common assumption behind CT is that underlying cognitive processes can be improved or maintained through training, and that training-related cognitive gains may be associated with gains in day-to-day activities, or functional cognition. CT is sometimes confused with the related but distinct approaches of general cognitive stimulation and cognitive rehabilitation (described elsewhere in this paper), and unfortunately these terms are still often used interchangeably.23 The treatment specification is summarized in Table 1.
TABLE 1.
Specification of cognitive training against the RTSS framework
| Cognitive Training. Ten-week home-based multidomain computer-based cognitive training for adults with mild cognitive impairment. | ||||
|---|---|---|---|---|
| Target population | Target | Target Group (Type) | Ingredients | Dose |
| Older adults with mild cognitive impairment | Increased knowledge and understanding of age-related changes in cognitive abilities and links with functional independence | R(D) | • Information about age-related changes in cognition included in Participant Information Sheet and provided verbally by researcher • Additional written information about various cognitive abilities and their links with everyday behaviors |
• When first expressing interest in the treatment study • Weekly during the treatment period |
| Increased capability to use the online training platform | S(D) | • Face-to-face orientation to online training platform • Demonstration of online training platform provided by clinician/trained research staff • Provision of written manual describing all necessary steps to access and complete a training session • Phone-based troubleshooting/technical support |
• Once in the first training session • Once in the first training session • Once in the first training session • Once per week |
|
| Improved performance on a composite global measure of cognitive function | S(D) | • Participant to practice on a set of 20 to 30 computerized tasks targeting multiple cognitive domains | • 30 training sessions (20 minutes each) over 10 weeks | |
| Motivation to adhere to the treatment | R(V) | • Verbally delivered information about potential short and long-term gains associated with process training • Feedback on performance delivered by the online training platform • Feedback on performance delivered over the phone by research staff • Written behavioral contract to increase commitment to the training • Barrier identification with problem solving |
• Once in the first training session • After each training session • Once per week • Once in the first training session • Once in the first training session |
|
Abbreviation: RTSS, Rehabilitation Treatment Specification System.
Common targets and aims of CT.
The primary/immediate target of CT interventions is typically cognition, as reflected in performance on standardized cognitive tests. Depending on the nature of the training, the target may be a specific cognitive ability or process (eg, divided attention), several inter-related processes, or global cognitive ability. Beyond cognitive ability, additional aims of CT treatments have included improvement on measures of mood and well-being of the person affected, subjective experience of everyday cognition, functional independence, quality of life, and caregiver burden.
Broader contexts of CT.
Process-based cognitive training has a long history of use in clinical and healthy populations, particularly with children and older people.26 In aging, interest in cognitive training increased at a rapid rate since the early 1980s, with numerous studies published in cognitively healthy people, people with acquired brain injury (eg, stroke), and people with neurodegenerative diseases, particularly dementia due to AD or vascular disease.
2.1.3 |. Amount and quality of evidence in relation to dementia and MCI
Numerous studies and reviews have been published on the effects of cognitive training on cognition and other outcomes in older adults with MCI and dementia. An influential and rigorous meta-analysis from 2017 (Hilletal.,21 AMSTAR = 12) found moderate effect on global cognition (k = 17, g = 0.35, 95% confidence interval [CI] = 0.20 to 0.51), as well as small to moderate effects on several specific cognitive processes, and on “psychosocial function.” A recently published Cochrane Review of computerized cognitive training (CCT) in people with MCI (Gates et al.,22 AMSTAR = 12.5) that focused on treatments lasting a minimum of 12 weeks, and that applied stricter inclusion criteria and risk of bias rating found, based on meta-analysis of five trials, a moderate effect of CCT on global cognition at the end of treatment relative to active control (k = 5, g = −0.53,95% CI = −1.06 to −0.01). Effects of CCT were found in several specific cognitive domains as well (episodic memory, speed of processing, working memory), but not on any non-cognitive outcome, based on a small number of meta-analyzed trials. Regarding people with mild to moderate dementia, a recent Cochrane Review (Bahar-Fuchs et al.,23 AMSTAR = 14), found that relative to active or passive control conditions, CT was associated with gains in global cognition (k = 33, g = 0.42, 95% CI = 0.23 to 0.62), and verbal category fluency (k = 9, g = 0.52, 95% CI = 0.23 to 0.81) at the end of treatment, and that these gains were maintained in the medium term (up to 12 months post-treatment). CT did not benefit non-cognitive outcomes, and no differences were found when CT was compared to an alternative treatment.
Evidence syntheses efforts of trials of CT in people with MCI and dementia have consistently identified methodological challenges in primary trials, which are often ranked at being at high or unclear risk of bias in several key domains, including lack of randomization concealment, incomplete outcome data, and selective reporting. Large statistical and clinical heterogeneity and imprecision of effect estimates has led authors of recent reviews to grade most of the evidence as being of “low” or even “very low” quality.
2.1.4 |. Main methodological challenges and the most pressing questions to be addressed
Consistent implementation of best practice standards in numerous aspects of trial planning (including registration and protocol publication), implementation (including adequate randomization, and appropriate control, as well as selection of outcomes and measures), analysis (including dealing with missing data, protocol deviations, and multiple comparisons), and reporting (clear, detailed, and transparent description of all key design features), remain significant barriers in the field of CT.
The methodological challenges discussed above notwithstanding, the weight of the evidence supports the view that structured process-based cognitive training leads to at least modest improvements in cognitive test performance in people with MCI and dementia, and that these improvements may be maintained over a short to medium period. What is now required is a sustained effort to improve our understanding of how to develop more personalized CT treatments that can be better integrated with everyday life and meaningful everyday activities, so that ongoing engagement is more likely and that transfer of gains from performance on standard tests of cognition to relevant functional domains is enabled. In addition, improved knowledge of the structural and functional effects of CT in key brain regions and networks would help ensure that task design and selection, as well as dosing parameters, are informed by such changes.
3 |. EXECUTIVE SUMMARIES FOR REMAINING TREATMENTS
3.1 |. Cognitive rehabilitation
Cognitive rehabilitation (CR) is a goal-oriented, problem-solving behavioral therapy aimed at optimizing ability to function in everyday life in relation to the person’s needs, wishes, and preferences.27 Robust evidence including findings from two large trials supports the potential of CR to improve management of functional disability.28,29 Evidence from pilot studies30,31 and qualitative evaluations32 suggests that people with dementia and caregivers can experience wider benefits such as improved adjustment or coping, but these have not yet been captured quantitatively in the larger trials. The typical length of follow-up in trials precludes identification of long-term benefits. Future efforts should focus on optimizing outcome measurement, including longer follow-up periods in trials, and implementing CR into health-care provision (see Text S2 and RTSS Table S2 in supporting information).
3.2 |. Cognitive stimulation therapy
Cognitive stimulation therapy (CST) is a brief, manualized intervention, based on the theory that appropriate and targeted mental stimulation can lead to the development of new neuronal pathways. CST aims to improve cognitive function, as well as quality of life and mood, through themed group activities, such as discussing current affairs, which implicitly stimulate memory, executive functioning, and language skills. There is a large, international evidence base for group CST demonstrating a significant beneficial effect on cognition and quality of life in dementia.33 A Cochrane systematic review of 15 randomized controlled trials (RCTs) found consistent benefits of CST on cognitive function.34 A systematic review of 12 studies evaluating the UK CST protocol found improvements in cognition, quality of life, depression, and impact on caregivers.35 Further research efforts may include investigating the relationship between adherence to outcome as well as the effectiveness of individual CST delivered by non-family caregivers (see Text S3 and RTSS Table S3 in supporting information).
3.3 |. Nutritional treatments
Nutritional treatments aim to modify dietary intake of micronutrients (vitamins and minerals) and/or macronutrients (proteins, fats, carbohydrates) through specific supplements or in combination through diet.36,37 Adequate nutrition is essential for brain health because of its involvement in biological pathways that reduce oxidative stress and inflammation, promote vascular health, as well as improve neuronal cell signaling and function of neuronal cells.38–40 Despite limited evidence on the effect of nutritional treatments on cognitive outcomes, and significant methodological challenges, data are promising.41–47 Single trials have shown a beneficial effect of B vitamin supplementation on memory performance in MCI patients with high homocysteine,41 as well as improvements in cognitive performance by supplementation of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), and flavonols.42–45 Large trials on vitamin E supplementation demonstrated delayed progression in functional decline and AD.46,47 Future research should replicate these findings by focusing on conducting clinical trials in sufficient size and duration, as well as rigorous procedures for randomization and blinded testing (see Text S4 in supporting information).
3.4 |. Physical exercise training
Physical exercise training is based on planned and/or structured activity which may be aerobic exercise, resistance training,48 or a combination (multimodal) or mind-body exercise (eg, tai chi). The evidence for a positive effect of aerobic training on global cognition is growing with consistent medium effect sizes reported from systematic reviews and meta-analyses.49–51 Resistance training usually requires supervision and therefore more resources and intensive treatments to achieve effective adherence. While promising, evidence on this type of training has yet to reach the same consistency, quality, and volume as that of aerobic training. Future research should investigate whether exercise modes are domain-specific and identification of strategies to enhance adherence to exercise training is warranted (see Text S5 and RTSS Table S4 in supporting information).
3.5 |. Sleep treatments
Insomnia is defined as problems with sleep quality or quantity, including difficulty initiating sleep, repeated prolonged awakenings, and/or nonrestorative sleep.52,53 Several meta-analyses of cognitive-behavioral therapy for insomnia (CBT-I) support the finding that sleep disturbance is very amenable to change in both young and older adults, with treatment resulting in robust improvements in sleep quantity and quality.54–56 Future efforts should study the neuronal overlap and causal mechanisms between sleep disturbance and cognitive decline in individuals ranging from cognitively normal to impaired, to decide which approaches should be used for which populations and at which point in time.
In addition to insomnia, sleep disturbances in persons with dementia (PWD) also include hypersomnia, excessive motor activity at night, and hallucinations or other behavioral problems. Sleep specialists aim to target nocturnal neurocognitive symptoms in PWD through implementation of sleep promotion strategies (eg, bright light, regulation of sleep-wake schedules, decreasing arousal, increasing daytime activity) based upon CBT of sleep. While studies have established the effectiveness of various environmental and multi-component treatments to improve sleep in PWD,57–62 there are currently no meta-analyses or systematic reviews of CBT-I in those with MCI or dementia. Specific CBT-related methodological challenges included lack of standard treatment components and measurements and need for caregivers who can oversee treatment recommendations and sleep assessments. Future research should focus on the understanding the role of sleep disturbances in the pathogenesis of dementia and the underlying mechanisms of sleep and cognitive decline, as well as how dementia diagnostic subtypes and age of onset may impact treatment response (see Text S6 and S7, and RTSS Table S5 in supporting information).
3.6 |. Meditation
Meditation refers to a family of emotional and attentional regulatory training exercises.63,64 Meditation training programs, usually including sessions with an instructor as well as daily home practice, have been shown to improve cognition, well-being, and health in older age and may contribute to delaying the onset of dementia.65–67 Potential mechanisms underlying the effect of meditation include effects on inflammation, stress and emotion regulation, brain microstructure and/or macroscopic brain structure, brain glucose metabolism and brain connectivity, and effects on telomere length and telomerase activity.67–70 There are currently no meta-analyses or systematic reviews specifically focused on meditation as a single domain treatment for MCI and dementia. Though there is limited formal evidence, pilot RCTs and cross-sectional studies showed effects on cognition,71,72 psycho-affective factors,71,73 sleep quality,73 and quality of life.73,74 Future research should include longitudinal studies and RCTs, with large samples and using clinically meaningful biological and neuroimaging biomarkers (see Text S8 and RTSS Table S6 in supporting information).
3.7 |. Reminiscence therapy
Reminiscence therapy is the recollection and sharing of personal memories and experiences. It aims to promote cognition, communication, identity, mood, social connectedness, mental health, wellbeing, and quality of relationships.75,76 It can be offered individually or in groups. Recently, systematic reviews and meta-analyses,77–80 and (an abridged) Cochrane review were published.76,81 Evidence showed small significant positive effects on quality of life, mood, BPSD, cognition, and communication in people with mainly mild to moderately severe dementia. Effects differed depending on treatment modality and setting. Future efforts should include large RCTs with detailed descriptions of the treatment protocols, targets, aims, and target groups (see Text S9 and RTSS Table S7 in supporting information).
3.8 |. Music-based treatments
Music-based treatments are classified as active or receptive musical activities that aim to address multiple non-musical outcomes (eg, cognitive, psychosocial, communication, and physical goals), often simultaneously. Active treatments include instrument playing, singing, songwriting, and moving to music. Receptive treatments involve listening to recorded or live music. Music therapy treatments (goal-based therapeutic musical interaction with a trained therapist), are distinguished from music activities that can be implemented by other clinicians, caregivers, or self-administered. A recent Cochrane review summarized the effect of multiple RCTs examining both active and receptive music treatments and reported evidence for reductions in depression and overall behavioral problems, but no effect on agitation, and low-quality evidence for reductions in anxiety and quality of life and little to no effect on cognition.82 For other areas, such as anxiety and social behavior, the Cochrane review found not enough evidence to determine effects. Other recent reviews have found evidence that music therapy is effective in reducing agitation, anxiety, depression, and other neuropsychiatric symptoms.83–85 There is disagreement between some published meta-analyses on whether active or receptive treatments are more effective for specific outcomes. The low quality of the current evidence highlights that improvements for future research are necessary, most specifically improving design quality and comparing different music-based treatments (receptive versus active) and dosage (see Text S10 and RTSS Table S8 in supporting information).
3.9 |. Communication treatments
Communication interventions target a construct referred to as “quality of communication life,” defined as the extent to which a person’s communication acts allow a meaningful participation in life situations.86 Communication treatments aim to maintain or improve quality and quantity of meaningful communication interactions. These treatments can be divided into direct and indirect treatments. Direct treatments are primarily language stimulation tasks and activities, delivered individually or in groups. Indirect treatments include training family and professional carers in strategies for effective communication, modifying environments to facilitate communication, and developing therapeutic routines and activities that promote communication. A current challenge is the tendency for late (or no) recognition of communication problems in MCI and dementia. The level of evidence is currently limited due to a lack of controlled studies, and future efforts should focus on this (see Text S11 and RTSS Table S9 in supporting information).
3.10 |. Multisensory treatments
Multisensory treatments stimulate the senses (sight, hearing, taste, touch, smell) to compensate for sensory deprivation or to restore an imbalance such as suboptimal pacing of stimulating and calming sensory input.87,88 These treatments mostly aim to improve behavior, quality of life, well-being, or functioning and are often suitable for moderate dementia. Snoezelen and sensory gardens involve supervised presence in environments equipped with tools to stimulate senses, and Sonas is a structured group program to stimulate all senses. Although all of these treatments have been studied in some RCTs, the treatments were heterogeneous and study samples small.89–94 Taken together, the evidence base for effects is still modest. At this point, well-designed RCTs that allow studying subgroup effects are necessary to advance the field (see Text S12 and RTSS Table S10 in supporting information).
3.11 |. Occupational therapy
The primary aim of occupational therapy is to optimize occupational performance by enabling people to participate in the activities that they want, need, or are expected to do.95 One of the theoretical models is the person-environment-occupation model,96 which portrays the relationship between the capabilities and characteristics of the person—the physical, social, and cultural environment—and the target occupation. The level of evidence for some outcomes is considered “moderate”, with a number of high quality RCTs supportive of improvements in activities of daily living (ADL), quality of life and reductions in problem behavior.97 Key challenges for the field of occupational therapy include determining the optimal characteristics of the treatment, dose, methods of service delivery, and subgroups most likely to benefit from treatment (see Text S13 and RTSS Table S11 in supporting information).
3.12 |. Neuromodulation
Neuromodulation involves the introduction of energy (eg, electrical, magnetic) into the brain with the goal of altering neurophysiology. Treatments include transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS). This treatment is currently not yet clinically approved for the use in cognitive deficits. Existing meta-analyses and reviews provide conflicting evidence for the effects of neuromodulation in those across the dementia spectrum but are limited by a small number of primary research studies with small sample sizes.98,99 Research into neuromodulation needs focus on dosing parameters, including dose-response relationships, and the combination with and timing of other (non)pharmacological agents. This will likely reduce heterogeneity in response and facilitate clinical translation efforts (see Text S14 and RTSS Table S12 in supporting information).
4 |. GENERAL METHODOLOGICAL CONSIDERATIONS
Several NPTs show a clear therapeutic potential, but the depth and breadth of the evidence base so far varies across the different treatments. The body of evidence concerning cognitive training21 and physical exercise10 appears to be more advanced relative to other treatments covered. A common thread through most of the primary research and associated systematic reviews is the need to improve the quality, transparency, and clinical relevance of NPT trials in aging and dementia. Realizing the potential of NPTs will strongly depend on future trials being developed against a well-articulated rationale, a stronger focus on hypothesis testing, and trials being more rigorously designed. Critical elements including treatment development, trial design, and outcome measurement should be carefully considered early in the planning phase to minimize risk of bias and to maximize generalizability of findings. Below, we provide recommendations concerning some of the key design questions that need to be considered by those designing such trials (for an overview, see Figure 4 andTable 2).
FIGURE 4.
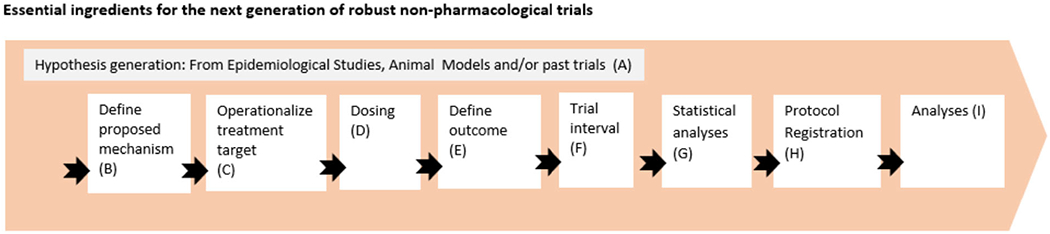
Overview of methodological considerations in the design of non-pharmacological treatments
TABLE 2.
Checklist for the design of a non-pharmacological treatment trial
| Stage | Checklist items | Considerations |
|---|---|---|
| (A) | 1. Duration of trial follow-up 2. Population selection |
Duration of follow-up should be reasonable in view of past studies and evidence. For the population selection, consider whether targeted sample characteristics are justifiable, in terms of age range, sex, genetics, and other characteristics. |
| (B) | 1. Conceptualization of mechanisms | Consider the alignment between treatment target and outcomes |
| (C) (D) | 1. Operationalize treatment targets 2. Dose considerations |
For specifying treatment targets, consider the use of the RTSS framework. For dose considerations, adequate dosing should be carefully considered, or multiple arms with different dosing might be part of the study design. |
| (E)(F) | 1. Operationalize outcomes 2. Trial intervals and duration of follow-up |
Adequately selected and operationalized outcomes are key to a treatment trial. Primary outcomes, co-primary outcomes, dual primary outcomes, exploratory outcomes, secondary outcomes should be carefully considered. For the operationalization of the outcomes it is recommended to use already validated measures, taking into account whether the scale is validated in the population of interest, as well as evidence for the sensitivity to capture changes over time (longitudinal validation) in relationship to the proposed assessment time period. When creating new outcome measures, consider an independent validation pilot study when planning your study. When planning trial intervals, consider how often the outcomes should be measured, based on past evidence. Clearly monitor and define adherence (eg, days completed?, % of duration completed?). |
| (G) | 1. Statistical analyses | Carefully consider what analytical models are best suited for establishing the hypothesized mechanisms, as well as the approach to minimize the required sample size. Consider whether changes are expected in a linear or non-linear fashion. Define threshold of statistical significance (consider multiple comparison adjustment) and define minimum effect size upfront. Consider potential drop-out mechanisms, and what kinds of sensitivity analyses need to be proposed. Intention to treat (ITT) analyses vs modified ITT analyses (eg, include those with at least one follow-up) vs per protocol analyses (PP). Both analyses are recommended with ITT analyses being the gold standard. Clearly propose up front what type of analyses will be conducted. Include sample size estimates and simulations. Is the sample size proposed adequate and feasible, provide evidence and justifications. |
| (H) | 1. IRB approval 2. Clinicaltrials.gov registration |
Consider how long will it take to receive ethical approval, whether you need site-specific approvals (multicenter studies), and other issues such as sponsor approval, confirmation of insurance, approval from health-care providers or regulatory bodies for allied health-care professionals, approvals from consumer groups, inclusion of people with lived experience on research committees, national or international approval or validation for device trials, secure data collection and data management plans. |
Existing evidence and pilot work should inform key factors such as treatment content and dose, ideally based on systematic reviews that investigate the moderating effects of design factors. The dose (intensity, frequency, and length) should consider not only possible dose responsiveness, but also matters of compliance and adherence (in particular for self-administered and unsupervised treatments) that should be measured using reliable methods.100,101 Ideally, a small-scale pilot study should be conducted to obtain the information before a “Phase I study” is carried out. A key issue of increasing importance in treatment development is creating opportunities for key stakeholders (patients, care partners, and clinicians) to be involved and provide input as appropriate into the development of the treatment protocol, and through the course of its evaluation.
Treatment targets (eg, cognition, behavior, or risk factor) and their operationalization should occur at the start of the planning process. Key questions to ask are whether the treatment is expected to affect proposed biological, clinical, or functional target(s) and aims, and the RTSS framework can be useful in thinking through these issues. Whether existing, validated measures of the proposed targets are available and can be used, and how frequently outcomes will be assessed, are questions of paramount importance. Where possible, selection of outcome measures should be based on standard diagnostic techniques and ensure measures have evidence of adequate psychometric properties, including test-retest reliability, validity, and sensitivity to detect change.
Researchers should ensure they carefully consider the research question(s) they would like the trial to address, and whether these imply trials that emphasize feasibility/acceptability of a treatment, dose-response issues, superiority or non-inferiority in relation to another treatment, an active control condition, or treatment as usual. These research questions are of great importance and should directly impact decisions regarding the most appropriate trial design. In addition to determining whether a randomized controlled design is feasible and appropriate, consideration should be given to whether assignment to treatment conditions, and outcome evaluations can be blinded. Moreover, considerations should include whether experimental and comparison conditions should take the form of parallel arms, cross-over or delayed start to one arm, or a factorial randomization to examine more than one treatment.
If hypotheses are made regarding more than one main outcome, it is important to determine whether the outcomes should be treated as co-primary, or whether one outcome should be considered primary and another secondary. These distinctions between primary and secondary outcomes are important for a range of reasons, including for establishing issues related to statistical power and interpreting the clinical relevance of the finding. Putative effect moderators need to be carefully considered to maximize the generalizability and clinical relevance of the findings (positive or negative).
4.1 |. Essential ingredients for the next generation of robust non-pharmacological trials
A power calculation should be conducted to inform recruitment and this should be based on the expected level of change in the primary outcome(s) based on pilot studies and/or past literature. The sources used to estimate the expected treatment effect for power calculations (eg, studies which provided the information on expected changes and its standard deviation or coefficient and its standard error) need to be clearly stated. In recent years, a growing number of trials include measurements of digital biomarkers (eg, motion sensors). Issues related to the frequency of such measurements (eg, continuously, daily, monthly) and processing of the data could have critical implications for power estimates102 and this needs to be carefully considered, especially if such markers are used as trial outcomes. Power and sample-size targets should also take into account expected rates of attrition, death, or discontinuation. If attrition is likely to be greater in one of the trial arms than other arms, this needs to be taken into account, and similarly if recruitment to one of the trial arms is expected to be more challenging, this too should betaken into account at the protocol stage. The Consolidated Standards of Reporting Trials Statement (CONSORT103), initially developed in the context of biomedical trials, was recently extended to accommodate the trials in behavioral and social sciences (CONSORT-SPI104). Researchers are encouraged to consider the items in the CONSORT-SPI statement at the protocol planning phase to make sure that all the basic requirements in RCTs are covered. Intention-to-treat (ITT) analyses are the gold standard because they eliminate potential selection bias associated with only analyzing data from participants who adhered to their assigned treatment arm. However, CONSORT guidelines recommend that both ITT and per protocol (PP) analyses be reported for all outcomes so that readers can interpret the effect of a treatment in a more informed manner.105 This practice is particularly relevant in NPT trials as personal preferences and individual differences often affect adherence levels and this has implications to whether a treatment can be sustained in a longer term. Understanding factors associated with treatment adherence is critical in considering the real-world application of a treatment and “dose-response” effects. The selection process from the ITT sample to the PP sample and the difference in effect sizes between the two analyses could provide key information in estimating translational effects or sustainability of a treatment in the real world. Planned subgroup and adjusted analyses should be clearly outlined at the protocol stage.
Finally, the planned trial should be considered pragmatically in a real-world context, in particular with regard to the (recruitment of) the target population. Regulatory aspects need to be taken into consideration, with the local regulatory agency for drug and device trials, the university, or sponsor’s research governance offices as good starting points for this information.
5 |. CONCLUDING REMARKS
The aim of this paper was to summarize the body of work available in relation to key types of NPT in the context of aging and dementia studies, identify key challenges for different NPTs, and provide methodological guidelines for the design of future trials of NPTs. Our work thereby represents an important step in the process of advancing the theoretical and methodological rigor of research into NPTs.
Numerous treatment approaches fall under the umbrella term “NPT.” The current paper focused on a sample of these approaches for which there is a relatively robust body of evidence, and applied the RTSS framework to identify targets, ingredients, and dosing parameters for these treatments. The importance of developing practices that promote the replicability of treatment studies cannot be overstated,106 and to do that we need systematic descriptions of those practices.18 The work in the present paper underscores some of the challenges and opportunities associated with using a single theoretical framework to describe, specify, and prescribe very diverse treatments. When examined using the RTSS framework, it can be clearly seen that many or most treatments involve targets that fall primarily into the skills and the representations target groups. This is not surprising given that NPTs typically (with some exceptions) do not have organ functions as their direct targets. The RTSS framework also revealed themes across treatments that go by different names and are offered by different health-care providers, including themes in targets (eg, improving memory), and ingredients (eg, physical and mental exercise in MCI, environmental modification in dementia). Articulating core targets, ingredients, and dosing parameters of given treatments was often challenging. Ingredients and dosing parameters were often omitted from treatments described in the literature, or described only in general terms (eg, “enabling ingredients,” weeks of treatment). Likewise, targets of a treatment were not always stated in published reports. Even for the expert contributors, distinguishing between treatment targets and downstream aims required some effort. A benefit of using the RTSS in this project was that it prompted critical thinking and discussion about what treatments were actually intended to achieve, and researchers’ theories about what aspects of treatment would effect that change.
Review of the evidence in relation to the different NPTs shows that while treatments may be effective in bringing about change in their immediate targets, effectiveness for changing downstream aims is less conclusive. The RTSS was helpful here as well, as it revealed treatments in which the primary outcome was actually an aim rather than the target of the treatment. A common example was cognitive training-type treatments that were hypothesized to improve everyday cognitive function, but had actual treatment targets like “improved accuracy of recall” on memory tasks, used ingredients designed to improve recall on those tasks, and measured outcomes via scores on memory tests and subjective everyday memory measures. Results of many studies showed improvements on tests but not in everyday life. Those results are not surprising because change in everyday life requires different ingredients, including ingredients and targets related to factors like family education and participant awareness. A treatment can therefore be dismissed as ineffective when the problem is a mismatch among ingredients, targets, and outcome measures. Using the RTSS can promote a better match across these variables and clearer specification of what the treatment is intended to achieve, and thus help reveal treatment effects. A further point that is also evident is that research to date has focused predominantly on the well-established clinical stages of MCI and dementia, with a very limited literature evaluating the benefits from NPTs on people with subjective cognitive decline.
Given accumulated supportive evidence in relation to some treatments (eg, cognitive training, music therapy), it is important that researchers collaborate effectively with industry partners, government, and non-government health-care organizations to design and carry out rigorous yet pragmatic implementation studies embedded within existing service frameworks. Such research should also aim to understand effective methods to shift attitudes of prescribing clinicians, so that appropriate NPTs be offered to individuals along the cognitive aging continuum. These studies are also important to further our understanding of the efficacy of different NPTs in the context of the etiological heterogeneity of dementia and MCI syndromes. Even in areas where the research is not mature enough to move to implementation studies (eg, mindfulness), increasing efforts should be now directed toward a better understanding of person- and treatment-related factors that moderate treatment efficacy and predict treatment response at the level of the individual patient. Common barriers that were reported included the inadequacy of reporting of relevant data regarding treatment adherence, motivation, and various personal characteristics that are likely to play a role in treatment adherence, persistence, and response. Along with improved specification of treatments, the field of NPTs will require continuously improving reporting standards, to make sure that evidence can be adequately synthesized and that replication efforts are not wasted.
The current study has several limitations. First, although we intended to provide a comprehensive overview of NPTs in aging and dementia, we acknowledge that several treatment areas were not covered. This was based on our decision to only include treatments for which substantial research evidence was available, as reflected primarily by the availability of at least one Cochrane review. Emerging treatment approaches for which there is growing interest, such as multidomain lifestyle interventions like the FINGER trial,107 and specific diets such as the Mediterranean and MIND diet108 were therefore not included. Regarding mindfulness, we are of the opinion that the current work can aid in an improved understanding of person and treatment related factors. A second limitation concerns the RTSS framework terminology used in the current paper, which in some cases might differ from conventions in this research field (eg, “target” instead of “outcome”). In our view, adopting an existing and well-established framework outweighed those limitations.
The many advantages of NPTs are well recognized. We hope that the theoretical framework proposed and the methodological guidelines offered in the current study would help researchers designing the next generation of NPT studies in such a way that more reliable evidence and evidence synthesis efforts are produced, and that the optimal approaches to NPTs, tailored to the specific characteristics of individuals, will be developed and lead to meaningful outcomes in the lives of people with dementia, or at risk of dementia.
Supplementary Material
RESEARCH IN CONTEXT.
1. Systematic review:
Using the Rehabilitation Treatment Specification Framework (RTSS), the authors defined and specified the targets and ingredients of 13 non-pharmacological treatments (NPTs) for older people at risk of or who live with dementia. Evidence of the effects of these treatments was summarized based on relevant synthesis efforts.
2. Interpretation:
The targets of most NPTs are changes in skills and habits and representations. Core/specific ingredients and dosing parameters are sometimes difficult to prescribe, and the distinction between targets and “downstream” aims is not always straightforward. The overall evidence supports the efficacy of most NPTs in relation to their primary targets.
3. Future directions:
Umbrella theoretical frameworks such as the RTSS are likely to make important contributions to our ability to specify existing treatment, design new treatment trials, and synthesize the evidence. To improve the quality of evidence behind NPTs, and accelerate clinical implementation, we offer several methodological principles and guidance for those planning and designing NPT trials.
ACKNOWLEDGMENTS
The NPI-PIA executive committee is grateful to the many contributors who have worked closely with us on this project. We would like to acknowledge in particular the guidance and vision of Prof Linda Clare and Henry Brodaty. We are very grateful to Prof Lyn Turkstra for generously giving much of her time to help the section contributors develop their work against the RTSS framework and for providing numerous insights and comments on the paper. We are grateful to the wider NPI-PIA membership and ISTAART for supporting and encouraging this effort.
This work is dedicated to the memory of Dr. Martha Clare Morris, a visionary researcher who shaped much of the work on the links between nutrition and dementia and who led the nutrition section of this paper. We are confident that she would have been proud of the outcome.
This manuscript was facilitated by the Alzheimer’s Association International Society to Advance Alzheimer’s Research and Treatment (ISTAART), through the Non-Pharmacological Interventions, Clinical Trial Advancement and Methods, and Nutrition, Metabolism and Dementia professional interest areas (PIAs). The views and opinions expressed by authors in this publication represent those of the authors and do not necessarily reflect those of the PIA membership, ISTAART or the Alzheimer’s Association.
FUNDING INFORMATION
ABF NHMRC Fellowship GNT1135605,SAMS JPND Zon-MW733051043, Alzheimer Nederland WE.26-2018-03,Zon-MW Memorabel 733050205, RJ Zon-MW Memorabel 733050205, LW JPND Zon-MW 733051043, GC European Union Horizon 2020 program, Inserm, Fondation d’entreprise MMAdes Entrepreneurs du Futur, Fondation Alzheimer, Programme Hospitalier de Recherche Clinique, Région Normandie, Association France Alzheimer etmaladies apparentées, Fondation Vaincre Alzheimer, RMD Association of Support VCVGZ for Chair Psychosocial care for people with dementia to stimulate further development, AL partially funded by a grant from the German Federal Ministry of Education and Research (BMBF) for industry collaboration with Synaptikon GmbH in the field of cognitive assessment and training (ID 13GW0212A,2017-2019). Received grants from the University of Melbourne and the Berlin University Alliance for development and piloting of systems for cognitive training personalization and delivery of simultaneous cognitive and physical exercise (2019-2021); KL NHMRC-ARC Dementia Research Development Fellowship GNT1097435.
Footnotes
CONFLICTS OF INTEREST
GC reports coordination of a European Union Horizon 2020 program (Silver-Sante Study-Medit-Ageing), with no stock options, patent or royalties. AL reports being co-investigator in clinical trials of computerized cognitive training using programs provided free-of-charge by HAPPYneuron Inc. and Synaptikon GmbH. GR and AS report offering training courses in CST and receiving occasional private income for training courses for dementia outside usual work. The remaining authors report no relevant conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Alzheimer’s Association, Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2015;11(3):332–384. [DOI] [PubMed] [Google Scholar]
- 2.Prince M, Herrera C, Knapp M, et al. , World Alzheimer report 2016: improving healthcare for people living with dementia: coverage, quality and costs now and in the future. London, UK: Alzheimer’s Disease International (ADI). 2016:131. [Google Scholar]
- 3.American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (5th ed.). Washington, DC: Author. [Google Scholar]
- 4.Petersen RC. Mild cognitive impairment. CONTINUUM: Lifelong Learning Neurol. 2016;22(2 Dementia):404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jessen F, Amariglio RE, Boxtel MV, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s Dement. 2014;10(6):844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scales K, Zimmerman S, Miller SJ. Evidence-Based nonpharmacological practices to address behavioral and psychological symptoms of dementia. Gerontologist. 2018;58(suppl_1):S88–S102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olazaran J, Reisberg B, Clare L, et al. Nonpharmacological therapies in Alzheimer’s disease: a systematic review of efficacy. Dement Geriatr Cogn Disord. 2010;30(2):161–178. [DOI] [PubMed] [Google Scholar]
- 8.Bahar-Fuchs A, Clare L, Woods B. Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer’s disease and vascular dementia. Cochrane Database Syst Rev. 2013(6):CD003260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Steen JT, van Soest-Poortvliet MC, van der Wouden JC, et al. Music-based therapeutic interventions for people with dementia (Review). Cochrane Database Syst Rev. 2017(5):CD003477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young J, Angevaren M, Rusted J, et al. Aerobic exercise to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2015(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–2734. [DOI] [PubMed] [Google Scholar]
- 12.Jack CR Jr, Albert MS, Knopman DS, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018;14(4):535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pharmacists Pharma Journal. http://www.pharmacistspharmajournal.org/. 2010. [cited May 14, 2019]. http://www.pharmacistspharmajournal.org/2010/11/definitions-of-drug-radioactive-drug_11.html#.XNoXoFszaUk.
- 14.Michie S, Abraham C. Advancing the science of behaviour change: a plea for scientific reporting. Addiction. 2008;103(9):1409–1410. [DOI] [PubMed] [Google Scholar]
- 15.Michie S, Prestwich A. Are interventions theory-based? Development of a theory coding scheme. Health Psychol. 2010;29(1): 1–8. [DOI] [PubMed] [Google Scholar]
- 16.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart T, Dijkers MP, Whyte J, et al. Toward a theory-driven classification of rehabilitation treatments. Arch Physical Med Rehabil. 2014;95(1):S33–S44.e2. [DOI] [PubMed] [Google Scholar]
- 18.Hart T, Dijkers MP, Whyte J, et al. A theory-driven system for the specification of rehabilitation treatments. Arch Physical Med Rehabil. 2019;100(1):172–180. [DOI] [PubMed] [Google Scholar]
- 19.Clare L, Woods RT, Moniz Cook ED, et al. Cognitive rehabilitation and cognitive training for early-stage Alzheimer’s disease and vascular dementia. Cochrane Database Syst Rev. 2003(4):CD003260. [DOI] [PubMed] [Google Scholar]
- 20.Gates NJ, Sachdev P. Is cognitive training an effective treatment for preclinical and early Alzheimer’s disease? J Alzheimer’s Dis. 2014. [DOI] [PubMed] [Google Scholar]
- 21.Hill NT, Mowszowski L, Psych D, et al. Computerized cognitive training in older adults with mild cognitive impairment or dementia: a systematic review and meta-analysis. Am J Psychiatry. 2017;174(4):329–340. [DOI] [PubMed] [Google Scholar]
- 22.Gates NJ, Vernooij RWM, Di Nisio M, et al. Computerised cognitive training for preventing dementia in people with mild cognitive impairment. Cochrane Database Syst Rev. 2019;3:Cd012279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bahar-Fuchs A, Barendse MEA, Bloom R, et al. Cognitive training for people with mild to moderate dementia. Cochrane Database Syst Rev. 2019;3:Cd013069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gavelin HM, Lampit A, Hallock H, et al. Cognition-oriented treatments for older adults: a systematic overview of systematic reviews. Neuropsychol Rev. 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simons DJ, Berger JO, Sellke TM, et al. Do “brain-training” programs work. Psychol Sci Public Interest. 2016;17(3):103–186. [DOI] [PubMed] [Google Scholar]
- 26.Katz B, Shah P, Meyer DE. How to play 20 questions with nature and lose: reflections on 100 years of brain-training research. Proc Natl Acad Sci. 2018;115(40):9897–9904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clare L, Teale JC, Toms G, et al. Cognitive rehabilitation, self-management, psychotherapeutic and caregiver support interventions in progressive neurodegenerative conditions: a scoping review. NeuroRehabilitation. 2018;43(4):443–471. [DOI] [PubMed] [Google Scholar]
- 28.Amieva H, Robert PH, Grandoulier AS, et al. Group and individual cognitive therapies in Alzheimer’s disease: the ETNA3 randomized trial. Int Psychogeriatr. 2016;28(5):707–717. [DOI] [PubMed] [Google Scholar]
- 29.Clare L, Aleksandra K, Oyebode JR, et al. Individual goal-oriented cognitive rehabilitation to improve everyday functioning for people with early-stage dementia: a multicentre randomised controlled trial (the GREAT trial). Int J Geriatr Psychiatry. 2019;34(5): 709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clare L, Linden DEJ, Woods RT, et al.Goal-oriented cognitive rehabilitation for people with early-stage Alzheimer disease: a single-blind randomized controlled trial of clinical efficacy. Am J Geriatr Psychiatry. 2010;18(10):928–939. [DOI] [PubMed] [Google Scholar]
- 31.Hindle JV, Watermeyer TJ, Roberts J, et al. Goal-orientated cognitive rehabilitation for dementias associated with Parkinson’s disease-A pilot randomised controlled trial. Int J Geriatr Psychiatry. 2018;33(5):718–728. [DOI] [PubMed] [Google Scholar]
- 32.Clare L, Kudlicka A, Oyebode JR, et al. Goal-oriented cognitive rehabilitation for early-stage Alzheimer’s and related dementias: the GREAT RCT. Health Technol Assess. 2019;23(10):1–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orrell M, Aguirre E, Spector A, et al. Maintenance cognitive stimulation therapy for dementia: single-blind, multicentre, pragmatic randomised controlled trial. Br J Psychiatry. 2014;204(6): 454–461. [DOI] [PubMed] [Google Scholar]
- 34.Woods B, Spector A, Orrell M,et al. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. 2012(2):Cd005562. [DOI] [PubMed] [Google Scholar]
- 35.Lobbia A, Carbone E, Faggia S, et al. The efficacy of cognitive stimulation therapy (CST) for people with mild-to-moderate dementia: a review. Eur Psychol. 2019;24(3):257–277. [Google Scholar]
- 36.Papanikolaou Y, Jones JM, Fulgoni VL. Several grain dietary patterns are associated with better diet quality and improved shortfall nutrient intakes in US children and adolescents: a study focusing on the 2015-2020 Dietary Guidelines for Americans. Nutr J. 2017;16(1): 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary guidelines for Americans, 2010. 7th Edition, Washington, DC: U.S. Government Printing Office. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller ER 3rd, Erlinge TP, Sacks FM, et al. , A dietary pattern that lowers oxidative stress increases antibodies to oxidized LDL: results from a randomized controlled feeding study. Atherosclerosis. 2005;183(1):175–182. [DOI] [PubMed] [Google Scholar]
- 39.Gadgil MD, Appel LJ, Yeung E, et al. The effects of carbohydrate, unsaturated fat, and protein intake on measures of insulin sensitivity: results from the OmniHeart trial. Diabetes Care. 2013;36(5):1132–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Azadbakht L, Surkan PJ, Esmaillzade A, et al.The dietary approaches to stop hypertension eating plan affects c-reactive protein, coagulation abnormalities, and hepatic function tests among type 2 diabetic patients. J Nutr. 2011;141(6):1083–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Jager CA, Oulhaj A, Jacoby R, et al. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: a randomized controlled trial. Int J Geriatr Psychiatry. 2012;27(6):592–600. [DOI] [PubMed] [Google Scholar]
- 42.Lee LK, Shahar S, Chin AV, ,et al. Docosahexaenoic acid-concentrated fish oil supplementation in subjects with mild cognitive impairment (MCI): a 12-month randomised, double-blind, placebo-controlled trial. Psychopharmacology. 2013;225(3):605–612. [DOI] [PubMed] [Google Scholar]
- 43.Bo Y, Zhang X, Wang Y, et al. The n-3 polyunsaturated fatty acids supplementation improved the cognitive function in the Chinese elderly with mild cognitive impairment: a double-blind randomized controlled trial. Nutrients. 2017;9(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang YP, Miao R, Li Q, et al. Effects of DHA supplementation on hippocampal volume and cognitive function in older adults with mild cognitive impairment: a 12-month randomized, double-blind, placebo-controlled trial. J Alzheimers Dis. 2017;55(2): 497–507. [DOI] [PubMed] [Google Scholar]
- 45.Desideri G, Kwik-Uribe C, Grassi D, et al. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: the cocoa, cognition, and aging (CoCoA) study. Hypertension. 2012;60(3):794–801. [DOI] [PubMed] [Google Scholar]
- 46.Dysken MW,Sano M,Asthana S,et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. Jama. 2014;311(1):33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sano M, Ernesto C, Thomas RG, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. Alzheimer’s Dis Cooperative Study. N Engl J Med. 1997;336(17):1216–1222. [DOI] [PubMed] [Google Scholar]
- 48.Garber CE, Blissmer B, Deschenes MR, et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Medi Sci Sports Exercise. 2011;43(7):1334–1359. [DOI] [PubMed] [Google Scholar]
- 49.Zheng G, Xia R, Zhou W, et al. Aerobic exercise ameliorates cognitive function in older adults with mild cognitive impairment: a systematic review and meta-analysis of randomised controlled trials. BrJ Sports Med. 2016;50(23):1443–1450. [DOI] [PubMed] [Google Scholar]
- 50.Song D, Yu DSF, Polly WCL, et al. The effectiveness of physical exercise on cognitive and psychological outcomes in individuals with mild cognitive impairment: a systematic review and meta-analysis. Int J Nursing Studies. 2018;79:155–164. [DOI] [PubMed] [Google Scholar]
- 51.Ströhle A, Schmidt DK, Schult F, et al. Drug and exercise treatment of Alzheimer disease and mild cognitive impairment: a systematic review and meta-analysis of effects on cognition in randomized controlled trials. Am J Geriatr Psychiatry. 2015;23(12):1234–1249. [DOI] [PubMed] [Google Scholar]
- 52.Association AP. Diagnostic and statistical manual of mental disorders (DSM-5®). 2013. Am Psychiatric Pub. 2013. [Google Scholar]
- 53.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine. 2014. [Google Scholar]
- 54.Geiger-Brown JM, Rogers VE, Liu W, et al. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. 2015;23:54–67. [DOI] [PubMed] [Google Scholar]
- 55.Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol. 2006;25(1):3–14. [DOI] [PubMed] [Google Scholar]
- 56.Trauer JM, Qian MY, Doyle JS, et al. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191–204. [DOI] [PubMed] [Google Scholar]
- 57.Cassidy-Eagle E,Siebern A, Unti L,et al. Neuropsychological functioning in older adults with mild cognitive impairment and insomnia randomized to CBT-I or Control Group. Clin Gerontol. 2018;41(2):136–144. [DOI] [PubMed] [Google Scholar]
- 58.Cassidy-Eagle E, Unti L, Glassman J, et al. Cognitive behavioral treatment for insomnia in older adults with mild cognitive impairment in independent living facilities: a pilot study. J Sleep Disorders Med Care. 2018;1(1):1–6. [Google Scholar]
- 59.deOliveira AM, Radanovic M, Homem de Mello PC, et al. An intervention to reduce neuropsychiatric symptoms and caregiver burden in dementia: Preliminary results from a randomized trial of the tailored activity program-outpatient version. Int. J. Geriatr. Psychiatry 2019;34(9):1301–1307. 10.1002/gps.4958. [DOI] [PubMed] [Google Scholar]
- 60.McCurry SM, Pike KC, Vitiello MV, et al. Increasing walking and bright light exposure to improve sleep in community-dwelling persons with Alzheimer’s disease: results of a randomized, controlled trial. J Am Geriatr Soc. 2011;59(8):1393–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Naismith SL, Mowszowski L. Sleep disturbance in mild cognitive impairment: a systematic review of recent findings. Curr Opin Psychiatry. 2018;31(2):153–159. [DOI] [PubMed] [Google Scholar]
- 62.Richards KC, Lambert C, Beck CK, et al. Strength training, walking, and social activity improve sleep in nursing home and assisted living residents: randomized controlled trial. J Am Geriatr Soc. 2011;59(2):214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lutz A, Dunne JD, Davidson RJ, et al. Attention regulation and monitoring in meditation. Trends Cognitive Sci. 2008;12(4):163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lutz A, Jha AP, Dunne JD, et al. Investigating the phenomenological matrix of mindfulness-related practices from a neurocognitive perspective. Am Psychol. 2015;70(7):632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malinowski P, Shalamanova L. Meditation and cognitive ageing: the role of mindfulness meditation in building cognitive reserve. J Cognitive Enhancement. 2017;1(2):96–106. [Google Scholar]
- 66.Luders E, Cherbuin N. Searching for the philosopher’s stone: promising links between meditation and brain preservation. Ann N Y Acad Sci. 2016;1373(1):38–44. [DOI] [PubMed] [Google Scholar]
- 67.Larouche E, Hudon C, Goulet S. Potential benefits of mindfulness-based interventions in mild cognitive impairment and Alzheimer’s disease: an interdisciplinary perspective. Behav Brain Res. 2015;276:199–212. [DOI] [PubMed] [Google Scholar]
- 68.Klimecki O, Marchant NL, Lutz A, et al. The impact of meditation on healthy ageing – the current state of knowledge and a roadmap to future directions. Curr Opin Psychol. 2019;28. [DOI] [PubMed] [Google Scholar]
- 69.Kurth F, Cherbuin N, Luders E. Reduced age-related degeneration of the hippocampal subiculum in long-term meditators. Psychiatry Res: Neuroimaging. 2015;232(3):214–218. [DOI] [PubMed] [Google Scholar]
- 70.Epel E, Blackburn E, Lin J, et al. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci. 2009;1172:34–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lenze EJ, Hickman S, Hershey T, et al. Mindfulness-based stress reduction for older adults with worry symptoms and co-occurring cognitive dysfunction. Int J Geriatr Psychiatr. 2014; 29(10): 991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smart CM, Segalowitz SJ, Mulligan BP, et al. Mindfulness training for older adults with subjective cognitive decline: results from a pilot randomized controlled trial. J Alzheimer’s Dis. 2016;52(2): 757–774. [DOI] [PubMed] [Google Scholar]
- 73.Innes KE, Selfe TK, Khalsa DS, Kandati S. Effects of meditation versus music listening on perceived stress, mood, sleep, and quality of life in adults with early memory loss: A pilot randomized controlled trial. J Alzheimer’s Dis, 2016;52(4):1277–1298. 10.3233/jad-151106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paller KA, Creery JD, Florczak SM, et al. Benefits of mindfulness training for patients with progressive cognitive decline and their caregivers. Am J Alzheimer’s. 2015;30(3):257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Woods B, O’Philbin L, Farrell EM, Spector AE, Orrell M. Reminiscence therapy for dementia. Cochrane Database Syst. Rev. 2018; 10.1002/14651858.cd001120.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.O’Philbin L, Woods B, Farrell EM, et al. Reminiscence therapy for dementia: an abridged Cochrane systematic review of the evidence from randomized controlled trials. Expert Rev Neurotherapeutics. 2018;18:715–727. [DOI] [PubMed] [Google Scholar]
- 77.Park K, Lee S, Yang JE, et al. A systematic review and meta-analysis on the effect of reminiscence therapy for people with dementia. Int Psychogeriatr. 2019;31:1581–1597. [DOI] [PubMed] [Google Scholar]
- 78.Elfrink TR, Zuidema SU, Kunz M, et al. Life story books for people with dementia: a systematic review. Int Psychogeriatr. 2018;30:1797–1811. [DOI] [PubMed] [Google Scholar]
- 79.Yen H-Y, Lin L-J. A systematic review of reminiscence therapy for older adults in Taiwan. J Nurs Res. 2018;26:138–150. [DOI] [PubMed] [Google Scholar]
- 80.Allen AP, Doyle C, Commins S, et al. Autobiographical memory, the ageing brain and mechanisms of psychological interventions. Ageing Res Rev. 2018;42:100–111. [DOI] [PubMed] [Google Scholar]
- 81.Woods B, Bruce E, Edwards RT, et al. Reminiscence therapy for dementia. Cochrane Database Syst Rev. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van der Steen JT, Smaling HJA, van der Wouden JC, et al. Music-based therapeutic interventions for people with dementia. Cochrane Database Syst Rev. 2018;7(7):CD003477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abraha I, Rimland JM, Trotta FM, et al. Systematic review of systematic reviews of nonpharmacological interventions to treat behavioural disturbances in older patients with dementia. SENATOR-OnTop series. BMJ Open. 2017;7(3):e012759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pedersen SKA, Andersen PN, Lugo RG, et al. Effects of music on agitation in dementia: a meta-analysis. Frontiers Psychol. 2017;8(742). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsoi KK, Joyce JYC, Ng YM, et al. Receptive music therapy is more effective than interactive music therapy to relieve behavioral and psychological symptoms of dementia: a systematic review and meta-analysis. J Am Med Directors Assoc. 2018;19(7):568–576. [DOI] [PubMed] [Google Scholar]
- 86.Paul DR, Yorkston KM, Klasner ER, et al. Quality of communication life scale. Manual. Rockville, MD: American Speech-Language-Hearing Association. 2004. [Google Scholar]
- 87.Behrman S, Chouliaras L, Ebmeier KP. Considering the senses in the diagnosis and management of dementia. Maturitas. 2014;77:305–310. [DOI] [PubMed] [Google Scholar]
- 88.Kovach CR. Sensoristasis and imbalance in persons with dementia. J Nurs Scholarship. 2000;32:379–384. [DOI] [PubMed] [Google Scholar]
- 89.Chung JCC, Lai CKY. Snoezelen for dementia. Cochrane Database of Systematic Reviews, 2002. 10.1002/14651858.cd003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Strøm BS, Ytrehus S, Grov EK. Sensory stimulation for persons with dementia: a review of the literature. J Clin Nurs. 2016;25: 1805–1834. [DOI] [PubMed] [Google Scholar]
- 91.Strøm BS, Engedal K, Benth JS, et al. Effect of the sonas programme on communication in people with dementia: a randomized controlled trial. Dement Geriatr Cognitive Disorders Extra. 2017;7:122–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lorusso LN, Bosch SJ. Impact of multisensory environments on behavior for people with dementia: a systematic literature review. Gerontologist. 2018;58:e168–e179. [DOI] [PubMed] [Google Scholar]
- 93.Gonzalez MT, Kirkevold M. Benefits of sensory garden and horticultural activities in dementia care: a modified scoping review. J Clin Nurs. 2014;23:2698–2715. [DOI] [PubMed] [Google Scholar]
- 94.Chang Y-S, Chu H, Yang CY, et al. The efficacy of music therapy for people with dementia: a meta-analysis of randomised controlled trials. J Clinl Nurs. 2015(23-24):3425. [DOI] [PubMed] [Google Scholar]
- 95.World Federation of Occupational Therapists, Statement on Occupational Therapy 2012. World Federation of Occupational Therapists. 2010. https://www.wfot.org/resources/statement-on-occupational-therapy. [Google Scholar]
- 96.Law M, Ku Y, Zanto TP, et al. The person-environment-occupation model: a transactive approach to occupational performance. Can J Occup Ther. 1996;63(1):9–23. [Google Scholar]
- 97.Bennett S, Laver K, Radloff SV, et al. Occupational therapy for people with dementia and their family carers provided at home: a systematic review and meta-analysis. BMJ Open. 2019;9(11): e026308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hsu W-Y, Ku Y, Zanto TP, et al. Effects of noninvasive brain stimulation on cognitive function in healthy aging and Alzheimer’s disease: a systematic review and meta-analysis. Neurobiol Aging. 2015;36:2348–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Inagawa T, Narita Z, Sugawara N, et al. A meta-analysis of the effect of multisession transcranial direct current stimulation on cognition in dementia and mild cognitive impairment. Clini EEG Neurosci. 2019;50:273–282. [DOI] [PubMed] [Google Scholar]
- 100.Wolinsky FD, Vander MW, Howre MB, et al. Interim analyses from a randomised controlled trial to improve visual processing speed in older adults: the Iowa Healthy and Active Minds Study. BMJ Open. 2011;1:e000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lampit A, Hallock H, Valenzuela M. Computerized cognitive training in cognitively healthy older adults: a systematic review and meta-analysis of effect modifiers. PLoS Med. 2014;11.(11):e100175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dodge HH,Campbell I, Hayes T,et al. Use of high-frequency in-home monitoring data may reduce sample sizes needed in clinical trials. PLoS One. 2015;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moher D, Altman DG, Schulz KF, et al. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001;285(15): 1987–1991. [DOI] [PubMed] [Google Scholar]
- 104.Montgomery P, Sean G, Hopewell S, et al. Protocol for CONSORT-SPI: an extension for social and psychological interventions. Implementation Sci. 2013;8(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schulz KF, Altman DG, Moher D, CONSORT2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nosek BA, Alter G, Banks GC, et al. Promoting an open research culture. Science. 2015;348(6242):1422–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rosenberg A, Ngandu T, Rusanen M, et al. Multidomain lifestyle intervention benefits a large elderly population at risk for cognitive decline and dementia regardless of baseline characteristics: the FINGER trial. Alzheimer’s Dement. 2018;14(3):263–270. [DOI] [PubMed] [Google Scholar]
- 108.Morris MC, Tangney CC, Wang Y, et al. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimer’s Dement. 2015;11(9):1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


