Abstract
In this study, we investigated whether the CHA2DS2-VASc score could be used to estimate the need for hospitalization in the intensive care unit (ICU), the length of stay in the ICU, and mortality in patients with COVID-19. Patients admitted to Merkezefendi State Hospital because of COVID-19 diagnosis confirmed by RNA detection of virus by using polymerase chain reaction between March 24, 2020 and July 6, 2020, were screened retrospectively. The CHA2DS2-VASc and modified CHA2DS2-VASc score of all patients was calculated. Also, we received all patients’ complete biochemical markers including D-dimer, Troponin I, and c-reactive protein on admission. We enrolled 1000 patients; 791 were admitted to the general medical service and 209 to the ICU; 82 of these 209 patients died. The ROC curves of the CHA2DS2-VASc and M-CHA2DS2-VASc scores were analyzed. The cut-off values of these scores for predicting mortality were ≥ 3 (2 or under and 3). The CHA2DS2-VASc and M-CHA2DS2-VASc scores had an area under the curve value of 0.89 on the ROC. The sensitivity and specificity of the CHA2DS2-VASc scores were 81.7% and 83.8%, respectively; the sensitivity and specificity of the M-CHA2DS2-VASc scores were 85.3% and 84.1%, respectively. Multivariate logistic regression analysis showed that CHA2DS2-VASc, Troponin I, D-Dimer, and CRP were independent predictors of mortality in COVID-19 patients. Using a simple and easily available scoring system, CHA2DS2-VASc and M-CHA2DS2-VASc scores can be assessed in patients diagnosed with COVID-19. These scores can predict mortality and the need for ICU hospitalization in these patients.
Keywords: CHA2DS2-VASc score, Modified CHA2DS2-VASc score, COVID-19, Mortality, Intensive care unit hospitalization
Highlights
Endothelial dysfunction and thrombosis are important causes of mortality in COVID-19 patients.
The CHA2DS2-VASc score has been used in different cardiovascular diseases to define thromboembolic risk and the need for anticoagulant treatment.
CHA2DS2-VASc score might predict mortality and the need for intensive care unit hospitalization in COVID-19 patients.
Introduction
Coronavirus disease (COVID-19) first emerged in Wuhan City in December 2019 and rapidly spread worldwide. On March 11, 2020, the World Health Organization (WHO) announced that it had become a pandemic [1]. COVID-19 is a single-strand RNA coronavirus that enters human cells mainly by binding to angiotensin-converting enzyme 2 receptors (ACE2R) [2], which are numerous in the lungs, heart, veins, and arteries [3]. Coronavirus enters the endothelial cells in the veins and arteries after it induces COVID-19-associated coagulopathy. COVID-19-associated coagulopathy (CC) is characterized by thrombin generation, fibrinolysis, and a hypercoagulable state [4]. The coagulopathy includes abnormalities that increase D-dimer concentration, modestly decrease platelet count, prolong prothrombin time, and decrease antithrombin [3, 5, 6]. The coagulation abnormalities are related to mortality and thrombotic complications [6] including acute myocardial infarction (MI), acute limb ischemia, stroke, pulmonary emboli, venous thromboembolism, and thrombosis in intravenous catheters, and they have been identified in COVID-19 patients [7–11]. Thrombosis is a result of inflammation in patients with COVID-19 and this inflammation causes endothelial dysfunction and procoagulant activity [12].
To the best of our knowledge, there are only a few simple scoring methods for predicting inflammation, a thrombotic state, mortality, and intensive care unit (ICU) hospitalization in COVID-19 patients. The CHA2DS2-VASc score is used to estimate thromboembolic stroke risk in atrial fibrillation as well as thrombotic risk in other cardiac diseases. The abbreviation stands for Congestive heart failure, Hypertension, Age (> 65 = 1 point, > 75 = 2 points), Diabetes, previous Stroke/transient ischemic attack (2 points). Yet a limited number of studies have considered CHA2DS2-VASc score in relation to COVID-19 patients [13].
In this study, we investigated whether the CHA2DS2-VASc score could be used to estimate the need for hospitalization in the ICU, the length of stay in the ICU, and mortality in patients with COVID-19.
Material and methods
The study was a retrospective study conducted at Merkezefendi State Hospital (Manisa, Turkey), which was designated for COVID-19 patients. The COVID-19 diagnosis was made according to the WHO definition [14]. Adult patients (18 years or older) with laboratory-confirmed COVID-19 [per RNA detection of the coronavirus using polymerase chain reaction (PCR)] between March 24 and July 6, 2020 were screened. This retrospective study was approved by the Republic of Turkey’s Ministry of Health (No. 2020-06-05T11_26_30) as well as the local ethics committee.
We received all patients’ complete biochemical markers including D-dimer, Troponin I, and c-reactive protein (CRP) on admission. We excluded patients with serious liver disease, hereditary coagulation disorders, active cancer or chemotherapy–radiotherapy treatment, rheumatologic disease (systemic lupus erythematosus, Behcet’s syndrome, etc.), use of oral contraceptive, and/or age < 18 years.
Every patient admitted to the ICU was included as an intensive care patient. These patients were considered intensive care patients even if they had been transferred from general medical service to the ICU. Patients who were admitted to the ICU and who had preserved renal function, defined as creatinine clearance (CrCl) > 50 mL/min according to the Cockroft–Gault formula [15] were treated with subcutaneous enoxaparin(Clexane®, Lovenox®; Sanofi‐Aventis, Berlin, Germany) 1 mg/kg body weight twice a day. Patients with renal impairment (CrCl 15–50 ml/min) were treated with enoxaparin 1 mg/kg body weight once a day. In patients who used vitamin K antagonists, enoxaparin treatment was delayed until their international normalized ratio was ˂ 2. Enoxaparin treatment was begun a day after discontinuing novel oral anticoagulant in patients who used these medications (apixaban, edoxaban, rivaroxaban, dabigatran). Enoxaparin treatment was continued during ICU hospitalization. Patients were followed-up from hospital admission to hospital discharge or in-hospital death.
Each patient’s CHA2DS2-VASc score was calculated using clinical data obtained from his or her electronic medical health record history and routine biochemical tests during hospitalizations. The CHA2DS2-VASc score was calculated (patients were given 1 point for congestive heart failure, hypertension, age 65 to 74 years, diabetes mellitus, vascular disease, and female sex and 2 points for age ≥ 75 years and previous stroke or transient ischemic attack) by two cardiologists who were blinded to patient-survival data [16]. Because male sex is a risk factor for COVID-19, we calculated modified CHA2DS2-VASc (M- CHA2DS2-VASc) to give 1 point for male sex and 0 points for female sex [13, 17].
Statistical analysis
IBM SPSS V25 (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp) and R Studio was used for the statistical analysis. The assumption of normality was tested by the Kolmogorov Smirnov. Non-normally distributed continuous variables were reported as median (minimum-maximum) values. Discrete variables were presented as the number of events and percentages. Categorical variables were compared using χ2 or Fishers’ exact test where expected frequencies were less than 20. The Mann–Whitney U test was used to compare median differences between two independent groups when the dependent variable is continuous, but not normally distributed. Spearman rank correlation was used to measure the degree of association between two variables. ROC-AUC analysis -Youden Index was used for determination of the optimal cut-off point of CHADS VASc and M-CHA2DS2-VASc scores. Sensitivity, specificity, AUC and percentage of correct classification values were presented. Multivariable logistic regression backward LR method was used to determine the risk factors of binary dependent variables. Odds ratios (OR) and 95% confidence intervals (CI) were calculated. Also, Hosmer-Lemeshow (HL) test results were presented to evaluate goodness of fit in logistic regression. The Brier scores has been used to evaluate predictive accuracy. Relationship between independent variables and time to event was compared using Kaplan–Meier methodology using the Log Rank test to determine statistical significancy. Type one error for the statistical tests was selected 5%.
Results
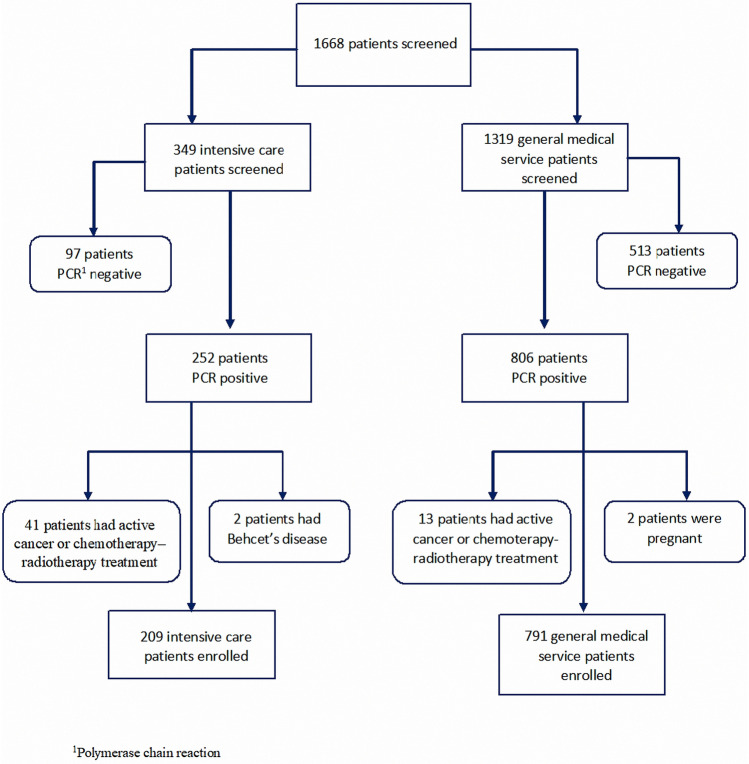
1668 patients were screened. 349 of them were intensive care patients, 1319 of them were general medical service patients. 140 of 349 intensive care patients and 528 of 1319 general medical service patients were excluded (Fig. 1). We enrolled 1000 patients; 791 were admitted to the general medical service and 209 to the ICU; 82 of these 209 patients died. The basic clinical characteristics of the patients in intensive care and in general medical service, including age, sex, and presence of hypertension, diabetes mellitus, previous stroke or transient ischemic attack, vascular disease, chronic obstructive pulmonary disease (COPD), and/or hyperlipidemia at admission are listed in Table 1. Patients in intensive care were older than those in general medical service. Hypertension, diabetes mellitus, previous stroke or transient ischemic attack, vascular disease, COPD, and hyperlipidemia were observed at a higher rate in ICU patients. Patients’ routine laboratory results are listed. ICU patients’ D-dimer, CRP, and Troponin I values were higher than those of the patients in general medical service. More renal failure was also seen in ICU patients than in general medical service patients. In addition, D-dimer, CRP, and Troponin I values of patients in the non-survivor group were higher than those in the survivor group in the ICU (Table 2).
Fig. 1.
Flow diagram of the study
Table 1.
Basic clinical characteristics stratifed according to the presence or absence of intensive care unit admission
| Patients in general medical service n (%) 791 (79.1) | Patients in intensive care unit n (%) 209 (20.9) | p value | |
|---|---|---|---|
| Age 18–64 years | 674 (85.2) | 71 (34) | < 0.001 |
| Age 65–74 years | 69 (8.7) | 67 (32) | |
| Age 75 and above years | 48 (6.1) | 71 (34) | |
| Sex male | 475 (60.1) | 119 (56.9) | 0.415 |
| Sex female | 316 (39.9) | 90 (43.1) | |
| Congestive heart failure | 13 (1.6) | 15 (7.2) | < 0.001 |
| Hypertension | 162 (20.5) | 148 (70.8) | < 0.001 |
| Stroke/TIA/thromboembolism history* | 3 (0.4) | 10 (4.8) | < 0.001 |
| Vascular disease history (prior MI, peripheral artery disease, or aortic plaque)** | 33 (4.2) | 59 (28.2) | < 0.001 |
| Diabetes mellitus | 58 (7.3) | 69 (33) | < 0.001 |
| COPD*** | 26 (3.3) | 28 (13.4) | < 0.001 |
| Hyperlipidemia | 29 (3.7) | 16 (7.7) | < 0.001 |
| Hydroxychloroquine | 791 (100) | 209 (100) | NA**** |
| Azithromycin | 537 (67.9) | 157 (75.1) | 0.059 |
| Favipiravir | 40 (5.1) | 68 (32.5) | < 0.001 |
| Death | 0(0) | 82(100) | < 0.001 |
*TIA Transient ıschaemic attack
**MI Myocardial ınfarction
***COPD Chronic obstructive pulmonary disease
****NA non-available
Table 2.
Laboratory results of all patients and intensive care unit patients
| Patients in general medical service [min–max] | Patients in intensive care unit [min–max] | p value | |
|---|---|---|---|
| ALT* | 22.0 [2.0–263.0] | 20.0 [1.0–2267.0] | 0.004 |
| AST** | 22.0 [4.0–140.0] | 28.0 [8.0–2500] | < 0.001 |
| Urea | 30.0 [8.0–128.0] | 53.0 [3.0–385.0] | < 0.001 |
| Creatinine | 0.81 [0.19–10.0] | 1.01 [0.15–13.7] | < 0.001 |
| CrCl (mL/min) | 103.0 [6.0–219.0] | 69.0 [3.0–158.0] | < 0.001 |
| CrCl*** 15 under | 3 (0.4%) | 23 (11.0%) | < 0.001 |
| CrCl 15–50 | 25 (3.2%) | 56 (26.8%) | |
| CrCl 50 above | 763 (95.6%) | 130 (62.2%) | |
| Troponin I | 0.00 [0–1.3] | 0.03 [0.0–2.12] | < 0.001 |
| Troponin I normal(< 0.002ng/ml) | 521 (65.9) | 38 (18.2) | < 0.001 |
| Troponin I high | 270 (34.1) | 171 (81.8) | |
| D-dimer | 150.0 [10.0–3329.0] | 709.0 [100.0–9094.0] | < 0.001 |
| D dimer normal (0–250ng/ml) | 652 (82.4) | 41 (19.6) | < 0.001 |
| D-dimer high | 139 (17.6) | 168 (80.4) | |
| CRP**** | 6.0 [1.0–255.0] | 107.8 [1.0–545.0] | < 0.001 |
| CRP normal (0–10mg/dl) | 491 (62.1) | 36 (17.2) | < 0.001 |
| CRP high | 300 (37.9) | 173 (82.8) |
| Patients in intensive care unit | |||
|---|---|---|---|
| Patients survivor [min–max] | Patients non-survivor [min–max] | ||
| ALT | 18.0 [1.0–2267.0] | 23.0 [2.0–2100.0] | 0.094 |
| AST | 23.0 [8.0–2500.0] | 36.0 [10.0–2500.0] | < 0.001 |
| Urea | 50.0 [14.0–258.0] | 60.0 [3.0–385.0] | 0.016 |
| Creatinine | 0.96 [0.4–11.6] | 1.07 [0.15–13.7] | 0.338 |
| CrCl (mL/min) | 71.0 [4.0–135.0] | 64.5 [3.0–158.0] | 0.275 |
| Troponin I | 0.01 [0.0–1.12] | 0.66 [0.0–2.12] | < 0.001 |
| Troponin I normal | 35 (27.6) | 3 (3.7) | < 0.001 |
| Troponin I high | 92 (72.4) | 79 (96.3) | |
| D-dimer | 382.0 [100.0–7216.0] | 1200.0 [150.0–9094.0] | < 0.001 |
| D-dimer normal | 37 (29.1) | 4 (4.9) | < 0.001 |
| D-dimer high | 90 (70.9) | 78 (95.1) | |
| CRP | 40.4 [1.0–369.7] | 204.2 [3.0–545.0] | < 0.001 |
| CRP normal | 32 (25.2) | 4 (4.9) | < 0.001 |
| CRP high | 95 (74.8) | 78 (95.1) | |
*ALT: Alanine Aminotransferase
**AST: Aspartate aminotransferase
***CrCl: Creatinine clearance
*****CRP: C-reactive protein
: Median, p < 0.05 significance level
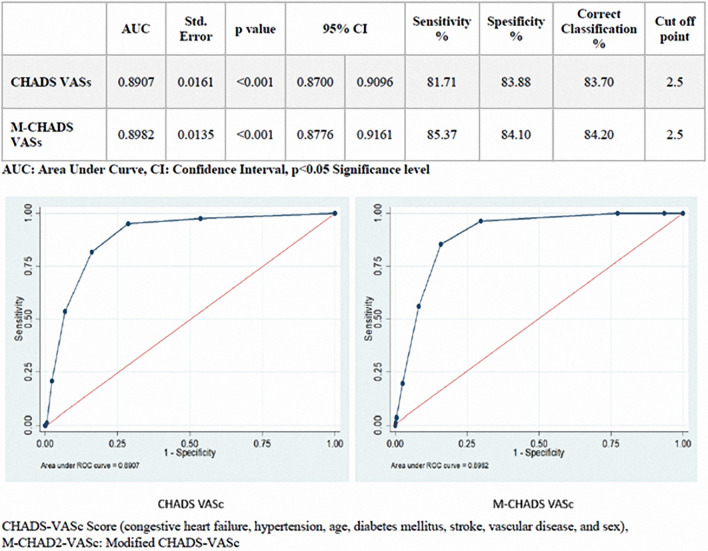
The ROC curves of the CHA2DS2-VASc and M-CHA2DS2-VASc scores were analysed. The cut-off values of these scores for predicting mortality were ≥ 3 (2 or under and 3 or above). The CHA2DS2-VASc and M-CHA2DS2-VASc scores had an area under the curve value of 0.89 on the ROC. The sensitivity and specificity of the CHA2DS2-VASc scores were 81.7% and 83.8%, respectively; the sensitivity and specificity of the M-CHA2DS2-VASc scores were 85.3% and 84.1%, respectively (Fig. 2).
Fig. 2.
The relationship between CHADS-VASc and M-CHADS-VASc mortality was evaluated by ROC curve. CHADS-VASc threshold value for mortality was found to be ≥ 3
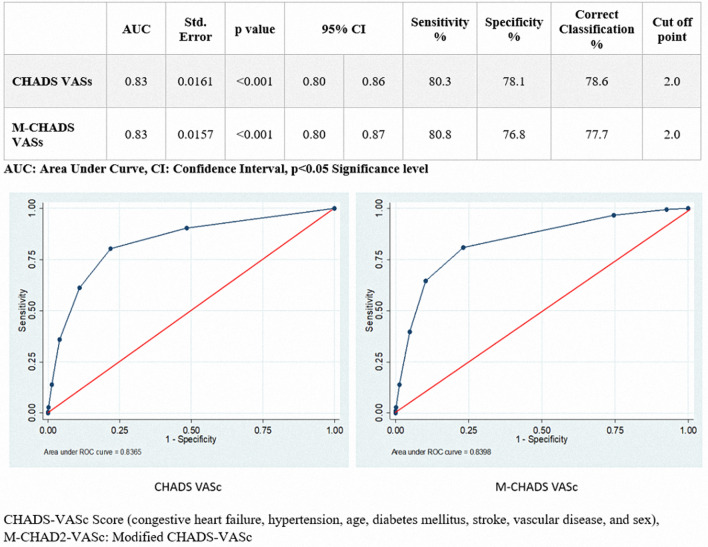
A total of 785 patients were in the 0–2 group of CHA2DS2-VASc scores; 81 (10.3%) of them were in the ICU and 704 (89.7%) in general medical service. The number of CHA2DS2-VASc scores 3 or above was 128 (59.5%) in the ICU and 87 (40.5%) in general medical service. A significant relationship was found between the patients with ICU hospitalization and CHA2DS2-VASc scores. Also, the ROC curves of the CHA2DS2-VASc and M-CHA2DS2-VASc scores were evaluated for intensive care unit hospitalizations. The cut-off values of these scores for predicting intensive care unit hospitalization were ≥ 2.0. AUC value was found out as 0.83 for both CHA2DS2-VASc and M-CHA2DS2-VASc scores (Fig. 3).
Fig. 3.
The relationship between CHADS-VASc and M-CHADS-VASc for intensive care unit hospitalizations was evaluated by ROC curve. CHADS-VASc threshold value for was found to be ≥ 2
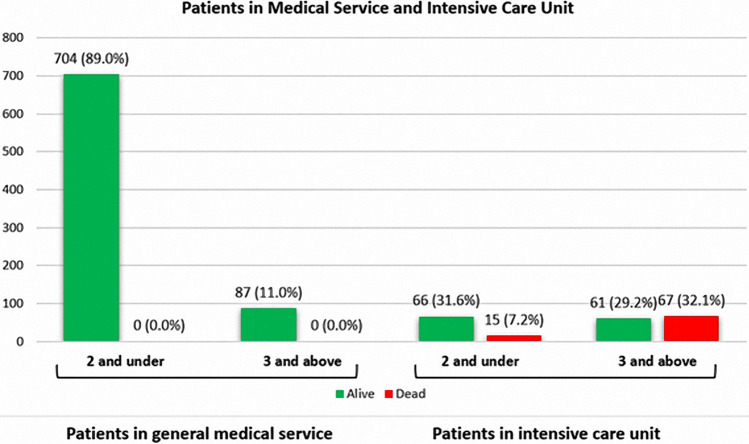
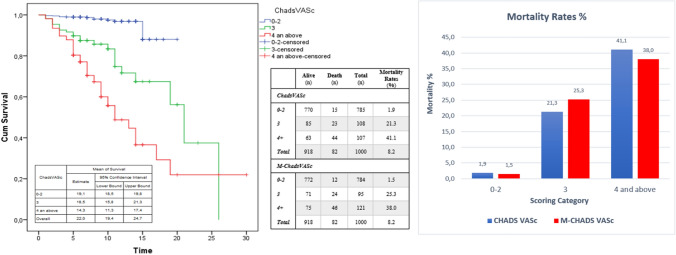
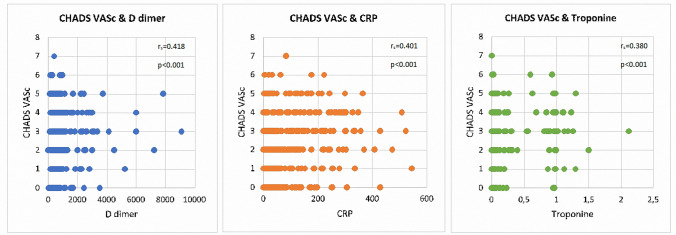
The number of patients with CHA2DS2-VASc scores 0–2 was 66 (81.5%) in the survivor group and 15 (18.5%) in the non-survivor group in the ICU. The number of patients with CHA2DS2-VASc scores 3 and above was 61 (47.7%) in the survivor group and 67 (52.3%) in the non-survivor group in the ICU (Fig. 4). The CHA2DS2-VASc and M-CHA2DS2-VASc scores are associated with mortality. The mortality rate of CHA2DS2-VASc and M-CHA2DS2-VASc scores 0–2 was (1.9% and 1.5%); for a score of 3, it was 21.3% and 25.3%, and for a score 4 and above, it was 41.1% and 38%, respectively. Higher CHA2DS2-VASc scores were associated with higher mortality. Survival was shown by Kaplan–Meier survival analysis according to the CHA2DS2-VASc score (Fig. 5). The results of univariate and multivariate logistic regression analysis of all patients (Table 3) and ICU patients (Table 4) for CHA2DS2-VASc were demonstrated. The goodness of fit for models -CHADVASc scores- showed no significant differences between predicted and observed mortality in hospital (HL- χ2 = 2.104; p = 0.910) and similarly in intensive care unit (HL- χ2 = 3.319; p = 0.768). Also, BS values showed acceptable calibration respectively 0.018 and 0.021 for overall mortality and in-hospital mortality for intensive care unit patients. Multivariate logistic regression analysis showed that CHA2DS2-VASc, Troponin I, D-Dimer, and CRP were independent predictors of mortality in COVID-19 patients. We categorized patients in three groups according to their CHA2DS2-VASc score. Group 1 had a CHA2DS2-VASc score ranging from 0 to 2; for group 2, the CHA2DS2-VASc score was 3; for group 3, the CHA2DS2-VASc score was 4 and above. The mortality rate increased 4.6-fold in the CHA2DS2-VASc score of 3 group and by 11-fold in the CHA2DS2-VASc score of 4 and above group, according to CHA2DS2-VASc 0–2 in all patients. The mortality rate increased 3.2-fold in the group with CHA2DS2-VASc scores of 3 and increased 6.2-fold in the group with CHA2DS2-VASc scores of 4 and above, according to CHA2DS2-VASc 0–2 in ICU patients (Table 3, 4). D-dimer, Troponin I, and CRP values correlated with CHA2DS2-VASc scores (Fig. 6). There was no correlation between the number of days in ICU hospitalization and CHA2DS2-VASc scores.
Fig. 4.
CHADS-VASc score of patients in according to intensive care unit hospitalizations
Fig. 5.
Kaplan Meier survival analysis and mortality rate according to CHA2DS2-VASc score
Table 3.
Univariable and multivariable predictors of in hospital mortality for all patients
| Univariate model | Multiple model | |||||||
|---|---|---|---|---|---|---|---|---|
| p value | Exp(β) | 95% CI for EXP(β) | p value | Exp(β) | 95% CI for EXP(β) | |||
| Lower | Upper | Lower | Upper | |||||
| CHA2DS2-VASc ref:0–2 | p < 0.001 | p < 0.001 | ||||||
| CHA2DS2-VASc 3 | p < 0.001 | 13.890 | 6.981 | 27.637 | p < 0.001 | 4.651 | 2.125 | 10.178 |
| CHA2DS2-VASc 4 and above | p < 0.001 | 35.852 | 18.908 | 67.980 | p < 0.001 | 11.092 | 5.240 | 23.479 |
| Troponin I ref: normal | p < 0.001 | 40.446 | 12.674 | 129.076 | p < 0.001 | 13.523 | 3.931 | 46.522 |
| D dimer ref: normal | p < 0.001 | 58.670 | 21.244 | 162.031 | p < 0.001 | 17.811 | 6.144 | 51.636 |
| CRP ref:normal | p < 0.001 | 25.819 | 9.373 | 71.120 | 0.005 | 5.051 | 1.639 | 15.567 |
| COPD ref: no | 0.001 | 3.153 | 1.558 | 6.381 | 0.034 | 2.734 | 1.079 | 6.928 |
| Constant | p < 0.001 | 0.001 | ||||||
Table 4.
Univariable and multivariable logistic predictors of in-hospital mortality for intensive care unit patients
| Patient in intensive care unit | Univariate model | Multiple model | ||||||
|---|---|---|---|---|---|---|---|---|
| p value | Exp(β) | 95% CI for Exp(β) | p value | Exp(β) | 95% CI for Exp(β) | |||
| Lower | Upper | Lower | Upper | |||||
| CHA2DS2-VASc ref:0–2 | < 0.001 | < 0.001 | ||||||
| CHA2DS2-VASc 3 | 0.002 | 3.373 | 1.545 | 7.363 | 0.007 | 3.244 | 1.386 | 7.594 |
| CHA2DS2-VASc 4 and above | < 0.001 | 6.245 | 3.025 | 12.894 | < 0.001 | 6.259 | 2.811 | 13.940 |
| Troponin I ref: normal | < 0.001 | 10.018 | 2.967 | 33.825 | 0.001 | 8.369 | 2.323 | 30.155 |
| D- dimer ref: normal | < 0.001 | 8.017 | 2.735 | 23.494 | 0.004 | 5.585 | 1.736 | 17.965 |
| CRP ref: normal | 0.001 | 6.568 | 2.227 | 19.375 | 0.028 | 3.815 | 1.157 | 12.578 |
| Constant | < 0.001 | 0.003 | ||||||
Fig. 6.
Correlation between CHA2DS2-VASc score and D-dimer, Troponin I, CRP
Discussion
Several biochemical markers, clinic comorbidities, and risk-score models can be used to predict ICU admission and hospital mortality in patients diagnosed with COVID-19. In COVID-19; age, comorbidities, lymphocytopenia, elevated alanine aminotransferase, D-dimer, creatine kinase, high-sensitivity cardiac Troponin, and prothrombin time were associated with ICU admission and disease severity. In addition, older age, higher Sequential Organ Failure Assessment (SOFA) score, D-dimer, Troponin, and CRP levels at admission were associated with hospital mortality [17–19].
In our study, patients in intensive care were older and had more comorbidities (hypertension, diabetes mellitus, vascular disease) and higher D-Dimer, Troponin I, and CRP values.
Troponin, D-dimer, and CRP signal inflammation severity and thrombotic risk for patients with COVID-19. This inflammation causes diffuse endothelial dysfunction and is associated with disease severity in these patients [12, 20].
Diffuse endothelial dysfunction can result in microvascular injury, thrombosis, and subsequent multisystem organ failure and death in patients with COVID-19 [21]. Endothelial dysfunction is an important factor in cardiovascular diseases such as COVID-19, and it is associated with age, male sex, smoking, hypertension, diabetes mellitus, obesity, and vascular disease. Advanced age, hypertension, diabetes, and coronary artery disease are risk factors for severe COVID-19 and are part of the CHA2DS2-VASc score. COVID-19 patients who have these risk factors are more vulnerable to adverse outcomes [17].
As a patient’s CHA2DS2-VASc score increases, endothelial dysfunction and susceptibility to thrombosis increases. But anticoagulant treatment could not reduce mortality for every adult hospitalized COVID-19 patient [Anti-thrombotics for Adults Hospitalized with COVID-19(ACTIV-4 trial)] due to the potential harm of anticoagulant therapy.1
The CHA2DS2-VASc score has been used in different cardiovascular diseases to define thromboembolic risk and the need for anticoagulant treatment. The efficacy of the CHA2DS2-VASc score has been demonstrated in cardiovascular diseases. Because endothelial dysfunction and thrombosis are important causes of mortality in COVID-19 patients, the CHA2DS2-VASc score can predict mortality in these patients as well [16, 21–23].
We found that, in multivariate and univariate analysis, D-dimer, CRP, Troponin I, and CHA2DS2-VASc score were independent predictors for mortality in patients with COVID-19. These markers and the score could predict mortality in all patients as well as in ICU patients only. The CHA2DS2-VASc score was moderately correlated with D-dimer, CRP, and Troponin I levels.
There are a number of risk scores, and a free risk calculator for COVID-19 can be found online The risk calculator is based on the Chinese Center for Disease Control and Prevention (CDC) and American CDC data. It includes age, cardiovascular disease, COPD, prior stroke, diabetes, hypertension, cancer, heart disease, and chronic kidney disease. Mortality rate according to scores is between < 1% and 27%.2
We compared scoring system for prediction of mortality in COVID-19 patients (Table 5). Knight et al. [24] developed the 4C Mortality Score to predict hospital mortality in patients with COVID-19. The score consists of eight variables: age, sex, number of comorbidities, respiratory rate, peripheral oxygen saturation, level of consciousness, urea level, and CRP. The score ranges from 0 to 21 points and has four risk categories for predicting mortality. The categories are low risk (score of 0–3, mortality rate 1.2%), intermediate risk (score of 4–8, mortality rate 9.9%), high risk (score of 9–14, mortality rate 31.4%) and very high risk (score ≥ 15, mortality rate 61.5%).
Table 5.
Comparing of scores for prediction of mortality in COVID-19 patients according to area under curve.
| Scorring system | Number of patients | AUC1 | |
|---|---|---|---|
| Gunduz et al. | CHA2DS2-VASc | 1000 | 0.89 95% CI 0.87–0.90 |
| Gunduz et al. | M-CHA2DS2-VASc | 1000 | 0.89 95% CI 0.87–0.91 |
| Liu et al. | SOFA | 140 | 0.89 95% CI 0.82–0.95 |
| Knight et al. | 4C | 22,361 | 0.79 95% CI 0.78–0.79 |
| Yadaw et al. | 3F model | 3841 | 0.91 95% CI 0.86–0.95 |
1AUC: Area under curve
Yadaw et al. [25] developed a risk-score model using machine-learning techniques to predict mortality in COVID-19 patients. They applied two different patients’ datasets. When the model was applied to dataset 1(961 patients) retrospectively and to dataset 2(249 patients) prospectively, a high accuracy rate (AUC = 0.91) was found in both datasets. The model first comprised 17 features of COVID-19 patients and having only three of them was enough to predict mortality. These features of the model are the patient’s age, minimum oxygen saturation at hospital admission, and type of patient encounter (inpatient/outpatient/telehealth).
We found that the CHA2DS2-VASc score could also be useful for predicting mortality. The cut-off value of the CHA2DS2-VASc score was found to be 3 in the ROC curve. When we categorized patients according to CHA2DS2-VASc and MCHA2DS2-VASc scores, scores of 0–2 were low risk (mortality rate 1.9% and 1.5%); a score of 3 indicated high risk (mortality rate 21.3% and 25.3%); and a score of 4 and above indicated very high risk (41.1% and 38%), respectively. Risk categories for the 4C score are similar to those for the CHA2DS2-VASc score but include an intermediate risk category. The 4C risk score is complex and not easily calculated. Several biochemical parameters and physical examination findings are needed to calculate the 4C risk score [24]. The risk score developed by Yadav et al. is simple, yet it has shown a high level of accuracy(AUC = 0.91) for mortality although it does not include risk categories and predicts mortality only [25].This risk calculator is based on clinical comorbidities in patients with COVID-19 and is calculated easily using a smartphone. However, the mortality rate is changing, according to CDC data, and it does not include risk categories.
The CHA2DS2-VASc score has been used for years, so physicians are familiar with its use. In addition, the score is easily calculated based on a patient’s anamnesis and does not require a radiological device, biochemical parameter, or computer application to calculate it. Using the CHA2DS2-VASc score, we were able to define low-risk COVID-19 patients with no need for intensive care unit admission or intensive treatment and high-risk COVID-19 patients.
We also found a correlation between the CHA2DS2-VASc score and ICU hospitalization. In general medical care patients, the CHA2DS2-VASc scores were lower than in ICU patients. But no correlation was found for number of days in the ICU. This could have resulted because some patients in the ICU died soon after admission.
Limitations
This study has several limitations. First, the study was not conducted in multiple centers. Thrombus could not be shown by autopsy in patients non-survivor. Second, all patients in the intensive care unit were treated with enoxaparin; therefore, it is not possible to determine which patients benefited from anticoagulant treatment.
Conclusion
The study is the first study of COVID-19 patients to evaluate CHA2DS2-VASc and M-CHA2DS2-VASc scores in relation to mortality, ICU hospitalization, and length of stay in the ICU.
Using a simple and easily available scoring system, CHA2DS2-VASc and M-CHA2DS2-VASc scores can be assessed in patients diagnosed with COVID-19. These scores might predict mortality and the need for ICU hospitalization in these patients.
Acknowledgments
We are sincerely grateful our medical secretary Bahriye Tay for support.
Abbreviations
- COVID-19
Coronavirus disease
- WHO
World health organisation
- CHA2DS2-VASc score
Congestive heart failure, hypertension, age, diabetes mellitus, stroke, vascular disease, and sex
- M-CHA2DS2-VASc score
Modified CHA2DS2-VASc
- CrCl
Creatinine clearance
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- CRP
c-Reactive protein
- COPD
Chronic obstructive pulmonary disease
- TIA
Transient ischaemic attack
- MI
Myocardial infarction
- IC
Intensive care unit
- PCR
Polymerase chain reaction
Funding
The authors have no funding.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Footnotes
Clinicaltrials.gov/ct2/show/NCT04505774.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ramazan Gunduz, Email: ramazankard@yahoo.com.
Bekir Serhat Yildiz, Email: bserhatyildiz@yahoo.com.
Ibrahim Halil Ozdemir, Email: dr.ibrahimhalilozdemir@gmail.com.
Nurullah Cetin, Email: nurullahct@hotmail.com.
Mehmet Burak Ozen, Email: mehmetburakozen@gmail.com.
Eren Ozan Bakir, Email: eren.ozan.bakir@gmail.com.
Su Ozgur, Email: suozgur35@gmail.com.
Ozgur Bayturan, Email: bayturanoz@hotmail.com.
References
- 1.Organization WH (2020) WHO Director-General's opening remarks at the media briefing on COVID-19-11 March 2020.
- 2.Walls AC, Park Y-J, Tortorici MA, Wall A, McGuire AT, Veesler DJC. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292.e286. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Der Nigoghossian C, Ageno W, Madjid M, Guo Y, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am College Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klok F, Kruip M, Van der Meer N, Arbous M, Gommers D, Kant K, Kaptein F, van Paassen J, Stals M, Huisman M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan W-J, Ni Z-y, Hu Y, Liang W-h, Ou C-q, He J-x, Liu L, Shan H, Lei C-l, Hui D. Clinical characteristics of coronavirus disease 2019 in China. New England J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yatskar L, Harari R, Shah B. ST-segment elevation in patients with covid-19: a case series. N Engl J Med. 2020;17(382):2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, Collange O, Boulay C, Fafi-Kremer S, Ohana M. Neurologic features in severe SARS-CoV-2 infection. New England J Med. 2020;382(323):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helms J, Tacquard C, Severac FJICM. (Clinical research in intensive care and sepsis trial group for global evaluation and research in sepsis). High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;4:1–10. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, De Leacy RA, Shigematsu T, Ladner TR, Yaeger K. Large-vessel stroke as a presenting feature of Covid-19 in the young. New England J Med. 2020;382(20):e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perini P, Nabulsi B, Massoni CB, Azzarone M, Freyrie AJTL. Acute limb ischaemia in two young, non-atherosclerotic patients with COVID-19. Lancet. 2020;395(10236):1546. doi: 10.1016/S0140-6736(20)31051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cetinkal G, Kocas BB, Ser OS, Kilci H, Keskin K, Ozcan SN, Verdi Y, Zeren MI, Demir T, Kilickesmez K. Assessment of the modified CHA2DS2VASc risk score in predicting mortality in patients hospitalized with COVID-19. Am J Cardiol. 2020;135:143–149. doi: 10.1016/j.amjcard.2020.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Organization WH. Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: interim guidance, 2 March 2020. World Health Organization; 2020. [Google Scholar]
- 15.Shoker A, Hossain M, Sengul T, Raju D, Cockcroft D. Performance of creatinine clearance equations on the original Cockcroft-Gault population. Clin Nephrol. 2006;66(2):89–97. doi: 10.5414/CNP66089. [DOI] [PubMed] [Google Scholar]
- 16.Kurtul A, Acikgoz S. Validation of the CHA2DS2-VASc score in predicting coronary atherosclerotic burden and in-hospital mortality in patients with acute coronary syndrome. Am J Cardiol. 2017;120(1):8–14. doi: 10.1016/j.amjcard.2017.03.266. [DOI] [PubMed] [Google Scholar]
- 17.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu J-b, Xu C, Zhang R-b, Wu M, Pan C-k, Li X-j, Wang Q, Zeng F-f, Zhu S. Associations of procalcitonin, C-reaction protein and neutrophil-to-lymphocyte ratio with mortality in hospitalized COVID-19 patients in China. Sci Rep. 2020;10(1):1–10. doi: 10.1038/s41598-019-56847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu S, Yao N, Qiu Y, He C, et al. Predictive performance of SOFA and qSOFA for in-hospital mortality in severe novel coronavirus disease. Am J Emerg Med. 2020;38(10):2074–2080. doi: 10.1016/j.ajem.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Yan X, Fan Q, Liu H, Liu X, LiuZhang ZZ, et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18(6):1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch HJTL. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levi M, Thachil J, Iba T, Levy JHJTLH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7(6):e438. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Çınar T, Hayıroğlu MI, Tanık VO, Aruğaslan E, Keskin M, Uluganyan M, Öz A, Çağdaş M, Alper AT, et al. The predictive value of the CHA2DS2-VASc score in patients with mechanical mitral valve thrombosis. J Thromb Thromb. 2018;45(4):571–577. doi: 10.1007/s11239-018-1640-3. [DOI] [PubMed] [Google Scholar]
- 24.Knight SR, Ho A, Pius R, Buchan I, Carson G, Drake TM, Dunning J, Fairfield CJ, Gamble C, Green CA, et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi: 10.1136/bmj.m3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yadaw AS, Li Y-C, Bose S, Iyengar R, Bunyavanich S, Pandey GJTLDH. Clinical features of COVID-19 mortality: development and validation of a clinical prediction model. Lancet Digit Health. 2020;2(10):e516–e525. doi: 10.1016/S2589-7500(20)30217-X. [DOI] [PMC free article] [PubMed] [Google Scholar]