Since the outbreak of COVID-19 pandemic, health care systems have focused on its containment,1 , 2 facing critical issues in treating patients with medical disorders other than COVID-19, such as cancer and cardiovascular diseases.2
During the first wave of the COVID-19 pandemic, an international panel of lung cancer experts recommended to delay both baseline lung cancer screening (LCS) rounds and any further evaluation of screen-detected pulmonary nodules with low probability of being malignant or likely representing indolent cancers.2 As screening programs dropped down in the hospitals’ priorities, a significant decline in the incidence of the six most frequent cancers was registered in the United States, including lung cancer.3 Delayed diagnosis and treatment of all lung cancers are well-known to lead to worse outcomes (eg, a surgical delay of 12 weeks is associated with a slightly decreased overall survival)4 that require lung cancer timely diagnosis and management to be ensured during the COVID-19 pandemic.
The Screening and Multiple Intervention on Lung Epidemics (SMILE) trial5 is an ongoing LCS trial that includes multiple smoking cessation and antiinflammatory interventions, is performed at the Fondazione IRCCS Istituto Nazionale dei Tumori of Milan (INTM) in Lombardy, Italy, and is offered to subjects from the whole country. The baseline round enrolled 978 heavy current or former smokers, starting in July 2019, and allowed the diagnosis of ten lung cancers (10/978, 1.0%; stage I disease: 7/10, 70%) to be treated with curative surgery. Enrolment was interrupted in early March 2020, when the first wave of the COVID-19 pandemic severely affected Northern Italy, and mainly Lombardy. To safely allow short-term and annual screening rounds, we expanded the INTM protocol that had been developed for all patients with cancer by adding to the workflow a rapid severe SARS-CoV-2 screening test (BIOCREDIT COVID-19 Ag). The designed COVID-19 SMILE algorithm was implemented after institutional review board and Ethics Committee approval (INT129/17; 28/09/2020).6
Screenees were contacted by phone for preliminary anamnestic evaluation. Subjects who reported no COVID-19 symptoms over the previous 2 weeks were invited to reach Milan by private transport, to allow those testing positive to return home and avoid physical contact with other people. If public transport was needed, we requested screenees to undergo viral screening tests before the scheduled appointment to travel safely to and from INTM. Of the 845 contacted subjects, 534 (63.2%; 221 women, 41.4%; mean age; 62.1 ± 5.4 y) agreed to participate and attended one of the 13 sessions scheduled between October 17, 2020, and January 30, 2021, whereas 277 (32.7%) requested to either postpone the appointment or withdraw from the project, similarly to other reports7 , 8 (Table 1 ). Participating subjects were evaluated with a median of only 1-month delay, as compared with their theoretic annual round. Unsurprisingly, long-distance travel to be covered by public transport reduced the adherence to the program, which increased the rate of rescheduled appointments from 16.7% for subjects living in Lombardy (median distance to cover, 17.6 km) to 89% for those from Southern Italy (median distance, 823.4 km).
Table 1.
Participation Rate of COVID-19 SMILE Screenees
| Zone | Contacted Screenees, No. (%) | Participants, No. (%) | Postponed/No-show/Withdrawn, No. |
|---|---|---|---|
| Lombardy | 436 (100) | 363 (83.3) | 51/15/7 |
| North | 198 (100) | 119 (60.1) | 68/9/2 |
| Centre | 129 (99.2) | 43 (33.3) | 74/9/3 |
| South | 82 (90.1) | 9 (11.0) | 71/1/1 |
| Totals | 845 | 534 (63.2) | 264/34/13 |
Italy was divided into four zones that represented different levels of ease for reaching Milan by private transport: 845 screenees were contacted; 534 of them agreed to participate; 264 of them requested that their appointment to be postponed; 34 of them did not show, and 13 of them withdrew from the project. SMILE = Screening and Multiple Intervention on Lung Epidemics.
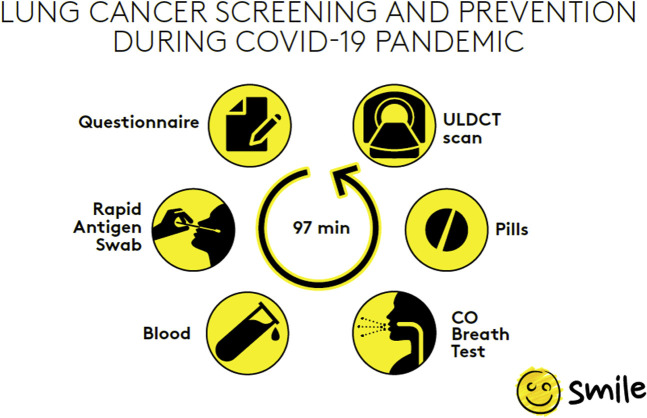
LCS feasibility during the COVID-19 pandemic was provided by an ad-hoc multistep framework that included detailed preliminary anamnestic evaluation for symptoms of COVID-19 and rapid antigenic testing. All LCS and prevention intervention-related costs were charged to the INTM, including the rapid antigenic test, which increased costs of about 10€ (12$) per screenee, whereas volunteers had to cover only travel expenses. We evaluated an average of 41 screenees per session, during which screenees first had to fill in a health questionnaire and to undergo a rapid antigenic nasopharyngeal swab test. Those who tested negative underwent a carbon monoxide test, smoking cessation and/or inflammatory status reduction intervention with antitobacco and antiinflammatory drugs, and an ultra-low-dose CT scan (SOMATOM Force; Siemens Healthineers) within an average hospital stay of 97 minutes (Fig 1 ).
Figure 1.
Workflow of the COVID-19 SMILE algorithm. Asymptomatic and apyretic screenees had to fill in a health questionnaire and to undergo rapid antigen nasopharyngeal swabbing and blood test. Those screenees who tested negative at the rapid antigenic swab were admitted and completed the lung cancer screening and prevention program interventions of the designed algorithm (carbon monoxide test, administration of antitobacco and antiinflammatory drugs, and ultra-low-dose CT scan). Ultra-low-dose CT scans were reviewed immediately to rule-out COVID-19 pneumonia-related findings. CO = carbon monoxide; SMILE = Screening and Multiple Intervention on Lung Epidemics; ULDCT = ultra-low-dose CT.
Safety of our approach is supported by the lack of COVID-19 cases among hospital staff and, to the best of our knowledge, screenees after the appointment. Only one asymptomatic subject (0.2%) was identified as positive by the rapid antigenic swab. The screenee was instructed to return home and contact the referring general practitioner; the positivity was then confirmed at the reverse transcriptase-polymerase chain reaction test. We performed a strict selection of subjects, admitting to the complete set of the SMILE trial activities only screenees with no COVID-19-related symptoms over the previous 2 weeks and those who tested negative at the rapid antigenic swab. During the whole stay within INTM, subjects had to maintain social distancing and use personal protective equipment. Furthermore, the SMILE staff (clinicians, nurses, biologists, and radiographers) was tested by rapid antigenic swab within 24 hours before the appointment. An on-call cleaning service was available for disinfection of rooms and equipment within 20 minutes after contact with potentially infected individuals. We also installed ultraviolet-C lamps to provide additional and fast disinfection of the CT scanner.
The COVID-19 SMILE algorithm shows that LCS and cigarette-smoking prevention measures can be performed feasibly and safely during the COVID-19 pandemic, with minimized risk of infection for both screenees and hospital staff.
Footnotes
FINANCIAL/NONFINANCIAL DISCLOSURES: None declared.
FUNDING/SUPPORT: Funding provided by Scientific Direction Fondazione IRCCS Istituto Nazionale Tumori (5x1000 2014-2015 Italian Ministry of Health for healthcare research) and Italian Association for Cancer Research (AIRC 5x1000 cod.12162, extension 2017-2020).
References
- 1.Tan B.S., Dunnick N.R., Gangi A., et al. RSNA International trends: a global perspective on the COVID-19 pandemic and radiology in late 2020. Radiology. 2021;299(1):E193–E203. doi: 10.1148/radiol.2020204267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazzone P.J., Gould M.K., Arenberg D.A., et al. Management of lung nodules and lung cancer screening during the COVID-19 pandemic: CHEST Expert Panel report. Chest. 2020;158(1):406–415. doi: 10.1016/j.chest.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaufman H.W., Chen Z., Niles J., Fesko Y. Changes in the ,umber of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Netw Open. 2020;3(8) doi: 10.1001/jamanetworkopen.2020.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson BA, Waddimba AC, Ogola GO, Fleshman JW Jr, Preskitt JT. A systematic review and meta-analysis of surgery delays and survival in breast, lung and colon cancers: implication for surgical triage during the COVID-19 pandemic [published online ahead of print December 8, 2020]. Am J Surg. 10.1016/j.amjsurg.2020.12.015. [DOI] [PMC free article] [PubMed]
- 5.Fondazione IRCCS Istituto Nazionale dei Tumori, Milano. Screening and Multiple Intervention on Lung Epidemics (SMILE). NCT03654105. ClinicalTrials.gov. Bethesda, MD: National Institutes of Health; 2018. https://clinicaltrials.gov/ct2/show/NCT03654105. Updated February 16, 2021.
- 6.Valenza F., Papagni G., Marchiano A., et al. Response of a comprehensive cancer center to the COVID-19 pandemic: the experience of the Fondazione IRCCS-Istituto Nazionale dei Tumori di Milano [published online ahead of print May 4, 2020] Tumori. 2020 doi: 10.1177/0300891620923790. [DOI] [PubMed] [Google Scholar]
- 7.Van Haren R.M., Delman A.M., Turner K.M., et al. Impact of the COVID-19 Pandemic on lung cancer screening program and subsequent lung cancer. J Am Coll Surg. 2021;232(4):600–605. doi: 10.1016/j.jamcollsurg.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang M., Yeung T., Shepard JO, et al. Operational Challenges of a low-dose CT lung cancer screening program during the coronavirus disease 2019 pandemic. Chest. 2021;159(3):1288–1291. doi: 10.1016/j.chest.2020.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]