Abstract
Hybrid molecules, furnished by combining two or more pharmacophores is an emerging concept in the field of medicinal chemistry and drug discovery that has attracted substantial traction in the past few years. Naturally occurring scaffolds such as coumarins display a wide spectrum of pharmacological activity including anticancer, antibiotic, antidiabetic and others, by acting on multiple targets. In this view, various coumarin-based hybrids possessing diverse medicinal attributes were synthesized in the last five years by conjugating coumarin moiety with other therapeutic pharmacophores. The current review summarizes the recent development (2014 and onwards) of these pharmacologically active coumarin hybrids and demonstrates rationale behind their design, structure-activity relationships (SAR) and mechanistic studies performed on these hybrid molecules. This review will be beneficial for medicinal chemist and chemical biologist, and in general to the drug discovery community and will facilitate the synthesis and development of novel, potent coumarin hybrid molecules serving as lead molecules for the treatment of complex disorders.
Keywords: coumarin hybrids, design strategies, structure-activity relationship, mechanistic insights, anticancer, antimicrobial, antidiabetic, antioxidant, anti-Alzheimer’s, anti-inflammatory
Graphical Abstract
1. Introduction
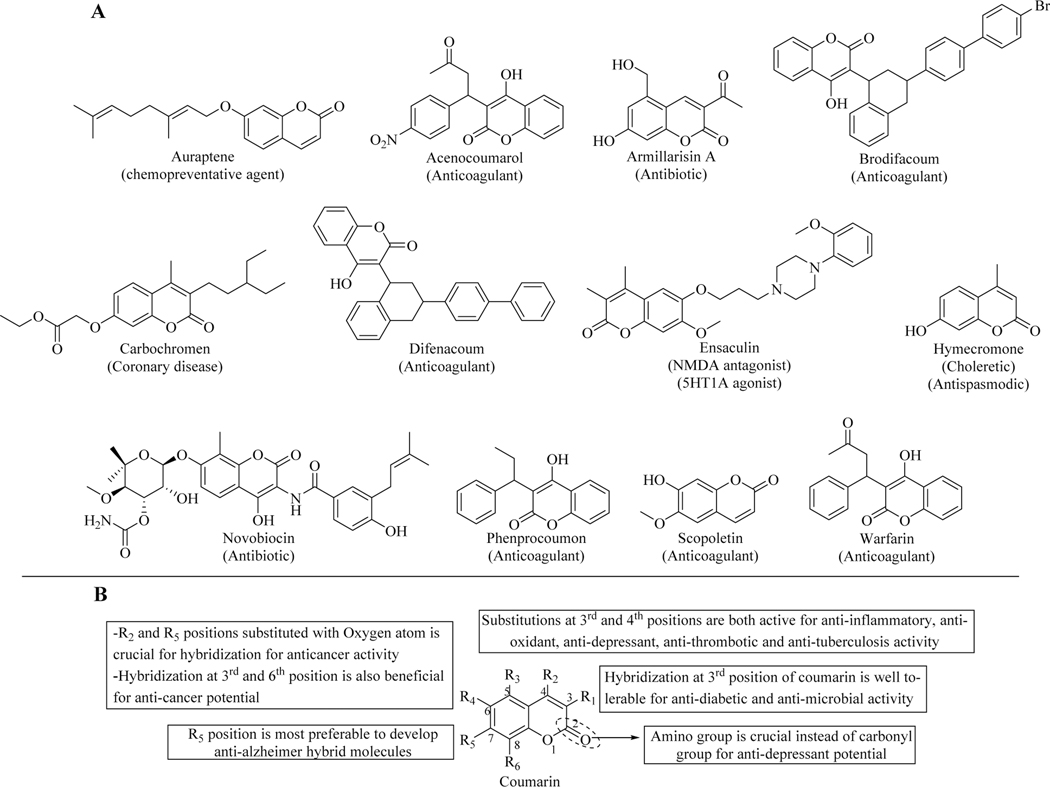
Since prehistoric times, natural products have played a significant role as traditional medicines for the therapeutic treatment of various diseases. Natural products have also played a critical role as lead molecules for drug discovery [1]. The synthetic and semi-synthetic derivatives of natural products have been found to exhibit various biological activities. It has been established that the heterocyclic compounds containing oxygen atom play an important role in designing novel molecular architectures for medical use [2]. Among oxygen-containing heterocyclic compounds, the chemistry of coumarin (2H-chromen-2-one) has been deeply explored owing to its wide range of biological activities. The parent coumarin was first isolated in 1820 by Vogel from Tonka beans [3]. Recently, about 150 species of coumarin have been found in 30 diverse plant families, such as Clusiaceae, Guttiferae, Rutaceae, Oleaceae, Umbelliferae [4]. Its diverse pharmacological activities like anti-bacterial [5], anticoagulant [6], anti-HIV [7,8], antioxidant [9], antitubercular [10], antihypertensive [11], anticonvulsant [12], anti-fungal [13], antihyperglycemic and anticancer [14] brought this class forefront. Some of the coumarin derivatives in clinical use are depicted in Figure 1A [15–17]. Its therapeutic applications depend upon the nature and position of substituents present on the basic nucleus. (Figure 1B)
Figure 1.
(A) Marketed formulations of coumarin derivatives and their clinical uses; (B) General structure-activity relationship of coumarins
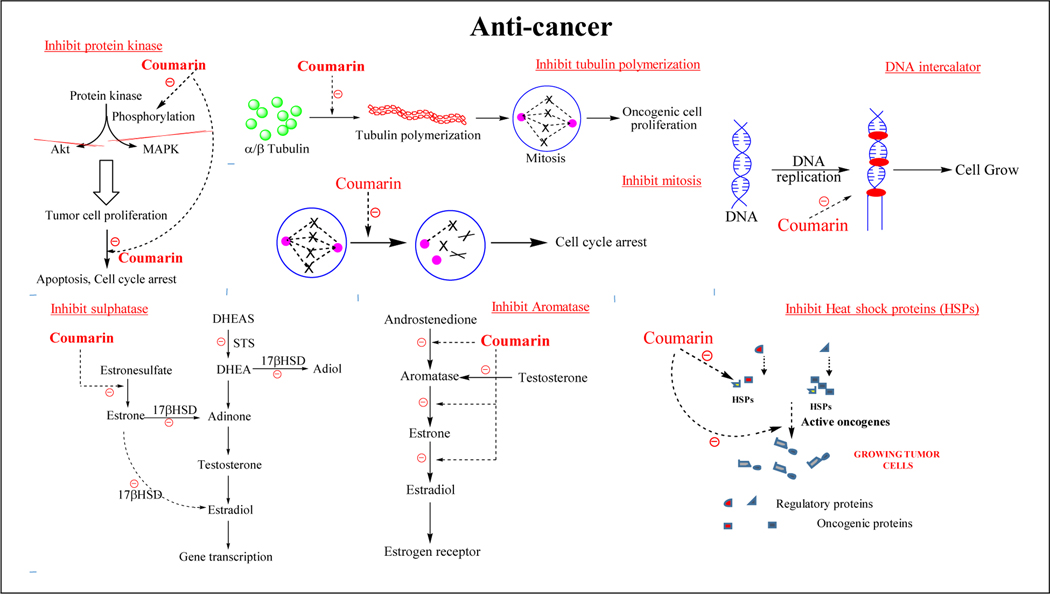
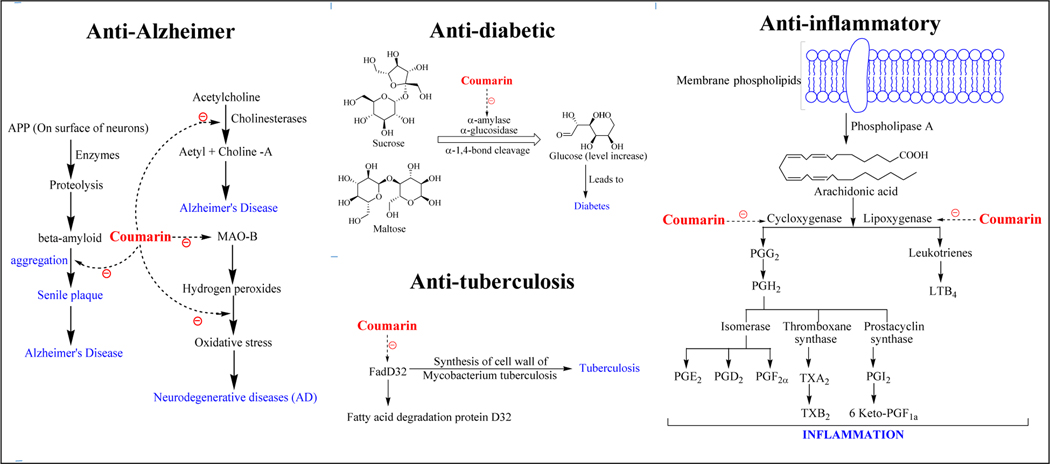
Coumarin derivatives follow a number of complex pathways to target various diseases. The detailed description is shown in Figure 2. To target cancer, coumarin derivatives directly or indirectly inhibit the process of tubulin polymerization, mitosis, and DNA replication by inhibiting various enzymes like protein kinases, sulphatases, aromatase, caspases and heat shock proteins [18–20]. Coumarin derivatives block the catalytic activity of cholinesterase enzymes (acetylcholinesterase and butyrylcholinesterase) thereby retaining the levels of acetylcholine in the brain and act as anti-Alzheimer’s agents. Coumarin derivatives were also developed as monoamine oxidase enzyme type B (MAO-B) inhibitors demonstrating neuroprotective activity [21,22]. α-amylase and α-glucosidase are other primary targeted enzymes of coumarin derivatives, the inhibition of which treats hyperglycemia [23,24]. Coumarin derivatives were also reported as inhibitors of fatty acid degradation protein D32 (FadD32) for the treatment of tuberculosis [25]. Several reports demonstrate the anti-inflammatory potential of these derivatives by targeting cyclooxygenase and lipoxygenase enzymes [26–29].
Figure 2a.
Mechanism of actions of coumarin derivatives against cancer
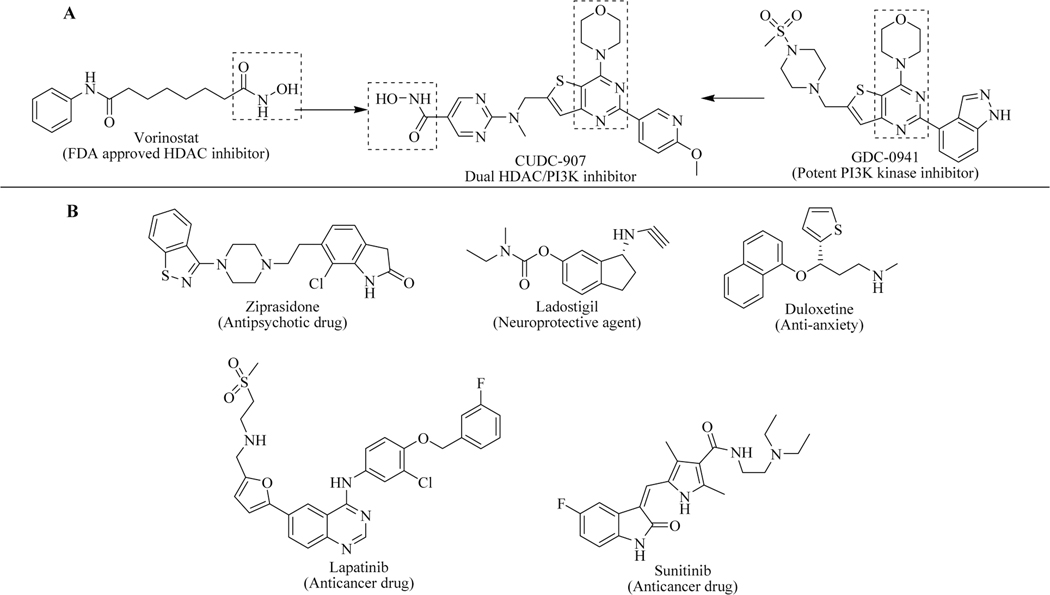
Recent trends in the field of medicinal chemistry suggest a change from the traditional “one molecule – one target – one disease” approach. Most disorders arise from a complex interplay of proteins, which is difficult to treat with single targeting agents. Targeting multiple pathways simultaneously in complex disorders such as cancer, CNS disorders and others is thus a promising approach. Vorinostat, a potent histone deacetylase inhibitor (HDAC inhibitor) was approved by the FDA in 2006 for the treatment of cutaneous T-cell lymphoma. CUDC-907, a hybrid molecule of vorinostat and GDC-0941 (a P13K kinase inhibitor) [30] is more efficient for the treatment of cancer both in in vitro and in vivo models without showing any systemic toxicity and resistance. This dual HDAC/PI3K inhibitor recently has passed the Phase I trial for the treatment of lymphoma or multiple myeloma and advanced solid tumours [31] and entered in Phase II trials (Figure 3A) [32–37]. Ziprasidone, ladostigil and duloxetine for multifactorial CNS diseases, and lapatinib and sunitinib for treatment of cancers are the other examples of prominent hybrid molecules (Figure 3B).
Figure 3.
(A) Hybrid molecule CUDC-907 in clinical trial; (B) Various hybrid molecules as drug candidates
Design and synthesis of these hybrids are typically performed using molecular hybridization techniques by identifying pharmacophoric sub-units in the molecular skeleton of two or more known biologically active derivatives. The recent interest among researchers in the discovery of hybrids that can concomitantly address more than one biological target is increasing day by day [38–40]. Additionally, hybrid molecules provide new dimensions by lowering the risk of drug-drug interactions and minimizing drug resistance [41].
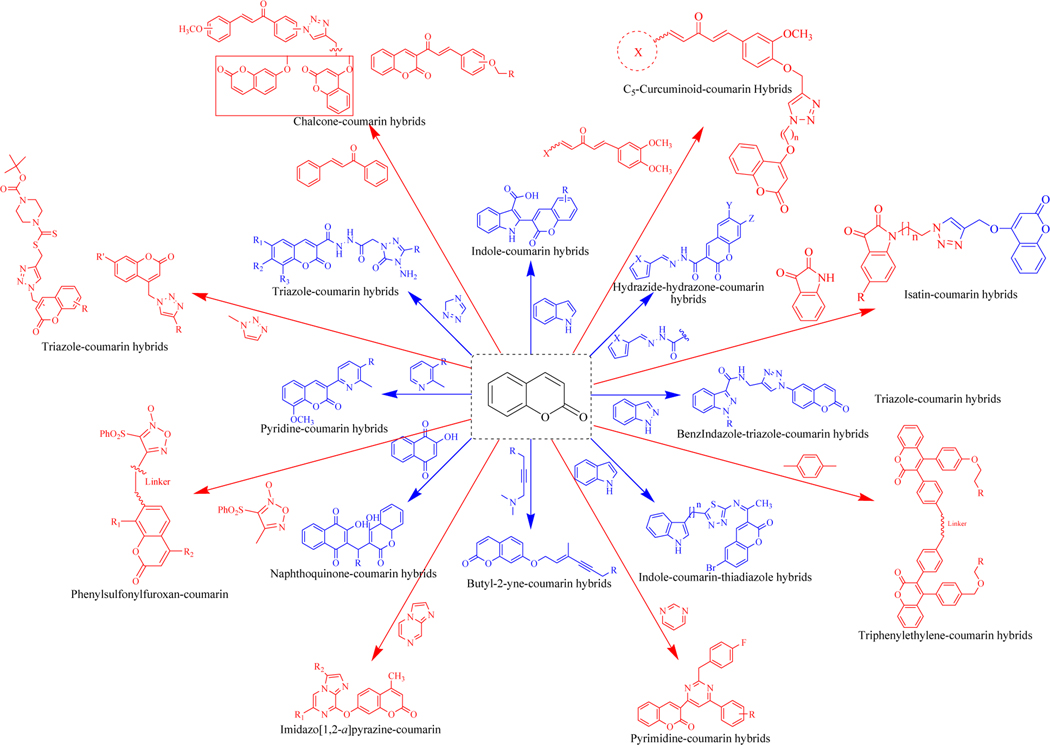
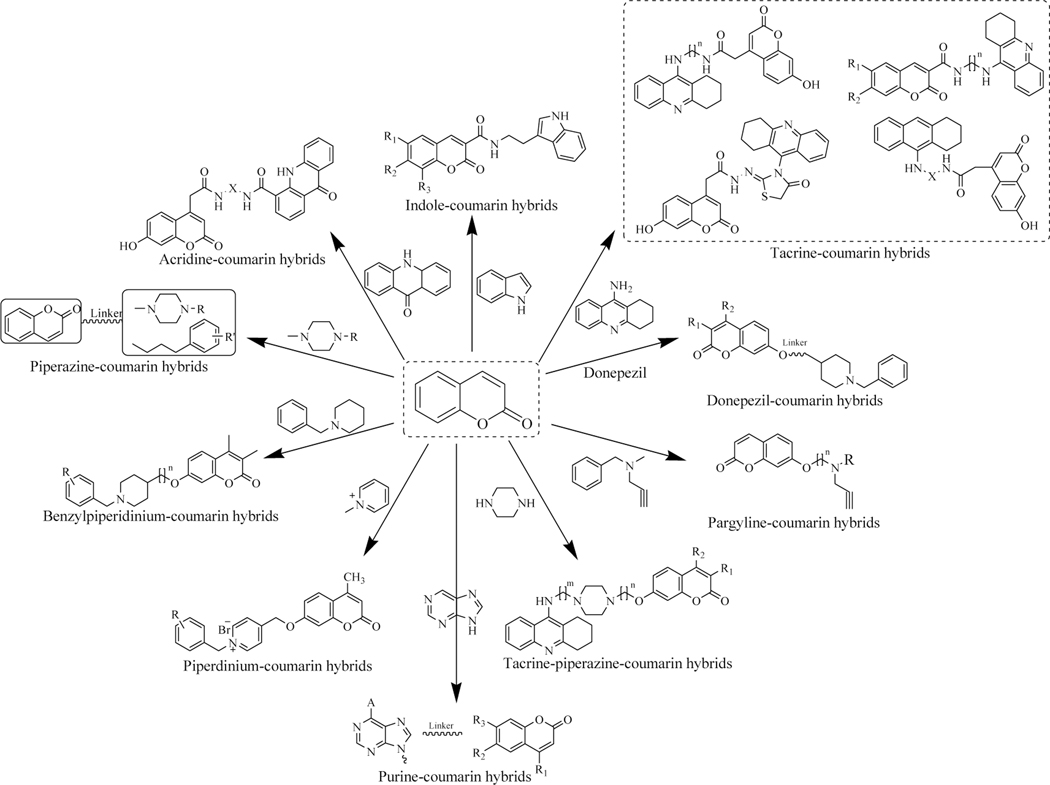
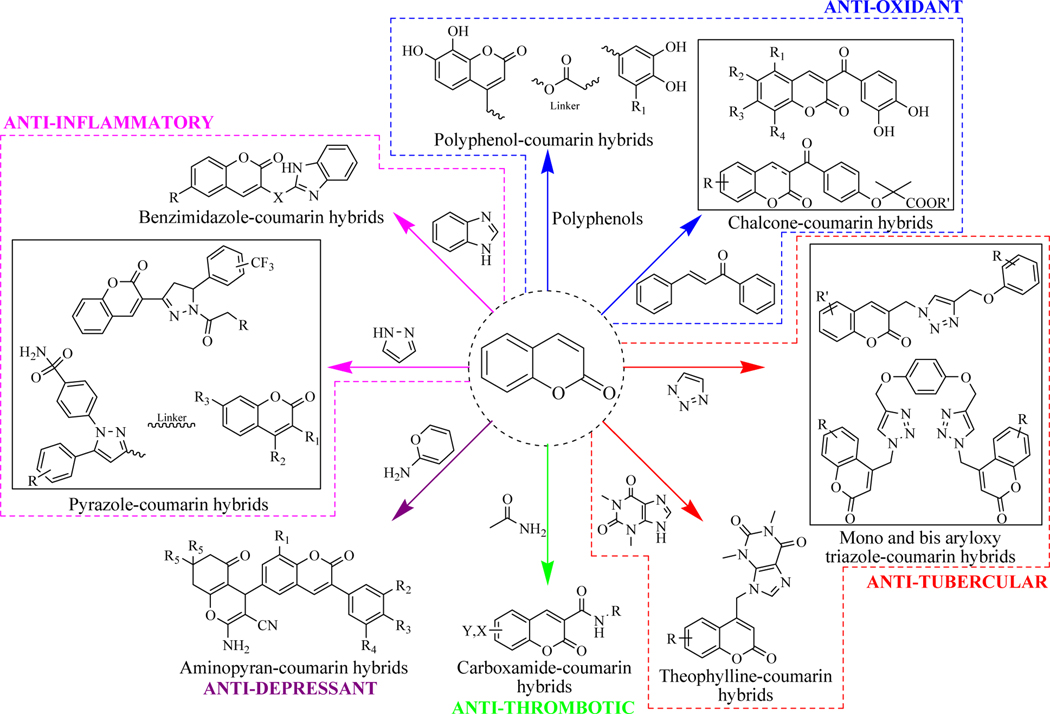
Coumarin-based molecular hybrids are developed by attaching the coumarin to pharmacologically active fragments with or without any tethering agents (Figures 4–7). Efforts have been conducted in the past to review coumarin derived hybrid molecules possessing pharmacological activity. This review sheds light on the recent work (2014 & onwards) conducted using coumarin hybrids active against various diseases. This compilation presents the in vitro as well as in vivo data of reported coumarin-based hybrids. The review also demonstrates some strategies for the design, structure-activity relationships (SAR) along with the mechanistic insights and the key interactions of the designed hybrids within their specific targets by computational studies. This compilation may help medicinal chemists to develop novel coumarin hybrids with varied therapeutic attributes.
Figure 4.
Recently reported coumarin-based anticancer hybrid molecules
Figure 6.
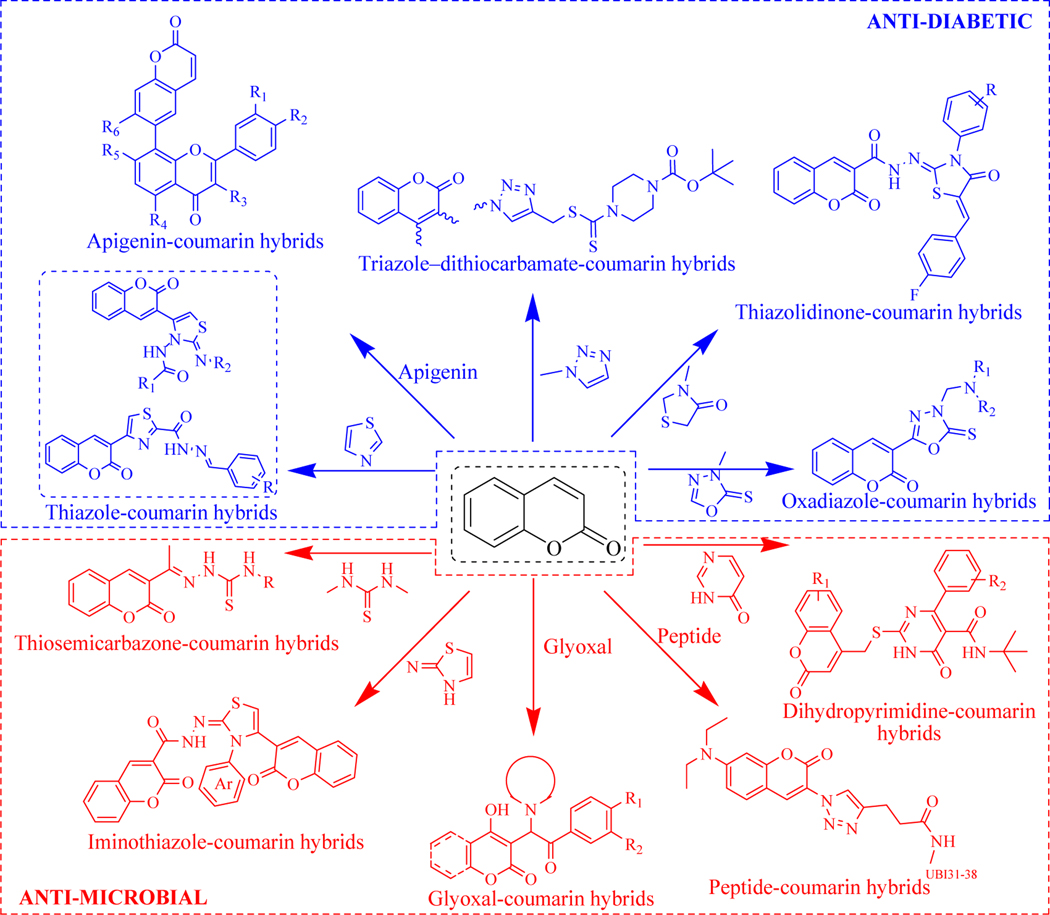
Coumarin based antidiabetic and anti-microbial hybrid molecules
2. Coumarin-based hybrids as anticancer agents:
A major affliction worldwide is a multifactorial disease, cancer, responsible for an estimated 9.6 million deaths in 2018 [42]. Multiple protein and enzymes are dysregulated in this devasting disease-causing difficulties for treatment with one target-based therapeutics. The initiation and progression of this complex disease depends on numerous receptors or signaling pathways indicating that multi-targeted therapy could have prominent efficacy as compared to solo-targeting therapies [43–48]. This can be achieved by using hybridization technique. In continuous efforts to fight against this tremendous problem, various research groups have developed coumarin-based hybrid molecules which are described below.
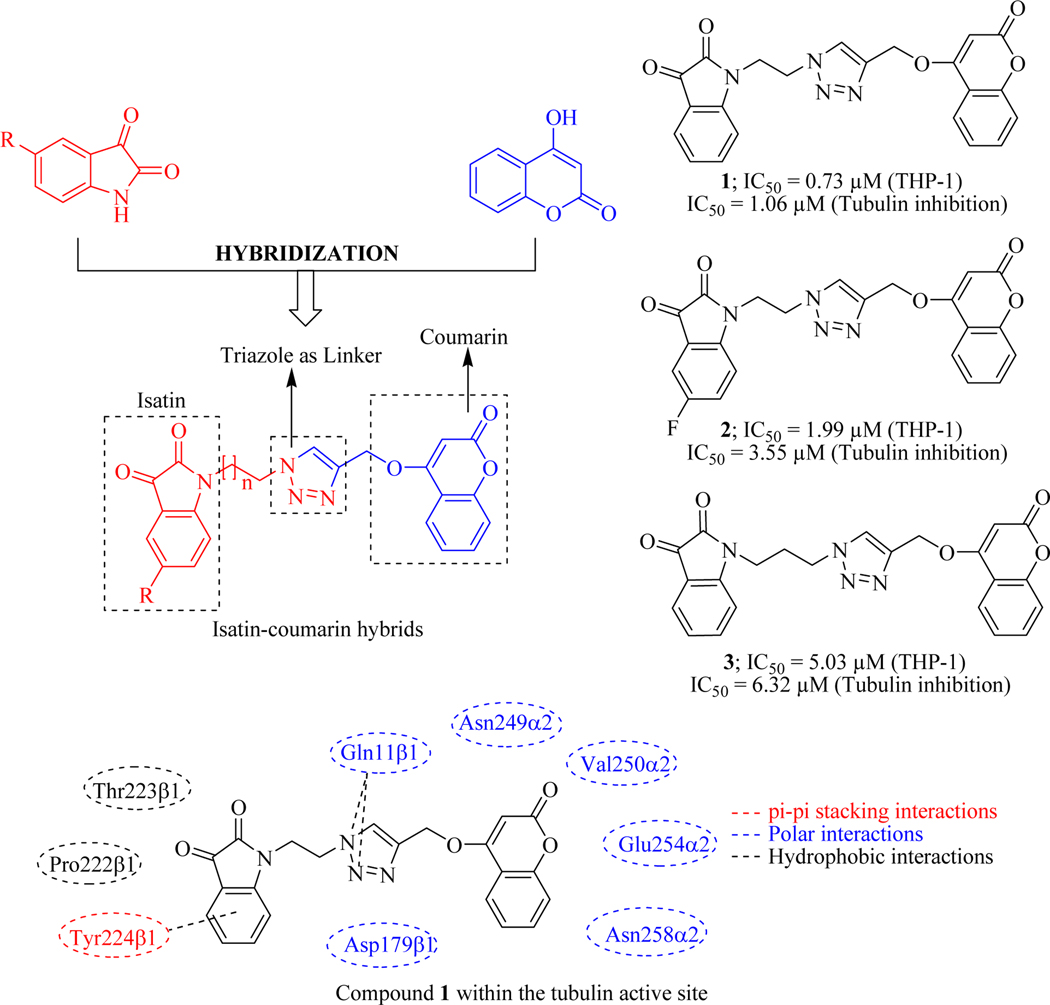
Keeping in view the success of isatin moiety as a potential anticancer agent [49], Singh et al. in 2017 rationally design and synthesized a series of novel isatin-coumarin hybrid molecules by conjoining through triazole ring. All the synthesized compounds were assessed to check their anticancer potential against four human cancer cell lines (Leukemia cancer cells: THP-1, Colon cancer cells: COLO-205, Colon cancer cells: HCT-116 and Prostate cancer cells: PC-3). Compounds were found active against three human cancer cells lines except for colon cancer cells (PC-3). Three compounds 1, 2 and 3 were found most active against leukaemia cancer cells. Careful examination of biological data with the structural features of compounds revealed that the substitution on the isatin moiety and length of the linker between isatin and triazole remarkably influenced the anticancer activity. Unsubstituted isatin (R = H) with two carbon chain (n = 1) was found to be crucial for activity. Additionally, electron withdrawing groups on isatin were well tolerable for anticancer activity as compared to electron donating groups. Because of the anticancer potential of isatin and coumarin derivatives is attributed to tubulin inhibition, tubulin inhibition was evaluated for most potent compounds by using western blotting analysis amongst which compound 1 showed best tubulin inhibition with the IC50 value of 1.06 μM (potent than combretastatin IC50 = 1.2 μM), which was further confirmed through confocal microscopy. It indicates that the anticancer potential of the synthesized hybrids is through tubulin inhibition. Possible molecular mechanism of binding within the tubulin active site of compound 1 was further revealed by using molecular modelling studies which exposed the binding pattern of the compound within the tubulin enzyme (Figure 8) [50].
Figure 8.
Isatin-coumarin hybrids as tubulin inhibitors
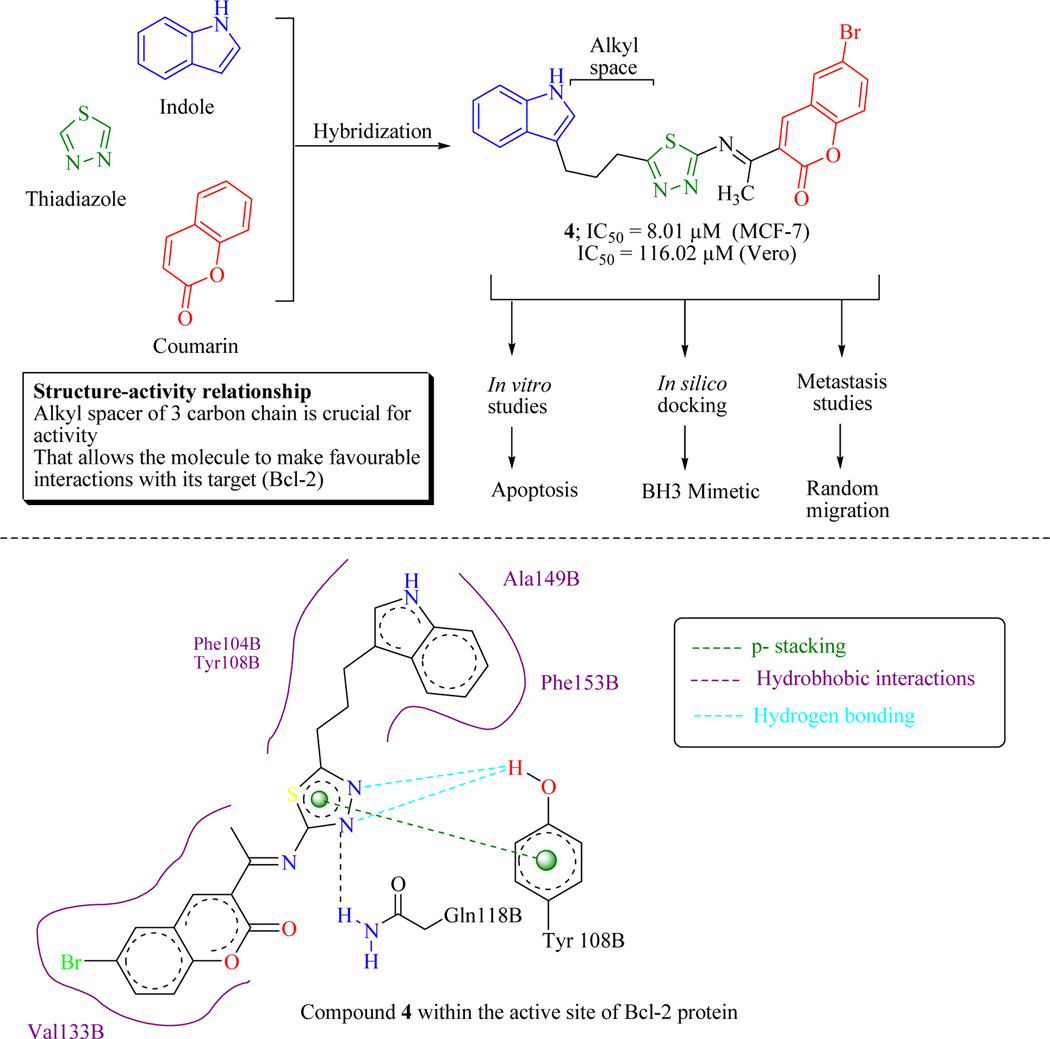
The avoidance of apoptosis is a leading hallmark of cancer and prompting apoptosis in specific cancer cells is one of the convenient and extensively used approaches for the development of anticancer chemotherapeutics. Kamath et al. designed and synthesized indole-coumarin hybrids tethered through thiadiazole moiety with alkyl spacers of varying lengths and evaluated them in vitro as anticancer chemotherapeutics. Hybrid 4 linking via a three methylene spacer specifically inhibit breast adenocarcinoma (MCF-7) cells and Vero cells with the IC50 values of 8.01 and 116.02 μM, respectively both by the induction of apoptosis and by preventing metastasis (confirmed through wound healing assay) in a dose-dependent manner. Bcl-2, an anti-apoptotic protein, is overexpressed in a range of cancers. Computational docking studies revealed that compound 4 has the ability to inhibit the antiapoptotic Bcl-2 protein by acting as a small molecule BH3 mimetic (Figure 9) [51].
Figure 9.
Indole-coumarin hybrids as anticancer agents
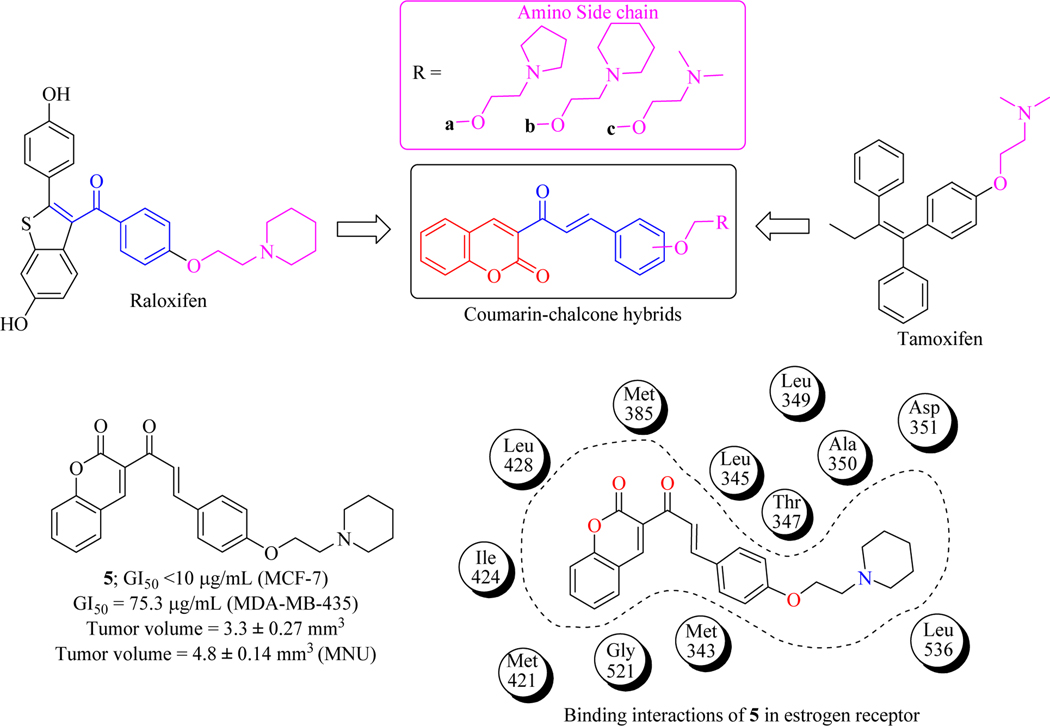
Estrogen receptor alpha plays an important role in breast cancer cell proliferation and various estrogen receptor modulator have been developed, but tumour develops resistance against these agents rapidly. Therefore, Mokale et al. designed and synthesized a series of hybrid molecules containing coumarin and chalcones and were evaluated for anti-proliferative activity both in vitro and in vivo methods. SAR revealed compound 5 as a hit. Administration of 5 (5 mg/kg, P.O.) showed a protective effect on mammary tumorigenesis in N-methyl nitrosourea (MNU) induced carcinoma female rat carcinoma model. Later on, compound 5 was docked and revealed that interactions were similar as that of standard compound (Raloxifene & Tamoxifen) with ER-α receptor and stabilized by various electrostatic interactions, thus concluding that 5 can be taken as lead and could be optimized further for better potency (Figure 10) [52].
Figure 10.
Coumarin-chalcone hybrids as anticancer agents
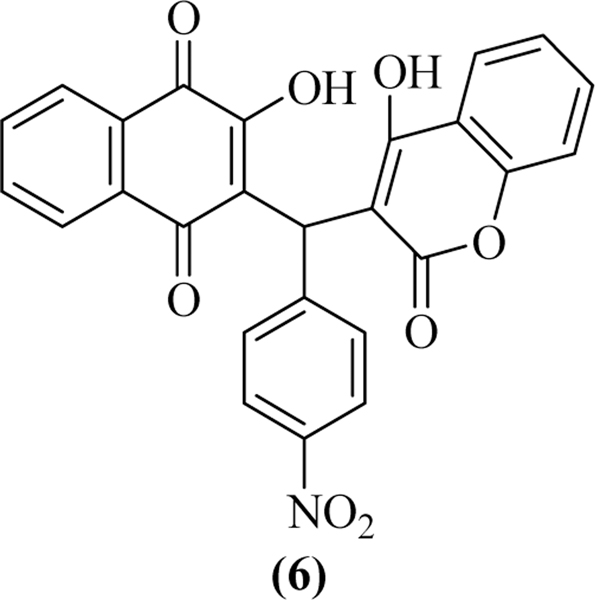
Falcon et al. synthesized naphthoquinone-coumarin molecular hybrids and evaluated their activity as topoisomerase II (Topo-II) inhibitors. Results demonstrate that all the synthesized compounds were capable of inhibiting the hTopoIIα-mediated relaxation of negatively supercoiled pRYG in the micromolar range (between 10 to 30 μM). ATPase inhibitory assay revealed that compound 6 showed higher inhibition among the synthesized compounds. These synthesized conjugates, possessing a chiral carbon and were obtained as racemic mixtures. Therefore, the binding interaction of these molecules into the ATP pocket of Topoisomerase II of both enantiomers was examined with the molecular docking studies. Results of the docking studies revealed that compounds were actively fit into the ATP-binding site and formed cation-π interactions between the aromatic ring of naphthoquinone moiety for S-enantiomers, while in R-enantiomers similar type of interactions were observed with the aromatic ring of coumarin moiety. Additionally, hydrogen bonding and hydrophobic interactions increase their effectiveness as topoisomerase II catalytic inhibitors (Figure 11) [53].
Figure 11.
Naphthoquinone-coumarin hybrid molecule topoisomerase II inhibitor
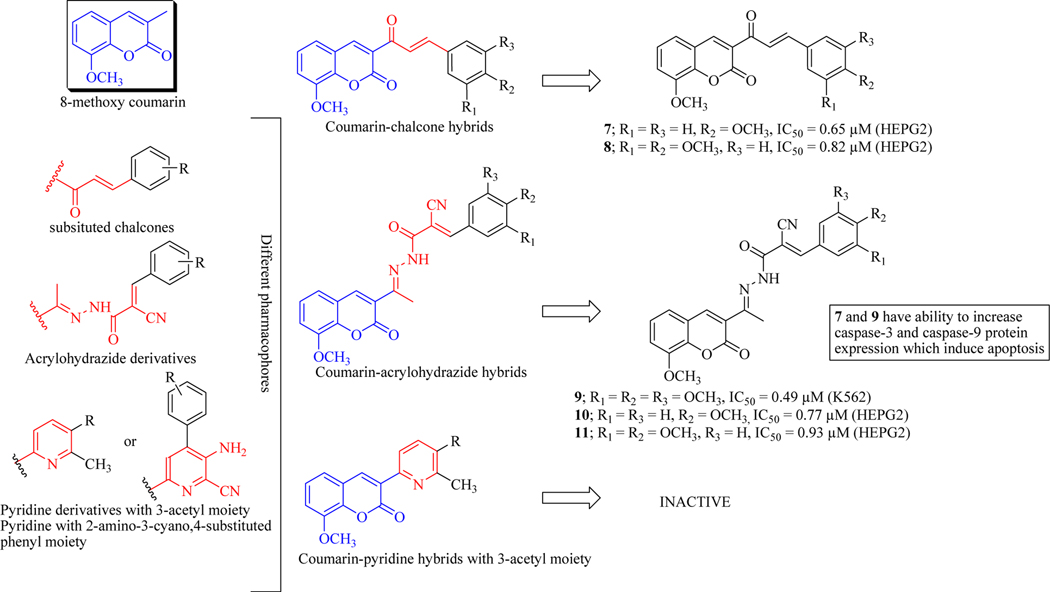
Elshemy et al. designed and synthesized new coumarin hybrids in which 8-methoxy coumarin scaffold was conjoined with various bioactive pharmacophores viz. chalcone, acrylohydrazide and pyridine. The anticancer activity was checked against two cancer cell lines (HEPG2 and K562) and toxicity against fibroblast cells (WI-38). Results indicated that chalcone related hybrids active as anticancer agents and compound 7 and 8 were the most potent in this series with IC50 values 0.65 μM and 0.82 μM, respectively against HEPG2 cells. Coumarin-acrylohydrazides series compounds were active as well; compound 9 demonstrated IC50 of 0.49 μM against leukaemia cancer cell line (K562) while compounds 10 and 11 demonstrated IC50 of 0.77 μM and 0.93 μM, respectively against HEPG2 cells. Coumarin-pyridine hybrids were found inactive while substituted pyridine derivatives showed valuable anticancer results but were lesser potent than former two series of hybrid molecules. SAR revealed that coumarin-chalcone and acrylohydrazide compounds comprising of 4-methoxy (at R2 position) substitution were most potent against HEPG2 cell line (compounds 7 & 10), followed by di-methoxy substitutions (compounds 8 & 11). Whereas, trimethoxy substitution on the phenyl ring (at R1 to R3) was found crucial against leukaemia cancer cells (compound 9). It was demonstrated that all the coumarin hybrids increase caspase-3 and caspase-9 protein levels along with the downregulation of Bcl-2 and up-regulation of Bax protein indicating activation of apoptotic signals, as a consequence of arrest in G2/M phase (Figure 12) [54].
Figure 12.
Coumarin-chalcone/acrylohydrazide/pyridine hybrids as anticancer agents
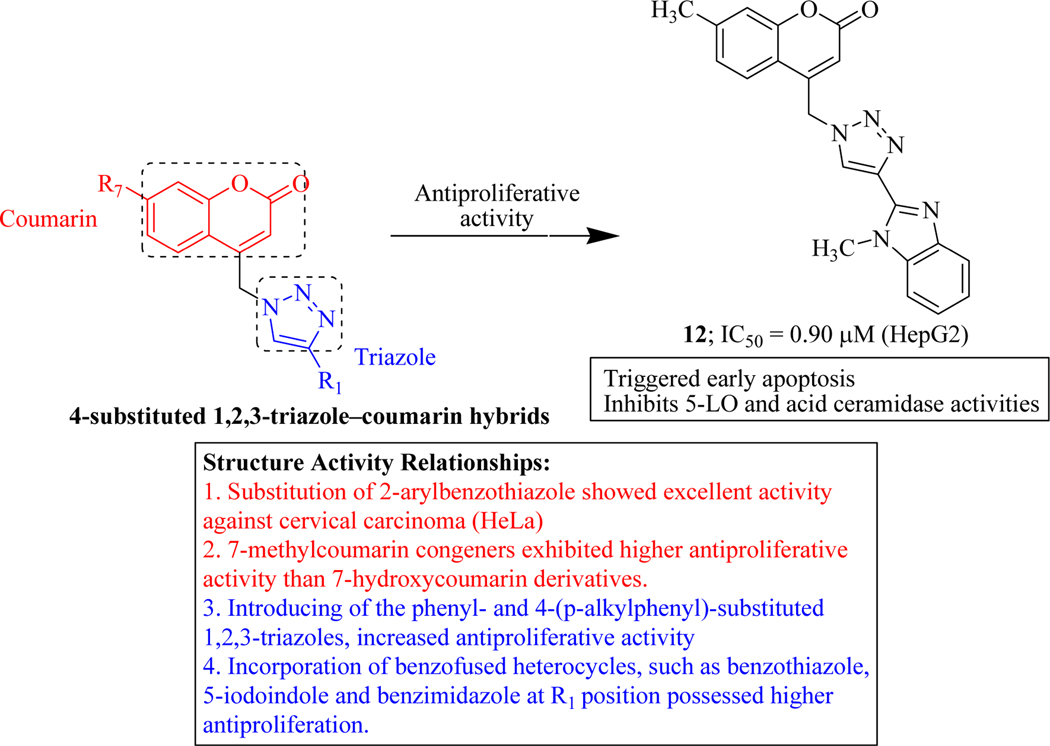
Kraljevic et al. intellectualized the new technique of molecular hybridization by merging 7-hydroxycoumarin (umbelliferone) or its 7-methyl analogue and 1,2,3-triazole entity with the aim to develop anticancer agents. The effect of these synthesized hybrids was also assessed on the regulation of specific lipid metabolic pathways which were facilitated by 5-lipoxygenase (5-LO), sphingosine kinase 1 and acid ceramidase, enzymes that control major aspects of cancer cell behaviour. Compounds containing phenyl- and 4-(p-alkylphenyl)-substituted 1,2,3-triazoles showed activity against lung adenocarcinoma (A549; IC50 = 8.87–32.34 μM) Incorporation of benzo-fused heterocycles such as benzothiazole, 5-iodoindole and benzimidazole, was responsible for the improvement in inhibitory activity. 7-methylcoumarin analogues such as compound 12 exhibited higher cytotoxicity against HepG2 cells (IC50 = 0.90 μM). The Prediction of Activity Spectra for Substances (PASS) revealed that compounds exhibited high cytotoxic potential for leukotriene synthesis with Pa values of and 0.49 [55–58]. Compound 12 in HepG2 cells could be associated with its ability to inhibit 5-LO and acid ceramidase activities that may, in turn, lead to accumulation of pro-apoptotic lipids arachidonic acid and ceramide, respectively leading to apoptosis (Figure 13) [59].
Figure 13.
Triazole-coumarin hybrids as 5-LO and ceramidase inhibitors
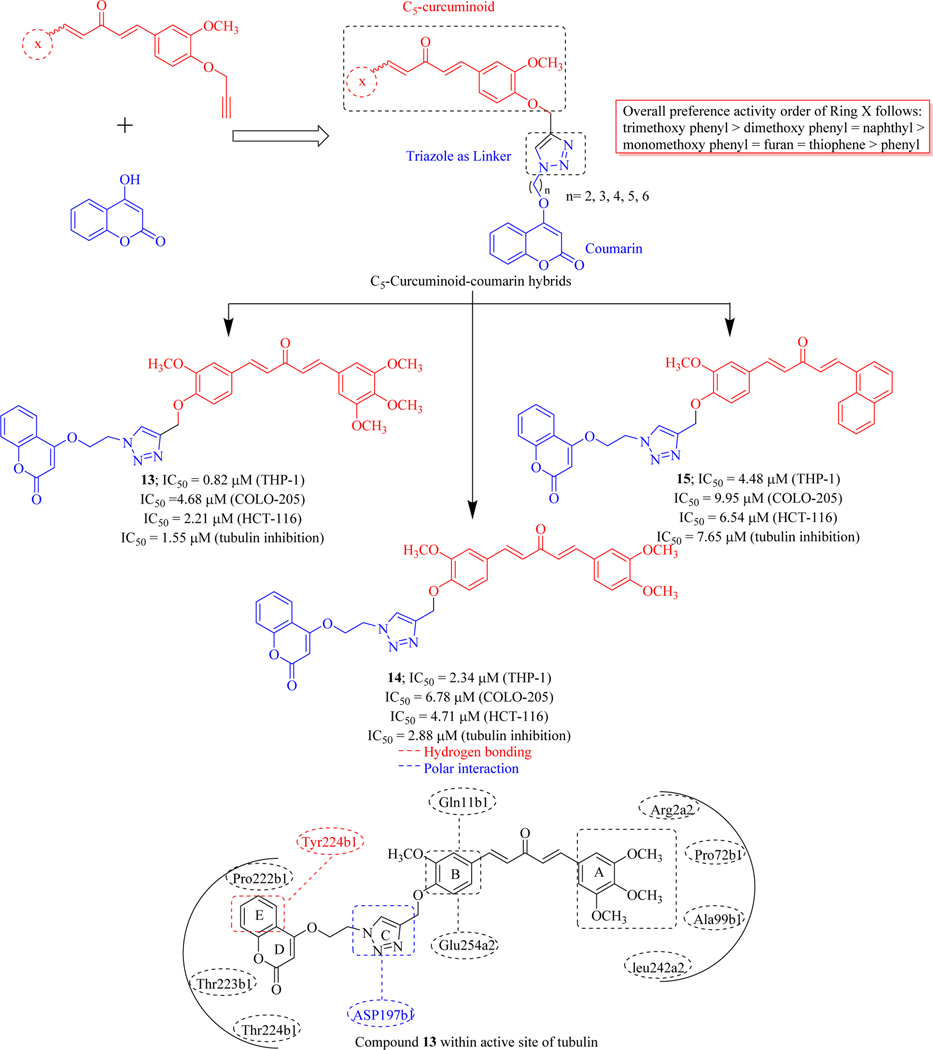
In 2015, Singh et al. Rationally designed and synthesized triazole tethered C5-curcuminoid coumarin bifunctional hybrids by using click chemistry approach and tested for their in vitro cytotoxicity against four human cancer cell lines (THP-1, COLO-205, HCT-116 and PC-3). Results of the inhibitory activity suggest that compounds 13, 14 and 15 exhibited cytotoxicity against THP-1, COLO-205, HCT-116 with IC50 values range from 0.82–4.68 μM, 2.34–6.78 μM, 4.48–9.95 μM respectively. The hybrid molecules were inactive against the prostate cancer cell line (PC-3). SAR revealed that methoxy substituted phenyl ring (Ring A) remarkably enhances the cytotoxic activity (compounds 13 and 14). The spacer length of two carbons between the coumarin and triazole moiety was found crucial for anticancer activity. The placement of naphthyl ring as Ring X acts as a surrogate for dimethoxy substituted phenyl ring. This set of compounds exert their cytotoxic potential through tubulin inhibition and compound 13 was found to be most promising in this series, exhibiting IC50 value of 1.55 μM against tubulin. Molecular docking studies compound 13 indicated that it completely blocks the catalytic assembly of tubulin by adhering at the interface of β1/α2 subunits (Figure 14) [60, 61].
Figure 14.
C5-curcuminoid-coumarin bifunctional hybrids as tubulin inhibitors
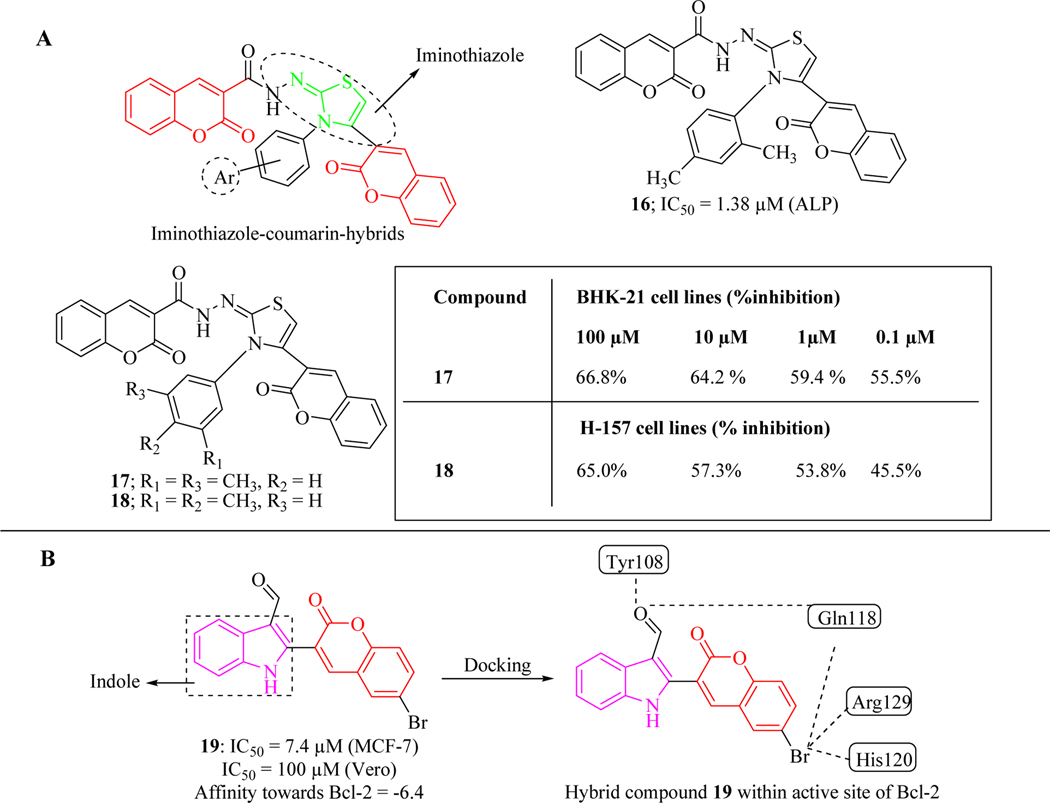
Keeping in view the imperative role of iminothiazole in various biological activities [62], Ibrar et al. reported a new series of bis-coumarin–iminothiazole hybrid molecules and evaluated for alkaline phosphatase inhibition and anticancer activity. Alkaline phosphatase inhibition assay revealed that 16 was the most potent compound among the series with an IC50 value of 1.38 μM. Synthesized series also evaluated against kidney fibroblast (BHK-21) and lung carcinoma (H-157) cell lines at four different concentrations (100, 10, 1.0 and 0.1 μM). Results showed that compounds 17 and 18 were the most active analogue in this series. All synthesized compounds were found to follow the Lipinski Rule of five and Veber’s Ro3. Various types of the binding interactions of compound 18 within the active site of the alkaline phosphatase enzyme were streamlined by using docking studies. The rigid and bulky structure favoured maximum exposure of the ligand to various amino acids of the active site (Figure 15A) [63].
Figure 15.
(A) bis-coumarin–iminothiazole hybrids as anticancer agents; (B) indole-coumarin hybrid molecules as anticancer agents
Kamath et al. designed and synthesized new indole-coumarin hybrid molecules. The hit compound, 19 exhibited inhibitory activity against MCF-7 and Vero cancer cell lines with the IC50 values of 7.4 μM and 100 μM by arresting the cell cycle at the G2/M phase. In order to understand the other possible mechanism to act as anticancer agent, binding behaviour of 19 was analyzed by using molecular docking studies within the catalytic active site of Bcl-2 protein (Figure 15B) [64].
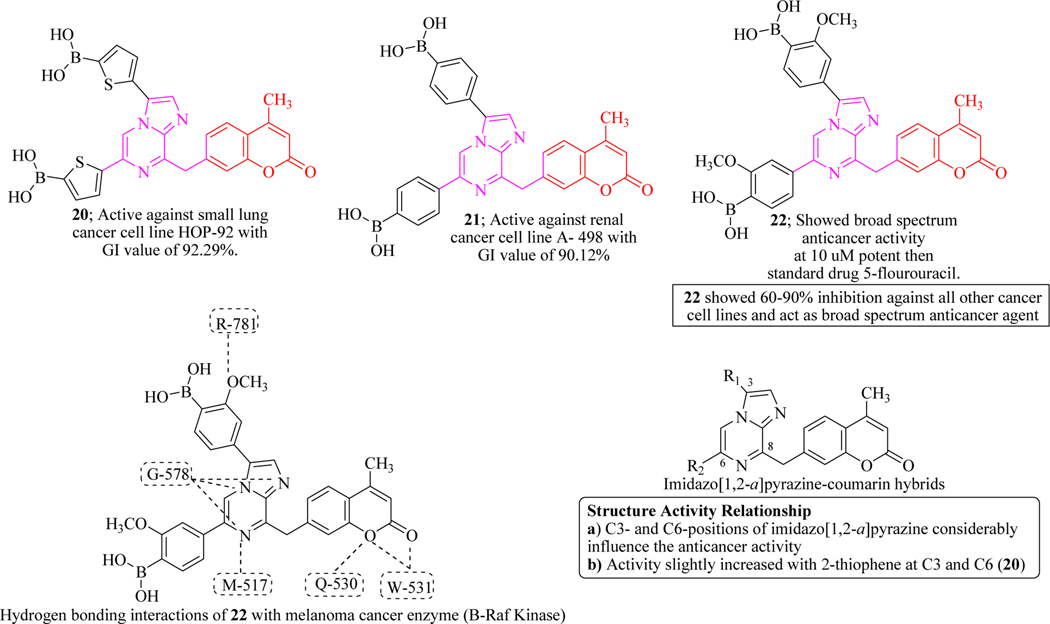
Keeping in mind the various therapeutic potential of heterocyclic moieties in pharmaceuticals, Goel et al. synthesized a new series of boronic acid-based imidazo[1,2-a]pyrazine-coumarin hybrids and screened for their in vitro anticancer activities against a panel of 60 human cancer cell lines (NCI-60). At 10 μM concentration, compounds 20, 21 and 22 were found excellent molecules against small lung cancer cell line. SAR indicated that C3- and C6-positions of imidazo[1,2-a]pyrazine considerably influenced the anticancer activity. The anticancer activity slightly increased with the substitution of 2-thiophene-3-boronic acid rings at C3 and C6 positions, as demonstrated by compound 20. Compound 22 containing a para boronic acid showed broad-spectrum anticancer activity with remarkable selectivity against healthy cells (Figure 16) [65].
Figure 16.
Imidazo[1,2-a]pyrazine-coumarin hybrids as B-Raf Kinase inhibitors
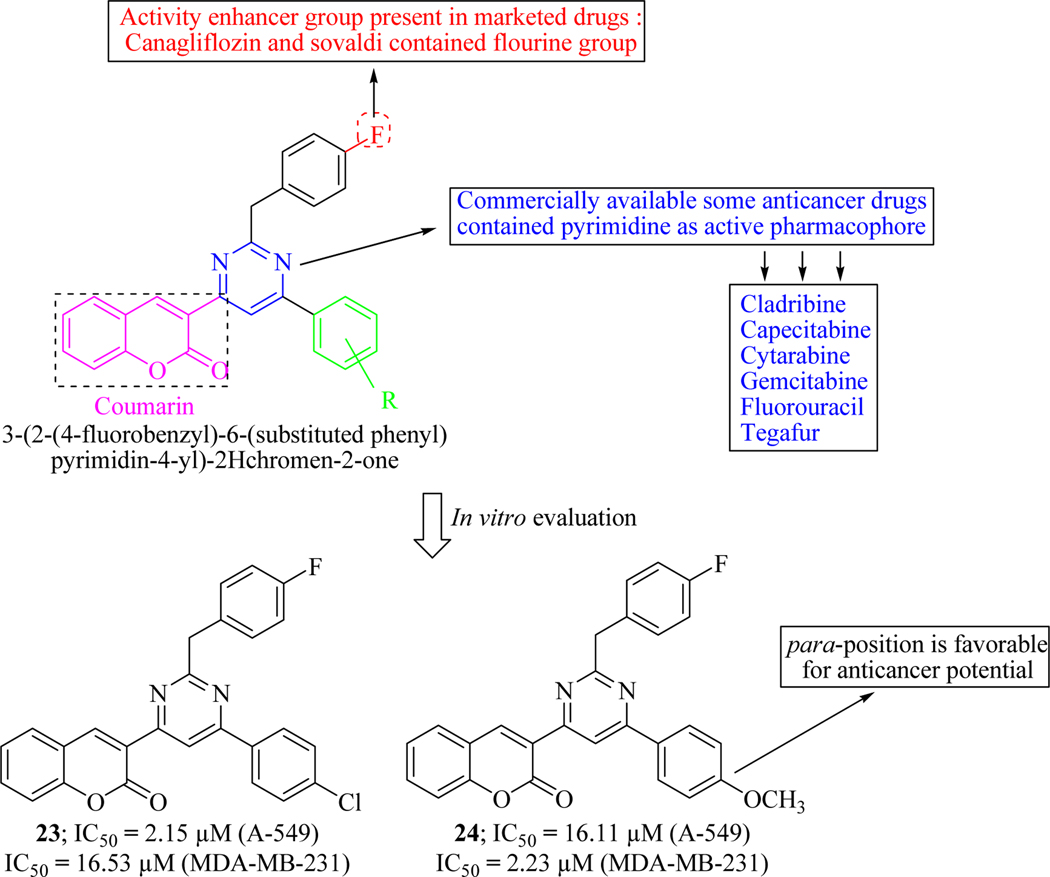
Inspired form various biological attributes of fluorine-containing organic compounds [66] and pyrimidine-based drug compounds [67], Hosamani et al. synthesized coumarin-pyrimidine based fluorinated compounds under microwave-irradiation in high yields and examined against two human cancer cell lines viz. A-549 (human lung carcinoma) and MDA-MB-231 (human adenocarcinoma mammary gland). In vitro results concluded that para substitution enhanced activity to deliver hits 23 and 24. SAR revealed that the halogenated compounds (at R) showed higher anticancer activity. It was also noteworthy that both the compounds cleave the DNA completely which could be a possible mechanism for their anticancer activity (Figure 17) [68].
Figure 17.
Coumarin-pyrimidine hybrids as anticancer agents
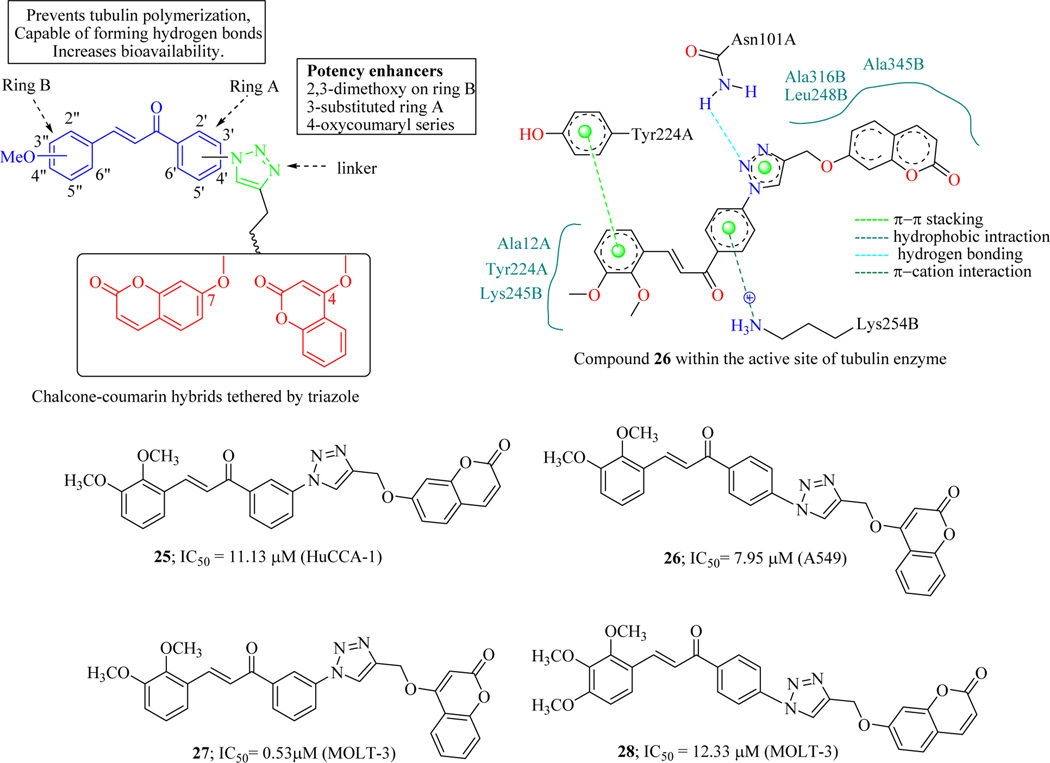
Pingaew et al. synthesized eleven novel chalcone-coumarin hybrids linked via 1,2,3-triazole using azide/alkyne dipolar cycloaddition reactions (click chemistry). Hybrid molecules were evaluated for their cytotoxic activity against four cancer cell lines (human bile duct epithelial cells: HuCCA-1, Hepatic cancer cells: HepG2, Lung cancer cells: A549 and Lymphoblastic T-cells: MOLT-3). The hybrid 27 was shown to be the most potent in this series (IC50 = 4.26 μM, HepG2), but toxic towards Vero cells while compound 26 displayed IC50 = 8.18 μM (HepG2) and was non-toxic to Vero cells. SAR investigation of the compounds revealed that the cytotoxicity depends on the substitution pattern of substituents on both rings A and B as well as coumaryl moieties. Compound 26 simultaneously was also found to bind with both α and β subunits of the tubulin, the probable mechanism for the anticancer potential of these hybrid molecules. This study provides novel chalcone-coumarin hybrids as potential lead molecules for further structural optimization (Figure 18) [69].
Figure 18.
Chalcone-coumarin hybrids linked via 1,2,3-triazole as tubulin inhibitors
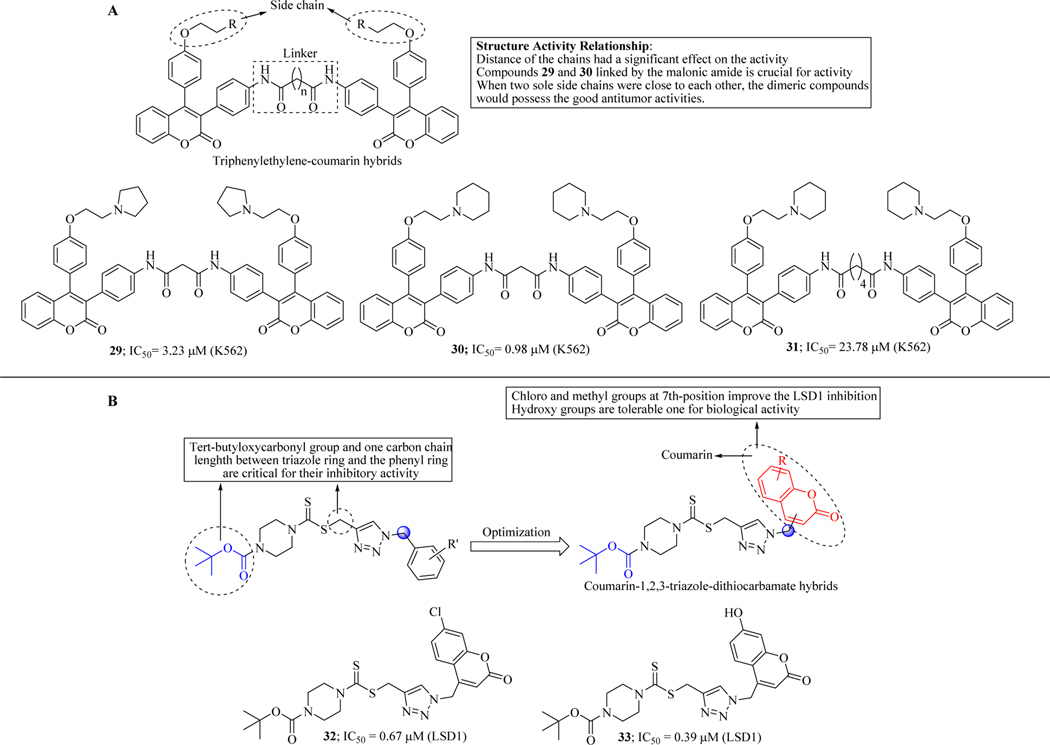
Tan et al. Rationally designed and synthesized novel dimers of triphenylethylene-coumarin hybrids with an amide side chain. The dimeric compounds 29 and 30 linked by the malonic acid, showed a broad-spectrum and good anti-proliferative activity against four tumour cell lines (MCF-7, A549, K562 and Hela) with approximate IC50 values of 10 μM, and low cytotoxicity in osteoblast. SARs suggested that the length of the linker (dicarboxylic acids) had profound effects on their anti-proliferative activities and DNA binding properties. UV–vis, fluorescence, and circular dichroism (CD) spectroscopies and thermal denaturation exhibited that compounds 30 and 31 had significant interactions with circulating tumor-DNA(Ct-DNA) by the intercalative mode of binding. Compound 30, possessing the malonic amide, had the highest binding constant, which suggest that the short length of the linker (n = 1) is beneficial to bind with DNA (Figure 19A) [70].
Figure 19.
(A) Triphenylethylene-coumarin hybrids active against leukemic cancer cell line; (B) Coumarin-1,2,3-triazole dithiocarbamate hybrids as LSD1 inhibitors
Ye et al., inspired by the significant activities of coumarins against lysine-specific demethylase enzymes (LSD1), designed and synthesized two series of coumarin–1,2,3-triazole dithiocarbamate hybrids and evaluated their in vitro inhibitory activity. LSD1 is a member of the monoamine oxidase (MAO) family, which shows homology with MAO-A and B. It has been reported that LSD 1 removes the methyl group from mono-, di-methylated Lys4 and Lys9 of histone H3 (H3K4 and H3K9) through flavin adenine dinucleotide (FAD) dependent enzymatic oxidation [71]. LSD1 also demethylates p53, DNA methyltransferase, E2F transcription factor 1 (E2F1) and regulate their cellular functions, therefore downregulation of LSD1 can inhibit cancer progression [72]. Initial work carried out by Liu and co-workers synthesized and evaluated a series of 1,2,3-triazole–dithiocarbamate hybrids for anticancer potential (Figure 19B). The compounds showed excellent activity and it has been reported that tert-butyloxycarbonyl group attached to the piperazine ring and only one carbon length between the phenyl ring and triazole ring were crucial for their anticancer activity (Figure 19B). Keeping intact the previous molecular architecture, coumarin moiety was introduced to develop a new series of hybrid molecules. Several compounds displayed activity against LSD1 in micromolar or submicromolar range. Compounds 32 and 33 showed potent and reversible inhibition against LSD1 with the IC50 values of 0.67 and 0.39 μM, respectively, without showing inhibition against MAO A and B. SAR studies revealed that substituents on coumarin moiety greatly influenced the LSD1 inhibitory activity. Substituted 7th position of coumarin moiety increased activity. The further mechanistic investigation revealed that compound upregulated the expression of H3K4me1, H3K4me2 and H3K9me2 (Figure 19B) [73].
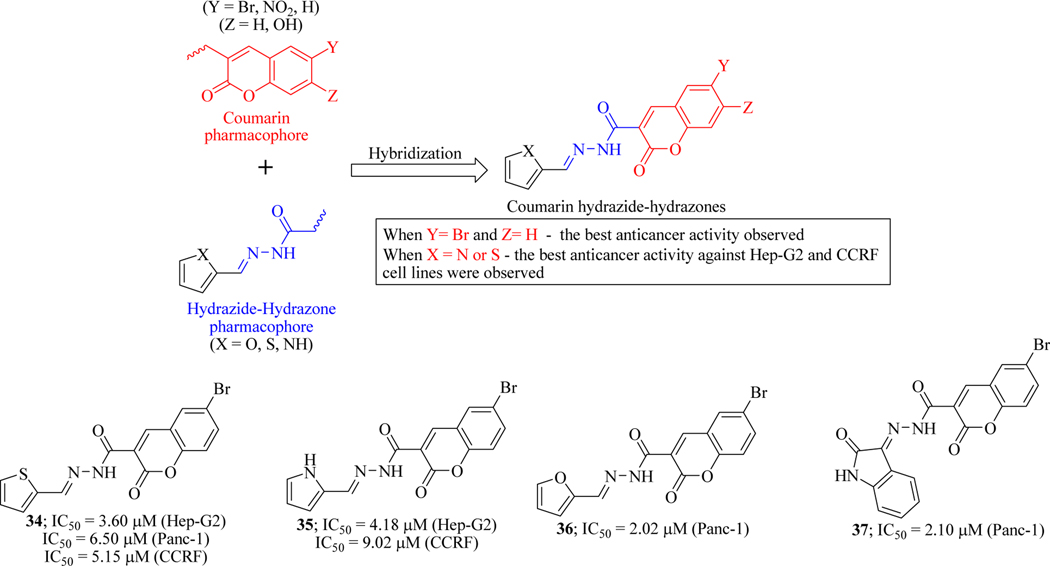
Coumarin derivatives suppress cancer cell proliferation by arresting the cell cycle in G0/G1, G2/M phases [74]. Literature survey revealed that the hydrazide-hydrazone moiety plays a role as an antitumor agent due to their ability to inhibit dihydrofolate reductase (DHFR) enzyme [75,76]. It was also reported that furan, thiophene, pyrrole and isatin derivatives possess biological activity [76]. Based on these findings, novel hybrid compounds having coumarin hydrazide-hydrazone backbone were designed and synthesized by Nasr and coworkers. The library was evaluated against human drug-resistant pancreatic carcinoma (Panc-1) cells and drug-sensitive (hepatic carcinoma; Hep-G2 and leukaemia; CCRF) cell lines. Bromocoumarin based hybrids (34-37) were found to be the most active antitumor agent against drug-resistant pancreatic carcinoma cells among this series of analogues. To get further insights to understand the mechanism of compounds to act as anticancer agents, cell cycle analysis and gene regulation was analyzed. Mechanistically, hybrid molecules activate caspases 3/7 and induced the expression of cell cycle arrest at the G2/M phase in resistant Panc-1 cells. Moreover, microarray-based gene expression analysis indicated the up-regulation of CDKN1A, DDIT4, GDF-15 and down-regulation of CDC2, CDC20, CDK2 genes, genes involved in apoptosis, cell cycle regulation and tumour suppression (Figure 20) [77].
Figure 20.
Coumarin-hydrazide-hydrazone hybrids as anticancer agents
3. Coumarin-based hybrids as anti-Alzheimer’s agents:
There are four major pathophysiological causes of Alzheimer’s disease: 1) aggregation of β-amyloids, 2) formation of tau proteins, 3) degradation of neurotransmitter (Acetylcholine) in the brain and 4) oxidative stress. The proteolysis of amyloid precursor proteins by β-secretase and γ-secretase generates β-amyloids (Aβ). The elevated levels of acetylcholinesterase (AChE) enzyme which breakdowns acetylcholine in the brain is also responsible for aggravation of Alzheimer’s disease [78–81]. Being a complex disorder, a multi-target directed ligand (MTDL) may be beneficial in reducing the rate of progression of this disease.
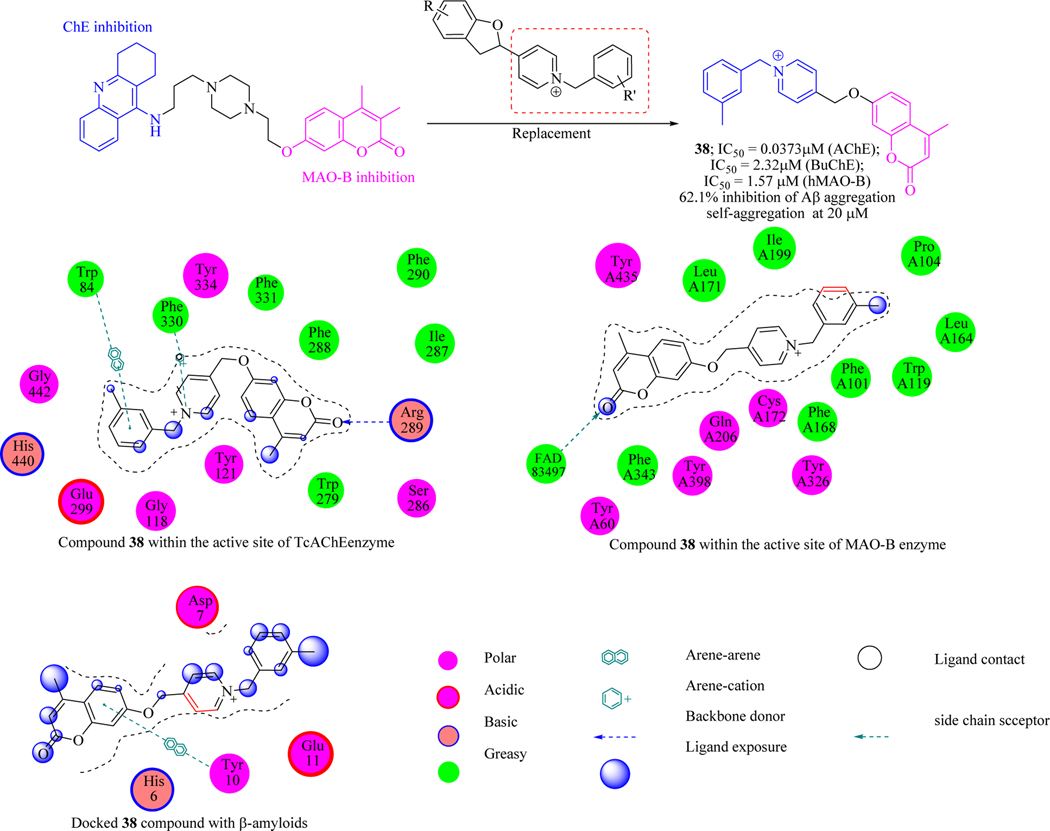
Lan et al. designed and synthesized a novel series of coumarin-N-benzyl pyridinium hybrids. The inhibition of compounds against AChE, MAO-B and β-amyloid aggregate ion was checked. Results concluded that all the compounds effectively inhibited the AChE and MAO-B enzymes and identified lead molecule 38 which displayed micromolar to submicromolar activity. SAR revealed that the AChE inhibitory activity greatly influenced by different substitutions on the benzyl group. The electron donating groups (like –CH3) on the benzyl ring is crucial for AChE inhibition. Enzyme kinetic studies and molecular docking validated that compound 38 was a mixed-type inhibitor, binding to both catalytic active site (CAS) and peripheral active site (PAS) of AChE & blocking the active site of MAO-B. In addition, 38 compound also penetrated the BBB, an essential requirement for a molecule targeting the central nervous system (Figure 21) [82].
Figure 21.
Coumarin-N-benzylpyridinium hybrids as anti-Alzheimer’s agents
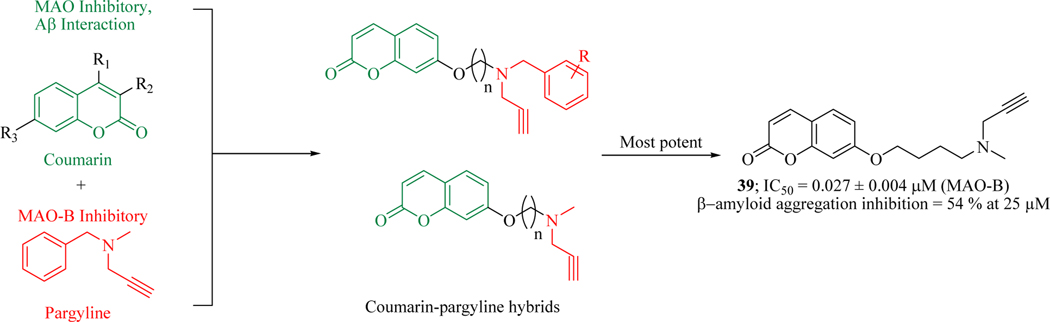
Pargyline is an irreversible selective MAO-B inhibitor and plays an important role in neuroprotection [83]. Based on these findings novel coumarin-pargyline hybrids were designed and evaluated to inhibit AChE, MAO-B and β-amyloid aggregation. The lead from this project, compound 39 exhibited inhibitory activities against monoaminoxidase with an IC50 value of 3.275 μM and 0.027 μM against MAO-A and MAO-B, respectively. About fifty percent reduction in β-amyloid aggregation was observed with compound 39 at 25 μM. SAR indicated that the nature and locality in the phenoxy ring of synthesized hybrids significantly influenced the β-amyloid aggregation inhibition. Hybrids with three to four carbon chain length were found most active against self-induced β-amyloid aggregation. PAMPA-assay (parallel artificial membrane permeation assay) revealed that compound 39 would be excellent molecule to cross the blood-brain barrier with no significant toxicity which demonstrates that compound 39 was an effective and promising candidate for AD therapy. Computational studies were performed to predict their binding modes in the active pocket of hMAO-B and illustrate that the size of compound 39 with a four-carbon spacer is better suited to completely fit the binding cavity compared to one & two carbon spacers (Figure 22) [84].
Figure 22.
Coumarin-pargyline hybrids as MAO-B inhibitors that prevent amyloid aggregation
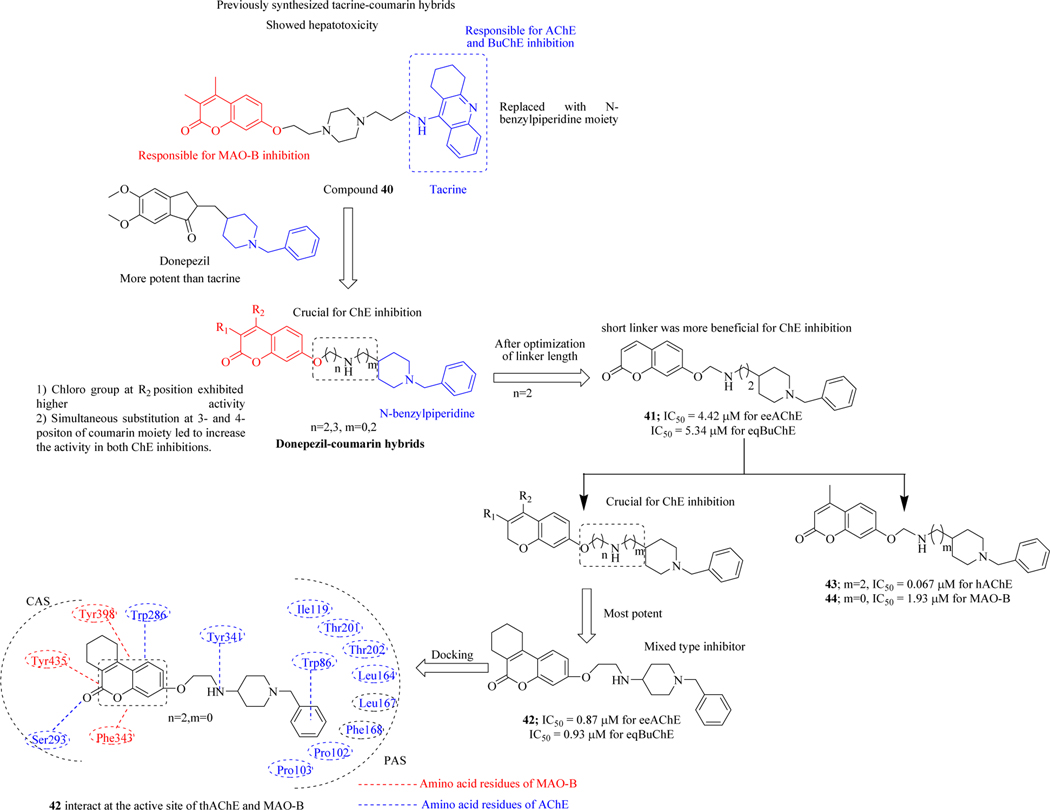
Previously Xie et al. synthesized series of multi-target compounds using tacrine (well-known ChE inhibitor) and -coumarin (MAO inhibitor) scaffolds [85], and identified compound 40 which showed inhibitory activity against AChE and BuChE and also exhibited selective inhibition for MAO-B. But compound 40 suffered from major drawback-severe hepatotoxicity due to the presence of tacrine moiety which hindered its further development. Based on these findings, Xie et al. designed and synthesized a new series of donepezil-coumarin hybrids as multi-target compounds for the treatment of AD. For the synthesis of these hybrids, tacrine moiety was replaced with an N-benzylpiperidine moiety of donepezil because donepezil was more potent and showed no hepatotoxicity [86–88] compared to tacrine. The N-benzylpiperidine moiety exhibited the same mechanism of inhibiting ChEs through binding to the catalytic anionic site (CAS) of ChEs whereas the alkyl chain between coumarin and N-benzylpiperidine moiety was also retained in the donepezil-coumarin hybrids. After the identification of optimal length of the linker compounds were synthesized and were subjected to their biological evaluation against eeAChE (from electric eel) and eqBuChE (from equine serum). Tacrine and Donepezil were used as reference compounds. Results of the inhibitory activity indicated that compound 41 exhibited IC50 = 4.42 μM against eeAChE and IC50 = 5.34 μM against eqBuChE. Among the series of 3,4-cyclohexane-fused coumarin, compound 42 showed potent inhibitory activity with the IC50 value of 0.87 μM and 0.93 μM against eeAChE and eqBuChE, respectively. The influence of inhibition was further assessed by replacing oxygen atom linked at 7th-position of coumarin moiety with carbon atom and the results showed that compound 43 having a two-carbon linker (m = 2) between amino group and N-benzyl piperidine moiety was more potent against human ChEs (IC50 = 0.067 μM for hAChE and IC50 = 3.45 μM for hBuChE). The investigational compound 44 demonstrated IC50 of 1.93 μM against MAO-B. The kinetic studies revealed that compound 42 was a mixed type inhibitor of both AChE and MAO-B. Various types of binding interactions of compound 42 within the active sites of AChE and MAO-B were also rationalized by using molecular modelling studies which revealed that the compound was stabilized within the active sites by various electrostatic interactions that were responsible for the inhibition of the enzyme. PAMPA-assay confirmed the BBB penetrability of compound 42 and it was noteworthy that no toxicity was observed with 42 on SH-SY5Y neuroblastoma cells when tested via in vitro toxicity assay (Figure 23) [89].
Figure 23.
Donepezil-coumarin hybrids as cholinesterase and MAO-B inhibitors
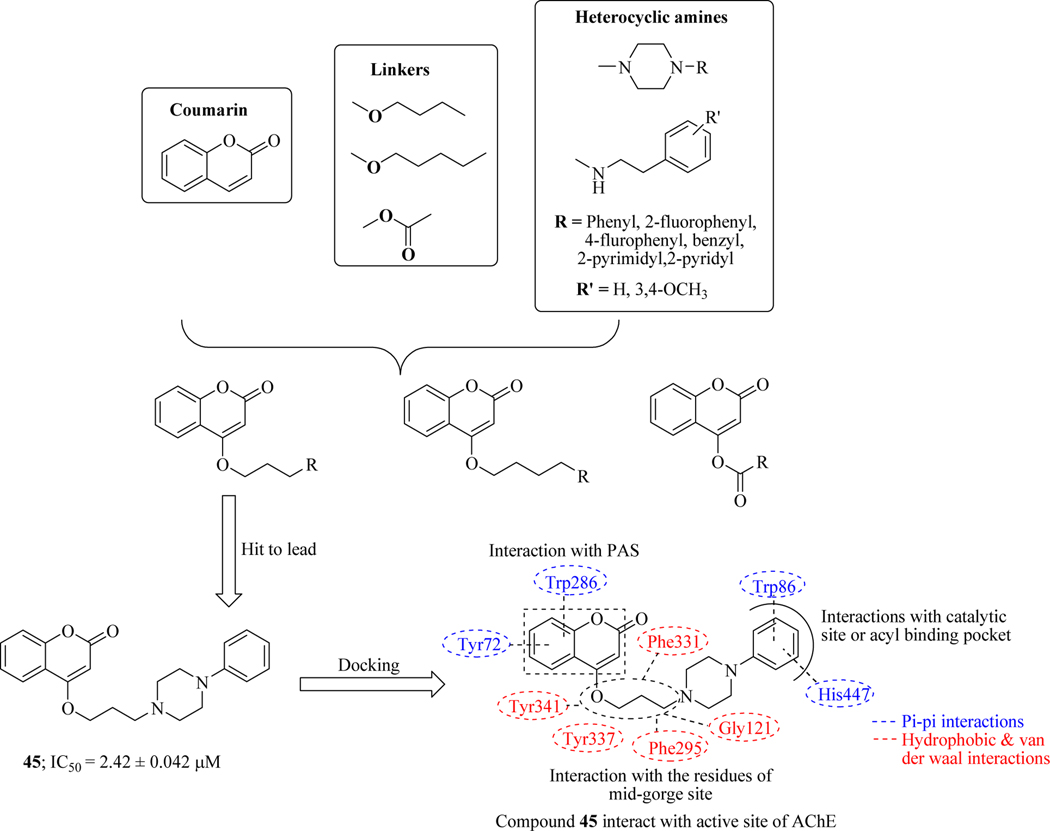
Singla et al. synthesized series of novel hybrids by linking coumarin moiety with various substituted heterocyclic amines with the help of an appropriate spacer. The library was tested for their cognition improving ability, AChE inhibitory potency and antioxidant activity. The influence of these synthesized hybrids on biochemical markers of oxidative stress- lipid peroxidation, superoxide dismutase, and plasma nitrite, were also evaluated and identified compound 45 as a hit. SAR revealed that the potency of AChE inhibition was mainly influenced by the catalytic site interacting moieties attached at the end of the linker, as well as on the length of the alkyl chain- three methylene units which were found optimal for AChE inhibition and compounds containing four methylene units, lead to a reduction in binding affinity towards AChE enzyme. While compounds containing carbamate linkage did not affect the binding affinity towards receptor sites, it was observed that compounds having same alkyl chain length but a variation of the functionality at the end of the linker led to a great change in their inhibitory potency. Conformational and electronic changes caused by the replacement of the phenyl ring present at piperazine with any other heterocyclic ring affects the interaction with the AChE enzyme and thereby, affecting its potency. Introduction of the o-fluorophenyl group showed higher activity than p-fluoro substituents because fluoro group at ortho-position might favour the π-π stacking interaction via the rotation of the phenyl ring. Limited space around the nitrogen atom leading to a decrease in conformational flexibility associated with the presence of a piperazine ring might be the possible reason for enhanced potency of 45. Furthermore, disubstituted compounds showed remarkably decrease in activity due to the steric hindrance which prevented proper alignment of the molecule within the enzyme. Molecular docking of compound 45 indicated that it interacts with all the crucial amino acids (Figure 24) present at the CAS, mid-gorge and PAS of TcAChE which ultimately results in increased inhibition of AChE enzyme (Figure 24) [90].
Figure 24.
Hybrid molecules of coumarin and various aromatic amines as AChE inhibitors
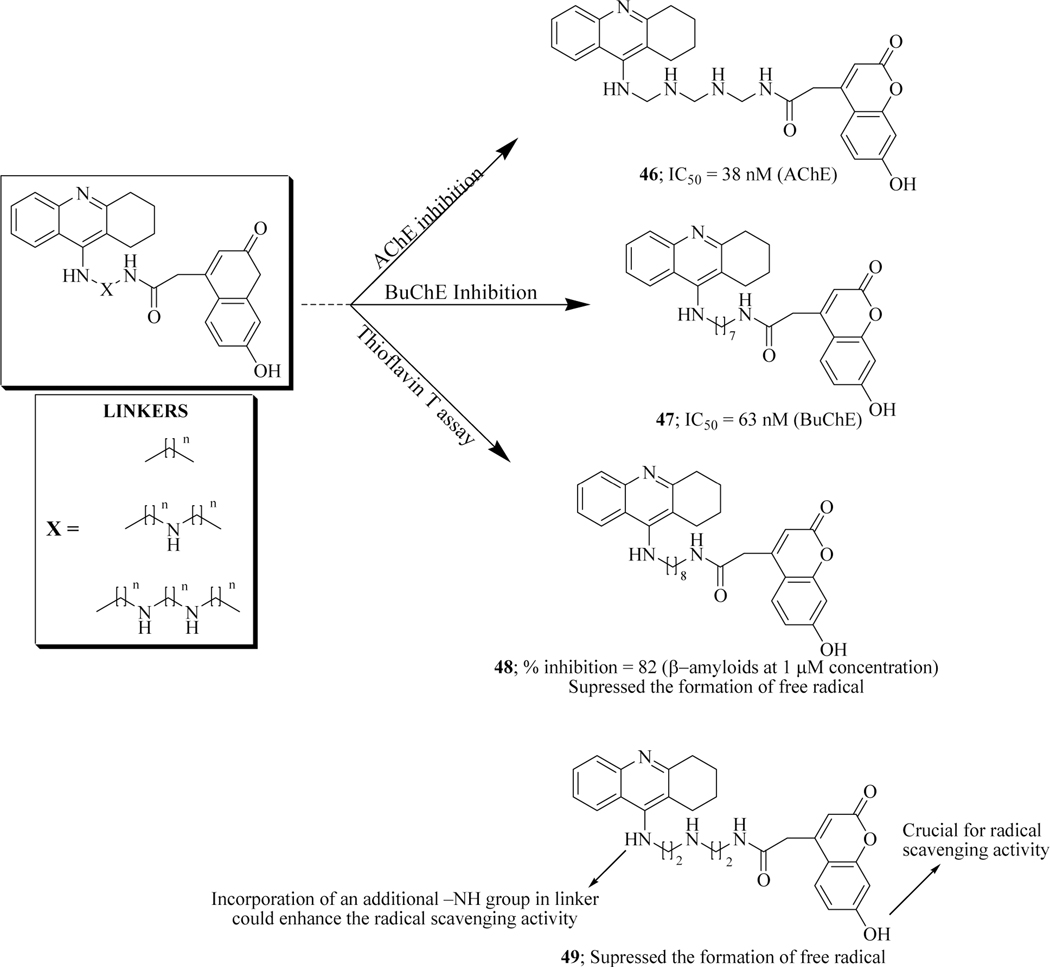
Hamulakova et.al. designed and synthesized novel conjugates of tacrine-7-hydroxycoumarin connected by a linker of different lengths as multifunctional cholinesterase inhibitors. Among the targeted compounds, 46 displayed acetylcholinesterase inhibitory activity with an IC50 value of 38 nM while compound 47 showed butyrylcholinesterase inhibition with the IC50 value of 63.5 nM. SAR studies indicate that with an increase in the length of the linker, AChE inhibitory activity remarkably decreased while β-amyloid aggregation inhibition increased, as shown by compound 48 (82% at 1 μM concentration) confirmed through Thioflavin T assay. Thioflavin T is a fluorescent dye which upon binding with β-amyloids generates a fluorescent product. Due to extensive metal (copper, iron)-induced oxidative stress in Alzheimer’s disease, they also evaluated the hybrid molecules to check their antioxidant activity. It was concluded that chelation of free copper(II) ions by tacrine-coumarin hybrids 48 and 49 suppressed the formation of reactive hydroxyl radicals. This was further confirmed by in vitro DNA damage protection experiments which suggested that compound 49 upon coordination to free copper ion prevents the decomposition of hydrogen peroxide decomposition catalyzed by Cu(II) (Figure 25) [91].
Figure 25.
Tacrine-coumarin hybrids as cholinesterase inhibitors
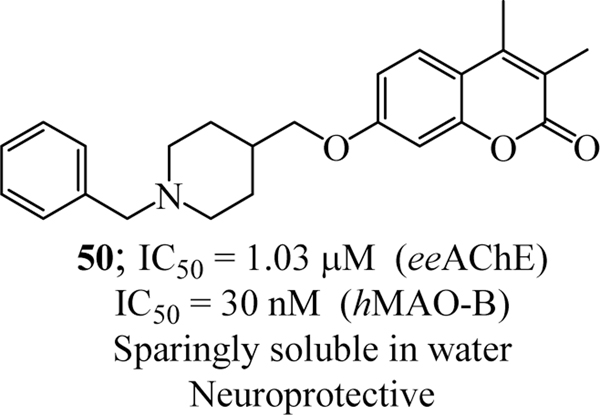
Leonardo et. al. synthesized a multi-target ligand series of 3,4-dimethylcoumarin-piperidine hybrid molecules and assessed them against ChEs and MAO enzymes. For hydrophilic/lipophilic balance, various modifications were done at 7th carbon of coumarin and spacer length connecting the coumarin to piperidine moiety. In vitro studies gave the promising multitarget hit compound 50 exhibiting high hMAO B inhibitory activity (IC50 = 30 nM) and good MAO B/A selectivity (SI = 94) along with eeAChE inhibition (IC50 = 1.03 μM). The study revealed that flexibility of basic side chain at the 7th position of coumarin and substitution on the piperidine ring greatly influenced the MAO-B inhibition while influenced AChE inhibition to a lesser extent (Figure 26) [92].
Figure 26.
3,4-dimethylcoumarin-piperidine hybrid as anti-Alzheimer’s agents
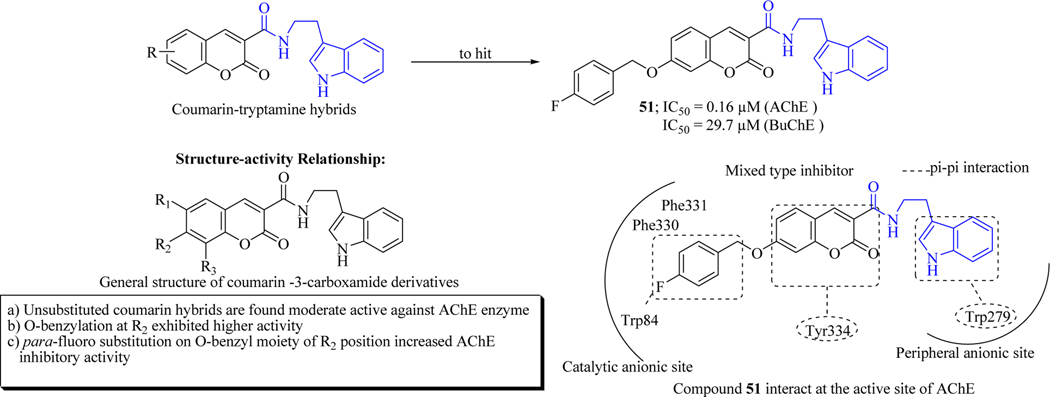
Inspired by the neuroprotective activity of tryptamine, Nasab et. al. synthesized a library of coumarin-tryptamine hybrids and evaluated them against both AChE and BuChE. Compound 51 containing a 4-fluorobenzyl group demonstrated sub-micromolar IC50 against AChE with the IC50 value of 0.16 μM. All synthesized compounds demonstrated moderate or no activity against BuChE. The SAR of these synthesized compounds concluded that unsubstituted compounds exhibited good inhibitory activity against AChE whereas 8-methoxy substitution on R2 position of coumarin was responsible for the improvement in activity. On the other hand, O-alkylation or O-benzylation in the same position maintained the AChE inhibitory activity. It was observed that the elongation of O-alkylation mainly abolishes the activity. In O-benzyl derivatives (at R2 position), fluoro substituent on benzyl moiety increased inhibitory activity. In particular, para fluoro substitutions increased activity in comparison to ortho- and meta-fluoro substitutions. The kinetic studies demonstrated that compound 51 showed a mixed type inhibition with their Ki value of 0.49 μM. Molecular modelling studies revealed that compound 51 was placed across the active site of the enzyme and also occupied both PAS and CAS of AChE enzyme (Figure 27) [93].
Figure 27.
Coumarin-tryptamine hybrids as cholinesterase inhibitors
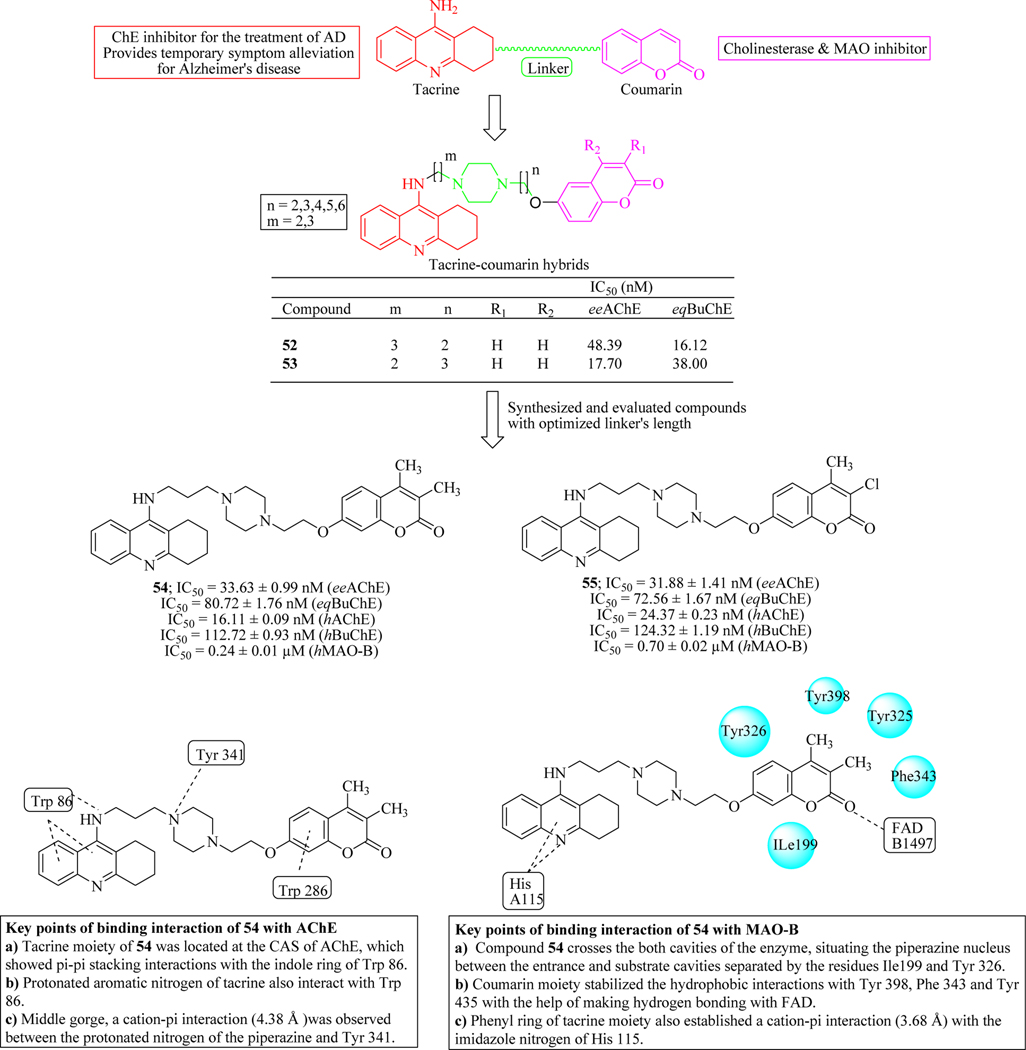
Xie et al. designed and synthesized a new series of tacrine-coumarin hybrids and evaluated the effect of linkers and substitutions on coumarin scaffold against cholinesterase enzymes. Piperazine linked compounds 52 and 53 exhibited good inhibitory activity against both the enzymes AChE and BuChE with IC50 values within the nanomolar range. Using these optimized linker lengths, another series of compounds were synthesized and evaluated against AChE, BuChE and MAO-B enzymes. Compound 54 showed IC50 values in the nanomolar range against human AChE (16 nM), BuChE (112 nM) and MAO-B (240 nM). Hybrid 55, a chloro substituted coumarin derivative was equipotent to compound 54. Compounds 54 and 55 displayed Pe value greater than 4.2 in the PAMPA-BBB assay suggesting that compounds have the ability to cross the blood-brain barrier. Kinetic behaviour of compound 54 against AChE inhibition which indicated its mixed-type inhibition. Docking studies revealed that compound 54 simultaneously interacted with both the catalytic sites (PAS and CAS) of AChE. In addition, compound 54 was non-toxic to the human neuroblastoma cell line SH-SY5Y (Figure 28) [94,95].
Figure 28.
Tacrine-coumarin hybrids tethered through piperazine as cholinesterase inhibitors
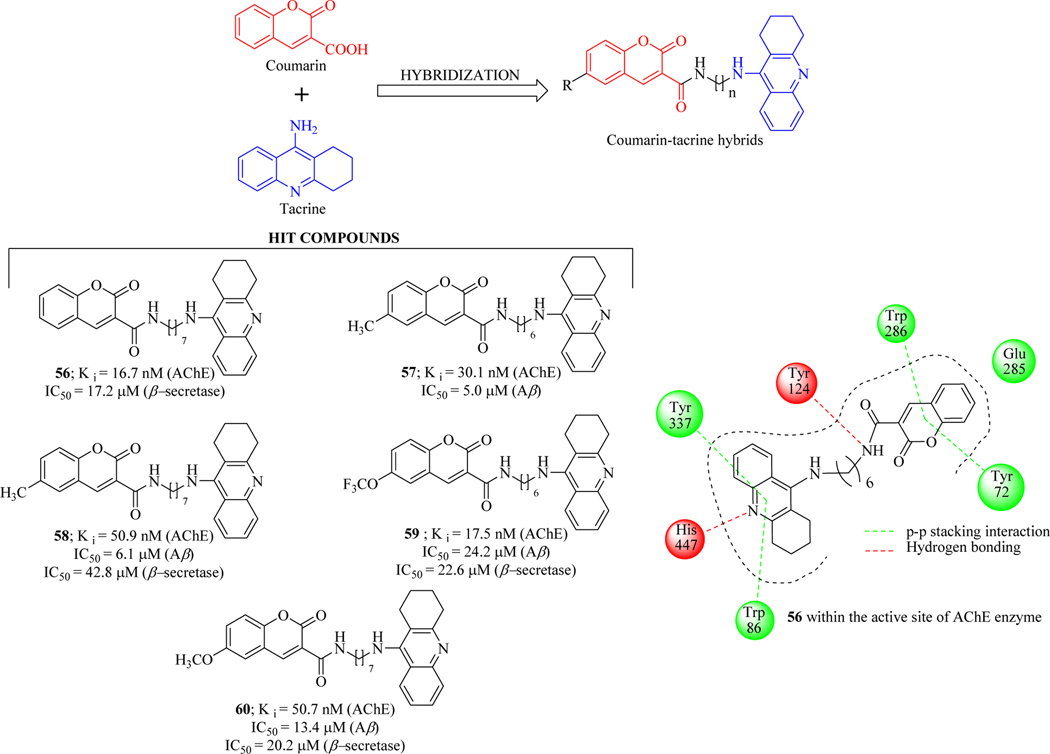
Sun et al. designed and synthesized eighteen novel tacrine-coumarin hybrids using methylene chain as a linker and evaluated against primary Alzheimer’s hallmarks AChE, BuChE, Aβ aggregation, and β-secretase inhibition. Inhibitors that simultaneously interact with multiple targets provide more effective candidates for neurodegenerative disorders including AD [96–99]. Compound 56 was identified as a potent dual-site AChE inhibitor with a Ki value of 16.7 nM, about 2-fold higher potency than tacrine. Compounds 57 and 58 revealed better activities against Aβ aggregation with IC50 values of ~5.0 μM and ~6.1 μM, while compounds 58, 59 and 60 displayed moderate to high activities for all the tested targets, which indicated that they could be multifunctional lead candidates for AD therapy. SAR investigation revealed that the linker length influences the inhibitory activities of hybrid compounds and six carbon chain/n-hexyl seemed to the best suitable length for AChE inhibition. Molecular docking and molecular dynamics (MD) studies suggested that the tacrine part and coumarin part of the hybrids bind with the catalytic active site (CAS) and peripheral active site with the conserved binding mode (Figure 29) [100].
Figure 29.
Coumarin-tacrine hybrids using methylene chain linker as dual AChE/β-secretase inhibitors
4. Coumarin-based hybrids as Antidiabetic agents
Diabetes mellitus is a metabolic disorder, which is characterized by hyperglycemia, and occurs due to insulin deficiency or resistance [101]. This dreadful disease can cause serious damage to many of the body’s organs like eyes, heart, nerves, kidneys and blood vessels [102,103]. The enzymes that play an imperative role in hyperglycemia are, α-glucosidase, α-amylase and aldose reductase which catalyze the cleavage of glucose from disaccharides. The inhibitors of these enzymes can significantly reduce the absorption of glucose and ultimately can decrease the blood glucose level. Thus, these enzymes are an important target to control the disease condition of diabetic patients.
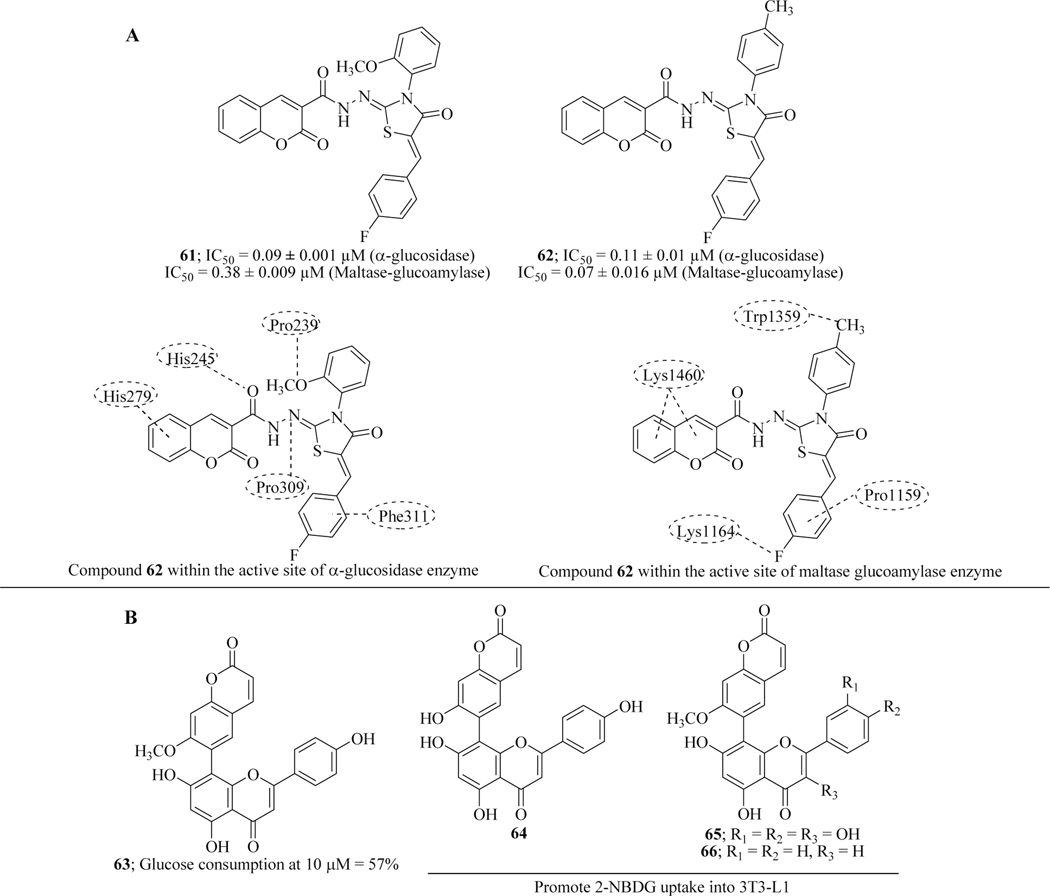
Ibrar et al. reported coumarin-thiazolidinediones hybrid molecules as novel glucosidase inhibitors based on the precedence that thiazolidinediones activate PPARɤ activation. In vitro screening of the molecules against α-glucosidase and maltase-glucoamylase revealed compounds (61 and 62) with submicromolar inhibitory activity. The enzyme kinetic studies suggested that both the compounds show the competitive mode of inhibition. SAR revealed that the change in position of the methoxy group in 61 from ortho to meta or para decreased activity. The inhibitory activity was greatly affected by the varied electronic environment on the N-substituted phenyl ring. Electronegative atom such as chlorine, at the ortho-position, was tolerated. Docking studies validated the binding of hits with α-glucosidase and maltase-glucoamylase (Figure 30A) [104].
Figure 30.
(A) Coumarin-thiazolidinediones hybrid molecules as novel glucosidase inhibitors; (B) Flavonoid-coumarin hybrids as antidiabetic agents
Pan et al. analyzed the antidiabetic effect of novel hybrid molecules of natural flavonoids and coumarin. Hybrids of apigenin, chrysin, quercetin and luteolin were synthesized and glucose disposal activity was measured. SAR indicated that the presence of coumarin at the 8th carbon atom of flavonoids (on chromone nucleus of flavone) is beneficial for activity. Hydroxyl groups of flavonoids were favourable for activity and the diminished activity was observed with the removal of these hydroxyl groups. Furthermore, compounds 63-66 promoted 2-NBDG uptake into 3T3-L1 which is generally responsible for the regulation of the glucose level in blood stream and could act as hit molecules for further development of antidiabetic agents (Figure 30B) [105].
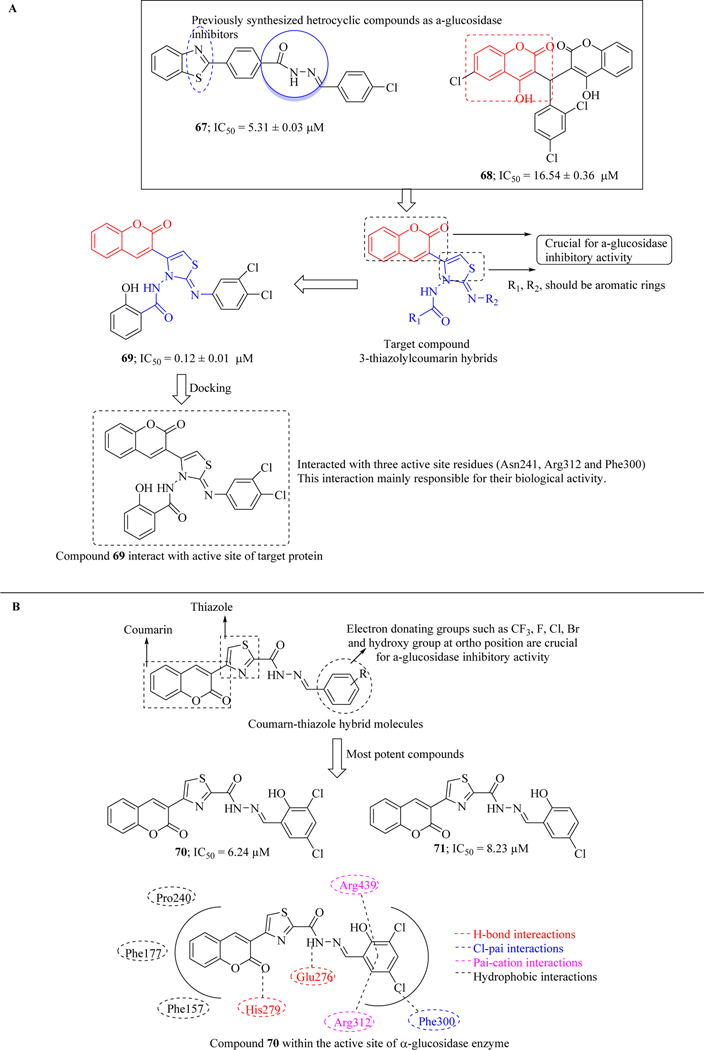
In order to increase the potency of previously synthesized compounds 67 and 68, Salar et al. designed and synthesized new series of 3-thiazolylcoumarin hybrids by incorporating three pharmacophores (Coumarin, thiazole, hydrazide) into a single entity. The library was evaluated for their in vitro α-glucosidase inhibitory activity and their results revealed that all compounds exhibited inhibitory activity within the range of IC50 = 0.12 ± 0.01 to 16.20 ± 0.23 μM. SAR suggested that 2H-chromen-2-one moiety, thiazole as well as arene rings were crucial for inhibitory activity. It was also found that compounds containing both electrons donating and electron withdrawing groups were well tolerated. Docking studies indicated that compound 69 interacted with three active site residues (Asn241, Arg312 and Phe300) and these interactions were mainly responsible for their biological activity. (Figure 31A) [106].
Figure 31.
Thiazole-coumarin hybrids as α-glucosidase inhibitors
Various pharmacological therapeutics based on thiazole moiety inspired Wang et al. to design and synthesize new hybrid molecules by joining thiazole with coumarin as α-glucosidase inhibitors. Majority of the compounds of this library were active against enzyme when tested in vitro. Two compounds 70 and 71 were found to be most active among the series with the IC50 value of 6.24 μM and 8.23 μM, respectively. SAR revealed that electron withdrawing groups and O-hydroxy group present on the terminal phenyl ring is crucial for inhibitory activity. Lineweaver-Burk plot of most potent compound 70 indicated that it non-competitively inhibits the enzyme. Various types of binding interactions of compound 70 within the active site of enzyme were also streamlined by docking studies which are depicted in Figure 31B [107].
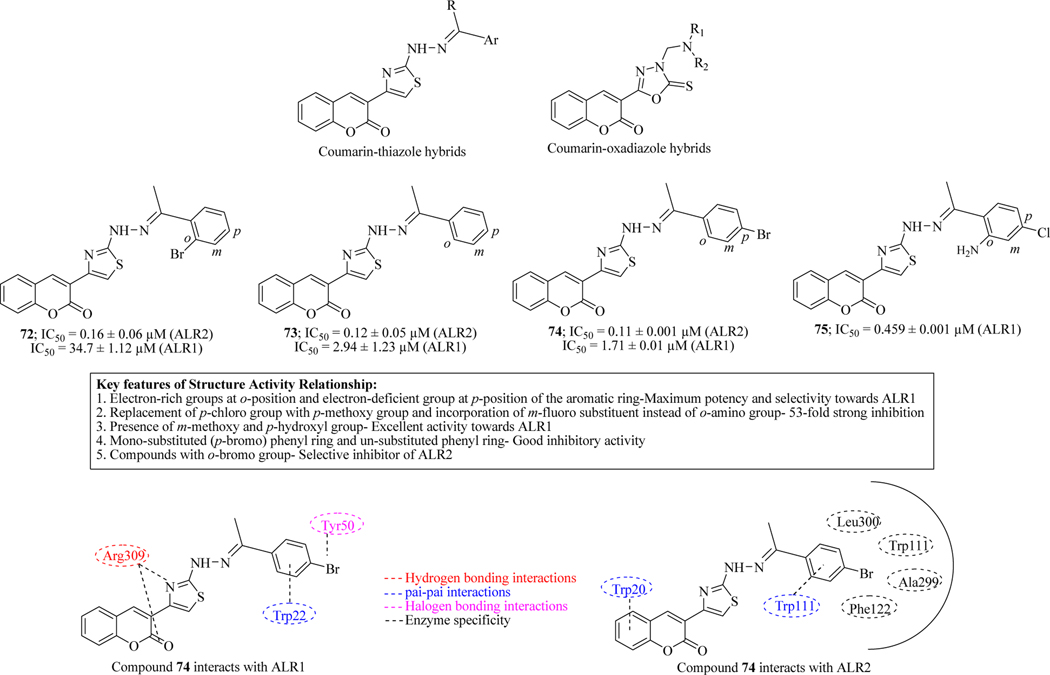
Inspired from the previous attempts towards the development of potent and selective aldose reductase (ALR2), and to control the diabetes mellitus (DM), Ibrar et al. synthesized novel coumarin-thiazole and coumarin-oxadiazole analogues. The inhibitory activity was evaluated against aldose reductase (ALR2) and the selectivity against aldehyde reductase (ALR1) was also determined. Most of the compounds were found selective inhibitors for ALR2 (72-75), amongst which 74 (IC50 = 0.11 μM) was the most potent. Molecular docking of compound 74 revealed its binding behaviour with various amino acid residues in the active sites (Figure 32) [108].
Figure 32.
Coumarin-thiazole and coumarin-oxadiazole hybrids as ALR inhibitors
5. Coumarin-based hybrids as Antimicrobial agents
Microbial resistance has become a serious threat to the future of human health, majorly due to increased drug resistance. The recent focus on the development of anti-microbial agents has failed to reach the expectations due to high risk of toxicity, insufficient anti-microbial activity as well as microbial resistance which led to a search for novel anti-microbial agents. Due to the multifactorial nature of microbial infections, the hybrid molecules could be beneficial to fight against these pathogens [109–118]. Following are some recent advancements made by various research groups in order to develop novel coumarin-based anti-microbial hybrid molecules.
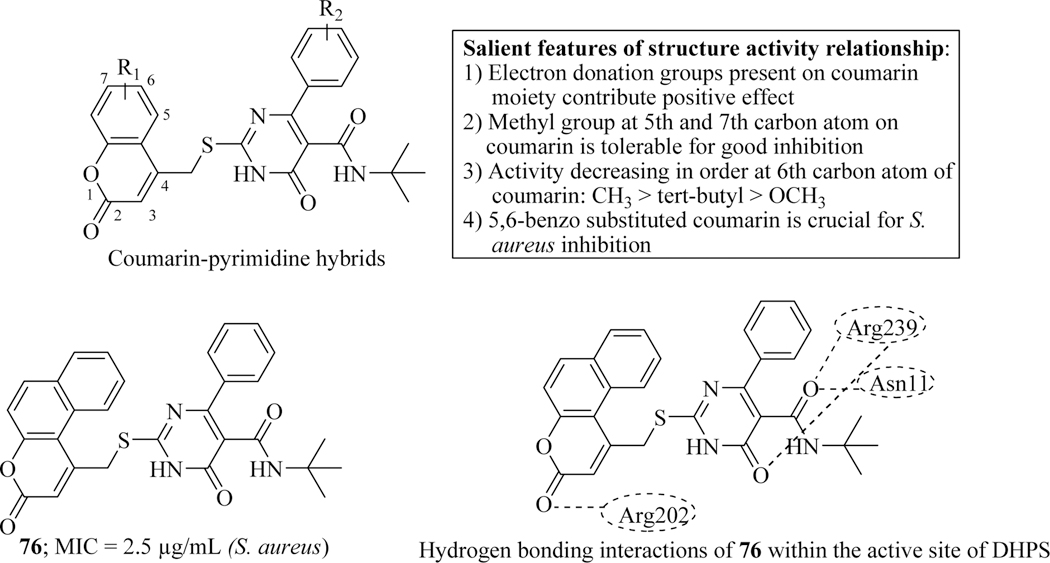
Chavan et al. reported a novel series of coumarin-pyrimidine hybrid molecules and evaluated their anti-bacterial property by using the agar plate method. Among the synthesized compounds, hit 76 exhibited better inhibition (MIC = 2.5 μg/mL) against gram-positive S. aureus bacterial strain as compared to ciprofloxacin (MIC = 10 μg/mL). SAR revealed that electron donating groups present on coumarin moiety enhanced the activity. While for potent inhibition against S. aureus, 5,6-benzo-coumarin moiety is crucial. Moreover, due to the imperative role of dihydropteroate synthase (DHPS) enzyme in folate metabolism (a valuable target for anti-bacterial drugs), molecular modelling studies were performed with the compound 76, within its active site which analyzed that four hydrogen bonds formed may be responsible for its potent anti-bacterial activity (Figure 33) [119].
Figure 33.
Coumarin-pyrimidine as anti-microbial agents
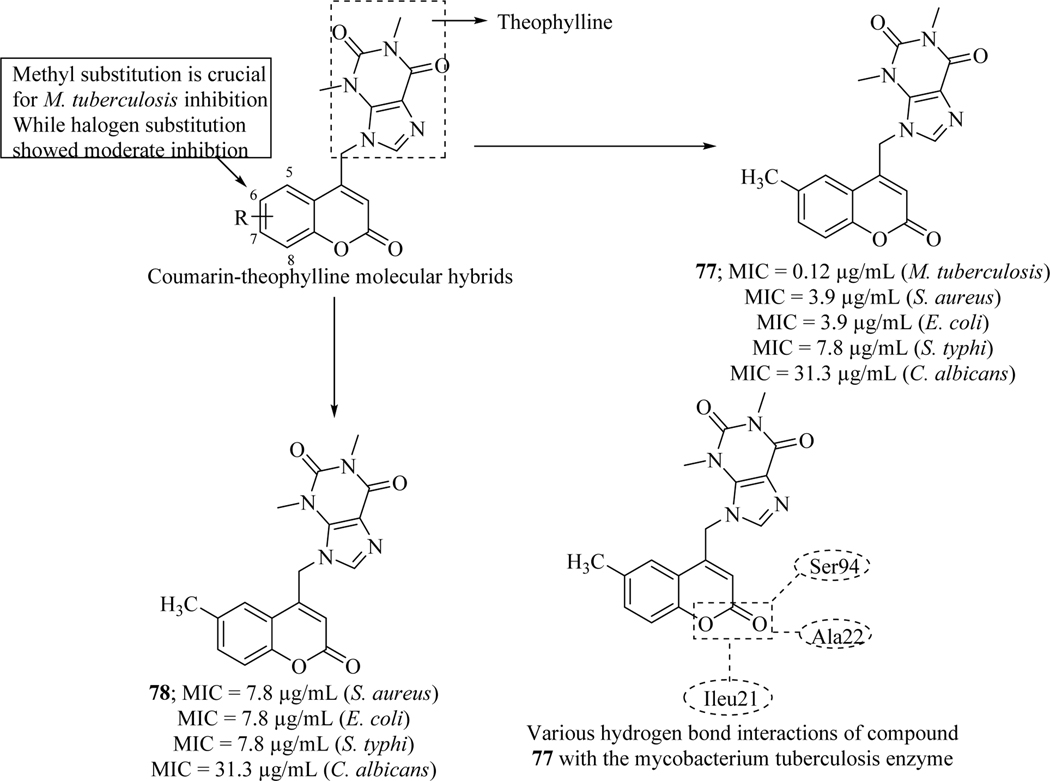
Recently Mangasuli et al. reported novel series of coumarin-theophylline hybrids as anti-microbial agents which were evaluated against gram-positive bacteria (S. aureus) and gram-negative bacteria (E. coli, S. Typhi) as well as fungi (C. albicans). Compounds with electron donating substituents (77 and 78) increased activity. It was found that the methyl and methoxy substitution at C-6 position on coumarin moiety is well tolerable for activity. Halogen substituted coumarin hybrids were found moderately active against Mycobacterium tuberculosis. Binding behaviour of 77 within the active site of Mycobacterium tuberculosis enzyme (Enoyl-[acyl-carrier-protein] reductase [NADH]) was also analyzed by molecular modelling studies (Figure 34) [120].
Figure 34.
Coumarin-theophylline hybrids as anti-microbial and antitubercular agents
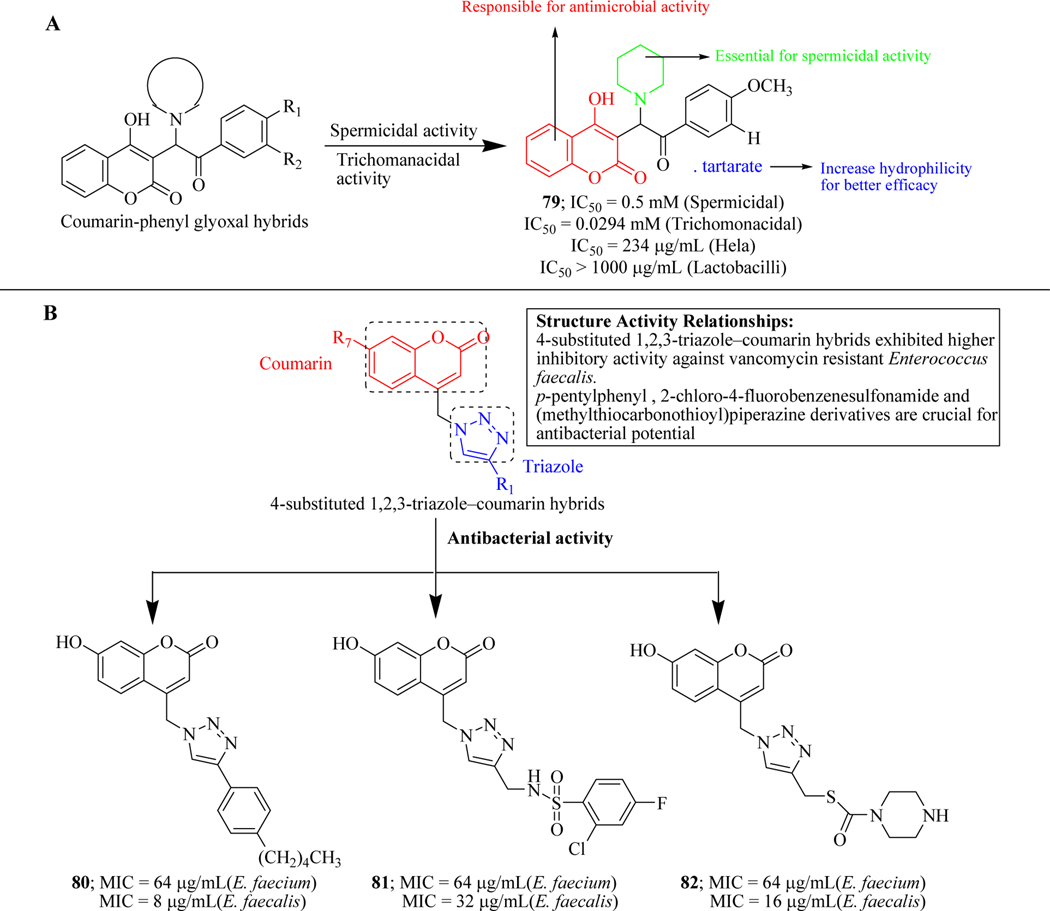
Due to its innumerable therapeutic potential, Gupta et. al. generated a novel series of scaffolds containing conjugates of 4-hydroxy coumarin and phenyl glyoxal and evaluated the effect of this library of hybrid molecules on the spermicidal and anti-microbial profile. A total of 11 compounds were active in both spermicidal and antitrichomonal assays, out of which, 79 was the most potent. These compounds were further evaluated by cytotoxicity and safety studies and were found safe in the cytotoxicity assay (HeLa cell lines) and safety assay (Lactobacillus jenseii strains). It was revealed that on the addition of polar groups like –OCH3 and –OH on 4th position of the glyoxal phenyl ring, the activity enhanced while the addition of –CH3 group on 2nd and 3rd position of the phenyl ring decreased activity (Figure 35A) [121].
Figure 35.
(A) Coumarin and phenyl glyoxal hybrids; (B) Triazole-coumarin hybrids as anti-microbial agents
Kraljevic et al. developed a series of 7-hydroxy coumarin-triazole molecular hybrids and accessed their anti-bacterial potential against Gram-positive bacteria including S. aureus, E. faecalis, vancomycin-resistant E. faecium (VRE) and Gram-negative bacteria including P. aeurigonsa, E., A. baumannii, extended-spectrum β-lactamase K. pneumoniae. The results revealed that the compounds could not show a considerable effect on any of the Gram-positive as well as Gram-negative strains except Enterococcus species. Hybrid 80 (containing p-pentylphenyl), 81 (containing 2-chloro-4-fluorobenzenesulfonamide) and 82 (containing dithiocarbamate) were found active with high selectivity against Enterococcus species with the MIC values ranging from 8–64 μg/mL (Figure 35B) [59].
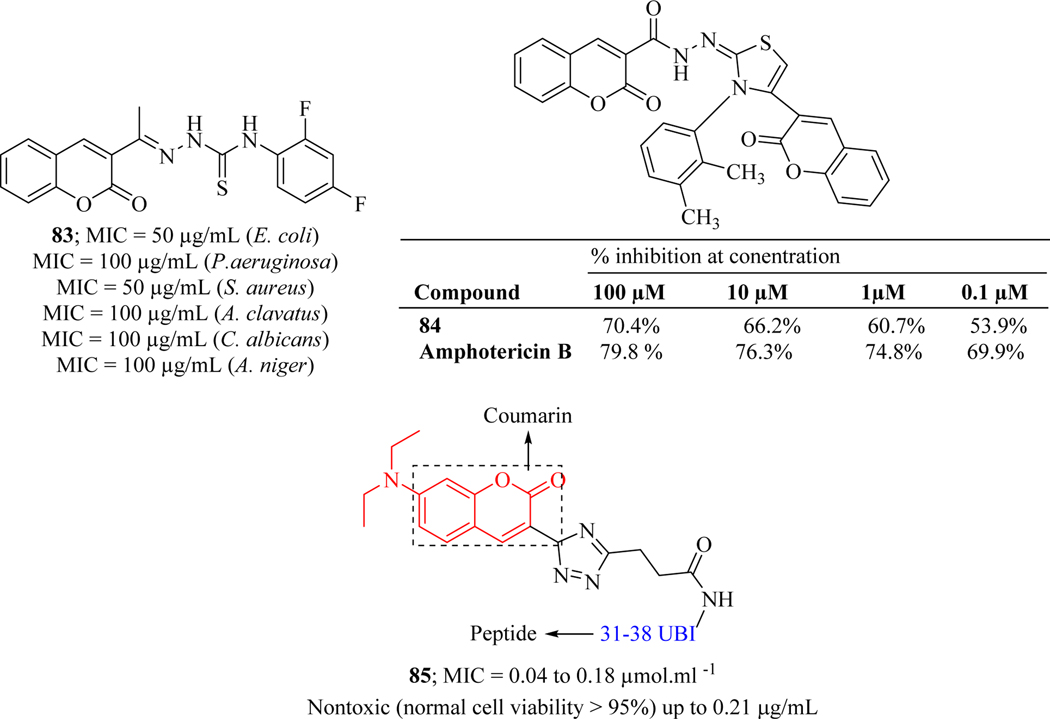
Inspired by vast biological activities of thiosemicarbazone, Vekariya et al. synthesized its hybrid molecules by combining with coumarin moiety and screened them for their anti-microbial activity. The screening was performed against two gram-positive bacterial strains (S. aureus, S. pyogenes), two gram-negative bacterial strains (E. coli, P. aeruginosa) and three fungal strains (A. clavatus, C. albicans and A. niger). The assay revealed hybrid molecule 83 as a hit which demonstrated broad-spectrum activity (Figure 36) [122].
Figure 36.
Various coumarin hybrid molecules as anti-microbial agents
Keeping in view the imperative role of iminothiazole in various biological activities [123–125], Ibrar et al. reported a new series of bis-coumarin-iminothiazole hybrid molecules and evaluated for antileishmanial activity. MTT assay revealed compound 84 as a potent hybrid which showed 70.4% inhibition at a concentration of 100 μM. SAR revealed that electron-rich (-OMe), as well as electron-poor (-Cl) groups, were tolerated which showed moderate inhibition of Leishmania major (Figure 36) [63].
Soraya et al. synthesized Ubiquicidine-peptide (UBI)-coumarin conjugates via copper(I)catalyzed azide-alkyne cycloaddition reaction (click chemistry). The conjugates were assessed for their in vitro anti-fungal activity against Cryptococcus gattii and Cryptococcus neoformans fungal strains. The results revealed that the analogue 85 exerted inhibitory potential against both the fungal strains with MIC values ranging from 0.04 to 0.18 μmol.mL−1. Additionally, conjugate 85 (with UBI 31–38) efficiently inhibited the growth of a fluconazole-resistant strain of C. gattii (L27/01F) at a concentration of 0.09 μmol.mL−1. The peptide-coumarin conjugate 85 was non-toxic to human cell line (lung fibroblast CCD-Lu). Compound 85 increases the number of ROS to exert anti-fungal activity (Figure 36) [126].
6. Coumarin-based hybrids as Antioxidants
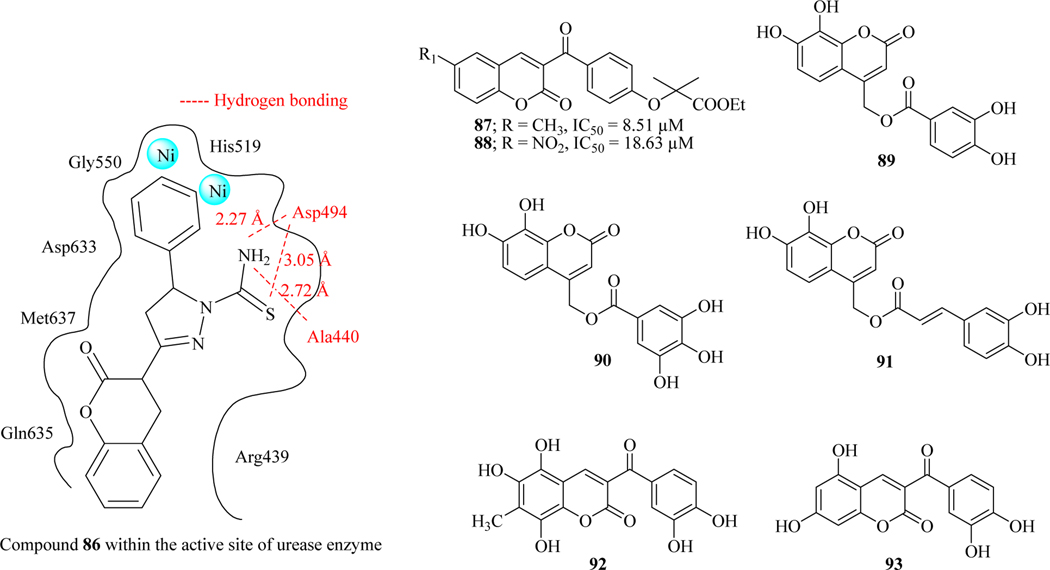
Adopting a hybrid pharmacophore approach, Saeed et al. designed and synthesized new series of coumarinyl pyrazolinyl thioamide derivatives as jack bean urease inhibitors and free radical scavengers. To overcome the scientific problems in designing of novel drugs, fusing Coumarinyl, pyrazolinyl and thioamide as potential pharmacophoric aptitude serves as a structural template in drug designing. Compound 86 was found to be the most effective derivative in the series with IC50 = 0.358 ± 0.017μM. Kinetic studies revealed that compound 86 inhibits urease enzyme by non-competitive mode of inhibition. The ADMET properties result justified that these novel synthesized compounds showed significant antioxidant with little hepatotoxic and skin sensitive effects. Molecular docking studies were performed within the urease enzyme which revealed that derivative 86 directly interacts with Asp494 and Ala440 amino acid residues through hydrogen bonding (Figure 37) [127].
Figure 37.
Various coumarin hybrid molecules with antioxidant activity
Niu et al. evaluated the antioxidant potential of coumarin-chalcone molecular hybrids using the 2,2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS·+) assay. Hit compounds 87 and 88 with the IC50 values of 8.51 μM and 18.63 μM, respectively were identified (Figure 37) [128].
Karina Perez-Cruz et al. designed and synthesized new hybrid compounds inserting hydroxybenzoic acids with common coumarin scaffold and evaluated their antioxidant activity against reactive oxygen species (ROS) using oxygen radical absorbance capacity-fluorescein (ORAC-FL), electron spin resonance (ESR) spin trapping, quenching of superoxide anion, cellular antioxidant activity (CAA) and ferric reducing ability of plasma (FRAP assay). Additionally, bond dissociation energies (BDE) values were calculated in order to obtain information about the antioxidant capacity for HAT (transfer of Hydrogen atom) mechanisms. Compound 89 exhibit synergy phenomenon for FRAP and ORAC assays. The superoxide scavenging results revealed that compound 90 was the most active molecule of synthesized hybrid series with an AI50 of 64 μM. CAA assay results showed the activity of the new compounds is limited to those oxidative processes in lipophilic media. From the theoretical calculations (Fukui index and BDE values) the different reactive sites were found within the molecules where the oxidative process actually occur (Figure 37) [129].
Previously reports of the various research articles concluded that coumarin-chalcone hybrids act as inhibitors for free radical over-production in oxidative stress-related diseases but there were no kinetic data and mechanism was reported on these hybrids [130]. So, based on this, Mazzone et al. provide quantitative data as well as their antioxidant behaviour of previously identified two coumarin-chalcone hybrids, compounds 92 [131] and 93 [132]. Kinetic calculations of these two coumarin-chalcone hybrids were performed and the results indicated that the compound 92 was a stronger radical scavenger than 93. Previously, it was also reported that the number and position of the hydroxyl substituents at catechol moiety of coumarin-chalcone hybrids directly related to their scavenging ability towards free radicals such as O2•, •CH3, and •OH [130]. Different reaction mechanisms of these hybrids were also investigated, and it was found that the largest contributions to the overall peroxyl radical scavenging activity of the studied compounds are the H transfer in lipid media, and the sequential proton loss hydrogen atom transfer (SPLHAT) in aqueous solution. It was also concluded that both hybrids are expected to be more efficient for scavenging peroxyl radicals in lipid media as compared to Trolox which was used as a reference drug. Peroxyl radical scavenging ability was also studied and the results suggested that the activity of the hybrids increases with the polarity of the environment and the number of phenolic sites (Figure 37) [133].
7. Coumarin hybrids as Anti-inflammatory agents
Today, most commonly used anti-inflammatory agents are non-steroidal anti-inflammatory drugs (NSAIDs) that exhibit their anti-inflammatory potential by inhibiting the release of prostaglandins (a potent mediator of inflammation). The primary target of these agents is cyclooxygenase (COX) enzyme which exists in two isoforms, namely, the constitutive form COX-1 and the inducible form COX-2. COX-1 is abundantly present in the body tissues, while COX-2 is expressed during inflammation. The NSAIDs were found to inhibit both the isoforms of COX enzyme, which is associated with gastrointestinal side effects [134–137]. Therefore to overcome this drawback, it is of great significance to develop novel compounds with improved profiles.
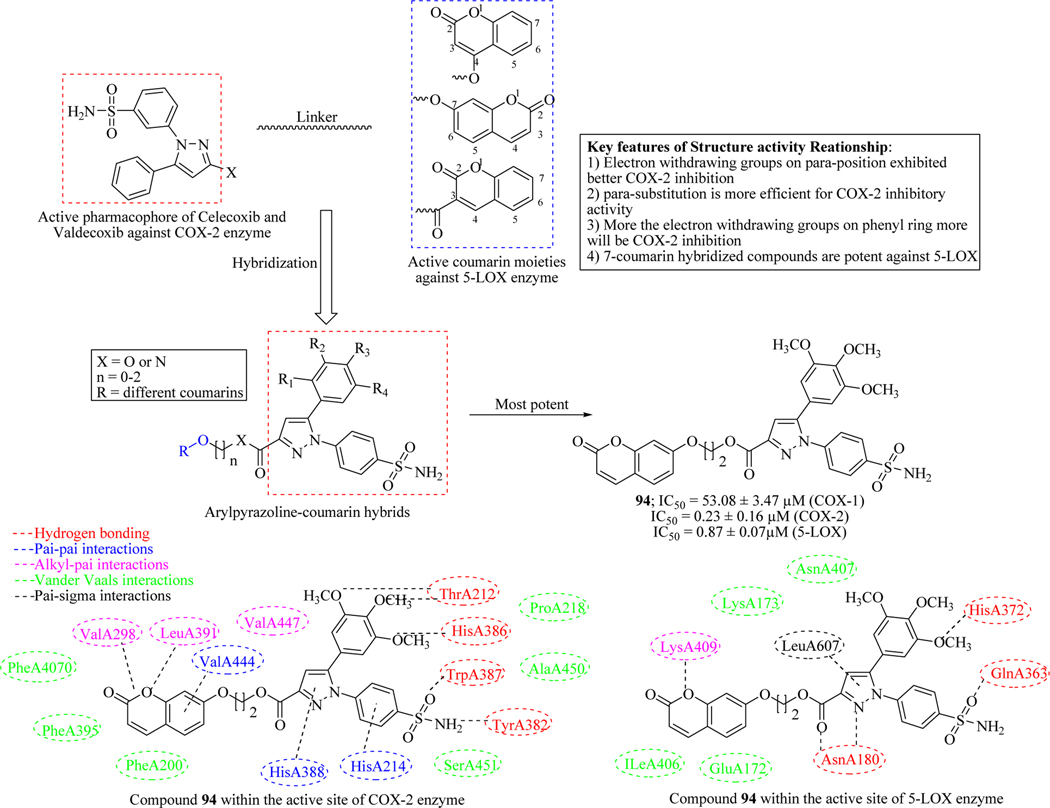
Shen et al. clubbed the active pharmacophores of COX-2 inhibitors (Celecoxib and Valdecoxib) and 5-LOX enzyme inhibitor coumarin derivatives into a single molecular architecture by using a molecular hybridization technique. All the designed compounds were evaluated against COX-2 and 5-LOX enzyme which revealed 94 as a most potent hybrid molecule in this library with the IC50 values of 0.23 μM and 0.87 μM, respectively for both the enzymes. Biological results also revealed an established SAR which indicated that electron withdrawing groups on para-position exhibited good COX-2 inhibitory activity. More the electron withdrawing groups present on the substituted phenyl ring, more the inhibitory activity against COX-2. The compound 94 showed strong binding interactions within the active sites of both 5-LOX and COX-2 enzymes which stated that the compound strongly binds with the enzymes (Figure 38) [138].
Figure 38.
Arylpyrazoline-coumarin hybrids as anti-inflammatory agents
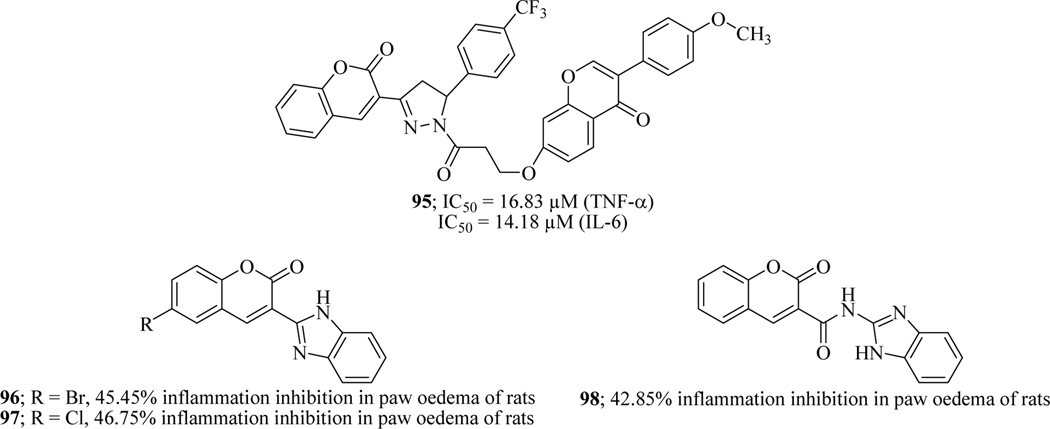
Tak-1, a primary LPS receptor was reported to cause activation of NF-κB through various signalling pathways. Therefore, Tak-1 inhibition prevents the generation of various interleukins and TNF-α. Chen et al. designed and synthesized a series of arylpyrazoline-coumarin hybrids and evaluated the library for anti-inflammatory activity using carrageenan-induced paw oedema by evaluating LPS-induced IL-6 release. The results demonstrated that all the compounds possess inhibitory activity among which compound 95 was found to be most potent with the IC50 values of 16.83 μM against TNF-α and 14.18 μM against IL-6. Further study also demonstrated this compound also reduced the expression of iNOS (Figure 39) [139].
Figure 39.
Various coumarin hybrid molecules as anti-inflammatory agents
Arora et al. Rationally designed and synthesized two series of coumarin-benzimidazole hybrid molecules and assessed their anti-inflammatory activity. The two active moieties were conjoined with or without amide linkage. In vitro assay identified hits 96, 97 and 98 which were further evaluated in vivo experiment using formalin-induced oedema rat model. SAR demonstrated that the compounds with electron withdrawing groups enhanced the activity profile, while compounds with electron donating groups diminished the activity. Series of compounds with amide linkage were found less active. It has been reported that NSAIDs are associated with gastric intolerance due to their acidic nature [140]. Thus, the gastric safety (gastric toxicity) profile of these most potent compounds was also evaluated which concluded that 97 and 98 could be used as clinical candidates due to their safety profile (Figure 39) [141].
8. Miscellaneous active coumarin-based hybrids
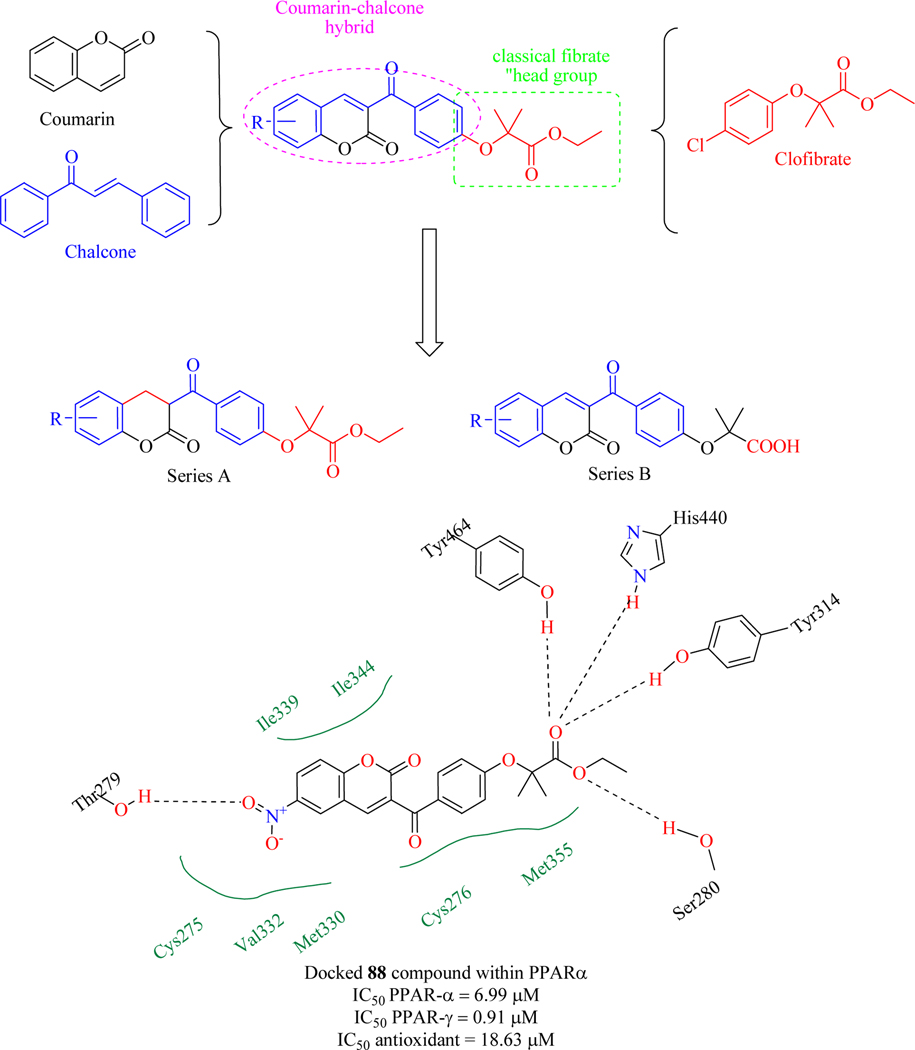
Niu H et al. utilized the multifarious coumarin molecule to develop a new hybrid with chalcones and fibrates to act as an agonist for PPAR-α (hyperlipidaemia) and PPAR-γ (Hyperglycaemia) with additional antioxidant activity. A novel series of molecules were synthesized and evaluated for PPAR-α/γ by transactivation assay using firefly luciferase reporter gene technology in HEK293 cells and antioxidant activity by ABTS evaluation method [142]. The results depicted that all compounds possess good agonistic activity for PPAR-α/γ and identified compound 88 as a hit for further development. Antioxidant activity revealed that compound 88 possesses free radical scavenging property. The SAR of the synthesized compounds confirmed that the C6 position of the benzopyran moiety was mandatory for agonistic activity and potency was improved by nitro group substitution. Moreover, PPARα/γ agonistic activity was enhanced by the presence of a double bond on the benzopyran moiety but the absence of a double bond enhanced the antioxidant activity. The hit compound 88 was docked, which stated that the hybrid showed hydrophobic interactions with Cys275, Met330, Val332, Ile339 and Ile344 and these interactions were potentiated by coumarin nucleus. In addition, a hydrogen bond is formed between the nitro group and thr279 amino residue, further potentiating the activity of compound 88 (Figure 40) [128].
Figure 40.
Coumarin-chalcone hybrid as a blood coagulator agent with antioxidant property
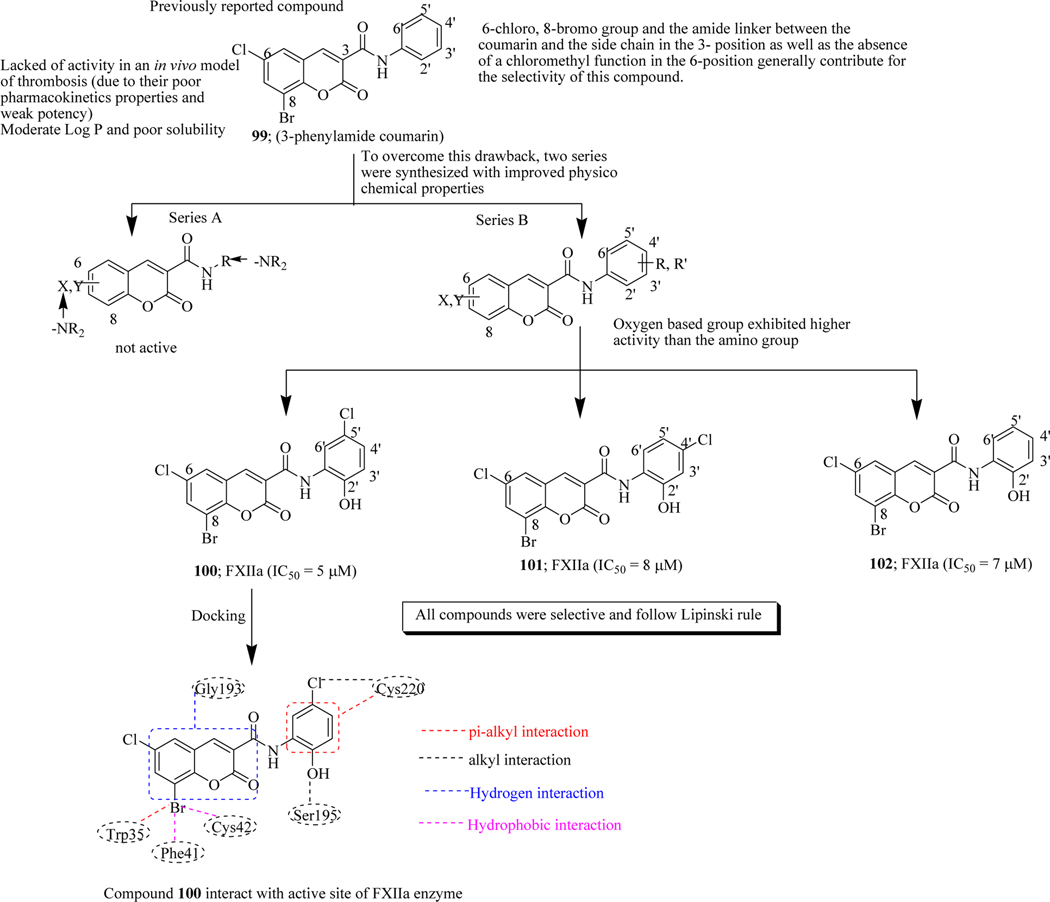
It has been reported that the coumarin-based protease inhibitors are associated with problems like lack of activity and poor pharmacokinetic properties within the in vivo model of thrombosis [143–147]. To overcome these drawbacks, Bauckaert et al. synthesized two series- A) containing the basic group (-NR2) and B) oxygen-containing groups (-OH or -COOH) of new 3-carboxamide derivatives by carrying out the structural modification of lead compound 99 (3-phenylamide coumarin). All the synthetics were screened on FXIIa at a concentration of 50 μM to determine their inhibitory potency and it was found that all the compounds displayed above 85% inhibition. Among the hybrids, 100–102 showed potent inhibitory activity with IC50 of 5, 8 and 7 μM, respectively. Moreover, the binding interactions between the synthesized compounds and the target molecule were also analyzed to understand the SAR. From these results, it was observed that oxygen-based group exhibited higher activity than the amino group such as compounds 100-102. The hit compounds were selective for FXIIa and did not demonstrate activity against thrombin, activated factor X (FXa), complex tissue factor/activated factor VII (TF/FVIIa) and plasma kallikrein at a concentration of 100 μM. Molecular docking studies of compound 100 indicated that π-alkyl interaction formed between halogens atom (bromine group) and indole of Trp35 from hydrophobic H1 pocket. This bromine atom also involved in two other hydrophobic interactions, one with Phe41 and other with Cys42 residues. Coumarin moiety formed a hydrogen bond with Gly193 whereas the hydroxyl group also showed hydrogen bond formation with Ser195. The phenyl and its chlorine atom both interact with Cys220 with π-alkyl and alkyl interactions. From the docking results, it was cleared that the H1 pocket was the typical feature of FXIIa which mainly responsible for the selectivity of compound 100 (Figure 41) [148].
Figure 41.
Phenylamide-coumarins as FXIIa enzyme inhibitors
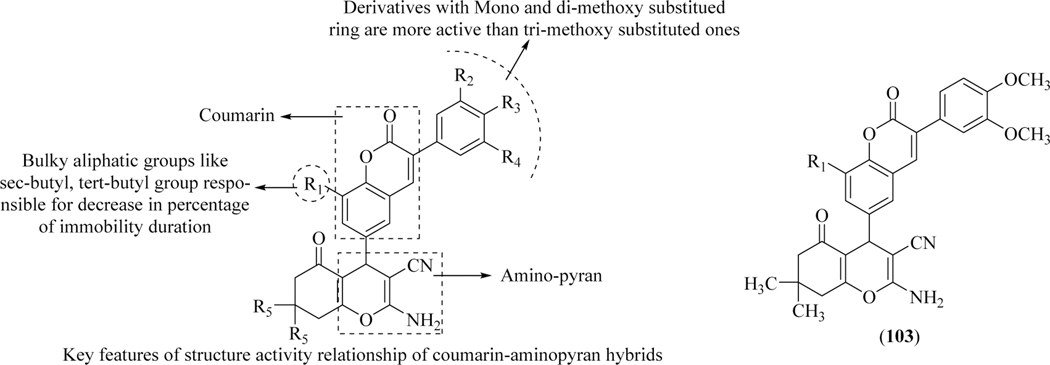
Due to serious side effects associated with current drug therapy to treat depression [149], Sashidhara et al. reported coumarin-aminopyran based hybrid molecules and assessed them in vivo to check their ability to treat depression. The compounds were evaluated after IP administration of lead compounds in adult male Swiss albino mice using Forced Swimming Test (FST). In in vivo test Compound, 103 demonstrated a dose-dependent decrease in immobility, even at a low dose of 0.25 mg/kg. These results were also confirmed by using the Tail Suspension Test (TST). Furthermore, it was also demonstrated that the compound did not affect the locomotor activity when mice were treated with an effective dose of 0.5 and 1.0 mg/kg IP (Figure 42) [150].
Figure 42.
Coumarin-aminopyran based hybrids as an antidepressant
9. Conclusion
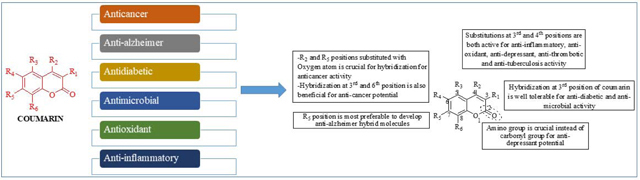
This review demonstrates that hybridization of coumarin with various active pharmacophores plays an important role in increasing the pharmacological profile of the novel molecules in the form of potency or pharmacokinetics or both. Hybrid molecules that can simultaneously engage more than one target will play a key role in developing small molecule therapeutics that can target complex disorders, where several key proteins are dysregulated [151]. Coumarin hybrids possessing anticancer activity or acting against neurodegenerative disorders such as Alzheimer’s disease have been the major focus of drug discovery since 2014. This review highlights the key SAR studies performed on coumarin hybrids and identifies key structural features on the coumarin scaffold, where, substitutions can increase or decrease the activity of the hit molecules. Molecules displaying polypharmacology are either in clinical trials or have been approved for the treatment of complex multifactorial disorders. The next few decades of drug discovery research will reveal the fate of hybrid molecules including coumarin hybrids in terms of drug molecules to treat diseases.
Figure 2b.
Mechanism of actions of coumarin derivatives against various diseases
Figure 5.
Recently reported Coumarin based hybrid molecules having anti-Alzheimer’s properties
Figure 7.
Various coumarin-based bioactive hybrid molecules
Rational approaches towards the design of pharmacologically active coumarin based hybrids
Structure-activity relationships and mechanistic insights for therapeutic coumarin hybrids
Chemotherapeutic potential of coumarin based hybrids
Coumarin hybrids as antioxidant and anti-Alzheimer’s agents
Antidiabetic and anti-inflammatory activity of coumarin based hybrids
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Li JWH, Vederas JC, Drug discovery and natural products: End of an era or an endless frontier? Science. 325 (2009) 161–165. 10.1126/science.1168243 [DOI] [PubMed] [Google Scholar]
- [2].Kaur P, Arora R, Gill NS, Review on oxygen heterocycles, Indo Am. J. Pharma. Res. 3 (2013) 9067–9084. 10.1044/1980-iajpr.01110 [DOI] [Google Scholar]
- [3].Feuer G, The metabolism and biological actions of coumarins. In Progress in Medicinal Chemistry (Elis GP, and West GB, Ed.), North Holland, New York. 1974, Vol 10, pp 86–87 [DOI] [PubMed] [Google Scholar]
- [4].Evans WC, Trease and Evans’ Pharmacognosy, Elsevier Health Sciences, 2009. [Google Scholar]
- [5].Rosselli S, Maggio AM, Faraone N, Spadaro V, Morris-Natschke SL, Bastow KF, Lee KH, Bruno M, The cytotoxic properties of natural coumarins isolated from roots of Ferulago campestris (Apiaceae) and of synthetic ester derivatives of aegelinol, Nat. Prod. Commun. 4 (2009) 1701–1706. [PubMed] [Google Scholar]
- [6].Poole SK, Poole CF, Thin-layer chromatographic method for the determination of the principal polar aromatic flavour compounds of the cinnamons of commerce, Analyst 119 (1994) 113–120. [Google Scholar]
- [7].Patil AD, Freyer AJ, Eggleston DS, Haltiwanger RC, Bean MF, Taylor PB, Caranfa MJ, Breen AL, Bartus HR, The inophyllums, novel inhibitors of HIV-1 reverse transcriptase isolated from the Malaysian tree, Calophyllum inophyllum Linn, J. Med. Chem. 36 (1993) 4131–4138. [DOI] [PubMed] [Google Scholar]
- [8].Spino C, Dodier M, Sotheeswaran S, Anti-HIV coumarins from Calophyllum seed oil, Bioorg. Med. Chem. Lett. 8 (1998) 3475–3478. [DOI] [PubMed] [Google Scholar]
- [9].Whang WK, Park HS, Ham I, Oh M, Namkoong H, Kim HK, Hwang DW, Hur SY, Kim TE, Park YG, Natural compounds, fraxin and chemicals structurally related to fraxin protect cells from oxidative stress, Exp. Mol. Med. 37 (2005) 436–446. [DOI] [PubMed] [Google Scholar]
- [10].Shin E, Choi KM, Yoo HS, Lee CK, Hwang BY, Lee MK, Inhibitory effects of coumarins from the stem barks of Fraxinus rhynchophylla on adipocyte differentiation in 3T3-L1 cells, Biol. Pharm. Bull. 33 (2010) 1610–1614. [DOI] [PubMed] [Google Scholar]
- [11].Crichton EG, Waterman PG, Dihydromammea C/OB: a new coumarin from the seed of Mammea africana, Phytochemistry 17 (1978) 1783–1786 [Google Scholar]
- [12].Baek NI, Ahn EM, Kim HY, Park YD, Furanocoumarins from the root of Angelica dahurica, Arch. Pharm. Res. 23 (2000) 467–470. [DOI] [PubMed] [Google Scholar]
- [13].Teng CM, Lin CH, Ko FN, Wu TS, Huang TF, The relaxant action of osthole isolated from Angelica pubescens in guinea-pig trachea, Schmiedeb. Arch. Pharmacol. 349 (1994) 202–208. [DOI] [PubMed] [Google Scholar]
- [14].Piller N, A comparison of the effectiveness of some anti-inflammatory drugs on thermal oedema, Br. J. Exp. Pathol. 56 (1975) 554. [PMC free article] [PubMed] [Google Scholar]
- [15].Guibourt NJBG, Histoire abregee des droques simples (Abridged history of simple drugs), (1820) Vol. 2, pp 160–161, L. Colas, Paris. [Google Scholar]
- [16].Lacy A, Kennedy RO, Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer, Curr. Pharm. Des. 10 (2004) 3797–3811. [DOI] [PubMed] [Google Scholar]
- [17].Heide L, The aminocoumarins: biosynthesis and biology, Nat. Prod. Rep. 26 (2009) 1241–1250. [DOI] [PubMed] [Google Scholar]
- [18].Ganina OG, Daras E, Bourgarel-Rey V, Peyrot V, Andresyuk AN, Finet JP, Fedorov AY, Beletskaya IP, Combes S, Synthesis and biological evaluation of polymethoxylated 4-heteroarylcoumarins as tubulin assembly inhibitor, Bioorg. Med. Chem. 16 (2008) 8806–8812. [DOI] [PubMed] [Google Scholar]
- [19].Bailly C, Bal C, Barbier P, Combes S, Finet JP, Hildebrand MP, Peyrot V, Wattez N, Synthesis and biological evaluation of 4-arylcoumarinanalogues of combretastatins, J. Med. Chem. 46 (2003) 5437–5444. [DOI] [PubMed] [Google Scholar]
- [20].Chen H, Li S, Yao Y, Zhou L, Zhao J, Gu Y, Wang K, Li X, Design, synthesis, and anti-tumor activities of novel triphenylethyleneecoumarin hybrids, and their interactions with Ct-DNA, Bioorg. Med. Chem. Lett. 23 (2013) 4785–4789. [DOI] [PubMed] [Google Scholar]
- [21].Soto-Ortega DD, Murphy BP, Gonzalez-Velasquez FJ, Wilson KA, Xie F, Wang Q, Moss MA, Inhibition of amyloid-b aggregation by coumarin analogs can be manipulated by functionalization of the aromatic center, Bioorg. Med. Chem. Lett. 19 (2011) 2596–2602. [DOI] [PubMed] [Google Scholar]
- [22].Pisani L, Muncipinto G, Miscioscia TF, Nicolotti O, Leonetti F, Catto M, Caccia C, Salvati P, Soto-Otero R, Mendez-Alvarez E, Passeleu C, Carotti A, Discovery of a Novel Class of Potent Coumarin Monoamine Oxidase B Inhibitors: Development and Biopharmacological Profiling of 7-[(3-Chlorobenzyl)oxy]-4-[(methylamino)methyl]-2H-chromen-2-one Methanesulfonate (NW-1772) as a Highly Potent, Selective, Reversible, and Orally Active Monoamine Oxidase B Inhibitor, J. Med. Chem. 52 (2009) 6685–6706. [DOI] [PubMed] [Google Scholar]
- [23].Shen Q, Shao J, Peng Q, Zhang W, Ma L, Chan ASC, Gu L, Hydroxycoumarin Derivatives: Novel and Potent r-Glucosidase Inhibitors, J. Med. Chem. 53 (2010) 8252–8259. [DOI] [PubMed] [Google Scholar]
- [24].Zhao DG, Zhou AY, Du Z, Zhang Y, Zhang K, Ma YY, Coumarins with α-glucosidase and α-amylase inhibitory activities from the flower of Edgeworthia gardneri, Fitoterapia. 107 (2015) 122–127. [DOI] [PubMed] [Google Scholar]
- [25].Stanley SA, Kawate T, Iwase N, Shimizu M, Clatworthy AE, Kazyanskay E, Sacchettini JC, Ioerger TR, Siddiqi NA, Minami S, Aquadro JA, Grant SS, Rubin EJ, Hung DT, Diarylcoumarins inhibit mycolic acid biosynthesis and kill Mycobacterium tuberculosis by targeting FadD32, PNAS. 110 (2013) 11565–11570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Revankar HM, Bukhari SNA, Kumar GB, Qin HL, Coumarins scaffolds as COX inhibitors, Bioorg. Chem. 71 (2017) 146–159. [DOI] [PubMed] [Google Scholar]
- [27].Kontogiorgis CA, Hadjipavlou-Litina DJ, Synthesis and Antiinflammatory Activity of Coumarin Derivatives, J. Med. Chem. 48 (2005) 6400–6408. [DOI] [PubMed] [Google Scholar]
- [28].Iranshahi M, Jabbari A, Orafaie A, Mehri R, Zeraatkar S, Ahmadi T, Alimardani M, Sadeghian H, Synthesis and SAR studies of mono O-prenylated coumarins as potent 15-lipoxygenase inhibitors, Eur. J. Med. Chem 57 (2012) 134–142. [DOI] [PubMed] [Google Scholar]
- [29].Srivastava P, Vyas VK, Variya B, Patel P, Qureshi G, Ghate M, Synthesis, anti-inflammatory, analgesic, 5-lipoxygenase (5-LOX) inhibition activities, and molecular docking study of 7-substituted coumarin derivatives, Bioorg. Chem. 67 (2016) 130–138. [DOI] [PubMed] [Google Scholar]
- [30].Qian C, Lai CJ, Bao R, Wang DG, Wang J, Xu GX, Atoyan R, Qu H, Yin L, Samson M, Zifcak B, Ma AWS, Rocca SD, Borek M, Zhai HX, Cai X, Voi M, Cancer Network Disruption by a Single Molecule Inhibitor Targeting Both Histone Deacetylase Activity and Phosphatidylinositol 3-Kinase Signaling, Clin. Cancer Res. 18 (2012) 4104–4113. [DOI] [PubMed] [Google Scholar]
- [31].Wu CP, Hsieh YJ, Hsiao SH, Su CY, Li YQ, Huang YH, Huang CW, Hsieh CH, Yu JS, Wu YS, Human ATP-Binding Cassette Transporter ABCG2 Confers Resistance to CUDC-907, a Dual Inhibitor of Histone Deacetylase and Phosphatidylinositol 3Kinase, Mol Pharmaceutics. 13 (2016) 784–794. [DOI] [PubMed] [Google Scholar]
- [32].Lai CJ, Bao R, Tao X, Wang J, Atoyan R, Qu H, Wang DG, Yin L, Samson M, Forrester J, Zifcak B, Xu GX, DellaRocca S, Zhai HX, Cai X, Munger WE, Keegan M, Pepicelli CV, Qian C, CUDC-101, a multitargeted inhibitor of histone deacetylase, epidermal growth factor receptor, and human epidermal growth factor receptor 2, exerts potent anticancer activity, Cancer Res. 70 (2010) 3647–3656. [DOI] [PubMed] [Google Scholar]
- [33].Shimizu T, LoRusso PM, Papadopoulos KP, Patnaik A, Beeram M, Smith LS, Rasco DW, Mays TA, Chambers G, Ma A, Wang J, Laliberte R, Voi M, Tolcher AW, Phase I first-inhuman study of CUDC-101, a multitargeted inhibitor of HDACs, EGFR, and HER2 in patients with advanced solid tumors, Clin Cancer Res. 20 (2010) 5032–5040. [DOI] [PubMed] [Google Scholar]
- [34].Wang J, Pursell NW, Samson ME, Atoyan R, Ma AW, Selmi A, Xu W, Cai X, Voi M, Savagner P, Lai CJ, Potential advantages of CUDC-101, a multitargeted HDAC, EGFR, and HER2 inhibitor, in treating drug resistance and preventing cancer cell migration and invasion, Mol Cancer Ther. 12 (2013) 925–936. [DOI] [PubMed] [Google Scholar]
- [35].Seo SY, Multi-targeted hybrids based on HDAC inhibitors for anti-cancer drug discovery, Arch Pharmacal Res. 35 (2012) 197–200. [DOI] [PubMed] [Google Scholar]
- [36].Cai X, Zhai HX, Wang J, Forrester J, Qu H, Yin L, Lai CJ, Bao C,R, Qian C, Discovery of 7-(4-(3-ethynylphenylamino)-7-methoxyquinazolin-6-yloxy)-N-hydroxyheptanamide (CUDC-101) as a potent multi-acting HDAC, EGFR, and HER2 inhibitor for the treatment of cancer, J. Med. Chem. 53 (2010) 2000–2009. [DOI] [PubMed] [Google Scholar]
- [37].Qian C, Lai CJ, Bao R, Wang DH, Wang J, Xu GX, Atoyan R, Qu H, Yin L, Samson M, Zifcak B, Ma AW, DellaRocca S, Borek M, Zhai HX, Cai X, Voi M, Cancer network disruption by a single molecule inhibitor targeting both histone deacetylase activity and phosphatidylinositol 3-kinase signaling, Clin. Cancer Res. 18 (2012) 4104–4113. [DOI] [PubMed] [Google Scholar]
- [38].Sharma S, Singh J, Ojha R, Singh H, Kaur M, Bedi PMS, Nepali K, Design strategies, structure activity relationship and mechanistic insights for purine as kinase inhibitors, Eur. J. Med. Chem. 112 (2016) 298–346. [DOI] [PubMed] [Google Scholar]
- [39].Nepali K, Sharm S, Sharma M, Bedi PMS, Dhar KL, Rational approaches, design strategies, structure activity relationship and mechanistic insights for anticancer hybrids, Eur. J. Med. Chem. 77 (2014) 422–487. [DOI] [PubMed] [Google Scholar]
- [40].Nepali K, Ojha R, Sharma S, Bedi PMS, Dhar KL, Tubulin inhibitors: a patent survey, Recent Pat. Anticancer Drug Discov. 9 (2014) 303–339. [DOI] [PubMed] [Google Scholar]
- [41].Marco-Contelles J, Soriano E, The medicinal chemistry of hybrid-based drugs targeting multiple sites of action, Curr. Med. Chem. 11(2011) 2714–2715. [DOI] [PubMed] [Google Scholar]
- [42].http://www.who.int/news-room/fact-sheets/detail/cancer (retrieved on 30-nov-2018)
- [43].Dong P, Rakesh KP, Manukumar HM, Mohammed YHE, Karthik CS, Sumathi S, Mallu P, Qin HL, Innovative nano-carriers in anticancer drug delivery-a comprehensive Review, Bioorg. Chem. 85 (2019) 325–336. [DOI] [PubMed] [Google Scholar]
- [44].Moku B, Ravindar L, Rakesh KP, Qin HL, The significance of N-methylpicolinamides in the development of anticancer therapeutics: Synthesis and structure-activity relationship (SAR) studies, Bioorg. Chem. 86 (2019) 513–537. [DOI] [PubMed] [Google Scholar]
- [45].Zhang X, Rakesh KP, Shantharam CS, Manukumar HM, Asiri AM, Marwani HM, Qin HL, Podophyllotoxin derivatives as an excellent anticancer aspirant for future chemotherapy: A key current imminent needs, Bioorg. Med. Chem. 26 (2018) 340–355. [DOI] [PubMed] [Google Scholar]
- [46].Zha GF, Qin HL, Youssif BGM, Amjad MW, Raja MAG, Abdelazeem AH, Bukhari SNA, Discovery of potential anticancer multi-targeted ligustrazine based cyclohexanone and oxime analogs overcoming the cancer multidrug Resistance, Eur. J. Med. Chem. 135 (2017) 34–48. [DOI] [PubMed] [Google Scholar]
- [47].Qin HL, Leng J, Zhang CP, Jantan I, Amjad MW, Sher M, Naeem-ul-Hassan M, Hussain MA, Bukhari SNA, Synthesis of α,β-Unsaturated Carbonyl-Based Compounds, Oxime and Oxime Ether Analogs as Potential Anticancer Agents for Overcoming Cancer Multidrug Resistance by Modulation of Efflux Pumps in Tumor Cells, J. Med. Chem. 59 (2016) 3549–3561. [DOI] [PubMed] [Google Scholar]
- [48].Qin HL, Leng J, Youssif BGM, Amjad MW, Raja MAG, Hussaine MA, Hussain Z, Kazmi SN, Bukhari SNA, Synthesis and mechanistic studies of curcumin analogs based oximes as potential anticancer agents, Chem. Biol. Drug. Des. 90 (2017) 443–449. [DOI] [PubMed] [Google Scholar]
- [49].(a) Singh J, Sharma S, Saxena AK, Nepali K, Bedi PMS, Synthesis of 1,2,3-triazole tethered bifunctional hybrids by click chemistry and their cytotoxic studies, Med. Chem. Res. 22 (2013) 3160–3169 [Google Scholar]; (b) Sharma S, Gupta MK, Saxena M,AK, Bedi PMS, Triazole linked mono carbonyl curcumin-isatin bifunctional hybrids as novel anti tubulin agents: Design, synthesis, biological evaluation and molecular modeling studies, Bioorg. Med. Chem. 23 (2015) 7165–7180 [DOI] [PubMed] [Google Scholar]; (c) Kumar K, Sagar S, Esau L, Kaur M, Kumar V, Synthesis of novel 1H-1,2,3-triazole tethered C-5 substituted uracil-isatin conjugates and their cytotoxic evaluation, Eur. J. Med. Chem. 58 (2012) 153–159. [DOI] [PubMed] [Google Scholar]
- [50].Singh H, Singh JV, Gupta MK, Saxena AK, Sharma S, Nepali K, Bedi PMS, Triazole tethered isatin-coumarin based molecular hybrids as novel antitubulin agents: Design, synthesis, biological investigation and docking studies, Bioorg. Med. Chem. Lett. 27 (2017) 3974–3979. [DOI] [PubMed] [Google Scholar]
- [51].Kamath PR, Sunil D, Josheph MM, Salam AAA, Sreelekha TT, Indole-coumarin-thiadiazole hybrids: An appraisal of their MCF-7 cell growth inhibition, apoptotic, antimetastatic and computational Bcl-2 binding potential, Eur. J. Med. Chem. 136 (2017) 442–451. [DOI] [PubMed] [Google Scholar]
- [52].Mokale SN, Begum A, Sakle NS, Shelke VR, Bhavale SA, Design, synthesis and anticancer screening of 3-(3-(substituted phenyl) acryloyl)-2H-chromen-2ones as selective anti-breast cancer agent, Biomed. Pharmacother. 89 (2017) 966–972. [DOI] [PubMed] [Google Scholar]
- [53].Falcon IH, Amesty A, Afonso LA, Castrillejo IL, Machin F, Estevez Braun A, Synthesis and Biological Evaluation of Naphthoquinone-Coumarin Conjugates as Topoisomerase II Inhibitors, Bioorg. Med. Chem. Lett. 27 (2017) 484–489. [DOI] [PubMed] [Google Scholar]
- [54].Elshemy HAH, Zaki MA, Design and synthesis of new coumarin hybrids and insight into their mode of antiproliferative action, Bioorg. Med. Chem. 25 (2017) 1066–1075. [DOI] [PubMed] [Google Scholar]
- [55].Poczobutt JM, Nguyen TT, Hanson D, Li H, Sippel TR, Weiser Evans MC, Gijon M, Murphy RC, Nemenoff RA, Deletion of 5-lipoxygenase in the tumor microenvironment promotes lung cancer progression and metastasis through regulating T cell recruitment, J. Immunol. 196 (2016) 891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Meng Z, Cao R, Yang Z, Liu T, Wang Y, Wang X, Inhibitor of 5-lipoxygenase, zileuton, suppresses prostate cancer metastasis by upregulating E-cadherin and paxillin, Urology. 82 (2013) 1452–1452. [DOI] [PubMed] [Google Scholar]
- [57].Xu XM, Deng JJ, Yuan GJ, Yang F, Guo HT, Xiang M, Ge W, Wu YG, 5-Lipoxygenase contributes to the progression of hepatocellular carcinoma, Mol. Med. Rep. 4 (2011) 1195–1200. [DOI] [PubMed] [Google Scholar]
- [58].Steele VE, Holmes CA, Hawk ET, Kopelovich L, Lubet RA, Crowell JA, Sigman CC, Kelloff GJ, Lipoxygenase inhibitors as potential cancer chemopreventives, Cancer Epidemiol. Biomarkers Prev. 8 (1999) 467–483. [PubMed] [Google Scholar]
- [59].Kraljevic TG, Harej A, Sedi M, Pavelic SK, Stepanic V, Drenjancevic D, Talapko J, Malic SR, Synthesis, in vitro anticancer and antibacterial activities and in silico studies of new 4-substituted 1,2,3-triazole-coumarin hybrids, Eur. J. Med. Chem. 124 (2016) 794–808. [DOI] [PubMed] [Google Scholar]
- [60].Burley SK, Petsko GA, Aromatic-aromatic interaction: a mechanism of protein structure stabilization, Science. 229 (1985) 23–28. [DOI] [PubMed] [Google Scholar]
- [61].Singh H, Kumar M, Nepali K, Gupta MK, Saxena AK, Sharma S, Bedi PMS, Triazole tethered C5-curcuminoid-coumarin based molecular hybrids as novel antitubulin agents: Design, synthesis, biological investigation and docking studies, Eur. J. Med. Chem. 116 (2016) 102–115. [DOI] [PubMed] [Google Scholar]
- [62].(a) Eren G, Unlu S, Nunez MT, Labeaga L, Ledo F, Entrena A, Banoglu E, Costantino G, Sahin MF, Synthesis, biological evaluation, and docking studies of novel heterocyclic diaryl compounds as selective COX-2 inhibitors, Bioorg. Med. Chem. 18 (2010) 6367–6376 [DOI] [PubMed] [Google Scholar]; (b) Saeed A, Ibrar A, Synthesis of Some New 3-(5-(Arylamino)-1,3,4-thiadiazol-2-yl)-2H-chromen-2-ones and 3-(4-Aryl-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-3-YL)-2H-chromen-2-ones, Phosphorus Sulfur Silicon, Relat. Elem. 186 (2010) 1801–1810 [Google Scholar]; (c) Raza A, Saeed A, Ibrar A, Muddassar M, Khan AA, Iqbal J, Pharmacological Evaluation and Docking Studies of 3-Thiadiazolyl- and Thioxo-1,2,4-triazolylcoumarin Derivatives as Cholinesterase Inhibitors, ISRN Pharmacol. 2012 (2012) 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Saeed A, Channar PA, Iqbal Q, Mahar J, C–H Arylation using acyl thiourea ligands: Applications in the synthesis of 3,6-diaryl-[1,2,4-]triazolo[3,4-b][1,3,4]thiadiazoles, Chin. Chem. Lett. 27 (2015) 37–40 [Google Scholar]; (e) Tsay SC, Hwua JR, Singha R, Huang WC, Chang YH, Hsu MH, Shieh FK, Lin CC, Hwang KC, Horng JC, Clercq ED, Vliegen I, Neyts J, Coumarins hinged directly on benzimidazoles and their ribofuranosides to inhibit hepatitis C virus, Eur. J. Med. Chem. 63 (2013) 290–298. [DOI] [PubMed] [Google Scholar]
- [63].Ibrar A, Zaib S, Khan I, Jabeen F, Iqbal J, Saeed A, Facile and expedient access to bis-coumarin–iminothiazole hybrids by molecular hybridization approach: synthesis, molecular modelling and assessment of alkaline phosphatase inhibition, anticancer and antileishmanial potential, RSC Adv. 5 (2015) 89919–89931. [Google Scholar]
- [64].Kamath PR, Sunil D, Ajees AA, Pai KS, Das S, Some new indole-coumarin hybrids; Synthesis, anticancer and Bcl-2 docking Studies, Bioorg. Chem. 63 (2015) 101–109. [DOI] [PubMed] [Google Scholar]
- [65].Goel R, Luxami V, Paul K, Synthesis, in vitro anticancer activity and SAR studies of arylated imidazo[1,2-a]pyrazine-coumarin hybrids, RSC Adv. 5 (2015) 37887–37895. [Google Scholar]
- [66].(a) Borowski E, Bontemps-Gracz MM, Piwkowska A, Strategies for overcoming ABC-transporters-mediated multidrug resistance (MDR) of tumor cells, Acta. Biochim. Pol. 52 (2005) 609–627 [PubMed] [Google Scholar]; (b) Zhang H, Li Y, Jiang ZX, Fluorine is flourishing in pharmaceuticals, J. Biomol. Res. Ther. Epub. 5 (2012) DOI: 10.4172/2167-7956.1000e107; [DOI] [Google Scholar]; (c) Elliott AJ, Fluorinated Pharmaceuticals. In Chemistry of Organic Fluorine Compounds II. A Critical Review, ACS Monograph 187 (Hudlicky M, and Pavlath AE, Ed.), pp 1119–1125, American Chemical Society, Washington, DC. [Google Scholar]
- [67].(a) Iyer VV, Griesgraber GW, Radmer MR, McIntee EJ, Wagner CR, Synthesis, in Vitro Anti-Breast Cancer Activity, and Intracellular Decomposition of Amino Acid Methyl Ester and Alkyl Amide Phosphoramidate Monoesters of 3’-Azido-3’-deoxythymidine (AZT), J. Med. Chem. 43 (2000) 2266–2274 [DOI] [PubMed] [Google Scholar]; (b) Focher F, Ubiali D, Pregnolato M, Zhi C, Gambino J, Wright GE, Spadari S, Novel Nonsubstrate Inhibitors of Human Thymidine Phosphorylase, a Potential Target for Tumor-Dependent Angiogenesis, J. Med. Chem. 43 (2000) 2601–2607 [DOI] [PubMed] [Google Scholar]; (c) Rostom SAF, Ashour HMA, Razik HAAE, Synthesis and biological evaluation of some novel polysubstituted pyrimidine derivatives as potential antimicrobial and anticancer agents, Arch. Pharm. 342 (2009) 299–310 [DOI] [PubMed] [Google Scholar]; (d) Lomax N, Narayanan VL, Chemical Structures of Interest to the Division of Cancer Treatment, In Developmental Therapeutics Program. National Cancer Institute, Vol 4 (1979) 32, [Google Scholar]; (e) Griffith R, Tracy T, Foye’s Principles of Medicinal Chemistry (Williams DA, and Lemke TL Ed.) 5 (2004) 900, Lippincott Williams and Wilkins, Baltimore, MD; [Google Scholar]; (f) Clercq ED, Antiviral drugs in current clinical use, J. Clin. Virol. 30 (2004) 115–133. [DOI] [PubMed] [Google Scholar]
- [68].Hosamani KM, Reddy DS, Devarajegowda HC, Microwave-assisted synthesis of new fluorinated coumarin–pyrimidine hybrids as potent anticancer agents, their DNA cleavage and X-ray crystal Studies, RSC Adv. 5 (2015) 1126–11271. [Google Scholar]
- [69].Pingaew R, Saekee A, Mandi P, Nantasenamat C, Prachayasittikul S, Ruchirawat S, Prachayasittikul V, Synthesis, biological evaluation and molecular docking of novel chalcone-coumarin hybrids as anticancer and antimalarial agents, Eur. J. Med. Chem. 85 (2014) 65–76. [DOI] [PubMed] [Google Scholar]
- [70].Tan G, Yao Y, Gu Y, Li S, Lv M, Wang K, Chen H, Li X, Cytotoxicity and DNA binding property of the dimers of triphenylethylene–coumarin hybrid with one amino side chain, Bioorg. Med. Chem. Lett. 24 (2014) 2825–30. [DOI] [PubMed] [Google Scholar]
- [71].Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y, Histone demethylation mediated by the nuclear amine oxidase homolog LSD1, Cell. 119 (2004) 941–953. [DOI] [PubMed] [Google Scholar]
- [72].(a) Jing H, Sengupta R, Espejo AB, Gyu LM, Dorsey JA, Richter M, Opravil S, Shiekhattar R, Bedford MT, Jenuwein T, Berger SL, p53 is regulated by the lysine demethylase LSD1, Nature. 449 (2007) 105–108; [DOI] [PubMed] [Google Scholar]; (b) Jing W, Hevi S, Kurash JK, Hong L, Gay F, Bajko J, Hui S, Weitao S, Hua C, Guoliang X, Gaudet F, En L, Taiping C, The lysine demethylase LSD1 (KDM1) is required for maintenance of global DNA methylation, Nat. Genet. 41(2009) 125–129. [DOI] [PubMed] [Google Scholar]
- [73].Ye XW, Zheng YC, Duan YC, Wang MM, Yu B, Ren JL, Ma JL, Zhanga E, Liu HM. Synthesis and biological evaluation of coumarin–1,2,3-triazole-dithiocarbamate hybrids as potent LSD1 inhibitors, Med. Chem. Commun. 5 (2014) 650. [Google Scholar]
- [74].(a) Chen Y, Liu HR, Liu HS, Cheng M, Xia P, Qian K, Wu PC, Lai CY, Xia Y, Yang ZY, Natschke SLM, Lee KH, Design, synthesis and pharmacological study of S- and O-substituted 7-mercapto- or hydroxy-coumarins and chromones as potent cytotoxic agents, Eur. J. Med. Chem. 49 (2012) 74–85. [DOI] [PubMed] [Google Scholar]; (b) Fong WF, Shen XL, Globisch C, Wiese M, Chen GY, Zhu GY, Yu ZL, Tse AKW, Hu YJ, Methoxylation of 3′,4′-aromatic side chains improves P-glycoprotein inhibitory and multidrug resistance reversal activities of 7,8-pyranocoumarin against cancer cells, Bioorg. Med. Chem. 16 (2008) 3694–3703; [DOI] [PubMed] [Google Scholar]; (c) Zhou T, Shi Q, Bastow KF, Lee KH, Antitumor Agents 286. Design, Synthesis, and Structure−Activity Relationships of 3′R,4′R-Disubstituted-2′,2′-dimethyldihydropyrano[2,3-f]chromone (DSP) Analogues as Potent Chemosensitizers to Overcome Multidrug Resistance, J. Med. Chem. 53 (2010) 8700–8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Vicini P, Incerti M, Doytchinova IA, Colla PL, Busonera B, Loddo R, Synthesis and antiproliferative activity of benzo[d]isothiazole hydrazones, Eur. J. Med. Chem. 41(2006) 624–632. [DOI] [PubMed] [Google Scholar]
- [76].Kumar D, Kumar NM, Ghosh S, Shah K, Novel bis(indolyl)hydrazide-hydrazones as potent cytotoxic agents, Bioorg. Med. Chem. Lett. 22 (2012) 212–215. [DOI] [PubMed] [Google Scholar]
- [77].Nasr T, Bondock S, Youns M, Anticancer activity of new coumarin substituted hydrazide-hydrazone derivatives, Eur. J. Med. Chem. 76 (2014) 539–548. [DOI] [PubMed] [Google Scholar]
- [78].Zhang X, Rakesh KP, Bukhari SNA, Balakrishna M, Manukumar HM, Qin HL, Multi-targetable chalcone analogs to treat deadly Alzheimer’s disease: Current view and upcoming advice, Bioorg. Chem. 80 (2018) 86–93. [DOI] [PubMed] [Google Scholar]
- [79].Zha GF, Zhang CP, Qin HL, Jantan I, Sher M, Amjad MW, Hussain MA, Hussain Z, Bukhari SNA, Biological evaluation of synthetic α,β-unsaturated carbonyl based cyclohexanone derivatives as neuroprotective novel inhibitors of acetylcholinesterase, butyrylcholinesterase and amyloid- β Aggregation, Bioorg. Med. Chem. 24 (2016) 2352–2359. [DOI] [PubMed] [Google Scholar]
- [80].Lenga J, Qin HL, Zhu K, Jantan I, Hussain MA, Sher M, Amjad MW, Naeemul-Hassan M, Ahmad W, Bukhari SNA, Evaluation of multifunctional synthetic tetralone derivatives for treatment of Alzheimer’s disease, Chem. Biol. Drug. Des. 88 (2016) 889–989. [DOI] [PubMed] [Google Scholar]
- [81].(a) Wang M, Qin HL, Leng J, Ameeduzzafar MW Amjad MAG Raja MA Hussain SNA Bukhari, Synthesis and biological evaluation of new tetramethylpyrazine-based chalcone derivatives as potential anti-Alzheimer Agents. Chem. Biol. Drug. Des. 92 (2018) 1859–1866. [DOI] [PubMed] [Google Scholar]; (b) Kinarivala N, Shah K, Abbruscato TJ, Trippier PC, Passage variation of PC12 cells results in inconsistent susceptibility to externally induced apoptosis, ACS Chem. Neurosci. 8 (2017) 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Lan JS, Ding Y, Liu Y, Kang P, Hou JW, Zhang XY, Xie SS, Zhang T, Design, synthesis and biological evaluation of novel coumarin-N-benzyl pyridinium hybrids as multi-target agents for the treatment of Alzheimer’s disease, Eur. J. Med. Chem. 139 (2017) 48–59. [DOI] [PubMed] [Google Scholar]
- [83].(a) Bolea I, Gella A, Unzeta M, Propargylamine-derived multitarget-directed ligands: fighting Alzheimer’s disease with monoamine oxidase inhibitors, J. Neural. Transm. 120 (2013) 893–902; [DOI] [PubMed] [Google Scholar]; (b) Zindo FT, Joubert J, Malan SF, Propargylamine as functional moiety in the design of multifunctional drugs for neurodegenerative disorders: MAO inhibition and beyond, Future Med. Chem. 7 (2015) 609–629. [DOI] [PubMed] [Google Scholar]
- [84].Yang HL, Cai P, Liu QH, Yang XL, Li F, Wang J, Wu JJ, Wang XB, Kong LY, Design, synthesis and evaluation of coumarin-pargyline hybrids as novel dual inhibitors of monoamine oxidases and amyloid-β aggregation for the treatment of Alzheimer’s disease, Eur. J. Med. Chem. 138 (2017) 715–728. [DOI] [PubMed] [Google Scholar]
- [85].Xie SS, Wang X, Jiang N, Yu W, Wang KD, Lan JS, Li ZR, Kong LY, Multi-target tacrine-coumarin hybrids: Cholinesterase and monoamine oxidase B inhibition properties against Alzheimer’s disease, Eur. J. Med. Chem. 95 (2015) 153–165. [DOI] [PubMed] [Google Scholar]
- [86].Sugimoto H, Yamanish Y, Iimura Y, Kawakami Y, Donepezil hydrochloride (E2020) and other acetylcholinesterase inhibitors, Curr. Med. Chem. 7 (2000) 303–339. [DOI] [PubMed] [Google Scholar]
- [87].Sepsova V, Karasova JZ, Tobin G, Jun D, Korabecny J, Cabelova P, Janska K, Krusek J, Skrenkova K, Kuca K, Soukup O, Cholinergic properties of new 7-methoxytacrine-donepezil derivatives, Gen. Physiol. Biophys. 34 (2015) 189–200. [DOI] [PubMed] [Google Scholar]
- [88].(a) Farina R, Pisani L, Catto M, Nicolotti O, Gadaleta D, Denora N, Soto-Otero R, Mendez-Alvarez E, Passos CS, Muncipinto G, Altomare CD, Nurisso A, Carrupt PA, Carotti A, Structure-Based Design and Optimization of Multitarget-Directed 2H-Chromen-2-one Derivatives as Potent Inhibitors of Monoamine Oxidase B and Cholinesterases, J. Med. Chem. 58 (2015) 5561–5578; [DOI] [PubMed] [Google Scholar]; (b) Pisani L, Catto M, Giangreco I, Leonetti F, Nicolotti O, Stefanachi A, Cellamare S, Carotti A, Design, Synthesis, and Biological Evaluation of Coumarin Derivatives Tethered to an Edrophonium-like Fragment as Highly Potent and Selective Dual Binding Site Acetylcholinesterase Inhibitors, Chem. Med. Chem. 5 (2010) 1616–1630; [DOI] [PubMed] [Google Scholar]; (c) Gnerre C, Catto M, Leonetti F, Weber P, Carrupt PA, Altomare C, Carotti A, Testa B, Inhibition of Monoamine Oxidases by Functionalized Coumarin Derivatives: Biological Activities, QSARs, and 3D-QSARs, J. Med. Chem. 43 (2000) 4747–4758. [DOI] [PubMed] [Google Scholar]
- [89].Xie SS, Lan JS, Wang X, Wang ZM, Jiang N, Li F, Wu JJ, Wang JLY, Design, Synthesis and Biological Evaluation of Novel Donepezil-Coumarin Hybrids as Multi-Target Agents for the Treatment of Alzheimer’s Disease, Bioorg Med Chem. 24 (2016) 1528–39. [DOI] [PubMed] [Google Scholar]
- [90].Singla S, Piplani P. Coumarin derivatives as potential inhibitors of acetylcholinesterase: Synthesis, molecular docking and biological studies, Bioorg Med. Chem. 24 (2016) 4587–4599. [DOI] [PubMed] [Google Scholar]
- [91].Hamulakova S, Poprac P, Jomova K, Brezova V, Lauro P, Drostinova L, Jun D, Sepsova V, Hrabinova M, Soukup O, Kristian P, Gazova Z, Bednarikova Z, Kuca K, Valko M, Targeting copper(II)-induced oxidative stress and the acetylcholinesterase system in Alzheimer’s disease using multifunctional tacrine-coumarin hybrid Molecules, J Inorg Biochem. 161 (2016) 52–62. [DOI] [PubMed] [Google Scholar]
- [92].Pisani L, Farina F, Catto M, Iacobazzi RM, Nicolotti O, Cellamare S, Mangiatordi GF, Denora N, Soto-Otero R, Siragusa L, Altomare CD, Carotti A, Exploring Basic Tail Modifications of Coumarin-Based Dual Acetylcholinesterase-Monoamine Oxidase B Inhibitors: Identification of Water-Soluble, Brain-Permeant Neuroprotective Multitarget Agents, J. Med. Chem. 59 (2016) 6791–6806. [DOI] [PubMed] [Google Scholar]
- [93].Ghanei-Nasab S, Khoobi M, Hadizadeh F, Marjani A, Moradi A, Nadri H, Emami S, Foroumadi A, Shafiee A, Synthesis and anticholinesterase activity of coumarin-3-carboxamides bearing tryptamine moiety, Eur. J. Med. Chem. 121 (2016) 40–46. [DOI] [PubMed] [Google Scholar]
- [94].Riederer P, Youdim MB, Monoamine Oxidase Activity and Monoamine Metabolism in Brains of Parkinsonian Patients Treated with l-Deprenyl, J. Neurochem. 46 (1986) 1359–1365. [DOI] [PubMed] [Google Scholar]
- [95].Xie SS, Wang X, Jiang N, Yu W, Wang KDG, Lan JS, Li ZR, Kong LY, Multi-target tacrine-coumarin hybrids: Cholinesterase and monoamine oxidase B inhibition properties against Alzheimer’s Disease. Eur. J. Med. Chem. 95 (2015) 153–165. [DOI] [PubMed] [Google Scholar]
- [96].Chaudhaery SS, Roy KK, Shakya N, Saxena G, Sammi SR, Nazir A, Nath C, Saxena AK, Novel Carbamates as Orally Active Acetylcholinesterase Inhibitors Found to Improve Scopolamine-Induced Cognition Impairment: Pharmacophore-Based Virtual Screening, Synthesis, and Pharmacology, J. Med. Chem. 53 (2010) 6490–6505. [DOI] [PubMed] [Google Scholar]
- [97].Rosini M, Simoni E, Bartolini M, Cavalli A, Ceccarini L, Pascu N, McClymont DW, Tarozzi A, Bolognesi ML, Minarini A, Tumiatti V, Andrisano V, Mellor IR, Melchiorre C, Inhibition of Acetylcholinesterase, β-Amyloid Aggregation, and NMDA Receptors in Alzheimer’s Disease: A Promising Direction for the Multi-target-Directed Ligands Gold Rush, J. Med. Chem. 51 (2008) 4381–4348. [DOI] [PubMed] [Google Scholar]
- [98].Kinarivala N, Patel R, Boustany RM, Al-Ahmad A, Trippier PC, Discovery of Aromatic Carbamates that Confer Neuroprotective Activity by Enhancing Autophagy and Inducing the Anti-Apoptotic Protein B-Cell Lymphoma 2 (Bcl-2), J. Med. Chem. 60 (2017) 9739–9756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Makouji J, Saadeh F, Mansou KA, El-Sitt S, Kinarivala N, Trippier PC, Boustany RM, Flupirtine Derivatives as Potential Treatment for the Neuronal Ceroid Lipofuscinoses, Ann. Clin. Transl. Neurol. 5 (2018) 1089–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Sun Q, Peng DY, Yang SG, Zhu XL, Yang WC, Yang GF, Syntheses of Coumarin–Tacrine Hybrids as Dual-site Acetylcholinesterase Inhibitors and Their activity against Butylcholinesterase, Aβ aggregation, and β-Secretase, Bioorg. Med. Chem. 22 (2014) 4784–4791. [DOI] [PubMed] [Google Scholar]
- [101].Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN, Hyperglycemic Crises in Adult Patients with Diabetes, Diabetes Care. 32 (2009) 1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Lopez-Candales A, Metabolic syndrome X: a comprehensive review of the pathophysiology and recommended therapy, J. Med. 32 (2001) 283–300. [PubMed] [Google Scholar]
- [103].Deshpande AD, Harris-Hayes M, Schootman M, Epidemiology of diabetes and diabetes-related complications. Phys. Ther. 88 (2008) 1254–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Ibrar A, Zaib S, Khan I, Shafique Z, Saeed A, Iqbal J, New prospects for the development of selective inhibitors of α-glucosidase based on coumarin-iminothiazolidinone hybrids: Synthesis, in-vitro biological screening and molecular docking analysis, J. Taiwan Inst. Chem. E. 81 (2017) 119–133. [Google Scholar]
- [105].Pan G, Zhao L, Xiao N, Yang K, Ma Y, Zhao X, Fan Z, Zhang Y, Yao Q, Lu K, Yu P, Total synthesis of 8-(600-umbelliferyl)-apigenin and its analogs as anti-diabetic reagents, Eur. J. Med. Chem. 122 (2016) 674–683. [DOI] [PubMed] [Google Scholar]
- [106].Salar U, Taha M, Khan KM, Ismail NH, Imran S, Perveen S, Gul S, Wadood A, Syntheses of new 3-thiazolyl coumarin derivatives, in vitro α-glucosidase inhibitory activity, and molecular modeling studies, Eur. J. Med. Chem. 122 (2016) 196–204. [DOI] [PubMed] [Google Scholar]
- [107].Wang G, He D, Li X, Li J, Peng Z, Design, synthesis and biological evaluation of novel coumarin thiazole derivatives as a-glucosidase inhibitors, Bioorg. Chem. 65 (2016) 167–174. [DOI] [PubMed] [Google Scholar]
- [108].Ibrar A, Tehseen Y, Khan I, Hameed A, Hameed A. Saeed, N. Furtmann, J. Bajorath, J. Iqbal, Coumarin-thiazole and -oxadiazole derivatives: Synthesis, bioactivity and docking studies for aldose/aldehyde reductase inhibitors, Bioorg. Chem. 68 (2016) 177–186. [DOI] [PubMed] [Google Scholar]
- [109].Zha GF, Leng J, Darshini N, Shubhavathi T, Vivek HK, Asiri AM, Marwani HM, Rakesh KP, Mallesha N, Qin HL, Synthesis SAR and molecular docking studies of benzo[d]thiazolehydrazones as potential antibacterial and antifungal agents, Bioorg. Med. Chem. Lett. 27 (2017) 3148–3155. [DOI] [PubMed] [Google Scholar]
- [110].Wang M, Rakesh KP, Leng J, Fang WY, Ravindar L, Gowda DC, Qin HL, Amino acids/peptides conjugated heterocycles: A tool for the recent development of novel therapeutic agents, Bioorg. Chem. 76 (2018) 113–129. [DOI] [PubMed] [Google Scholar]
- [111].Ravindara L, Bukharia SNA, Rakesha KP, Manukumarb HM, Vivekc HK, Malleshad N, Xiea ZZ, Qin HL, Aryl fluorosulfate analogues as potent antimicrobial agents: SAR, cytotoxicity and docking studies, Bioorg. Chem. 81 (2018) 107–118. [DOI] [PubMed] [Google Scholar]
- [112].Zha GF, Wang SM, Rakesh KP, Bukhari SNA, Manukumar HM, Vivek HK, Mallesha N, Qin HL, Discovery of novel arylethenesulfonyl fluorides as potential candidates against methicillin-resistant of Staphylococcus aureus (MRSA) for overcoming multidrug resistance of bacterial infections, Eur. J. Med. Chem. 162 (2019) 364–377. [DOI] [PubMed] [Google Scholar]
- [113].Fang WY, Ravindar L, Rakesh KP, Manukumar HM, Shantharam CS, Alharbi NS, Qin HL, Synthetic approaches and pharmaceutical applications of chloro-containing molecules for drug discovery: A critical review, Eur. J. Med. Chem. 173 (2019) 117–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Zhang X, Manukumar HM, Rakesh KP, Karthik CS, Prasad HSN, Nanjunda Swamy S, Mallu P, Mohammed YHE, Qin HL, Role of BP*C@AgNPs in Bap-dependent multicellular behavior of clinically important methicillin-resistant Staphylococcus aureus (MRSA) biofilm adherence: A key virulence study, Microb. Pathog. 123 (2018) 275–284. [DOI] [PubMed] [Google Scholar]
- [115].Zhang X, Marichannegowdab MH, Rakesh KP, Qin HL, Master mechanisms of Staphylococcus aureus: consider its excellent protective mechanisms hindering vaccine development!, Microb. Res. 212 (2018) 59–66. [DOI] [PubMed] [Google Scholar]
- [116].Rakesh KP, Marichannegowda MH. Srivastava S, Chen X, Long S, Karthik CS, Mallu P, Qin HL, Combating a Master Manipulator: Staphylococcus aureus Immunomodulatory Molecules as Targets for Combinatorial Drug Discovery, ACS Comb. Sci. 20 (2018) 681–693. [DOI] [PubMed] [Google Scholar]
- [117].Manukumar HM, Chandrasekhar B, Rakesh KP, Anand AP, Nandhini M, Lalitha P, Sumathi S, Qin HL, Umesha S, Novel T-C@AgNPs mediated biocidal mechanism against 1 biofilm associated 2 methicillin-resistant Staphylococcus aureus (Bap-MRSA) 090, cytotoxicity and 3 its molecular docking studies, Med. Chem. Commun. 8 (2017) 2181–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Rakesh KP, Shantharam CS, Sridhara MB, Manukumar HM, L Qin H, Benzisoxazole: a privileged scaffold for medicinal Chemistry, Med. Chem. Commun. 8 (2017) 2023–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Rakesh R, Chavan M. Kallappa, Hosamani D. Badarinath, Kulkarni D. Shrinivas, Joshi, Molecular docking studies and facile Synthesis of most potent biologically active N-tert-butyl-4-(4-substituted phenyl)-2-((substituted-2-oxo-2H-chromen-4-yl)methylthio)-6-oxo-1,6-dihydropyrimidine-5-carboxamide hybrids: An approach for microwave assisted syntheses and biological evaluation, Bioorg. Chem. 78 (2018) 185–194. [DOI] [PubMed] [Google Scholar]
- [120].Sumitra N, Mangasuli, Kallappa M, Hosamani, Hirihalli C, Devarajegowda, Mahantesh M, Kurjogi, Shrinivas D, Joshi (2018) Synthesis of coumarin-theophylline hybrids as a new class of antitubercular and anti-microbial agents, Eur. J. Med. Chem. 146, 747–756. [DOI] [PubMed] [Google Scholar]
- [121].Gupta S, Kushwaha B, Srivastava A, Maikhuri JP, Sankhwar SN, Gupta G, Dwivedi AK, Design and synthesis of coumarin–glyoxal hybrids for spermicidal and antimicrobial actions: a dual approach to contraception, RSC Adv. 6 (2016) 76288–76297. [Google Scholar]
- [122].Rajesh H, Vekariya D. Kinjal, Patel P. Dhanji, Rajani D. Smita, Rajani D. Hitesh, Patel, A one pot, three component synthesis of coumarin hybrid thiosemicarbazone derivatives and their antimicrobial evolution, J. Assoc. Arab Univ. Basic Appl. Sci. 23 (2017) 10–19. [Google Scholar]
- [123].Eren G, Unlu S, Nunez MT, Labeaga L, Ledo F, Entrena A, Lu EB, Costantin G, Sahin MF, Synthesis, biological evaluation, and docking studies of novel heterocyclic diaryl compounds as selective COX-2 inhibitors. Bioorg. Med. Chem. 18 (2010) 6367–6376. [DOI] [PubMed] [Google Scholar]
- [124].(a) Saeed A, Ibrar A, Synthesis of Some New 3-(5-(Arylamino)-1,3,4-thiadiazol-2-yl)-2H-chromen-2-ones and 3-(4-Aryl-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-3-YL)-2H-chromen-2-ones, Phosphorus, Sulfur Silicon Relat. Elem. 186 (2011) 1801–1810; [Google Scholar]; (b) Raza A, Saeed A, Ibrar A, Muddassar M, Khan AA, Iqbal J, Pharmacological Evaluation and Docking Studies of 3-Thiadiazolyl- and Thioxo-1,2,4-triazolylcoumarin Derivatives as Cholinesterase Inhibitors, ISRN Pharmacol. 2012 (2012) 1–11; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Saeed A, Channar PA, Iqbal Q, Mahar J, C–H arylation using acyl thiourea ligands: Applications in the 4 synthesis of 3,6-diaryl-[1,2,4-]triazolo[3,4-b][1,3,4]thiadiazoles, Chin. Chem. Lett. 27 (2016) 37–40. [Google Scholar]
- [125].Tsay SC, Hwua JR, Singha R, Huang WC, Chang YH, Hsu MH, Shieh FK, Lin CC, Hwang KC, Horng JC, de-Clercq E, Vliegen I, Neyts J, Coumarins hinged directly on benzimidazoles and their ribofuranosides to inhibit hepatitis C virus, Eur. J. Med. Chem. 63 (2013) 290–298. [DOI] [PubMed] [Google Scholar]
- [126].Soraya Z, Ferreira C. Hellem, Carneiro, Hugo A, Lara, Rosemeire B, Alves, Jarbas M, Resende, Heloisa M, Oliveira, Luciana M, Silva, Daniel A, Santos, Rossimiriam P, Freitas, Soraya Z, Ferreira, Hellem C, Carneiro, Hugo A, Lara, Rosemeire B, Alves, Jarbas M, Resende, Heloisa M, Oliveira, Luciana M, Silva, Daniel A, Santos, Rossimiriam P, Freitas, Synthesis of a New Peptide-Coumarin Conjugate: A Potential Agent against Cryptococcosis, ACS Med. Chem. Lett. 6 (2015) 271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Saeeda A, Mahesara PA, Channara PA, Larika FA, Abbasb Q, Hassanb M, Razab H, Seob S,Y, Hybrid Pharmacophoric Approach in the Design and Synthesis of Coumarin Linked Pyrazolinyl as Urease Inhibitors, Kinetic Mechanism and Molecular Docking, Chem. Biodivers. 14 (2017) 1–14. [DOI] [PubMed] [Google Scholar]
- [128].Niu H, Wang W, Li J, Lei Y, Zhao Y, Yang W, Zhao C, Lin B, Song S, Wang S, A novel structural class of coumarin-chalcone fibrates as PPARa/g agonists with potent antioxidant activities: Design, synthesis, biological evaluation and molecular docking studies, Eur. J. Med. Chem. 138 (2017) 212–220. [DOI] [PubMed] [Google Scholar]
- [129].Perez-Cruz K, Moncada-Basualto M, Morales-Valenzuela J, Barriga-Gonza lez G, Navarrete-Encina P, ez-Vergara LN, Squella JA, Olea-Azar C, Synthesis and antioxidant study of new polyphenolic hybrid-coumarins, Arab. J. Chem. 11 (2018) 525–537. [Google Scholar]
- [130].(a) Sashidhara KV, Kumar A, Kumar M, Sarkar J, Sinha S, Synthesis and in vitro Evaluation of Novel Coumarin-Chalcone Hybrids as Potential Anticancer Agents, Bioorg. Med. Chem. Lett. 20 (2010) 7205–7211; [DOI] [PubMed] [Google Scholar]; (b) Hamdi N, Fischmeister C, Carmen Puerta M, Valerga PA, Rapid Access to New Coumarinyl Chalcone and Substituted chromeno[4,3-c]pyrazol-4(1H)-ones and their Antibacterial and DPPH Radical Scavenging Activities, Med. Chem. Res. 20 (2011) 522–530; [Google Scholar]; (c) Perez-Cruz F, Vazquez-Rodriguez S, Joao Matos M, Herrera- Morales A, Villamena FA, Das A, Gopalakrishnan B, Olea-Azar C, Santana L, Uriarte E, Synthesis and Electrochemical and Biological Studies of Novel Coumarin-Chalcone Hybrid Compounds, J. Med. Chem. 56 (2013) 6136–6145; [DOI] [PubMed] [Google Scholar]; (d) Xi GL, Liu ZQ, Coumarin Moiety Can Enhance Abilities of Chalcones to Inhibit DNA Oxidation and to Scavenge Radicals, Tetrahedron 70 (2014) 8397–8404. [Google Scholar]
- [131].Mazzone G, Malaj N, Galano A, Russo N, Toscano M, Antioxidant Properties of Several Coumarin-Chalcone Hybrids from Theoretical Insights, RSC Adv. 5 (2015) 565–575. [Google Scholar]
- [132].Vazquez-Rodriguez S, Figueroa-Guinez R, Joao-Matos M, Santana L, Uriarte E, Lapier M, Diego Maya J, Olea-Azar C, Synthesis of Coumarin-Chalcone Hybrids and Evaluation of their Antioxidant and Trypanocidal Properties, Med. Chem. Comm. 4 (2013) 993–1000. [Google Scholar]
- [133].Mazzone G, Galano A, Alvarez-Idaboy JR, Russo N, Coumarin-Chalcone Hybrids as Peroxyl Radical Scavengers: Kinetics and Mechanisms, J. Chem. Inf. Model. 56 (2016) 662–670. [DOI] [PubMed] [Google Scholar]
- [134].Lia C, Sridhara MB, Rakesh KP, Vivek HK, Manukumar HM, Shantharam CS, Qin HL, Multi-targeted dihydrazones as potent biotherapeutics, Bioorg. Chem. 81 (2018) 389–395. [DOI] [PubMed] [Google Scholar]
- [135].Chen X, Leng J, Rakesh KP, Darshini N, Shubhavathi T, Vivek HK, Mallesh N, Qin HL, Synthesis and molecular docking studies of xanthone attached amino acids as potential antimicrobial and anti-inflammatory agents, Med. Chem. Commun. 8 (2017) 1706–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Karthik CS, Manukumar HM, Sandeep S, Sudarshan BL, Nagashree S, Mallesha L, Rakesh KP, Sanjay KR, Mallu P, Qin HL, Development of piperazine-1-carbothioamide chitosan silver nanoparticles (P1C-Tit*CAgNPs) as a promising anti-inflammatory candidate: a molecular docking validation, Med. Chem. Commun. 9 (2018) 713–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Wang SM, Zha GF, Rakesh KP, Darshini N, Shubhavathi T, Vivek HK, Mallesh N, Qin HL, Synthesis of benzo[d]thiazole-hydrazone analogues: molecular docking and SAR studies of potential H+/K+ ATPase inhibitors and anti-inflammatory Agents, Med. Chem. Commun. 8 (2017) 1173–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Shen FQ, Wang ZC, Wu SY, Ren SZ, Man RJ, Wang BZ, Zhu HL, Synthesis of novel hybrids of pyrazole and coumarin as dual inhibitors of COX-2 and 5-LOX. Bioorg. Med. Chem. Lett. 27 (2017) 3653–3660. [DOI] [PubMed] [Google Scholar]
- [139].Chen LZ, Sun WW, Bo L, Wang JQ, Xiu C, Tang WJ, Shi JB, Zhou HP, Liu XH, New arylpyrazoline-coumarins: Synthesis and anti-inflammatory activity. Eur. J. Med. Chem. 138 (2017) 170–181. [DOI] [PubMed] [Google Scholar]
- [140].El-Nezhawy AO, Biuomy AR, Hassan FS, Ismaiel AK, Omar HA, Design, synthesis and pharmacological evaluation of omeprazole-like agents with anti-inflammatory activity, Bioorg. Med. Chem. 21 (2013) 1661–1670. [DOI] [PubMed] [Google Scholar]
- [141].Arora RK, Kaur N, Bansal Y, Bansal G, Novel coumarin–benzimidazole derivatives as antioxidants and safer anti-inflammatory agents. Acta. Pharmaceutica. Sinica. B. 4 (2014) 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Kinarivala N, Suh JH, Botros M, Webb P, Trippier PC, Pharmacophore elucidation of phosphoiodyn A - Potent and selective peroxisome proliferator-activated receptor β/δ agonists with neuroprotective activity, Bioorg. Med. Chem. 26 (2016) 1889–93. [DOI] [PubMed] [Google Scholar]
- [143].(a) Pochet L, Doucet C, Dive G, Wouters J, Masereel B, Reboud-Ravaux M, Pirotte B, Coumarinic derivatives as mechanism-based inhibitors of alpha-chymotrypsin and human leukocyte elastase, Bioorg. Med. Chem. 8 (2000) 1489–1501; [DOI] [PubMed] [Google Scholar]; (b) Pochet L, Frederick R, Masereel B, Coumarin and isocoumarin as serine protease inhibitors, Curr. Pharm. Des. 10 (2004) 3781–3796. [DOI] [PubMed] [Google Scholar]
- [144].Frederick R, Charlier C, Robert S, Wouters J, Masereel B, Pochet L, Investigation of mechanism-based thrombin inhibitors: Implications of a highly conserved water molecule for the binding of coumarins within the S pocket, Bioorg. Med. Chem. Lett. 16 (2006) 2017–2021. [DOI] [PubMed] [Google Scholar]
- [145].Frederick R, Robert S, Charlier C, Wouters J, Masereel B, Pochet L, Mechanism-Based Thrombin Inhibitors: Design, Synthesis, and Molecular Docking of a New Selective 2-Oxo-2H-1-benzopyran Derivative, J. Med. Chem. 50 (2007) 3645–3650. [DOI] [PubMed] [Google Scholar]
- [146].Frederick R, Robert S, Charlier C, de Ruyck J, Wouters J, Pirotte B, Masereel B, Pochet L, 3,6-disubstituted coumarins as mechanism-based inhibitors of thrombin and factor Xa, J. Med. Chem. 48 (2005) 7592–7603. [DOI] [PubMed] [Google Scholar]
- [147].Kraft P, Schwarz T, Pochet L, Stoll G, Kleinschnitz C, COU254, a specific 3-carboxamide-coumarin inhibitor of coagulation factor XII, does not protect mice from acute ischemic stroke, Exp. Transl. Stroke. Med. 2 (2010) 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Bouckaert C, Serra S, Rondelet G, Dolusic E, Wouters J, Dogne JM, Frederick R, Pochet L, Synthesis, evaluation and structure-activity relationship of new 3-carboxamide coumarins as FXIIa inhibitors. Eur. J. Med. Chem. 110 (2016) 181–94 [DOI] [PubMed] [Google Scholar]
- [149].(a) Check E, Antidepressant bitter pills, Nature. 431 (2004) 122–124; [DOI] [PubMed] [Google Scholar]; (b) Licinio J, Wong ML, Depression, antidepressants and suicidality: a critical appraisal, Nat. Rev. Drug Discov. 4 (2005) 165–171. [DOI] [PubMed] [Google Scholar]
- [150].Sashidhara KV, Modukuri RK, Singh S, Rao KB, Teja GA, Gupta S, Shukla S, Design and synthesis of new series of coumarin–aminopyran derivatives possessing potential anti-depressant-like activity, Bioorg. Med. Chem. Lett. 25 (2015) 337–341. [DOI] [PubMed] [Google Scholar]
- [151].Singh H, Kinarivala N, Sharma S, Multi-targeting Anticancer Agents: Rational Approaches, Synthetic Routes and Structure Activity Relationship, Anticancer Agents Med. Chem. doi: 10.2174/1871520619666190118120708. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]