Abstract
In combination with current sociological trends, the maturing development of IoT devices is projected to revolutionize healthcare. A network of body-worn sensors, each with a unique ID, can collect health data that is orders-of-magnitude richer than what is available today from sporadic observations in clinical/hospital environments. When databased, analyzed, and compared against information from other individuals using data analytics, HIoT data enables the personalization and modernization of care with radical improvements in outcomes and reductions in cost. In this paper, we survey existing and emerging technologies that can enable this vision for the future of healthcare, particularly in the clinical practice of healthcare. Three main technology areas underlie the development of this field: (a) sensing, where there is an increased drive for miniaturization and power efficiency; (b) communications, where the enabling factors are ubiquitous connectivity, standardized protocols, and the wide availability of cloud infrastructure, and (c) data analytics and inference, where the availability of large amounts of data and computational resources is revolutionizing algorithms for individualizing inference and actions in health management. Throughout the paper, we use a case study to concretely illustrate the impact of these trends. We conclude our paper with a discussion of the emerging directions, open issues, and challenges.
Keywords: health management, clinical IoT, health monitoring, healthcare analytics, digital health, medical decision support
I. INTRODUCTION
A strong synergy exists between the technological advances in Internet of Things (IoT) and the emerging needs and directions of healthcare applications. With a rapid expansion in the deployment of IoT devices and increasing desire to make healthcare more cost-effective, personalized, and proactive, IoT is poised to play a strong role in all aspects of health management, and for our discussion, we refer to this important segment of IoT as the Healthcare Internet of Things (HIoT). HIoT can be broadly classified into two sub-categories: personal and clinical. Personal HIoT includes devices such as activity/heart rate trackers, smart clothes and smartwatches (e.g., Fitbit [1], Apple watch [2]) that are used by consumers for self-monitoring. These general purpose devices are not strictly regulated and are intended for use by consumers without involvement/guidance from physicians. Clinical HIoT devices are built specifically for health monitoring under the guidance —and with the involvement— of a physician. Examples include smart continuous glucose monitors [3] and connected inhalers [4]. These devices are intended for use in either clinical or home environments and are strictly regulated and approved for use only after clinical validation. This paper surveys the emerging field of clinical HIoT.
A confluence of social and technological trends is motivating the adoption of IoT in the clinical setting. On the social side, aging populations in the West are straining clinical institutions and healthcare costs are rising at significantly higher rates than baseline inflation [5]. On the technology side, most healthcare institutions are already connected to the internet and are, therefore, well poised to take advantage of the increasing availability of high bandwidth connectivity, inexpensive cloud storage and computation, and large-scale data analytics. HIoT technologies are attractive in this emerging scenario because they allow personalization of clinical healthcare enabling not only significant cost reductions but also improved outcomes through higher responsiveness, customization, and effective exploitation of aggregated data. HIoT can reduce the time taken to diagnose a health condition [6], provide efficient high-quality care, and help to reduce hospitalization costs [7] as well as the chance of readmission for the same health issue [8], [9]. Being connected with HIoT enables patients to provide continuous feedback to the doctors and monitor their own progress, which enhances patient engagement and satisfaction. Rich collections of longitudinal data from heterogeneous sources, made possible by HIoT adoption, also opens up new avenues for augmenting traditional diagnostic approaches employed by physicians. Specifically, data analytics can automatically flag physiological anomalies for further investigation and visualization technologies can summarize salient trends, without cognitively overloading physicians and interfering with their patient interactions in the clinic.
Several recent surveys address specific aspects of HIoT systems. Medical IoT devices focusing on personalized healthcare systems [10], and applications with toddlers/kids [11] have been reviewed. Applications of remote health monitoring systems such as body temperature monitoring and elderly care [12], and applications focusing on healthcare solutions using smartphones, ambient assisted living (AAL), and wearable devices have been studied in detail [13]. HIoT applications in rural healthcare focusing on improving healthcare in developing countries have also been reviewed [14]. Other survey papers include studies focusing on communication [15], security, and data privacy aspects of HIoT systems [16], [17]. HIoT in smart homes [18], high-risk environments, and safety industries [19], and HIoT based services for mental health [20] have also been the focus of several reviews. Distinct from these prior surveys, the present paper focuses particularly on —and provides end-to-end coverage for— the clinical side of HIoT. Additionally, the ideas discussed are concretely illustrated throughout the paper by using an example from the clinical setting, specifically from neurology.
The rest of the paper is organized as follows. Section II presents a discussion of trends, challenges, and application requirements that IoT adoption in the clinical settings faces. To motivate the subsequent discussions, example IoT-based health management applications are presented in Section III. This section also introduces a case study from the neurology field, which is used as a running example throughout the paper to concretely highlight the concepts presented in the paper. In Section IV, the overall architecture of a health management system is presented and its components, data sensing, acquisition and communication, aggregation, and pre-processing are further discussed in Section V and Section VI, respectively. Analytics and inference in healthcare applications are surveyed in Section VII, followed by a discussion of medical data visualization techniques in Section VIII. Section IX investigates security and privacy considerations of HIoT. Finally, Section X concludes the paper with our vision for the future.
II. TRENDS, APPLICATION DEMANDS, AND CHALLENGES
The majority of prior surveys on smart healthcare limit their discussion to specific aspects of the field such as sensing [21], [22], communication [23]–[25], data processing [26], and security [27]. Taking full advantage of the smart healthcare concept is contingent upon understanding the synergy of multiple mega-trends happening concurrently in the smart healthcare ecosystem [28] and how these trends affect the clinical practice of medicine and healthcare. In this section, we first highlight some of the major technological and societal trends that are driving HIoT adoption and then summarize the demands and challenges of HIoT applications.
A. Technological and Societal Trends
Data Acquisition
Unobtrusive, inexpensive, and accurate sensors are the work-horses for smart healthcare systems. Such sensors can replace the current practice of in-clinic sporadic sensing with continuous monitoring. Although the problem of building ideal smart healthcare sensors is far from being solved, several recent advances in sensing technologies have alleviated many of the existing challenges. Continuously diminishing feature sizes, integrated circuits have reduced the physical dimensions and power consumption of on-chip sensing devices while offering impressive computational capability.
Ambient energy harvesting proposals enable practical in-vivo sensors that no longer require a battery [29], [30]. Radio Frequency-based (RF-based) ambient sensors are working toward the measurement of multiple biomarkers such as respiration [31], [32], heartbeat [33], and motion detection [34], [35], although practical designs are still an active research area.
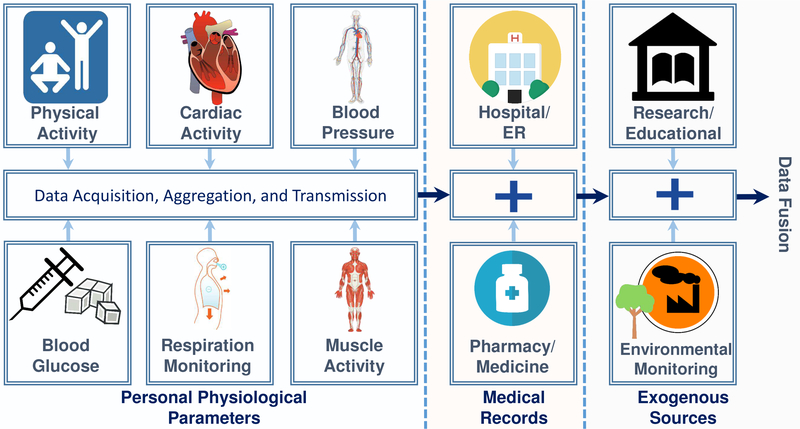
The first generation of personal health monitoring devices, such as smartwatches [2], has addressed several system integration issues and a software-user ecosystem has emerged that can be re-purposed to effectively integrate HIoT into clinical healthcare to enable better-informed decisions and care. In an important development, illustrated in Fig. 1, such data is also being complemented by data collected from alternative sensors —that are not necessarily designed for healthcare applications, e.g., ambient sensors— can be fused with information from personal health monitoring devices to provide context awareness.
Fig. 1.
Modern smart healthcare applications are intricate multidimensional systems that not only focus on the personalized acquisition of physiological data but also incorporate information from various external sources such as past records of patients from their hospitals, research and educational resources, and even environmental information from smart city applications.
Data Communication
Low-delay, high-throughput, and low-power communication is an integral requirement of many smart healthcare applications. Two factors are crucial to the evolution of HIoT: (i) hierarchical network structuring (e.g., using cloudlets [36]) (ii) and the maturity of Wireless Body Area Networks (WBANs) [37]. A complete review of healthcare communication technologies can be found in [15].
Data Processing
By generating an unprecedented volume of information, burgeoning IoT services have significantly contributed to the big data phenomenon. Advances in parallel computation architectures, such as Graphics Processing Units (GPUs), promise to substantially reduce the amount of time necessary to perform sophisticated computations on acquired data. Furthermore, advances in data analytics and inference promise to extract information that can open the door to new cures for diseases and drastically improve diagnostic quality by providing superior decision support to healthcare professionals. ML algorithms have specifically made it feasible to predict the onset of fatal incidents such as seizures and heart attacks [38]. The impact of these advances has reached far beyond data processing; for example, signal processing and ML algorithms can now be used as an effective defense against noise [39], [40], offsetting the imperfections in data acquisition and communication.
Security and Privacy
Security and privacy considerations in smart healthcare systems have long been overshadowed by the design objectives of other system components; application-oriented services often trade off security and privacy considerations for shortened design time. However, the focus is returning to security and privacy in the wake of a surge in large scale cyberattacks targeting a vast range of IoT services. It is now evident that the lack of cybersecurity in different components of smart healthcare (and other IoT applications) is manifested as multi-faceted security flaws with ramifications ranging from privacy violations to endangering patients’ health. Consistent implementation of security and privacy preservation measures is now the primary trend in the field, as it is now known that one-dimensional communication security protocols do not provide immunity against complicated attacks. Researchers are constantly working to enhance the security of data acquisition (in areas such as countermeasures against physical attacks, side channel attacks, and malicious firmware overrides [41]) and data processing (in areas such as cloud-oriented data privacy and security).
Societal Trends
In parallel to the aforementioned technological advances, emerging societal trends are also driving HIoT adoption. These technological and societal developments are indeed the systole and diastole of modern healthcare infrastructure; the former assures feasibility and practicality, while the latter promotes HIoT from a mere nicety to an absolute necessity. At a societal level, the requirement for personalized and continuous healthcare is chiefly fueled by worldwide population aging [42]–[46], which is expected to further increase ever-soaring healthcare expenses. While the impact of aging populations is being felt in most societies, developed countries are facing this most urgently.
In parallel to these demographic changes, two additional societal trends further drive the demand for HIoT. The first of these is the expansion of middle-class families worldwide [47], particularly in countries such as China, India, and Brazil. With education and continuous access to the information resources of the Internet, this technologically savvy class is becoming more and more cognizant about personal healthcare. This directly translates to a growing market for a variety of devices spanning from smartphone-based services to smart homes, smart wearables, Air Quality (AQ) monitoring, etc. A second trend involves the increasing concentration of physicians and medical care facilities in large urban areas leaving sparse coverage in large geographically spread rural areas. In these latter settings, HIoT technology can effectively expand the geographic footprint of clinics and provide effective remote health management solutions.
B. Application Demands and Challenges
While HIoT systems should be specifically designed to fulfill the requirements of their target application, system designers should be aware of the limitations that will act as a barrier to proper functionality. We now highlight some of the most common demands and restrictions of HIoT devices, which may or may not apply to every possible application.
Physiological and Environmental Signals
The first demand of any HIoT system is determining the type(s) of physiological/environmental signals required for its intended application(s). A healthcare management system inherently requires a certain level of accuracy for acquired signals, and clear boundaries for the noise imposed on those signals [48]. Some of the major physiological attributes used in more common applications are shown in Fig. 1, though it should be noted that different applications generally require various types of signals and data.
Decision Support
Decision support plays a crucial role in an HIoT management system. The data collected from various sources should be analyzed by the machine and presented to healthcare professionals in a comprehensive format, making the machine a support tool for the professionals [49]. The type(s) of decision support provided by an application can vary based on its purpose. For example, an application may require an automated warning system that issues an alert on critical conditions. This alert may be issued to the healthcare organization (HCO), to the doctor, to the patient’s caregiver, or directly to the patient.
Latency Tolerance
Latency tolerance can also affect the design of a system. Applications that deal with patients in critical condition, who need constant real-time monitoring, must be able to issue alerts with minimal delay [50]. Other applications targeted for less critical conditions, however, may be able to tolerate higher delay.
Computational Intensity
The volume of data gathered by the system requires a proportional amount of computation power to analyze it. Factors such as the number of sensors, signal sampling frequency, sample accuracy, and overhead imposed by encryption schemes all affect the computational power necessary for the system [51]. In addition, applications requiring lower latency need higher computational capabilities. Machine learning algorithms used in a system may also impose additional computational intensity, independent from the absolute volume of data; for example, two algorithms working on the same input data may require different computational intensity based on their nature.
Power Consumption
The sensors used to gather physiological signals are generally wearable, meaning they will be powered by batteries. Optimizing power consumption to extend battery life (using both hardware configurations and energy-aware algorithms [52], [53]) is a necessary step toward ensuring continuous signal acquisition and monitoring.
Data Communication Rate
In most HIoT systems, physiological signals are transmitted to a local concentrator through a WBAN [54]–[57]. The communication link between the WBAN and the concentrator has a bandwidth restriction, imposing a limit on the amount of data acquired per unit time. Some pre-processing may need to be performed on the acquired data before transmission to a local concentrator. and, in some cases, the size of the data after pre-processing can be greater than when it was transmitted from the WBAN (e.g., some encryption schemes impose a significant overhead).
III. HIOT EXAMPLE APPLICATIONS
We now highlight some common HIoT management applications and introduce a case-study that we will use for highlighting the relevance of HIoT in the clinical healthcare setting.
Activity Recognition
Activity recognition is prevalent in various areas of the healthcare domain, where multiple techniques are used for this purpose such as computer vision [58], active sensor beacons, passive radio-frequency identification (RFID) [59]–[62], WiFi [63], radar [64], etc. Most traditional activity recognition platforms, however, suffer from a high rate of false-positives when detecting abnormal activity. Applying learning techniques, such as Support Vector Machines (SVMs) [65] or dictionaries [66], can help mitigate this problem. Some examples of activity detection applications include: fall detection [34], [67], [68], fitness tracking [69], [70], human localization and tracking [71], [72], posture recognition [73], and gait abnormality detection [74], and multiuser activity recognition [75].
Stroke Rehabilitation
Rehabilitation among stroke patients is an important task that has been studied in many aspects. Recent trends have increasingly turned to self-managed rehabilitation [76]. Providing a virtual environment for patient rehabilitation [77], [78], predicting the strength of muscles based on kinematics [79], and monitoring a patient’s activities to provide feedback and assess the patient’s recovery process [80] are all examples of how HIoT can help with stroke rehabilitation.
Blood Glucose Monitoring
According to the Centers for Disease Control and Prevention (CDC), approximately 30.3 million people (or 9.4% of the US population) across all ages are currently living with diabetes [81]. Blood glucose monitoring can be especially useful for diabetic patients, as it can provide important information related to managing the disease [82]. In addition to regular monitoring, IoT devices can issue warnings in extreme/dangerous cases [83], provide suggestions on adherence to treatment regimens [84], and even help track patients’ meals or make healthy eating suggestions [85].
Cardiac Monitoring
One out of three deaths in the US are associated with cardiovascular disease, making it the leading cause of death in the nation [86]. Personalized and continuous cardiac monitoring (as opposed to conventional in-hospital monitoring) plays an integral role in lowering the fatality rate and associated expenses of cardiovascular diseases [87]. Existing studies investigate the efficacy of in-vivo and exvivo sensors in prediction and detection of various cardiac hazards such as arrhythmia [88], [89], long QT syndrome [90], and sudden cardiac arrest (SCA) [91]. These efforts have resulted in the recent emergence of noninvasive Food and Drug Administration (FDA)-cleared cardiac monitoring systems that can help diagnose a variety of heart rate variations (HRV)-related syndromes [92]. Regardless of their increasing commercial popularity, cardiac monitoring devices have recently been subject to scrutiny due to their potential security and privacy flaws. New regulations are expected to emerge to enforce more strict requirements on these systems [93].
Respiration Monitoring
Patient respiratory activity is a clear indicator of their overall health. Respiration monitoring devices are categorized as either contact (most common [94]) or non-contact [95]. Monitoring newborns for sudden infant death syndrome [96], monitoring the effects of medication(s) on respiration [97], and asthma patient monitoring [98] are all examples of IoT respiration monitoring devices.
Sleep Monitoring
Monitoring patients during sleep is another useful implementation of IoT devices. Some applications include monitoring and classifying sleep stages [99], monitoring vital signs during sleep [100], and detecting sleep disorders such as obstructive sleep apnea [101], [102].
Blood Pressure Monitoring
High blood pressure currently impacts approximately 45.6% of US adults [103] and, as a result, is a crucially-important health concern to address. IoT-based general hypertension monitoring systems are already in use [104], while some applications can even take control of hypertension decision-making processes [105]. Other blood pressure related issues, such as hypotension (low blood pressure), can also be monitored by IoT devices [106].
Stress Monitoring
Many systems have been developed to monitor various types of stress, and even intervene if necessary. While low-stress levels are normal, high-stress levels can lead to serious health issues [107]. Some applications attempt to reduce stress by offering suggestions on a mobile device [107], [108], monitoring post-traumatic stress disorder patients [109], and helping people on the autism spectrum manage their emotions [110].
Medical Adherence
Ensuring that patients adhere to their healthcare/medicinal regimen is a grand challenge in healthcare [111], [112]. Monitoring adherence for elderly patients [113], people with dementia [114], or general medical adherence monitoring [115]show how IoT devices can help ensure that regimens are followed properly.
Alzheimer’s Disease (AD) Monitoring
AD affects 5.3 million people in the US and incurs an estimated annual cost of $200 billion [116]. AD patients generally require constant, round-the-clock care. IoT-based devices can provide considerable assistance to caregivers in many areas, such as early detection of dementia [117], reporting anomalous activities [118], monitoring patient location, and providing task reminders [119].
Parkinson’s and Huntington’s Disease (PD/HD) Monitoring (Case Study)
Parkinson’s and Huntington’s are neurological diseases characterized by movement disorders. It is estimated that more than 900,000 individuals in North America will suffer from PD by 2020 [120] while HD currently affects more than 20,000 individuals in the US [121]. Body worn HIoT sensors provide an effective mechanism for monitoring the movement symptoms associated with PD/HD and are an promising option for assessing disease status, progression, and medication efficacy.
To provide concrete examples of the ideas discussed throughout the paper, we use a PD/HD case study from our recent and ongoing research [124], [125]. Motivating background information on these disease conditions and the related demands and challenges are summarized here. Subsequent sections in the paper highlight examples of relevant components from the case study through brief remarks.
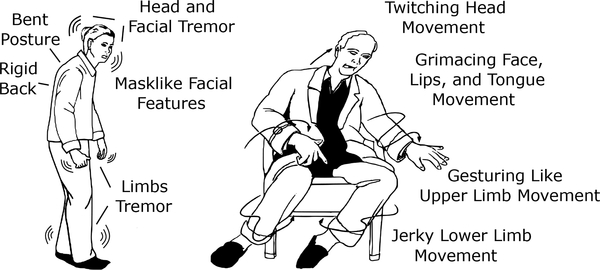
Typical symptoms of PD/HD are depicted in Fig. 2. Parkinson’s is characterized by rest tremors, slowness in movement, rigidity, and postural instability. Huntington’s is a genetic disease marked by jerky movements in the body (referred to as chorea), unsteady gait, and cognitive impairments [126]. Both diseases are progressive; after onset, patients encounter increasingly severe symptoms as time advances.
Fig. 2.
Graphics showing typical symptoms of Parkinson’s (left) and Huntington’s (right) disease that are the focus of the multisensor case study that we will use to illustrate the ideas discussed in this paper. Based on [122], [123].
PD and HD are currently considered incurable. Although medications can be used to manage symptoms, their effect is neither universal nor complete. The overall quality of life is often severely degraded for PD/HD subjects. Disease progression and efficacy of medications are currently assessed subjectively by physicians via in-clinic-tests that assign patients a score on the Unified Parkinson’s Disease Rating Scale (UPDRS) [127] or the Unified Huntington’s Disease Rating Scale (UHDRS) [128]. These ratings are subject to variability because they are based on sporadic observations that sample relatively short durations of time and because of inherent variability in human assessments. There is, therefore, a strong desire to develop sensor-based quantitative measures and scales that can be used to objectively assess disease progression and efficacy of treatments. This clinical application is ideally suited for HIoT because miniaturized unobtrusive sensors for movement have become ubiquitous and cheap due to the smartphone revolution, as have circuits for low power wireless communication and Internet connectivity infrastructure.
IV. SYSTEM ARCHITECTURE
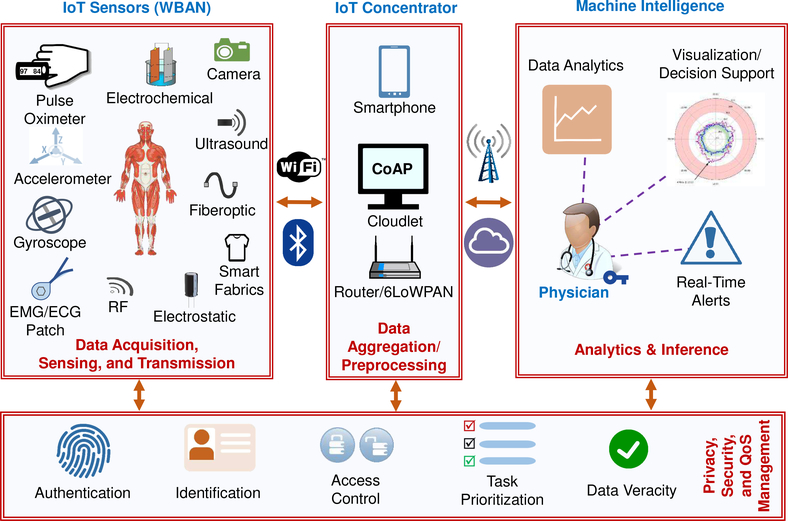
The realization of inexpensive, unobtrusive, and reliable systems that can meet the requirements discussed in Section II–B necessitates a robust and inclusive design framework. To this end, various HIoT system architectures have been discussed in the literature [129]–[133]. In this section, we investigate the general architecture of typical HIoT systems [10] (as seen in Fig. 3) and outline each of the layers. Each layer is then discussed in more detail in subsequent sections.
Fig. 3.
High-level system architecture illustrating HIoT integration into clinical healthcare. IoT sensors record measurements for a range of physiological and health-related physical attributes. The data is communicated over the network and aggregated in the cloud by IoT concentrators. Cloud-based analytics and inference algorithms operating on the data provide decision support to physicians via visualization interfaces, dashboards, and real-time alerts to individual users. Security and privacy solutions must be implemented to include other components, ensuring data protection from acquisition to storage.
Data Acquisition, Sensing, and Transmission
The first HIoT component is data acquisition, where IoT devices and sensors measure physiological and environmental signals. These devices are connected to a WBAN, generally through an intermediate data aggregator such as a smartphone [36]. The primary function of the sensors is to sense and gather data, but many are also now able to preprocess data before transmission. Data acquisition is further detailed in Section V.
Data Aggregation/Preprocessing Cloudlet
The data gathered in the acquisition and sensing layer are transmitted to an IoT concentrator through a WBAN (using mediums such as WiFi, Bluetooth, or ZigBee Standard). These concentrators are responsible for gathering all the sensed data and transmitting it to the HCO data center within the delay tolerance requirements of the application. To fulfill this task, concentrators need to be more computationally capable (compared to devices in the previous layer) and must have a certain amount of local storage for data. We detail data aggregation and preprocessing in Section VI.
Cloud Processing and Storage
Once the data arrives at the HCO data center, it can be processed using advanced algorithms to provide decision support including data summarization and creation of visual representations. Since the cloud processing and storage layer is responsible for various tasks, we discuss it in multiple sections. Analysis of data using ML algorithms to extract useful insights is studied in Section VII, and data visualization is explored in Section VIII.
Privacy, Security, and Quality of Service (QoS) Management
As highlighted in Fig. 3, the entire system must provide robust security and privacy guarantees while satisfying the QoS requirements of applications. Overemphasizing security within a single component (typically communication) does not translate to improved overall security because adversaries can exploit any susceptibility to infiltrate not only the HIoT system but also other connected services. The implementation of such an inclusive security structure, however, is challenging. Aside from the common limitations of IoT applications (such as meager energy budget, on-site deployment, and large-scale), HIoT services are subject to domain-specific complications, such as, the inherent sensitivity of health data, highly dynamic roles of stakeholders, heterogeneity in electronic health records (EHRs), and potentially grave consequences from failures. Section IX further lays out the details of HIoT security.
V. DATA ACQUISITION AND SENSING
In this section, we review some of the most commonly used sensors and investigate their invasiveness, cost, and accuracy.
A. Activity Detection Sensors
Activity detection is typically conducted through Inertial Measurement Units (IMUs), which are composed of multiaxis accelerometers, gyroscopes, magnetometers, and force sensors. IMUs can be implemented as wearable sensors [134] or sensors integrated into the environment [135]. Wearable sensors are typically inexpensive and can cater to a broad range of applications; multiple wearable sensors can easily be deployed to determine the relative position of body parts [134]. In some cases, wearable sensors are inconvenient for long-term use as their bulk and weight, coupled with their requirement to be in close proximity to the patients, impede the acquisition of continuous and real-time data. In these situations, ambient sensing techniques can be employed (e.g., cameras and RF sensing). Cameras are relatively easy to set-up and employ, as they do not require extensive changes to the environment. They, however, suffer from limited line-of-sight and pose privacy concerns.
B. Respiration Sensors
Respiratory motion can be captured using accelerometers or piezoelectric materials. The solid-state nature of piezoelectric sensors enables them to be reliable, small, low-power, and non-invasive. Authors in [136] demonstrate a complementary metal-oxide-semiconductor (CMOS) sensor for respiration monitoring that is implemented using a polyvinylidene-fluoride (PVDF) material to ensure bio-compatibility. The sensor consumes up to 800 μW and, since direct contact with the skin is not required, can be embedded inside a jacket or worn around the chest. Alternatively, camera-based sensing solutions apply image processing algorithms to videos, capturing patients’ respiratory motions. Instead of tracking the slight motions of the torso (which has been demonstrated to be a demanding task), the majority of the proposed works rely on analyzing variations in the ambient light caused by body movement [137], [138].
C. Heart Beat Monitoring Sensors
Generally, heartbeat monitoring techniques are classified into four groups: (i) electrocardiography (ECG) (ii) ballistocardiography (BCG) (iii) phonocardiography (PCG), and (iv) photoplethysmography (PPG).
Holter devices [139] are common tools that capture ECG data through electrodes over long periods of time with various sampling frequencies. Non-contact, capacitive-coupled electrodes are also commonly used for ECG signal acquisition as they do not require direct contact with the skin, enabling their use as wearable sensors embedded in clothing [140]. These less invasive methods mean trading accuracy for comfort/wearability. While BCG signals can be captured in numerous ways, accelerometers are typically the primary choice [141]. Accelerometers can be used as wearable sensors if worn in proximity to the heart. While PCG signals are typically captured by microphones, piezoelectric sensors installed on the throat [142] and fiber optic sensors [143] can also be used. Finally, due to low-cost, non-invasiveness, and high reliability, pulse oximeters are generally used to capture PPG signals. Various physiological parameters, including Heart Rate Variability and even ECG signals, can be extracted from PPG signals [144]. Pulse oximeters, however, are not suitable for long-term continuous monitoring as they are typically worn on a fingertip.
D. Blood Pressure Sensors
Authors in [145] propose a wireless and battery-less sensor to be surgically implanted in the femoral artery to monitor hypertension patients. A second, bulkier device needs to be worn by the patient to power and interrogate this in-vivo sensor. Although invasive, the proposed solution is comfortable and suitable for long-term monitoring. Less intrusive techniques, which operate based on fundamentals of wave propagation dynamics in fluids, are also proposed in the literature. These techniques typically measure Pulse Wave Velocity (PWV) or Pulse Transit Time (PTT) to calculate blood pressure (BP). Data samples are collected using cuffless PPG, ECG, and Impedance Cardiography (ICG) sensors, which can be used as wrist bands and/or be worn around the chest [146], [147]. Relying on PTT poses additional challenges to BP monitoring; one challenge arises from the dependence of PTT on heart rate, age, gender, and body shape of the patients. These obstacles, however, can be partially addressed by employing suitable signal processing solutions [147], [148].
E. Blood Glucose Monitoring Sensors
Blood Glucose Monitoring (BGM) can be categorized into two different classes: (i) electrochemical-based and (ii) optical-based. The former analyzes the chemical content of interstitial fluids, while the latter involves spectroscopy techniques. Electrochemical sensors are typically more accurate but are more invasive. Common models of electrochemical sensors require blood samples, typically obtained from the fingertips of patients. Electrochemical-based sensors can also be implanted underneath the skin [149]. Because of the correlation between glucose level in sweat and blood, less invasive monitoring can be implemented by deploying flexible, small, and stretchable sensing patches that analyze sweat samples [150].
Spectroscopy is widely used as an alternative technique to provide a less invasive but less accurate BGM solution. Chemical composition of the blood (including glucose level) changes its ability to absorb, reflect, and scatter light beams. Spectroscopy uses this to estimate the blood glucose level. Therefore, optical-based spectroscopy sensors typically encompass a Near Infra Red (NIR) light source and a photon counter, which are installed on opposite sides of the tissue [151]. Table I provides a summary of aforementioned sensing technologies along with their primary strengths and shortcomings.
TABLE I.
A list of commonly used sensors in various clinical HIoT applications. The qualities listed under ‘characteristics’ are relative. For example, for activity detection applications, RF-based sensing typically yields lower accuracy than wearables. Hence, it includes the ‘low accuracy’ attribute. This should not imply that RF accuracy is not practical.
| Application | Sensor | Characteristics |
|---|---|---|
| Activity Detection | Wearable & Environment IMU | ✓ Inexpensive X Obtrusive |
| RF | ✓ Unobtrusive X Low Accuracy |
|
| Camera | ✓ Easy-to-Setup X LoS Limitation X Privacy Concerns |
|
| Respiration Detection | Accelerometer & Piezoelectric Materials | ✓ Low Power ✓ Accurate X Obtrusive |
| Camera | ✓ Inexpensive X Low Accuracy X Noise Sensitivity |
|
| Heartbeat Monitoring | Wearable ECG Electrodes | ✓ No Skin Contact X Low Accuracy |
| Accelerometers (BCG) | ✓ Inexpensive X Noise Sensitivity |
|
| Accelerometer, Fiber Optics, & Microphone (PCG) | ✓ Unobtrusive X Noise Sensitivity |
|
| Pulse Oximeter | ✓ Versatile X Obtrusive |
|
| Blood Pressure Monitoring | In-Vivo | ✓ Unobtrusive X Invasive |
| PWV and PTT Sensors | ✓ Non-Invasive X Noise Sensitivity |
|
| Blood Glucose Monitoring | Electro-Chemical Sensors | ✓ Accurate X Invasive |
| Optical Sensors | ✓ Non-Invasive X Low Accuracy |
|
F. Wearable Multisensors in the PD/HD Clinical Study
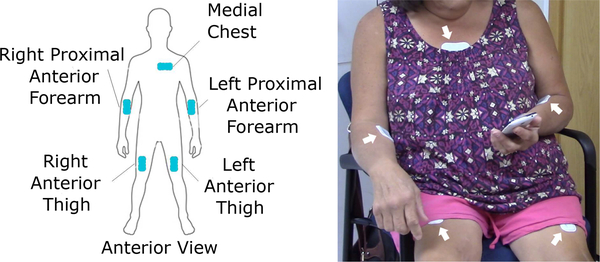
The PD/HD case study, outlined in Section III provides an excellent example of how multiple sensing modalities can be combined in one sensor. In this study, BioStampRC sensors from MC10 Inc. are used, which are lightweight (≈ 7 grams) and unobtrusive devices capable of operating in multiple modalities. The sensor (specifications shown in Table II) operates with various sampling rates and dynamic ranges with high-power and long-duration recording capabilities. The sensors are applied to subjects’ body at five different locations as depicted in Fig. 4. We primarily utilize the sensors’ accelerometer to obtain data, sampled at 31.25Hz, over the duration of 46 hours.
TABLE II.
Capabilities of the BioStampRC sensor from MC10, Inc. used in our case study. The sensor operates in 6 modes. g indicates the acceleration due to gravity (9.81 m/s2).
| Mode | Sampling Rate (Hz) | Dynamic Range | Recording time (h) | |
|---|---|---|---|---|
| I | Accel. | 31.25, 50, 100, 200 | ±2, ±4, or ±8 g | 8–35 |
| II | ECG | 125, 250 | ±0.2 V | 17–35 |
| III | EMG | 250 | ±0.2 V | 17 |
| IV | Accel. | 50 | ±2, ±4, or ±8 g | 11–22 |
| ECG | 125, 250 | ±0.2 V | ||
| V | Accel. | 50 | ±2, ±4, or ±8 g | 11 |
| EMG | 250 | ±0.2 V | ||
| VI | Accel. | 25, 50, 100, 250 | ±2, ±4, ±8, ±16 g | 2–4 |
| Gyro | Off, ±250, ±500, ±1000, ±2000 °/sec | |||
Fig. 4.
A graphic showing five different locations on the body for applying BioStampRC sensors in our PD/HD case study [152] (left) and a participant wearing sensors at these locations for in-clinic assessment [125] (right).
VI. DATA COMMUNICATION, AGGREGATION, AND PRE-PROCESSING
In this section, we investigate two main aspects of HIoT communication: (i) connectivity, and (ii) data aggregation.
A. Data Communication
A WBAN consists of multiple low-power, resource-constrained devices that are connected to a more computationally capable device, such as an Access Point (AP), through a low-range and low-rate wireless link. The AP performs multiple pre-processing, data aggregation, and data-fusion operations on the collected data. Most importantly, it provides Internet connectivity. Broad trends in communications for HIoT have been surveyed in [15]. The majority of implementations in healthcare applications rely on either BLE [153]–[155] or ZigBee [156], [157].
BLE specifically targets low-rate, low-power, and low-range IoT communications [158]. It operates in the 2.4 GHz Industrial, Scientific, and Medical (ISM) frequency band, and can provide up to 2 Mbps bandwidth. To suppress the adverse effects of interference and fading, the protocol leverages adaptive frequency hopping techniques. Many existing portable devices such as smartphones and laptops are shipped with embedded BLE modules [159]. BLE, however, does not provide multicast communication, which can be crucial to many applications. Furthermore, as it only supports single-hop star topology, it cannot be adopted by multi-level hierarchal architectures. This limits its scalability and raises security concerns [158].
ZigBee is developed atop the IEEE 802.15.4 standard. It is designed for low-power, short-range, and low-rate data connectivity. Unlike BLE, ZigBee supports mesh architecture, which results in more robust implementations. Furthermore, it supports multicast [158]. Table III provides a comparison between ZigBee and WiFi in the context of smart healthcare applications.
TABLE III.
A comparison of two commonly used communication protocols for WBAN implementation, along with with their advantages and disadvantages within the context of smart healthcare.
| Protocol/Characteristics | Advantages/Disadvantages | Example Applications |
|---|---|---|
| BLE / 2.4 GHz ISM 2 Mbps ≤100 m Low-Energy | ✓ Ubiquitous ✓ Low-Power ✓ Low-Delay X Single-Hop X No Multicasting X MAC Inflexibility |
EEG [160] IMU [155] PPG [154] EEG [161] EMG [162] |
| ZigBee / 2.4 GHz ISM ≤250 kbps ≤100 m | ✓ Multi-Hop ✓ 6LoWPAN Compatibility ✓ Self-Healing ✓ Multicasting X Static Power Management X Rerouting Delay X Crowded 2.4 GHz Band X Poor Coexistence with WiFi |
EEG [160] ECG [160] PPG [163] IMU [164] |
Note that the composition of this network should be defined prior to the application and verified after the setup. In our PD/HD case study, the number of sensors, their locations on the body, recording modes, and sampling rates are determined through the Investigator application before the network setup and their functionality is verified after the sensors are attached.
B. Data Aggregation and Pre-processing: Front-end
For data aggregation and pre-processing, an aggregator (typically added as a functionality of the AP) is used to collect and combine all sensed data before transmission. This step also includes performing preliminary computations on the data. This concept has seen more interest recently, especially with the introduction of fog computing [165]. Fog computing provides multiple benefits including low latency in some applications (more critical in emergency medical situations) [166], mobility support and location awareness [167], and reduced power consumption by replacing cloud communication with local computation (as communication consumes orders-of-magnitude more power than computation) [168].
Other roles and benefits of this stage include condensing data from multiple sensors into single packets to reduce communication overhead, removing redundant data that do not provide useful information for the system, and representing data from multiple sources using a single value (such as their arithmetic mean or median). For example, in a 12-lead ECG data acquisition system, it is shown that the same intervals from different leads can be median filtered to provide a final, single value, thereby reducing data volume by a factor of 12 [90]. Additionally, in many applications, data from many different sources complement each other to provide a bigger and better picture of the situation. The aggregation process keeps data from all sources in sync with each other so that the concurrency of events recorded by different sensors are maintained. The aggregation and pre-processing stage tends to lose information.
C. Data Aggregation and Pre-processing: Back-end
Many HIoT applications resort to cloud-based solutions not only to circumvent the challenges of processing large data volumes but also to take full advantage of cloud’s compatibility with off-the-shelf data analytics, always-on property, scalability, and affordability [169]. Particularly, the cloud can provide permanent data storage services (ultimate data aggregation), which are the basis of data analytics and inference. Long-term data storage is also a prerequisite for history-based verification mechanisms that can evaluate the veracity of data by comparing them to the expected values. Long-term data are also valuable assets to HCOs and other entities. For example, health insurance agencies use such data to evaluate the overall health of applicants and charge them accordingly [170]. Cloud storage also provides a point of access to retrieve information.In our PD/HD case study, all of the data recorded by the sensors are eventually transferred to and stored in the cloud. After the data is uploaded to the cloud, researchers can access, preview, or download it through the Investigator portal on demand, as seen in Fig. 5. Each subject’s information can easily be downloaded without any loss of information.
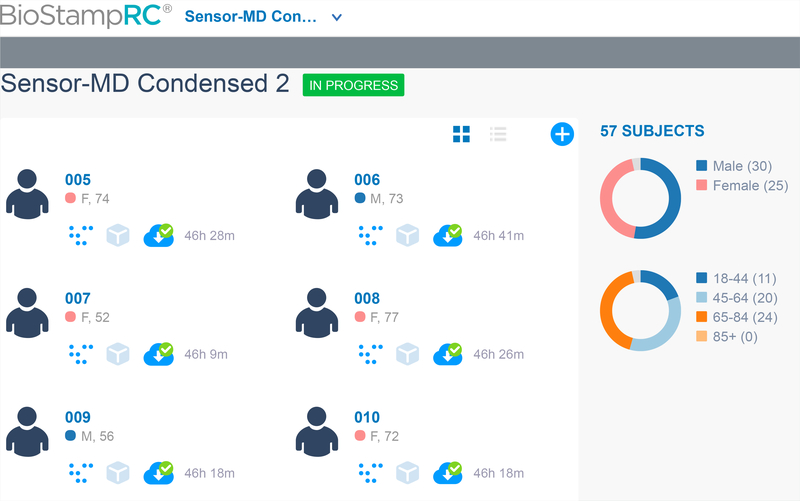
Fig. 5.
Screen shot of MC10 web portal used in our PD/HD case study (named Sensor-MD Condensed 2). We can identify six subjects with unique subject ID (005), sex (M/F), and age (years) indicated on the right side. Total duration of recorded data is indicated on the right of an icon with a green tick mark. Clicking on the green tick marked icon downloads the data which can be utilized for analysis. On the far right, we can observe the statistics of the study showing the total number of subjects in the study followed by graphs showing number of male and female participants and age distribution.
HCOs can opt for public, private, or hybrid cloud services. Public servers can simultaneously host multiple applications from different entities. This reduces operational expenses but increases the risks of privacy leakage. Alternatively, HCOs can set up and maintain their private servers trading off additional expenses for improved security. As a compromise between the two implementations, it is also feasible to outsource demanding computations to public servers while processing sensitive data locally in a private setup. From another perspective, cloud-based servers can be centralized or distributed. The latter can reduce maintenance costs by taking advantage of geo-diversity. However, distributed servers are known to be more difficult to manage and require more complicated task distribution and resource allocation [171].
VII. ANALYTICS & INFERENCE
The volume of healthcare data generated in recent years from bio-sensors, EHRs, computerized physician order entry, social media, and administrative entries, was estimated to be 153 Exabytes in 2013 and is expected to reach 2000 Exabytes in 2020 [172]. This impressive accumulation of data in HIoT has created a conducive environment for data analytics and inference algorithms, which are now used in a variety of healthcare applications for anomaly detection, prediction of future health events, early detection of diseases, cost reduction, improved accuracy in clinical diagnosis, and clinical decision support [173], [174].
Despite its potential to revolutionize clinical HIoT, integration of data analytics and statistical inference techniques in clinical practice has been slow. The few documented successful examples are localized within HCOs with rich datasets. We dedicate the remainder of this section to providing an analysis of the two key drivers, which can enable a widespread adoption for clinical HIoT. In Section VII–A, we provide a brief overview of the existing and emerging algorithms that can form the backbone of clinical HIoT, followed by Section VII–B, where we study the issues arising due to the availability of the data that can be used in these algorithms.
A. Algorithms
Data analytics and inference algorithms can make HIoT an indispensable decision support tool for healthcare professionals. Although a good portion of the algorithms used today has existed for decades (e.g., Support Vector Machines and decision trees), they have not seen widespread use until the recent explosive growth in the computational capabilities of computing hardware [175]. This growth made extremely computationally-intensive algorithms, which were previously considered unusable, practical in cloud computing platforms with vast resources (e.g., Amazon EC2). Application of these algorithms has also been accelerated due to the wide availability of open-source software toolboxes that provide rapid development environments. Most of these algorithms are not only good at discovering explicit relations among data but also the latent and hidden features that are very difficult to detect by human specialists [176].
Most of the algorithms that existed in the previous decade required intricate knowledge of the features that were associated with a health condition; for example, the study in [90] attempts to use inference algorithms to determine the existence of known cardiac conditions in patients. They start their application by extracting features from ECG signals. This implies that the success of the inference depends strongly on the understanding of the features. The emerging deep learning (DL) network-based inference techniques largely eliminate this feature extraction step by utilizing a network that not only provides inference, but also extracts the features. DL-based inference applications have seen significant recent success in clinical practice [175], [177], however, their success has been restricted to applications that can provide the vast amount of data that deep networks demand to achieve acceptable accuracy.
Algorithmic nuances of data analytics and inference techniques pose additional obstacles against their commercialized use in clinical HIoT. A major challenge concerns the interpretability of an algorithm’s decisions. In fact, physicians are often more interested in the algorithm’s thought process as opposed to its final decision as this enables them to better assess the reliability of the inference [178]. Additionally, the reliance of analytics and inference techniques on statistical analysis sometimes limits their ability to model outliers, which are often of interest in the medical diagnosis of rare conditions. The evolving nature of progressive learning algorithms also complicates approval processes for clinical deployment; although the FDA has recently passed new regulations, where algorithms are cleared based on their developing teams [179].
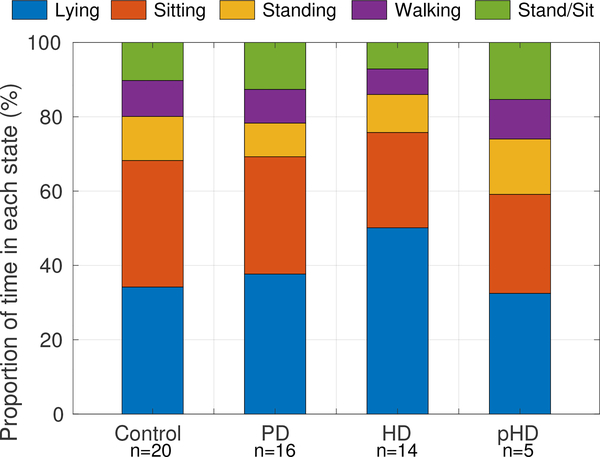
Data analytics for our PD/HD case study involved both classification and regression [125]. The former involves categorizing patient activity into one of four classes: {Lying down, Sitting, Standing, Walking}. In our study, activities were classified every five seconds and the activity states for each interval was identified by determining the dominant acceleration axis (the axis with the largest mean acceleration). Based on the dominant orientations in chest and thigh sensors, activity states were categorized as lying down, sitting, and upright. A normalized auto-correlation-based analysis [124] was performed on the upright state intervals, which were further classified upright durations into standing and walking intervals. Figure 6 shows the proportion of time (over the full duration) subjects spent in different activity states.
Fig. 6.
Classification of the activity states in our PD/HD case study as percentage of time spent (total duration ≈ 46 hours) lying down, sitting, standing, and walking for Control, PD, HD, and prodromal Huntington’s disease (pHD) participants. “n” is the number of participants analyzed [125].
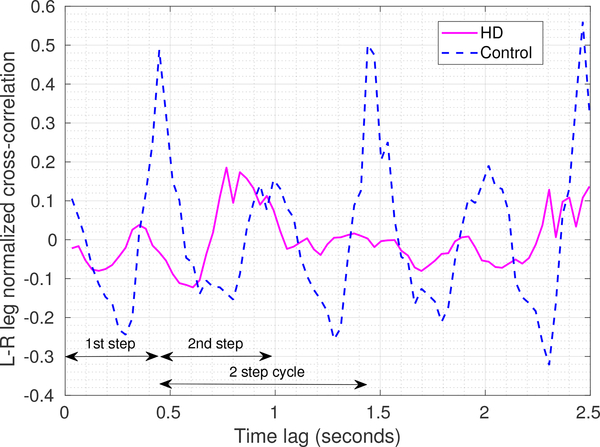
Our PD/HD case study uses regression to analyze (poor) inter-limb coordination in HD subjects. Sensor data from a 10-meter walk test was used. A normalized cross-correlation of recorded acceleration from the left and right leg sensors indicated coordination between the legs while walking. As shown in Fig. 7, for the control participant, the strong peaks in the cross-correlations at the 1-step and 2-step durations are indicative of the rhythmic nature of normal walking, whereas the HD participant’s peaks are much smaller and poorly defined due to lack of coordination between the legs [124]. Apart from quantifying and visualizing leg coordination, this example specifically illustrates the benefit of using multiple body-worn sensors for the analysis.
Fig. 7.
Data analysis performed for our PD/HD case study to assess inter-leg coordination. The plot shows the normalized vector cross-correlation [124] of the recorded accelerations from left leg and right leg sensors as a function of time lags for a HD and a control participant. A 2-step cycle is annotated and highlights stronger peaks for the controls at the one and two step intervals compared with participants with HD.
B. Data Availability
The algorithms described in the previous subsection require a significant amount of well-structured clinical data to achieve useful levels of accuracy. Providing such data presents multi-faceted challenges, specifically, with respect to widespread data availability and heterogeneity.
A major data-related challenge in clinical HIoT is assuring the generality of results. Most available pilot studies in the literature are developed and tested on small datasets (relative to the actual patient population size) [180]. Therefore, their applicability and efficacy in real-world scenarios remain uncertain; especially, considering that health and disease states can be highly correlated with many personal parameters such as gender, age, and ethnicity. This problem is even more acute for rare diseases. Consequently, data analytics approaches have the best chances for success if clinics and hospitals share their data with algorithm developers. The flow of such information is, however, fraught with unresolved legal and ethical complications. An example of this is the collaboration between Google’s DeepMind and the British healthcare provider, Royal Free, which shared its acute kidney injury dataset with DeepMind without acquiring patients’ explicit consent. The decision led to official scrutiny, which concluded that the deal was illegal [181], [182].
To take full advantage of the existing medical knowledge, it is common sense to use historical medical data as input to inference algorithms. Combined with the data that is being streamed from the HIoT platforms, this amalgamation of data can provide an effective data source for modern inference algorithms. However, historical data sources are diverse and highly unstructured as part of the data must be inferred from EHRs, insurance claims, medical textbooks, and scientific papers [183]. The language used in these documents can be vague, out of chronicle order, and incomplete. To cope with these challenges, NLP is often used. However, regardless of their remarkable progress in recent years, current NLP technologies are not efficient enough in these applications, even when supported by the resources of tech giants [180].
This data heterogeneity problem is exacerbated when we consider the fact that even the data obtained from the same HIoT platform can be highly-heterogeneous within itself. For example, the MC10 sensors used in our case study continuously evolve and additional clinically relevant attributes can be measured with every new generation of sensors, which effectively adds a new ”input dimension” to the existing data. Therefore, algorithms must be designed to cope with not only an increasing volume of data, but also an increasing variety.
VIII. VISUALIZATION
The primary objective of visualization is to present the results of data analytics and inference algorithms in the form of intuitive tables, charts, graphs, etc. to enable rapid and intuitive absorption and interpretation of the patient data by healthcare professionals. Compared to sporadic in-clinic measurements, HIoT datasets are large and visualization is an absolute necessity. Physicians seeing between 20–40 patients a day have no way to absorb and interpret the reams of numerical data that HIoT sensors can generate, effectively presenting the results via visualizations is also a challenging task [36]. Including too much detail can distract the attention of the user from critical information, while vital data can be omitted in aggressive summarization. Although visualization in clinical HIoT applications is primarily directed toward physicians, there is also a need to visually present some information to patients to facilitate understanding and improve engagement.
Visualization schemes can be static or interactive. Static visualization techniques range from lists/tables, plots/charts, graphs/trees, and pictograms to more complex formats showing spatial data, multidimensional data, or causal relationships [184]. Interactive methods are especially useful when visualizing information that simultaneously incorporates multidimensional temporal signals and generic static information.
A. Static Visualization
Systems such as hGraph [185] and its related programming libraries (such as the one introduced in [186]) provide visualizations by combining in-clinic, activity, sleep, blood pressure, and nutrition data. Other systems such as TimeLine [187] provide an EHR visualization, which shows all related static data (such as a list of medical problems, general information, and patient status) on a timeline. Another study targeting clinical information is presented in [188], where a real-time bedside graphic display is developed. The display includes personal information, lab results, vital signs for specific periods of time (e.g., over the past 24 hours), and Intensive Care Unit (ICU) information.
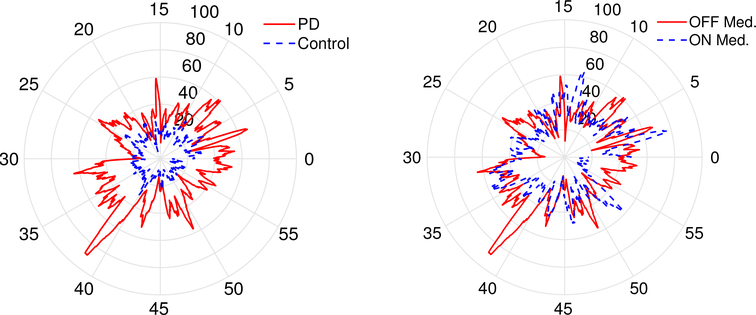
Choosing the proper visualization format ensures that medical professionals do not miss critical information. In our PD/HD case study, patient tremors over the period of one hour are plotted using a radial plot (as shown in Fig. 8). This allows easy comparison of tremors between healthy subjects and PD patients while identifying the difference in patient tremors when they are on vs off their medication. As more data becomes available, both for the individual, and across individuals, this figure can be further enhanced by adding in bounds for the “normal” range for tremors, or by customizing the presentation for each individual. Compared with the raw sensor data which for a single sensor is 8.2 MB over an hour and 196.1 MB over a 24 hour period (total across 5 sensors is approximately 1GB per day), the summarized presentation in the visualization is much easier for physicians to absorb and interpret.
Fig. 8.
Visualization example from our PD/HD case study. A clock based visualization shows the variation in at-rest tremor magnitude in individuals with PD and compares it against controls and between on and off medication states. The tremor magnitude is quantified as the fraction of power in the 4 to 6.5 Hz frequency band (radial axis) for the principal axis acceleration recorded from MC 10 sensors affixed to the forearm over an hour duration (angular axis): (a) PD vs control (left), and b) PD ON vs OFF medication (right).
Studies such as [189]–[191] focus on providing feedback on personal health status through visualization. This information may be presented in different forms, such as an abstract art display of physical activity [192], charts and graphs [190], or even through a physical form with data sculptures [193].
B. Interactive Visualization
Many applications focus on providing an interactive environment in their visualization scheme. For example, Care-Cruiser is an interactive system that visualizes the effects of applying patient treatment plans [194]. The system shows the treatment plan progress and depicts the patient’s condition at any given point during treatment. The system also shows hierarchical data (nesting of treatment plans and sub-plans), temporal data, and qualitative data, and provides a means to compare multiple patients. Another interactive system is Medical Information Visualization Assistant [195] that allows medical experts to obtain relevant information in a context-related environment. A more specific visualization called Interactive Parallel Bar Chart (IPBC) is proposed in [196], which depicts data gathered from multiple hemodialysis sessions in a 3D bar chart. Numerical data over time of each hemodialysis session is shown in one row and different sessions are bundled together to create the final plot.
IX. SECURITY AND PRIVACY CONSIDERATIONS
System security and data privacy are the highest priorities in a medical system and should be considered during every phase of system design. Additionally, designers must comply with the Health Insurance Portability and Accountability Act (HIPAA) [197], which mandates that all service providers ensure the privacy of their clients. In this section, we discuss security and privacy considerations for each layer of our proposed HIoT system.
Data Acquisition and Sensing
Strict energy-budget, on-field deployment, and limited computational resources leave data acquisition level vulnerable to a wide spectrum of cyber threats such as eavesdropping and man-in-the-middle attacks, making it an easy target for adversaries (particularly insiders [198]). Lightweight cryptography is the main countermeasure against attacks. It is very effective in protecting both the security and privacy of smart healthcare sensing; simple but effective algorithms such as Advanced Encryption Standard (AES) and Elliptic Curve Cryptography (ECC) can be tailored for resource-constrained devices. These techniques, however, leave the system vulnerable to side channel and hardware attacks. Hence, in addition to cryptography-based solutions, HIoT devices must be equipped with platform integrity attestation mechanisms for protection against hardware/software tampering attacks [199].
Communications
Adversaries primarily target smart healthcare communication because (i) its properties are better-known (in comparison to other components), (ii) it can be addressed remotely, (iii) due to its interconnection with other networks (e.g., home network) it can provide a launchpad for targeting non-HIoT applications, and (iv) well-known attacks such as Denial-of-Service (DoS) can result in grave consequences.
Cryptography is the premise of many existing communication security measures. Particularly, AES and ECC are suitable for the resource-constrained nodes in smart healthcare systems [200]. Both BLE and ZigBee utilize AES in their link layers. The network layer can be effectively protected using IPv6 over low power wireless personal area networks (6LoWPAN), since it can incorporate the Internet protocol security (IPSec) protocol. IPSec improves communication security, regardless of the protective mechanisms implemented in the application layer [201]. Finally,to achieve end-to-end encryption, the application layer must use the constrained Application Protocol (CoAP), as it utilizes Datagram Transport Layer Security (DTLS) and IPSec. DTLS can be considered as the User Datagram Protocol (UDP)-compliant implementation of the transport layer security (TLS) protocol.
Cloud Storage and Processing
Modern authentication mechanisms aim to replace conventional password-based solutions, as passwords are susceptible to many attacks (e.g., brute force and dictionary attack) and can become an inconvenience (especially, in HIoT, where a portion of users are elderly and disabled people). Two-factor authentication (TFA) is a common methodology today for healthcare systems to avoid a breach in case one key is compromised. TFA requires the user to enter a password and a secondary piece of information (e.g., a code obtained through a cell phone call or a dedicated device) for successful login. Due to the sensitivity of collected data and EHRs, HIoT implementations must ensure secure access to data. The conventional access control techniques, however, are proven inefficient in HIoT as it involves a large number of stakeholders with many dynamic roles. Attribute-based access control can circumvent many of these limitations but it fails to dynamically adjust privileges (say) in an emergency situation [202].
To protect data during processing [203], Advanced encryption schemes, such as Fully Homomorphic Encryption (FHE) have been proposed. While there is research showing the feasibility of such schemes, their computational intensity renders them impractical. Additionally, applying multi-layer isolation (Operating System (OS), Virtual Machine (VM), and hardware) can reduce the odds of data leakage in public servers both in the data processing and data storage layers [169]. Finally, it is equally important to stress the non-technical aspects of HIoT security. HCOs must form security teams to oversee the security of the system and provide timely responses to threats and attacks. Stakeholders must be trained, and HCOs must be ready to negotiate with attackers to protect the data [204].
In the PD/HD case study, participant privacy is carefully managed. Within the MC10 portal where the sensor data for the participants is stored and made available for analytics, participants are identified only by assigned ID numbers. The portal provides basic demographic information such as sex and age (see Fig. 5) but does not provide any other personally identifying information. EHRs with personally identifying information used in the clinic are maintained in a separate Red-Cap database [205] that is HIPPA compliant. This partitioning of data effectively ensures the availability of the sensor data for analytics without compromising the privacy of the participants. Of course, the partitioning of the data notwithstanding, the study is performed in compliance with the Institutional Review Board (IRB) requirements: informed consent is required from all participants and everyone accessing the data is required to undergo human subjects training certification.
X. CONCLUSION AND VISION FOR THE FUTURE
This paper reviewed the state-of-the-art in Healthcare IoT (HIoT) technologies, particularly focusing on clinical applications of HIoT. It presented HIoT through the lens of three of its primary components: (i) sensing and data acquisition, (ii) communication, and (iii) data analytics and inference. As the underlying IoT technologies in HIoT become more mature, each one of these three components will independently witness rapid progress within their own domain. Data acquisition and sensing technologies will benefit from future VLSI technologies that require lower battery power for their operation, while communication standards will continuously advance to provide higher communication throughput with decreasing power consumption demands from the sensing networks. Intelligent energy-aware operating systems will also be critical to harnessing the energy demand of end-devices [206]. Future cloud platforms will take advantage of the ever-increasing computing power of the CPU and GPUs to enable more sophisticated machine learning algorithms to be run in the cloud with higher accuracy [207], [208]. As a consequence of this dizzying pace of progress, we envision significant opportunities that will eventually make HIoT an indispensable part of clinical practice in the coming decade. However, we also expect several barriers to entry that can slow the pace of adoption. In this section, we first review the challenges and then discuss opportunities.
Challenges that may prevent rapid adoption of HIoT can be broadly categorized as legal, regulatory, and ethnographic [209]. From a legal point of view, we particularly expect legal accountability to pose a challenge. Suppose a highly-trusted machine learning-based decision support system were to fail, causing bodily harm or fatality among patients. Can the machine be held responsible? If so, specifically, does the responsibility lie with the programmer, adopting institution, or the business entity that sells the program [210]? Questions of this type that were previously irrelevant, must be addressed to eliminate uncertainties and allow organizations adopting HIoT to better understand their legal exposure and risks.
The second set of challenges we envision are in the regulatory domain. HIoT devices are likely to span a wide range of applications and sensing/actuation modalities that vary in invasiveness. Accordingly, there are likely to be different classes of devices with different regulatory requirements [211]. While a majority of the non-invasive sensors may be made available for purchase over-the-counter (for instance, for gathering data prior to a routine check-up with a physician), other devices that are more invasive —and/or have potentially adverse side effects— will likely be available only upon prescription and will likely be subjected to regulatory approval after clinical trials [212]. As such, approval and adoption rates, as well as pricing of devices, will exhibit significant heterogeneity and the synergistic benefit from multiple HIoT sensors working in unison may take longer than anticipated [177], [213].
Finally, ethnographic challenges involve the reluctance of the medical community to adopt HIoT, due to its perceived risks vs. marginal added-utility in day-to-day clinical practice [214]. We anticipate the adoption may be slow until some avant-garde healthcare organizations start gaining significant advantage due to HIoT adoption and build a history of operation without glitches. If early adopters are able to exhibit significant operating advantages quickly, there is also the possibility that HIoT penetration may accelerate quickly and virtually become the norm within a short period of time.
Despite the challenges, the HIoT also presents significant opportunities. One of the biggest opportunities is the potential for much higher diagnostic accuracy that can be achieved by using statistical inference and data analytics algorithms with the increasing availability of clinical data [215]. Currently, limited public datasets are available for training ML algorithms and HCOs rely on their individual databases [216], [217]. Large datasets that are required to train sophisticated algorithms (such as ones that use Deep Learning) are not freely available. Widespread adoption of HIoT in clinical settings and the aggregation of the resulting data, with appropriate anonymization, can create shared large-scale datasets from which all organizations can benefit and improve diagnostics and health management [218]. We envision that large-scale anonymous medical data sharing networks, enabled by HIoT, will vastly improve ML accuracy, much like data availability is currently revolutionizing computer and machine vision applications. Once established, the trend is likely to be self-reinforcing with continued acceleration in data accumulation driving improved inference and finer-grained/personalized analyses. Such a positive feedback cycle is also likely to speed up the mainstreaming of HIoT.
Another opportunity lies in the potential future use of new sensing modalities and actuators. For example, while today’s sensor technology requires a blood draw for accurate measurement of blood glucose levels, future sensor technology promises to perform the same measurements far less invasively, without a blood draw [219]. Availability of such conveniently-deployable technologies (e.g., non-invasive blood pressure, blood glucose, and oxygenation sensors) can drastically increase their use in clinical settings. Future actuator technologies can also improve the automation of routine medical tasks, such as drug delivery. Advances in Micro-Electro-Mechanical Systems (MEMS) technologies promise to create actuators that can deliver drugs (e.g., insulin) into the bloodstream directly, eliminating the need for the patient to manually do so [220]. This can result in substantial improvements in patient health, as the need for the patient to perform routine measurements, followed by drug intake, are eliminated and the drug dosage and administration regimen can be customized based on both the specific medication involved and the individual [221]. Benefits of MEMS-based technologies are even more pronounced for patients that cannot administer the drugs themselves due to motor deficiencies.
In summary, although the HIoT roadmap into the future is not free from bumps, we expect the pace to quicken once the ecosystem is in place and early adopters demonstrate the benefits it can provide.
ACKNOWLEDGMENT
The authors thank the anonymous reviewers for several constructive suggestions that have significantly improved the presentation in the paper. This work was supported in part by the U.S. National Science Foundation grants CNS-1239423 and CNS-1464273 and by the U.S. National Institute of Health grant NINDS-1P50NS108676-01.
Biography
Hadi Habibzadeh (S’17) received his B.S. in CE from Isfahan University of Technology in Iran in 2015 and his M.S. degree from University of Rochester, USA in 2016. He is currently pursuing his PhD degree in the ECE department at SUNY Albany, under the supervision of Dr. Tolga Soyata in the field of Cyber Physical systems and embedded systems with applications in Internet of Things and Smart Cities.

Karthik Dinesh received B.E in Electronics and Communication from National Institute of Engineering, Mysore, India in 2010 and M.Tech from Indian Institute of Technology, Kanpur, India in 2013. He is currently pursuing PhD in ECE department, University of Rochester under the supervision of Dr. Gaurav Sharma.

Omid Rajabi Shishvan (S’16) received his B.Sc. in EE from Sharif University of Technology in 2012 and his M.Sc. degree in ECE from University of Rochester in 2015. He is currently pursuing his PhD degree at the ECE department of SUNY Albany.

Andrew Boggio-Dandry (S’17) graduated summa cum laude from The University at Albany, State University of New York (SUNY Albany) with his B.S. in Computer Engineering in 2018. He is currently pursuing his PhD degree at the ECE department of SUNY Albany.

Gaurav Sharma (S’88-M’96-SM’00-F’13) is a professor at the University of Rochester in the Department of Electrical and Computer Engineering, in the Department of Computer Science, and in the Department of Biostatistics and Computational Biology. He received the BE degree in Electronics and Communication Engineering from Indian Institute of Technology Roorkee (formerly Univ. of Roorkee), India in 1990; the ME degree in Electrical Communication Engineering from the Indian Institute of Science, Bangalore, India in 1992; and the MS degree in Applied Mathematics and PhD degree in Electrical and Computer Engineering from North Carolina State University, Raleigh in 1995 and 1996, respectively. Dr. Sharma’s research interests include data analytics, cyberphysical systems, signal and image processing, computer vision, media security, and communications. He is a fellow of the IEEE, of SPIE, and of the Society of Imaging Science and Technology (IS&T) and a member of Sigma Xi. He is the Editor-in-Chief (EIC) for the IEEE Transactions on Image Processing and previously served as the EIC for the Journal of Electronic Imaging from 2011 through 2015. He is a 2020 Distinguished Lecturer for the IEEE Signal Processing Society.

Tolga Soyata (M’08 SM’16) received his B.S. degree in ECE from Istanbul Technical University in 1988, M.S. degree in ECE from Johns Hopkins University in 1992 and Ph.D. in ECE from University of Rochester in 2000. He is an Associate Professor in the ECE Department of SUNY Albany. His teaching interests include CMOS VLSI ASIC Design, FPGA-and GPU-based Parallel Computation. His research interests include Cyber Physical Systems and Digital Health. He is a senior member of both IEEE and ACM.

Contributor Information
Hadi Habibzadeh, Department of Electrical and Computer Engineering, SUNY Albany, Albany NY, 12203.
Karthik Dinesh, Department of Electrical and Computer Engineering, University of Rochester, Rochester, NY 14627.
Omid Rajabi Shishvan, Department of Electrical and Computer Engineering, SUNY Albany, Albany NY, 12203.
Andrew Boggio-Dandry, Department of Electrical and Computer Engineering, SUNY Albany, Albany NY, 12203.
Gaurav Sharma, Department of Electrical and Computer Engineering, University of Rochester, Rochester, NY 14627.
Tolga Soyata, Department of Electrical and Computer Engineering, SUNY Albany, Albany NY, 12203.
REFERENCES
- [1].Fitbit Inc. (2019) Fitbit technology. Accessed on: Oct. 4, 2019. [Online]. Available: https://www.fitbit.com/technology
- [2].Apple Inc. (2019) Apple watch. Accessed on: Oct. 4, 2019. [Online]. Available: https://www.apple.com/watch/
- [3].Abbott Diabetes Care Inc. (2019) Continuous glucose monitoring–FreeStyle libre system. Accessed on: Oct. 4, 2019. [Online]. Available: https://www.freestylelibre.us/
- [4].Teva Pharmaceutical Industries Ltd. (2019) ProAir Digihaler with connected mobile app. Accessed on: Oct. 4, 2019. [Online]. Available: https://www.proairdigihaler.com/
- [5].Murthy V and Okunade A, “Is the health care price inflation in US urban areas stationary? evidence from panel unit root tests,” Journal of Economics, Finance and Administrative Science, vol. 23, no. 44, pp. 77–94, 2018. [Google Scholar]
- [6].Reddy S. (2017, March) Can tech speed up emergency room care. Wall Street Journal. Accessed on: Oct. 4, 2019. [Online]. Available: https://www.wsj.com/articles/can-tech-speed-upemergency-room-care-1490629118
- [7].Maeng DD, Starr AE, Tomcavage JF, Sciandra J, Salek D, and Griffith D, “Can telemonitoring reduce hospitalization and cost of care? a health plan’s experience in managing patients with heart failure,” Population Health Management, vol. 17, no. 6, pp. 340–344, 2014. [DOI] [PubMed] [Google Scholar]
- [8].Couturier J, Sola D, Borioli GS, and Raiciu C, “How can the internet of things help to overcome current healthcare challenges,” Communications & Strategies, No. 87, 3rd Quarter, 2012. [Google Scholar]
- [9].Rosner BI, Gottlieb M, and Anderson WN, “Effectiveness of an automated digital remote guidance and telemonitoring platform on costs, readmissions, and complications after hip and knee arthroplasties,” The Journal of Arthroplasty, vol. 33, no. 4, pp. 988–996.e4, 2018. [DOI] [PubMed] [Google Scholar]
- [10].Qi J, Yang P, Min G, Amft O, Dong F, and Xu L, “Advanced internet of things for personalised healthcare systems: A survey,” Pervasive and Mobile Computing, vol. 41, pp. 132–149, 2017. [Google Scholar]
- [11].Yeole AS and Kalbande DR, “Use of internet of things (IoT) in healthcare: A survey,” in Proc. ACM Symp. Women in Research 2016, ser. WIR ‘16. New York, NY, USA: ACM, 2016, pp. 71–76. [Online]. Available: http://doi.acm.org/10.1145/2909067.2909079 [Google Scholar]
- [12].Thakar AT and Pandya S, “Survey of IoT enables healthcare devices,” in Int. Conf. Computing Methodologies and Communication (ICCMC). IEEE, 2017, pp. 1087–1090. [Google Scholar]
- [13].Rodrigues JJ, Segundo DBDR, Junqueira HA, Sabino MH, Prince RM, Al-Muhtadi J, and De Albuquerque VHC, “Enabling technologies for the internet of health things,” IEEE Access, vol. 6, pp. 13 129–13 141, 2018. [Google Scholar]
- [14].Anandhavijayalaxmy E, Dhanalakshmi V, and Sivasankaran S, “Trends and technologies in IoT based healthcare system-a survey,” Biometrics and Bioinformatics, vol. 10, no. 3, pp. 41–47, 2018. [Google Scholar]
- [15].Alam MM, Malik H, Khan MI, Pardy T, Kuusik A, and Le Moullec Y, “A survey on the roles of communication technologies in IoT-based personalized healthcare applications,” IEEE Access, vol. 6, pp. 36 611–36 631, 2018. [Google Scholar]
- [16].Al-Garadi MA, Mohamed A, Al-Ali A, Du X, and Guizani M, “A survey of machine and deep learning methods for internet of things (IoT) security,” arXiv preprint arXiv:1807.11023, 2018. [Google Scholar]
- [17].Hameed S, Khan FI, and Hameed B, “Understanding security requirements and challenges in internet of things (IoT): A review,” Journal of Computer Networks and Communications, vol. 2019, 2019. [Google Scholar]
- [18].Talal M, Zaidan A, Zaidan B, Albahri A, Alamoodi A, Albahri O, Alsalem M, Lim C, Tan K, Shir W et al. , “Smart home-based IoT for real-time and secure remote health monitoring of triage and priority system using body sensors: Multi-driven systematic review,” Journal of medical systems, vol. 43, no. 3, p. 42, 2019. [DOI] [PubMed] [Google Scholar]
- [19].Thibaud M, Chi H, Zhou W, and Piramuthu S, “Internet of things (IoT) in high-risk environment, health and safety (EHS) industries: A comprehensive review,” Decision Support Systems, vol. 108, pp. 79–95, 2018. [Google Scholar]
- [20].de la Torre Díez I, Alonso SG, Hamrioui S, Cruz EM, Nozaleda LM, and Franco MA, “IoT-based services and applications for mental health in the literature,” Journal of medical systems, vol. 43, no. 1, p. 11, 2019. [DOI] [PubMed] [Google Scholar]
- [21].Darwish A and Hassanien AE, “Wearable and implantable wireless sensor network solutions for healthcare monitoring,” Sensors, vol. 11, no. 6, pp. 5561–5595, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Patel S, Park H, Bonato P, Chan L, and Rodgers M, “A review of wearable sensors and systems with application in rehabilitation,” Journal of neuroengineering and rehabilitation, vol. 9, no. 1, p. 21, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen M, Gonzalez S, Vasilakos A, Cao H, and Leung VCM, “Body area networks: A survey,” Mobile Networks and Applications, vol. 16, no. 2, pp. 171–193, April 2011. [Google Scholar]
- [24].Movassaghi S, Abolhasan M, Lipman J, Smith D, and Jamalipour A, “Wireless body area networks: A survey,” EEE Commun. Surveys Tuts, vol. 16, no. 3, pp. 1658–1686, Third 2014. [Google Scholar]
- [25].Alemdar H and Ersoy C, “Wireless sensor networks for healthcare: A survey,” Computer Networks, vol. 54, no. 15, pp. 2688–2710, 2010. [Google Scholar]
- [26].Rahmani AM, Thanigaivelan NK, Gia TN, Granados J, Negash B, Liljeberg P, and Tenhunen H, “Smart e-health gateway: Bringing intelligence to internet-of-things based ubiquitous healthcare systems,” in 12th Annu. IEEE Consumer Communications and Networking Conf. (CCNC; ), January 2015, pp. 826–834. [Google Scholar]
- [27].Kumar P and Lee H-J, “Security issues in healthcare applications using wireless medical sensor networks: A survey,” Sensors, vol. 12, no. 1, pp. 55–91, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Islam SMR, Kwak D, Kabir MH, Hossain M, and Kwak KS, “The internet of things for health care: A comprehensive survey,” IEEE Access, vol. 3, pp. 678–708, 2015. [Google Scholar]
- [29].Cheng X, Xue X, Ma Y, Han M, Zhang W, Xu Z, Zhang H, and Zhang H, “Implantable and self-powered blood pressure monitoring based on a piezoelectric thinfilm: Simulated, in vitro and in vivo studies,” Nano Energy, vol. 22, no. Supplement C, pp. 453–460, 2016. [Google Scholar]
- [30].Zhang Y, Zhang F, Shakhsheer Y, Silver JD, Klinefelter A, Nagaraju M, Boley J, Pandey J, Shrivastava A, Carlson EJ, Wood A, Calhoun BH, and Otis BP, “A batteryless 19μw MICS/ISM-band energy harvesting body sensor node SoC for ExG applications,” IEEE J. Solid-State Circuits, vol. 48, no. 1, pp. 199–213, January 2013. [Google Scholar]
- [31].Wang H, Zhang D, Ma J, Wang Y, Wang Y, Wu D, Gu T, and Xie B, “Human respiration detection with commodity Wifi devices: Do user location and body orientation matter?” in Proc. 2016 ACM Int. Joint Conf. Pervasive and Ubiquitous Computing, ser. UbiComp ‘16, 2016, pp. 25–36. [Google Scholar]
- [32].Abdelnasser H, Harras KA, and Youssef M, “UbiBreathe: A ubiquitous non-invasive WiFi-based breathing estimator,” in Proc. 16th ACM Int. Symp. Mobile Ad Hoc Networking and Computing, ser. MobiHoc ‘15, 2015, pp. 277–286. [Google Scholar]
- [33].Liu J, Wang Y, Chen Y, Yang J, Chen X, and Cheng J, “Tracking vital signs during sleep leveraging off-the-shelf WiFi,” in Proc. 16th ACM Int. Symp. Mobile Ad Hoc Networking and Computing, ser. MobiHoc ‘15, 2015, pp. 267–276. [Google Scholar]
- [34].Wang Y, Wu K, and Ni LM, “WiFall: Device-free fall detection by wireless networks,” IEEE Trans. Mobile Comput, vol. 16, no. 2, pp. 581–594, February 2017. [Google Scholar]
- [35].Wang H, Zhang D, Wang Y, Ma J, Wang Y, and Li S, “RT-Fall: A real-time and contactless fall detection system with commodity WiFi devices,” IEEE Trans. Mobile Comput, vol. 16, no. 2, pp. 511–526, February 2017. [Google Scholar]
- [36].Hassanalieragh M, Page A, Soyata T, Sharma G, Aktas MK, Mateos G, Kantarci B, and Andreescu S, “Health monitoring and management using internet-of-things (IoT) sensing with cloud-based processing: Opportunities and challenges,” in 2015 IEEE Int. Conf. Services Computing (SCC), New York, NY, June 2015, pp. 285–292. [Google Scholar]
- [37].Qu Y, Zheng G, Ma H, Wang X, Ji B, and Wu H, “A survey of routing protocols in WBAN for healthcare applications,” Sensors, vol. 19, no. 7, p. 1638, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kumar PM and Gandhi UD, “A novel three-tier internet of things architecture with machine learning algorithm for early detection of heart diseases,” Computers & Electrical Engineering, vol. 65, pp. 222–235, 2018. [Google Scholar]
- [39].Lin YD and Hu YH, “Power-line interference detection and suppression in ECG signal processing,” IEEE Trans. Biomed. Eng, vol. 55, no. 1, pp. 354–357, January 2008. [DOI] [PubMed] [Google Scholar]
- [40].Zhang Y, Meratnia N, and Havinga PJ, “Distributed online outlier detection in wireless sensor networks using ellipsoidal support vector machine,” Ad Hoc Networks, vol. 11, no. 3, pp. 1062–1074, 2013. [Google Scholar]
- [41].Arias O, Wurm J, Hoang K, and Jin Y, “Privacy and security in internet of things and wearable devices,” IEEE Trans. Multi-Scale Comput. Syst, vol. 1, no. 2, pp. 99–109, April 2015. [Google Scholar]
- [42].Flaherty JH, Liu ML, Ding L, Dong B, Ding Q, Li X, and Xiao S, “China: The aging giant,” Journal of the American Geriatrics Society, vol. 55, no. 8, pp. 1295–1300, 2007. [DOI] [PubMed] [Google Scholar]
- [43].Jacobsen LA, Kent M, Lee M, and Mather M, “America’s aging population,” Population Bulletin, vol. 66, no. 1, pp. 1–20, 2011. [Google Scholar]
- [44].Bhattacharya P, “Implications of an aging population in India: Challenges and opportunities,” Living to, vol. 100, pp. 12–14, 2005. [Google Scholar]
- [45].Chanana H and Talwar P, “Aging in India: its socio-economic and health implications,” Asia-Pacific Population Journal, vol. 2, no. 3, pp. 23–38, 1987. [PubMed] [Google Scholar]
- [46].Chatterji S, Kowal P, Mathers C, Naidoo N, Verdes E, Smith JP, and Suzman R, “The health of aging populations in China and India,” Health Affairs, vol. 27, no. 4, pp. 1052–1063, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kharas H and Gertz G, “The new global middle class: A cross-over from west to east,” Wolfensohn Center for Development at Brookings, pp. 1–14, 2010. [Google Scholar]
- [48].Li S, Da Xu L, and Wang X, “Compressed sensing signal and data acquisition in wireless sensor networks and internet of things,” EEE Trans. Ind. Informat, vol. 9, no. 4, pp. 2177–2186, 2013. [Google Scholar]
- [49].Zois D-S, “Sequential decision-making in healthcare IoT: Real-time health monitoring, treatments and interventions,” in 2016 IEEE 3rd World Forum Internet of Things (WF-IoT). IEEE, 2016, pp. 24–29. [Google Scholar]
- [50].Kruger CP and Hancke GP, “Implementing the internet of things vision in industrial wireless sensor networks,” in 2014 12th IEEE Int. Conf. Industrial Informatics (INDIN). IEEE, 2014, pp. 627–632. [Google Scholar]
- [51].Spencer B, Al-Obeidat F, and Alfandi O, “Selecting sensors when forecasting temperature in smart buildings,” Procedia Computer Science, vol. 109, pp. 777–784, 2017. [Google Scholar]
- [52].Chen X, Ma M, and Liu A, “Dynamic power management and adaptive packet size selection for IoT in e-healthcare,” Computers & Electrical Engineering, vol. 65, pp. 357–375, 2018. [Google Scholar]
- [53].Ghanei F, Tipnis P, Marcus K, Dantu K, Ko S, and Ziarek L, “OS-based resource accounting for asynchronous resource use in mobile systems,” in Proc. 2016 Int. Symp. Low Power Electronics and Design. ACM, 2016, pp. 296–301. [Google Scholar]
- [54].Pantelopoulos A and Bourbakis NG, “A survey on wearable sensor-based systems for health monitoring and prognosis,” IEEE Trans. Syst., Man, Cybern. Syst., Part C (Applications and Reviews), vol. 40, no. 1, pp. 1–12, 2010. [Google Scholar]
- [55].Bazzani M, Conzon D, Scalera A, Spirito MA, and Trainito CI, “Enabling the IoT paradigm in e-health solutions through the VIRTUS middleware,” in 2012 IEEE 11th Int. Conf. Trust, Security and Privacy in Computing and Communications (TrustCom). IEEE, 2012, pp. 1954–1959. [Google Scholar]
- [56].Benharref A and Serhani MA, “Novel cloud and SOA-based framework for e-health monitoring using wireless biosensors,” IEEE J. Biomed. Health Inform, vol. 18, no. 1, pp. 46–55, 2014. [DOI] [PubMed] [Google Scholar]
- [57].Mitra U, Emken BA, Lee S, Li M, Rozgic V, Thatte G, Vathsangam H, Zois D-S, Annavaram M, Narayanan S et al. , “KNOWME: a case study in wireless body area sensor network design,” IEEE Commun. Mag, vol. 50, no. 5, 2012. [Google Scholar]
- [58].Yeung S, Russakovsky O, Jin N, Andriluka M, Mori G, and Fei-Fei L, “Every moment counts: Dense detailed labeling of actions in complex videos,” International Journal of Computer Vision, vol. 126, no. 2–4, pp. 375–389, 2018. [Google Scholar]
- [59].Smith JR, Fishkin KP, Jiang B, Mamishev A, Philipose M, Rea AD, Roy S, and Sundara-Rajan K, “RFID-based techniques for human-activity detection,” Communications of the ACM, vol. 48, no. 9, pp. 39–44, 2005. [Google Scholar]
- [60].Wang L, Gu T, Xie H, Tao X, Lu J, and Huang Y, “A wearable RFID system for real-time activity recognition using radio patterns,” in Int. Conf. Mobile and Ubiquitous Systems: Computing, Networking, and Services. Springer, 2013, pp. 370–383. [Google Scholar]
- [61].Wang L, Gu T, Tao X, and Lu J, “Toward a wearable RFID system for real-time activity recognition using radio patterns,” IEEE Trans. Mobile Comput, vol. 16, no. 1, pp. 228–242, 2017. [Google Scholar]
- [62].Yao L, Sheng QZ, Li X, Wang S, Gu T, Ruan W, and Zou W, “Freedom: Online activity recognition via dictionary-based sparse representation of RFID sensing data,” in 2015 IEEE Int. Conf. Data Mining (ICDM). IEEE, 2015, pp. 1087–1092. [Google Scholar]
- [63].Li H, Chen X, Du H, He X, Qian J, Wan P-J, and Yang P, “Wi-Motion: A robust human activity recognition using WiFi signals,” arXiv preprint arXiv:1810.11705, 2018. [Google Scholar]
- [64].Zhu S, Xu J, Guo H, Liu Q, Wu S, and Wang H, “Indoor human activity recognition based on ambient radar with signal processing and machine learning,” in 2018 IEEE Int. Conf. Communications (ICC). IEEE, 2018, pp. 1–6. [Google Scholar]
- [65].Yin J, Yang Q, and Pan JJ, “Sensor-based abnormal human-activity detection,” IEEE Trans. Knowl. Data Eng, vol. 20, no. 8, pp. 1082–1090, 2008. [Google Scholar]
- [66].Yao L, Sheng QZ, Li X, Gu T, Tan M, Wang X, Wang S, and Ruan W, “Compressive representation for device-free activity recognition with passive RFID signal strength,” IEEE Trans. Mobile Comput, vol. 17, no. 2, pp. 293–306, 2018. [Google Scholar]
- [67].Gia TN, Tcarenko I, Sarker VK, Rahmani AM, Westerlund T, Liljeberg P, and Tenhunen H, “IoT-based fall detection system with energy efficient sensor nodes,” in 2016 IEEE Nordic Circuits and Systems Conf. (NORCAS). IEEE, 2016, pp. 1–6. [Google Scholar]
- [68].Ruan W, Yao L, Sheng QZ, Falkner N, Li X, and Gu T, “Tagfall: Towards unobstructive fine-grained fall detection based on UHF passive RFID tags,” in Proc. 12th EAI Int. Conf. Mobile and Ubiquitous Systems: Computing, Networking and Services. ICST (Institute for Computer Sciences, Social-Informatics and Telecommunications Engineering), 2015, pp. 140–149. [Google Scholar]
- [69].Kolamunna H, Hu Y, Perino D, Thilakarathna K, Makaroff D, Guan X, and Seneviratne A, “AFit: Adaptive fitness tracking by application function virtualization,” in Proc. 2016 ACM Int. Joint Conf. Pervasive and Ubiquitous Computing: Adjunct. ACM, 2016, pp. 309–312. [Google Scholar]
- [70].Bender CG, Hoffstot JC, Combs BT, Hooshangi S, and Cappos J, “Measuring the fitness of fitness trackers,” in 2017 IEEE Sensors Applications Symp. (SAS). IEEE, 2017, pp. 1–6. [Google Scholar]
- [71].Ruan W, Yao L, Sheng QZ, Falkner NJ, and Li X, “Tagtrack: Device-free localization and tracking using passive RFID tags,” in Proc. 11th Int. Conf. Mobile and Ubiquitous Systems: Computing, Networking and Services. ICST (Institute for Computer Sciences, Social-Informatics and Telecommunications Engineering), 2014, pp. 80–89. [Google Scholar]
- [72].Ruan W, Sheng QZ, Yao L, Li X, Falkner NJ, and Yang L, “Device-free human localization and tracking with UHF passive RFID tags: A data-driven approach,” Journal of Network and Computer Applications, vol. 104, pp. 78–96, 2018. [Google Scholar]
- [73].Yao L, Sheng QZ, Ruan W, Gu T, Li X, Falkner N, and Yang Z, “RF-care: Device-free posture recognition for elderly people using a passive RFID tag array,” in Proc. 12th EAI Int. Conf. Mobile and Ubiquitous Systems: Computing, Networking and Services. ICST (Institute for Computer Sciences, Social-Informatics and Telecommunications Engineering), 2015, pp. 120–129. [Google Scholar]
- [74].Abtahi M, Gyllinsky JV, Paesang B, Barlow S, Constant M, Gomes N, Tully O, D’Andrea SE, and Mankodiya K, “MagicSox: An E-textile IoT system to quantify gait abnormalities,” Smart Health, vol. 5–6, pp. 4–14, 2018. [Google Scholar]
- [75].Gu T, Wang L, Chen H, Tao X, and Lu J, “Recognizing multiuser activities using wireless body sensor networks,” IEEE Trans. Mobile Comput, vol. 10, no. 11, pp. 1618–1631, 2011. [Google Scholar]
- [76].da Silva Cameirão M, Bermúdez i Badia S, Duarte E, and Verschure PF, “Virtual reality based rehabilitation speeds up functional recovery of the upper extremities after stroke: a randomized controlled pilot study in the acute phase of stroke using the rehabilitation gaming system,” Restorative neurology and neuroscience, vol. 29, no. 5, pp. 287–298, 2011. [DOI] [PubMed] [Google Scholar]
- [77].Standen P, Threapleton K, Richardson A, Connell L, Brown D, Battersby S, Platts F, and Burton A, “A low cost virtual reality system for home based rehabilitation of the arm following stroke: A randomised controlled feasibility trial,” Clinical rehabilitation, vol. 31, no. 3, pp. 340–350, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Saposnik G, Cohen LG, Mamdani M, Pooyania S, Ploughman M, Cheung D, Shaw J, Hall J, Nord P, Dukelow S et al. , “Efficacy and safety of non-immersive virtual reality exercising in stroke rehabilitation (EVREST): a randomised, multicentre, single-blind, controlled trial,” The Lancet Neurology, vol. 15, no. 10, pp. 1019–1027, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Hoda M, Hoda Y, Hafidh B, and El Saddik A, “Predicting muscle forces measurements from kinematics data using Kinect in stroke rehabilitation,” Multimedia Tools and Applications, vol. 77, no. 2, pp. 1885–1903, January 2018. [Online]. Available: 10.1007/s11042-016-4274-5 [DOI] [Google Scholar]
- [80].Bobin M, Anastassova M, Boukallel M, and Ammi M, “SyMPATHy: Smart glass for monitoring and guiding stroke patients in a home-based context,” in Proc. 8th ACM SIGCHI Symp. Engineering Interactive Computing Systems, ser. EICS ‘16. New York, NY, USA: ACM, 2016, pp. 281–286. [Online]. Available: http://doi.acm.org/10.1145/2933242.2935870 [Google Scholar]
- [81].National Center for Chronic Prevention and Health Promotion. (2014) National diabetes statistics report, 2014. Accessed on: Oct. 4, 2019. [Online]. Available: https://www.cdc.gov/diabetes/pdfs/data/2014-report-estimates-of-diabetes-and-its-burden-in-the-united-states.pdf
- [82].Evans JM, Newton RW, Ruta DA, MacDonald TM, Stevenson RJ, and Morris AD, “Frequency of blood glucose monitoring in relation to glycaemic control: Observational study with diabetes database,” BMJ, vol. 319, no. 7202, pp. 83–86, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Al-Taee MA, Al-Nuaimy W, Muhsin ZJ, and Al-Ataby A, “Robot assistant in management of diabetes in children based on the internet of things,” IEEE Internet Things J, vol. 4, no. 2, pp. 437–445, 2017. [Google Scholar]
- [84].Fioravanti A, Fico G, Salvi D, García-Betances RI, and Arredondo MT, “Automatic messaging for improving patients engagement in diabetes management: an exploratory study,” Medical & Biological Engineering & Computing, vol. 53, no. 12, pp. 1285–1294, 2015. [DOI] [PubMed] [Google Scholar]
- [85].Kaiya K and Koyama A, “Design and implementation of meal information collection system using IoT wireless tags,” in 2016 10th Int. Conf. Complex, Intelligent, and Software Intensive Systems (CISIS). IEEE, 2016, pp. 503–508. [Google Scholar]
- [86].Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R et al. , “Heart disease and stroke statistics-2018 update: a report from the American Heart Association.” Circulation, vol. 137, no. 12, p. e67, 2018. [DOI] [PubMed] [Google Scholar]
- [87].Schmier JK, Ong KL, and Fonarow GC, “Cost-effectiveness of remote cardiac monitoring with the cardioMEMS heart failure system,” Clinical Cardiology, vol. 40, no. 7, pp. 430–436, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kiranyaz S, Ince T, and Gabbouj M, “Personalized monitoring and advance warning system for cardiac arrhythmias,” Scientific reports, vol. 7, no. 1, p. 9270, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Xia Y, Zhang H, Xu L, Gao Z, Zhang H, Liu H, and Li S, “An automatic cardiac arrhythmia classification system with wearable electrocardiogram,” IEEE Access, vol. 6, pp. 16 529–16 538, 2018. [Google Scholar]
- [90].Hijazi S, Page A, Kantarci B, and Soyata T, “Machine learning in cardiac health monitoring and decision support,” IEEE Computer Mag, vol. 49, no. 11, pp. 38–48, November 2016. [Google Scholar]
- [91].Ousaka D, Sakano N, Morita M, Shuku T, Sanou K, Kasahara S, and Oozawa S, “A new approach to prevent critical cardiac accidents in athletes by real-time electrocardiographic tele-monitoring system: Initial trial in full marathon,” Journal of Cardiology Cases, vol. 20, no. 1, pp. 35–38, 2019. [Online]. Available: http://www.sciencedirect.com/science/article/pii/S1878540919300386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Cardiac Insight Inc. (2019) Cardea SOLO wireless ECG werable monitor. Accessed on: Oct. 4, 2019. [Online]. Available: https://www.cardiacinsightinc.com/cardea-solo
- [93].Baranchuk A, Refaat MM, Patton KK, Chung MK, Krishnan K, Kutyifa V, Upadhyay G, Fisher JD, and Lakkireddy DR, “Cybersecurity for cardiac implantable electronic devices: What should you know?” Journal of the American College of Cardiology, vol. 71, no. 11, pp. 1284–1288, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Tan KS, Saatchi R, Elphick H, and Burke D, “Real-time vision based respiration monitoring system,” in 2010 7th Int. Symp. Communication Systems Networks and Digital Signal Processing (CSNDSP). IEEE, 2010, pp. 770–774. [Google Scholar]
- [95].AL-Khalidi FQ, Saatchi R, Burke D, Elphick H, and Tan S, “Respiration rate monitoring methods: A review,” Pediatric pulmonology, vol. 46, no. 6, pp. 523–529, 2011. [DOI] [PubMed] [Google Scholar]
- [96].Ferreira AG, Fernandes D, Branco S, Monteiro JL, Cabral J, Catarino AP, and Rocha AM, “A smart wearable system for sudden infant death syndrome monitoring,” in 2016 IEEE Int. Conf. Industrial Technology (ICIT). IEEE, 2016, pp. 1920–1925. [Google Scholar]
- [97].Hao T, Bi C, Xing G, Chan R, and Tu L, “MindfulWatch: A smartwatch-based system for real-time respiration monitoring during meditation,” Proc. ACM on Interactive, Mobile, Wearable and Ubiquitous Technologies, vol. 1, no. 3, p. 57, 2017. [Google Scholar]
- [98].Raji A, Devi PK, Jeyaseeli PG, and Balaganesh N, “Respiratory monitoring system for asthma patients based on IoT,” in 2016 Online Int. Conf. Green Engineering and Technologies (IC-GET). IEEE, 2016, pp. 1–6. [Google Scholar]
- [99].Nguyen A, Alqurashi R, Raghebi Z, Banaei-kashani F, Halbower AC, and Vu T, “A lightweight and inexpensive in-ear sensing system for automatic whole-night sleep stage monitoring,” in Proc. 14th ACM Conf. Embedded Network Sensor Systems CD-ROM. ACM, 2016, pp. 230–244. [Google Scholar]
- [100].Yang Z, Pathak PH, Zeng Y, Liran X, and Mohapatra P, “Vital sign and sleep monitoring using millimeter wave,” ACM Transactions on Sensor Networks (TOSN), vol. 13, no. 2, p. 14, 2017. [Google Scholar]
- [101].Alberto MC, Ruano MG, Herrero M, Jiménez A, García J, and Díaz E, “Sensory system for the sleep disorders detection in the geriatric population,” in 2017 4th Experiment@ Int. Conf. (exp. at’17). IEEE, 2017, pp. 329–334. [Google Scholar]
- [102].Tal A, Shinar Z, Shaki D, Codish S, and Goldbart A, “Validation of contact-free sleep monitoring device with comparison to polysomnography,” Journal of Clinical Sleep Medicine, vol. 13, no. 3, pp. 517–522, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT, and Whelton PK, “Potential US population impact of the 2017 ACC/AHA high blood pressure guideline,” Circulation, vol. 137, no. 2, pp. 109–118, 2018. [Online]. Available: https://www.ahajournals.org/doi/abs/10.1161/CIRCULATIONAHA.117.032582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Janjua G, Guldenring D, Finlay D, and McLaughlin J, “Wireless chest wearable vital sign monitoring platform for hypertension,” in 2017 39th Annu. Int. Conf. IEEE Engineering in Medicine and Biology Society (EMBC). IEEE, 2017, pp. 821–824. [DOI] [PubMed] [Google Scholar]
- [105].Ruiz-Fernández D, Marcos-Jorquera D, Gilart-Iglesias V, Vives-Boix V, and Ramírez-Navarro J, “Empowerment of patients with hypertension through BPM, IoT and remote sensing,” Sensors, vol. 17, no. 10, p. 2273, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Iakovakis D and Hadjileontiadis L, “Standing hypotension prediction based on smartwatch heart rate variability data: a novel approach,” in Proc. 18th Int. Conf. Human-Computer Interaction with Mobile Devices and Services Adjunct. ACM, 2016, pp. 1109–1112. [Google Scholar]
- [107].Clarke S, Jaimes LG, and Labrador MA, “mStress: A mobile recommender system for just-in-time interventions for stress,” in 2017 14th IEEE Annu. Consumer Communications & Networking Conf. (CCNC). IEEE, 2017, pp. 1–5. [Google Scholar]
- [108].Dillon A, Kelly M, Robertson IH, and Robertson DA, “Biofeedback and gaming-style smartphone applications as a stress reduction intervention,” in ACM Conf. Human Factors in Computing Systems: Computing and Mental Health Workshop, 2016. [Google Scholar]
- [109].McWhorter J, Brown L, and Khansa L, “A wearable health monitoring system for posttraumatic stress disorder,” Biologically inspired cognitive architectures, vol. 22, pp. 44–50, 2017. [Google Scholar]
- [110].Torrado Vidal JC, Montoro G, and Gómez J, “The potential of smartwatches for emotional self-regulation of people with autism spectrum disorder,” BIOSTEC 2016. 9th Int. Joint Conf. Biomedical Engineering Systems and Technologies: HealthInf. Rome, Italy, 20160221, 2016. [Google Scholar]
- [111].Glasgow RE, McCaul KD, and Schafer LC, “Barriers to regimen adherence among persons with insulin-dependent diabetes,” Journal of Behavioral Medicine, vol. 9, no. 1, pp. 65–77, 1986. [DOI] [PubMed] [Google Scholar]
- [112].Farmer KC, “Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice,” Clinical Therapeutics, vol. 21, no. 6, pp. 1074–1090, 1999. [DOI] [PubMed] [Google Scholar]
- [113].Toh X, Tan H-X, Liang H, and Tan H-P, “Elderly medication adherence monitoring with the internet of things,” in 2016 IEEE Int. Conf. Pervasive Computing and Communication Workshops (PerCom Workshops). IEEE, 2016, pp. 1–6. [Google Scholar]
- [114].Moshnyaga V, Koyanagi M, Hirayama F, Takahama A, and Hashimoto K, “A medication adherence monitoring system for people with dementia,” in 2016 IEEE Int. Conf. Systems, Man, and Cybernetics (SMC). IEEE, 2016, pp. 000 194–000 199. [Google Scholar]
- [115].Wu Q, Wu Q, Zeng Z, Zeng Z, Lin J, Lin J, Chen Y, and Chen Y, “AI empowered context-aware smart system for medication adherence,” International Journal of Crowd Science, vol. 1, no. 2, pp. 102–109, 2017. [Google Scholar]
- [116].Alzheimer’s Association, “2015 Alzheimer’s disease facts and figures.” Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, vol. 11, no. 3, p. 332, 2015. [DOI] [PubMed] [Google Scholar]
- [117].Ishii H, Kimino K, Aljehani M, Ohe N, and Inoue M, “An early detection system for dementia using the M2M/IoT platform,” Procedia Computer Science, vol. 96, pp. 1332–1340, 2016. [Google Scholar]
- [118].Tamamizu K, Tokunaga S, Saiki S, Matsumoto S, Nakamura M, and Yasuda K, “Towards person-centered anomaly detection and support system for home dementia care,” in Int. Conf. Digital Human Modeling and Applications in Health, Safety, Ergonomics and Risk Management. Springer, 2016, pp. 274–285. [Google Scholar]
- [119].Szeles J and Kubota N, “Location monitoring support application in smart phones for elderly people, using suitable interface design,” in Int. Conf. Intelligent Robotics and Applications. Springer, 2016, pp. 3–14. [Google Scholar]
- [120].Marras C, Beck J, Bower J, Roberts E, Ritz B, Ross G, Abbott R, Savica R, Van Den Eeden S, Willis A et al. , “Prevalence of Parkinson’s disease across North America,” NPJ Parkinson’s disease, vol. 4, no. 1, p. 21, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Rawlins MD, Wexler NS, Wexler AR, Tabrizi SJ, Douglas I, Evans SJ, and Smeeth L, “The prevalence of Huntington’s disease,” Neuroepidemiology, vol. 46, no. 2, pp. 144–153, 2016. [DOI] [PubMed] [Google Scholar]
- [122].Body-Disease.com. Nervous system diseases and disorders, Parkinson’s disease. Accessed on: Oct. 4, 2019. [Online]. Available: http://bodydisease.com/parkinson-disease/
- [123].Al-Fayez M. Basal nuclei or basal ganglia. Accessed on: Oct. 4, 2019. [Online]. Available: http://slideplayer.com/slide/7992667/
- [124].Dinesh K, Xiong M, Adams J, Dorsey R, and Sharma G, “Signal analysis for detecting motor symptoms in Parkinson’s and Huntington’s disease using multiple body-affixed sensors: A pilot study,” in 2016 IEEE Western New York Image and Signal Processing Workshop (WNYISPW). IEEE, 2016, pp. 1–5. [Google Scholar]
- [125].Adams J, Dinesh K, Xiong M, Tarolli C, Sharma S, Sheth N, Aranyosi AJ, Zhu W, Goldenthal S, Biglan K, Dorsey ER, and Sharma G, “Multiple wearable sensors in Parkinson and Huntington disease individuals: A pilot study in clinic and at home,” Digital Biomarkers, vol. 1, pp. 52–63, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Hayden MR, Huntingtons Chorea. Berlin: Springer-Verlag, 1981. [Google Scholar]
- [127].Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R et al. , “Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): Scale presentation and clinimetric testing results,” Movement disorders, vol. 23, no. 15, pp. 2129–2170, 2008. [DOI] [PubMed] [Google Scholar]
- [128].Kieburtz K, Penney JB, Corno P, Ranen N, Shoulson I, Feigin A, Abwender D, Greenarnyre JT, Higgins D, Marshall FJ et al. , “Unified Huntington’s disease rating scale: Reliability and consistency,” Movement Disorders, vol. 11, no. 2, pp. 136–142, 1996. [DOI] [PubMed] [Google Scholar]
- [129].Catarinucci L, De Donno D, Mainetti L, Palano L, Patrono L, Stefanizzi ML, and Tarricone L, “An IoT-aware architecture for smart healthcare systems,” IEEE Internet Things J, vol. 2, no. 6, pp. 515–526, 2015. [Google Scholar]
- [130].Manogaran G, Varatharajan R, Lopez D, Kumar PM, Sundarasekar R, and Thota C, “A new architecture of internet of things and big data ecosystem for secured smart healthcare monitoring and alerting system,” Future Generation Computer Systems, vol. 82, pp. 375–387, 2018. [Google Scholar]
- [131].Jabbar S, Ullah F, Khalid S, Khan M, and Han K, “Semantic interoperability in heterogeneous IoT infrastructure for healthcare,” Wireless Communications and Mobile Computing, vol. 2017, 2017. [Google Scholar]
- [132].Zgheib R, Conchon E, and Bastide R, “Engineering IoT healthcare applications: towards a semantic data driven sustainable architecture,” in eHealth 360. Springer, 2017, pp. 407–418. [Google Scholar]
- [133].Pace P, Aloi G, Gravina R, Caliciuri G, Fortino G, and Liotta A, “An edge-based architecture to support efficient applications for healthcare industry 4.0,” IEEE Trans. Ind. Informat, vol. 15, no. 1, pp. 481–489, 2018. [Google Scholar]
- [134].Mller P, Bgin MA, Schauer T, and Seel T, “Alignment-free, self-calibrating elbow angles measurement using inertial sensors,” IEEE J. Biomed. Health Inform, vol. 21, no. 2, pp. 312–319, March 2017. [DOI] [PubMed] [Google Scholar]
- [135].Daher M, Diab A, Najjar MEBE, Khalil MA, and Charpillet F, “Elder tracking and fall detection system using smart tiles,” IEEE Sensors J, vol. 17, no. 2, pp. 469–479, January 2017. [Google Scholar]
- [136].Mahbub I, Pullano SA, Wang H, Islam SK, Fiorillo AS, To G, and Mahfouz MR, “A low-power wireless piezoelectric sensor-based respiration monitoring system realized in CMOS process,” IEEE Sensors J, vol. 17, no. 6, pp. 1858–1864, March 2017. [Google Scholar]
- [137].Reyes BA, Reljin N, Kong Y, Nam Y, and Chon KH, “Tidal volume and instantaneous respiration rate estimation using a volumetric surrogate signal acquired via a smartphone camera,” IEEE J. Biomed. Health Inform, vol. 21, no. 3, pp. 764–777, May 2017. [DOI] [PubMed] [Google Scholar]
- [138].Jorge J, Villarroel M, Chaichulee S, Guazzi A, Davis S, Green G, McCormick K, and Tarassenko L, “Non-contact monitoring of respiration in the neonatal intensive care unit,” in 2017 12th IEEE Int. Conf. Automatic Face & Gesture Recognition (FG 2017). IEEE, 2017, pp. 286–293. [Google Scholar]
- [139].Jovanov E, Gelabert P, Adhami R, Wheelock B, and Adams R, “Real time Holter monitoring of biomedical signals,” in DSP Technology and Education Conf. DSPS, vol. 99, 1999, pp. 4–6. [Google Scholar]
- [140].Rachim VP and Chung WY, “Wearable noncontact armband for mobile ECG monitoring system,” IEEE Trans. Biomed. Circuits Syst, vol. 10, no. 6, pp. 1112–1118, December 2016. [DOI] [PubMed] [Google Scholar]
- [141].Lahdenoja O, Hurnanen T, Iftikhar Z, Nieminen S, Knuutila T, Saraste A, Kiviniemi T, Vasankari T, Airaksinen J, Pänkäälä M et al. , “Atrial fibrillation detection via accelerometer and gyroscope of a smartphone,” IEEE J. Biomed. Health Inform, vol. 22, no. 1, pp. 108–118, 2017. [DOI] [PubMed] [Google Scholar]
- [142].Popov B, Sierra G, Telfort V, Agarwal R, and Lanzo V, “Estimation of respiratory rate and heart rate during treadmill tests using acoustic sensor,” in 2005 IEEE Engineering in Medicine and Biology 27th Annu. Conf., 2005, pp. 5884–5887. [DOI] [PubMed] [Google Scholar]
- [143].Martinek R, Nedoma J, Fajkus M, Kahankova R, Konecny J, Janku P, Kepak S, Bilik P, and Nazeran H, “A phonocardiographic-based fiber-optic sensor and adaptive filtering system for noninvasive continuous fetal heart rate monitoring,” Sensors, vol. 17, no. 4, p. 890, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Moreno EM, Luján MJA, Rusiñol MT, Fernández PJ, Manrique PN, Triviño CA, Miquel MP, Rodríguez MA, and Burguillos MJG, “Type 2 diabetes screening test by means of a pulse oximeter,” IEEE Trans. Biomed. Eng, vol. 64, no. 2, pp. 341–351, February 2017. [DOI] [PubMed] [Google Scholar]
- [145].Cleven NJ, Mntjes JA, Fassbender H, Urban U, Grtz M, Vogt H, Grfe M, Gttsche T, Penzkofer T, Schmitz-Rode T, and Mokwa W, “A novel fully implantable wireless sensor system for monitoring hypertension patients,” IEEE Trans. Biomed. Eng, vol. 59, no. 11, pp. 3124–3130, November 2012. [DOI] [PubMed] [Google Scholar]
- [146].Lin H, Xu W, Guan N, Ji D, Wei Y, and Yi W, “Noninvasive and continuous blood pressure monitoring using wearable body sensor networks,” IEEE Intelligent Systems, vol. 30, no. 6, pp. 38–48, November 2015. [Google Scholar]
- [147].Seeberg TM, Orr JG, Opsahl H, Austad HO, Red MH, Dalgard SH, Houghton D, Jones DEJ, and Strisland F, “A novel method for continuous, noninvasive, cuff-less measurement of blood pressure: Evaluation in patients with nonalcoholic fatty liver disease,” IEEE Trans. Biomed. Eng, vol. 64, no. 7, pp. 1469–1478, July 2017. [DOI] [PubMed] [Google Scholar]
- [148].Kachuee M, Kiani MM, Mohammadzade H, and Shabany M, “Cuffless blood pressure estimation algorithms for continuous healthcare monitoring,” IEEE Trans. Biomed. Eng, vol. 64, no. 4, pp. 859–869, April 2017. [DOI] [PubMed] [Google Scholar]
- [149].Xiao Z, Tan X, Chen X, Chen S, Zhang Z, Zhang H, Wang J, Huang Y, Zhang P, Zheng L, and Min H, “An implantable RFID sensor tag toward continuous glucose monitoring,” IEEE J. Biomed. Health Inform, vol. 19, no. 3, pp. 910–919, May 2015. [DOI] [PubMed] [Google Scholar]
- [150].Lee H, Song C, Hong YS, Kim MS, Cho HR, Kang T, Shin K, Choi SH, Hyeon T, and Kim D-H, “Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module,” Science Advances, vol. 3, no. 3, p. e1601314, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Ali H, Bensaali F, and Jaber F, “Novel approach to non-invasive blood glucose monitoring based on transmittance and refraction of visible laser light,” IEEE Access, vol. 5, pp. 9163–9174, 2017. [Google Scholar]
- [152].MC10 Inc. (2019) Wearable health systems. Accessed on: Oct. 4, 2019. [Online]. Available: https://www.mc10inc.com/our-products/biostamprc
- [153].Mokhtari G, Zhang Q, Nourbakhsh G, Ball S, and Karunanithi M, “BLUESOUND: A new resident identification sensor–using ultrasound array and BLE technology for smart home platform,” IEEE Sensors J, vol. 17, no. 5, pp. 1503–1512, March 2017. [Google Scholar]
- [154].Lee DS, Chong TW, and Lee BG, “Stress events detection of driver by wearable glove system,” IEEE Sensors J, vol. 17, no. 1, pp. 194–204, January 2017. [Google Scholar]
- [155].Lin F, Wang A, Zhuang Y, Tomita MR, and Xu W, “Smart insole: A wearable sensor device for unobtrusive gait monitoring in daily life,” IEEE Trans. Ind. Informat, vol. 12, no. 6, pp. 2281–2291, December 2016. [Google Scholar]
- [156].Wang LH, Chen TY, Lin KH, Fang Q, and Lee SY, “Implementation of a wireless ECG acquisition SoC for IEEE 802.15.4 (ZigBee) applications,” IEEE J. Biomed. Health Inform, vol. 19, no. 1, pp. 247–255, January 2015. [DOI] [PubMed] [Google Scholar]
- [157].Kumar A and Hancke GP, “A Zigbee-based animal health monitoring system,” IEEE Sensors J, vol. 15, no. 1, pp. 610–617, January 2015. [Google Scholar]
- [158].Bello O, Zeadally S, and Badra M, “Network layer interoperation of device-to-device communication technologies in internet of things (IoT),” Ad Hoc Networks, vol. 57, pp. 52–62, 2017, special Issue on Internet of Things and Smart Cities security, privacy and new technologies. [Online]. Available: http://www.sciencedirect.com/science/article/pii/S1570870516301597 [Google Scholar]
- [159].Palattella MR, Dohler M, Grieco A, Rizzo G, Torsner J, Engel T, and Ladid L, “Internet of things in the 5G era: Enablers, architecture, and business models,” IEEE J. Sel. Areas Commun, vol. 34, no. 3, pp. 510–527, 2016. [Google Scholar]
- [160].Chua E and Fang WC, “Mixed bio-signal lossless data compressor for portable brain-heart monitoring systems,” IEEE Trans. Consum. Electron, vol. 57, no. 1, pp. 267–273, February 2011. [Google Scholar]
- [161].Wang Y, Doleschel S, Wunderlich R, and Heinen S, “Evaluation of digital compressed sensing for real-time wireless ECG system with Bluetooth low energy,” Journal of Medical Systems, vol. 40, no. 7, p. 170, May 2016. [DOI] [PubMed] [Google Scholar]
- [162].Raj M, Wei P, Patel S, Wang X, McGrane B, Klinker L, De-Petrillo P, and Ghaffari R, “Bio-integrated systems with stretchable designs for skin-mounted wearable health monitoring,” in 2014 IEEE Int. Electron Devices Meeting, December 2014, pp. 31.7.1–31.7.3. [Google Scholar]
- [163].Kim Y and Cho I, “Wearable ECG monitor: Evaluation and experimental analysis,” in 2011 Int. Conf. Information Science and Applications, April 2011, pp. 1–5. [Google Scholar]
- [164].Benocci M, Tacconi C, Farella E, Benini L, Chiari L, and Vanzago L, “Accelerometer-based fall detection using optimized ZigBee data streaming,” Microelectronics Journal, vol. 41, no. 11, pp. 703–710, 2010. [Google Scholar]
- [165].Bonomi F, Milito R, Zhu J, and Addepalli S, “Fog computing and its role in the internet of things,” in Proc. 1st edition of the MCC workshop on Mobile cloud computing. ACM, 2012, pp. 13–16. [Google Scholar]
- [166].Azimi I, Anzanpour A, Rahmani AM, Pahikkala T, Levorato M, Liljeberg P, and Dutt N, “HiCH: Hierarchical fog-assisted computing architecture for healthcare IoT,” ACM Transactions on Embedded Computing Systems (TECS), vol. 16, no. 5s, p. 174, 2017. [Google Scholar]
- [167].Gia TN, Rahmani AM, Westerlund T, Liljeberg P, and Tenhunen H, “Fog computing approach for mobility support in internet-of-things systems,” IEEE Access, vol. 6, pp. 36 064–36 082, 2018. [Google Scholar]
- [168].Lu Y, Kuonen P, Hirsbrunner B, and Lin M, “Benefits of data aggregation on energy consumption in wireless sensor networks,” IET Communications, vol. 11, no. 8, pp. 1216–1223, 2017. [Google Scholar]
- [169].Singh J, Pasquier T, Bacon J, Ko H, and Eyers D, “Twenty security considerations for cloud-supported internet of things,” IEEE Internet Things J, vol. 3, no. 3, pp. 269–284, June 2016. [Google Scholar]
- [170].Henze M, Hermerschmidt L, Kerpen D, Häußling R, Rumpe B, and Wehrle K, “A comprehensive approach to privacy in the cloud-based internet of things,” Future Generation Computer Systems, vol. 56, pp. 701–718, 2016. [Google Scholar]
- [171].Endo PT, de Almeida Palhares AV, Pereira NN, Goncalves GE, Sadok D, Kelner J, Melander B, and Mangs JE, “Resource allocation for distributed cloud: Concepts and research challenges,” IEEE Netw, vol. 25, no. 4, pp. 42–46, July 2011. [Google Scholar]
- [172].Harmony Healthcare IT. (2018, August) Health data volumes skyrocket, legacy data archives on the rise. Accessed on: Oct. 4, 2019. [Online]. Available: https://www.healthdataarchiver.com/healthdata-volumes-skyrocket-legacy-data-archives-rise-hie/
- [173].Banaee H, Ahmed MU, and Loutfi A, “Data mining for wearable sensors in health monitoring systems: a review of recent trends and challenges,” Sensors, vol. 13, no. 12, pp. 17 472–17 500, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Aggarwal CC, Managing and Mining Sensor Data. Springer Science & Business Media, 2013. [Google Scholar]
- [175].Beam AL and Kohane IS, “Big data and machine learning in health care,” JAMA, vol. 319, no. 13, pp. 1317–1318, 2018. [DOI] [PubMed] [Google Scholar]
- [176].Weng SF, Reps J, Kai J, Garibaldi JM, and Qureshi N, “Can machine-learning improve cardiovascular risk prediction using routine clinical data?” PLOS ONE, vol. 12, no. 4, pp. 1–14, 04 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Mehta N and Pandit A, “Concurrence of big data analytics and healthcare: A systematic review,” International Journal of Medical Informatics, vol. 114, pp. 57–65, 2018. [DOI] [PubMed] [Google Scholar]
- [178].Ahmad MA, Eckert C, and Teredesai A, “Interpretable machine learning in healthcare,” in Proc. 2018 ACM Int. Conf. Bioinformatics, Computational Biology, and Health Informatics, ser. BCB ‘18. New York, NY, USA: ACM, 2018, pp. 559–560. [Google Scholar]
- [179].Harrington SG and Johnson MK, “The FDA and artificial intelligence in radiology: defining new boundaries,” Journal of the American College of Radiology, vol. 16, no. 5, pp. 743–744, 2019. [DOI] [PubMed] [Google Scholar]
- [180].Strickland E, “IBM watson, heal thyself: How IBM overpromised and underdelivered on AI health care,” IEEE Spectr, vol. 56, no. 04, pp. 24–31, April 2019. [Google Scholar]
- [181].Powles J and Hodson H, “Google DeepMind and healthcare in an age of algorithms,” Health and Technology, vol. 7, no. 4, pp. 351–367, December 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Suleyman M and King D. (2017, July) The information commissioner, the royal free, and what we’ve learned. Accessed on October 4, 2019. [Online]. Available: https://deepmind.com/blog/ico-royal-free
- [183].Jiang F, Jiang Y, Zhi H, Dong Y, Li H, Ma S, Wang Y, Dong Q, Shen H, and Wang Y, “Artificial intelligence in healthcare: past, present and future,” Stroke and Vascular Neurology, vol. 2, no. 4, pp. 230–243, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Bui AA and Hsu W, “Medical data visualization: Toward integrated clinical workstations,” in Medical Imaging Informatics. Springer, 2010, pp. 139–193. [Google Scholar]
- [185].Jonathan F and Sonin J. (2012, April) hGraph: An open system for visualizing personal health metrics. Involution studios, Arlington, Massachusetts, Tech. Rep [Online]. Available: http://hgraph.org/docs/hGraph_Whitepaper.pdf [Google Scholar]
- [186].Ledesma A, Al-Musawi M, and Nieminen H, “Health figures: an open source JavaScript library for health data visualization,” BMC Medical Informatics and Decision making, vol. 16, no. 1, p. 38, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Bui AA, Aberle DR, and Kangarloo H, “TimeLine: Visualizing integrated patient records,” IEEE Trans. Inf Technol. Biomed, vol. 11, no. 4, pp. 462–473, 2007. [DOI] [PubMed] [Google Scholar]
- [188].Engelman D, Higgins TL, Talati R, and Grimsman J, “Maintaining situational awareness in a cardiac intensive care unit,” The Journal of Thoracic and Cardiovascular Surgery, vol. 147, no. 3, pp. 1105–1106, 2014. [DOI] [PubMed] [Google Scholar]
- [189].Christmann CA, Zolynski G, Hoffmann A, and Bleser G, “Effective visualization of long term health data to support behavior change,” in Int. Conf. Digital Human Modeling and Applications in Health, Safety, Ergonomics and Risk Management. Springer, 2017, pp. 237–247. [Google Scholar]
- [190].Cuttone A, Petersen MK, and Larsen JE, “Four data visualization heuristics to facilitate reflection in personal informatics,” in Int. Conf. Universal Access in Human-Computer Interaction. Springer, 2014, pp. 541–552. [Google Scholar]
- [191].Bentley F and Tollmar K, “The power of mobile notifications to increase wellbeing logging behavior,” in Proc. SIGCHI Conf. Human Factors in Computing Systems. ACM, 2013, pp. 1095–1098. [Google Scholar]
- [192].Fan C, Forlizzi J, and Dey AK, “A spark of activity: Exploring informative art as visualization for physical activity,” in Proc. 2012 ACM Conf. Ubiquitous Computing. ACM, 2012, pp. 81–84. [Google Scholar]
- [193].Stusak S, Tabard A, Sauka F, Khot RA, and Butz A, “Activity sculptures: Exploring the impact of physical visualizations on running activity,” IEEE Trans. Vis. Comput. Graphics, vol. 20, no. 12, pp. 2201–2210, 2014. [DOI] [PubMed] [Google Scholar]
- [194].Gschwandtner T, Aigner W, Kaiser K, Miksch S, and Seyfang A, “CareCruiser: Exploring and visualizing plans, events, and effects interactively,” in 2011 IEEE Pacific Visualization Symp. (PacificVis). IEEE, 2011, pp. 43–50. [Google Scholar]
- [195].Faiola A and Newlon C, “Advancing critical care in the ICU: a human-centered biomedical data visualization systems,” Ergonomics and Health Aspects of Work with Computers, pp. 119–128, 2011. [Google Scholar]
- [196].Chittaro L, Combi C, and Trapasso G, “Data mining on temporal data: a visual approach and its clinical application to hemodialysis,” Journal of Visual Languages & Computing, vol. 14, no. 6, pp. 591–620, 2003. [Google Scholar]
- [197].104th Congress. (1996) Heath insurance portability and accountability act of 1996. Accessed on: Oct. 4, 2019. [Online]. Available: https://www.gpo.gov/fdsys/pkg/PLAW-104publ191/html/PLAW-104publ191.htm
- [198].Kolias C, Stavrou A, Voas J, Bojanova I, and Kuhn R, “Learning internet-of-things security “hands-on”,” IEEE Security Privacy, vol. 14, no. 1, pp. 37–46, January 2016. [Google Scholar]
- [199].Broström T, Zhu J, Robucci R, and Younis M, “IoT boot integrity measuring and reporting,” SIGBED Rev, vol. 15, no. 5, pp. 14–21, November. 2018. [Google Scholar]
- [200].Moosavi SR, Nigussie E, Levorato M, Virtanen S, and Isoaho J, “Performance analysis of end-to-end security schemes in healthcare IoT,” Procedia computer science, vol. 130, no. C, pp. 432–439, 2018. [Google Scholar]
- [201].Hennebert C and Santos JD, “Security protocols and privacy issues into 6LoWPAN stack: A synthesis,” IEEE Internet Things J, vol. 1, no. 5, pp. 384–398, October 2014. [Google Scholar]
- [202].Yang Y, Liu X, and Deng RH, “Lightweight break-glass access control system for healthcare internet-of-things,” IEEE Trans. Ind. Informat, vol. 14, no. 8, pp. 3610–3617, August 2018. [Google Scholar]
- [203].Zhang K, Ni J, Yang K, Liang X, Ren J, and Shen XS, “Security and privacy in smart city applications: Challenges and solutions,” IEEE Commun. Mag, vol. 55, no. 1, pp. 122–129, January 2017. [Google Scholar]
- [204].Yaqoob I, Ahmed E, ur Rehman MH, Ahmed AIA, Al-garadi MA, Imran M, and Guizani M, “The rise of ransomware and emerging security challenges in the internet of things,” Computer Networks, vol. 129, pp. 444–458, 2017. [Google Scholar]
- [205].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, and Conde JG, “Research electronic data capture (REDCap) –a metadatadriven methodology and workflow process for providing translational research informatics support,” Journal of Biomedical Informatics, vol. 42, no. 2, pp. 377–381, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Ghanei F, Tipnis P, Marcus K, Dantu K, Ko SY, and Ziarek L, “OS-based energy accounting for asynchronous resources in IoT devices,” IEEE Internet Things J, vol. 6, no. 3, pp. 5841–5852, June 2019. [Google Scholar]
- [207].Yamato Y, Demizu T, Noguchi H, and Kataoka M, “Automatic GPU offloading technology for open IoT environment,” IEEE Internet Things J, vol. 6, no. 2, pp. 2669–2678, April 2019. [Google Scholar]
- [208].Wu C and Zhang Y, “Toward efficient transparent computing for IoT apps by on-chip kernel offload,” IEEE Internet Things J, vol. 6, no. 3, pp. 4085–4097, June 2019. [Google Scholar]
- [209].Alraja MN, Farooque MMJ, and Khashab B, “The effect of security, privacy, familiarity, and trust on users’ attitudes toward the use of the IoT-based healthcare: The mediation role of risk perception,” IEEE Access, vol. 7, pp. 111 341–111 354, 2019. [Google Scholar]
- [210].AboBakr A and Azer MA, “IoT ethics challenges and legal issues,” in 2017 12th Int. Conf. Computer Engineering and Systems (ICCES), December 2017, pp. 233–237. [Google Scholar]
- [211].Food and Drug Adminstration (FDA). (2019) Digital health innovation action plan. Accessed on: Oct. 4, 2019. [Online]. Available: https://www.fda.gov/medical-devices/digital-health
- [212].Izmailova ES, Wagner JA, and Perakslis ED, “Wearable devices in clinical trials: hype and hypothesis,” Clinical Pharmacology & Therapeutics, vol. 104, no. 1, pp. 42–52, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Firouzi F, Rahmani AM, Mankodiya K, Badaroglu M, Merrett G, Wong P, and Farahani B, “Internet-of-things and big data for smarter healthcare: From device to architecture, applications and analytics,” Future Generation Computer Systems, vol. 78, pp. 583–586, 2018. [Google Scholar]
- [214].Alaiad A and Zhou L, “Patients’ adoption of WSN-based smart home healthcare systems: An integrated model of facilitators and barriers,” IEEE Prof. Commun, vol. 60, no. 1, pp. 4–23, March 2017. [Google Scholar]
- [215].Gous N, Boeras DI, Cheng B, Takle J, Cunningham B, and Peeling RW, “The impact of digital technologies on point-of-care diagnostics in resource-limited settings,” Expert Review of Molecular Diagnostics, vol. 18, no. 4, pp. 385–397, 2018. [DOI] [PubMed] [Google Scholar]
- [216].Purushotham S, Meng C, Che Z, and Liu Y, “Benchmarking deep learning models on large healthcare datasets,” Journal of Biomedical Informatics, vol. 83, pp. 112–134, 2018. [DOI] [PubMed] [Google Scholar]
- [217].HealthData.gov. (2019) About HealthData.gov. Accessed on: May 23, 2019. [Online]. Available: https://healthdata.gov/content/about
- [218].Bradley CJ, Penberthy L, Devers KJ, and Holden DJ, “Health services research and data linkages: Issues, methods, and directions for the future,” Health Services Research, vol. 45, no. 5p2, pp. 1468–1488, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Lipani L, Dupont BG, Doungmene F, Marken F, Tyrrell RM, Guy RH, and Ilie A, “Non-invasive, transdermal, path-selective and specific glucose monitoring via a graphene-based platform,” Nature nanotechnology, vol. 13, no. 6, p. 504, 2018. [DOI] [PubMed] [Google Scholar]
- [220].Lee SH, Ahn JW, Cho YC, Kim S-N, Lee C, Ku GW, Choy YB, and Kim HC, “Wirelessly controlled implantable system for on-demand and pulsatile insulin administration,” Scientific reports, vol. 9, no. 1, p. 5009, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Lee HJ, Choi N, Yoon E-S, and Cho I-J, “MEMS devices for drug delivery,” Advanced Drug Delivery Reviews, vol. 128, pp. 132–147, 2018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The algorithms described in the previous subsection require a significant amount of well-structured clinical data to achieve useful levels of accuracy. Providing such data presents multi-faceted challenges, specifically, with respect to widespread data availability and heterogeneity.
A major data-related challenge in clinical HIoT is assuring the generality of results. Most available pilot studies in the literature are developed and tested on small datasets (relative to the actual patient population size) [180]. Therefore, their applicability and efficacy in real-world scenarios remain uncertain; especially, considering that health and disease states can be highly correlated with many personal parameters such as gender, age, and ethnicity. This problem is even more acute for rare diseases. Consequently, data analytics approaches have the best chances for success if clinics and hospitals share their data with algorithm developers. The flow of such information is, however, fraught with unresolved legal and ethical complications. An example of this is the collaboration between Google’s DeepMind and the British healthcare provider, Royal Free, which shared its acute kidney injury dataset with DeepMind without acquiring patients’ explicit consent. The decision led to official scrutiny, which concluded that the deal was illegal [181], [182].
To take full advantage of the existing medical knowledge, it is common sense to use historical medical data as input to inference algorithms. Combined with the data that is being streamed from the HIoT platforms, this amalgamation of data can provide an effective data source for modern inference algorithms. However, historical data sources are diverse and highly unstructured as part of the data must be inferred from EHRs, insurance claims, medical textbooks, and scientific papers [183]. The language used in these documents can be vague, out of chronicle order, and incomplete. To cope with these challenges, NLP is often used. However, regardless of their remarkable progress in recent years, current NLP technologies are not efficient enough in these applications, even when supported by the resources of tech giants [180].
This data heterogeneity problem is exacerbated when we consider the fact that even the data obtained from the same HIoT platform can be highly-heterogeneous within itself. For example, the MC10 sensors used in our case study continuously evolve and additional clinically relevant attributes can be measured with every new generation of sensors, which effectively adds a new ”input dimension” to the existing data. Therefore, algorithms must be designed to cope with not only an increasing volume of data, but also an increasing variety.