Abstract
Genomic divergence was studied in 10 small insular populations of the endangered Balearic Islands lizard (Podarcis lilfordi) using double digest restriction-site associated DNA sequencing. The objectives were to establish levels of divergence among populations, investigate the impact of population size on genetic variability and to evaluate the role of different environmental factors on local adaptation. Analyses of 72,846 SNPs supported a highly differentiated genetic structure, being the populations with the lowest population size (Porros, Foradada and Esclatasang islets) the most divergent, indicative of greater genetic drift. Outlier tests identified ~ 2% of loci as candidates for selection. Genomic divergence-Enviroment Association analyses were performed using redundancy analyses based on SNPs putatively under selection, detecting predation and human pressure as the environmental variables with the greatest explanatory power. Geographical distributions of populations and environmental factors appear to be fundamental drivers of divergence. These results support the combined role of genetic drift and divergent selection in shaping the genetic structure of these endemic island lizard populations.
Subject terms: Ecology, Evolution, Genetics
Introduction
Insular populations are naturally isolated systems that harbour high levels of biodiversity and endemism1. Their characteristic isolation leads to a reduction in gene flow and generates population divergence and speciation2. High levels of genetic structuring also result from frequent physical events combined with the impact of rapid fixation rates in often small populations subject to genetic drift and selection3,4.
Understanding the relative roles of selection and drift are key to understanding the divergence of insular populations. Drift is expected to be considerable due to low migration rates and small population sizes5–7. Nonetheless, morphological divergence and environmental heterogeneity between islands suggests that divergent selection may also play a key role8–10. The interplay between local adaptation and genetic drift in moulding variation in these environments is often not clear and requires more research11–13. Genetic and genomic approaches provide additional value as an important basis for conservation decisions14,15.
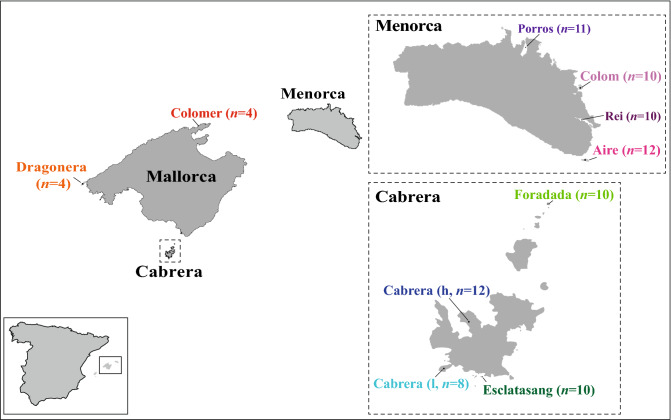
The Balearic lizard, Podarcis lilfordi, as an insular endemism inhabiting a large group of coastal islands and islets of Mallorca and Menorca (Balearic Islands, Spain), provides a suitable system for studying selection and genetic drift as mechanisms of evolution. Podarcis lilfordi likely became extinct from the main islands of Mallorca and Menorca during the Holocene (~ 2000 years ago), presumably as a consequence of the introduction of foreign terrestrial predators by humans who arrived 2000–3000 years prior to this16,17. Small populations managed to survive on the coastal islands and islets situated around Menorca and Mallorca, as well as the uninhabited Cabrera archipelago (Fig. 1). The sizes of these populations varies considerably, ranging from fewer than 100 individuals, to over 100,000 individuals18.
Figure 1.
Locations of each coastal island and islet in Mallorca, Menorca and Cabrera and number (n) of samples used in this study. h harbour, l lighthouse.
Figure source: Wikimedia commons.
Previous phylogeographical analyses using mitochondrial DNA (mtDNA) have indicated that P. lilfordi separated from the Ibizan lizard, Podarcis pityusensis, when the Mediterranean refilled at the end of the Messinian Salinity Crisis (~ 5.3 Ma ago). Subsequently, the P. lilfordi populations of the islands of Menorca began to diverge from the populations of the islands of Mallorca at the beginning of the Quarternary period, ~ 2.6 Ma ago19–21. Despite subsequent glacial events causing sea-level fluctuations22, no evidence of historical gene flow or migration can be detected between present-day Mallorcan (including the Cabrera archipelago), and Menorcan populations20. Within Mallorca, the earliest split (~ 2 Ma ago) separates the populations of the islands of Western Mallorca from the other populations. The next split within the latter group occurred 1.2 Ma ago and separates northern, southern Mallorcan and northern Cabrera populations from other Cabrera populations. Splits within the latter Cabrera populations are also quite old, with the first estimated at 0.8 Ma19. Changes in sea level during the Quarternary were apparently insufficient to reconnect the main islands (Mallorca, Menorca and Cabrera) but would have allowed connections between islets and islands within groups20. It is particularly interesting the phylogenetic position of the Colomer Island, an isolated population in northern Mallorca with a steep orography and almost inaccessible nature, that make introductions extremely unlikely. Its closer relationship with populations from the south of Mallorca and Cabrera archipelago seems more probable to be explained by the recent extinction of populations that once inhabited the main island of Mallorca.
The extensive genetic, morphological, ecological and behavioural differences between P. lilfordi populations have led to the proposal that they should each be recognized as Evolutionarily Significant Units (ESUs). The range of this species is restricted to a limited geographical area within the Western Mediterranean basin, across which climatic and altitude characteristics vary only slightly23. Nonetheless, other environmental traits, such as food availability, habitat structure, orography, predation pressure, the presence of potential competitors and human pressure or some parameter correlated with it, show substantial differences across populations. Here, we aimed to reveal whether these aspects of the environment had led to population divergence.
These well-known populations provide us with a rare opportunity to obtain insight on the effect of short-term environmental changes, most of them driven by humans, in adaptive traits of individuals from a common origin, but now living in different environmental conditions. There are several examples of rapid evolution of species, quickly responding to new selective pressures as human pressure24,25. In addition, it is clear that most of the selective pressures associated with humans can be extremely strong and microevolutionary changes can occur on time frames comparable to human disturbance and anthropogenic changes. Such knowledge is crucial to the conservation of biodiversity26.
We used double digest restriction-site associated DNA sequencing (ddRADseq), to obtain single-nucleotide polymorphims (SNP) data from across the genome27–29. This enabled us to reexamine the population history of P. lilfordi, previously described using mtDNA20, and explore the roles of genetic drift and divergent selection in shaping genome diversity among these endangered populations.
Results
A total of 6.8 billion paired-end reads of 101 bp length were generated from the 91 individuals. Following application of denovo_map.pl and described filtering steps, 288,286 SNPs were called from 80,091 ddRAD contigs, with a mean coverage of 28.6 per site. The first SNP for each locus was retained leaving 72,846 SNPs for analysis (this number is fewer than the number of loci due to removal of SNPs present in only 20% of individuals).
Population structure
Nucleotide diversity ranged between 0.120 (Porros islets) and 0.182 (Cabrera harbour). Foradada, Esclatasang and Porros presented the highest number of private alleles (746, 475, and 945, respectively) indicating considerable genetic divergence, with little or no gene flow between them and the other populations, probably due to their strong geographical isolation. In general, inbreeding coefficients (FIS) were low (less than 10%) (Supplementary Table 1). Patterns of divergence based on FST distance analysis were highly congruent with previous results, with the populations of the islands of Menorca showing a clear differentiation with respect to the populations of the islands of Mallorca together with Cabrera populations (Supplementary Figure 1). Using all 72,846 SNPs, the greatest divergence was between Porros islet (Menorca) and all other populations from Mallorca and Cabrera and between the two Cabrera islets (Foradada and Esclatasang) and Menorcan populations. Lowest divergence was found between the two locations within Cabrera island (harbour and lighthouse), between the populations of the islands of Mallorca (Dragonera and Colomer) and Cabrera main island, and among all Menorcan islands (with the exception of Porros). The divergent position of Porros, Foradada and Esclatasang was less pronounced when only outlier SNPs (1,355 SNPs) were considered, while Mallorca populations were more divergent with respect to Cabrera populations (Supplementary Figure 1).
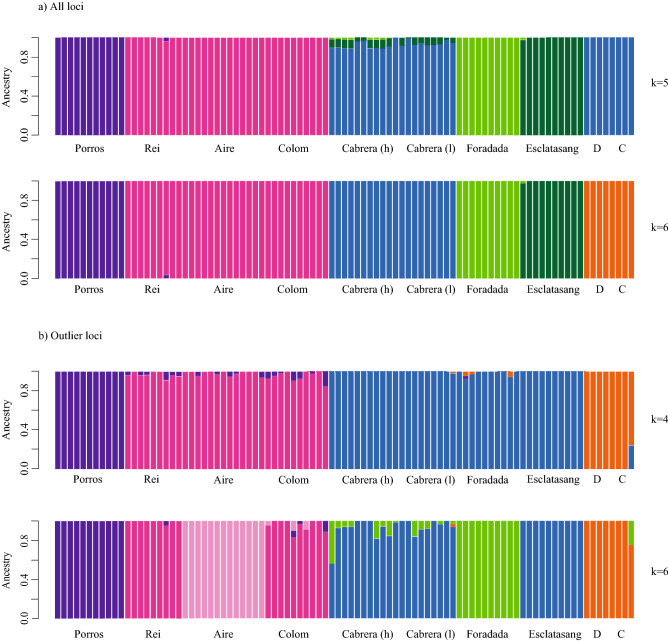
The best-supported values of K in the Admixture analysis were K = 5 (CV = 0.372) or K = 6 (CV = 0.388) for the first single SNPs dataset. The divergent positions of Porros, Foradada and Esclatasang islets was corroborated by these results; Dragonera and Colomer grouped with Cabrera main island with K = 5 or formed an independent group with K = 6 (Fig. 2). When only outlier SNPs were used, Admixture analyses supported separation into three geographic groups (Menorca, Mallorca and Cabrera), with the exception of Porros islet, when K was set to four (CV = 0.288). When K = 6 (CV = 0.294), Porros, Aire and Foradada were revealed as independent groups (Fig. 2).
Figure 2.
Admixture analysis results using all SNPs dataset (a) at K = 5 and K = 6, and only outlier loci (b) at K = 4 and K = 6. D Dragonera, C Colomer.
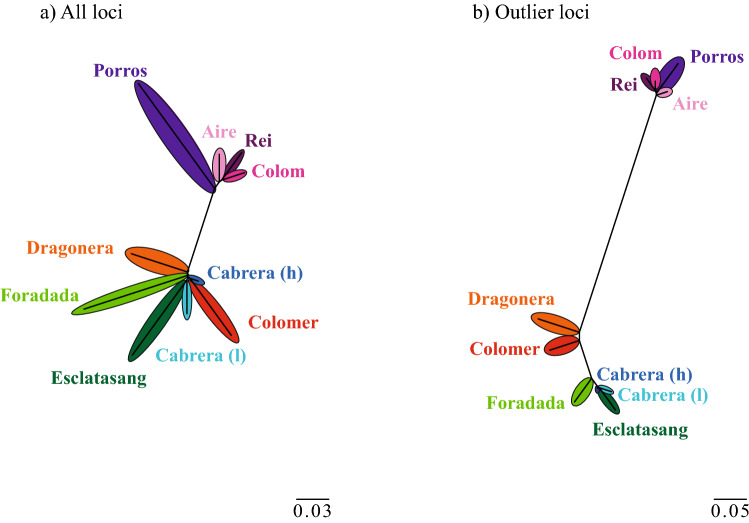
Patterns of differentiation observed in the previous analysis match with the population structure obtained with DAPC analyses. The k-means clustering algorithm, used prior to DAPC analyses revealed lowest BIC values (637.3) for 10 clusters. Cross-validation showed that use of the first 15 PCs (55.3% of variance) provided higher assignment rates (99.5%) and the lowest root mean squared error (RMSE) (0.016), justifying the use of this subset of PCs in the analysis. The first PC (51.2% of variance) separated all populations into two major groups: Menorcan populations and all the remaining populations from Mallorca and Cabrera. All lizard populations were grouped by island (Cabrera main island, Dragonera, Porros, Aire, Foradada, Esclatasang and Colomer), except for Rei and Colom islets in Menorca that grouped together. Ten clusters were also favored when analyses were carried out using only SNPs that were candidates for selection, and variance was best explained by 25 PCs (90.2% of variance). In this case, the first PC (91.4%) reinforced the clear separation between Menorca islands and Mallorca islands and Cabrera populations. The populations grouped geographically (Menorca, Mallorca and Cabrera), except for Porros islet which continued showing a divergent position (Supplementary Figure 2). NJ tree based on FST distances (Fig. 3) confirmed the results found using the admixture analysis.
Figure 3.
NJ tree based on FST distances based on all SNPs dataset (a) and only outlier SNPs (b). NJ trees were inferred using Mega 760 and modified with Adobe Illustrator 2020.
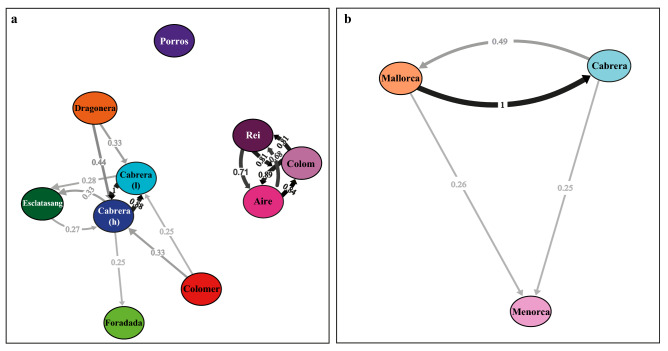
As expected, positive association had been obtained between N and Ne, and between N and nucleotide diversity (pi) and Ne and pi. Negative correlations had been achieved between mean FST and N, but not with Ne (data not shown). Migration rates (estimated by divMigrate) did not show gene flow between Menorca islands and Mallorca islands and Cabrera populations (Fig. 4a). The highest migration rates were observed between Aire, Colom and Rei islands in Menorca (0.68–0.89) and between the two localities situated in Cabrera main island (harbour and lighthouse) (0.88–1.00). These migration rates are almost symmetrical. The population from the smallest islet (Porros) did not showed gene flow even with other proximate populations. Directional migration from the populations of the islands of Mallorca (Dragonera and Colomer) to Cabrera archipelago was also observed (0.25–0.44). The Fig. 4b, showed an asymmetric and high migration rate from Mallorca islands to Cabrera archipelago, and low values between Mallorca islands/Cabrera and populations of the islands of Menorca.
Figure 4.
Migration networks for the Podarcis lilfordi based on all SNPs (72,846) among the 10 populations (a) and between the three main islands (Menorca, Mallorca, Cabrera) (b), obtained with the Nei’s GST estimate using divMigrate. Only migration rates ≥ 0.25 are indicated, circles represent the localities, and arrows indicate the direction of migration.
Candidate regions under selection
A total of 1,355 candidate sites for selection from 72,846 RAD tags were determined by BayeScan under a prior of 1:100 for selected:neutral sites. This increased to 2,884 sites when a ratio of 1:10 was used, and decreased to 732 sites when the prior ratio was 1:1000. Comparison of prior and posterior proportions suggests a true ratio between 1:10 and 1:100 and so our use of a 1:100 prior provides quite conservative results. After filtering, a total of 141 of the 184 RAD sites that contained outlying SNPs produced hits on BLASTn and hits with < 30% query coverage were discarded (Table 1).
Table 1.
Gene ID, definition, Kegg pathway, GO-molecular function and GO-biological process found in Podarcis or Anolis annotated genomes of the 1,355 outliers SNPs obtained by BayeScan analysis and the posterior filters. References of studies related with specific biological functions are included.
| Gene | Definition | Kegg pathway | GO-molecular function | GO-biological process | References |
|---|---|---|---|---|---|
| ACACB | Acetyl-CoA carboxylase 2 | Fatty acid biosynthesis, pyruvate metabolism, propanoate metabolism, metabolic pathways, insulin signalling pathway, adipocytokine signalling pathway | Acetyl-CoA carboxylase activity, ATP binding, identical protein binding, metal ion binding | Acetyl-CoA metabolic process, fatty acid biosynthesis process, malonyl-CoA biosynthetic process, protein homotetramerization | Lipid metabolism, hibernation69 |
| ACSBG1 | Acyl-CoA synthetase bubblegum family member 1 | Fatty acid biosynthesis, fatty acid degradation, metabolic pathways, fatty acid metabolism, PPAR signalling pathway, adipocytokine signalling pathway | CoA-ligase activity, long-chain fatty acid-CoA ligase activity, very long-chain fatty acid-CoA ligase activity | Long-chain fatty acid biosynthesis process, response to glucocorticoid, very long-chain fatty acid metabolic process | Lipid metabolism, hibernation70 |
| ADAM2 | ADAM metallopeptidase domain 2 |
Disintegrins and metallopeptidase activity, metal ion binding, metalloendopeptidase activity, toxin activity |
Integrin-mediated signalling pathway | Fertility71 | |
| ADAM9 | ADAM metallopeptidase domain 9 |
Disintegrins and metallopeptidase activity, collagen binding, metal ion binding, toxin activity Collagen, integrin, laminin. Metal ion and SH3 domain binding, metalloendopeptidase activity, toxin activity |
Activation of MAPKK activity, cell–cell adhesion mediated by integrin, cell–matrix adhesion, cellular response to lipopolysaccharide, keratinocyte differentiation, membrane protein ectodomain proteolysis, monocyte activation, positive regulation of cell adhesion mediated by integrin, keratinocyte migration, macrophage fusion and protein secretion, response to calcium ion, hydrogen peroxide, manganese ion, tumor necrosis factor, transforming growth factor beta receptor signalling pathway | Fertility, tail regeneration71,72 | |
| ADAMTS17 | ADAM metallopeptidase with thrombospondin type 1 motif 17 | Metal ion binding and metallopeptidase activity | Extracellular matrix organization | 73 | |
| ADCY1 | Adenylate cyclase 1 | Purine metabolism, metabolic pathways, calcium signalling pathway, oocyte meiosis, adrenergic signalling in cardiomyocytes, vascular smooth muscle contraction, apelin signalling pathway, gap junction, GnRH signalling pathway, progesterone-mediated oocyte maturation, melanogenesis | Adenylate cyclase activity, ATP binding, metal ion binding | Adenylate cyclase-activating G protein-coupled receptor signalling pathway, axonogenesis, cAMP biosynthetic process, long-term memory, neuroinflammatory response, positive regulation of CREB transcription factor activity and long-term synaptic potentiation, reulation of circadian rhythm and synaptic vesicle exocytosis | Circadian rhythm74 |
| ADCY2 | Adenylate cyclase 2 | Purine metabolism, metabolic pathways, calcium signalling pathway, oocyte meiosis, adrenergic signalling in cardiomyocytes, vascular smooth muscle contraction, apelin signalling pathway, gap junction, GnRH signalling pathway, progesterone-mediated oocyte maturation, melanogenesis | Adenlylate cyclase activity, ATP binding, metal ion binding | Adenylate cyclase-activating G protein-coupled receptor signalling pathway, axonogenesis, cAMP biosynthetic process | 73 |
| ANK1 | Ankyrin 1 | ATPase binding, cytoskeletal anchor activity, ion channel binding, protein phosphatase binding, spectrin binding | Endoplasmic reticulum to Golgi vesicle-mediated transport, protein localization to plasma membrane | Transcriptional factors, cell regulators, cytoskeletal, ion transporters and signal transducers75 | |
| ANKRD13A | Ankyrin repeat domain 13A | ||||
| CACNA1G | Calcium voltage-gated channel subunit alpha1 G | MAPK and calcium signalling pathway | Voltage-gated calcium and sodium channel activity, scaffold protein binding, cation channel activity | Calcium ion import, cardiac muscle cell action potential involved in contraction, chemical synaptic transmission, membrane depolarization during action potential, neuronal action potential, positive regulation of calcium ion-dependent exocytosis, regulation of atrial cardiac muscle cell membrane depolarization, regulation of heart rate by cardiac conduction, regulation of ion transmembrane transport, response to nickel cation | Sperm storage76 |
| CAMK1D | Calcium/calmodulin dependent protein kinase 1D | Calcium signalling pathway. ATP binding, calmodulin binding, calmodulin-dependent protein kinase activity, protein serine/threonine kinase activity | Peptidyl-serine phosphorylation, negative regulation of apoptotic process, positive regulation of apoptotic process, CREB transcription factor activity, neuron projection development, neutrophil chemotaxis, phagocytosis and respiratory burst, regulation of dendrite development | 73 | |
| CNKSR2 | Connector enhancer of kinase suppressor of Ras 2 | Protein kinase binding | Intracellular signal transduction, regulation of signal transduction | 73 | |
| COL5A3 | Collagen alpha-1(XI) chain | ||||
| COLGALT1 | Collagen beta(1-O)galactosyltransferase 1 | Lysine degradation, O-glycan biosynthesis, metabolic pathways | Procollagen galactosyltransferase activity | Positive regulation of collagen fibril organization | Skin development77 |
| FGFR1 | Fibroblast growth factor receptor 1 | MAPK and calcium signalling pathway, adherens junction, regulation of actin cytoskeleton | ATP binding, fibroblast growth factor-activated receptor activity | Positive regulation of cell population proliferation | Tail regeneration78,79 |
| GPC1 | Glypican 1 | Copper ion binding, fibroblast growth factor binding, laminin binding | Cell migration, heparan sulfate proteoglycan catabolic process, negative regulation of fibroblast growth factor receptor signalling pathway, positive regulation of skeletal muscle cell differentiation, regulation of protein localization to membrane | 73 | |
| GPC4 | Glypican 4 | Wnt signalling pathway | Cell migration, regulation of neurotransmitter receptor localization to postsynaptic specialization membrane, regulation of presynapse assembly, regulation of protein localization to membrane, regulation of signal transduction, synaptic membrane adhesion, Wnt signalling pathway | Adipocyte differentiation80 | |
| HS6ST2 | Heparan-sulfate 6-O-sulfotransferase 2 | Glycosaminoglycan biosynthesis—heparan sulfate/heparin | Sulfotransferase activity | Cell proliferation and differentiation | Cell proliferation81 |
| ITPR2 | Inositol 1,4,5-trisphosphate receptor type 2 isoform X1 | Calcium signaling pathway, phosphatidylinositol signalling system, oocyte meiosis, apoptosis, cellular senescence, vascular smooth muscle contraction, apelin signalling pathway, Gap junction, NOD-like receptor signalling pathway, C-type lectin receptor signalling pathway, GnRH signalling pathway | Calcium ion binding, ion channel binding, phosphatidylinositol binding, scaffold protein binding | Cellular response to cAMP and ethanol, release of sequestered calcium ion into cytosol, response to hypoxia | Egg shell quality, muscle contraction, response to hypoxia82–84 |
| MAP2 | Microtubule associated protein 2 | Dystroglycan and microtubule binding | Axonogenesis, cellular response to organic substance, central nervous system neuron development, dendrite morphogenesis, establishment of cell polarity, microtubule bundle formation, microtubule cytoskeleton organization, negative regulation of axon extension, neuron projection development, regulation of cellular protein localization | Neuronal development85 | |
| MAP7D3 | MAP7 domain-containing protein 3 isoform X1 | Microtubule cytoskeleton organization | Sex determination86 | ||
| MYO18B | Myosin-XVIIIb | Actin and ATP binding, motor activity | 73 | ||
| MYO7B | Myosin VIIb | Actin-dependent ATPase activity, actin filament binding, ATP binding, microfilament motor activity | Actin filament organization, brush border assembly, sensory organ development, sensory perception of sound, vesicle transport along actin filament | 73 | |
| OLFM2 | Olfactomedin 2 | Positive regulation of smooth muscle cell differentiation, protein secretion | 73 | ||
| PBX3 | Pre-B-cell leukemia transcription factor 3 | DNA binding, DNA-binding transcription factor activity, RNA polymerase II-specific | Animal organ morphogenesis, brain development, embryonic organ development, eye development, neuron development, regulation of transcription by RNA polymerase II | Embryonic development87 | |
| PCDH17 | Protocadherin 17 | Calcium ion binding | Adult behaviour, cell adhesion, homophilic cell adhesion via plasma membrane adhesion molecules, negative regulation of synaptic transmission, presynaptic active zone assembly, regulation of synaptic vesicle clustering | 73 | |
| PCDH7 | Protocadherin 7 | Calcium ion binding | Cell adhesion, homophilic cell adhesion via plasma membrane adhesion molecules | 73 | |
| TACC1 | Transforming acidic coiled-coil containing protein 1 | Estrogen receptor binding, glucocorticoid receptor binding, peroxisome proliferator activated receptor binding, retinoid X receptor binding, thyroid hormone receptor binding | Cell population proliferation, microtubule cytoskeleton organization, mitotic spindle organization, positive regulation of nuclear receptor transcription coactivator activity | 73 | |
| WNT10A | Protein Wnt-10a | mTOR and Wnt signalling pathway, melanogenesis | Signaling receptor binding | Multicellular organism development, Wnt signalling pathway | Tail regeneration, epidermis morphogenesis88 |
| ZNF516 | Zinc finger protein 516 | Activating transcription factor binding, DNA-binding transcription factor activity, RNA polymerase II-specific, RNA polymerase II cis-regulatory region sequence-specific DNA binding | Adipose tissue development, brown fat cell differentiation, positive regulation of cold-induced thermogenesis and transcription, response to cold | Thermogenesis89 | |
| ZNF711 | Zinc finger protein 711 | DNA binding, metal ion binding | Regulation of transcription | 73 |
Environmental association analysis
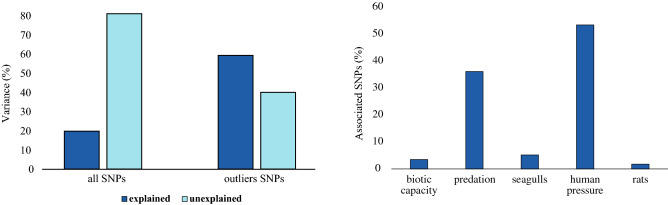
The RDA analysis that used all SNPs indicated that the variation explained by the environmental variables (20.1%) was lower than the unexplained variance (79.9%) (Fig. 5). However, when the analysis was based on only outlier SNPs (1,355), environmental variables explained most of the variation (60.4%). The low explanatory power obtained with all SNPs is not surprising given that we expect that most of the SNPs in our global dataset to be neutral and not associated with environmental predictors. A total of 58 loci with associations with environmental variables were detected, most of which were related to human pressure (53.5%) and predation (36.2%). Some of these associated SNPs have been found to be related to locomotory and feeding behavior (NEGR1, GRM1), perception of pain (GRM1), lipid metabolism (GDPD2) or ion transport (FHL1, FTH1, SLC9A6), microtubule formation (CLIP1), myoblast differentiation (MBNL3), embryonic development (INTS6L), pH regulation (SLC9A6), toxin transport (DNAJC17), cell adhesion (ESAM, NEGR1), hormone regulation (TG, NCOA1), brain development and cognition (SHROOM4).
Figure 5.
Variation explained by ecological variables computed in the RDA analysis based on all SNPs (72,846) and outlier SNPs only (1355) is indicated on the left graph. The percentage of associated SNPs for the retained variables after RDA analysis based on SNPs under selection is indicated on the right graph.
Discussion
The RADseq methodology has been applied in other studies of squamate (lizards and snakes), increasing understanding of the processes related to genetic divergence and the identification of genomic regions of interest. The total number of SNPs obtained in this study (288,286) agree with the SNP density found in other RADseq studies of reptiles, with relatively high levels of diversity detected30,31. Population structure analysis revealed a clear genetic structure among all the populations of P. lilfordi, independent of whether we used SNPs from all RAD tags or just candidates for selection32. Major genetic structuring mirrors that found using mtDNA, with high levels of divergence between Menorca islands and Mallorca islands/Cabrera populations19,20. However, analyses of outlier SNPs revealed greater similarity between the northern Mallorca Colomer population and other Mallorca islands (Dragonera), which differs from the pattern found in mtDNA20. These results together with the high migration rate detected between Mallorca islands and the Cabrera archipelago populations supports a previous proposal20 that the Colomer Island could be home of a relict population representative of the early population that once colonized Mallorca Island.
The populations with the smallest population sizes (Porros, Foradada and Esclatasang islets) were most divergent with highest FST values and the greatest number of private alleles relative to other populations, which supports previous findings30,33 and is suggestive of genetic drift. Long-term isolation and small population size should lead to decreased genetic diversity and increased inbreeding coffecients34–37. While nucleotide diversity was low38, inbreeding values were under 10%39,40 which is not indicative of an inbreeding effect.
It is worth highlighting evidence of adaptive divergence among lizard populations based on FST outlier tests. Almost 2% of total SNPs were candidates for selection. These loci were related to several functions with direct survival value such as tail regeneration, reproduction, lipid metabolism and circadian rhythm. Nonetheless, the still incomplete annotation of the available Podarcis genome makes necessary a more in-depth analysis to elucidate the molecular mechanisms of adaptation in this genus. Other studies of lizards have revealed links between genetic variation of candidate genes and geographical distributions, patterns of colonization and/or landscapes gradients13,41–43.
We show that environmental variables appear to be an important driver of divergence between lizard populations after taking into account the effect of historical divergence. The RDA analysis revealed most SNPs that were influenced by the environment were associated with levels of predation and human pressure. These SNPs were involved in diverse functions most notably with feeding and locomotory behavior. The explanatory power of the remaining environmental predictors, such as the biotic capacity of islands, the presence of rats, or the existence of breeding colonies of gulls, is negligible. Some behavioral and physiological differences between populations can be related to differences in predation and human pressures, as in the case of escape behavior in lizard populations with or without terrestrial predators. For example, predation pressure has previously been shown to influence flight initiation distance, distance fled, or hiding time in Balearic lizard populations44–47.
Predation has traditionally been identified as a major selective factor shaping the morphological and demographic evolution of animal species48. Unlike many terrestrial vertebrates that have evolved in the presence of these selection pressures over millions of years, P. lilfordi has evolved for ~ 5.3 Ma in a pristine environment, free from terrestrial predators16. The subsequent arrival of humans ~ 5000 years ago caused a major change as allochthonous predators were introduced. Hence there is a strong association between indices of human pressure and predation pressure as a result of this Holocenic arrival16,17,49.
It is interesting that this selection has had a strong and detectable effect on the genomic structure of these populations in a relatively short time. This has been described in a few other studies50–52. However, to our knowledge, this is the first case where predator and human pressures have been functionally linked with possible selection on loci involved in physiological functions that are directly involved with locomotor and escape behaviors. Same human-driven factors are often responsible of rapid adaptation and current extinction crisis53. This fact implies that the study of rapid adaptation to novel environment changes, especially those related with humans, has an inmediate relevance to conservation biology. For this reason, the study of adaptive evolution need to be incorporate into conservation strategies of insular terrestrial vertebrates populations and specifically in the Balearic lizard. In this way, Ashley et al.25 proposed the promotion of an evolutionary enlightened management in which conservation decisions need to take into account the evolutionary effects of anthropogenic changes.
Overall, our results reveal that both evolutionary processes, associated with isolation and small population size, and selective factors, related to environmental patterns (specifically human pressure and level of predation) have played a role in shaping divergence between Balearic lizard populations.
Methods
Sample collection, DNA extraction, library preparation, and sequencing
Tissue samples were collected from 94 lizards (P. lilfordi) from 10 different sampling locations across the Balearic archipelago (Fig. 1 and Table 2). Populations were selected to cover a diverse range of substrates, orographies, plant cover, presence of terrestrial predator and human pressure, as well as different population sizes and different mtDNA clades (Table 2). Total genomic DNA was extracted from each tissue sample using DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) following the manufacturer’s standard protocol with a specific RNase copurification step. DNA was quantified using the Thermo Fisher Scientific Qubit 3.0 Fluorometer (ThermoFisher Scientific) and quality evaluated using agarose gel and Nanovue Plus Spectrophotometer (GE Healthcare, UK Limited). Paired-end ddRADseq libraries were prepared and sequenced by Floragenex (Eugene, Oregon, USA), following Peterson et al.28 and Truong et al.54 protocols. Full details are provided in Supplementary Methods.
Table 2.
Characteristics and environmental variables of the studied populations. n number of samples used for every population, S island surface area in hectares, predation indexes absence of terrestrial predators = 0; one occasional predators in the island = 1; one widespread predator was or is present in the island = 2; two frequent predators present in the island = 3, human pressure uninhabited island and very difficult access = 0; sporadic human presence and easy access = 1; regular human presence and easy access = 2; previous permanent human presence with constructions but with an actual protection = 3; present and past human presence = 4.
| Population | n | Population size (N) | S (ha) | Biotic capacity | Vascular plants | Predation | Human pressure | Rats | Seagulls |
|---|---|---|---|---|---|---|---|---|---|
| Menorca | |||||||||
| Aire | 12 | 77,500 | 29.8 | 6.10 | 94 | 0 | 2 | No | Yes |
| Colom | 10 | 58,107 | 51.14 | 7.62 | 267 | 1 | 3 | Yes | Yes |
| Porros | 10 | 54 | 0.05 | −2.66 | 32 | 0 | 1 | No | No |
| Rei | 10 | 1845 | 4.08 | 4.08 | 204 | 2 | 4 | Yes | No |
| Cabrera | |||||||||
| Cabrera (harbour) | 12 | 534,888 | 1137.24 | 12.18 | 486 | 3 | 3 | Yes | Yes |
| Cabrera (lighthouse) | 10 | 5171 | 10.6 | 7.16 | 486 | 3 | 2 | Yes | Yes |
| Esclatasang | 11 | 714 | 0.42 | 2.69 | 23 | 0 | 0 | No | Yes |
| Foradada | 10 | 1356 | 1.61 | 3.77 | 19 | 0 | 1 | No | Yes |
| Mallorca | |||||||||
| Dragonera | 4 | 132,875 | 267.81 | 11.29 | 300 | 0 | 2 | Yes | Yes |
| Colomer | 4 | 10,017 | 3.05 | 5.74 | 8 | 0 | 0 | No | Yes |
Data processing and variant calling
Stacks v2.455 pipelines were used to process the sequence reads and call SNPs for each individual. First, a demultiplexing and quality filtering step was carried out using process_radtags with the default parameters. Clean reads were used to perform a de novo RAD assembly using the denovo_map.pl pipeline. The percentage of missing genotypes for each individual was calculated using the -missing-indv in VCFtools v0.1.1556 and three individuals with more than 79% of missing data were removed. SNPs present in RAD tags found in at least 80% (R) of individuals (Supplementary Figure 3) and with a minimum allele frequency (MAF) of 0.05 were selected and exported into a VCF file using populations. One single SNP per RAD tag was called using populations to reduce the effects of linkage disequilibrium. See Supplementary Methods.
Population structure
Several analyses were used to characterize population structure of island lizard populations based on all RAD-tag information (single SNP selected from each tag, referred to as the all-SNP dataset: VCF file in Appendix S1) and using only outlier SNPs (see later for identification of outliers: VCF file in Appendix S2). First, two different programs, Stacks v4.255 and hierfstat R package57, were used to estimate levels of genetic variability among different lizard populations. Second, population structure was examined with Admixture v1.3.0 program58 based on both datasets, for K = 2 to K = 10 co-ancestry clusters. Third, patterns of genetic divergence on both datasets were analyzed using two approaches. Discriminant Analysis of Principal Components (DAPC) was performed using the R package adegenet59 to obtain an overall representation of the divergence between populations and Neighbor-Joining (NJ) trees were inferred using Mega 760 based on pairwise FST distances.
Effective population size (Ne) for each population has been estimated with the software NeEstimator v2.0.161 using the molecular coancestry method. Linear regression analyses between N and Ne, pi and N, pi and Ne, and FST with N and with Ne, was performed with Pearson correlation. To investigate migration rates between each locality and between each island (Mallorca, Menorca and Cabrera), migration networks were generated usind divMigrate function62 in the R package diveRsity63 based on GST genetic distance64 with 1000 bootstrap repetitions and a filter threshold of 0.25. More information is provided in the Supplementary Methods.
Test of selection and environmental association analysis
Tests of selection was carried out to explore the role of divergent selection using BayeScan65. This program identifies candidate loci under selection using an FST outlier approach across all sampled populations. The BayeScan algorithm is based on an island model in which subpopulations differ from a common migrant pool. Thus, a departure from neutrality is identified at a SNP when the overall genome divergence between different subpopulations is insufficient to explain its diversity across these subpopulations.
Genome-environment association (GEA) is an important tool for the examination of local adaptation to heterogeneous landscapes66,67. Climatic variables were not used as environmental predictors because the Balearic lizard inhabits a reduced geographical range with minimal climatic variation23. Six environmental traits were considered: biotic capacity, number of vascular plants species, predation pressure, human pressure, and presence/absence of rats and gulls. All of these traits are related to natural resources on the islands and factors that potentially affect the lizards’ survival and were known to show clear differences among the populations studied. Partial redundancy analysis (RDA) was used as a GEA method to identify adaptive loci based on associations between genetic data and environmental predictors68. See Supplementary Methods.
Ethical statement
All tail tips samples used in this study were obtained in accordance with Ethical Guidelines of the Universities of Balearic Islands and Salamanca, particularly, following the Bioethics Committee Guidelines of the University of Salamanca. The Ethical Committee from the University of Salamanca publishes general Guidelines concerning the experimental protocols with laboratory animals. These general Guidelines for laboratory animals can be read in http://www.usal.es. According to these Guidelines, only the requirements applicable to our study were implemented simply because we did not perform any experiment with lizards in captivity. Field protocols for the capture, handling and release of lizards (which was done at the site of capture a few minutes after the sampling of tail tips) were approved by the competent authority: the Nature Conservation Agency (Conselleria de Medi Ambient) of the Government of Balearic Island (permits: CEP 02/2018 and CEP 10/2016 to V.P.-M. and A. P.-C.).
Supplementary Information
Acknowledgements
This work was financed by the research grant CGL-2015-68139-C2 of the Ministerio Español de Economía y Competitividad and European Regional Development Fund (ERDF). MB (FPI/1772/2015) was funded by fellowship from the Conselleria d’Educació, Cultura i Universitats (Govern de les Illes Balears, Spain), co-financed by the ERDF.
Author contributions
M.B. carried out the laboratory work, data analysis and interpretation, and paper writing. C.R., A.P. and R.P.B. designed the study, C.R., A.P. and J.A.C. participated in the interpretation of the data and the discussion of the manuscript and R.P.B. in data analysis and interpretation and writing of the paper. V.P.M. and A.P.C. collected samples, environmental data, and participated in data interpretation, and paper elaboration. All authors read and approved the final manuscript.
Data availability
Individual raw sequences are available at the Sequence Read Archive (SRA) (BioProject ID: PRJNA645796). The VCF files with first single SNPs and only with outlier SNPs putatively under selection are found on Appendices S1 and S2.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-85591-x.
References
- 1.Stuart YE, Losos JB, Algar AC. The island-mainland species turnover relationship. Proc. R. Soc. B Biol. Sci. 2012;279:4071–4077. doi: 10.1098/rspb.2012.0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant PR. Evolution on Islands. Oxford University Press; 1998. [Google Scholar]
- 3.Garant D, Forde SE, Hendry AP. The multifarious effects of dispersal and gene flow on contemporary adaptation. Funct. Ecol. 2007;21:434–443. doi: 10.1111/j.1365-2435.2006.01228.x. [DOI] [Google Scholar]
- 4.Armstrong C, et al. Genomic associations with bill length and disease reveal drift and selection across island bird populations. Evol. Lett. 2018;2(1):22–36. doi: 10.1002/evl3.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eldridge MDB, et al. Unprecedented low levels of genetic variation and inbreeding depression in an island population of the black-footed rock-wallaby. Conserv. Biol. 1999;13:531–541. doi: 10.1046/j.1523-1739.1999.98115.x. [DOI] [Google Scholar]
- 6.Wright S. Isolation by distance under diverse systems of mating. Genetics. 1946;31:39–59. doi: 10.1093/genetics/31.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimura M, Crow JF. The number of alleles that can be maintained in a finite population. Genetics. 1964;49(4):725. doi: 10.1093/genetics/49.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luikart G, England PR, Tallmon D, Jordan S, Taberlet P. The power and promise of population genomics: From genotyping to genome typing. Nat. Rev. Genet. 2003;4(12):981–994. doi: 10.1038/nrg1226. [DOI] [PubMed] [Google Scholar]
- 9.Nei M, Suzuki Y, Nozawa M. The neutral theory of molecular evolution in the genomic era. Annu. Rev. Genom. Hum. Genet. 2010;11:265–289. doi: 10.1146/annurev-genom-082908-150129. [DOI] [PubMed] [Google Scholar]
- 10.Weigelt P, Jetz W, Kreft H. Bioclimatic and physical characterization of the world's islands. Proc. Natl. Acad. Sci. U.S.A. 2013;110(38):15307–15312. doi: 10.1073/pnas.1306309110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huey RB, Gilchrist GW, Carlson ML, Berrigan D, Serra L. Rapid evolution of a geographic cline in size in an introduced fly. Science. 2000;287(5451):308–309. doi: 10.1126/science.287.5451.308. [DOI] [PubMed] [Google Scholar]
- 12.Prates I, Angilleta MJ, Wilson RS, Niehaus AC, Navas CA. Dehydration hardly slows hopping toads (Rhinella granulosa) from xeric and mesic environments. Physiol. Biochem. Zool. 2013;86(4):451–457. doi: 10.1086/671191. [DOI] [PubMed] [Google Scholar]
- 13.Prates I, Penna A, Trefaut M, Carnaval AC. Local adaptation in mainland anole lizards: Integrating population history and genome-environment associations. Ecol. Evol. 2018;8:11932–11944. doi: 10.1002/ece3.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funk WC, et al. Adaptive divergence despite strong genetic drift: Genomic analysis of the evolutionary mechanisms causing genetic differentiation in the island fox (Urocyonlittoralis) Mol. Ecol. 2016;25(10):2176–2194. doi: 10.1111/mec.13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friis G, et al. Genome-wide signals of drift and local adaptation during rapid lineage divergence in a songbird. Mol. Ecol. 2018;27(24):5137–5153. doi: 10.1111/mec.14946. [DOI] [PubMed] [Google Scholar]
- 16.Bover P, Quintana J, Alcover JA. Three islands, three worlds: Paleogeography and evolution of the vertebrate fauna from the Balearic Islands. Quatern. Int. 2008;182:135–144. doi: 10.1016/j.quaint.2007.06.039. [DOI] [Google Scholar]
- 17.Pérez-Mellado V. Les sargantanes de les Balears. Edicions Quaderns de Natura de les Balears, Documenta Balear; 2009. [Google Scholar]
- 18.Pérez-Mellado V, et al. Population density in Podarcis lilfordi (Squamata, Lacertidae), a lizard species endemic to small islets in the Balearic Islands (Spain) Amphibia-Reptilia. 2008;29(1):49–60. doi: 10.1163/156853808783431587. [DOI] [Google Scholar]
- 19.Brown RP, et al. Bayesian estimation of post-Messinian divergence times in Balearic Island lizards. Mol. Phylogenet. Evol. 2008;48(1):350–358. doi: 10.1016/j.ympev.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Terrasa B, et al. Foundations for conservation of intraspecific genetic diversity revealed by analysis of phylogeographical structure in the endangered endemic lizard Podarcis lilfordi. Divers. Distrib. 2009;15(2):207–221. doi: 10.1111/j.1472-4642.2008.00520.x. [DOI] [Google Scholar]
- 21.Terrasa B, et al. Use of NCPA to understanding genetic sub-structuring of Podarcis lilfordi from the Balearic archipelago. Amphibia-Reptilia. 2009;30(4):505–514. doi: 10.1163/156853809789647130. [DOI] [Google Scholar]
- 22.Emig CC, Geistdoerfer P. The Mediterranean deep-sea fauna: Historical evolution, bathymetric variations and geographical changes. Carnets Geol. 2004 doi: 10.4267/2042/3230. [DOI] [Google Scholar]
- 23.Pérez-Cembranos A, et al. Morphological and genetic diversity of the Balearic lizard, Podarcis lilfordi (Günther, 1874): Is it relevant to its conservation? Divers. Distrib. 2020;26:1122–1141. doi: 10.1111/ddi.13107. [DOI] [Google Scholar]
- 24.Palumbi SR. The Evolution Explosion: How Humans Cause Rapid Evolutionary Change. W. W. Norton & Company; 2002. [Google Scholar]
- 25.Ashley MV, et al. Evolutionary enlightened management. Biol. Conserv. 2003;111:115–123. doi: 10.1016/S0006-3207(02)00279-3. [DOI] [Google Scholar]
- 26.Stockwell CA, Hendry AP, Kinnison MT. Contemporary evolution meets conservation biology. Trends Ecol. Evol. 2003;18:94–101. doi: 10.1016/S0169-5347(02)00044-7. [DOI] [Google Scholar]
- 27.Baird NA, et al. Rapid SNP discovery and genetic mapping using sequenced RAD markers. PLoS ONE. 2008;3(10):e3376. doi: 10.1371/journal.pone.0003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson BK, Weber JN, Kay EH, Fisher HS, Hoekstra HE. Double digest RADseq: An inexpensive method for de novo SNP discovery and genotyping in model and non-model species. PLoS ONE. 2012;7(5):e37135. doi: 10.1371/journal.pone.0037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrews KR, Good JM, Miller MR, Luikart G, Hohenlohe PA. Harnessing the power of RADseq for ecological and evolutionary genomics. Nat. Rev. Genet. 2016;17(2):81. doi: 10.1038/nrg.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown RP, Paterson S, Risse J. Genomic signatures of historical allopatry and ecological divergence in an island lizard. Genome Biol. Evol. 2016;8(11):3618–3626. doi: 10.1093/gbe/evw268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin Y, Brown RP. Morphological species and discordant mtDNA: A genomic analysis of Phrynocephalus lizard lineages on the Qinghai-Tibetan Plateau. Mol. Phylogenet. Evol. 2019;139:106523. doi: 10.1016/j.ympev.2019.106523. [DOI] [PubMed] [Google Scholar]
- 32.Yang W, et al. Spatial variation in gene flow across a hybrid zone reveals causes of reproductive isolation and asymmetric introgression in wall lizards. Evolution. 2020;74(7):1289–1300. doi: 10.1111/evo.14001. [DOI] [PubMed] [Google Scholar]
- 33.Li YL, Xue DX, Zhang BD, Liu JX. Population genomic signatures of genetic structure and environmental selection in the catadromous roughskin sculpin Trachidermus fasciatus. Genome Biol. Evol. 2019;11(7):1751–1764. doi: 10.1093/gbe/evz118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hedrick PW, Kalinowski ST. Inbreeding depression in conservation biology. Annu. Rev. Ecol. Syst. 2000;31(1):139–162. doi: 10.1146/annurev.ecolsys.31.1.139. [DOI] [Google Scholar]
- 35.Willi Y, Van Buskirk J, Hoffmann AA. Limits to the adaptive potential of small populations. Annu. Rev. Ecol. Evol. Syst. 2006;37:433–458. doi: 10.1146/annurev.ecolsys.37.091305.110145. [DOI] [Google Scholar]
- 36.Perrier C, Ferchaud AL, Sirois P, Thibault I, Bernatchez L. Do genetic drift and accumulation of deleterious mutations preclude adaptation? Empirical investigation using RAD seq in a northern lacustrine fish. Mol. Ecol. 2017;26(22):6317–6335. doi: 10.1111/mec.14361. [DOI] [PubMed] [Google Scholar]
- 37.Sovic M, Fries A, Martin SA, Lisle Gibbs H. Genetic signatures of small effective population sizes and demographic declines in an endangered rattlesnake, Sistrurus catenatus. Evol. Appl. 2019;12(4):664–678. doi: 10.1111/eva.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao R, et al. Genetic structure and diversity of Australian freshwater crocodiles (Crocodylus johnstoni) from the Kimberley, Western Australia. Conserv. Genet. 2020;21:421–429. doi: 10.1007/s10592-020-01259-5. [DOI] [Google Scholar]
- 39.Lowe WH, Allendorf FW. What can genetics tell us about population connectivity? Mol. Ecol. 2010;19(15):3038–3051. doi: 10.1111/j.1365-294X.2010.04688.x. [DOI] [PubMed] [Google Scholar]
- 40.Ralls K, et al. Call for a paradigm shift in the genetic management of fragmented populations. Conserv. Lett. 2018;11(2):e12412. doi: 10.1111/conl.12412. [DOI] [Google Scholar]
- 41.Benestan L, et al. Seascape genomics provides evidence for thermal adaptation and current-mediated population structure in American lobster (Homarus americanus) Mol. Ecol. 2016;25(20):5073–5092. doi: 10.1111/mec.13811. [DOI] [PubMed] [Google Scholar]
- 42.Campbell-Staton SC, Edwards SV, Losos JB. Climate mediated adaptation after mainland colonization of an ancestrally subtropical island lizard, Anolis carolinensis. J. Evol. Biol. 2016;29(11):2168–2180. doi: 10.1111/jeb.12935. [DOI] [PubMed] [Google Scholar]
- 43.Rodríguez A, et al. Genomic and phenotypic signatures of climate adaptation in an Anolis lizard. Ecol. Evol. 2017;7(16):6390–6403. doi: 10.1002/ece3.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cooper WE, Hawlena D, Pérez-Mellado V. Islet tameness: Escape behavior and refuge use in populations of the Balearic lizard (Podarcis lilfordi) exposed to differing predation pressure. Can. J. Zool. 2009;87(10):912–919. doi: 10.1139/Z09-077. [DOI] [Google Scholar]
- 45.Cooper WE, Hawlena D, Pérez-Mellado V. Influence of risk on hiding time by Balearic lizards (Podarcis lilfordi): Predator approach speed, directness, persistence, and proximity. Herpetologica. 2010;66(2):131–141. doi: 10.1655/08-084R.1. [DOI] [Google Scholar]
- 46.Cooper WE, Pérez-Mellado V. Island tameness: Reduced escape responses and morphological and physiological antipredatory adaptations related to escape in lizards. In: Pérez-Mellado V, Ramon MM, editors. Islands and Evolution. Institut Menorquí d’Estudis; 2010. pp. 231–253. [Google Scholar]
- 47.Cooper WE, Pérez-Mellado V. Historical influence of predation pressure on escape by Podarcis lizards in the Balearic Islands. Biol. J. Linn. Soc. 2012;107:254–268. doi: 10.1111/j.1095-8312.2012.01933.x. [DOI] [Google Scholar]
- 48.Mayr E. Animal Species and Evolution. The Belknap Press, Harvard University Press; 1963. [Google Scholar]
- 49.Bover P, et al. Late Miocene/Early Pliocene vertebrate fauna from Mallorca (Balearic Islands, Western Mediterranean): An update. Integr. Zool. 2014;9:183–196. doi: 10.1111/1749-4877.12049. [DOI] [PubMed] [Google Scholar]
- 50.Vervust B, Grbac I, Van Damme R. Differences in morphology, performance and behaviour between recently diverged populations of Podarcissicula mirror differences in predation pressure. Oikos. 2007;116(8):1343–1352. doi: 10.1111/j.0030-1299.2007.15989.x. [DOI] [Google Scholar]
- 51.Marques DA, Jones FC, Di Palma F, Kingsley DM, Reimchen TE. Experimental evidence for rapid genomic adaptation to a new niche in an adaptive radiation. Nat. Ecol. Evol. 2018;2(7):1128–1138. doi: 10.1038/s41559-018-0581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johannesson K, Le Moan A, Perini S, André C. A Darwinian laboratory of multiple contact zones. Trends Ecol. Evol. 2020;35:1021–1036. doi: 10.1016/j.tree.2020.07.015. [DOI] [PubMed] [Google Scholar]
- 53.Stockwell CA, Ashley MV. Rapid adaptation and conservation. Conserv. Biol. 2004;18:272–273. doi: 10.1111/j.1523-1739.2004.00307.x. [DOI] [Google Scholar]
- 54.Truong HT, et al. Sequence-based genotyping for marker discovery and co-dominant scoring in germplasm and populations. PLoS ONE. 2012;7(5):e37565. doi: 10.1371/journal.pone.0037565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Catchen J, Hohenlohe PA, Bassham S, Amores A, Cresko WA. Stacks: An analysis tool set for population genomics. Mol. Ecol. 2013;22(11):3124–3140. doi: 10.1111/mec.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Danecek P, et al. The variant call format and VCFtools. Bioinformatics. 2011;27(15):2156–2158. doi: 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goudet, J. & Jombart, T. hierfstat: Estimation and tests of hierarchical F-statistics. R package version 0.5-7. Available from http://github.com/jgx65/hierfstat (2015).
- 58.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19(9):1655–1664. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jombart T, Ahmed I. Adegenet 1.3-1: New tools for the analysis of genome-wide SNP data. Bioinformatics. 2011;27(21):3070–3071. doi: 10.1093/bioinformatics/btr521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Do C, et al. NeEstimator v2: Re-implementation of software for the estimation of contemporary effective population size (Ne) from genetic data. Mol. Ecol. Resour. 2014;14(1):209–214. doi: 10.1111/1755-0998.12157. [DOI] [PubMed] [Google Scholar]
- 62.Sundqvist L, Keenan K, Zackrisson M, Prodöhl P, Kleinhans D. Directional genetic differentiation and relative migration. Ecol. Evol. 2016;6(11):3461–3475. doi: 10.1002/ece3.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Keenan K, McGinnity P, Cross TF, Crozier WW, Prodöhl PA. diveRsity: An R package for the estimation and exploration of population genetics parameters and their associated errors. Methods Ecol. Evol. 2013;4(8):782–788. doi: 10.1111/2041-210X.12067. [DOI] [Google Scholar]
- 64.Nei M. The Theory and Estimation of Genetic Distance. Genetic Structure of Populations. University of Hawaii Press; 1973. pp. 45–54. [Google Scholar]
- 65.Foll M, Gaggiotti O. A genome-scan method to identify selected loci appropriate for both dominant and codominant markers: A Bayesian perspective. Genetics. 2008;180(2):977–993. doi: 10.1534/genetics.108.092221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frichot E, Schoville SD, Bouchard G, François O. Testing for associations between loci and environmental gradients using latent factor mixed models. Mol. Biol. Evol. 2013;30(7):1687–1699. doi: 10.1093/molbev/mst063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rellstab C, Gugerli F, Eckert AJ, Hancock AM, Holderegger R. A practical guide to environmental association analysis in landscape genomics. Mol. Ecol. 2015;24(17):4348–4370. doi: 10.1111/mec.13322. [DOI] [PubMed] [Google Scholar]
- 68.Forester BR, Lasky JR, Wagner HH, Urban DL. Comparing methods for detecting multilocus adaptation with multivariate genotype-environment associations. Mol. Ecol. 2018;27(9):2215–2233. doi: 10.1111/mec.14584. [DOI] [PubMed] [Google Scholar]
- 69.Jin L, Yu JP, Yang ZJ, Merilä J, Liao WB. Modulation of gene expression in liver of hibernating Asiatic Toads (Bufo gargarizans) Int. J. Mol. Sci. 2018;19(8):2363. doi: 10.3390/ijms19082363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Secor SM, Carey HV. Integrative physiology of fasting. Compr. Physiol. 2011;6(2):773–825. doi: 10.1002/cphy.c150013. [DOI] [PubMed] [Google Scholar]
- 71.Bahudhanapati H, Bhattacharya S, Wei S. Evolution of vertebrate adam genes; duplication of testicular adams from ancient adam9/9-like loci. PLoS ONE. 2015;10(8):e0136281. doi: 10.1371/journal.pone.0136281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alibardi L. Immunolocalization of matrix metalloproteinases in regenerating lizard tail suggests that an intense remodelling activity allows for apical tail growth. Acta Zool. 2020;101(2):124–132. doi: 10.1111/azo.12277. [DOI] [Google Scholar]
- 73.The UniProt Consortium UniProt: The universal protein knowledgebase. Nucleic Acids Res. 2017;45:D158–D169. doi: 10.1093/nar/gkw1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tosini G, Baba K, Hwang CK, Iuvone PM. Melatonin: An underappreciated player in retinal physiology and pathophysiology. Exp. Eye Res. 2012;103:82–89. doi: 10.1016/j.exer.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Voronin DA, Kiseleva EV. Functional role of proteins containing ankyrin repeats. Tsitologiia. 2007;49(12):989–999. [PubMed] [Google Scholar]
- 76.Yang L, et al. Transcriptome analysis and identification of genes associated with chicken sperm storage duration. Poult. Sci. 2020;99(2):1199–1208. doi: 10.1016/j.psj.2019.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Geng X, et al. Proteomic analysis of the skin of Chinese giant salamander (Andrias davidianus) J. Proteomics. 2015;119:196–208. doi: 10.1016/j.jprot.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 78.Subramaniam N, Petrik JJ, Vickaryous MK. VEGF, FGF-2 and TGF β expression in the normal and regenerating epidermis of geckos: Implications for epidermal homeostasis and wound healing in reptiles. J. Anat. 2018;232(5):768–782. doi: 10.1111/joa.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pillai A, Desai I, Balakrishnan S. Pharmacological inhibition of FGFR1 signaling attenuates the progression of tail regeneration in the northern house gecko Hemidactylus flaviviridis. Int. J. Life Sci. Biotechnol. Pharma Res. 2013;2:263–278. [Google Scholar]
- 80.Schoettl T, Fischer IP, Ussar S. Heterogeneity of adipose tissue in development and metabolic function. J. Exp. Biol. 2018;221:jeb162958. doi: 10.1242/jeb.162958. [DOI] [PubMed] [Google Scholar]
- 81.Wang X, et al. Identification of a novel 43-bp insertion in the heparan sulfate 6-O-sulfotransferase 3 (HS6ST3) gene and its associations with growth and carcass traits in chickens. Anim. Biotechnol. 2019;30(3):252–259. doi: 10.1080/10495398.2018.1479712. [DOI] [PubMed] [Google Scholar]
- 82.Sun C, et al. Genome-wide association study revealed a promising region and candidate genes for eggshell quality in an F2 resource population. BMC Genomics. 2015;16(1):565. doi: 10.1186/s12864-015-1795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ng CS, et al. Transcriptomic analyses of regenerating adult feathers in chicken. BMC Genomics. 2015;16(1):756. doi: 10.1186/s12864-015-1966-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qu Y, et al. Ground tit genome reveals avian adaptation to living at high altitudes in the Tibetan plateau. Nat. Commun. 2013;4(1):1–9. doi: 10.1038/ncomms3071. [DOI] [PubMed] [Google Scholar]
- 85.Fischer I, Kosik KS, Sapirstein VS. Heterogeneity of microtubule-associated protein (MAP2) in vertebrate brains. Brain Res. 1987;436(1):39–48. doi: 10.1016/0006-8993(87)91554-X. [DOI] [PubMed] [Google Scholar]
- 86.Singchat W, et al. Chromosome map of the Siamese cobra: Did partial synteny of sex chromosomes in the amniote represent “a hypothetical ancestral super-sex chromosome” or random distribution? BMC Genomics. 2018;19(1):939. doi: 10.1186/s12864-018-5293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tosches MA, et al. Evolution of pallium, hippocampus, and cortical cell types revealed by single-cell transcriptomics in reptiles. Science. 2018;360(6391):881–888. doi: 10.1126/science.aar4237. [DOI] [PubMed] [Google Scholar]
- 88.Vitulo N, Dalla Valle L, Skobo T, Valle G, Alibardi L. Transcriptome analysis of the regenerating tail vs. the scarring limb in lizard reveals pathways leading to successful vs. unsuccessful organ regeneration in amniotes. Dev. Dyn. 2017;246(2):116–134. doi: 10.1002/dvdy.24474. [DOI] [PubMed] [Google Scholar]
- 89.Carobbio S, Guénantin AC, Samuelson I, Bahri M, Vidal-Puig A. Brown and beige fat: From molecules to physiology and pathophysiology. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2019;1864(1):37–50. doi: 10.1016/j.bbalip.2018.05.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual raw sequences are available at the Sequence Read Archive (SRA) (BioProject ID: PRJNA645796). The VCF files with first single SNPs and only with outlier SNPs putatively under selection are found on Appendices S1 and S2.