The Hippo signaling pathway controls the maturation and effector differentiation of iNKT cells.
Abstract
Invariant natural killer T (iNKT) cells are innate-like lymphocytes with unique signaling requirements for their development and differentiation. In this issue of JEM, Raynor et al. (https://doi.org/10.1084/jem.20191157) report that the Hippo signaling pathway controls the maturation and effector differentiation of iNKT cells by modulating cellular metabolism.
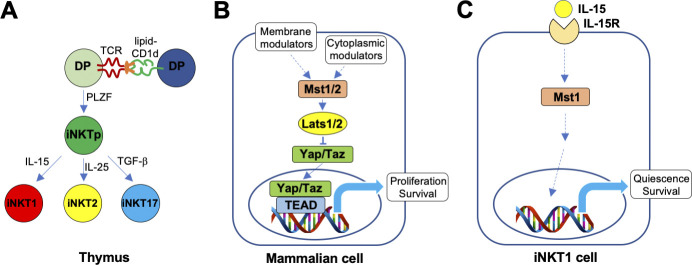
Invariant natural killer T (iNKT) cells are a subset of T lymphocytes that express semi-invariant TCRs specific for glycolipid antigens bound with the MHC class I–related protein CD1d (Iwabuchi and Van Kaer, 2019). iNKT cells exhibit characteristics of innate immune cells such as rapid effector responses to infection and sterile inflammation, with potent effects on both innate and adaptive immune responses. Consequently, these cells influence a broad range of diseases and hold promise for the development of immunotherapies. To fully harness the therapeutic properties of iNKT cells, it is critically important to understand how these cells mature and develop their effector functions. Like conventional T cells, iNKT cells develop in the thymus predominantly from CD4+CD8+ double-positive (DP) precursors, but in contrast with conventional T cells, which are selected by weak interactions with peptide–MHC complexes on thymic epithelial cells, iNKT cells are selected by relatively strong interactions with lipid–CD1d complexes on other DP cells. This so-called agonist selection process turns on the transcription factor PLZF (promyelocytic leukemia zinc finger), which functions as a master regulator of the innate-like properties of iNKT cells. From there, immature iNKT cell precursors undergo proliferative expansion and differentiate into three distinct, terminally differentiated effector cell subsets (Krovi and Gapin, 2018; Hogquist and Georgiev, 2020): (1) iNKT1 cells express the transcription factor T-bet and produce IFN-γ and low levels of IL-4; (2) iNKT2 cells express GATA-3 and produce IL-4; and (3) iNKT17 cells express RORγt and produce IL-17. iNKT1 cells are most abundant, and many take up long-term residence in the thymus. The signaling pathways that influence the maturation and effector cell differentiation of iNKT cell subsets remain incompletely understood. Emerging evidence has shown that metabolic pathways such as the PI3K-mTOR (phosphoinositide 3-kinase–mechanistic target of rapamycin) and autophagy pathways can influence these cell fate decisions (Yang et al., 2018). In this issue of JEM, Raynor et al. (2020) demonstrate that the Hippo signaling pathway controls the metabolic regulation of iNKT cell maturation and effector lineage decisions.

Insights from Luc Van Kaer and Sebastian Joyce.
The Hippo pathway was originally identified as an evolutionarily conserved signaling cascade that controls tissue growth (Ma et al., 2019). Fruit flies with mutations in this pathway displayed enormously sized organs and resembled a hippopotamus. Since then, this pathway has been implicated in cell proliferation, survival, migration, and differentiation. The Hippo pathway responds to a wide variety of intracellular and extracellular cues, such as mechanical force, cell–cell contact, energy status, stress, and hormones and other growth factors. The core of the mammalian Hippo pathway consists of a kinase cascade, including the Mst1/2 (mammalian STE20-like 1/2) and Lats1/2 (large tumor suppressor 1/2) kinases and the downstream transcriptional coactivators Yap/Taz (Yes-associated protein 1/WW domain-containing transcription regulator 1), which induce a host of transcription factors, primarily TEA domain family members. Using mice conditionally deficient in Mst1 and/or Mst2 starting from the DP stage of thymocyte development, Raynor et al. (2020) found a critical role for Hippo signaling in the maturation of terminally differentiated iNKT1 cells. The development of Mst1-deficient iNKT1 cells was stalled at an immature stage, whereas Mst2 deficiency only caused modest effects, and combined Mst1/2 deficiency led to an exacerbated phenotype. This maturation defect appeared to be unique to the iNKT1 subset, as iNKT2 and iNKT17 cell terminal differentiation proceeded unimpeded. Rather, Mst1 ablation caused an expansion of iNKT17 cells, which was absent from conditional Mst2- and Mst1/2-deficient animals, suggesting that this phenotype might be due to a compensatory increase in Mst2 activity in the absence of Mst1. These alterations seen in thymic iNKT cell maturation and effector cell differentiation were also reflected in peripheral organs such as spleen and liver. These effects of Mst1 deficiency were iNKT cell intrinsic and not due to alterations in the capacity of DP cells to present endogenous lipids to the developing iNKT cells. Mst1 deficiency did not affect the thymic development of conventional T cells, although mature T cells accumulated in the thymus of these animals, consistent with prior studies identifying a role for Hippo signaling in the egress of mature T cells from the thymus (Hong et al., 2018). While exploring Hippo signaling modules downstream of Mst1/2, the investigators failed to identify a role for Lats1/2 or Yap/Taz, implying that the effects of Mst1/2 on iNKT cell maturation involve a noncanonical Hippo pathway.
iNKT cells coopt the evolutionarily conserved Hippo signaling pathway to control their maturation and terminal differentiation. (A) Salient features of thymic iNKT cell maturation and effector subset differentiation. (B) Core components of the mammalian canonical Hippo signaling pathway, and common cellular inputs and outcomes. (C) Model for the consequences of Mst signaling on the maturation and terminal effector differentiation of iNKT1 cells and its association with IL-15R signaling, based on the new work from Raynor et al. (2020).
When searching for upstream cues that activate Mst1, the investigators focused on IL-15, which is critically important for the terminal maturation and survival of iNKT1 cells (Gordy et al., 2011), but not iNKT2 or iNKT17 cells, which rely partly on IL-25 and TGF-β, respectively, for their intrathymic survival. They found that IL-15 induced Mst1 signaling activity in iNKT1 cells and that Mst1-deficient iNKT1 cells were resistant to the cell survival activities of IL-15, which involves induction of anti-apoptotic members of the Bcl-2 family of proteins. In addition to IL-15R, other surface receptors might be involved in the induction of Mst activity in iNKT cells. For example, TCR engagement induces Mst signaling (Hong et al., 2018), and TCR signaling strength during iNKT cell maturation has been shown to potently impact iNKT cell terminal differentiation, with low signaling favoring iNKT1 differentiation and high signaling favoring iNKT2 and iNKT17 differentiation (Tuttle et al., 2018; Zhao et al., 2018). However, as Mst1 deficiency resulted in reduced iNKT1 and enhanced iNKT17 differentiation, alterations in signaling downstream from the TCR cannot explain the phenotype observed in the conditional Mst1-deficient animals. Instead, Raynor et al. (2020) found a sharp increase in the levels of the ICOS (inducible T cell costimulator) costimulatory receptor in Mst1-deficient iNKT cells, and combined Mst1 and ICOS deficiency mitigated the increased iNKT17 development seen in the conditional Mst1 mutant animals, suggesting that in the absence of Mst1, a compensatory increase in Mst2 activity induces ICOS–mTORC2 (mTOR complex 2) signaling to promote iNKT17 cell differentiation. Additionally, TGF-β, previously implicated in Mst signaling (Hong et al., 2018) and critical for iNKT17 cell differentiation (Hogquist and Georgiev, 2020), is another cytokine that might contribute to the increase of iNKT17 cells in conditional Mst1-deficient animals, a possibility that remains to be explored.
Immature, post-selection iNKT cells undergo extensive proliferation with high rates of glycolytic activity (Salio et al., 2014). In sharp contrast, mature iNKT1 cells are maintained in a quiescent state characterized by low glycolytic activity and predominantly employ mitochondrial oxidative phosphorylation to generate ATP (Kumar et al., 2019). In this context, iNKT1 cells resemble memory CD8+ T cells, which are similarly metabolically quiescent but poised for rapid activation (Sukumar et al., 2013). Raynor et al. (2020) found that mature iNKT1 cells, but not immature iNKT or mature iNKT2 and iNKT17 cells, display a transcriptional profile characterized by down-regulated proliferation and metabolic programs and enriched for naive/quiescence programs, which was mitigated by Mst1 deficiency. Consistent with these findings, iNKT1 cells displayed low mitochondrial mass and capacity and low levels of reactive oxygen species, which was overcome by Mst1 deficiency.
The findings reported by Raynor et al. (2020) raise a number of new questions. First, how is IL-15R signaling linked to Mst activity and downstream transcriptional programs? The heterotrimeric IL-15R complex signals through multiple pathways, including JAK-STAT to promote survival, Grb2-PI3K-Akt-mTORC to increase proliferation and survival, and Grb2-MAPK to enhance proliferation. Raynor et al. (2020) showed that Mst deficiency in iNKT cells resulted in impaired STAT5 phosphorylation, which might contribute to their reduced survival. Additionally, the phenotype of Mst-deficient iNKT cells might be related to defective autophagy, as a recent study showed that IL-15 elicits autophagy in iNKT cells by inducing Tbkbp1 (TBK-binding protein 1) to antagonize mTORC1. Consistent with this possibility, iNKT cells deficient in Tbkbp1, mTORC1, or autophagy factors exhibited defective iNKT cell development, predominantly involving iNKT1 cells (Yang et al., 2018). Additionally, mice lacking the mTOR signaling negative regulator Tsc1 (tuberous sclerosis 1) displayed defective iNKT1 and enhanced iNKT17 effector cell differentiation (Wu et al., 2014), similar to the phenotype seen in conditional Mst1-deficient mice. How these IL-15R signaling modules might be connected to Mst signaling remains to be explored. Second, in addition to the thymic iNKT cell subsets already discussed, other functional iNKT cell subsets that arise in the periphery have been identified (Iwabuchi and Van Kaer, 2019). IL-10–producing iNKT10 cells with regulatory properties are enriched in adipose tissues and IL-21–producing iNKTFH cells can provide help to B cells in germinal centers. The differentiation of these additional effector iNKT cell subsets remains incompletely understood. The finding that Mst signaling modulates the polarization of regulatory T and Th17 cells (Hong et al., 2018) raises the possibility that this pathway similarly influences the generation of iNKT10 and iNKTFH cells. Third, how Mst signaling modulates iNKT cell–mediated effector functions and diseases remains to be investigated. Fourth, in addition to its effects on iNKT cells, IL-15 plays an important role in the development and/or survival of memory CD8+ T cells, natural killer cells, and a subset of intestinal intraepithelial lymphocytes (Castillo and Schluns, 2012). Considering the contribution of Mst activity to IL-15R signaling, these findings raise the possibility that Mst signaling is involved in the development and function of additional lymphocyte subsets. Finally, the reported findings raise the question of whether the Hippo signaling pathway can be manipulated to optimize therapeutic iNKT cell targeting in human diseases.
References
- Castillo, E.F., and Schluns K.S.. 2012. Cytokine. 10.1016/j.cyto.2012.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordy, L.E., et al. 2011. J. Immunol. 187:6335–6345. 10.4049/jimmunol.1003965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogquist, K., and Georgiev H.. 2020. F1000 Res. 10.12688/f1000research.21378.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, L., et al. 2018. Cell. Mol. Immunol. 10.1038/s41423-018-0007-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabuchi, K., and Van Kaer L.. 2019. Front. Immunol. 10.3389/fimmu.2019.01837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krovi, S.H., and Gapin L.. 2018. Front. Immunol. 10.3389/fimmu.2018.01393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, A., et al. 2019. Proc. Natl. Acad. Sci. USA. 10.1073/pnas.1901376116 [DOI] [Google Scholar]
- Ma, S., et al. 2019. Annu. Rev. Biochem. 10.1146/annurev-biochem-013118-111829 [DOI] [PubMed] [Google Scholar]
- Raynor, J.L., et al. 2020. J. Exp. Med. 10.1084/jem.20191157 [DOI] [Google Scholar]
- Salio, M., et al. 2014. Proc. Natl. Acad. Sci. USA. 10.1073/pnas.1413935112 [DOI] [Google Scholar]
- Sukumar, M., et al. 2013. J. Clin. Invest. 10.1172/JCI69589 [DOI] [Google Scholar]
- Tuttle, K.D., et al. 2018. Nat. Commun. 10.1038/s41467-018-05026-6 [DOI] [Google Scholar]
- Wu, J., et al. 2014. J. Clin. Invest. 10.1172/JCI69780 [DOI] [Google Scholar]
- Yang, G., et al. 2018. Front. Immunol. 10.3389/fimmu.2018.02653 [DOI] [Google Scholar]
- Zhao, M., et al. 2018. Nat. Commun. 10.1038/s41467-018-05095-7 [DOI] [Google Scholar]