Abstract
Background
Immunosuppression is a known risk factor for anal human papillomavirus (HPV) disease, including anal squamous cell carcinoma. Additional risk factors for HPV-related disease have not been studied in the renal transplant population. The demographics of anal HPV and associated risk factors were investigated in this population.
Methods
Anal cytology and polymerase chain reaction were used to assess anal HPV disease in a cohort of transplant recipients at the Royal London Hospital. Risk factors associated with increased immunosuppression and HPV exposure were collated to determine any association with anal disease.
Results
Anal dysplasia was associated with anal oncogenic HPV infection (P < 0·001), duration of immunosuppression (P = 0·050), previous genital warts (P = 0·018) and receptive anal intercourse (P = 0·013).
Conclusion
Anal dysplasia was related to immunosuppression and patient factors in this cohort.
Low prevalence in at-risk group
Introduction
Anal cancer (anal squamous cell carcinoma, ASCC) is a rare malignancy of the gastrointestinal tract, which accounts for fewer than 1·5 per cent of gastrointestinal tumours and has an age-adjusted incidence of 1·4–2 per 100 000. It is, however, a significant disease in high-risk populations such as men who practise anal intercourse and immunosuppressed individuals, including those infected with human immunodeficiency virus (HIV) and transplant recipients1. Epidemiological studies performed before the acquired immune deficiency syndrome epidemic established that homosexual men were 33 times more likely to develop anal cancer than the general population; this risk doubles to 66 in HIV-positive homosexual patients2. Immunosuppression is a recognized risk factor for ASCC and precancerous lesions (anal intraepithelial neoplasia, AIN). Consequently, organ transplant recipients requiring long periods of immunosuppression may be at increased risk of anal lesions. A large study of 5931 transplant recipients from the Swedish Cancer Registry estimated the excess risk of anal cancer to be tenfold compared with the general population3, whereas a similar study from Denmark proposed an excess risk of 14·4 in 5113 transplant recipients4.
Immunosuppression is a strong risk factor for human papillomavirus (HPV) infection, and HPV is the aetiological agent for AIN and ASCC in both immunocompetent and immunosuppressed individuals5. AIN was reported in 20·3 per cent of renal transplant recipients in 1994 (27 of 133; AIN grade I, 20; AIN II, 3; AIN III, 4)6 and in 18 per cent (7 of 40) in 20087. Malignant progression from high-grade AIN (AIN III) to ASCC was reported in 9 per cent (3 of 35) and 11 per cent (8 of 72) of other cohorts8,9, and was more likely in the presence of immunosuppression. The prevalence of anal high-risk HPV infection was 47 per cent (36 of 76) in the renal transplant cohort from 19946 and 9 per cent (4 of 43) in 200410. The latter study examined transplant recipients within 24 h of transplantation and reduced HPV infection was to be expected owing to the short duration of immunosuppressant therapy. It is difficult to compare these two studies and further investigation of the demographics of anal HPV infection in immunosuppressed patients is required.
Screening programmes for AIN were initiated in the 1990s after large observational studies of HIV-positive patients and homosexual men revealed a high prevalence of AIN and oncogenic anal HPV infection11. In this context, anal cytology is the preferred test for detection of AIN (anal dyskaryosis or anal predicted pathological dysplasia) and has 60–90 per cent sensitivity for anal lesions of any grade but only modest discrimination between grades of AIN12,13. Cost-effectiveness has been demonstrated for high-risk men and is comparable to that of cervical cancer screening14.
The deficit of knowledge regarding risk factors for HPV-related anal disease in transplant recipients was addressed in the present single-centre investigation of patients with a fairly uniform immunosuppressive regimen. The main objectives were to determine the prevalence of anal dysplasia and anal HPV infection in renal transplant recipients. Additional risk factors for HPV infection were identified and the perianal region was sampled to determine the presence of HPV infection as a regional field effect.
Methods
Renal transplant recipients were recruited from the Renal Transplant Department at the Royal London Hospital between December 2004 and August 2006. A single renal centre was chosen for this study to minimize differences in immunosuppressive regimens among patients. Patients less than 18 years of age were excluded and ethics approval confirmed. All patients were approached in a consecutive manner during an outpatient appointment without previous knowledge of transplant history and co-morbidities. Patients who declined involvement in the study were asked to give their reasons. Data on risk factors for anogenital disease, including history of cutaneous or genital warts, coitarche, number of sexual partners and previous receptive anal intercourse, were collected. Indices of immunosuppression such as previous rejection episodes and malignancies were also noted.
The perianal region was inspected and an anal smear taken. A Dacron® swab (Medical Wire & Equipment, Corsham, UK) was inserted into the anal canal to a depth of more than 4 cm to sample the anal canal and anal transition zone; this was placed into PreservCyt ThinPrep® solution (Hologic UK, Crawley, UK) for processing. Between five and ten pubic hairs were plucked from the perianal region. Consenting women also underwent a standard cervical smear.
Sample processing
Anal and cervical liquid-based samples were processed on a ThinPrep® 2000 Processor (Hologic UK) under the ‘gynae’ setting for cervical and ‘mucoid’ setting for anal specimens. Adequacy for anal samples was based on a semiquantitative scoring system for overall cellularity (0 to + + +) along with presence or absence of transitional cells. The presence of dyskaryosis was assessed and graded according to the British Society of Clinical Cytologists' guidelines (http://www.clinicalcytology.co.uk). There was less than 5 per cent interobserver variation. Smear results also indicated whether endocervical or metaplastic cells were present to indicate sampling of the cervical transformation zone and anal transition zone respectively.
DNA extraction
Samples were processed according to the tissue protocol for the QIAamp DNA mini kit (Qiagen, Valencia, California, USA). DNA was extracted from pubic hair samples by first cutting the hair shaft at the follicle and then immersing follicles in a solution of 180 µl Automated Tissue Lysis buffer and 40 µl 20 per cent proteinase K. Samples were then processed as above and extracted DNA was stored at − 20 °C.
Human papillomavirus analysis
DNA from anal and cervical samples and pubic hair was subjected to polymerase chain reaction (PCR) with the MY09/11 primer set15. Positive control samples of HPV plasmid DNA were included in each PCR plate as were five negative controls of PCR-grade water in random wells. HPV-positive samples were sequenced on an ABI 3100 analyser (Applied Biosystems, Warrington, UK) after purification with ExoSAP-It® (Affymetrix UK, High Wycombe, UK). Sequences were examined with Four Peaks software (Four Peaks Software Developers, Owatonna, Minnesota, USA) and the NCBI Blastn (nucleotide) search program (http://blast.ncbi.nlm.nih.gov/Blast.cgi?CMD=Web&PAGE_TYPE=BlastHome) to identify the HPV type.
Statistical analysis
Patient demographics were collected, including indices of immunosuppression and sexual characteristics, to determine any association with anal HPV infection or anal dyskaryosis. Statistical analyses included Fisher's exact test (two-tailed tests only) and logistic regression analysis. Logistic regression was performed using Stata® version 7 software (StataCorp, College Station, Texas, USA) after a goodness-of-fit test had ensured that the data were compatible for this form of analysis. Test variables were examined against anal dysplasia and anal HPV infection. P < 0·050 was considered significant.
Results
Of 189 patients approached (116 men and 73 women), 81 declined to participate in the study for various reasons (Table 1). A total of 108 patients agreed to participate (57·1 per cent; 68 men and 40 women). Mean age at the time of transplantation was 51·8 years. Data on several parameters were missing for a few patients. Thirteen (12·4 per cent) of 105 patients were current smokers, 24 (22·9 per cent) were ex-smokers and 68 (64·8 per cent) non-smokers.
Table 1.
Reasons for refusal to participate in study
| No. of patients | |
|---|---|
| Examination too invasive | 46 (57) |
| Patient unwell | 12 (15) |
| Unable to contact | 8 (10) |
| Did not attend appointment | 6 (7) |
| Transplant failure | 3 (4) |
| Poor mobility | 3 (4) |
| Existing benign anal disease | 2 (2) |
| No free time | 1 (1) |
Values in parentheses are percentages.
The main index of immunosuppression was the length of time since transplantation, which was a mean of 92·8 (median 75, range 1–381) months. The more frequently employed immunosuppressants were ciclosporin, prednisolone and mycophenolate mofetil, which was prescribed in 42 (38·9 per cent) of the 108 patients. A previous rejection reaction had occurred in 32 patients (29·6 per cent). Immunosuppression-related malignancies had developed previously in 26 patients (24·1 per cent), including non-anal squamous cell carcinoma (16), basal cell carcinoma (8), Kaposi's sarcoma (1) and lymphoproliferative disease (1).
Only five patients (4·6 per cent) had engaged in receptive anal intercourse; all were women who described this practice as infrequent and had had fewer than five partners. Nine patients (8·3 per cent; 6 men and 3 women) reported a previous genital wart infection and 65 (60·2 per cent; 43 men and 22 women) had had cutaneous warts. Five patients (4·6 per cent; 3 men and 2 women) recalled a previous sexually transmitted infection (STI)—one case of herpes, two of gonorrhoea and two not specified.
Anal dysplasia and human papillomavirus infection
Anal cytology demonstrated HPV-related borderline changes in 3·8 per cent of samples (4 of 104) and dysplastic changes in 5·8 per cent (6 of 104; 2 mild dyskaryosis predicting AIN I, 1 moderate dyskaryosis predicting AIN II and 3 severe dyskaryosis predicting AIN III). Four samples were reported as inadequate because of lack of transitional cells and low cellularity. All patients with anal dysplasia (dyskaryosis) were referred to a colorectal surgeon at the Royal London Hospital at the end of the study for 6-monthly or annual anoscopy.
HPV PCR on anal smears (Fig. 1) revealed HPV DNA in 23 (21·3 per cent) of 108 patients. HPV typing was possible in 22 of 23 samples; a wide variety of HPV types was identified, including high-risk oncogenic HPV types (16, 18, 53, 61 and 67) in ten of the 22 positive samples and in 9·3 per cent of the whole group (Table 2). HPV 6 was the most prevalent type (5 of 22) and cutaneous HPV types were present in seven samples (Table 2). Seven of the ten abnormal anal cytology results were positive for mucosal HPV DNA, five with high-risk types 16, 18, 61 and 67, and two with the low-risk type 6. A strong association was seen between the presence of anal dysplasia and oncogenic HPV infection (P < 0·001) (Table 3).
Figure 1.
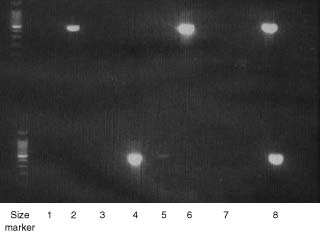
Representative example of human papillomavirus (HPV) polymerase chain reaction (PCR) of anal smears with MY09/11 primer. Lanes 1–6, extracted DNA; lane 7, negative control (PCR-grade water); lane 8, positive control (HPV 18, 450 base pairs). DNA size ladder is shown on the left (brightest band is 500 base pairs)
Table 2.
Prevalence of human papillomavirus types in anal smears
High-risk mucosal human papillomavirus (HPV) types;
non-mucosal epidermodysplasia verriciformis HPV types. With the exception of HPV 6, the remainder are cutaneous HPV types.
Table 3.
Association between oncogenic anal human papillomavirus infection and anal dysplasia
| Anal dysplasia | ||
|---|---|---|
| Oncogenic anal HPV infection | Yes | No |
| Present | 5 | 5 |
| Absent | 5 | 93 |
HPV, human papillomavirus. P < 0·001 (Fisher's two-tailed exact test).
Human papillomavirus infection in additional perineal sites
Twenty-six of 40 women had cervical examination and all samples were either normal (23) or inadequate (3). HPV DNA was present in six of 26 and half of the positive samples harboured high-risk mucosal HPV types (18, 33 and 53). The remaining three were cutaneous (HPV 62 and SIBX3) and low-risk mucosal (HPV 6). There was no association between anal dysplasia or anal HPV infection and cervical HPV infection (P = 0·628). HPV DNA was identified in 12 (18 per cent) of 68 pubic hair samples and HPV typing revealed three mucosal HPV types (HPV 6, 16 and 61) and six cutaneous types (HPV 17, 22, 84 and SIBX3). There was no association between anal HPV infection and a positive HPV pubic hair result (P = 0·074).
Risk factors for anal human papillomavirus disease
The median duration of immunosuppression differed significantly between patients with and without anal dysplasia (P = 0·050) (Table 4). Similarly, patients with anal HPV infection had a longer duration of immunosuppression than those with no evidence of infection (P = 0·058).
Table 4.
Duration of immunosuppression
| No. of patients | Duration of immunosuppression (months)* | |
|---|---|---|
| Anal HPV infection | 23 | 131 (10–294)† |
| No HPV infection | 84 | 60 (1–381) |
| Anal dysplasia | 10 | 135 (33–223)‡ |
| No dysplasia | 94 | 72 (1–381) |
| Whole group | 107 | 75 (1–381) |
Values are median (range).
P = 0·058 for presence versus absence of human papillomavirus (HPV) infection;
P = 0·050 for anal dysplasia versus no dysplasia (two-sample Mann–Whitney U test).
Regression analysis for risk factors associated with anal dysplasia identified receptive anal intercourse (P = 0·013) and genital warts (P = 0·018) as significant factors (Table 5). Significant risk factors for anal HPV infection were receptive anal intercourse (P = 0·030), genital warts (P = 0·010), previous transplant rejection (P = 0·048) and multiple sexual partners (P = 0·021) (Table 6). A history of cutaneous warts or smoking did not increase risk of acquiring anal HPV infection. Duration of immunosuppression was not included in regression analysis as continuous variables are better assessed by Mann–Whitney testing.
Table 5.
Results of regression analysis to identify risk factors for anal dysplasia
| Odds ratio | P | |
|---|---|---|
| Female patient | 0·32 (0·04, 2·45) | 0·271 |
| Receptive anal intercourse | 56·36 (2·35, 1348·91) | 0·013 |
| Genital warts | 19·96 (1·67, 239·16) | 0·018 |
| Cutaneous warts | 4·01 (0·39, 41·10) | 0·242 |
| Previous STI | 12·46 (0·39, 400·55) | 0·154 |
| Previous rejection | 3·09 (0·53, 17·92) | 0·208 |
| Ex-smoker | 1·70 (0·06, 44·64) | 0·750 |
| Sexual partners > 5 | 0·17 (0·01, 2·61) | 0·205 |
Values in parentheses are 95 per cent confidence intervals. STI, sexually transmitted infection.
Table 6.
Results of regression analysis to identify risk factors for anal human papillomavirus infection
| Odds ratio | P | |
|---|---|---|
| Female patient | 4·08 (0·80, 20·89) | 0·091 |
| Receptive anal sex | 18·11 (1·33, 245·86) | 0·030 |
| Genital warts | 12·90 (1·83, 90·80) | 0·010 |
| Cutaneous warts | 2·50 (0·70, 8·98) | 0·159 |
| Previous STI | 0·23 (0·00, 11·85) | 0·463 |
| Previous rejection | 3·88 (1·01, 14·89) | 0·048 |
| Ex-smoker | 7·03 (0·56, 87·58) | 0·130 |
| Sexual partners > 5 | 10·14 (1·41, 72·87) | 0·021 |
Values in parentheses are 95 per cent confidence intervals. STI, sexually transmitted infection.
Discussion
There was a relatively high rate of non-participation in this study, mainly because of the invasive nature of anal examination. Anal dysplasia or dyskaryosis was detected in 9·6 per cent, half the prevalence reported in a previous study using random biopsies of all transplant recipients in 19946. Anal cytology was a robust method of sampling the anal transition zone in the present study, and has a sensitivity of between 60 and 90 per cent for dysplasia12,13. Histological specimens were not collected from patients with dysplasia as anal cytology results became available only at the end of the study period. Random biopsies were not performed on all patients. It should be stressed that no particular investigation has been validated for anal cancer screening but most studies on prevalence of AIN in high-risk groups use a combination of anal cytology and high-resolution anoscopy-guided biopsy. Anal cytology is also repeated 3 or 6 monthly in follow-up studies, but this was not possible in the present study owing to limited human resources and time.
A strong correlation was seen between anal dyskaryosis and oncogenic anal HPV infection, supporting a causal link. The lack of association between anal and cervical HPV infection was probably due to a type II error. No association was found between anal HPV infection and pubic hair follicle HPV infection. This is the first report of cutaneous HPV infection in the anal canal, cervix and perineal region of renal transplant recipients, and may indicate a change in the spectrum of HPV types that infect the region. The significance of non-mucosal types in the anal canal is uncertain; they may arise as a result of iatrogenic immunosuppression16. Importantly, a substantial proportion of the group had evidence of infection with a genital HPV type that could progress to anal cancer.
The duration of immunosuppression was longer in patients with anal HPV infection and in those with AIN. This suggests an increase in risk of anal HPV-associated disease with protracted immunosuppression. The mean duration of immunosuppression in patients with anal disease was approximately 5 years, in keeping with the known peak incidence of anogenital neoplasia of between 5 and 10 years6,17. This time interval is probably more than sufficient for establishment of HPV infection and neoplastic transformation. A relationship was demonstrated between anal HPV infection, dysplasia and previous genital warts, but not for remote cutaneous HPV infection (warts). This is expected as mucosal HPV viruses (not cutaneous types) cause anogenital disease. The numbers are too small for firm conclusions. Factors that might increase the risk of HPV infection, such as early coitarche, smoking, previous STI and number of sexual partners, were also examined, but were not associated with the presence of dysplasia or anal HPV infection.
There was a low prevalence of anal dysplasia and oncogenic anal HPV infection in the study population. Even though the relative risk of anal cancer in transplant recipients (10–14)3,4 is increased compared with the general population, the case for screening transplant recipients for this condition is weak. The natural history of the progression from AIN to invasive malignancy remains unclear, although it appears to be a less aggressive process than in cervical neoplasia. There are no randomized data on the effect of cytological screening on survival from anal cancer in transplant recipients or any other group. Any such trial would have to be very large, and therefore could not be mounted in practice owing to the small at-risk population and the rarity of the target lesion. There are no clear strategies for treating AIN and investigation of potential therapeutic agents has been limited. This question also arises in those immunocompromised by HIV infection, and has given rise to animated debate. Stringent criteria need to be applied before screening should be adopted in any at-risk group18.
Acknowledgements
The Renal Institute, Royal London Hospital, and the Colorectal Cancer Unit, St Mark's Hospital, are acknowledged for assistance in patient recruitment and sample processing respectively. This study was funded by Cancer Research UK. The authors declare no conflict of interest.
References
- 1. Clark MA, Hartley A, Geh JI. Cancer of the anal canal. Lancet Oncol 2004; 5: 149–157. [DOI] [PubMed] [Google Scholar]
- 2. Chin-Hong PV, Vittinghoff E, Cranston RD, Browne L, Buchbinder S, Colfax G et al. Age-related prevalence of anal cancer precursors in homosexual men: the EXPLORE study. J Natl Cancer Inst. 2005; 97: 896–905. [DOI] [PubMed] [Google Scholar]
- 3. Adami J, Gäbel H, Lindelöf B, Ekström K, Rydh B, Glimelius B et al. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer 2003; 89: 1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sunesen KG, Nørgaard M, Thorlacius-Ussing O, Laurberg S. Immunosuppressive disorders and risk of anal squamous cell carcinoma: a nationwide cohort study in Denmark, 1978–2005. Int J Cancer 2009; 127: 675–684. [DOI] [PubMed] [Google Scholar]
- 5. Laytragoon-Lewin N, Nilsson PJ, Castro J, Gharizadeh B, Nyren P, Glimelius B et al. Human papillomavirus (HPV), DNA aberrations and cell cycle progression in anal squamous cell carcinoma patients. Anticancer Res 2007; 27: 4473–4479. [PubMed] [Google Scholar]
- 6. Ogunbiyi OA, Scholefield JH, Raftery AT, Smith JH, Duffy S, Sharp F et al. Prevalence of anal human papillomavirus infection and intraepithelial neoplasia in renal allograft recipients. Br J Surg 1994; 81: 365–367. [DOI] [PubMed] [Google Scholar]
- 7. Ogilvie JW, Park IU, Downs LS, Anderson KE, Hansberger J, Madoff RD. Anal dysplasia in kidney transplant recipients. J Am Coll Surg 2008; 207: 914–921. [DOI] [PubMed] [Google Scholar]
- 8. Scholefield JH, Castle MT, Watson NF. Malignant transformation of high-grade anal intraepithelial neoplasia. Br J Surg 2005; 92: 1133–1136. [DOI] [PubMed] [Google Scholar]
- 9. Watson AJ, Smith BB, Whitehead MR, Sykes PH, Frizelle FA. Malignant progression of anal intra-epithelial neoplasia. ANZ J Surg 2006; 76: 715–717. [DOI] [PubMed] [Google Scholar]
- 10. Roka S, Rasoul-Rockenschaub S, Roka J, Kirnbauer R, Mühlbacher F, Salat A. Prevalence of anal HPV infection in solid-organ transplant patients prior to immunosuppression. Transpl Int 2004; 17: 366–369. [DOI] [PubMed] [Google Scholar]
- 11. Wilkin TJ, Palmer S, Brudney KF, Chiasson MA, Wright TC. Anal intraepithelial neoplasia in heterosexual and homosexual HIV-positive men with access to antiretroviral therapy. J Infect Dis 2004; 190: 1685–1691. [DOI] [PubMed] [Google Scholar]
- 12. Nahas CS, da Silva Filho EV, Segurado AA, Genevcius RF, Gerhard R, Gutierrez EB et al. Screening anal dysplasia in HIV-infected patients: is there an agreement between anal pap smear and high-resolution anoscopy-guided biopsy? Dis Colon Rectum 2009; 52: 1854–1860. [DOI] [PubMed] [Google Scholar]
- 13. Cranston RD, Hart SD, Gornbein JA, Hirschowitz SL, Cortina G, Moe AA. The prevalence, and predictive value, of abnormal anal cytology to diagnose anal dysplasia in a population of HIV-positive men who have sex with men. Int J STD AIDS 2007; 18: 77–80. [DOI] [PubMed] [Google Scholar]
- 14. Mathews WC. Screening for anal dysplasia associated with human papillomavirus. Top HIV Med 2003; 11: 45–49. [PubMed] [Google Scholar]
- 15. Milutin Gasperov N, Sabol I, Matovina M, Spaventi S, Grce M. Detection and typing of human papillomaviruses combining different methods: polymerase chain reaction, restriction fragment length polymorphism, line probe assay and sequencing. Pathol Oncol Res 2008; 14: 355–363. [DOI] [PubMed] [Google Scholar]
- 16. Porro AM, Alchorne MM, Mota GR, Michalany N, Pignatari AC, Souza IE. Detection and typing of human papillomavirus in cutaneous warts of patients infected with human immunodeficiency virus type 1. Br J Dermatol 2003; 149: 1192–1199. [DOI] [PubMed] [Google Scholar]
- 17. Penn I. Cancers of the anogenital region in renal transplant recipients. Analysis of 65 cases. Cancer 1986; 58: 611–616. [DOI] [PubMed] [Google Scholar]
- 18. Chiao EY, Giordano TP, Palefsky JM, Tyring S, El Serag H. Screening HIV-infected individuals for anal cancer precursor lesions: a systematic review. Clin Infect Dis 2006; 43: 223–233. [DOI] [PubMed] [Google Scholar]


