Abstract
TAM family tyrosine kinase receptors including Tyro3, Axl, and MerTK are the key efferocytosis receptors presenting on antigen-presenting cell that mediate the clearance of apoptotic cells. They are thought to regulate inflammatory diseases by modulating inflammatory response and efferocytosis. Recent studies have revealed novel roles of TAM receptors in the biosynthesis of specialized pro-resolving mediators (SPMs) and inflammation resolution. In this chapter, we discuss the central roles of TAM signaling in atherosclerosis focusing on their regulation in efferocytosis and inflammation resolution and highlight the unique therapeutic potential of SPMs in blocking the progression of atherosclerosis.
1. Introduction
Atherosclerosis, which is driven by lipoprotein disease, is characterized by non-resolving chronic inflammatory of the arterial intima and activation of both the innate and adaptive immune system. Atherosclerosis is the underlying cause of fatal heart attacks and strokes, which are responsible for the majority of deaths in industrialized countries (Lusis, 2000). Many atherosclerosis studies using different genetically modified or pharmacologically intervened mouse models and clinical data have demonstrated strong evidence that immune responses have a role in the development of atherosclerosis (Hansson et al., 2015).
In addition to established molecules affecting immune responses,
TAM family tyrosine kinase receptors including Tyro3, Axl, and MerTK have been shown to influence the immune system in the steady state and pathology of atherosclerosis. Accordingly, a deeper understanding of how TAM receptors induced cellular mechanisms in pathology of atherosclerosis may increase investigator focus on them as a therapeutic target. The TAM family tyrosine kinase receptors contain an extracellular N-terminal region carrying two immunoglobulin (Ig)-like domains, which mediate ligand’s binding, followed by two fibronectin type III (FNIII) repeats, a hydrophobic transmembrane domain, and an intracellular tyrosine kinase C-terminal domain (Lemke, 2013; Rothlin et al., 2015). The activation of TAM receptors is initiated by binding to their ligands: vitamin K-mediated γ-carboxylated Growth Arrest-Specific gene 6 (Gas6) (Geng et al., 2017) and Protein S (ProS1) (Nagata et al., 1996; Stitt et al., 1995). However, these ligands bind to their receptors with different affinities. Gas6 can engage with all the receptors with strongest affinity to Axl, then Tyro3, and with lower affinity to MerTK (Axl>Tyro3≫Mer) (Nagata et al., 1996). Conversely, Pros1 does not bind to Axl, and has stronger affinity binding with Tyro3 than MerTK (Lew et al., 2014; Zagorska et al., 2014). The TAM receptors promote efferocytosis, a process of apoptotic cell (AC) clearance, by binding to their ligands that serve as the bridging molecules that bind externalized phosphatidylserine on ACs (Geng et al., 2017; Lemke and Burstyn-Cohen, 2010). However, these receptors are expressed in myeloid efferocytes differently: MerTK is highly expressed in macrophages (Mϕs), while Axl and Tyro3 are prominently expressed in dendritic cells (Zagorska et al., 2014). TAM-mediated efferocytosis elicits the production of immunosuppressive cytokines and drives immune evasion by inhibiting T-cell priming and activation. Hence, pan-inhibition of TAM receptors have been suggested as cancer therapeutics to promote an inflammatory tumor microenvironment and improve host antitumor immunity (Davra et al., 2016; Kasikara et al., 2017, 2019; Kimani et al., 2016). While the role of TAM receptors has long been recognized in AC clearance and consequent regulation of immune response (Lemke and Burstyn-Cohen, 2010; Lemke and Rothlin, 2008; Rothlin et al., 2007), recent work by a number of groups has also identified defects in TAM-induced efferocytosis and inflammation resolution as key causal factors for atherosclerosis progression (Ait-Oufella et al., 2008; Cai et al., 2016, 2017, 2018). Here, we present an overview of how dysfunction of TAM receptors drives atherosclerosis and discuss the therapeutic implications of this concept.
2. Defective efferocytosis and inflammation resolution in atherosclerosis
2.1. Defective efferocytosis in atherosclerosis
Atherosclerosis begins with the retention of apolipoprotein B (apoB)-containing lipoproteins within the subendothelial layer of arteries. These subendothelial lipoproteins, particularly after oxidation, generate an inflammatory stimulus that drives leukocyte influx into the vessel wall (Williams and Tabas, 1995, 1998). These infiltrating cells are primarily monocyte-derived Mϕs, which internalize cholesterol-rich lipoproteins and give rise to foam cells. Foam cells secrete an extracellular matrix that further promotes lipoprotein retention as well as pro-inflammatory cytokines that increase the recruitment of additional monocytes, T cells, and neutrophils. Because of these sustained inflammatory stimuli and other cytotoxic factors, many lesional cells become apoptotic. In early atherosclerotic lesions, these lesional ACs are cleared by neighboring macrophages in order to limit lesion cellularity (Tabas, 2005). However, in advanced atherosclerosis, efferocytosis becomes defective and the ACs cannot be cleared up efficiently, leading to the accumulation of secondarily necrotic cells and the formation of a highly inflammatory “necrotic core” (Linton et al., 2016; Schrijvers et al., 2005; Tabas, 2010). Large necrotic cores are a hallmark of advanced atherosclerotic disease that can trigger plaque rupture and acute thrombotic cardiovascular events (Hansson et al., 2015; Yahagi et al., 2016). Therefore, the efficient clearance of dead cells plays an essential role in preventing the development of clinically significant atherosclerotic plaques.
2.2. Defective inflammation resolution in atherosclerosis
Inflammation resolution is an active process to counterbalance excessive inflammation and restore tissue homeostasis after injury without compromising host defense (Serhan, 2014). Failure of resolution contributes to the pathology of numerous chronic inflammatory diseases including atherosclerosis (Back et al., 2019). Inflammation resolution is mediated by bioactive lipids such as lipoxins, resolvins, protectins, and maresins, which are referred to as specialized pro-resolving mediators (SPMs) (Fredman et al., 2016; Serhan, 2014; Viola et al., 2016). These SPMs are derived from long-chain fatty acids, by lipoxygenase (LOX) enzymes such as 5-LOX and 12/15-LOX. The LOX enzymes promote the conversion of arachidonic acid (AA) to lipoxins, like LXA4, or docosahexaenoic acid (DHA) to resolvins, like RvD1 (Serhan et al., 1984, 2002). SPMs block inflammatory cell influx and promote the egress of inflammatory cells, serving to limit tissue damage and to promote tissue repair (Perretti and D’Acquisto, 2009; Serhan, 2010). Defective inflammation resolution is another hallmark of advanced atherosclerosis, which has been characterized by an imbalance between pro-resolving and pro-inflammatory mediators, such as leukotrienes (Fredman et al., 2016; Merched et al., 2008). The ratio of SPMs to leukotrienes is significantly decreased in advanced versus early atherosclerotic plaques in humans and mice (Fredman et al., 2016; Viola et al., 2016). The impaired resolution is often associated with the clinically dangerous features of plaques including defective efferocytosis, damage associated molecular pattern (DAMP)-mediated inflammation, formation of a necrotic core, and thinning of the protective collagen cap that overlies the core (Kojima et al., 2017; Kolodgie et al., 2004; Merched et al., 2008; Tabas, 2010). Consequently, there is evidence showing that administration of SPMs decreases plaque necrosis and inflammation (Fredman et al., 2016; Hasturk et al., 2015; Viola et al., 2016). For instance, RvD1 led to smaller necrotic cores, enhanced rates of lesional efferocytosis, and thicker collagen fibrous caps when administered to high-fat-fed Ldlr−/− mice (atherosclerosis mouse model) (Fredman et al., 2016). Similarly, administration of RvD5 to high-fat-fed Apoe−/− (atherosclerosis mouse model) mice decreased atherosclerotic lesion size and reduced leukocyte and platelet activation (Colas et al., 2018). Treatment of Apoe−/− mice with RvD2 and MaR1 also promoted plaque stability by decreasing lesional macrophage numbers, halting expansion of necrotic cores, and thickening collagen fibrous caps (Viola et al., 2016).
2.3. MerTK-mediated efferocytosis and inflammation resolution
As an important efferocytosis receptor in Mϕs, MerTK plays a protective role in atherosclerosis by limiting the formation of the inflammatory necrotic core (Ait-Oufella et al., 2008; Thorp et al., 2008). Both whole MerTK-deficient and bone marrow-transplanted mice (Mertk−/−/Apoe−/− and Mertk− bone marrow-transplanted-Ldlr−/−) had marked decreases in the clearance of apoptotic bodies and the subsequent accelerated plaque necrosis and inflammation in their advanced atherosclerotic lesions compared with the control mice (Ait-Oufella et al., 2008; Thorp et al., 2008). Consistent with the role of MerTK-mediated efferocytosis in atherosclerosis, Mϕ Ca2+/calmodulin-dependent protein kinase IIγ (CaMKIIγ) promoted the development of necrotic atherosclerotic plaques by preventing MerTK expression through the inhibition of the transcription factors ATF6 and LXRα (Doran et al., 2017). Similarly, the immunoproteasome subunit β5i was recently shown to decrease MerTK expression in Mϕs and enhance necrotic core area in atherosclerotic lesions (Liao et al., 2020). In terms of the mechanism of MerTK-mediated efferocytosis, MerTK binds apoptotic cells through the bridging molecules Gas6 or protein S and mediates their uptake via actin signaling pathways (Lemke and Burstyn-Cohen, 2010; Tibrewal et al., 2008). Axl, the other TAM receptor, has been shown to have no effect in atherosclerosis disease progression that transfer of Axl deficient bone marrow cells to Ldlr−/− mice did not affect the lesional efferocytosis, size of necrotic core and lesion compared to wild-type bone marrow-transplanted Ldlr−/− mice (Subramanian et al., 2016). Hence, this study may suggest that MerTK is a dominantly acting efferocytosis receptor during lesional AC clearance whereas Axl, prominently expressed on dendritic cells, does not have significant effect on efferocytosis.
Efferocytosis can stimulate SPM formation (Dalli and Serhan, 2012; Schwab et al., 2007) and consistent with this concept, we have elucidated a novel role for MerTK in SPM biosynthesis (Cai et al., 2016, 2017, 2018). MerTK activation with ligands or ACs stimulates SPM biosynthesis by promoting the cytoplasmic localization of nonphosphorylated 5-LOX (Cai et al., 2016, 2018) in Mϕs. Mechanistically, MerTK activates ERK in Mϕs, leading to an increase in the expression of sarcoplasmic/endoplasmic reticulum calcium ATPase 2 (SERCA2), which decreases the cytosolic Ca2+ concentration and suppresses the activity of CaMKII. This in turn reduces the activities of p38 MAP kinase and MAPKAP kinases MK2, resulting in the increased abundance of the nonphosphorylated, cytoplasmic form of 5-LOX and enhances SPM biosynthesis (Fig. 1) (Cai et al., 2018). Our in vivo studies showed that MerTK deficiency delayed the resolution of sterile peritonitis and hind limb ischemia-reperfusion (I/R)-induced lung injury by suppressing the biosynthesis of SPMs including lipoxins and resolvins (Cai et al., 2016).
Fig. 1.
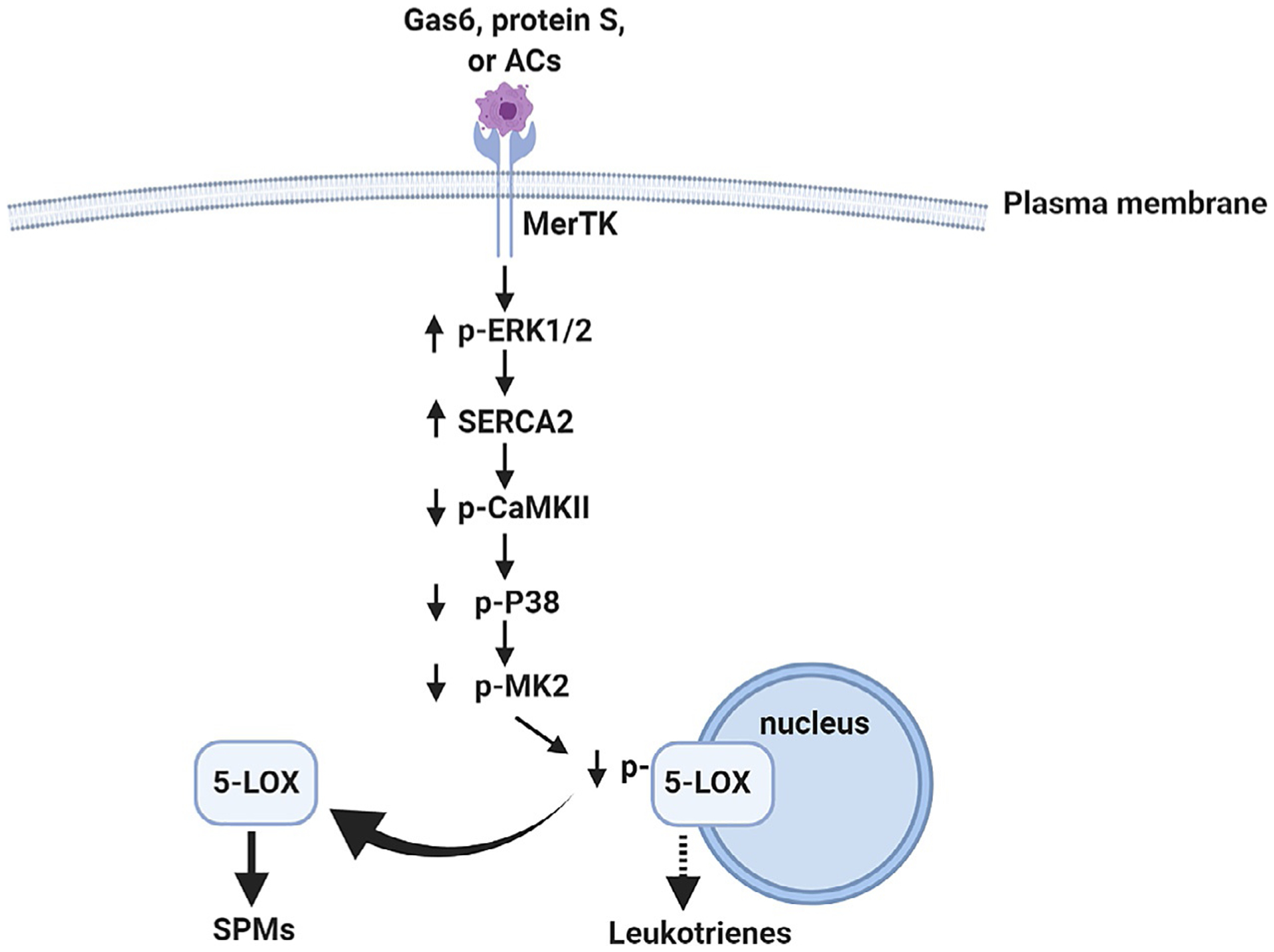
The signaling pathway of MerTK-mediated inflammation resolution. MerTK activation by Gas6, protein S, or apoptotic cells activates ERK, which is followed by the induction of SERCA2 expression. The decrease in the concentration of intracellular Ca2+ (Ca2+ i) caused by the increased SERCA abundance suppresses CaMKII activity, which then reduces the activities of the kinases p38 and MK2. The decrease in MK2 activity promotes the cytoplasmic localization of 5-LOX, leading to SPM production and inflammation resolution.
2.4. MerTK cleavage by ADAM17
MerTK is known to undergo ectodomain cleavage mediated by the metalloproteinase ADAM17 in the presence of inflammatory stimuli (Sather et al., 2007; Thorp et al., 2011). In the atherosclerotic plaques, the byproducts of polyunsaturated fatty acid oxidation and inflammatory mediators can activate ADAM17 (Garbin et al., 2013). Interestingly, Mϕs near the necrotic core of human atheromas have lower MerTK and higher ADAM17 than peripheral lesional Mϕs (Garbin et al., 2013). Therefore, MerTK cleavage may contribute to defective efferocytosis and failed inflammation resolution in advanced atherosclerosis. We recently showed that soluble Mer (sol-Mer), the product of MerTK cleavage from the human carotid artery was positively correlated with necrosis and that plaques of symptomatic patients had higher levels of sol-Mer compared with those of asymptomatic patients, while cell-surface MerTK in Mϕ-rich areas in plaques from symptomatic patients was less than that in asymptomatic patients (Cai et al., 2017). To test the role of MerTK cleavage in vivo, we first identified proline-485 as the MerTK cleavage site and showed that cells expressing MerTK lacking residues 483–488 maintained MerTK-dependent efferocytosis under cleavage-inducing conditions (Thorp et al., 2011), and then we created a mouse in which the Mertk locus was replaced by cleavage-resistant MertkΔ483–488 (MertkCR). As expected, MertkCR is protective in different mouse models: In the sterile peritonitis model, efferocytosis and inflammation resolution were improved in MertkCR mice, as indicated by the increased Mϕ-internalized apoptotic neutrophils and SPM levels in the exudates (Cai et al., 2016); In the I/R-induced lung injury model, MertkCR mice had increased circulating levels of SPMs and less lung injury (Cai et al., 2016); In the diet-induced atherosclerosis model, MertkCR mice exhibited improved efferocytosis, smaller necrotic cores, thicker fibrous caps, and increased ratio of pro-resolving versus pro-inflammatory lipid mediators in the atherosclerotic lesions (Fig. 2) (Cai et al., 2017). Consistent with our studies, MertkCR mice displayed improved levels of efferocytosis, reduced infarct size, and improved cardiac function following myocardial ischemia-reperfusion (DeBerge et al., 2017). Moreover, a recent study showed that preventing MerTK cleavage plays a beneficial role in aging as aged MertkCR mice restored senescence-reduced efferocytosis and increased the ratio of pro-resolving versus pro-inflammatory lipid mediators compared with the aged control mice (Rymut et al., 2020).
Fig. 2.
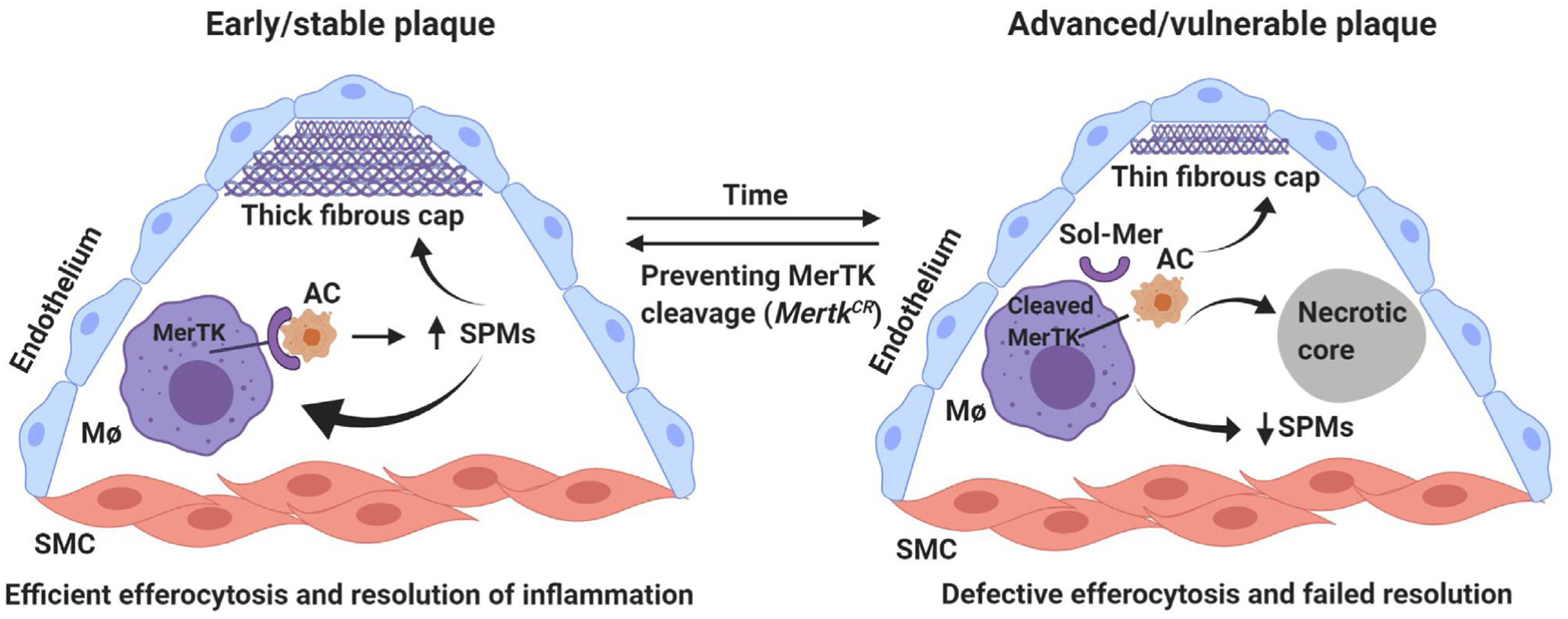
MerTK-mediated efferocytosis and inflammation resolution in atherosclerosis. In early plaque, intact MerTK promotes efferocytosis and SPM biosynthesis, favoring the formation of stable plaque. However, in advanced atherosclerosis, cleavage of MerTK results in decreased efferocytosis and decreased SPMs, leading to the formation of necrotic core and unstable plaque. SMC, smooth muscle cell; AC, apoptotic cell; Sol-Mer, soluble MerTK.
In addition to sol-Mer, increased soluble Axl (sol-Axl), the product of Axl cleavage has been detected in plasma of acute coronary syndrome patients compared to control individuals (Liu et al., 2015). Interestingly, sol-Axl associates with Gas6 levels in mouse serum (Budagian et al., 2005). These findings suggest that sol-Axl may neutralize Gas6 in serum and block its interaction to TAM receptors, especially to MerTK and decrease MerTK-AC interaction, consequently efferocytosis. As mentioned above, deficiency of myeloid Axl does not show any significant effect in the progression of atherosclerotic lesion, therefore sol-Axl may influence pathology of atherosclerosis not through Axl-mediated efferocytosis but through sol-Axl/Gas6/MerTK axis.
3. Conclusions and future directions
MerTK is fundamentally protective in cardiovascular diseases by facilitating the clearance of apoptotic cell debris and enhancing the inflammation resolution response. Therefore, enhancing MerTK synthesis or blocking its cleavage may represent novel therapeutic approaches to cardiovascular diseases. However, this approach must consider the possible adverse effects, given that MerTK is detrimental in cancer and pathologic liver fibrosis (Caetano et al., 2019; Cai et al., 2020; Petta et al., 2016). Future studies will be required to understand why MerTK signaling fails to stimulate resolution in non-atherosclerosis inflammatory diseases including in liver fibrosis.
Given that chronic inflammation plays an important role in the progression of atherosclerosis, therapeutic targeting of inflammation has been considered as a complementary strategy to LDL reduction for lowering the risk of atherosclerotic vascular disease. For instance, the CANTOS trial (Canakinumab Anti-inflammatory Thrombosis and Outcomes Study) has recently demonstrated the efficacy of targeting interleukin IL1β for reducing cardiovascular events in patients with a history of myocardial infarction independently of the role of lipids, highlighting the promise of therapy directed toward inflammation in cardiovascular disease (Ridker et al., 2011). However, the anti-inflammatory drug therapy has the potential to compromise host defense (Fallahi-Sichani et al., 2012; St Clair et al., 2004), and in CANTOS there was a significant increase in the number of fatal infections associated with canakinumab therapy (Ridker et al., 2017). Therefore, administration of exogenous SPMs that actively boost resolution without compromising host defense may present a novel promising therapeutic strategy for cardiovascular diseases. Indeed, some SPMs have already been tested in clinical trials for other chronic inflammatory diseases. For example, RvE1 analogue has been used to treat ocular inflammation and pain (ClinicalTrials.gov, NCT00799552 and NCT00941018) (Basil and Levy, 2016). LXA4 decreases LTC4−initiated bronchoprovocation in patients with asthma and reduces the severity of infantile eczema (Christie et al., 1992; Wu et al., 2013). Despite our understanding of the protective roles of SPMs in atherosclerosis, more mechanistic studies still need to be done to fully understand how SPMs are synthesized and metabolized and how they work on their effector cells to carry out the resolution processes.
Acknowledgments
This work was supported by NIH grant R00 DK115778 (to B.C.) and AHA Post-Doctoral Fellowship grant 20POST35210962 (to C.K.).
References
- Ait-Oufella H, Pouresmail V, Simon T, Blanc-Brude O, Kinugawa K, Merval R, Offenstadt G, Leseche G, Cohen PL, Tedgui A, Mallat Z, 2008. Defective mer receptor tyrosine kinase signaling in bone marrow cells promotes apoptotic cell accumulation and accelerates atherosclerosis. Arterioscler. Thromb. Vasc. Biol 28, 1429–1431. [DOI] [PubMed] [Google Scholar]
- Back M, Yurdagul A Jr., Tabas I, Oorni K, Kovanen PT, 2019. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat. Rev. Cardiol 16, 389–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basil MC, Levy BD, 2016. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat. Rev. Immunol 16, 51–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budagian V, Bulanova E, Orinska Z, Duitman E, Brandt K, Ludwig A, Hartmann D, Lemke G, Saftig P, Bulfone-Paus S, 2005. Soluble Axl is generated by ADAM10-dependent cleavage and associates with Gas6 in mouse serum. Mol. Cell. Biol 25, 9324–9339. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Caetano MS, Younes AI, Barsoumian HB, Quigley M, Menon H, Gao C, Spires T, Reilly TP, Cadena AP, Cushman TR, Schoenhals JE, Li A, Nguyen QN, Cortez MA, Welsh JW, 2019. Triple therapy with MerTK and PD1 inhibition plus radiotherapy promotes abscopal antitumor immune responses. Clin. Cancer Res 25, 7576–7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai B, Thorp EB, Doran AC, Subramanian M, Sansbury BE, Lin CS, Spite M, Fredman G, Tabas I, 2016. MerTK cleavage limits proresolving mediator biosynthesis and exacerbates tissue inflammation. Proc. Natl. Acad. Sci. U. S. A 113, 6526–6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai B, Thorp EB, Doran AC, Sansbury BE, Daemen MJ, Dorweiler B, Spite M, Fredman G, Tabas I, 2017. MerTK receptor cleavage promotes plaque necrosis and defective resolution in atherosclerosis. J. Clin. Invest 127, 564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai B, Kasikara C, Doran AC, Ramakrishnan R, Birge RB, Tabas I, 2018. MerTK signaling in macrophages promotes the synthesis of inflammation resolution mediators by suppressing CaMKII activity. Sci. Signal 11, eaar3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai B, Dongiovanni P, Corey KE, Wang X, Shmarakov IO, Zheng Z, Kasikara C, Davra V, Meroni M, Chung RT, Rothlin CV, Schwabe RF, Blaner WS, Birge RB, Valenti L, Tabas I, 2020. Macrophage MerTK promotes liver fibrosis in nonalcoholic steatohepatitis. Cell Metab. 31 (406–421), e407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie PE, Spur BW, Lee TH, 1992. The effects of lipoxin A4 on airway responses in asthmatic subjects. Am. Rev. Respir. Dis 145, 1281–1284. [DOI] [PubMed] [Google Scholar]
- Colas RA, Souza PR, Walker ME, Burton M, Zaslona Z, Curtis AM, Marques RM, Dalli J, 2018. Impaired production and diurnal regulation of vascular RvDn-3 DPA increase systemic inflammation and cardiovascular disease. Circ. Res 122, 855–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Serhan CN, 2012. Specific lipid mediator signatures of human phagocytes: micro-particles stimulate macrophage efferocytosis and pro-resolving mediators. Blood 120, e60–e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davra V, Kimani SG, Calianese D, Birge RB, 2016. Ligand activation of TAM family receptors-implications for tumor biology and therapeutic response. Cancer 8, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerge M, Yeap XY, Dehn S, Zhang S, Grigoryeva L, Misener S, Procissi D, Zhou X, Lee DC, Muller WA, Luo X, Rothlin C, Tabas I, Thorp EB, 2017. MerTK cleavage on resident cardiac macrophages compromises repair after myocardial ischemia reperfusion injury. Circ. Res 121, 930–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran AC, Ozcan L, Cai B, Zheng Z, Fredman G, Rymond CC, Dorweiler B, Sluimer JC, Hsieh J, Kuriakose G, Tall AR, Tabas I, 2017. CAMKIIgamma suppresses an efferocytosis pathway in macrophages and promotes atherosclerotic plaque necrosis. J. Clin. Invest 127, 4075–4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallahi-Sichani M, Flynn JL, Linderman JJ, Kirschner DE, 2012. Differential risk of tuberculosis reactivation among anti-TNF therapies is due to drug binding kinetics and permeability. J. Immunol 188, 3169–3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredman G, Hellmann J, Proto JD, Kuriakose G, Colas RA, Dorweiler B,Connolly ES, Solomon R, Jones DM, Heyer EJ, Spite M, Tabas I, 2016. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat. Commun 7, 12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbin U, Baggio E, Stranieri C, Pasini A, Manfro S, Mozzini C, Vallerio P, Lipari G, Merigo F, Guidi G, Cominacini L, Fratta Pasini A, 2013. Expansion of necrotic core and shedding of Mertk receptor in human carotid plaques: a role for oxidized polyunsaturated fatty acids? Cardiovasc. Res 97, 125–133. [DOI] [PubMed] [Google Scholar]
- Geng K, Kumar S, Kimani SG, Kholodovych V, Kasikara C, Mizuno K, Sandiford O, Rameshwar P, Kotenko SV, Birge RB, 2017. Requirement of gammacarboxyglutami acid modification and phosphatidylserine binding for the activation of Tyro3, Axl, and Mertk receptors by growth arrest-specific 6. Front. Immunol 8, 1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson GK, Libby P, Tabas I, 2015. Inflammation and plaque vulnerability. J. Intern. Med 278, 483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasturk H, Abdallah R, Kantarci A, Nguyen D, Giordano N, Hamilton J, Van Dyke TE, 2015. Resolvin E1 (RvE1) attenuates atherosclerotic plaque formation in diet and inflammation-induced atherogenesis. Arterioscler. Thromb. Vasc. Biol 35, 1123–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasikara C, Kumar S, Kimani S, Tsou WI, Geng K, Davra V, Sriram G, Devoe C, Nguyen KN, Antes A, Krantz A, Rymarczyk G, Wilczynski A, Empig C, Freimark B, Gray M, Schlunegger K, Hutchins J, Kotenko SV, Birge RB, 2017. Phosphatidylserine sensing by TAM receptors regulates AKT-dependent chemoresistance and PD-L1 expression. Mol. Cancer Res 15, 753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasikara C, Davra V, Calianese D, Geng K, Spires TE, Quigley M, Wichroski M, Sriram G, Suarez-Lopez L, Yaffe MB, Kotenko SV, De Lorenzo MS, Birge RB, 2019. Pan-TAM tyrosine kinase inhibitor BMS-777607 enhances anti-PD-1 mAb efficacy in a murine model of triple-negative breast cancer. Cancer Res. 79, 2669–2683. [DOI] [PubMed] [Google Scholar]
- Kimani SG, Kumar S, Davra V, Chang YJ, Kasikara C, Geng K, Tsou WI, Wang S, Hoque M, Bohac A, Lewis-Antes A, De Lorenzo MS, Kotenko SV, Birge RB, 2016. Normalization of TAM post-receptor signaling reveals a cell invasive signature for Axl tyrosine kinase. Cell Commun. Signal 14, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y, Weissman IL, Leeper NJ, 2017. The role of efferocytosis in atherosclerosis. Circulation 135, 476–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodgie FD, Virmani R, Burke AP, Farb A, Weber DK, Kutys R, Finn AV, Gold HK, 2004. Pathologic assessment of the vulnerable human coronary plaque. Heart 90, 1385–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke G, 2013. Biology of the TAM receptors. Cold Spring Harb. Perspect. Biol 5, a009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke G, Burstyn-Cohen T, 2010. TAM receptors and the clearance of apoptotic cells. Ann. N. Y. Acad. Sci 1209, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke G, Rothlin CV, 2008. Immunobiology of the TAM receptors. Nat. Rev. Immunol 8, 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew ED, Oh J, Burrola PG, Lax I, Zagorska A, Traves PG, Schlessinger J, Lemke G, 2014. Differential TAM receptor-ligand-phospholipid interactions delimit differential TAM bioactivities. eLife 3, e03385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J, Xie Y, Lin Q, Yang X, An X, Xia Y, Du J, Wang F, Li HH, 2020. Immunoproteasome subunit beta5i regulates diet-induced atherosclerosis through altering MERTK-mediated efferocytosis in Apoe knockout mice. J. Pathol 250, 275–287. [DOI] [PubMed] [Google Scholar]
- Linton MF, Babaev VR, Huang J, Linton EF, Tao H, Yancey PG, 2016. Macrophage apoptosis and efferocytosis in the pathogenesis of atherosclerosis. Circ. J 80, 2259–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YW, Yang QF, Zuo PY, Xiao CL, Chen XL, Liu CY, 2015. Elevated serum levels of soluble Axl in acute coronary syndrome. Am. J. Med. Sci 349, 124–129. [DOI] [PubMed] [Google Scholar]
- Lusis AJ, 2000. Atherosclerosis. Nature 407, 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L, 2008. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 22, 3595–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K, Ohashi K, Nakano T, Arita H, Zong C, Hanafusa H, Mizuno K, 1996. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, sky, and Mer receptor tyrosine kinases. J. Biol. Chem 271, 30022–30027. [DOI] [PubMed] [Google Scholar]
- Perretti M, D’Acquisto F, 2009. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat. Rev. Immunol 9, 62–70. [DOI] [PubMed] [Google Scholar]
- Petta S, Valenti L, Marra F, Grimaudo S, Tripodo C, Bugianesi E, Camma C, Cappon A, Di Marco V, Di Maira G, Dongiovanni P, Rametta R, Gulino A, Mozzi E, Orlando E, Maggioni M, Pipitone RM, Fargion S, Craxi A, 2016. MERTK rs4374383 polymorphism affects the severity of fibrosis in non-alcoholic fatty liver disease. J. Hepatol 64, 682–690. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Thuren T, Zalewski A, Libby P, 2011. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab anti-inflammatory thrombosis outcomes study (CANTOS). Am. Heart J 162, 597–605. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, Group CT, 2017. Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N. Engl. J. Med 377, 1119–1131. [DOI] [PubMed] [Google Scholar]
- Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G, 2007. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell 131, 1124–1136. [DOI] [PubMed] [Google Scholar]
- Rothlin CV, Carrera-Silva EA, Bosurgi L, Ghosh S, 2015. TAM receptor signaling in immune homeostasis. Annu. Rev. Immunol 33, 355–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymut N, Heinz J, Sadhu S, Hosseini Z, Riley CO, Marinello M, Maloney J, MacNamara KC, Spite M, Fredman G, 2020. Resolvin D1 promotes efferocytosis in aging by limiting senescent cell-induced MerTK cleavage. FASEB J 34, 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather S, Kenyon KD, Lefkowitz JB, Liang X, Varnum BC, Henson PM, Graham DK, 2007. A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood 109, 1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W, 2005. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler. Thromb. Vasc. Biol 25, 1256–1261. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Chiang N, Arita M, Serhan CN, 2007. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 447, 869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, 2010. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am. J. Pathol 177, 1576–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, 2014. Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Hamberg M, Samuelsson B, 1984. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc. Natl. Acad. Sci. U. S. A 81, 5335–5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL, 2002. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med 196, 1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair EW, van der Heijde DM, Smolen JS, Maini RN, Bathon JM, Emery P, Keystone E, Schiff M, Kalden JR, Wang B, Dewoody K, Weiss R, Baker D, Active-Controlled Study of Patients Receiving Infliximab for the Treatment of Rheumatoid Arthritis of Early Onset Study Group, 2004. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum. 50, 3432–3443. [DOI] [PubMed] [Google Scholar]
- Stitt TN, Conn G, Gore M, Lai C, Bruno J, Radziejewski C, Mattsson K, Fisher J, Gies DR, Jones PF, et al. , 1995. The anticoagulation factor protein S and its relative, Gas6, are ligands for the tyro 3/Axl family of receptor tyrosine kinases. Cell 80, 661–670. [DOI] [PubMed] [Google Scholar]
- Subramanian M, Proto JD, Matsushima GK, Tabas I, 2016. Deficiency of AXL in bone marrow-derived cells does not affect advanced atherosclerotic lesion progression. Sci. Rep 6, 39111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I, 2005. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler. Thromb. Vasc. Biol 25, 2255–2264. [DOI] [PubMed] [Google Scholar]
- Tabas I, 2010. Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol 10, 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I, 2008. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe−/− mice. Arterioscler. Thromb. Vasc. Biol 28, 1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorp E, Vaisar T, Subramanian M, Mautner L, Blobel C, Tabas I, 2011. Shedding of the Mer tyrosine kinase receptor is mediated by ADAM17 protein through a pathway involving reactive oxygen species, protein kinase Cdelta, and p38 mitogen-activated protein kinase (MAPK). J. Biol. Chem 286, 33335–33344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibrewal N, Wu Y, D’Mello V, Akakura R, George TC, Varnum B, Birge RB, 2008. Autophosphorylation docking site Tyr-867 in Mer receptor tyrosine kinase allows for dissociation of multiple signaling pathways for phagocytosis of apoptotic cells and down-modulation of lipopolysaccharide-inducible NF-kappaB transcriptional activation. J. Biol. Chem 283, 3618–3627. [DOI] [PubMed] [Google Scholar]
- Viola JR, Lemnitzer P, Jansen Y, Csaba G, Winter C, Neideck C, Silvestre-Roig C, Dittmar G, Doring Y, Drechsler M, Weber C, Zimmer R, Cenac N, Soehnlein O, 2016. Resolving lipid mediators maresin 1 and resolvin D2 prevent atheroprogression in mice. Circ. Res 119, 1030–1038. [DOI] [PubMed] [Google Scholar]
- Williams KJ, Tabas I, 1995. The response-to-retention hypothesis of early atherogenesis. Arterioscler. Thromb. Vasc. Biol 15, 551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KJ, Tabas I, 1998. The response-to-retention hypothesis of atherogenesis reinforced. Curr. Opin. Lipidol 9, 471–474. [DOI] [PubMed] [Google Scholar]
- Wu SH, Chen XQ, Liu B, Wu HJ, Dong L, 2013. Efficacy and safety of 15(R/S)-methyl-lipoxin A(4) in topical treatment of infantile eczema. Br. J. Dermatol 168, 172–178. [DOI] [PubMed] [Google Scholar]
- Yahagi K, Kolodgie FD, Otsuka F, Finn AV, Davis HR, Joner M, Virmani R, 2016. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat. Rev. Cardiol 13, 79–98. [DOI] [PubMed] [Google Scholar]
- Zagorska A, Traves PG, Lew ED, Dransfield I, Lemke G, 2014. Diversification of TAM receptor tyrosine kinase function. Nat. Immunol 15, 920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]


