Abstract
This study examined the effect of high-temperature conditions and uniform wear time durations (expeditionary, 33 h continuous wear; garrison, 3 days, 8 h/day wear) on permethrin exposure, assessed by urinary permethrin biomarkers, from wearing post-tailored, factory-treated military uniforms. Four group study sessions took place over separate 11-day periods, involving 33 male Soldiers. Group 1 (n = 10) and Group 2 (n = 8) participants wore a study-issued permethrin-treated Army uniform under high heat environment (35 °C, 40% relative humidity (rh)) and expeditionary and garrison wear-time conditions, respectively. For comparison, Group 3 (n = 7) and Group 4 (n = 8) participants wore study-issued permethrin-treated uniforms in cooler ambient conditions under operational and garrison wear-time conditions, respectively. Urinary biomarkers of permethrin (3-phenoxybenzoic acid, and the sum of cis- and trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane-1-carboxylic acid) were significantly higher under high temperature compared to ambient conditions, regardless of wear-time situations (Group 1 vs. Group 3; Group 2 vs. Group 4; p < 0.001, for both). Under high-temperature conditions, expeditionary (continuous) compared to garrison wear-time resulted in significantly (p < 0.001) higher urinary biomarker concentrations (Group 1 vs. Group 2). Differences related to wear-time under the ambient conditions (Group 3 vs. Group 4) were not statistically significant. Findings suggest that wearing permethrin-treated clothing in heat conditions results in higher internal dose of permethrin above that observed under ambient conditions.
Keywords: Permethrin, Urinary biomarkers, Absorbed dose, Heat, Military, Pesticides
Introduction
Use of permethrin ((+/−)-3-phenoxybenzyl-3-(2,2-dicholorovinyl)-2,2-dimethylcyclopropanecarboxylate) by U.S. military personnel has been required historically when on duty or deployed to environments with biting insects for protection from the annoyance and pain of mosquito, sand fly, tick, and/or chigger bites and to protect from vector-borne diseases, such as malaria, leishmaniasis, and Lyme disease [1, 2]. Moreover, in a policy change effective in 2013, uniforms worn by all non-deployed Active Duty Army personnel were replaced with post-tailoring, factory-treated permethrin uniforms. Therefore, currently, all U.S. Army personnel (>470,000 persons annually) [3] regardless of deployment status, military job or location, wear permethrin-treated uniforms. Each military service requires permethrin-treated clothing to meet U.S. Environmental Protection Agency (EPA) and Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) standards, along with their own service permethrin-treated fabric levels and efficacy (% bite) protection requirements. For example, U.S. Army requires all new, unwashed uniform permethrin concentrations to be within a minimum of 0.095 mg/cm2 and maximum of 0.135 mg/cm2. This treatment provides 99–100% bite protection out to 50 launderings (which is considered the life of the Army uniform). Although the EPA label on the uniforms reads 25 launderings, the US Army has strict requirements for permethrin treatment out to 50 launderings and testing is completed to ensure that those requirements are met (personal communication, Natick Textile Materials Evaluation Team).
Wear of permethrin-treated clothing is not limited to just military personnel, as permethrin-treated outdoor camping and sportswear gear have been manufactured and sold commercially for wear by the general public [4]. As such, there are more opportunities for the general population to be exposed to permethrin through dermal routes from direct clothing-to-skin contact, as well as through oral routes, due to ingestion from dietary sources [5], and likely hand-to-mouth transmission, through eating and smoking following hand contact with treated clothing. While the exact reason is not delineated, the concentration of permethrin biomarkers in U.S. national monitoring studies has demonstrated increases [6, 7] since the early 2000s.
Several studies have reported data on general population background permethrin exposure, as measured by concentration of urinary permethrin biomarkers, namely 3-phenoxybenzoic acid (3-PBA), cis-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane-1-carboxylic acid (cis-DCCA), and trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane-1-carboxylic acid (trans-DCCA) [6–10]. Additional studies have examined biomarker concentrations in military populations wearing permethrin-treated uniforms, including investigations of the effects of factors, such as clothing usage (i.e., number of washings and length of wear), environmental temperature conditions, and physical activity factors [11–15]. Biomarker concentrations in studies involving wear of treated clothing have been reported as 100–200 times higher than U.S. general population reference range concentrations [7, 16]. However, on average, daily estimated doses calculated from biomarker concentrations are 4–5 times lower [11, 13, 14, 17] than the WHO acceptable daily intake (ADI; 50 μg/kg/day) [18] for oral ingestion assuming 50% absorption of oral dose.
There are limited reports of acute human health impacts related to the increased number of persons wearing permethrin-treated clothing [11, 19], presumably because of permethrin’s low mammalian toxicity [20]. However, permethrin has been classified by the EPA as a potential carcinogen because there is limited evidence to suggest permethrin may cause cancer in animals [21, 22]. Also, permethrin is a neurotoxicant [23] based on its mechanism of action. As such, examination of potential health symptoms would contribute additional knowledge to the risk assessment and medical literature.
Determination of the significant factors that influence permethrin exposure is critical to enable accurate interpretation and prediction of potential human health impacts. In this study, the primary research objective is to examine whether operating in high-temperature environments compared to ambient conditions when wearing permethrin-treated clothing was associated with higher urinary concentrations of permethrin biomarkers (3-PBA, cis-DCCA, trans-DCCA). U.S. Army personnel are regularly exposed to high heat conditions [24]. Increased heat stress in humans produce consequent changes in metabolic rates and thermoregulatory mechanisms [25], as well as sweat rates, all of which may impact the toxicology of environmental exposures and have a direct impact on dermal absorption. Therefore, we hypothesize that high-temperature conditions affect the dermal absorption of permethrin and result in higher urinary permethrin metabolite concentrations. In addition, we predict that expeditionary (continuous) vs. garrison wear-time conditions result in significantly higher permethrin exposure under high heat conditions. We also explored whether permethrin exposure was associated with significant health symptoms or neurocognitive performance effects.
Method and procedures
The protocol was reviewed and approved by the US Army Research Institute of Environmental Medicine Institutional Review Board (USARIEM IRB) and the US Army Medical Research and Materiel Command IRB. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory did not constitute engagement in human subjects research. The investigators adhered to the policies for protection of human subjects as prescribed in Army Regulation 70–25 and the research was conducted in adherence with the provisions of 32 CFR Part 219. All participants gave their informed consent prior to the research study.
Study design
Thirty-three participants were recruited from a pool of Soldier Human Research Volunteers at Natick Soldier Systems Center (NSSC), Natick, MA and all participant volunteers were male. Females were not excluded from the study, however, no females volunteered to participate. Persons having a history of heat intolerance (heat exhaustion or heat stroke) or currently taking any medications that might affect heat/exercise tolerance (such as antihistamines or blood pressure medication) were excluded from participation. Soldier participants volunteered to participate in one of four groups (Fig. 1) which, by design, permitted examination of the two primary study objectives: (i) whether there are differences in permethrin exposure under different wear-time durations (expeditionary: 33 h continuous wear vs. garrison: 3 consecutive day, 8 h/day wear) in high-temperature conditions and (ii) whether wearing permethrin-treated uniforms in high temperature compared to ambient temperatures, under the same wear-time conditions, resulted in higher permethrin exposure as measured through urinary permethrin biomarkers. We determined the a priori sample size determination based on results observed in an earlier study [13]. Group sizes between n = 8–11 were determined to be sufficient to test the study hypotheses with at least 80% power of showing associations at the two-tailed p < 0.05 level.
Fig. 1.
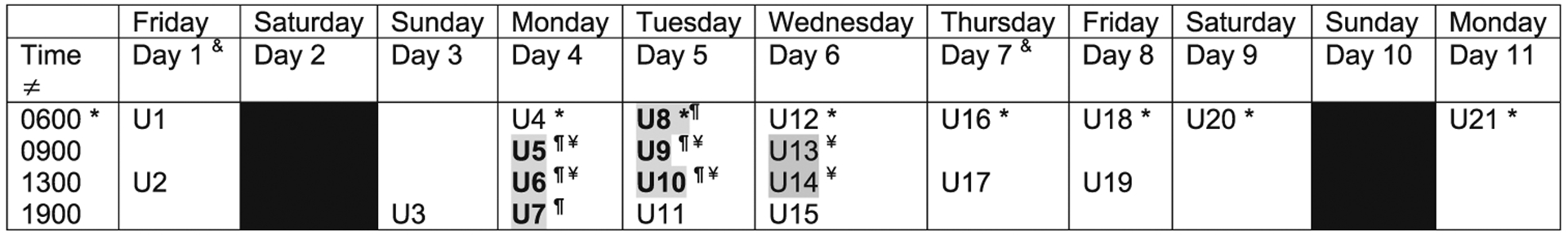
Study timeline. ǂCollection times are approximate, urine sample collected within hour from given time. &Demographic/health questionnaires and cognitive battery administered; physiological measurements recorded at first morning session. *First morning void. ¶Groups 1 and 3 (operational/expeditionary: 33 h continuous wear): Group 1: in simulated hot environment (35 °C, 40% rh), Group 3: in comparison, ambient environment (average conditions 3 °C, 80% rh). ¥Groups 2 and 4 (garrison: 3 day, 8 h/day wear) Group 2: in simulated hot environment (35 °C, 40% rh), Group 4: in comparison, ambient environment (average conditions 13 °C, 60% rh). Gray-shading: Time under study environmental conditions; urine (U) samples collected during time when study uniforms worn. Black: No study activities on Day 2 and 10
Different Group sessions were conducted randomly over the study time frame. So, different groups of Soldiers had the potential to participate in a particular Study session condition. There was no random assignment of participants to a particular session.
Each Group was tested separately between February 2014 and December 2016, however, no Groups were tested during any of the summer months (June–September) to minimize any uncontrolled high ambient outside temperature conditions between the study groups. Other than the permethrin-treated uniform, no other pesticide use was permitted during the study period. Group 1 participants (n = 10) wore a study issued permethrin-treated Army Combat Uniform for 33 h consecutively in a high heat environment (35 °C, 40% relative humidity (rh)). Participants in Group 2 (n = 8) wore a study issued permethrin-treated uniform for 8 h/day, over 3 consecutive days in high heat conditions (35 °C, 40% rh). The specific high heat conditions were selected as comparable to summer month conditions observed in the mid-Atlantic region of the U.S. For comparison, participants in Group 3 (n = 7) wore a study issued permethrin-treated uniform for 33 h consecutively during periods when the average outdoor conditions were 3 °C, 80% rh, and participants in Group 4 (n = 8) wore a study issued permethrin-treated uniform for 8-h/day over 3 days when the outdoor conditions averaged 13 °C, 60% rh.
Each group completed an 11-day study session, where study activities were scheduled on nine of the 11 days (Fig. 1). On Day 1, participants completed a baseline questionnaire, battery of neurocognitive tests, and a set of anthropometric measurements (details below). No study activities took place on Day 2 or Day 10.
On the evening of Day 3, participants reported to the NSSC Doriot Climatic Chambers [26] to sleep overnight in the building dormitory, supervised by study staff. For all study groups, study-issued uniforms were worn beginning the morning of Day 4 (Groups 1 and 3 wore the study-issued uniforms for 33 h continuously until 1600 on Day 5, while Groups 2 and 4 wore the study-issued uniforms for 3 consecutive days, 8 h/day on Day 4–6 between approximately 0800 and 1600 (Fig. 1)). All study-issued uniforms had been washed once before wearing. (See further description of study uniforms below.)
For Groups 1 and 2, the high heat environmental conditions were set at 35 °C with 40% rh within the Tropical Chamber (60′ × 15′ = 900 ft2) of the NSSC Doriot Climatic Chambers building. Group 1 participants were in the chamber from ~0730 on Day 4 until 1600 on Day 5. Group 2 participants were in the chamber from ~0730 to 1600 on Days 4, 5 and 6. During the prescribed time in the high heat conditions, participants were only allowed to leave the chamber for bathroom breaks; all meals and water were brought to the study participants in the chambers. Per human use and safety requirements, heart rate and core temperature were monitored and recorded every 30 min while participants were in the chambers. In an effort to standardize physical activity between groups, supervised 1-h ruck marches/walks were conducted at a slow speed (2 mph, with no treadmill incline) while wearing a 50–55 lb load. Group 1 completed a ruck march in the chamber at 1000 and 1400 on Days 4 and 5; Group 2 completed the ruck march at 1000 and 1400 on Days 4 and 5 and at 1000 on Day 6.
Bunkbeds were available in the chamber for Group 1 for sleeping during their 33 h wear-time period. Overnight conditions were set at 30 °C with 50% rh to simulate cooler conditions that naturally occur at night. During the study period when participants wore study-issued uniforms, Groups 2, 3, and 4 participants slept overnight in the building dormitory under ambient conditions. Participants wearing the study issued uniforms under the ambient conditions (Groups 3 and 4) performed their regular Soldier daily activities at NSSC during Days 4–6. For consistency between all study groups, participants were not permitted to shower from the start of Day 4 through ~1600 on Day 6, however, for personal hygiene, participants were permitted to wash their hands, feet, and faces during this time.
On Days 7–11 of the study sessions, participants wore their own personal permethrin-treated uniforms during required time periods. The age of these personal uniforms and number of times they had been washed was collected through daily survey questions. On the weekend (Days 2, 3, 9, and 10), participants wore non-permethrin-treated civilian clothing. All participants were provided logbooks to record the time of each urine collection, along with times of other non-study urine voids, smoking breaks, eating, physical exercise, showers, and medication use.
Anthropometric measurements and activity levels
Height (cm) (Day 1 only) and weight (kg) (Day 1 and 7) were measured and used to calculate body mass index (BMI) according to CDC guidance [27]. To determine percent body fat, skinfold measurements were taken in duplicate at three locations on the body (chest, abdomen, and thigh) using a Harpenden skinfold caliper [28]. Physical activity levels were monitored (Days 4–6), by having participants wear an activity monitor (Philips brand-Actical) [29] on the right leg at the ankle or above their boots. These monitors recorded data on the participant’s movement and computed the estimated energy expenditure (kcals, in 1 min intervals). Raw data from the devices were used to calculate individual-level average daily activity levels (kcals) during the time when study-issued uniforms were worn to provide assessment of energy expenditure or workload.
Baseline and daily surveys
A self-administered questionnaire was given to participants on their initial day (Day 1) in which participants were asked about current age, prior occupational and hobby exposure history (including personal use of pesticides), educational history, and about their alcohol and caffeine consumption, smoking habits, and physical job demands. For analytical purposes, a current smoker was categorized inclusively as smoking traditional cigarettes, e-cigarettes, and/or using chewing tobacco.
Functional health and current health symptoms were assessed, respectively, with the Veterans RAND 12-item Health Survey (VR-12) [30] and with a 25-item health symptom checklist adapted from prior studies with Gulf War veterans [31] and used in prior military environmental exposure studies [32]. The VR-12 queries for self-appraisal of somatic (“physical”) health and emotional (“mental”) impact on day-to-day functioning (e.g., accomplishing less than usual). Functional health is computed through the physical and mental component summary (PCS, MCS, respectively) scores; PCS and MCS scores are standardized to U.S. population norms (mean = 50, SD = 10). The Medical Outcomes Study (MOS) cognitive functioning scale assesses the functional impacts of thinking and attention on day-to-day functioning [33]. Higher VR-12 and MOS cognitive functioning scores indicate better functioning. The frequency of individual health symptoms reports were assessed on Day 1 and Day 7, and changes over the study period were examined.
Neurocognitive assessment
The battery of neurocognitive function tests was administered two times over the study period (morning of Day 1 and Day 7). The test battery included the Automated Neuropsychological Assessment Metrics (ANAM) Military Battery administered on a laptop computer, as well as the Grooved Pegboard test, which examines fine motor skills [34]. The ANAM Military Battery is a computer-based battery of tests designed to measure cognitive performance across several functional domains, including executive functioning, attention, memory, response time, and information processing speed. Previous studies have documented the psychometric properties of the Grooved Pegboard test [35] and of the ANAM tests, with normative data for military personnel provided for the latter [36–40].
Uniforms
Army uniforms are factory-treated using a polymer-coating method to apply permethrin (cis/trans ratio 35:65) to the blouse and trousers of the uniform. Study-issued permethrin-treated uniforms (blouse and trousers) were newly purchased for the purpose of the study through online Army supply sites or at local installation exchange stores. Cut-out section from uniform sets were analyzed for permethrin content by the Natick Textile Materials Evaluation Team, per Army Combat Uniform purchase descriptions (personal communication), after laundering once to provide an estimate of the range of permethrin levels: blouse values ranged between 0.073 and 0.096 mg/cm2 (average = 0.080 mg/cm2) and trousers ranged between 0.064 and 0.093 mg/cm2 (average = 0.082 mg/cm2). These levels are ~20–60% lower than concentrations reported in new, unlaundered Army treated uniforms and about 33% lower than levels measured in the Marine Corps Combat Utility Uniforms (MCCUUs) that had been washed once and utilized in a prior study [13]. Study-issued uniform sets from each Group were also analyzed for permethrin content following the respective study sessions.
Biological samples
During each 11-day study period, 21 urine samples were collected from each of the participants at scheduled time points (Fig. 1). On Day 1 of the study, before the collection of any samples, participants were given instructions on how to properly collect the urine to minimize contamination of the sample. All samples received at ~0600 were the first morning void. Participants provided urine samples at the Doriot Climatic Chambers building with the exception of the evening (1900) and morning (0600) samples collected on Days 7–11. For these latter samples, participants were given pre-labeled specimen cups and cooler bags containing ice packs to keep samples cold until they were turned in to study staff at the next study visit.
Laboratory analyses
Each urine sample was tested for creatinine (mg/dL), specific gravity, and osmolality at the Central Laboratory at USARIEM. Five milliliters of aliquots were frozen and sent in batches to the CDC for the quantification of permethrin metabolites: 3-PBA, cis-DCCA, and trans-DCCA. Permethrin biomarkers were measured via semi-automated solid phase extraction followed by high-performance liquid chromatography-isotope dilution tandem mass spectrometry using a method described in Davis et al. [41]. Limits of detection (LOD) across all batches were 0.1 μg/L (3-PBA) and 0.6 μg/L (trans-DCCA); for cis-DCCA, LODs were 0.6 μg/L (Groups 1 and 2) and 0.5 μg/L (Groups 3 and 4).
Statistical analyses
For descriptive comparisons across Groups at analyses of variance (ANOVA) with the Tukey correction to compare differences between Groups were performed. To evaluate whether there was change between Day 1 and Day 7 neurocognitive performances, paired t-tests were performed; Fisher’s exact test was run to examine health symptom reporting differences between Day 1 and Day 7.
Biomarker concentrations below the LOD were replaced with a value of LOD/√2 [42]. In addition to examining the cis-DCCA and trans-DCCA concentrations separately, cis-DCCA and trans-DCCA concentrations were summed to yield a total DCCA concentration (ΣDCCA). Samples with creatinine concentrations < 20 mg/dL (2.6% of samples or n = 18: Group 1, n = 3; Group 2, n = 4; Group 3, n = 2; Group 4, n = 9) or >350 mg/dL (3.9% of samples or n = 27: Group 1, n = 11, Group 2, n = 5; Group 3, n = 4, Group 4, n = 7) were excluded from the analyses because samples were either too dilute or too concentrated. Biomarker concentrations were adjusted for creatinine (μg/g creatinine) for the graphical presentation over the course of the study sessions.
To examine the study main hypotheses, biomarker concentrations from five urine samples collected before, during, and after wearing the study-issued uniform were used in repeated measures linear mixed models (LMMs) (Groups 1 and 3: U7, U8, U11, U12, U14; Groups 2 and 4: U7, U8, U15, U16, U17) (Fig. 1), similar to the approach in our earlier study [13]. Samples U7 and U8 were collected within the first 12-h and 24-h of wearing the study uniform, respectively, while Groups 1 and 2 were in the high heat and Groups 3 and 4 were in ambient conditions. Sample U11 in Groups 1 and 3 and U15 in Groups 2 and 4 was the first sample collected after removing the study-issued uniform (~1900) following completion of their respective wear-time conditions. For Groups 1 and 2, U11 and U15, respectively, were also the first sample collected after exiting the chamber and the high heat conditions. Samples U12 and U14 in Groups 1 and 3 and samples U16 and U17 in Groups 2 and 4 were collected within 12 and 24 h after removing the study-issued uniform, respectively.
Repeated measures LLMs account for both the within individual correlation and the between group covariance in order to produce robust estimates of the mean responses to the different group conditions over time. In this study, LMMs were used to determine differences in biomarker concentrations based on environmental conditions (high heat, 35 °C, 40% rh vs. ambient conditions, average 13 °C, 60% rh) and wear-time conditions (33 h continuous vs. 3 days, 8 h/day wear-times).
To examine the effects of high heat compared to ambient conditions, study groups that wore the study-issued uniform under the same wear-time conditions but in different environmental conditions were compared: 33 h continuous wear, Groups 1 and 3 (Model 1), and 3 consecutive days, 8 h/day, Groups 2 and 4 (Model 2). To examine the effect of wear-time in the heat, biomarker concentrations in the study groups that experienced the same simulated environmental conditions were compared: heat Groups 1 and 2 (Model 3), and ambient condition Groups 3 and 4 (Model 4). Each model comparison scenario was run for log-transformed unadjusted 3-PBA, cis-DCCA, trans-DCCA, and ΣDCCA.
The models were fit using an unstructured covariance matrix assumption. Parameter estimates were obtained using maximum-likelihood estimation to provide relative comparison of effects. Since the outcome variables were log transformed, exponentiated beta coefficients estimated the change associated with the predictors in the model. The main effect of ‘Group’ on biomarker concentration was adjusted for creatinine (mg/dL) and % body fat. Model 4 was also adjusted for dichotomized use of cigarettes, e-cigarettes, and chewing tobacco (0 = no, 1 = yes). Smoking and/or chewing tobacco was not included as a covariate in the other models because participants were not allowed to smoke or use other tobacco products while in the simulated heat (Groups 1 and 2). LMM analyses were performed using SAS 9.3/9.4 (SAS, Carey, NC).
Daily dose estimates (μg/kg/day) for permethrin were calculated for each participant using a method outlined in Appel et al. [11] from cis-DCCA and trans-DCCA concentrations. For computation of the highest estimate of permethrin dose during the 11-day study, we used peak excretion concentrations which are generally measured 12-h after removing the study uniform [13]. For these comparisons, first, an estimate of daily creatinine excretion was calculated for each participant based on their Day 1 weight, age, and race using an equation outlined by Ix and colleagues [43] estimated creatinine excretion rate (g/day) = 879.89 +12.51*weight (kg) − 6.19*age (years) + (34.51 if black) − (379.42 if female). Next, creatinine adjusted ΣDCCA was multiplied by estimated daily creatinine excretion. The daily metabolite dose (μg/day) was converted using a parent compound to metabolite molar mass ratio (391/209 = 1.87) to estimate daily permethrin dose (μg/day). Finally, daily permethrin dose was divided by each participant’s body weight (kg) to compute daily dose estimates (μg/kg/day). Daily dose estimates were averaged by Group to determine differences in permethrin exposure by study condition.
Results
Across the four study Groups, ages ranged between 18 and 27 years; all 33 participants were male (Table 1). The majority (72%, 23/33) of the Army Soldier participants were in combat arms/infantry occupational specialties (Group 1: 100%; Group 2: 12.5%; Group 3: 57.1%; Group 4: 100%); other occupational specialties included mechanics and repairmen. There were no significant differences in age, years of education, rank, race/ethnicity, height, weight, BMI, % body fat, smoking status, current caffeine or alcohol use, or previous use of pesticides among groups. Differences in months of Army service were noted between groups (F = 6.81, df = 3, p = 0.001). No one reported taking prescription medications immediately prior to or during the study period. There were no significant differences in permethrin metabolite concentrations between Groups on the morning of Day 4, just before they put on the study issued uniforms.
Table 1.
Descriptive characteristics of study groups
| Group 1 (n = 10) | Group 2 (n = 8) | Group 3 (n = 7) | Group 4 (n = 8) | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Age (years) | 20.8 (3.6) | 19.1 (1.0) | 19.7 (3.4) | 18.8 (1.0) |
| Education (years) | 12.6 (1.3) | 12.0 (0) | 12.6 (1.5) | 12.1 (0.4) |
| Active army (months) | 6.4 (0.7) | 9.25 (1.7) | 7.1 (2.4) | 6.5 (0.8) |
| Height (m) | 1.79 (0.1) | 1.80 (0.04) | 1.79 (0.1) | 1.84 (0.1) |
| Day 1 weight (kg) | 78.8 (12.3) | 85.4 (10.4) | 74.9 (10.5) | 83.7 (9.9) |
| Day 1 BMI | 24.5 (3.1) | 26.2 (2.3) | 23.4 (2.3) | 24.6 (2.5) |
| Day 1 % body fat | 12.3 (4.5) | 11.6 (2.6) | 12.9 (5.9) | 11.9 (4.5) |
| Day 4 3-PBA (μg/g creatinine), 1st morning void | 15.1 (11.9) | 14.5 (3.5) | 12.5 (5.2) | 12.9 (10.5) |
| N (%) | N (%) | N (%) | N (%) | |
| Race | ||||
| Black | 2 (20) | 1 (12.5) | 1 (14.3) | 1 (12.5) |
| White | 8 (80) | 5 (62.5) | 6 (85.7) | 5 (62.5) |
| Other | – | 2 (25) | – | 2 (25) |
| Current rank | ||||
| E1 | – | 2 (25) | 2 (28.6) | 4 (50) |
| E2 | 8 (80) | 4 (50) | 4 (57.1) | 4 (50) |
| E3 | 1 (10) | 2 (25) | – | – |
| E4 | 1 (10) | – | 1 (14.3) | – |
| Regular exercise (% yes) | 8 (80) | 8 (100) | 7 (100) | 8 (100) |
| Smoker (% yes) | 4 (40) | 5 (62.5) | 3 (42.9) | 4 (50) |
| Current alcohol use (% yes) | 3 (30) | 1 (12.5) | 5 (71.4) | 4 (50) |
| Current caffeine use (% yes) | 9 (90) | 8 (100) | 7 (100) | 8 (100) |
| Ever had job with pesticides (% yes) | 1 (10) | 1 (12.5) | 1 (14.3) | 1 (12.5) |
| History of pesticide use around house/garden (% yes) | 2 (20) | 2 (25) | 2 (28.6) | 3 (37.5) |
There were no significant difference between Groups at Day 1 in terms of reported physical or mental health status or performance on neurocognitive tests (Table 2). Reported health symptoms were minimal with 2 or fewer participants in each group reporting any of the 25 symptoms at a rate greater than ‘sometimes’ or once/week. Symptoms reported included headaches, muscle aches, flu, physical fatigue, and itching of skin. On Day 7, three participants from Group 4 reported ‘difficulty paying attention because of some experience at work’, which was statistically significant relative to the other Groups (Fisher’s exact test, p = 0.013). No significant differences in health symptom reporting were noted between Day 1 and Day 7. Cognitive performance did not change in Groups 2, 3, and 4 between Days 1 and 7. There was a significant decrease in time (faster) to complete the Grooved Pegboard with the dominant hand between Day 1 (mean (SD) 71.0 s (11.0)) and Day 7 (64.7 s (5.6)) (paired t-test, p = 0.017).
Table 2.
Baseline (Day 1) functional health status and neurocognitive test performances
| Group 1 (n = 10) | Group 2 (n = 8) | Group 3 (n = 7) | Group 4 (n = 8) | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Functional health | ||||
| VR12 Physical Component Score | 54.94 (1.1) | 55.82 (1.6) | 54.93 (3.4) | 54.80 (3.9) |
| VR12 Mental Component Score | 56.92 (4.7) | 60.07 (2.1) | 57.97 (4.9) | 56.05 (4.3) |
| MOS Cognitive score | 91.50 (10.6) | 94.38 (8.2) | 89.29 (9.8) | 88.75 (8.4) |
| ANAM4TBI, throughput scores: | ||||
| Code substitution | 52.37 (17.3) | 58.66 (11.3) | 37.28 (14.0) | 45.90 (20.4) |
| Code substitution—delayed | 61.24 (10.0) | 64.23 (3.9) | 52.28 (12.5) | 55.33 (10.4) |
| Match to sample | 39.65 (10.1) | 40.56 (15.9) | 36.89 (9.1) | 37.60 (13.6) |
| Math processing | 18.27 (6.2) | 19.77 (5.7) | 17.93 (7.2) | 18.62 (5.9) |
| Procedural reaction time | 87.26 (32.0) | 100.71 (12.2) | 91.20 (9.7) | 96.35 (18.2) |
| Simple reaction time | 234.4 (46.7) | 221.15 (35.7) | 225.5 (39.8) | 210.9 (19.3) |
| Simple reaction time 2 | 245.6 (34.6) | 217.99 (23.7) | 240.2 (23.0) | 230.0 (16.7) |
| Grooved Pegboard: | ||||
| Dominant hand, time (s) | 71.0 (11.0) | 75.25 (7.6) | 75.1 (8.1) | 71.3 (10.0) |
| Dominant hand, # drops | 0.5 (0.5) | 0.38 (0.5) | 0.7 (1.1) | 0.4 (0.5) |
| Non-dominant hand, time (s) | 75.3 (6.9) | 83.88 (23.0) | 73.4 (6.2) | 76.9 (10.7) |
| Non-dominant hand, # drops | 0.7 (0.8) | 1.0 (1.3) | 0.1 (0.4) | 0.4 (0.5) |
The mean (SD) activity levels in kcals measured while wearing the study-issued uniform were: Group 1 = 2077.6 (388.8), Group 2 = 2544.0 (505.5), Group 3 = 1422.9 (228.8), Group 4 = 2135.4 (732.6). Activity levels differed by Group (F = 6.38, df = 3, p = 0.002) with significant differences between Group 2 and Group 3 (p = 0.001) and Group 3 and Group 4 (p = 0.046). On Days 5 and 6, when participants were not wearing the study-issued uniforms, 50% of Group 1, 100% of Group 2, 71% of Group 3, and 100% of Group 4 reported wearing their personal uniforms during the workday; otherwise, they wore civilian or work-out clothing. Their personal uniforms were all of comparable age as they received them at the start of service and all participants reported having 6–9 months of Army service.
In a subset of study-issued uniforms measured for permethrin levels after the heat sessions, levels were0.069–0.082 mg/cm2 (average = 0.076 mg/cm2) for blouses and 0.062–0.092 mg/cm2 (average = 0.080 mg/cm2) for trousers, and following non-heat sessions were 0.072–0.12 mg/cm2 (average = 0.090 mg/cm2) for blouses, and 0.081–0.092 mg/cm2 (average = 0.087 mg/cm2) for trousers. There were subtle differences in permethrin concentrations in the uniform after the study sessions, but the differences did not reach the same magnitude as the onetime wash before the start of the study.
Permethrin biomarkers were detected in most samples across all Groups (98.7–100% [3-PBA]; 95.2–99.5% [cis-DCCA]; 98.6–100% [trans-DCCA]). For each Group, permethrin biomarker concentrations over the study sessions are presented graphically in Fig. 2a–c. Biomarker concentrations are lowest at U4 and U21, which are Monday mornings (Day 4 and 11) after a weekend of not wearing any permethrin-treated uniforms. In general, after Days 4–5 (Groups 1 and 3) or Days 4–6 (Groups 2 and 4), following the wearing of the study-issued permethrin uniforms, biomarker concentrations begin to gradually decrease. The relatively high concentration of the three permethrin metabolites measured in Group 4 on the morning of Day 6 (U12) was not expected and did not fit the pattern observed. Based on review of the individual log books from the study, there were no obvious explanations. However, participants did go straight to the dining hall following removal of the study uniform and it is possible that not washing their hands before eating may have contributed to the results.
Fig. 2.
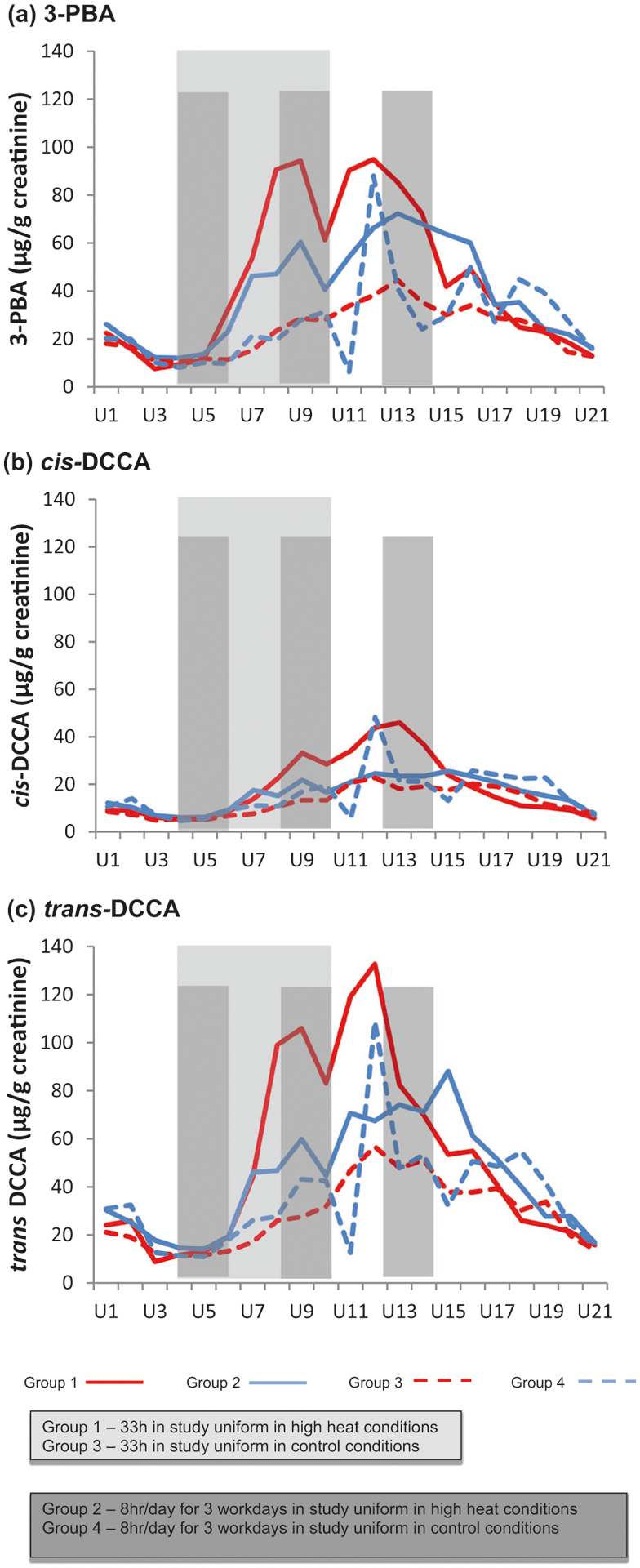
a, b, c Median creatinine-adjusted permethrin metabolite concentrations.
The results of the linear mixed effects models are summarized in Table 3. Under both expeditionary and garrison wear conditions, significantly higher biomarker concentrations were found in the Groups under high heat conditions compared to those exposed to ambient conditions: concentrations were 3.6 times higher for 3-PBA and 1.7 times higher for ΣDCCA in Model 1 (comparing Group 1 and Group 3) and were 1.9 times higher for 3-PBA and 1.6 times higher for ΣDCCA in Model 2, (comparing Group 2 and Group 4). In high heat conditions, expeditionary compared to garrison wear-times resulted in significantly higher permethrin biomarker concentrations. Concentrations were 1.6 times higher for 3-PBA and 1.3 times higher for ΣDCCA in Model 3 (comparing Groups 1 and 3). Also, % body fat was independently and significantly associated with higher permethrin biomarker concentrations. There were no significant differences between expeditionary compared to garrison wear-time in ambient conditions (Model 4, comparing Groups 3 and 4). (Similar findings were observed when examining cis-DCCA and trans-DCCA separately (see Supplement Table 1).)
Table 3.
Results from mixed models evaluating effects of wear time on urinary concentrations of permethrin exposure biomarkers
| LN(3-PBA)β (p- value) | LN(ΣDCCA)β (p- value) | |
|---|---|---|
| Model 1 (expeditionary conditions—33 h consecutive; comparing heat (Group 1) to ambient (Group 3) conditions) | ||
| Group 1 | 1.267 (<0.0001) | 0.550 (<0.0001) |
| Group 3 | Reference | Reference |
| Creatinine (mg/dL) | 0.008 (<0.0001) | 0.007 (<0.0001) |
| % Body fat | 0.0004 (0.974) | 0.005 (0.602) |
| Model 2 (Garrison conditions—8 h/day over 3 days; comparing heat (Group 2) to ambient (Group 4) conditions) | ||
| Group 2 | 0.654 (0.0003) | 0.474 (0.001) |
| Group 4 | Reference | Reference |
| Creatinine (mg/dL) | 0.010 (<0.0001) | 0.009 (<0.0001) |
| % Body fat | 0.002 (0.913) | −0.027 (0.121) |
| Model 3 (in heat conditions; comparing 33 h consecutive (Group 1) 8 h/day over 3 days (Group 2) wear-time durations) | ||
| Group 1 | 0.437 (0.0002) | 0.275 (< 0.0001) |
| Group 2 | Reference | Reference |
| Creatinine (mg/dL) | 0.010 (<0.0001) | 0.008 (< 0.0001) |
| % Body fat | 0.049 (0.0009) | 0.050 (< 0.0001) |
| Model 4 (in ambient conditions; comparing 33 h consecutive (Group 3) 8 h/day over 3 days (Group 4) wear-time durations) | ||
| Group 3 | 0.083 (0.568) | −0.020 (0.852) |
| Group 4 | Reference | Reference |
| Creatinine (mg/dL) | 0.009 (<0.0001) | 0.007 (<0.0001) |
| % Body fat | −0.013 (0.405) | −0.010 (0.383) |
| Smoker | 0.116 (0.431) | 0.154 (0.187) |
| Non-smoker | Reference | Reference |
Groups 1 and 3 (operational/expeditionary: 33 h continuous uniform wear):
Group 1: in simulated hot environment (35 °C, 40% rh)
Group 3: in comparison, ambient environment (average conditions 3 °C, 80% rh)
Groups 2 and 4 (garrison: 3 day, 8 h/day uniform wear)
Group 2: in simulated hot environment (35 °C, 40% rh)
Group 4: in comparison, ambient environment (average conditions 13 °C, 60% rh)
LN natural log, β beta coefficient, 3-PBA 3-phenoxybenzoic acid, ΣDCCA sum of cis-3-(2,2-dichlorovinyl)-2,2-dimethyl-cyclopropane-1-carboxylic acid and trans-3-(2,2-dichlorovinyl)-2,2-dimethyl-cyclopropane-1-carboxylic acid
The daily dose estimates (Table 4) significantly differed by Group (F = 8.79, df = 3, p < 0.001), with average dose significantly higher for Group 1 compared to Group 2 (p =0.005), Group 3 (p = 0.003), and Group 4 (p < 0.001). There were no significant differences between any other Group comparisons (Groups 2 and 3, Groups 2 and 4, and Groups 3 and 4).
Table 4.
Estimated daily dose at approximate peak excretion (12 h post heat/wear time)
| Daily dose estimate (μg/kg/day) geometric mean (range) | |
|---|---|
| Group 1 (n = 9)a | 6.88 (3.31–10.01) |
| Group 2 (n = 8) | 2.60 (0.02–8.49) |
| Group 3 (n = 6)a | 3.33 (2.62–4.43) |
| Group 4 (n = 8) | 2.81 (0.99–4.97) |
Groups 1 and 3 (operational/expeditionary: 33 h continuous uniform wear):
Group 1: in simulated hot environment (35 °C, 40% rh)
Group 3: in comparison, ambient environment (average conditions 3 °C, 80% rh)
Groups 2 and 4 (garrison: 3 day, 8 h/day uniform wear)
Group 2: in simulated hot environment (35 °C, 40% rh)
Group 4: in comparison, ambient environment (average conditions 13 °C, 60% rh)
One sample excluded because creatinine value was outside accepted range
Compared to the U.S. general population from the 2009 to 2010 National Health and Nutrition Examination Survey (NHANES) [6], permethrin biomarker concentrations in this study sample were orders of magnitude higher among Soldiers wearing permethrin-treated clothing (Supplement Table 2).
Discussion
High heat conditions significantly affect permethrin exposure through the wearing of permethrin-treated clothing, resulting in 60–360% higher urinary biomarker concentrations and significantly higher estimated daily doses compared to ambient conditions. In addition, exposure to high temperatures under expeditionary compared to garrison-type conditions resulted in significantly higher concentrations of permethrin urinary biomarkers. These results support previous research findings that continuous wear-time durations affect human permethrin exposure [13]. However, in contrast to earlier findings, no significant wear-time differences were noted under ambient conditions.
It is important to note that the degree of increase related to wear-time conditions is not directly comparable between this study and our earlier study, as the permethrin content of the uniforms was higher at the start of the prior study [13] compared to this study. Also, in our previous study, Soldiers were not regularly wearing permethrin-treated clothing as that study was conducted before the 2013 Army policy change and the baseline concentrations at the start of that study were lower (all <5 μg/g creatinine with the majority of samples with non-detectable concentrations) compared to the baseline concentrations in this study (8–12 μg/g creatinine). Relatedly, it is suspected that higher baseline concentrations may partially explain why no significant differences in biomarker concentrations were observed when comparing wear-time conditions within the ambient setting (Model 4).
Study findings support those of Rossbach and colleagues [17] who reported significantly increased urine biomarker concentrations within a group of male volunteers wearing permethrin-treated clothing (designed for forestry workers) under simulated high-temperature conditions around 27 °C, 60% rh. However, it is difficult to directly compare biomarker concentrations due to methodological differences across studies. Specifically, in the Rossbach study, temperature conditions were not similar, clothing were worn only over an 8 h period, and results were not presented as creatinine-adjusted. Despite these differences, Rossbach and colleagues reported estimated daily permethrin dose levels of about 5 μg/kg/day, which were comparable to the mean Group 2 levels in this study.
No evidence of acute health impacts in terms of health symptom reporting or cognitive performance was observed. While there is a lack of occupational limit values, such as NIOSH or American Conference of Governmental Industrial Hygenists (ACGIH) exposure regulations, no estimated dose reached levels above the ADI; they ranged 7–15 times lower than the 50 μg/kg/day ADI for oral ingestion (and 2–8 times lower than 25 μg/kg/day, the acceptable level assuming 50% absorption of oral dose). Whether there are longer-term health effects related to routine wear of permethrin-treated clothing remains an unknown and could not be evaluated in the present investigation as it fell outside the scope of the project. Further research is warranted given the potential for increased use of permethrin treated clothing in both military and civilian settings.
The strength of this study is that the four Groups were clearly comparable across a number of factors and only differed by environmental and wear-time conditions. This allowed direct analysis of the hypotheses of interest with minimal bias (confounding) or effect modification by other factors. Also, conducting of the study within the unique climatic chamber setting permitted standardized temperature conditions for Groups 1 and 2. Within this controlled design, it is unlikely that there were other major sources of exposure to permethrin that would have resulted in the high concentrations (such as, through particular foods or particular military activities).
One limitation of the study is the inability to generalize the findings directly to the general public or recreational users as daily, long-term wear of permethrin-treated clothing is typically limited to military personnel. Also, the study only included young, physically active males, with 6–9 months of Army service so it was not possible to address potential sex or age-based differences or longer range of durations wearing permethrin-treated clothing.
As variability in the range of estimated daily doses was observed across individuals even within Groups, further study is encouraged to address additional personal factors that may impact dose, such as genetic profiles and co-exposures with other chemicals (e.g., DEET, jet fuel (JP8)) [44, 45] or common medications (e.g., cytochrome p450 enzyme inhibitors [7]). In conclusion, findings suggest that wearing permethrin-treated clothing in heat conditions results in a higher internal dose of permethrin above that observed under ambient conditions and confirm previous research emphasizing the role of wear-time duration on exposure. Additional environmental factors, such as humidity, solar load, and precipitation, may also play a role, especially in extended-wear, occupational settings.
Supplementary Material
Acknowledgements
We acknowledge the contribution of additional USARIEM study team members who assisted in the data collection for this project and the laboratory technicians at the USARIEM Central Laboratory who performed sample analyses of creatinine. We also greatly appreciate the experience and input from Melynda Perry and Amy Johnson (Natick Textile Materials Evaluation Team, Natick Soldier Research, Development and Engineering Center, Natick, MA) who consulted on the project and conducted the permethrin analyses of the uniforms, respectively. We thank Mark Davis and Isuru Vidanage at the Centers for Disease Control and Prevention for technical assistance in the quantification of the pyrethroid metabolites in urine.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Supplementary information The online version of this article (https://doi.org/10.1038/s41370-019-0120-y) contains supplementary material, which is available to authorized users.
Extended author information available on the last page of the article
Publisher's Disclaimer: Department of the Army or the Department of Defense. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
References
- 1.Armed Forces Pest Management Board (AFPMB). Technical guide no. 36. Personal protective measures against insects and other arthropods of military significance. 2015. https://www.acq.osd.mil/eie/afpmb/docs/techguides/tg36.pdf. Accessed 14 May 2018.
- 2.United States Army Public Health Center (USAPHC). DoD insect repellant system. 2018. https://phc.amedd.army.mil/topics/envirohealth/epm/Pages/DoD-Insect-Repellent-System.aspx. Accessed 14 May 2018.
- 3.United States Army Public Health Center (USAPHC). 2017 Health of the force report. 2018. https://phc.amedd.army.mil/Periodical%20Library/2017HealthoftheForceweb.pdf. Accessed 27 Mar 2018.
- 4.United States Environmental Protection Agency (EPA). Repellant-treated clothing. https://www.epa.gov/insect-repellents/repellent-treated-clothing. Accessed 14 May 2018.
- 5.Riederer AM, Bartell SM, Barr DB, Ryan PB. Diet and nondiet predictors of urinary 3-phenoxybenzoic acid in NHANES 1999–2002. Environ Health Perspect. 2008;116:1015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Center for Disease Control and Prevention (CDC). Fourth national report on human exposure to environmental chemicals, updated tables, January 2017: vol. 1. 2017. https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Jan2017.pdf. Accessed 16 Feb 2018. [Google Scholar]
- 7.Barr DB, Olsson AO, Wong L, Udunka S, Baker SE, Whitehead RD, et al. Urinary concentrations of metabolites of pyrethroid insecticides in the general U.S. population: National Health and Nutrition Examination Survey 1999–2002. Environ Health Perspect. 2010;118:742–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Health Canada. Second report on human biomonitoring of environmental chemicals in Canada: Results of the Canadian Health Measures Survey Cycle 2 (2009–2011). 2013. https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/ewhsemt/alt_formats/pdf/pubs/contaminants/chmsecms-cycle2/chmsecms-cycle2-eng.pdf. Accessed 16 Feb 2018.
- 9.Heudorf U, Angerer J. Metabolites of pyrethroid insecticides in urine specimens: current exposure in an urban population in Germany. Environ Health Perspect. 2001;109:213–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trunnelle KJ, Bennett DH, Tulve NS, Clifton MS, Davis MD, Calafat AM, et al. Urinary pyrethroid and chlorpyrifos metabolite concentrations in Northern California families and their relationship to indoor residential insecticide levels, part of the Study of Use of Products and Exposure Related Behavior (SUPERB). Environ Sci Technol. 2014;48:1931–9. [DOI] [PubMed] [Google Scholar]
- 11.Appel KE, Gundert-Remy U, Fischer H, Faulde M, Mross KG, Letzel S, et al. Risk assessment of Bundeswehr (German Federal Armed Forces) permethrin-impregnated battle dress uniforms (BDU). Int J Hyg Environ Health. 2008;211:88–104. [DOI] [PubMed] [Google Scholar]
- 12.Kegel P, Letzel S, Rossbach B. Biomonitoring in wearers of permethrin impregnated battle dress uniforms in Afghanistan and Germany. Occup Environ Med. 2014;71:112–7. [DOI] [PubMed] [Google Scholar]
- 13.Proctor SP, Maule AL, Heaton KJ, Adam GE. Permethrin exposure from fabric-treated military uniforms under difference wear-time scenarios. J Exp Sci Environ Epidemiol. 2014;24:572–8. [DOI] [PubMed] [Google Scholar]
- 14.Proctor SP, Scarpaci MM, Maule AL, Heaton KJ, Taylor K, Dillon C, et al. Role of body composition and physical activity on permethrin urinary biomarker levels while wearing treated military uniforms. Toxicol Lett. 2018;299:210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossbach B, Appel KE, Mross KG, Letzel S. Uptake of permethrin from impregnated clothing. Toxicol Lett. 2010;192:50–55. [DOI] [PubMed] [Google Scholar]
- 16.Maule AL, Scarpaci MM, Proctor SP. Urinary concentrations of metabolites of permethrin in US Army personnel and US adult population: a review of occupational and population cohorts. Unpublished results; manuscript currently under review at journal. [Google Scholar]
- 17.Rossbach B, Niemietz A, Kegel P, Letzel S. Uptake and elimination of permethrin related to the use of permethrin treated clothing for forestry workers. Toxicol Lett. 2014;231:147–53. [DOI] [PubMed] [Google Scholar]
- 18.FAO/WHO. Pesticide residues in food—1999. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group. Geneva: World Health Organization; 1999. [Google Scholar]
- 19.Macedo PA, Peterson RKD, Davis RS. Risk assessments for exposure of deployed military personnel to insecticides and personal protective measures used for disease-vector management. J Toxicol Environ Health Part A. 2007;70:1758–71. [DOI] [PubMed] [Google Scholar]
- 20.National Research Council. Health effects from permethrin-impregnated army battle dress uniforms. Washington, DC: National Academy Press; 1994. [PubMed] [Google Scholar]
- 21.United States Environmental Protection Agency (EPA). Permethrin. Reprot of the Cancer Assessment Review Committee (Third Evaluation). 2002. https://www3.epa.gov/pesticides/chem_search/cleared_reviews/csr_PC-109701_23-Oct-02_a.pdf. Accessed 15 May 2018. [Google Scholar]
- 22.United States Environmental Protection Agency (EPA). Reregistration Eligibility Decision (RED) for Permethrin. 2009. https://archive.epa.gov/pesticides/reregistration/web/pdf/permethrin-red-revised-may2009.pdf. Accessed 16 Feb 2018.
- 23.Vijverberg HP, van den Bercken J. Neurotoxicological effects and the mode of action of pyrethroid insecticides. Crit Rev Toxicol. 1990;21:105–26. [DOI] [PubMed] [Google Scholar]
- 24.TBMED 507. Heat stress control and heat casualty management. In: Technical Bulletin Medical 507/air force pamphlet. Washington, DC: Department of the Army and Air Force; 2003. p. 5–10. [Google Scholar]
- 25.Gordon CJ. Role of environmental stress in the physiological response to chemical toxicants. Environ Res. 2003;92:1–7. [DOI] [PubMed] [Google Scholar]
- 26.Doriot Climatic Chambers. 2009. http://doriot.natick.army.mil/. Accessed 30 Apr 2018.
- 27.Center for Disease Control and Prevention (CDC) Division of Nutrition, Physical Activity, and Obesity, National Center for Chronic Disease Prevention and Health Promotion. About BMI for Adults. 2017. http://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html#Interpreted. Accessed 29 Apr 2018. [Google Scholar]
- 28.Baty International. The harpenden skinfold caliper HSB-BI. West Sussex, UK: Baty International Ltd.; 2010. [Google Scholar]
- 29.Heil DP. Predicting activity energy expenditure using the actical activity monitor. Res Q Exerc Sport. 2006;77:64–80. [DOI] [PubMed] [Google Scholar]
- 30.Kazis LE, Wilson N, Skinner K, Lee A, Rogers W, Ren XS, et al. Health status and outcomes of veterans: physical and mental component summary scores (Veterans SF-12). 1998 National Survey of Hospitalized Patients. Executive Report. Washington, DC; Bedford, MA: Office of Performance and Quality, Health Assessment Project, Health Service Research and Development Service Field Program; 1999. [Google Scholar]
- 31.Proctor SP, Heeren T, White RF, Wolfe J, Borgos MS, Davis JD. Health status of Persian Gulf War veterans: self-reported symptoms, environmental exposures and the effect of stress. Int J Epidemiol. 1998;27:1000–10. [DOI] [PubMed] [Google Scholar]
- 32.Proctor SP, Heaton KJ, Smith KW, Rodrigues ER, Widing DE, Herrick R, et al. The occupational JP8 exposure neruoepidemiology study (OJENES): repeated workday exposure and central nervous system functioning among US Air Force personnel. Neurotoxicology. 2011;32:799–808. [DOI] [PubMed] [Google Scholar]
- 33.Stewart AL, Ware JE. Measuring functioning and well-being: the Medical Outcomes Study approach. Durham, NC: Duke University Press; 1992. [Google Scholar]
- 34.Matthews C, Klove K. Instruction manual for the adult neuropsychological test battery. Madison, WI: University of Wisconsin Medical Center; 1964. [Google Scholar]
- 35.Strauss E, Sherman EMS, Spreen O. A compendium of neuropyschological tests. 3rd ed. New York City, NY: Oxford University Press; 2006. [Google Scholar]
- 36.Kaminski TW, Groff RM, Glutting JJ. Examining the stability of Automated Neuropsychological Assessment Metric (ANAM) baseline test scores. J Clin Exp Neuropsychol. 2009;31:689–97. [DOI] [PubMed] [Google Scholar]
- 37.Roebuck-Spencer TM, Reeves DL, Bleiberg J, Cernich AN, Schwab K, Ivins B, et al. Influence of demographics on computerized cognitive testing in a military sample. Mil Psychol. 2008;20:187–203. [Google Scholar]
- 38.Center for the Study of Human Operator Performance (C-SHOP). ANAM4: software user manual. Norman, OK: University of Oklahoma; 2007. [Google Scholar]
- 39.Vincent A, Bleiberg J, Yan S, Ivins B, Reeves DL, Schwab K, et al. Reference data from the Automated Neuropsychological Assessment Metrics for use in traumatic brain injury in an active duty military sample. Mil Med. 2008;173:836–52. [DOI] [PubMed] [Google Scholar]
- 40.Vincent AS, Roebuck-Spencer T, Gilliland K, Schlegel R. Automated Neuropsychological Assessment Metrics (v4) Traumatic Brain Injury Battery: military normative data. Mil Med. 2012; 177:256–69. [DOI] [PubMed] [Google Scholar]
- 41.Davis MD, Wade EL, Restrepo PR, Roman-Esteva W, Bravo R, Kuklenyik P, et al. Semi-automated solid phase extraction method for the mass spectrometric quantification of 12 specific metabolites of organophosphorus pesticides, synthetic pyrethroids, and select herbicides in human urine. J Chromatogr B. 2013;929:18–26. [DOI] [PubMed] [Google Scholar]
- 42.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- 43.Ix JH, Wassel CL, Stevens LA, Beck GJ, Froissart M, Navis G, et al. Equations to estimate creatinine excretion rate: the CKD Epidemiology Collaboration. Clin J Am Soc Nephrol. 2011;6:184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abou-Donia MB, Dechkovskaia AM, Goldstein LB, Abdel-Rahman A, Bullman SL, Khan WA. Co-exposure to pyridostygmine bromide, DEET, and/or permethrin causes sensor-imotor deficit and alterations in brain acetylcholinesterase activity. Pharmacol Biochem Behav. 2004;77:253–62. [DOI] [PubMed] [Google Scholar]
- 45.Riviere JE, Monteiro-Riviere NA, Baynes RE. Gulf War related exposure factors influencing topical absorption of 14C-permethrin. Toxicol Lett. 2002;135:61–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


