Abstract
BACKGROUND:
Conventional platelet (PLT) storage at room temperature under continuous agitation results in a limited shelf life (5 days) and an increased risk of bacterial contamination. However, both of these aspects can be ameliorated by cold storage. Preliminary work has suggested that PLTs can be cold stored for up to 3 weeks, while preserving their metabolic activity longer than in PLTs stored at room temperature. As such, in the present study, we hypothesized that the metabolic phenotypes of PLTs stored at 4°C for 3 weeks could be comparable to that of room temperature–stored PLTs at 22°C for 5 days.
Study Design and Methods:
Metabolomics analyses were performed on nine apheresis PLT concentrates stored either at room temperature (22°C) for 5 days or refrigerated conditions (4°C) for up to 3 weeks.
RESULTS:
Refrigeration did not impact the rate of decline in glutamine or the intracellular levels of Krebs cycle metabolites upstream to fumarate and malate. It did, however, decrease oxidant stress (to glutathione and purines) and slowed down the activation of the pentose phosphate pathway, glycolysis, and fatty acid metabolism (acyl-carnitines).
CONCLUSION:
The overall metabolic phenotypes of 4°C PLTs at Storage Day 10 are comparable to PLTs stored at 22°C at the end of their 5-day shelf life, while additional changes in glycolysis, purine, and fatty acid metabolism are noted by Day 21.
Platelet (PLT) transfusion is an essential therapy in modern medicine, as it allows prevention or correction of pathology in the thrombocytopenic or bleeding patient, respectively.1 Despite decades of PLT transfusion therapies, PLT transfusion-related complications still represent a major risk. Indeed, while originally PLTs were stored under refrigerated conditions, in the 1970’s banking practices for this component started to change,2 owing to the appreciation that PLTs stored at room temperature would circulate longer in the bloodstream of the recipient.3 Transfusion of PLTs with longer circulation times are beneficial in the prevention of bleeding and reduce the frequency of prophylactic interventions based on PLT transfusion, thus mitigating the risk of alloimmunization in patients with hypoproliferative thrombocytopenia.4 On the other hand, storage at 20 to 24°C under constant agitation for 5 to 7 days increases the risk of bacterial growth, resulting in 1:5000 units at risk for bacterial contamination.5
Refrigeration of PLTs (4°C storage) is associated with multiple potential advantages. First, cold storage of PLTs is significantly less conducive to bacterial growth, thereby reducing the risk of transfusion-transmitted infections as well as the costs associated with bacterial culture testing.6 Second, cold storage would also likely improve PLT inventory logistics if the storage duration can be increased past the current standard of 5 to 7 days for room temperature stored platelets. Current estimates suggest that close to 9% of PLT inventories are wasted due to expiration beyond the current room temperature 5-day shelf life, which is estimated to cause a loss of approximately US$89 million annually.7 Moreover, extending the shelf life of PLTs would also make inventories more cost effective and manageable at non–tertiary care centers. This is important, as a large number (approx. 30,000) of traumatic hemorrhage deaths occur per year before arrival at tertiary care centers.8 Increased availability of a cold-stored platelet in non–tertiary care centers would allow for the use of PLTs in patients who present to these facilities with life-threatening hemorrhage. Approximately 20% of UK hospitals keep less than 10 PLT units per year in their blood product inventories9; this type of data is currently unknown in the United States. Importantly, due to the increased rural and austere areas in the United States compared to the United Kingdom, it is probable that the percentage of hospitals that do not keep PLTs in inventory is higher.
Finally, and perhaps most importantly, cold storage of PLT results in an altered PLT phenotype associated with enhanced in vitro1,2,10-12 and in vivo13,14 hemostatic capacity. Refrigerated PLTs more highly express apoptotic markers compared to room temperature PLTs (hence the lower posttransfusion life span in circulation).15 In addition, cold temperatures promote PLT activation by mechanisms of increased intracellular calcium, reorganization of the actin cytoskeleton, and an increase in F-actin and degranulation.16 These features of cold storage–induced activation might thus be beneficial in the context of acute bleeding (e.g., in the trauma patient with platelet dysfunction17). Notably, the US Food and Drug Administration has recently approved the use of cold-stored apheresis PLTs for actively bleeding patients.18 In addition, two clinical trials are under way, with one assessing recovery of transfused cold-stored PLTs (NCT02754414) and the other assessing cold-stored PLT function in cardiac surgery patients (NCT02495506). To this end, a better understanding of the platelet storage lesion (PSL) formed during cold storage would be beneficial for identifying those PLT features associated with improved quality.
Despite decades of advancements in the field, PLT storage results in the consistent loss of up to 30% of transfused PLTs upon transfusion.19 An improved understanding of the PSL could drive the development of novel storage strategies, including, for example, novel PLT storage additives that minimize PSL formation and maximize the benefits of PLT transfusion. Preliminary work seems to suggest that metabolic intervention (e.g., by means of metabolic suppression via incubation in the absence of glucose or low gas exchange, either at 22 or 4°C20,21) may be an effective means by which to mitigate PSL development. Omics technologies, in particular metabolomics,22 have recently contributed a significant amount of novel data about the impact of manufacturing methods on the quality of blood components. In particular, metabolomics studies have helped in defining the impact of PLT additive solution23 and processing (e.g., buffy coat vs. apheresis24 or pathogenreduced PLTs25) on quality metrics for platelets. Ultimately, these studies revealed a two-phase decay phenotype: Days 0 to 3 were characterized by an active glycolysis, pentose phosphate pathway (PPP) and glutathione (GSH) metabolism; Days 4 to 5 were associated with an altered Krebs cycle and purine metabolism,26 at least in part explained by a compromised capacity to metabolize substrates like pyruvate/acetate and palmitate.27
Despite these studies, and preliminary evidence about a potential impact of cold storage on glucose consumption rates15 and mitochondrial function,28 little is known about whether and to what extent PLT metabolism is altered by refrigerated storage. Indeed, while lower storage temperature are expected to slow enzymatic reactions, as in the case of other stored blood components, only one metabolomics study has been performed to date to investigate this with stored platelets.29 As such, in the present study, we performed a metabolomics study on PLT concentrates, either stored at room temperature (22°C) or under refrigerated conditions (4°C). While the main focus of the study was to compare the metabolic phenotypes of cold and room temperature–stored PLTs within the clinically relevant time span of Storage Days 0 to 5, prior work seems to suggest that refrigerated PLTs could preserve their hemostatic properties for up to 3 weeks.2 Based on this rationale, we also extended the storage duration of refrigerated PLTs to 3 weeks and assessed the metabolic changes at Days 10 and 21.
MATERIALS AND METHODS
Platelet collection and storage
Apheresis PLT concentrates (Trima Accel, TerumoBCT) were collected from nine healthy donor volunteers at a Mississippi Valley Regional Blood Center, according to Washington University in St. Louis Institutional Review Board Protocol 201710149. All PLTs were stored in plasma and then either under standard conditions (22 ± 2°C) with continuous agitation for up to 5 days, or under refrigerated conditions (4 ± 2°C) with continuous agitation for up to 21 days. Agitation was performed using a PLT agitator (PF15i, Helmer Scientific) stored in either an incubator (PC100, Helmer Scientific) held at 22°C or a walk-in cold room (2-6°C). Units were sterilely sampled at Storage Days 0 and 5 (all units), 10 and 21 (only cold-stored platelets). Samples were immediately snap frozen in liquid nitrogen and preserved at −80°C before shipment to the University of Colorado Denver–Anschutz Medical Campus for metabolomics testing.
Sample processing and metabolite extraction
A volume of 50 μL of PLTs was extracted in 450 μL of lysis buffer (methanol: acetonitrile: water 5:3:2), before ice cold extraction by vortexing for 30 minutes at 4°C.22,30 Insoluble proteins were pelleted by centrifugation (10 minutes at 4°C and 10,000 × g) and supernatants were collected and stored at −80°C until analysis.
Ultra-high-performance liquid chromatography–mass spectroscopy metabolomics
Analyses were performed using an ultra-high-performance liquid chromatography system (UHPLC; Vanquish, Thermo Fisher Scientific) coupled online with mass spectrometry (MS; Q Exactive, Thermo Fisher Scientific). Samples were resolved over a C18 column (2.1 × 150 mm, 1.7 μm; Kinetex, Phenomenex) at 25°C with a 3-minute isocratic condition of 5% acetonitrile, 95% water, and 0.1% formic acid flowing at 250 μL/min,31 or using a 9-minute gradient at 400 μL/min from 5% to 95% B (A: water/0.1% formic acid; B: acetonitrile/0.1% formic acid).1 MS analysis and data elaboration was performed as described.30 Metabolite assignments were performed with computer software (MAVEN, Princeton University), as described.31 While extractions were normalized by PLT volumes, metabolite levels from raw data were normalized post hoc on PLT counts. Graphs and statistical analyses (either t test or repeated measures analysis of variance [ANOVA]) were prepared with computer software (Prism 5.0, GraphPad Software; and Metaboanalyst 3.0, Wishart Research Group).32 Specifically, in Metaboanalyst raw data were normalized by a pooled sample from the Day 0 samples stored at 22°C and scaled with Auto Scaling (i.e., mean centered and divided by the standard deviation of each variable).
RESULTS
Overview of the metabolic impact of storage temperature on apheresis platelet concentrates
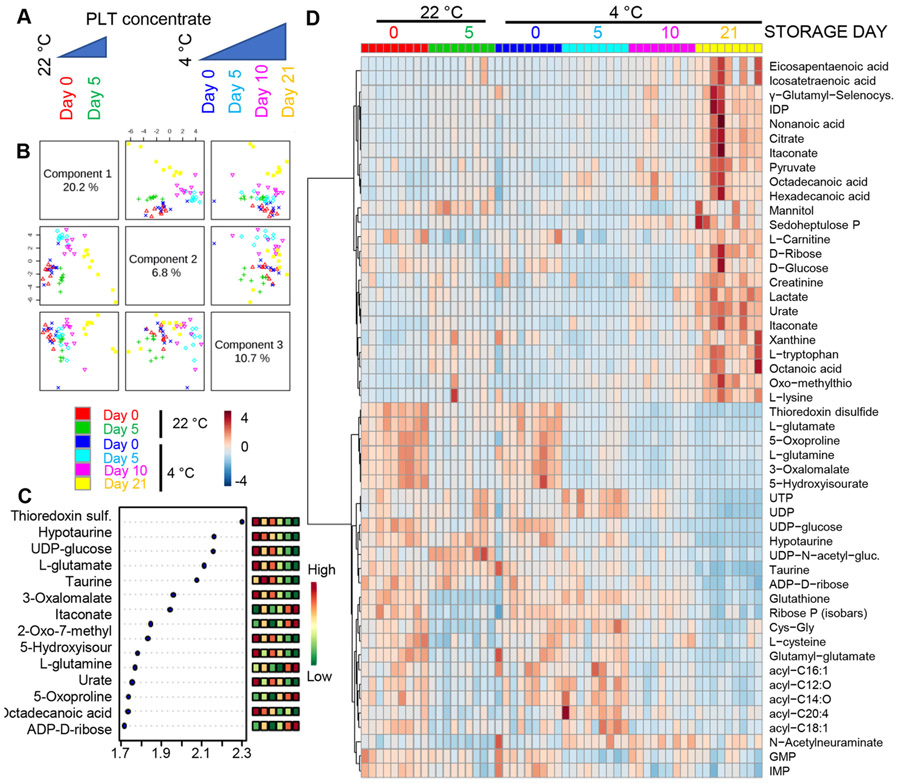
To determine the impact of storage temperature on PLT metabolism, apheresis PLT concentrates (n = 9) were stored under either standard room temperature conditions (22°C) or refrigerated conditions (4°C) for up to 5 days (room temperature) or 21 days, respectively (cold storage; Fig. 1A). Metabolomics analyses were performed via UHPLC–MS, and the results are extensively reported in tabulated form in Table S1, available as supporting information in the online version of this paper, which also includes PLT counts for each one of the samples tested in this study. Multivariate analyses were performed including partial least square–discriminant analysis (Fig. 1B) and related determination of variable importance in projection (Fig. 1C), and hierarchical clustering analysis (top 50 metabolites by ANOVA in Fig. 1D; a vectorial version is provided as Fig. S1, available as supporting information in the online version of this paper). Principal component 1, which explained 20.2% of the total metabolic variance across samples, highlighted a pattern of sample clustering based on storage time. On the other hand, principal component 3 (10.7% of the total variance) showed sample clustering based on storage temperature (Fig. 1B). Notably, Day 21 samples from 4°C PLTs clustered close to Day 5 22°C PLTs with respect to principal component 1. Top metabolites informing this clustering scheme were mostly related to glutaminolysis/GSH metabolism, carboxylic acids, and PPP metabolites, glycolysis, purine metabolism, arginine metabolism (including polyamines and creatine), acyl-carnitines, and free fatty acids (Fig. 1D; Fig. S1, available as supporting information in the online version of this paper).
Fig. 1.
Metabolic changes in PLTs stored under standard temperature (22°C) or refrigeration (4°C) at Storage Days 0, 5, 10, and 21. (A) An overview of the experimental design and (B) a partial least square–discriminant analysis of platelet metabolic phenotypes during room temperature or refrigerated storage. (C) The top 15 metabolites from the variable importance in projection plot. (D) The top 50 metabolic changes by ANOVA.
Refrigerated storage decreased glutathione consumption without impacting glutaminolysis
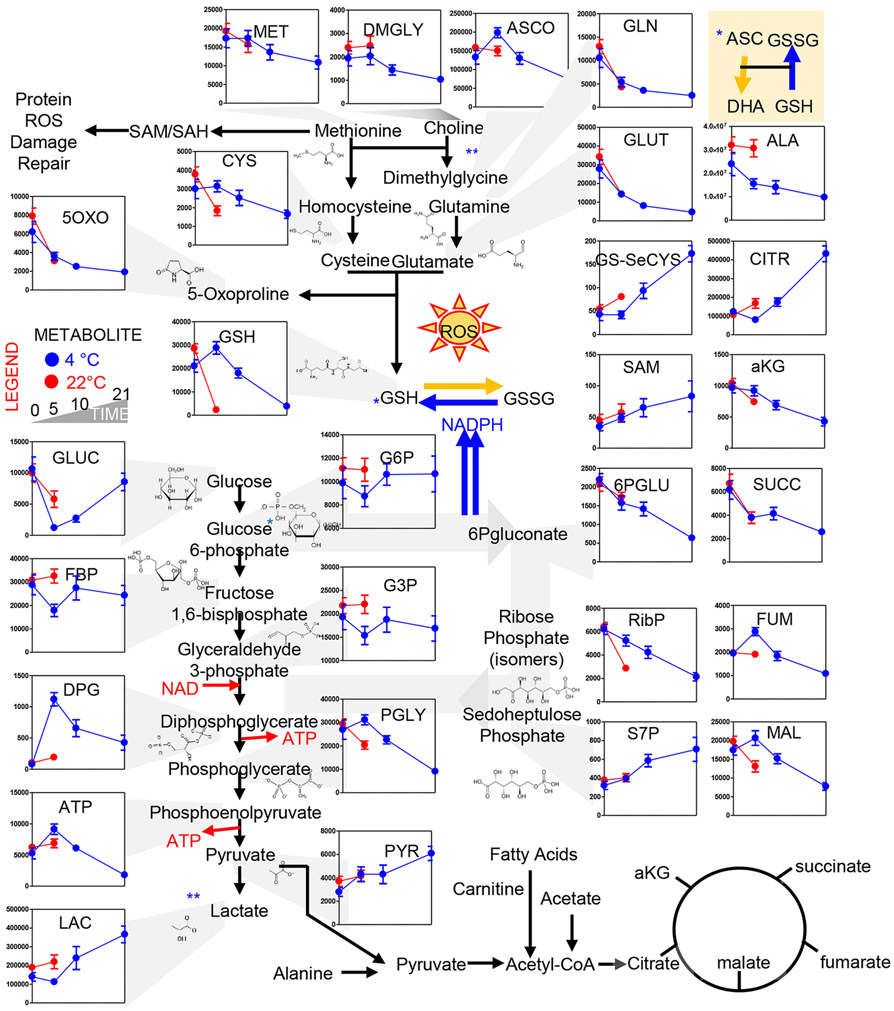
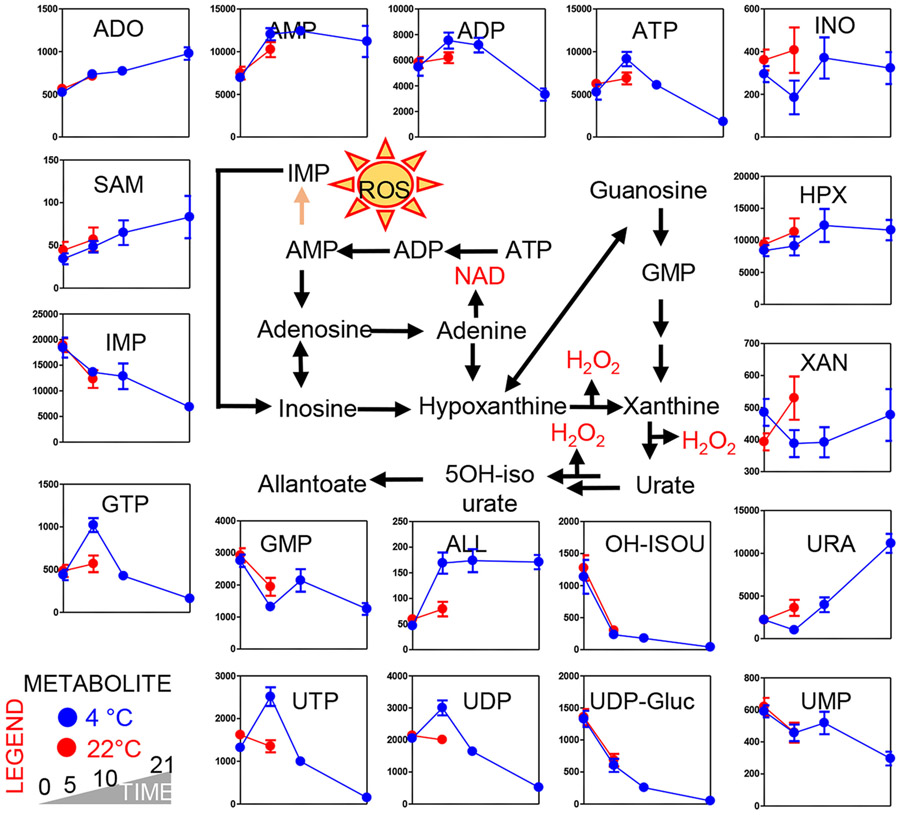
Refrigerated storage did not impact the rate of glutamine consumption, glutamate generation, α-ketoglutarate consumption and 5-oxoproline decrease, as the trends of Day 0 and Day 5 22°C and 4°C PLTs overlapped perfectly (Fig. 2, top panels). However, reduced glutathione levels dropped drastically in 22°C PLTs within the first 5 days of storage, while 4°C PLTs maintained high levels of reduced glutathione until Storage Day 10 and only by Storage Day 21 did GSH levels drop to levels comparable to Day 5 22°C PLTs (Fig. 2, top left panels). These changes were accompanied by similar rates of methionine consumption between the two arms of the study, comparable increases in S-adenosylmethionine and comparable decreases in the levels of the PPP intermediate 6-phosphogluconate. However, the PPP end-product ribose phosphate (isobaric isomers) was significantly lower in Day 5 22°C PLTs versus refrigerated PLTs at Day 5. These observations were paralleled by comparable decreased in Day 5 levels of the mitochondrial metabolites α-ketoglutarate and succinate between PLTs stored at 4°C and 22°C PLTs (Fig. 2). On the other hand, higher levels of fumarate and malate were observed in refrigerated PLTs at Day 5 in comparison to fresh PLTs and Day 5 22°C PLTs (Fig. 2), suggestive of differential rates of complex I activity between the two groups. Interestingly, steady-state measurements suggest that PLT storage resulted in activation of glycolysis, with faster glucose consumption in the first 5 days of 22°C than 4°C storage, accompanied by accumulation of lactate and pyruvate, respectively. However, past Day 5, 4°C PLTs started to show an accumulation of lactate as well (Fig. 2). Lactate accumulation was accompanied by a decline in glucose consumption rates in 4°C PLTs past Day 5. At Day 5, high-energy phosphate compounds adenosine triphosphate (ATP) and diphosphoglycerate (DPG) were significantly higher in refrigerated PLTs in comparison to 22°C PLTs (Fig. 2). ATP decline at Day 5 in warm PLTs or past Day 10 in cold-stored PLTs resulted in the accumulation of its breakdown products: ADP, adenosine monophosphate (AMP), and adenosine (ADO; Fig. 3). The total adenylate pool (adenylate binding entrance and ADO) was overall higher in refrigerated PLTs at Day 10 than in Day 5 warm PLTs. AMP conversion into its oxidized purine products (hypoxanthine, xanthine, urate) was faster in 22°C PLTs, with Day 5 values comparable to Day 10 and 21 for 4°C PLTs (Fig. 3). However, trends of downstream products 5-hydroxyisourate were comparable in both arms of the study. On the other hand, 4°C PLTs accumulated significantly higher levels of allantoate by Storage Day 5 compared to Day 5 PLTs (independent of storage temperature). Storage duration and storage temperatures significantly impacted the levels of other purines (guanine derivatives) and pyrimidines (uridine derivatives), with refrigerated PLTs showing higher levels of guanosine triphosphate (GTP), uridine triphosphate (UTP), and uridine diphosphate (UDP) at Day 5; Fig. 3).
Fig. 2.
Metabolic changes in glycolysis, Krebs cycle, PPP, glutathione, and methionine homeostasis. Platelets stored either under refrigerated (blue) or room temperature (red) conditions for 0, 5 (both groups), 10, and 21 days (only cold stored).
Fig. 3.
Metabolic changes in purine metabolism. Platelets stored either under refrigerated (blue) or room temperature (red) conditions for 0, 5 (both groups), 10, and 21 days (only cold stored).
Arginine metabolism was storage dependent but only in part temperature dependent
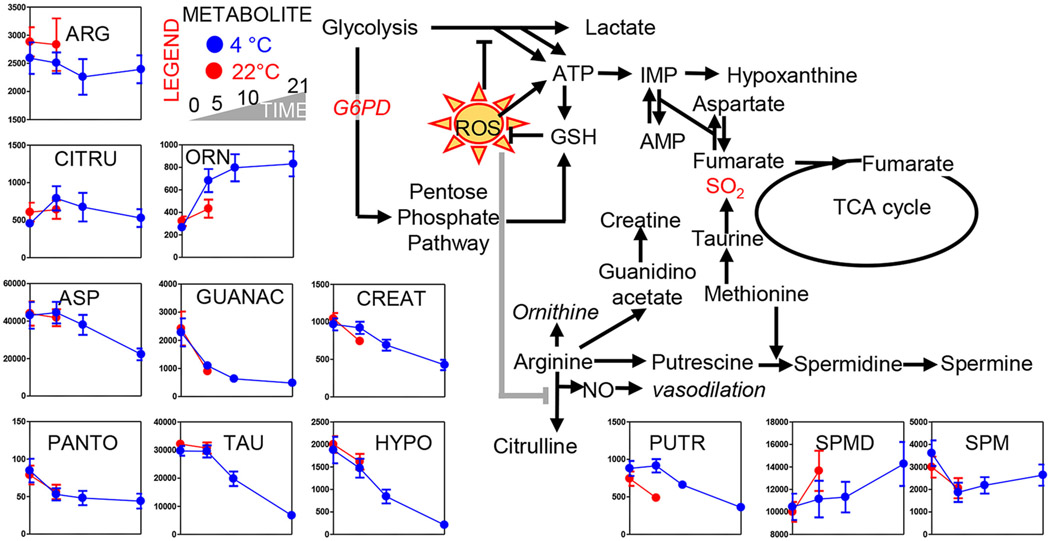
Arginine consumption by Day 5 was notable in 4°C PLTs, but not 22°C PLTs (Fig. 4). Citrulline levels were comparable between the two arms of the study, while significant increases in ornithine (product of arginase) were observed in refrigerated PLTs at Day 5 and kept increasing as a function of storage duration (Fig. 4). On the other hand, creatine synthesis (and related metabolic intermediates of that pathway like guanidinoacetate) decreased over storage in a temperature-independent fashion (Fig. 4). Polyamines—especially spermidine, but not putrescine—increased significantly over storage, the former accumulating at a faster rate in 22°C PLTs (Fig. 4). However, the final product of the polyamine synthesis pathway, spermine, decreased significantly in both arms of the study by Storage Day 5, though it started accumulating again in 4°C PLTs past Storage Day 5. Polyamine synthesis is controlled by methyl-group donors such as methionine and S-adenosylmethionine, the former decreasing and the latter increasing in both arms of the study (Figs. 2 and 3). Methionine and cysteine metabolism and, in general, sulfur metabolism are further intertwined with arginine metabolism (cysteine at a slower rate in cold-stored PLTs; Fig. 2). Taurine and hypotaurine are mitochondrial metabolites that are part of 1) an SO2-generating pathway that is connected to arginine metabolism,33,34 and 2) mitochondrial function by mechanism of regulation of mitochondrial protein translation.35 These metabolites decreased at comparable rates in both arms of the study by Day 5 and further declined in refrigerated PLTs as storage progressed to Day 21 (Fig. 4).
Fig. 4.
Metabolic changes in arginine and taurine metabolism. Platelets stored either under refrigerated (blue) or room temperature (red) conditions for 0, 5 (both groups), 10, and 21 days (only cold stored).
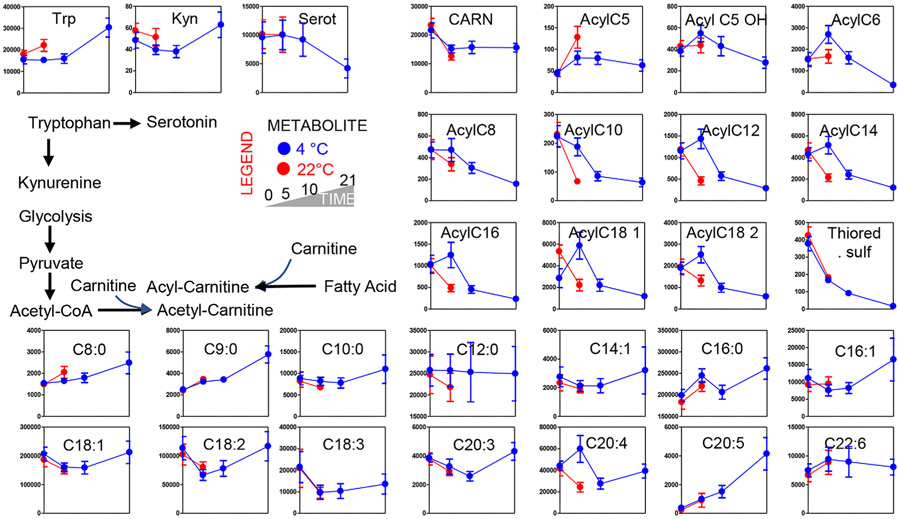
Consistent with a phenotype of storage- and temperature-induced mitochondrial dysfunction, acyl-carnitine levels were significantly impacted by storage (Acyl-C8) and/or temperature (Acyl-C10, C12, C14, C16, 18:1, 18:2; Fig. 5). This observation was likely a result of increased fatty acid mobilization or decreased consumption. However, no significant differences in free fatty acid levels were noted at Day 5 between the two groups, with the exception of the polyunsaturated fatty acid eicosatetraenoate (Fig. 5). Most medium- and long-chain fatty acids tended to decrease by Day 5 in both arms of the study before showing trends toward accumulation in refrigerated PLTs (Fig. 5). Shorter-chain fatty acids, including C8 (octanoate), whose import and metabolism in mitochondria does not require carnitine conjugation27 showed increases already at Day 5 at a faster rate in 22°C PLTs (Fig. 5).
Fig. 5.
Metabolic changes in acyl-carnitines and free fatty acids. Platelets stored either under refrigerated (blue) or room temperature (red) conditions for 0, 5 (both groups), 10, and 21 days (only cold stored).
Finally, the levels of tryptophan and its bioactive catabolite kynurenine, but not serotonin, were significantly higher in Day 5 22°C PLTs than in 4°C counterparts, where comparable levels of these metabolites were only measured at Storage Day 21 for tryptophan and kynurenine (Fig. 5).
DISCUSSION
Recent metabolomics studies have investigated the impact of storage duration on apheresis PLT concentrates.23,24,26 In those studies, storage was accompanied by a progressive loss of mitochondrial activity, which was accompanied by increased glutamine and glucose consumption and lactate accumulation, especially after Storage Day 3.23,24,26 Though less granular in the investigation of the earlier time points, the present study confirms and expands on these observations, by further noticing a quick drop in intracellular glutathione and decline in PPP activation, which was accompanied by a significantly deregulated mitochondrial metabolism (all carboxylic acids decreased in Day 5 PLTs both at 22 and 4°C) at least in part explained by altered fatty acid metabolism (decline in acyl-carnitines, faster in warm-stored PLTs). Of note, refrigerated storage for 5 days was accompanied by increases, instead of decreases, in fumarate and malate, suggestive of altered complex I and II activity at lower storage temperature. Though only based on steady state measurements, our data suggest either increased lipid mobilization (but decreased carnitine conjugation) or decreased fatty acid oxidation, an observation consistent with prior observations on decreased palmitate and octanoate metabolism as gleaned by stable isotope-labeled tracing experiments.27 This is of potential relevance because of the well-established role of acyl-carnitines as anticoagulants,36 and PLT mitochondrial metabolism in the processes of PLT activation.37 However, it is worth noticing that purine metabolism was sufficient to stabilize the adenylate pool (including ATP, ADP, AMP, adenosine, and S-adenosylmethionine) until Storage Day 5 in both arms of the study, especially in refrigerated units (until Storage Day 10). However, Day 5 warm-stored PLTs showed markers of purine oxidation (including inosine, hypoxanthine, xanthine, and allantoate), suggestive of high-energy phosphate purine breakdown and deamination in this group, a phenomenon that was observed only past Storage Day 10 in refrigerated units. Finally, arginine consumption fueled ornithine and polyamine synthesis, especially spermidine, which may be relevant in the light of the role of polyamines in blood coagulation and fibrinolysis,38 as they can serve as substrate for cross-linking enzymes in the coagulation cascade (e.g., factor XIIIa39) or interact with kringle domain–enriched components of the fibrinolytic cascade.40 Interestingly, citrulline levels were comparable between the two arms of the study at Day 5. In the absence of tracing experiments, it is interesting to speculate that increases in ornithine/arginine ratios without increases in citrulline levels are consistent with the higher activation of arginase in cold-stored PLTs, instead of mitochondrial nitric oxide synthase. Nitric oxide levels are well-established inhibitors of PLT activity,41 providing a potential linkage between our metabolic observation and an increased activation of cold-stored PLTs.
Beyond these considerations that are merely based on the impact of storage duration on PLT metabolism, the main goal of the present study was to determine the impact of refrigerated storage on PLT metabolism in comparison to standard (room temperature) storage. Specifically, we hypothesized that by depressing metabolism as a function of slower enzymatic rates at 4°C compared to 22°C storage temperatures, the metabolic phenotypes of refrigerated PLTs after prolonged storage would be comparable to those of Day 5 conventional PLTs stored at room temperature. Our results in part confirm this specific hypothesis but also highlight three categories of metabolic pathways in 4°C PLTs in comparison to 22°C PLT:
Metabolic pathways that fully overlapped with conventional stored PLTs during the first 5 days of storage. This category included glutamine and intracellular levels of Krebs cycle metabolites upstream to fumarate and malate, as well as carnitine, and some free fatty acids (C9:0; C14:1: C18:1; C18:2; C18:3; C20:5; C20:6).
Slower metabolic pathways, as gleaned by metabolic intermediates that were lower in 4°C PLTs than in 22°C PLTs. This category included some abundant free fatty acids, including octanoate, tryptophan, and polyamines (putrescine and spermidine). Cold-stored PLTs reached comparable levels of these metabolites only after 10 days or 3 weeks of storage. In addition, some purine breakdown and deamination products like xanthine, urate, and hypoxanthine, reached levels comparable to Day 5 22°C PLTs only after 10 or 21 days of refrigerated storage, suggesting similar trends of—albeit slower—activation rates for the same pathways in refrigerated units. In this group, we also include the oxidative PPP metabolite ribose phosphate.
Pathways with opposite directionality in refrigerated units in comparison to room temperature PLTs, either throughout the whole storage period (e.g., dimethylglycine) or transiently (e.g., ascorbate, fumarate, malate, and most acyl-carnitines). One key example in the latter group is characterized by glycolytic metabolites, for example, glucose 6-phosphate (and hexose phosphate isobaric isomers), glyceraldehyde 3-phosphate, fructose bisphosphate, and lactate, initially decreasing in refrigerated units, before increasing again to reach and pass Day 5 levels of lactate detected in 22°C units after 10 and 21 days of refrigerated storage, respectively. In this group also, the main intracellular antioxidant, reduced GSH, purine metabolites (ATP, ADP, AMP, GTP, inosine) and pyrimidines (UTP, UDP).
Notably, the latter group is the most interesting one, in that it suggests that refrigerated PLTs still preserve the capacity to metabolize pyruvate and fatty acids in mitochondria, with increases in lactate and decreases in acyl-carnitines starting at Day 10 in 4°C PLTs after a transient opposite trend compared to warm PLTs. In this view, it is worth noting that the refrigerated storage resulted in evident metabolic benefits with respect to (i.e., higher levels of) high-energy phosphate compounds such as DPG, but also ATP and other triphosphate compounds (GTP and UTP) at Day 5 in comparison to warm PLTs.
A limitation of this study relates to the size of the population investigated here, with only 9 apheresis units from different subjects being tested in a double-arm study. Future studies will investigate whether factors such as donor sex, age, and ethnicity can impact the metabolism of stored PLTs under room temperature or refrigerated conditions, as prior proteomics work on apheresis PLT supernatants42 and extensive work on stored red blood cells seems to suggest.43 In addition, the present study did not correlate any of the metabolomics measurements to PLT functional assays, such as viscoelastic measurements that could be telling, at least from a correlative standpoint, whether any of the three metabotypes observed in refrigerated units in this study (identical to, slower than, or inverse to conventional storage) may be related to functionally critical aspects. One potential marker of this could be, for example, the appreciation of a peculiar tryptophan metabolism in refrigerated units. Tryptophan is an essential amino acid whose metabolism generates a compound critical for PLT activation, serotonin, which is routinely stored in PLT-dense granules and released as a mediator of immune-thrombotic effects.44,45 Of note, in response to oxidant stress or inflammation (e.g., via indole 2,3-dioxygenase that is in turn controlled by interferon signaling46), as well as in response to metabolic starvation, tryptophan can be converted to kynurenine (and downstream to it, into nicotinamide to generate the critical cofactor nicotinamide adenine dinucleotide) instead of serotonin. In this view, it is worth noting that kynurenine levels decreased in Day 5 4°C PLTs, while they increased in 22°C counterparts at Day 21 after an initial, more marked decrease compared to warm PLTs.
Finally, pathogen reduction technologies are an emerging tool in the mitigation of the risk of bacterial (and viral) contamination of PLT products. However, their impact on PLT physiology and metabolism25 not to mention the costs associated with the procedure47,48 may slow the implementation of these approaches. Future studies will have to investigate the compatibility and the metabolic cost of cold (4°C) storage in combination with pathogen reduction technologies, if appropriate, given the decreased likelihood of bacterial contamination under refrigerated temperature.
CONCLUSION
In the present study, we investigated the metabolic impact of refrigerated storage on apheresis PLTs. As a result, we conclude that refrigeration either 1) did not impact some pathways (including glutaminolysis); 2) slowed down other pathways, including oxidant stress (PPP, as suggested by the levels of ribose phosphate and isobaric pentose phosphate compounds); 3) had peculiar trends, decreasing the steady-state levels of metabolites in a given pathway during the first 5 days of storage, while promoting their accumulation during the subsequent 2 weeks, when levels became comparable to or higher than those measured in Day 5 room temperature units (e.g., glycolytic metabolites and byproducts, acyl-carnitines, glutathione, mitochondrial metabolites fumarate, and malate). Altogether, these results are indicative of a higher energetic stage of Day 5 cold-stored PLTs (ATP, DPG, GTP, UTP) and higher antioxidant potential (GSH, cysteine) in comparison to warm PLTs, though these trends are reverted to those observed in warm PLTs by Storage Day 10 (purines, fumarate, and malate) or 21 (reduced glutathione, ribose phosphate).
Supplementary Material
Table S1. Supporting information.
Figure S1. Supporting information.
ACKNOWLEDGMENTS
Research reported in this publication was supported in part by funds from the Boettcher Webb-Waring Biomedical Research Award – Early Career grant (ADA) and RM1GM131968 from the National Institute of General and Medical Sciences (ADA). The authors would also like to acknowledge the team at Mississippi River Valley Blood Center for their help in acquiring the apheresis platelet units.
ABBREVIATIONS:
- ADO
adenosine
- AMP
adenosine monophosphate
- ANOVA
analysis of variance
- ATP
adenosine triphosphate
- DPG
diphosphoglycerate
- GSH
glutathione
- GTP
guanosine triphosphate
- MS
mass spectrometry
- PLT
platelet
- PPP
pentose phosphate pathway
- PSL
platelet storage lesion
- UHPLC
ultra-high-performance liquid chromatography
- UDP
uridine diphosphate
- UTP
uridine triphosphate
Footnotes
CONFLICT OF INTEREST
KAT, DS, FG, SMS, and JAR have disclosed no conflicts of interest. Though unrelated to the contents of the manuscript, the authors declare that ADA is a founder of Omix Technologies Inc. ADA and PCS are consultants for Hemanext Inc. PCS is a consultant for Secure Transfusion Services, and Cerus.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Apelseth TO, Cap AP, Spinella PC, et al. Cold stored platelets in treatment of bleeding. ISBT Science Series. 2017;12:488–95. [Google Scholar]
- 2.Pidcoke HF, Spinella PC, Ramasubramanian AK, et al. Refrigerated platelets for the treatment of acute bleeding: a review of the literature and reexamination of current standards. Shock 2014;41(Suppl 1):51–3. [DOI] [PubMed] [Google Scholar]
- 3.Murphy S, Gardner FH. Effect of storage temperature on maintenance of platelet viability–deleterious effect of refrigerated storage. N Engl J Med 1969;280:1094–8. [DOI] [PubMed] [Google Scholar]
- 4.Haddaway K, Bloch EM, Tobian AAR, et al. Hemostatic properties of cold-stored whole blood leukoreduced using a plateletsparing versus a non-platelet-sparing filter. Transfusion 2019; 59:1809–17. [DOI] [PubMed] [Google Scholar]
- 5.Food and Drug Administration UDoHaHS. Bacterial risk control strategies for blood collection establishments and transfusion services to enhance the safety and availability of platelets for transfusion. 2018. Last accessed on December 23, 2019. Available at https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bacterial-risk-control-strategies-blood-collection-establishments-and-transfusion-services-enhance.
- 6.Ketter PM, Kamucheka R, Arulanandam B, et al. Platelet enhancement of bacterial growth during room temperature storage: mitigation through refrigeration. Transfusion 2019;59 (S2):1479–89. [DOI] [PubMed] [Google Scholar]
- 7.Ellingson KD, Sapiano MRP, Haass KA, et al. Continued decline in blood collection and transfusion in the United States-2015. Transfusion 2017;57(Suppl 2):1588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spinella PC, Cap AP. Prehospital hemostatic resuscitation to achieve zero preventable deaths after traumatic injury. Curr Opin Hematol 2017;24:529–35. [DOI] [PubMed] [Google Scholar]
- 9.Ponting L, Cotton S, Hyam C, Jordan C. A new method for calculating platelet usage categories in the Blood Stocks Management Scheme. Vol. 2019. National Health Services; 2011. Available from https://www.bloodstocks.co.uk/resources/publications/ [Google Scholar]
- 10.Manno C, Hedberg K, Kim H, et al. Comparison of the hemostatic effects of fresh whole blood, stored whole blood, and components after open heart surgery in children. Blood 1991;77:930–6. [PubMed] [Google Scholar]
- 11.Reddoch KM, Pidcoke HF, Montgomery RK, et al. Hemostatic function of apheresis platelets stored at 4 degrees C and 22 degrees C. Shock 2014;41(Suppl 1):54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montgomery RK, Reddoch KM, Evani SJ, et al. Enhanced shear-induced platelet aggregation due to low-temperature storage. Transfusion 2013;53:1520–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker GA, Tuccelli M, Kunicki T, et al. Studies of platelet concentrates stored at 22 C nad 4 C. Transfusion 1973;13:61–8. [DOI] [PubMed] [Google Scholar]
- 14.Torres Filho IP, Torres LN, Valdez C, et al. Refrigerated platelets stored in whole blood up to 5 days adhere to thrombi formed during hemorrhagic hypotension in rats. J Thromb Haemost 2017;15:163–75. [DOI] [PubMed] [Google Scholar]
- 15.Marini I, Aurich K, Jouni R, et al. Cold storage of platelets in additive solution: the impact of residual plasma in apheresis platelet concentrates. Haematologica 2019;104:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egidi MG, D’Alessandro A, Mandarello G, et al. Troubleshooting in platelet storage temperature and new perspectives through proteomics. Blood Transfus 2010;8(Suppl 3):S73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore HB, Moore EE, Chapman MP, et al. Viscoelastic measurements of platelet function, not fibrinogen function, predicts sensitivity to tissue-type plasminogen activator in trauma patients. J Thromb Haemost 2015;13:1878–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stubbs JR, Tran SA, Emery RL, et al. Cold platelets for traumaassociated bleeding: regulatory approval, accreditation approval, and practice implementation-just the "tip of the iceberg.". Transfusion 2017;57:2836–44. [DOI] [PubMed] [Google Scholar]
- 19.Mays JA, Hess JR. Modelling the effects of blood component storage lesions on the quality of haemostatic resuscitation in massive transfusion for trauma. Blood Transfus 2017;15:153–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badlou BA, Ijseldijk MJ, Smid WM, et al. Prolonged platelet preservation by transient metabolic suppression. Transfusion 2005;45:214–22. [DOI] [PubMed] [Google Scholar]
- 21.Badlou BA, van der Meer PF, Akkerman JW, et al. Metabolic energy reduction by glucose deprivation and low gas exchange preserves platelet function after 48 h storage at 4 degrees C. Vox Sang 2007;92:311–8. [DOI] [PubMed] [Google Scholar]
- 22.Nemkov T, Hansen KC, Dumont LJ, et al. Metabolomics in transfusion medicine. Transfusion 2016;56:980–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johannsson F, Guethmundsson S, Paglia G, et al. Systems analysis of metabolism in platelet concentrates during storage in platelet additive solution. Biochem J 2018;475:2225–40. [DOI] [PubMed] [Google Scholar]
- 24.Paglia G, Sigurjonsson OE, Rolfsson O, et al. Metabolomic analysis of platelets during storage: a comparison between apheresis- and buffy coat-derived platelet concentrates. Transfusion 2015;55:301–13. [DOI] [PubMed] [Google Scholar]
- 25.Marrocco C, D’Alessandro A, Girelli G, et al. Proteomic analysis of platelets treated with gamma irradiation versus a commercial photochemical pathogen reduction technology. Transfusion 2013;53:1808–20. [DOI] [PubMed] [Google Scholar]
- 26.Paglia G, Sigurjonsson OE, Rolfsson O, et al. Comprehensive metabolomic study of platelets reveals the expression of discrete metabolic phenotypes during storage. Transfusion 2014; 54:2911–23. [DOI] [PubMed] [Google Scholar]
- 27.Sims C, Salliant N, Worth AJ, et al. Metabolic tracing analysis reveals substrate-specific metabolic deficits in platelet storage lesion. Transfusion 2017;57:2683–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bynum JA, Meledeo MA, Getz TM, et al. Bioenergetic profiling of platelet mitochondria during storage: 4 degrees C storage extends platelet mitochondrial function and viability. Transfusion 2016;56(Suppl 1):S76–84. [DOI] [PubMed] [Google Scholar]
- 29.Jóhannsson F, Yurkovich JT, Guðmundsson S, et al. Temperature dependence of platelet metabolism. bioRxiv 2019. 10.1101/802660v1.full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Alessandro A, Nemkov T, Yoshida T, et al. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion 2017;57:325–36. [DOI] [PubMed] [Google Scholar]
- 31.Nemkov T, Hansen KC, D’Alessandro A. A three-minute method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways. Rapid Commun Mass Spectrom 2017;31: 663–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia J, Sinelnikov IV, Han B, et al. MetaboAnalyst 3.0–making metabolomics more meaningful. Nucleic Acids Res 2015;43 (W1):W251–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nittynen L, Nurminen ML, Korpela R, et al. Role of arginine, taurine and homocysteine in cardiovascular diseases. Ann Med 1999;31:318–26. [DOI] [PubMed] [Google Scholar]
- 34.Jiang H, Stabler SP, Allen RH, et al. Altered hepatic sulfur metabolism in cystathionine beta-synthase-deficient homocystinuria: regulatory role of taurine on competing cysteine oxidation pathways. FASEB J 2014;28:4044–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansen SH, Andersen ML, Cornett C, et al. A role for taurine in mitochondrial function. J Biomed Sci 2010;17(Suppl 1):S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zimring JC. Does AC stand for acylcarnitine, anticoagulant, or both? Blood 2015;126:1524–5. [DOI] [PubMed] [Google Scholar]
- 37.Zharikov S, Shiva S. Platelet mitochondrial function: from regulation of thrombosis to biomarker of disease. Biochem Soc Trans 2013;41:118–23. [DOI] [PubMed] [Google Scholar]
- 38.Homma R, Mase A, Toida T, et al. Modulation of blood coagulation and fibrinolysis by polyamines in the presence of glycosaminoglycans. Int J Biochem Cell Biol 2005;37: 1911–20. [DOI] [PubMed] [Google Scholar]
- 39.Mosher DF, Schad PE, Kleinman HK. Inhibition of blood coagulation factor XIIIa-mediated cross-linking between fibronectin and collagen by polyamines. J Supramol Struct 1979;11:227–35. [DOI] [PubMed] [Google Scholar]
- 40.Castellino FJ, McCance SG. The kringle domains of human plasminogen. Ciba Found Symp 1997;212:46–60. [DOI] [PubMed] [Google Scholar]
- 41.Wang GR, Zhu Y, Halushka PV, et al. Mechanism of platelet inhibition by nitric oxide: in vivo phosphorylation of thromboxane receptor by cyclic GMP-dependent protein kinase. Proc Natl Acad Sci U S A 1998;95:4888–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dzieciatkowska M, D’Alessandro A, Hill RC, et al. Plasma QconCATs reveal a gender-specific proteomic signature in apheresis platelet plasma supernatants. J Proteomics 2015;120: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanias T, Lanteri MC, Page GP, et al. Ethnicity, sex, and age are determinants of red blood cell storage and stress hemolysis: results of the REDS-III RBC-Omics study. Blood Adv 2017;1:1132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White JG, Hagert K, Nipper JH, et al. Functional platelets after storage in vitro for 15–21 days. Am J Pathol 1980;101: 613–34. [PMC free article] [PubMed] [Google Scholar]
- 45.Duerschmied D, Suidan GL, Demers M, et al. Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice. Blood 2013;121:1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Powers RK, Culp-Hill R, Ludwig MP, et al. Trisomy 21 activates the kynurenine pathway via increased dosage of interferon receptors. Nat Commun 2019;10:4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cicchetti A, Coretti S, Sacco F, et al. Budget impact of implementing platelet pathogen reduction into the Italian blood transfusion system. Blood Transfus 2018;16:483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCullough J, Goldfinger D, Gorlin J, et al. Cost implications of implementation of pathogen-inactivated platelets. Transfusion 2015;55:2312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Supporting information.
Figure S1. Supporting information.