ABSTRACT
Non-negligible nighttime transpiration rates (TRN) have been identified in grasses such as wheat and barley. Evidence from the last 30 years indicate that in drought-prone environments with high evaporative demand, TRN could amount to 8–55% of daytime TR, leading several investigators to hypothesize that reducing TRN might represent a viable water-saving strategy that minimizes seemingly ‘wasteful’ water loss that is not traded for CO2 fixation. More recently however, evidence suggests that actual increases in TRN during pre-dawn hours, which are presumably controlled by the circadian clock, mediate drought tolerance – not through water conservation – but by enabling maximized gas exchange early in the morning before midday depression sets in. Finally, new findings point to a previously undocumented role for leaf sheaths as substantial contributors (up to 45%) of canopy TRN, although the extent of their involvement in these two strategies remains unknown. In this paper, we synthesize and reconcile key results from experimental and simulation-based modeling efforts conducted at scales ranging from the leaf tissue to the field plot on wheat and barley to show that both strategies could in fact concomitantly enable yield gains under limited water supply. We propose a simple framework highlighting the role played by TRN dynamics in drought tolerance and provide a synthesis of potential research directions, with an emphasis on the need for further examining the role played by the circadian clock and leaf sheath gas exchange.
KEYWORDS: Circadian clock, climate change, food security, nocturnal transpiration, sheath gas exchange, stem photosynthesis, stomatal conductance, vapor pressure deficit, water conservation
Although still not considered in most crop models, nighttime transpiration in crops has been documented for nearly 100 years now. One of the earliest reports of this phenomenon was made by J.V.G Loftfield in his monograph titled “The Behavior of Stomata”, in which he reported nighttime stomatal opening in field-grown alfalfa, a puzzling observation that led him to conclude that “If the stomatal mechanism depends upon light alone for its action, neither night opening nor day closure should occur”.1 Since then, a substantial body of experimental evidence confirmed this observation on a number of crops, particularly grasses, yet the mechanism and purpose of such phenomenon is still unclear.2–4
Additionally, this nighttime water loss is near-universally assumed to be a product of nocturnal transpiration through stomata located on the leaf blades. In grasses however, due to the complex arrangement of leaves into three distinct organs, namely the blade, ligule and sheath, the potential gas exchange capability of the canopy also extends to stems. This is, in fact, supported by a century-old evidence indicating that sheath photosynthesis accounts for 22–55% of grain yields in wheat and barley,5,6 and follow-up investigations in the 60s-70s showing that barley sheath photosynthesis accounted for 7 to 60% that of blade, a contribution that increases as plants progress toward seed fill.7–9 However, no information was available about the contribution of sheaths to transpirational water loss, particularly during the nighttime. In this paper, we present new evidence on wheat and barley documenting a key role of nocturnal transpiration dynamics in drought tolerance with a previously undocumented role of sheath-based nighttime water losses.
Nighttime transpiration dynamics contribute to drought tolerance
In grass crops such as wheat and barley, nighttime transpiration rates (TRN) could represent non-negligible rates of water loss, anywhere from 8 to 55% of daytime TR depending on the species, genotype and experimental conditions.10–15 Intriguingly, a quite significant number of observations on crops were made in arid or semi-arid environments such as Australia, Arizona or the Mediterranean region, suggesting a direct role of nighttime water use in drought-tolerance.10,11,16–18 A number of studies attributed these high levels of TRN to high nighttime evaporative demand taking place in such environments, and to genotypic factors, where “profligate” species or cultivars could lose as much as 0.5 to 2 mm of evapotranspiration per night, with reports of seasonal nighttime water losses of up to 40–60 mm.11,16 It is therefore reasonable to assume that due to TRN not being associated with CO2 fixation as happens during the daytime, minimizing nighttime water losses would be a viable drought-tolerance strategy particularly for crops grown on stored soil moisture.
Alternative evidence seems to be at odds with the benefits of such water-conservation hypothesis, with results indicating that a pre-dawn increase in TRN leads to enhanced productivity under drought among Eucalyptus genotypes.19 This was attributed to a circadian regulation of stomatal conductance, which enabled a faster stomatal response to light at sunrise leading to maximizing early morning gas exchange and radiation use efficiency (RUE) before the onset of midday depression. Recently, a similar behavior was identified on several grass crops including wheat, maize and barley, with observations confirming a positive relationship between pre-dawn increase in TRN and maximal canopy conductance.14,15,20 More unexpectedly, evidence from these studies point to natural and “unconscious” artificial selection pressures favoring the expression of such pre-dawn increase in drought-prone environments.14,15
How to reconcile these two seemingly opposite strategies? In other words, do plants grown under limited water supply need to reduce TRN to save water or increase its predawn increase to maximize early morning gas exchange? To address this question, we used geospatially explicit crop simulation modeling as a way to simulate these behaviors and examine their consequences on wheat yields, expressed in terms of gains/penalties and their probabilities, as a function of historical weather, soil and crop management data for the conditions of Tunisia. This key Mediterranean country was chosen given the critical importance of wheat production as a source for food security and socioeconomic stability, which is often threatened by severe and recurrent drought events that could reduce yields by up to 50% in the region.21
We used SSM-wheat, a process-based crop model,22,23 which was modified to enable for simulating these nighttime transpiration traits, based on experimental data assembled on wheat.12,14 The simulations confirmed the hypothesis that a profligate TRN behavior will inevitably lead to yield penalties across all production regions of the country. Specifically, simulating the level of TRN of 0.5 mm/night11 (or 15% of daytime TR in our dataset) resulted in yield losses of 10–20% across locations, which were also associated with increases in yield variability of up to 10%. Such findings indicate that Tunisian production environments are water-limited enough for a reduction in TRN to be effective as a water conservation strategy, particularly if local genotypes are not selected for reduced TRN. More surprisingly, keeping the same level of TRN (15% of daytime TR) while simulating a circadian, pre-dawn increase in TRN leading to an increase in radiation use efficiency by 25% (based on14) resulted in offsetting these yield penalties in most regions. In fact, this behavior even resulted in small yield gains (5%) in the north of country where the largest portion of wheat acreage is located. Combined, these results reconciliate – at least theoretically – the apparent contradiction between the need for reducing TRN for water conservation and for a circadian increase of TRN toward the end of the night, indicating that a genotype combining both behaviors would maximize productivity under water-limited conditions.24 Even more importantly, they point to the critical importance of examining the dynamics or patterns of water use in investigating drought tolerance, in opposition to just the assessing the total amount of water used by the plant (Figure 1).
Figure 1.
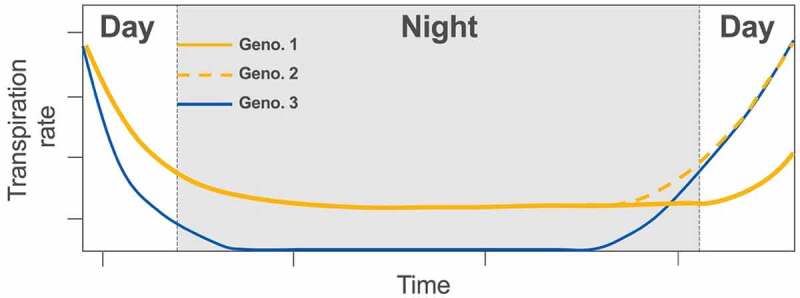
Examples of time courses liking nighttime transpiration rate (TRN) dynamics to differential drought tolerance for three hypothetical genotypes. The orange time course represents a standard line with a non-negligible TRN and no pre-dawn increase in stomatal conductance (genotype 1). The dotted orange line represents a variation of this behavior (genotype 2) which exhibits an identical baseline TRN relative to genotype 1, while expressing a pre-dawn increase in TRN. The blue line represents a genotype with a negligible or null baseline TRN, with a pre-dawn increase in TRN. Genotypes 2 and 3 are considered to be more drought tolerant compared to genotype 1 since they express a pre-dawn increase in TRN which enables maximizing early morning gas exchange. Genotype 3 is the most drought tolerant since it also exhibits a negligible TRN during most of the night, leading to water conservation. Time courses assume a non-zero VPD throughout the night with early morning dynamics amplified for illustration purposes
Leaf sheaths: a new player in nighttime transpiration
We investigated on barley the hypothesis that leaf sheaths would be non-negligible contributors to transpiration, particularly during the nighttime. To this end, we conducted experiments on two different genotypes under growth chamber and greenhouse conditions using a combination of measurements conducted at the leaf and canopy levels using anatomical observations, infrared gas exchange analyzers, a lysimetric approach for estimating whole-plant and ecophysiological modeling. We found that sheaths are equipped with outward-facing, functional stomates with densities that were on average 58% those of the abaxial side of the blade. In addition, light response curves of sheath stomatal conductance (gs) revealed high levels of sheath minimal gs (gs,Min) at zero PAR, which were approximated using this approach to be nearly 16 times that of the blades. Consistent with this, estimating whole-plant sheath TR via blade removal or treatment using a transpiration inhibitor revealed that sheath TRN accounted for up to 45% of that of whole canopy (see example in Figure 2), a value that was consistent with estimates computed based on a modified energy balance model. Overall, these results strongly point to a previously undocumented role for sheaths as substantial contributors to nighttime water loss in grasses.25
Figure 2.
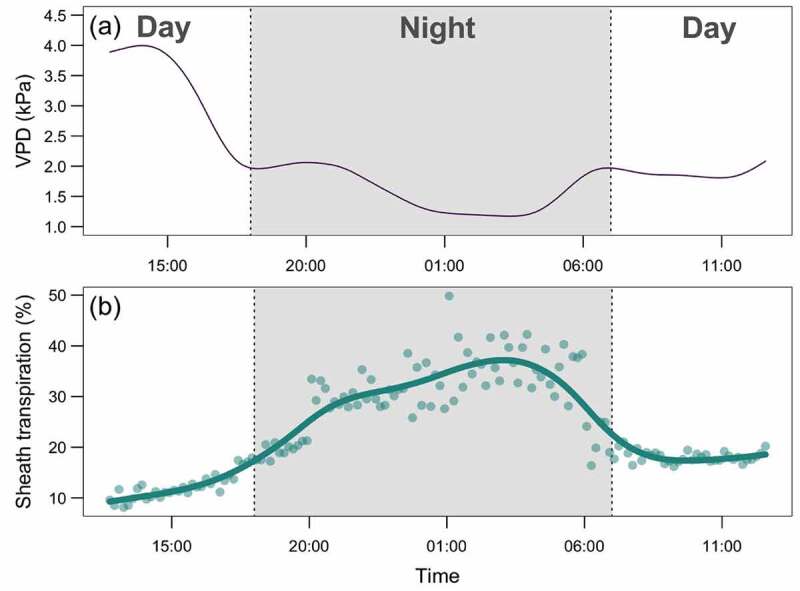
Examples of time courses of vapor pressure deficit (VPD, a) and sheath transpiration in barley (b), expressed as a percentage relative to whole-plant transpiration rate during a 24-h greenhouse experiment (unpublished data). The relative contribution of sheath-based transpiration was estimated gravimetrically, as a ratio of transpiration rates of plants with excised blades to those measured on intact plants, under the same conditions. Each datapoint on panel (b) represents a 10-minute average of three replicate plants
Taken together, the simulation-based and experimental approaches from the two studies highlighted in this paper offer new insights into the relevance of TRN dynamics in enabling drought tolerance, revealing a previously unsuspected role played by leaf sheaths. Most crop models do not incorporate or simulate nighttime water losses and even less – if any – capture sheath gas exchange parameters. Future research efforts, particularly those dedicated to identifying drought-tolerance traits or predicting productivity outcomes in response to climate change would benefit from a reexamination and deeper understanding of the mechanistic basis of nighttime transpiration. Particularly, the role played by the circadian regulation of nighttime stomatal movements and its 1) impact on morning gas exchange, 2) partition between blades and sheaths and 3) its response to environmental variables (e.g., VPD, temperature and soil moisture) deserve more attention. This is all the more relevant considering that most key staple crops are from the grass family and that nighttime warming is a major threat to food security3.
Funding Statement
This work was funded by the Minnesota Department of Agriculture (Contract No. 138815), USDA-NIFA through the Minnesota Agricultural Experiment Station (project# MIN-13-124) and National Science Foundation/Civilian Research and Development Foundation (award# OISE-16–62788-0).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Loftfield JVG. The behavior of stomata. Washington: The Carnegie Institution of Washington; 1921. [Google Scholar]
- 2.Fricke W. Night-time transpiration – favouring growth? Trends Plant Sci. 2019;24:1–4. doi: 10.1016/j.tplants.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Sadok W, Jagadish SVK.. The hidden costs of nighttime warming on yields. Trends Plant Sci. 2020;25:644–651. doi: 10.1016/j.tplants.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Monnens D, Sadok W. Whole‐plant hydraulics, water saving, and drought tolerance: a triptych for crop resilience in a drier world. Annu Plant Rev. 2020;3:661–698. [Google Scholar]
- 5.Boonstra AERH. Invloed van de verschillende assimileerende deelen op de korrelproducie bij Wilhelminatarwe. Meded LandbHoogesch Wageningen. 1929;33:3–21. [Google Scholar]
- 6.Archbold HK. Physiological studies in plant nutrition XIII. Experiments with barley on defoliation and shading of the ear in relation to sugar metabolism. Ann Bot. 1942;8:487–531. doi: 10.1093/oxfordjournals.aob.a088419. [DOI] [Google Scholar]
- 7.Thorne GN. Photosynthesis of lamina and sheath of barley leaves. Ann Bot. 1959;8:365–370. doi: 10.1093/aob/23.3.365. [DOI] [Google Scholar]
- 8.Angus JF, Jones R, Wilson JH. A comparison of barley cultivars with different leaf inclinations. Aust J Agric Res. 1972;23:945–957. doi: 10.1071/AR9720945. [DOI] [Google Scholar]
- 9.Takeda G, Udagawa T. Ecological studies on the photosynthesis of winter cereals III. Changes of the photosynthetic ability of various organs with growth. Proc Crop Sci Soc Jpn. 1976;45:357–368. doi: 10.1626/jcs.45.357. [DOI] [Google Scholar]
- 10.Richards RA, Rawson HM, Johnson DA. Glaucousness in wheat: its development and effect on water use efficiency, gas exchange and photosynthetic tissue temperatures. Aust J Plant Physiol. 1986;13:465–473. [Google Scholar]
- 11.Rawson HM, Clarke JM. Nocturnal transpiration in wheat. Aust J Plant Physiol. 1988;15:397–406. [Google Scholar]
- 12.Schoppach R, Claverie E, Sadok W. Genotype-dependent influence of night-time vapour pressure deficit on night-time transpiration and daytime gas exchange in wheat. Funct Plant Biol. 2014;41:963–971. doi: 10.1071/FP14067. [DOI] [PubMed] [Google Scholar]
- 13.Even M, Sabo M, Meng D, Kreszies T, Schreiber L, Fricke W. Night-time transpiration in barley (Hordeum vulgare) facilitates respiratory carbon dioxide release and is regulated during salt stress. Ann Bot. 2018;122:569–582. doi: 10.1093/aob/mcy084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamang BG, Schoppach R, Monnens D, Steffenson BJ, Anderson JA, Sadok W. Variability in temperature-independent transpiration responses to evaporative demand correlate with nighttime water use and its circadian control across diverse wheat populations. Planta. 2019;250:115–127. doi: 10.1007/s00425-019-03151-0. [DOI] [PubMed] [Google Scholar]
- 15.Sadok W, Tamang BG. Diversity in daytime and night-time transpiration dynamics in barley indicates adaptation to drought regimes across the Middle-East. J Agron Crop Sci. 2019;205:372–384. doi: 10.1111/jac.12331. [DOI] [Google Scholar]
- 16.Tolk JA, Howell TA, Evett SR. Nighttime evapotranspiration from alfalfa and cotton in a semiarid climate. Agron J. 2006;98:730–736. doi: 10.2134/agronj2005.0276. [DOI] [Google Scholar]
- 17.Resco de Dios V, Roy J, Ferrio JP, Alday JG, Landais D, Milcu A, Gessler A. Processes driving nocturnal transpiration and implications for estimating land evapotranspiration. Sci Rep. 2015;5:10975. doi: 10.1038/srep10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coupel-Ledru A, Lebon E, Christophe A, Gallo A, Gago P, Pantin F, Doligez A, Simonneau T. Reduced nighttime transpiration is a relevant breeding target for high water-use efficiency in grapevine. Proc Natl Acad Sci USA. 2016;113:8963–8968. doi: 10.1073/pnas.1600826113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Resco de Dios V, Loik ME, Smith R, Aspinwall MJ, Tissue DT. Genetic variation in circadian regulation of nocturnal stomatal conductance enhances carbon assimilation and growth. Plant Cell Environ. 2016;39:3–11. doi: 10.1111/pce.12598. [DOI] [PubMed] [Google Scholar]
- 20.Tamang BG, Sadok W. Nightly business: links between daytime canopy conductance, nocturnal transpiration and its circadian control illuminate physiological trade-offs in maize. Environ Exp Bot. 2018;148:192–202. doi: 10.1016/j.envexpbot.2017.11.016. [DOI] [Google Scholar]
- 21.Sadok W, Schoppach R, Ghanem ME, Zucca C, Sinclair TR. Wheat drought-tolerance to enhance food security in Tunisia, birthplace of the Arab spring. Eur J Agron. 2019;107:1–9. doi: 10.1016/j.eja.2019.03.009. [DOI] [Google Scholar]
- 22.Sinclair TR, Amir J. A model to assess nitrogen limitations on the growth and yield of spring wheat. Field Crops Res. 1992;30:63–78. doi: 10.1016/0378-4290(92)90057-G. [DOI] [Google Scholar]
- 23.Soltani A, Sinclair TR. Modeling physiology of crop development, growth and yield. Wallingford (UK): CAB International; 2012. [Google Scholar]
- 24.Schoppach R, Sinclair TR, Sadok W. Sleep tight and wake-up early: nocturnal transpiration traits to increase wheat drought tolerance in a Mediterranean environment. Funct Plant Biol. 2020;47:1117–1127. doi: 10.1071/FP20044. [DOI] [PubMed] [Google Scholar]
- 25.Sadok W, Lopez JR, Zhang Y, Tamang BG, Muehlbauer GJ. Sheathing the blade: significant contribution of sheaths to daytime and nighttime gas exchange in a grass crop. Plant Cell Environ. 2020;43:1844–1861. doi: 10.1111/pce.13808. [DOI] [PubMed] [Google Scholar]


