ABSTRACT
Mobile wound signals transmitted from local damaged to distal undamaged sites induce upsurge of jasmonic acid (JA) and activation of core JA signaling, priming the whole plant for broad-spectrum resistance/immunity against future challenges. We recently characterized two jasmonate importers AtJAT3 and AtJAT4 in Arabidopsis thaliana jasmonate transporter (JAT) family that cooperatively regulate the transmission of JA from leaf-to-leaf in this wound-induced systemic response/resistance (WSR). As half-molecule ATP-binding cassette transporters, AtJAT3 and AtJAT4 need to form homodimers or/and heterodimer to function. Here we show interactions in AtJAT3-AtJAT3, AtJAT3-AtJAT4, and AtJAT4-AtJAT4 pairs by both yeast two-hybrid and bimolecular fluorescent complementation assays. Furthermore, we propose a model in which the homo-/hetero-dimers of AtJAT3/AtJAT4 mediated cell-cell transport of JA drives long-distance transmission of JA signal in a self-propagation mode and give perspectives on future works to reinforce this model.
KEYWORDS: Wound-induced systemic resistance (WSR), Jasmonate importers, Protein interaction, Cell-cell transport, Long-distance transmission
Jasmonic acid (JA) and related oxylipins, referred as jasmonates (JAs), play essential roles in activation of plant systemic resistance/immunity, broad-spectrum resistance in the whole plant triggered by prior challenges at local sites/tissues. This JA-regulated systemic resistance includes wound-induced systemic resistance (WSR) triggered by herbivores or necrotrophic pathogens1 and induced systemic resistance (ISR) triggered by beneficial, in particular root-associated, microbes.2 Remarkably, mobile signals have to transmit from the local challenged to distal unchallenged sites to activate WSR and ISR. Less has been known on the nature and transmission mode of mobile signals in ISR. Transmission of the mobile wound signals from local damaged older to distal younger/systemic leaves has been intriguing scientists for decades.3–8 Stem-stem grafting experiments in tomato (Solanum esculentum)1,9 show that production of JA in local leaves and perception of JA in systemic leaves are both essential to activate WSR, indicating that JAs could be the mobile wound signal. Recent grafting experiments in Nicotiana attenuata7 and Arabidopsis thaliana (Arabidopsis hereafter)7,10 revealed both JA-dependent and -independent mobile wound signals. In Arabidopsis, experiments to manipulate synthesis of JA in local damaged and/or systemic leaves revealed that a JA-independent rapid wound-signal could trigger the de novo JA as well as the bioactive jasmonoyl-L-isoleucine (JA-Ile) production in distal leaves.4 The rapid electrical signal mediated by clade three GLUTAMATE RECEPTOR-LIKE (GLR3) regulates propagation of Calcium (Ca2+) waves to trigger the JA/JA-Ile upsurge in the distal leaves,11,12 possibly by activation of lipoxygenase activity13 and/or the inactivation of the repressor JAV1 of wound-induced JA biosynthesis in the JAV1-JAZ8-WRKY51 complex.14 Grafting experiments show JAs can act as the transmissible wound signal, the specific molecular form, however, remains to be determined, which requires identification of mutants at each step in JA biosynthesis after OPDA production.10,15 Although previous studies in tomato and tobacco plants revealed that exogenous radiolabeled JA and JA-Ile could both be translocated from local damaged to systemic leaves, difficulties in determination of the absorbed concentration of these tracers in cells has hindered interpreting of these results.7,16–19 Thus, whether and how JA functions as the mobile wound signal remains unknown.
By setting up rosette-petiole grafting experiments in Arabidopsis, we show that JA could relocate from local damaged to systemic leaves.20 Direct evidence showing that JA is the mobile form of JA-dependent wound signals came from tracing leaf-to-leaf translocation of D5-JA. The absorbed D5-JA in the local damaged leaves was calculated from the amount of D5-JA-Ile, which is derived from the absorbed D5-JA in the cytosol, and was in a physiologically relevant range that does not significantly affect wound response. Thus, D5-JA could be utilized to trace the translocation of wound-induced endogenous JA. Remarkably, we reveal a predominant role of two members of jasmonate transporter (JAT) family, AtJAT3 and AtJAT4, in modulation of leaf-to-leaf translocation of JA. In atjat3;4 mutants, the translocated JA decreased ~70%, leading to ~50% reduction in wound-induced JA/JA-Ile upsurge in the systemic leaves, which, however, results in significant compromise or abolished wound-induced systemic response/immunity as determined by the expression of JA-responsive marker genes and resistance to necrotrophic fungal pathogen, Botrytis cinerea. Both AtJAT3 and AtJAT4 are localized at the plasma membrane and mediate cellular import preferentially of JA. Therefore, these two importers modulate long distance translocation of JA to maintain a critical systemic JA/JA-Ile level essential for wound-induced systemic response/immunity.
Similar promoter activities in the core phloem cells20 indicate a functional redundancy between AtJAT3 and AtJAT4. Furthermore, as half molecule ABCG transporters, AtJAT3 and AtJAT4 should form either homodimers or heterodimers for their transport activities. A cooperation between AtJAT3 and AtJAT4 in mediating JA import suggested that AtJAT3 and AtJAT4 may form heterodimers. However, overexpression of either AtJAT3 or AtJAT4 could rescue the defect of atjat3;4 cells in JA import,20 supporting the view that AtJAT3 and AtJAT4 may also form homodimers. To provide evidence for AtJAT3/4 interactions, we first performed yeast two-hybrid (Y2H) assays by fusion AtJAT3 and AtJAT4 with prey or bait in various combinations as shown in Figure 1a. Yeast cells co-transformed with bait construct Cub-AtJAT3/4 in combination with prey construct Nub-AtJAT3/4 could grow on the synthetic dropout media without Leu, Trp, and His and Ade (SD-LWHA), revealing physical interactions between AtJAT3 and AtJAT3, AtJAT4 and AtJAT4, as well as AtJAT3 and AtJAT4 (Figure 1a). These interactions were also confirmed by bimolecular fluorescence complementation (BiFC) experiments in tobacco cells (Figure 1b). The specificity of AtJAT3-AtJAT4 interaction was confirmed by replacing AtJAT3 with the jasmonate exporter AtJAT1, another member in JAT family, which exhibited no interactions with AtJAT4 in yeast (Figure 1a) as well as tobacco (Figure 1b) cells. We thus propose a model in which cell-cell transport of JA mediated by AtJAT3/4 homodimers and heterodimer drives leaf-to-leaf transmission of JA in a self-propagation mode (Figure 1c). The transport properties (e.g. substrate specificity, affinity) of AtJAT3-AtJAT3, AtJAT3-AtJAT4, and AtJAT4-AtJAT4 dimmers in mediating JA import may vary and their particular roles in driving long distance transmission of JA remain to be addressed in the future. Besides, this cell-cell transport also requires exporter(s) mediating cellular JA export. AtJAT1, which is localized at both plasma membrane as well as nuclear envelope and mediates cellular export of JA and nuclear entry of JA-Ile,21 is the mostly likely candidate. However, decoupling of AtJAT1-mediated cellular JA export with nuclear entry of JA-Ile is critical to demonstrate a role of AtJAT1-mediated cellular JA export in driving long distance transmission of JA.
Figure 1.
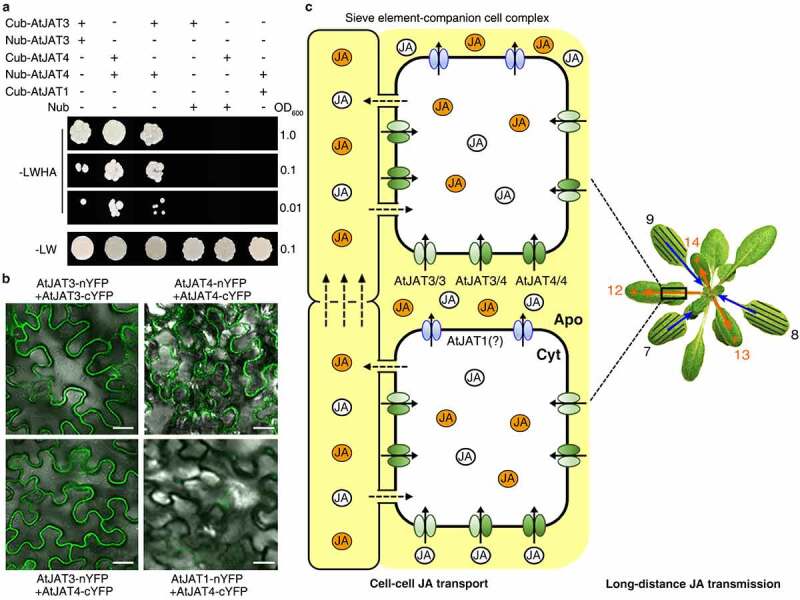
AtJAT3 and AtJAT4 interactions in driving long distance leaf-to-leaf translocation of JA. (a) Y2H analysis of interactions between AtJAT3 and AtJAT3, AtJAT4 and AtJAT4, and AtJAT3 and AtJAT4. The yeast strain NMY51 was co-transformed with bait construct Cub-AtJAT3/4 or Cub-AtJAT1, and prey construct Nub-AtJAT3/4. The transformants were grown on synthetic SD without Leu and Trp (SD-LW) plate or synthetic SD without Leu, Trp and His and Ade (SD-LWHA). (b) Confocal microscopy analysis of BiFC in Nicotiana benthamiana epidermal leaf cells revealed physical interactions between AtJAT3 and AtJAT3, AtJAT4 and AtJAT4, and AtJAT3 and AtJAT4, while no AtJAT1-AtJAT4 interaction was observed. Scale bar, 25 μm. (c) A model showing that cell-cell transport drives long-distance transmission of JA along the phloem in WSR. Cellular import of wound-induced JA (indicated by white cycles) from apoplast by AtJAT3-AtJAT3 and AtJAT4-AtJAT4 homodimers and AtJAT3-AtJAT4 heterodimer, as well as export of JA to apoplast by exporter(s) such as AtJAT1 is essential to mediate cell-cell transport along the phloem. Furthermore, de novo biosynthesis of JA (indicated by orange cycles) by positive feedback enables self-propagation of JA during cell-cell transport, generating JA waves to trigger systemic immunity in distal leaves. The local damaged leaves (leaf 7, 8 and 9 indicated) and systemic leaves (the parastichy leaf 12, 13 and 14) are indicated by black lines and orange stars respectively
Materials and methods
Yeast two-hybrid (Y2H) analysis
Y2H assays were performed as previously described.22 The coding sequences of AtJAT3, AtJAT4 and AtJAT1 were cloned into the SfiI sites of pBT3-N (Cub) bait or pPR3-N (Nub) prey vector to generate Cub-AtJAT3/4, Nub-AtJAT3/4 and Cub-AtJAT1 constructs. Combinations of constructs were co-transformed into the yeast cells of strain NMY51. Colonies grown on synthetic dropout media without Leu and Trp (SD-LW) were cultured for 20 h in SD-LW liquid media. The cultures were then serially diluted and 2 μL of each dilution (OD600 = 1.0, 0.1, 0.01) were plated on SD-LW and SD without Leu, Trp, His and Ade (SD-LWHA). Plates were kept at 28°C for 2 days before taking photos.
Transient expression in N. benthamiana and confocal microscopy analysis
Transient expression in N. benthamiana assays were performed as previously described.22 The coding sequences of AtJAT3, AtJAT4, and AtJAT1 were cloned into the BamHI/XmaI, StuI/SacI or XhoI/XmaI sites of pSPYCE (MR) or pSPYNE (R) vector to generate AtJAT3/4-cYFP, AtJAT3/4-nYFP and AtJAT1-nYFP constructs. Agrobacterium tumefaciens strain EHA105 carrying the BiFC constructs at OD600 = 0.5 and p19 at OD600 = 0.3 were co-infiltrated into 5-6-week-old N. benthamiana leaves. Plants were cultivated in soil at 22°C and ~70% relative humidity with 12 h/12 h light/dark photoperiod for 2 to 3 days after infiltration. Confocal imaging analysis was performed using Leica TCS SP5 laser scanning confocal microscope. GFP was excited at 488 nm, and the fluorescence emissions were detected at 498 to 538 nm.
Funding Statement
This work was supported by the Natural Science Foundation of China [31970310]; Natural Science Foundation of China [31470326]; Natural Science Foundation of China [30870358]; Major Research Plan from the Ministry of Science and Technology of China [2013CB945100]; Program for New Century Excellent Talents in University [NECT-08-0529].
Acknowlegments
This work was supported by the Natural Science Foundation of China (NSFC) (No. 31970310, No. 31470326 and 30870358), Major Research Plan from the Ministry of Science and Technology of China (No. 2013CB945100), and Program for New Century Excellent Talents in University (NECT-08-0529) to P. L.
References
- 1.Schilmiller AL, Howe GA.. Systemic signaling in the wound response. Curr Opin Plant Biol. 2005;8:1–4. doi: 10.1016/j.pbi.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Pieterse CM, Leon-Reyes A, Van der Ent S, Van Wees SC.. Networking by small-molecule hormones in plant immunity. Nat Chem Biol. 2009;5:308–316. doi: 10.1038/nchembio.164. [DOI] [PubMed] [Google Scholar]
- 3.Mielke K, Forner S, Kramell R, Conrad U, Hause B.. Cell-specific visualization of jasmonates in wounded tomato and Arabidopsis leaves using jasmonate-specific antibodies. New Phytol. 2011;190:1069–1080. doi: 10.1111/j.1469-8137.2010.03638.x. [DOI] [PubMed] [Google Scholar]
- 4.Koo AJ, Gao X, Jones AD, Howe GA. A rapid wound signal activates the systemic synthesis of bioactive jasmonates in Arabidopsis. Plant J. 2009;59:974–986. doi: 10.1111/j.1365-313X.2009.03924.x. [DOI] [PubMed] [Google Scholar]
- 5.Hilleary R, Gilroy S. Systemic signaling in response to wounding and pathogens. Curr Opin Plant Biol. 2018;43:57–62. doi: 10.1016/j.pbi.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Chauvin A, Caldelari D, Wolfender JL, Farmer EE. Four 13-lipoxygenases contribute to rapid jasmonate synthesis in wounded Arabidopsis thaliana leaves: a role for lipoxygenase 6 in responses to long-distance wound signals. New Phytol. 2013;197:566–575. doi: 10.1111/nph.12029. [DOI] [PubMed] [Google Scholar]
- 7.Bozorov TA, Dinh ST, Baldwin IT. JA but not JA-Ile is the cell-nonautonomous signal activating JA mediated systemic defenses to herbivory in Nicotiana attenuata. J Integr Plant Biol. 2017;59:552–571. doi: 10.1111/jipb.12545. [DOI] [PubMed] [Google Scholar]
- 8.Choi WG, Hilleary R, Swanson SJ, Kim SH, Gilroy S. Rapid, long-distance electrical and calcium signaling in plants. Annu Rev Plant Biol. 2016;67:287–307. doi: 10.1146/annurev-arplant-043015-112130. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Li C, Lee GI, Howe GA. Distinct roles for jasmonate synthesis and action in the systemic wound response of tomato. Proc Natl Acad Sci U S A. 2002;99:6416–6421. doi: 10.1073/pnas.072072599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gasperini D, Chauvin A, Acosta IF, Kurenda A, Stolz S, Chetelat A, Wolfender JL, Farmer EE. Axial and radial oxylipin transport. Plant Physiol. 2015;169:2244–2254. doi: 10.1104/pp.15.01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mousavi SA, Chauvin A, Pascaud F, Kellenberger S, Farmer EE. GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature. 2013;500:422–426. doi: 10.1038/nature12478. [DOI] [PubMed] [Google Scholar]
- 12.Toyota M, Spencer D, Sawai-Toyota S, Jiaqi W, Zhang T, Koo AJ, Howe GA, Gilroy S. Glutamate triggers long-distance, calcium-based plant defense signaling. Science. 2018;361:1112–1115. doi: 10.1126/science.aat7744. [DOI] [PubMed] [Google Scholar]
- 13.Chauvin A, Lenglet A, Wolfender JL, Farmer EE. Paired hierarchical organization of 13-lipoxygenases in Arabidopsis. Plants (Basel). 2016:5. doi: 10.3390/plants5020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan C, Fan M, Yang M, Zhao J, Zhang W, Su Y, Xiao L, Deng H, Xie D. Injury activates Ca(2+)/calmodulin-dependent phosphorylation of JAV1-JAZ8-WRKY51 complex for jasmonate biosynthesis. Mol Cell. 2018;70:136–149.e137. doi: 10.1016/j.molcel.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Schulze A, Zimmer M, Mielke S, Stellmach H, Melnyk CW, Hause B, Gasperini D. Wound-induced shoot-to-root relocation of JA-Ile precursors coordinates Arabidopsis growth. Mol Plant. 2019;10:1383–1394. doi: 10.1016/j.molp.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Matsuura H, Takeishi S, Kiatoka N, Sato C, Sueda K, Masuta C, Nabeta K. Transportation of de novo synthesized jasmonoyl isoleucine in tomato. Phytochemistry. 2012;83:25–33. doi: 10.1016/j.phytochem.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Sato C, Aikawa K, Sugiyama S, Nabeta K, Masuta C, Matsuura H. Distal transport of exogenously applied jasmonoyl-isoleucine with wounding stress. Plant Cell Physiol. 2011;52:509–517. doi: 10.1093/pcp/pcr011. [DOI] [PubMed] [Google Scholar]
- 18.Sato C, Seto Y, Nabeta K, Matsuura H. Kinetics of the accumulation of jasmonic acid and its derivatives in systemic leaves of tobacco (Nicotiana tabacum cv. Xanthi nc) and translocation of deuterium-labeled jasmonic acid from the wounding site to the systemic site. Biosci Biotechnol Biochem. 2009;73:1962–1970. doi: 10.1271/bbb.90119. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z, Baldwin IT. Transport of [2-14C]jasmonic acid from leaves to roots mimics wound-induced changes in endogenous jasmonic acid pools in Nicotiana sylvestris. Planta. 1997;201:436–441. [Google Scholar]
- 20.Li M, Wang F, Li S, Yu G, Wang L, Li Q, Zhu X, Li Z, Yuan L, Liu P. Importers drive leaf-to-Leaf jasmonic acid transmission in wound-Induced systemic immunity. Mol Plant. 2020;13:1485–1498. doi: 10.1016/j.molp.2020.08.017. [DOI] [PubMed] [Google Scholar]
- 21.Li Q, Zheng J, Li S, Huang G, Skilling SJ, Wang L, Li L, Li M, Yuan L, Liu P. Transporter-mediated nuclear entry of jasmonoyl-isoleucine is essential for jasmonate signaling. Mol Plant. 2017;10:695–708. doi: 10.1016/j.molp.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Wang T, Liang L, Xue Y, Jia PF, Chen W, Zhang MX, Wang YC, Li HJ, Yang WC. A receptor heteromer mediates the male perception of female attractants in plants. Nature. 2016;531:241–244. doi: 10.1038/nature16975. [DOI] [PubMed] [Google Scholar]


