ABSTRACT
Ammonium (NH4+) is known to produce alterations in root-system architecture, notably, by inhibiting primary root elongation and stimulating lateral root branching. This stimulation is associated with higher auxin transport promoted by apoplast acidification. Recently, we showed that MYB28 and MYB29 transcription factors play a role in ammonium tolerance, since its double mutant (myb28myb29) is highly hypersensitive toward ammonium nutrition in relation to altered Fe homeostasis. In the present work, we observed that primary root elongation was lower in the mutant with respect to wild-type plants under ammonium nutrition. Moreover, ammonium-induced lateral root branching was impaired in myb28myb29 in a Fe-supply dependent manner. Further research is required to decipher the link between MYB28 and MYB29 functions and the signaling pathway leading to root-system architecture modification by NH4+ supply.
KEYWORDS: Ammonium stress, Arabidopsis thaliana, glucosinolate, iron, lateral root, myb28myb29, primary root, signaling
Plants shape their root-system architecture (RSA) in order to adapt to soil heterogeneity and therefore, to optimize nutrient uptake. Ammonium (NH4+) is a major inorganic nitrogen (N) source readily available in most natural and agricultural soils for plant uptake. However, a high concentration of NH4+ in the soil is a cause of stress in most plant species. NH4+ competes with the uptake of other essential cations, leads to changes in hormonal homeostasis and may inhibit photosynthesis, produce oxidative stress and generate osmotic unbalance. These alterations commonly entail visible symptoms such as the general loss of biomass and leaf chlorosis.1–5 Furthermore, NH4+ provokes changes in RSA by 1) inhibiting primary root (PR) and lateral roots (LR) elongation, through the repression of cell growth and proliferation, and 2) stimulating root branching.4,6
Reduced PR length is a common marker of ammonium stress. This inhibition responds to a number of different ammonium-related processes. Among others, NH4+ stress is known to generate the overproduction of reactive oxygen species (ROS), and this increase could have a negative impact on root meristem cells proliferation.7 Indeed, Xie et al.8 showed ammonium-induced root shortening alleviation in the rice overexpressor OsSE5/HO1 (heme-heme oxygenase 1), which has a higher ROS-detoxifying capacity. Besides, Arabidopsis vtc1-1 mutant, which is deficient in GDP-mannose pyrophosphorylase (GMPase), shows a dramatic PR inhibition under NH4+ supply. Still unclear, the reasons underlying vtc1-1 phenotype appear associated with disturbed protein N-glycosylation, perturbed auxin and ethylene homeostases and pH alterations associated with NH4+ uptake.9–11
LR branching is induced under local low-NH4+ concentrations in an AMT1;3 NH4+ transporter-dependent manner.12 Very recently, Meier et al.13 demonstrated that ammonium-induced LR branching is a consequence of root apoplast acidification mediated by NH4+ uptake via AMTs. This acidification increases the protonation of indole-3-acetic acid and thus, its transport into the epidermal cell to finally promote LR formation.13
In a recent work, we reported that Arabidopsis double mutant for MYB28 and MYB29 transcription factors (myb28myb29) is highly hypersensitive to ammonium nutrition.14MYB28 and MYB29 are master regulators of aliphatic glucosinolate (GSL) biosynthesis.15 The hypersensitivity of myb28myb29 to ammonium nutrition was associated with a clear leaf chlorosis and reduced biomass. These symptoms appeared independent of the absence of aliphatic GSLs but were related to a defective Fe homeostasis.14 In the present work, we analyzed whether RSA was also affected in myb28myb29 in response to NH4+. To do so, wild-type Col-0 (WT) and myb28myb29 plants grown as described in Coleto et al.14 were scanned using an EPSON expression 10000 XL scanner. PR length was analyzed using ImageJ software and LR density calculated by dividing PR length by the number of LRs.
As expected, ammonium nutrition provoked PR elongation inhibition with respect to control plants grown with nitrate, being this inhibition stronger when the concentration of NH4+ supplied was higher (Figure 1a,b). In agreement with the reported myb28myb29 sensitivity, PR length in the mutant was shorter with respect to WT plants (Figure 1a,b). Regarding LR number and density, moderate ammonium stress (5 mM NH4+) provoked the reported stimulation of LR emergence in WT plants. However, the stimulation was not observed under severe ammonium stress (10 mM NH4+) (Figure 1a,c,d). Apart from the stress severity, this absence of stimulation could be due to the repressive effect that Gln levels derived from NH4+ assimilation have on LR formation.9
Figure 1.
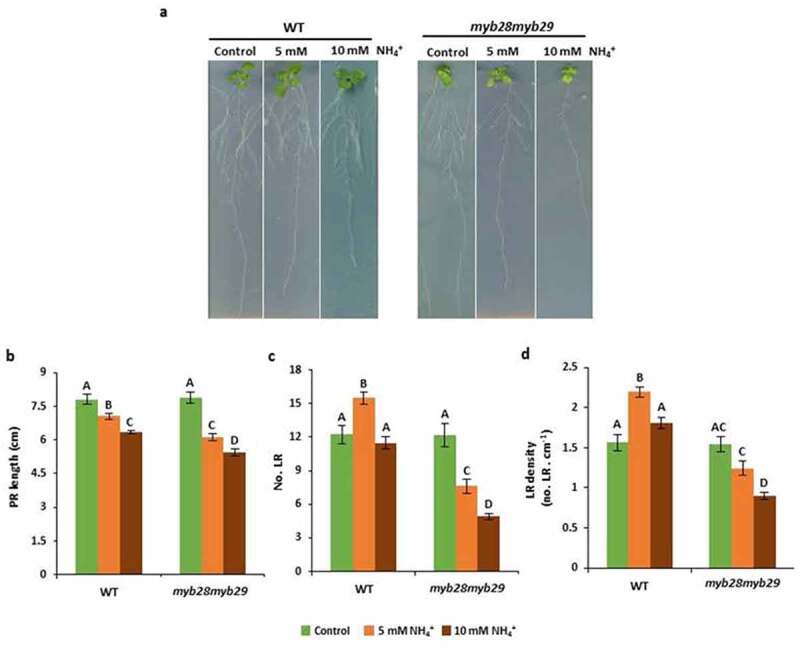
Root-system architecture is affected in myb28myb29 under ammonium nutrition. (a) Representative images, (b) PR length, (c) number of LR and (d) LR density of WT and myb28myb29 plants grown with 5 mM NO3− (control), 5 mM NH4+, or 10 mM NH4+ for 15 days. Values represent mean ± SE (n = 20). Different letters indicate significant differences between treatments and genotypes (one-way ANOVA followed by Duncan´s post hoc test, p ≤ 0.05)
In myb28myb29, ammonium-induced stimulation of LR number and density did not occur (Figure 1a,c,d); therefore, opening the question of whether MYB28, MYB29 function might be somehow associated with pH-related auxin transport. In the same line, as reported for myb28myb29 hypersensitivity,14 the observed effect on RSA was also independent of the lack of aliphatic GSLs, since myc234 mutant, which is also devoid of aliphatic GSLs,16 did not display any RSA difference with respect to WT plants (data not shown). In addition, in Coleto et al.,14 we also reported that when myb28myb29 was grown with a higher Fe supply (200 µM respect to 100 µM) its hypersensitivity to ammonium was restored. In this work, we also show that the differences in RSA observed between WT and myb28myb29 disappeared when growing the plants with 200 µM Fe supply (Figure 2). Besides MYB28 and MYB29, the disruption of other genes including GMPase10 and AMT1;3,12 as described above, and others such as GSA-1/ARG1 (GRAVITROPISM-SENSITIVE-TO-AMMONIUM-1), that encodes a DnaJ-like protein, and AMOT1 (AMMONIUM TOLERANCE 1) that encodes an EIN3 transcription factor, provokes alteration in RSA in response to ammonium nutrition. In particular, root gravitropism is affected in gsa-1 mutant and a lower inhibition of lateral root formation by ammonium stress was reported in amot117,18. In these works, the potential association with Fe supply or GLS metabolism was not studied and thus, at present, it is difficult to predict a potential link between the function of MYB28 and MYB29 and these genes. Future studies will be of great interest to analyze the potential interconnection among these genes in RSA response to ammonium.
Figure 2.
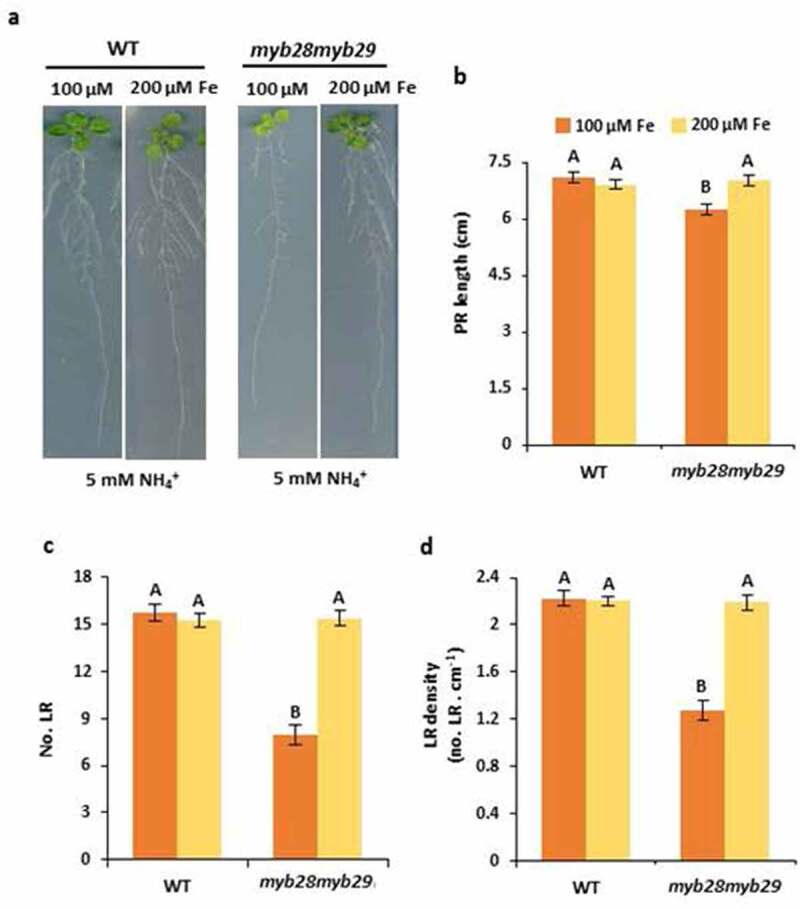
Myb28myb29 root-system architecture is restored by higher Fe supply. (a) Representative images, (b) PR length (c) number of LR and (d) LR density of WT and myb28myb29 plants grown with 5 mM NH4+ and 100 µM or 200 µM Fe supply for 15 days. Values represent mean ± SE (n = 20). Different letters indicate significant differences between treatments and genotypes (one-way ANOVA followed by Duncan´s post hoc test, p ≤ 0.05)
Altogether, our data indicate that the disruption of Fe homeostasis, as a consequence of the functional lack of MYB28 and MYB29 transcription factors, may also be involved in ammonium-mediated changes in RSA.
Acknowledgments
We thank Masami Hirai and Andrea Chini for kindly providing seeds for the myb28myb29 and myc234 mutants, respectively.
Funding Statement
The research leading to these results has received funding from the Basque Government, IT-932-16 and the Spanish Government (BIO2014-56271-R and BIO2017-84035-R, co-funded by FEDER). IB holds a fellowship from the Basque Government.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Britto DT, Kronzucker HJ.. NH4+ toxicity in higher plants: a critical review. J Plant Physiol. 2002;159:1–4. [Google Scholar]
- 2.Li B, Li G, Kronzucker HJ, Baluška F, Shi W.. Ammonium stress in Arabidopsis: signaling, genetic loci, and physiological targets. Trends Plant Sci. 2014;19:107–114. doi: 10.1016/j.tplants.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Esteban R, Ariz I, Cruz C, Morán JF. Mechanisms of ammonium toxicity and the quest for tolerance. Plant Sci. 2016;248:92–101. doi: 10.1016/j.plantsci.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, von Wirén N. Ammonium as signal for physiological and morphological responses in plants. J Exp Bot. 2017;68:2581–2592. doi: 10.1093/jxb/erx086. [DOI] [PubMed] [Google Scholar]
- 5.Marino D, Morán JF. Can ammonium stress be positive for plant performance? Front Plant Sci. 2019;10:1103. doi: 10.3389/fpls.2019.01103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buezo J, Esteban R, Cornejo A, López-Gómez P, Marino D, Champizo-Ampudia A, Gil MJ, Martínez-Merino V, Morán JF. IAOx induces the SUR phenotype and differential signaling from IAA under different types of nitrogen nutrition in Medicago truncatula roots. Plant Sci. 2019;287:110176. doi: 10.1016/j.plantsci.2019.110176. [DOI] [PubMed] [Google Scholar]
- 7.Tsukagoshi H, Busch W, Benfey PN. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell. 2010;143:606–616. doi: 10.1016/j.cell.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 8.Xie Y, Mao Y, Xu S, Zhou H, Duan X, Cui W, Zhang J, Xu G. Heme-heme oxygenase 1 system is involved in ammonium tolerance by regulating antioxidant defence in Oryza sativa. Plant Cell Environ. 2015;38:129–143. doi: 10.1111/pce.12380. [DOI] [PubMed] [Google Scholar]
- 9.Qin C, Qian WQ, Wang WF, Wu Y, Yu CM, Jiang XH, Wang DW, Wu P. GDP-mannose pyrophosphorylase is a genetic determinant of ammonium sensitivity in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2008;105:18308–18313. doi: 10.1073/pnas.0806168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barth C, Gouzd ZA, Steele HP, Imperio RM. A mutation in GDP-mannose pyrophosphorylase causes conditional hypersensitivity to ammonium, resulting in Arabidopsis root growth inhibition, altered ammonium metabolism, and hormone homeostasis. J Exp Bot. 2010;61:379–394. doi: 10.1093/jxb/erp310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kempinski CF, Haffar R, Barth C. Toward the mechanism of NH4+ sensitivity mediated by Arabidopsis GDP-mannose pyrophosphorylase. Plant Cell Environ. 2011;34:847–858. doi: 10.1111/j.1365-3040.2011.02290.x. [DOI] [PubMed] [Google Scholar]
- 12.Lima JE, Kojima S, Takahashi H, von Wirén N. Ammonium triggers lateral root branching in Arabidopsis in an AMMONIUM TRANSPORTER1;3-dependent manner. Plant Cell. 2010;22:3621–3633. doi: 10.1105/tpc.110.076216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meier M, Liu Y, Lay-Pruitt KS, Takahashi H, von Wirén N. Auxin-mediated root branching is determined by the form of available nitrogen. Nat Plants. 2020;6:1136–1145. doi: 10.1038/s41477-020-00756-2. [DOI] [PubMed] [Google Scholar]
- 14.Coleto I, Bejarano I, Marín-Peña AJ, Medina J, Rioja C, Burow M, Marino D. Arabidopsis thaliana transcription factors MYB28 and MYB29 shape ammonium stress responses by regulating Fe homeostasis. New Phytol. 2020. doi: 10.1111/nph.16918. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Sawada Y, Hirai A, Sato M, Kuwahara A, Yan X, Hirai MY. Novel insights into the function of Arabidopsis R2R3-MYB transcription factors regulating aliphatic glucosinolate biosynthesis. Plant Cell Physiol. 2013;54:1335–1344. doi: 10.1093/pcp/pct085. [DOI] [PubMed] [Google Scholar]
- 16.Schweizer F, Fernández-Calvo P, Zander M, Diez-Díaz M, Fonseca S, Glauser G, Lewsey MG, Ecker JR, Solano R, Reymond P. Arabidopsis basic helix-loop-helix transcription factors MYC2, MYC3, and MYC4 regulate glucosinolate biosynthesis, insect performance, and feeding behavior. Plant Cell. 2013;25:3117–3132. doi: 10.1105/tpc.113.115139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou N, Li B, Chen H, Su Y, Kronzucker HJ, Xiong L, Baluška F, Shi W. GSA-1/ARG1 protects root gravitropism in Arabidopsis under ammonium stress. New Phytol. 2013;200:97–111. doi: 10.1111/nph.12365. [DOI] [PubMed] [Google Scholar]
- 18.Li G, Zhang L, Wang M, Di D, Kronzucker HJ, Shi W. The Arabidopsis AMOT1/EIN3 gene plays an important role in the amelioration of ammonium toxicity. J Exp Bot. 2019;70:1375–1388. doi: 10.1093/jxb/ery457. [DOI] [PMC free article] [PubMed] [Google Scholar]


