Abstract
Background
Inflammation and immune surveillance evasion are cancer hallmarks. Peripheral blood leukocytes (PBLs) represent both. The aim of this study is to examine PBLs as predictors of outcomes in oral cavity squamous cell carcinoma (OSCC), and to find specific cutoffs with the goal of including PBLs as host factor in patients’ preoperative risk assessment.
Methods
Previously established head and neck squamous cell carcinoma (HNSCC) cutoffs were examined in an independent cohort of 1369 OSCC patients. Then optimal OSCC cutoffs were found and validated in the subset of patients with OSCC (n = 119) from the external HNSCC cohort. The PBLs analyzed were neutrophils, monocytes and lymphocytes individually, neutrophil-to-lymphocyte ratio (NLR), and a combined index using all PBLs called Systematic Inflammation Response Index (SIRI).
Results
All parameters were significant predictors of survival using previous cutoffs. However, OSCC cutoffs stratified survival outcomes better. Considering neutrophils ≤ 4.8 ×109/L as reference, patients with 4.8–9.1 ×109/L neutrophils had 1.536 times higher risk of death (95% CI: 1.295–1.822), and patients with ≥ 9.1 ×109/L had 3.076 times higher risk (95% CI: 2.170–4.360). All PBLs maintained independent prognostic capacity in multivariable analysis. Neutrophils, NLR, and SIRI were significant predictors of survival when validating OSCC cutoffs in the external validation cohort.
Conclusions
Pretreatment peripheral blood neutrophils, NLR and SIRI are the most robust independent predictors of overall survival amongst all PBLs in OSCC. We report externally validated cutoffs that demonstrate the feasibility of including PBLs as host features in the preoperative prognostication of OSCC.
Keywords: Neutrophils, Monocytes, Lymphocytes, NLR, SIRI, Mouth Neoplasms, Biomarkers
Precis for use in the Table of Contents:
Pretreatment peripheral blood neutrophils, NLR and SIRI are the most robust independent predictors of overall survival amongst all PBLs in OSCC. We report externally validated cutoffs that demonstrate the feasibility of including PBLs as host features in the preoperative prognostication of OSCC.
INTRODUCTION
Inflammation and immune surveillance have been recently established as cancer hallmarks, emphasizing the role of the host when analyzing the complexity of cancer, its treatment, and outcomes. Inflammation promotes tumor initiation and progression. Immune surveillance evasion reflects the immune system’s failure to detect and eradicate tumor cells, allowing cancer to develop and spread.1–3 It has been shown in different tumor models that higher infiltration by neutrophils and macrophages in the tumor microenvironment is associated with worse oncological outcomes.4–7 On the contrary, higher lymphocyte infiltration is associated with better outcomes.8
At a systemic level, the same correlations have been studied in the peripheral blood. The most analyzed parameters in the literature are the pretreatment absolute count of neutrophils, monocytes, and lymphocytes, as well as the ratio between neutrophils and lymphocytes (NLR). These have been reported to be strong predictors of outcomes in a wide range of tumor models, including head and neck tumors.9–20 Moreover, to integrate the influence of all these variables, Qi et al. have recently proposed an index called Systemic Inflammation Response Index (SIRI), which combines all the leukocytes of interest (neutrophils, monocytes, and lymphocytes), showing that it has prognostic capacity in pancreatic cancer.21
Multiple studies have been published analyzing the prognostic capacity of pretreatment peripheral blood leukocytes (PBLs) using different cutoffs found with different methods. However, a limited number of studies aimed to validate established cutoffs or define universal cutoffs that can be used on a daily clinical basis when preoperatively assessing patient’s risk for worse outcomes.22
A previous study showed that higher pretreatment peripheral neutrophils, monocytes, NLR, and lower lymphocytes are associated with worse outcomes in head and neck squamous cell carcinomas (HNSCC).23 The previous cohort included patients treated both surgically and non-surgically, and tumors from different head and neck sites, with a limited number of patients with oral cavity tumors (n = 119, 14.4%).
Since oral cavity cancer is mainly treated surgically and is a distinct entity, the first aim of this study is to examine the role of PBLs as predictors of outcomes in an independent cohort of patients with oral cavity squamous cell carcinoma (OSCC). The second aim is to find the optimal cutoffs for OSCC, with the goal of analyzing the feasibility of including PBLs as host features in the preoperative assessment of prognosis for OSCC patients.
MATERIALS AND METHODS
The previous study was performed in 824 patients with biopsy-proven squamous cell carcinoma of the oral cavity, oropharynx, larynx, and hypopharynx diagnosed and treated in Hospital de la Santa Creu i Sant Pau (HSCSP) from 2000 to 2012. Optimal cutoffs for each PBL were found using a recursive-partitioning analysis (RPA), with disease-specific survival (DSS) as the outcome of interest.23
We first validated these previously reported cutoffs in 1369 patients with OSCC treated at Memorial Sloan Kettering Cancer Center (MSK) from 1998 to 2015 (Group A: test set). The cohort was selected from MSK’s departmental database of patients who had a biopsy-proven invasive OSCC treated with primary surgery. Exclusion criteria were synchronous HNSCC, prior treatment of the reference carcinoma, distant metastasis at presentation, and prior history of non-endocrine head and neck cancer. Patients without available leukocyte counts within a month prior to start of treatment were excluded (n = 8). The retrospective study design was approved by MSK’s Institutional Review Board.
Optimal cutoffs for the MSK OSCC cohort were identified using an RPA (CART-method) with overall survival (OS) as outcome of interest.24 Recursive-partitioning analyses create a decision tree by finding the optimal cutoffs of the independent variable (leukocyte counts in this study) that will classify the cohort into groups with significant differences in the dependent variable (overall survival in this study). This method provides different number of cutoffs for each independent variable tested depending on the stratification that will more accurately group the cohort in terms of the dependent variable. The PBLs analyzed were the absolute counts of neutrophils, monocytes, and lymphocytes, NLR, and SIRI, the recently published index that combines the 3 absolute counts using the following formula: neutrophils*monocytes/lymphocytes. The established cutoffs from both studies are shown in Supplementary Table 1.
A multivariable analysis for each PBL as predictor of OS was conducted including the clinicopathologic characteristics that were significant in the univariable analysis and the PBL categories obtained with the RPA as independent variables.
The cutoffs identified in the test set (Group A) were externally validated in the subset of 119 patients with OSCC from the previous HSCSP cohort (Group B: validation set). The study was approved by HSCSP’s Institutional Review Board. The description of clinicopathologic characteristics and the comparison between Group A and Group B are shown in Table 1. Median values of each PBL for both groups are shown in Supplementary Table 2. The average count of each PBL according to the clinicopathologic characteristics are shown in Supplementary Table 3.
Table 1.
Clinicopathologic characteristics of the test set (Group A) and validation set (Group B)
| Characteristics | MSKa OSCCb GROUP A (n = 1369) | HSCSPc OSCC GROUP B (n = 119) | P value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age Mean (SDd, range) years | 61.9 (14.4, 18.3–100.4) | 66.2 (12.9, 31.1–91.7) | 0.002 | ||
| Sex | 0.032 | ||||
| Female | 599 | 43.8% | 40 | 33.6% | |
| Male | 770 | 56.2% | 79 | 66.4% | |
| Tobacco | 0.301 | ||||
| Never | 514 | 37.5% | 39 | 32.8% | |
| Ever | 855 | 62.5% | 80 | 67.2% | |
| Alcohol | 0.096 | ||||
| Never | 417 | 30.5% | 45 | 37.8% | |
| Ever | 952 | 69.5% | 74 | 62.2% | |
| WUHNCIe | 0.013 | ||||
| 0 | 977 | 71.4% | 72 | 60.5% | |
| ≥ 1 | 392 | 28.6% | 47 | 39.5% | |
| Subsite | |||||
| Oral tongue | 734 | 53.6% | 49 | 41.2% | < 0.001 |
| Lower gum | 181 | 13.2% | 7 | 5.9% | |
| Floor of mouth | 162 | 11.8% | 28 | 23.5% | |
| Buccal mucosa | 105 | 7.7% | 14 | 11.8% | |
| Upper gum | 93 | 6.8% | 3 | 2.5% | |
| Retromolar trigone | 68 | 5.0% | 12 | 10.1% | |
| Hard palate | 26 | 1.9% | 6 | 5.0% | |
| pTf stage (AJCCg 8th edition) | 0.003 | ||||
| pT1 | 446 | 32.6% | 32 | 26.9% | |
| pT2 | 342 | 25.0% | 35 | 29.4% | |
| pT3 | 258 | 18.8% | 18 | 15.1% | |
| pT4 | 256 | 18.7% | 34 | 28.6% | |
| Not recorded | 67 | 4.9% | 0 | 0.0% | |
| pNh stage (AJCC 8th edition) | 0.014 | ||||
| pN0 | 936 | 68.4% | 70 | 58.8% | |
| pN1 | 124 | 9.1% | 17 | 14.3% | |
| pN2 | 132 | 9.6% | 21 | 17.6% | |
| pN3 | 158 | 11.5% | 11 | 9.2% | |
| Not recorded | 19 | 1.4% | 0 | 0.0% | |
| Overall stage (AJCC 8th edition) | 0.013 | ||||
| Stage I | 406 | 29.7% | 28 | 23.5% | |
| Stage II | 241 | 17.6% | 22 | 18.5% | |
| Stage III | 210 | 15.3% | 21 | 17.6% | |
| Stage IV | 441 | 32.2% | 48 | 40.3% | |
| Not recorded | 71 | 5.2% | 0 | 0.0% | |
| Grade | < 0.001 | ||||
| Well differentiated | 231 | 16.9% | 30 | 25.2% | |
| Moderately differentiated | 870 | 63.6% | 85 | 75.4% | |
| Poorly differentiated | 204 | 14.9% | 4 | 3.4% | |
| Not recorded | 64 | 4.7% | 0 | 0.0% | |
| Treatment | < 0.001 | ||||
| Surgery | 851 | 62.2% | 53 | 44.5% | |
| Surgery + radiotherapy | 394 | 28.8% | 23 | 19.4% | |
| Surgery + chemoradiotherapy | 124 | 9.1% | 6 | 5.0% | |
| Radiotherapy/chemoradiotherapy | 0 | 0.0% | 30 | 25.2% | |
| Palliative | 0 | 0.0% | 7 | 5.9% | |
MSK: Memorial Sloan Kettering;
OSCC: oral cavity squamous cell carcinoma;
HSCSP: Hospital de la Santa Creu i Sant Pau;
SD: standard deviation;
WUHNCI: Washington University Head and Neck Comorbidity Index;
pT: pathological tumor;
AJCC: American Joint Committee on Cancer;
pN: pathological nodal
Student’s t-test, Pearson’s chi-squared test, or Fisheŕs exact test were used to compare the clinicopathological characteristics. We evaluated the relationship between the leukocyte counts and patient characteristics using student’s t test or 1-way ANOVA. Survival curves were calculated according to the Kaplan-Meier method, and differences in survival were compared using the log-rank test. Hazard ratios were calculated according to Cox’s proportional hazard regression model, also used to perform the multivariable analyses. A P value of less than 0.05 was considered statistically significant. All statistical analyses were conducted using SPSS (v25.0, IBM Corporation; Somers, NY) and Stata (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC).
RESULTS
Description of the test set (Group A; n = 1369)
The clinicopathologic characteristics of the cohort are shown in Table 1 (Group A). The mean age was 62 years (range 18–100), and 56.2% were men. History of tobacco and alcohol use was reported by 62.5% and 69.5%, respectively. Comorbidities were recorded according to the Washington University Head and Neck Comorbidity Index (WUHNCI), with 28.6% of the patients having a WUHNCI ≥ 1 at time of diagnosis.25 The most common primary tumor subsite was tongue (53.6%). A total of 47.5% of patients had an advanced pathological stage (III–IV), according to American Joint Committee on Cancer 8th Edition TNM classification.26 Median follow-up time was 39 months (range, 1–221). Five-year OS and DSS were 64.1% and 79.8%, respectively.
Validation of the previous cutoffs in the test set (Group A)
We first analyzed the 5-year OS and DSS in the MSK cohort (n = 1369) using the previously published cutoffs (Supplementary Table 4).23 All PBLs were significant predictors of OS. Regarding DSS, neutrophils and NLR were significant predictors of outcomes (P < 0.001) and monocytes showed nearly significant differences (P = 0.099). Neutrophils showed the best discrimination for both OS and DSS. Survival curves according to neutrophil count categories defined by the previous cutoffs are shown in Supplementary Figure 1.
Optimal PBL cutoffs for the test set (Group A; n = 1369)
Differences in OS and DSS using the optimal cutoffs found in our test cohort were analyzed (Table 2). All PBLs categorized by the new cutoffs were found to be good predictors of outcomes for both OS and DSS. Only the absolute count of lymphocytes showed no significant differences in DSS (P = 0.246).
Table 2.
Survival outcomes in Group A patients according to leukocyte counts using the new specific OSCCa cutoffs
| Number of patients (n=1369) | 5-year OSb % | HRc (95% CId) | P value | 5-year DSSe % | HR (95% CI) | P value | |
|---|---|---|---|---|---|---|---|
| Neutrophils1 | |||||||
| ≤ 4.8 | 770 | 69.7 | 1 | < 0.001 | 83.2 | 1 | < 0.001 |
| 4.8–9.1 | 549 | 59.4 | 1.536 (1.295–1.822) | 76.4 | 1.539 (1.185–1.999) | ||
| ≥ 9.1 | 50 | 31.5 | 3.076 (2.170–4.360) | 59.9 | 3.069 (1.816–5.186) | ||
| Monocytes1 | |||||||
| ≤ 0.3 | 577 | 71.3 | 1 | < 0.001 | 83.3 | 1 | 0.014 |
| > 0.3 | 792 | 58.6 | 1.473 (1.240–1.749) | 76.9 | 1.387 (1.068–1.802) | ||
| Lymphocytes1 | |||||||
| ≤ 0.8 | 69 | 41.4 | 1 | < 0.001 | 75.6 | 1 | 0.246 |
| > 0.8 | 1300 | 65.3 | 0.427 (0.314–0.579) | 80.0 | 0.709 (0.396–1.267) | ||
| NLRf | |||||||
| ≤ 2.9 | 783 | 69.5 | 1 | < 0.001 | 83.1 | 1 | < 0.001 |
| 2.9–5.7 | 481 | 61.9 | 1.376 (1.151–1.646) | 77.8 | 1.462 (1.114–1.919) | ||
| ≥ 5.7 | 105 | 35.6 | 3.083 (2.396–3.967) | 61.6 | 2.941 (1.976–4.377) | ||
| SIRIg | |||||||
| ≤ 1.0 | 675 | 72.5 | 1 | < 0.001 | 84.0 | 1 | < 0.001 |
| 1.0–1.9 | 450 | 62.2 | 1.543 (1.273–1.871) | 79.0 | 1.404 (1.046–1.885) | ||
| ≥ 1.9 | 244 | 44.5 | 2.485 (2.017–3.061) | 67.9 | 2.261 (1.644–3.111) | ||
OSCC: oral cavity squamous cell carcinoma;
OS: overall survival;
HR: hazard ratio;
CI: confidence interval;
DSS: disease-specific survival;
NLR: neutrophil-to-lymphocyte ratio;
SIRI: Systemic Inflammation Response Index.
Units × 109/L
Again, neutrophils seemed to be the variable with the best discrimination for OS and DSS, with a 1.5 times higher risk of death (overall and disease-specific) in patients with 4.8–9.1 × 109/L neutrophils and a 3 times higher risk in patients with ≥ 9.1 × 109/L compared to patients with neutrophils ≤ 4.8 × 109/L (P < 0.001). Survival curves according to the neutrophil count categories defined by the new cutoffs are shown in Figure 1. OS curves for the other PBLs are shown in Figure 2, and DSS curves are shown in Supplementary Figure 2. Additionally, receiver operating characteristic (ROC) curves for all the parameters analyzed are shown in Supplementary Figure 3.
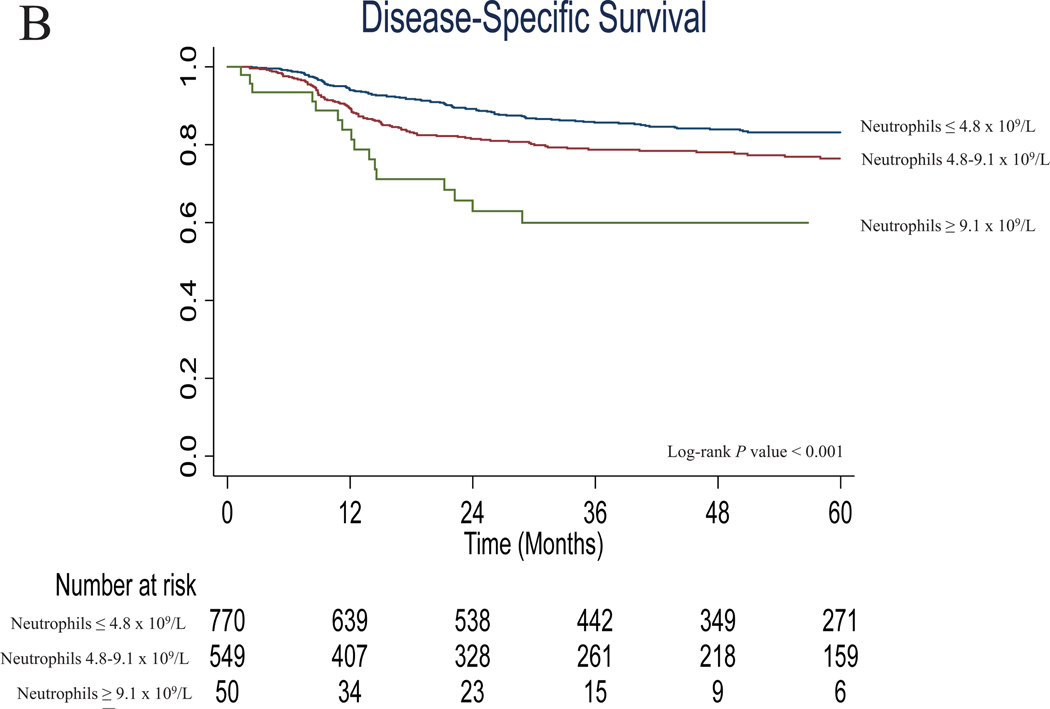
Figure 1.
(a) Overall survival, and (b) disease-specific survival in Group A according to neutrophil count categories defined by the new oral cavity cutoffs
Figure 2.
Overall survival in Group A according to (a) Monocytes, (b) Lymphocytes, (c) Neutrophil-to-Lymphocyte Ratio (NLR), and (d) Systemic Inflammation Response Index (SIRI) categories defined by the new oral cavity cutoffs
Finally, we carried out an individual multivariable analysis for each PBL as predictors of OS. All PBLs maintained their prognostic capacity in the multivariable analysis and were therefore independent prognostic factors. The multivariable analysis including SIRI is shown in Table 3. The multivariable analyses for neutrophils, monocytes, lymphocytes, and NLR are not shown.
Table 3.
Multivariable analysis in Group A including SIRIa as independent variable for predicting overall survival
| Variable | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| HRb | 95% CIc | P value | HR | 95% CI | P value | |
| Age | < 0.001 | < 0.001 | ||||
| ≤ 60 years | 1 | 1 | ||||
| > 60 years | 2.017 | 1.688–2.410 | 1.904 | 1.569–2.311 | ||
| Sex | 0.490 | |||||
| Female | 1 | |||||
| Male | 0.943 | 0.800–1.113 | ||||
| Tobacco use | 0.004 | 0.841 | ||||
| Never | 1 | 1 | ||||
| Ever | 1.297 | 1.089–1.545 | 1.020 | 0.841–1.237 | ||
| Alcohol use | 0.269 | |||||
| Never | 1 | |||||
| Ever | 0.906 | 0.759–1.080 | ||||
| WUHNCId | < 0.001 | 0.078 | ||||
| 0 | 1 | 1 | ||||
| ≥ 1 | 1.614 | 1.359–1.917 | 1.187 | 0.981–1.436 | ||
| Vascular invasion | < 0.001 | 0.080 | ||||
| Absent | 1 | 1 | ||||
| Present | 2.119 | 1.713–2.621 | 1.242 | 0.975–1.583 | ||
| Perineural invasion | < 0.001 | 0.013 | ||||
| Absent | 1 | 1 | ||||
| Present | 2.143 | 1.806–2.543 | 1.310 | 1.060–1.620 | ||
| Margin status | < 0.001 | 0.001 | ||||
| Negative | 1 | 1 | ||||
| Close | 1.531 | 1.252–1.871 | 1.282 | 1.025–1.603 | ||
| Positive | 2.962 | 2.287–3.836 | 1.794 | 1.317–2.445 | ||
| Histologic grade | < 0.001 | 0.613 | ||||
| Well differentiated | 1 | 1 | ||||
| Moderately differentiated | 1.542 | 1.207–1.970 | 1.149 | 0.863–1.531 | ||
| Poorly differentiated | 2.203 | 1.647–2.946 | 1.174 | 0.826–1.670 | ||
| pTestatus (AJCCf 8th Edition) | 1 | < 0.001 | 1 | < 0.001 | ||
| pT1 | 1.632 | 1.266–2.104 | 1.323 | 1.001–1.749 | ||
| pT2 | 2.582 | 2.008–3.320 | 1.543 | |||
| pT3 | 4.076 | 3.212–5.173 | 2.183 | 1.129–2.111 | ||
| pT4 | 1.588–3.001 | |||||
| pNg status (AJCC 8th Edition) | < 0.001 | < 0.001 | ||||
| pN0 | 1 | 1 | ||||
| pN1 | 1.527 | 1.148–2.031 | 1.622 | 1.179–2.232 | ||
| pN2 | 2.206 | 1.710–2.846 | 2.138 | |||
| pN3 | 5.461 | 4.409–6.765 | 3.628 | 1.578–2.896 2.708–4.860 | ||
| Adjuvant treatment | < 0.001 | < 0.001 | ||||
| None | 1 | 1 | ||||
| Radiotherapy | 1.412 | 1.181–1.689 | 0.576 | 0.456–0.727 | ||
| Chemoradiotherapy | 2.208 | 1.686–2.892 | 0.523 | 0.374–0.733 | ||
| SIRI | < 0.001 | < 0.001 | ||||
| ≤ 1.0 | 1 | 1 | ||||
| 1.0–1.9 | 1.543 | 1.273–1.871 | 1.323 | 1.074–1.630 | ||
| ≥ 1.9 | 2.485 | 2.017–3.061 | 1.685 | 1.329–2.135 | ||
SIRI: Systemic Inflammation Response Index;
HR: hazard ratio;
CI: confidence interval;
WUHNCI: Washington University Head and Neck Comorbidity Index;
pT: pathological tumor;
AJCC: American Joint Committee on Cancer;
pN: pathological nodal.
Validation of the new cutoffs in the validation set (Group B; n = 119)
In order to validate the new OSCC cutoffs identified upon analysis of the test set (Group A), the cutoffs were tested in the validation set (Group B), which was formed by the subset of patients from HSCSP that had OSCC (n = 119, 14.4%). The clinicopathologic characteristics are shown in Table 1 (Group B). Compared to the MSK OSCC cohort (Group A), the validation set (Group B) had a higher percentage of males, patients were older, had more comorbidities, and had higher stage tumors. Regarding treatment, Group A’s inclusion criteria only considered primary surgically treated patients. On the other hand, Group B considered all treatment modalities, where 25% of patients were treated non-surgically, and 6% of patients had palliative treatment.
We analyzed if there were differences in OS using the new cutoffs from Group A in the validation set (Group B). The cutoffs for neutrophils, NLR, and SIRI reached statistically significant differences (P < 0.001). Monocytes and lymphocytes did not, even though there were trends for both. Among all parameters analyzed, SIRI was the factor that had the best balance in the number of patients included in each category using the new cutoffs, emphasizing that patients in the highest category (SIRI ≥ 1.9) had 3.069 times higher risk of death (95% CI: 1.655–5.691), compared to patients in the lowest category (SIRI ≤ 1.0) (Table 4). Survival curves are shown in Supplementary Figure 4.
Table 4.
Validation of the cutoffs developed in Group A in the subset of OSCCa from the previous cohort (Group B)
| Number of patients (n = 119) | 5-year OSb % | Log-rank P value | HRc (95% CId) | P value | |
|---|---|---|---|---|---|
| Neutrophils1 | |||||
| ≤ 4.8 | 70 | 60.8 | < 0.001 | 1 | 0.002 |
| 4.8–9.1 | 47 | 37.7 | 1.578 (0.984–2.531) | ||
| ≥ 9.1 | 2 | 0.0 | 11.387 (2.622–49.448) | ||
| Monocytes1 | |||||
| ≤ 0.3 | 8 | 62.5 | 0.314 | 1 | 0.321 |
| > 0.3 | 111 | 49.8 | 1.797 (0.565–5.712) | ||
| Lymphocytes1 | |||||
| ≤ 0.8 | 1 | 0.0 | 0.250 | 1 | 0.275 |
| > 0.8 | 118 | 51.1 | 0.330 (0.045–2.413) | ||
| NLRe | |||||
| ≤ 2.9 | 76 | 65.3 | < 0.001 | 1 | < 0.001 |
| 2.9–5.7 | 39 | 27.2 | 2.382 (1.465–3.871) | ||
| ≥ 5.7 | 4 | 0.0 | 7.815 (2.644–23.098) | ||
| SIRIf | |||||
| ≤ 1.0 | 33 | 69.7 | < 0.001 | 1 | < 0.001 |
| 1.0–1.9 | 46 | 59.8 | 1.292 (0.677–2.464) | ||
| ≥ 1.9 | 40 | 24.0 | 3.069 (1.655–5.691) | ||
OSCC: oral cavity squamous cell carcinoma;
OS: overall survival;
HR: hazard ratio;
CI: confidence interval;
NLR: neutrophil-to-lymphocyte ratio;
SIRI: Systemic Inflammation Response Index.
: Units × 109/L
DISCUSSION
It has been shown in several studies that PBLs predict oncological outcomes in various tumor models, including OSCC.9–20 However, these biomarkers have not gained acceptance for routine use in preoperative prognostication. To add a new factor as a widely used prognostic indicator, the variable must be universally evaluable, easily available, reproducible, have a low cost, and be easy to standardize. PBLs meet all these criteria. The main challenge is to set the optimal cutoffs that can be used universally. To our knowledge, only Cho et al. have tried to set the optimal cutoffs for HNSCC.22 Our aim was to establish the utility of pretreatment PBLs as prognostic factors in OSCC and to find optimal cutoffs that can be translated to routine clinical use.
To evaluate the prognostic capacity of PBLs in OSCC, the previously published cutoffs for HNSCC accurately predicted OS in the test set (Group A). When analyzing DSS, lymphocytes did not show a significant prognostic capacity. This correlated with the previous findings, where lymphocytes had a limited prognostic capacity.
Since OSCC is a separate entity among HNSCC, and is mainly treated with primary surgery, we believe that specific cutoffs for OSCC should be established. In the previous study, there were limited OSCC within the cohort (n = 119, 14.4%), and only 70% of them were primarily surgically treated.23 Therefore, we decided to define optimal cutoffs for OSCC in a large series of 1369 OSCC treated with primary surgery.
Additionally, we included SIRI in the analyses, an index defined by Qi et al., which combines all PBLs.21 We agree with these authors that incorporating the value added by each leukocyte count makes an even more integrative biomarker than analyzing the individual counts. A limited number of studies have been published analyzing the prognostic capacity of SIRI.27–31 To the best of our knowledge, this is the first study analyzing SIRI as a prognostic factor in a large series of OSCC.
When analyzing the prognostic capacity of PBLs using the new cutoffs, we obtained similar results to the previous study.23 Neutrophils, monocytes, NLR, and SIRI were consistent predictors of OS and DSS. Lymphocytes showed a more limited predictive capacity in terms of DSS.
If we compare the optimal cutoffs for the test set (Group A) with the previous cutoffs for HNSCC (Supplementary Table 1), the results for neutrophils were similar. However, for lymphocytes and monocytes, the cutoffs were lower in the OSCC cohort. It follows that NLR cutoffs were higher in the OSCC cohort. Interestingly, the NLR cutoffs were very similar to the optimal NLR cutoffs defined by Cho et al. (2 and 6 vs 2.9 and 5.7 in our study, respectively).22
Finally, we validated the cutoffs identified in the test set (Group A) using an independent subset of patients with OSCC (Group B) from the previous study. Only neutrophils, NLR, and SIRI predicted outcomes in the validation cohort. To explain the lack of validation of monocytes and lymphocytes, we compared the median values of the PBLs between the 2 groups (Supplementary Table 2). The test cohort (Group A) had a higher median for neutrophils and a lower median for monocytes and lymphocytes. These lower thresholds for monocytes and lymphocytes meant that only 8 patients were eligible in the lowest monocyte category, and 1 patient was eligible in the lowest monocyte category. This limited number of patients may explain why the results are not significant, even though we observed the expected trend. The discrepancies in the median values of the leukocytes may also explain the differences in the cutoffs found between the 2 groups.
Baseline differences in PBLs exist depending on clinical characteristics such as age. Valiathan et al. showed that neutrophil and monocyte counts increase with aging, and lymphocyte counts decrease.32 We have seen correlations between PBLs and patient’s clinicopathologic characteristics such as age, sex, history of tobacco and/or alcohol consumption, comorbidities, subsite, stage, and histological grade (Supplementary Table 3). As shown in Table 1, there were differences in these clinicopathologic characteristics between the 2 groups that can account for the different cutoffs and median values. These differences between populations are emblematic of the challenge in establishing universal cutoffs for the less robust predictors such as monocytes and lymphocytes. It is also conceivable that the prognostic influence of leukocytes may be driven by the associations between leukocytes and clinicopathologic characteristics. However, we have shown that all PBLs maintained independent prognostic capacity for OS when analyzed in multivariable analyses including clinicopathologic characteristics.
This study has inherent limitations due to its retrospective nature, and since PBLs values were only analyzed at a single time point prior to initial treatment. PBLs values can be influenced by multiple factors, such as infections, treatment with steroids, or hematologic malignancies. These could not be controlled in this study, and a single snap-shot assessment may not be truly representative of the patient’s immune status. Notwithstanding these limitations, our observations are consistent with previously published studies.
The current staging system for OSCC only considers tumor features. Even though risk stratification of patients is overall accurate, there is considerable heterogeneity within staging groups, especially with increasing stage.33 Differences in host characteristics can partially explain this heterogeneity. Inflammation and immune system evasion are fundamental pillars when trying to understand carcinogenesis, tumor progression, and oncological outcomes. The interplay between these opposing forces is an important host feature that can be easily evaluated using pretreatment PBLs.
Studies developing nomograms including PBLs to predict outcomes in OSCC have recently been used to stratify patients considering both tumor and host factors. However, none of the studies performed an external validation.34, 35 The results of our study provide the basis for considering inclusion of PBLs in the preoperative assessment of prognosis in patients with OSCC using a larger cohort of patients, and aims to further analyze and externally validate the feasibility of including PBLs as host factors in a nomogram-based system.36
CONCLUSIONS
Pretreatment peripheral blood neutrophils, NLR and SIRI are the most robust independent predictors of OS amongst all PBLs in OSCC. We report externally validated cutoffs that demonstrate the feasibility of including PBLs as host features in the preoperative prognostication of OSCC.
Supplementary Material
Acknowledgments:
The authors are thankful to Jessica Massler for editorial support and Laura Pardo for her contribution to the study.
Funding: This study was funded by Fundación Alfonso Martín Escudero and the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Conflict of interest: The authors declare no conflicts of interest pertinent to this work.
REFERENCES
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 2.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. [DOI] [PubMed] [Google Scholar]
- 4.Shen M, Hu P, Donskov F, Wang G, Liu Q, Du J. Tumor-associated neutrophils as a new prognostic factor in cancer: a systematic review and meta-analysis. PLoS One. 2014;9:e98259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao X, Qu J, Sun Y, et al. Prognostic significance of tumor-associated macrophages in breast cancer: a meta-analysis of the literature. Oncotarget. 2017;8:30576–30586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu P, Wu D, Zhao L, et al. Inverse role of distinct subsets and distribution of macrophage in lung cancer prognosis: a meta-analysis. Oncotarget. 2016;7:40451–40460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin S, Huang J, Li Z, et al. The Prognostic and Clinicopathological Significance of Tumor-Associated Macrophages in Patients with Gastric Cancer: A Meta-Analysis. PLoS One. 2017;12:e0170042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Ruiter EJ, Ooft ML, Devriese LA, Willems SM. The prognostic role of tumor infiltrating T-lymphocytes in squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. Oncoimmunology. 2017;6:e1356148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218–230. [DOI] [PubMed] [Google Scholar]
- 10.Paramanathan A, Saxena A, Morris DL. A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg Oncol. 2014;23:31–39. [DOI] [PubMed] [Google Scholar]
- 11.Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. [DOI] [PubMed] [Google Scholar]
- 12.Chai YD, Zhang L, Yang Y, et al. Discovery of potential serum protein biomarkers for lymph node metastasis in oral cancer. Head Neck. 2016;38:118–125. [DOI] [PubMed] [Google Scholar]
- 13.Tsai YD, Wang CP, Chen CY, et al. Pretreatment circulating monocyte count associated with poor prognosis in patients with oral cavity cancer. Head Neck. 2014;36:947–953. [DOI] [PubMed] [Google Scholar]
- 14.Rassouli A, Saliba J, Castano R, Hier M, Zeitouni AG. Systemic inflammatory markers as independent prognosticators of head and neck squamous cell carcinoma. Head Neck. 2015;37:103–110. [DOI] [PubMed] [Google Scholar]
- 15.Sun W, Zhang L, Luo M, et al. Pretreatment hematologic markers as prognostic factors in patients with nasopharyngeal carcinoma: Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio. Head Neck. 2016;38 Suppl 1:E1332–1340. [DOI] [PubMed] [Google Scholar]
- 16.Rosculet N, Zhou XC, Ha P, et al. Neutrophil-to-lymphocyte ratio: Prognostic indicator for head and neck squamous cell carcinoma. Head Neck. 2017;39:662–667. [DOI] [PubMed] [Google Scholar]
- 17.Mascarella MA, Mannard E, Silva SD, Zeitouni A. Neutrophil-to-lymphocyte ratio in head and neck cancer prognosis: A systematic review and meta-analysis. Head Neck. 2018;40:1091–1100. [DOI] [PubMed] [Google Scholar]
- 18.Takenaka Y, Oya R, Kitamiura T, et al. Prognostic role of neutrophil-to-lymphocyte ratio in head and neck cancer: A meta-analysis. Head Neck. 2018;40:647–655. [DOI] [PubMed] [Google Scholar]
- 19.Tham T, Bardash Y, Herman SW, Costantino PD. Neutrophil-to-lymphocyte ratio as a prognostic indicator in head and neck cancer: A systematic review and meta-analysis. Head Neck. 2018;40:2546–2557. [DOI] [PubMed] [Google Scholar]
- 20.Huang SH, Waldron JN, Milosevic M, et al. Prognostic value of pretreatment circulating neutrophils, monocytes, and lymphocytes in oropharyngeal cancer stratified by human papillomavirus status. Cancer. 2015;121:545–555. [DOI] [PubMed] [Google Scholar]
- 21.Qi Q, Zhuang L, Shen Y, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. 2016;122:2158–2167. [DOI] [PubMed] [Google Scholar]
- 22.Cho JK, Kim MW, Choi IS, et al. Optimal cutoff of pretreatment neutrophil-to-lymphocyte ratio in head and neck cancer patients: a meta-analysis and validation study. BMC Cancer. 2018;18:969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valero C, Pardo L, Lopez M, et al. Pretreatment count of peripheral neutrophils, monocytes, and lymphocytes as independent prognostic factor in patients with head and neck cancer. Head Neck. 2017;39:219–226. [DOI] [PubMed] [Google Scholar]
- 24.Puten WV. CART: Stata module to perform Classification and Regression Tree analysis Boston College Department of Economics Statistical Software Compontents S456776, 2006. [Google Scholar]
- 25.Piccirillo JF, Lacy PD, Basu A, Spitznagel EL. Development of a new head and neck cancer-specific comorbidity index. Arch Otolaryngol Head Neck Surg. 2002;128:1172–1179. [DOI] [PubMed] [Google Scholar]
- 26.Amin MB, Edge SB, Greene FL, et al. (eds). AJCC Cancer Staging Manual 8th ed. New York: Springer, 2017. [Google Scholar]
- 27.Li S, Lan X, Gao H, et al. Systemic Inflammation Response Index (SIRI), cancer stem cells and survival of localised gastric adenocarcinoma after curative resection. J Cancer Res Clin Oncol. 2017;143:2455–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geng Y, Zhu D, Wu C, et al. A novel systemic inflammation response index (SIRI) for predicting postoperative survival of patients with esophageal squamous cell carcinoma. Int Immunopharmacol. 2018;65:503–510. [DOI] [PubMed] [Google Scholar]
- 29.Chen Z, Wang K, Lu H, et al. Systemic inflammation response index predicts prognosis in patients with clear cell renal cell carcinoma: a propensity score-matched analysis. Cancer Manag Res. 2019;11:909–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y, Jiang W, Xi D, et al. Development and validation of nomogram based on SIRI for predicting the clinical outcome in patients with nasopharyngeal carcinomas. J Investig Med. 2019;67:691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu L, Yu S, Zhuang L, et al. Systemic inflammation response index (SIRI) predicts prognosis in hepatocellular carcinoma patients. Oncotarget. 2017;8:34954–34960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valiathan R, Ashman M, Asthana D. Effects of Ageing on the Immune System: Infants to Elderly. Scand J Immunol. 2016;83:255–266. [DOI] [PubMed] [Google Scholar]
- 33.Montero PH, Yu C, Palmer FL, et al. Nomograms for preoperative prediction of prognosis in patients with oral cavity squamous cell carcinoma. Cancer. 2014;120:214–221. [DOI] [PubMed] [Google Scholar]
- 34.Kao HK, Lofstrand J, Loh CY, et al. Nomogram based on albumin and neutrophil-to-lymphocyte ratio for predicting the prognosis of patients with oral cavity squamous cell carcinoma. Sci Rep. 2018;8:13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattavelli D, Lombardi D, Missale F, et al. Prognostic Nomograms in Oral Squamous Cell Carcinoma: The Negative Impact of Low Neutrophil to Lymphocyte Ratio. Front Oncol. 2019;9:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel SG, Lydiatt WM. Staging of head and neck cancers: is it time to change the balance between the ideal and the practical? J Surg Oncol. 2008;97:653–657. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.