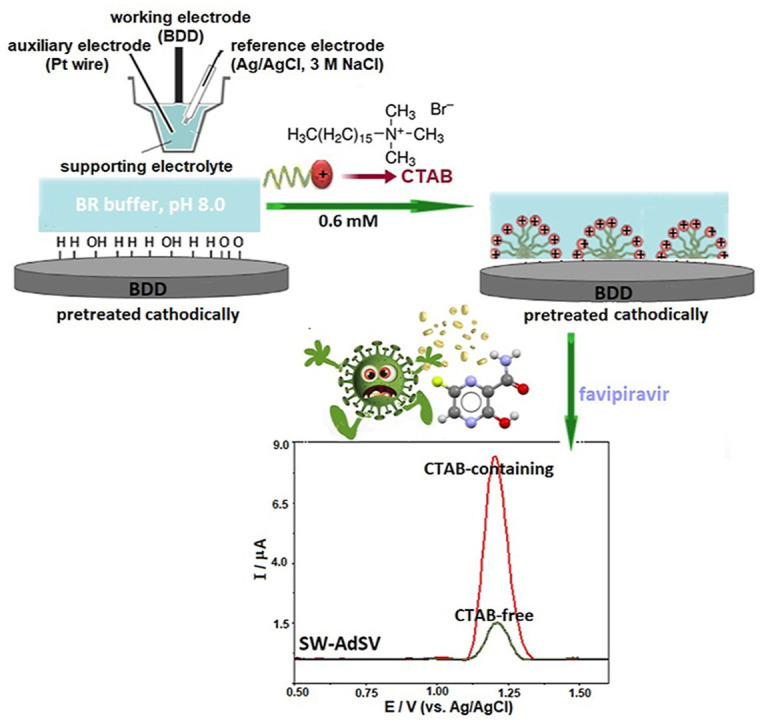
Abstract
Favipiravir, a promising antiviral agent, is undergoing clinical trials for the potential treatment of the novel coronavirus disease 2019 (COVID-19). This is the first report for the electrochemical activity of favipiravir and its electroanalytical sensing. For this purpose, the effect of cationic surfactant, CTAB was demonstrated on the enhanced accumulation of favipiravir at the surface of cathodically pretreated boron-doped diamond (CPT-BDD) electrode. At first, the electrochemical properties of favipiravir were investigated in the surfactant-free solutions by the means of cyclic voltammetry. The compound presented a single oxidation step which is irreversible and adsorption controlled. A systematic study of various operational conditions, such as electrode pretreatment, pH of the supporting electrolyte, concentration of CTAB, accumulation variables, and instrumental parameters on the adsorptive stripping response, was examined using square-wave voltammetry. An oxidation signal at around +1.21 V in Britton-Robinson buffer at pH 8.0 containing 6 × 10−4 M CTAB allowed to the adsorptive stripping voltammetric determination of favipiravir (after 60 s accumulation step at open-circuit condition). The process could be used in the concentration range with two linear segments of 0.01–0.1 μg mL−1 (6.4 × 10−8-6.4 × 10−7 M) and 0.1–20.0 μg mL−1 (6.4 × 10−7-1.3 × 10−4 M). The limit of detection values were found to be 0.0028 μg mL−1 (1.8 × 10−8 M), and 0.023 μg mL−1 (1.5 × 10−7 M) for the first and second segments of calibration graph, respectively. The feasibility of developed methodology was tested to the analysis of the commercial tablet formulations and model human urine samples.
Keywords: Favipiravir, Cathodically pretreated boron-doped diamond, Cetyltrimethylammonium bromide, Square-wave adsorptive stripping voltammetry, Real samples
Graphical abstract
1. Introduction
Coronaviruses are enveloped, single-stranded RNA viruses that can infect a wide variety of hosts, including avian, wild, domesticated mammal species, and humans. Six of these well-known viruses have been reported since the 1960s to cause disease in humans. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a novel type of coronavirus that gives rise to acute respiratory infections known as coronavirus disease 2019 (COVID-19). This respiratory disease is characterized with common symptoms including fever, dry cough, fatigue, body pain, nasal congestion, conjunctivitis, sore throat, loss of taste and smell, and headache. It was first identified in Wuhan (Central China) in December 2019, and was reported as a global pandemic by the World Health Organization (WHO) on March 11, 2020. As of February 19, 2021, the disease affected approximately 219 countries, infected more than 109 million people, and resulted in at least 2.4 million deaths worldwide. Currently, it appears that SARS-CoV-2 may pose a significant risk to the health system, due to its high contagiousness. However, to date, there is no specific effective approved drug for use in treating or preventing COVID 19, since there is not enough evidence. For this purpose, either a few recommended antiviral drugs approved for different infections or new alternatives that are still under trials, are being tested in various parts of the world [[1], [2], [3], [4], [5], [6], [7], [8]].
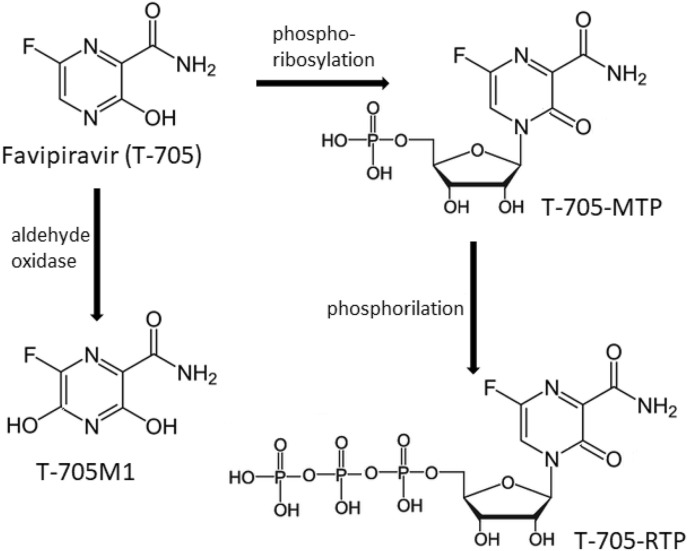
Favipiravir (T-705), a purine nucleic acid analog, is a novel antiviral pro-drug with a broad activity against influenza virus and diverse RNA viruses (such as arena-, phlebo-, hanta-, flavi-, noro-viruses, Western equine encephalitis virus, and Ebola virus). First introduced in Japan in 2014 as a cure for influenza, this compound has been extensively investigated for its possible use to treat and/or prevent infections caused by SARS-CoV-2. Chemically, favipiravir is a pyrazine carboxamide derivative (6-fluoro-3-hydroxy-2-pyrazinecarboxamide) with a lower molecular weight (see Fig. 1 for its chemical structure). It has been shown that 3-hydroxyl group of the pyrazinecarboxamide derivatives is responsible for their phosphoribosylation (as will be explained below) and antiviral activity but does not require the presence of 6-fluoro substituent. After oral administration of favipiravir (formulation: tablet, 0.2 g), it is incorporated into the cells, and then ribosylated and phosphorylated by host cellular enzymes to form the active metabolite T-705-ribofuranosyl-5′-triphosphate (T-705-RTP). Possible routes for conversion of favipiravir into its active and inactive metabolites are presented in Fig. 1. This triphosphate form, T-705-RTP selectively inhibits the transcription and replication of the enzyme RNA dependent RNA polymerase (RdRp) of influenza and many other RNA viruses by incorporation into the virus RNA at low concentrations. However, it does not inhibit RNA and DNA synthesis in humans up to the half-maximal inhibitory concentration (IC50) of 100 μg mL−1. Following its oral administration, favipiravir reaches peak plasma concentrations in approximately 0.5 h, and is eliminated significantly by the urinary system with a mean plasma elimination half-life (t½) of 1.5 h. The inactive and major metabolite T-705M1 of favipiravir is generated as a result of liver oxidation, and is excreted in the hydroxylated form [[9], [10], [11], [12], [13], [14], [15]].
Fig. 1.
Possible routes for conversion of favipiravir (T-705) into its active and inactive metabolites (adaptation from Ref. [13]).
Given the facts mentioned above, the development of suitable analytical methodologies for the determination of promising antiviral agents used for COVID 19, such as favipiravir, is strongly required not only for routine quality control in pharmaceutical formulations but also to support pharmacokinetic and metabolic studies in biological samples of both human and animal origin. Apart from these requirements, such studies in biological matrices will assist in the development of new drugs [16]. Survey of the literature revealed that only two studies have been made on the quantification of favipiravir by using high performance liquid chromatography coupled with ultraviolet (UV) detection [17], and spectrofluorimetric method [18].
Among the most widely used instrumental techniques, electroanalytical approaches, especially voltammetric ones, may be suitable alternatives with simplicity of operation, low instrument cost, rapid response, portability, allowing the use of low toxicity reagents (usually aqueous buffer solutions), sufficient sensitivity accompanied by satisfactory selectivity, precision and accuracy. Undoubtedly, another remarkable feature of voltammetry is that it can provide useful information about the oxidation-reduction behavior of the analytes with redox active groups to design new strategies for their additional therapeutic effects.
It is very important to develop an outstanding electrode material to improve the performance of the voltammetric method. As reported in several extensive reviews over the past two decades, the boron-doped diamond (BDD), a relatively new form of carbon, received great attention as an eco-friendly electrode material in many fields, e.g., analytical chemistry, environmental science, biological science, materials science, and so on [[19], [20], [21], [22], [23], [24], [25], [26], [27], [28]]. In general, BDD provides exceptionally useful properties (without the need of any chemical modifications) among all metal (gold, platinum) and conventional sp2 carbon (glassy carbon, carbon paste, pyrolytic graphite, etc.) electrode materials. This electrode is known to have the widest electrochemical potential window available without the interference of oxygen and hydrogen evolution, lower and stable voltammetric background current which gives it an advantage for potential scan analyses, good physical and chemical robustness which is very useful for maintaining the signal reproducibility, as well as high resistance to adsorption of most contaminants (due to the presence of sp3 hybridized carbon atoms in diamond structure). However, it should be highlighted that for many analytes, the analytical performance of the BDD is extremely affected by three essential factors, such as the boron doping concentration in diamond (for a good electrical conductivity), the content of non-diamond sp2 carbon (sp2 impurities), and its surface treatment (hydrogen or oxygen terminated surface functionalities). Surface termination of BDD is considered to be probably the most complex of all factors that strongly affects its physical, chemical and electronic surface properties. The as-prepared (untreated) surface of the BDD (both made in the lab and commercially available in polycrystalline form) is initially hydrophobic (free of C-O functionalities) with noticeable surface conductivity and negative electron affinity. It is mainly hydrogen-terminated as it is produced by hydrogen plasma process. Its surface can be altered to an oxygen-terminated surface using an anodic polarization process (anodic pretreatment ‒ APT) by applying high positive potentials in the region of oxygen evolution reaction. This procedure introduces high oxygen-bonded carbon content such as ethers, ketones, alcoholic and/or carboxylic groups. Therefore BDD becomes hydrophilic with a relatively negative surface charge, lower conductivity and positive electron affinity depending on the polarization potential and period. However, the hydrophobic nature of the BDD surface with noticeable surface conductivity can be restored by applying a cathodic polarization (cathodic pretreatment ‒ CPT) in the region of hydrogen evolution reaction. In order to increase the sensitivity, provide the better selectivity, reduce the fouling effect and ensure the adequate repeatability, BDD properties should be tailored specifically for each electroanalytical application by selecting an appropriate electrochemical pretreatment step [28].
In order to improve the analytical performance of voltammetric method, another simple and inexpensive way compared to the modified electrodes is the use of surfactant-containing solutions. As mentioned above, the bare BDD surface has assumed to be relatively inert to the adsorption of organic pollutants, which is considered one of its most important advantages [20]. However, the authors of the present work have demonstrated the capability of the BDD electrode to adsorb a wider spectrum of organic compounds in the presence of surfactants (anionic and/or cationic) as pre-concentration agents, and thus to increase the sensitivity of the voltammetric method [[29], [30], [31], [32], [33], [34], [35], [36]].
Taking these facts listed above into account, and considering the lack of electrochemical investigation on favipiravir, therefore, for the first time in the present study an effort will be made to examine the redox properties of this compound using voltammetric techniques and modification-free BDD electrode (only pretreated electrochemically) in the absence and the presence of cationic surfactant (cetyltrimethylammonium bromide, CTAB). For the following part, the practical applicability of the proposed voltammetric method will be tested in commercial pharmaceutical and model (spiked) human urine samples. Additionally, the results obtained for pharmaceutical formulation will be compared with those obtained by applying the UV-spectrophotometric method.
2. Experimental
2.1. Apparatus
Electrochemical measurements were performed using a computer driven μAutolab Type III potentiostat/galvanostat (Metrohm Autolab B.V., The Netherlands) controlled by the GPES software (Version 4.9). For baseline correction, the raw signals of the square-wave (SW) voltammograms were mathematically processed by applying the moving average method (peak width: 0.01 V), and then the voltammograms were corrected using the Savicky and Golay algorithm. A conventional three-electrode electrochemical cell (volume of 10 mL) was used, consisting of a commercially available BDD working electrode (with the inner diameter of 3 mm, boron doping level of 1000 ppm, Windsor Scientific Ltd., UK), a platinum wire auxiliary electrode (BAS, MW-4130, USA), an Ag/AgCl reference electrode (3 M NaCl, Model RE-1, BAS, USA), to which all working electrode potentials are referred. When necessary (reduction), the solution was bubbled with purified nitrogen to remove the dissolved oxygen.
The pH measurements were conducted using a WTW inoLab pH 720 m with a combined glass-reference electrode (Xylem, New York, USA).
Furthermore, to obtain comparative results, the quantitative assay of favipiravir in tablets by UV spectrophotometric method was carried out using an AE-S90 UV–Vis spectrophotometer with 1 cm quartz cell. Its absorption spectra in methanol, in the wavelength range from 200 to 800 nm, were recorded using methanol as a blank. Calibration graph was plotted using the absorbance value of favipravir at λmax 320 nm and its corresponding concentrations in μg mL−1, and the regression equation was derived.
2.2. Chemicals and solutions
The reference standard of favipiravir was kindly provided by Atabay Pharmaceuticals and Fine Chemicals Inc. (Turkey) and used as received. Commercially available tablet samples containing the active compound were supplied from a local hospital in city of Van (Turkey). Since favipiravir possesses poor aqueous solubility, its stock standard solution at 1 mg mL−1 was prepared by dissolving of the calculated amount in methanol and kept refrigerated when not in use. Analytical-grade reagents (acetic, boric and orthophosphoric acids) and highly pure deionized water from a Milli-Q water purifying system (Millipore, resistivity ≥ 18.2 MΩ cm) were used for the preparation of Britton-Robinson (BR) buffer (0.04 M in each constituent, pH 2–12). The lower concentrations of favipiravir working solutions were prepared, just before use, by dilution of appropriate volume using this supporting electrolyte at selected pH values. The potentially interfering compounds (glucose, lactose, sucrose, fructose, povidone, polyethylene glycol, ascorbic acid, dopamine and uric acid), potassium ferrocyanide (K4[Fe(CN)6] × 3H2O), potassium ferricyanide (K3[Fe(CN)6]) and potassium chloride (KCl) were purchased from Sigma-Aldrich. The surfactant used was cationic type, cetyltrimethylammonium bromide, CTAB (99%, Sigma). Stock solutions of CTAB (1 × 10−2 M) were prepared in a water-methanol mixture (90:10, v/v). For spectrophotometric experiments, calibration standards of favipiravir were prepared in methanol.
2.3. Pretreatment of BDD surface
Before starting any other electrochemical experiments, a cathodic or anodic (for comparative purposes) pretreatment of BDD electrode was carried out for its activation by applying either −1.8 or +1.8 V (both for period of 180 s) in an independent electrochemical cell containing 0.5 M H2SO4 without solution stirring. Before each new scan, 60-s activation procedure was applied under the same conditions. Between individual measurements in the presence of surfactant, the electrode was first rubbed very gently on a damp smooth polishing cloth (BAS velvet polishing pad) (a simple manual cleaning step with minimal possibility of mechanical surface damage) and then activated electrochemically (applying a period of 60 s) as in their absence [37]. This coupled pretreatment procedure (mechanical/electrochemical) guarantees reproducible results and enhanced electrochemical response in surfactant-containing solutions.
2.4. Measurement procedures
Cyclic voltammetry (CV) was carried out for preliminary studies to investigate the electrochemical behavior of favipiravir. Afterwards, it was followed by utilization of square-wave adsorptive stripping voltammetry (SW-AdSV) to broaden the knowledge on its electrochemical behavior at different pH values and in surfactant-containing solutions. The analytical performance and practical applicability in the real samples were demonstrated using SW-AdSV in the presence of CTAB.
The general procedure for the determination of favipiravir using SW-AdSV was as follows: The appropriate volume of the previously prepared favipiravir working solutions was placed in the electrochemical cell containing BR buffer at pH 8.0 in the presence of 6 × 10−4 M CTAB. The pre-concentration of favipiravir was performed by immersing the electrochemically treated BDD electrode in its solution stirred at a speed of 500 rpm for a given period of time (60 s), at a selected accumulation potential (open-circuit). After a fixed rest period (5 s), voltammograms were recorded by scanning the potential towards the positive direction over the range +0.50 to +1.60 V using SW waveform. The successive measurements were carried out by repeating the above assay protocol on the working electrode. Each voltammogram was repeated three times under laboratory conditions. It is important to mention that the methanol concentration in the working solutions was kept at the level of ≤2% (v/v).
2.5. Sample preparation
For sampling of commercial favipiravir tablet, Favimol® tablets labeled as containing 200.0 mg of favipiravir per tablet were used for the present analytical applications. According to the information listed on the drug leaflet the excipients present in the tablets were as follows: povidone, colloidal silicon dioxide, crospovidone, sodium stearil fumarate, hypromellose, titanium dioxide (E171), polyethylene glycol/macrogol, yellow iron oxide. Five pieces of tablets were weighed, and reduced to a homogeneous fine powder in a mortar. An adequate amount of the collected powder which is equivalent to 6.25 mg favipiravir was weighed, transferred into a 25-mL volumetric flask, and completed to the volume with methanol. The content of the flask was sonicated for about 10 min to ensure complete dissolution (solution A). A certain volume of the upper clear sample solution (20 μL) was transferred to the electrochemical cell containing 10 mL of BR buffer at pH 8.0 in the presence of 6 × 10−4 M CTAB, and analyzed via SW-AdSV. The contents of favipiravir in sample solutions were calculated by means of the calibration curve method from the related regression equation.
For the spectrophotometric assay applied to the analysis of tablet samples, the solution A (after filtration through paper) was diluted by a factor 1:4 (v/v) with methanol in order to fit into linear range of calibration curve previously determined, and analyzed by this technique.
In the next step, the human urine samples spiked with favipiravir were used in order to assess the feasibility of the voltammetric method for clinical trials. Drug-free urine samples were collected from a healthy laboratory co-worker (male, age 30 years) immediately before the experiments, and analyzed after resting for 30 min. Aliquot volume of fresh urine (500 μL) was directly transferred into the electrochemical cell already containing 10 mL of BR buffer at pH 8.0 in the presence of 6 × 10−4 M CTAB (dilution factor 1:20, v/v). Further, the solution in electrochemical cell was enriched with 50 μL of favipiravir stock solution (1 mg mL−1 prepared in methanol) to form model (spiked) human urine sample with a final concentration level of 0.05 μg mL−1 favipiravir. The quantification of favipiravir in spiked urine samples was carried out using standard addition method with respective volumes of 25, 50, 75, 100 μL of favipiravir solution used above, and the analyses were undertaken. The favipiravir-free part of the urine sample was used as a blank. Each determination was repeated three times.
2.6. Data analysis
The described method was validated for the parameters such as linearity, limits of detection (LOD) and quantification (LOQ), precision and accuracy. LOD and LOQ values were calculated using formulas 3 s/m and 10 s/m, respectively, where s represents standard deviation of the peak current (three runs) of the lowest level concentration (0.01 and 0.1 μg mL−1 for the 1st and 2nd linear segments of calibration graph, respectively) of the analytical curve, and m the slope of the related calibration equation [38]. To determine the precision, 0.01 or 0.1 μg mL−1 favipiravir was determined ten times within the same day (intra-day repeatability) and on three consecutive days (inter-day repeatability). Data were reported as the mean values and relative standard deviations (RSD %).
3. Results and discussion
3.1. Electrochemistry of favipiravir
In order to understand the electrochemical response of favipiravir at the surface of BDD electrode, the experiments were performed in surfactant-free and surfactant-containing solutions by means of the CV and AdSV.
3.1.1. Cyclic voltammetry in the absence of surfactant
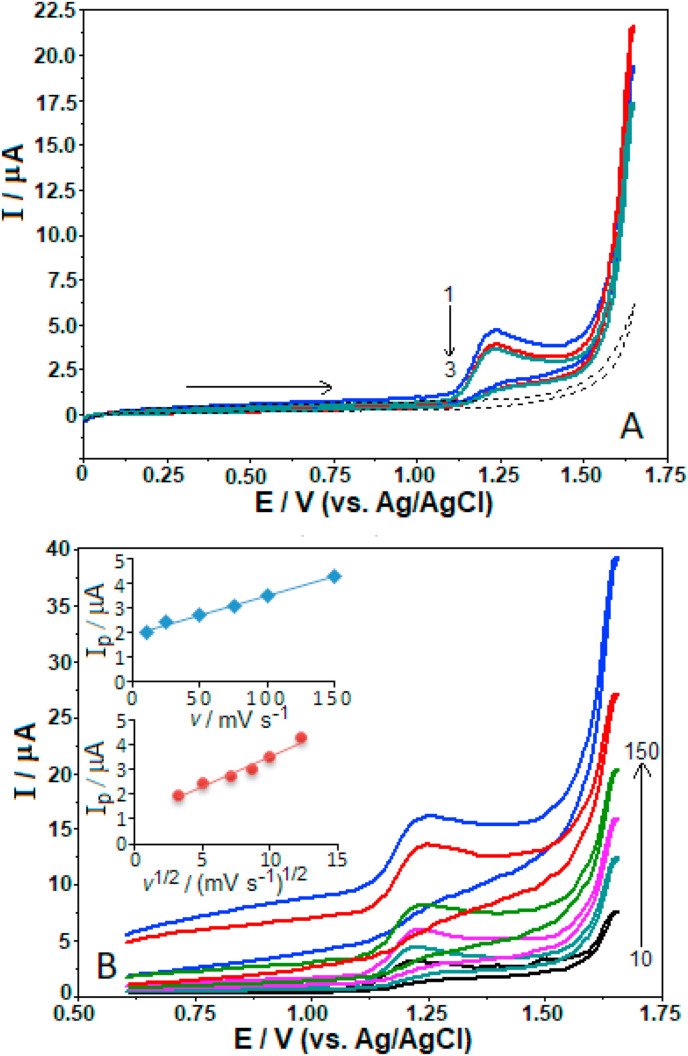
At first, the experiments were focused on the CV behavior of favipiravir using a cathodically pretreated boron-doped diamond electrode (here referred as CPT-BDD, see Experimental Section 2.3). Fig. 2 A shows the three consecutive CV curves (CVs) of 50 μg mL−1 (3.2 × 10−4 M) favipiravir in BR buffer at pH 8.0 (optimized response for analytical purposes, as shown later) recorded at a scan rate of 100 mV s−1 (note that the methanol degree in the working solutions was 5%, v/v). During the anodic scan from 0.0 to +1.6 V, favipiravir presented a broad oxidation step at around +1.23 V while no reduction step was observed in the reverse cathodic scan. It suggests the totally irreversible electrode reaction of this compound. By recording consecutive CVs, it was observed a current decrease of oxidation signal in the second cycle (however less distinct in the third cycle), indicating that it may cause partial fouling and inactivation of the available electrode surface area.
Fig. 2.
The repetitive CVs at the scan rate of 100 mV s−1 for 50 μg mL−1 favipiravir (A), and CVs at different scan rates (10, 25, 50, 75, 100 and 150 mV s−1) for 100 μg mL−1 favipiravir (B). Electrode, CPT-BDD; supporting electrolyte, surfactant-free BR buffer at pH 8.0. A: Arrow indicates order of the recorded scans. Dashed lines represent background current. B: Linear dependences ip vs. v and ip vs. v1/2 are appended in the inset.
It is also worth noting that when the electrode was scanned to a potential value of −0.8 V, no reduction peak was observed.
3.1.1.1. Effect of scan rate
In order to determine the mass transport nature of favipiravir at the electrode/solution interface, the next step was an investigation of influence of scan rate on the electrooxidation response for 100 μg mL−1 (3.2 × 10−4 M) favipiravir using CV technique in BR buffer at pH 8.0. Fig. 2B depicts the respective CVs for the potential scan rates from 10 to 150 mV s−1 (n = 6). It should be noted at this point that worse current response was observed at a scan rate higher than 150 mV s−1, making accurate measurement difficult.
As can be seen from the following equations, there is a linear relationship between the peak current (i p) and the scan rate (ν):
| ip (μA) = (0.016 ± 0.0003) ν (mV s−1) + (1.904 ± 0.071) (r = 0.996) |
It indicates an adsorption-controlled mechanism of the electrode reaction of favipiravir. Meanwhile, i p linearly increased with ν 1/2 expressed as follow:
| ip (μA) = (0.245 ± 0.004) ν1/2 (mV s−1) + (1.108 ± 0.031) (r = 0.987) |
Such dependence indicated that the favipiravir oxidation process can be controlled by both adsorption and diffusion (less effective), i.e., there is a mixing mechanism. Similar findings have also been reported in the previous investigations for some compounds using not only the BDD electrode [33,34,36] but also any other carbon-based electrodes [[39], [40], [41]].
On the other hand, there was a shift of the oxidation peak potential (E p) towards more positive values as the scan rate gradually increased, confirming the above-mentioned irreversible behavior of the electrode reaction [42]. The plot of E p versus log v can be expressed by the following equation:
| Ep (V) = 0.027 log ν (mV s−1) + 1.184 (r = 0.998) |
For an adsorption-controlled and irreversible electrode process [43], the relationship between E p and v is defined according to the equation:
| Ep = E0 + (2.303RT / αnF) log (RTk0 / αnF) + (2.303RT / αnF) log v |
where α is charge transfer coefficient, k 0 the standard heterogeneous rate constant of the reaction, n the number of transferred electrons involved in favipiravir oxidation process, and E 0 the formal redox potential. Other symbols have their usual meanings. The slope obtained from the E p vs log v was 0.027; thus, by means of the above equation using T = 298 K, R = 8.314 J K−1mol−1, and F = 96480 Cmol-1, the value equals to about 2.33 was calculated for αn. Generally α is assumed to be 0.5 for most organic molecules in total irreversible electrode process. Further, the value of n was calculated to be 4.3 (≈4). These results indicate that the oxidation of favipiravir involves four electrons per molecule.
3.1.2. Stripping voltammetry in the absence and the presence of surfactant
Considering the above experimental data, it was examined whether favipiravir could be pre-concentrated onto the surface of CPT-BDD, which may have a positive effect on its sensitivity for analytical use. To verify this, medium exchange technique (transfer voltammetry) prior to the recording of voltammograms was initially carried out. For this purpose, CPT-BDD electrode (see Experimental Section 2.3) was kept in contact with a stirred solution of favipiravir (25 μg mL−1) prepared in BR buffer at pH 8.0 for a low accumulation period (30 s) under open-circuit condition. The electrode was then transferred into the blank supporting electrolyte solution to continue the stripping step (Fig. S1 in the Supplementary Material file). This experiment demonstrated clearly that after medium exchange which leaves behind compounds with poor adsorption ability in the accumulation solution, there was a very slight decrease in the peak height compared to the original value. This behavior could be indicative of the adsorptive accumulation of favipiravir on the electrode surface. Following this initial investigation, the measurements were performed by AdSV without applying a medium exchange step in the continuation of the study.
3.1.2.1. Effect of BDD pretreatment
A preliminary study showed that suitable electroanalytical responses in terms of reproducibility and sensitivity of the measurements could not be obtained when using the unpretreated BDD electrode. In order to obtain the improved electrochemical response of the BDD electrode, two pretreatment procedures such as anodic polarization (APT, +1.8 V for 180 s) and cathodic polarization (CPT, −1.8 V for 180 s) were tested. The pretreatments were carried out as described in Experimental Section. Although a strong anodic polarization in the region of water decomposition reaction is required to complete oxygenation of the BDD surface, in the case of BDD electrode used in this study (with the diameter of 3 mm and boron content 1000 ppm), a distortion in the peak shape of favipiravir was observed at potential higher than +2.0 V. It has been demonstrated in some of our reports that anodic polarization potential between +1.6 and + 1.8 V provides remarkable improvements for electroanalytical purposes [30,33,34]. On the other hand, a recently published study from our laboratory [33] showed that when negative potentials higher than −1.0 V are applied, cathodic pretreatment involves high value of current density, indicating that the magnitude of the applied potential polarization is sufficient for the surface of BDD to become completely hydrophobic. Regarding studies on the pretreatment period (20–180 s), 180 s was found to be suitable for the highest current response of favipiravir compared to shorter pretreatment times.
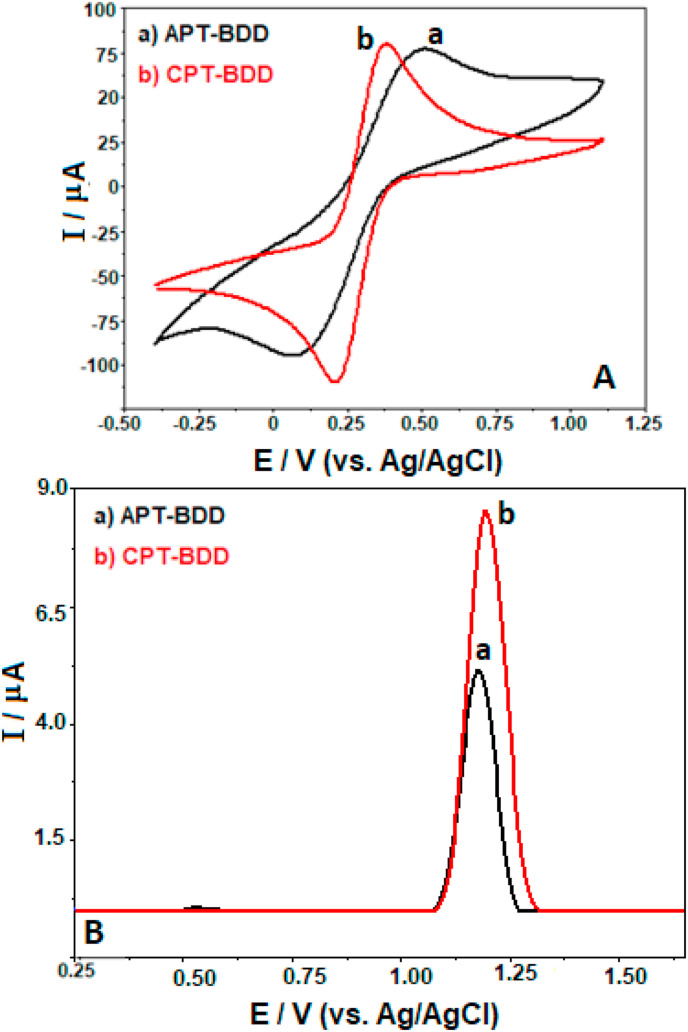
The effect of both pretreatment procedures on the electrochemical activity of BDD electrode was first examined by recording CV measurements at scan rate of 100 mV s−1 in 0.1 M KCl containing the redox couple of 0.4 mM [Fe(CN)6]3-/4-, which are presented in Fig. 3 A. The oxidation and reduction peak separations (ΔE p) of [Fe(CN)6]3-/4- for APT-BDD and CPT-BDD are about 365 and 167 mV, respectively. Moreover, the use of APT-BDD presents the lower voltammetric signals for both the oxidation and reduction peaks when compared to CPT-BDD. The remarkably nonreversible behavior of APT-BDD due to the difficulty of electron transfer is in agreement in previously reported studies for BDD electrodes treated anodically [[44], [45], [46]]. Although the ΔE p is quite different from the theoretical value of 59 mV for reversible systems, the findings indicate improved surface activity of BDD after cathodical pretreatment as reported in Ref. [47]. Furthermore, both pretreatment procedures were also tested for 25 μg mL−1 favipiravir in surfactant-free BR buffer, at pH 8.0 by SW-AdSV with an accumulation period of 30 s at an open-circuit condition. It is clearly seen from Fig. 3B that CPT-BDD offers higher peak intensity of the analyte than APT-BDD (the reason will be explained later). Therefore, the cathodic activation protocol was chosen in the continuation of this study and was conducted at the beginning of every experiment day. However, it should be underlined that prior to every voltammetric experiment in solutions containing CTAB, a simple mechanical polishing step (as described in the Experimental Section) is required prior to this activation protocol to remove favipiravir that has strongly adsorbed on the electrode surface by interacting with the surfactant.
Fig. 3.
CVs of 0.4 mM [Fe(CN)6]3-/4- in 0.1 M KCl at a scan rate of 100 mV s−1 (A), and SW stripping voltammograms of 25 μg mL−1 favipiravir in surfactant-free BR buffer at pH 8.0 (B) recorded using cathodically (CPT-) or anodically (APT-) pretreated BDD electrodes. Accumulation period, 30 s at open-circuit condition; SWV parameters: frequency, 50 Hz; step potential, 8 mV; pulse amplitude, 30 mV.
3.1.2.2. Effect of solution pH
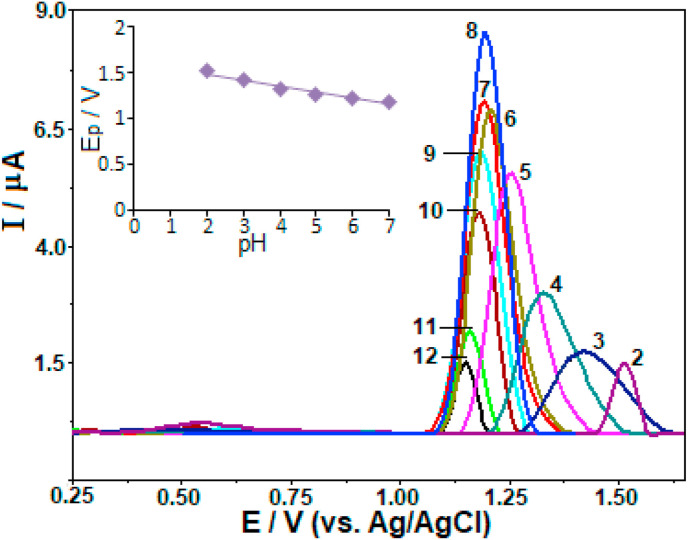
With the aim of obtaining the best voltammetric results for analytical purposes, the next stage of experimental work was dedicated towards examining the effect of different pH values. In Fig. 4 , the baseline corrected SW-AdSV voltammograms are depicted in surfactant-free BR buffer varying the pH in the interval 2.0–12.0 by carrying out stripping measurement in a stirred favipiravir solution (25 μg mL−1, methanol degree was 2.5%, v/v), with an open-circuit accumulation for 30 s. For the whole pH range mentioned above, only a single oxidation peak of favipiravir was recorded. It is clearly seen from the figure that by increasing the solution pH, an increase in favipiravir peak height over the pH range 2.0–8.0 was observed. In more alkaline solutions, the peak height decreased.
Fig. 4.
SW stripping voltammograms for 25 μg mL−1 favipiravir in surfactant-free BR buffer with different pHs (2.0–12.0) recorded using CPT-BDD. Inset: the plots of Ep vs. pH. Accumulation period 30 s at open-circuit condition; SWV parameters: frequency, 50 Hz; step potential, 8 mV; pulse amplitude, 30 mV.
On the other hand, the peak potential (E p) shifted towards less positive values with the increase pH from 2.0 to 7.0, revealing the proton-dependent nature of favipiravir electrochemical oxidations, which can be expressed by the following equations:
| Ep (V) = −0.067 pH + 1.619 (r = 0.987) |
The slope of 67 mV/pH, which is close to the theoretical value of 59 mV suggests that the equal numbers of electrons and protons take part in the electrode reaction. Bearing the previous scan rate results of favipiravir in mind, this finding demonstrates that the electrode process for this compound is four-proton coupled four-electron transfer. Above pH 7.0, the position of the oxidation peak is not remarkably sensitive to the pH, indicating that a proton transfer step does not occur prior to the electron transfer rate-determination step at these pH values.
Until now there is no agreement on the assignment of pK a values of favipiravir available in literature. In the Drugbank database [48], its experimental pK a value has been reported to be 5. However in the same database, two predicted values such as pK a = 9.39 (strongest acidic) and pK a = −3.7 (strongest basic) are also mentioned. Moreover, very recently published paper reported eight different pK a values calculated using the direct, modified direct, and proton exchange thermodynamic cycles [49]. As seen from Fig. 1, favipiravir (T-705, more stable enol form) and its analogues (T-705-MTP and T-705-RTP) (ketone form) belong to the potentially tautomeric pyrazinecarboxamide family. Thus, the conflicting results regarding pK a values can be due to the complexity of the proton transfer (ionization) process, which may involve several complicated steps to generate numerous products (keto and enol tautomers of favipiravir) after a series of tautomerization [49]. It should be emphasized that there is vital importance to know the ionization and tautomeric states of clinically important compounds for new drug design studies. At this moment of urgent need for COVID-19 treatment solutions, more experimental and theoretical research could be useful to investigate the tautomerism of favipiravir. In the light of findings from two very recently published papers on favipiravir tautomerism [49,50], the intersection point of the E p/pH curves and clear change in the peak intensity at about pH 7.0–8.0 may be explained by a replacement of one mechanism (reaction with enol form) by another (reaction with the ketone form) in the tautomeric equilibrium of the favipiravir.
Thus, it may be assumed that favipiravir is in nonionized form in aqueous media at pH 8.0. From the results given in previous section, the surface of CPT-BDD electrode is completely hydrophobic in our cathodic pretreatment condition (−1.8 V for 180 s). However, by applying a mild polarization potential of +1.8 V, the complete transformation of the BDD surface to the negative form is not sufficient, thus its surface is still partly hydrophobic. Based on this fact, the higher current intensity of favipiravir at the surface of CPT-BDD is not surprising in comparison with APT-BDD since the former one can interact better with this neutral molecule.
It is beyond the scope of the present study to describe the oxidation process of favipiravir in detail. On the other hand, more work needs to be carried out for collecting more information by using galvanostatic/potentiostatic coulometry with subsequent spectral analysis. However, a short comment can be made from the above results and considering the electrochemical behavior of the model compound pyrazinamide (pyrazinecarboxamide) used for tuberculosis treatment. Favipiravir differs from pyrazinamide by having a hydroxyl group in the position C3 of the pyrazine ring. As stated in the scientific literature, pyrazinamide does not undergo oxidation. In previously published reports, the electrochemical properties based on the reduction of this compound were investigated using several chemically modified electrodes [[51], [52], [53]]. With this knowledge in mind, we may assume that the hydroxyl group on this molecule also plays a role in the oxidation process of favipiravir.
As can be clearly verified from Fig. 4, highest current response was obtained at pH 8.0, which was chosen for further studies and development of the methodology. Under the same experimental conditions, favipiravir signal recorded after applying an accumulation process was almost 2.0 times higher than that recorded without an accumulation step (see Fig. S1).
3.1.2.3. Effect of accumulation variables and other operating parameters
Based on the adsorptive properties of favipiravir on the surface of CPT-BDD, which would make AdSV technique applicable for developing a sensitive analytical methodology, the effect of accumulation parameters (accumulation period of time, t acc and accumulation potential, E acc) was tested for 25 μg mL−1 favipiravir under the above experimental conditions (in the absence of surfactant). The effect of the t acc was examined in the range 0–240 s at open-circuit condition. While there was a linear increase in the peak current of favipiravir in the range of 0–60 s, no significant change was observed beyond this range. After fixing the t acc at 60 s, the effect of E acc was also investigated either at open-circuit condition or over the potential range +0.1 to +0.9 V. In this case, the oxidation peak current remained almost unchanged in the whole range, revealing that this parameter had no effect on the detection of this compounds, thus open-circuit accumulation was used.
Next, the effect of SWV operating parameters such as frequency (f = 50–125 Hz), step potential (ΔE s = 8–14 mV) and pulse amplitude (ΔE sw = 30–70 mV) was also investigated under the above conditions to obtain the highest sensitivity and the best reproducibility of measurements (results not presented). The optimization was performed in such a manner that one parameter was always changed while keeping the other two parameters constant. For entire analysis, the optimized values were: f, 75 Hz; ΔE s, 10 mV; and ΔE sw, 50 mV.
3.1.2.4. Effect of cationic surfactant
Considering some studies from our laboratory on the significant role of CTAB towards the accumulation of the poorly water-soluble compounds at the hydrophobic surface of CPT-BDD [29,31,35,36], the attention was then turned to the effect of this surfactant in order to obtain more information for the adsorption process.
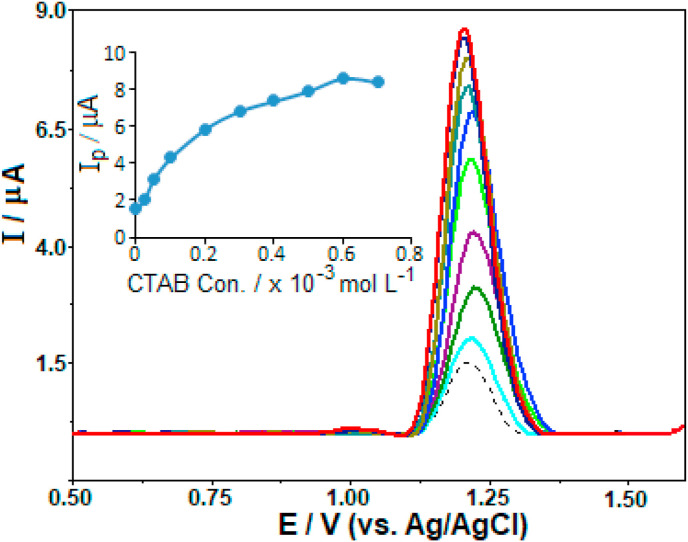
For this purpose, keeping the favipiravir concentration constant at 7.5 μg mL−1 (4.8 × 10−5 M), the concentration of CTAB was increased from 2.5 × 10−5 to 7 × 10−4 M (note that CTAB was electrochemically inactive in the selected potential range). As can be seen in Fig. 5 , the peak potential remained practically constant when the electrolyte solution contains CTAB. Regarding the peak currents, a remarkable enhancement was observed in the presence of CTAB. The peak current increased gradually with CTAB concentration up to 6.0 × 10−4 M. At its higher concentration values, no significant change in the signal was observed (Fig. 5 inset). To sum up, the following experiments for the analytical application (see Section 3.2) were carried out by fixing the concentration of CTAB at 6.0 × 10−4 M. It should be highlighted that in the case of the accumulation process after CTAB addition, favipiravir stripping signals were almost 5.5 times higher compared to the measurements in CTAB-free solutions. This finding demonstrates the possibility that CTAB can be used as a pre-concentration agent on the performance of the CPT-BDD electrode for the sensitive determination of favipiravir.
Fig. 5.
SW stripping voltammograms for 7.5 μg mL−1 favipiravir in BR buffer solution at pH 8.0 in the presence of different CTAB concentrations (2.5 × 10−5-7 × 10−4 M). Dashed line represents the voltammograms without CTAB. Inset: the plot of ip vs. the concentration of CTAB. Electrode, CPT-BDD; accumulation period 60 s at open-circuit condition; SWV parameters: frequency, 75 Hz; step potential, 10 mV; pulse amplitude, 50 mV.
In solutions containing a certain concentration of CTAB (below critical micelle concentration in water, CMC = 0.92–1.0 mM) [54,55], its long hydrophobic tails are attracted to the hydrophobic surface of the CPT-BDD through the hydrophobic interaction. On the other hand, according to an accepted adsorption scenario [[56], [57], [58], [59], [60]], small molecules with a low solubility like favipiravir may be interacted with adsorbed surfactant aggregates on the electrode surface, by an interaction mechanism very similar to the classical micellar solubilisation observed above the equilibrium CMC. As shown in the previous sections, favipiravir can be adsorbed on the surface of CPT-BDD when it is alone. However, in the presence of CTAB molecules, there is an enhancement of its surface concentration. This can be explained by the fact that a large part of the adsorption is the solubilisation of the favipiravir molecule inside surfactant aggregates. This phenomenon has been referred to as coadsorption. In some cases, the hydrophobic solute molecule normally is not adsorbed by the solid surface in the absence of surfactant but is adsorbed when a surfactant molecule is adsorbed, which is called adsolubilization. In general, the medium pH, the ionic strength of the aqueous phase, the surfactant and solute structures play an important role on the coadsorption/adsolubilization-oriented processes [58]. Reported studies have demonstrated that especially the hydrophobic tail length of surfactants (from C12 to C18) significantly affects the coadsorption/adsolubilization capacity of surfactant surface aggregates [61]. Both of these interaction mechanisms have recently found many applications in pharmacology (drug carrier targeting), engineering (chromatographic separation), materials science (surface modification), and environmental science (wastewater treatment).
Based on this fact, it is expected an adsorption of CTAB and subsequent coadsorption of favipiravir onto the surface of CPT-BDD, resulting in an increase in analytical signal of favipiravir.
3.2. Quantification of favipiravir in the presence of CTAB
3.2.1. Analytical performance evaluation
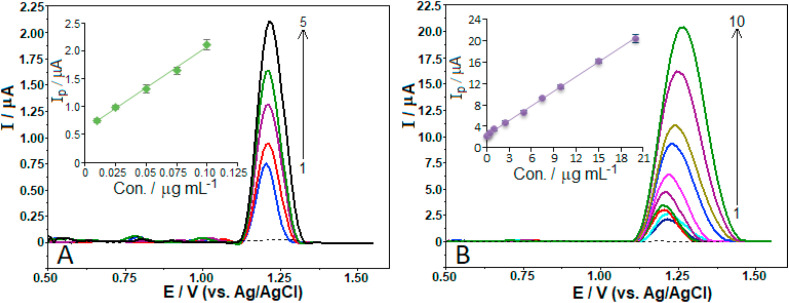
Using the previously optimized experimental and operating conditions, the applicability of the CPT-BDD in combination with AdSV technique was tested by plotting the peak current against favipiravir concentration. Fig. 6 A and B depicts the respective stripping voltammograms recorded for additions of aliquots of standard solution (in triplicate) containing increasing concentrations of favipiravir. The corresponding analytical curves with the obtained linear relationship between oxidation peak current and concentration of favipiravir are presented in the insets of Fig. 6. It is seen from the figure that the calibration graph consists of two linear segments, from 0.01 to 0.1 μg mL−1 (6.4 × 10−8‒6.4 × 10−7 M), and from 0.1 to 20.0 μg mL−1 (6.4 × 10−7‒1.3 × 10−4 M) with good linearities. The related analytical parameters regarding the both segments of analytical curve are summarized in Table 1 . The observation of the two linear segments may be a result of the adsorption effect that can occur in the presence of higher favipiravir concentration levels at the electrode surface. Therefore, the slope value and hence the sensitivity corresponding to the second linear segment was much lower than that recorded for the first linear segment. Moreover, it is clearly shown from Fig. 6B that the increase in concentration caused the potentials to shift slightly to more positive values.
Fig. 6.
SW stripping voltammograms for favipiravir levels of (1–5) 0.01, 0.025, 0.05, 0.075 and 0.10 μg mL−1 (A), and (1–10) 0.1, 0.25, 0.5, 1.0, 2.5, 5.0, 7.5, 10.0, 15.0 and 20 μg mL−1 (B) in BR buffer solution at pH 8.0 containing 6 × 10−4 M CTAB. Inset shows the corresponding calibration plot for the quantitation of favipiravir. The other operating conditions as indicated in Fig. 5.
Table 1.
Analytical parameters for the determination of favipiravir in BR buffer, pH 8.0 + 6 × 10−4 M CTAB using by SW-AdSV.
| Analytical parameter | 1st linear segment | 2nd linear segment |
|---|---|---|
| Linear working range | 0.01–0.1 μg mL−1 (6.4 × 10−8‒6.4 × 10−7 M) | 0.1–20.0 μg mL−1 (6.4 × 10−7‒1.3 × 10−4 M) |
| Linear regression equation |
ip (μA) = (14.760 ± 0.305) C (μg mL−1) + (0.598 ± 0.053) (n = 5) |
ip (μA) = (0.912 ± 0.006) C (μg mL−1) + (2.317 ± 0.004) (n = 10) |
| Correlation coefficient (r) | 0.997 | 0.999 |
| LOD | 0.0028 μg mL−1 (1.8 × 10−8 M) | 0.023 μg mL−1 (1.5 × 10−7 M) |
| LOQ | 0.0093 μg mL−1 (5.9 × 10−8 M) | 0.076 μg mL−1 (4.9 × 10−7 M) |
| intra-day precision/RSD% | 4.6 (peak current) | 7.3 (peak current) |
| inter-day precision/RSD% | 5.7 (peak current) | 8.8 (peak current) |
LOD values achieved by the proposed method appears to be sufficient for favipiravir sensing in routine quality control analysis of commercial products and even in some biological samples such as urine (the principal route of excretion).
As it was mentioned earlier, a survey of the literature revealed that there are only two studies for the quantitative determination of favipiravir. In the first attempt, it was reported the use of high performance liquid chromatography with UV detection [17]. LOD value equaled to 1.20 mg mL−1 has been estimated in a mobile phase consisting potassium dihydrogen phosphate 50 mM (pH 2.3) and acetonitrile (90:10, v/v). The method was applied to the determination of favipiravir in pharmaceutical preparations. In the second published work [18], spectrofluorimetric method has also been found to be applicable for its determination in pharmaceutical formulations and spiked human plasma with its LOD values of 9.44 ng mL−1. From these data, it can be clearly seen that the proposed voltammetric method exhibits slightly better or even superior performance for the detection of favipiravir compared to other two techniques. In addition, the present electroanalytical methodology meets almost required characteristics such as simple and rapid treatment of the sample, minimum use of organic reagent, low cost, sufficient sensitivity and acceptable repeatability for application to real samples.
3.2.2. Effect of interfering compounds
Before analyzing real samples, the effect of some possible interfering compounds, usually present in the pharmaceutical tablets and/or human urine, was investigated for 6.5 × 10−6 M (∼1.0 μg mL−1) favipiravir at the concentration ratios of 1:1, 1:10, and 1:50 (analyte:interfering compound) under the optimum experimental conditions. The maximum concentration of the selected interfering compounds, which caused an approximately ±5% relative error for the oxidation peak current of favipiravir was considered as the tolerance limit.
It was found that 50-fold excess of several excipients commonly co-formulated in tablet samples such as some metal ions (K+, Na+, Zn2+, Mg2+, Ca2+, Cu2+, Al3+, Fe3+, Ti4+), some small biomolecules (lactose, sucrose, fructose, glucose), and some agents (microcrystalline cellulose, starch) did not show any interfering effects on the determination of favipiravir. Although in case of some polyvalent cations, a precipitation may be observed in solutions at around pH 8.0, these ions are not or less extracted from methanol in real sample analysis. Nevertheless, its detection in the presence of povidone (polyvinylpyrrolidone) and polyethylene glycol at a concentration ratio of 1:50 was somewhat complicated, which can be explained by the polymeric film formation on the surface of the BDD electrode. However, the interferences of these excipients are not expected since their contents in tablets are much lower than that here investigated.
In order to show the possibility of monitoring this compound in biological samples such as urine with more complicated matrix compared to pharmaceutical dosage forms, the interferences of the common urinary interfering compounds, uric acid, ascorbic acid and dopamine were also examined. Their oxidation signals appeared at less positive potentials than favipiravir. The obtained results revealed that there was no remarkable effect on favipiravir signal even their 50-times higher concentration levels (Fig. S2 in the Supplementary Material file).
As a result, it can be concluded that the developed strategy presents sufficient selectivity and offers possibility for applying to the pharmaceutical formulations and biological matrices.
3.2.3. Real sample analysis
Based on obtained results, in the final step, the applicability of the CPT-BDD electrode in cooperation with the cationic surfactant, CTAB was tested for the determination of favipiravir in commercially available tablet forms and spiked human urine samples. The samples were prepared as described in Section 2.5. It should be noted at this point that these experiments were performed using linear concentration range with the second linear segment for tablet formulation, and with the first linear segment for urine samples.
The analysis of the tablet samples was carried out exploiting the calibration curve method from the related regression equation. The mean value of favipiravir was found as 0.475 μg mL−1 in the measurement cell. Taking into account the successive dilution of the sample, the favipiravir content was calculated to be 190.0 mg (RSD of 3.82%) approaching the label value of 200 mg declared by the manufacturer.
In order to testify the interference effect of filling materials present in the analyzed tablets, recovery studies were carried out by adding the standard favipiravir solutions (final concentrations of 0.1–1.0 μg mL−1) to 10 mL of the earlier analyzed tablet sample solution in electrochemical cell (Fig. S3 in the Supplementary Material file). The recovery of the drug was calculated by comparing the concentration obtained from the spiked mixtures with those of the pure drug. According to Table 2 , satisfactory recovery values manifested the fact that the proposed protocol did not suffer from any considerable matrix effect.
Table 2.
The analysis of the pharmaceutical tablets (Favimol®) fortified with favipiravir standard solutions using the proposed voltammetric method.
| Addeda (μg mL−1) | Expecteda (μg mL−1) | Founda,b (μg mL−1) | Recovery (%) ± RSD (%) |
|---|---|---|---|
| 0 | – | 0.475 | 0 ± 3.9 |
| 0.1 | 0.575 | 0.568 | 93.1 ± 3.9 |
| 0.5 | 0.975 | 0.957 | 96.4 ± 3.6 |
| 1.0 | 1.475 | 1.460 | 98.5 ± 3.4 |
Concentration in the measured solution.
Average of three replicate measurements.
The results obtained by the proposed voltammetric procedure for commercial tablet formulations were also compared to those obtained by a simple UV spectrophotometric method which was developed in the present study. The representative absorption spectrum of favipiravir prepared in methanol is presented in Fig. S4 (Supplementary Material file). The calibration studies were performed in the favipiravir concentration range of 1.0–25.0 μg mL−1 (6.4 × 10−6‒1.6 × 10−4 M) The measurements were recorded at 320 nm. The results showed that the linearity range of calibration plot obtained by the proposed voltammetric method could be expanded to about 100 times lower concentration than that obtained by UV spectrophotometric method. The regression equation of the calibration graph was computed to be [A = (0.063 ± 7 × 10−5) C (μg mL−1) + (0.044 ± 8 × 10−5) (r = 0.999, n = 7)]; where A is the absorbance value of favipiravir, and C the favipiravir concentration. The average value of favipiravir content was calculated to be 185.3 mg per tablet (RSD of 1.2%) after three replicate measurements. By evaluation of the findings, it may be concluded that both methods give similar results for favipiravir content. Besides, to check the validity, the Student’s t-test was applied to these results obtained by both proposed and comparison method. At the confidence interval for 95% probability, the calculated (experimental) t value (2.29) was less than the tabulated (theoretical) one (2.78, for α = 0.05), indicating insignificant differences between the values obtained using two methods and the true (labeled) values. These results show that the herein-proposed method can be used easily and conveniently for routine quality control purposes. Both methods do not require complicated sample preparation, but for the UV spectrophotometric studies in commercial tablet formulations, a filtration was necessary.
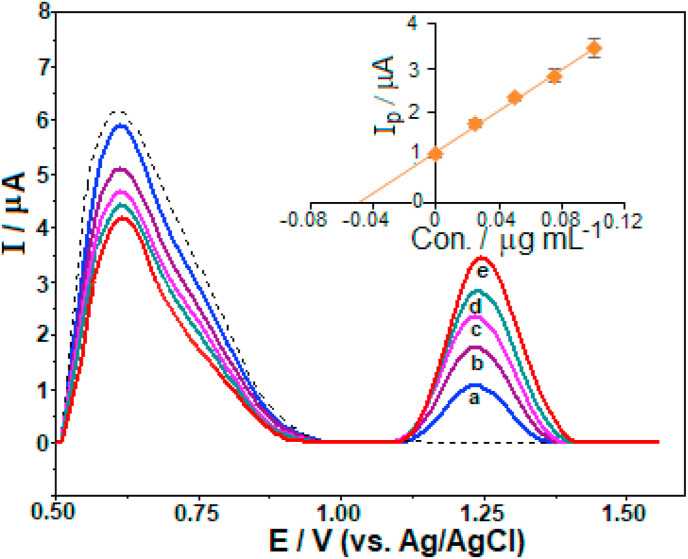
The ability of the proposed method for the analysis of more complex matrices was also verified via the determination of favipiravir in spiked human urine samples without any complex treatment or separation. As an illustrative example of analysis of model human urine sample, Fig. 7 illustrates the SW stripping voltammograms with the graphical evaluation of multiple standard addition method. The related results are presented in Table 3 . In the absence of favipiravir, there were no detectable peaks in the working potential range where its peak appeared. However, a broad peak at about +0.65 V was recorded, which might be probably due to the oxidation of common urinary compounds such as uric acid (see Fig. S2). This peak did not prevent the detection of favipiravir as it differs very well from that of analyte. In the presence of favipiravir, the distinct peak occurring at about +1.22 V could be attributed to its oxidation since its current increased proportionally after each addition of the stock favipiravir solutions. As can be seen in Table 3, satisfactory recovery and RSD values indicate the potential applicability of the developed approach for analysis of these types of matrices.
Fig. 7.
SW stripping voltammograms of model human urine sample from volunteer (diluted with BR buffer at pH 8.0 containing 6 × 10−4 M CTAB in the ratio of 1:20, v/v): (dashed line) in the absence of favipiravir, (a) in the presence of 0.05 μg mL−1 favipiravir, (b–e) after standard additions to obtain final concentration levels of 0.025, 0.050, 0.075, 0.100 μg mL−1 favipiravir. Inset depicts the result of analysis by standard addition method. The other operating conditions as indicated in Fig. 5.
Table 3.
Measurement results for addition and recovery of favipiravir from urine sample using the proposed voltammetric method.
Calculated by the use of standard addition method. Values reported are the average of three independent analysis of the same sample.
Calculated as: (determined concentration/expected concentration) × 100.
Bearing the major urinary hydroxylated metabolite T-705M1 of favipiravir in mind (as explained above), the determination of this metabolite is necessary for closer examination and clarification of the analysis of human urine samples.
4. Conclusions
As mentioned in Introduction, this study is the first attempt describing the electrochemical investigation of favipiravir that is used as an antiviral option in the treatment of COVID-19. Taking advantage of a distinct enhancement effect of the cationic surfactant, CTAB on the electrochemical responses of favipiravir at the hydrophobic surface of CPT-BDD electrode, this electrode in combination with SW-AdSV could be used to develop a voltammetric method for favipiravir determination. The proposed approach was applicable directly to the routine quality control of commercial tablet formulations and to the analysis of model urine samples, without any use of sophisticated sample preparation, or relatively expensive apparatus.
Bearing in mind that the no data on the electrochemical investigation of favipiravir are still available, the promising experimental findings reported here could serve as a good reference point for future electrochemical studies focusing on the use of different electrode materials and modifying agents.
CRediT authorship contribution statement
Shabnam Allahverdiyeva: Methodology, Software, Validation, Formal analysis. Oruc Yunusoğlu: Data curation, Data collecting, Writing – original draft. Yavuz Yardım: Validation, Data curation, Writing – original draft. Zühre Şentürk: Writing – original draft, Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank the pharmaceutical company Atabay Pharmaceuticals and Fine Chemicals Inc., (Turkey) for the favipravir standard material.
Footnotes
This article is dedicated to the loving memory of Professor O. Yavuz Ataman, a brilliant Turkish scientist working in Analytical Chemistry, who passed away on August 15, 2020.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aca.2021.338418.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol. Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. Humana Press, New York, NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sohrabi C., Alsafi Z., O’Neill N., Khan M., Kerwan A., Al-Jabir A., Iosifidis C., Agha, World Health Organization declares global emergency R. A review of the 2019 novel coronavirus (COVID-19) Int. J. Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helmy Y.A., Fawzy M., Elaswad A., Sobieh A., Kenney S.P., Shehata A.A. The COVID-19 pandemic: a comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control. J. Clin. Med. 2020;9:1225. doi: 10.3390/jcm9041225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Udugama B., Kadhiresan P., Kozlowski H.N., Malekjahani A., Osborne M., Li V.Y.C., Chen H., Mubareka S., Gubbay J.B., Chan W.C.W. Diagnosing COVID-19: the disease and tools for detection. ACS Nano. 2020;14:3822–3835. doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- 6.Jin Y., Yang H., Ji W., Wu W., Chen S., Zhang W., Duan G. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12:372. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO Coronavirus Disease (COVID-2019) Dashboard. Available online: https://covid19.who.int.
- 8.Furuta Y., Takahashi K., Shiraki K., Sakamoto K., Smee D.F., Barnard D.L., Gowen B.B., Julander J.G., Morrey J.D. T-705 (favipiravir) and related compounds: novel broad-spectrum inhibitors of RNA viral infections. Antivir. Res. 2009;82:95–102. doi: 10.1016/j.antiviral.2009.02.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furuta Y., Gowen B.B., Takahashi K., Shiraki K., Smee D.F., Barnard D.L. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antivir. Res. 2013;100:446–454. doi: 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naesens L., Guddat L.W., Keough D.T., van Kuilenburg A.B.P., Meijer J., Voorde J.V., Balzarini J. Role of human hypoxanthine guanine phosphoribosyltransferase in activation of the antiviral agent T-705 (Favipiravir) Mol. Pharmacol. 2013;84:615–629. doi: 10.1124/mol.113.087247. [DOI] [PubMed] [Google Scholar]
- 11.Madelain V., Nguyen T.H., Olivo A., de Lamballerie X., Guedj J., Taburet A.M., Mentre F. Ebola virus infection: review of the pharmacokinetic and pharmacodynamics properties of drugs considered for testing in human efficacy trials. Clin. Pharmacokinet. 2016;55:907–923. doi: 10.1007/s40262-015-0364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boretti A. Favipiravir use for SARS CoV-2 infection. Pharmacol. Rep. 2020;72:1542–1552. doi: 10.1007/s43440-020-00175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du Y.-X., Chen X.-P. Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection. Clin. Pharmacol. Ther. 2020;108:242–247. doi: 10.1002/cpt.1878. [DOI] [PubMed] [Google Scholar]
- 14.Joshia S., Parkarb J., Ansaric A., Vorad A., Talware D., Tiwaskarf M., Patilg S., Barkate H. Role of favipiravir in the treatment of COVID-19. Int. J. Infect. Dis. 2021;102:501–508. doi: 10.1016/j.ijid.2020.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kivrak A., Kivrak B. Ulas H. A comparative analysis for anti-viral drugs: their efficiency against SARS-CoV-2. Int. Immunopharm. 2021;90 doi: 10.1016/j.intimp.2020.107232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acquavia M.A., Foti L., Pascale R., Nicolò A., Brancaleone V., Cataldi T.R.I., Martelli G., Scrano L., Bianco G. Detection and quantification of Covid-19 antiviral drugs in biological fluids and tissues. Talanta. 2021;224 doi: 10.1016/j.talanta.2020.121862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bulduk I. HPLC-UV method for quantification of favipiravir in pharmaceutical formulations. Acta Chromatogr. 2020 doi: 10.1556/1326.2020.00828. (accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.M Megahed S., Habib A.A., Hammad S.F., Kamal A.H. Experimental design approach for development of spectrofluorimetric method for determination of favipiravir; a potential therapeutic agent against COVID-19 virus: application to spiked human plasma. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021;249 doi: 10.1016/j.saa.2020.119241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chailapakul O., Siangproh W., Tryk D.A. Boron-doped diamond-based sensors: a review. Sens. Lett. 2006;4:99–119. doi: 10.1166/sl.2006.008. [DOI] [Google Scholar]
- 20.Peckova K., Musilova J., Barek J. Boron-doped diamond film electrodes-new tool for voltammetric determination of organic substances. Crit. Rev. Anal. Chem. 2009;39:148–172. doi: 10.1080/10408340903011812. [DOI] [Google Scholar]
- 21.luong J.H.T., Male K.B., Glennon J.D. Boron-doped diamond electrode: synthesis, characterization, functionalization and analytical applications. Analyst. 2009;134:1965–1979. doi: 10.1039/b910206j. [DOI] [PubMed] [Google Scholar]
- 22.Einaga Y. Diamond electrodes for electrochemical analysis. J. Appl. Electrochem. 2010;40:1807–1816. doi: 10.1007/s10800-010-0112-z. [DOI] [Google Scholar]
- 23.Yang N., Foord J.S., Jiang X. Diamond electrochemistry at the nanoscale: a review. Carbon. 2016;99:90–110. doi: 10.1016/j.carbon.2015.11.061. [DOI] [Google Scholar]
- 24.Cobb S.J., Ayres Z.J., Macpherson J.V. Boron doped diamond: a designer electrode material for the twenty-first century. Annu. Rev. Anal. Chem. 2018;11:463–484. doi: 10.1146/annurev-anchem-061417-010107. [DOI] [PubMed] [Google Scholar]
- 25.Muzyka K., Sun J., Fereja T.H., Lan Y., Zhang W., Xu G. Boron-doped diamond: current progress and challenges in view of electroanalytical applications. Anal. Methods. 2019;11:397–414. doi: 10.1039/C8AY02197J. [DOI] [Google Scholar]
- 26.Baluchova S., Danhel A., Dejmkova H., Ostatna V., Fojta M., Schwarzova-Peckova K. Recent progress in the applications of boron doped diamond electrodes in electroanalysis of organic compounds and biomolecules–A review. Anal. Chim. Acta. 2019;1077:30–66. doi: 10.1016/j.aca.2019.05.041. [DOI] [PubMed] [Google Scholar]
- 27.Trellu C., Chakraborty S., Nidheesh P.V., Oturan M.A. Environmental applications of boron-doped diamond electrodes: 2. soil remediation and sensing applications. ChemElectroChem. 2019;6:2143–2156. doi: 10.1002/celc.201801877. [DOI] [Google Scholar]
- 28.Lourencao B.C., Brocenschi R.F., Medeiros R.A., Fatibello-Filho O., Rocha-Filho R.C. Analytical applications of electrochemically pretreated boron-doped diamond electrodes. ChemElectroChem. 2020;7:1291–1311. doi: 10.1002/celc.202000050. [DOI] [Google Scholar]
- 29.Yigit A., Yardım Y., Selcuk Zorer O., Şentürk Z. Electrochemical determination of pterostilbene at a cathodically pretreated boron-doped diamond electrode using square-wave adsorptive anodic stripping voltammetry in cationic surfactant media. Sensor. Actuator. B Chem. 2016;231:688–695. doi: 10.1016/j.snb.2016.03.113. [DOI] [Google Scholar]
- 30.Saadi Ali H., Abdullah A.A., Talay Pınar P., Yardım Y., Şentürk Z. Simultaneous voltammetric determination of vanillin and caffeine in food products using an anodically pretreated boron-doped diamond electrode: its comparison with HPLC-DAD. Talanta. 2017;170:384–391. doi: 10.1016/j.talanta.2017.04.037. [DOI] [PubMed] [Google Scholar]
- 31.Abdullah A.A., Yardım Y., Şentürk Z. The performance of cathodically pretreated boron-doped diamond electrode in cationic surfactant media for enhancing the adsorptive stripping voltammetric determination of catechol-containing flavonoid quercetin in apple juice. Talanta. 2018;187:156–164. doi: 10.1016/j.talanta.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 32.Talay Pınar P., Allahverdiyeva S., Yardım Y., Şentürk Z. Voltammetric sensing of dinitrophenolic herbicide dinoterb on cathodically pretreated boron-doped diamond electrode in the presence of cationic surfactant. Microchem. J. 2020;155 doi: 10.1016/j.microc.2020.104772. [DOI] [Google Scholar]
- 33.Allahverdiyeva S., Talay Pınar P., Keskin E., Yunusoğlu O., Yardım Y., Şentürk Z. Adsorptive stripping voltammetric determination of higenamine on a boron-doped diamond electrode improved by the use of an anionic surfactant. Sensor. Actuator. B Chem. 2020;303 doi: 10.1016/j.snb.2019.127174. [DOI] [Google Scholar]
- 34.Allahverdiyeva S., Yardım Y., Şentürk Z. Electrooxidation of tetracycline antibiotic demeclocycline at unmodified boron-doped diamond electrode and its enhancement determination in surfactant-containing media. Talanta. 2021;223 doi: 10.1016/j.talanta.2020.121695. [DOI] [PubMed] [Google Scholar]
- 35.Talay Pınar P., Şentürk Z. Electrochemical and analytical performance of cathodically pretreated boron-doped diamond electrode for the determination of oxazolidinone antibiotic linezolid in cationic surfactant media. J. Electroanal. Chem. 2020;878 doi: 10.1016/j.jelechem.2020.114681. [DOI] [Google Scholar]
- 36.Hoshyar S.A., Barzani H.A.H., Yardım Y., Şentürk Z. The effect of CTAB, a cationic surfactant, on the adsorption ability of the boron-doped diamond electrode: application for voltammetric sensing of Bisphenol A and Hydroquinone in water samples. Colloids Surf. A Physicochem. Eng. Asp. 2021;610 doi: 10.1016/j.colsurfa.2020.125916. [DOI] [Google Scholar]
- 37.Brycht M., Lochyński P., Barek J., Skrzypek S., Kuczewski K., Schwarzova-Peckova K. Electrochemical study of 4-chloro-3-methylphenol on anodically pretreated boron-doped diamond electrode in the absence and presence of a cationic surfactant. J. Electroanal. Chem. 2016;771:1–9. doi: 10.1016/j.jelechem.2016.03.031. [DOI] [Google Scholar]
- 38.Gumustas M., Ozkan S.A. The role of and the place of method validation in drug analysis using electroanalytical techniques. Open Anal. Chem. J. 2011;5:1–21. doi: 10.2174/1874065001005010001. [DOI] [Google Scholar]
- 39.Rahayu R.S., Noviandri I., Buchari B., Abdullah M., Hinoue T. The effects of laser pulse irradiation at glassy carbon electrode on the electrochemistry of dopamine and ascorbic acid. Int. J. Electrochem. Sci. 2012;7:8255–8265. [Google Scholar]
- 40.Shankar S.S., Kumara Swamy B.E., Mahanthesha K.R., Sathisha T.V., Vishwanath C.C. Acetanilide modified carbon paste electrode for the electrochemical detection of dopamine: a cyclic voltammetric study. Anal. Bioanal. Electrochem. 2013;5:19–31. [Google Scholar]
- 41.Silva L.P., Vicentini F.C., Lourencao B.C., Oliveira G.G., Lanza M.R.V., Fatibello-Filho O. A new sensor architecture based on carbon Printex 6L to the electrochemical determination of ranitidine. J. Solid State Electrochem. 2016;20:2395–2402. doi: 10.1007/s10008-016-3143-5. [DOI] [Google Scholar]
- 42.Aristov N., Habekost A. Cyclic voltammetry-A versatile electrochemical method investigating electron transfer processes. World J. Chem. Edu. 2015;3:115–119. doi: 10.12691/wjce-3-5-2. [DOI] [Google Scholar]
- 43.Laviron E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979;101:19–28. doi: 10.1016/S0022-0728(79)80075-3. [DOI] [Google Scholar]
- 44.Suffredini H.B., Pedrosa V.A., Codognoto L., Machado S.A.S., Rocha-Filho R.C., Avaca L.A. Enhanced electrochemical response of boron-doped diamond electrodes brought on by a cathodic surface pre-treatment. Electrochim. Acta. 2004;49:4021–4026. doi: 10.1016/j.electacta.2004.01.082. [DOI] [Google Scholar]
- 45.Carlos S., Oliveira B., Oliveira-Brett A.M. Voltammetric and electrochemical impedance spectroscopy characterization of a cathodic and anodic pre-treated boron doped diamond electrode. Electrochim. Acta. 2010;55:4599–4605. doi: 10.1016/j.electacta.2010.03.016. [DOI] [Google Scholar]
- 46.Brocenschi R.F., Hammer P., Deslouis C., Rocha-Filho R.C. Assessments of the effect of increasingly severe cathodic pretreatments on the electrochemical activity of polycrystalline boron-doped diamond electrodes. Anal. Chem. 2016;88:5363–5368. doi: 10.1021/acs.analchem.6b00676. [DOI] [PubMed] [Google Scholar]
- 47.Sarakhman O., Dubenska L., Švorc Ľ. First voltammetric behavior study of non-narcotic analgesic drug nefopam and its reliable determination on boron-doped diamond electrodes. J. Electroanal. Chem. 2020;858 doi: 10.1016/j.jelechem.2019.113759. [DOI] [Google Scholar]
- 48.Favipiravir https://go.drugbank.com/drugs/DB12466.
- 49.da Silva G. Protonation, tautomerism, and base pairing of the antiviral favipiravir (T-705) ChemRxiv. 2020 doi: 10.26434/chemrxiv.12229122.v1. Preprint. [DOI] [Google Scholar]
- 50.Antonov L. Favipiravir tautomerism: a theoretical insight. Theor. Chem. Acc. 2020;139:145. doi: 10.1007/s00214-020-02656-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bergamini M.F., Santos D.P., Zanoni M.V.B. Electrochemical behavior and voltammetric determination of pyrazinamide using a poly-histidine modified electrode. J. Electroanal. Chem. 2013;690:47–52. doi: 10.1016/j.jelechem.2012.11.032. [DOI] [Google Scholar]
- 52.Ferraz B.R.L., Leite F.R.F., Malagutti A.R. Simultaneous determination of ethionamide and pyrazinamide using poly(l-cysteine) film-modified glassy carbon electrode. Talanta. 2016;154:197–207. doi: 10.1016/j.talanta.2016.03.058. [DOI] [PubMed] [Google Scholar]
- 53.Chokkareddy R., Kanchi S., Inamuddin Simultaneous detection of ethambutol and pyrazinamide with IL@CoFe2O4NPs@MWCNTs fabricated glassy carbon electrode. Sci. Rep. 2020;10:13563. doi: 10.1038/s41598-020-70263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Linke D. Detergents: an overview. Methods Enzymol. 2009;463:603–617. doi: 10.1016/S0076-6879(09)63034-2. [DOI] [PubMed] [Google Scholar]
- 55.Goronja J.M., Janošević Ležaić A.M., Dimitrijević B.M., Malenović A.M., Stanisavljev D.R., Pejić N.D. Determination of critical micelle concentration of cetyltrimethylammonium bromide: different procedures for analysis of experimental data. Hem. Ind. 2016;70:485–492. doi: 10.2298/HEMIND150622055G. [DOI] [Google Scholar]
- 56.Esumi K., Matoba M., Yamanaka Y. Characterization of adsorption of quaternary ammonium cationic surfactants and their adsolubilization behaviors on silica. Langmuir. 1996;12:2130–2133. doi: 10.1021/la950545k. [DOI] [Google Scholar]
- 57.Kovacs L., Warr G.G. Changes in the adsorbed layer structure of cationic surfactants on mica induced by adsolubilized aromatic molecules. Langmuir. 2002;18:4790–4794. doi: 10.1021/la0118558. [DOI] [Google Scholar]
- 58.Asvapathanagul P., Malakul P., O’Haver J. Adsolubilization of toluene and acetophenone as a function of surfactant adsorption. J. Colloid Interface Sci. 2005;292:305–311. doi: 10.1016/j.jcis.2005.05.064. [DOI] [PubMed] [Google Scholar]
- 59.Li L., Wang L., Du X., Lu Y., Yang Z. Adsolubilization of dihydroxybenzenes into CTAB layers on silica particles. J. Colloid Interface Sci. 2007;315:671–677. doi: 10.1016/j.jcis.2007.06.078. [DOI] [PubMed] [Google Scholar]
- 60.Wang L.-C., Ni X.-J., Cao Y.-H., Cao G.-Q. Adsorption behavior of bisphenol A on CTAB-modified graphite. Appl. Surf. Sci. 2018;428:165–170. doi: 10.1016/j.apsusc.2017.07.093. [DOI] [Google Scholar]
- 61.Aloulou F., Boufi S., Belgacem N., Gandini A. Adsorption of cationic surfactants and subsequent adsolubilization of organic compounds onto cellulose fibers. Colloid Polym. Sci. 2004;283:344–350. doi: 10.1007/s00396-004-1143-y. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.