Abstract
Patients with end-stage renal failure depend on hemodialysis indefinitely without renal transplantation, requiring a long-term patent vascular access. Although an arteriovenous fistula (AVF) remains the preferred vascular access for hemodialysis because of its longer patency and fewer complications compared with other vascular accesses, the primary patency of AVF is only 50% to 60%, presenting a clinical need for improvement. AVF mature by developing a thickened vascular wall and increased diameter to adapt to arterial blood pressure and flow volume. Inflammation plays a critical role during vascular remodeling and fistula maturation; increased shear stress triggers infiltration of T cells and macrophages that initiate inflammation, with involvement of several different subsets of T cells and macrophages. We review the literature describing distinct roles of the various subsets of T cells and macrophages during vascular remodeling. Immunosuppression with sirolimus or prednisolone decreases neointimal hyperplasia during AVF maturation, suggesting novel approaches to enhance vascular remodeling. However, M2 macrophages and CD4+ T cells play essential roles during AVF maturation, suggesting that total immunosuppression may suppress adaptive vascular remodeling. Therefore, it is likely that regulation of inflammation during fistula maturation will require a balanced approach to coordinate the various inflammatory cell subsets. Advances in immunosuppressive drug development and delivery systems may allow for more targeted regulation of inflammation to improve vascular remodeling and enhance AVF maturation. (JVS–Vascular Science 2020;1:207-18.)
Keywords: Arteriovenous fistula, Inflammation, T-cells, Macrophages, Vascular remodeling
Clinical Relevance
Patients with end-stage renal failure depend on successful AVF maturation to have a useful access. Inflammation occurs during fistula maturation and may be a therapeutic target to improve fistula maturation and utilization. Inflammation is mainly caused by T cells and macrophages, which have several subsets with distinct phenotypes and roles during vascular remodeling. Regulation of inflammation during fistula maturation requires a balanced approach to coordinate the various inflammatory cell subsets.
Graphical abstract
Article Highlights.
-
•
Type of Research: Literature review
-
•
Key Findings: T cells and macrophages accumulate and create inflammation in the vascular wall during arteriovenous fistula (AVF) maturation. Inflammation is classically detrimental to AVF success; however, depletion of T cells and macrophages leads to AVF failure. This contradiction may be explained by the diversity of T cells and macrophages, because they have several phenotypes and distinct roles in vascular remodeling.
-
•
Take Home Message: Inflammation from T cells and macrophages needs coordinated regulation for successful AVF maturation.
Arteriovenous fistulae (AVF) are the preferred vascular access for hemodialysis despite maturation success rates of only 50% to 60%,1 creating a clinical need to find additional therapeutic targets to improve AVF maturation. Inflammation is one potential therapeutic target as it is associated with various effects during venous remodeling.2, 3, 4, 5 A retrospective observational study showed that high C-reactive protein and fibrinogen levels, indicating increased inflammation, were associated with unsuccessful AVF maturation.3 Similarly, another study showed that C-reactive protein was an independent risk factor for late AVF failure.6 Therefore, inhibiting inflammation may offer benefits to improve AVF maturation and patency. Although several clinical studies investigated the ability of several drugs to improve AVF maturation or patency, no study has shown that inhibiting inflammation improves AVF patency or success.7, 8, 9, 10 This lack of efficacy may be due to the complex cellular and cytokine regulation of inflammation.
Inflammation in the maturing AVF is characterized by accumulation of T cells and macrophages.11, 12, 13, 14, 15 Because inhibition of T cell and macrophage accumulation in the maturing AVF leads to AVF failure, accumulation of T cells and macrophages is critically important for AVF maturation.11, 12, 13 T cells and macrophages have several subsets that play distinct roles to regulate inflammation during vascular remodeling.16, 17, 18, 19, 20, 21, 22, 23 Regulation of inflammation may need a more balanced approach to account for the diversity of T cells and macrophages that contribute to vascular remodeling. This review examines the roles of several types of T cells and macrophages during venous remodeling such as occurs during AVF maturation, supplements this with data from vein graft adaptation, and proposes several potential approaches to regulate distinct inflammatory cell types to improve AVF maturation.
Distinct subsets of inflammatory cells during venous remodeling
AVF maturation
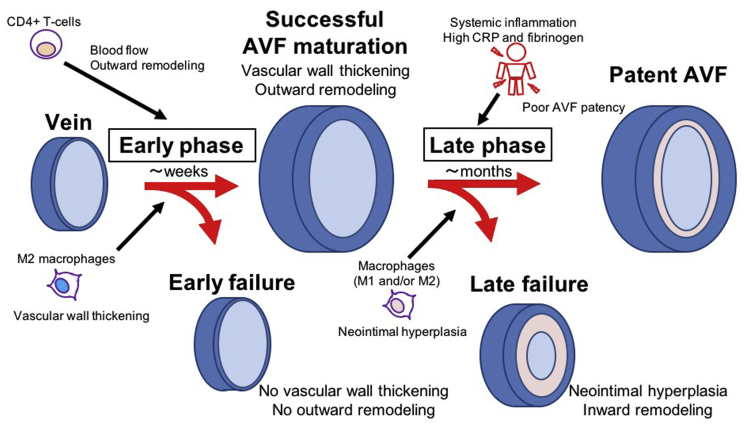
After surgical creation of an AVF, the vein is exposed to the new environment of the fistula, including arterial pressure, shear stress and oxygen tension.24,25 Accordingly, the fistula adapts to this environment, characterized by an early phase and a late phase (Fig 1). The early phase occurs during the first several weeks after AVF creation during which the AVF undergoes adaptive remodeling, characterized by diameter expansion and wall thickening, enabling the vein to become usable for hemodialysis.24 Failure of adaptive remodeling to occur, that is, failure of venous maturation, may be due to a lack of either outward remodeling or of wall thickening, although these processes are likely coordinated. Little is known about the normal physiology of the late phase of AVF remodeling, the time during which the AVF is used for hemodialysis; however, failure during the late phase is characterized by neointimal hyperplasia that can be associated with inward remodeling, and causes lumen loss, decreased flow, and a loss of access function. These two different times and mechanisms of AVF failure contribute to the high rate of access failure.
Fig 1.
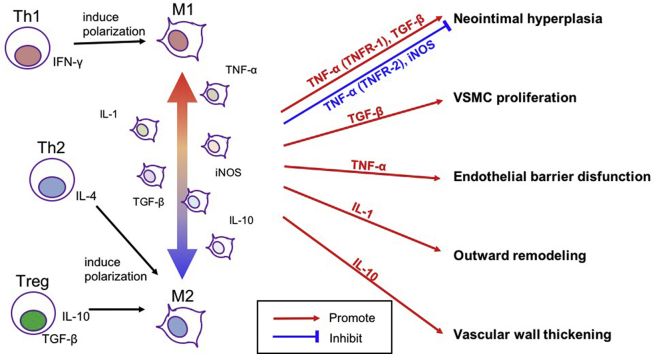
Associations between arteriovenous fistula (AVF) remodeling and inflammation. There are two phases of AVF remodeling, the early phase and the late phase. In the early phase, the remodeling AVF develops wall thickening and outward remodeling to become useable for hemodialysis. CD4+ T cells contribute to increased blood flow and outward remodeling, whereas M2 macrophages contribute to wall thickening. In the later phase, some AVF develop neointimal hyperplasia and inward remodeling and can eventually become unusable for hemodialysis. Systemic inflammation is associated with poor AVF patency. Macrophages are associated with neointimal hyperplasia. CRP, C-reactive protein.
Inflammation critically regulates both early and late phases of AVF adaptation to the fistula environment. Several types of inflammatory cells play a role during venous remodeling such as occurs during AVF maturation (Fig 1). Early and eccentric macrophage infiltration may play an important role in the pathogenesis of early AVF failure.14,15 In addition, low serum albumin and high C-reactive protein and fibrinogen, indicators of systemic inflammation, are found in patients with failed AVF maturation.3,6 Therefore, inflammation may be associated with poor Fig early AVF patency. However, recent studies have also shown the necessity of CD4+ T cell and M2 macrophage-driven inflammation for successful AVF maturation,11, 12, 13,26 suggesting that there are subsets of inflammatory cells with diverse contributions to venous remodeling. CD4+ T cells are necessary to maintain proper blood flow and outward remodeling during AVF maturation, as transfer of CD4+ T cells improved blood flow and outward remodeling in a T-cell-deficient rat AVF model.13 Different from CD4+ T cells, M2 macrophages have important roles in vascular wall thickening; reduction of M2 macrophages was associated with less vascular wall thickening.11,12 Thus, different subtypes of inflammatory cells may have different roles in AVF remodeling. Although it is still unclear about the roles of other inflammatory cells such as CD8+ T cells, M1 macrophages, circulating macrophages, natural killer T cells, monocytes, granulocytes, dendritic cells, and mast cells during AVF maturation, these inflammatory cells have distinct roles in vascular remodeling. Given the diversity of these inflammatory cells and cytokines that are involved in AVF maturation, it is likely that approaches to regulate inflammation should not focus on general promotion or suppression of inflammation, but rather will need regulation of specific cell types as well as of specific dosing and timing of therapeutics during different phases of venous remodeling.
Vein graft adaptation
Adaptation of a vein graft to the arterial environment is both similar to and different than fistula maturation.25 Vein grafts are exposed to arterial flow, pressure, and oxygen tension distinctly different from the venous environment and subsequently develop vascular wall thickening and outward remodeling. Although vein graft adaptation is adaptive venous remodeling similar to AVF maturation, vein graft adaptation is different from AVF maturation, with vein grafts developing thicker vascular walls and less outward remodeling compared with fistulae; however, accumulation of T cells and macrophages in the venous wall are observed in both vein grafts as well as in AVF.25,27
Vein graft remodeling that is predominantly characterized by wall thickening is, in part, regulated by locally released cytokines; however, differences in hemodynamics between the environments of vein grafts and AVF lead to the production of different cytokine profiles during vein graft adaptation and AVF maturation.28,29 During the early phase of vein graft remodeling, macrophages and T cells infiltrate through the endothelial layer into the vein wall.27 In some patients, the macrophages and T cells in the vein wall then promote development of areas that form stenoses in vein grafts via crosstalk between macrophages and vascular smooth muscle cells (SMC).27 Infiltrated T cells and macrophages secrete proinflammatory cytokines, and chemokines such as tumor necrosis factor-α (TNF-α) and monocyte chemotactic protein-1 (MCP-1) to enhance migration and proliferation of SMC that leads to neointimal hyperplasia,30,31 and depletion of macrophages significantly inhibits neointimal hyperplasia that forms in a rat vein graft model.32 In contrast, macrophages also produce inducible nitric oxide synthase (iNOS) that attenuates constrictive vascular remodeling and neointimal hyperplasia, and thus iNOS may have the potential to promote long-term patency of vein grafts.33, 34, 35 Altered shear stress affects early and transient interleukin (IL)-1 synthesis and delayed and persistent IL-10 production.36 IL-1 contributes to outward remodeling36, 37, 38 and IL-10 contributes to vascular wall thickening12,36,39; thus, there is a diversity of inflammatory cells and cytokines that regulate vein graft remodeling, similar to that which occurs in the venous wall during AVF maturation.
Phenotypes of T cells and macrophages
Helper, regulatory, and cytotoxic T cells
Distinct subsets of T cells are characterized by different cell surface markers, intracellular markers and secreted cytokines (Table I).16, 17, 18, 19 CD4 is the common marker of helper (Th) cells and regulatory (Treg) T cells, and CD8 is the marker of cytotoxic T cells (CTL).17,55,56 Arterial magnitudes of shear stress after AVF creation increases expression and secretion of interferon (IFN)-γ on the endothelial cells, and IFN-γ activates major histocompatibility complex (MHC) II to activate both circulating and resident CD4+ T cells.57 Activated CD4+ T cells are characterized as Th or Treg cells by their functions that are distinct with different effects on inflammation.
Table I.
Types of T cells involved in vascular remodeling
| Cell surface and intracellular markers | Secreted cytokines causing vascular remodeling | Association with macrophages | |
|---|---|---|---|
| Th140, 41, 42 | CD3, CD4, STAT4 | IFN-γ, TNF-α | Activate M1 |
| Th243,44 | CD3, CD4, STAT5 | IL-4 | Activate M2 |
| Treg19,45, 46, 47, 48, 49 | CD3, CD4, CD25, Foxp3 | IL-10, TGF-β | Suppress M1 Activate M2 |
| CTL50, 51, 52, 53, 54 | CD3, CD8 | IFN-γ, TNF-α | – |
CTL, Cytotoxic T cells; IFN-γ, interferon-γ; IL, interleukin; STAT, signal transducer and activator of transcription; Th1, T helper-1 cells; Th2, T helper-2 cells; Treg, regulatory T cells; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α.
The main function of Th cells is to produce cytokines, and Th cells are divided into several subtypes such as Th1 or Th2, depending on the secreted cytokines.40, 41, 42,45 Th1 cells secrete IFN-γ and activate other inflammatory cells including M1 macrophages,40, 41, 42,45 and IFN-γ is involved in vascular remodeling (Fig 2).58 M1 macrophages produce proinflammatory cytokines such as IFN-γ and TNF-α, which may damage vasculature integrity and promote inflammation.59, 60, 61, 62, 63
Fig 2.
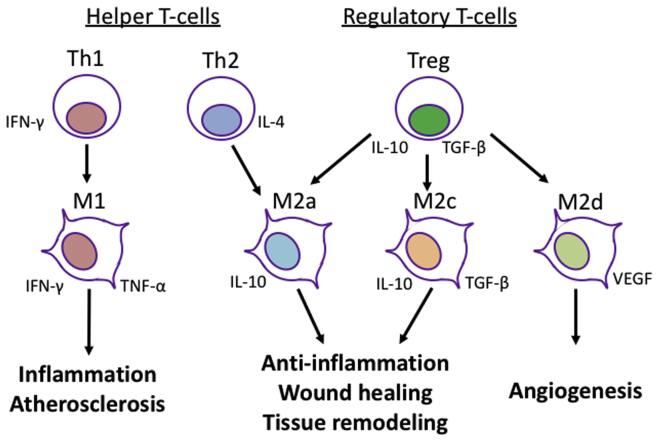
Associations between CD4+ T cells and macrophages. T-helper (Th) 1 cells secrete interferon-γ (IFN-γ) to induce M1 macrophages. M1 macrophages secrete IFN-γ and tumor necrosis factor-α (TNF-α) to promote inflammation. Th2 cells secrete IL-4 to induce M2a macrophages. Regulatory T cells (Treg) secrete interleukin (IL)-10 and transforming growth factor-β (TGF-β) to induce M2a, M2c and M2d macrophages. M2a and M2c macrophages secrete IL-10 and TGF-β to contribute to anti-inflammation, wound healing, and tissue remodeling. M2d secrete vascular endothelial growth factor (VEGF) to promote angiogenesis.
One of the primary functions of Th2 cells is secretion of IL-4.40, 41, 42,45 Interestingly, IL-4 exacerbates inflammation and decreases the integrity of the vascular endothelial barrier64 that occurs with atherosclerosis,65 yet Th2 cells seem to lessen the inflammation that occurs in diseased blood vessels.43 In particular, Th2 cells promote polarization of M2 macrophages that promote wound healing (Fig 2).44,66 M2 macrophages have been shown to be necessary for AVF maturation12,43; therefore, it is likely that a major role for Th2 cells to improve vascular remodeling is via induction of M2 macrophage polarization and differentiation.
Treg cells are anti-inflammatory T cells that are characterized by CD25+ and Foxp3+ markers (Table I).19,46 Treg cells are induced by IL-2 that is derived from other inflammatory cells; therefore, Treg cell differentiation functions as a negative feedback system to resolve inflammation.19,47 Treg cells secrete IL-10 and TGF-β not only to suppress other inflammatory cells including Th1, Th2, CTL, and M1 macrophages,48,49 but also to induce M2 macrophage polarization so that inflammation resolution, tissue healing and vascular remodeling are promoted (Fig 2).20 Thus, Treg cells have important roles in maintaining the homeostasis of the vasculature during vascular remodeling.
CTL are characterized by expression of CD8 antigen on their cell surface. There are several phenotypes of CD8+ T cells, including naïve CD8+ T cells, CTL, exhausted T cells, and memory CD8+ T cells.50 Different from CD4+ T cells, CD8+ T cells function independently from macrophages. Once CD8+ T cells recognize MHC I and pathogens, CD8+ T cells differentiate into CTL which secrete perforin, granzyme, and TNF-α to induce cell apoptosis.51 CTL are typically initially activated by coexistence of MHC I and pathogenic antigens that are presented by dendritic cells.52 In baseline conditions, vascular cells have MHC I, but are not activated because there are no dendritic cells presenting antigens; therefore, CTL are rarely detectable in the normal vascular wall. However, once the vascular wall is exposed to inflammation, CTL infiltrate into the vascular wall.53 Because apoptosis of vascular cells causes reduced vascular wall barrier function, leading to vulnerability of the diseased vasculature, CTL promote vulnerable atherosclerotic plaques in apoE-deficient mice.53,54 In contrast, CD8+ cells protect against the development of neointimal hyperplasia and failure of vein grafts53; CD8+ T cells improved vein graft patency whereas CD8+ T-cell-depleted mice showed increased apoptosis and higher rates of vein graft failure; surprisingly, the protective effect of CD8+ T cells on vein graft patency was independent of MHC and other antigens that are necessary for inducing CD8+ T cells to differentiate into CTL.53 There may be other subsets of CD8+ T cells that contribute to vascular remodeling; methods to activate specific groups of CD8+ T cells during AVF maturation or vein graft adaptation that allows taking advantage of their protective effects are still unknown.
M1 and M2 macrophages
Different from circulating inflammatory cells that contribute to systemic inflammation, macrophages work within tissues to contribute to local inflammation. Macrophages are classically divided into one of two major subtypes, M1 or M2, and the polarization into a particular subset phenotype depends on the cytokine environment of the tissue5,23; the commonly described macrophage subsets are summarized in Table II. M1 macrophage polarization is induced by Th1 cytokines such as IFN-γ, whereas M2 macrophage polarization is induced by Th2 and Treg cytokines such as IL-4, IL-10, and TGF-β (Fig 2). M1 macrophages are known as inflammatory macrophages because they secrete proinflammatory cytokines, such as IFN-γ and TNF-α, and may affect vascular remodeling. The proinflammatory cytokines may induce vascular cell apoptosis, dissolve extracellular matrix, induce breaks in elastic fibers, promote SMC apoptosis, and impair endothelial barrier function,59,60 resulting in accelerated atherosclerosis61,62 and aneurysm formation.63 Although proinflammatory cytokines may be associated with adverse effects on the vascular wall to stimulate some vascular pathologies, however, proinflammatory cytokines play important roles in dissolving the venous wall extracellular matrix to enable outward remodeling during AVF maturation.68,69 iNOS is also produced by M1 macrophages, and iNOS attenuates constrictive vascular remodeling and neointimal hyperplasia of vein grafts in a rabbit model.33,34 From these data, the role of M1 macrophages during venous remodeling is unclear, because M1 macrophages may both inhibit AVF primary maturation as well as improve AVF long-term patency.
Table II.
Types of macrophages involved in vascular remodeling
| Cell surface and intracellular markers | Secreted cytokines | Main functions | |
|---|---|---|---|
| M159, 60, 61, 62, 63 | CD68, iNOS, STAT1, IFN-γ receptor | TNF-α, IFN-γ, IL-1 | Proinflammation |
| M2a20, 21, 22, 23,67 | CD68, CD206, TGM2, Arginase 1, STAT6 | IL-10, TGF-β | Anti-inflammation Wound healing |
| M2b20, 21, 22, 23 | CD68, IL-4 receptor | IL-1β, IL-6, IL-10, TNF-α | Immunoregulation |
| M2c20, 21, 22, 23,67 | CD68, CD206, CCR2, Arginase 1 | IL-10, TGF-β | Immunosuppression Tissue remodeling |
| M2d20, 21, 22, 23 | CD68, iNOS | IL-10, VEGF | Angiogenesis |
CCR, Chemokine receptor; IFN-γ, interferon-γ; IL, interleukin; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; M1, M1-type macrophages; M2, M2-type macrophages; TNF-α, tumor necrosis factor-α; TGF-β, transforming growth factor-β; TGM, transglutaminase; TLR, toll like receptor; VEGF, vascular endothelial growth factor.
M2 macrophages, also known as prerepair macrophages, are polarized into the M2 phenotype by IL-4 secreted from Th2 cells and by IL-10 and TGF-β secreted from Treg cells.66,67,70 The main role of M2 macrophages is to resolve inflammation and promote tissue remodeling and wound healing. M2 macrophages are further categorized into M2a, M2b, M2c, and M2d macrophages (Table II).20, 21, 22, 23 M2a and M2c macrophages seem to have important roles in vascular remodeling because they produce IL-10, TGF-β, and arginase 1, which have critical roles in vascular remodeling. IL-10 and TGF-β have important roles in maintaining the integrity of the vasculature as described elsewhere in this article (Table III). M2a and M2c macrophages also produce arginase 1, which promotes SMC proliferation and inhibits inflammation.80 During the early phase of AVF maturation, SMC proliferation and vascular wall thickening are critically regulated by M2 macrophage infiltration,12,24 and thus arginase 1 may be associated with AVF primary maturation. In contrast, arginase 1 might have an adverse effect during the later phase of AVF maturation because arginase 1 also promotes neointimal formation in a rat common carotid injury model.81 Although arterial remodeling is different from AVF remodeling, arginase 1 may critically regulate the balance of vascular wall thickening and neointimal formation, and may be either beneficial or deleterious during AVF maturation.
Table III.
Effector cytokines secreted by T cells and macrophages during vascular remodeling
| Effects on vascular wall | M1 | M2 | CTL | Th1 | Th2 | Treg | |
|---|---|---|---|---|---|---|---|
| IL-1 | Outward remodeling37 Vein graft wall adaptation38 |
++ | + | + | + | + | - |
| IL-10 | Maintenance of normal vasculature12,36,39 | - | ++ | - | - | - | ++ |
| TNF-α | Endothelial barrier dysfunction71 Increase vein graft neointimal hyperplasia (TNFR-1)72 Reduce vein graft neointimal hyperplasia (TNFR-2)73 |
++ | - | + | + | - | - |
| TGF-β | Promote SMC proliferation74, 75, 76, 77, 78, 79 Promotes neointimal hyperplasia79 |
- | ++ | - | - | - | ++ |
CTL, Cytotoxic T cells; IL, interleukin; M1, M1-type macrophages; M2, M2-type macrophages; SMC, smooth muscle cell; TGF-β, transforming growth factor-β; Th1, T helper-1 cells; Th2, T helper-2 cells; TNF-α, tumor necrosis factor-α; TNFR, tumor necrosis factor receptor; Treg, regulatory T-cells.
Etiology of inflammation during venous remodeling
Shear stress as a trigger of inflammation
Shear stress is a hemodynamic force tangential to the endothelium whereas blood pressure is a hemodynamic force perpendicular to the vessel wall.82,83 The magnitude of shear stress in arteries is approximately 1 Pa, which is more than 300,000 times less than typical magnitudes of arterial blood pressure (300,000-500,000 Pa). Nevertheless, the effects of shear stress on the vascular wall are not negligible.82,83 Normal veins are typically exposed to less blood flow with lower magnitudes of shear stress compared with arteries, but veins are exposed to increased magnitudes of shear stress after AVF creation.25 Exposure of veins to arterial magnitudes of shear stress and mechanical stretch promotes inflammation and remodeling.84,85 Because AVF are exposed to higher magnitudes of shear stress and lower arterial pressure compared with vein grafts,25 AVF maturation and vein graft adaptation show different patterns of remodeling to normalize the hemodynamic forces, with AVF maturation characterized by more outward remodeling and less wall thickening compared with vein graft adaptation.25 The arterial magnitudes of shear stress promote production of anti-inflammatory cytokines in vein grafts.28 Because anti-inflammatory cytokines are necessary for maintenance of the normal architecture of vessels, it is possible that increased shear stress contributes to venous remodeling.25,28 In contrast, nonlaminar shear stress induces a proinflammatory response.86 Low and oscillatory shear stress is characteristic of most vascular anastomoses and is associated with clinically significant stenosis in some patients.87 Nonlaminar shear stress induces changes in endothelial cell gene expression, cytokine production, and cytoskeleton organization to increase secretion of MCP-1, and decrease nitric oxide secretion to cause activation and proliferation of SMC within the vein wall.86 Thus, maintaining arterial magnitudes and laminar character of shear stress is important to regulate inflammatory cells for AVF maturation without excessive neointimal thickening.
Plasticity of endothelial identity
Veins and arteries have different molecular identity that is most easily identified in the endothelium. Both embryonic and adult arterial endothelial cells express Ephrin-B2 and delta-like protein 4 (dll-4) whereas venous endothelial cells express Eph-B4.88,89 After vein grafts are exposed to the arterial environment, they lose Eph-B4 expression, but do not express Ephrin-B2 and dll-4, consistent with venous endothelial cells losing venous identity but not acquiring arterial identity during adaptation to an arterial environment.89 Interestingly, changes of endothelial identity during AVF maturation are different from those that occur during vein graft adaptation; the AVF expresses Ephrin-B2 and dll-4 arterial markers in addition to increasing Eph-B4 expression, consistent with the AVF gaining arterial as well as venous identities.90 This change in endothelial identity is likely to affect the inflammatory response during vascular remodeling because ephrin-Eph signaling regulates cell proliferation, survival and migration,91 as well as regulation of T-cell and monocyte activation.92 Monocytes also express Eph-B4 on their cell surface, and Eph-B4 binds to endothelial Ephrin-B2 to induce adhesion of monocytes.93 Monocytes then migrate into the vascular wall and differentiate into macrophages,93 promoting inflammation. Additionally, dll-4, which is an arterial marker, simulates macrophage activation, MCP-1 production and nuclear factor-kappa B activation. In particular, dll-4 induces polarization of macrophages to the M1 phenotype.94 Thus, acquiring arterial identity may play an important role in regulating inflammation during AVF maturation.
Adhesion molecules promote inflammatory cell infiltration into the vascular wall
Mechanical stretch, endothelial injury and arterial shear stress also lead to vascular inflammation.95,96 Arterial shear stress regulates expression of cell surface molecules that stimulate leukocyte adhesion and migration, such as vascular cell adhesion molecule 1 (VCAM-1) and E-selectin.97 In a mouse venous arterialization model, expression of endothelial VCAM-1 and E-selectin was present early after surgery, concomitant with the presence of neutrophils and monocytes/macrophages in the vascular wall.98 In AVF that fail owing to thrombosis, there is greater infiltration of macrophages and higher expression of adhesion molecules such as VCAM-1; VCAM-1 and proinflammatory macrophages may be associated with impaired endothelial antithrombotic functions.99 E-selectin initiates rolling of leukocytes including monocytes and T cells followed by infiltration of these cells into the vascular wall.100, 101, 102 Infiltrated monocytes and T cells will then differentiate into different subtypes of macrophages and T cells, depending on the cytokines and chemokines present in the vessel wall.16, 17, 18, 19, 20, 21, 22, 23 Unlike VCAM-1, which is associated with endothelial barrier functions, E-selectin is associated with adventitial inflammation103; inhibition of E-selectin expression decreases adventitial inflammatory cells and attenuates neointimal hyperplasia in a rat carotid balloon injury model.103 These data suggest that E-selectin regulates infiltration of inflammatory cells into the vascular adventitia after an endothelial injury and contributes to neointimal hyperplasia during the process of vascular wall healing. Moreover, E-selectin also contributes to leukocyte recruitment in a mouse vein graft model.95 Although the vascular environment within an AVF is different from the arterial environment,25 E-selectin may also have an important role during AVF remodeling to promote infiltration of inflammatory cells and contribute to vascular wall thickening or neointimal hyperplasia during AVF maturation.
Effector cytokines during venous remodeling
An important role of infiltrating inflammatory cells during venous remodeling is the production of effector cytokines that promote inflammation. Although some data are available regarding the types of these cytokines as well as their effects on venous remodeling (Table III; Fig 3), additional mechanistic understanding of how these cytokines regulate remodeling is still needed.
Fig 3.
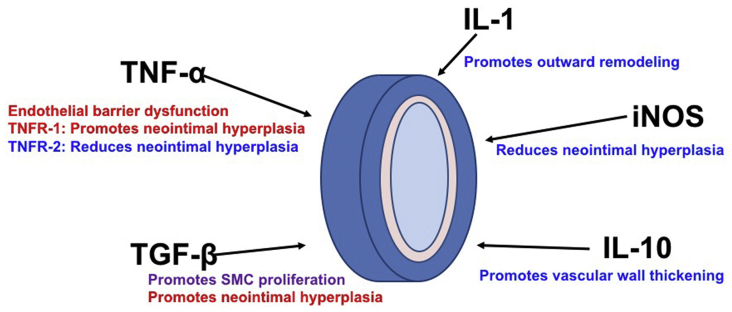
Associations between effector cytokines and venous remodeling. Tumor necrosis factor-α (TNF-α) causes endothelial barrier dysfunction. Tumor necrosis factor receptor (TNFR)-1 signaling promotes neointimal hyperplasia, but TNFR-2 signaling reduces neointimal hyperplasia. Transforming growth factor-β (TGF-β) promotes vascular smooth muscle cell (SMC) proliferation and neointimal hyperplasia. Interleukin (IL)-1 promotes outward remodeling. Inducible nitric oxide synthase (iNOS) decreases neointimal hyperplasia. IL-10 promotes vascular wall thickening. Factors promoting adaptive venous remodeling are shown in blue and factors inhibiting adaptive venous remodeling are shown in red. Factors having both effects on venous remodeling are shown in purple.
After exposure of a vein to arterial blood flow, increased wall shear stress affects early IL-1 production and delayed IL-10 signaling during vein graft remodeling.36 IL-1 regulates matrix metalloproteinases expression to promote outward remodeling,37 and a lack of IL-1 signaling results in perturbed early vein graft wall adaptation in a mouse vein graft model.38 Although there is no study that shows the significance of IL-1 during AVF maturation, early production of IL-1 may have a critical role during AVF maturation because outward remodeling at early time points is essential for AVF maturation. IL-10 is an anti-inflammatory cytokine secreted by M2 macrophages and Treg cells. IL-10 inhibits the adhesion of leukocytes to endothelial cells.104 In addition to preventing leukocyte adhesion, it appears that IL-10 is essential for the maintenance of normal vasculature.39 IL-10 is also an essential cytokine that regulates vascular wall thickening during AVF maturation.12
TNF-α is a proinflammatory cytokine secreted by Th1 and M1 macrophages. TNF-α contributes to endothelial dysfunction and neointimal hyperplasia.71 Interestingly, vein graft neointimal hyperplasia is exacerbated by TNF receptor-1 signaling, but attenuated by TNF receptor-2 signaling.72,73 Because TNF-α plays a role in both exacerbation and attenuation of neointimal hyperplasia, direct inhibition of TNF-α may not be a translatable therapy; however, selective inhibition of TNF receptor-1 may be a potential strategy to inhibit neointimal hyperplasia, and targeting TNF receptors may therefore be a strategy to improve AVF patency.
TGF-β is an anti-inflammatory cytokine produced by Treg cells and M2 macrophages. TGF-β can suppress Th1 and Th2 cell differentiation, proliferation and cytokine secretion, but induces differentiation of Treg cells.105, 106, 107 TGF-β also independently contributes to vascular remodeling by promoting SMC proliferation and extracellular matrix and collagen production to maintain the integrity of the vasculature.74, 75, 76 Therefore, TGF-β has functions that may promote AVF primary maturation. In contrast, TGF-β is also associated with AVF failure in the late phase of AVF remodeling77, 78, 79; TGF-β stimulates vascular SMC proliferation and promotes neointimal hyperplasia formation in AVF.78 Because neointimal hyperplasia is a risk factor for late AVF failure, elevated expression of TGF-β may be a risk factor for AVF failure.79 Thus, although TGF-β may promote early venous remodeling, such as occurs during AVF maturation, higher levels of TGF-β were associated with increased risk of AVF failure79; this may be secondary to TGF-β′s role in promotion of neointimal hyperplasia. The exact roles of TGF-β in early or late venous remodeling are currently under investigation.
Future directions to improve AVF patency
Targeting inflammation
Sirolimus and tacrolimus are immunosuppressant drugs that decrease neointimal hyperplasia in animal models. Sirolimus improves experimental AVF patency by decreasing wall thickening while attenuating the Akt1-mTORC1 signaling pathway in SMC and macrophages.11 Because sirolimus has been used clinically in human patients in conjunction with coronary stents to preserve lumen diameter and decrease restenosis after coronary artery stenting,108 sirolimus should have potential to decrease neointimal hyperplasia in AVF; because the arterial and venous environments are different, additional research in this area will be needed. Interestingly, the combination of sirolimus and tacrolimus is associated with a greater decrease in vascular narrowing109; because tacrolimus acts specifically on T cells, these data also suggest that T cells are associated with excessive SMC proliferation, and that macrophages are inhibited by sirolimus. Prednisolone is another anti-inflammatory drug used in human patients and that could be used to modulate AVF maturation; a murine AVF model showed prednisolone attenuated infiltration of lymphocytes and M1 macrophages and enhanced outward remodeling of the AVF wall.110
Because immunosuppressive drugs have multiple effects on inflammatory cells, additional evidence is still required to determine which subset of inflammatory cells is the optimal cell type to target to improve AVF maturation. Treg cells are one potential therapeutic target.48,49 Treg cells secrete IL-10 and TGF-β to suppress inflammation that is associated with AVF failure3,6,111; therefore, inducing the number of Treg cells and/or their function could be considered a potential treatment to improve the success of AVF in human patients. Interestingly, because sirolimus can induce Treg cell differentiation,112 sirolimus's success in improving AVF patency might be due to its upregulation of Treg cell activity.11
Antibodies targeting inflammation have therapeutic potential during AVF remodeling. For example, a selective anti-CD4+ T-cell epithelial growth factor receptor antibody induces T-cell anergy and reduces atherosclerosis.113 Because antibodies can specifically target single cell types such as antigen expressing cells or M1 macrophages, targeted therapeutic antibodies may be able to both provide additional mechanistic understanding of venous remodeling as well as regulate inflammation during AVF maturation in more sophisticated ways.
Local therapy to control inflammation
Local delivery of anti-inflammatory agents may help to minimize adverse effects such as immunosuppression and severe infection that are associated with systemic administration of these therapies. Local injection of virus vectors has been successful in gene transduction without an inflammatory response.114, 115, 116 Sendai virus causes less inflammation than other viruses, and has been used in patients with peripheral arterial disease.117 The use of nanoparticles for local drug delivery is also effective in humans.118 In animal models, treatment of vein grafts with imatinib nanoparticles before graft implantation inhibits development of focal areas of stenosis,119 and nanoparticle-mediated delivery of irbesartan promotes cardioprotection from myocardial ischemia-reperfusion injury.120 Thus, local drug delivery systems with virus vectors and nanoparticles show efficacy in some cardiovascular diseases, and therefore could potentially be used for patients with AVF. Pluronic gel, a thermoreversible hydrogel, is another medium used for local drug delivery to the adventitia; pluronic gel is delivered as a liquid at room temperature and hardens at body temperature. After pluronic gel is applied around the perivascular wall, there is sustained release of drugs.121 Animal models have shown the usefulness of this delivery system in AVF maturation and vein graft remodeling.11,12,122
An endovascular delivery system can be used to infuse drugs locally into the arterial adventitia.123 Although this system has been used to deliver dexamethasone into an arterial wall,123 treatment of the thinner venous wall may not be possible, especially prior to maturation; however, since perivascular inflammatory cells play an important role during AVF maturation, immunosuppressive drug delivery into the venous adventitia might help treat AVF stenoses to increase secondary patency. A double balloon catheter system has been used to deliver drugs directly to the intima124; the balloons are inflated to isolate a vessel segment, and drugs are infused between the two balloons.124 Because endothelial cells regulate the infiltration of inflammatory cells, local drug delivery with the double balloon catheter may be feasible to locally control endothelial function to improve AVF remodeling. Although balloon catheters induce neointimal hyperplasia, the double balloon catheter may deliver drugs to inhibit neointimal hyperplasia and therefore contribute to improved secondary AVF patency. With local drug delivery systems, patients with an AVF may be treated more specifically to maximize therapeutic effects and minimize systemic adverse effects.
Conclusions
T cells and macrophages play critical roles during AVF maturation with distinct effects attributed to each of these cells. From the limited evidence to date, Th2, Treg, and M2 macrophages are necessary for primary AVF maturation, but are also associated with late AVF failure. Th1 and M1 macrophages are associated with primary AVF failure, but may also contribute to long-term patency by inhibiting neointimal hyperplasia. The nuanced roles of T cells and macrophages during venous remodeling such as occurs during AVF maturation and vein graft adaptation have yet to be adequately understood owing to the great functional diversity of these cells. Regulation of specific inflammatory cells and their functions during the different phases of AVF remodeling with selective immunosuppressive drugs and local drug delivery systems may offer therapeutic promise to improve AVF maturation, the numbers of working fistulae and long-term AVF patency.
AUTHOR CONTRIBUTIONS
Conception and design: YM, GK
Analysis and interpretation: YM, GK, AF, JL, AD
Data collection: YM, GK, AF, JL, AD
Writing the article: YM, GK, AF, JL
Critical revision of the article: AD
Final approval of the article: YM, GK, AF, JL, AD
Statistical analysis: Not applicable Obtained funding: YM, AD
Overall responsibility: AD
Footnotes
Supported by US National Institute of Health (NIH) grant R01-HL144476 [to A.D.] and the Uehara Memorial Foundation postdoctoral fellowship [to Y.M.] as well as with the resources and the use of facilities at the VA Connecticut Healthcare System, West Haven, CT.
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS-Vascular Science policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Disbrow D.E., Cull D.L., Carsten C.G., 3rd, Yang S.K., Johnson B.L., Keahey G.P. Comparison of arteriovenous fistulas and arteriovenous grafts in patients with favorable vascular anatomy and equivalent access to health care: is a reappraisal of the Fistula First Initiative indicated? J Am Coll Surg. 2013;216:679–685. doi: 10.1016/j.jamcollsurg.2012.12.021. discussion: 685-6. [DOI] [PubMed] [Google Scholar]
- 2.Christiansen J.F., Hartwig D., Bechtel J.F., Kluter H., Sievers H., Schonbeck U. Diseased vein grafts express elevated inflammatory cytokine levels compared with atherosclerotic coronary arteries. Ann Thorac Surg. 2004;77:1575–1579. doi: 10.1016/j.athoracsur.2003.10.107. [DOI] [PubMed] [Google Scholar]
- 3.Kaygin M.A., Halici U., Aydin A., Dag O., Binici D.N., Limandal H.K. The relationship between arteriovenous fistula success and inflammation. Ren Fail. 2013;35:1085–1088. doi: 10.3109/0886022X.2013.815100. [DOI] [PubMed] [Google Scholar]
- 4.Moreno K., Murray-Wijelath J., Yagi M., Kohler T., Hatsukami T., Clowes A. Circulating inflammatory cells are associated with vein graft stenosis. J Vasc Surg. 2011;54:1124–1130. doi: 10.1016/j.jvs.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parma L., Baganha F., Quax P.H.A., de Vries M.R. Plaque angiogenesis and intraplaque hemorrhage in atherosclerosis. Eur J Pharmacol. 2017;816:107–115. doi: 10.1016/j.ejphar.2017.04.028. [DOI] [PubMed] [Google Scholar]
- 6.Stirbu O., Gadalean F., Pitea I.V., Ciobanu G., Schiller A., Grosu I. C-reactive protein as a prognostic risk factor for loss of arteriovenous fistula patency in hemodialyzed patients. J Vasc Surg. 2019;70:208–215. doi: 10.1016/j.jvs.2018.10.100. [DOI] [PubMed] [Google Scholar]
- 7.Tanner N.C., Da Silva A. Medical adjuvant treatment to increase patency of arteriovenous fistulae and grafts. Cochrane Database Syst Rev. 2015;2015:CD002786. doi: 10.1002/14651858.CD002786.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irish A.B., Viecelli A.K., Hawley C.M., Hooi L.S., Pascoe E.M., Paul-Brent P.A. Effect of fish oil supplementation and aspirin use on arteriovenous fistula failure in patients requiring hemodialysis: a randomized clinical trial. JAMA Intern Med. 2017;177:184–193. doi: 10.1001/jamainternmed.2016.8029. [DOI] [PubMed] [Google Scholar]
- 9.Paulson W.D., Kipshidze N., Kipiani K., Beridze N., DeVita M.V., Shenoy S. Safety and efficacy of local periadventitial delivery of sirolimus for improving hemodialysis graft patency: first human experience with a sirolimus-eluting collagen membrane (Coll-R) Nephrol Dial Transplant. 2012;27:1219–1224. doi: 10.1093/ndt/gfr667. [DOI] [PubMed] [Google Scholar]
- 10.do Sameiro Faria M., Ribeiro S., Rocha-Pereira P., Miranda V., Quintanilha A., Reis F. Vascular access versus the effect of statins on inflammation and fibrinolysis in renal dialysis patients. J Vasc Access. 2013;14:335–341. doi: 10.5301/jva.5000132. [DOI] [PubMed] [Google Scholar]
- 11.Guo X., Fereydooni A., Isaji T., Gorecka J., Liu S., Hu H. Inhibition of the Akt1-mTORC1 Axis alters venous remodeling to improve arteriovenous fistula patency. Sci Rep. 2019;9:11046. doi: 10.1038/s41598-019-47542-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuwahara G., Hashimoto T., Tsuneki M., Yamamoto K., Assi R., Foster T.R. CD44 promotes inflammation and extracellular matrix production during arteriovenous fistula maturation. Arterioscler Thromb Vasc Biol. 2017;37:1147–1156. doi: 10.1161/ATVBAHA.117.309385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duque J.C., Martinez L., Mesa A., Wei Y., Tabbara M., Salman L.H. CD4(+) lymphocytes improve venous blood flow in experimental arteriovenous fistulae. Surgery. 2015;158:529–536. doi: 10.1016/j.surg.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorecka J., Fereydooni A., Gonzalez L., Lee S.R., Liu S., Ono S. Molecular targets for improving arteriovenous fistula maturation and patency. Vasc Investig Ther. 2019;2:33–41. doi: 10.4103/VIT.VIT_9_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy-Chaudhury P., Khan R., Campos B., Wang Y., Kurian M., Lee T. Pathogenetic role for early focal macrophage infiltration in a pig model of arteriovenous fistula (AVF) stenosis. J Vasc Access. 2013;15:25–28. doi: 10.5301/jva.5000151. [DOI] [PubMed] [Google Scholar]
- 16.Fang D., Zhu J. Molecular switches for regulating the differentiation of inflammatory and IL-10-producing anti-inflammatory T-helper cells. Cell Mol Life Sci. 2019;77:289–303. doi: 10.1007/s00018-019-03277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konjar S., Veldhoen M. Dynamic metabolic state of tissue resident CD8 T cells. Front Immunol. 2019;10:1683. doi: 10.3389/fimmu.2019.01683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ou H.X., Guo B.B., Liu Q., Li Y.K., Yang Z., Feng W.J. Regulatory T cells as a new therapeutic target for atherosclerosis. Acta Pharmacol Sin. 2018;39:1249–1258. doi: 10.1038/aps.2017.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 20.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Shapouri-Moghaddam A., Mohammadian S., Vazini H., Taghadosi M., Esmaeili S.A., Mardani F. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 22.Wang L.X., Zhang S.X., Wu H.J., Rong X.L., Guo J. M2b macrophage polarization and its roles in diseases. J Leukoc Biol. 2019;106:345–358. doi: 10.1002/JLB.3RU1018-378RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray P.J., Wynn T.A. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu H., Patel S., Hanisch J.J., Santana J.M., Hashimoto T., Bai H. Future research directions to improve fistula maturation and reduce access failure. Semin Vasc Surg. 2016;29:153–171. doi: 10.1053/j.semvascsurg.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu D.Y., Chen E.Y., Wong D.J., Yamamoto K., Protack C.D., Williams W.T. Vein graft adaptation and fistula maturation in the arterial environment. J Surg Res. 2014;188:162–173. doi: 10.1016/j.jss.2014.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duque J.C., Martinez L., Tabbara M., Salman L.H., Vazquez-Padron R.I., Dejman A. Arteriovenous fistula outcomes in human immunodeficiency virus-positive patients. Saudi J Kidney Dis Transpl. 2018;29:1350–1357. doi: 10.4103/1319-2442.248312. [DOI] [PubMed] [Google Scholar]
- 27.Koga J.I., Nakano T., Dahlman J.E., Figueiredo J.L., Zhang H., Decano J. Macrophage notch ligand delta-like 4 promotes vein graft lesion development: implications for the treatment of vein graft failure. Arterioscler Thromb Vasc Biol. 2015;35:2343–2353. doi: 10.1161/ATVBAHA.115.305516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozaki C.K. Cytokines and the early vein graft: strategies to enhance durability. J Vasc Surg. 2007;45(Suppl A):A92–A98. doi: 10.1016/j.jvs.2007.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brahmbhatt A., Remuzzi A., Franzoni M., Misra S. The molecular mechanisms of hemodialysis vascular access failure. Kidney Int. 2016;89:303–316. doi: 10.1016/j.kint.2015.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Vries M.R., Quax P.H.A. Inflammation in vein graft disease. Front Cardiovasc Med. 2018;5:3. doi: 10.3389/fcvm.2018.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Owens C.D., Gasper W.J., Rahman A.S., Conte M.S. Vein graft failure. J Vasc Surg. 2015;61:203–216. doi: 10.1016/j.jvs.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoch J.R., Stark V.K., van Rooijen N., Kim J.L., Nutt M.P., Warner T.F. Macrophage depletion alters vein graft intimal hyperplasia. Surgery. 1999;126:428–437. [PubMed] [Google Scholar]
- 33.Yogo K., Shimokawa H., Funakoshi H., Kandabashi T., Miyata K., Okamoto S. Different vasculoprotective roles of NO synthase isoforms in vascular lesion formation in mice. Arterioscler Thromb Vasc Biol. 2000;20:e96–e100. doi: 10.1161/01.atv.20.11.e96. [DOI] [PubMed] [Google Scholar]
- 34.Meng Q.H., Irvine S., Tagalakis A.D., McAnulty R.J., McEwan J.R., Hart S.L. Inhibition of neointimal hyperplasia in a rabbit vein graft model following non-viral transfection with human iNOS cDNA. Gene Ther. 2013;20:979–986. doi: 10.1038/gt.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kibbe M.R., Tzeng E., Gleixner S.L., Watkins S.C., Kovesdi I., Lizonova A. Adenovirus-mediated gene transfer of human inducible nitric oxide synthase in porcine vein grafts inhibits intimal hyperplasia. J Vasc Surg. 2001;34:156–165. doi: 10.1067/mva.2001.113983. [DOI] [PubMed] [Google Scholar]
- 36.Jiang Z., Berceli S.A., Pfahnl C.L., Wu L., Goldman D., Tao M. Wall shear modulation of cytokines in early vein grafts. J Vasc Surg. 2004;40:345–350. doi: 10.1016/j.jvs.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 37.Alexander M.R., Moehle C.W., Johnson J.L., Yang Z., Lee J.K., Jackson C.L. Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. J Clin Invest. 2012;122:70–79. doi: 10.1172/JCI43713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu P., Nguyen B.T., Tao M., Jiang T., Mauro C.R., Wang Y. Lack of interleukin-1 signaling results in perturbed early vein graft wall adaptations. Surgery. 2013;153:63–69. doi: 10.1016/j.surg.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dammanahalli J.K., Wang X., Sun Z. Genetic interleukin-10 deficiency causes vascular remodeling via the upregulation of Nox1. J Hypertens. 2011;29:2116–2125. doi: 10.1097/HJH.0b013e32834b22a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cosmi L., Maggi L., Santarlasci V., Liotta F., Annunziato F. T helper cells plasticity in inflammation. Cytometry A. 2014;85:36–42. doi: 10.1002/cyto.a.22348. [DOI] [PubMed] [Google Scholar]
- 41.Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227–257. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- 42.Zhu J., Yamane H., Paul W.E. Differentiation of effector CD4 T cell populations (∗) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Engelbertsen D., Andersson L., Ljungcrantz I., Wigren M., Hedblad B., Nilsson J. T-helper 2 immunity is associated with reduced risk of myocardial infarction and stroke. Arterioscler Thromb Vasc Biol. 2013;33:637–644. doi: 10.1161/ATVBAHA.112.300871. [DOI] [PubMed] [Google Scholar]
- 44.Gordon S., Martinez F.O. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Tang Q., Adams J.Y., Tooley A.J., Bi M., Fife B.T., Serra P. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hori S., Nomura T., Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 47.Gershon R.K., Kondo K. Infectious immunological tolerance. Immunology. 1971;21:903–914. [PMC free article] [PubMed] [Google Scholar]
- 48.Kearley J., Barker J.E., Robinson D.S., Lloyd C.M. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med. 2005;202:1539–1547. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakamura K., Kitani A., Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harty J.T., Badovinac V.P. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 51.Henkart P.A. Lymphocyte-mediated cytotoxicity: two pathways and multiple effector molecules. Immunity. 1994;1:343–346. doi: 10.1016/1074-7613(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 52.Germain R.N. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 53.Simons K.H., de Vries M.R., Peters H.A.B., Jukema J.W., Quax P.H.A., Arens R. CD8+ T cells protect during vein graft disease development. Front Cardiovasc Med. 2019;6:77. doi: 10.3389/fcvm.2019.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kyaw T., Winship A., Tay C., Kanellakis P., Hosseini H., Cao A. Cytotoxic and proinflammatory CD8+ T lymphocytes promote development of vulnerable atherosclerotic plaques in apoE-deficient mice. Circulation. 2013;127:1028–1039. doi: 10.1161/CIRCULATIONAHA.112.001347. [DOI] [PubMed] [Google Scholar]
- 55.Chapman N.M., Boothby M.R., Chi H. Metabolic coordination of T cell quiescence and activation. Nat Rev Immunol. 2020;20:55–70. doi: 10.1038/s41577-019-0203-y. [DOI] [PubMed] [Google Scholar]
- 56.Smith-Garvin J.E., Koretzky G.A., Jordan M.S. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muhlethaler-Mottet A., Otten L.A., Steimle V., Mach B. Expression of MHC class II molecules in different cellular and functional compartments is controlled by differential usage of multiple promoters of the transactivator CIITA. EMBO J. 1997;16:2851–2860. doi: 10.1093/emboj/16.10.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gupta S., Pablo A.M., Jiang X., Wang N., Tall A.R., Schindler C. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest. 1997;99:2752–2761. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 60.Sukhova G.K., Schonbeck U., Rabkin E., Schoen F.J., Poole A.R., Billinghurst R.C. Evidence for increased collagenolysis by interstitial collagenases-1 and -3 in vulnerable human atheromatous plaques. Circulation. 1999;99:2503–2509. doi: 10.1161/01.cir.99.19.2503. [DOI] [PubMed] [Google Scholar]
- 61.Smith J.D., Trogan E., Ginsberg M., Grigaux C., Tian J., Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci USA. 1995;92:8264–8268. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khallou-Laschet J., Varthaman A., Fornasa G., Compain C., Gaston A.T., Clement M. Macrophage plasticity in experimental atherosclerosis. PLoS One. 2010;5:e8852. doi: 10.1371/journal.pone.0008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dale M.A., Xiong W., Carson J.S., Suh M.K., Karpisek A.D., Meisinger T.M. Elastin-derived peptides promote abdominal aortic aneurysm formation by modulating M1/M2 macrophage polarization. J Immunol. 2016;196:4536–4543. doi: 10.4049/jimmunol.1502454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chalubinski M., Wojdan K., Luczak E., Gorzelak P., Borowiec M., Gajewski A. IL-33 and IL-4 impair barrier functions of human vascular endothelium via different mechanisms. Vascul Pharmacol. 2015;73:57–63. doi: 10.1016/j.vph.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 65.Davenport P., Tipping P.G. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein e-deficient mice. Am J Pathol. 2003;163:1117–1125. doi: 10.1016/S0002-9440(10)63471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martinez F.O., Helming L., Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 67.Martinez F.O., Gordon S., Locati M., Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 68.Hall M.R., Yamamoto K., Protack C.D., Tsuneki M., Kuwahara G., Assi R. Temporal regulation of venous extracellular matrix components during arteriovenous fistula maturation. J Vasc Access. 2015;16:93–106. doi: 10.5301/jva.5000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Satish M., Gunasekar P., Agrawal D.K. Pro-inflammatory and pro-resolving mechanisms in the immunopathology of arteriovenous fistula maturation. Expert Rev Cardiovasc Ther. 2019;17:369–376. doi: 10.1080/14779072.2019.1612745. [DOI] [PubMed] [Google Scholar]
- 70.Sica A., Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fenyo I.M., Gafencu A.V. The involvement of the monocytes/macrophages in chronic inflammation associated with atherosclerosis. Immunobiology. 2013;218:1376–1384. doi: 10.1016/j.imbio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 72.Zhang L., Peppel K., Brian L., Chien L., Freedman N.J. Vein graft neointimal hyperplasia is exacerbated by tumor necrosis factor receptor-1 signaling in graft-intrinsic cells. Arterioscler Thromb Vasc Biol. 2004;24:2277–2283. doi: 10.1161/01.ATV.0000147766.68987.0d. [DOI] [PubMed] [Google Scholar]
- 73.Zhang L., Sivashanmugam P., Wu J.H., Brian L., Exum S.T., Freedman N.J. Tumor necrosis factor receptor-2 signaling attenuates vein graft neointima formation by promoting endothelial recovery. Arterioscler Thromb Vasc Biol. 2008;28:284–289. doi: 10.1161/ATVBAHA.107.151613. [DOI] [PubMed] [Google Scholar]
- 74.Suwanabol P.A., Seedial S.M., Shi X., Zhang F., Yamanouchi D., Roenneburg D. Transforming growth factor-beta increases vascular smooth muscle cell proliferation through the Smad3 and extracellular signal-regulated kinase mitogen-activated protein kinases pathways. J Vasc Surg. 2012;56:446–454. doi: 10.1016/j.jvs.2011.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van der Pluijm I., van Vliet N., von der Thusen J.H., Robertus J.L., Ridwan Y., van Heijningen P.M. Defective connective tissue remodeling in Smad3 mice leads to accelerated aneurysmal growth through disturbed downstream TGF-beta signaling. EBioMedicine. 2016;12:280–294. doi: 10.1016/j.ebiom.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bai H., Lee J.S., Hu H., Wang T., Isaji T., Liu S. Transforming growth factor-beta1 inhibits pseudoaneurysm formation after aortic patch angioplasty. Arterioscler Thromb Vasc Biol. 2018;38:195–205. doi: 10.1161/ATVBAHA.117.310372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pushevski V., Dejanov P., Gerasimovska V., Petrushevska G., Oncevski A., Sikole A. Severe endothelial damage in chronic kidney disease patients prior to haemodialysis vascular access surgery. Pril (Makedon Akad Nauk Umet Odd Med Nauki) 2015;36:43–49. doi: 10.1515/prilozi-2015-0077. [DOI] [PubMed] [Google Scholar]
- 78.Cai C., Zhao C., Kilari S., Sharma A., Singh A.K., Simeon M.L. Effect of sex differences in treatment response to angioplasty in a murine arteriovenous fistula model. Am J Physiol Renal Physiol. 2020;318:f565–f575. doi: 10.1152/ajprenal.00474.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heine G.H., Ulrich C., Sester U., Sester M., Kohler H., Girndt M. Transforming growth factor beta1 genotype polymorphisms determine AV fistula patency in hemodialysis patients. Kidney Int. 2003;64:1101–1107. doi: 10.1046/j.1523-1755.2003.00176.x. [DOI] [PubMed] [Google Scholar]
- 80.Wang X.P., Zhang W., Liu X.Q., Wang W.K., Yan F., Dong W.Q. Arginase I enhances atherosclerotic plaque stabilization by inhibiting inflammation and promoting smooth muscle cell proliferation. Eur Heart J. 2014;35:911–919. doi: 10.1093/eurheartj/eht329. [DOI] [PubMed] [Google Scholar]
- 81.Peyton K.J., Ensenat D., Azam M.A., Keswani A.N., Kannan S., Liu X.M. Arginase promotes neointima formation in rat injured carotid arteries. Arterioscler Thromb Vasc Biol. 2009;29:488–494. doi: 10.1161/ATVBAHA.108.183392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yamamoto K., Protack C.D., Kuwahara G., Tsuneki M., Hashimoto T., Hall M.R. Disturbed shear stress reduces Klf2 expression in arterial-venous fistulae in vivo. Physiol Rep. 2015;3:e12348. doi: 10.14814/phy2.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kwak B.R., Back M., Bochaton-Piallat M.L., Caligiuri G., Daemen M.J., Davies P.F. Biomechanical factors in atherosclerosis: mechanisms and clinical implications. Eur Heart J. 2014;35:3013–3020. doi: 10.1093/eurheartj/ehu353. 20a-20d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Browne L.D., Bashar K., Griffin P., Kavanagh E.G., Walsh S.R., Walsh M.T. The role of shear stress in arteriovenous fistula maturation and failure: a systematic review. PLoS One. 2015;10:e0145795. doi: 10.1371/journal.pone.0145795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Anwar M.A., Shalhoub J., Lim C.S., Gohel M.S., Davies A.H. The effect of pressure-induced mechanical stretch on vascular wall differential gene expression. J Vasc Res. 2012;49:463–478. doi: 10.1159/000339151. [DOI] [PubMed] [Google Scholar]
- 86.Remuzzi A., Bozzetto M., Brambilla P. Is shear stress the key factor for AVF maturation? J Vasc Access. 2017;18(Suppl 1):10–14. doi: 10.5301/jva.5000686. [DOI] [PubMed] [Google Scholar]
- 87.Ene-Iordache B., Remuzzi A. Disturbed flow in radial-cephalic arteriovenous fistulae for haemodialysis: low and oscillating shear stress locates the sites of stenosis. Nephrol Dial Transplant. 2012;27:358–368. doi: 10.1093/ndt/gfr342. [DOI] [PubMed] [Google Scholar]
- 88.Iso T., Maeno T., Oike Y., Yamazaki M., Doi H., Arai M. Dll4-selective Notch signaling induces ephrinB2 gene expression in endothelial cells. Biochem Biophys Res Commun. 2006;341:708–714. doi: 10.1016/j.bbrc.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 89.Kudo F.A., Muto A., Maloney S.P., Pimiento J.M., Bergaya S., Fitzgerald T.N. Venous identity is lost but arterial identity is not gained during vein graft adaptation. Arterioscler Thromb Vasc Biol. 2007;27:1562–1571. doi: 10.1161/ATVBAHA.107.143032. [DOI] [PubMed] [Google Scholar]
- 90.Protack C.D., Foster T.R., Hashimoto T., Yamamoto K., Lee M.Y., Kraehling J.R. Eph-B4 regulates adaptive venous remodeling to improve arteriovenous fistula patency. Sci Rep. 2017;7:15386. doi: 10.1038/s41598-017-13071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kania A., Klein R. Mechanisms of ephrin-Eph signalling in development, physiology and disease. Nat Rev Mol Cell Biol. 2016;17:240–256. doi: 10.1038/nrm.2015.16. [DOI] [PubMed] [Google Scholar]
- 92.Funk S.D., Orr A.W. Ephs and ephrins resurface in inflammation, immunity, and atherosclerosis. Pharmacol Res. 2013;67:42–52. doi: 10.1016/j.phrs.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 93.Korff T., Braun J., Pfaff D., Augustin H.G., Hecker M. Role of ephrinB2 expression in endothelial cells during arteriogenesis: impact on smooth muscle cell migration and monocyte recruitment. Blood. 2008;112:73–81. doi: 10.1182/blood-2007-12-128835. [DOI] [PubMed] [Google Scholar]
- 94.Fukuda D., Aikawa E., Swirski F.K., Novobrantseva T.I., Kotelianski V., Gorgun C.Z. Notch ligand delta-like 4 blockade attenuates atherosclerosis and metabolic disorders. Proc Natl Acad Sci USA. 2012;109:e1868–e1877. doi: 10.1073/pnas.1116889109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tseng C.N., Karlof E., Chang Y.T., Lengquist M., Rotzius P., Berggren P.O. Contribution of endothelial injury and inflammation in early phase to vein graft failure: the causal factors impact on the development of intimal hyperplasia in murine models. PLoS One. 2014;9:e98904. doi: 10.1371/journal.pone.0098904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu J., Thabet S.R., Kirabo A., Trott D.W., Saleh M.A., Xiao L. Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circ Res. 2014;114:616–625. doi: 10.1161/CIRCRESAHA.114.302157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reglero-Real N., Marcos-Ramiro B., Millan J. Endothelial membrane reorganization during leukocyte extravasation. Cell Mol Life Sci. 2012;69:3079–3099. doi: 10.1007/s00018-012-0987-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kwei S., Stavrakis G., Takahas M., Taylor G., Folkman M.J., Gimbrone M.A. Early adaptive responses of the vascular wall during venous arterialization in mice. Am J Pathol. 2004;164:81–89. doi: 10.1016/S0002-9440(10)63099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chang C.-J., Ko Y.-S., Ko P.-J., Hsu L.-A., Chen C.-F., Yang C.-W. Thrombosed arteriovenous fistula for hemodialysis access is characterized by a marked inflammatory activity. Kidney Int. 2005;68:1312–1319. doi: 10.1111/j.1523-1755.2005.00529.x. [DOI] [PubMed] [Google Scholar]
- 100.Silva M., Videira P.A., Sackstein R. E-selectin ligands in the human mononuclear phagocyte system: implications for infection, inflammation, and immunotherapy. Front Immunol. 2017;8:1878. doi: 10.3389/fimmu.2017.01878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kulidjian A.A., Issekutz A.C., Issekutz T.B. Differential role of E-selectin and P-selectin in T lymphocyte migration to cutaneous inflammatory reactions induced by cytokines. Int Immunol. 2002;14:751–760. doi: 10.1093/intimm/dxf045. [DOI] [PubMed] [Google Scholar]
- 102.Alon R., Rossiter H., Wang X., Springer T.A., Kupper T.S. Distinct cell surface ligands mediate T lymphocyte attachment and rolling on P and E selectin under physiological flow. J Cell Biol. 1994;127:1485–1495. doi: 10.1083/jcb.127.5.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gotoh R., Suzuki J., Kosuge H., Kakuta T., Sakamoto S., Yoshida M. E-selectin blockade decreases adventitial inflammation and attenuates intimal hyperplasia in rat carotid arteries after balloon injury. Arterioscler Thromb Vasc Biol. 2004;24:2063–2068. doi: 10.1161/01.ATV.0000145942.31404.20. [DOI] [PubMed] [Google Scholar]
- 104.Krakauer T. IL-10 inhibits the adhesion of leukocytic cells to IL-1-activated human endothelial cells. Immunol Lett. 1995;45:61–65. doi: 10.1016/0165-2478(94)00226-h. [DOI] [PubMed] [Google Scholar]
- 105.Chen W., Jin W., Hardegen N., Lei K.J., Li L., Marinos N. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gorelik L., Constant S., Flavell R.A. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J Exp Med. 2002;195:1499–1505. doi: 10.1084/jem.20012076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gorelik L., Fields P.E., Flavell R.A. Cutting edge: TGF-beta inhibits Th type 2 development through inhibition of GATA-3 expression. J Immunol. 2000;165:4773–4777. doi: 10.4049/jimmunol.165.9.4773. [DOI] [PubMed] [Google Scholar]
- 108.Schofer J., Schlüter M., Gershlick A.H., Wijns W., Garcia E., Schampaert E. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double-blind, randomised controlled trial (E-SIRIUS) Lancet. 2003;362:1093–1099. doi: 10.1016/S0140-6736(03)14462-5. [DOI] [PubMed] [Google Scholar]
- 109.Waller J.R., Murphy G.J., Bicknell G.R., Toomey D., Nicholson M.L. Effects of the combination of rapamycin with tacrolimus or cyclosporin on experimental intimal hyperplasia. Br J Surg. 2002;89:1390–1395. doi: 10.1046/j.1365-2168.2002.02271.x. [DOI] [PubMed] [Google Scholar]
- 110.Wong C., Bezhaeva T., Rothuizen T.C., Metselaar J.M., de Vries M.R., Verbeek F.P. Liposomal prednisolone inhibits vascular inflammation and enhances venous outward remodeling in a murine arteriovenous fistula model. Sci Rep. 2016;6:30439. doi: 10.1038/srep30439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wasse H., Huang R., Naqvi N., Smith E., Wang D., Husain A. Inflammation, oxidation and venous neointimal hyperplasia precede vascular injury from AVF creation in CKD patients. J Vasc Access. 2012;13:168–174. doi: 10.5301/jva.5000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chapman N.M., Chi H. mTOR signaling, Tregs and immune modulation. Immunotherapy. 2014;6:1295–1311. doi: 10.2217/imt.14.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zeboudj L., Maitre M., Guyonnet L., Laurans L., Joffre J., Lemarie J. Selective EGF-receptor inhibition in CD4(+) T cells induces anergy and limits atherosclerosis. J Am Coll Cardiol. 2018;71:160–172. doi: 10.1016/j.jacc.2017.10.084. [DOI] [PubMed] [Google Scholar]
- 114.Kume M., Komori K., Matsumoto T., Onohara T., Takeuchi K., Yonemitsu Y. Administration of a decoy against the activator protein-1 binding site suppresses neointimal thickening in rabbit balloon-injured arteries. Circulation. 2002;105:1226–1232. doi: 10.1161/hc1002.104903. [DOI] [PubMed] [Google Scholar]
- 115.Matsumoto T., Komori K., Yonemitsu Y., Morishita R., Sueishi K., Kaneda Y. Hemagglutinating virus of Japan-liposome-mediated gene transfer of endothelial cell nitric oxide synthase inhibits intimal hyperplasia of canine vein grafts under conditions of poor runoff. J Vasc Surg. 1998;27:135–144. doi: 10.1016/s0741-5214(98)70300-3. [DOI] [PubMed] [Google Scholar]
- 116.Ohta S., Komori K., Yonemitsu Y., Onohara T., Matsumoto T., Sugimachi K. Intraluminal gene transfer of endothelial cell-nitric oxide synthase suppresses intimal hyperplasia of vein grafts in cholesterol-fed rabbit: a limited biological effect as a result of the loss of medial smooth muscle cells. Surgery. 2002;131:644–653. doi: 10.1067/msy.2002.124878. [DOI] [PubMed] [Google Scholar]
- 117.Yonemitsu Y., Matsumoto T., Itoh H., Okazaki J., Uchiyama M., Yoshida K. DVC1-0101 to treat peripheral arterial disease: a Phase I/IIa open-label dose-escalation clinical trial. Mol Ther. 2013;21:707–714. doi: 10.1038/mt.2012.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nakano K., Matoba T., Koga J.I., Kashihara Y., Fukae M., Ieiri I. Safety, tolerability, and pharmacokinetics of NK-104-NP. Int Heart J. 2018;59:1015–1025. doi: 10.1536/ihj.17-555. [DOI] [PubMed] [Google Scholar]
- 119.Koga J., Matoba T., Egashira K. Anti-inflammatory nanoparticle for prevention of atherosclerotic vascular diseases. J Atheroscler Thromb. 2016;23:757–765. doi: 10.5551/jat.35113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nakano Y., Matoba T., Tokutome M., Funamoto D., Katsuki S., Ikeda G. Nanoparticle-mediated delivery of irbesartan induces cardioprotection from myocardial ischemia-reperfusion injury by antagonizing monocyte-mediated inflammation. Sci Rep. 2016;6:29601. doi: 10.1038/srep29601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li J., Chu M.K., Lu B., Mirzaie S., Chen K., Gordijo C.R. Enhancing thermal stability of a highly concentrated insulin formulation with Pluronic F-127 for long-term use in microfabricated implantable devices. Drug Deliv Transl Res. 2017;7:529–543. doi: 10.1007/s13346-017-0381-8. [DOI] [PubMed] [Google Scholar]
- 122.Wu B., Werlin E.C., Chen M., Mottola G., Chatterjee A., Lance K.D. Perivascular delivery of resolvin D1 inhibits neointimal hyperplasia in a rabbit vein graft model. J Vasc Surg. 2018;68:188S–200S.e4. doi: 10.1016/j.jvs.2018.05.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Owens C.D., Gasper W.J., Walker J.P., Alley H.F., Conte M.S., Grenon S.M. Safety and feasibility of adjunctive dexamethasone infusion into the adventitia of the femoropopliteal artery following endovascular revascularization. J Vasc Surg. 2014;59:1016–1024. doi: 10.1016/j.jvs.2013.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brieger D., Topol E. Local drug delivery systems and prevention of restenosis. Cardiovasc Res. 1997;35:405–413. doi: 10.1016/s0008-6363(97)00155-7. [DOI] [PubMed] [Google Scholar]