Abstract
A 35-year-old myopic woman developed right-eye optic disc edema with normal visual function. The presence of a subtle crescent-shaped peripapillary subretinal hemorrhage in addition to the disc edema raised concern for a peripapillary choroidal neovascular membrane, which was confirmed by enhanced depth optical coherence tomography.
Keywords: Choroidal neovascularization, myopia, optic nerve, optic nerve diseases, optical coherence tomography, papilledema
Introduction
A peripapillary choroidal neovascular membrane (CNVM) is a classic cause of visual loss. Most CNVM occur in association with age-related macular degeneration (AMD) and other underlying inflammatory or degenerative chorioretinal disorders, but CNVM may also occur following ocular trauma or in association with high myopia.[1] We report a case of peripapillary CNVM causing optic disc edema in a young myopic woman.
Case Report
A 35-year-old myopic woman was referred for assessment of right-eye optic disc edema. The patient had a past medical history of migraine which had been stable for many years; her past ocular history was significant only for anisometropic myopia (−3.50 right eye; −5.00 left eye). Several months prior, the patient was assessed by an outside eye care provider due to blurred vision in her right eye and was found to have right optic disc edema. Neuro-ophthalmology consultation was requested.
Her best-corrected Snellen visual acuity was 20/20 in both eyes. Color vision was full, as assessed by Ishihara pseudoisochromatic plates. There was no relative afferent pupillary defect. Her anterior segment examination was normal, with no findings of active or previous intraocular inflammation. Intraocular pressures were 22 mmHg in the right eye and 21 mmHg in the left eye. Dilated fundus examination showed elevation of the superonasal right optic nerve with mild swelling and a crescent-shaped peripapillary subretinal hemorrhage in the same area [Figure 1a]. The left optic nerve was tilted and elevated nasally without disc edema [Figure 1b]. The remainder of the ocular fundus examination in both eyes was normal.
Figure 1.
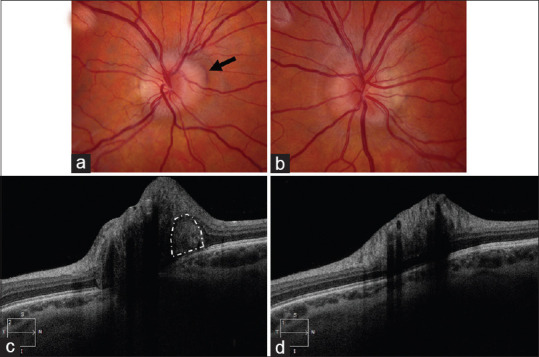
Fundus photograph of the right eye (a) and the left eye (b)centered on the optic nerves show tilted optic discs with mild superonasal elevation in both eyes and mild swelling of the right optic nerve head. In the right eye, a peripapillary hemorrhage (black arrow) is seen along the superonasal margin of the disc. (c) Enhanced depth imaging-optical coherence tomography centered on the optic nerve shows a hyperreflective area (white outline) adjacent to the optic nerve head, suggesting a choroidal neovascular membrane. (d) Enhanced depth imaging-optical coherence tomography centered on the left optic nerve was normal.
Optical coherence tomography (OCT) of the retinal nerve fiber layer (RNFL) confirmed increased peripapillary thickness of the right eye but did not demonstrate intra- or subretinal fluid near the optic nerve head. OCT-RNFL was normal in the left eye. OCT of the ganglion cell complex was normal in both eyes. B-scan ultrasonography showed elevation of the right optic nerve without hyperechoic signal to suggest optic disc drusen. Enhanced depth imaging-OCT showed a hyperreflective area in the peripapillary subretinal space [Figure 1c], suggesting a peripapillary CNVM. Suggesting a peripapillary CNVM but normal in the left eye [Figure 1d]. Intravenous fluorescein angiography (IVFA) in the right eye showed subtle late leakage at the superonasal margin of the optic disc, consistent with disc edema [Figure 2]. No disc leakage was noted in the left eye.
Figure 2.
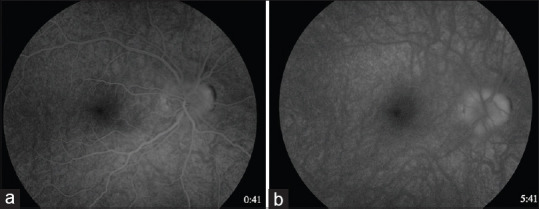
Intravenous fluorescein angiography in the right eye in early (left image; 41 s) and late phases (right image; 5 min and 41 s), showing late leakage most apparent at the superonasal margin of the optic disc, indicating disc edema
Discussion
Choroidal neovascular membranes are new, abnormal blood vessels that originate from the choroidal circulation and enter the subretinal space via a break in Bruch's membrane; they may be associated with a number of underlying inflammatory or degenerative chorioretinal disorders or may occur following ocular trauma.[2]
Some CNVMs develop in the peripapillary area, within 1 disc diameter of the optic disc, as a result of disorders affecting the optic nerve head and adjacent chorioretinal areas.[2] Peripapillary CNVMs have a predilection for women[2,3] and comprise 10% of all CNVMs.[2] They have been most commonly described in patients diagnosed with exudative or “wet” AMD[1,4,5] who are usually over 50 years of age.[1] Other ocular pathologies including optic disc drusen, optic pits, chronic papilledema, myopia, angioid streaks, ocular histoplasmosis syndrome, trauma, and uveitis may also cause peripapillary CNVMs.[1,4] Idiopathic CNVM, or CNVM without an apparent underlying cause, represent up to 17% of cases in some series[4,6,7] and is more common in younger patients.[6] Because of their close relationship with the optic nerve head, peripapillary CNVM may present as unilateral optic disc edema, which may create diagnostic confusion and prompt unnecessary neurologic diagnostic investigations.
In our patient, the presence of a peripapillary crescent subretinal hemorrhage raised concern for a peripapillary CNVM, especially due to the patient's myopia. Sibony et al. described a case series of patients with peripapillary CNVM and subretinal hemorrhage and described average myopia of − 4.50 D in nine patients and as high as − 8.63 D.[3] Peripapillary CNVM is presumed to occur even more commonly in patients with pathological myopia (spherical correction of − 6.00 D or greater),[7,8] presumably due to progressive elongation of the globe and the presence of abnormal choroidal vasculature and posterior staphyloma.[6] Our patient did not have a posterior staphyloma, but was noted to have tilting of her optic nerves, which was a finding also described by Sibony et al.[3]
As in our patient, peripapillary CNVM may cause mild optic disc edema. However, peripapillary CNVM may also be the result of severe chronic disc edema, particularly in patients with papilledema due to intracranial hypertension. Cases involving both of these clinical scenarios have been reported in the literature and are summarized in [Table 1]. The pathogenesis of peripapillary CNVM in the setting of chronic papilledema is presumed to be secondary to pressure deformity of the border of Bruch's membrane at the edge of the optic disc, resulting in discontinuity of the normal anatomic apposition of the chorioretinal layers. This anatomic dehiscence, hypoxia created by axonal swelling, as well as resultant vascular perfusion of tissues, are believed to promote angiogenesis with the resultant formation of subretinal neovascular membranes.[9]
Table 1.
Reported cases of chronic disc edema causing a secondary peripapillary choroidal neovascular membrane and of peripapillary choroidal neovascular membrane causing secondary disc edema
| Author, year | Patients | CNVM laterality | BCVA in affected eye | Examination findings | Ophthalmic investigations | Treatment | Visual outcome |
|---|---|---|---|---|---|---|---|
| CNVM secondary to papilledema | |||||||
| Morse et al., 1981[9] | 1 | Bilateral | 20/20 OU with worsening to 20/200 OS after 1 month | Disc edema OU, SRH, SRF, subretinal fibrosis extending to fovea OS | IVFA | Observation | 20/25 OU (11 months) |
| Wendel et al., 2006[10] | 6 | Unilateral | Unknown in 5 cases; 1 patient CF (eye unspecified) | Disc edema OU | IVFA | Observation (3), ALP (2), PDT (1) | Variable (20/20– 20/200) (mean 11 months) |
| Sathornsumetee et al., 2006[11] | 1 | Unilateral (OS) | 20/30 OD 20/300 OS | Disc edema OU; SRH OS | IVFA | Declined treatment | 20/30 OD 20/300 OS (9 months) |
| Jamerson et al., 2009[12] | 1 | Unilateral (OS) | 20/20 OD 20/25 OS with worsening to 20/25 OD 20/400 after 1 week | Disc edema OU; SRF, SRH in OS | SD-OCT, IVFA | IVB | 20/20 OD 20/25 OS (10 months) |
| Belliveau et al., 2013[13] | 1 | Unilateral (OD) | 20/200 OD 20/30 OS | Disc edema OU; SRH, IRH, SFH OS | SD-OCT, IVFA | IVB | 20/40 OD 20/30 OS |
| Lee et al., 2013[14] | 1 | Unilateral (OS) | 20/20 OD 20/80 OS | Disc edema OU; SRH and SFH OS | SD-OCT, IVFA | IVB | 20/20 OU (3.5 years) |
| Kumar et al., 2018[15] | 1 | Bilateral | 20/30 CF at 1 foot OS | Disc edema OU; SRF, SRH in OS | SS-OCT, IVFA | IVR | 20/20 OU (3 months) |
| Ozgonul et al., 2019[16] | 10 | 3 bilateral, 7 unilateral (13 eyes) | Variable (20/20 to HM) | Disc edema OU; SRH, SRF | SD-OCT only (5), SD-OCT + IVF (2), IVF only (3) | Observation (7), IVB (4), IVA and IVB (1), IVR (1) | Variable (20/20– 20/400) (3–56 months) |
| Disc edema secondary to CNVM | |||||||
| Browning and Fraser, 2005[4] | 1 | Unilateral (OS) | 20/25 OD 20/100 OS | Peripapillary SRH and disc edema | IVFA | ALP | 20/20 OD 20/60 OS (7 months) |
| Hawy et al.[17] In press | 1 | Unilateral (OS) | 20/20 OU | Disc edema OS | SD-OCT, IVFA | Observation | 20/20 OU (40 months) |
BCVA=Best-corrected Snellen visual acuity at distance, OD=Right eye, OS=Left eye, OU=Both eyes, CF=Counting Fingers, HM=Hand motion, ft=Feet; for examination findings, SRH=Subretinal hemorrhage, SRF=Subretinal fluid, SFH=Subfoveal hemorrhage, IRH=Intraretinal hemorrhage, for ophthalmic investigations, SS-OCT=Swept-source ocular coherence tomography, SD-OCT=Spectral domain-optical coherence tomography, IVFA=Intravenous fluorescein angiogram; for treatment, IVA=Intravitreal aflibercept, IVB=intravitreal bevacizumab, IVR=Intravitreal ranibizumab, PDT=Photodynamic therapy, ALP=Argon laser photocoagulation, CNVM=Choroidal neovascular membrane
Recognition of peripapillary CNVM as a cause of mild disc edema is important.[18] Ancillary ophthalmic investigations including optic disc photographs, B-scan ultrasonography to screen for optic disc drusen,[18] OCT, and IVFA are helpful in making the diagnosis.[4,18] Peripapillary CNVM are often observed when asymptomatic and, similar to other CNVM, may be treated with intravitreal anti-vascular endothelial growth factor if symptomatic or enlarging.[2,12,13,14,19] Retinal laser,[2,7] photodynamic therapy,[2,7] or vitreoretinal surgery are alternative treatment options but are rarely used.[19]
Financial support and sponsorship
Nil.
Conflicts of interest
The authors declare that there are no conflicts of interests of this paper.
References
- 1.Alharthi RA, Almalki AM, Alotibi FA. Case of idiopathic peripapillary subretinal neovascular membrane in an otherwise healthy young male: A case report. Egypt J Hosp Med. 2018;70:1421–3. [Google Scholar]
- 2.Jutley G, Jutley G, Tah V, Lindfield D, Menon G. Treating peripapillary choroidal neovascular membranes: A review of the evidence. Eye (Lond) 2011;25:675–81. doi: 10.1038/eye.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sibony P, Fourman S, Honkanen R, El Baba F. Asymptomatic peripapillary subretinal hemorrhage: A study of 10 cases. J Neuro Ophthalmol. 2008;28:114–9. doi: 10.1097/WNO.0b013e318175cd90. [DOI] [PubMed] [Google Scholar]
- 4.Browning DJ, Fraser CM. Ocular conditions associated with peripapillary subretinal neovascularization, their relative frequencies, and associated outcomes. Ophthalmology. 2005;112:1054–61. doi: 10.1016/j.ophtha.2004.11.062. [DOI] [PubMed] [Google Scholar]
- 5.Xiaoyan D, Mrinali P, Chan CC. Molecular pathology of AMD. Prog Retin Eye Res. 2009;28:1–18. doi: 10.1016/j.preteyeres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung CM, Arnold JJ, Holz FG, Park KH, Lai TY, Larsen M, et al. Myopic choroidal neovascularization: Review, guidance, and consensus statement on management. Ophthalmology. 2017;124:1690–711. doi: 10.1016/j.ophtha.2017.04.028. [DOI] [PubMed] [Google Scholar]
- 7.Cohen SY, Laroche A, Leguen Y, Soubrane G, Coscas GJ. Etiology of choroidal neovascularization in young patients. Ophthalmology. 1996;103:1241–4. doi: 10.1016/s0161-6420(96)30515-0. [DOI] [PubMed] [Google Scholar]
- 8.Moriyama M, Ohno-Matsui K, Futagami S, Yoshida T, Hayashi K, Shimada N, et al. Morphology and long-term changes of choroidal vascular structure in highly myopic eyes with and without posterior staphyloma. Ophthalmology. 2007;114:1755–63. doi: 10.1016/j.ophtha.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 9.Morse PH, Leveille AS, Antel JP, Burch JV. Bilateral juxtapapillary subretinal neovascularization associated with pseudotumor cerebri. Am J Ophthalmol. 1981;91:312–7. doi: 10.1016/0002-9394(81)90282-8. [DOI] [PubMed] [Google Scholar]
- 10.Wendel L, Lee AG, Boldt HC, Kardon RH, Wall M. Subretinal neovascular membrane in idiopathic intracranial hypertension. Am J Ophthalmol. 2006;141:573–4. doi: 10.1016/j.ajo.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 11.Sathornsumetee B, Webb A, Hill DL, Newman NJ, Biousse V. Subretinal hemorrhage from a peripapillary choroidal neovascular membrane in papilledema caused by idiopathic intracranial hypertension. J Neuro Ophthalmol. 2006;26:197–9. doi: 10.1097/01.wno.0000235583.10546.0a. [DOI] [PubMed] [Google Scholar]
- 12.Jamerson SC, Arunagiri G, Ellis BD, Leys MJ. Intravitreal bevacizumab for the treatment of choroidal neovascularization secondary to pseudotumor cerebri. Int Ophthalmol. 2009;29:183–5. doi: 10.1007/s10792-007-9186-y. [DOI] [PubMed] [Google Scholar]
- 13.Belliveau MJ, Xing L, Almeida DR, Gale JS, Ten Hove MW. Peripapillary choroidal neovascular membrane in a teenage boy: Presenting feature of idiopathic intracranial hypertension and resolution with intravitreal bevacizumab. J Neuro Ophthalmol. 2013;33:48–50. doi: 10.1097/WNO.0b013e318281b7b9. [DOI] [PubMed] [Google Scholar]
- 14.Lee IJ, Maccheron LJ, Kwan AS. Intravitreal bevacizumab in the treatment of peripapillary choroidal neovascular membrane secondary to idiopathic intracranial hypertension. J Neuro Ophthalmol. 2013;33:155–7. doi: 10.1097/WNO.0b013e31827c6b49. [DOI] [PubMed] [Google Scholar]
- 15.Kumar N, Tigari B, Dogra M, Singh R. Successful management of peripapillary choroidal neovascular membrane secondary to idiopathic intracranial hypertension with intravitreal ranibizumab. Indian J Ophthalmol. 2018;66:1358–60. doi: 10.4103/ijo.IJO_419_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozgonul C, Moinuddin O, Munie M, Lee MS, Bhatti MT, Landau K, et al. Management of peripapillary choroidal neovascular membrane in patients with idiopathic intracranial hypertension. J Neuro Ophthalmol. 2019;39:451–7. doi: 10.1097/WNO.0000000000000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawy E, Sharma RA, Peragallo JH, Dattilo M, Newman NJ, Biousse V. Isolated unilateral paucisymptomatic optic nerve head edema. J Neuro Ophthalmol [In press] doi: 10.1097/WNO.0000000000001136. [DOI] [PubMed] [Google Scholar]
- 18.Rebolleda G, Kawasaki A, de Juan V, Oblanca N, Muñoz-Negrete FJ. Optical coherence tomography to differentiate papilledema from pseudopapilledema. Curr Neurol Neurosci Rep. 2017;17:74. doi: 10.1007/s11910-017-0790-6. [DOI] [PubMed] [Google Scholar]
- 19.Gelisken F, Szurman P, Bartz-Schmidt KU, Aisenbrey S. Surgical treatment of peripapillary choroidal neovascularisation. Br J Ophthalmol. 2007;91:1027–30. doi: 10.1136/bjo.2006.108118. [DOI] [PMC free article] [PubMed] [Google Scholar]


