Abstract
Optical coherence tomography (OCT) is a noninvasive imaging technique used to qualitatively and quantitatively analyze various layers of the retina. OCT of the retinal nerve fiber layer (RNFL) and ganglion cell–inner plexiform layer (GCIPL) is particularly useful in neuro-ophthalmology for the evaluation of patients with optic neuropathies and retrochiasmal visual pathway disorders. OCT allows for an objective quantification of edema and atrophy of the RNFL and GCIPL, which may be evident before obvious clinical signs and visual dysfunction develop. Enhanced depth imaging OCT allows for visualization of deep structures of the optic nerve and has emerged as the gold standard for the detection of optic disc drusen. In the evaluation of compressive optic neuropathies, OCT RNFL and GCIPL thicknesses have been established as the most important visual prognostic factor. There is increasing evidence that inclusion of OCT as part of the diagnostic criteria for multiple sclerosis (MS) increases its sensitivity. Moreover, OCT of the RNFL and GCIPL may be helpful in the early detection and monitoring the treatment of conditions such as MS and Alzheimer's disease. OCT is an important aspect of the neuro-ophthalmologic assessment and its use is likely to increase moving forward.
Keywords: Diagnostic techniques, ophthalmological, ophthalmology, optical coherence, tomography
Introduction
Optical coherence tomography (OCT) is a noninvasive imaging technique that provides high resolution images of the retina and optic nerve.[1] While OCT was originally used in diagnosing diseases of the retina and then in glaucoma, this technology is increasingly being used to evaluate patients with afferent neuro-ophthalmic conditions and has become the standard of care for evaluating patients with various optic neuropathies.[2] The retina is composed of multiple layers of neural tissue which can be differentiated by varying levels of signal on OCT. Two important retinal layers in evaluating patients with optic neuropathies are the ganglion cell layer (GCL) and retinal nerve fiber layer (RNFL). The former contains the cell bodies of the retinal ganglion cells, whereas the latter contains the axons which eventually synapse with the lateral geniculate nucleus. The inner plexiform layer and the GCL are often segmented together on OCT devices and collectively referred to as the ganglion cell–inner plexiform layer (GCIPL).
Recent advancements in OCT include new modalities such as swept-source (SS) OCT, enhanced depth imaging (EDI) OCT, en face OCT, and OCT angiography (OCT-A).[3,4,5,6] Initial OCT methods had poor visualization of the deeper layers of the retina beyond Bruch's membrane due to scattering of light at the retinal pigment epithelium (RPE). EDI-OCT allows for visualization of the deep portions of the choroid, which is implicated in many retinal diseases, and the deep structures of the optic nerve.[6] SS-OCT allows for penetrance to the level of the choroid at the expense of slightly reduced axial resolution.[7] En face OCT produces transverse images of the retinal layers allowing for evaluation of morphological changes in the coronal view similar to fundus photography.[5] OCT-A visualizes the retinal vasculature without the use of fluorescein dye with much greater resolution than traditional angiography.[8] Collectively, these advancements have expanded the utility of OCT, making it one of the most important ancillary tests in ophthalmology. In this study, we review the utility of OCT in the evaluation of various optic neuropathies and afferent visual pathway disorders seen in neuro-ophthalmology.
Optical Coherence Tomography in the Evaluation of Optic Neuropathies
Optic disc drusen
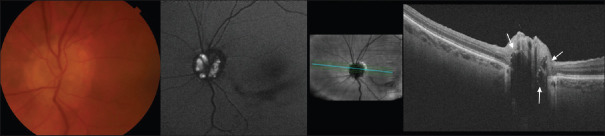
Optic disc drusen (ODD) are calcified deposits of axonal metabolic products within the prelaminar tissue of the optic nerve.[9] Many patients with ODD are asymptomatic, but ODD can cause transient visual obscurations, be mistaken for papilledema, or increase the risk for nonarteritic anterior ischemic optic neuropathy (NAION) due to crowding at the optic nerve head (ONH).[10,11,12] Historically, ODD were diagnosed using imaging modalities such as B-scan ultrasound, fundus autofluorescence (FAF), and computed tomography (CT).[13] EDI-OCT, however, has been shown to be superior compared to previous methods when evaluating ODD due to better resolution when imaging the deep ONH structures.[10,14,15,16] A primary limitation was that the majority of ODD are “buried” and difficult to visualize on examination and on B-scan ultrasound. For visible ODD, EDI-OCT and B-scan ultrasound have similar sensitivity, but there is recent evidence to suggest that EDI-OCT can identify buried ODD not seen on B-scan ultrasound.[14,17,18] EDI-OCT has offered improvements to the detection of noncalcified and buried drusen compared to B-scan ultrasound, FAF, and CT.[14,15,19,20] Therefore, EDI-OCT is particularly useful in younger populations where a greater proportion of ODD are buried.[11,15,20,21] An example of ODD seen with FAF and EDI-OCT is shown in Figure 1.
Figure 1.
Optic disc drusen can be seen in color fundus photos and autofluorescence. Optical coherence tomography HD-5 line raster using the enhanced depth imaging protocol shows three regions of hyporeflectivity with hyperreflective margins corresponding to drusen seen in the photos
EDI-OCT has changed the way ODD are diagnosed, has further elucidated other peripapillary structures, such as peripapillary hyperreflective ovoid mass-like structures (PHOMS) and hyperreflective horizontal lines, and has provided a way to predict visual prognosis in patients with ODD. The Optic Disc Drusen Studies (ODDS) Consortium has made recommendations for consistent diagnostic criteria using OCT.[10] ODD are defined as hyporeflective structures that are located above the lamina cribrosa and have a hyperreflective margin.[10] Hyperreflective horizontal lines often seen on OCT of the optic nerve, with and without ODD, were previously thought to be nascent ODD. However, because there are still questions if the horizontal lines were from the lamina cribrosa, and or if they represent some other peripapillary finding, the ODDS Consortium does not include these findings in the definition of ODD.
PHOMS, hyperreflective structures seen on OCT, received increasing attention with the introduction of EDI-OCT as they were not previously seen on B-scan ultrasound or FAF due to lack of calcifications.[15] They were originally thought to be precursors to ODD. Histological analysis of PHOMS, however, demonstrated that they are more similar to the distended axons seen in papilledema.[22] Studies have suggested that PHOMS are caused by prelaminar axonal distension due to anomalies of the ONH and are not specific to ODD.[22] PHOMS have been seen in a number of other pathologies of the ONH including the numerous causes of optic disc edema.[23] Most recently, OCT-A showed that PHOMS contain a complex vascular structure, which represent a divergence from the pathogenesis of ODD.[24] This highlights the growing sentiment that PHOMS should be viewed as a separate phenomenon and not diagnosed as ODD.[10]
Visual prognosis in patients with ODD has been suspected to correlate with the volume of the ODD, but was difficult to predict prior to the availability of EDI due to challenges identifying buried ODD.[25] In several studies using EDI-OCT to evaluate ODD, it has been found that larger ODD are correlated with reduced RNFL thickness and worsening visual field loss.[26,27] Recent studies have suggested that the association with GCIPL thinning could be even greater and may result in a greater emphasis on the GCIPL as a predictor of functional decline in ODD.[26] EDI-OCT has also showed that ODD are highly associated with the development of NAION in younger patients without vascular risk factors.[11,21] This is likely the result of ODD predisposing them to an axonal compartment syndrome.[11,21]
Optic neuritis, multiple sclerosis, and neuromyelitis optica spectrum disorders
Optic neuritis can be idiopathic or related to multiple sclerosis (MS) or neuromyelitis optica spectrum disorders (NMOSD), aquaporin-4-immunoglobulin (Ig) G, and myelin oligodendrocyte glycoprotein (MOG)-IgG.[28] It has long been known that RNFL and GCIPL thinning occurs in optic neuritis and MS, and that these changes correlate with visual dysfunction.[29] OCT is able to quantify the thickness of different retinal layers impacted by optic neuritis and may be helpful in the acute and chronic period.[30,31]
Spontaneous recovery without treatment is typically expected in optic neuritis, but residual thinning of the RNFL occurs due to retrograde degeneration [Figure 2].[32] Monitoring absolute measures of RNFL or GCIPL layers are used, but this method can be problematic as some individuals may have variation in thickness at baseline.[33,34] Recent studies have shown that intereye differences in RNFL and GCIPL can accurately diagnose previous cases of unilateral optic neuritis.[31,35,36] In addition, MOG-IgG-related optic neuritis has been shown to result in relative preservation of the GCIPL, whereas AQP4-IgG-related optic neuritis results in significant GCIPL loss.[37,38] These findings suggest that OCT may have a role in diagnosing these patients when they are seen after the acute period. The various causes of optic neuritis, however, have overlapping features that preclude OCT from being clinically useful alone at this time.
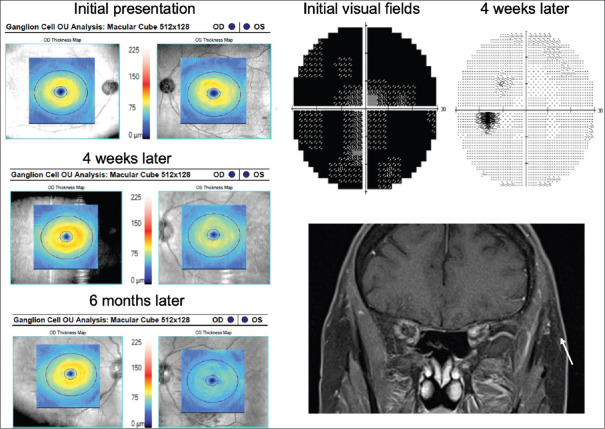
Figure 2.
Left idiopathic optic neuritis. Initial visual fields were diffusely depressed with full recovery after 4 weeks. However, on optical coherence tomography, early ganglion cell–inner plexiform layer thinning is seen. Despite visual field improvement, there was significant ganglion cell–inner plexiform layer thinning at 6 months
NMOSD is characterized by optic neuritis, longitudinally extensive transverse myelitis in the spinal cord, and brain stem encephalitis.[39,40] NMOSD shares many similarities with MS, such as presenting with optic neuritis, but carries distinct immunopathogenesis.[39,40] The majority of patients with NMOSD have detectable AQP-4 or MOG antibodies, which assists in making the diagnosis.[39] The optic neuritis associated with NMOSD often has atypical features such as being recurrent, bilateral, and more severe.[39,40] Hence, while many of the OCT changes described in idiopathic or MS-related optic neuritis will also be seen in NMOSD, recurrent episodes of optic neuritis in NMOSD can result in severe optic nerve atrophy with RNFL values <30 μm and flooring effects of the RNFL and GCIPL.[41] Bilateral OCT changes are often seen due to involvement of the optic nerve near the chiasm which can result in carryover effects.[39,42] The presence of microcystic macular edema on OCT is more prevalent in patients with NMOSD (20%–26%), even more so in those with the AQP-4 positive phenotype (40%) relative to MS patients (5%), although this feature can be seen in other optic neuropathies.[41,43] These features on OCT can help differentiate from NMOSD from other demyelinating optic neuropathies, which has historically been difficult to do.
Diagnosis of MS is based on the MacDonald criteria, which requires dissemination in time (DIT) and dissemination in space (DIS) for diagnosis.[44] The optic nerve is not currently a lesion site listed in the 2017 revisions to the MacDonald criteria despite a high prevalence of acute optic neuritis in MS.[44,45] This criteria did not use OCT testing and acknowledged that further studies using OCT to prove DIS would be helpful.[44] Since then, studies have shown that inclusion of asymptomatic patients with optic nerve lesions to satisfy DIS can improve sensitivity of the MacDonald criteria while maintaining specificity.[46] Studies have shown that RNFL thickness can be used as a clinical biomarker of visual function, response to disease-modifying therapies, and overall disease progression in MS.[47,48]
Increasingly, there is an emphasis on axonal loss being a main contributor to permanent disability in progressive forms of MS.[49] Magnetic resonance imaging (MRI) is limited in its specificity to detect axonal loss, and use of OCT to quantify retinal layers is likely the most accessible way to determine axonal loss in MS patients. Recent work has shown thinning of the RNFL and GCIPL on OCT as a marker of MS disease activity independent of optic neuritis.[32] The thinning is known to be dependent on the duration of disease, showing the greatest effect in early disease with a plateau effect.[50] Thinning of the RNFL in MS has also been associated with worse functional outcomes and decreased quality of life.[51] These data suggest that OCT changes could reflect global axonal loss and that neuroprotective interventions may have the most impact early in the disease. This is supported by the observation that RNFL and GCIPL atrophy were faster in progressive MS compared to relapsing–remitting MS.[52]
There is increasing interest in the volume of the inner nuclear layer (INL) as a marker of central nervous system (CNS) inflammatory disease activity. The INL is deep to the GCIPL and is a network of bipolar, amacrine, and horizontal cells.[32] The INL is not subject to retrograde degeneration nor does it thin in optic neuritis. The mechanism of INL thickening in MS optic neuritis is still being studied, but is postulated to be due to dynamic fluid shifts from adjacent vascular plexuses.[30] A study of disease-modifying therapies in MS showed a reduction with INL volume that correlated with therapeutic activity and overall CNS inflammation.[53] Future directions may look at how the INL volume changes during the acute phases of optic neuritis and how it correlates with radiological signs of inflammatory activity in MS.
OCT-A has been used to study optic neuritis despite ischemia or changes in circulation not being the primary etiology.[19] The ONH flow index, a marker of radial peripapillary capillary (RPC) circulation, was significantly lower in patients with a history of optic neuritis compared to healthy controls.[54] Reduction in RPC circulation in optic neuropathies of a nonischemic etiology may simply be related to the reduction in the retinal nerve fiber and GCLs.[19]
Nonarteritic anterior ischemic optic neuropathy
While there are no diagnostic features of NAION on OCT, both spectral-domain (SD) OCT and more recently OCT-A have demonstrated utility in monitoring disease course through the acute and postacute phases.[55,56] Segmentation of the RNFL using SD-OCT in acute NAION is limited by edema, and studies have suggested that GCIPL thinning is a better indicator of visual impairment.[57] GCIPL thinning was present within the 1st month following acute NAION and was found to precede reliable RNFL changes which can take months.[57] NAION has also been found to have characteristic “altitudinal” changes of the GCIPL, where the one horizontal hemisphere thins greater than the other, which has been used to differentiate it from optic neuritis at 2 weeks post-onset [Figure 3].[58,59] After an initial episode of NAION, patients are known to be at an increased risk of developing NAION in the fellow unaffected eye.[56] A study by Duman et al. compared fellow unaffected eyes to controls using SD-OCT and found subclinical retinal changes, specifically mean GCIPL thinning and RNFL thinning in the superior and nasal quadrants.[51] The exact pathogenesis of NAION is not known, but the leading hypothesis relates to impairment of perfusion to the ONH via the short posterior ciliary arteries. OCT-A has increasingly become a useful tool in NAION by advancing our understanding of its pathophysiology and prediction of functional outcomes. Multiple recent studies of OCT-A in NAION have established a structure–function relationship, demonstrating that bidirectional changes in the vascular flow density of superficial capillaries surrounding the ONH are positively correlated with a degree of visual improvement.[55,60,61] OCT-A has also been used to differentiate NAION from unaffected eyes or other forms of optic disc edema, such as optic neuritis and papilledema, with NAION having a significantly lower peripapillary vessel density.[62,63]
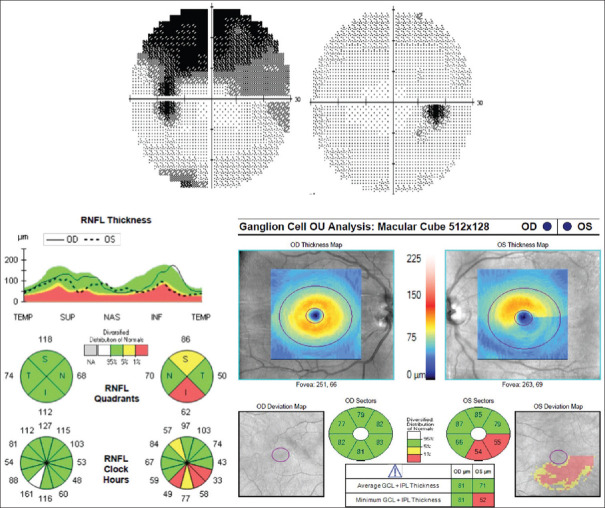
Figure 3.
Left nonarteritic anterior ischemic optic neuropathy with typical arcuate visual field loss. Optical coherence tomography shows mostly inferior retinal nerve fiber and the ganglion cell–inner plexiform layer thinning both corresponding to the visual field loss
Papilledema
Papilledema has been characterized on OCT by the elevation of the peripapillary RNFL, which obscures the optic disc margins on fundoscopy and is seen in idiopathic intracranial hypertension (IIH). OCT has shown utility in differentiating papilledema and pseudopapilledema, an anomalous elevation of the optic disc, through its ability to identify edema within the nerve fiber layers, presence of vitreous traction, and visualization of deep ODD using EDI-OCT.[64] Quantification of retinal layer volumes with OCT has shown that patients with papilledema have larger outer macular RNFL ring volumes in the inferior and nasal quadrants, while the macular GCIPL shows loss in the outer temporal region.[64] These OCT changes are similar to patterns seen in glaucoma where a pressure gradient also exists across the lamina cribrosa, leading to stasis of axoplasmic flow.[64,65] In addition to the diagnosis of papilledema and IIH, OCT has also been shown to predict visual outcomes through quantification of the GCIPL. Thinning of the GCIPL measured in the initial months following IIH diagnosis was correlated with poor visual outcomes at 1-year follow-up and preceded detectable visual field changes.[66,67] Other studies have suggested that in addition to single values, early changes in GCIPL thickness following diagnosis of IIH, specifically thinning >10 μm in the first 2–3 weeks following diagnosis, are also correlated with poor visual outcomes.[68] Papilledema also results in retinal changes outside of the optic nerve including submacular fluid, choroidal neovascular membranes, and choroidal and retinal folds, which may be difficult to appreciate on clinical examination and can be better assessed with OCT [Figure 4].[69] This can help explain reduced central visual acuity or visual field defects that may not be directly attributable to papilledema.
Figure 4.
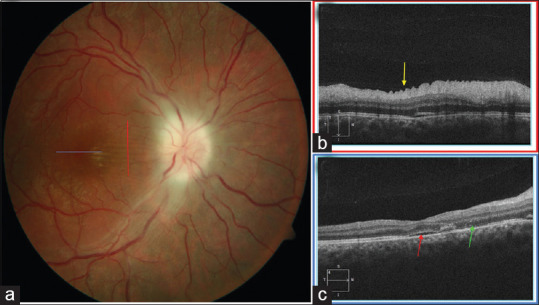
Optic atrophy from papilledema. (a) Vertical raster optical coherence tomography scan (red line) highlights the (b) radial retinal folds (yellow arrow). (c) Horizontal raster optical coherence tomography scan through the fovea demonstrates mild subretinal fluid (red arrow) and dropout of the ellipsoid zone (green arrow)
OCT is also being used as a noninvasive method of monitoring changes in ICP and response to treatment for underlying causes of papilledema. The shape of the peripapillary retinal pigment epithelium and/or Bruch's membrane (pRPE/BM) seen on OCT can often be anteriorly displaced due to the translaminar pressure differences in increased ICP.[70,71] Reduction in the anterior displacement of the pRPE/BM was associated with better treatment outcomes in IIH.[72] Future directions include improvement of techniques to efficiently and accurately label the BM as the edges can often be obscured by disc edema.[72] A recent pilot study showed that en face OCT could be used to monitor papilledema as objective parameters such as diameter of edema and subjective ranking by neuro-ophthalmologists were well correlated with RNFL thickness in patients being treated for IIH.[73] There have been advancements in the software used in OCT-A to quantify the vessels and demonstrated a decrease in ONH capillary density in both NAION and papilledema.[55,74] Peripapillary vessel density seen on OCT-A has also been shown to be correlated with grading of papilledema and may also have potential as a clinical marker of early optic nerve damage due to correlations found with choroidal blood flow and GCL thickness.[75]
Toxic and nutritional deficiencies optic neuropathies
Optic neuropathies can be secondary to toxic effects of medications or tobacco and nutritional deficiencies such as Vitamin B12. Ethambutol, an antituberculosis drug, is a well-established cause of severe toxic optic neuropathy that is often irreversible even with immediate discontinuation of the medication.[76] However, early stage ethambutol optic neuropathy (EON) is often associated with a normal appearing fundus.[77] OCT has been used in retrospective studies to identify subclinical early EON through both increases and decreases in RNFL thickness.[78,79] A recent study found that GCIPL changes preceded fundus abnormalities and thinning was negatively correlated with a degree of visual recovery.[80] GCIPL changes after discontinuation of ethambutol could be used to predict recovery at 12 months after stoppage.[80] There is likely value in regular screening and clinical investigation of the RNFL and GCIPL after initiation of ethambutol treatment.[78] Optic neuropathies and RNFL thickening are a relatively rare, but potentially the only, manifestation of nutritional deficiencies such as thiamine.[81] In general, it has been described that OCT of nutritional and toxic optic neuropathies presents with thinning of the temporal RNFL and diffuse GCIPL thinning with central field loss [Figure 5].[82] The former of which has been suggested to be related to Wallerian degeneration and preferential effects on the papillomacular fibers.[83,84] These studies suggest that a multimodal imaging approach, including OCT, is useful in the potential early identification and confirmation of optic neuropathies related to nutritional deficiencies or adverse drug reactions.
Figure 5.
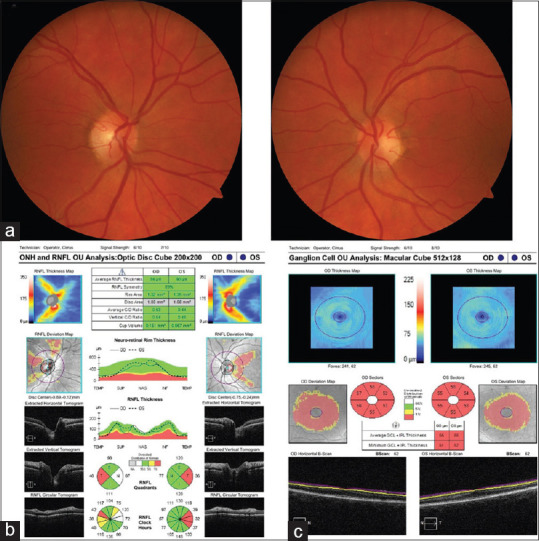
Vitamin B12 deficiency optic neuropathy. (a) Fundus photographs demonstrated temporal pallor of the optic nerves. (b) Optical coherence tomography showed temporal retinal nerve fiber layer thinning. (c) Optical coherence tomography of the ganglion cell–inner plexiform layer showed diffuse thinning
Hereditary optic neuropathies
Hereditary optic neuropathies are caused by inherited nuclear or mitochondrial DNA point mutations which affect cellular metabolism and may present at any point in life. Leber's hereditary optic neuropathy (LHON) is a rare condition resulting in bilateral optic neuropathies which have typically been characterized on OCT with initial thickening of the RNFL and choroid followed by thinning.[2] Choroidal remodeling and vascular changes seen in chronic LHON have led to recent studies using SS-OCT to better visualize the GCIPL and deep choroidal structures with better spatial resolution.[85] Thinning of the GCIPL has been found to precede RNFL swelling in acute LHON and provides a more sensitive marker of disease progression in known disease carriers.[85,86,87] Dominant optic atrophy (DOA) is similar to LHON, but typically presents earlier in life with a slow, progressive loss of vision. While the natural history of LHON and DOA is quite different, these conditions can sometimes have overlapping features. OCT has demonstrated that DOA shows RNFL thinning in the superior and inferior quadrants, whereas LHON has RNFL thickening in the acute stage, consistent with what is clinically observed.[86]
Compressive Optic Neuropathies and the Optic Chiasm
Compressive optic neuropathies typically present with slowly progressive, painless vision loss, and etiologies include pituitary macroadenomas, craniopharyngiomas, and aneurysms.[2] Chronic compression of the optic chiasm resulting in a bitemporal hemianopia shows characteristic RNFL fiber loss on OCT that has been coined “bow-tie atrophy.”[2] This comes from the arcuate radiations of the slightly nasally positioned optic disc. A similar pattern can also be seen in OCT-A where loss of the superficial retinal capillary network can be seen in the areas of classic bow-tie atrophy.[88] On the other hand, atrophy of the GCIPL in chiasmal lesions affecting nasal optic fibers typically respects the vertical meridian of the retina and can be more easily correlated with visual field loss [Figure 6]. Suprasellar masses may also affect the anterior optic chiasm resulting on a junctional scotoma or the optic tract, producing a homonymous hemianopia and corresponding OCT GCIPL changes [Figures 7 and 8]. Visualization of GCIPL atrophy using OCT is important as it is a predictor of worse visual prognosis after surgical decompression.[89]
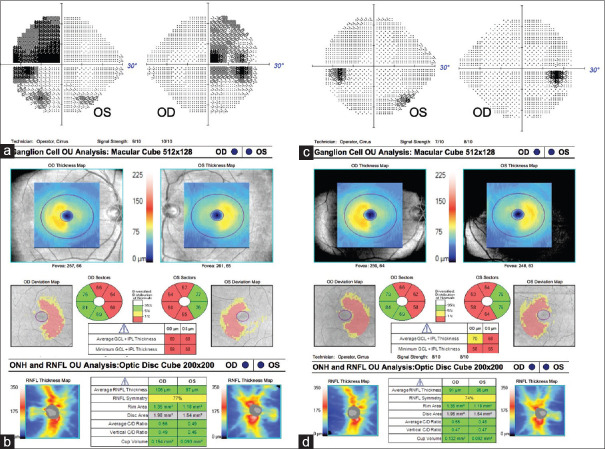
Figure 6.
Bitemporal hemianopia secondary to prolactinoma. (a) Optical coherence tomography of the ganglion cell–inner plexiform layer showing binasal thinning while the (b) retinal nerve fiber layer showing temporal thinning. (c) After medical treatment, the visual field defect resolved, but (d) the binasal ganglion cell–inner plexiform layer thinning persisted
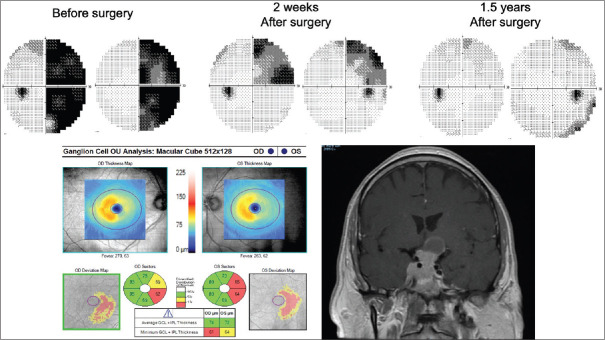
Figure 7.
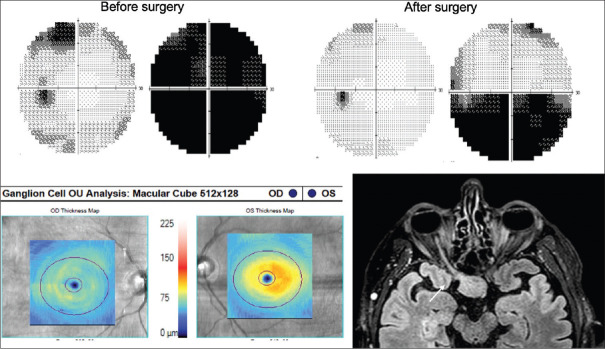
Pituitary macroadenoma causing a right junctional scotoma. Corresponding ganglion cell–inner plexiform layer thinning is seen on optical coherence tomography. There was some recovery of visual field loss in the right eye and full recovery in the left after surgery
Figure 8.
Pituitary macroadenoma extending into the cavernous sinus and left optic tract. Prior to surgery, there was a right homonymous visual field loss and corresponding ganglion cell–inner plexiform layer loss on optical coherence tomography. After surgery, he regained vision despite persistent corresponding ganglion cell–inner plexiform layer loss
OCT assessment of RNFL and GCIPL thicknesses is recommended in the preoperative evaluation of patients with sellar/suprasellar masses.[90] Earlier studies using OCT in this context focused mainly on RNFL thickness.[91,92] More recent evidence has suggested that the GCIPL is also important in evaluating compressive chiasmal lesions.[93,94] This is particularly true in early or mild chiasmal compression where distinct patterns of binasal GCIPL thinning were seen even before the RNFL.[93,95] Thinning of the GCIPL may be the first sign of early chiasmal compression affecting the anterior visual pathways and can be present in the absence of visual field deficits or compression on MRI.[95] In addition to the GCIPL, recent studies in patients with pituitary tumors have also shown that nasal and temporal RNFL thinning can occur in chiasmal compression without visual field loss.[96]
Preservation of RNFL and GCIPL thickness prior to medical or surgical treatment has been shown to be predictive of visual recovery.[92] The mechanism of recovery includes initial removal of the conduction block due to the compression, secondary remyelination, followed by restoration of axoplasmic flow.[2] Early or chronic cases of mild compression causing only a conduction block without atrophy of the ganglion cells are thus more likely to make a faster and more complete recovery. In patients with suprasellar tumors, normal thickness of the RNFL (≥70 μm) preoperatively was found to be the only significant predictor of improved postoperative visual acuity and fields among other non-OCT clinical characteristics in a multivariate analysis.[97] Recent studies have used preoperative inferior and superior RNFL thickness as part of a risk prediction model that accurately prognosticates long-term visual recovery and maintenance following pituitary tumor surgery.[98] While many patients show persistent GCIPL loss even after visual recovery, thickness of the GCIPL was positively correlated with post-operative visual field outcomes.[93] Similar findings have been replicated in thyroid eye disease looking at RNFL thickness as a predictor of surgical outcomes in individuals with significant visual field defects due to compressive optic neuropathy.[99] There is emerging evidence to suggest in some cases that nasal GCIPL thickness may have superior prognostic power to the RNFL in postoperative visual outcomes in sellar tumours.[100]
It should be noted that changes in the RNFL and GCIPL on OCT are not always seen, particularly in the case of chronic chiasmal compression.[101] However, preserved RNFL and GCIPL in the case of chronic chiasmal compression seem to suggest good visual prognosis adding to its value as a predictor of visual recovery.[101] Since optic disc pallor is a subjective sign that is open to interpretation, RNFL and GCIPL thickness provides an objective measure that can easily be documented and compared at each visit and can be more reliable than visual field testing.[102]
Retrochiasmal Visual Pathways
Recent studies in the use of OCT in retrochiasmal lesions have looked at the temporal evolution, morphology, and frequency of GCIPL thinning in patients with homonymous hemianopia and retrochiasmal lesions.[103] In contrast to RNFL thinning which produces “bow-tie” atrophy in the contralateral eye, Mühlemann et al. proposed referring to the pattern of GCIPL thinning as homonymous hemiatrophy to illustrate its respect for vertical meridian.[98] Within the retrochiasmal pathway, lesions can further be classified relative to where the retinal ganglion cells synapse at lateral geniculate nucleus, either pregeniculate or postgeniculate.[103] Atrophy of RNFL and GCIPL still occurs even in postgeniculate lesions despite no direct lesions to the axon and is due to retrograde transsynaptic degeneration (RTSD).[104] GCIPL thinning was found to have higher sensitivity for detecting RTSD and occurs earlier after lesion onset compared to RNFL thinning.[103] However, analyses using a combination of RNFL and GCIPL thinning were superior to either layer on its own.[103]
Previous studies have found that RNFL thinning can be detected immediately for pre-geniculate lesions whereas this process takes longer for post-geniculate lesions, being first detectable at approximately 5 months.[103] Additionally, there is recent evidence to suggest that GPICL thinning in post-geniculate lesions may occur in a biphasic fashion, in contrast to previous studies that reported an exponential decline.[103] Other studies have also found GCIPL homonymous hemiatrophy in patients without detectable visual field deficits suggestive of retrochiasmal lesions.[105] In a case series, these GCIPL changes were due to demyelinating processes with resolved visual field deficits and remote traumatic brain injury.[106] These data suggest that homonymous hemiatrophy of the GCIPL could be used as a tool for documenting previous lesions to the retrochiasmal visual pathways and be helpful for establishing DIT or DIS. Similar to chiasmal lesions, homonymous hemiatrophy of the GCIPL on OCT may be the first sign that a neoplastic lesion is affecting the retrochiasmal visual pathways.[107]
Optical Coherence Tomography and Neurodegenerative Disease
The ability to quantify the RNFL and GCIPL using OCT is being studied as a noninvasive means of evaluating neurodegenerative diseases such as Alzheimer's disease (AD) and Parkinson's disease (PD). A meta-analysis found that the GCIPL, RNFL, and choroid were all thinner in individuals with AD.[108] These findings support the hypothesis that AD affects both cerebral neurons and the ganglion cells of the retina.[108] While a similar trend was found in individuals with mild cognitive impairment (MCI), often a precursor to AD, this effect did not reach statistical significance.[108] However, a study in amyloid-proven AD cases only found an association with macular thinning, not the RNFL.[109] Another study in “preclinical AD,” defined as cognitively normal individuals with amyloid pathology on positron emission tomography, also found no association with RNFL thinning.[110] A cross-sectional study of seniors without AD found that changes in GCIPL were associated with decreased global cognition and may represent an early marker of progression to AD.[111] Future studies could confirm the findings that suggest that GCIPL thinning is more prevalent compared to RNFL thinning in conditions thought to precede AD. Involvement of the RNFL and GCIPL could be due to pathological cerebral changes similar to retrochiasmal lesions, leading to RTSD or direct neurotoxic effects to the retina. Involvement of the choroid could be explained by cerebral vascular impairment, being one of the earliest features of AD and deposition of amyloid-beta could occur in the choroidal vasculature.[112] The theory of vascular impairment is also supported by OCT-A studies that showed significant changes in macular vessel and perfusion density in AD compared to MCI and controls. Future directions may include prospective studies to understand how OCT changes may predate cognitive changes and its temporal relationship to development of AD. Longitudinal studies could also be more sensitive to subtle changes over time and reduce the effect of interindividual differences.[109]
There is also interest in the use of OCT in patients with parkinsonism, which can be separated into PD and atypical parkinsonism such as progressive supranuclear palsy (PSP). Retinal ganglion cells are known to in part use dopaminergic transmission to modulate visual processing.[113] Parkinsonism is the result of decreased dopaminergic transmission in the basal ganglia. It is thought that the various mechanisms that reduce dopaminergic transmission in the basal ganglia can also affect the retina.[113] Therefore it is speculated that retinal changes measured by OCT could be used as a surrogate marker of progression in conditions such as Parkinson's disease.[113] OCT changes consistent with nonspecific neurodegeneration such as RNFL and macular thinning have long been described in patients with PD.[114] Recent work has focused on looking at OCT changes seen in those with rapid eye movement sleep behavior disorders (RBD) as a surrogate for prodromal PD and have seen RNFL and GCIPL thinning in these individuals.[115,116] Other studies have found different patterns of RNFL thinning on OCT when comparing different forms of parkinsonism such as PSP.[117] However, at this time, many of the changes described are also seen in general neurodegeneration, thus limiting the clinical use of OCT in the prediction or evaluation of parkinsonism. Future work could involve longitudinal studies to determine if OCT could be used as a screening tool in RBD patients to predict progression to parkinsonism.
Conclusions
OCT has become an important tool in the evaluation of neuro-ophthalmologic diseases of the afferent visual pathway through its ability to directly visualize and quantify retinal tissues such as the RNFL and GCIPL. Recent work in the use of OCT has included the localization of lesions in the afferent visual system based on patterns of RNFL and GCIPL thinning such as in compressive chiasmal and retrochiasmal lesions. OCT has shown that patients with transient autoimmune or inflammatory lesions of the optic nerve can have lasting changes on OCT that can be used reliably to detect previous episodes of optic neuritis or retrochiasmal lesions. Moving forward, OCT may be formally included in the diagnostic criteria of MS as one tool to demonstrate dissemination of demyelination in space and time. The utility of OCT is also expanding beyond ophthalmology to other specialties such as neurosurgery and neurology to help prognosticate diseases with specific manifestations in the visual system such as pituitary tumors or as a surrogate marker of more global neurodegeneration as seen in AD. The scope of OCT is likely to continue to expand and will be an increasingly important aspect in assessing neuro-ophthalmologic disease moving forward.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors declared that there are no conflicts of interests of this paper.
References
- 1.Sakata LM, DeLeon-Ortega J, Sakata V, Girkin CA. Optical coherence tomography of the retina and optic nerve – A review. Clin Experiment Ophthalmol. 2009;37:90–9. doi: 10.1111/j.1442-9071.2009.02015.x. [DOI] [PubMed] [Google Scholar]
- 2.Micieli JA, Newman NJ, Biousse V. The role of optical coherence tomography in the evaluation of compressive optic neuropathies. Curr Opin Neurol. 2019;32:115–23. doi: 10.1097/WCO.0000000000000636. [DOI] [PubMed] [Google Scholar]
- 3.Chinn SR, Swanson EA, Fujimoto JG. Optical coherence tomography using a frequency-tunable optical source. Opt Lett. 1997;22:340–2. doi: 10.1364/ol.22.000340. [DOI] [PubMed] [Google Scholar]
- 4.Jia Y, Morrison JC, Tokayer J, Tan O, Lombardi L, Baumann B, et al. Quantitative OCT angiography of optic nerve head blood flow. Biomed Opt Express. 2012;3:3127–37. doi: 10.1364/BOE.3.003127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosen RB, Hathaway M, Rogers J, Pedro J, Garcia P, Laissue PP, et al. Multidimensional en-face OCT imaging of the retina. Opt Express. 2009;17:4112–33. doi: 10.1364/oe.17.004112. [DOI] [PubMed] [Google Scholar]
- 6.Spaide RF, Koizumi H, Pozzoni MC, Pozonni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496–500. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 7.Adhi M, Liu JJ, Qavi AH, Grulkowski I, Lu CD, Mohler KJ, et al. Choroidal analysis in healthy eyes using swept-source optical coherence tomography compared to spectral domain optical coherence tomography. Am J Ophthalmol. 2014;157:1272–81. doi: 10.1016/j.ajo.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 8.Spaide RF, Klancnik JM, Cooney MJ. Retinal Vascular Layers Imaged by Fluorescein Angiography and Optical Coherence Tomography Angiography. JAMA Ophthalmol. 2015;133:45–50. doi: 10.1001/jamaophthalmol.2014.3616. [DOI] [PubMed] [Google Scholar]
- 9.Spencer WH. Drusen of the Optic Disc and Aberrant Axoplasmic Transport. Ophthalmology. 1978;85:21–38. doi: 10.1016/s0161-6420(78)35696-7. [DOI] [PubMed] [Google Scholar]
- 10.Malmqvist L, Bursztyn L, Costello F, Digre K, Fraser JA, Fraser C, et al. The Optic Disc Drusen Studies Consortium Recommendations for Diagnosis of Optic Disc Drusen Using Optical Coherence Tomography. J Neuro-Ophthalmol Off J North Am Neuro-Ophthalmol Soc. 2018;38:299–307. doi: 10.1097/WNO.0000000000000585. [DOI] [PubMed] [Google Scholar]
- 11.Hamann S, Malmqvist L, Wegener M, Biousse V, Bursztyn L, Citirak G, et al. Young Adults with Anterior Ischemic Optic Neuropathy: A Multicenter Optic Disc Drusen Study. Am J Ophthalmol. 2020 doi: 10.1016/j.ajo.2020.03.052. [DOI] [PubMed] [Google Scholar]
- 12.Bassi ST, Mohana KP. Optical coherence tomography in papilledema and pseudopapilledema with and without optic nerve head drusen. Indian J Ophthalmol. 2014;62:1146–51. doi: 10.4103/0301-4738.149136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurz-Levin MM, Landau K. A Comparison of Imaging Techniques for Diagnosing Drusen of the Optic Nerve Head. Arch Ophthalmol. 1999;117:1045–9. doi: 10.1001/archopht.117.8.1045. [DOI] [PubMed] [Google Scholar]
- 14.Loft FC, Malmqvist L, Wessel Lindberg A-S, Hamann S. The Influence of Volume and Anatomic Location of Optic Disc Drusen on the Sensitivity of Autofluorescence. J Neuroophthalmol. 2019;39:23–7. doi: 10.1097/WNO.0000000000000654. [DOI] [PubMed] [Google Scholar]
- 15.Teixeira FJ, Marques RE, Mano SS, Couceiro R, Pinto F. Optic disc drusen in children: morphologic features using EDI-OCT. Eye. 2019:1–8. doi: 10.1038/s41433-019-0694-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skougaard M, Heegaard S, Malmqvist L, Hamann S. Prevalence and histopathological signatures of optic disc drusen based on microscopy of 1713 enucleated eyes. Acta Ophthalmol (Copenh) 2020;98:195–200. doi: 10.1111/aos.14180. [DOI] [PubMed] [Google Scholar]
- 17.Merchant KY, Su D, Park SC, Qayum S, Banik R, Liebmann JM, et al. Enhanced Depth Imaging Optical Coherence Tomography of Optic Nerve Head Drusen. Ophthalmology. 2013;120:1409–14. doi: 10.1016/j.ophtha.2012.12.035. [DOI] [PubMed] [Google Scholar]
- 18.Wang DD, Leong JCY, Gale J, Wells AP. Multimodal imaging of buried optic nerve head drusen. Eye. 2018;32:1145–6. doi: 10.1038/s41433-017-0009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen JJ, AbouChehade JE, Iezzi R, Leavitt JA, Kardon RH. Optical Coherence Angiographic Demonstration of Retinal Changes From Chronic Optic Neuropathies. Neuro-Ophthalmol. 2017;41:76–83. doi: 10.1080/01658107.2016.1275703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maccora KA, Sheth S, Ruddle JB. Optical coherence tomography in paediatric clinical practice. Clin Exp Optom. 2019;102:300–8. doi: 10.1111/cxo.12909. [DOI] [PubMed] [Google Scholar]
- 21.Fraser JA, Rueløkke LL, Malmqvist L, Hamann S. Prevalence of Optic Disc Drusen in Young Patients With Nonarteritic Anterior Ischemic Optic Neuropathy: A 10-Year Retrospective Study. J Neuro-Ophthalmol Off J North Am Neuro-Ophthalmol Soc. 2020 doi: 10.1097/WNO.0000000000000974. [DOI] [PubMed] [Google Scholar]
- 22.Malmqvist L, Sibony PA, Fraser CL, Wegener M, Heegaard S, Skougaard M, et al. Peripapillary Ovoid Hyperreflectivity in Optic Disc Edema and Pseudopapilledema. Ophthalmology. 2018;125:1662–4. doi: 10.1016/j.ophtha.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 23.Lee KM, Hwang J-M, Woo SJ. Optic Disc Drusen Associated with Optic Nerve Tumors. Optom Vis Sci. 2015;92(4S):S67. doi: 10.1097/OPX.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 24.Borrelli E, Barboni P, Battista M, Sacconi R, Querques L, Cascavilla ML, et al. Peripapillary hyperreflective ovoid mass-like structures (PHOMS): OCTA may reveal new findings. Eye. 2020:1–4. doi: 10.1038/s41433-020-0890-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yi K, Mujat M, Sun W, Burnes D, Latina MA, Lin DT, et al. Imaging of Optic Nerve Head Drusen: Improvements With Spectral Domain Optical Coherence Tomography. J Glaucoma. 2009;18:373–8. doi: 10.1097/IJG.0b013e31818624a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costello F, Malmqvist L, Hamann S. The Role of Optical Coherence Tomography in Differentiating Optic Disc Drusen from Optic Disc Edema. Asia-Pac J Ophthalmol. 2018;7:271–9. doi: 10.22608/APO.2018124. [DOI] [PubMed] [Google Scholar]
- 27.Traber GL, Weber KP, Sabah M, Keane PA, Plant GT. Enhanced Depth Imaging Optical Coherence Tomography of Optic Nerve Head Drusen: A Comparison of Cases with and without Visual Field Loss. Ophthalmology. 2017;124:66–73. doi: 10.1016/j.ophtha.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 28.Hickman S, Dalton C, Miller D, Plant G. Management of acute optic neuritis. The Lancet. 2002;360(9349):1953–62. doi: 10.1016/s0140-6736(02)11919-2. [DOI] [PubMed] [Google Scholar]
- 29.Parisi V, Manni G, Spadaro M, Colacino G, Restuccia R, Marchi S, et al. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci. 1999;40:2520–7. [PubMed] [Google Scholar]
- 30.Balk LJ, Coric D, Knier B, Zimmermann HG, Behbehani R, Alroughani R, et al. Retinal inner nuclear layer volume reflects inflammatory disease activity in multiple sclerosis; a longitudinal OCT study. Mult Scler J - Exp Transl Clin [Internet] 2019. [Last cited on 2020 May 30]. p. 5. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6728683/ [DOI] [PMC free article] [PubMed]
- 31.Nolan-Kenney RC, Liu M, Akhand O, Calabresi PA, Paul F, Petzold A, et al. Optimal intereye difference thresholds by optical coherence tomography in multiple sclerosis: An international study. Ann Neurol. 2019;85:618–29. doi: 10.1002/ana.25462. [DOI] [PubMed] [Google Scholar]
- 32.Costello F, Burton JM. Retinal imaging with optical coherence tomography: A biomarker in multiple sclerosis? Eye Brain. 2018;10:47–63. doi: 10.2147/EB.S139417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costello F, Pan YI, Yeh EA, Hodge W, Burton JM, Kardon R. The temporal evolution of structural and functional measures after acute optic neuritis. J Neurol Neurosurg Psychiatry. 2015;86:1369–73. doi: 10.1136/jnnp-2014-309704. [DOI] [PubMed] [Google Scholar]
- 34.Kupersmith MJ, Garvin MK, Wang J-K, Durbin M, Kardon R. Retinal ganglion cell layer thinning within one month of presentation for optic neuritis. Mult Scler Houndmills Basingstoke Engl. 2016;22:641–8. doi: 10.1177/1352458515598020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu SC, Kardon RH, Leavitt JA, Flanagan EP, Pittock SJ, Chen JJ. Optical coherence tomography is highly sensitive in detecting prior optic neuritis. Neurology. 2019;92:e527–35. doi: 10.1212/WNL.0000000000006873. [DOI] [PubMed] [Google Scholar]
- 36.Outteryck O, Lopes R, Drumez É, Labreuche J, Lannoy J, Hadhoum N, et al. Optical coherence tomography for detection of asymptomatic optic nerve lesions in clinically isolated syndrome. Neurology. 2020;95:e733–44. doi: 10.1212/WNL.0000000000009832. [DOI] [PubMed] [Google Scholar]
- 37.Oertel FC, Outteryck O, Knier B, Zimmermann H, Borisow N, Bellmann-Strobl J, et al. Optical coherence tomography in myelin-oligodendrocyte-glycoprotein antibody-seropositive patients: A longitudinal study. J Neuroinflammation. 2019;16:154. doi: 10.1186/s12974-019-1521-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oertel FC, Havla J, Roca-Fernández A, Lizak N, Zimmermann H, Motamedi S, et al. Retinal ganglion cell loss in neuromyelitis optica: a longitudinal study. J Neurol Neurosurg Psychiatry. 2018;89:1259–65. doi: 10.1136/jnnp-2018-318382. [DOI] [PubMed] [Google Scholar]
- 39.Oertel FC, Zimmermann H, Paul F, Brandt AU. Optical coherence tomography in neuromyelitis optica spectrum disorders: potential advantages for individualized monitoring of progression and therapy. EPMA J. 2018;9:21–33. doi: 10.1007/s13167-017-0123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mateo J, Esteban O, Martínez M, Grzybowski A, Ascaso FJ. The Contribution of Optical Coherence Tomography in Neuromyelitis Optica Spectrum Disorders. Front Neurol [Internet] 2017. p. 8. [DOI] [PMC free article] [PubMed]
- 41.Costello F. Optical Coherence Tomography in Neuro-ophthalmology. Neurol Clin. 2017;35:153–63. doi: 10.1016/j.ncl.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 42.Ramanathan S, Prelog K, Barnes EH, Tantsis EM, Reddel SW, Henderson AP, et al. Radiological differentiation of optic neuritis with myelin oligodendrocyte glycoprotein antibodies, aquaporin-4 antibodies, and multiple sclerosis. Mult Scler J [Internet] 2015. [Last cited on 2020 Sep 21]. Available from: https://journals.sagepub.com/ doi/10.1177/1352458515593406 . [DOI] [PubMed]
- 43.Bennett J, de Seze J, Lana-Peixoto M, Palace J, Waldman A, Schippling S, et al. Neuromyelitis optica and multiple sclerosis: Seeing differences through optical coherence tomography. Mult Scler J. 2015;21:678–88. doi: 10.1177/1352458514567216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–73. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 45.Percy AK, Nobrega FT, Kurland LT. Optic Neuritis and Multiple Sclerosis: An Epidemiologic Study. Arch Ophthalmol. 1972;87:135–9. doi: 10.1001/archopht.1972.01000020137004. [DOI] [PubMed] [Google Scholar]
- 46.Brownlee WJ, Miszkiel KA, Tur C, Barkhof F, Miller DH, Ciccarelli O. Inclusion of optic nerve involvement in dissemination in space criteria for multiple sclerosis. Neurology. 2018;91:e1130–4. doi: 10.1212/WNL.0000000000006207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fisher JB, Jacobs DA, Markowitz CE, Galetta SL, Volpe NJ, Nano-Schiavi ML, et al. Relation of Visual Function to Retinal Nerve Fiber Layer Thickness in Multiple Sclerosis. Ophthalmology. 2006;113:324–32. doi: 10.1016/j.ophtha.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 48.Nolan RC, Akhand O, Rizzo J-R, Galetta SL, Balcer LJ. Evolution of Visual Outcomes in Clinical Trials for Multiple Sclerosis Disease-Modifying Therapies. J Neuro-Ophthalmol Off J North Am Neuro-Ophthalmol Soc. 2018;38:202–9. doi: 10.1097/WNO.0000000000000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Costello F. The Afferent Visual Pathway: Designing a Structural-Functional Paradigm of Multiple Sclerosis. ISRN Neurol [Internet] 2013. 2013. [Last cited on 2020 May 30]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3830872/ [DOI] [PMC free article] [PubMed]
- 50.Balk LJ, Cruz-Herranz A, Albrecht P, Arnow S, Gelfand JM, Tewarie P, et al. Timing of retinal neuronal and axonal loss in MS: A longitudinal OCT study. J Neurol. 2016;263:1323–31. doi: 10.1007/s00415-016-8127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcia-Martin E, Ara JR, Martin J, Almarcegui C, Dolz I, Vilades E, et al. Retinal and Optic Nerve Degeneration in Patients with Multiple Sclerosis Followed up for 5 Years. Ophthalmology. 2017;124:688–96. doi: 10.1016/j.ophtha.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Sotirchos ES, Gonzalez Caldito N, Filippatou A, Fitzgerald KC, Murphy OC, Lambe J, et al. Progressive Multiple Sclerosis Is Associated with Faster and Specific Retinal Layer Atrophy. Ann Neurol. 2020;87:885–96. doi: 10.1002/ana.25738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knier B, Schmidt P, Aly L, Buck D, Berthele A, Mühlau M, et al. Retinal inner nuclear layer volume reflects response to immunotherapy in multiple sclerosis. Brain J Neurol. 2016;139:2855–63. doi: 10.1093/brain/aww219. [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Jia Y, Spain R, Potsaid B, Liu JJ, Baumann B, et al. Optical coherence tomography angiography of optic nerve head and parafovea in multiple sclerosis. Br J Ophthalmol. 2014;98:1368–73. doi: 10.1136/bjophthalmol-2013-304547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaier ED, Wang M, Gilbert AL, Rizzo JF, Cestari DM, Miller JB. Quantitative analysis of optical coherence tomographic angiography (OCT-A) in patients with non-arteritic anterior ischemic optic neuropathy (NAION) corresponds to visual function. PLoS ONE [Internet] 2018. [Last cited on 2020 Jun 12]. p. 13. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6023180/ [DOI] [PMC free article] [PubMed]
- 56.Duman R, Yavas GF, Veliyev I, Dogan M, Duman R. Structural changes of macula and optic disk of the fellow eye in patients with nonarteritic anterior ischemic optic neuropathy. Int Ophthalmol. 2019;39:1293–8. doi: 10.1007/s10792-018-0942-y. [DOI] [PubMed] [Google Scholar]
- 57.Akbari M, Abdi P, Fard MA, Afzali M, Ameri A, Yazdani-Abyaneh A, et al. Retinal Ganglion Cell Loss Precedes Retinal Nerve Fiber Thinning in Nonarteritic Anterior Ischemic Optic Neuropathy. J Neuroophthalmol. 2016;36:141–6. doi: 10.1097/WNO.0000000000000345. [DOI] [PubMed] [Google Scholar]
- 58.Erlich-Malona N, Mendoza-Santiesteban CE, Hedges TR, Patel N, Monaco C, Cole E. Distinguishing ischaemic optic neuropathy from optic neuritis by ganglion cell analysis. Acta Ophthalmol (Copenh) 2016;94:e721–6. doi: 10.1111/aos.13128. [DOI] [PubMed] [Google Scholar]
- 59.MacIntosh PW, Kumar SV, Saravanan VR, Shah VM. Acute changes in ganglion cell layer thickness in ischemic optic neuropathy compared to optic neuritis using optical coherence tomography. Int J Ophthalmol. 2020;13:120–3. doi: 10.18240/ijo.2020.01.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma S, Ang M, Najjar RP, Sng C, Cheung CY, Rukmini AV, et al. Optical coherence tomography angiography in acute non-arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol. 2017;101:1045–51. doi: 10.1136/bjophthalmol-2016-309245. [DOI] [PubMed] [Google Scholar]
- 61.Wright Mayes E, Cole ED, Dang S, Novais EA, Vuong L, Mendoza-Santiesteban C, et al. Optical Coherence Tomography Angiography in Nonarteritic Anterior Ischemic Optic Neuropathy. J Neuro-Ophthalmol Off J North Am Neuro-Ophthalmol Soc. 2017;37:358–64. doi: 10.1097/WNO.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 62.Fard MA, Yadegari S, Ghahvechian H, Moghimi S, Soltani-Moghaddam R, Subramanian PS. Optical Coherence Tomography Angiography of a Pale Optic Disc in Demyelinating Optic Neuritis and Ischemic Optic Neuropathy. J Neuroophthalmol. 2019;39:339–344. doi: 10.1097/WNO.0000000000000775. [DOI] [PubMed] [Google Scholar]
- 63.Fard MA, Jalili J, Sahraiyan A, Khojasteh H, Hejazi M, Ritch R, et al. Optical Coherence Tomography Angiography in Optic Disc Swelling. Am J Ophthalmol. 2018;191:116–23. doi: 10.1016/j.ajo.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 64.Aghsaei Fard M, Okhravi S, Moghimi S, Subramanian PS. Optic Nerve Head and Macular Optical Coherence Tomography Measurements in Papilledema Compared With Pseudopapilledema. J Neuroophthalmol. 2019;39:28–34. doi: 10.1097/WNO.0000000000000641. [DOI] [PubMed] [Google Scholar]
- 65.Fard MA, Afzali M, Abdi P, Yasseri M, Ebrahimi KB, Moghimi S. Comparison of the Pattern of Macular Ganglion Cell-Inner Plexiform Layer Defect Between Ischemic Optic Neuropathy and Open-Angle Glaucoma. Invest Ophthalmol Vis Sci. 2016;57:1011–6. doi: 10.1167/iovs.15-18618. [DOI] [PubMed] [Google Scholar]
- 66.Athappilly G, García-Basterra I, Machado-Miller F, Hedges TR, Mendoza-Santiesteban C, Vuong L. Ganglion Cell Complex Analysis as a Potential Indicator of Early Neuronal Loss in Idiopathic Intracranial Hypertension. Neuro-Ophthalmol. 2018;43:10–7. doi: 10.1080/01658107.2018.1476558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marzoli SB, Ciasca P, Curone M, Cammarata G, Melzi L, Criscuoli A, et al. Quantitative analysis of optic nerve damage in idiopathic intracranial hypertension (IIH) at diagnosis. Neurol Sci. 2013;34:143–5. doi: 10.1007/s10072-013-1373-1. [DOI] [PubMed] [Google Scholar]
- 68.Chen JJ, Thurtell MJ, Longmuir RA, Garvin MK, Wang J-K, Wall M, et al. Causes and Prognosis of Visual Acuity Loss at the Time of Initial Presentation in Idiopathic Intracranial Hypertension. Invest Ophthalmol Vis Sci. 2015;56:3850. doi: 10.1167/iovs.15-16450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nichani P, Micieli JA. Retinal manifestations of idiopathic intracranial hypertension. Ophthalmol Retina. 2020 doi: 10.1016/j.oret.2020.08.016. S2468-6530(20)30348-1. [DOI] [PubMed] [Google Scholar]
- 70.Kupersmith MJ, Sibony P, Mandel G, Durbin M, Kardon RH. Optical coherence tomography of the swollen optic nerve head: deformation of the peripapillary retinal pigment epithelium layer in papilledema. Invest Ophthalmol Vis Sci. 2011;52:6558–64. doi: 10.1167/iovs.10-6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sibony P, Kupersmith MJ, Rohlf FJ. Shape analysis of the peripapillary RPE layer in papilledema and ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2011;52:7987–95. doi: 10.1167/iovs.11-7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang J-K, Kardon RH, Ledolter J, Sibony PA, Kupersmith MJ, Garvin MK. Peripapillary Retinal Pigment Epithelium Layer Shape Changes From Acetazolamide Treatment in the Idiopathic Intracranial Hypertension Treatment Trial. Invest Ophthalmol Vis Sci. 2017;58:2554–65. doi: 10.1167/iovs.16-21089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carey AR, Bosley TM, Miller NR, McCulley TJ, Henderson AD. Use of En Face Optical Coherence Tomography to Monitor Papilledema in Idiopathic Intracranial Hypertension: A Pilot Study. J Neuro-Ophthalmol Off J North Am Neuro-Ophthalmol Soc. 2020 doi: 10.1097/WNO.0000000000000940. [DOI] [PubMed] [Google Scholar]
- 74.Gandhi U, Chhablani J, Badakere A, Kekunnaya R, Rasheed MA, Goud A, et al. Optical coherence tomography angiography in acute unilateral nonarteritic anterior ischemic optic neuropathy: A comparison with the fellow eye and with eyes with papilledema. Indian J Ophthalmol. 2018;66:1144–8. doi: 10.4103/ijo.IJO_179_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rodriguez Torres Y, Lee P, Mihlstin M, Tomsak RL. Correlation Between Optic Disc Peripapillary Capillary Network and Papilledema Grading in Patients With Idiopathic Intracranial Hypertension: A Study of Optical Coherence Tomography Angiography. J Neuroophthalmol. 2020 doi: 10.1097/WNO.0000000000000877. doi: 10.1097/ WNO.0000000000000877. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 76.Tsai R-K, Lee Y-H. Reversibility of Ethambutol Optic Neuropathy. J Ocul Pharmacol Ther. 1997;13:473–7. doi: 10.1089/jop.1997.13.473. [DOI] [PubMed] [Google Scholar]
- 77.Chan RYC, Kwok AKH. Ocular toxicity of ethambutol. Hong Kong Med J Xianggang Yi Xue Za Zhi. 2006;12:56–60. [PubMed] [Google Scholar]
- 78.Jin KW, Lee JY, Rhiu S, Choi DG. Longitudinal evaluation of visual function and structure for detection of subclinical Ethambutol-induced optic neuropathy.In: Tzekov R, editor. PLOS ONE. 2019;14:e0215297. doi: 10.1371/journal.pone.0215297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pavan Taffner BM, Mattos FB, da Cunha MC, Saraiva FP. The use of optical coherence tomography for the detection of ocular toxicity by ethambutol. PLoS ONE [Internet. 2018. [Last cited on 2020 Jun 12]. p. 13. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6224029/ [DOI] [PMC free article] [PubMed]
- 80.Lee J-Y, Choi JH, Park K-A, Oh SY. Ganglion Cell Layer and Inner Plexiform Layer as Predictors of Vision Recovery in Ethambutol-Induced Optic Neuropathy: A Longitudinal OCT Analysis. Invest Ophthalmol Vis Sci. 2018;59:2104–9. doi: 10.1167/iovs.17-22988. [DOI] [PubMed] [Google Scholar]
- 81.Sia PI, Sia DIT, Crompton JL, Casson RJ. Nerve Fiber Layer Infarcts in Thiamine Deficiency. J Neuroophthalmol. 2015;35:274–6. doi: 10.1097/WNO.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 82.Walker C, Terveen D, Bailey J. Nutritional Optic Neuropathy. EyeRoundsOrg [Internet] 2018. Available from: https:// EyeRounds.org/cases/273-nutritional-optic-neuropathy.htm .
- 83.Xiao H, Liu X, Lian P, Liao L-L, Zhong Y-M. Different damage patterns of retinal nerve fiber layer and ganglion cell-inner plexiform layer between early glaucoma and non-glaucomatous optic neuropathy. Int J Ophthalmol. 2020;13:893–901. doi: 10.18240/ijo.2020.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pasol J. Neuro-ophthalmic disease and optical coherence tomography: glaucoma look-alikes. Curr Opin Ophthalmol. 2011;22:124–32. doi: 10.1097/ICU.0b013e328343c1a3. [DOI] [PubMed] [Google Scholar]
- 85.Borrelli E, Triolo G, Cascavilla ML, La Morgia C, Rizzo G, Savini G, et al. Changes in Choroidal Thickness follow the RNFL Changes in Leber's Hereditary Optic Neuropathy. Sci Rep. 2016;6:37332. doi: 10.1038/srep37332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Darvizeh F, Asanad S, Falavarjani KG, Wu J, Tian JJ, Bandello F, et al. Choroidal thickness and the retinal ganglion cell complex in chronic Leber-s hereditary optic neuropathy: a prospective study using swept-source optical coherence tomography. Eye. 2019:1–7. doi: 10.1038/s41433-019-0695-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Asanad S, Tian JJ, Frousiakis S, Jiang JP, Kogachi K, Felix CM, et al. Optical Coherence Tomography of the Retinal Ganglion Cell Complex in Leber's Hereditary Optic Neuropathy and Dominant Optic Atrophy. Curr Eye Res. 2019;44:638–44. doi: 10.1080/02713683.2019.1567792. [DOI] [PubMed] [Google Scholar]
- 88.Hedges TR, Gobuty M, Manfready RA, Erlich-Malona N, Monaco C, Mendoza-Santiesteban CE. The Optical Coherence Tomographic Profile of Leber Hereditary Optic Neuropathy. Neuro-Ophthalmol. 2016;40:107–12. doi: 10.3109/01658107.2016.1173709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Micieli JA, Newman NJ, Biousse V. Retinal Imaging of an Optic Tract Lesion: OCT Angiography of Structural and Functional Defects. Ophthalmology. 2018;125:756. [Google Scholar]
- 90.Micieli JA, Newman NJ, Biousse V. The role of optical coherence tomography in the evaluation of compressive optic neuropathies. Curr Opin Neurol. 2019;32:115–23. doi: 10.1097/WCO.0000000000000636. [DOI] [PubMed] [Google Scholar]
- 91.Newman SA, Turbin RE, Bodach ME, Tumialan LM, Oyesiku NM, Litvack Z, et al. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guideline on Pretreatment Ophthalmology Evaluation in Patients With Suspected Nonfunctioning Pituitary Adenomas. Neurosurgery. 2016;79:E530–2. doi: 10.1227/NEU.0000000000001388. [DOI] [PubMed] [Google Scholar]
- 92.Jacobs DA, Galetta SL. Multiple sclerosis and the visual system. Ophthalmol Clin. 2004;17:265–73. doi: 10.1016/j.ohc.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 93.Danesh-Meyer HV, Papchenko T, Savino PJ, Law A, Evans J, Gamble GD. In Vivo Retinal Nerve Fiber Layer Thickness Measured by Optical Coherence Tomography Predicts Visual Recovery after Surgery for Parachiasmal Tumors. Invest Ophthalmol Vis Sci. 2008;49:1879–85. doi: 10.1167/iovs.07-1127. [DOI] [PubMed] [Google Scholar]
- 94.Tieger MG, Hedges TR, Ho J, Erlich-Malona NK, Vuong LN, Athappilly GK, et al. Ganglion Cell Complex Loss in Chiasmal Compression by Brain Tumors. J Neuro-Ophthalmol Off J North Am Neuro-Ophthalmol Soc. 2017;37:7–12. doi: 10.1097/WNO.0000000000000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Monteiro MLR. Macular Ganglion Cell Complex Reduction Preceding Visual Field Loss in a Patient With Chiasmal Compression With a 21-Month Follow-Up. J Neuroophthalmol. 2018;38:124–7. doi: 10.1097/WNO.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 96.Blanch RJ, Micieli JA, Oyesiku NM, Newman NJ, Biousse V. Optical coherence tomography retinal ganglion cell complex analysis for the detection of early chiasmal compression. Pituitary. 2018;21:515–23. doi: 10.1007/s11102-018-0906-2. [DOI] [PubMed] [Google Scholar]
- 97.Orman G, Sungur G, Culha C. Assessment of inner retina layers thickness values in eyes with pituitary tumours before visual field defects occur. Eye [Internet] 2020. [Last cited on 2020 Jun 25]. Available from: http://www.nature.com/articles/s41433-020-1032-8 . [DOI] [PMC free article] [PubMed]
- 98.Jeon C, Park K-A, Hong SD, Choi JW, Seol HJ, Nam D-H, et al. Clinical Efficacy of Optical Coherence Tomography to Predict the Visual Outcome After Endoscopic Endonasal Surgery for Suprasellar Tumors. World Neurosurg. 2019;132:e722–31. doi: 10.1016/j.wneu.2019.08.031. [DOI] [PubMed] [Google Scholar]
- 99.Wang MTM, King J, Symons RCA, Stylli SS, Meyer J, Daniell MD, et al. Prognostic utility of optical coherence tomography for long-term visual recovery following pituitary tumor surgery. Am J Ophthalmol [Internet] 2020. [Last cited on 2020 Jun 25]. Available from: http://www.sciencedirect.com/science/article/pii/S0002939420302890 . [DOI] [PubMed]
- 100.Rajabi MT, Ojani M, Riazi Esfahani H, Tabatabaei SZ, Rajabi MB, Hosseini SS. Correlation of peripapillary nerve fiber layer thickness with visual outcomes after decompression surgery in subclinical and clinical thyroid-related compressive optic neuropathy. J Curr Ophthalmol. 2019;31:86–91. doi: 10.1016/j.joco.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yoo YJ, Hwang J-M, Yang HK, Joo J-D, Kim Y-H, Kim C-Y. Prognostic value of macular ganglion cell layer thickness for visual outcome in parasellar tumors. J Neurol Sci. 2020;414:116823. doi: 10.1016/j.jns.2020.116823. [DOI] [PubMed] [Google Scholar]
- 102.Lukewich MK, Micieli JA. Chronic chiasmal compression and persistent visual field defect without detectable changes in optical coherence tomography of the macular ganglion cell complex. Am J Ophthalmol Case Rep. 2019;16:100533. doi: 10.1016/j.ajoc.2019.100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Al-Louzi O, Prasad S, Mallery RM. Utility of optical coherence tomography in the evaluation of sellar and parasellar mass lesions. Curr Opin Endocrinol Diabetes Obes. 2018;25:274–84. doi: 10.1097/MED.0000000000000415. [DOI] [PubMed] [Google Scholar]
- 104.Mühlemann F, Grabe H, Fok A, Wagner F, Brügger D, Sheldon CA, et al. Homonymous hemiatrophy of ganglion cell layer from retrochiasmal lesions in the visual pathway. Neurology. 2020;94:e323–9. doi: 10.1212/WNL.0000000000008738. [DOI] [PubMed] [Google Scholar]
- 105.Jindahra P, Petrie A, Plant GT. The time course of retrograde trans-synaptic degeneration following occipital lobe damage in humans. Brain. 2012;135:534–41. doi: 10.1093/brain/awr324. [DOI] [PubMed] [Google Scholar]
- 106.Lukewich MK, Schlenker MB, Micieli JA. Homonymous hemi-macular atrophy of the ganglion cell-inner plexiform layer with preserved visual function? J Neurol Sci. 2020;417:117072. doi: 10.1016/j.jns.2020.117072. doi: 10.1016/j.jns.2020. Epub 2020 Aug 1. [DOI] [PubMed] [Google Scholar]
- 107.Momen AI, Muir RT, Barnett C, Sundaram ANE. Homonymous Retinal Ganglion Cell Layer Atrophy With Asymptomatic Optic Tract Glioma in Neurofibromatosis Type I. Front Neurol [Internet] 2020. [Last cited on 2020 Jun 25]. p. 11. Available from: https://www.frontiersin.org/ articles/10.3389/fneur.2020.00256/full . [DOI] [PMC free article] [PubMed]
- 108.Chan VTT, Sun Z, Tang S, Chen LJ, Wong A, Tham CC, et al. Spectral-Domain OCT Measurements in Alzheimer's Disease: A Systematic Review and Meta-analysis. Ophthalmology. 2019;126:497–510. doi: 10.1016/j.ophtha.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.den Haan J, van de Kreeke JA, Konijnenberg E, ten Kate M, den Braber A, Barkhof F, et al. Retinal thickness as a potential biomarker in patients with amyloid-proven early- and late-onset Alzheimer's disease. Alzheimers Dement Diagn Assess Dis Monit. 2019;11:463–71. doi: 10.1016/j.dadm.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kreeke JA van de, Nguyen H-T, Haan J den, Konijnenberg E, Tomassen J, Braber A den, et al. Retinal layer thickness in preclinical Alzheimer's disease. Acta Ophthalmol (Copenh) 2019;97:798–804. doi: 10.1111/aos.14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu Y-L, Hsieh Y-T, Chen T-F, Chiou J-M, Tsai M-K, Chen J-H, et al. Retinal ganglion cell–inner plexiform layer thickness is nonlinearly associated with cognitive impairment in the community-dwelling elderly. Alzheimers Dement Diagn Assess Dis Monit. 2019;11:19–27. doi: 10.1016/j.dadm.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yoon SP, Grewal DS, Thompson AC, Polascik BW, Dunn C, Burke JR, et al. Retinal Microvascular and Neurodegenerative Changes in Alzheimer's Disease and Mild Cognitive Impairment Compared with Control Participants. Ophthalmol Retina. 2019;3:489–99. doi: 10.1016/j.oret.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mailankody P, Lenka A, Pal PK. The role of Optical Coherence Tomography in Parkinsonism: A critical review. J Neurol Sci. 2019;403:67–74. doi: 10.1016/j.jns.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 114.Inzelberg R, Ramirez JA, Nisipeanu P, Ophir A. Retinal nerve fiber layer thinning in Parkinson disease. Vision Res. 2004;44:2793–7. doi: 10.1016/j.visres.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 115.Yang Z, Wei J, Mao C, Zhang J, Chen J, Ji X, et al. Retinal nerve fiber layer thinning: a window into rapid eye movement sleep behavior disorders in Parkinson's disease. Sleep Breath. 2016;20:1285–92. doi: 10.1007/s11325-016-1366-4. [DOI] [PubMed] [Google Scholar]
- 116.Lee J-Y, Ahn J, Oh S, Shin JY, Kim YK, Nam H, et al. Retina Thickness as a Marker of Neurodegeneration in Prodromal Lewy Body Disease. Mov Disord. 2020;35:349–54. doi: 10.1002/mds.27914. [DOI] [PubMed] [Google Scholar]
- 117.Alkabie S, Lange A, Manogaran P, Stoessl AJ, Costello F, Barton JJS. Optical coherence tomography of patients with Parkinson's disease and progressive supranuclear palsy. Clin Neurol Neurosurg. 2020;189:105635. doi: 10.1016/j.clineuro.2019.105635. [DOI] [PubMed] [Google Scholar]