Abstract
Objective
Lumbar radiofrequency ablation is a commonly used intervention for chronic back pain. However, the pain typically returns, and though retreatment may be successful, the procedure involves destruction of the medial branch nerves, which denervates the multifidus. Repeated procedures typically have diminishing returns, which can lead to opioid use, surgery, or implantation of permanent neuromodulation systems. The objective of this report is to demonstrate the potential use of percutaneous peripheral nerve stimulation (PNS) as a minimally invasive, nondestructive, motor-sparing alternative to repeat radiofrequency ablation and more invasive surgical procedures.
Design
Prospective, multicenter trial.
Methods
Individuals with a return of chronic axial pain after radiofrequency ablation underwent implantation of percutaneous PNS leads targeting the medial branch nerves. Stimulation was delivered for up to 60 days, after which the leads were removed. Participants were followed up to 5 months after the start of PNS. Outcomes included pain intensity, disability, and pain interference.
Results
Highly clinically significant (≥50%) reductions in average pain intensity were reported by a majority of participants (67%, n = 10/15) after 2 months with PNS, and a majority experienced clinically significant improvements in functional outcomes, as measured by disability (87%, n = 13/15) and pain interference (80%, n = 12/15). Five months after PNS, 93% (n = 14/15) reported clinically meaningful improvement in one or more outcome measures, and a majority experienced clinically meaningful improvements in all three outcomes (i.e., pain intensity, disability, and pain interference).
Conclusions
Percutaneous PNS has the potential to shift the pain management paradigm by providing an effective, nondestructive, motor-sparing neuromodulation treatment.
Keywords: Low Back Pain, Percutaneous Peripheral Nerve Stimulation, Radiofrequency Ablation, Chronic Pain
Introduction
As the leading cause of disability worldwide, chronic low back pain (LBP) represents a significant societal challenge and economic burden for patients and health care systems [1–3]. When LBP becomes chronic, a cycle of intensified pain and disability can occur as the result of central sensitization (i.e., primarily nociceptive pain can lead to sustained changes in central pain processing in the spinal cord and supraspinal centers that take on nociplastic characteristics), which produces hypersensitivity to normal inputs as well as pain [4–11]. Disability resulting from chronic LBP is known to commonly interfere with and reduce activities of daily living (e.g., walking, housework, personal care) and decreases quality of life [12–14]. Chronic LBP can be difficult to treat with existing approaches [2, 15], and the limited efficacy and drawbacks associated with existing approaches, such as medication management, radiofrequency ablation, or surgery, highlight the need for new non-opioid, nondestructive, and nonsurgical pain management strategies.
Existing non-opioid treatments for chronic LBP include procedures such as radiofrequency ablation (RFA), open surgery, and permanently implanted neurostimulation systems. RFA may provide relief in well-selected patients, but outcomes are highly dependent on physician expertise and may be followed by a return or worsening of pain [16–18]. Collateral effects of RFA include denervation atrophy of the multifidus, a key stabilizer of the spine, and possibly other paraspinal muscles, including the erector spinae [19]. Surgical procedures for axial back pain (e.g., spinal fusion, disc replacement) may not reduce pain or disability (e.g., in up to 50% [16–18]) and regularly lead to persistent pain and disability (e.g., in up to 40% [20]), worsening pain from biomechanical alterations and violation of the spinal architecture (e.g., adjacent segment disease, muscle atrophy, in >25% [21–23]), or reoperation (in up to 32% [24–27]). Whereas permanently implanted neurostimulation leads (e.g., spinal cord stimulation) can provide clinically significant reductions in back pain, opioid use, and disability [28–37], the systems are typically used late in the care continuum in approximately 5% of candidates with primary axial pain in the United States [28, 37–39]. Limited use of permanently implanted systems is perhaps largely due to the risks of and patient aversion to such systems (especially when the implantation of leads near the spinal cord is considered), as well as the frequency of hardware complications or adverse events that may require additional medical attention, surgical revision, or explantation in a significant number of patients [40–46]. Peripheral nerve stimulation (PNS) is a promising treatment for axial back pain, but the use of conventional permanently implanted PNS systems has been limited by the invasiveness of the procedure and is technically challenging because of the lack of dedicated hardware. A percutaneous PNS system has been designed to prevent the need for invasive surgery and permanent implantation, obviating the need for frequent use of neuro-destructive procedures such as RFA that denervate key paraspinal musculature.
Multiple clinical trials have demonstrated clinically significant reductions in pain, disability, and opioid analgesic use with percutaneous PNS in patients with chronic pain conditions. Across 16 publications representing 12 studies (three randomized controlled trials, six prospective case series, and three case reports) evaluating percutaneous PNS for up to 60 days for the treatment of chronic pain, including shoulder pain, LBP, and pain after amputation, the aggregate responder rate (≥50% pain relief and/or ≥50% improvement in pain interference) with PNS was 77% (75/98), with an average of 81% reduction in pain intensity and 90% reduction in pain interference among responders [47–62]. Similar percentages of participants experienced sustained relief of pain and/or pain interference at 3 months (77%, 62/81) and 1 year (76%, 35/46). Responders at 1 year reported an average 82% reduction in pain and 87% reduction in pain interference. Percutaneous PNS offers a safe, minimally invasive, and effective non-opioid, motor-sparing treatment option for chronic pain treatment, designed to be used earlier in the care continuum than traditional neuromodulation systems (e.g., fully implanted PNS or spinal cord stimulation systems) to reduce pain and in turn reduce disability. The objective of the present report is to demonstrate the potential use of percutaneous PNS as an alternative to RFA or more invasive surgical procedures in patients with chronic LBP and a history of RFA to guide the clinical application of percutaneous PNS.
Methods
Individuals with chronic LBP were screened for enrollment in an institutional review board (IRB) (Quorum Review IRB, Seattle, Washington)–approved prospective, multicenter study (registered on ClinicalTrials.gov). All IRB approvals were granted before the study began, and institutional guidelines were followed. Written informed consent was obtained from each individual before participation. Participants were required to have a history of chronic axial LBP (i.e., pain lasting ≥12 weeks that was confined to the lumbar region and did not radiate to the lower extremities) and at least 4 weeks of stable analgesic medication use. After a physical exam to confirm eligibility and collect a back pain–related history, participants completed a written 7-day baseline diary by recording their daily average back pain scores (on a 0–10 numeric rating scale, via Question #5 of the validated Brief Pain Inventory Short Form [BPI-5]). To qualify for enrollment, participants had a baseline average pain intensity ≥4 (via BPI-5) and previously had used at least two different categories of LBP treatments (e.g., medications, physical therapy, injections). Key exclusion criteria included predominant radicular leg pain, prior lumbar surgery, lumbar anesthetic injections within 3 months of baseline, lumbar RFA within 6 months of baseline, lumbar scoliosis, pending litigation or secondary gain issues, allergy to adhesives, body mass index ≥40, or active depression (evidenced by a score >20 on the Beck Depression Inventory [BDI-II]). Aside from the listed exclusion criteria, there were no requirements for a specific etiology of back pain for enrollment, and participants with various or multiple etiologies of axial pain were potentially eligible for inclusion (e.g., spondylosis, degenerative disc disease, nonspecific back pain, etc.). Participants who had a history of RFA of the lumbar medial branches that had occurred more than 6 months prior were enrolled as part of a prospective substudy to explore the effects of percutaneous PNS of the medial branches after RFA. Follow-up data collection continues for participants in the multicenter trial who were not enrolled in the substudy (i.e., those without a history of RFA before PNS), with results to be reported in future publications.
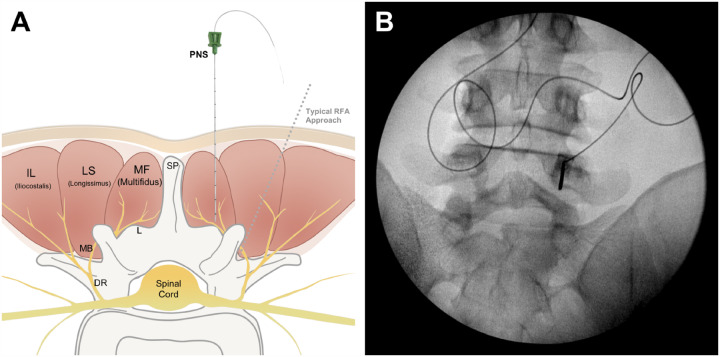
Participants underwent placement of bilateral percutaneous open-coil PNS leads (Figure 1C) with ultrasound and/or fluoroscopy, targeting the medial branch nerves at the vertebral level in the center of the painful region (Figure 2). Needle insertion was located approximately 2 cm lateral from midline at an approximately 90° angle (see Table 2), targeting the medial branch or its parent as it lies over the lamina medial and inferior to the facet joint (i.e., using a different approach from the traditional RFA approach; Figure 3). Selective activation of the lumbar multifidi, evidenced by visualization with ultrasound and the generation of comfortable sensations covering the region of pain, confirmed successful stimulation of the medial branch nerves. After confirmation of the activation of multifidi, the needle introducer was removed, leaving the percutaneous leads in the tissue. The percutaneous leads were then secured with surgical glue and a waterproof dressing and were connected to miniature wearable stimulators (SPRINT® PNS System and Microlead®, SPR Therapeutics®, Cleveland, Ohio; Figure 1A). Stimulation therapy was programmed to produce comfortable cyclical activation of the multifidus for 6–12 hours/day for up to 60 days, during which time participants were encouraged to continue their normal activities. At the end of the 2-month therapy period, leads were withdrawn by using gentle traction. Participants recorded daily pain levels and analgesic medication consumption in weekly diaries. Secondary outcomes were assessed with validated questionnaires (e.g., disability with the Oswestry Disability Index [ODI]; pain interference with the Brief Pain Inventory [BPI-9]; patient global impression of change, etc.), and adverse events were assessed up to 3 months after lead removal (5 months after start of PNS).
Figure 1.
PNS system and lead. Participants received PNS via a rechargeable, body-worn stimulator (A), controllable by each patient by using a wireless handheld remote (B), delivered to the lumbar medial branch nerves through fine-wire, open-coil leads (C).
Figure 2.
Anatomic target and lead placement approach for medial-branch PNS. PNS leads were placed bilaterally to target the medial branch of the dorsal ramus as it courses over the lamina toward the multifidus muscle, medial and inferior to the facet joint, at the spinal level in the center of the participant’s region of pain (A). An example of lead placement insertion is shown on fluoroscopic image with an anteroposterior (AP) view (B).
Table 2.
PNS lead placement details
| Participant | Time Between RFA and PNS, years | Level(s) of Previous RFA | Spinal Level of PNS Lead Placement |
Image Guidance for PNS Lead Placement |
Method Used to Confirm Multifidus Contractions | PNS Insertion Distance from Midline, cm | PNS Insertion Angle, ° from Skin Surface | PNS Lead Placement Depth, cm | % Reduction in Back Pain with PNS | Patient Global Impression of Change (PGIC) with PNS |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.7 | L3/L4, L4/L5, L5/S1 | L4 | Ultrasound | Ultrasound | 2.0 | 90 | 3.8 | 56% | 6: Much Improved |
| 2 | 3.5 | L2/L3, L3/L4, L4/L5 | L5 | Ultrasound | Ultrasound | 1.0 | 88 | 4.5 | 100% | 6: Much Improved |
| 3 | 1.5 | L3/L4, L4/L5 | L3 | Ultrasound | Ultrasound | 0.9 | 80 | 3.2 | 73% | 7: Very Much Improved |
| 4 | 1.8 | L5/S1 | L5 | Fluoroscopy | Ultrasound | 1.8 | 90 | 5.9 | 58% | 6: Much Improved |
| 5 | 1.5 | L4/L5 | L4 | Ultrasound | Ultrasound | 3.5 | 50 | 5.0 | 45% | 5: Minimally Improved |
| 6 | 0.5 | Unknown | S1 | Fluoroscopy | Ultrasound | 2.0 | 90 | 7.0 | 76% | 7: Very Much Improved |
| 7 | 0.7 | L3/L4, L4/L5 | L5 | Ultrasound | Ultrasound | 1.0 | 85 | 5.0 | 75% | 6: Much Improved |
| 8 | 1.0 | L3/L4, L4/L5, L5/S1 | L5 | Fluoroscopy | Ultrasound | 2.0 | 90 | 5.0 | 27% | 6: Much Improved |
| 9 | 0.9 | L3/L4, L4/L5 | L4 | Fluoroscopy | Ultrasound | 2.0 | 75 | 5.5 | 30% | 5: Minimally Improved |
| 10 | 1.0 | Unknown | L4 | Fluoroscopy | Ultrasound | 5.0 | 75 | 5.8 | 100% | 6: Much Improved |
| 11 | 17.0 | Unknown | L4 | Fluoroscopy | Ultrasound | 5.0 | 80 | 6.5 | 43% | 6: Much Improved |
| 12 | 0.6 | L2/L3, L3/L4, L4/L5 | L4 | Ultrasound | Ultrasound | 1.5 | 85 | 4.0 | 71% | 6: Much Improved |
| 13 | 1.0 | Unknown | S1 | Fluoroscopy | Ultrasound | 2.0 | 90 | 6.0 | 14% | 6: Much Improved |
| 14 | 0.7 | L3/L4, L4/L5, L5/S1 | L5 | Fluoroscopy | Ultrasound | 1.5 | 90 | 5.5 | 86% | 6: Much Improved |
| 15 | 0.7 | L3/L4, L4/L5, L5/S1 | L4 | Fluoroscopy | Ultrasound | 1.5 | 90 | 9.0 | 72% | 7: Very Much Improved |
|
Mean (Median) |
2.2 years (1.0 year) |
- |
L3 (7%) L4 (47%) L5 (33%) S1 (13%) |
60% Placed with Fluoroscopy, 40% Placed with Ultrasound |
100% Confirmed with Ultrasound |
2.2 cm (2.0 cm) |
83.2° (87.5°) |
5.4 cm (5.5 cm) |
62% (71%) |
6.1 (6: Much Improved) |
Figure 3.
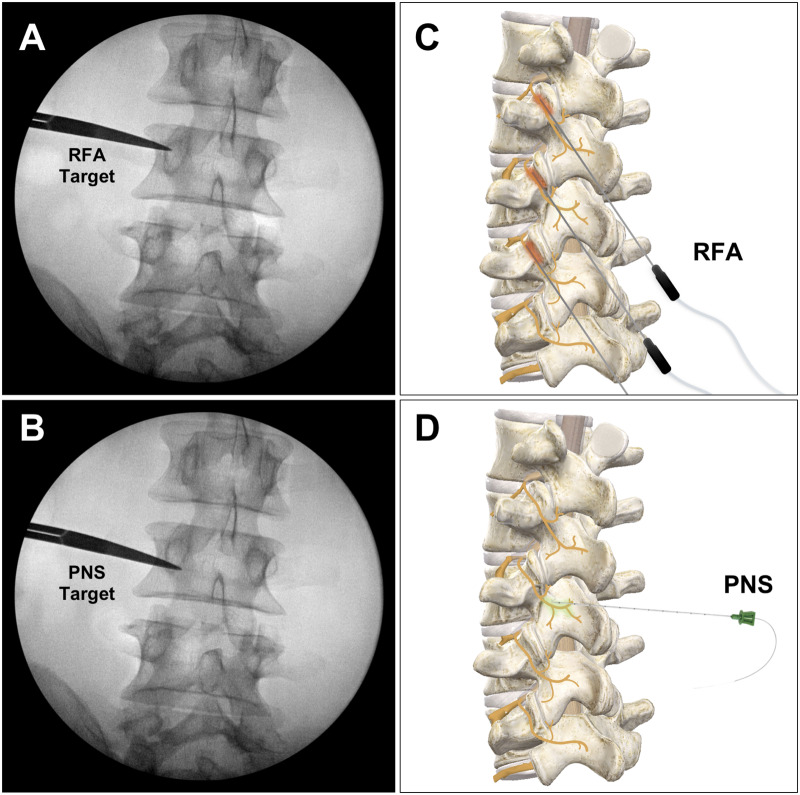
Comparison of needle insertion approach for medial-branch PNS and medial-branch RFA. Although the same nerve (medial branch of the dorsal ramus) is targeted with PNS as for RFA, compared with the traditional target for RFA probe placement at the “eye of the Scottie Dog” (A), the PNS lead is placed medial and inferior to the facet joint (B). The design of the PNS lead avoids the requirement to place the stimulating electrode in intimate contact with the nerve via a parallel placement at the facet joint (as is done with RFA) (C) and instead enables the PNS electrode to be positioned remote to the nerve (approximately 0.5–1.0 cm away), targeting the medial branch nerve as it courses over the lamina (D). Furthermore, a single PNS lead is typically placed on each side of the back at the spinal level in the center of the region of pain to provide relief of pain across the entire region, whereas RFA requires ablation at multiple vertebral levels.
Results
Seventeen participants were enrolled as part of the prospective substudy of patients with a history of RFA of the lumbar medial branch nerves; however, two participants were later found after enrollment to not meet eligibility criteria and were excluded from this analysis (one was excluded after it was found that the participant met the exclusion criteria of lumbar scoliosis, and the other was excluded because the duration of time between RFA and enrollment was less than 6 months). The 15 qualifying participants were on average 57.5 (standard deviation [SD] = 17.4) years of age, with a mean body mass index of 29.1, and had experienced chronic axial LBP for an average of 12.5 (SD = 12.8) years (Table 1). All participants had previously undergone lumbar medial branch RFA for treatment of their axial LBP a median of 1.0 year before PNS lead placement (average: 2.2 years; range: 0.5–17 years; Table 2). At baseline, participants reported an average LBP intensity of 6.3 (SD = 1.0; BPI-5, scale 0–10). The most common diagnoses or proposed etiologies for the participants’ axial LBP were lumbar spondylosis (40%, n = 6/15), degenerative disc disease (27%, n = 4/15), and nonspecific LBP (20%, n = 3/15) (Table 1). Although prior imaging was not required for enrollment, findings from previous magnetic resonance imaging were available in a majority of participants and most commonly included multilevel degenerative changes (73%, n = 8/11) and facet arthrosis (27%, n = 3/11) (Table 1). To understand which treatments could possibly be avoided or delayed by successful relief with PNS, the physician investigators were asked to indicate what LBP treatment they would have recommended for each participant if the participant were not receiving PNS as part of the clinical trial. The most commonly recommended next treatments for the participants’ back pain included spinal cord stimulation (53%, n = 8/15), repeat RFA (40%, n = 6/15), lumbar surgery (20%, n = 3/15), anesthetic injections (20%, n = 3/15), and other conservative treatments, such as physical therapy or medication management (27%, n = 4/15), as shown in Table 1.
Table 1.
Participant demographics and baseline information
| Participant | Age | Sex | BMI | LBP Duration, years | Baseline Pain (BPI-5) |
LBP Diagnosis or Proposed LBP Etiology |
MRI Findings | Treatment That Physician Recommends Next for Participant If Not Receiving PNS |
|---|---|---|---|---|---|---|---|---|
| 1 | 57.6 | F | 26.3 | 2.2 | 5.14 | Lumbosacral spondylosis |
Multilevel facet arthrosis and severe right L4–L5 foraminal stenosis |
Medication management, SCS |
| 2 | 38.6 | M | 30.7 | 6.6 | 5.14 |
Lumbar discogenic pain, facet arthropathy |
Mild facet arthropathy, disc degeneration at L5–S1 |
Repeat RFA |
| 3 | 67.0 | M | 20.3 | 1.4 | 7.43 |
Degenerative disc disease and fasciitis |
Mild multilevel degenerative disc disease, moderate multilevel facet arthropathy | SIJ injection, SCS |
| 4 | 29.4 | F | 27.0 | 3.6 | 6.14 | Degenerative disc disease |
Loss of disc height and degenerative disc disease |
Surgery, SCS |
| 5 | 82.1 | M | 25.6 | 8.8 | 5.43 | Degenerative disc disease, spinal stenosis with neurogenic claudication | Degenerative disc disease | Surgery |
| 6 | 35.3 | M | 28.8 | 9.4 | 7.14 | Bulging disc | Mild lateral bulging at L5–S1 | SIJ injection, LBB |
| 7 | 74.8 | F | 27.0 | 2.1 | 6.29 | Lumbar spondylosis | N/A | SCS |
| 8 | 53.2 | F | 30.9 | 36.1 | 7.14 | Degenerative disc disease | Degenerative lumbar disc disease and facet arthrosis | Repeat RFA, SCS |
| 9 | 79.5 | M | 31.7 | 6.3 | 7.57 | Nonspecific LBP (unknown) | Mild degenerative changes | SCS, surgery |
| 10 | 60.3 | F | 25.0 | 34.5 | 7.29 | Nonspecific LBP (unknown) | Central disc protrusion | MBB, repeat RFA |
| 11 | 77.3 | M | 37.3 | 22.6 | 5.00 | Nonspecific LBP (unknown) | N/A | Repeat RFA |
| 12 | 44.6 | F | 33.7 | 3.2 | 6.00 | Lumbar spondylosis | N/A | Physical therapy |
| 13 | 46.6 | F | 26.6 | 10.6 | 5.14 | Lumbar spondylosis | Mild disc degenerative changes and small annular tear at L5 |
Facet joint injection, physical therapy |
| 14 | 72.1 | M | 25.2 | 34.7 | 7.14 | Lumbar spondylosis | Mild to moderate foraminal narrowing | Repeat RFA, SCS |
| 15 | 44.0 | M | 39.7 | 5.8 | 7.14 | Lumbar spondylosis | N/A |
Conservative treatment, repeat RFA, SCS |
| Mean | 57.5 | - | 29.1 | 12.5 | 6.3 | - | - | - |
| SD | 17.4 | – | 5.1 | 12.8 | 1.0 | – | – | – |
BPI-5 = Brief Pain Inventory, Question 5; BMI = body mass index; F = female; LBB = lateral branch block; M = male; MBB = medial branch block; SCS = spinal cord stimulation; SIJ = sacroiliac joint; N/A = no previous MRI.
With percutaneous PNS of the lumbar medial branch nerves, the average pain intensity score was reduced from 6.3 (SD = 1.0) at baseline to 2.4 (SD = 1.6; 62% reduction; P < 0.0001 by analysis of variance [ANOVA] Tukey post hoc; Table 3) after 2 months of treatment. A majority of participants (67%, n = 10/15) experienced highly clinically meaningful (≥50%) reductions in average pain intensity (BPI-5) with PNS (Figure 4). Furthermore, 87% (n = 13/15) experienced a clinically meaningful (≥30%) reduction in average pain intensity (Figure 5) [63]. The mean reduction in average pain intensity was 77% among responders experiencing ≥50% reduction in pain (Figure 4). Participants also experienced clinically and statistically significant improvements in functional outcomes as measured by disability (ODI, P = 0.0002, mean 21-point reduction) and pain interference (BPI-9, P < 0.0001, mean 61% reduction) (Table 3). Clinically meaningful reductions in disability (≥10-point reduction on ODI) were reported by 87% of participants (n = 13/15), and clinically significant reductions (≥30% reduction of BPI-9 score) in pain interference were reported by 80% (n = 12/15) [63, 64]. Participants rated their quality of life as “much improved” on average with percutaneous PNS (patient global impression of change; Table 2).
Table 3.
Statistically significant reductions in pain intensity, disability, and pain interference
| Timepoint | Mean (SD) |
P Value (1-Way ANOVA) |
P Value (Tukey Post hoc) |
|
|---|---|---|---|---|
| Pain Intensity (BPI-5) | Baseline | 6.3 (1.0) | <.0001 | - |
| 2 Months | 2.4 (1.6) | <0.0001 | ||
| 5 Months | 3.1 (1.9) | <0.0001 | ||
|
Disability (ODI) |
Baseline | 43.1 (12.7) | 0.0002 | – |
| 2 Months | 21.8 (13.9) | 0.0002 | ||
| 5 Months | 26.1 (13.2) | 0.003 | ||
|
Pain Interference (BPI-9) |
Baseline | 6.2 (1.8) | <0.0001 | – |
| 2 Months | 2.4 (2.1) | <0.0001 | ||
| 5 Months | 3.2 (2.7) | 0.0016 |
Figure 4.
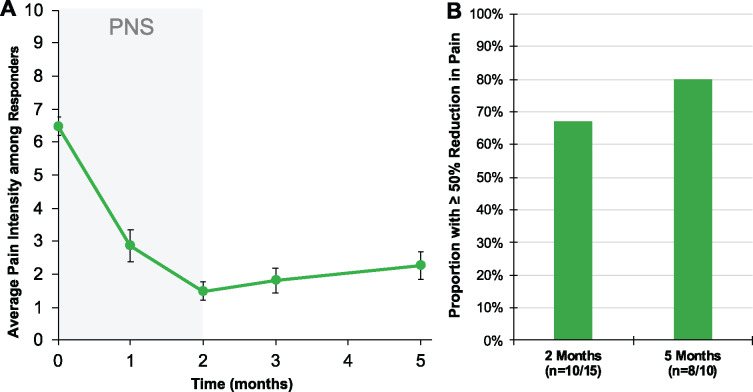
Sustained reductions in average pain intensity among responders. (A) Time course of pain relief among participants who experienced ≥50% reduction in pain intensity after 2 months of PNS (n = 10/15). Participants experienced sustained reductions in average pain intensity at 5 months after start of treatment (3 months after lead removal). (B) Proportion of participants responding with ≥50% reduction in pain intensity with PNS after 2 months of PNS (67%, n = 10/15) and the proportion of responders who experienced sustained highly clinically significant reductions in pain intensity at 5 months (80%, n = 8/10).
Figure 5.
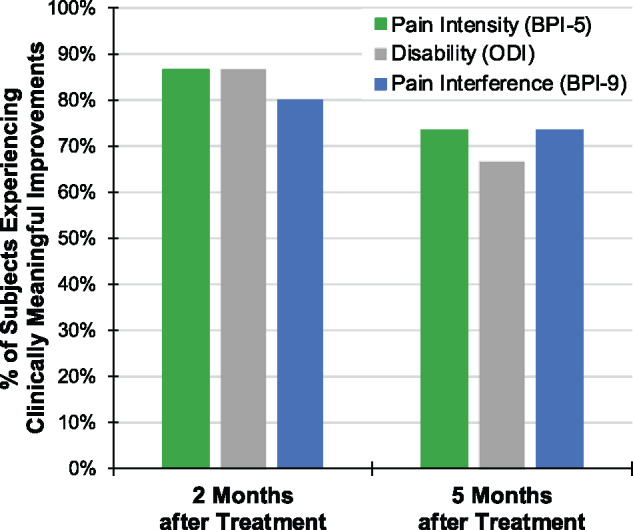
Proportion of participants with clinically meaningful improvements with PNS. A majority of participants (n = 15) experienced clinically significant improvements in pain intensity (BPI-5), disability (ODI), and/or pain interference (BPI-9). After 2 months of PNS, 87% of participants experienced ≥30% reduction in average pain intensity, 87% experienced ≥10-point reduction in disability, and 80% experienced ≥30% reduction in pain interference. Five months after the start of PNS treatment (3 months after PNS lead removal), the results were sustained across all three clinical outcomes.
Five months after the start of PNS (i.e., 3 months after PNS lead removal), participants experienced sustained clinically and statistically significant reductions in average pain intensity, disability, and pain interference (Table 3). Eighty percent of responders (80%, n = 8/10) had sustained highly clinically meaningful (≥50%) reductions in pain 5 months after the start of treatment (average 71% reduction). Seventy-three percent (73%, n = 11/15) of all participants experienced clinically significant reductions in pain intensity at 5 months after the start of treatment (Figure 4). Improvements in functional outcomes measured by disability and pain interference were also sustained in the long term, as 67% of participants reported clinically significant improvements in disability and 73% of participants reported clinically significant improvements in pain interference (Figure 5). All but one participant, i.e., 93% (n = 14/15), reported clinically significant improvements in at least one outcome measure (i.e., pain intensity, disability, or pain interference) at both 2 and 5 months after PNS (Table 4), and a majority of participants experienced improvements in all three outcome measures at both 2 and 5 months after PNS, respectively (Table 4). Clinically meaningful improvements in at least two outcome measures were experienced by 87% (n = 13/15) and 73% (n = 11/15) of participants at 2 and 5 months after PNS, respectively.
Table 4.
Proportion of participants experiencing clinically significant improvements with percutaneous PNS
| Proportion Experiencing Clinically Significant Improvements in Pain, Disability, and/or Pain Interference | 2 Months | 5 Months |
|---|---|---|
| Success in at least one outcome | 93% (14/15) | 93% (14/15) |
| Success in at least two outcomes | 87% (13/15) | 73% (11/15) |
| Success in all three outcomes | 73% (11/15) | 53% (8/15) |
There were no serious or unanticipated adverse events. The most common adverse events were mild skin irritation or itching at the site of the waterproof dressings or stimulator’s hydrogel mounting pad. One participant experienced a superficial skin infection at one lead exit site that was resolved by removal of the lead in the week before the end of treatment and by use of an oral antibiotic. Four participants experienced lead migration or dislodgement and received lead replacements per protocol (e.g., because of lead dislodgement during bandage change).
Discussion
This report demonstrates the effectiveness of percutaneous PNS of the medial branch nerves for the treatment of chronic LBP to provide clinically significant and sustained reductions in pain and improvements in disability and quality of life for patients who experienced a return of pain or failed to derive relief after lumbar RFA. Although lumbar RFA is commonly used for LBP, a recent consensus statement asserted that paraspinal muscle degeneration is a risk of RFA [65], and others have demonstrated changes (e.g., muscle atrophy, increased fatty infiltration, degenerative changes) [19, 66] after RFA that may cause recurrent LBP. This opens the door for the use of a nondestructive, motor-sparing approach like percutaneous PNS [65, 67, 68].
Percutaneous PNS for Axial Back Pain
The results described here for participants with a history of lumbar RFA of the medial branch nerves demonstrate that percutaneous PNS, when applied at least 6 months after RFA, provides clinically meaningful improvements in pain, disability, and pain interference. After two months of PNS, all but one participant, i.e., 93% (n = 14/15), reported clinically significant improvements in at least one outcome measure (pain, disability, or pain interference), and 87% (n = 13/15) reported clinically significant improvements in at least two. A majority of participants (n = 8/15; Table 4) experienced clinically significant reductions improvements in all three outcomes (pain, disability, and pain interference) that were sustained for 5 months (3 months after PNS lead removal), demonstrating the broad, sustained impact of percutaneous PNS on clinical outcomes.
Percutaneous PNS, with its innovative fine-wire, open-coil lead design and short-term treatment, was designed to be a safe, effective, non-opioid, neurostimulation option for patients earlier on the treatment continuum. There were no serious or unanticipated adverse events. The most common adverse events were mild skin irritation or itching. In one participant, a superficial skin infection at one lead exit site resolved after removal of the lead after 7 weeks of stimulation. Although the reported infection was not confirmed by culture, this is the first apparent infection reported to date across the literature during the use of percutaneous PNS leads [47–62]. Overall, an analysis of literature demonstrated that the percutaneous PNS leads with a coiled design have a statistically significantly lower risk of infection (i.e., approximately 1 infection for every 30,000 indwelling days) than that observed with noncoiled neurostimulation leads (i.e., 1 infection for every 1,200 indwelling days; P = 0.006) [69].
For the few participants who did not experience sustained clinically significant improvements with percutaneous PNS, a review of the LBP characteristics and PNS lead placement details may provide some clarity underpinning those outcomes. A history of spinal stenosis with neurogenic claudication likely affected the potential for success with percutaneous PNS in Subject 5, as leg pain outside of the axial low back stemming from stenosis is unlikely to respond to stimulation of the medial branches, suggesting that future studies should be adjusted to exclude participants with a history of this nature. Subject 13 experienced minimal improvement with PNS (14%), possibly because of the presence of pain in the sacral region and/or lead placement at the S1 vertebral level (i.e., the innervation and size of the multifidus at S1 is different from at the lumbar levels, which could have made effective lead placement and treatment more difficult). Two participants were found after enrollment to not meet inclusion/exclusion criteria and were excluded from analysis. One was excluded because of failure to meet the eligibility criterion of an RFA done at least 6 months before enrollment (i.e., RFA occurred 4 months before PNS). This patient failed to experience benefit with PNS, suggesting that sufficient nerve regeneration may be necessary to enable successful PNS. Notably, the other participant, who was excluded from analysis because of lumbar scoliosis, did successfully experience long-term clinically significant relief with PNS (83% reduction in pain at 5 months), suggesting that mild scoliosis may not impede improvement with PNS. However, idiopathic scoliosis may predispose patients to facet arthropathy, and degenerative lumbar scoliosis may be a consequence [70].
Description of PNS Approach in Comparison with Traditional RFA Approach
Optimizing RFA outcomes requires that the probe and electrode tip be positioned in intimate contact with the target nerves in a near-parallel approach, with a large probe size and extended lesioning time to increase the likelihood of successful denervation [65, 71–73]. The percutaneous PNS lead was designed to enable remote stimulation of target nerves, and previous studies have demonstrated successful activation of peripheral nerves at distances of up to 3.0 cm away from the electrode for relief of chronic pain [74].
Because of the wide area of activation in which peripheral nerves can be stimulated in relation to this particular percutaneous PNS lead, a new approach to target the medial branch nerves was used in the present study to avoid lead placement in areas where stimulation could activate off-target structures (e.g., structures that could be activated by PNS but are outside the range of the typical RFA lesioned area, such as the lateral branch of the dorsal ramus, spinal nerve, or erector spinae). Furthermore, because this percutaneous PNS lead does not need to be placed in intimate contact with the medial branches as in RFA, insertion of the PNS lead to target the medial branch nerves as they course over lamina, medial and inferior to the facet joint, enables selective activation of the medial branch nerves (see PNS lead placement details in Table 2). The key bony anatomic landmarks used for this approach are the lamina and spinous process, enabling placement with a more straightforward approach by using either ultrasound (e.g., using a transverse probe position with out-of-plane needle insertion) or fluoroscopy with an anteroposterior view (i.e., no need to use an oblique view or target the nerve at the eye of Scottie dog with a near-parallel nerve approach; Figure 3). To supplement patient-reported sensations and muscle contractions, ultrasound was used to confirm that the new approach resulted in selective activation of the multifidus for all participants because it has been previously reported that visualization of skin movement cannot be used to distinguish activation of multifidi from the erector spinae [75, 76].
Another key difference in the PNS approach is the number of joints or spinal levels targeted to produce pain relief. Polysegmental innervation of the multifidus and other paraspinal muscles has previously been described [77, 78], and RFA, which seeks to block transmission of sensory signals from the region of pain, typically requires insertion of probes and ablation at multiple levels to be effective (e.g., L2/L3, L3/L4, L4/L5). With percutaneous PNS, placement of a single lead at the spinal level within the center of the region of pain generates comfortable multifidus activation and sensations that are mediated by the medial branch nerves across the entire region of pain. With percutaneous PNS, one lead placed on each side of the back at the spinal level in the center of the region of pain (e.g., L3; Figure 3) may be used to activate the multifidus, spanning multiple spinal levels in order to generate focal and robust stimulation and proprioceptive signaling, which may be crucial to providing sustained pain relief.
Mechanism of Action
Whereas RFA seeks to treat chronic back pain by destruction and denervation of the nerve fibers carrying painful signals from the facet joints and paraspinal musculature, PNS is theorized to treat chronic pain via a neuromodulatory mechanism. Ablative lesions are thought to interrupt transmission of nociceptive (A-delta and C-fiber) inputs to the central nervous system through heating of the nerve and tissue adjacent to the radiofrequency probe [71, 72, 79]. After lesioning, tissue coagulation and acute inflammatory responses typically occur, along with iatrogenic neural degeneration, necrosis, and scar formation [79, 80]. RFA has also been shown to disrupt axonal continuity and degenerate nerve fibers distal to the lesion (Wallerian degeneration) [79]. However, as re-innervation or regeneration occurs, this can result in a return of pain or the formation of abnormal reconnections, leading to a need for repeat RFA or other pain treatments.
Percutaneous PNS is designed to be a minimally invasive, nondestructive treatment for chronic pain that uses electrical stimulation of nerve fibers to modulate central sensitization. Electrical stimulation applied via percutaneous PNS activates neural signals in the medial branch nerves that innervate the multifidus across the region of axial back pain [81]. Stimulation of afferent, sensory fibers directly engages the gate mechanism to decrease pain signals, while stimulation of efferent nerve fibers simultaneously activates muscles (i.e., the multifidi) and indirectly generates physiological, proprioceptive afferent signals that are known to occur with muscle activity [82, 83]. Conventional stimulation therapies for chronic pain have historically sought to avoid efferent fiber activation [84–87], and percutaneous PNS of the medial branch nerves notably contrasts with these prior approaches by intentionally targeting both sensory and motor fibers. Together, the resulting afferent signals may modulate synaptic transmission via gate control [88–91] and result in the normalization of membrane excitability of neurons and circuits in nociceptive pathways in the central nervous system. PNS may further disrupt the cycle of centrally maintained pain by decreasing pain and abnormal processing for an extended period of time, thus permitting greater levels of physical activity and engaging activity-dependent neuroplasticity (i.e., reversal of sensitization) [92–96], which may underpin the sustained reductions in pain that persist after the removal of percutaneous PNS leads.
Clinical Takeaways
Percutaneous PNS offers the potential to be an effective clinical alternative for the treatment of chronic axial back pain in patients in whom at least 6 months have passed since RFA. Percutaneous PNS offers a nondestructive, nonsurgical, non-opioid, motor-sparing treatment for axial back pain that is capable of providing durable relief. Percutaneous PNS may also be considered for patients with axial back pain who have failed medial branch blocks, wherein RFA is not indicated and the next treatment option is often unclear. Percutaneous PNS has the potential to shift the pain management paradigm by providing an effective neuromodulation treatment earlier on the care continuum than has previously been considered.
Conclusion
This work explores the use of percutaneous PNS in participants with prior RFA of the lumbar medial branch nerves, as an alternative to repeat RFA or other therapies for the treatment of chronic LBP. Clinically significant reductions in pain, disability, and pain interference were reported with percutaneous PNS among participants with chronic axial LBP after lumbar RFA, although additional studies are needed to further explore the comparative efficacy of RFA and percutaneous PNS. Percutaneous PNS has the potential to shift the pain management paradigm by providing an effective, nondestructive, motor-sparing neuromodulation treatment to patients earlier on the care continuum than has previously been considered.
Acknowledgments
The authors thank Lauren Easley, Rosemary Zang, Sara Weigel, and Kristin Murray for their significant contributions to the design and conduct of the clinical trial.
Authorship Statement
All authors have made substantial contribution to the conception and design, conduct, and/or analysis and interpretation of the study. All authors contributed to reviewing and revising the draft manuscript and approved the final version to be published.
Funding: These results were collected as part of an ongoing Institutional Review Board–approved study sponsored by SPR Therapeutics.
Conflicts of interest: Drs. Deer, Gilmore, Desai, Li, DePalma, Hopkins, and Burgher are investigators with research funded by SPR Therapeutics. Drs. Deer, Gilmore, Desai, Spinner, and Cohen are consultants to SPR. Drs. Deer and Desai have equity ownership in SPR. Drs. McGee and Boggs are employees of SPR with equity ownership and inventors on patents relating to the peripheral nerve stimulation technology.
Trial registration: ClinicalTrials.gov Id: NCT03179202.
References
- 1. Spears CA, Hodges SE, Kiyani M, et al. Healthcare resource utilization and management of chronic, refractory low back pain in the United States. Spine 2020;45(20):E1333–E1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cohen SP, Argoff CE, Carragee EJJB. Management of low back pain. 2008;337(dec22 1):a2718. [DOI] [PubMed] [Google Scholar]
- 3. Gore M, Sadosky A, Stacey BR, Tai K-S, Leslie D. The burden of chronic low back pain: Clinical comorbidities, treatment patterns, and health care costs in usual care settings . Spine 2012;37(11):E668–E77. [DOI] [PubMed] [Google Scholar]
- 4. Nijs J, Van Houdenhove B, Oostendorp RAB. Recognition of central sensitization in patients with musculoskeletal pain: Application of pain neurophysiology in manual therapy practice. Man Ther 2010;15(2):135–41. [DOI] [PubMed] [Google Scholar]
- 5. Roussel NA, Nijs J, Meeus M, et al. Central sensitization and altered central pain processing in chronic low back pain: Fact or myth? Clin J Pain 2013;29(7):625–38. [DOI] [PubMed] [Google Scholar]
- 6. Casey Greenberg YC, Nicassio MA, Harpin RE,, Hubbard D. Transition from acute to chronic pain and disability: A model including cognitive, affective, and trauma factors. Pain 2008;134(1–2):69–79. [DOI] [PubMed] [Google Scholar]
- 7. Giesecke T, Gracely RH, Grant MA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum 2004;50(2):613–23. [DOI] [PubMed] [Google Scholar]
- 8. Kregel J, Meeus M, Malfliet A, et al. Structural and functional brain abnormalities in chronic low back pain: A systematic review. Semin Arthritis Rheum 2015;45(2):229–37. [DOI] [PubMed] [Google Scholar]
- 9. Nijs J, Apeldoorn A, Hallegraeff H, et al. Low back pain: Guidelines for the clinical classification of predominant neuropathic, nociceptive, or central sensitization pain. Pain Physician 2015;18(3):E333–46. [PubMed] [Google Scholar]
- 10. Deyo RA, Dworkin SF, Amtmann D, et al. Report of the NIH Task Force on Research Standards for Chronic Low Back Pain. Phys Ther 2015;95(2):e1–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Staats P, Deer T, Desai M, et al. Toward a practical definition of chronic intractable pain. 2021: Manuscript submitted for publication.
- 12. Fairbank JC, Pynsent PB. The Oswestry disability index. Spine 2000;25(22):2940–52. [DOI] [PubMed] [Google Scholar]
- 13. Bentsen SB, Hanestad BR, Rustoen T, Wahl AK. Quality of life in chronic low back pain patients treated with instrumented fusion. J Clin Nurs 2008;17(15):2061–9. [DOI] [PubMed] [Google Scholar]
- 14. Anderson VC, Carlson C, Shatin D. Outcomes of spinal cord stimulation: Patient validation. Neuromodulation 2001;4(1):11–7. [DOI] [PubMed] [Google Scholar]
- 15. Maher C, Underwood M, Buchbinder RJTL. Non-specific low back pain. 2017;389(10070):736–47. [DOI] [PubMed] [Google Scholar]
- 16. Chotai S, Parker SL, Sivaganesan A, et al. Effect of complications within 90 days on patient-reported outcomes 3 months and 12 months following elective surgery for lumbar degenerative disease. Neurosurgl Focus 2015;39(6):E8. [DOI] [PubMed] [Google Scholar]
- 17. Parker SL, Mendenhall SK, Shau DN, et al. Minimally invasive versus open transforaminal lumbar interbody fusion for degenerative spondylolisthesis: Comparative effectiveness and cost-utility analysis. World Neurosurg 2014;82(1–2):230–8. [DOI] [PubMed] [Google Scholar]
- 18. Abbott AD, Tyni-Lenné R, Hedlund R. Early rehabilitation targeting cognition, behavior, and motor function after lumbar fusion: A randomized controlled trial. Spine 2010;35(8):848–57. [DOI] [PubMed] [Google Scholar]
- 19. Bonython M, Nottage T, Xu L, et al. Magnetic resonance imaging morphology of lumbar paraspinal muscles following successful bilateral facet joint denervation. European Congress of Radiology/New Zealand College of Radiologists-2019 ASM. Auckland, New Zealand; October 17–20, 2019.
- 20. Shapiro CM. The failed back surgery syndrome: Pitfalls surrounding evaluation and treatment. Phys Med Rehabil Clin N Am 2014;25(2):319–40. [DOI] [PubMed] [Google Scholar]
- 21. Okuda S, Yamashita T, Matsumoto T, et al. Adjacent segment disease after posterior lumbar interbody fusion: A case series of 1000 patients. Glob Spine J 2018;8(7):722–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ekman P, Möller H, Shalabi A, Yu YX, Hedlund R. A prospective randomised study on the long-term effect of lumbar fusion on adjacent disc degeneration. Eur Spin J 2009;18(8):1175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Keller A, Brox JI, Gunderson R, et al. Trunk muscle strength, cross-sectional area, and density in patients with chronic low back pain randomized to lumbar fusion or cognitive intervention and exercises. Spine (Phila Pa 1976) 2004;29(1):3–8. [DOI] [PubMed] [Google Scholar]
- 24. Fritzell P, Hagg O, Wessberg P, Nordwall A. Volvo Award Winner in Clinical Studies: Lumbar fusion versus nonsurgical treatment for chronic low back pain: A multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine (Phila Pa 1976) 2001;26(23):2521–32. 2001; discussion 32–4. [DOI] [PubMed] [Google Scholar]
- 25. Martin BI, Mirza SK, Comstock BA, et al. Reoperation rates following lumbar spine surgery and the influence of spinal fusion procedures. Spine (Phila Pa 1976) 2007;32(3):382–7. [DOI] [PubMed] [Google Scholar]
- 26. Cloyd JM, Acosta FL Jr, Ames CP. Complications and outcomes of lumbar spine surgery in elderly people: A review of the literature. J Am Geriatr Soc 2008;56(7):1318–27. [DOI] [PubMed] [Google Scholar]
- 27. Brox JI, Reikerås O, Nygaard Ø, et al. Lumbar instrumented fusion compared with cognitive intervention and exercises in patients with chronic back pain after previous surgery for disc herniation: A prospective randomized controlled study. Pain 2006;122(1–2):145–55. [DOI] [PubMed] [Google Scholar]
- 28. Sharan A, Riley J, Falowski S, et al. Association of opioid usage with spinal cord stimulation outcomes. North American Neuromodulation Society 20th Annual Meeting. Las Vegas, NV, USA; January 23–26, 2020.
- 29. Rainville J, Hartigan C, Martinez E, et al. Exercise as a treatment for chronic low back pain. Spine J 2004;4(1):106–15. [DOI] [PubMed] [Google Scholar]
- 30. van der Velde G, Mierau D. The effect of exercise on percentile rank aerobic capacity, pain, and self-rated disability in patients with chronic low-back pain: A retrospective chart review. Arch Phys Med Rehabil 2000;81(11):1457–63. [DOI] [PubMed] [Google Scholar]
- 31. Rainville J, Ahern DK, Phalen L, Childs LA, Sutherland R. The association of pain with physical activities in chronic low back pain. Spine (Phila Pa 1976) 1992;17(9):1060–4. [DOI] [PubMed] [Google Scholar]
- 32. Mayer TG, Gatchel RJ, Mayer H, et al. A prospective two-year study of functional restoration in industrial low back injury. An objective assessment procedure. JAMA 1987;258(13):1763–7. [PubMed] [Google Scholar]
- 33. Hazard RG, Fenwick JW, Kalisch SM, et al. Functional restoration with behavioral support. A one-year prospective study of patients with chronic low-back pain. Spine (Phila Pa 1976) 1989;14(2):157–61. [PubMed] [Google Scholar]
- 34. Wittink H, Rogers W, Gascon C, et al. Relative contribution of mental health and exercise-related pain increment to treadmill test intolerance in patients with chronic low back pain. Spine (Phila Pa 1976) 2001;26(21):2368–74. [DOI] [PubMed] [Google Scholar]
- 35. Edwards BC, Zusman M, Hardcastle P, et al. A physical approach to the rehabilitation of patients disabled by chronic low back pain. Med J Aust 1992;156(3):167–72. [DOI] [PubMed] [Google Scholar]
- 36. Frost H, Moffett JAK, Moser JS, Fairbank JCT. Randomised controlled trial for evaluation of fitness programme for patients with chronic low back pain. BMJ 1995;310(6973):151–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barolat G, Oakley JC, Law JD, et al. Epidural spinal cord stimulation with a multiple electrode paddle lead is effective in treating intractable low back pain. Neuromodulation 2001;4(2):59–66. [DOI] [PubMed] [Google Scholar]
- 38. Vakkala M, Järvimäki V, Kautiainen H, Haanpää M, Alahuhta S. Incidence and predictive factors of spinal cord stimulation treatment after lumbar spine surgery. J Pain Res 2017;10:2405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oakley JC. Spinal cord stimulation in axial low back pain: solving the dilemma. Pain Med 2006;7(suppl 1):S58–S63. [Google Scholar]
- 40. Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: A 20-year literature review. J Neurosurg 2004;100(3):254–67. [DOI] [PubMed] [Google Scholar]
- 41. Turner JA, Hollingworth W, Comstock BA, Deyo RA. Spinal cord stimulation for failed back surgery syndrome: Outcomes in a workers’ compensation setting. Pain 2010;148(1):14–25. [DOI] [PubMed] [Google Scholar]
- 42. Turner JA, Loeser JD, Deyo RA, Sanders SB. Spinal cord stimulation for patients with failed back surgery syndrome or complex regional pain syndrome: A systematic review of effectiveness and complications. Pain 2004;108(1–2):137–47. [DOI] [PubMed] [Google Scholar]
- 43. Mekhail NA, Mathews M, Nageeb F, et al. Retrospective review of 707 cases of spinal cord stimulation: Indications and complications. Pain Pract 2011;11(2):148–53. [DOI] [PubMed] [Google Scholar]
- 44. Rosenow JM, Stanton-Hicks M, Rezai AR, Henderson JM. Failure modes of spinal cord stimulation hardware. J Neurosurg 2006;5(3):183–90. [DOI] [PubMed] [Google Scholar]
- 45. Eldabe S, Buchser E, Duarte RV. Complications of spinal cord stimulation and peripheral nerve stimulation techniques: A review of the literature. Pain Med 2015;17(2):325–36. [DOI] [PubMed] [Google Scholar]
- 46.Gulati A, Deer T, Ottestad E, , , et al. Patient Preferences Among Various Interventional Pain Management Treatment Options. 2021 North American Neuromodulation Society Annual Meeting 2021. January 15–16, 2021 (Virtual).
- 47. Yu DT, Chae J, Walker ME, Fang ZP. Percutaneous intramuscular neuromuscular electric stimulation for the treatment of shoulder subluxation and pain in patients with chronic hemiplegia: A pilot study. Arch Phys Med Rehabil 2001;82(1):20–5. [DOI] [PubMed] [Google Scholar]
- 48. Yu DT, Chae J, Walker ME, et al. Intramuscular neuromuscular electric stimulation for poststroke shoulder pain: A multicenter randomized clinical trial. Arch Phys Med Rehabil 2004;85(5):695–704. [DOI] [PubMed] [Google Scholar]
- 49. Chae J, Yu DT, Walker ME, et al. Intramuscular electrical stimulation for hemiplegic shoulder pain. Am J Phys Med Rehabil 2005;84(11):832–42. [DOI] [PubMed] [Google Scholar]
- 50. Wilson RD, Bennett ME, Lechman TE, Stager KW, Chae J. Single-lead percutaneous peripheral nerve stimulation for the treatment of hemiplegic shoulder pain: A case report. Arch Phys Med Rehabil 2011;92(5):837–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chae J, Wilson RD, Bennett ME, Lechman TE, Stager KW. Single-lead percutaneous peripheral nerve stimulation for the treatment of hemiplegic shoulder pain: A case series. Pain Pract 2013;13(1):59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wilson RD, Gunzler DD, Bennett ME, Chae J. Peripheral nerve stimulation compared with usual care for pain relief of hemiplegic shoulder pain: A randomized controlled trial. Am J Phys Med Rehabil 2014;93(1):17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wilson RD, Harris MA, Bennett ME, Chae J. Single-lead percutaneous peripheral nerve stimulation for the treatment of shoulder pain from subacromial impingement syndrome. PM & R 2012;4(8):624–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wilson RD, Harris MA, Gunzler DD, Bennett ME, Chae J. Percutaneous peripheral nerve stimulation for chronic pain in subacromial impingement syndrome: A case series. Neuromodulation 2014;17(8):771–6; discussion 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rauck RL, Kapural L, Cohen SP, et al. Peripheral nerve stimulation for the treatment of postamputation pain-A case report. Pain Pract 2012;12(8):649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rauck RL, Cohen SP, Gilmore CA, et al. Treatment of post-amputation pain with peripheral nerve stimulation. Neuromodulation 2014;17(2):188–97. [DOI] [PubMed] [Google Scholar]
- 57. Cohen SP, Gilmore CA, Rauck RL, et al. Percutaneous peripheral nerve stimulation for the treatment of chronic pain following amputation. Mil Med 2019;184(7–8):e267–e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gilmore CA, Ilfeld BM, Rosenow JM, et al. Percutaneous 60-day peripheral nerve stimulation implant provides sustained relief of chronic pain following amputation: 12-month follow-up of a randomized, double-blind, placebo-controlled trial. Reg Anesth Pain Med 2020;45(1):44–51. [DOI] [PubMed] [Google Scholar]
- 59. Kapural L, Gilmore CA, Chae J, et al. Percutaneous peripheral nerve stimulation for the treatment of chronic low back pain: Two clinical case reports of sustained pain relief. Pain Pract 2018;18(1):94–103. [DOI] [PubMed] [Google Scholar]
- 60. Gilmore CA, Kapural L, McGee MJ, Boggs JW. percutaneous peripheral nerve stimulation (PNS) for the treatment of chronic low back pain provides sustained relief. Neuromodulation 2019;22(5):615–20. [DOI] [PubMed] [Google Scholar]
- 61. Gilmore CA, Kapural L, McGee MJ, Boggs J. Percutaneous peripheral nerve stimulation for chronic low back pain: Prospective case series with one year of sustained relief following short‐term implant. 2020;20(3):310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cohen S, Gilmore C, Kapural L, et al. Percutaneous peripheral nerve stimulation for pain reduction and improvements in functional outcomes in chronic low back pain. Mil Med 2019;184(Supplement_1):537–41. [DOI] [PubMed] [Google Scholar]
- 63. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008;9(2):105–21. [DOI] [PubMed] [Google Scholar]
- 64. Ostelo RW, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain: Towards international consensus regarding minimal important change. Spine 2008;33(1):90–4. [DOI] [PubMed] [Google Scholar]
- 65. Cohen SP, Bhaskar A, Bhatia A, et al. Consensus practice guidelines on interventions for lumbar facet joint pain from a multispecialty, international working group. Reg Anesth Pain Med 2020;45(6):424–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Smuck M, Crisostomo RA, Demirjian R, et al. Morphologic changes in the lumbar spine after lumbar medial branch radiofrequency neurotomy: A quantitative radiological study. Spine J 2015;15(6):1415–21. [DOI] [PubMed] [Google Scholar]
- 67. Kanchiku T, Imajo Y, Suzuki H, et al. Percutaneous radiofrequency facet joint denervation with monitoring of compound muscle action potential of the multifidus muscle group for treating chronic low back pain: A preliminary report. J Spinal Disord Tech 2014;27(7):E262–E7. [DOI] [PubMed] [Google Scholar]
- 68. Sadeghi S, Bible JE, Cortes DH. Quantifying Dysfunction of the Lumbar Multifidus Muscle After Radiofrequency Neurotomy and Fusion Surgery: A Preliminary Study. J Eng Sci Med Diagn Ther 2020;3(4) (doi: 10.1115/1.4047651). [DOI] [PMC free article] [PubMed]
- 69. Ilfeld BM, Gabriel RA, Saulino MF, et al. Infection rates of electrical leads used for percutaneous neurostimulation of the peripheral nervous system. Pain Pract 2017;17(6):753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kotwal S, Pumberger M, Hughes A, Girardi FJHJ. Degenerative scoliosis: A review. HSS J 2011;7(3):257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Goldberg SN, Gazelle GS, Dawson SL, et al. Tissue ablation with radiofrequency: Effect of probe size, gauge, duration, and temperature on lesion. Acad Radiol 1995;2(5):399–404. [DOI] [PubMed] [Google Scholar]
- 72. Kapural L, Mekhail N. Radiofrequency ablation for chronic pain control. Curr Pain Headache Rep 2001;5(6):517–25. [DOI] [PubMed] [Google Scholar]
- 73. Cosman ER Jr, Dolensky JR, Hoffman RA. Factors that affect radiofrequency heat lesion size. Pain Med 2014;15(12):2020–36. [DOI] [PubMed] [Google Scholar]
- 74. Gilmore CA, Ilfeld BM, Rosenow JM, et al. Percutaneous peripheral nerve stimulation for the treatment of chronic neuropathic post-amputation pain: a multi-center randomized placebo-controlled trial. Reg Anesth Pain Med 2019;44(6):637–45. [DOI] [PubMed] [Google Scholar]
- 75. Baek SO, Ahn SH, Jones R, et al. Activations of deep lumbar stabilizing muscles by transcutaneous neuromuscular electrical stimulation of lumbar paraspinal regions. Ann Rehabil Med 2014;38(4):506–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Koh JC, Kim DH, Lee YW, et al. Relationship between paravertebral muscle twitching and long-term effects of radiofrequency medial branch neurotomy. Korean J Pain 2017;30(4):296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Macintosh JE, Valencia F, Bogduk N, Munro R. The morphology of the human lumbar multifidus. Clin Biomech (Bristol, Avon) 1986;1(4):196–204. [DOI] [PubMed] [Google Scholar]
- 78. Kottlors M, Glocker F. Polysegmental innervation of the medial paraspinal lumbar muscles. Eur Spine J 2008;17(2):300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Choi EJ, Choi YM, Jang EJ, et al. Neural ablation and regeneration in pain practice. Korean J Pain 2016;29(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kapural L, Mekhail NJCP. adiofrequency ablation for chronic pain control. Reports H. R 2001;5(6):517–25. [DOI] [PubMed] [Google Scholar]
- 81. Kapural L, Gilmore C, Chae J, et al. Percutaneous peripheral nerve stimulation for the treatment of chronic low back pain: Two clinical case reports of sustained pain relief. Pain Practice 2018;18(1):94–103. [DOI] [PubMed] [Google Scholar]
- 82. Proske U. What is the role of muscle receptors in proprioception? Muscle Nerve 2005;31(6):780–7. [DOI] [PubMed] [Google Scholar]
- 83. Windhorst U. Muscle proprioceptive feedback and spinal networks. Brain Res Bull 2007;73(4-6):155–202. [DOI] [PubMed] [Google Scholar]
- 84. Huntoon MA, Burgher AH. Review of ultrasound-guided peripheral nerve stimulation. Tech Reg Anesth Pain Manag 2009;13(3):121–7. [Google Scholar]
- 85. Law JD, Swett J, Kirsch WM. Retrospective analysis of 22 patients with chronic pain treated by peripheral nerve stimulation. J Neurosurg 1980;52(4):482–5. [DOI] [PubMed] [Google Scholar]
- 86. Molnar G, Barolat G. Principles of cord activation during spinal cord stimulation. Neuromodulation 2014;17(Suppl 1):12–21. [DOI] [PubMed] [Google Scholar]
- 87. Novak CB, Mackinnon SE. Outcome following implantation of a peripheral nerve stimulator in patients with chronic nerve pain. Plast Reconstr Surg 2000;105(6):1967–72. [DOI] [PubMed] [Google Scholar]
- 88. Melzack R, Wall PD. Pain mechanisms: A new theory. Science 1965;150(3699):971–9. [DOI] [PubMed] [Google Scholar]
- 89. Guo D, Hu J. Spinal presynaptic inhibition in pain control. Neuroscience 2014;283:95–106. [DOI] [PubMed] [Google Scholar]
- 90. Braz J, Solorzano C, Wang X, Basbaum AI. Transmitting pain and itch messages: A contemporary view of the spinal cord circuits that generate gate control. Neuron 2014;82(3):522–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mendell LM. Constructing and deconstructing the gate theory of pain. Pain 2014;155(2):210–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Buonomano DV, Merzenich MM. Cortical plasticity: From synapses to maps. Annu Rev Neurosci 1998;21(1):149–86. [DOI] [PubMed] [Google Scholar]
- 93. Hummel FC, Cohen LG. Drivers of brain plasticity. Curr Opin Neurol 2005;18(6):667–74. [DOI] [PubMed] [Google Scholar]
- 94. Jenkins WM, Merzenich MM, Ochs MT, Allard T, Guic-Robles E. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophys 1990;63(1):82–104. [DOI] [PubMed] [Google Scholar]
- 95. Moseley GL, Flor H. Targeting cortical representations in the treatment of chronic pain: A review. Neurorehabil Neural Repair 2012;26(6):646–52. [DOI] [PubMed] [Google Scholar]
- 96. Wu CW, van Gelderen P, Hanakawa T, Yaseen Z, Cohen LG. Enduring representational plasticity after somatosensory stimulation. Neuroimage 2005;27(4):872–84. [DOI] [PubMed] [Google Scholar]